Abstract
Background
Biliary complications are common causes of morbidity and mortality after liver transplantation.
Material/Methods
From 2013 to 2018, 102 whole-organ liver transplantations were conducted in our department. Patients were closely monitored for biliary complication development. In all suspected cases, patients underwent either endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangial drainage. Patients’ demographic characteristics, preexisting conditions, and perioperative characteristics, as well as morbidity and mortality, were analyzed. Risk factors for 1-year survival were calculated.
Results
Of the 102 patients, 43 (42%) experienced biliary complications. In comparison with patients without biliary complications, patients with biliary complications exhibited the following risk factors: underlying liver disease (viral hepatitis; P=0.009), blood group A (P=0.005), and previous abdominal surgery (P=0.037). Neither perioperative characteristics, especially duration of cold ischemia (P=0.86), nor postoperative course differed between patients with and without biliary complications. Risk factors for mortality within 1 year were cirrhosis caused by entities other than viral hepatitis (P=0.017), cardiac comorbidities (P=0.019), re-transplantation (P=0.032), and reduced organ weight (P=0.002). Biliary complications, postoperative hemorrhage, primary nonfunction, and repeated surgery worsened outcome; moreover, serum bilirubin trough in the first 30 days after transplantation might be prognostic for mortality (P=0.043).
Conclusions
Biliary complications adversely affect outcome after liver transplantation. Neither frequency nor outcome of biliary complications was improved by intensified endoscopic evaluation. Patients on the waiting list for liver transplants should also be closely monitored for cardiac comorbidities.
Keywords: Anastomotic Leak, Biliary Fistula, Biliary Tract, Endoscopy, Liver Cirrhosis, Liver Transplantation
Background
Liver transplantation (LTx) is the main therapy for end-stage liver disease. The 1-year survival rate is excellent (~90%), according to European and United States registries [1,2]. Biliary complications (BCs) commonly cause morbidity, graft loss, and even mortality in transplant recipients [3]. Early in the history of LTx, morbidity and mortality rates (34–50% and 24–30%, respectively) from BCs were high [4,5]. Although those rates have decreased over the last decades, 10–30% of BCs still result in morbidity [6–8]. In recent years, a renewed rise in BCs has been noted, possibly as a result of the increased use of marginal organs [3,9]. The discovery and media coverage of compliance violations in several transplantation centers has significantly reduced the willingness to donate organs after brain death in Germany. In 2010, 1271 donations were registered; this number dropped to 769 in 2017. Although national guidelines have been adapted since 2017 and intense audits of transplant centers have been conducted, the number of donations has not yet recovered (899 in 2019; annual reports at www.eurotransplant.org). Because 80% of the donated livers are nationally allocated in the Eurotransplant area (of the 1571 donated livers, 1264 were nationally allocated in total, according to the 2019 annual report), marginal organs have been increasingly accepted for transplantation, with a simultaneous increase of BC in our personal experience.
BCs after LTx are various problems arising from the intrahepatic or extrahepatic bile ducts or the papillary region. Bile leaks mainly occur at the anastomosis, with a reported incidence of 1–25% [8,10]. Risk factors for bile leaks include partial graft transplantations, hepaticojejunostomy, insufficient surgical technique, and ischemia; t-tube use remains under debate [8,10]. Anastomotic strictures (Figures 1, 2) occur within 1 cm of the surgical anastomosis, with an incidence of 1–18% [6,8]. Risk factors for anastomotic strictures are postoperative bile leaks, sex mismatch, prolonged duration of warm and cold ischemia, insufficient surgical technique, ischemia, and hepaticojejunostomy anastomosis and partial graft use [6,8,10]. Nonanastomotic strictures (NASs; Figure 3) arise outside the anastomotic region and are regarded the most challenging BC [8,11]. Because of different definitions and various manifestations, the reported incidence ranges widely (between 1% and 20%) [6,7,10]. The presumed causes of NASs are ischemia, ABO incompatibility, chronic rejection, recurrent primary sclerosing cholangitis, older donor age, and, especially, organ quality [6,10].
Figure 1.
