Abstract
Background
A better understanding of non-structural carbohydrate (NSC) dynamics in trees under drought stress is critical to elucidate the mechanisms underlying forest decline and tree mortality from extended periods of drought. This study aimed to assess the contribution of ectomycorrhizal (ECM) fungus (Suillus variegatus) to hydraulic function and NSC in roots, stems, and leaves of Pinus tabulaeformis subjected to different water deficit intensity. We performed a continuous controlled drought pot experiment from July 10 to September 10, 2019 using P. tabulaeformis seedlings under 80, 40, and 20% of the field moisture capacity that represented the absence of non-drought, moderate drought, and severe drought stress, respectively.
Results
Results indicated that S. variegatus decreased the mortality rate and increased height, root biomass, and leaf biomass of P. tabulaeformis seedlings under moderate and severe drought stress. Meanwhile, the photosynthetic rates, stomatal conductance, and transpiration rates of P. tabulaeformis were significantly increased after S. variegatus inoculation. Moreover, the inoculation of S. variegatus also significantly increased the NSC concentrations of all seedling tissues, enhanced the soluble sugars content, and increased the ratios of soluble sugars to starch on all tissues under severe drought. Overall, the inoculation of S. variegatus has great potential for improving the hydraulic function, increasing the NSC storage, and improving the growth of P. tabulaeformis under severe drought.
Conclusions
Therefore, the S. variegatus can be used as a potential application strain for ecological restoration on arid regions of the Loess Plateau, especially in the P. tabulaeformis woodlands.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-021-02945-3.
Keywords: Ectomycorrhizal fungi, Pinus tabulaeformis, Drought stress, Non-structural carbohydrate, Forest restoration
Background
Water deficit is one of the most important environmental stresses affecting plants productivity and reducing grain yield around the world [1–4]. The drought-induced tree mortality throughout the world have been documented over the last several years [5, 6], and an even greater drought-induced loss of net ecosystem productivity is the nonlethal reductions in growth [7–9]. The Loess Plateau is the most severe drought and soil erosion area in the world [10]. In the past 30 years, Pinus tabulaeformis, which can form a symbiotic relationship with ectomycorrhizal (ECM) fungi, has been planted on a large area on the Loess Plateau to maintain soil and water and to improve the ecological environment [11–13]. However, a large number of P. tabulaeformis died due to the drought on the Loess Plateau. Since plants cannot relocate, their survival largely depends on the tolerance and rapid responses to counter the stress effects [14]. Consequently, it is urgent to conduct study to improve the survival rate of P. tabulaeformis and increase its drought resistance in this area.
To alleviate the negative effects of drought on plants, the influence of microorganisms on host plants has received extensive attention. Mycorrhizal fungi improve the ability of their hosts to resist, tolerate, and recover from drought [15, 16]. The extrametrical fungal hyphae can deeply extend into the rhizosphere of the soil leading to the absorption of large amounts of various nutrients, increasing the availability and uptake of micro-nutrients, and alleviating the stress effects on plant [17]. Previous studies have shown that the water transport via extraradical mycelium of ECM fungi to host plant can be sufficient to make a difference between the survival and death of a tree seedling [18]. Lehto and Zwiazek [16] concluded that the influences of ECM fungi on host-plant–water relations were manifested in increased stomatal conductance to water vapor, altered hydraulic conductance of mycorrhizal roots, and facilitated osmotic adjustments. However, the physiological responses of plants to inoculation with ECM fungi were highly variable and dependent upon the species and even genotype of fungus [7].
Previous studies have shown that P. tabulaeformis could form a mycorrhizal symbiosis relationship with Suillus, Tomentella, Tuber, Handkea etc. [19–21]. Meanwhile, we investigated the fungal resources of the P. tabulaeformis forests on the Loess Plateau and found that Suillus variegatus (Swartz ex Fr.) O. Kuntze and P. tabulaeformis had a close symbiotic relationship. S. variegatus is an epigeous ECM fungal species that is widespread and common in pine forests in Europe and in parts of Asia [22, 23]. And S. variegatus can form an ECM symbiosis system with Pinus sylvestris L. seedlings [24], promoting the growth of roots and shoots of seedlings [25]. Inoculation with S. variegatus also increased the chitin content of P. sylvestris seedlings and the contents of Mg and K in the rhizosphere soil. However, few studies have clarified the specific effects of S. variegatus on the growth of P. tabulaeformis.
As the photosynthesis is one of the most important physiological processes for plants to obtain carbon [26]. When drought stress causes a decrease in photosynthesis, trees are vulnerable to carbon starvation [27]. Prolonged drought stress will significantly reduce the storage of non-structural carbohydrates (NSC) in the trees, and eventually lead to death [8, 28]. The NSC, which play different roles in plant energy metabolism, transportation and osmotic adjustment, is mainly starch and soluble sugars [29]. Starch is the most prevalent and abundant storage carbohydrate in woody tissues [30, 31]. When plants suffered from severe drought stress that causes carbohydrate deficiencies, starch would be consumed in large quantities and be preferentially transported to growing parts. Soluble sugars also play an important role in osmotic regulation and are considered to be important physiological indicators related to drought tolerance [2, 32]. Therefore, the NSC could make a buffer between carbohydrate supply and demand, and allow trees to resist drought [33]. Mycorrhizal hyphal are dynamic and play a crucial role in forest ecosystem functioning and carbon dioxide fluxes [34, 35]. However, mycorrhizal hyphal usually come at the cost of increased carbohydrate costs [18]. In ECM systems, fungi have been reported to receive up to 19 times more carbohydrates, which results in a strong belowground carbon sink [33], from the host tree compared with the normal exudation of root systems [36]. However, the effect of ECM on plant’s NSC content under different levels of drought intensity has not been fully understood.
Though there are many studies on the effect of different ECM fungi on different plant species, no information is available on the effect of S. variegatus inoculation on the growth and NSC content of P. tabulaeformis under drought conditions. The hypothesis of the present study was that the S. variegatus could form a close symbiotic relationship with P. tabulaeformis seedlings, promote the growth, reduce mortality, and participate in regulating the content of NSC in tissues under drought conditions. Therefore, to test this hypothesis, we conducted a 6-month drought-simulating indoor pot experiment. The biomass and mortality of P. tabulaeformis were used to evaluate plant growth. The starch and soluble sugar content, and their ratio in various tissues of P. tabulaeformis were used to study the dynamic changes of NSC. In addition, the gas exchange parameters were used to interpret the mechanisms involved in NSC variations caused by S. variegatus under drought stress.
Results
The S. variegatus colonization of P. tabulaeformis
At harvest, non-inoculated P. tabulaeformis seedlings did not been colonized and all inoculated seedlings been colonized by S. variegatus in their root systems. The seedlings in the severe drought soil had the lowest and in the moderate drought soil had the highest ECM fungus colonization, at 38 and 60%, respectively. (Table 1).
Table 1.
