Abstract
Human papillomavirus (HPV)-related oropharyngeal squamous cell cancer (OPSCC) has emerged as a distinct clinical entity of head and neck cancer with expected high survival. This recognition has led to the investigation of whether a population of patients can be identified who can safely undergo treatment de-escalation, in an effort to minimize long-term treatment toxicity while maintaining excellent survival. The purpose of this review is to describe the rationale for treatment deintensification for HPV-related OPSCC, summarize available results from published clinical trials, explore the methods by which risk groups are assigned, and provide context for the multitude of clinical trials that are currently underway.
Keywords: Human papillomavirus, Oropharyngeal squamous cell carcinoma, Treatment, Deintensification, De-escalation, Review
Introduction
In the last few decades, human papillomavirus (HPV) has emerged as the key etiologic factor driving the rising incidence of oropharyngeal squamous cell cancer (OPSCC) in North America, Europe, and areas of South America and Asia [1,2]. In contrast to traditional tobacco- and alcohol-associated head and neck squamous cell cancers (HNSCC), for which incidence in the United States (US) is declining, patients with HPV-related OPSCC have demonstrated greatly improved survival and significantly higher cure rates [3–8]. Indeed, HPV-related OPSCC and HPV-negative OPSCC are now recognized as distinct clinical and biologic entities that merit individualized study. However, by and large, treatment recommendations do not yet differ based upon tumor HPV status, leading to the hypothesis that treatment approaches developed for poorer-prognosis tobacco-associated OPSCC may not be suitable for HPV-related OPSCC.
Under current guidelines, standard-of-care treatment for patients with bulky or locally advanced OPSCC or adverse pathologic features dictates multimodality therapy, comprised of primary surgery and postoperative (chemo)radiotherapy or definitive concurrent cisplatin-based chemoradiotherapy (CRT). Multimodality treatment is known to cause significant toxicities, both acute and late [9–11]. This means that HPV-related OPSCC survivors – who may be diagnosed at a younger age, are healthier, and have longer life expectancies compared to tobacco-associated OPSCC [8,12] – will live with treatment toxicities for decades. This has motivated considerable efforts directed towards treatment de-escalation, with the hypothesis that deintensified treatment will result in less treatment-related harm and better quality of life while maintaining the excellent survival observed under traditional treatment practices. However, the key to successful treatment de-escalation lies in selecting appropriate patient populations, and therefore deintensification should be undertaken only within the context of clinical trials, as recently emphasized by the American Society of Clinical Oncology (ASCO) [13]. Here, we review concepts salient to therapeutic deintensification including determination of HPV status, its prognostic influence, risk stratification and the determination of populations best suited for deintensification, and the current state of deintensification studies.
HPV-positivity and diagnostic methods
The accurate determination of oncogenic tumor HPV status is necessary for any successful de-escalation protocol. Several diagnostic methods are available, including p16 immunohistochemistry (IHC), detection of viral DNA or mRNA using quantitative polymerase chain reaction (qPCR), HPV type-specific in-situ hybridization (ISH) using viral DNA or RNA, or detection of serum antibodies against HPV antigens [14]. The multitude of techniques for the diagnosis of HPV-driven cancer using formalin-fixed paraffin-embedded tumor sections and cytologic aspirates was recently reviewed by the College of American Pathologists and endorsed by ASCO, with resultant consensus recommendations identifying p16 IHC as the preferred diagnostic test for all OPSCC tumors or level II/III cervical lymph nodes with an unknown primary site, with additional confirmatory HPV testing conducted at the pathologist or clinician’s discretion [15,16].
P16 IHC has emerged as the preferred diagnostic surrogate for HPV-related OPSCC due to its low cost, high sensitivity and ease of use. P16 positivity is defined as ≥70% nuclear and cytoplasmic staining. P16 is a key cell cycle regulatory protein that accumulates in HPV-driven tumors as a result of high-risk HPV E7 protein expression causing dys-regulation of the tumor suppressor retinoblastoma protein pathway. However, p16 overexpression is not exclusive to HPV-related tumors and can be present in non-HPV-related tumors in 5–7% of cases; therefore, assay performance is influenced by prevalence of HPV-positive tumors and pre-test probability [17–19]. In a recent study that examined p16 as a surrogate marker for ISH positivity among OPSCC sub-sites (namely tonsils and base of tongue), p16 IHC was found to have sensitivity 100%, specificity 91%, positive predictive value (PPV) 93%, and negative predictive value (NPV) 100% [20]. In contrast, among non-oropharynx sub-sites both sensitivity (83%) and PPV (40%) were reduced (though specificity and NPV remained high). Among OPSCC tumor samples that were p16-positive but HPV16 ISH negative, approximately a third were positive for a different oncogenic HPV type using RNA ISH probes, consistent with other reports [21].
HPV, survival, and defining risk
The effect of HPV on survival has been analyzed within the context of several multi-institutional phase II and III clinical trials. These include Eastern Cooperative Oncology Group (ECOG) 2399 [3], Radiation Therapy Oncology Group (RTOG) 0129 [4], RTOG 0522 [8], Trans-Tasman Radiation Oncology Group (TROG) 02.02 [6], TAX324 [5] and DAHANCA 6&7 [7]. These were large prospective studies testing varied CRT protocols for patients with locoregionally advanced HNSCC, with either prospective or retrospective subset analyses studying the association of HPV status with survival (Table 1). Each of these studies found HPV-positive tumor status to be significantly associated with improved survival.
Table 1.
Summary of prospective clinical trials that have demonstrated survival benefit for HPV-related OPSCC.
| Trial | Design | Result |
|---|---|---|
| ECOG 2399 | Phase II trial of locoregionally advanced HNC treated with two cycles of IC (paclitaxel + carboplatin) followed by concomitant weekly paclitaxel and standard fractionation RT | HPV-related OPSCC had better OS (p = 0.004) and PFS (p = 0.05) |
| RTOG 0129 | Phase III RCT of locoregionally advanced OPSCC comparing standard-fractionation RT vs. accelerated-fractionation RT, each concurrent with cisplatin | HPV-related OPSCC had better 3-year OS (82.4% vs. 57.1%, p < 0.001) |
| RTOG 0522 | Phase III RCT of locoregionally advanced HNC treated with concurrent RT with cisplatin, with or without cetuximab | HPV-related OPSCC had better 3-year PFS (72.8% vs. 49.2%, p < 0.001) and 3-year OS (85.6% vs. 60.1%, p < 0.001) |
| TROG 02.02 | Phase III trial of locoregionally advanced HNC RCT with concurrent RT with cisplatin, with or without tirapazamine | HPV-related OPSCC had better 2-year PFS (87% vs. 72%, p = 0.003) and OS (91% vs. 74%, p = 0.004) |
| TAX324 | Phase III RCT of locoregionally advanced HNC testing IC with either TPF or PF | HPV-related OPSCC had better 5-year OS (82% vs. 35%, p < 0.0001) |
| DAHANCA 6&7 | Phase III RCT of locoregionally advanced HNC treated with accelerated vs. standard RT | HPV-related HNC had better 5-year OS (62% vs. 47%); did not perform analysis of OP-only |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; RTOG = Radiation Therapy Oncology Group; TROG = Trans-Tasman Radiation Oncology Group; HNC = head and neck cancer; IC = induction chemotherapy; RT = radiation therapy; CRT = chemoradiation therapy; RCT = randomized clinical trial; OS = overall survival; PFS = progression-free survival; TPF = docetaxel, cisplatin, 5-fluorouracil.
Of note, while RTOG0129 was a negative study, recursive partitioning analysis (RPA) identified a low-risk group who might benefit from less therapy. Concurrent with this was the recognition that the American Joint Committee on Cancer (AJCC) 7th edition staging system may not be apropos to HPV-related OPSCC, given that most patients had advanced overall stage 7th edition at diagnosis and para-doxically high survival. This led to an effort to refine staging in order to accurately reflect prognosis for this population.
The risk groups established by Ang et al. in RTOG 0129 were defined using HPV status, tobacco smoking pack-years, tumor stage, and nodal stage [4]. Patients with HPV-related tumors fell into the low-risk group (93% 3-year OS) if they had ≤10 pack-years or, in the case of > 10 pack-years, if they had AJCC 5th ed. N0-N2a disease. However, HPV-positive patients with > 10 pack-years and N2b-N3 disease were at “intermediate” risk of death (70.8% 3-year OS) – higher than the low-risk group, though still lower than most patients with HPV-negative disease (high-risk; 46.2% 3-year OS). This has been validated using another prospective cohort treated uniformly with (chemo)radiotherapy in the US [22] and a heterogeneously treated cohort in Italy [23], however variable failure rates for the low-risk group have been found.
O’Sullivan et al. used RPA to retrospectively analyze 505 patients with p16-positive OPSCC treated with primary RT or CRT at a single institution between 2001 and 2009 [24]. Among HPV-positive patients, the low-risk group was comprised of patients with 6th edition T1-T3N0-N2c disease (93% 3-year distant control [DC]), whereas patients with T4N0-N2c or T(any)N3 disease were in the high-risk group (76% 3-year DC). Of note, patients with N2c disease treated with RT alone had poorer DC than those who received CRT (3-year DC 73% vs. 92%, respectively).
Huang et al. examined OPSCC survival in the context of tumor HPV status using RPA and multivariable models to identify patients at high-risk of metastasis and subsequently propose refined TNM groupings [25]. Thereafter, Horne et al. validated the RPA-generated TNM staging using National Cancer Database data [26], and criteria were refined further with multivariable analysis of a multi-institutional dataset compiled by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S) group [27]. These efforts and subsequent validation studies led to the adoption of the ICON-S criteria for the 8th edition AJCC Cancer Staging Manual for HPV-related OPSCC [28,29]. While the updated guidelines addressed the conundrum of discrepantly good survival of HPV-related patients with typically later AJCC 7th stage disease, there is concern regarding further risk stratification among HPV-related patients.
The updated guidelines resulted in down-staging a majority of patients compared to previous guidelines. Some validation studies demonstrated improved ability to differentiate survival between stages for HPV-related OPSCC, though of note, many studies observed over-lapping survival curves for stages I and II [30–36]. Importantly, the new staging system was not intended to make differential treatment decisions; for example, despite potential reclassification from 7th edition stage IVa to 8th edition stage I, treatment should be guided by 7th edition staging until alternative treatments based on 8th edition guidelines have been prospectively validated.
