Abstract
Background
The XALIA and XALIA‐LEA prospective, noninterventional studies investigated the safety and effectiveness of rivaroxaban versus standard anticoagulation for venous thromboembolism (VTE) treatment in routine clinical practice across global regions.
Objectives
This pooled analysis combined their data to determine the incidence of thromboembolic and bleeding events in both treatment groups and addressed specific bleeding patterns in a broad range of patients.
Methods
Patients with objectively confirmed VTE and an indication for ≥3 months’ anticoagulation treatment received rivaroxaban or standard anticoagulation (eg, initial treatment with heparin/fondaparinux, followed by a vitamin K antagonist [VKA]). Treatment choice, dose, management, and duration were at the physician’s discretion. Primary outcomes (major bleeding, recurrent VTE, and all‐cause mortality) were compared between the two treatment groups. Propensity score stratification, and matching were used to reduce bias due to confounding variables.
Results
Overall, 7129 patients were enrolled from 36 countries; 6445 and 2714 patients were included in the propensity score–stratified and –matched analyses, respectively. Major bleeding and incidences of recurrent VTE were similar between treatment groups; all‐cause mortality was lower with rivaroxaban than with standard anticoagulation. The incidences of genitourinary bleeding were higher with rivaroxaban than with standard anticoagulation therapy (46 and 23 events in the matched analysis, respectively). VKA management in real‐world practice was suboptimal.
Conclusion
XALIA and XALIA‐LEA show similar safety and effectiveness profiles of rivaroxaban and standard anticoagulation for VTE treatment in routine practice in many parts of the world. The observations are consistent with results from the phase III EINSTEIN randomized controlled trials.
Keywords: anticoagulants, direct oral anticoagulants, genitourinary bleeding, noninterventional study, propensity score, rivaroxaban, venous thromboembolism treatment
Essentials.
Rivaroxaban is used for VTE treatment, but real‐world data in several global regions are lacking.
XALIA and XALIA‐LEA compared rivaroxaban versus standard anticoagulation in clinical practice.
Pooled analyses suggest similar safety and effectiveness profiles in both treatment groups.
The findings of these phase IV studies are consistent with the phase III EINSTEIN trials.
1. INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major health problem potentially leading to severe short‐ and long‐term sequelae and is associated with increased mortality. 1 Standard treatment for acute VTE has been a parenteral anticoagulant (eg, low‐molecular‐weight heparin [LMWH]) overlapping with and followed by a vitamin K antagonist (VKA). However, as an outcome of successful phase III clinical trials, the direct oral anticoagulants (DOACs; apixaban, dabigatran, edoxaban, and rivaroxaban) are now the guideline‐preferred treatment options for most patients. 2
Phase III clinical trials have clearly defined study protocols, with strict inclusion and exclusion criteria, which may limit their applicability to broader, unselected patient populations. Consequently, for the DOACs, the focus has now shifted to data collection from routine clinical practice, specifically “real‐world” evidence, which aims to establish whether the outcomes of clinical trials are replicated in more diverse patient populations and to provide information on how drugs are used by physicians in day‐to‐day practice. Furthermore, noninterventional phase IV studies may help formulate hypotheses to be tested in subsequent experiments, and they are of great educational value.
XA inhibition with rivaroxaban for Long‐term and Initial Anticoagulation in venous thromboembolism (XALIA) and XALIA in Latin America, Eastern Europe, the Middle East, Africa, and Asia‐Pacific (XALIA‐LEA) were large, multicenter, prospective, noninterventional studies that investigated the safety and effectiveness profile of rivaroxaban versus standard anticoagulation therapy for the treatment of VTE in everyday clinical practice. 3 , 4 XALIA enrolled 5142 patients with objectively confirmed DVT between June 2012 and March 2014 from Europe, Canada, and Israel. 3 Patients with DVT and concomitant PE (but not isolated PE) were eligible for enrollment after a protocol amendment in August 2013 after the European approval of rivaroxaban for the treatment of PE. XALIA‐LEA enrolled 1987 patients with objectively confirmed DVT and/or PE between June 2014 and October 2015 from Eastern Europe, the Middle East, Africa, Asia‐Pacific, and Latin America. 4 Both studies reported low rates of major bleeding and recurrent VTE with rivaroxaban, demonstrating that rivaroxaban may be a reasonable alternative to standard anticoagulation for the treatment of VTE in a broad range of patients in routine clinical practice. 3 , 4
This pooled analysis of XALIA and XALIA‐LEA reports outcomes with rivaroxaban and standard anticoagulation in an expanded sample of patients and encompasses more global regions and countries (36 countries in total) than those in XALIA. Pooling the data from these two studies not only allows for a larger and broader population but also enables analyses of outcomes that were not feasible in either individual study. For example, PE was significantly underrepresented in XALIA, 3 and previous analyses of randomized controlled trials suggested the need for further investigation of genitourinary bleeding in patients receiving anticoagulation for the treatment of VTE. 5 , 6 However, the sample sizes of the individual XALIA and XALIA‐LEA studies did not allow these issues to be addressed. The combined analysis provides an opportunity to explore the topics further.
2. METHODS
A pooled analysis of the prospective, observational XALIA and XALIA‐LEA studies of unselected patients with VTE initiating treatment with rivaroxaban or standard anticoagulation was performed. XALIA and XALIA‐LEA had the same basic study design; the methods for which have been described in detail previously. 3 , 4
2.1. Study design and participants
XALIA and XALIA‐LEA were multicenter, prospective, noninterventional studies enrolling adult patients with objectively confirmed VTE and an indication to receive anticoagulation treatment for ≥3 months: XALIA included patients with DVT alone or DVT and concomitant PE 3 ; XALIA‐LEA included patients with DVT and/or PE. 4 Patients received rivaroxaban or standard anticoagulation (initial treatment with unfractionated heparin, LMWH, or fondaparinux, usually overlapping with and followed by a VKA). Both XALIA and XALIA‐LEA were registered with ClinicalTrials.gov (NCT01619007 and NCT02210819, respectively).
2.2. Procedures
Treatment, dose, and duration were at the attending physician’s discretion. Patients who received rivaroxaban alone and those who had received heparin/fondaparinux for ≤48 hours before enrollment were included in the rivaroxaban cohort. “Early switchers” were those who initially received heparin/fondaparinux for >2‐14 days and/or a VKA for 1‐14 days before switching to rivaroxaban. Detailed analyses of patients classed as early switchers have been published recently 7 ; thus, they were excluded from these analyses. The planned follow‐up period was 12 months from the final patient enrollment.
2.3. Outcomes
The primary outcomes in XALIA and XALIA‐LEA were the incidences of treatment‐emergent major bleeding, recurrent VTE, and all‐cause mortality. Treatment‐emergent outcomes were defined as those occurring in the at‐risk period, that is, from the start of anticoagulation therapy until 2 days after discontinuation of treatment. In case of switching between rivaroxaban and standard of care, only the time on the initial regimen was considered.
Secondary outcomes included major adverse cardiovascular events (cardiovascular death, stroke, myocardial infarction, unstable angina, and acute coronary syndromes), other symptomatic thromboembolic events (Budd‐Chiari syndrome, retinal vein thrombosis, sinus vein thrombosis, portal vein thrombosis, catheter‐associated thrombosis, and upper‐limb thrombosis [if the initial DVT was not an upper‐limb thrombosis]), health care resource use (admissions to hospital, length of stay, and adverse events [AEs] leading to prolonged hospitalization during the study period), and other AEs. All reported primary outcomes, major adverse cardiovascular events, and any other thromboembolic events were adjudicated by a central adjudication committee, who were blinded to treatment decisions.