The soil water content and ECM colonization of Pinus tabulaeformis seedlings under different drought intensity treatments. (mean ± standard error)
| Parameter | T1 | T2 | T3 | Significance |
|---|---|---|---|---|
| Soil water content (%) | 80 | 40 | 20 | |
| ECM colonization (%) | 52 ± 6 b | 60 ± 3 a | 38 ± 6 c | ** |
Note: T1 = non-drought stress, T2 = moderate drought stress, T3 = severe drought stress. Data expressed as mean ± standard error (n = 6). Different lowercase letters indicate significant differences between the means by Tukey (HSD) test (P < 0.05); “*” indicates that the interaction is significant (P<0.05); “**” indicates that the interaction is extremely significant (P<0.01); “ns” indicates no interaction (P ≥ 0.05)
The mortality rate and growth of P. tabulaeformis
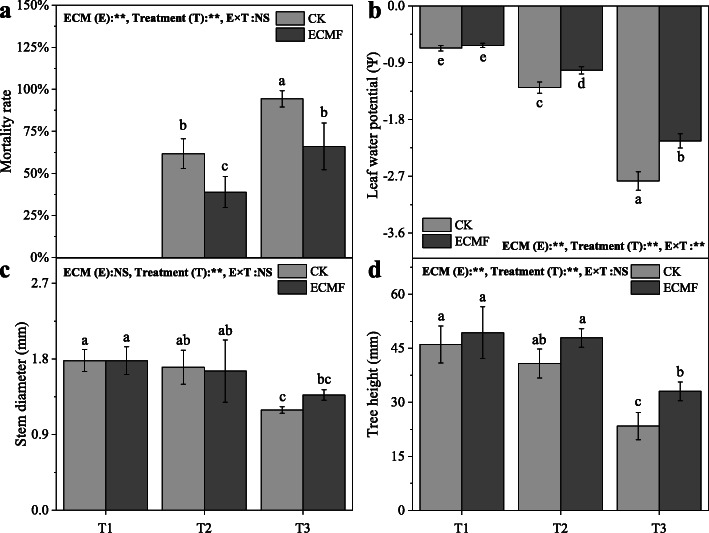
The mortality rate of P. tabulaeformis seedlings increased significantly (P < 0.05) as the drought gradient increased (Fig. 1A). In non-drought (T1) soil, all seedlings survived. In the T2, T3 treatment, the inoculation of S. variegatus could significantly reduce (P < 0.05) the mortality, by 20%, compared with the mortality of non-inoculation.
Fig. 1.
The mortality rate (a), leaf water potential (b), stem diameter (c) and tree height (d) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
The leaf water potential of P. tabulaeformis seedlings decreased significantly (P < 0.05) as the drought intensity increased (Fig. 1B). The inoculation of S. variegatus could significantly increase (P < 0.05) the leaf water potential among the T2 and T3 drought intensity.
The stem diameter of P. tabulaeformis seedlings decreased significantly (P < 0.05) in T3 treatment (Fig. 1C). There was no significant difference in the stem diameter of seedlings between non-inoculated and inoculated in all treatments. Like stem diameter, the height of P. tabulaeformis seedlings decreased significantly (P < 0.05), but the inoculation of S. variegatus could significantly increase (P < 0.05) the height compared with non-inoculated in T3 treatment (Fig. 1D). With the drought gradient increased, seedlings stem diameter and height showed a significant downward trend (P < 0.05).
The root index of P. tabulaeformis
The drought gradient and S. variegatus inoculation had a significant effect on the root index of P. tabulaeformis seedlings (Table 2). Data showed with the increase of drought intensity, all root indexes decreased except the root average diameter. The inoculation of S. variegatus could significantly increase the root length, surface area and root volume under non-drought (T1 treatment) compared with CK group. Under T2 treatment, the root length, surface area and forks of seedlings with S. variegatus inoculation were significantly higher than CK group. And the root length and surface area were greatly increased by S. variegatus inoculation under severe drought (T3 treatment).
Table 2.
Effect of ECM fungi on root indicators of Pinus tabulaeformis seedlings under different drought intensity treatments. (mean ± standard error)
| Drought intensity treatments (T) | ECM fungi (E) | Length (cm) | Surface area (cm2) | Average diameter (mm) | Root volume (cm3) | Tips | Forks |
|---|---|---|---|---|---|---|---|
| T1 | Non-ECM | 338.6 ± 13.91 b | 55.95 ± 1.28 b | 0.54 ± 0.02 c | 0.74 ± 0.06 b | 845 ± 284 ab | 1398 ± 113 a |
| ECM | 460.94 ± 59.85 a | 76.55 ± 12.07 a | 0.53 ± 0.01 c | 1.01 ± 0.19 a | 1009 ± 197 a | 1728 ± 322 a | |
| T2 | Non-ECM | 194.59 ± 29.15 c | 40.76 ± 8.37 c | 0.66 ± 0.05 a | 0.68 ± 0.18 b | 572 ± 25 bcd | 835 ± 127 b |
| ECM | 346.8 ± 43.1 b | 56.75 ± 6.9 b | 0.53 ± 0.02 c | 0.74 ± 0.08 bc | 826 ± 126 abc | 1500 ± 296 a | |
| T3 | Non-ECM | 107.95 ± 5.18 d | 20.9 ± 1.6 d | 0.63 ± 0.01 ab | 0.32 ± 0.03 c | 299 ± 57 c | 487 ± 41 b |
| ECM | 173.74 ± 26.92 c | 31.83 ± 3.92 c | 0.59 ± 0.02 bc | 0.47 ± 0.04 cd | 409 ± 98 cd | 708 ± 127 b | |
| Effect (P value) | T | ** | ** | ** | ** | ** | ** |
| E | ** | ** | ** | * | * | ** | |
| T & E | ns | ns | ** | ns | ns | ns |
Note: T1 = non-drought stress, T2 = moderate drought stress, T3 = severe drought stress. Data expressed as mean ± standard error (n = 6). Different lowercase letters indicate significant differences between the means by Tukey (HSD) test (P < 0.05); “*” indicates that the interaction is significant (P<0.05); “**” indicates that the interaction is extremely significant (P<0.01); “ns” indicates no interaction (P ≥ 0.05)
The biomass of various tissues of P. tabulaeformis
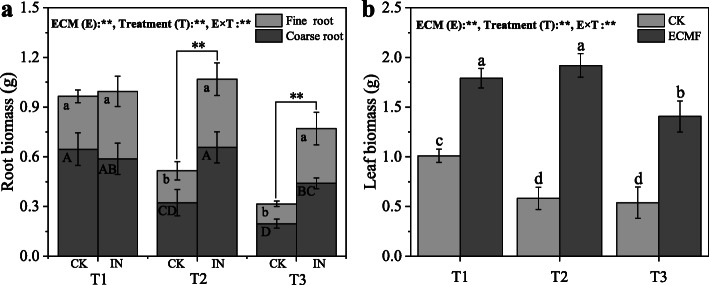
The seedlings in severe drought soil had lower root and leaf biomass compared with the seedlings in the T1 and T2 treatment (Fig. 2). The inoculation of S. variegatus could significantly increase the fine root biomass, by 116 and 175%, respectively, compare with CK group in the T2, T3 treatments (Fig. 2A). And it also could significantly increase the coarse roots biomass, by 97 and 120%, respectively, compare with CK group in the T2 and T3 treatment. Therefore, the root biomass was significantly increased (P < 0.01) after S. variegatus inoculation, by 106 and 140%, respectively, compare with non-inoculation in the T2 and T3 treatment. Like fine root biomass, the leaf biomass also greatly increased, by 63, 210 and 160%, respectively, after S. variegatus inoculation in three treatments (Fig. 2B). However, drought and S. variegatus inoculation had no significant effect on the biomass of the stem (Table 3).
