Abstract
Background and Objectives:
Robotic surgery data need a setback on many years of practice with high-volume surgeons to evaluate its real value. Our main objective was to study the impact of a decade of robotic surgery on minimally-invasive hysterectomies for benign indications. Our secondary objectives were to evaluate our results for high-volume surgeons and complex cases.
Methods:
In this retrospective cohort study, we reviewed medical records at Foch Hospital, from 2010 to 2019, to evaluate the outcomes of robotic hysterectomies for benign disease. We compared the trends of benign hysterectomies done by laparoscopy and laparotomy during this period. We analyzed the proficiency group (≥ 75 cases per surgeon) and complex cases including obese patients and large uteri (>250 g).
Results:
495 hysterectomies were performed by robotic, 275 by laparotomy, and 130 by laparoscopy. The laparotomy approach decreased from 62% to 29%, whereas the robotic approach increased from 26% to 61%. The operating room (OR) time decreased in the proficiency group (157.3 ± 43.32 versus 178.6 ± 48.05, P = 0.005); whereas the uterine weight was higher (194.6 ± 158.6 versus 161.3 ± 139.4, P = 0.04). Lower EBL and shorter OR time were seen with uteri ≤ 250 g subgroup (64.24 ± 110.2 ml versus 116.63 ± 146.98 ml, P = 0.0004) (169.62 ± 47.50 min versus 192.44 ± 45.82 min, P = 0.0001). The estimated blood loss (EBL) was less in the BMI ≤ 30 subgroup (68.83 ± 119.24 ml versus 124.53 ± 186.14 ml, P = 0.0005).
Conclusion:
A shift was observed between the laparotomy and robotic approaches. High-volume surgeons were more efficient and showed a decrease in OR time after 75 cases despite an increase in uterine weight.
Keywords: Hysterectomy, Robotic-assisted, Benign, Laparoscopy, Minimally invasive
INTRODUCTION
Hysterectomy is the most common surgery performed in women with millions of procedures done annually throughout the world.1 Approximately 90% of hysterectomies are performed for benign conditions, such as fibroids, endometriosis/adenomyosis, and prolapse among others. Despite the progress of minimally invasive techniques, abdominal hysterectomy is still performed in 46%–46% of cases.2,3 According to ACOG, vaginal hysterectomy should be performed “whenever is feasible”.4 Whenever it is not feasible, surgeons are left to choose a different surgical approach. Prior to the introduction of the robotic surgery, a laparoscopic approach was the sole minimally invasive remaining approach. A steep learning curve and advanced training and skills limit its adoption especially for complex cases. Since the introduction of the da Vinci System (Intuitive Surgical, Sunnyvale, CA) with the United States Food and Drug Administration approval for hysterectomy in 2005, robotic minimally invasive surgery has gained popularity because of its potential benefits: “wrist like” motions offer better precision, mobility, and dexterity. Its 3D vision enhances the surgeon’s view of the operative field. All these factors may contribute to reduce the surgeon’s fatigue, seated at a console, remote from the patient.5,6 Several randomized studies and meta-analyses were not able to prove the superiority of robotic surgery compared to laparoscopic surgery for benign hysterectomies.7–10 However, in complex cases including obesity and large uteri, robotic surgery have shown a benefit.11–16 Robotic surgery demonstrated a rapid adoption in minimally invasive hysterectomy because of the shorter learning curve.17,18 The increase in minimally invasive surgery attributed to robotic surgery could be one of the most interesting aspects of its use, especially for benign conditions, offering patients shorter hospital stay, smaller incisions, less postoperative pain, and fewer complications when compared to laparotomy.19 The impact of highly skilled surgeons in robotic surgery on different peri-operative parameters should be further evaluated.20
Our main objective was to study the impact of robotic surgery on benign hysterectomies routes since its implementation in our hospital over the course of a decade. Our secondary objectives were to analyze our results for high volume surgeons and complex cases including obese patients BMI > 30 and large uteri >250 g.
