Abstract
Background
While the number of individuals with hemophilia who are expected to be or have already been included in gene therapy trials has been regularly reported, the number of unscreened or excluded individuals, in addition to the reasons for exclusion, is mostly not reported.
Methods
We conducted an eligibility assessment of all people with severe hemophilia for gene therapy trials in one large Belgian hemophilia treatment center based on patient selection criteria of gene therapy trials and patients’ profiling.
Results
Among 87 adult patients with severe hemophilia A and B, 11 aged ≥65 years and two women were excluded from analysis. Six patients were excluded because of inhibitor development. One patient exhibited active hepatitis C infection, one had insufficient exposure to factor VIII, and five had uncontrolled comorbidities, while two were enrolled in other trials and two abused alcohol. Overall, 43 patients were not screened owing to psychosocial factors. Among 14 patients accepting gene therapy, six had adeno‐associated virus type 5 neutralizing antibodies and one had liver fibrosis. The number of patients who would accept gene therapy in the absence of strict clinical trial requirements was estimated at 36 (41.4%), irrespective of any exclusion criteria.
Conclusion
The majority of individuals with severe hemophilia could not be enrolled in gene therapy trials, almost half of them because of partly modifiable psychosocial reasons (49.4%). The proportion of candidates should substantially increase in the future, as eligibility criteria are likely to change and as more data on long‐term efficacy and safety of gene therapy will become available.
Keywords: adeno‐associated virus, gene therapy, hemophilia A, hemophilia B, patient selection
Essentials.
Gene therapy for hemophilia is available only in clinical trials with strict inclusion criteria.
There is no information on unscreened and excluded people with hemophilia.
Reasons for exclusion were studied in 87 individuals with severe hemophilia from a single center.
Safety‐efficacy data and patient support appear critical to enhance adoption of gene therapy.
1. BACKGROUND
Gene therapy is one of the most promising therapeutic innovations in the hemophilia field. Hemophilia is an X‐linked recessive bleeding disorder, caused by deficiencies in either coagulation factor VIII (FVIII) (hemophilia A [HA]) or factor IX (FIX) (hemophilia B [HB]), resulting in severe hemorrhagic complications, typically involving joint bleeds, if left untreated. Gene therapy has the potential to provide optimized bleeding protection in people with HA and HB by initiating the production of missing coagulation factors (FVIII and FIX, respectively) in human cells. This new approach can be performed either by delivering functional factor genes to cells or by editing the existing genome. Two options for factor gene delivery are currently studied, the most common being the transfer of FVIII/FIX genes into hepatocytes by adeno‐associated viruses (AAVs). Another option is gene transfer with lentiviral vectors into the genome of autologous stem cells, which are then delivered intravenously. 1
The first successful gene therapy trial in HB was reported by Nathwani et al in 2011. 2 , 3 Since then, numerous gene therapy trials have been initiated in both HA and HB populations. To date, 46 gene therapy trials for HA and HB have been registered on clinicaltrials.gov. 4 First results on gene therapy efficacy and safety appear promising. 1 Although Pasi et al 5 reported in 2020 sustained gene therapy efficacy for HA at 3 years after injection, the US Food and Drug Administration (FDA) delayed its approval of valoctocogene roxaparvovec for HA in August of the same year, claiming that more data on long‐term efficacy and safety are required. 6 Moreover, whether multiple injections of vectors (redosing) will be needed to sufficiently sustain high factor levels must still be determined.
Current gene therapy trials in the hemophilia field present with numerous well‐defined inclusion and exclusion criteria, with only slight variations among protocols. 4 The expected number of patients to be enrolled has generally been reported, whereas the exact number of patients enrolled per hemophilia treatment center (HTC) has mostly not been reported in the context of large‐scale international and multicenter studies. The accurate number of patients deemed ineligible by the investigators, invited to take part in a gene therapy study, selected for screening, and effectively screened is often left unreported. Some people who are unable to participate in a trial may still benefit from gene therapy at a later time. This explains why it is difficult to estimate the proportion of people who would likely be eligible for undergoing gene therapy once it has become commercially available.