Additionally of potential concern, 8th edition staging guidelines have divergent criteria for clinical and pathologic nodal staging, with clinical staging determined by node size and laterality but pathologic staging reliant solely on the number of involved nodes; different survival curves have been observed between clinical and pathologic nodal stages [37,38].
Therefore, despite the substantial improvement in prognostication afforded by the updated staging guidelines, AJCC 8th edition does not provide sufficient information as to which patients may benefit from deintensified treatment approaches.
Investigators have sought to further refine risk populations and identify prognostic factors that can be used in conjunction with tumor HPV status to predict outcomes and identify candidate deintensification sub-groups. Several studies have identified tobacco history as a prognostic factor among patients with HPV-related OPSCC. HPV-related OPSCC was originally characterized as a disease of non-smokers, but it has become increasingly recognized that smoking – though indeed statistically less frequent among HPV-related OPSCC patients as compared to HPV-negative OPSCC patients – is still common and confers worse survival [24,39]. Prospective data from radiation platform studies (RTOG 9003 and RTOG 0129) showed that each year of smoking increased risk of death by 2% among p16-positive patients [12]. Other retrospective studies have shown worse survival among current and former smokers with HPV-related OPSCC, although some controversy exists whether the ideal cutpoint should be 10 or 20 pack-years [25,40,41].
Alternative methods of risk stratification have also been explored using imaging. One ongoing prospective observational study (NCT03342378) is assessing the use of mid-treatment PET-MRI to identify a low-risk subset among HPV-related OPSCC patients undergoing definitive CRT. Other studies have retrospectively examined whether radiographic characteristics can inform risk stratification, such as the use of quantitative tumor imaging techniques to predict treatment response [42], or the identification of retropharyngeal lymph nodes [43] or radiographic extranodal extension (ENE) [44].
Therefore, several risk stratification methods have been proposed, yet there is no consensus on which patients are truly at low-risk for failure. It is imperative to continue efforts to identify patient populations appropriate for testing de-escalation approaches within the context of clinical trials with appropriate safeguards and monitoring at higher-volume centers.
Completed de-escalation trials
Currently, two phase III and several phase II trials have been reported that assessed reduced radiation therapy (RT) doses or fields and/or reduced or alternative chemotherapy regimens for patients with HPV-related OPSCC (Fig. 1a). Five trials stratified patients into risk groups based on response to induction chemotherapy (IC), two trials tested de-escalation of adjuvant therapy after surgery, and the remainder enrolled patients defined as low-risk based on clinical enrollment criteria.
Fig. 1.
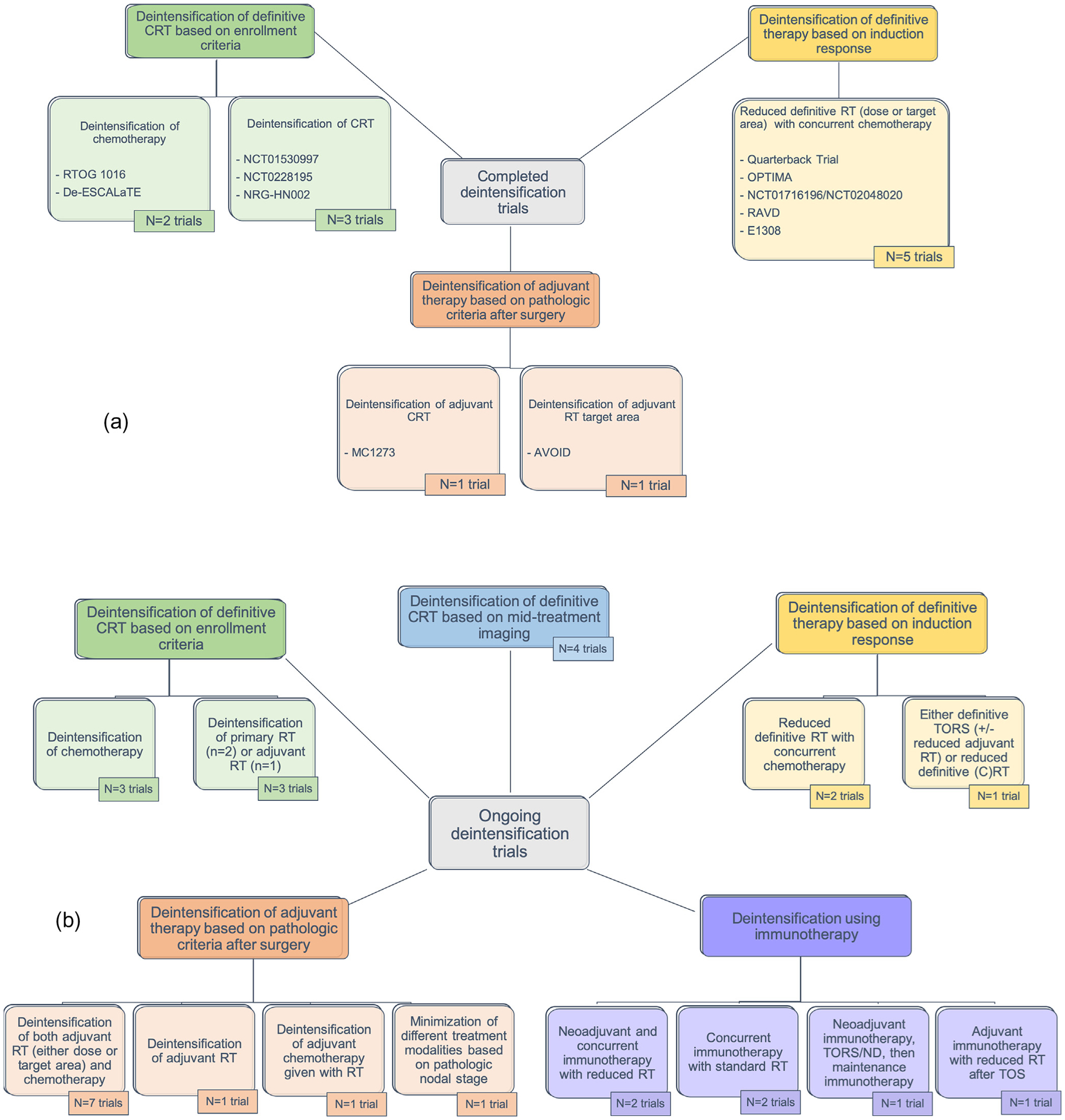
Overview of (a) published and (b) ongoing treatment deintensification clinical trials for HPV-associated OPSCC, Abbreviations: RT = radiation therapy; CRT = chemoradiotherapy; TORS = transoral robotic surgery; ND = neck dissection.
De-escalation of definitive CRT after induction chemotherapy
Several trials have evaluated the use of reduced-dose RT and varied chemotherapy in populations stratified based on clinical response to IC, including E1308, the Quarterback trial, OPTIMA, NCT02048020/NCT01716195 (dually registered), and the RAVD trial, each of which demonstrated rates of progression-free survival (PFS) and/or overall survival (OS) at 2- or 3-year timepoints that are comparable to previously published rates after standard therapy (Fig. 2a).
Fig. 2.
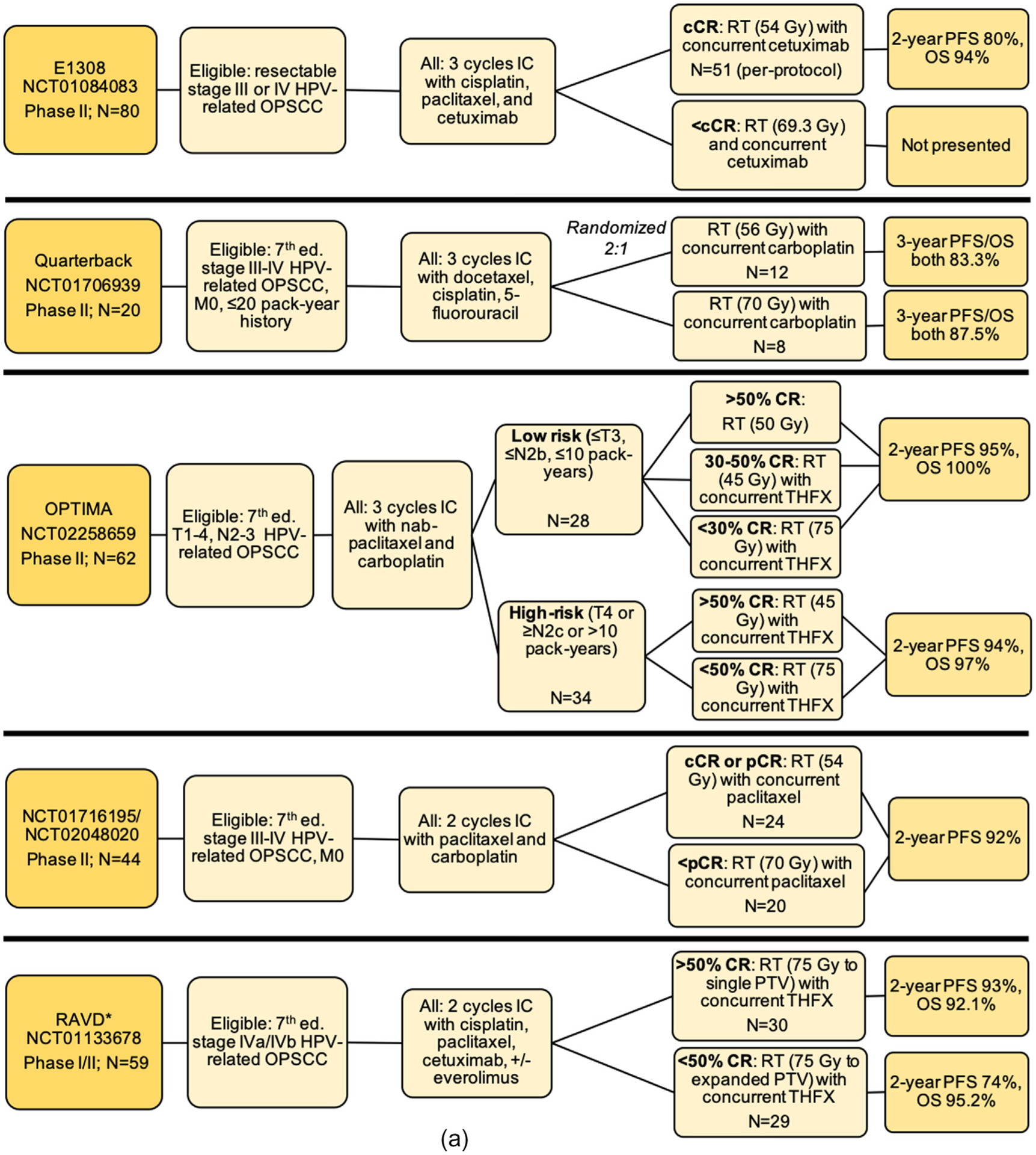
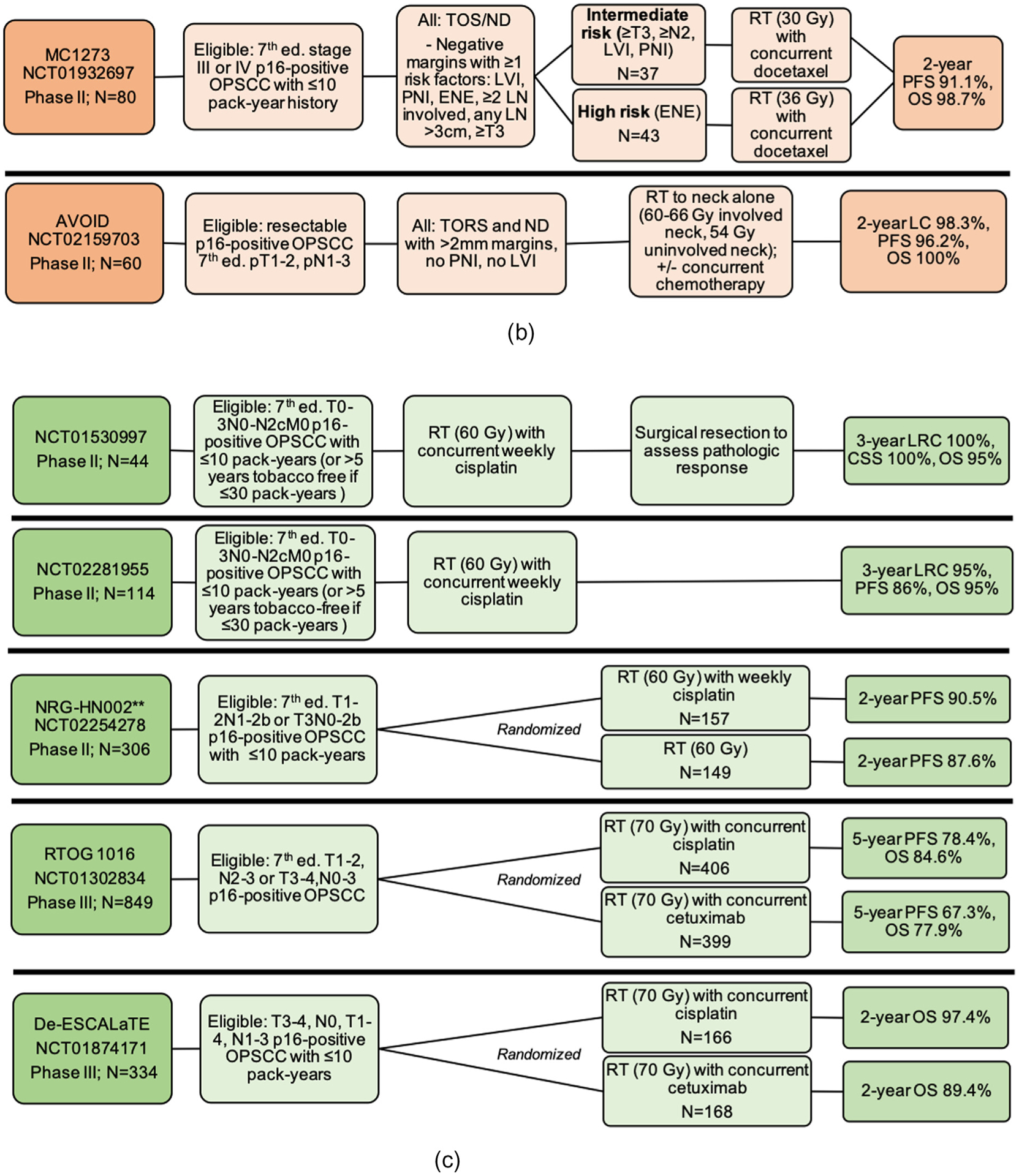