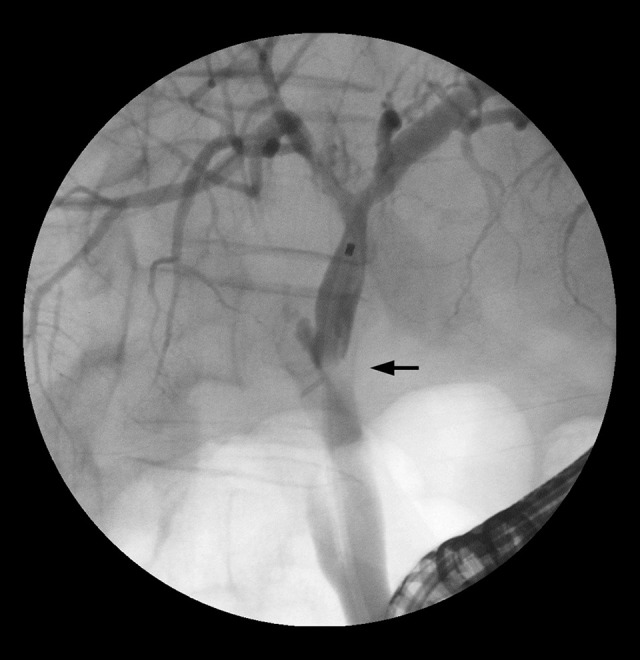
Anastomotic stricture (arrow) visualized via endoscopic retrograde cholangiopancreatography (ERCP).
Figure 2.
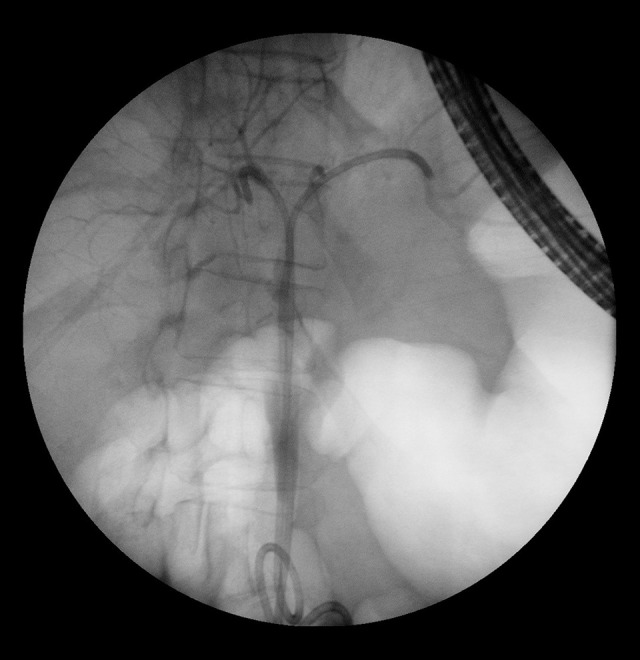
Same patient as in Figure 1. After dilatation and stent placement.
Figure 3.
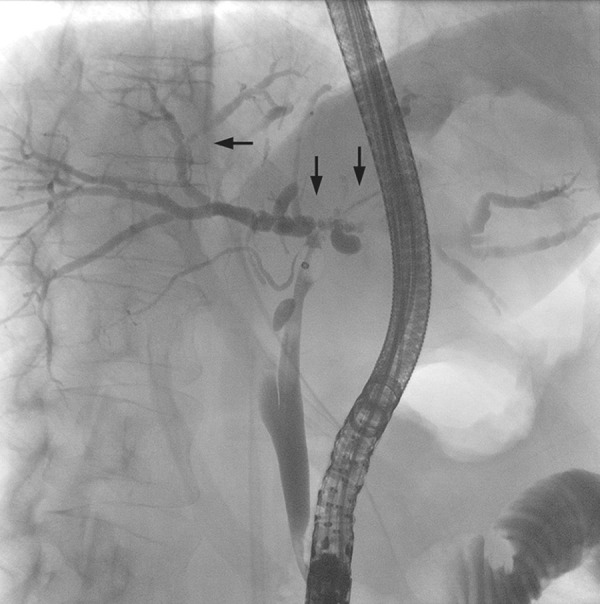
Nonanastomotic strictures (arrows) visualized via endoscopic retrograde cholangiopancreatography (ERCP).