There are multiple patient characteristics, nonmodifiable physical factors as well as psychosocial factors, that determine whether a patient is eligible for gene therapy, whether within or outside of a clinical trial. The nonmodifiable parameters include age (individuals aged ≥65 years are excluded, and current gene therapy trials do not yet include minors), inhibitor status (current presence or past history of inhibitors), liver disease (including fibrosis and active hepatitis C infection), uncontrolled HIV infection, and other poorly controlled or severe comorbidities, as well as insufficient FVIII/FIX exposure in the past. At present, women with hemophilia are not yet included in trials either. 4 , 7 The possibly modifiable variables that render the individual unsuitable or unwilling to undergo gene therapy include lack of motivation to participate in a trial, fear, lack of trust in the gene therapy concept, conservatism, geographic factors, socioeconomic issues, language barrier, and inability or unwillingness to comply with study requirements.
1.1. Objectives of the current study
This study aimed to estimate the number of individuals with severe hemophilia from a single HTC who have recently been candidates in gene therapy trials or could benefit from gene therapy in the future when it has become commercially available. Additionally, we attempted to identify which modifiable and nonmodifiable patient characteristics recently led to nonscreening for, noninclusion into, or exclusion from gene therapy trials.
2. METHODOLOGY
At the HTC of the Cliniques universitaires Saint‐Luc in Brussels, a total of 260 people with hemophilia have been registered, and currently, 87 adults with severe HA and HB are actively followed up. The HTC has previously been selected to recruit patients for two gene therapy trials, focused on HA and HB, respectively. 8 , 9 All individuals with severe hemophilia had been previously informed on several occasions about gene therapy modalities, including routine follow‐up visits, educational meetings, newsletters, surveys, and local patient associations (Belgian Haemophilia Society/AHVH), in addition to materials provided through the World Federation of Hemophilia and European Haemophilia Consortium.
Eligibility for a gene therapy trial was evaluated individually for each patient, considering the patient selection criteria of current gene therapy hemophilia trials. Patient profiling, that is, assessment of potential participation in a gene therapy trial from a hematologist’s perspective, was then performed by two experienced adult hematologists from the HTC, who are this paper’s co‐authors. Both were actively involved in the care of all enrolled patients and were therefore able to identify psychosocial exclusion criteria for each patient. The opportunity to take part in such a trial was discussed face‐to‐face with all patients eligible from the hematologists’ point of view during review clinics. In addition, attempts were made to estimate the number of patients who would be interested in gene therapy without the burden of a clinical trial. The latter was performed without taking into account a potentially limited access to gene therapy because of clinical, financial, and psychosocial parameters.
3. RESULTS
A total of 75 adults with severe HA and 12 adults with severe hemophilia HB were considered for this study. Results are displayed in Figure 1. Detailed information on inclusion and exclusion criteria for the two ongoing gene therapy trials for hemophilia at our center can be found on the website of the Belgian Federal Agency for Medicines and Health Products. 8 , 9 As only male patients aged ≥18 years are eligible for current gene therapy trials, we first excluded two female patients. Eleven patients aged ≥65 years were also excluded. Six patients displayed either a history or current presence of inhibitors against FVIII or FIX, while one patient exhibited active hepatitis C infection and two abused alcohol. None of our patients had uncontrolled HIV infection, which has been defined in most trials as a CD4 count <200/mm3 or viral load >20 copies/mL. One of our patients displayed insufficient FVIII exposure; indeed, despite his very mild bleeding phenotype, he was affected by severe HA. It should be noted that two patients were affected by liver cancer, though both were already among the patients excluded from participation because of their age (≥65 years). No further patients were affected by other active cancers. Five patients were excluded because of uncontrolled comorbidities, such as heart disease, seizures, and Crohn disease. Two patients who were currently enrolled in other clinical trials were also excluded.
FIGURE 1.