Schemas for completed treatment deintensification trials for HPV-associated OPSCC including (a) trials testing de-escalation based on induction response; (b) trials testing de-escalation of adjuvant therapy after surgery; (c) trials testing primary chemoradiation de-escalation, Notes: *RAVD enrolled stage IVa/IVb HNSCC inclusive of all primary sites and without regard to HPV status. However, a subset analysis of HPV-OPSCC patients was performed. Of 94 total enrolled patients, 71 patients had an oropharynx primary site, out of whom 59 patients had positive HPV-status. The data presented here are specific to the HPV-associated OPSCC patients only and is not inclusive of the larger study population. **NRG-HN002: results have been presented and published as an abstract, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; IC = induction chemotherapy; RT = radiation therapy; CRT = chemoradiotherapy; CR = clinical response; cCR = complete clinical response; pCR = partial clinical response; OS = overall survival; PFS = progression free survival; LRC = locoregional control; LC = local control; CSS = cause-specific survival; LVI = lymphovascular invasion; PNI = perineural invasion; ENE = extranodal extension; LN = lymph node; TOS = transoral surgery; TORS = transoral robotic surgery; ND = neck dissection; THFX: chemotherapy regimen consisting of paclitaxel, 5-fluorouracil, and hydroxyurea; PTV = primary target volume.
E1308 (NCT01084083) was a single-arm phase II trial evaluating reduced-dose RT (54 Gy) with weekly cetuximab 250 mg/m2 for patients who achieved complete clinical response (cCR) after IC [45]. The IC regimen consisted of three 21-day cycles of cisplatin 75 mg/m2 on day 1, paclitaxel 90 mg/m2 on days 1, 8, and 15, and cetuximab 400 mg/m2 on day 1 of cycle 1, followed by weekly cetuximab 250 mg/m2. Eligible patients had resectable HPV-positive AJCC 7th edition stage III or IV OPSCC. HPV status was determined using p16 IHC and/or HPV16 ISH. Of 80 evaluable patients, 56 (70%) demonstrated cCR after IC and 51 then continued to reduced-dose RT with weekly cetuximab. Two-year PFS and OS for this group were 80% and 94%, respectively. In a post-hoc analysis, PFS and OS improved to 96% and 96%, respectively, when analysis was restricted to patients (n = 27) with ≤10 pack-year smoking history, < T4, and < N2c disease, reinforcing the need to identify which patients are at low-risk for recurrence and conversely suitable for de-escalation. Patient-reported outcomes demonstrated that at 12 months, patients in the reduced-radiation group had significantly fewer swallowing and nutrition problems.
The phase II Quarterback trial (NCT01706939) also tested outcomes of reduced-dose RT (54 Gy) after IC [46]. Eligibility criteria included AJCC 7th edition stage III and IV HPV-related OPSCC with ≤20 pack-years and no distant metastasis (DM). HPV status was determined by p16 IHC and type-specific ISH. IC consisted of three 21-day cycles of docetaxel 75 mg/m2 and cisplatin mg/m2 on day 1 and fluorouracil 750 mg/m2 for 4 days by continuous infusion. Patients with partial clinical response (pCR) or cCR to IC were randomized 2:1 to reduced-dose (56 Gy) or standard-dose (70 Gy) RT, both with weekly carboplatin. 20 patients were randomized, with 12 assigned to reduced-dose RT. At 3 years, there was statistically equivalent survival, with PFS/OS stable at 87.5% and 83.3% for the standard-dose and reduced-dose groups, respectively. No functional outcomes are reported.
OPTIMA (NCT02258659) was a phase II trial that categorized HPV-related OPSCC patients into risk groups at enrollment based on tumor staging and tobacco history, and then further stratified treatment based on response to IC [47]. Tumor HPV status was determined with p16 IHC and confirmatory E6/E7 DNA PCR testing. IC consisted of three 21-day cycles of carboplatin (area under the curve (AUC) = 6 on day 1) with nab-paclitaxel 100 mg/m2 on days 1, 8, and 15. Low-risk patients (≤T3, ≤N2b, ≤10 pack-years; n = 28) were stratified to receive 45, 50, or 75 Gy with or without concurrent chemotherapy, whereas high-risk patients (T4 or ≥ N2c or > 10 pack-years; n = 34) received either 45 or 75 Gy with concurrent chemotherapy. Concurrent chemotherapy consisted of three 14-day cycles of paclitaxel 100 mg/m2 on day 1, 5-fluorouracil given via continuous infusion at 600 mg/m2/day on days 0–5, and hydroxyurea 500 mg orally twice daily on days 0–5 with 11 doses per cycle. 2-year PFS and OS were 95% and 100% among the low-risk patients, respectively, and 94% and 97% for the high-risk patients. Rates of acute toxicity (dermatitis, mucositis) and gastrostomy tube use significantly decreased with lower doses of radiation.
NCT02048020/NCT01716195 was a phase II trial testing reduced RT after IC consisting of two 21-day cycles of paclitaxel 175 mg/m2 and carboplatin (AUC = 6) [48]. HPV status was determined by p16 IHC. Patients with cCR or pCR were categorized as low-risk (N = 24) and went on to receive reduced RT (54 Gy), concurrently with weekly paclitaxel 30 m/m2, whereas patients with less than pCR (N = 20) underwent higher (but still reduced) RT (60 Gy), also with weekly paclitaxel. Overall, 2-year PFS was 92% for both groups. Rates of gastrostomy tube dependence after therapy completion were 2% at 3-months and 0% at 6 months.
