Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of COVID-19, a disease that as of July 10, 2020, has infected >12 million people and killed >500,000. COVID-19 infection leads to acute respiratory distress syndrome in a subset of patients and is a primary driver of acute morbidity in infected persons. However, it is becoming increasingly clear that SARS-CoV-2 infection drives dysfunction and pathology outside the lungs, including reports of renal, cardiac, and neurological complications. In this study, we summarize the known incidence and evidence of neurological complications associated with SARS-CoV-2 infection and other pathogenic coronaviruses. These studies describe a poorly understood spectrum of COVID-19 central nervous system symptoms, ranging from common and subclinical issues such as anosmia and headache to more concerning reports of stroke and encephalopathy. We discuss potential mechanisms of pathogenesis, including a discussion of how the understanding of neurological complications known to occur in HIV-1 patients may provide insight into SARS-CoV-2-associated neurological manifestations. Specifically, three hypotheses are discussed that are informed by decades of knowledge about HIV pathogenesis in the brain, which include a potential direct viral effect, an indirect viral effect, and/or a neuroimmune axis effect. Individually or in combination these potential effects may contribute to COVID-19 neurological complications.
Keywords: SARS-CoV-2, brain, COVID-19, HIV, neuroinvasion, autopsy studies
Emergence of SARS-CoV-2
Coronaviruses (CoVs) are zoonotic in origin and cause respiratory disease in humans. Although a number of common seasonal CoVs cause mild illness, three highly pathogenic beta-coronaviruses jumped from bats to humans, with or without an intermediate animal host, causing three epidemics/pandemics in the past 18 years, all predominately characterized by severe acute respiratory syndrome (SARS). In 2002, the first SARS-CoV infection spread from China to 29 countries, infecting ∼8,000 people with a 9% fatality rate.1,2 The second infection, Middle East respiratory syndrome (MERS), emerged in 2012 and although it infected considerably fewer people, infection is associated with a mortality rate of 35%.3 MERS-CoV is transmitted through Arabian camels, presumably initially infected by bats then transmitted to humans through camel/human interactions.2,4–6 The latest zoonotic CoV, SARS-CoV-2 emerged in Wuhan, China, in December 2019, and is characterized by a lower mortality rate than SARS and MERS but a much more efficient human to human transmission rate.7 As of July 10, 2020, SARS-CoV-2 infected 12,375,147 people worldwide, with 556,895 deaths (https://coronavirus.jhu.edu/map.html). Coronavirus disease 2019 (COVID-19) has now become the leading cause of death in the United States (https://www.cdc.gov/coronavirus/2019). SARS-CoV-2 shares 79.5% of its single-stranded ribonucleic acid (ssRNA) genome with SARS-CoV and 96% with RaTG131, a bat coronavirus.8 Based on further viral genomic analyses of coronaviruses across species, SARS-CoV-2 appears to either have been transmitted from bats to an intermediate species, such as pangolins, before infecting humans, or alternatively from bats directly to humans.8–10 Regardless of the zoonotic path to humans, the virus quickly spread throughout Asia to the rest of the word, crippling economies and health care systems worldwide.