2.4. Statistical analysis
XALIA and XALIA‐LEA were postauthorization safety studies not designed for formal hypothesis testing with respect to superiority or noninferiority of specific study outcomes. The safety analysis included a descriptive analysis of the primary and secondary outcomes in patients who received at least one dose of the anticoagulant treatment (the safety population). Propensity score stratification (with 10 subclasses) and propensity score matching were used to adjust for imbalances in baseline characteristics between treatment groups (propensity score–stratified and –matched analysis sets). Propensity score stratification was the main method in each case because it allows most patients to be included in the analysis, that is, only those patients who have extreme propensity scores are excluded. Propensity score–matched analysis allows a simple direct comparison between groups and was, therefore, performed as a sensitivity analysis. Baseline characteristics used for the derivation of the propensity scores included alcohol consumption, baseline creatinine clearance, body mass index, weight, country, clinical characteristics of index VTE (eg, presentation/location/diagnosis/symptoms), recent hospitalization or hospitalization for current VTE, history of chronic inflammatory disease/chronic obstructive pulmonary disease/diabetes mellitus/liver cirrhosis/tuberculosis, patient’s insurance, race, reason for choice of initial VTE treatment, relevant pretrial medications (eg, acetylsalicylic acid, nonsteroidal anti‐inflammatory drugs, cytochrome P450 3A4 inhibitor/inducers, P‐glycoprotein inhibitors, and other anticoagulants/antithrombotics), presence of VTE risk factors, sex, smoking status, study (ie, XALIA or XALIA‐LEA), and type of VTE (ie, DVT only/PE with or without DVT). To compare the treatment groups with respect to the primary and secondary variables, hazard ratios (HRs) from stratified Cox regression and corresponding 95% confidence intervals (CIs) were calculated. Models for major bleeding, major or nonmajor clinically relevant bleeding, and recurrent VTE used active cancer at baseline as a covariate; study and type of VTE were included in the model as stratification variables in the safety population; propensity score subclass was additionally included as a stratification variable in Cox regressions of the propensity score–stratified analysis set.
The model for all‐cause mortality used the same variables as above, with the only difference being that active cancer was used as a stratification variable. Because of the low numbers of events, models for major adverse cardiovascular events and other thromboembolic events were neither adjusted nor stratified for active cancer, but were stratified by subclass, study, and type of VTE. Time in therapeutic range (TTR) in the patients who received VKA treatment was ascertained using the method described by Rosendaal and colleagues 8 and adjusted by removing unreliable periods (ie, periods where additional anticoagulants were used, periods after major bleeding or venous thromboembolic events, and VKA interruption of >3 days). SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
3. RESULTS
A total of 7129 patients were enrolled between June 26, 2012, and October 3, 2015, from hospital and community centers across 36 countries worldwide.
In total, 21 patients (0.3%) who did not take any study medication were excluded. A further 653 patients (9.2%) were defined as early switchers and were ineligible for the safety analysis set. A total of 6455 patients (90.5%) were valid for the safety analysis set: 3904 received rivaroxaban and 2551 received standard anticoagulation (Figure 1). Overall, 6445 of the 6455 patients in the safety analysis set were included in the propensity score–stratified analysis set (90.4% of the enrolled population and 99.8% of the safety analysis set). A total of 2714 patients in the safety analysis set were included in the propensity score–matched analysis set (38.1% of the enrolled population and 42.0% of the safety analysis set). Ten patients with HIV, who were included in the safety analysis set, were excluded from the propensity score analysis sets because of their low numbers (two patients in the rivaroxaban group and eight patients in the standard anticoagulation group) and severity of the disease. The proportions of patients who prematurely discontinued from the study were 14.8% (579/3904) in the rivaroxaban group and 18.8% (479/2551) in the standard anticoagulation group. Reasons for premature discontinuation are summarized in Table S1. Overall, 425/7108 patients (6.0%) were lost to follow‐up.
FIGURE 1.
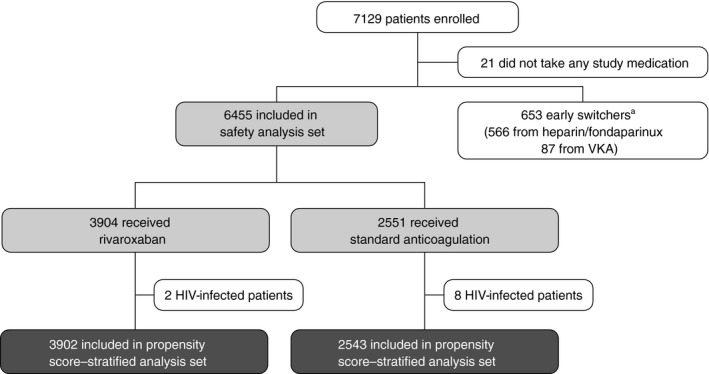
Flow of patients through the study from enrollment to treatment allocation and inclusion in the propensity score–stratified analysis set. aEarly switchers were defined as patients for whom rivaroxaban was planned, but who initially received heparin or fondaparinux for ≥2‐14 days, a VKA for 1‐14 days, or both before switching to rivaroxaban. This patient group was ineligible for the safety analysis set. HIV, human immunodeficiency virus; VKA, vitamin K antagonist
3.1. Baseline demographics and clinical characteristics
Baseline demographics and clinical characteristics by treatment group are shown in Table 1. Patients treated with rivaroxaban were younger and were less likely to have creatinine clearance <50 mL/min, cancer, provoked VTE, or be fragile compared with those treated with standard anticoagulation.
TABLE 1.