MATERIALS AND METHODS
This manuscript met the STROBE reporting guidelines for observational studies. This study was approved by the institutional review board in March 2020. All the patients who underwent a robotic hysterectomy for benign pathologies at Foch Hospital (France) over a 10-year period (from implementation in January 1, 2010 to December 31, 2019) were included. Patients with malignant indications were excluded. The surgeries were performed with the Da Vinci® SI Surgical System, available in our center since January 2010. All the procedures were done by two senior surgeons: one on the robotic console as main operator and the other one as a bedside assistant. Throughout the study period, a total of 10 surgeons performed all the robotic-assisted hysterectomies as the main surgeon. The remaining OR team was dedicated to robotic surgery. Three surgeons had performed more than 75 cases. All the cases were total hysterectomies with salpingectomies and all surgical specimens were extracted through the vagina. Patients received standardized fluid during the surgery: between 4 and 5 ml/kilogram/hour in addition to fluid repletion depending on the EBL.
Postoperative modalities were the same for all patients.
All the required data was collected from the patient’s electronic medical records. Age, body mass index (BMI), gravidity and parity, menopausal status, history of abdominal surgery, indications for surgery, surgeon, surgery time (skin to skin time), and OR time (from the intubation time to transfer to the recovery unit), uterine weight obtained from the final pathology record, objective EBL, concurrent procedures, hemoglobin differential (the difference between hemoglobin at day 1 compared to the hemoglobin collected prior to surgery during the preoperative checkup), blood transfusions, length of stay (LOS), rate of conversion to laparotomy, intra- and postoperative complications, and reoperation within 6 months after, were included in the data collected.
To study the impact of the route of hysterectomy, all the cases performed by laparotomy or laparoscopy for benign indications were included during the same period. We studied the trends of robotic surgery related to the surgeon’s volume. Proficiency was defined as 75 cases or more per surgeon. Proficiency Group included the surgeons with ≥ 75 cases per surgeon and nonproficiency group < 75 cases based on the literature.21 We also studied subgroups of patients with uteri weighing >250 g as well as obese patients with BMI > 30. These cases were defined as complex cases.
The statistical analysis was conducted using the T-test for continuous variables and the χ–2 test for categorical variables with a statistical significance set for a P value < 0.05. To assess the trends of the LOS during the 10-year period a linear regression was carried out. Microsoft Excel software was used for storing data and analyses were performed using SAS v90.4.
RESULTS
We identified 495 cases of benign robotic hysterectomies during the study period. The population’s mean age was 48.18 ± 12.68 years with 143 (29%) of patients were postmenopausal. 82.44% of the population had a BMI ≤ 30. The population’s overall mean number of gestations was 2.02 ± 1.91 and mean parity 1.58 ± 1.52. Indications were: 192 leiomyomas (39%), 89 adenomyosis (18%), 88 transgenders (18%), 50 endometrial hyperplasia with or without atypia (150.5%), 14 endometriosis (3%), 8 uterine prolapse (2%), and 26 others (ovarian cysts, prophylactic BRCA 1 and 2 and benign tumors) (40.5%).