The onset time of BCs differs for leaks and anastomotic/nonanastomotic strictures; most BCs occur during the first year after transplantation [10]. Diagnosis is mainly accomplished with imaging. Abdominal ultrasonography is not reliable for the diagnosis of biliary obstruction, but in combination with Doppler examination, it is crucial for ruling out hepatic artery occlusion [6,11,12]. Both biliary leakage and strictures can be diagnosed with endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), both of which have high sensitivity and specificity (>90%) [8,10,11]. MRCP is noninvasive, but its availability might be limited. ERCP has the advantage of combining diagnostic and therapeutic measures in the same session [6]. Because contrast media can be injected into the bile ducts with pressure, even small insufficiencies that might be missed with MRCP can be detected on ERCP. If Roux-en-Y reconstruction has been performed, percutaneous cholangiography might be indicated [8,10]. Liver biopsy is helpful for diagnosing other causes of graft dysfunction, such as rejection, recurrent hepatitis C virus infection, or cytomegalovirus-induced hepatitis [10,13].
We hypothesized that obligatory evaluation of BCs with ERCP after LTx and early treatment can help improve morbidity and mortality rates. All cases of BCs that were suspected on the basis of results of isolated cholestatic liver tests, bilious secretion into the drains, and severe graft dysfunction without other suspected causes during the study period were therefore evaluated by ERCP.
Material and Methods
All patients eligible for LTx were described to a transplant board before transplantation, in accordance with the guidelines of the German Medical Association as amended (DOI: 10.3238/arztebl.2019.rili_baek_OrgaWlOvLeberTx20190924). Because of German legislation, only organs donated after brain death were used in transplantation. Partial transplantations increase the risk of BCs [8,10] and were therefore not included in this analysis.
At our institution, bile duct anastomosis is automatically performed in a duct-to-duct, end-to-end manner using a double-armed 5-0 polydioxanone suture. Hepaticojejunostomy via Roux-en-Y reconstruction was only used in selected cases (primary sclerosing cholangitis involving the common bile duct, bile duct atresia, and Todani cyst). We do not routinely insert a T-drain.
When a BC was suspected (cholestatic liver tests, bilious secretion into the drains, and severe graft dysfunction without other suspected causes) during a patient’s hospital stay or during ambulatory aftercare, the patient automatically underwent ERCP in the Department of Gastroenterology. If ERCP failed, percutaneous transhepatic cholangial drainage was performed.
Statistical Analysis
All patient data were prospectively recorded and anonymously stored in our liver transplant database in an Excel file (Microsoft Corporation, Redmond, WA, USA). For this study, SPSS 24 (IBM Corporation, Armonk, NY, USA) was used to retrospectively analyze the data. Continuous and normally distributed variables were analyzed with the t test and are expressed as means±standard deviations. Non-normally distributed data were analyzed with the Mann-Whitney U test and are expressed as medians and interquartile ranges. Pearson’s chi-square or Fisher’s exact test, as appropriate, was used to analyze categorical data, which are expressed as proportions and percentages. Factors for which P<0.1 in the univariable analysis were subjected to multivariable stepwise logistic regression analysis. Relative risk is described as the estimated odds ratio with 95% confidence intervals. A P value of <0.05 was considered significant.
Results
Between 2013 and 2018, LTx with whole-liver grafts was performed in 102 patients. Of these, 43 experienced some degree of BCs. Mild forms included cholestasis, detected in laboratory tests, without symptoms. Every form of bilious secretion indicated BCs. In severe cases, parenchymal decompensation occurred as a result of biliary strictures. In uncertain cases, liver biopsy was performed. In 41 patients, ERCP was successful. In 2 patients, ERCP could not be conducted because of Roux-en-Y reconstruction, and percutaneous transhepatic cholangial drainage was performed. The first ERCP was conducted on a median of postoperative day 56. In 2 patients, no biliary problem could be detected; all others were diagnosed with either bile leaks, anastomotic, or nonanastomotic strictures. More than 1 ERCP was needed in 78% of the patients. In patients with anastomotic strictures, the success rate of endoscopic treatment was 72%. Nonanastomotic strictures were treated successfully in only 24% of cases, with many patients still under recurrent endoscopic treatment at the end of follow-up.
