Abstract
Introduction
The purpose of this study was to review and update the content of the Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool version 2.0 (CHO‐KLAT), in the context of extended half‐life (EHL) factor concentrates (FCs) and to establish the validity and reliability of the updated CHO‐KLAT.
Methods
Focus groups were conducted with boys with hemophilia, their parents, and health care providers across Canada to review the CHO‐KLAT v2.0 and determine if any modifications were required. The validity of the revised CHO‐KLAT (version 3.0) was then determined in a sample of boys with hemophilia and their parents by calculating its correlation with the Pediatric Quality of Life Core Module (PedsQL‐Core). Test‐retest reliability was assessed using an intraclass correlation coefficient (ICC).
Results
Thirteen focus groups at 5 pediatric hemophilia treatment centers (HTCs) (n = 71) resulted in 19 changes to the CHO‐KLAT v2.0, generating a revised 40‐item CHO‐KLAT, the CHO‐KLAT v3.0. Thirty‐five boys with hemophilia (median age, 14; range, 7–17 years) and 47 parents participated in the validation of the CHO‐KLAT v3.0. There was a moderate correlation between the CHO‐KLAT v3.0 child self‐report and PedsQL‐Core (r = 0.56, P = .01), and a strong correlation between the CHO‐KLAT v3.0 parent‐proxy and PedsQL‐Core (r = .79, P = .0007). The test‐retest reliability ICC was 0.90 for the child self‐report CHO‐KLAT v3.0 and 0.68 for the parent‐proxy CHO‐KLAT v3.0.
Conclusion
The CHO‐KLAT v3.0 is a reliable and valid child‐centric tool that effectively measures health‐related quality of life in boys with hemophilia who are receiving standard half‐life or EHL FCs.
Keywords: hemophilia, patient reported outcome measure, Pediatrics, quality of life, questionnaire
Essentials.
The CHO‐KLAT 2.0 was developed before availability of extended half‐life (EHL) factor concentrates (FCs).
This study updated the CHO‐KLAT (now version 3.0) and tested its measurement properties.
The CHO‐KLAT 3.0 is valid to measure quality of life in boys aged 4‐18 years with hemophilia.
Data shows the CHO‐KLAT 3.0 may differentiate between extended and standard half‐life FCs.
1. INTRODUCTION
For persons with hemophilia, regular infusions of factor concentrates (prophylaxis) is proven superior to episodic (“on‐demand”) therapy in preserving long‐term joint health and quality of life (QoL). 1 Consequently, the World Federation of Hemophilia strongly recommends the early initiation of prophylaxis (referred to as primary prophylaxis) and that prophylaxis be continued long term in all boys with hemophilia and a severe bleeding phenotype. 2 However, the short half‐life of standard half‐life (SHL) factor concentrates (FCs) necessitates frequent intravenous infusions and is a barrier to the initiation and adherence to long‐term prophylaxis regimens. 3 The development of extended half‐life (EHL) CFCs and novel nonfactor therapies provide the potential for fewer injections and improved adherence and, consequently, may improve health‐related quality of life (HRQoL) in young boys with hemophilia.
With the advent of novel hemostatic therapies for use in persons with hemophilia, assessing patient‐reported outcomes is paramount to evaluate efficacy, assess cost‐benefit, and advocate for funding of these expensive hemostatic agents.
The Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool (CHO‐KLAT) version (v) 2.0 is a disease‐specific, child‐centric HRQoL measure that was developed in 2004 and updated in 2008. 4 , 5 It contains 35 questions for boys with hemophilia between the ages of 7 and 18 years, and includes boys as young as 4 years via a parent‐proxy version. The measure is scored on a scale of 0‐100, with 100 indicating best HRQoL. This tool is reliable and valid 6 , 7 and has been cross‐culturally adapted for use in many regions of the world. 8 , 9 , 10 , 11
The purpose of this study was to review the CHO‐KLAT v2.0, in the context of new and emerging therapies, to determine which revisions are required with the availability of EHL FC, and the promise of nonfactor therapies, for use in boys with hemophilia. The secondary objective was to establish the validity and reliability of any revisions made to the existing CHO‐KLAT v2.0.
2. METHODS
The study was divided into two phases. Phase I examined the relevance of the CHO‐KLAT v2.0 in the era of EHL FCs, via semistructured focus groups, including a review of the item content and the domain structure. Phase II evaluated the validity and reliability of the revised CHO‐KLAT version 3.0.
Consistent with previous CHO‐KLAT development work, 5 , 10 , 11 participants were included if they met the following criteria: boys aged 7‐18 years with moderate or severe hemophilia A or B, receiving prophylaxis or episodic therapy with SHL or EHL FCs; parents of boys aged 0‐18 years with hemophilia A or B; or experienced health care providers (HCPs) who manage patients with hemophilia A or B. Boys with mild hemophilia A or B (factor VIII [FVIII]/factor IX [FIX] level >5%) and those with active inhibitors (defined as neutralizing alloantibodies to FVIII/FIX indicated by an inhibitor level ≥0.6 Bethesda Units within the previous 12 months) were excluded. 12
2.1. Phase I
Three types of focus groups were conducted: (i) boys aged 7‐18 years with moderate or severe hemophilia without inhibitors, (ii) parents of boys with hemophilia; and (iii) hemophilia HCPs. Focus groups were held in 5 pediatric comprehensive care hemophilia treatment centers (HTCs) in Canada located in Halifax, Toronto, Hamilton, Ottawa, and Edmonton.