The mean uterine weight was 171.39 ± 145.79 g. No cases of supracervical hysterectomy were performed. The mean surgery time was 127.21 ± 42.37 min and OR time was 172.03 ± 47.62 min, the estimated blood loss (EBL) was 78.38 ± 134.26 mL with a differential of Hb of 1.13 ± 0.80 and 1 case of blood transfusion reported (EBL= 700 ml with a hemoglobin differential of 30.3). LOS was of 3.26 ± 1.55 days. It decreased with time from 30.5 ± 00.5 in 2010 to 2.49 ± 0.98 in 2019. There were 104 cases (21%) with concurrent procedures, such as extensive adhesiolysis, ureterolysis, adnexectomy, Richter procedure, transobturator tape (TOT), sacrocolpopexy, and umbilical hernia repair. Intraoperative complications were 2.22% (11 cases). Five bladder injuries and 6 bowel injuries were repaired by an urologist and a general surgeon. One conversion to laparotomy was reported for extensive adhesions. Postoperative complications were 3.63% (18 cases). One case of ureteral injury, four cases of vaginal dehiscence, eight cases of pelvic hematomas, one case of active bleeding, one case of pelvic abscess, one case of small bowel obstruction managed with medical treatment, and two cases of pulmonary embolism. Seven of these complications needed reoperation, all of which were done laparoscopically (3b according to Dindo-Clavien classification): four vaginal dehiscences, one pelvic abscess, one case of active hemorrhage, and a ureteral injury that was managed with double J stent and ureteral reimplantation a few months later.22
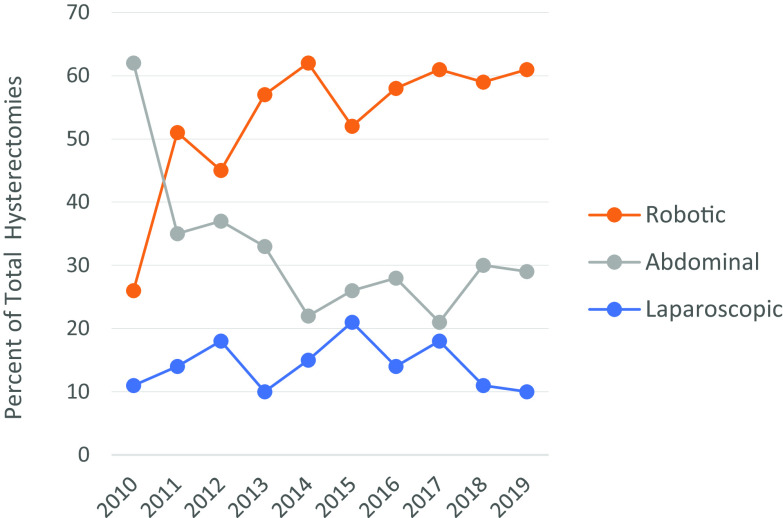
During the same period, 275 hysterectomies for benign indications were performed with laparotomy and 130 with laparoscopy. Table 1 and Figure 1 show the evolution of the route for hysterectomy during 10 years. In 2010, laparotomy was significantly more common than the robotic approach. As early as 2011, the trend was reversed, and from 2014 the robotic route was used significantly more than laparotomy, which decreased during the decade. The laparoscopy route was stable throughout. Robotic surgery volume increased, starting with 14 cases the first year of acquisition of the robot and reached 72 cases per year at the end of our study in 2019.
Table 1.
Percent of Hysterectomies by Route
| Year | Laparoscopy n (%) | Robotic n (%) | Laparotomy n (%) | Total |
|---|---|---|---|---|
| 2010 | 6 (11) | 14 (26) | 33 (62)a | 53 |
| 2011 | 11 (14) | 40 (51) | 28 (35) | 79 |
| 2012 | 13 (18) | 32 (45) | 26 (37) | 71 |
| 2013 | 7 (10) | 41 (57) | 24 (33) | 72 |
| 2014 | 13 (15) | 53 (62) | 19 (22)a | 85 |
| 2015 | 22 (21) | 54 (52) | 27 (26)a | 103 |
| 2016 | 12 (14) | 51 (58) | 25 (28)a | 88 |
| 2017 | 22 (18) | 74 (61) | 26 (21)a | 122 |
| 2018 | 12 (11) | 64 (59) | 33 (30)a | 109 |
| 2019 | 12 (10) | 72 (61) | 34 (29)a | 118 |
Percentages are rounded up to the nearest unit place: statistical significance, P < .05 between laparotomy and robotic route.
Figure 1.
Evolution of hysterectomies by route.
Characteristics of patients in the proficiency group (149) and nonproficiency group (346) are summarized in Table 2. There was no statistical difference between the two groups except uterine weight. Global results are summarized in Table 3.
Table 2.