Patients’ demographic characteristics are listed in Table 1. Most patients with BCs had not undergone transplantation for viral hepatitis (P=0.009); other causes (polycystic liver degeneration, veno-occlusive disease, acute liver failure, autoimmune hepatitis, bile duct atresia, Todani cysts, and alpha-1-antitrypsin deficiency) were more common (P=0.001). Except for blood group A (P=0.005) and previous abdominal surgery (P=0.037), no other differences between patients with and without BCs were found. Patients with a lab MELD score <15 were transplanted according to matchMELD for diseases in which parenchymal decompensation does not determine the clinical course (eg, hepatocellular carcinoma). Cardiac comorbidities are any known history of coronary artery disease, congestive heart failure, arrhythmias, and valvular heart disease. No perioperative characteristics of patients with and without BCs were different (Table 2). In particular, durations of cold and warm ischemia were not risk factors for BCs in our cohort. The surgical technique, proportions of positive microbiological results, and rates of transfusion were the same in both groups, and the amounts of blood loss did not differ. BCs significantly prolonged the index hospital stay (23 vs 36 days; P=0.034). Complications are listed in Table 3. Serum bilirubin level is a good indicator of BCs, with significant trough (P=0.015) and peak (P=0.001) values in the first 30 days after LTx. Other complications that accompanied BCs were equally frequent in the 2 groups of patients. The use of different immunosuppressants was also equal in both groups. No peri-interventional complications occurred; 3 patients exhibited slightly elevated serum lipase levels but needed no specific treatment.
Table 1.
Demographics.
| No biliary complications | Biliary complications | P | |
|---|---|---|---|
| n=59 | n=43 | ||
| Age (a) | 54 (45–63) | 58 (51–63) | 0.18 |
| Gender | 0.38 | ||
| Male | 38 (64%) | 21 (49%) | |
| Female | 21 (36%) | 19 (51%) | |
| BMI | 25.30±4.04 | 25.99±5.05 | 0.45 |
| Cause of cirrhosis* | |||
| Alcohol | 17 (29%) | 9 (21%) | 0.37 |
| Viral hepatitis | 22 (37%) | 6 (14%) | 0.009 |
| Primary sclerosing cholangitis | 10 (17%) | 4 (9%) | 0.27 |
| Other | 15 (25%) | 25 (58%) | 0.001 |
| Hepatocellular carcinoma | 17 (29%) | 10 (23%) | 0.53 |
| labMELD | 20 (9–32) | 28 (12–36) | 0.17 |
| High urgent transplant | 8 (14%) | 5 (12%) | 0.77 |
| Child-Turcotte-Pugh | |||
| A | 17 (29%) | 10 (23%) | 0.53 |
| B | 20 (34%) | 12 (28%) | 0.52 |
| C | 22 (37%) | 21 (49%) | 0.24 |
| Blood group | |||
| 0 | 25 (42%) | 12 (28%) | 0.13 |
| A | 18 (31%) | 25 (58%) | 0.005 |
| Previous abdominal surgery | 22 (37%) | 25 (58%) | 0.037 |
| Redo transplantation | 5 (8%) | 6 (14%) | 0.52 |
| TIPS prior to surgery | 10 (17%) | 8 (19%) | 0.83 |
| Pre-existing conditions | |||
| Cardiac | 9 (15%) | 11 (26%) | 0.2 |
| Pulmonary | 9 (15%) | 10 (23%) | 0.31 |
| Renal | 22 (37%) | 22 (51%) | 0.16 |
| Diabetes | 11 (19%) | 13 (30%) | 0.17 |
| CMV donor positive | 19 (32%) | 18 (42%) | 0.35 |
| CMV recipient positive | 27 (46%) | 17 (40%) | 0.48 |
Data are expressed as number (%), mean±standard deviation or median (interquartile range). TIPS – transjugular intrahepatic portosystemic shunt.
Multiple responses possible.
Table 2.
Perioperative characteristics.
| No biliary complications | Biliary complications | P | |
|---|---|---|---|
| n=59 | n=43 | ||
| Donor organ weight (g) | 1565 (1339–1918) | 1550 (1300–1825) | 0.57 |
| Time of operation (min) | 326 (280–384) | 312 (290–404) | 1.0 |
| Cold ischemia time (min) | 566 (499–622) | 560 (485–632) | 0.86 |
| Anast. suture time (min) | 37 (30–45) | 38 (28–47) | 0.71 |
| Warm ischemia time (min) | 40 (32–50) | 42 (32–49) | 0.59 |
| Choledocho-choledochostomy | 49 (83%) | 39 (91%) | 0.27 |
| Modified Belgithi IVC | 35 (59%) | 30 (70%) | 0.28 |
| CPR during surgery | 2 (3%) | 1 (2%) | 1.0 |
| Vascular reconstruction | 14 (24%) | 7 (16%) | 0.36 |
| Microbiological swab positive | |||
| donor | 2 (3%) | 2 (5%) | 1.0 |
| recipient | 20 (34%) | 15 (35%) | 0.92 |
| Transfusions | |||
| Red blood cells (units) | 6 (0–13) | 6 (2–12) | 0.65 |
| Fresh frozen plasma (units) | 8 (2–16) | 6 (4–16) | 0.82 |
| Platelet concentrate (units) | 1 (0–4) | 2 (0–3) | 0.58 |
| Blood loss (ml) | 1100 (593–4000) | 1500 (588–2725) | 0.82 |
| Peritransplant hospital stay | 23 (16–42) | 36 (22–75) | 0.034 |
| Peritransplant ICU stay | 5 (3–13) | 7 (3–38) | 0.16 |
Data are expressed as number (%), or median (interquartile range). ICV – inferior caval vein; CPR – cardiopulmonary resuscitation.