The Response-Adjusted Volume De-escalation (RAVD) trial (NCT01133678) was a phase I/II clinical trial testing reduced RT target volume areas among 7th edition stage IVa/IVb HNSCC patients after IC [49]. A subset analysis was performed for the 59 patients with HPV-related OPSCC (out of 94 total patients). IC regimens varied, but generally consisted of two 21-day cycles of cisplatin, paclitaxel, and cetuximab, with some patients also receiving everolimus. Patients with > 50% CR to IC were considered “good responders” (GR) and received concurrent CRT (75 Gy) with reduced planning target volume (targeting gross disease only), whereas patients with < 50% CR underwent concurrent CRT (75 Gy) with a planning target volume including elective nodal basins. For the GR group, 2-year PFS and OS were 93.1% and 92.1%, respectively, compared to 2-year PFS and OS of 74% and 95.2%, respectively, for the non-responder group. Rates of acute toxicities were similar between groups. However, rates of gastrostomy tube use and dependence during follow-up were lower among the reduced target volume group.
De-escalation of adjuvant therapy after surgery
Two trials tested de-escalation of adjuvant therapy after surgery (Fig. 2b). MC1273 (NCT01932697) was a phase II trial investigating markedly reduced adjuvant RT doses (30–36 Gy) based on pathologic risk factors after curative-intent surgery with neck dissection [50]. Eligible patients had HPV-related OPSCC with ≤10 pack-year smoking history who had negative margins but at least one pathologic risk factor (such as ENE, lymphovascular invasion [LVI], perineural invasion [PNI], or advanced 7th edition T and N criteria). Tumor HPV status was determined using p16 IHC. Patients who were ≥T3, ≥N2, or had LVI or PNI (N = 37) were considered the lower risk group and were pre-scribed RT (30 Gy over 12 days) with concurrent docetaxel 15 mg/m2 on days 1 and 8. Patients with ENE were considered high-risk (N = 43) and received 36 Gy RT (over 12 days) with concurrent docetaxel. Overall 2-year PFS and OS for both groups combined were 91.1% and 98.7%, respectively, with low reported toxicities and very low gastrostomy tube use. While this was a small phase II study potentially prone to bias, it was noted that fewer low-risk patients developed DM compared to the high-risk group (defined by ENE).
AVOID (NCT02159703) was a phase II study that tested the delivery of adjuvant RT to the neck only (avoiding the primary site) for 60 patients with 7th edition pT1–2 N1–3 HPV-associated OPSCC who were treated with transoral robotic surgery (TORS) and neck dissection [51]. Patients with PNI, LVI, or close (< 2mm) or positive margins were not eligible. Adjuvant RT was delivered as 60–66 Gy to the involved neck and 54 Gy to the uninvolved neck, with avoidance of the resected primary site. Patients with ENE (n = 13, 21.7%) also received concurrent chemotherapy. With a median follow-up of 2.4 years, 2-year local control was 98.3%; OS 100%; and PFS 92.1%. There were 1 local, 1 regional, and 2 distant recurrences. The median measured radiation dose to the primary site was 36.9 Gy. Reported treatment toxicities were low, with no patients requiring a feeding tube during RT or long-term (though two required temporary feeding tubes related to additional procedures).
De-escalation of definitive chemoradiotherapy
Several trials have tested de-escalation of primary CRT (Fig. 2c). NCT01530997 was a phase II trial that tested the de-escalation of definitive RT (60 Gy) and concurrent reduced-dose cisplatin (30 mg/m2 weekly) among patients with 7th edition T0–3N0-N2c HPV-related OPSCC with ≤10 pack-years smoking history (or > 5 years tobacco free if ≤30 pack-years) [52]. HPV status was established using p16 IHC and HPV ISH. After treatment completion, patients (n = 43) underwent at least neck dissection with primary site biopsy: 86% showed complete pathologic response. On long-term follow-up, 3-year locoregional control (LRC), cause-specific survival, and DC were all 100%, with 3-year OS 95% [53]. Gastrostomy tubes were used in 39%, with a median duration of 15 weeks and none permanent.
NCT02281955 was a phase II follow-up to this study, with the same enrollment criteria and treatment but with post-treatment PET-CT scan in place of neck dissection and biopsy [54]. With 114 enrolled patients, 2-year LRC was 95%, DM-free survival (DMFS) 91%, PFS 86%, and OS 95%. Functional results were similar to the pilot study, with 34% of patients requiring a gastrostomy tube for a median of 10 weeks with none permanent.
NRG-HN002 (NCT02254278) is a phase II trial testing reduced-dose RT (60 Gy) with or without concurrent cisplatin among 306 randomized patients with 7th edition stage T1–2N1-N2bM0 or T3N0-N2bM0 OPSCC with ≤10 pack-year smoking history [55]. Patients were stratified by need for unilateral or bilateral radiation, and then randomized to either concurrent CRT (60 Gy) over 6 weeks with cisplatin 40 mg/m2 weekly (N = 157) or to 60 Gy RT alone delivered over 5 weeks. For the cisplatin-RT arm, 2-year PFS was 90.5%, which met acceptability criteria per pre-defined study parameters, compared to 87.6% for the RT-alone arm, which did not meet acceptability criteria. Estimated 2-year OS were similar between groups (96.7% for the cisplatin-RT group compared to 97.3% for the RT-alone group).
Two recently concluded phase III randomized trials tested the substitution of cetuximab in place of cisplatin as a de-escalation strategy [56,57]. Cetuximab, an epidermal growth factor receptor inhibitor, was posed as an attractive alternative to cisplatin due to its hypothesized lower toxicity profile. However, the results of RTOG 1016 and De-ES-CALaTE HPV demonstrated that treatment with cetuximab resulted in inferior OS and PFS, with both higher locoregional and distant failure rates compared to cisplatin [56,57]. Additionally, though specific toxicity profiles differed between cetuximab and cisplatin, the overall burden of moderate, severe, and late toxicities proved largely similar between groups.
RTOG 1016 was a phase III randomized non-inferiority trial in the US in which 849 patients with locoregionally advanced HPV-related OPSCC were randomized 1:1 to receive standard-dose RT (70 Gy) given concurrently with either cisplatin (100 mg/m2 on days 1 and 22) or cetuximab (400 mg/m2 loading dose 5–7 days prior to RT, followed by 250 mg/m2 weekly for 7 doses) [56]. Eligible patients had AJCC 7th edition T1–2N2a-N3M0 or T3–4N0-N3M0 p16-positive OPSCC. At a median follow-up of 4.5 years, cetuximab treatment resulted in inferior OS and PFS, with estimated 5-year OS of 77.9% vs. 84.6% for the cetuximab vs. cisplatin groups, respectively, and 5-year PFS of 67.3% vs. 78.4%, respectively. Of note, in a post-hoc analysis, for patients with a Zubrod performance score of 0 (74% of the study population), OS estimates were similar at 5-years for the cisplatin and cetuximab treatment groups (HR 1.08, one-sided 95% upper CI 1.55), whereas among patients with a Zubrod performance score of 1, those who received cetuximab had worse survival compared to cisplatin (HR 2.66, one-sided 95% upper CI 4.32). However, this effect was statistically not significant after adjustment for multiple comparisons, without report of the effect on direction and magnitude of the hazard ratio.
Similarly, De-ESCALaTE HPV was a phase III randomized controlled trial conducted in western Europe that randomized 334 patients to definitive standard-dose RT (70 Gy) given concurrently with either cisplatin (100 mg/m2 on days 1, 22, and 43) or cetuximab (400 mg/m2 loading dose followed by 7 weekly 250 mg/m2 doses) [57]. Eligibility criteria were defined using the characteristics of the low-risk group established by Ang et al.:[4] AJCC 7th edition T3N0-T4N0, or T1N1-T4N3 HPV-related OPSCC with ≤10 pack-years. HPV status was determined using p16 IHC and type-specific ISH. In accordance with the RTOG 1016 findings, patients treated with cetuximab had inferior 2-year OS compared to the cisplatin group (89.4% vs. 97.5%, respectively) and higher 2-year recurrence risk (16.1% vs. 6%, respectively).
The results of RTOG 1016, De-ESCALaTE, and NRG-HN002 support the therapeutic role of cisplatin in HPV-related OPSCC and inferiority of either cetuximab or RT alone, even in defined low-risk populations.
Ongoing de-escalation trials
To better understand the spectrum of treatment de-escalation efforts, we queried the National Institute of Health Clinical Trials database (https://clinicaltrials.gov/) using the search terms “oropharyngeal cancer,” “HPV-positive oropharyngeal squamous cell carcinoma,” and related synonyms to identify relevant studies as of November 2019. Each entry was reviewed to select ongoing clinical trials testing de-escalation protocols for newly diagnosed HPV-related OPSCC.
This search revealed a multitude of ongoing clinical trials that vary both in how risk groups are assigned and in the ways by which therapy is de-escalated (Fig. 1b). Methods of treatment de-escalation include reductions in primary or adjuvant RT dosing or target area; reduction, substitution, or omission of chemotherapy; or combinations thereof.
In the following sections, trials are categorized by the strategies with which risk is assigned using information gleaned from ClinicalTrials.gov. These include: use of clinical enrollment criteria; use of pathologic criteria after surgery; use of pre- or mid-treatment imaging characteristics; and use of response to IC. Trials studying the use of immunotherapy are also included.
De-escalation based on clinical enrollment criteria
Several trials are testing deintensification of definitive CRT based on clinical eligibility criteria (Fig. 3). Of note, two trials from this group, TROG12.01 and NCT01663259, which are not currently recruiting but outcomes are pending, were designed to examine the substitution of cetuximab for cisplatin given concurrently with RT - similar to the already reported RTOG1016 and De-ESCALaTE trials.
Fig. 3.