Characteristics of Patients
| Overall Population (n = 495) | Proficiency Group(n = 149) | Non-proficiency Group(n = 346) | P | BMI ≤ 30(n = 403) | BMI > 30(n = 86) | P | Uterine Weight ≤ 250 (n = 337) | Uterine Weight > 2 50 (n = 80) | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age at surgery (years) [mean (SD)]a | 48.18 ± 12.68 | 49.14 ± 12.02 | 47.77 ± 12.95 | NS | 47.61 ± 12.65 | 50.59 ± 12.78 | 0.04 | 47.38 ± 13.83 | 48.88 ± 6.06 | NS |
| BMI ≤ 30 | 82.44% (409) | 81% (121) | 83% (288) | NS | NA | NA | NS | 84% (284) | 78 % (61) | NS |
| BMI > 30 | 17.55% (86) | 19% (28) | 17% (58) | NS | NA | NA | NS | 16% (50) | 22% (17) | NS |
| Menop. | 29% (143) | 30% (44) | 29% (99) | NS | 25.55% (103) | 43% (37) | NS | 30.2% (102) | 15% (12) | NS |
| Gravity (mean (SD)) | 2.02 ± 1.91 | 2.15 ± 1.99 | 1.96 ± 1.87 | 0.05 | 1.98±1.85 | 2.11± 2.16 | NS | 2.39±1.85 | 2.11±2.50 | NS |
| Parity (mean (SD)) | 1.58 ± 1.52 | 1.74 ± 1.67 | 1.51 ± 1.40 | NS | 1.53±1.39 | 1.75±1.85 | NS | 2.05±1.65 | 1.57±1.09 | 0.01 |
| History of L. | 47.67% (236) | 52% (77) | 46% (149) | NS | 47.64% (192) | 48.83% (42) | NS | 49.2% (166) | 32.5% (26) | NS |
| Uterine weight (mean (SD)) | 171.39 ± 145.79 (419) | 194.6 ± 158.6 | 161.3 ± 139.4 | 0.04 | 165.79 ± 140.03 (346) | 195.63 ± 172.33 (30) | NS | NA | NA | NS |
Results in % (n =) or mean ± SD, standard deviation; Menop, menopause; L, laparotomy; Hb, hemoglobin; NA, nonapplicable; NS, nonsignificant; Proficiency group, ≥ 75 cases/surgeon; Nonproficiency group, < 75 cases/surgeon.
Table 3.
Results
| Overall Population (n = 495) | Proficiency Group | Non-proficiencyGroup | P | BMI ≤ 30(n = 403) | BMI > 30(n = 86) | P | Uterine Weight ≤ 250 (n = 337) | Uterine Weight > 250 g (n = 80) | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Surgerytime (min) (mean (SD))a | 127.21 ± 42.37 | 117.2 ± 40.24 | 131.6 ± 42.59 | < 0.0001 | 125.73 ± 42.26 | 133.20 ± 42.77 | NS | 124.11 ± 42.34 | 146.20 ± 43.34 | 0.0001 |
| OR time (min) (mean (SD)) | 172.03 ± 47.62 | 157.3 ± 43.32 | 178.6 ± 48.05 | 0.0005 | 170.68 ± 48.19 | 177.71 ± 45.27 | NS | 169.62 ± 47.50 | 192.44 ± 45.82 | 0.0001 |
| EBL (mean (SD)) | 78.38 ± 134.26 | 75.77 ± 125.8 | 79.51 ± 137.9 | NS | 68.83±119.24 | 124.53±186.14 | 0.0005 | 64.24±110.2 | 116.63±146.98 | 0.0004 |
| Laparoconversion | 1 | 0 | 1 | NS | 0 | 1 | NS | 0 | 1 | NS |
| Concurrent procedures | 21% (104) | 23% (35) | 20% (69) | NS | 81 | 23 | NS | 76 | 13 | NS |
| Transfusion | 1 | 0 | 1 | NS | 1 | 0 | NS | 0 | 0 | NS |
| Hb differential (mean (SD)) | 1.13 ± 0.80 | 1.17 ± 0.70 | 1.12 ± 0.84 | NS | 1.15 ± 0.83 | 1.07 ± 0.63 | NS | 1.14 ± 0.76 | 1.11 ± 0.88 | NS |
| Intraoperative complications | 2.22% (11) | 1% (2) | 3% (9) | NS | 1.7% (7) | 4.6% (4) | NS | 2% (7) | 2.5% (2) | NS |
| LOS (mean (SD)) | 3.26 ± 1.55 | 3.32 ± 1.89 | 3.29 ± 1.22 | NS | 3.25 ± 1.63 | 3.29 ± 1.08 | NS | 3.26 ± 1.61 | 3.34 ± 1.36 | NS |
| Postoperative complications | 3.63% (18) | 3% (5) | 4% (13) | NS | 4.4% (18) | 0 | NS | 2.67% (9) | 5% (4) | NS |
Results in % (n =) or mean ± SD, standard deviation; NS, nonsignificant; Proficiency group, ≥ 75 cases/surgeon; Nonproficiency group, < 75 cases/surgeon.