Fig. 2.
The root (a) and leaf biomass (b) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
Table 3.
Effect of ECM fungi on some indicators of Pinus tabulaeformis seedlings under different drought intensity treatments. (mean ± standard error)
| Drought intensity treatments (T) | ECM fungi (E) | Stem biomass (mg) | Stem water content (%) | Stomatal conductance (mmol H2O m−2 s−1) | Intercellular CO2 concentration (μmol CO2 mol− 1) |
|---|---|---|---|---|---|
| T1 | Non-ECM | 108 ± 10 a | 56 ± 15 b | 0.28 ± 0.10 a | 362 ± 19 a |
| ECM | 195 ± 77 a | 56 ± 3.6 b | 0.29 ± 0.10 a | 357 ± 11 a | |
| T2 | Non-ECM | 110 ± 58 a | 61 ± 10 a | 0.04 ± 0.01 b | 315 ± 66 a |
| ECM | 168 ± 91 a | 63 ± 4.3 a | 0.12 ± 0.05 b | 325 ± 26 a | |
| T3 | Non-ECM | 119 ± 10 a | 32 ± 6.2 c | 0.02 ± 0.01 c | 308 ± 67 a |
| ECM | 191 ± 20 a | 37 ± 7.3 c | 0.04 ± 0.01 b | 329 ± 48 a | |
|
Effect (P value) |
T | ns | ** | ** | * |
| E | ns | ns | * | ns | |
| T & E | ns | ns | ns | ns |
Note: T1 = non-drought stress, T2 = moderate drought stress, T3 = severe drought stress. Data expressed as mean ± standard error (n = 6). Different lowercase letters indicate significant differences between the means by Tukey (HSD) test (P < 0.05); “*” indicates that the interaction is significant (P<0.05); “**” indicates that the interaction is extremely significant (P<0.01); “ns” indicates no interaction (P ≥ 0.05)
The water content of root and leaf of P. tabulaeformis
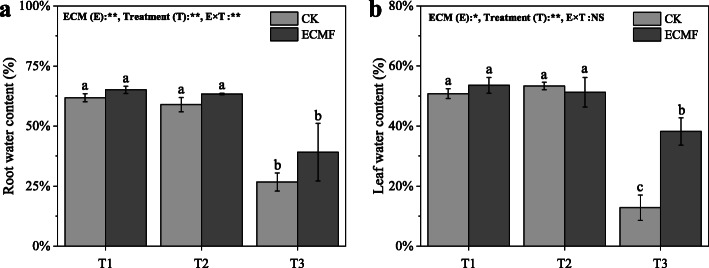
The seedlings in severe drought soil had lower root and leaf water content compared with the seedlings in the T1 and T2 treatment (Fig. 3). The inoculation of S. variegatus could significantly increase the leaf water content, by 216%, compare with CK group in T3 treatment. However, the highest and lowest stem water content was in T2 and T3 treatment, respectively (Table 3). There was no significant effect on the water content of the stem after S. variegatus inoculation.
Fig. 3.
The root (a) and leaf water content (b) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
The leaf gas exchange parameters of P. tabulaeformis
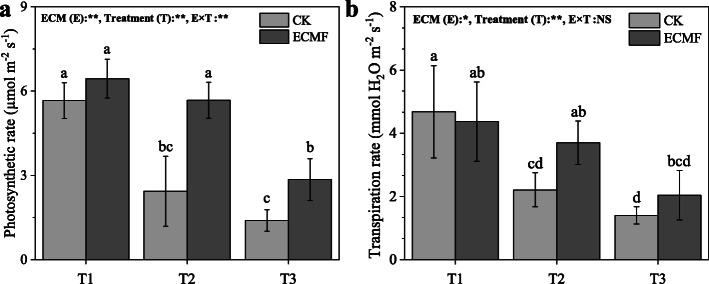
The seedlings in severe drought soil had lower photosynthetic rate and transpiration rate compared with the seedlings in the T1 and T2 treatment (Fig. 4). The inoculation of S. variegatus could significantly increase photosynthetic rate, by 133 and 100%, respectively, compare with CK group in T2 and T3 treatment (Fig. 4A). Meanwhile, the inoculation of S. variegatus also increased transpiration rate, by 67%, compare with CK group in T2 treatment (Fig. 4B).
Fig. 4.
The photosynthetic (a) and transpiration rate (b) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
Stomatal conductance also greatly increased with S. variegatus inoculation in T3 treatment (Table 3). However, no significant effect of ECM inoculation on intercellular CO2 concentration was found under all treatments.
The non-structural carbohydrate of P. tabulaeformis
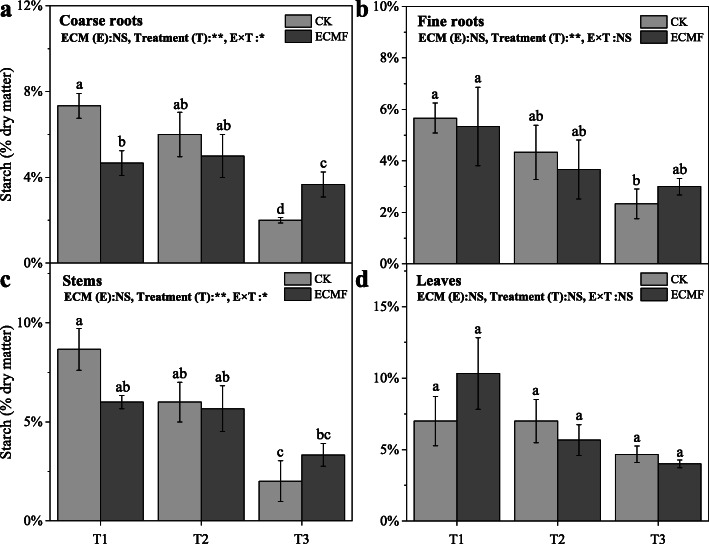
As the drought intensity increased, the starch content of seedling all tissues decreased significantly (Fig. 5). The seedlings tissues in severe drought soil had lower starch content compared with the seedlings in the T1 and T2 treatment. The inoculation of S. variegatus could significantly increase the coarse roots starch content, by 80%, compare with CK group in T3 treatment (Fig. 5A). However, no significant effect of S. variegatus inoculation on other tissues was found under all treatments.
Fig. 5.