Table 3.
Complications and immunosuppressive regimens.
| No biliary complications | Biliary complications | P | |
|---|---|---|---|
| n=59 | n=43 | ||
| Bilirubin (mg/dl) first 30 days | |||
| Trough | 0.78 (0.52–1.12) | 1.02 (0.67–1.96) | 0.015 |
| Peak | 8.25 (4.58–13.34) | 10.98 (8.02–22.95) | 0.001 |
| Bile duct strictures | 41 (95%) | ||
| Anastomotic/nonanastomotic | 20 (46%)/21 (49%) | ||
| Biliary leakage | 7 (16%) | ||
| Early/late (>1 year) onset | 37 (86%)/6 (14%) | ||
| Postoperative hemorrhage | 12 | 7 (16%) | 0.6 |
| SSI superficial | 8 (14%) | 4 (9%) | 0.51 |
| SSI deep (subfascial) | 3 (5%) | 2 (5%) | 1.0 |
| Hepatic artery thrombosis | 1 (2%) | 3 (7%) | 0.31 |
| Primary nonfunction | 2 (3%) | 3 (7%) | 0.65 |
| Organ rejection | 4 (8%) | 7 (16%) | 0.2 |
| Pneumonia | 9 (15%) | 13 (30%) | 0.07 |
| Repeated surgery | 28 (47%) | 15 (35%) | 0.51 |
| Immunosuppressant* | |||
| Tacrolimus | 41 (69%) | 32 (74%) | 0.59 |
| Ciclosporin | 21 (36%) | 17 (40%) | 0.68 |
| CellCept | 22 (37%) | 23 (53%) | 0.1 |
| Everolimus | 17 (29%) | 11 (26%) | 0.72 |
Data are expressed as number (%), or median (interquartile range).
Multiple responses possible.
The overall 1-year mortality rate was 12.7% and the 3-year mortality rate was 17.6%. Table 4 lists significant risk factors for 1-year mortality. No patients with viral hepatitis died during the first postoperative year (P=0.017), whereas preexisting cardiac disease of any kind (P=0.019) and re-transplantation (P=0.032) were significant risk factors for mortality. In our cohort, the median organ weight was lower in deceased patients (1357±261 g vs 1655±392 g; P=0.002). Postoperative hemorrhage (P=0.014), primary nonfunction (P=0.014), BCs (P=0.034), and repeated surgery (P=0.022) were postoperative risk factors for 1-year mortality. Interestingly, serum bilirubin trough values during the first 30 days were significantly lower in patients who survived the first year (0.86 mg/dL) than in those who did not survive (1.07 mg/dL; P=0.043). As shown in Table 5, preexisting cardiac comorbidity (P=0.02) and re-transplantation (P=0.031) were significant multivariable risk factors for 1-year mortality. Viral hepatitis could not be included in the regression analysis because no patients with this disease died.
Table 4.
Risk factors for one-year mortality.
| Alive | Deceased | P | |
|---|---|---|---|
| n=89 | n=13 | ||
| Cause of cirrhosis: viral hepatitis | 28 (31%) | 0 (0%) | 0.017 |
| Pre-existing cardial comorbidity | 14 (16%) | 6 (46%) | 0.019 |
| Redo transplantation | 7 (8%) | 4 (31%) | 0.032 |
| Donor organ weight (g) | 1655±392 | 1357±261 | 0.002 |
| Bilirubin trough (mg/dl) first 30 days | 0.86 (0.54–1.29) | 1.07 (0.77–4.07) | 0.043 |
| ICU stay | 5 (3–13) | 26 (9–182) | 0.003 |
| Postoperative hemorrhage | 13 (15%) | 6 (46%) | 0.014 |
| Biliary complications | 34 (38%) | 9 (69%) | 0.034 |
| Primary nonfunction | 2 (2%) | 3 (23%) | 0.014 |
| Repeated surgery | 24 (27%) | 8 (62%) | 0.022 |
Data are expressed as number (%), mean±standard deviation or median (interquartile range).