The surgery time decreased significantly in the proficiency group (1170.2 ± 40.24 versus 1310.6 ± 42.59, P < .0001) as the OR time (1570.3 ± 43.32 versus 1780.6 ± 48.05, P = .005). Uterine weight was significantly higher in the proficiency group (1940.6 ± 1580.6 versus 1610.3 ± 1390.4, P = .04). Other results were not different between the groups. Linear regression showed that the length of stay significantly decreased through the years from 3.36 ± 0.63 in 2010 to 2.49 2.49 ± 0.98 in 2019 (P = .02).
Uterine weight ranged from 11 to 900 g for 417 procedures, 78 weight measurements were missing. 337 patients (80%) had a uterus ≤ 250 g and 80 patients (20%) had a uterus > 250 g. No statistical difference was found between the two subgroups characteristics except a lower parity for bigger uteri (Table 2). Lower EBL was seen with uteri ≤250 g subgroup (64.24 ± 1100.2 mL versus 116.63 ± 146.98 ml, P = .0004). The surgery time was shorter with the uteri ≤ 250 g subgroup (124.11 ± 42,34 min versus 146.20 ± 43.34 min) (P = .0001). So was the OR time (169.62 ± 47.50 min versus 192.44 ± 45.82 min, P = .0001). There was no statistical difference between the two subgroups in terms of LOS, complications, conversions, transfusion or hemoglobin differential. Results are summarized in Table 3.
403 patients (82.44%) were in the BMI ≤ 30 subgroup and 86 (17.55%) in the BMI > 30 subgroup (6 missing data). There was no statistical difference in the characteristics between the 2 subgroups except older patients in obese subgroup (Table 2).
The EBL was significantly less in the BMI ≤ 30 subgroup (68.83 ± 119.24 mL versus 124.53 ± 186.14 mL, P value= 0.0005). There was no statistical difference in the surgery and OR time, LOS, uterine weight, hemoglobin differential, and complications (Table 3).
DISCUSSION
Our robotic OR time results were concordant with the published literature. Swenson et al. have reported in their robotic group a mean OR time and mean uterine weight similar to ours in our nonproficiency group but they reported higher EBL values.23 The reduced OR time in our proficiency group is comparable to the values found by Landeen24 as well as by Sarlos et al.9,25 Other studies have shown lower OR times but they didn’t include large uteri.
The LOS was high in our series but decreased during the course of this study and reached 2.49 ± 0.98 in 2019. Initially, patients were hospitalized the day before the surgery, which could explain the longer LOS during the first period. Gradually, patients were hospitalized the same day of the surgery. Depending on our robotic experience, we decreased the LOS but it was still longer than literature, most often 2 days or less14,23 and this is due to the standard of care in our community. This could be explained by the differences in practice across global healthcare systems. For instance, in France the government covers all hospitalization’s fees, whereas the United States health system is more private. So the same day discharge after minimally invasive hysterectomy is commonly done in the United States, it appears to be more beneficial for patients, feasible and safe when adequate emphasis is placed on presurgical planning, careful patient selection, and increased postoperative monitoring at home, and allows costs saving.26
One of the goals of the introduction of the robotic surgery at our institution was to ultimately increase the proportion of hysterectomies safely completed via a minimally invasive approach, by enabling the surgeons to perform more complex hysterectomies that would otherwise require a laparotomy. Since its introduction at our hospital, robotic surgery has had a significant impact on the routes of hysterectomies. There was a significant decline in the percentage of abdominal hysterectomies performed coupled with an increase in robotic hysterectomies, over the same time period. This is consistent with previous reports.17,18,27,28 We did not observe a decrease in traditional laparoscopy route. Robotic surgery introduction shifted complex laparotomies to a minimally invasive approach, which benefited our patients by reducing complications, LOS, pain, and postoperative recovery.29
Robotic surgery has significant economic implications related to the capital cost and the per-case additional cost of hysterectomy.30 Data about hospital costs were lacking in our analysis, which is a weakness of the study. In most cases, hysterectomies for benign pathologies may be more expensive with the robotic approach, especially with nonproficient surgeons in comparison to laparoscopic24 or vaginal approaches.26 Some authors have found that complex indications like big uteri could be less expensive and reduce operative time with the robot compared to laparoscopy.13 Same day discharge practices could add to the cost reduction. However, total cost of care including hospital stay and recovery time is lower than open surgery.29
We have previously showed the short learning curve for robotic-assisted surgeries, with 20 cases needed to master basic surgical tasks and stabilize the operative time.31 Existing studies investigating the learning curve of robotic hysterectomy are limited by a small sample size and inconsistent reporting of complication rates.31–34 In fact, 20 to 26 interventions were enough to stabilize operative time versus 75 for laparoscopy.31,35,36 Few studies have evaluated the learning curve associated with attaining proficiency.20,21 After 75 cases per surgeon, we noticed another decrease in surgery and operating room time. Efficiency continued to improve with experience and case volume. Furthermore, it was associated with more complex cases: uterine weight was higher in our proficiency group, which indicates that this group probably performed more minimally invasive surgery for larger uteri than the nonproficiency group of surgeons, as these patients were not particularly referred to proficient surgeons. We didn’t find a difference in patient morbidity, which should be the true measure of the quality of surgical care. Our complication rate was higher than the literature and it was not increased in our nonproficient group nor for complex cases, although there is a statistical trend but no statistical significance, probably due to a lack of power for this cohort. Woelk et al20 found a decrease in complications after 44 cases and per-operative injuries after 91 cases.