Baseline patient characteristics in the safety analysis set
| Characteristic | Rivaroxaban (n = 3904) | Standard anticoagulation (n = 2551) | P value |
|---|---|---|---|
| Age, y, mean ± SD | 58.0 ± 16.9 | 62.2 ± 17.1 | <.0001 |
| <60 | 1958 (50.2) | 1021 (40.0) | <.0001 |
| ≥60 | 1946 (49.8) | 1530 (60.0) | |
| Sex, male | 2051 (52.5) | 1296 (50.8) | .17 |
| Body weight, kg, mean ± SD | 78.6 ± 18.4 | 79.4 ± 18.1 | .16 |
| <50 kg | 95 (2.4) | 57 (2.2) | NA |
| ≥50 to ≤70 kg | 1037 (26.6) | 671 (26.3) | |
| >70 to <90 kg | 1234 (31.6) | 837 (32.8) | |
| ≥90 kg | 796 (20.4) | 564 (22.1) | |
| Missing | 742 (19.0) | 422 (16.5) | |
| BMI, kg/m2, mean ± SD | 27.4 ± 5.2 | 28.2 ± 6.8 | <.0001 |
| <25 kg/m2 | 975 (25.0) | 499 (19.6) | .007 |
| ≥25 to ≤35 kg/m2 | 1538 (39.4) | 923 (36.2) | |
| >35 kg/m2 | 228 (5.8) | 163 (6.4) | |
| Missing | 1163 (29.8) | 966 (37.9) | |
| First available CrCl, mL/min, mean ± SD | 97.8 ± 42.3 | 91.9 ± 47.0 | <.0001 |
| <30 mL/min | 35 (0.9) | 80 (3.1) | <.0001 |
| ≥30 to <50 mL/min | 191 (4.9) | 198 (7.8) | |
| ≥50 to <80 mL/min | 694 (17.8) | 488 (19.1) | |
| ≥80 mL/min | 1574 (40.3) | 928 (36.4) | |
| Missing | 1410 (36.1) | 857 (33.6) | |
| Fragile a | 768 (19.7) | 763 (29.9) | <.0001 |
| Type of VTE | .62 | ||
| DVT only | 3281 (84.0) | 2132 (83.6) | |
| PE ± DVT | 623 (16.0) | 419 (16.4) | |
| DVT location | |||
| Upper limb | 168 (4.3) | 158 (6.2) | |
| Lower limb | 3633 (93.1) | 2342 (91.8) | |
| Active cancer | 362 (9.3) | 480 (18.8) | <.0001 |
| Known thrombophilia | 206 (5.3) | 124 (4.9) | .46 |
| AT deficiency | 4 (0.1) | 6 (0.2) | |
| PC deficiency | 46 (1.2) | 24 (0.9) | |
| PS deficiency | 17 (0.4) | 7 (0.3) | |
| aPCR | 9 (0.2) | 4 (0.2) | |
| Lupus anticoagulant | 13 (0.3) | 8 (0.3) | |
| Hyperhomocysteinemia | 12 (0.3) | 8 (0.3) | |
| Factor V Leiden | 75 (1.9) | 50 (2.0) | |
| Prothrombin G20210A | 15 (0.4) | 9 (0.4) | |
| Antiphospholipid antibodies | 12 (0.3) | 13 (0.5) | |
| Other | 35 (0.9) | 19 (0.7) | |
| Provoked VTE | 1376 (35.2) | 1015 (39.8) | .0002 |
| Previous VTE | 780 (20.0) | 536 (21.0) | .31 |
| Previous major bleeding | 65 (1.7) | 73 (2.9) | .001 |
| Geographic region | <.0001 | ||
| Western Europe/Canada/Israel | 2318 (59.4) | 1913 (75.0) | |
| Eastern Europe | 490 (12.6) | 283 (11.1) | |
| Middle East/Africa | 284 (7.3) | 149 (5.8) | |
| Asia‐Pacific | 720 (18.4) | 167 (6.5) | |
| Latin America | 92 (2.4) | 39 (1.5) | |
Data are n (%) unless stated otherwise.
Abbreviations: aPCR, activated protein C resistance; AT, antithrombin; BMI, body mass index; CrCl, creatinine clearance; DVT, deep vein thrombosis; NA, not available; PC, pyruvate carboxylase; PE, pulmonary embolism; PS, protein S; SD, standard deviation; VTE, venous thromboembolism.
Fragile patients were defined as aged >75 years old or weight ≤50 kg or first available CrCl <50 mL/min; note that some patients had missing CrCl values.
Regarding type of index VTE, of the 6455 patients in the safety population, there were 5413 patients (83.9%) with DVT alone and 1042 (16.1%) with PE with or without DVT. Of these, 3281/3904 patients (84.0%) in the rivaroxaban group and 2132/2551 (83.6%) in the standard anticoagulation group had DVT alone, whereas 623/3904 (16.0%) and 419/2551 (16.4%) had PE with or without DVT, respectively. The proportions of patients with upper‐limb DVT were higher in the standard anticoagulation group, whereas proportions of lower‐limb DVT were slightly higher in the rivaroxaban group.
3.2. Treatment patterns
Treatment patterns in patients in the safety population receiving rivaroxaban or standard anticoagulation are shown in Tables 2 and 3, respectively. Of the 3904 patients treated with rivaroxaban, a single‐drug approach was used in the majority (75.9%; 2962/3904). Most rivaroxaban‐treated patients (90.8%) received an initial dose in accordance with the label (ie, 15 mg twice daily) and planned to switch to 20 mg once daily after 21 days, whereas 359 patients (9.2%) received an initial rivaroxaban dose of 20 mg once daily, 15 mg once daily, or “other.” Patient characteristics at baseline that increased the use versus nonuse of initial rivaroxaban 15 mg twice daily dosing included being male, aged <60 years, and enrolled outside of Latin America, as well as the absence of venous catheter placement, cardiovascular disease, and “fragile” characteristics (Table S2).
TABLE 2.
Treatment patterns in patients in the safety population receiving rivaroxaban
| Treatment details | Rivaroxaban (n = 3904) |
|---|---|
| Initial parenteral treatment for ≤48 h before enrollment | 942 (24.1) |
| Rivaroxaban only | 2962 (75.9) |
| Treatment duration, d, median (IQR) | 183 (95‐274) |
| Follow‐up duration, d, median (IQR) | 225 (152‐374) |
| Rivaroxaban initial dosing | |
| 15 mg twice daily | 3545 (90.8) |
| 20 mg once daily | 199 (5.1) |
| 15 mg once daily | 53 (1.4) |
| Other | 107 (2.7) |
| Planned switch to rivaroxaban dose after 21 ± 3 days | |
| 20 mg once daily | 2633 (67.4) |
| 15 mg once daily | 38 (1.0) |
| Rivaroxaban doses received at any time during study | |
| 15 mg twice daily | 3572 (91.5) |
| 20 mg once daily | 3358 (86.0) |
| 15 mg once daily | 174 (4.5) |
| Other | 194 (5.0) |
Data are n (%) unless stated otherwise.
Abbreviation: IQR, interquartile range.
TABLE 3.
Treatment patterns in patients in the safety population receiving standard anticoagulation
| Treatment details | Standard anticoagulation (n = 2551) |
|---|---|
| Heparin/fondaparinux only | 607 (23.8) |
| VKA | 1944 (76.2) |
| Initial heparin/fondaparinux | 1586 (62.2) |
| No heparin/fondaparinux | 358 (14.0) |
| Treatment duration, d, median (IQR) | 186 (94‐365) |
| Follow‐up duration, d, median (IQR) | 263 (172‐401) |
| Patients receiving VKA with available INR values | 989 (50.9) |
| TTR, mean, % (SD) | |
| INR <2.0 | 33.5 (39.3) |
| INR 2.0‐3.0 | 53.5 (39.0) |
| INR >3.0 | 13.0 (25.5) |
Data are n (%) unless stated otherwise.
Abbreviations: INR, international normalized ratio; IQR, interquartile range; SD, standard deviation; TTR, time in therapeutic range; VKA, vitamin K antagonist.
Of the 2551 patients treated with standard anticoagulation, 1944 (76.2%) received treatment that included a VKA (358 [14.0%] received a VKA without initial heparin/fondaparinux); 607 patients (23.8%) received parenteral anticoagulants only. VKA management was suboptimal; international normalized ratio (INR) values in the therapeutic range of 2.0 to 3.0 were observed only 53.5% of the time. The INR was <2.0 for 33.5% of the time and >3.0 for 13.0% of the time (Table 3). Patient characteristics at baseline that increased the use versus nonuse of initial heparin/fondaparinux before transitioning to a VKA included presence of active cancer, catheter venous placement, immobilization, provoked DVT, and recent surgery (Table S3).
3.3. Outcome events
Treatment‐emergent clinical outcomes for the safety analysis set and the propensity score–stratified and –matched analyses sets are presented in Tables 4, 5, 6.
TABLE 4.