Similar to SARS-CoV, SARS-CoV-2 requires angiotensin converting enzyme II (ACE2), a membrane-associated aminopeptidase, to attach to and enter target cells.11,12 However, the Spike (S) protein of SARS-CoV-2 binds to ACE2 with higher affinity than SARS-CoV,13,14 which may in part explain differences in spread and pathogenesis between SARS-CoV and SARS-CoV-2. Furthermore, the SARS-CoV-2 S protein also contains a furin cleavage site that may promote its shedding protein in the extracellular milieu,13 which may play a role in immunological and other cellular responses associated with the cytokine storm known to underly pathogenesis among severely ill COVID-19 patients.15–17 However, although ACE2 is widely distributed in human tissue, not all ACE2 positive cells are infected by SARS-CoV-2.18 This is consistent with findings from other coronaviruses, including SARS-CoV, where ACE2 expression alone was not sufficient to promote viral entry. For example, muscle cells highly express ACE2, yet they are not infected by SARS-CoV.19,20 Similar to other CoVs, SARS-CoV-2 S protein requires protease activity of the target cell to mediate membrane fusion during infection.11,12 The cell surface protease TMPRSS2 can mediate this function for SARS-CoV-2, and its activity is required for pathogenesis in animal models.11,12 However, other CoV Spike proteins, including the S proteins of SARS and MERS, can conditionally utilize other cellular proteases, such as furin, or endosomal cathepsins to mediate entry in some cell types,21 and this seems to be the case for SARS-CoV-2 S protein in some cell types.13 It is also possible that virus internalization, mediated by S/ACE2 interactions or through nonspecific endocytosis, could drive cellular responses associated with disease pathogenesis in the absence of fusion and infection. Coronavirus may also drive cellular responses in the absence of functional entry into the target cell cytoplasm, as viral degradation in the endosome or lysosome could trigger the activation of pattern recognition receptors and molecules, pathogen-associated molecular patterns, and/or danger-associated molecular patterns to induce potent immune activation. ACE2 was recently identified as an interferon-stimulated gene.22 How this impacts COVID-19 pathology remains unclear. Studies of SARS-CoV and other coronaviruses reveal that these viruses have developed numerous mechanisms to directly antagonize interferon pathways.23,24 However, these mechanisms delay, but do not eliminate interferon responses during infection, so it will be important to understand how infection leads to changes in ACE2 levels throughout the course of infection. In addition, upregulation of ACE2 expression by interferon may also be important to consider in the context of vaccine design, as any vaccine that would prime an immune response and trigger interferon-stimulated genes may also increase the targets for SARS-CoV-2 infection.
As a cytoplasmic RNA virus with its own RNA proof reading enzyme, the rate of SARS-CoV-2 mutation is not high, estimated at 25 mutations per year (https://nextstrain.org/ncov/global?l=clock). These mutations, however, are sufficient to trace the virus between various geographical areas. For example, the virus in New York, the hardest hit state in the United States to date, shares extensive homology to SARS-CoV-2 isolates from Italy, whereas in Washington state, the virus likely entered the United States through China (https://nextstrain.org/ncov). It is also unclear if there are certain strains of SARS-CoV-2 that may be more pathogenic and associated with severe illness, independent of clinical comorbidities. An earlier report identified two strains of SARS-CoV-2, an ancestral S strain and a predominant L strain.25 Initially the authors concluded that the L strain's predominance was due to a higher transmission rate and more “aggressive” phenotype.25 Owing to confounding effects of genetic drift, founder effects, and small sample size of the study, it has been argued that a conclusion cannot be made that L is more aggressive and the authors have accepted this counter argument (http://virological.org/t/response-to-on-the-origin-and-continuing-evolution-of-sars-cov-2/418). Furthermore, as more SARS-CoV-2 genomes have been incorporated into worldwide phylogenetic analyses, many prominent clades have now emerged, suggesting the so-called S and L strains represented an oversimplified assessment of SARS-CoV-2 genomes. Mutation in SRAS CoV-2 S at D614G is associated with higher rate of transmission, yet not higher pathogenesis and patients may be infected with multiple strains of the virus, the implications of which is not clear.26
COVID-19 and Neurological Manifestations
Although SARS-CoV-2 is primarily known for its rapid spread and potentially deadly respiratory syndrome, increasing evidence points to effects in the nervous system. Data are emerging from several countries and case reports documenting the presence of neurological manifestations among COVID-19 patients (Table 1). Neurological signs and symptoms appear to be common, ranging from mild to severe, and appear to involve the central and/or peripheral nervous system (CNS, PNS). Little is known whether these neurological signs/symptoms are related to direct viral infection or indirect effects of the virus (e.g., postinfectious encephalomyelitis, cytokine storm, or systemic inflammatory reactions), associated systemic dysfunction (e.g., hepatic failure, renal failure, disseminated intravascular coagulation) or intensive care unit (ICU) hospitalization (e.g., delirium). In this study, we summarize some of the emerging reports of these neurological manifestations associated with COVID-19.