Complex cases defined by large uteri and obese patients could be a better indication for robotic surgery.21 Robotic surgery optimizes the conditions in which the surgeon operates: good exposure, decreased fatigue, enhanced mobility, and increased dexterity. It is in line with the literature confirming that robotic surgery is a preferable route for obese patients undergoing hysterectomy.11,37 In our series, no significant statistical difference was noted in terms of complications, LOS, and conversions between the obese and nonobese groups. There was, however, a statistically significant increase in estimated blood loss in the group with BMI > 30 compared to the group with BMI ≤ 30. Although, this statistical difference is found but it is not clinically significant.
Most studies showed that hysterectomies for large uteri (> 250 g) took longer than those with small uteri.2,6,22 Our study findings showed significant differences in surgery and OR time between the subgroup with uteri ≤ 250 g compared to uteri > 250 g. Our study population did not undergo laparoscopic morcellation. Tissue extraction was always performed vaginally, which might increase the operating time in larger specimens.23 The robotic approach appears to be associated with shorter operative time compared to the laparoscopic route for large uteri, this led some authors to advocate for the robotic approach for this particular indication.3
We reported only one case of conversion to laparotomy. It was due to extensive adhesions. We did not have any additional complications or increase in LOS in our large uteri group. Our results match existing data supporting a significant increase in EBL with larger uteri with no increase in the length of stay nor the mortality and morbidity rate.6,16,17
Recent data showed that high-volume robotic surgeons could have shorter operative time and decreased blood loss for complex cases in comparison with laparoscopy.21
This study is limited by its retrospective nature. The data had some missing information that was not filled in the charts. Moreover, within our surgical team, 10 surgeons performed the surgeries during 10 years and fewer surgeons had reached their proficiency curve, which could impact the statistical analysis. Additional robotic procedures performed by our surgeons during the study period were not reported. In our study, we could not compare robotic hysterectomies studied to laparoscopic hysterectomies because the volume of laparoscopic hysterectomies was significantly less.
The strength of this study is that it includes the largest European robotic benign database of 495 patients on a 10-year period of time since the implementation of a robotic surgery program.
CONCLUSION
Our results confirm that robotic surgery for benign hysterectomy is a feasible and safe approach even in “complex cases” including patients with high BMI and big uteri.
Although most studies compare the robotic technique with the laparoscopic one, our study focused on the trends of the robotic surgery and its impact on shifting cases from laparotomy to a minimally invasive approach. A decreased OR time with an increase in complexity of the cases was observed with high-volume surgeons. Further studies are needed to assess the impact of robotic assistance on the total cost of care in the general gynecology practice.
Footnotes
Disclosure: Gabby Moawad is a speaker for Intuitive Surgical and Applied Medical.
Contributor Information
Marie Carbonnel, Department of Obstetrics and, Gynecology, Foch Hospital, Suresnes, Faculty of Medicine, Paris Ouest (UVSQ), France..
Gaby N. Moawad, Department of Obstetrics & Gynecology, George Washington University School of Medicine and Health Sciences, 2150 Pennsylvania Ave. NW, Ste 6A429, 20037 Washington, DC, USA..
Mia Maria Tarazi, Department of Obstetrics and, Gynecology, Foch Hospital, Suresnes, Faculty of Medicine, Paris Ouest (UVSQ), France..