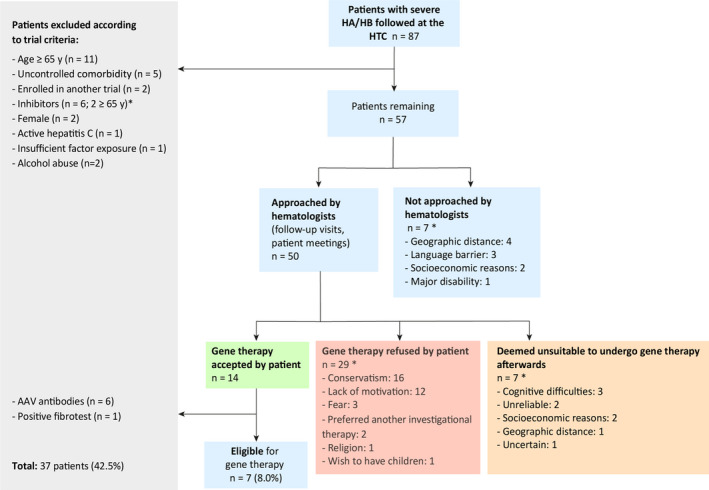
Application of exclusion/nonselection criteria for gene therapy trials at the HTC of the Cliniques Universitaires Saint‐Luc, Brussels. * Some patients combined two or more of these criteria. AAV, adeno‐associated virus; HA, hemophilia A; HB, hemophilia B; HTC, hemophilia treatment center
After application of the trial criteria, 57 patients remained. Seven patients were deemed unsuitable to participate in a gene therapy trial by the hematologists and were therefore not approached. Reasons for this were geographic distance (n = 4), language barrier (n = 3), socioeconomic difficulties (n = 2), and major disability rendering participation in a trial too burdensome (n = 1). Two or more of these reasons were present in some patients. Of the 50 patients who were informed and interviewed about the possibility of gene therapy, 14 agreed to undergo the screening process, and 29 declined to participate in a gene therapy trial; two of these preferred to receive another investigational therapy. Seven patients were approached by hematologists initially but were later deemed unsuitable to participate in a trial due to cognitive difficulties (n = 3), unreliability (n = 2), socioeconomic difficulties (n = 2), geographic distance (n = 1), and uncertainty about the patient’s willingness and reliability (n = 1). There was often more than one reason for exclusion. When adding together patients who refused to participate in a gene therapy trial and those deemed ineligible by their hematologist, 43 patients, or 49.4% of our study cohort, were excluded from participation in a gene therapy trial due to psychosocial factors.
Among the patients accepting gene therapy (n = 14), six were found positive for adeno‐associated virus type 5 (AAV5) neutralizing antibodies (NAbs), and one had a fibrotest and fibroscan compatible with liver fibrosis. Seven patients were thus still eligible for gene therapy trial participation, accounting for 8.0% of the 87 initially assessed patients. Two candidates withdrew their consent because of fear. Two individuals with severe HB have undergone gene therapy, while another with HB patient will soon be included in a gene therapy trial.
The number of candidates for gene therapy, if it was widely available, was estimated at 36 (41.4%). For this estimation, all 87 patients were considered, regardless of potential clinical trial–related exclusion criteria and based on the hypothesis that no particular burden would be associated with follow‐up.
4. DISCUSSION
Our study demonstrates that in a fairly large cohort of individuals with severe hemophilia, <10% were motivated and eligible to participate in a clinical gene therapy study. However, this observation must be further nuanced, given that patients aged ≥65 years are not systematically excluded from any clinical trials.
With regard to nonmodifiable patient characteristics, we can easily assume that, in the future, we would be able to treat pediatric patients using gene therapy or those aged ≥65 years, in addition to women affected by severe hemophilia. The recent uniQure AMT‐061 study using an AAV5 vector containing FIX Padua DNA did not exclude patients with NAbs, yet still obtained good results (FIX activity of 30%‐54% at 36 weeks after infusion); hence, gene therapy may be considered for these patients. 1 , 10 Of note is that in our study, around 60% of individuals with severe HA and eligible for gene therapy trial participation displayed NAbs, and they were, therefore, excluded from participation.