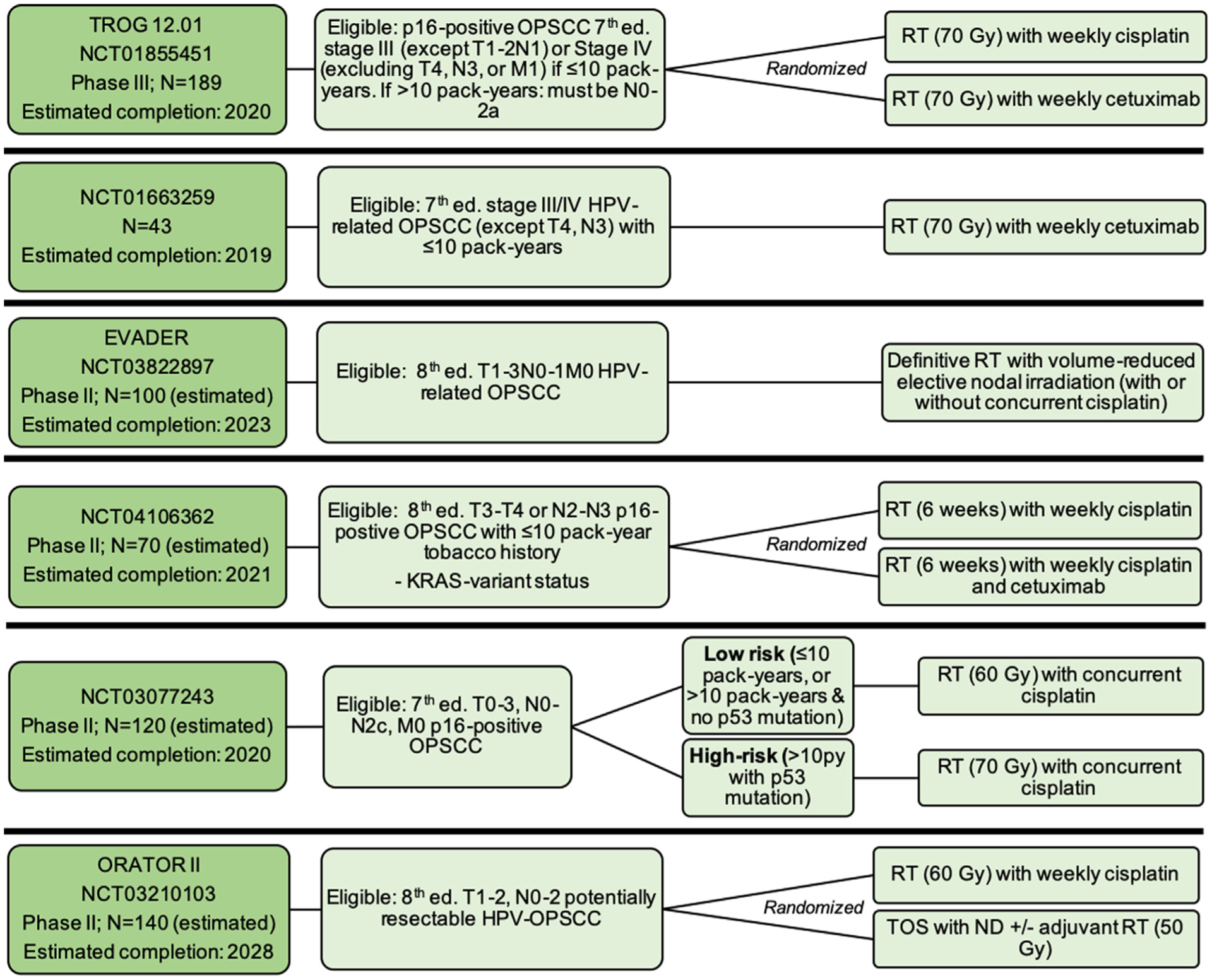
Ongoing chemoradiation de-escalation trials based on clinical eligibility criteria, Notes: For Figs. 3–7, the listed “estimated completion” dates are the estimated primary completion date listed on clinicaltrials.gov. Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; RT = radiation therapy; LVI = lymphovascular invasion; extension; LN = lymph node; TOS = transoral surgery; ND = neck dissection.
EVADER (NCT03822897) is a phase II study enrolling patients with AJCC 8th edition T1–3N0–1 HPV-related OPSCC and testing whether RT to some lymph node areas can be safely omitted from definitive CRT. Two trials are testing de-escalation based on molecular criteria. NCT04106362 will enroll patients based on KRAS-variant status, which is theorized to confer greater response to cetuximab treatment [58]. Patients will be randomized to 6 weeks of concurrent RT and cisplatin, either with or without cetuximab. NCT03077243 will assign treatment based on tumor p53 mutation status in conjunction with smoking history, hypothesizing that patients with wild-type p53 may respond favorably to deintensified treatment (even with > 10 pack-years tobacco smoking history), compared to patients with > 10 pack-year smoking history and p53 mutations (who will receive standard-dose CRT).
ORATOR-II (NCT03210103) will directly compare primary de-intensified CRT to primary transoral surgery (TOS). Patients with potentially resectable 8th edition T1–2 N0–2 HPV-related OPSCC will be stratified by smoking history and then randomized to either primary deintensified RT (60 Gy) (with or without concurrent cisplatin) or primary TOS, with or without de-intensified adjuvant RT (50–60 Gy) [59].
De-escalation of adjuvant therapy
Several trials are testing adjuvant treatment de-escalation based on pathologic criteria after surgery (Fig. 4). These protocols generally call for TOS (sometimes minimally invasive) with at least ipsilateral neck dissection (ND), followed by a range of de-escalated adjuvant therapies. Several studies restrict eligibility based on tobacco history.
Fig. 4.
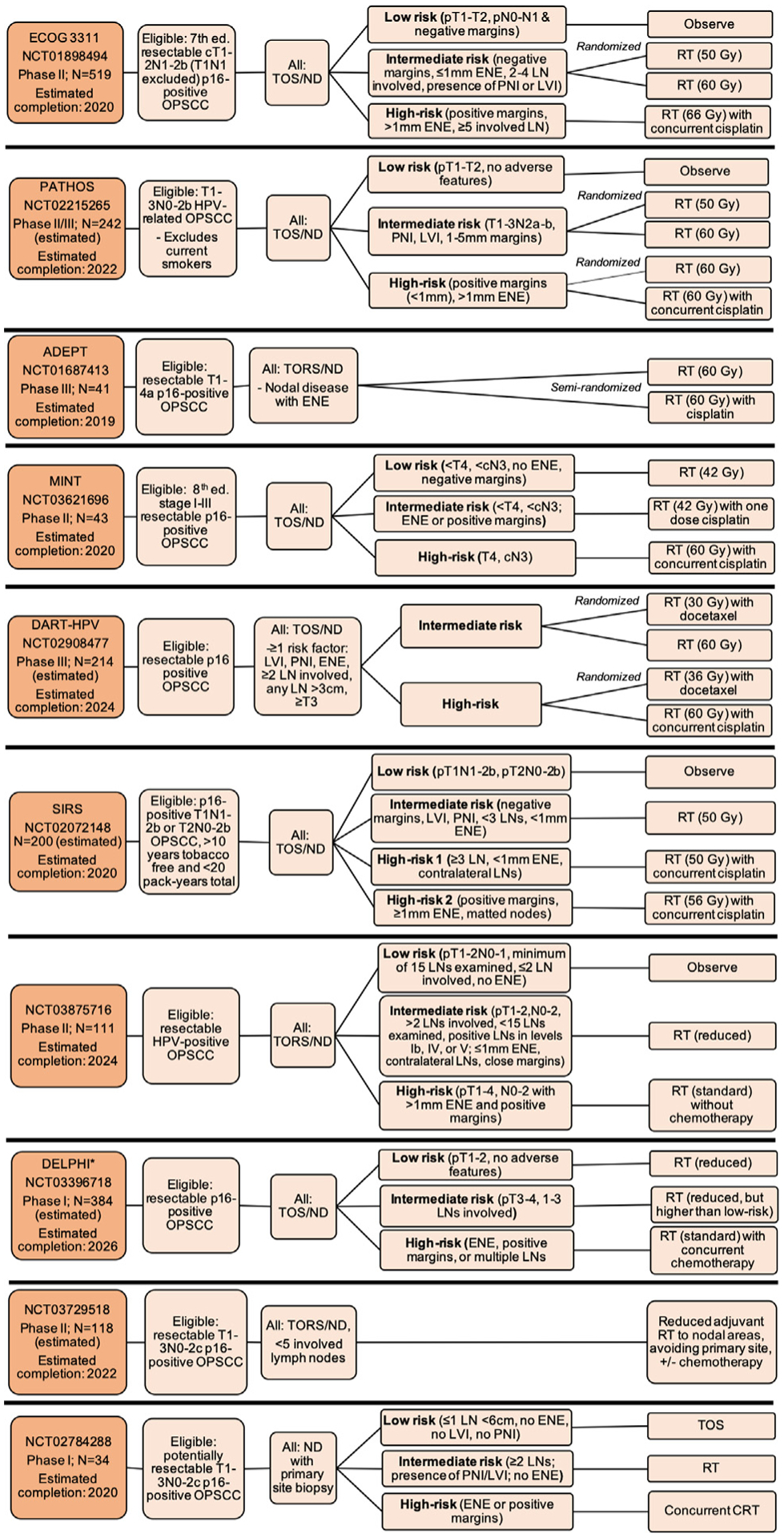
Ongoing trials testing de-escalation of adjuvant therapy after surgery, Notes: *DELPHI study also includes HPV-negative OPSCC patients in the high-risk treatment group, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; RT = radiation therapy; CRT = chemoradiotherapy; LVI = lymphovascular invasion; PNI = perineural invasion; ENE = extranodal extension; LN = lymph node; TOS = transoral surgery; TORS = transoral robotic surgery; ND = neck dissection; THFX: chemotherapy regimen consisting of paclitaxel, 5-fluorouracil, and hydroxyurea; PTV = primary target volume.
E3311 (NCT01898494) is a phase II multicenter trial with 519 enrolled patients with locoregionally advanced p16-positive OPSCC eligible for TORS with ND. After surgery, patients deemed to be low-risk (pT1–2N0-N1 with negative margins) were observed; patients in the intermediate-risk group (clear margins, ≤1mm ENE, 2–4 involved lymph nodes, or presence of PNI or LVI) were randomized to post-operative RT, either 50 or 60 Gy; and patients in the high-risk group (positive margins, > 1mm ENE, or ≥5 involved lymph nodes) received 66 Gy and concurrent cisplatin. Read-out is pending.
PATHOS (NCT02215265) is a phase III, currently accruing trial in the United Kingdom with similar structure to E3311, though with variations in eligibility, risk assignment, and treatment. Current smokers are ineligible. The low- and intermediate-risk groups’ assigned treatment mirrors that of E3311; however, the high-risk group is randomized 1:1 to RT (60 Gy) alone or concurrently with cisplatin.