Aurelie Revaux, Department of Obstetrics and, Gynecology, Foch Hospital, Suresnes, Faculty of Medicine, Paris Ouest (UVSQ), France..
Titouan Kennel, Department of Clinic Research, Foch Hospital, Suresnes, France..
Angéline Favre-Inhofer, Department of Obstetrics and, Gynecology, Foch Hospital, Suresnes, Faculty of Medicine, Paris Ouest (UVSQ), France..
Jean Marc Ayoubi, Department of Obstetrics and, Gynecology, Foch Hospital, Suresnes, Faculty of Medicine, Paris Ouest (UVSQ), France..
References:
- 1.Garry R. Health economics of hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19(3):451–465. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby VL, Autry A, Jacobson G, Domush R, Nakagawa S, Jacoby A. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114(5):1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhvani K, Curnow T, Carpenter T. Route of hysterectomy: a retrospective, cohort study in English NHS Hospitals from 2011 to 2017. BJOG. 2019;126(6):795–802. [DOI] [PubMed] [Google Scholar]
- 4.ACOG Committee Opinion 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158. [DOI] [PubMed] [Google Scholar]
- 5.Carbonnel M, Goetgheluck J, Frati A, Even M, Ayoubi JM. Robot-assisted laparoscopy for infertility treatment: current views. Fertil Steril. 2014;101(3):621–626. [DOI] [PubMed] [Google Scholar]
- 6.Carbonnel M, Abbou H, N’Guyen HT, et al. Robotically assisted hysterectomy versus vaginal hysterectomy for benign disease: a prospective study. Minim Invasive Surg. 2013;(2013):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albright BB, Witte T, Tofte AN, et al. Robotic versus laparoscopic hysterectomy for benign disease: a systematic review and meta-analysis of randomized trials. J Minim Invasive Gynecol. 2016;23(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paraiso MF, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotically assisted total laparoscopic hysterectomy. Am J Obstet Gynecol. 2013;208(5):368e1–7. [DOI] [PubMed] [Google Scholar]
- 9.Sarlos D, Kots L, Stevanovic N, von Felten S, Schär G. Robotic compared with conventional laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;120(3):604–611. [DOI] [PubMed] [Google Scholar]
- 10.Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015(8):CD003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iavazzo C, Gkegkes ID. Robotic assisted hysterectomy in obese patients: a systematic review. Arch Gynecol Obstet. 2016;293(6):1169–1183. [DOI] [PubMed] [Google Scholar]
- 12.Nawfal AK, Orady M, Eisenstein D, Wegienka G. Effect of body mass index on robotic-assisted total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2011;18(3):328–332. [DOI] [PubMed] [Google Scholar]
- 13.Moawad GN, Abi Khalil ED, Tyan P, et al. Comparison of cost and operative outcomes of robotic hysterectomy compared to laparoscopic hysterectomy across different uterine weights. J Robotic Surg. 2017;11(4):433–439. [DOI] [PubMed] [Google Scholar]
- 14.Payne TN, Dauterive FR, Pitter MC, et al. Robotically assisted hysterectomy in patients with large uteri: outcomes in five community practices. Obstet Gynecol. 2010;115(3):535–542. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Bana R, Sanjay M. Comparison of robotic and laparoscopic hysterectomy for the large uterus. JSLS. 2019;23(1):e2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orady ME, Karim Nawfal A, Wegienka G. Does size matter? The effect of uterine weight on robot-assisted total laparoscopic hysterectomy outcomes. J Robotic Surg. 2011;5(4):267–272. [DOI] [PubMed] [Google Scholar]
- 17.Smorgick N, Patzkowsky KE, Hoffman MR, Advincula AP, Song AH, As-Sanie S. The increasing use of robot-assisted approach for hysterectomy results in decreasing rates of abdominal hysterectomy and traditional laparoscopic hysterectomy. Arch Gynecol Obstet. 2014;289(1):101–105. [DOI] [PubMed] [Google Scholar]