Table 1.
Neurological Complications in COVID-19
| Reported neurological complication | References |
|---|---|
| Dizziness | 27 |
| Headache | 27 |
| Impaired consciousness | 27 |
| Acute cerebrovascular disease | 27 |
| Ataxia | 27 |
| Seizure | 27 |
| Gustatory impairment | 27,31–36 |
| Olfactory impairment | 27,31–36 |
| Visual impairment | 27 |
| Nerve pain impairment | 27 |
| Delirium | 19,55 |
| Agitation | 19,55 |
| Corticospinal tract signs | 19,55 |
| Dysexecutive syndrome | 19 |
| Coagulopathy | 45–49,51,52,55 |
| Guillain-Barré syndrome | 38,55,111,112 |
| Acute necrotizing hemorrhagic encephalopathy | 41 |
| Acute myelitis | 43,55 |
| Progressive encephalopathy | 29 |
| Acute necrotizing encephalopathy | 53 |
| Diffuse hemorrhagic disease | 53 |
In a case series in Wuhan China, of 214 confirmed cases of SARS-CoV-2 infections, neurological complications were identified in 78 (36.4%).27 They were more common (45.5%) in persons with severe COVID-19. CNS complications were present in 24.7% and included dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, and seizure. PNS complications were present in 8.9% and included gustatory, olfactory, and visual impairments. Nerve pain and skeletal muscle injury also occurred. Interestingly, some patients initially tested negative for SARS-CoV-2 and presented with only neurological symptoms; a repeat test a few days later was positive for SARS-CoV-2. This finding suggests that in some patients the initial presentation of COVID-19 may be neurological and these patients in particular carry a risk to health care workers as they are presumed to be SARS-CoV-2 negative without testing. Moreover, this may suggest a more direct effect of the virus on the nervous system. A case series from Strasbourg, France, reviewed 58 COVID-19 patients diagnosed with acute respiratory distress syndrome between March 3, 2020 and April 3, 2020, in an ICU.28 A total of 49 (84%) had neurological manifestations, which included delirium (65%), agitation (69%), corticospinal tract signs (67%), and dysexecutive syndrome (36%). Of a subset who underwent neuroimaging; 8 of 13 showed leptomeningeal enhancement, all showed perfusion abnormalities, and 3 of 13 had evidence of an ischemic infarction. Seven patients underwent removal and analysis of cerebrospinal fluid (CSF), SARS-CoV-2 was not detected in any of the seven patients tested, although a case study of a COVID-19 patient with meningitis did detect SARS-CoV-2 in CSF.29 A large study probing for SARS-CoV-2 in CSF also did not detect the virus in the CSF.30 This may indicate that the infection may not be active/productive in the brain but that initial viral neuroinvasion may set the stage for inflammation or necrosis (which will be highlighted as follows). Furthermore, it is unclear if reverse transcriptase PCR (RT-PCR) assay for detection of SARS-CoV-2 was optimized for spinal fluid detection or the lower limit of assay detection to preclude detection of SARS-CoV-2 in CSF in some of these studies. Finally, oligoclonal bands were seen in two cases with a pattern similar to serum. One case had elevated IgG and CSF protein and four had low albumin.28
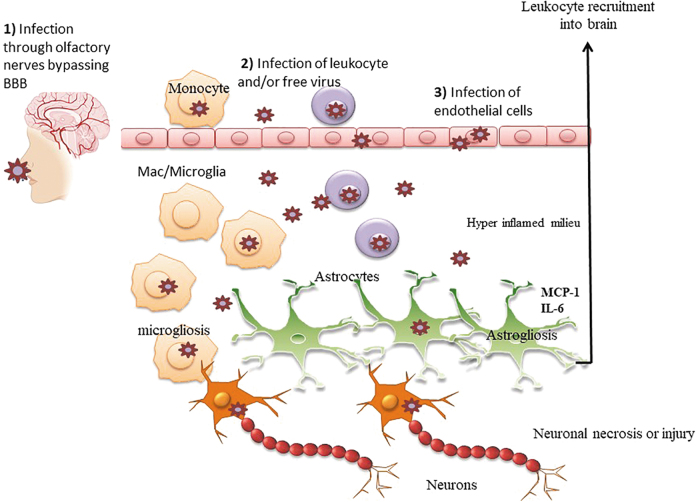