Other trials include: ADEPT (NCT01687413), a recently terminated trial that assigned 41 patients with nodal involvement and ENE to 60 Gy RT alone or with concurrent weekly cisplatin; the Minimalist trial (MINT; NCT03621696), which stratified 43 patients with p16-positive OPSCC into low-, intermediate-, and high-risk groups for adjuvant treatment with reduced (42 Gy) or standard (60 Gy) RT, given with or without cisplatin; DART-HPV (NCT02908477), a follow-up phase III randomized clinical trial to MC1273 that will examine significantly reduced adjuvant RT (30 or 36 Gy) with concurrent docetaxel compared to standard adjuvant therapy; SIRS (NCT02072148), a phase II study testing observation vs. combinations of de-escalated adjuvant RT given with or without cisplatin based on low-, intermediate-, or high-risk assignment; NCT03875716, a phase II study testing observation vs. reduced or standard RT delivered without concurrent cisplatin; the DELPHI study (NCT03396718), testing reduced doses of adjuvant RT for low-risk patients; NCT03729518, a phase II study testing reduced adjuvant RT dose and target areas; and NCT02784288, testing whether up-front ND for patients with potentially resectable disease can be used to establish pathologic nodal burden and thereafter minimize the number of treatment modalities required.
De-escalation based on imaging
Four studies are testing de-escalation based on pre- or mid-treatment imaging characteristics (Fig. 5). NCT03323463 is a phase II study exploring the use of pre- and mid-treatment 18F-FMISO (fluoromisonidazole) PET imaging to stratify patients based on presence of tumor hypoxia, after data from a pilot study of 33 patients proved promising, with 100% 2-year OS and 97% DMFS [60]. Patients without tumor hypoxia are theorized to be more responsive to treatment [61] and will receive significantly reduced RT with concurrent chemotherapy.
Fig. 5.
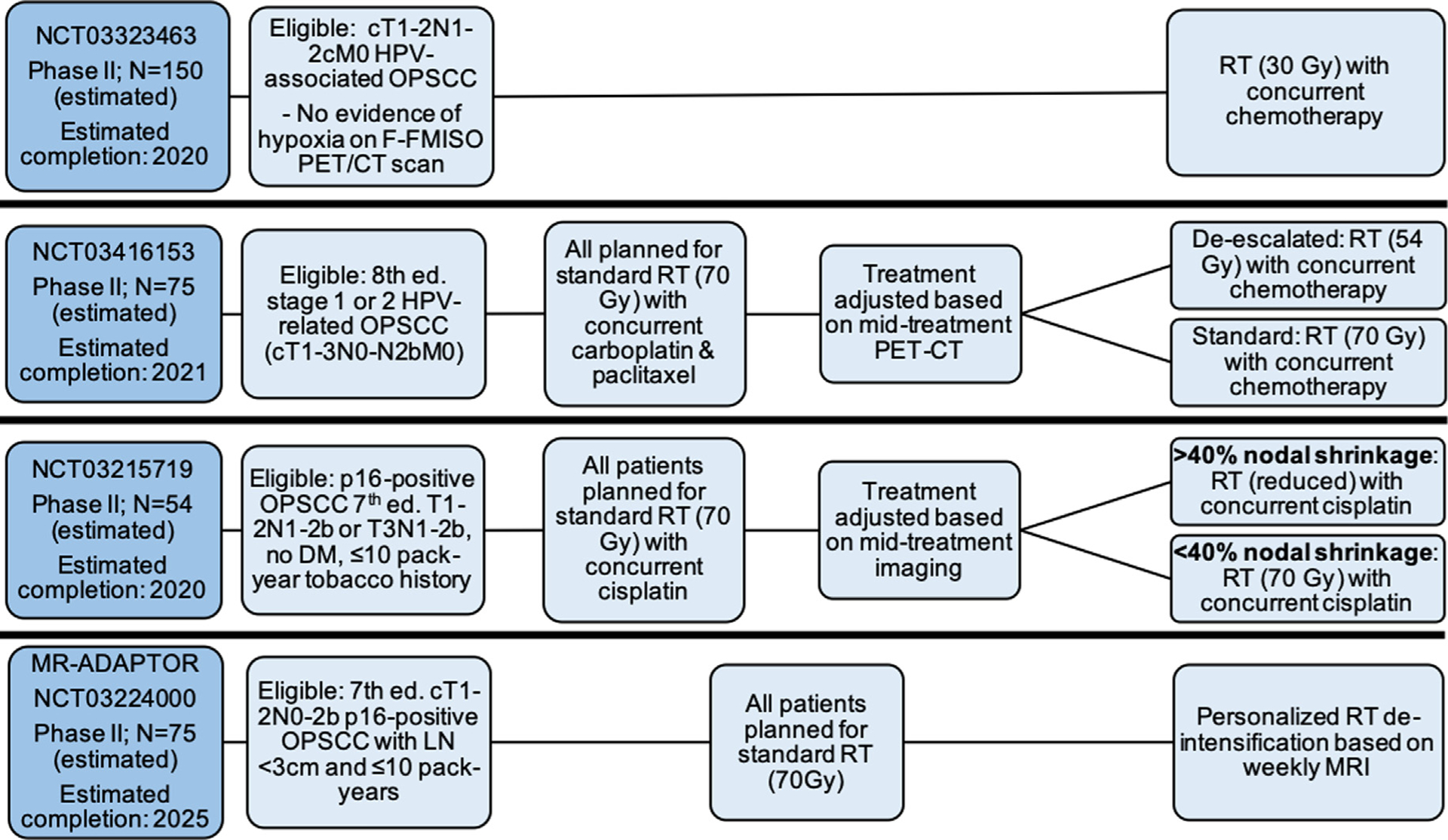
Ongoing trials of de-escalation based on imaging, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; F-FMISO = fluoromisonidazole; PET/CT = positron emission tomography/computed tomography; MRI = magnetic resonance imaging; LN = lymph nodes; DM = distant metastasis; RT = radiation therapy.
Two phase II studies, NCT03416153 and NCT03215719, test the use of mid-treatment computed tomography to de-escalate the remainder of planned CRT based on pre-determined clinical response criteria. A final study, MR-ADAPTOR (NCT03224000) is testing the use of individualized RT dose-adjustment based on weekly MRI assessment during treatment [62].
De-escalation after induction chemotherapy
Three trials are investigating treatment de-escalation after IC (Fig. 6). OPTIMA-II (NCT03107182) is a follow-up to OPTIMA that will deliver 3 cycles of IC with nab-paclitaxel and carboplatin with the addition of nivolumab, and subsequently stratifies patients based on induction response to either definitive surgical resection or definitive deintensified or standard RT, with or without concurrent chemotherapy.
Fig. 6.
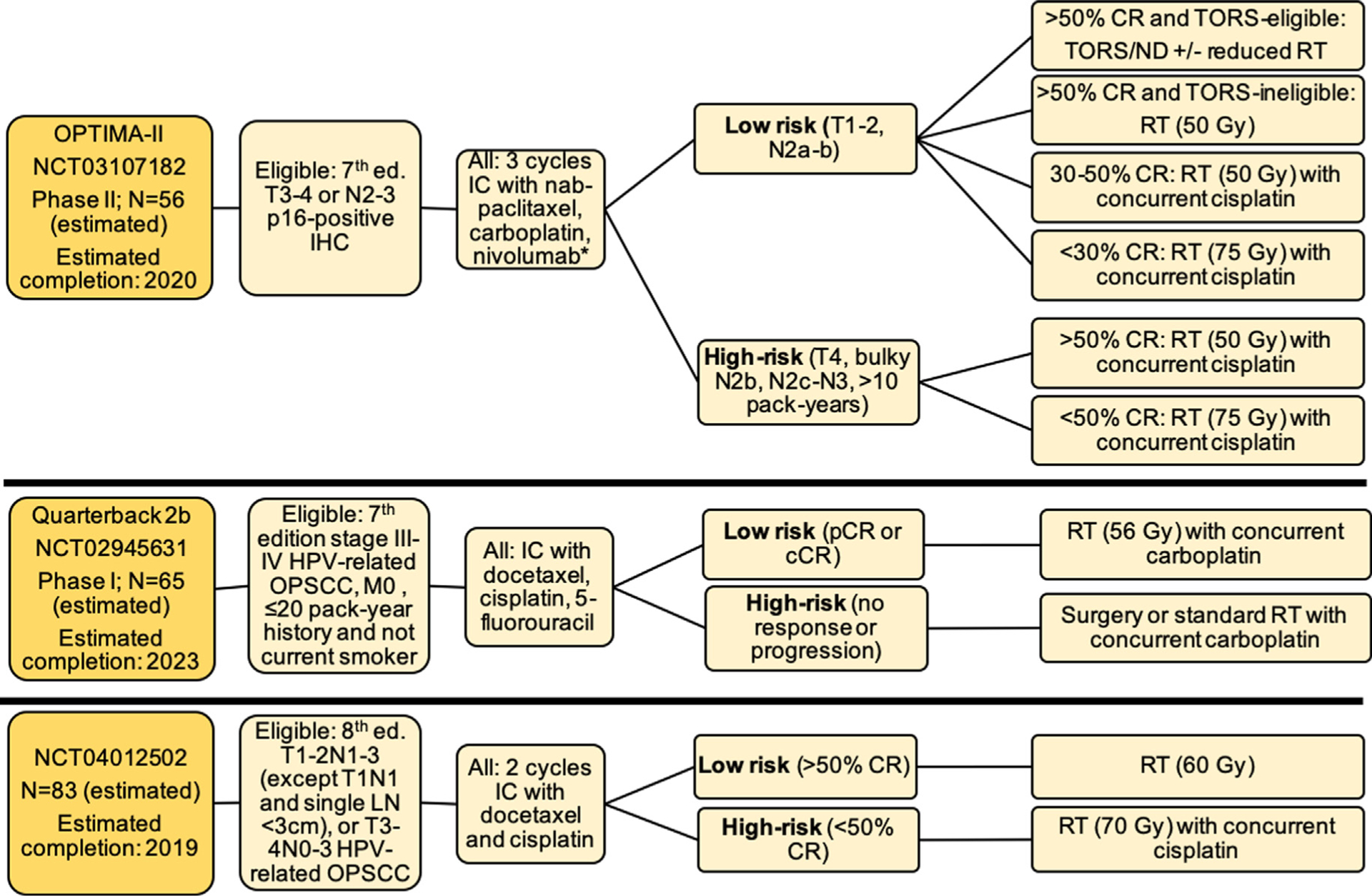
Ongoing trials of de-escalation based on response to induction chemotherapy, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; IC = induction chemotherapy; RT = radiation therapy; CR = clinical response; cCR = complete clinical response; pCR = partial clinical response; LN = lymph node; TORS = transoral robotic surgery; ND = neck dissection.