Table 5.
Preoperative risk factors for one-year mortality: multivariate logistic regression analysis.
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Pre-existing cardial comorbidity | 4.556 | 1.265–16.414 | 0.02 |
| Redo transplantation | 5.153 | 1.165–22.785 | 0.031 |
Discussion
Since the early days of LTx, the bile duct has been called the “Achilles heel” of this procedure [4,5]. BCs represent important risk factors for morbidity, graft loss, and even mortality. In particular, NAS are challenging to treat. Graft loss rate of up to 46% in the first 2 postoperative years and a mortality rate of 22.2% among patients with NAS have been reported [14]. Patients with the worst prognosis seem to be those in whom NAS occurs during the first year after transplantation [11,15]. Therefore, all transplant recipients were intensively monitored for BC and underwent ERCP. Bile leaks can be interventionally treated; success rates are 85–100% [6,10]. For anastomotic strictures, ERCP treatment yields success rates of 70–90% [8,11,12], and even NAS can be successfully treated in 50–75% of cases [6,8,12]. With this intensified endoscopic diagnostic regimen, we diagnosed BCs in 42% of our patients after LTx. This percentage is higher than those reported previously [6–8], but this finding can be explained by several factors: ERCP was undertaken at the slightest suspicion of BC, even mild cholestasis detected in laboratory tests prompted invasive diagnostic measures, and perhaps these BCs would not have been diagnosed under normal circumstances. After introduction of the Model for End-Stage Liver Disease organ allocation system, the incidence of BCs after LTx increased [9,16]; this is attributable to the reduced quality of donor organs. In our experience, the shortage of liver grafts in Germany has clearly necessitated the increased use of marginal organs. Other transplant centers have also observed a rise in BCs in recent years [16]. Apart from poorer organ quality, the rising frequency of BCs can be explained by an increased awareness of the signs of BCs in transplant centers and by the improvement in diagnostic modalities [3].
Some risk factors for the development of BCs could not be confirmed in our study. Primary sclerosing cholangitis was described by other investigators as a risk factor [14,17]. Of our patients, 14% underwent transplantation for this indication, but we found no difference in the incidence of BCs between these patients and others. Hepatic artery thrombosis is an important risk factor [18]; a total of 21 vascular reconstructions had to be performed during LTx among our patients. Although these reconstructions were more frequently performed in patients without BCs (24%) than in those with BCs (16%), occlusion of the hepatic artery was more common in those with BCs (7%) than in those without (2%; P=0.31), but the difference was not significant, perhaps because of the low number of events. Duct-to-duct biliary anastomosis is standard in LTx because the rate of BCs afterward is lower than after Roux-en-Y hepaticojejunostomy [19,20]. In our cohort, the incidence of BCs did not differ between the 2 types of reconstruction. The same result was found in other reports [16], and some investigators even reported a higher rate of BCs after duct-to-duct anastomosis [9]. The duration of cold ischemia has been repeatedly described as a risk factor for BCs, especially for NAS [6,14,21]. In our cohort, the duration of cold ischemia was relatively short: 566 min in patients with no BCs and 560 min in patients with BCs. It was shown that the duration of cold ischemia was correlated with the risk for BC [22], and if it was kept short, the risk for BCs did not increase [3]. Warm ischemia time was comparable to other transplant centers (37–56 min) [3,19,23,24] and was not a risk factor for BCs. BCs occur at much longer warm ischemia times of more than 1 h [14]. Blood group A and previous abdominal surgery have not been previously described as risk factors. ABO incompatibility is known to increase the risk for BCs [25,26], although specific blood groups were not previously described. ABO blood types as risk factors have been linked to several diseases, such as pancreatic cancer [27] or venous thromboembolism [28]; therefore, blood group A could be a risk factor for BC development after LTx. Patients with BCs had more often undergone previous surgery (58%) than had those without BCs (37%; P=0.037). Operation time did not differ between patients who had not undergone previous abdominal surgery (median, 321 min; range, 287–402 min) and those who had (median, 322 min; range, 290–385 min; P=0.83), and neither did the duration of cold ischemia (median, 550 min [range, 470–630 min] vs 578 min [range, 515–622]; P=0.45); Roux-en-Y frequency also did not differ between groups (P=0.141). It is possible that greater unspecific trauma from adhesiolysis might contribute to the increased risk for BCs.