The starch content of different tissues (coarse roots (a); fine roots (b); stems (c); leaves (d)) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
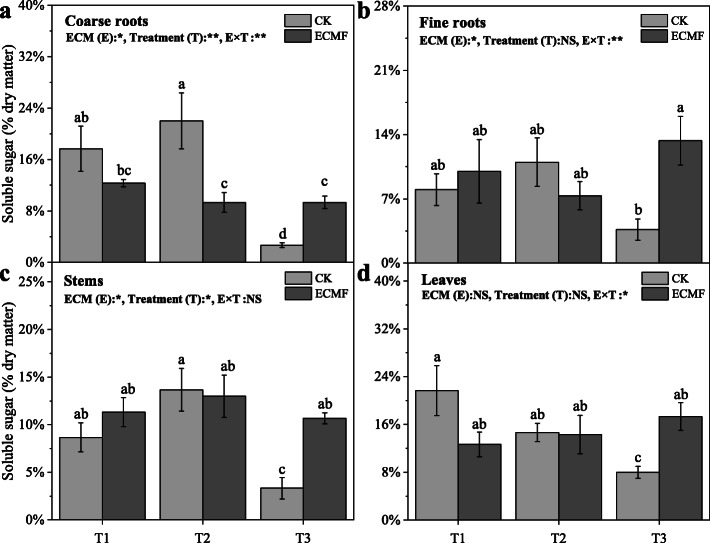
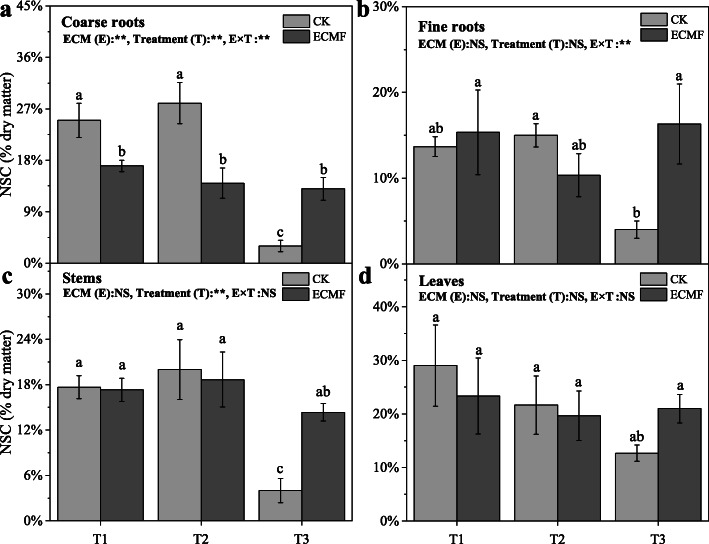
The soluble sugar content of seedling all tissues decreased significantly in non-inoculation group, remained stable in inoculation group under three treatments (Fig. 6). The inoculation of S. variegatus could significantly increase the soluble sugar content of coarse roots, fine roots, stems and leaves, by 800, 550, 614 and 113%, respectively, compare with non-inoculation group in T3 treatment (Fig. 6). This change trend was similar to the change in NSC content, the NSC content of seedling all tissues decreased significantly in non-inoculation group, remained stable in inoculation group under three treatments. The inoculation of S. variegatus could significantly increase the NSC content of coarse roots, fine roots, stems and leaves, by 333, 300, 250 and 62%, respectively, compare with CK group in T3 treatment (Fig. 7).
Fig. 6.
The soluble sugar content of different tissues (coarse roots (a); fine roots (b); stems (c); leaves (d)) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
Fig. 7.
The non-structural carbohydrate (NSC) content of different tissues (coarse roots (a); fine roots (b); stems (c); leaves (d)) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
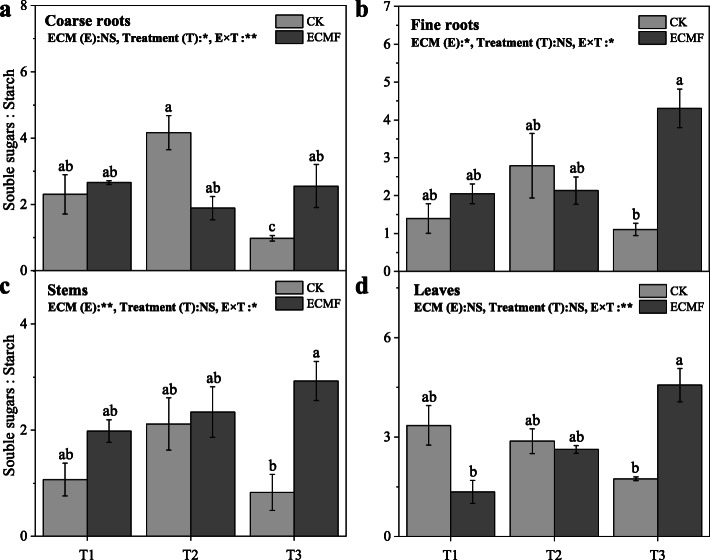
The non-inoculation seedling tissues in severe drought soil had lower the ratios of soluble sugars to starch compared with the seedlings in the T1 and T2 treatment (Fig. 8). The inoculation of S. variegatus could significantly increase the ratios of soluble sugars to starch of coarse roots, fine roots, stems, and leaves compare with non-inoculation group in T3 treatment. Despite drought intensity, the ratios of soluble sugars to starch in coarse roots tended to stable throughout the experiment, while that in all other tissues tended to increase (Fig. 8).
Fig. 8.
The ratios of soluble sugars to starch of different tissues (coarse roots (a); fine roots (b); stems (c); leaves (d)) of Pinus tabulaeformis with ECM fungi inoculation in three drought levels. The data are the means ± standard deviation (n = 3). Different lowercase above the columns indicate significant difference between the means by Tukey (HSD) test (P < 0.05). CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation.; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress
Discussion
Severe drought causes significant declines in the productivity and survival of plants [37–39]. In our study, the mortality of P. tabulaeformis seedlings was more than 95% under severe drought condition, and was decreased by the S. variegatus inoculation. Two mechanisms have been proposed to explain drought-induced tree mortality: hydraulic failure and carbon starvation [40]. Therefore, the present experimental results show that the inoculation of S. variegatus may alleviate hydraulic failure and carbon starvation caused by drought. The present study also showed that S. variegatus inoculation increased plant height, stem diameter, root (both coarse and fine roots) biomass, and leaf biomass. This might be due to the increased absorption area of the plant roots in soil by establishment of the ECM symbiosis [41, 42].