Treatment‐emergent outcomes in the propensity score–stratified analysis set
| Outcome | Rivaroxaban (n = 3902) | Standard anticoagulation (n = 2543) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| n (%) | Events per 100 patient‐years (95% CI) | n (%) | Events per 100 patient‐years (95% CI) | |||
| Safety | ||||||
| Major bleeding | ||||||
| Any | 39 (1.00) | 1.74 (1.24‐2.38) | 63 (2.48) | 3.95 (3.03‐5.05) | 0.65 (0.39‐1.08) a | .10 |
| Head and neck | 1 (<0.1) | … | 4 (0.2) | … | … | |
| CNS | 8 (0.2) | … | 6 (0.2) | … | … | … |
| Thorax | 2 (0.1) | … | 1 (<0.1) | … | – | … |
| Gastrointestinal | 14 (0.4) | … | 24 (0.9) | … | … | … |
| Abdominal | 1 (<0.1) | … | 7 (0.3) | … | … | … |
| Genitourinary | 9 (0.2) | … | 8 (0.3) | … | … | … |
| Uterine | 8 (0.2) | … | 3 (0.1) | … | … | … |
| Macroscopic (gross) hematuria | 1 (<0.1) | … | 4 (0.2) | … | … | … |
| Other urogenital | 0 | … | 2 (0.1) | … | … | … |
| Musculoskeletal | 0 | … | 3 (0.1) | … | … | … |
| Skin | 2 (0.1) | … | 7 (0.3) | … | … | … |
| Other | 4 (0.1) | … | 10 (0.4) | … | … | … |
| Effectiveness | ||||||
| Recurrent VTE | 55 (1.41) | 2.47 (1.86‐3.21) | 71 (2.79) | 4.49 (3.51‐5.67) | 0.85 (0.54‐1.32) a | .46 |
| Other | ||||||
| Major adverse cardiovascular events b | 15 (0.38) | 0.67 (0.37‐1.10) | 15 (0.59) | 0.94 (0.52‐1.54) | 1.47 (0.61‐3.55) c | .39 |
| Other thromboembolic events d | 4 (0.10) | 0.18 (0.05‐0.46) | 5 (0.20) | 0.31 (0.10‐0.73) | 1.12 (0.27‐4.75) c | .88 |
| All‐cause mortality | 41 (1.05) | 1.83 (1.31‐2.48) | 117 (4.60) | 7.28 (6.02‐8.72) | 0.55 (0.33‐0.91) e | .02 |
Abbreviations: CI, confidence interval; CNS, central nervous system; HR, hazard ratio; MedDRA, Medical Dictionary for Regulatory Activities; SMQ, standardized MedDRA queries; VTE, venous thromboembolism.
HRs and corresponding 95% CIs calculated from Cox regression using active cancer at baseline as a covariate and study, type of VTE, and subclass as stratification variables.
Including cardiovascular death, stroke, myocardial infarction, and unstable angina or acute coronary syndrome.
HR and corresponding 95% CIs calculated from Cox regression using study, type of VTE, and subclass as stratification variables.
Defined by MedDRA SMQ “embolic and thromboembolic events” excluding postthrombotic syndrome and events assessed as major adverse cardiovascular events or symptomatic VTE.
HR and corresponding 95% CI calculated from Cox regression using active cancer, study, type of VTE, and subclass as stratification variables.
TABLE 5.
Treatment‐emergent outcomes in the propensity score–matched analysis set
| Outcome | Rivaroxaban (n = 1357) | Standard anticoagulation (n = 1357) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| n (%) | Events per 100 patient‐years (95% CI) | n (%) | Events per 100 patient‐years (95% CI) | |||
| Safety | ||||||
| Major bleeding | ||||||
| Any | 17 (1.25) | 2.11 (1.23‐3.38) | 26 (1.92) | 3.08 (2.01‐4.52) | 0.65 (0.35‐1.20) a | .17 |
| Head/neck | 1 (0.1) | … | 2 (0.1) | … | … | … |
| CNS | 6 (0.4) | … | 3 (0.2) | … | … | … |
| Thorax | 2 (0.1) | … | 0 (0.0) | … | … | … |
| Gastrointestinal | 2 (0.1) | … | 12 (0.9) | … | … | … |
| Abdominal | 1 (0.1) | … | 2 (0.1) | … | … | … |
| Genitourinary | 4 (0.3) | – | 2 (0.1) | … | … | … |
| Uterine | 4 (0.3) | – | 0 (0.0) | … | … | … |
| Macroscopic (gross) hematuria | 0 (0.0) | … | 1 (0.1) | … | … | … |
| Other genitourinary | 0 (0.0) | … | 1 (0.1) | … | … | … |
| Musculoskeletal | 0 (0.0) | – | 1 (0.1) | … | … | … |
| Skin | 0 (0.0) | – | 2 (0.1) | … | … | … |
| Other | 2 (0.1) | … | 5 (0.4) | … | … | … |
| Major or nonmajor bleeding | ||||||
| Any | 154 (11.3) | … | 128 (9.4) | … | 1.22 (0.97‐1.54) a | 0.096 |
| Head/neck | 44 (3.2) | … | 37 (2.7) | … | … | … |
| CNS | 6 (0.4) | … | 3 (0.2) | … | … | … |
| Thorax | 7 (0.5) | … | 2 (0.1) | … | … | … |
| Gastrointestinal | 16 (1.2) | … | 31 (2.3) | … | … | … |
| Abdominal | 1 (<0.1) | – | 2 (0.1) | … | … | … |
| Genitourinary | 46 (3.4) | – | 23 (1.7) | … | … | … |
| Uterine | 28 (2.1) | – | 5 (0.4) | … | … | … |
| Macroscopic (gross) hematuria | 17 (1.3) | – | 14 (1.0) | … | … | … |
| Other genitourinary | 3 (0.2) | – | 4 (0.3) | … | … | … |
| Musculoskeletal | 3 (0.2) | – | 1 (<0.1) | … | … | … |
| Skin | 41 (3.0) | – | 37 (2.7) | … | … | … |
| Other | 17 (1.3) | – | 13 (1.0) | … | … | … |
| Effectiveness | ||||||
| Recurrent VTE | 21 (1.55) | 2.62 (1.62‐4.01) | 27 (1.99) | 3.23 (2.13‐4.70) | 0.79 (0.44‐1.39) a | .41 |
| Other | ||||||
| Major adverse cardiovascular events b | 6 (0.44) | 0.74 (0.27‐1.62) | 9 (0.66) | 1.06 (0.49‐2.02) | 0.71 (0.25‐2.00) c | .52 |
| Other thromboembolic events d | 4 (0.29) | 0.5 (0.14‐1.27) | 4 (0.29) | 0.47 (0.13‐1.21) | 1.06 (0.27‐4.26) c | .93 |
| All‐cause mortality | 19 (1.40) | 2.36 (1.42‐3.68) | 34 (2.51) | 4.00 (2.77‐5.59) | 0.55 (0.31‐0.97) e | .04 |
Abbreviations: CI, confidence interval; CNS, central nervous system; DVT, deep vein thrombosis; HR, hazard ratio; PE, pulmonary embolism; SMQ, standardized MedDRA queries; VTE, venous thromboembolism.
HRs and corresponding 95% CIs calculated from Cox regression using active cancer at baseline as a covariate and study and type of VTE (DVT only/PE with or without DVT) as stratification variables.
Including cardiovascular death, stroke, myocardial infarction, and unstable angina or acute coronary syndrome.