Quarterback IIb (NCT02945631) is a follow-up to the Quarterback Trial, also testing RT dose reduction (56 Gy) after IC. Finally, NCT04012502 will enroll locoregionally advanced HPV-related OPSCC patients within a Chinese population and treat with de-intensified RT (60 Gy) or standard concurrent CRT, based on response after 2 cycles of IC with docetaxel and cisplatin.
De-escalation using immunotherapy
The final category includes studies investigating the use of immunotherapy (Fig. 7). The role of immunotherapy has been more completely explored in recurrent/metastatic (R/M) HNSCC [63]. Briefly, three randomized phase III clinical trials have indicated that treatment with PD-1 inhibitors (e.g. pembrolizumab or nivolumab) prolong survival in patients with R/M HNSCC in both the platinum-refractory and platinum-sensitive setting [64–67]. Some trials suggest that HPV-related OPSCC may have more immunologically active tumor profiles and may experience improved response rates with immunotherapy [63,68].
Fig. 7.
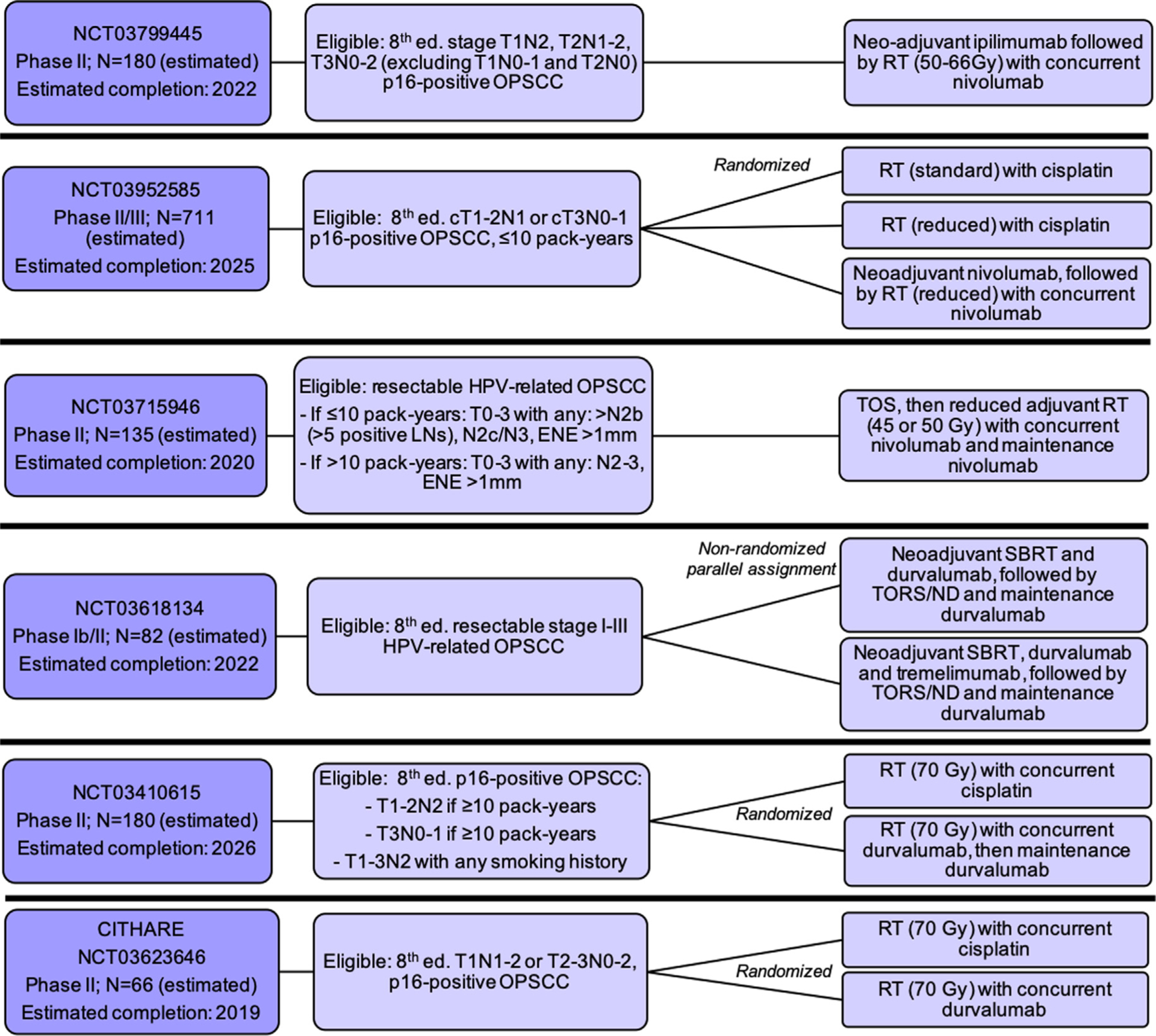
Ongoing trials of de-escalation involving immunotherapy, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; IC = induction chemotherapy; RT = radiation therapy; SBRT = stereotactic body radiation therapy LN = lymph node; TOS = transoral surgery; ENE = extranodal extension.
NCT03799445 is a phase II study testing the use of neo-adjuvant ipilimumab (monoclonal antibody targeting CTLA-4) followed by reduced-dose definitive RT concurrently with nivolumab for patients with p16-positive 8th edition T1N2, T2N1-N2, or T3N0-N2 OPSCC patients. NRG-HN005 (NCT03952585) is a multicenter phase II/III trial randomizing p16-positive OPSCC patients with ≤10 pack-years smoking history to one of three arms: standard-dose RT with concurrent cisplatin, reduced-dose RT with concurrent cisplatin, or neoadjuvant nivolumab followed by reduced-dose RT with concurrent nivolumab. NCT03715946 is a phase II study enrolling patients with resectable p16-positive OPSCC with specified intermediate risk factors and smoking histories. After TOS, patients will undergo deintensified adjuvant RT (45–50 Gy) with concurrent and then maintenance nivolumab.
Three studies are testing the use of durvalumab (a monoclonal antibody targeting PD-L1). NCT03618134 is enrolling resectable 8th edition stage I-III HPV-related OPSCC. Arm 1 will undergo neoadjuvant stereotactic body RT with durvalumab, followed by TORS and ND, followed by 4 cycles of maintenance durvalumab starting 12 weeks after surgery; arm 2 will undergo the same treatment with the addition of neoadjuvant tremelimumab (a monoclonal antibody targeting CTLA-4). NCT03410615 will randomize locoregionally advanced HPV-related OPSCC patients to either definitive standard-dose RT with concurrent cisplatin or standard-dose RT with concurrent and then maintenance durvalumab. The CITHARE study (NCT03623646) will similarly randomize locoregionally advanced HPV-related OPSCC patients within a French population to definitive standard-dose RT with either concurrent cisplatin or durvalumab.
Conclusion
With several clinical trials already reported and dozens more underway, the topic of treatment de-escalation for patients with HPV-related OPSCC is gaining necessary attention. Some early results seem promising, with results from phase II studies suggesting that de-escalated definitive and adjuvant RT doses or reduced chemotherapy regimens may provide comparable early oncologic results among appropriately selected patients. However, by contrast, cetuximab-based de-escalation approaches have proven inferior in two phase III studies, therefore supporting the continued vital role for cisplatin and high-lighting the need for de-escalation to be done solely in clinical trials at experienced centers.
In the coming years, the challenge will lie in the interpretation of the results of each clinical trial and de-escalation approach in context with one another as we move towards potential new standards of care for HPV-related OPSCC patients. As detailed in the previous sections, there are a multitude of trials testing deintensified treatment protocols, many with distinct risk-assignment criteria and varying eligibility factors. For example, while many trials are enrolling patients with similarly locoregionally advanced tumors, some restrict enrollment by tobacco smoking history; therefore, results of each study may not be generalizable to broader HPV-OPSCC populations, instead only to those who were eligible for that specific trial. Additionally, some methods of stratification (for example, weekly MRI or use of dynamic biomarkers), while potentially effective, may be resource- or cost-intensive, raising questions of feasibility for wide implementation.