The strategies of plants resist drought rely on hydraulic mechanisms: reducing water loss, and increasing water uptake [38, 43]. Under drought conditions, plants would significantly reduce leaf water potential and gas exchange [44, 45]. As an isohydric species, P. tabulaeformis can reduce stomatal conductance and maintain a relatively constant leaf water potential during low water availability periods [27, 46, 47]. Previous studies had shown that the leaf water potential values between − 1.4 and − 1.5 MPa can represent the carbon safety margin of isotonic tree species [48]. In our study, the average of leaf water potential of P. tabulaeformis in the control group was − 1.29 (± 0.09) MPa and − 2.78 (± 0.15) MPa under moderate and severe drought conditions, respectively. This means that P. tabulaeformis was already in a state of carbon starvation under moderate drought conditions, while under severe drought conditions, it would easily die from severe carbon starvation. These were consistent with our mortality test results. However, the leaf water potential of inoculation group was significantly higher than that of the control, maintaining at − 1.02 (± 0.06) MPa and − 2.14 (± 0.11) MPa under moderate and severe drought conditions, respectively. Therefore, we found that the inoculation of S. variegatus could increase the leaf water potential to maintain a safe range (especially under moderate drought conditions), thereby alleviating the damage caused by drought and reducing the mortality of trees. At the same time, S. variegatus inoculation in moderate and severe droughts could significantly increase the stomatal conductance, transpiration rate, and photosynthetic rate of plants. The influence suggested the participation of S. variegatus in the hydraulic adjustments of P. tabulaeformis under drought. The high stomatal conductance at low soil moisture content might be due to the extraction of soil moisture by mycorrhizal system or the expansion of exploration area by extra-root hyphae [49]. At the same time, the increment of stomatal conductance increases the absorption of CO2, and consequently increases the photosynthesis rate of plants [7, 50]. In this way, although the P. tabulaeformis is still restricted by the stomatal conductance under drought conditions, it would produce more carbohydrates required for respiration than the control, thereby maintaining basic metabolic and defensive capabilities. Taken together, we suggested that S. variegatus inoculation improves the photosynthesis of P. tabulaeformis under drought through increased water absorption and transportation.
Trees are vulnerable to carbon starvation induced by drought stress [51]. Previous studies have shown that severe, but not moderate, drought stress would significantly influence NSC concentration [52, 53], which is similar to our results. When P. tabulaeformis suffers severe drought, the starch content of other tissues except the leaves will be significantly reduced. As NSC have been identified as key carbon sources under severe drought [54, 55], decreasing starch concentrations of tissues indicate the remobilization of starch reserves in P. tabulaeformis seedlings in order to meet C requirements when drought response reduces C assimilation [56]. The insignificant decrease in the content of starch in leaves may be due to it initially declined, and then increased above pre-drought concentrations before mortality [57]. The inoculation of S. variegatus could significantly increase the NSC concentrations of all seedling tissues compared with the control group. This phenomenon suggested that S. variegatus could improve the NSC storage and alleviate carbon starvation of P. tabulaeformis under severe drought. This may be due to the close relationship between plant stomatal conductance, leaf hydraulic regulation and C reserve under drought conditions [58]. In our study, inoculation with S. variegatus could directly increase the stomatal conductance and leaf water potential, which means that seedlings could perform more photosynthesis. Although the increase in stomatal conductance may increase the loss of water, from our experimental results, the increase of photosynthesis was beneficial to seedlings under drought. Meanwhile, the inoculation of S. variegatus had different effects on the composition of seedling NSC. That maybe due to the NSC reserves can adjust their tissue concentrations in response to changes in the balance of C sources or sinks [59, 60]. On the one hand, the inoculation of S. variegatus could increase the starch content, which can be preferentially transported to the growing part of the plant roots to ensure root growth, in crude roots under severe drought conditions. On the other hand, the inoculation of S. variegatus could increase all tissues’ soluble sugar content and the ratio of soluble sugar to starch under severe drought conditions, which can reduce the water potential, maintain cell expansion, and increase water absorption [61]. Therefore, we believe that S. variegatus inoculation would play an important role in alleviating C starvation caused by drought in P. tabulaeformis.
Conclusion
The inoculation of S. variegatus could greatly promote the growth and survival rate of P. tabulaeformis by increasing the water absorption and transportation, and improving the NSC storage under severe drought. In general, the S. variegatus have the potential to be used as biological modifiers in ecological restoration on arid regions of the Loess Plateau, especially in the P. tabulaeformis woodlands.
Materials and methods
Plant material and growth substrate
P. tabulaeformis trees were grown from seed in nursery trays at the microbiology lab of the Forestry College, Northwest A&F University. The seeds were provided by the Forestry Technology Extension Station of the Forestry Department, Shaanxi Province, China. The seeds were sterilized on the surface with 0.05% KMnO4 for 30 min, washed 3 times with sterilized water, and immersed in sterilized water at 45 °C for 1 h. The sterilized seeds are placed in sterile gauze and cultured under sterile dark conditions. The sterilized seeds were germinated in sterile gauze at 25 °C under sterile dark conditions. The germinated seeds were transplanted into seedling trays (50 mL per hole) filled with sterilized vermiculite. The seeds were fertilized every week with 10 mL of 1/2-strength Hoagland’s solution [62]. In March 2019, 180 2-month seedlings with a height to meristem of 30 ± 5 mm and a root collar diameter of 1 ± 0.4 mm were selected and transplanted into plastic pots ( 10 cm in diameter, 10 cm in depth) containing 2 kg of growth substrate. Each pot was planted with 3 seedlings, for a total of 60 pots. The seedlings were kept under optimal growth conditions (ambient light, 24 °C, RH = 70%, ultra-optimal irrigation) for a 4-week acclimation period.
The growth substrate of P. tabulaeformis was composed of a mixture of soil, sand, and vermiculite (1: 1: 1, v/v/v). Soil was collected from the top layer of the Northwest A&F University campus nursery in Yangling city, Shaanxi province, China. The main soil nutrient characteristics were as follows: 16.15 g kg− 1 organic matter, 30.35 mg kg− 1 available nitrogen, 20.40 mg kg− 1 available phosphorus and 126.36 mg kg− 1 available potassium. Soil was ground, passed through a 2-mm sieve, and mixed with thoroughly washed river sand and vermiculite. The mixture was autoclaved at 0.11 MPa and 121 °C for 2 h, after which it sat for 1 week before use.
Fungal preparation and inoculation
ECM fungal inoculum (S. variegatus) was stored in the microbiology lab of the Forestry College, Northwest A&F University. The strain was originally isolated from the ectomycorrhizae of P. tabulaeformis on the Loess Plateau. The fungus was firstly cultivated on potato dextrose agar (PDA) solid medium. After 2 weeks of growth, four blocks of media (1 cm in diameter) were inoculated in every 300 ml potato dextrose liquid medium. After 14 days of culture shaking (25 °C, 120 rpm), the mixture of fungal mycelia was homogenized by blender and then used as an inoculum.
Experimental design
The pot experiment was performed using one inoculation status treatment (inoculated with S. variegatus or sterile inoculum) as a single-factor experiment under three drought intensity levels (non-drought, moderate drought, and severe drought). When the seedlings have finished acclimation period, all pots were randomly divided into 2 inoculation treatments: inoculated with S. variegatus and sterile inoculum, 30 pots per treatment. For ectomycorrhizal fungal inoculation, 30 mL inoculum was applied for the roots of P. tabulaeformis, whereas the non-mycorrhizal treatment received 30 mL autoclaved inoculum. Then all the pots were well watered and kept at 85–90% of the field capacity in the greenhouse at 25 °C with 12 h light per day for 4 weeks. In our previous field trials, we found that the field capacity was 20–40% under natural drought conditions on the Loess Plateau. After 4 weeks, each inoculation treatment was subjected to three levels (T1 non-drought: 80% of the field capacity, T2 moderate drought: 40% of the field capacity, and T3 severe drought: 20% of the field capacity) of drought intensity (Table 1), 10 pots per level. All pots were weighed and watered every day at 09:00 AM to maintain the water content at the desired levels. Trees were grown in the same greenhouse (average temperature was 25 °C and average relative humidity was 70%) and kept at stable water content for 60 days and then harvested.