- 18.Papalekas E, Fisher J. Trends in route of hysterectomy after the implementation of a comprehensive robotic training program. Minim Invasive Surg. 2018;2018:7362489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Settnes A, Topsoee MF, Moeller C., Dueholm M, Kopp TI, et al. Reduced complications following implementation of laparoscopic hysterectomy: a Danish population-based cohort study of minimally invasive benign gynecologic surgery between 2004 and 2018. J Minim Invasive Gynecol. 2020;27(6):1344–1353. [DOI] [PubMed] [Google Scholar]
- 20.Woelk JL, Casiano ER, Weaver AL, Gostout BS, Trabuco EC, Gebhart JB. The learning curve of robotic hysterectomy. Obstet Gynecol. 2013;121(1):87–95. [DOI] [PubMed] [Google Scholar]
- 21.Herrinton LJ, Raine-Bennett T, Liu L, Alexeeff SE, Ramos W, Suh-Burgmann B. Outcomes of robotic hysterectomy for treatment of benign conditions: influence of patient complexity. Perm J. 2020;24:19.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. [DOI] [PubMed] [Google Scholar]
- 23.Swenson CW, Kamdar NS, Harris JA, Uppal S, Campbell DA, Jr., Morgan DM. Comparison of robotic and other minimally invasive routes of hysterectomy for benign indications. Am J Obstet Gynecol. 2016;215(5):650.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deimling TA, Eldridge JL, Riley KA, Kunselman AR, Harkins GJ. Randomized controlled trial comparing operative times between standard and robot-assisted laparoscopic hysterectomy. Int J Gynaecol Obstet. 2017;136(1):64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landeen LB, Bell MC, Hubert HB, Bennis LY, Knutsen-Larson SS, Seshadri-Kreaden U. Clinical and cost comparisons for hysterectomy via abdominal, standard laparoscopic, vaginal and robot-assisted approaches. S D Med. 2011;64(6):197–199. [PubMed] [Google Scholar]
- 26.Korsholm M, Mogensen O, Jeppesen MM, Lysdal VK, Traen K, Jensen PT. Systematic review of same-day discharge after minimally invasive hysterectomy. Int J Gynaecol Obstet. 2017;136(2):128–137. [DOI] [PubMed] [Google Scholar]
- 27.Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–698. [DOI] [PubMed] [Google Scholar]
- 28.Moawad G, Liu E, Song C, Fu AZ. Movement to outpatient hysterectomy for benign indications in the United States, 2008-2014. PLoS One. 2017;12(11):e0188812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsdottir GM, Jorgensen S, Cohen SL, et al. Increasing minimally invasive hysterectomy: effect on cost and complications. Obstet Gynecol. 2011;117(5):1142–1149. [DOI] [PubMed] [Google Scholar]
- 30.Wu CZ, Klebanoff JS, Tyan P, Moawad GN. Review of strategies and factors to maximize cost-effectiveness of robotic hysterectomies and myomectomies in benign gynecological disease. J Robot Surg. 2019;13(5):635–642. [DOI] [PubMed] [Google Scholar]
- 31.Favre A, Huberlant S, Carbonnel M, Goetgheluck J, Revaux A, Ayoubi JM. Pedagogic approach in the surgical learning: the first period of “assistant surgeon” may improve the learning curve for laparoscopic robotic-assisted hysterectomy. Front Surg. 2016;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenihan JP, Jr., Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008;15(5):589–594. [DOI] [PubMed] [Google Scholar]
- 33.Pitter MC, Anderson P, Blissett A, Pemberton N. Robotic-assisted gynaecological surgery-establishing training criteria; minimizing operative time and blood loss. Int J Med Robotics Comput Assist Surg. 2008;4(2):114–120. [DOI] [PubMed] [Google Scholar]
- 34.Bell MC, Torgerson JL, Kreaden U. The first 100 da Vinci hysterectomies: an analysis of the learning curve for a single surgeon. S D Med. 2009;62(3):93–95. [PubMed] [Google Scholar]
- 35.Terzi H, Biler A, Demirtas O, Guler OT, Peker N, Kale A. Total laparoscopic hysterectomy: Analysis of the surgical learning curve in benign conditions. Int J Surg. 2016;35:51–57. [DOI] [PubMed] [Google Scholar]
- 36.Tang FH, Tsai EM. Learning curve analysis of different stages of robotic-assisted laparoscopic hysterectomy. Biomed Res Int. 2017;2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida OD., Jr. Robotic hysterectomy strategies in the morbidly obese patient. JSLS. 2013;17(3):418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]