HR and corresponding 95% CI calculated from Cox regression using study and type of VTE (DVT only/PE with or without DVT) as stratification variables.
Defined by MedDRA SMQ “embolic and thromboembolic events” excluding postthrombotic syndrome and events assessed as major adverse cardiovascular events or symptomatic VTE.
HR and corresponding 95% CI calculated from Cox regression using active cancer, study, and type of VTE (DVT only/PE with or without DVT) as stratification variables.
TABLE 6.
Treatment‐emergent outcomes in the safety analysis set
| Outcome | Rivaroxaban (n = 3904) | Standard anticoagulation (n = 2551) | ||
|---|---|---|---|---|
| n (%) | Events per 100 patient‐years (95% CI) | n (%) | Events per 100 patient‐years (95% CI) | |
| Safety | ||||
| Major bleeding (adjudicated) | ||||
| Any | 39 (1.0) | 1.74 (1.24‐2.38) | 63 (2.5) | 3.94 (3.03‐5.04) |
| Fatal | 2 (0.1) | 0.09 (0.01‐0.32) | 3 (0.1) | 0.19 (0.04‐0.54) |
| Nonfatal critical site | 10 (0.3) | 0.45 (0.21‐0.82) | 14 (0.5) | 0.87 (0.48‐1.46) |
| Gastrointestinal | 14 (0.4) | NR | 24 (0.9) | NR |
| CNS | 8 (0.2) | NR | 6 (0.2) | NR |
| Genitourinary | 9 (0.2) | NR | 8 (0.3) | NR |
| Uterine | 8 (0.2) | NR | 3 (0.1) | NR |
| Macroscopic (gross) hematuria | 1 (<0.1) | NR | 4 (0.2) | NR |
| Other | 0 (0.0) | NR | 2 (0.1) | NR |
| Major or nonmajor bleeding (as reported by the investigator) | 396 (10.14) | 18.89 (17.07‐20.84) | 270 (10.58) | 18.00 (15.91‐20.28) |
| Effectiveness | ||||
| Recurrent VTE | 55 (1.4) | 2.47 (1.86‐3.21) | 71 (2.8) | 4.48 (3.50‐5.65) |
| Type of recurrent VTE | ||||
| Fatal PE | 1 (<0.1) | 0.04 (0.00‐0.25) | 5 (0.2) | 0.31 (0.10‐0.72) |
| Death in which PE could not be ruled out | 8 (0.2) | 0.36 (0.15‐0.70) | 8 (0.3) | 0.50 (0.21‐0.98) |
| Nonfatal PE | 17 (0.4) | 0.76 (0.44‐1.21) | 20 (0.8) | 1.25 (0.76‐1.93) |
| DVT and PE | 1 (<0.1) | 0.04 (0.00‐0.25) | 5 (0.2) | 0.31 (0.10‐0.72) |
| DVT | 27 (0.7) | 1.21 (0.80‐1.76) | 33 (1.3) | 2.07 (1.42‐2.91) |
| Other | 1 (<0.1) | 0.04 (0.00‐0.25) | 1 (<0.1) | 0.06 (0.00‐0.35) |
| Other | ||||
| Major adverse cardiovascular events a | 15 (0.4) | 0.67 (0.37‐1.10) | 15 (0.6) | 0.94 (0.52‐1.54) |
| Other thromboembolic events b | 4 (0.1) | 0.18 (0.05‐0.46) | 5 (0.2) | 0.31 (0.10‐0.73) |
| All‐cause mortality | 41 (1.1) | 1.83 (1.31‐2.48) | 117 (4.6) | 7.26 (6.00‐8.70) |
| Cause of death | ||||
| VTE‐related death | ||||
| PE | 2 (0.1) | … | 7 (0.3) | … |
| PE not ruled out | 7 (0.2) | … | 7 (0.3) | … |
| Bleeding (including ICH and hemorrhagic stroke) | 1 (<0.1) | … | 3 (0.1) | … |
| Cancer | 22 (0.6) | … | 69 (2.7) | … |
| Cardiovascular | 3 (0.1) | … | 11 (0.4) | … |
| Infectious disease | 4 (0.1) | … | 16 (0.6) | … |
| Other | 2 (0.1) | … | 4 (0.2) | … |
Abbreviations: CI, confidence interval; CNS, central nervous system; DVT, deep vein thrombosis; ICH, intracranial hemorrhage; MedDRA, Medical Dictionary for Regulatory Activities; NR, not reported; PE, pulmonary embolism; SMQ, standardized MedDRA queries; VTE, venous thromboembolism.
Including cardiovascular death, stroke, myocardial infarction, and unstable angina or acute coronary syndrome.
Defined by MedDRA SMQ “embolic and thromboembolic events” excluding postthrombotic syndrome.
3.3.1. Primary outcomes in the safety population
Major bleeding events occurred less frequently in the rivaroxaban group (39/3904 [1.0%]) than in the standard anticoagulation group (63/2551 [2.5%]). There were two fatal bleeding events in the rivaroxaban group and three in the standard anticoagulation group. Most major bleeding events were in the central nervous system, gastrointestinal tract, or genitourinary tract (Table 6). In the rivaroxaban group, a smaller proportion of major bleeding events occurred in those aged ≥60 years than in the standard anticoagulation group (59.0% vs 71.4%). Similar proportions of major bleeding events in each treatment group were seen in patients with active cancer at baseline (35.9% with rivaroxaban and 36.5% with standard anticoagulation). Finally, a smaller proportion of major bleeding events was seen in fragile patients in the rivaroxaban group than in the standard anticoagulation group (33.3% vs 39.7%).
Recurrent VTE occurred less frequently in the rivaroxaban group (55/3904 [1.4%]) than the standard anticoagulation group (71/2551 [2.8%]). In terms of type of venous thromboembolic events, nonfatal PE occurred in 17 patients (0.4%) in the rivaroxaban group versus 20 patients (0.8%) treated with standard anticoagulation; similarly, DVT without PE occurred in 27 rivaroxaban‐treated patients (0.7%) and 33 (1.3%) standard anticoagulation–treated patients, whereas rates of fatal PE and recurrent DVT with concomitant PE were relatively low in both groups (1 [<0.1%] and 5 [0.2%], respectively) (Table 6). All‐cause mortality was lower in the rivaroxaban group (41/3904 patients [1.1%]) than in the standard anticoagulation group (117/2551 patients [4.6%]). The most frequent cause of death was cancer in both treatment groups; the proportion of patients dying from cancer was lower in patients treated with rivaroxaban versus standard anticoagulation (0.6% and 2.7%, respectively). The cause of death was VTE related in 9 (0.2%) and 14 (0.5%) patients in the rivaroxaban and standard anticoagulation groups, respectively (Table 6).
3.3.2. Primary outcomes in the propensity score–stratified analysis set
Propensity score–stratified analyses (with 10 equally sized subclasses) were done for 3902 patients who received rivaroxaban and 2543 patients who received standard anticoagulation. Within each subclass, baseline characteristics were similar between treatment arms. The incidence of major bleeding was 39 of 3902 (1.0%) in patients treated with rivaroxaban and 63 of 2543 (2.5%) with standard anticoagulation, while the incidence of recurrent VTE was 55 of 3902 (1.4%) and 71 of 2543 (2.8%), respectively. However, propensity score–stratified analyses showed no statistically significant differences between the treatment groups for either of these outcomes (HR, 0.65 [95% CI, 0.39‐1.08] for major bleeding and HR, 0.85 [95% CI, 0.54‐1.32] for recurrent VTE) (Table 4). The adjusted HR for all‐cause mortality was significant in comparing patients treated with rivaroxaban versus standard anticoagulation (41/3902 [1.1%] and 117/2543 [4.6%] patients, respectively; HR, 0.55 [95% CI, 0.33‐0.91]; P = .02) (Table 4).