Each of the focus groups followed a semistructured format as previously described by Young and colleagues. 4 In the first part, each of the 35 questions from the CHO‐KLAT v2.0 were displayed on cardstock for participants to review and determine if they continued to be relevant and applicable, given the changing therapeutic landscape for persons with hemophilia. The participants then discussed potential new items or ideas that could be added to the CHO‐KLAT to address missing concepts that they felt were necessary to accurately assess the HRQoL. These ideas were recorded and added to the existing questions. The new ideas were considered alongside the existing questions for the remaining exercises.
In the second part, each participant was given 20 stickers (votes), 10 green and 10 red, to apply to any of the existing 35 CHO‐KLAT items or the new items generated during part 1. They were instructed to use their 10 green stickers to vote for items that they felt were important and should be retained, and their 10 red stickers to vote for items that they felt were not relevant. There were no limits on the number of votes that could be applied to a single item. Following voting, the group shared thoughts and ideas about their motivation for their voting decisions and their opinions on the new ideas that were generated.
In the third part, participants were given another set of 20 stickers, 10 blue and 10 yellow, this time to indicate whether they felt the items would be sensitive to a change in therapy, specifically the use of EHL FCs (parents and HCPs were instructed to also consider other potential emerging therapies at the time not yet available in Canada, such as non–factor‐based subcutaneously administered therapies and gene therapy). They were instructed to place 10 yellow stickers on items that, in their opinion, were likely to be highly sensitive to change in therapy from a SHL to an EHL FC or to a nonfactor hemostatic agent, and 10 blue stickers on items that were unlikely to be affected/influenced by such a change in therapy.
2.2. Phase II
Phase II determined the validity and reliability of the CHO‐KLAT v3.0. Validity was determined by administering the CHO‐KLAT v3.0 together with the Pediatric Quality of Life Core Module (PedsQL‐Core) to a sample of boys with hemophilia and their parents, with the a priori hypothesis that the correlation between these two measures would be in the range of 0.4‐0.6. The PedsQL‐Core is a generic quality‐of‐life measure with both child self‐ and parent‐proxy report options scored on a scale of 0‐100, with 100 representing the best quality of life. 13 The sample size required to show that the correlation is in the desired range (α = 0.05, β = 0.20) was a minimum of 19 participants. 14
The questionnaires were completed during a routine clinic visit, and again after 3 months at home. The 3‐month follow‐up was completed twice, spaced 1 week apart, to improve the precision of the measure and to assess test‐retest reliability.
2.3. Analysis
Phase I analysis was completed primarily by the focus group facilitators (SD, VP, NY) with the goal of generating a list of modifications required to improve the relevance of the CHO‐KLAT v2.0 in the era of EHL FCs and novel hemostatic therapies. The results of the voting by each group were summarized using descriptive statistics. These results were used to inform the current relevance of the questions and to determine which questions should be removed and which should be retained and/or modified. In keeping with the child‐centric focus of the measure, in the event of discrepancies, the perspectives of the boys were prioritized over those of the parents and HCPs. 4
To determine new items that should be added to the measure, related ideas from across the focus groups were examined using content analysis, based on Charmaz’s analysis methods. 15 An expert panel determined the final wording and content of the new items, again giving priority to the ideas from the boys, but using input from the parents and HCPs to help guide decisions.
Using the domain framework initially proposed for the CHO‐KLAT version 1.0, 4 and guided by expert consensus, the team developed a revised domain structure for use with the current measure. The items on the revised CHO‐KLAT were grouped by core themes and mapped onto the revised domain scoring structure.
Cognitive debriefing was completed with boys and parents in Toronto and Halifax, to confirm that the suggested revisions to the CHO‐KLAT were clear and relevant. Thereafter, the revised version, CHO‐KLAT v3.0, was used for phase II.
Phase II data analysis included descriptive statistics to summarize the participant characteristics and the distributions of the CHO‐KLAT v3.0, the CHO‐KLAT v3.0 domains, and the PedsQL‐Core. The validity of the CHO‐KLAT v3.0 was assessed by calculating Pearson’s correlation coefficient in relation to the PedsQL‐Core summary scores. The reliability of the CHO‐KLAT v3.0 was assessed using a random‐effects intraclass correlation coefficient (ICC) 16 using results from the two assessments at the 3‐month follow‐up. An ICC <0 was defined as evidence of poor reliability, with progressively increasing ICC scores defining slight (0.00‐0.20), fair (0.21‐0.40), moderate (0.41‐0.60), strong (0.61‐0.80), and excellent (>0.80) reliability. 17
The change in CHO‐KLAT v3.0 scores were then examined in the context of the PedsQL‐Core. For the purpose of this study, stable disease was defined by a change in PedsQL‐Core score between −4 and +4, with a change in score of more than 4 indicating improvement and a change in score of more than −4 indicating worsening. 13 The changes in CHO‐KLAT v3.0 scores relative to the PedsQL‐Core were explored by generating point estimates with standard errors and presenting them graphically.
Qualitative analyses were organized using Microsoft Excel (Microsoft Corporation,Redmond, WA, USA). Quantitative analyses were conducted using R version 3.6.2 (R Core Team, Vienna, Austria).
