Abstract
Background and Objectives:
Gastroesophageal reflux disease is a common disease and there is little known about the role family history plays in its disease process and incidence. Our study was designed to compare the patients with first degree relatives with and without the disease and see if there was any difference in patients needing antireflux surgery, the outcomes after antireflux surgery, and whether they needed redo surgery.
Methods:
An institutional review board approved registry for patients undergoing antireflux surgery at a single institution was used. Patients were asked specific questions about their family history of gastroesophageal reflux disease at their pre-operative visit. Patients with a family history and those without were compared.
Results:
There was no statistical difference between the patients with family history of gastroesophageal reflux disease for likelihood to undergo surgery, outcomes from surgery, or the need for redo surgery. There were more females than males in the study and there were more patients with a positive family history in the study than those without.
Conclusion:
Since there is no impact of family history of gastroesophageal reflux disease on antireflux surgery, patients can be counseled that their decision to undergo antireflux surgery is independent from the response of their first degree relatives.
Keywords: Gastroesophageal reflux disease (GERD), Anti-reflux surgery (ARS), Hiatal hernia, Family history
INTRODUCTION
The prevalence of gastroesophageal reflux disease (GERD) is an estimated 10% – 20% in the United States making it one of the most common gastrointestinal tract disorders.1,2 GERD is associated with significant morbidity and a substantial amount of health care dollars spent in the diagnosis, surveillance, and treatment of the disease.3 Current therapy includes both lifestyle modifications such as weight loss and medical therapy mainly with proton-pump inhibitors.4,5 When adequate control of symptoms is not achieved with the aforementioned therapies, antireflux surgery (ARS) should be considered.6 The gold standard ARS is laparoscopic fundoplication, either complete or partial configuration.7 ARS outcomes have been shown to be impacted by risk factors such as body mass index, depression, and race in several studies.8–10 Other risk factors have been less well established in the literature. For instance, there is little known regarding the role family history plays in the setting of antireflux surgery, though it has been clearly established as a significant risk factor for longer disease duration and eventual diagnosis in Barrett’s esophagus.11,12 A previous study examining the ultrastructure in the crural muscle fibers demonstrates a difference in patients with hiatal hernias versus the control group. This may imply a genetic predisposition to formation of a hiatal hernia leading to GERD, which may be related to collagen formation.13 Understanding family history impacts a numerous diseases, thus we decided to study the role of patient-reported history of GERD in first degree relatives with regard to needing ARS, outcomes from ARS, and needing to redo ARS.
METHODS
A prospectively gathered institutional review board approved registry for all patients undergoing antireflux surgery at a single institution was queried to find patients for the study. All patients have consented to participate in the registry. A commonly used assessment, the Gastroesophageal Reflux Disease – Health Related Quality of Life (GERD-HRQL) was administered to all patients to assess them pre-operatively and at each of the postoperative endpoints.14 Patients were specifically asked at their pre-operative visit about their family history of GERD. This included asking a question if there were any GERD diagnoses in the family and was followed by a question asking if any of those family members had ARS. A positive answer to either of these questions was considered inclusion to the study group. Patients that answered no to both questions were considered the control group. Additional inclusion criteria to the study, in either group, was objectively confirmed GERD as determined by DeMeester score > 140.7, esophagogastroduodenoscopy confirmed Grade C or D esophagitis, or para-esophageal hernia. Subjects were excluded for failure to obtain the completed family history questions, and patients not proven to have GERD as determined by the criteria listed above. A total of 337 patients were seen in the office from January 2014 to December 2018 with the presumptive diagnosis of GERD. Thirty-eight patients were excluded for incomplete family history leaving 299 patients meeting inclusion criteria. Another 37 patients were excluded in the analysis portion for the endpoints of outcomes and redo surgery for lacking a complete data set.
Patients with positive family history were compared to the control group of negative family history. Our primary endpoints were a family history of GERD in first degree relatives predicted whether a patient was more likely to undergo ARS. Also, the same family history was used to see if there was a correlation with their outcomes from undergoing ARS. GERD-HQRL scores were used to assess surgery outcomes which were obtained pre-operatively and at 6 and 12 months postoperatively.14 Lastly, we did a subset analysis of the study and control groups dividing them into primary repair versus redo surgery. There was no standardized surgical intervention (complete vs. partial fundoplication) for patients who underwent antireflux surgery.
Patient characteristics and outcomes were summarized by mean and standard deviation for continuous variables, and frequency and percent for categorical variables. Independent sample t test was used to compare continuous variables between positive and negative family history groups. Differences between categorical variables were assessed by χ2 test. Statistical analysis was performed using R statistical software (R foundation for Statistical Computing, Vienna, Austria). P-value < 0.05 was considered statistically significant.