3.3.3. Primary outcomes in the propensity score–matched analysis set
Propensity score–matched analyses were carried out for 1357 patients who received rivaroxaban and 1357 patients who received standard anticoagulation. Baseline characteristics were similar between treatment arms. Results are shown in Table 5. As per propensity score–stratified analysis, there were no statistically significant differences between the treatment groups for incidences of major bleeding or recurrent VTE, but all‐cause mortality was significantly lower with rivaroxaban versus standard anticoagulation (Table 5).
3.3.4. Secondary outcomes
Incidences of major adverse cardiovascular events and other thromboembolic events were similar between the rivaroxaban and standard anticoagulation groups in both the safety analysis set (Table 6) and the propensity score–stratified and –matched analysis sets (Tables 4 and 5, respectively).
3.3.5. Genitourinary bleeding events
The incidences of major or nonmajor genitourinary bleeding differed between the treatment groups in the propensity score–matched analysis set. In total, there were 46 events in the rivaroxaban group and 23 events in the standard anticoagulation group. In the rivaroxaban group, 28 of 46 events (60.9%) were a result of increased or prolonged menstrual or abnormal vaginal bleeding, 17 of 46 (37.0%) were hematuria, and 3 of 46 (6.5%) were categorized as “other urogenital” bleeding. In the standard anticoagulation group, 5 of 23 events (21.7%) were related to increased or prolonged menstrual or abnormal vaginal bleeding, 14 of 23 (60.9%) were hematuria, and 4 of 23 (17.4%) were other urogenital bleeding events (Table 5). For females aged <55 years, 216 were treated with rivaroxaban, and 216 received standard anticoagulation. The incidences of prolonged menstrual or abnormal vaginal bleeding events that occurred in these patients were 25 of 216 (11.6%) and 4 of 216 (1.9%) in the rivaroxaban and standard anticoagulation groups, respectively (Table S4). In the overall propensity score–matched analysis set, prolonged menstrual or abnormal vaginal bleeding events occurring in females aged <55 years were classed as treatment‐emergent serious AEs in 4 of 1357 (1.9%) patients who received rivaroxaban and 1 of 1357 (0.5%) patients who received standard anticoagulation.
3.3.6. Health care resource use
In the propensity score–stratified analysis set, 1339 patients (34.3%) treated with rivaroxaban and 1286 patients (50.6%) treated with standard anticoagulation were admitted to hospital for their index VTE. The least‐squares mean duration of the initial hospital stay was 6.5 days (standard error [SE] of logarithmized data, 0.04) for the rivaroxaban group and 9.7 days (SE, 0.03) for the standard anticoagulation group, with a geometric mean ratio of 0.67 (95% CI, 0.63‐0.73; P < .001). In the propensity score–matched analysis set, 602 patients (44.4%) treated with rivaroxaban and 604 patients (44.5%) treated with standard anticoagulation were admitted to the hospital for their index VTE. The least‐squares mean duration of initial hospital stay was 7.1 days (SE, 0.04) for the rivaroxaban group and 10.7 days (SE, 0.04) for the standard anticoagulation group, with a geometric mean ratio of 0.66 (95% CI, 0.61‐0.72; P < .001).
In the propensity score–matched analysis set (across both treatment arms), the most frequent AEs leading to prolonged hospitalization were gastrointestinal disorders (38 patients; 1.4%); respiratory, thoracic, and mediastinal disorders (33 patients; 1.2%); and infections and infestations (29 patients; 1.1%). More patients treated with rivaroxaban than those treated with standard anticoagulation were hospitalized for AEs related to nervous system disorders (1.0% and 0.6%, respectively), and fewer were hospitalized for AEs related to the presence of a tumor (0.6% and 1.0%, respectively).
3.3.7. Other AEs
Data on other AEs in the safety population are presented in Table S5.
4. DISCUSSION
This pooled analysis of the XALIA and XALIA‐LEA studies included >7000 patients and provides the largest prospective data set on the use of rivaroxaban for the treatment of VTE in routine clinical practice. The low rates of major bleeding, recurrent VTE, and all‐cause mortality support the safety and effectiveness of rivaroxaban for the treatment of VTE in a broad range of patients. In addition, the analyses provide novel information on anticoagulation treatment in specific patient groups such as those with PE and genitourinary bleeding events. These novel insights contribute to an improved understanding of the effectiveness and safety of rivaroxaban across a broad range of patients in day‐to‐day practice.
In the pooled data set of the XALIA and XALIA‐LEA studies, patients prescribed rivaroxaban were typically younger and less likely to have renal impairment or cancer than those prescribed standard anticoagulation. This was consistent with other real‐world studies that have described baseline characteristics in patients receiving DOACs versus standard anticoagulation. 9 , 10 This may indicate a cautious approach to novel therapies because the XALIA program had been initiated only shortly after the registration of rivaroxaban.
As was the case in two other real‐world studies, the prospective Dresden DOAC Registry and the Registro Informatizado Enfermedad TromboEmbolica (RIETE) Registry, most patients (91%) received the approved initial daily dose of rivaroxaban 15 mg twice daily. 11 , 12 Approximately two‐thirds of patients switched to the label‐recommended rivaroxaban 20 mg once‐daily regimen after 21 days. For comparison, in RIETE 89% of patients receiving rivaroxaban for long‐term treatment of VTE received the recommended dose. 12
The incidence proportion of major bleeding in rivaroxaban‐treated patients in this analysis was consistent with incidences reported in the pooled analysis of the Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism (EINSTEIN PE) and Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis (EINSTEIN DVT) phase III trials (1.0% and 1.0%, respectively), 13 while the mean rivaroxaban treatment durations were the same for the pooled data of XALIA and XALIA‐LEA (208.4 days) and the pooled data of EINSTEIN DVT and EINSTEIN PE (207.6 days). Incidences for recurrent VTE and all‐cause mortality with rivaroxaban were numerically lower than those reported in the pooled analysis of EINSTEIN PE and EINSTEIN DVT 13 : recurrent VTE, 1.4% and 2.1%, respectively; all‐cause mortality, 1.1% and 2.3%, respectively. After both propensity score matching and propensity score stratification, patients treated with rivaroxaban had fewer major bleeding and recurrent venous thromboembolic events and lower all‐cause mortality than those treated with standard anticoagulation, although the differences in major bleeding and recurrent VTE were not statistically significant. Considering that DOACs have not been associated with a mortality benefit versus VKAs for the treatment of VTE in randomized clinical trials, 14 the significant reduction in mortality with rivaroxaban observed in the current study should be interpreted with caution and may reflect limitations of propensity score matching; for example, severity of cancer could not be considered in the propensity score generation. However, lower mortality associated with DOAC use was corroborated by findings from another large (N = 10 870), independent, prospective real‐world database. The Global Anticoagulant Registry in the FIELD ‐ Venous Thromboembolism (GARFIELD‐VTE) Registry also reported a significantly reduced risk of all‐cause mortality in patients receiving DOACs compared with VKAs over a 12‐month follow‐up period. 15 Overall, the findings of this analysis support the applicability of the EINSTEIN PE and EINSTEIN DVT results for the wider VTE population treated in routine clinical practice; however, direct comparisons between the EINSTEIN phase III and the XALIA phase IV programs cannot be made.