Several psychosocial and environmental exclusion criteria are directly related to specific constraints, including travel, time spent at the clinic, or psychological stress, which are associated with gene therapy–related study protocols. Despite not being detailed in study protocols, they still play an important role for patient selection. 11 The study protocol for gene therapy consists of a single intravenous injection, yet associated with regular follow‐up consultations (weekly at the beginning) with collection of blood, saliva, and semen specimens, thereby rendering study participation time‐intensive and burdensome for several patients. 12 Gene therapy administration outside of a clinical study should reduce this burden, thereby increasing the proportion of patients who are likely to receive gene therapy. Regular monitoring, particularly involving liver tests to detect liver damage, will still be essential; these tests, however, can be carried out by a peripheral treatment center close to the patient’s home, in strict collaboration with the HTC.
Of the 29 patients who have refused to participate in a trial, three patients reported fear and lack of confidence concerning the gene therapy setting, while 16 patients were not willing to change their usual treatment. This latter observation certainly reflects the need for security and stability. We sincerely hope that strong scientific evidence concerning long‐term gene therapy safety and efficacy for the hemophilia indication will be obtained in the future, thus providing answers to several persisting concerns with respect to patient safety, attainable factor levels, durability, and possible redosing requirements. 13 In our clinical practice, it appears essential to provide exhaustive information on potential gene therapy risks and benefits, while worries about this new treatment should be taken seriously. We must also pay attention to our patients’ expectations regarding gene therapy. It should be made absolutely clear to the candidates that gene therapy neither provides a cure for existing joint damage, nor does it abolish the hereditary nature of hemophilia. 12 The possible need for immunosuppressive therapy during the study should also be discussed with patients. At our HTC, no patient has been excluded on the basis of a refusal to be treated with an immunosuppressive agent or a formal medical contraindication to such treatment.
Lack of motivation to participate in a gene therapy trial was reported by 12 patients, six of whom were excluded for this sole reason. We expect that most of these patients could be motivated to consider gene therapy outside of a clinical trial setting, provided that they are sufficiently informed and accompanied by their HTC team. Furthermore, based on clinical data, our knowledge of patients and patients’ claims, we anticipated that 36 of our 87 patients with severe hemophilia disease would accept gene therapy, yet outside of a clinical trial, without considering potential exclusionary factors. The PAVING study that was conducted in 2019 and involved a small Belgian patient sample (n = 20) demonstrated a more positive attitude of individuals with hemophilia toward gene therapy. This study, however, may have been subject to a sampling bias, and thus its participants may not be representative of all Belgian people with hemophilia. 14
Upon follow‐up visits, at patient meetings, and as part of a comprehensive approach to hemophilia care, the hematologists should individually assess gene therapy benefits for each patient and regularly inform them about advances in gene therapy. Scores and algorithms providing guidance for treatment choice in hemophilia must still be developed; these should integrate the main gene therapy efficacy and safety outcomes, which were reported by the coreHEM project in 2018. 7 , 15
5. CONCLUSIONS
Although the vast majority of individuals with hemophilia with severe disease at our HTC could not be enrolled in gene therapy trials, the proportion of individuals who may actually undergo gene therapy is likely to significantly increase in the future, given that eligibility criteria will change upon commercialization of products. We emphasize that almost half of our patient cohort was excluded primarily due to psychosocial factors, some of which are clearly modifiable, thus constituting leverage points that are able to increase gene therapy adoption. Solid scientific evidence on long‐term gene therapy efficacy and safety is urgently needed to extend its availability and adoption to a broader population of individuals with hemophilia, including women and pediatric patients, as well as those with inhibitors of FVIII/FIX or AAV NAbs.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
ETHICS APPROVAL
The data on patient eligibility for gene therapy collected in this article are part of the patient selection process for hemophilia A and B gene therapy studies conducted at our center and approved by the local ethics committee.
AUTHOR CONTRIBUTIONS
All authors wrote and approved the final version of the manuscript.
Handling Editor: Pantep Angchaisuksiri.
Contributor Information
Evelien Krumb, @krumbeve.
Cedric Hermans, Email: cedric.hermans@uclouvain.be, @HermansCedric.