3. RESULTS
3.1. Phase I: Focus groups
Data were obtained from 13 focus groups (with a combined total of 71 participants) between October 2017 and April 2018. In Canada, FVIII/FIX FCs are provided free of personal cost to persons with hemophilia, and EHL FVIII/FIX FCs became available for use in boys with hemophilia registered at the 5 participating HTCs in February 2016. Emicizumab (Hemlibra, Hoffmann‐La Roche, Basel, Switzerland), the only approved nonfactor hemostatic agent at present was not available in Canada during the time period that the focus groups were conducted; it only became available for boys with hemophilia and inhibitors in Canada in late 2019. There were five focus groups with boys with hemophilia (n = 28), three with parents (n = 23), and four with HCPs (n = 20). Table 1 provides the characteristics of the boys with hemophilia. Of the 28 boys, 25 (89%) had severe hemophilia, defined as a baseline FVIII/FIX level of <1 IU/dL; 27 (96%) were on long‐term prophylaxis; and 14 (50%) were receiving EHL FCs.
TABLE 1.
Baseline characteristics of study participants
| Participant type |
Phase I Focus groups |
Phase II Validation |
Phase II Reliability |
|---|---|---|---|
| Boys with hemophilia a | |||
| N | 28 | 35 | 25 |
| Age (years), median (range) | 13 (7‐16) | 14 (7‐17) | 13.5 (6‐17) |
| Severe b hemophilia, n (%) | 25 (89) | 22 (63) | 19 (79) |
| Moderate c hemophilia, n (%) | 3 (11) | 13 (37) | 5 (31) |
| Treatment regimen, n (%) | |||
| Prophylaxis | 27 (96) | 33 (94) | 23 (96) |
| On‐demand | 1 (4) | 2 (6) | 1 (4) |
| Factor concentrate type, n (%) | |||
| Extended half‐life | 14 (50) | 26 (74) | 18 (75) |
| Standard half‐life | 14 (50) | 9 (26) | 6 (25) |
| Parent of child with hemophilia | |||
| N | 23 d | 47 | 24 |
| Child aged ≥7, n (%) | 16 (70) | 35 (75) | 19 (79) |
| Child aged 4 to <7, n (%) | 4 (17) | 10 (21) | 4 (17) |
| Child aged <4, n (%) | 7 (30) | … | … |
| Child age unknown, n (%) | — | 2 (4) | 1 (4) |
| Hemophilia health care provider | |||
| N | 20 | … | … |
| Total participants | |||
| N | 71 | 84 | 24 |
Boys with hemophilia were a mix of those on prophylaxis with standard and extended half‐life FCs.
Severe hemophilia = baseline circulating factor level <1%.
Moderate hemophilia = baseline circulating factor level 1%‐5%.
Total number of children represented does not equal the number of parents due to >1 parent of some children participating or some parents having >1 child with hemophilia.
The duration of the focus groups ranged from 60 to 120 minutes. All participants were given the opportunity to express their opinions and engage in active discussion. Parents and HCPs reinforced the need to consider all available and potentially soon‐to‐be‐available factor‐ and non–factor‐based hemostatic therapies in the revision of the CHO‐KLAT, since the HCPs were aware of the impressive results of clinical trials with these novel hemostatic therapies, 18 , 19 and parents were aware of the promise of new therapies that would not require intravenous infusions. Therefore, the results that follow reflect the impacts of available hemostatic therapies in Canada at the time of this study (i.e., SHL and EHL FCs) and the anticipated impact of hemostatic therapies that were expected to become available in Canada at a later date, such as emicizumab.
Based on the results of the voting process and discussions in the focus groups, 19 changes were made to the existing items in the CHO‐KLAT v2.0. Of these changes, 11 were minor changes to the wording of items that did not significantly change their meaning, 6 items underwent major changes to their meaning, and 2 items were deleted. In addition, 7 new items were added—all generated from ideas/suggestions identified by the boys. This process resulted in a revised 40‐item CHO‐KLAT, referred to as the CHO‐KLAT v3.0. Examples of some of the changes are shown in Table 2.
TABLE 2.
Sample of questions from the Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool (CHO‐KLAT) v2.0 that were changed for the CHO‐KLAT v3.0 as a result of the focus groups
| Question on CHO‐KLAT v2.0 | Question on CHO‐KLAT v3.0 |
|---|---|
| Minor changes a | |
| It bothered me when strangers were nosy about my hemophilia. | It bothered me when people asked or talked about my hemophilia. |
| I was able to talk to others about my hemophilia. | I was able to talk to people other than my family and friends about my hemophilia. |
| Factor infusions were a bother. | Product infusions/injections were a bother. |
| Major changes b | |
| I felt like I had some control of my life. | I felt like I had some control over the activities I did in my spare time. |
| I felt as well as other kids my age. | I felt different from my friends because of my hemophilia. |
| New questions c | |
| Taking care of my hemophilia (treatment, rest, diaries) took time away from other activities. | |
| It bothered me that I had to get needles as part of my treatment. | |
Minor changes were defined as a change to the wording of the question without altering the intended meaning.
Major changes were defined as a change to the intended meaning of the question.
New questions added to the CHO‐KLAT v3.0 covering concepts not addressed in the CHO‐KLAT v2.0.
Because there were substantive changes to more than 25% of the existing CHO‐KLAT v2.0 (6/35 major changes and the addition of 7 new items), cognitive debriefing was conducted with four boys and eight parents for those items at two HTCs resulting in minor wording changes to two items to improve clarity. There was also one major revision to align participants’ understanding with the intended meaning of the item.
Seven domains were identified through the content analysis using the domains originally proposed in earlier work developing the CHO‐KLAT. 4 The domains are activities (five items), autonomy (seven items), bleeding (four items), emotional health (four items), hemophilia knowledge (three items), social functioning (nine items), and treatment (eight items). These are compared to the eight originally proposed domains for the CHO‐KLAT v1.0 in Table 3.