ECM fungal colonization
The ECM fungal colonization was measured on the basis of the method used in previous study [63], with minor modifications. Part of the fine roots were carefully washed with distilled water, bleached with 10% KOH at 90 °C for 24 h and then rinsed with distilled water, after which alkaline H2O2 was added to soften for 5 min. After the fine roots were washed with distilled water again, 1% HCl was added to acidify for 5 min. The washed fine roots were added with trypan blue dye (250 mL of lactic acid and glycerol, 0.5 g of trypan blue and 500 mL of water) and placed it in a water bath (90 °C) for 30 min. The roots were subsequently decolorized with lactic acid-glycerol (1:1) at 90 °C for 30 min. The colonization was counted according to the grid line intersection method under an optical microscope [63, 64].
Leaf gas exchange parameters and water potential
Before the end of the experiment, seedlings with the same growth conditions were used to determine the gas exchange parameters including stomatal conductance (Gs), transpiration rate (T), and photosynthesis rate (A). The gas exchange parameters of the seedlings were measured using a LI-6400 Photosynthesis System (Licor Inc., Lincoln, NE, USA), and the photosynthesis system parameters were set as follows: the chamber area 2 cm2, the reference CO2 concentration was 385 ppm, the light environment was 1000 l mol m− 2 s− 1 using the 6400-2B red/blue LED light source. On sunny days from 9:00 to 11:30 AM, the youngest fully expanded leaves were selected for measurement. The water potential (Ψ) of P. tabulaeformis leaves was measured using the pressure chamber method [65].
Morphology and biomass determination
After measuring the gas exchange parameters, the seedlings height and ground diameter of the P. tabulaeformis were measured, and the mortality was calculated. All the seedlings were divided into three parts, one part was used for root scanning, the other part was used for biomass determination, and another part is used for NSC content determination. One part of seedlings was divided into two parts (leaves and roots system) after harvested, placed them in a clear plastic bag and scanned it with a root scanner (STD1600 Epson, Long Beach, CA, USA). The root system index we measured were root length, root surface area, root average diameter, root volume, root forks and root tips. The scan results were analyzed by the software WinRHIZO™. The other part of seedlings was quickly cleansed after harvest, and the roots, stems, and leaves were collected separately for biomass determination. These tissues were dried at 75 °C for 48 h until constant weight.
Non-structural carbohydrate determination
Another part of seedlings was divided into four parts: leaves, stems, coarse roots (> 2 mm), and fine roots (< 2 mm) after harvested. To limit the photosynthesis consumption on concentration of NSC in seedlings, the sampling time was completed within 2 h. After washing and thoroughly removing surface moisture, they were weighed and used to determine non-structural carbohydrate content. The collected samples were quickly heated under a microwave of 650 W for 90 s to prevent enzymatic carbohydrate reactions [66]. They were then dried to constant weight in an oven at 80 °C, and the dry weight was recorded. All dried tissues were ground until they passed smoothly through a 40-mesh sieve.
NSC concentration was defined as the sum of soluble sugar and starch content. According to the anthrone method [67, 68], some modifications were made to determine the NSC content. Precise 0.1000 g of samples from different organs was weighted and placed in 10 mL centrifuge tubes. Two milliliter 80% ethanol solution was added to the centrifuge tube, and then water bath at 80 °C for 30 min. After the solution was cooled to room temperature, the solution was centrifuged at 4800 rmin− 1 for 10 min. The supernatant was retained for determination of soluble sugar content. The precipitate was retained for determination of starch content [67]. The extraction was repeated 3 times. Added 2 mL of distilled water to the precipitate, gelatinized in a boiling water bath for 15 min. After the solution was cooled to room temperature, added 2 mL of 9.2 M HClO4 solution, shaken for 15 min, added 4 mL of distilled water, mixed and centrifuged at 4800 rmin− 1 for 10 min. After aspiration of the supernatant, there was a further extracted with 2 ml of 4.6 M HClO4. All supernatants were collected for determination of starch content. The absorbance of the solution after the sugar and starch reacted with the anthrone reagent was measured using a spectrophotometer at 625 nm. The content was calculated according to the standard curve and expressed as a % relative to the dry weight of the organ.
Statistical analysis
All data were statistically analyzed using SPSS 25 (IBM® SPSS® Statistics) software. Repeated-measures analysis of variance (ANOVA) was used to determine the effects of drought intensity and ECM fungus (S. variegatus) on morphology, biomass, leaf gas exchange parameters, water potential, and NSC concentrations in each organ. Before ANOVA, levene’s test was used to test the homogeneity of the variance. The Tukey (HSD) test was used to determine significant differences (P < 0.05) in each indicator.
Supplementary Information
Additional file 1: Figure S1. The phenotypes of Pinus tabulaeformis as influenced by ECM inoculation under different drought gradients. CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress.
Additional file 2: Figure S2. The colonization of Pinus tabulaeformis by ECM at different levels of drought. CK = No ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress.
Acknowledgments
Not applicable.
Abbreviations
- ECM
Ectomycorrhizal
- NSC
Non-structural carbohydrate
- S. variegatus
Suillus Variegatus
- P. tabulaeformis
Pinus tabulaeformis
- PDA
Potato dextrose agar
- CK
No ECM fungi inoculation
- ECMF
ECM fungi inoculation
- T1
Non-drought stress
- T2
Moderate drought stress
- T3
Severe drought stress
- A
Photosynthesis rate
- Gs
Stomatal conductance
- T
Transpiration rate
Authors’ contributions
Jiaxing Wang: Methodology, Conceptualization, Writing – original draft, Writing - review & editing. Haoqiang Zhang: Writing - review & editing. Jing Gao: Investigation. Yu Zhang: Investigation. Yaqin Liu: Investigation. Ming Tang: Supervision, Funding acquisition, Project administration. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32071639, 31700530), and the National Key Research and Development Program of China (2018YFD0600203-3).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxing Wang and Haoqiang Zhang contributed equally to this work.
References
- 1.Ganie SA, Ahammed GJ. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice. Plant Cell Rep. 2021;40(3):437–459. doi: 10.1007/s00299-020-02649-2. [DOI] [PubMed] [Google Scholar]
- 2.Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot. 2009;67(2):312–319. doi: 10.1016/j.envexpbot.2009.06.010. [DOI] [Google Scholar]
- 3.Kaya C, Şenbayram M, Akram NA, Ashraf M, Alyemeni MN, Ahmad P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci Rep. 2020;10(1):1–13. 10.1038/s41598-020-62669-6. [DOI] [PMC free article] [PubMed]
- 4.Petrov P, Petrova A, Dimitrov I, Tashev T, Olsovska K, Brestic M, et al. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J Agron Crop Sci. 2018;204(3):219–27. 10.1111/jac.12255.