REFERENCES
- 1. Croteau SE, Wang M, Wheeler AP. 2021 clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2021;96(1):128–144. 10.1002/ajh.26018 [DOI] [PubMed] [Google Scholar]
- 2. Nathwani AC, Tuddenham EG, Rangarajan S et al. Adenovirus‐associated virus vector‐mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357‐2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nathwani AC, Tuddenham EGD. Haemophilia, the journey in search of a cure. 1960–2020. Br J Haematol. 2020;191(4):573‐578. [DOI] [PubMed] [Google Scholar]
- 4. US National Library of Medicine, ClinicalTrials.gov, database of privately and publicly funded clinical studies conducted around the world. Accessed January 25, 2021. Available from https://clinicaltrials.gov
- 5. Pasi KJ, Rangarajan S, Mitchell N et al. Multiyear follow‐up of AAV5‐hFVIII‐SQ Gene Therapy for Hemophilia A. N Engl J Med. 2020;382(1):29‐40. [DOI] [PubMed] [Google Scholar]
- 6. World Federation of Hemophilia . Update on FDA approval for valoctocogene roxaparvovec gene therapy for severe hemophilia A. World Federation of Hemophilia website. August 19, 2020. Accessed November 12, 2020. Available from https://news.wfh.org/update‐on‐fda‐approval‐for‐valoctocogene‐roxaparvovec‐gene‐therapy‐for‐severe‐hemophilia‐a/
- 7. Sidonio RF Jr, Pipe SW, Callaghan MU, Valentino LA, Monahan PE, Croteau SE. Discussing investigational AAV gene therapy with hemophilia patients: a guide. Blood Rev. 2020;100759. [DOI] [PubMed] [Google Scholar]
- 8. A Phase 3 Open‐Label, Single‐Arm Study To Evaluate The Efficacy and Safety of BMN 270, an Adeno‐Associated Virus Vector‐Mediated Gene Transfer of Human Factor VIII in Hemophilia A Patients with Residual FVIII Levels ≤1 IU/dL Receiving Prophylactic FVIII Infusions. Federal agency for medicines and health products website. Accessed January 18, 2021. Available from https://clinicaltrialsdatabase.be/en/trials/8e19282e‐2a67‐439f‐a614‐1203000750c3
- 9. Phase III, open‐label, single‐dose, multi‐center multinational trial investigating a serotype 5 adeno‐associated viral vector containing the Padua variant of a codon‐optimized human factor IX gene (AAV5‐hFIXco‐Padua, AMT‐061) administered to adult subjects with severe or moderately severe hemophilia B. Federal Agency for Medicines and Health Products website. Accessed January 18, 2021. Available from https://clinicaltrialsdatabase.be/en/trials/f9d4473a‐bba6‐471d‐bae8‐9a4c65d96deb
- 10. Pipe S, Giermasz A, Castaman G et al. One year data from a phase 2b trial of AMT‐061 (AAV5‐Padua hFIX variant), an enhanced vector for gene transfer in adults with severe or moderate‐severe hemophilia B. Blood. 2019;134(Suppl 1):3348. [Google Scholar]
- 11. Evaluating inclusion and exclusion criteria in clinical trials. FDA public workshop: workshop report. Washington, DC: The National Press Club. 16 Apr 2018.
- 12. Miesbach W, O'Mahony B, Key NS, Makris M. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. 2019;25(4):545‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pierce GF, Kaczmarek R, Noone D, O’Mahony B, Page D, Skinner MW. Gene therapy to cure haemophilia: is robust scientific inquiry the missing factor? Haemophilia. 2020;26(6):931‐933. [DOI] [PubMed] [Google Scholar]
- 14. Overbeeke E, Michelsen S, Hauber B et al. Patient perspectives regarding gene therapy in haemophilia: interviews from the PAVING study. Haemophilia. 2021;27(1):129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iorio A, Skinner MW, Clearfield E et al. Core outcome set for gene therapy in haemophilia: results of the coreHEM multistakeholder project. Haemophilia. 2018;24(4):e167‐e172. [DOI] [PubMed] [Google Scholar]