TABLE 3.
Domains adopted for use in the Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool (CHO‐KLAT) v3.0 compared to original domains proposed in early work with the CHO‐KLAT v1.0
| Domains for CHO‐KLAT v3.0 | Original CHO‐KLAT v1.0 Domains | |
|---|---|---|
| Domain | Number of items | Domain |
| Activities | 5 | Other people and friends |
| Autonomy | 7 | Control over your life |
| Bleeding | 4 | Physical health |
| Emotional health | 4 | Feelings |
| Future | ||
| Hemophilia Knowledge | 3 | Understanding of hemophilia |
| Social Functioning | 9 | Family |
| Treatment | 8 | Treatment |
3.2. Phase II: Validation and reliability
Between October 2018 and November 2019, 35 boys with hemophilia and 47 parents participated in the validation of the CHO‐KLAT v3.0: 34 child‐parent dyads, 1 child, and 13 additional parents of boys who were too young or unable/unwilling to complete the self‐reported CHO‐KLAT v3.0. The median age of the boys who completed the self‐reported CHO‐KLAT v3.0 was 14 years (range, 7‐17). The number of boys participating in each part of phase II is detailed in Table 1. Of the 35 boys who participated in the validation process of phase II, 22 (63%) had severe hemophilia, 33 (94%) were on long‐term prophylaxis, and 26 (74%) were receiving EHL FCs. Of the 24 boys who participated in the reliability portion of phase II, 19 (79%) had severe hemophilia, 23 (96%) were on long‐term prophylaxis, and 18 (75%) were receiving EHL FCs. Of note, no significant differences in CHO‐KLAT scores were observed between boys with hemophilia A and hemophilia B; therefore, the results were combined for the two subgroups.
At baseline, there was a moderate correlation between the CHO‐KLAT v3.0 child self‐report and PedsQL‐Core (r = .56, P = .01) and a strong correlation between the CHO‐KLAT v3.0 parent‐proxy and PedsQL‐Core (r = .79, P = .0007).
Three of the domains of the CHO‐KLAT v3.0 covered similar topics as domains in the PedsQL‐Core. Moderate correlations were found between the Activity domain of the CHO‐KLAT v3.0 and the Health and Activities domain of the PedsQL‐Core (r = .50, P = .002) and the Emotional Health domain of the CHO‐KLAT v3.0 and the Feelings domain of the PedsQL‐Core (r = .43, P = .009). The Social domain of the CHO‐KLAT v3.0 was poorly correlated with the “How I get along with others” domain of the PedsQL‐Core (r = .11, P = .51).
Overall, there was no significant difference in the mean child self‐reported scores on the CHO‐KLAT v3.0 for boys using SHL (mean = 77.2 ± SD = 13.6) or EHL (77.7 ± 10.5) FCs (P = .98). However, there was a significant difference in the parent‐proxy–reported mean summary scores for those with boys on SHL versus EHL therapy (64.2 ± 13.7 vs 81.7 ± 15.9, P = .009). The greatest differences were in the domains of autonomy, hemophilia knowledge, social functioning, and treatment as reported by parents of boys using SHL and EHL FCs (Table 4). Similarly, there were no significant differences in the child‐reported PedsQL‐Core scores between those using SHL and EHL FCs, but there was a difference in the parent‐proxy–reported scores (Table 4).
TABLE 4.
Baseline scores with domain breakdown for matched pairs of child‐parent scores and comparison between those using standard half‐life (SHL)and extended half‐life (EHL) clotting factor replacement therapies
| Child (n = 34) | Parent (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | EHL | SHL |
P value (EHL vs SHL) |
All | EHL | SHL |
P value (EHL vs SHL) |
|
| CHO‐KLAT v3.0, mean (SD) | 77.7 (10.5) | 78.0 (9.5) | 77.2 (13.6) | .88 | 77.6 (15.9) | 81.7 (14.3) | 64.2 (13.7) | .009 |
| Activities | 77.2 (17.5) | 78.2 (16.0) | 74.4 (22.0) | .64 | 77.9 (19.6) | 81.8 (18.7) | 65.6 (18.2) | .05 |
| Autonomy | 78.8 (14.4) | 79.7 (14.1) | 76.2 (16.0) | .57 | 80.8 (15.9) | 87.3 (10.3) | 62.9 (15.0) | .002 |
| Bleeding | 83.1 (17.5) | 83.0 (17.2) | 83.3 (19.3) | .96 | 80.7 (22.4) | 85.1 (19.6) | 66.4 (26.3) | .09 |
| Emotional Health | 82.3 (12.3) | 81.9 (13.2) | 83.8 (9.5) | .79 | 80.5 (19.1) | 82.5 (17.9) | 72.5 (24.0) | .42 |
| Hemophilia Knowledge | 80.4 (17.5) | 80.3 (17.0) | 80.6 (20.0) | .98 | 81.6 (16.9) | 84.9 (16.2) | 70.8 (15.4) | .05 |
| Social Functioning | 70.3 (14.7) | 70.1 (12.3) | 71.0 (21.2) | .91 | 69.8 (21.9) | 75.3 (17.3) | 49.2 (26.0) | .04 |
| Treatment | 81.0 (13.0) | 82.6 (13.6) | 75.6 (9.7) | .24 | 81.5 (12.0) | 85.9 (10.6) | 71.4 (8.5) | .004 |
| PedsQL‐Core, mean (SD) | 80.7 (12.4) | 82.1 (10.8) | 76.4 (16.1) | .34 | 84.3 (16.5) | 89.7 (10.6) | 67.9 (21.1) | .05 |
Note. CHO‐KLAT, Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool; PedsQL‐Core, Pediatric Quality of Life Core Module.