- 5.Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag. 2010;259(4):660–84. 10.1016/j.foreco.2009.09.001.
- 6.Williams AP, Allen CD, Macalady AK, Griffin D, Woodhouse CA, Meko DM, et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Chang. 2013;3(3):292–7. 10.1038/nclimate1693.
- 7.Gehring CA, Swaty RL, Deckert RJ. Mycorrhizas, drought, and host-plant mortality. In: Johnson NC, Gehring C, Jansa J, editors. Mycorrhizal Mediation of Soil. Elsevier; 2017. pp. 279–298. [Google Scholar]
- 8.Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci. 2009;106(17):7063–6. 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed]
- 9.Williams AP, Allen CD, Millar CI, Swetnam TW, Michaelsen J, Still CJ, et al. Forest responses to increasing aridity and warmth in the southwestern United States. Proc Natl Acad Sci. 2010;107(50):21289–94. 10.1073/pnas.0914211107. [DOI] [PMC free article] [PubMed]
- 10.Shi H, Shao M. Soil and water loss from the loess plateau in China. J Arid Environ. 2000;45(1):9–20. doi: 10.1006/jare.1999.0618. [DOI] [Google Scholar]
- 11.Zhou Z, Shangguan Z. Vertical distribution of fine roots in relation to soil factors in Pinus tabulaeformis Carr. Forest of the loess plateau of China. Plant Soil. 2007;291(1–2):119–129. doi: 10.1007/s11104-006-9179-z. [DOI] [Google Scholar]
- 12.Guo Y, Tang M, Yj W, Yj Y. Effect of inoculating pinus tabulaeformis with ectomycorrhizal fungi. J Northwest Forestry Univ. 2006;21:116–119. [Google Scholar]
- 13.Wang YQ, Shao MA. Spatial variability of soil physical properties in a region of the loess plateau of PR China subject to wind and water erosion. Land Degrad Dev. 2013;24(3):296–304. doi: 10.1002/ldr.1128. [DOI] [Google Scholar]
- 14.Ahammed GJ, Li X, Liu A, Chen S. Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul. 2020;39(4):1451–1464. doi: 10.1007/s00344-020-10098-0. [DOI] [Google Scholar]
- 15.Augé RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11(1):3–42. doi: 10.1007/s005720100097. [DOI] [Google Scholar]
- 16.Lehto T, Zwiazek JJ. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza. 2011;21(2):71–90. doi: 10.1007/s00572-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 17.Gao C, El-Sawah AM, Ali DFI, Alhaj Hamoud Y, Shaghaleh H, Sheteiwy MS. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.) Agronomy. 2020;10(3):319. doi: 10.3390/agronomy10030319. [DOI] [Google Scholar]
- 18.Smith SE, Read DJ. Mycorrhizal symbiosis. London: Academic press; 2010. [Google Scholar]
- 19.Zhang H, Yu H, Tang M. Prior contact of Pinus tabulaeformis with ectomycorrhizal fungi increases plant growth and survival from damping-off. New For. 2017;48(6):855–866. doi: 10.1007/s11056-017-9601-9. [DOI] [Google Scholar]
- 20.Zhang H, Tang M, Chen H, Zheng C. Effects of inoculation with ectomycorrhizal fungi on microbial biomass and bacterial functional diversity in the rhizosphere of Pinus tabulaeformis seedlings. Eur J Soil Biol. 2010;46(1):55–61. doi: 10.1016/j.ejsobi.2009.10.005. [DOI] [Google Scholar]
- 21.Wang Q, Guo L-D. Ectomycorrhizal community composition of Pinus tabulaeformis assessed by ITS-RFLP and ITS sequences. Botany. 2010;88(6):590–595. doi: 10.1139/B10-023. [DOI] [Google Scholar]
- 22.Gumińska B, Wojewoda W. Grzyby i ich oznaczanie. Państwowe Wydawnicto Rolnicze i Leśne: Warszawa; 1985. [Google Scholar]
- 23.Ohenoja E. Effect of weather conditions on the larger fungi at different forest sites in northern Finland in 1976–1988. Finland: Oulu University; 1993. [Google Scholar]
- 24.Heinonsalo J, Buée M, Vaario L-M. Root-endophytic fungi cause morphological and functional differences in scots pine roots in contrast to ectomycorrhizal fungi. Botany. 2017;95(2):203–210. doi: 10.1139/cjb-2016-0161. [DOI] [Google Scholar]
- 25.Niemi K, Julkunen-Tiitto R, Häggman H, Sarjala T. Suillus variegatus causes significant changes in the content of individual polyamines and flavonoids in scots pine seedlings during mycorrhiza formation in vitro. J Exp Bot. 2006;58(3):391–401. doi: 10.1093/jxb/erl209. [DOI] [PubMed] [Google Scholar]
- 26.Hajihashemi S, Brestic M, Kalaji HM, Skalicky M, Noedoost F. Environmental pollution is reflected in the activity of the photosynthetic apparatus. Photosynthetica. 2020;58(Special Issue):529–539. doi: 10.32615/ps.2019.179. [DOI] [Google Scholar]
- 27.McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008;178(4):719–39. 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed]
- 28.Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010;186(2):274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann H, Trumbore S. Understanding the roles of nonstructural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytol. 2016;211(2):386–403. doi: 10.1111/nph.13955. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowski TT, Pallardy SG. Physiology of woody plants: Elsevier. 1996. [Google Scholar]
- 31.Keller J, Loescher W. Nonstructural carbohydrate partitioning in perennial parts of sweet cherry. J Am Soc Horticultural Sci (USA) 1989;114(6):969–975. [Google Scholar]
- 32.Martinez JP. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J Exp Bot. 2005;56(419):2421–2431. doi: 10.1093/jxb/eri235. [DOI] [PubMed] [Google Scholar]
- 33.Schiestl-Aalto P, Ryhti K, Mäkelä A, Peltoniemi M, Bäck J, Kulmala L. Analysis of the NSC storage dynamics in tree organs reveals the allocation to belowground symbionts in the framework of whole tree carbon balance. Front Forests Glob Chang. 2019;2:17. doi: 10.3389/ffgc.2019.00017. [DOI] [Google Scholar]
- 34.Heinemeyer A, Hartley IP, Evans SP, Carreira De La Fuente JA, Ineson P. Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Chang Biol. 2007;13(8):1786–1797. doi: 10.1111/j.1365-2486.2007.01383.x. [DOI] [Google Scholar]
- 35.Malcolm GM, JC LÓP-GÉR, Koide RT, Eissenstat DM. Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi. Glob Chang Biol. 2008;14(5):1169–1180. doi: 10.1111/j.1365-2486.2008.01555.x. [DOI] [Google Scholar]
- 36.Nehls U. Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. J Exp Bot. 2008;59(5):1097–1108. doi: 10.1093/jxb/erm334. [DOI] [PubMed] [Google Scholar]
- 37.Anderegg WRL, Kane JM, Anderegg LDL. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat Clim Chang. 2013;3(1):30–36. doi: 10.1038/nclimate1635. [DOI] [Google Scholar]
- 38.Ahammed GJ, Li X, Mao Q, Wan H, Zhou G, Cheng Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol Plant. 2020:1–11. 10.1111/ppl.13243. [DOI] [PubMed]