RESULTS
A total of 299 patients met inclusion criteria of objectively confirmed GERD and complete family history (Table 1). There were 172 (58%) patients reporting positive family history of GERD or ARS, while 127 (42%) patients reporting negative family history. There was no difference in age between the two groups, 55.0 years and 56.9 years (P = .249) respectively. The gender distribution between positive and negative family history of GERD was statistically significant, where 197 (66%) patients were female and 102 (34%) patients were male. A total of 246 (82%) patients were White, 26 (9%) were Black, and 27 (9%) were listed as Other.
Table 1.
Demographic Information
| Family History |
||||
|---|---|---|---|---|
| Total (N = 299) | Negative (N = 127) | Positive (N = 172) | P-Value | |
| Age (years) | 0.249 | |||
| N | 299 | 127 | 172 | |
| Mean (SD) | 55.8 (16.0) | 56.9 (16.6) | 55.0 (15.5) | |
| Gender | 0.032 | |||
| Female | 197 (65.9%) | 75 (59.1%) | 122 (70.9%) | |
| Male | 102 (34.1%) | 52 (40.9%) | 50 (29.1%) | |
| Race | 0.339 | |||
| 1. White | 246 (82.3%) | 102 (80.3%) | 144 (83.7%) | |
| 2. Black | 26 (8.7%) | 10 (7.9%) | 16 (9.3%) | |
| 3. Other | 27 (9.0%) | 15 (11.8%) | 12 (7.0%) | |
| BMI | 0.108 | |||
| N | 138 | 60 | 78 | |
| Mean (SD) | 29.2 (5.2) | 29.8 (5.0) | 28.6 (5.3) | |
SD, standard deviation; BMI, body mass index.
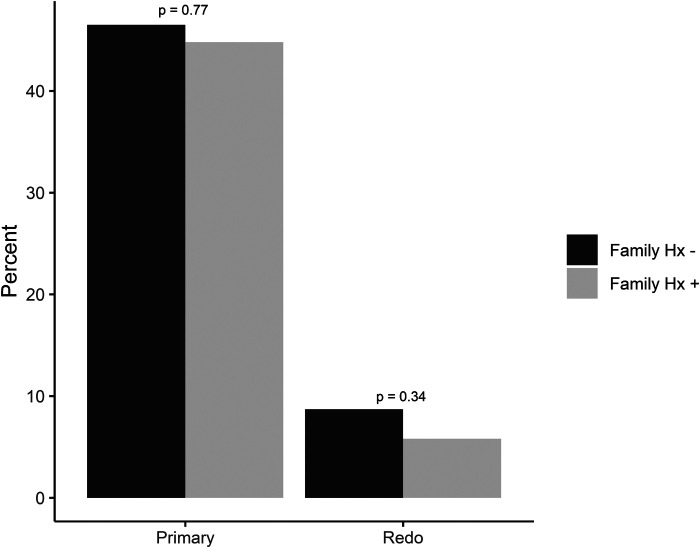
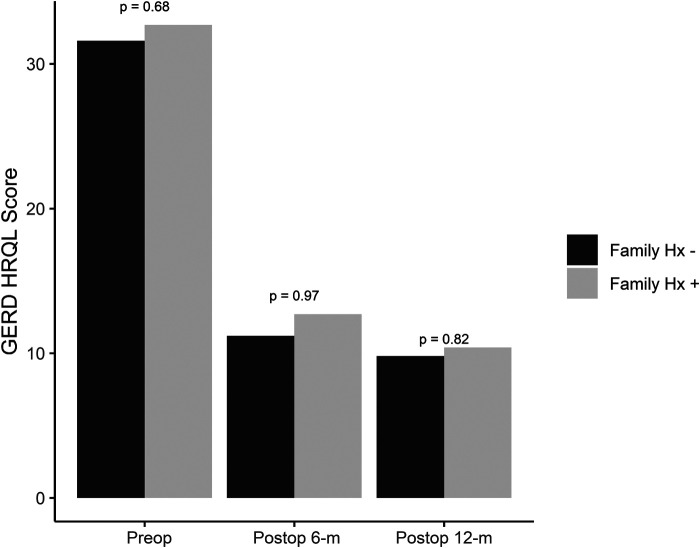
Of the subjects with positive family history, 87 (51%) underwent antireflux surgery, while 70 (55%) with negative family history underwent surgery (Table 2). GERD-HQRL values in the positive family history group versus negative family history groups were 32.7 and 31.6 pre-operatively, 12.7 and 11.2 at 6 months, and 10.4 and 9.8 at 12 months (Figure 1). No significant difference was found in GERD-HQRL scores between groups preoperatively or at either 6-month or 12-month follow-up. During subset analysis, 10 of the 87 patients who had a positive family history and underwent ARS subsequently underwent redo ARS. When examining the population with negative family history, 11 of the 70 patients had to undergo redo surgery ARS (Figure 2). There were no significant differences between the two groups.