The reliability statistics for the CHO‐KLAT v3.0 are presented in Table 5. The median time between time 1 and time 2 was 14 (8‐50) days. The test‐retest reliability ICC for the child self‐report CHO‐KLAT v3.0 was excellent (ICC = 0.90) and substantial for the parent‐proxy CHO‐KLAT v3.0 (ICC = 0.68).
TABLE 5.
Summary of reliability statistics for scores obtained at 3 months–time 1 and 3 months–time 2
| Intrarater | |
|---|---|
| Child self‐report concordance CHO‐KLAT |
ICC = 0.90 Lower limit of 95% CI =0.74 |
| Parent report concordance CHO‐KLAT |
ICC = 0.68 Lower limit of 95% CI = 0.32 |
Abbreviations: CHO‐KLAT, Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool; CI, confidence interval; ICC, intraclass correlation coefficient.
The paired child self‐report and parent‐proxy scores at baseline and 3 months are presented in Table 6. The child self‐reported PedsQL‐Core scores indicated a stable subgroup of 10 of 23 (43%) boys, with 8 of 23 (35%) showing improvement of >4 points and 5 of 23 (22%) showing worsening of >4 points. The parent proxy‐reported PedsQL‐Core scores indicated a stable subgroup of 5 of 18 (28%) boys, with 3 of 18 (17%) showing improvement of >4 points, and 7 of 18 (39%) showing worsening of >4 points. Figure 1 shows the mean change in CHO‐KLAT v3.0 score between baseline and 3‐month follow‐up relative to the change as defined by the change in PedsQL‐Core score. Preliminary assessment shows that the CHO‐KLAT v3.0 scores generally increased where the PedsQL‐Core score improved, and remained stable where the PedsQL‐Core score remained stable for both the child self‐ and parent‐proxy reports. When the PedsQL‐Core scores suggested an interval worsening, the CHO‐KLAT v3.0 scores tended to decrease for the parent‐proxy reports but remain stable for the child self‐reported scores. The mean (SD) change in CHO‐KLAT v3.0 scores for the child self‐report and parent‐proxy report between baseline and 3 months was 3.11 (10.7) and 1.3 (11.3) respectively.
TABLE 6.
Paired child self‐reported and parent‐proxy–reported scores for the CHO‐KLAT v3.0 and the PedsQL‐Core at baseline and 3 months (n = 19)
| Child self‐report | Parent‐proxy report | |||||||
|---|---|---|---|---|---|---|---|---|
|
Baseline |
3 months | Mean change | P value | Baseline | 3 months | Mean change | P value | |
| CHO‐KLAT v3.0 | 78.1 (10.6) | 81.3 (12.0) | 3.2 | .18 | 78.1 (18.5) | 79.5 (13.8) | 1.4 | .61 |
| PedsQL‐Core | 81.1 (13.1) | 84.9 (15.0) | 3.8 | .22 | 86.8 (15.8) | 81.2 (11.5) | −5.6 | .21 |
Abbreviations: All scores are presented as mean (SD); the 3‐month score represents the average of the 2 at‐home administrations.
FIGURE 1.
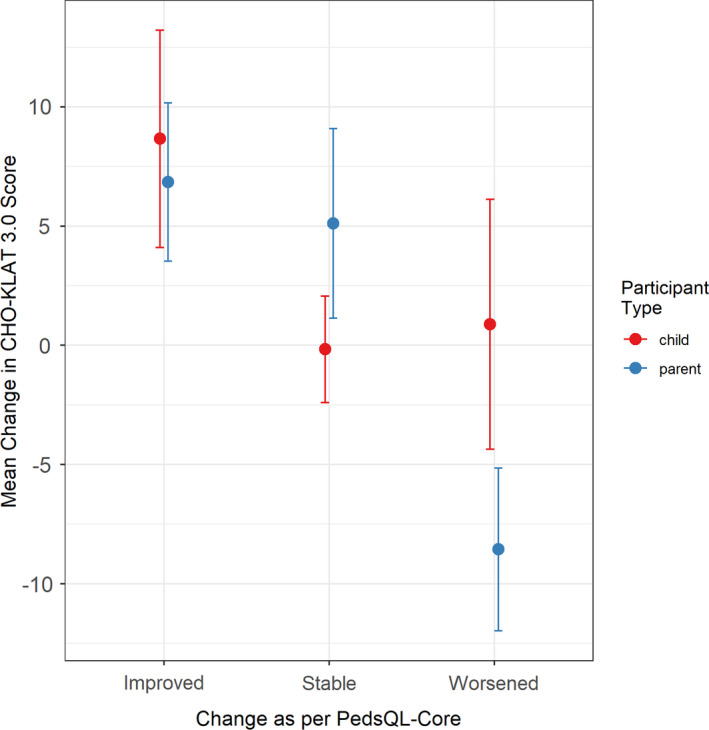
Mean change in CHO‐KLAT v3.0 score (baseline score minus 3‐month score) vs change in PedsQL‐Core score. Bars represent standard error of the means. Improved was defined as a change in PedsQL‐Core score of >4; stable was defined as a change in PedsQL‐Core score between –4 and 4; and worsened was defined as a worsening of the PedsQL‐Core score of more than –4. CHO‐KLAT, Canadian Hemophilia Outcomes–Kids’ Life Assessment Tool; PedsQL‐Core, Pediatric Quality of Life Core Module
4. DISCUSSION
This study reports the methodology and results of revising a disease‐specific, child‐centric questionnaire to measure quality of life in boys aged 4‐18 years with hemophilia, the CHO‐KLAT v3.0, for use in the therapeutic clinical milieu of EHL FCs and potentially emerging non–factor‐based hemostatic therapies. We demonstrate that the CHO‐KLAT v3.0 remains a reliable and valid tool. During this revision process, the previous version of the questionnaire, the CHO‐KLAT v2.0, underwent substantial changes, and a domain scoring structure was developed. The revised CHO‐KLAT v3.0 is a 40‐item questionnaire, with the items categorized into seven domains.