In patients treated with standard anticoagulation, incidence proportions were numerically higher than those reported in the standard anticoagulation group of the pooled analysis of the EINSTEIN PE and EINSTEIN DVT studies: The incidence proportions for major bleeding, recurrent VTE, and all‐cause mortality were 2.5% versus 1.7%, 2.8% versus 2.3%, and 4.6% versus 2.4%, respectively. 13 In this context, it is worth mentioning that the control of VKA, as assessed with TTR, in real‐world practice was much worse than under the stringent conditions of a randomized controlled trial. In the pooled analysis of XALIA and XALIA‐LEA, INR values were in the therapeutic range of 2.0‐3.0 for 53.5% of the time, <2.0 for 33.5% of the time, and >3.0 for 13.0% of the time. In contrast, the pooled analysis of EINSTEIN PE and EINSTEIN DVT reported more optimal TTRs: An INR of 2.0‐3.0 was observed for 61.7% of the time, an INR <2.0 for 22.3% of the time, and an INR >3 for 16% of the time. 13 In addition to being driven by a longer standard anticoagulation mean treatment duration (229.4 days in XALIA and XALIA‐LEA vs 203.8 days in EINSTEIN DVT and EINSTEIN PE), this may reflect the fact that more complex patient populations who would have been excluded from the pivotal randomized phase III trials were treated in real‐world situations.
Other outcomes of interest included the frequency of uterine bleeding events in younger women (aged <55 years). The incidence of these events that were attributable to increased or prolonged menstrual or abnormal vaginal bleeding was higher in the rivaroxaban versus the standard anticoagulation group. This observation was not unexpected because similar findings have been reported previously showing incidences of heavy menstrual bleeding with rivaroxaban and other DOACs, including higher incidences with rivaroxaban versus VKAs and LMWH. 5 , 16 , 17 , 18 , 19 , 20 One of the reasons why increased menstrual bleeding is more frequently seen with DOACs than with conventional anticoagulation therapy may relate to their respective modes of action. In contrast to VKAs, heparins, and other indirect anticoagulants, these DOACs are small molecules that bind directly to the active site of the coagulation factors and can penetrate the clot. Thus, DOACs can inhibit circulating clotting factors and clot‐bound factor Xa or thrombin, respectively. Therefore, local clot formation is more potently inhibited by these agents than is the case with indirect anticoagulants. Suggested management strategies include omitting or halving the dose of rivaroxaban in the first 2 days of menses, both of which reduced rates of heavy menstrual bleeding and facilitated continued anticoagulation treatment. 18 In the Reduced‐dosed Rivaroxaban in the Long‐term Prevention of Recurrent Symptomatic VTE (EINSTEIN CHOICE) study, lower rates of abnormal menstrual bleeding were observed with rivaroxaban 10 mg once daily versus acetylsalicylic acid, so the rivaroxaban 10 mg once‐daily regimen could be considered as a potential therapeutic strategy after 6 months of anticoagulation treatment in this situation. 21 Other management strategies of abnormal uterine bleedings, such as oral contraceptive use, were not systematically captured in these patients. In a subanalysis of the EINSTEIN DVT and EINSTEIN PE studies, a nonsignificant difference in the rates of abnormal uterine bleeding or uterine bleeding leading to blood transfusion was observed between patients with and without hormone therapy. 5
Annualized event rates of major bleeding, recurrent VTE, and all‐cause mortality with rivaroxaban in this analysis were 1.7, 2.5, and 1.8 events per 100 patient‐years, respectively; the major bleeding rate was lower than for rivaroxaban‐treated patients in the prospective Dresden NOAC Registry (1.7 and 3.3 events per 100 patient‐years, respectively), whereas the recurrent VTE rate was higher (2.5 and 1.0 events per 100 patient‐years, respectively). 11 On average, rivaroxaban‐treated patients in the Dresden NOAC Registry were older than those in this analysis (61.4 and 58.0 years, respectively) and had a longer mean treatment duration (358 and 208 days, respectively). In the RIETE Registry, in 2348 patients who received long‐term rivaroxaban therapy and would likely have been eligible for the phase III DOAC trials, major bleeding rates were similar (1.6 events per 100 patient‐years), with lower rates of recurrent VTE (1.9 events per 100 patient‐years), versus this analysis. 22 In terms of health care resource use, length of hospital stay was shorter with rivaroxaban than standard anticoagulation in this analysis. This was consistent with the pooled analysis of EINSTEIN PE and EINSTEIN DVT, 23 and similar to the findings reported in several retrospective database analyses. 24 , 25 , 26
In terms of limitations, because XALIA and XALIA‐LEA were noninterventional studies, selection bias likely impacted on the treatment allocations, resulting in imbalances in baseline characteristics between treatments, which was as expected. Sicker patients (eg, those with renal impairment or cancer) were more likely to receive standard anticoagulation therapy; this may be because physicians were more familiar with the use of standard anticoagulation and/or there was a reluctance to use a newer therapy in these higher‐risk patients. In addition, at the time of enrollment in the XALIA and XALIA‐LEA studies, LMWH was the recommended first‐line treatment for cancer‐associated thrombosis. 2 , 27 Despite this, in the time between XALIA and XALIA‐LEA, this phenomenon diminished because physicians gained more experience with rivaroxaban. Several guidelines now recommend certain DOACs as alternatives to LMWH for the treatment of VTE in certain patients with cancer. 28 , 29 Another potential limitation was that patients with isolated PE were eligible only for XALIA‐LEA, but not XALIA, and so were not represented in some geographic regions/countries. The propensity score–stratified and –matched analyses aimed to address these differences in baseline characteristics; however, although the use of propensity score stratification and matching can balance baseline covariates between treatment groups, the potential effects of unmeasured characteristics and confounders cannot be excluded. This, along with selection bias and patient channeling, may account for the significant difference in mortality observed between the treatment groups with propensity score–stratified and –matched analyses.
5. CONCLUSIONS
These pooled analyses of the phase IV XALIA and XALIA‐LEA studies expand on the original XALIA study and complement the results of the phase III EINSTEIN program in real‐world clinical routine. It is the largest prospective data set on the use of rivaroxaban for the treatment of VTE in routine clinical practice, covering several global regions. Rivaroxaban had low incidences of major bleeding and recurrent VTE, which were similar to those observed with standard anticoagulation.
RELATIONSHIP DISCLOSURE
SH has received consultancy fees from Aspen Pharmacare, Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, Pfizer Inc., and Sanofi SA. LGM has received consultancy fees from Bayer, Pfizer Inc., Daiichi Sankyo, and Boehringer Ingelheim; and research support from Bayer, Daiichi Sankyo, and Boehringer Ingelheim. RK has received consultancy fees from Bayer, Berlin‐Chemie Menarini, Daiichi Sankyo, Lundbeck Ltd, and Servier Laboratories Ltd; and speaker’s honoraria from Bayer, Bristol‐Myers Squibb, and Daiichi Sankyo. DM, JS, MT, MG, and J‐PB are employees of Bayer AG. EZ has received consultancy fees from Bayer. WA has received speaker’s honoraria from, and participated in scientific advisory boards for, Aspen Pharmacare, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, CSL Behring, Daiichi Sankyo, Portola, and Stago; and has received research support from Bayer. AGGT has received speaker’s honoraria and consultancy fees from, and participated in scientific advisory boards for, Bayer and Janssen Research & Development, LLC.