Table 2.
Between Group Comparison of Incidence of Antireflux Surgery
| Positive Family History | Negative Family History | P-Value | |
|---|---|---|---|
| Total Number of Patients | 172 | 127 | |
| Antireflux Surgery | 87 (50.6%) | 70 (55.1%) | 0.44 |
| Primary Antireflux surgery | 77 (44.8%) | 59 (46.5%) | 0.77 |
Figure 1.
Between group comparisons of gastroesophageal reflux disease – health related quality of life scores at pre-operative, 6 months postoperative, and 12 months postoperative timepoints.
Figure 2.
Between group comparisons of primary versus redo antireflux surgery.
DISCUSSION
We aimed to determine the significance of family history of GERD in first degree relatives and the relationship to ARS, specifically the need for ARS, its outcomes, and need to undergo redo ARS. We examined this relationship retrospectively and each patient included in this study was asked specific questions about family history of GERD at his or her pre-operative appointment. We were able to show that family history does not affect the need for ARS or the outcomes following ARS. We were also able to show that a family history of GERD did not change the chances of needing redo ARS. These were unexpected outcomes and disproved our hypothesis. Ultimately, this is one less variable to worry about when determining if a patient is a good surgical candidate for ARS and recognizes that patients respond to ARS the same regardless of family history.
GERD is a widely prevalent disease seen both in the out-patient primary care, in-patient, and surgical settings. It is known that family history of certain diseases puts patients at a risk of developing the same disease. We initially hypothesized that patients who have a family history of GERD, mainly in a first degree relative, would be more likely to undergo ARS or even redo ARS, but this was not the case. However, we did have a greater percentage of our patient population report positive family history of GERD, 57% (P = .01). This shows that more patients present for ARS with a family history of GERD than those who lack a family history. This can be attributed to patients having an overall better understanding of the disease having seen family members go through the same disease process and who are looking for a more permanent or superior solution. This can also point to the realization that medical therapy has its limitations and with good clinical guidance, patients can be counseled on surgical repair to improve symptoms and quality of life.
When comparing demographic information from our study (Table 1), it is noted that there was no significant difference between the positive and negative family history groups. However, it is interesting to note that there were significantly more females in the study (66% female, P < .0001) as well as more females than males who reported positive family history of GERD, which was statistically significant (61.9% female vs. 49.0% male, P = .03). There is not a well-defined consensus that females are more likely to have GERD than males1,2, but we made the assumption that our patient population saw more females with positive family history due to a larger percentage of female patients who had knowledge of family history. In this sample a larger percentage of patients identified as White as opposed to Black or other in both positive and negative family history groups. This is supported by a similar study in Michigan that showed fewer patients that underwent ARS to be Black as opposed to White.10 This would reinforce that a predominantly White population may have more ARS than a different racially dispersed group where their family history does not play a role.
The basis of this study was formulated on the premise that there are hereditary features of GERD that may guide its disease course or incidence. Previous publications show that patients with hiatal hernias have an ultrastructural abnormality that predisposes them to such hernias.13 This is an idea that has been studied previously in the inguinal15–17 and ventral hernia literature.18 What used to be postulated because of prior surgery or weakness in abdominal tissue has now been shown to be directly related to a genetic predisposition of increased type III collagen thus making the overall muscle weaker.19 Due to the retrospective design of this study, the data available did not allow for us to investigate a potential genetic predisposition based on genetic markers. Future studies which collect such data regarding the genetic make-up of GERD patients and may be beneficial to better define the role of family history in the development of GERD and need for ARS.
There are limitations to our study. First, this was a single institution study. Although the demographics did not differ between our main cohorts, this may not represent populations outside of the location for this study. A multicenter, more diverse patient population sample may provide a different outcome when comparing those having ARS and their outcomes. Secondly, the sample may have been underpowered to detect a statistically significant difference. In addition, our data came from patient-reported family history. While this type of reporting is less reliable than objective measures of GERD, this preliminary data can inform future research into the genetic contribution to GERD. Lastly, we could have extended our follow up period beyond 12 months to help capture the patients with failing ARS and seeing if the family history played a role at those time points.