The child self‐report CHO‐KLAT v3.0 maintained a moderate correlation to the PedsQL‐Core, similar to the CHO‐KLAT v2.0, 5 confirming the continued relevance of a hemophilia‐specific tool over a generic tool in this population. The baseline mean summary scores of the CHO‐KLAT v3.0 were high, and remained in the range of approximately 75‐80, similar to previous findings using the CHO‐KLAT v2.0. 5 , 10 , 11 The CHO‐KLAT v3.0 scores were high in boys with hemophilia using either SHL or EHL FCs. This is to be expected, as the sample was limited to boys with hemophilia receiving prophylaxis and who experienced very little bleeding at the time of this study. It will be important to explore the relationship between scores and treatment type in more diverse groups of participants in the future.
The parent‐proxy–reported CHO‐KLAT v3.0 summary scores showed a higher correlation to the PedsQL‐Core than that of the boys’ self‐reported scores. Given the child‐centric approach to the development of the CHO‐KLAT, it is not unexpected that the boys’ scores deviated from the PedsQL‐Core more so than the parent‐reported scores. This is consistent with previous studies using the CHO‐KLAT v2.0, where the parent‐proxy–reported CHO‐KLAT v2.0 scores had a higher correlation to the PedsQL‐Core than the boys’ self‐reported scores. 10 It is important to consider this difference, particularly when relying on proxy scores for the younger age group.
In our experience, the CHO‐KLAT v2.0 was unable able to detect significant changes in HRQoL scores in groups of boys with hemophilia on long‐term prophylaxis who switched from a SHL to an EHL FC and who had very low spontaneous bleeding rates. 20 However, improvements in HRQoL have been reported using other HRQoL tools in populations of boys who switched from SHL to EHL FCs and who had high bleeding rates at the time of the switch in product. 21 To the best of our knowledge, no existing disease‐specific HRQoL tools have been able to demonstrate improvement in total scores in the absence of high bleeding rates before the switch. We suspect that the reason for this is the paucity of questions directed at treatment‐related burden in existing HRQoL tools. For example, only 10% of questions in the CHO‐KLAT v2.0 specifically addressed the burden of treatment administration, while the Haemo‐QoL only has 10% of questions in the Hemophilia and Treatment domain, only half of which specifically target the burden of infusions. 22
To address the issue of burden of administration of therapies, a key area where SHL and EHL FCs and non–factor‐based therapies differ substantially, several items were added to the CHO‐KLAT v3.0 and a domain scoring structure was adopted. The “treatment” domain contains eight items, which represents 20% of the total number of questions. Whether the current CHO‐KLAT v3.0 will be able to detect a change in HRQoL in boys with low bleeding rates who switch from SHL to EHL FVIII/FIX concentrates and nonfactor hemostatic agents is currently unknown, and more research is required.
The mean baseline summary score of the parent‐proxy report was very similar to that of the boys’ self‐report. Interestingly, while there was no difference in CHO‐KLAT v3.0 scores for the boys on EHL versus SHL FCs, the parents of boys using EHL FCs reported a higher mean score than the parents of boys using SHL FCs. This may reflect the parental confidence in the use of EHL FCs compared to SHL FCs, to provide higher FVIII/FIX activity levels for longer periods of time, conferring protection from risk of bleeding. We suggest that this difference is not seen in the boys’ self‐report since this cohort of boys experienced very few joint bleeds irrespective of factor type. This finding is consistent with literature that suggests that children tend to perceive their quality of life in terms of daily/repeated positive experiences rather than small numbers of major negative events (ie, spontaneous or activity‐related musculoskeletal bleeds), whereas parents may be more concerned about life‐threatening bleeds, such as intracranial hemorrhages, and long‐term joint health. 23 , 24 Furthermore, the burden of administration of repeated intravenous infusions of FVIII/IX falls to the parents in young boys with severe hemophilia who are therefore most impacted by a switch from a SHL to an EHL FVIII/FIX concentrate that allows a significant decrease in the number of weekly infusions while retaining equivalent protective FVIII/FIX coverage.
In our exploratory analysis of the sensitivity to change over time for the CHO‐KLAT v3.0, we showed that on average, the CHO‐KLAT v3.0 scores tend to change as expected based on the change in PedsQL‐Core score. The groups of boys who were classified as improved and stable on the basis of the Peds‐QL‐Core also showed improvement and stability on the CHO‐KLAT v3.0. However, while parents reported a subgroup of boys with interval worsening between baseline and 3‐month follow‐up that aligned with a decrease in CHO‐KLAT v3.0, the boys’ self‐reported scores on the CHO‐KLAT v3.0 showed no change. More research in a larger group of boys with hemophilia is needed in this area.