Finally, long-term functional and quality-of-life results will provide insight into acute and late treatment toxicities, as trials to date have already hinted. Many trials will report objective measures of swallowing function and patient-reported quality of life outcomes in the years after treatment; however, the eventual long-term 5-to-10-year functional outcomes of these treatment protocols will be invaluable.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–42. 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 Patients. J Natl Cancer Inst 2016;108(6):djv403. 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- [3].Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100(4):261–9. 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- [4].Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med 2010;363(1):24–35. 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol 2011;22(5):1071–7. 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rischin D, Young RJ, Fisher R, et al. Prognostic Significance of p16 INK4A and Human Papillomavirus in Patients With Oropharyngeal Cancer Treated on TROG 02.02 Phase III Trial. J Clin Oncol 2010;28(27):4142–8. 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lassen P, Eriksen JG, Krogdahl A, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: Evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol 2011;100(1):49–55. 10.1016/j.radonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [8].Ang KK, Zhang Q, Rosenthal DI, et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J Clin Oncol 2014;32(27):2940–50. 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Survivorship Ringash J. and quality of life in head and neck cancer. J Clin Oncol 2015;33(29):3322–7. 10.1200/JCO.2015.61.4115. [DOI] [PubMed] [Google Scholar]
- [10].O’Neill CB, Baxi SS, Atoria CL, et al. Treatment-related toxicities in older adults with head and neck cancer: A population-based analysis. Cancer 2015;121(12):2083–9. 10.1002/cncr.29262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 2019;20(10):1349–59. 10.1016/S1470-2045(19)30410-3. [DOI] [PubMed] [Google Scholar]
- [12].Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol 2012;30(17):2102–11. 10.1200/jco.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adelstein DJ, Ismaila N, Ku JA, et al. Role of treatment deintensification in the management of p16+ oropharyngeal cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37(18):1578–89. 10.1200/JCO.19.00441. [DOI] [PubMed] [Google Scholar]
- [14].Bussu F, Ragin C, Boscolo-Rizzo P, et al. HPV as a marker for molecular characterization in head and neck oncology: Looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck 2019;41(4):1104–11. 10.1002/hed.25591. [DOI] [PubMed] [Google Scholar]
- [15].Lewis JS, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of american pathologists. Arch Pathol Lab Med 2018;142(5):559–97. 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- [16].Fakhry C, Lacchetti C, Rooper LM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the college of american pathologists guideline. J Clin Oncol 2018;36(31):3152–61. 10.1200/JCO.18.00684. [DOI] [PubMed] [Google Scholar]
- [17].Liang C, Marsit CJ, McClean MD, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res 2012;72(19):5004–13. 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity and prognostic discrimination. Clin Cancer Res 2011;17(19):6262–71. 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seiwert TY. Ties that bind: p16 as a prognostic biomarker and the need for high-accuracy human papillomavirus testing. J Clin Oncol 2014;32(35):3914–6. 10.1200/JCO.2014.57.9268. [DOI] [PubMed] [Google Scholar]
- [20].D’Souza G, Westra WH, Wang SJ, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol 2017;3(2):169–77. 10.1001/jamaoncol.2016.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a national cancer institute state of the science meeting, november 9–10, 2008, Washington, D.C. Head Neck 2009;31(11):1393–422. 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- [22].Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer 2019;125(12):2027–38. 10.1002/cncr.32025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Granata R, Miceli R, Orlandi E, et al. Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: an Italian validation study. Ann Oncol 2012;23(7):1832–7. 10.1093/annonc/mdr544. [DOI] [PubMed] [Google Scholar]
- [24].O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol 2013;31(5):543–50. 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- [25].Huang SH, Xu W, Waldron J, et al. Refining american joint committee on cancer/union for international cancer control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 2015;33(8):836–45. 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- [26].Horne ZD, Glaser SM, Vargo JA, et al. Confirmation of proposed human papillo-mavirus risk–adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer 2016;122(13):2021–30. 10.1002/cncr.30021. [DOI] [PubMed] [Google Scholar]
- [27].O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. LancetOncol 2016;17(4):440–51. 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- [28].Amin MB, Edge SB, Greene FL, editors. AJCC Cancer Staging Manual. 8th ed.Springer; 2017. [Google Scholar]
- [29].Lydiatt W, O’Sullivan B, Patel S. Major Changes in Head and Neck Staging for 2018. Am Soc Clin Oncol Edu Book 2018;38:505–14. 10.1200/EDBK_199697. [DOI] [PubMed] [Google Scholar]
- [30].Malm I-J, Fan CJ, Yin LX, et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer 2017;123(10):1768–77. 10.1002/cncr.30512. [DOI] [PubMed] [Google Scholar]
- [31].Zhan KY, Eskander A, Kang SY, et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral Oncol 2017;73:152–9. 10.1016/j.oraloncology.2017.08.020. [DOI] [PubMed] [Google Scholar]
- [32].Beltz A, Gösswein D, Zimmer S, et al. Staging of oropharyngeal squamous cell carcinoma of the head and neck: Prognostic features and power of the 8th edition of the UICC staging manual. Eur J Surg Oncol 2019;45(6):1046–53. 10.1016/j.ejso.2019.02.032. [DOI] [PubMed] [Google Scholar]
- [33].Geltzeiler M, Bertolet M, Albergotti W, et al. Staging HPV-related oropharyngeal cancer: Validation of AJCC-8 in a surgical cohort. Oral Oncol 2018;84:82–7. 10.1016/j.oraloncology.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Würdemann N, Wagner S, Sharma SJ, et al. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Front Oncol 2017;7. 10.3389/fonc.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizumachi T, Homma A, Sakashita T, Kano S, Hatakeyama H, Fukuda S. Confirmation of the eighth edition of the AJCC/UICC TNM staging system for HPV-mediated oropharyngeal cancer in Japan. Int J Clin Oncol 2017;22(4):682–9. 10.1007/s10147-017-1107-0. [DOI] [PubMed] [Google Scholar]
- [36].Nauta IH, Rietbergen MM, van Bokhoven AAJD, et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann Oncol 2018;29(5):1273–9. 10.1093/annonc/mdy060. [DOI] [PubMed] [Google Scholar]
- [37].Fakhry C, Zevallos JP, Eisele DW. Imbalance Between Clinical and Pathologic Staging in the Updated American Joint Commission on Cancer Staging System for Human Papillomavirus-Positive Oropharyngeal Cancer. J Clin Oncol 2018;36(3):217–9. 10.1200/JCO.2017.75.2063. [DOI] [PubMed] [Google Scholar]
- [38].Haughey B, Sinha P, Kallogjeri D, et al. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral Oncol 2016;62:11–9. 10.1016/j.oraloncology.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res 2010;16(4):1226–35. 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mirghani H, Leroy C, Chekourry Y, et al. Smoking impact on HPV driven head and neck cancer’s oncological outcomes? Oral Oncol 2018;82:131–7. 10.1016/j.oraloncology.2018.05.007. [DOI] [PubMed] [Google Scholar]
- [41].Vawda N, Banerjee RN, Debenham BJ. Impact of smoking on outcomes of HPV-related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiation Oncol*Biol*Phys 2019;103(5):1125–31. 10.1016/j.ijrobp.2018.11.046. [DOI] [PubMed] [Google Scholar]
- [42].Kwan JYY, Su J, Huang SH, et al. Radiomic Biomarkers to Refine Risk Models for Distant Metastasis in HPV-related Oropharyngeal Carcinoma. Int J Radiation Oncol*Biol*Phys 2018;102(4):1107–16. 10.1016/j.ijrobp.2018.01.057. [DOI] [PubMed] [Google Scholar]
- [43].Lin TA, Garden AS, Elhalawani H, et al. Radiographic retropharyngeal lymph node involvement in HPV-associated oropharyngeal carcinoma: Patterns of involvement and impact on patient outcomes. Cancer 2019;125(9):1536–46. 10.1002/cncr.31944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Billfalk-Kelly A, Yu E, Su J, et al. Radiologic Extranodal Extension Portends Worse Outcome in cN+ TNM-8 Stage I Human Papillomavirus-Mediated Oropharyngeal Cancer. Int J Radiation Oncol*Biol*Phys 2019;104(5):1017–27. 10.1016/j.ijrobp.2019.03.047. [DOI] [PubMed] [Google Scholar]
- [45].Marur S, Li S, Cmelak AJ, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol 2017;35(5):490–7. 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Misiukiewicz K, Gupta V, Miles BA, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: The Quarterback trial. Oral Oncol 2019;95:170–7. 10.1016/j.oraloncology.2019.06.021. [DOI] [PubMed] [Google Scholar]
- [47].Seiwert TY, Foster CC, Blair EA, et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol 2019;30(2):297–302. 10.1093/annonc/mdy522. [DOI] [PubMed] [Google Scholar]
- [48].Chen AM, Felix C, Wang P-C, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. LancetOncol 2017;18(6):803–11. 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Villaflor VM, Melotek JM, Karrison TG, et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol 2016;27(5):908–13. 10.1093/annonc/mdw051. [DOI] [PubMed] [Google Scholar]
- [50].Ma DJ, Price KA, Moore EJ, et al. Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus-associated oropharynx squamous cell carcinoma. J Clin Oncol 2019;37(22):1909–18. 10.1200/JCO.19.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Swisher-McClure S, Lukens JN, Aggarwal C, Lin, et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus–Related Squamous Cell Carcinoma of the Oropharynx. Int J Radiation Oncol*Biol*Phys 2020;106(4):725–32. 10.1016/j.ijrobp.2019.11.021. [DOI] [PubMed] [Google Scholar]
- [52].Chera BS, Amdur RJ, Tepper J, et al. Phase 2 Trial of De-intensified Chemoradiation Therapy for Favorable-Risk Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. Int J Radiation Oncol*Biol*Phys 2015;93(5):976–85. 10.1016/j.ijrobp.2015.08.033. [DOI] [PubMed] [Google Scholar]
- [53].Chera BS, Amdur RJ, Tepper JE, et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2018;124(11):2347–54. 10.1002/cncr.31338. [DOI] [PubMed] [Google Scholar]
- [54].Chera BS, Amdur RJ, Green R, et al. Phase II trial of de-intensified chemor-adiotherapy for human papillomavirus-associated oropharyngeal squamous cell carcinoma. J Clin Oncol 2019. 10.1200/JCO.19.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yom SS, Torres-Saavedra P, Caudell JJ, et al. NRG-HN002: A Randomized Phase II Trial for Patients With p16-Positive, Non-Smoking-Associated Locoregionally Adv Oropharyngeal Cancer. Int J Radiation Oncol Biol Phys 2019;105(3):684–5. [Google Scholar]
- [56].Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. The Lancet 2019;393(10166):40–50. 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019;393(10166):51–60. 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weidhaas JB, Harris J, Schaue D, et al. The KRAS-variant and cetuximab response in head and neck squamous cell cancer: a secondary analysis of a randomized clinical trial. JAMAOncol 2017;3(4):483–91. 10.1001/jamaoncol.2016.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nichols AC, Lang P, Prisman E, et al. Treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma with radiotherapy vs. trans-oral surgery (ORATOR2): study protocol for a randomized phase II trial. BMC Cancer 2020;20(1):125. 10.1186/s12885-020-6607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee N, Schoder H, Beattie B, et al. A strategy of using intra-treatment hypoxia imaging to selectively and safely guide radiation dose deescalation concurrent with chemotherapy for loco-regionally advanced human papillomavirus-related oropharyngeal carcinoma. Int J Radiation Oncol Biol Phys 2016;96(1):9–17. 10.1016/j.ijrobp.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Søvik A, Malinen E, Bruland ØS, Bentzen SM, Olsen DR. Optimization of tumour control probability in hypoxic tumours by radiation dose redistribution: a modelling study. Phys Med Biol 2007;52(2):499–513. 10.1088/0031-9155/52/2/013. [DOI] [PubMed] [Google Scholar]
- [62].Bahig H, Yuan Y, Mohamed ASR, et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a–2b/Bayesian phase II trial. Clin Transl Radiat Oncol 2018;13:19–23. 10.1016/j.ctro.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol 2019;99:104460. 10.1016/j.oraloncology.2019.104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37(15_suppl):6000. 10.1200/JCO.2019.37.15_suppl.6000. [DOI] [Google Scholar]
- [65].Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet 2019;393(10167):156–67. 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- [66].Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375(19):1856–67. 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. LancetOncol 2016;17(7):956–65. 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]