BCs were a risk factor for mortality within 1 year after LTx in our cohort (69% of patients with BCs vs 38% of those without; P=0.034). This emphasizes the importance of efforts to minimize BCs. Serum bilirubin trough values, which are easily obtained during the first 30 postoperative days, were prognostic for 1-year survival (P=0.043). Other risk factors for 1-year mortality are listed in Table 4. In patients with virus-related cirrhosis, rates of patient and graft survival are excellent; only patients receiving transplants for metabolic disease survive longer [29]. Re-transplantation is a univariable and multivariable risk factor for 1-year mortality; this finding is consistent with published data from European registries, in which the rate of 1-year survival after re-transplantation was only 53% [29]. To our astonishment, donor organs in patients who died during the first year weighed significantly less than those in survivors; body mass index did not differ between the groups. Preventing small-for-size syndrome is essential, and in most studies of the estimation of liver volume, cadaveric livers were used [30,31]. This is especially interesting because the formulas with references from White populations were criticized for overestimating liver volume [30]. Bearing this finding in mind might help surgeons choose organs with the correct weight rather than those on the upper limit of the weight range.
Preexisting cardiac comorbidity was another significant risk factor for 1-year mortality, with an odds ratio of 4.556 (P=0.02). Cardiovascular disease is an important cause of morbidity in transplant recipients. Twenty-six percent of transplant recipients have unknown moderate to severe coronary narrowing, 30% have hypertension, and 36% have diabetes mellitus [32]. The 10-year risk of coronary heart disease after liver transplantation is 11.5% compared to 6.5% in the matched local population [33]. The relative risk for ischemic cardiac events and cardiovascular deaths after transplantation is 3.07 and 2.56, respectively [34]. Reducing the effects of cardiovascular disease (and posttransplant metabolic syndrome) is 1 of 3 strategies for improving survival after LTx, according to the 10-year Roadmap in 2016 [2]. This strategy is explicitly supported by our data. For patients on the waiting list for transplants, we already intensively screen for preexisting cardiac diseases. If these are not improvable (eg, fixed pulmonary hypertension that does not respond to medication), patients are ineligible for transplantation. Diagnostic testing is repeated annually in patients on the waiting list. Whether this helps fulfill the objectives of the 10-year Roadmap must be assessed by prospective evaluation.
Conclusions
The incidence of BCs after LTx is even higher than previously reported. At our institution, every case of suspected BCs is checked with endoscopic- or radiologically-guided intervention. This is of utmost importance because BCs represent a significant risk factor for posttransplantation morbidity and mortality. An intensified focus on cardiac comorbidity is needed for patients on the waiting list for LTx.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–13. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 2.Charlton MR. Roadmap for improving patient and graft survival in the next 10 years. Liver Transpl. 2016;22(S1):71–78. doi: 10.1002/lt.24602. [DOI] [PubMed] [Google Scholar]
- 3.Verdonk RC, Buis CI, Porte RJ, et al. Anastomotic biliary strictures after liver transplantation: Causes and consequences. Liver Transpl. 2006;12(5):726–35. doi: 10.1002/lt.20714. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Putnam CW, Hansbrough JF, et al. Biliary complications after liver transplantation: With special reference to the biliary cast syndrome and techniques of secondary duct repair. Surgery. 1977;81(2):212–21. [PubMed] [Google Scholar]
- 5.Calne RY, McMaster P, Portmann B, et al. Observations on preservation, bile drainage and rejection in 64 human orthotopic liver allografts. Ann Surg. 1977;186(3):282–90. doi: 10.1097/00000658-197709000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdonk RC, Buis CI, Porte RJ, et al. Biliary complications after liver transplantation: A review. Scand J Gastroenterol Suppl. 2006;243:89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 7.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: A review. Dig Surg. 2008;25(4):245–57. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 8.Roos FJM, Poley J-W, Polak WG, et al. Biliary complications after liver transplantation; Recent developments in etiology, diagnosis and endoscopic treatment. Best Pract Res Clin Gastroenterol. 2017;31(2):227–35. doi: 10.1016/j.bpg.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Sundaram V, Jones DT, Shah NH, et al. Posttransplant biliary complications in the pre- and post-model for end-stageliver disease era. Liver Transpl. 2011;17(4):428–35. doi: 10.1002/lt.22251. [DOI] [PubMed] [Google Scholar]