A limitation of this study was the focus on boys aged 4‐18 years. Development of a tool that measures the impact of hemophilia on a family in boys aged ≤4 should be a priority for future research given the recommendation that primary prophylaxis be introduced before the age of 3 years in boys with severe hemophilia 2 and because this is also the period when inhibitors to FVIII/FIX more frequently appear. 25 A second limitation is that since this study was conducted in a single country (Canada), our results may not be generalizable globally, and research with the CHO‐KLAT v3.0 tool in other cultures/countries is needed.
A final limitation of this study is that there were no participants who were receiving treatment with emicizumab, as this novel nonfactor hemostatic therapy was not available for use in Canada at the time of the study. However, both parents of boys with hemophilia and HCPs in Canada were aware of the impressive reduction of bleeding events reported in the prelicensure trials of emicizumab in both inhibitor‐positive and ‐negative subjects with hemophilia A, including the important fact that emicizumab can be administered subcutaneously with a frequency that varies between once weekly and once every 4 weeks. 18 , 19 Future prospective studies of the CHO‐KLAT v3.0 questionnaire in adolescent and young boys with hemophilia A switched from long‐term prophylaxis with “bypassing” hemostatic agents (inhibitor‐positive cases) or SHL and EHL FVIII FCs (inhibitor‐negative cases) to emicizumab are merited.
5. CONCLUSION
The results of this study confirmed that most of the CHO‐KLAT v2.0 items remain relevant and important to boys with hemophilia, their parents, and their health care providers, in the current therapeutic environment. However, there are improvements required in the CHO‐KLAT v2.0 questionnaire that focus on the reduced burden of administration afforded by newer and emerging hemostatic therapies for use in boys with hemophilia. To address this need, a revised version of the CHO‐KLAT v2.0 tool, the CHO‐KLAT v3.0, was developed and its measurement properties studied. The CHO‐KLAT v3.0 is a valid, child‐centric tool that is designed to measure quality of life in boys with hemophilia, incorporating a subdomain scoring structure to facilitate assessment of concepts important to emerging therapies, such as burden of administration. It is a reliable HRQoL tool suitable for use in boys aged 4‐18 years with hemophilia. Future studies with this tool should determine the minimal important clinical difference and develop a conversion scoring algorithm for the CHO‐KLAT v2.0 to the CHO‐KLAT v3.0.
RELATIONSHIP DISCLOSURE
VEP has a patent on the CHO‐KLAT, with royalties paid to the Hospital for Sick Children, Laurentian University, University of Manitoba, and Dr. Victoria Price. VSB reports that he is chair of the International Prophylaxis Study Group, a cooperative study group that is funded by education grants from Bayer Healthcare, Bioverativ/Sanofi, Novo Nordisk, Pfizer, Shire/Takeda, and Spark Therapeutics to the Hospital for Sick Children (“SickKids”) Foundation. He has received fees for participation in advisory boards/education events supported by Amgen, Bayer, Novo Nordisk, Pfizer, Hoffmann‐La Roche, and Shire/Takeda and for participation in Data Safety Monitoring Boards for Octapharma and Shire/Takeda. He has received investigator‐initiated, industry‐supported research grants from Novo Nordisk, Bioverativ/Sanofi, and Shire/Takeda. In addition, he has a patent on the CHO‐KLAT, with royalties paid to the Hospital for Sick Children, Laurentian University, University of Manitoba, and Dr. Victoria Price. RJK reports receiving speaker and/or consultant fees from Agios Pharmaceuticals Inc., Amgen, Hoffmann‐La Roche LTD, Shire Pharma Canada ULC, Novo Nordisk Canada Inc, Octapharma AG, Takeda, and Sanofi‐Genzyme. MB has received fees for participation in Advisory Boards from Hoffmann‐La Roche, Novo Nordisk, and Takeda, and has received grants from Octapharma AG. MC reports having received research support from Bayer, Bioverativ/Sanofi, CSL‐Behring, Novo Nordisk, Octapharma, Pfizer, and Shire. He has also received honoraria for speaking/participating in advisory boards from Bayer, Bioverativ/Sanofi, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Hoffmann‐La Roche, and Shire. NLY has a patent on the CHO‐KLAT, with royalties paid to the Hospital for Sick Children, Laurentian University, University of Manitoba, and Dr. Victoria Price. The remaining authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
VEP designed the study, assisted with data analysis and interpretation, and wrote the first draft of the manuscript. SD assisted with the design of the study, conducted data analysis, assisted with data interpretation and drafting the manuscript, and critically revised the manuscript. VSB designed the study, assisted with data analysis and interpretation, and critically revised the manuscript. RJK provided study participants, assisted with data analysis and interpretation, and critically revised the manuscript. MB provided study participants and critically revised the manuscript. AAKB provided study participants and critically revised the manuscript. AKC provided study participants and critically revised the manuscript. CW assisted with data interpretation and critically revised the manuscript. MC provided study participants and critically revised the manuscript. VB provided study participants and critically revised the manuscript. NLY designed the study; assisted with data analysis, interpretation, and drafting the manuscript; and critically revised the manuscript. All authors approved the final version to be submitted for consideration of publication.
Funding information
Funding for this study was provided by Sanofi‐Genzyme. The funders did not have any role in study design, data collection, analysis, interpretation, or preparation of the manuscript.