AUTHOR CONTRIBUTIONS
SH created the initial draft of this report. LM, RK, JS, MT, J‐PB, WA, and AGGT provided input into the content and direction of the manuscript. MG performed the statistical analyses, and EZ did the propensity score design. DM was responsible for data management. All authors had full access to the source data and participated in writing and reviewing the report and accept full responsibility for its overall content.
Supporting information
Table S1‐5
ACKNOWLEDGMENTS
The authors thank Hayley Dawson of Chameleon Communications International, who provided editorial support with funding from Bayer AG and Janssen Scientific Affairs, LLC.
Haas S, Mantovani LG, Kreutz R, et al. Anticoagulant treatment for venous thromboembolism: A pooled analysis and additional results of the XALIA and XALIA‐LEA noninterventional studies. Res Pract Thromb Haemost. 2021;5:426–438. 10.1002/rth2.12489
Handling Editor: Cihan Ay
Contributor Information
Sylvia Haas, Email: sylvia@sylviahaas.com.
Reinhold Kreutz, @KreutzReinhold.
Alexander G. G. Turpie, @turpiea.
REFERENCES
- 1. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235–42. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 3. Ageno W, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep‐vein thrombosis (XALIA): an international, prospective, non‐interventional study. Lancet Haematol. 2016;3:e12–21. [DOI] [PubMed] [Google Scholar]
- 4. Kreutz R, Mantovani LG, Haas S, Monje D, Schneider J, Bugge JP, et al. XALIA‐LEA: an observational study of venous thromboembolism treatment with rivaroxaban and standard anticoagulation in the Asia‐Pacific, Eastern Europe, the Middle East, Africa and Latin America. Thromb Res. 2019;176:125–32. [DOI] [PubMed] [Google Scholar]
- 5. Martinelli I, Lensing AWA, Middeldorp S, Levi M, Beyer‐Westendorf J, van Bellen B, et al. Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use. Blood. 2016;127:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alotaibi GS, Almodaimegh H, McMurtry MS, Wu C. Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex‐based meta‐analysis. Thromb Res. 2013;132:185–9. [DOI] [PubMed] [Google Scholar]
- 7. Turpie AGG, Mantovani LG, Haas S, Kreutz R, Monje D, Schneider J, et al. Analysis of patients with deep vein thrombosis switched from standard therapy to rivaroxaban in the non‐interventional XALIA study. Thromb Res. 2017;155:23–7. [DOI] [PubMed] [Google Scholar]
- 8. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PubMed] [Google Scholar]
- 9. Cohen AT, Gitt AK, Bauersachs R, Fronk EM, Laeis P, Mismetti P, et al. The management of acute venous thromboembolism in clinical practice. Results from the European PREFER in VTE Registry. Thromb Haemost. 2017;117:1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman CI, Bunz TJ, Turpie AGG. Effectiveness and safety of rivaroxaban versus warfarin for treatment and prevention of recurrence of venous thromboembolism. Thromb Haemost. 2017;117:1841–7. [DOI] [PubMed] [Google Scholar]
- 11. Keller L, Marten S, Hecker J, Werth S, Tittl L, Beyer‐Westendorf J. Treatment of acute VTE with rivaroxaban ‐ results of the prospective Dresden NOAC Registry (NCT01588119). Blood. 2016;128:2618; Abstract 332. [Google Scholar]
- 12. Trujillo‐Santos J, Di Micco P, Dentali F, Douketis J, Diaz‐Peromingo JA, Nunez MJ, et al. Real‐life treatment of venous thromboembolism with direct oral anticoagulants: the influence of recommended dosing and regimens. Thromb Haemost. 2017;117:382–9. [DOI] [PubMed] [Google Scholar]
- 13. Prins MH, Lensing AWA, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J. 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:320–8. [DOI] [PubMed] [Google Scholar]
- 15. Bounameaux H, Haas S, Farjat AE, Ageno W, Weitz JI, Goldhaber SZ, et al. Comparative effectiveness of oral anticoagulants in venous thromboembolism: GARFIELD‐VTE. Thromb Res. 2020;191:103–12. [DOI] [PubMed] [Google Scholar]
- 16. Myers B, Webster A. Heavy menstrual bleeding on rivaroxaban ‐ comparison with apixaban. Br J Haematol. 2017;176:833–5. [DOI] [PubMed] [Google Scholar]
- 17. Bryk AH, Pirog M, Plens K, Undas A. Heavy menstrual bleeding in women treated with rivaroxaban and vitamin K antagonists and the risk of recurrent venous thromboembolism. Vascul Pharmacol. 2016;87:242–7. [DOI] [PubMed] [Google Scholar]
- 18. Ferreira M, Barsam S, Patel JP, Czuprynska J, Roberts LN, Patel RK, et al. Heavy menstrual bleeding on rivaroxaban. Br J Haematol. 2016;173:314–5. [DOI] [PubMed] [Google Scholar]
- 19. Beyer‐Westendorf J, Michalski F, Tittl L, Hauswald‐Dorschel S, Marten S. Management and outcomes of vaginal bleeding and heavy menstrual bleeding in women of reproductive age on direct oral anti‐factor Xa inhibitor therapy: a case series. Lancet Haematol. 2016;3:e480–8. [DOI] [PubMed] [Google Scholar]
- 20. Winans SA, Ademolu A. Case report: dabigatran‐associated gynecologic bleeding. Hosp Pharm. 2013;48:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weitz JI, Lensing AWA, Prins M, Bauersachs R, Beyer‐Westendorf J, Brighton TA, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- 22. Moustafa F, Pesavento R, di Micco P, Gonzalez‐Martinez J, Quintavalla R, Peris ML, et al. Real‐life use of anticoagulants in venous thromboembolism with a focus on patients with exclusion criteria for direct oral anticoagulants. Clin Pharmacol Ther. 2017;103:684–91. [DOI] [PubMed] [Google Scholar]
- 23. van Bellen B, Bamber L, Correa de Carvalho F, Prins M, Wang M, Lensing AWA. Reduction in the length of stay with rivaroxaban as a single‐drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin. 2014;30:829–37. [DOI] [PubMed] [Google Scholar]
- 24. Kohn CG, Lyman GH, Beyer‐Westendorf J, Spyropoulos AC, Bunz TJ, Coleman CI. Effectiveness and safety of rivaroxaban in patients with cancer‐associated venous thromboembolism. Blood. 2017;130: Abstract 3717. [DOI] [PubMed] [Google Scholar]
- 25. Desai A, Desai A, Calixte R, Aparnath M, Hindenburg A, Salzman S, et al. Comparing length of stay between patients taking rivaroxaban and conventional anticoagulants for treatment of venous thromboembolism. Lung. 2016;194:605–11. [DOI] [PubMed] [Google Scholar]
- 26. Deitelzweig S, Laliberte F, Crivera C, Germain G, Bookhart BK, Olson WH, et al. Hospitalizations and other health care resource utilization among patients with deep vein thrombosis treated with rivaroxaban versus low‐molecular‐weight heparin and warfarin in the outpatient setting. Clin Ther. 2016;38:1803–16.e3. [DOI] [PubMed] [Google Scholar]
- 27. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 29. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐5