- 39.Brzostek ER, Dragoni D, Schmid HP, Rahman AF, Sims D, Wayson CA, et al. Chronic water stress reduces tree growth and the carbon sink of deciduous hardwood forests. Glob Chang Biol. 2014;20(8):2531–9. 10.1111/gcb.12528. [DOI] [PubMed]
- 40.Gattmann M, Birami B, Nadal Sala D, Ruehr NK. Dying by drying: timing of physiological stress thresholds related to tree death is not significantly altered by highly elevated CO2. Plant Cell Environ. 2021;44(2):356–370. doi: 10.1111/pce.13937. [DOI] [PubMed] [Google Scholar]
- 41.Cairney JWG. Ectomycorrhizal fungi: the symbiotic route to the root for phosphorus in forest soils. Plant Soil. 2011;344(1–2):51–71. doi: 10.1007/s11104-011-0731-0. [DOI] [Google Scholar]
- 42.Vayssières A, Pěnčík A, Felten J, Kohler A, Ljung K, Martin F, et al. Development of the poplar-Laccaria bicolor ectomycorrhiza modifies root auxin metabolism, signaling, and response. Plant Physiol. 2015;169(1):890–902. 10.1104/pp.114.255620. [DOI] [PMC free article] [PubMed]
- 43.Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C. How tree roots respond to drought. Front Plant Sci. 2015;6(547):1–16. 10.3389/fpls.2015.00547. [DOI] [PMC free article] [PubMed]
- 44.Waseem M, Nie ZF, Yao GQ, Hasan M, Xiang Y, Fang XW. Dew absorption by leaf trichomes in Caragana korshinskii : an alternative water acquisition strategy for withstanding drought in arid environments. Physiol Plant. 2021. 10.1111/ppl.13334. [DOI] [PubMed]
- 45.Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ. 2009;32(11):1584–1595. doi: 10.1111/j.1365-3040.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Zhang Z, Chen L, Cai Y, Zhang H, Lou J, et al. Sparse Pinus tabuliformis stands have higher canopy transpiration than dense stands three decades after thinning. Forests. 2020;11(1):70. 10.3390/f11010070.
- 47.Wu X, Tang Y, Chen Y, Wen J, Xie Y, Lu S. Sap flow characteristics and responses to summer rainfall for Pinus tabulaeformis and Hippophae rhamnoides in the loess hilly region of China. Ecol Evol. 2018;8(1):617–630. doi: 10.1002/ece3.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell PJ, O'Grady AP, Tissue DT, Worledge D, Pinkard EA. Co-ordination of growth, gas exchange and hydraulics define the carbon safety margin in tree species with contrasting drought strategies. Tree Physiol. 2014;34(5):443–458. doi: 10.1093/treephys/tpu014. [DOI] [PubMed] [Google Scholar]
- 49.Duan X, Neuman DS, Reiber JM, Green CD, Saxton AM, Augé RM. Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. J Exp Bot. 1996;47(10):1541–1550. doi: 10.1093/jxb/47.10.1541. [DOI] [Google Scholar]
- 50.Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25(1):13–24. doi: 10.1007/s00572-014-0585-4. [DOI] [PubMed] [Google Scholar]
- 51.McDowell NG. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011;155(3):1051–1059. doi: 10.1104/pp.110.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiley E, Huepenbecker S, Casper BB, Helliker BR. The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013;33(11):1216–1228. doi: 10.1093/treephys/tpt093. [DOI] [PubMed] [Google Scholar]
- 53.Zhang T, Cao Y, Chen Y, Liu G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees. 2015;29(6):1837–49. 10.1007/s00468-015-1265-5.
- 54.Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhäusser SM, Tissue DT, et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat Ecol Evol. 2017;1(9):1285–91. 10.1038/s41559-017-0248-x. [DOI] [PubMed]
- 55.Blackman CJ, Creek D, Maier C, Aspinwall MJ, Drake JE, Pfautsch S, et al. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol. 2019;39(6):910–24. 10.1093/treephys/tpz016. [DOI] [PubMed]
- 56.Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, et al. Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol Monogr. 2016;86(4):495–516. 10.1002/ecm.1231.
- 57.Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Guardiola-Claramonte M, Zou CB, et al. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013;197(4):1142–51. 10.1111/nph.12102. [DOI] [PubMed]
- 58.Woodruff DR, Meinzer FC, Marias DE, Sevanto S, Jenkins MW, McDowell NG. Linking nonstructural carbohydrate dynamics to gas exchange and leaf hydraulic behavior in Pinus edulis and Juniperus monosperma. New Phytol. 2015;206(1):411–421. doi: 10.1111/nph.13170. [DOI] [PubMed] [Google Scholar]
- 59.Lyu M, Sun M, Peñuelas J, Sardans J, Sun J, Chen X, et al. Temperature controls growth of Pinus taiwanensis along an elevational gradient. Trees. 2020.
- 60.Canham CD, Kobe RK, Latty EF, Chazdon RL. Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia. 1999;121(1):1–11. doi: 10.1007/s004420050900. [DOI] [PubMed] [Google Scholar]
- 61.Silva EN, Ferreira-Silva SL, Viégas RA, Silveira JAG. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ Exp Bot. 2010;69(3):279–285. doi: 10.1016/j.envexpbot.2010.05.001. [DOI] [Google Scholar]
- 62.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stat. 1950;347(2nd edit):32. [Google Scholar]
- 63.Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. Working with mycorrhizas in forestry and agriculture. In. Australian Centre for International Agricultural Research: Canberra; 1996. [Google Scholar]
- 64.Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84(3):489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x. [DOI] [Google Scholar]
- 65.Holbrook NM, Burns MJ, Field CB. Negative xylem pressures in plants: a test of the balancing pressure technique. Science. 1995;270(5239):1193–1194. doi: 10.1126/science.270.5239.1193. [DOI] [Google Scholar]
- 66.Hoch G, Popp M, Körner C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos. 2002;98(3):361–374. doi: 10.1034/j.1600-0706.2002.980301.x. [DOI] [Google Scholar]
- 67.Hajihashemi S, Skalicky M, Brestic M, Pavla V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol Biochem. 2020;154:657–664. doi: 10.1016/j.plaphy.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The phenotypes of Pinus tabulaeformis as influenced by ECM inoculation under different drought gradients. CK = No ECM fungi inoculation; ECMF = ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress.
Additional file 2: Figure S2. The colonization of Pinus tabulaeformis by ECM at different levels of drought. CK = No ECM fungi inoculation; T1 = non-drought stress; T2 = moderate drought stress; T3 = severe drought stress.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.