Contributor Information
Victoria E. Price, Email: vicky.price@iwk.nshealth.ca.
Anthony K. Chan, @noclots.
REFERENCES
- 1. Manco‐Johnson MJ, Abshire TC, Shapiro AD et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535‐544. [DOI] [PubMed] [Google Scholar]
- 2. Carcao M, van den Berg HM, Gouider E, Khair K, Baarslag MA, Bagley L, de Paula CF, Ljung RCR, Ragni MV, Santagostino E, Pierce GE, Srivastava A. Chapter 6: Prophylaxis in Hemophilia. In Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV, Windyga J. WFH Guidelines for the Management of Hemophilia. Haemophilia. 2020;26:1‐158. [DOI] [PubMed]
- 3. Dover S, Blanchette VS, Wrathall D et al. Hemophilia prophylaxis adherence and bleeding using a tailored, frequency‐escalated approach: The Canadian Hemophilia Primary Prophylaxis Study. Res Pract Thrombosis Haemostasis. 2020;4:318‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young NL, Bradley CS, Blanchette V et al. Development of a health‐related quality of life measure for boys with haemophilia: the Canadian Haemophilia Outcomes–Kids’ Life Assessment Tool (CHO‐KLAT). Haemophilia. 2004;10:34‐43. [DOI] [PubMed] [Google Scholar]
- 5. Young NL, Wakefield C, Burke TA, Ray R, McCusker PJ, Blanchette V. Updating the Canadian Hemophilia Outcomes–Kids Life Assessment Tool (CHO‐KLAT Version2.0). Value in Health. 2013;16(5):837–841 [DOI] [PubMed] [Google Scholar]
- 6. Usuba K, Price VE, Blanchette V et al. Impact of prophylaxis on health‐related quality of life of boys with hemophilia: An analysis of pooled data from 9 countries. Res Pract Thromb Haemost. 2019;3:397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Limperg PF, Terwee CB, Young NL et al. Health‐related quality of life questionnaires in individuals with haemophilia: a systematic review of their measurement properties. Haemophilia. 2017;23:497‐510. [DOI] [PubMed] [Google Scholar]
- 8. Wu R, Zhang J, Luke KH et al. Cross‐cultural adaptation of the CHO‐KLAT for boys with hemophilia in rural and urban China. Health Qual Life Outcomes. 2012;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young NL, St‐Louis J, Burke TA, Hershon L, Blanchette V. Cross‐cultural validation of the CHO‐KLAT and HAEMO‐QoL‐A in Canadian French. Haemophilia. 2012;18:353‐437. [DOI] [PubMed] [Google Scholar]
- 10. McCusker PJ, Burke TA, Holzhauer S et al. International cross cultural validation study of the Canadian Hemophilia Outcomes–Kids Life Assessment Tool (CHO‐KLAT). Haemophilia. 2015;21:351‐357. [DOI] [PubMed] [Google Scholar]
- 11. Villaça PR, Carneiro JDA, D’Amico EA et al. Process and experience of cross‐cultural adaptation of a quality of life measure (CHO‐KLAT) for boys with hemophilia in São Paulo, Brazil. Haemophilia. 2013;19:861‐865. [DOI] [PubMed] [Google Scholar]
- 12. Blanchette V, Key N, Ljung L, Manco‐Johnson M, Van Den Berg H, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost: JTH. 2014;12:1935. [DOI] [PubMed] [Google Scholar]
- 13. Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;800‐812. [DOI] [PubMed] [Google Scholar]
- 14. Designing HS. Clinical Research: an Epidemiologic Approach, 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 15. Charmaz K. Grounded theory: Objectivist and constructivist methods. In: Denzin N and Lincoln Y editors Strategies of qualitative inquiry. 2nd edn. SAGE Publications; 2003. [Google Scholar]
- 16. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420. [DOI] [PubMed] [Google Scholar]
- 17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 18. Oldenburg J, Mahlangu JN, Kim B et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 19. Mahlangu J, Oldenburg J, Paz‐Priel I et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811‐822. [DOI] [PubMed] [Google Scholar]
- 20. Carcao M, Zunino L, Young NL et al. Measuring the impact of changing from standard half‐life (SHL) to extended half‐life (EHL) FVIII prophylaxis on health‐related quality of life (HRQoL) in boys with moderate/severe haemophilia A: lessons learned with the CHO‐KLAT tool. Haemophilia. 2020;26:73‐78. [DOI] [PubMed] [Google Scholar]
- 21. Kearney S, Raffini LJ, Pham TP et al. Health‐related quality‐of‐life and treatment satisfaction of individuals with hemophilia A treated with turoctocog alfa pegol (N8‐GP): a new recombinant extended half‐life FVIII. Patient Preference Adherence. 2019;13:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Mackensen S, Bullinger M., Group HQ . Development and testing of an instrument to assess the quality of life of children with haemophilia in Europe (Haemo‐QoL). Haemophilia. 2004;10:17‐25. [DOI] [PubMed] [Google Scholar]
- 23. Fayed N, Davis AM, Streiner DL et al. Children's perspective of quality of life in epilepsy. Neurology. 2015;84:1830‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huebner ES, Suldo SM, Smith LC, McKnight CG. Life satisfaction in children and youth: empirical foundations and implications for school psychologists. Psychol Sch. 2004;41:81‐93. [Google Scholar]
- 25. van den Berg HM, Fischer K, Carcao M et al.; PedNet Study Group . Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood. 2019;134:317‐320. [DOI] [PubMed] [Google Scholar]