- 10.Seehofer D, Eurich D, Veltzke-Schlieker W, et al. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13(2):253–65. doi: 10.1111/ajt.12034. [DOI] [PubMed] [Google Scholar]
- 11.Larghi A, Tringali A, Rimbaş M, et al. Endoscopic management of benign biliary strictures after liver transplantation. Liver Transpl. 2019;25(2):323–35. doi: 10.1002/lt.25358. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl. 2008;14(6):759–69. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 13.Gopal DV, Pfau PR, Lucey MR. Endoscopic management of biliary complications after orthotopic liver transplantation. Curr Treat Options Gastroenterol. 2003;6(6):509–15. doi: 10.1007/s11938-003-0053-2. [DOI] [PubMed] [Google Scholar]
- 14.Guichelaar MMJ, Benson JT, Malinchoc M, et al. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3(7):885–90. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 15.Verdonk RC, Buis CI, van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13(5):725–32. doi: 10.1002/lt.21165. [DOI] [PubMed] [Google Scholar]
- 16.Kaltenborn A, Gutcke A, Gwiasda J, et al. Biliary complications following liver transplantation: Single-center experience over three decades and recent risk factors. World J Hepatol. 2017;9(3):147–54. doi: 10.4254/wjh.v9.i3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sankary HN, McChesney L, Frye E, et al. A simple modification in operative technique can reduce the incidence of nonanastomotic biliary strictures after orthotopic liver transplantation. Hepatology. 1995;21(1):63–69. [PubMed] [Google Scholar]
- 18.Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9(6):612–20. doi: 10.1053/jlts.2003.50098. [DOI] [PubMed] [Google Scholar]
- 19.Buis CI, Verdonk RC, Van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13(5):708–18. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 20.Thethy S, Thomson BN, Pleass H, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18(6):647–53. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 21.Heidenhain C, Pratschke J, Puhl G, et al. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23(1):14–22. doi: 10.1111/j.1432-2277.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Urdazpal L, Gores GJ, Ward EM, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16(1):49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 23.Iacob S, Cicinnati VR, Dechêne A, et al. Genetic, immunological and clinical risk factors for biliary strictures following liver transplantation. Liver Int. 2012;32(8):1253–61. doi: 10.1111/j.1478-3231.2012.02810.x. [DOI] [PubMed] [Google Scholar]
- 24.Mehrabi A, Mood ZA, Fonouni H, et al. A single-center experience of 500 liver transplants using the modified piggyback technique by Belghiti. Liver Transpl. 2009;15(5):466–74. doi: 10.1002/lt.21705. [DOI] [PubMed] [Google Scholar]
- 25.Rull R, Garcia Valdecasas JC, Grande L, et al. Intrahepatic biliary lesions after orthotopic liver transplantation. Transpl Int. 2001;14(3):129–34. doi: 10.1007/s001470100320. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Urdazpal L, Batts KP, Gores GJ, et al. Increased bile duct complications in liver transplantation across the ABO barrier. Ann Surg. 1993;218(2):152–58. doi: 10.1097/00000658-199308000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Xu H, Gao P. ABO blood group and diabetes mellitus influence the risk for pancreatic cancer in a population from China. Med Sci Monit. 2018;24:9392–98. doi: 10.12659/MSM.913769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baudouy D, Moceri P, Chiche O, et al. B blood group: A strong risk factor for venous thromboembolism recurrence. Thromb Res. 2015;136(1):107–11. doi: 10.1016/j.thromres.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Adam R, McMaster P, O’Grady JG, et al. Evolution of liver transplantation in Europe: Report of the European Liver Transplant Registry. Liver Transpl. 2003;9(12):1231–43. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Yoshizumi T, Taketomi A, Kayashima H, et al. Estimation of standard liver volume for Japanese adults. Transplant Proc. 2008;40(5):1456–60. doi: 10.1016/j.transproceed.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 31.Heinemann A, Wischhusen F, Püschel K, et al. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5(5):366–68. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- 32.Tiukinhoy-Laing SD, Rossi JS, Bayram M, et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98(2):178–81. doi: 10.1016/j.amjcard.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 33.Neal DAJ, Tom BDM, Luan J, et al. Is there disparity between risk and incidence of cardiovascular disease after liver transplant? Transplantation. 2004;77(1):93–99. doi: 10.1097/01.TP.0000100685.70064.90. [DOI] [PubMed] [Google Scholar]
- 34.Johnston SD, Morris JK, Cramb R, et al. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–6. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]