In conclusion, although family history of GERD is documented to be a predictor of whether patients have GERD, there is no correlation with outcomes in patients who undergo ARS. We did note that many patients with positive family history of GERD do subsequently undergo ARS, but this was not statistically significant in comparison to those patients who have a negative family history of GERD. However, in the pre-operative appointments for patients who do report a positive family history of GERD, it is vital to counsel these patients to understand that they may fail traditional proton-pump inhibitors therapy and thus may need antireflux surgery in the future. In the time that has passed since this study, we have taken the approach of educating patients that any family response to ARS and redo ARS is independent to their response to the same or similar surgery. Such education allows patients to feel at ease pursuing surgical intervention if they have negative associations with ARS due to family members experiences.
Contributor Information
Jennifer J. Misenhimer, Division of Minimally Invasive Surgery, Baylor University Medical Center, Dallas, TX..
Marc A. Ward, Division of Minimally Invasive Surgery, Baylor University Medical Center, Dallas, TX.; Center for Advanced Surgery, Baylor Scott & White Health, Dallas, TX. Texas A&M College of Medicine, Bryan, TX.
Christine E. Sanchez, Division of Minimally Invasive Surgery, Baylor University Medical Center, Dallas, TX.; Research Institute, Baylor Scott & White Health, Dallas, TX.
Andrew Ngov, Division of Minimally Invasive Surgery, Baylor University Medical Center, Dallas, TX.; Research Institute, Baylor Scott & White Health, Dallas, TX.
Rehma Shabbir, Research Institute, Baylor Scott & White Health, Dallas, TX..
Gerald O. Ogola, Research Institute, Baylor Scott & White Health, Dallas, TX..
Carolina Orsi, Texas A&M College of Medicine, Bryan, TX..
Stephen G. Leeds, Division of Minimally Invasive Surgery, Baylor University Medical Center, Dallas, TX.; Center for Advanced Surgery, Baylor Scott & White Health, Dallas, TX. Texas A&M College of Medicine, Bryan, TX.
References:
- 1.Dent J, El-Serag HB, Wallander M-A, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–386. [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Lee J, Gupta N, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity. 2013;21(2):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galmiche J-P, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS Randomized Clinical Trial. JAMA. 2011;305(19): [DOI] [PubMed] [Google Scholar]
- 6.Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513–1523. [DOI] [PubMed] [Google Scholar]
- 7.Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24(11):2647–2669. [DOI] [PubMed] [Google Scholar]
- 8.Bashir Y, Chonchubhair HN, Duggan SN, et al. Systematic review and meta-analysis on the effect of obesity on recurrence after laparoscopic anti-reflux surgery. The Surgeon. 2019;17(2):107–118. [DOI] [PubMed] [Google Scholar]
- 9.Kamolz T, Pointner R, Velanovich V. The impact of gastroesophageal reflux disease on quality of life. Surg Endosc. 2003;17(8):1193–1199. [DOI] [PubMed] [Google Scholar]
- 10.Haithcock B, Velanovich V. Comparison of antireflux surgery among ethnicity. J Natl Med Assoc. 2004;96(4):7. [PMC free article] [PubMed] [Google Scholar]
- 11.Kulig M, Nocon M, Vieth M, et al. Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol. 2004;57(6):580–589. [DOI] [PubMed] [Google Scholar]
- 12.Bakr O, Zhao W, Corley D. Gastroesophageal reflux frequency, severity, age of onset, family history and acid suppressive therapy predict Barrett esophagus in a large population. J Clin Gastroenterol. 2018;52(10):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei L, del Genio G, Rossetti G, et al. Hiatal hernia recurrence: surgical complication or disease? Electron microscope findings of the diaphragmatic pillars. J Gastrointest Surg. 2009;13(3):459–464. [DOI] [PubMed] [Google Scholar]
- 14.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130–134. [DOI] [PubMed] [Google Scholar]
- 15.Lau H, Fang C, Yuen WK, Patil NG. Risk factors for inguinal hernia in adult males: a case-control study. Surgery. 2007;141(2):262–266. [DOI] [PubMed] [Google Scholar]
- 16.Liem MSL, van der Graaf Y, Zwart RC, Geurts I, van Vroonhoven TJMV. Risk factors for inguinal hernia in women: a case-control study. The Coala Trial Group. Am J Epidemiol. 1997;146(9):721–726. [DOI] [PubMed] [Google Scholar]
- 17.Jansen PL, Klinge U, Jansen M, Junge K. Risk factors for early recurrence after inguinal hernia repair. BMC Surg. 2009;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sazhin A, Zolotukhin I, Seliverstov E, et al. Prevalence and risk factors for abdominal wall hernia in the general Russian population. Hernia. 2019;23(6):1237–1242. [DOI] [PubMed] [Google Scholar]
- 19.Koruth S, Narayanaswamy Chetty YV. Hernias is it a primary defect or a systemic disorder? Role of collagen III in all hernias: a case control study. Ann Med Surg. 2017;19:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]