Among the most common neurological manifestations of COVID-19 are olfactory and gustatory dysfunction. These can be the presenting clinical manifestation of the disease in mild COVID-19 infections. In a multicenter European study, of 417 who completed a standard self-report assessment, 85.6% and 88.0% of patients reported olfactory and gustatory dysfunctions.31 These patients were evaluated an average of 9 days postinfection. The olfaction deficits included the full range of anosmia (smell blindness), hyposmia (incomplete loss), phantosmia (presence of smell where none exists), and parosmia (distortion of smells). The gustatory deficits reduced/discontinued or distorted ability to all four tastes (salt, bitter, sweet, and sour). A study of 59 patients with severe COVID-19 in Milan, Italy, found that a third of patients had either olfactory or gustatory dysfunction by self-report.32 Another study from the Treviso and Belluno provinces of Italy conducted a telephone screen of 202 patients 5–6 days after positive testing for COVID-19.33 A total of 130 (64.4%) reported olfactory and/or gustatory dysfunction. A study in Madrid, Spain, examined the specificity of olfactory and/or gustatory function by comparing 79 hospitalized cases of COVID-19 with 40 cases of influenza (the vast majority with A-H1).34 New onset self-report deficits were present in 31 (39.2%) of those with COVID-19. By contrast, only 5 (12.5%) of those with influenza reported such deficits. Finally, a study from San Diego, CA, found that anosmia was associated with a lower odd of hospitalization.35 A total of 26 of 128 patients with COVID-19 required hospitalization. Only 26% and 23% of those reported olfactory dysfunction or dysgeusia, compared with 66.7% and 62.7% of those not admitted. The authors suggested that these deficits might be associated with a milder course of disease. Finally, in a study of 60 COVID-19 patients administered the 40-item University of Pennsylvania Smell Identification Test (UPSIT) in Tehran, Iran, 59 (98%) were abnormal.36 Anosmia was present in 58% and hyposmia in 33% of participants, all were inpatients at the time of the testing awaiting to be discharged. To our knowledge, this was the only study that objectively assessed olfaction. The involvement of loss of smell would suggest that the virus may gain entry into the brain through the olfactory bulb, a route of transmission that has been described for neurotropic viruses such as herpes, borna, and rabies.37 The exact mechanism of neuroinvasion is not clearly understood, but the virus could potentially travel retrograde through the olfactory nerves to gain access to the mesial temporal lobe, including the piriform and entorhinal cortices, and the hippocampus, and potential other regions without a break in blood–brain barrier (BBB), causing neuronal damage/death and/or neuroinflammation (Fig. 1). Finally, a recent study reported that those patients presenting with anosmia are less likely to be hospitalized and those who are hospitalized present without loss of smell,35 suggesting that loss of smell is associated with a different pathological course of disease. If this is the case, it may be that the neurological complications observed in severe cases28 are driven by mechanisms other than direct infection of the CNS through the olfactory bulb route. A better understanding of which cells of the olfactory bulb are infected by SARS-CoV-2 and how infection of these cells is related to CNS pathology and neurological complications in patients and/or animal models should provide better insight into the relationship between these presentations in patients.
FIG. 1.
Potential route of SARS-CoV-2 transmission into the brain and neuroinflammatory pathways: (1) SARS-CoV-2 could enter the brain through the olfactory nerves to gain access to the mesial temporal lobe, including the piriform and entorhinal cortices, and the hippocampus, and potential other regions without a break in BBB. (2) SARS-CoV-2 could enter the brain through trafficking of a Trojan horse, either infected monocytes or another leukocyte or possibly as a free virion. (3) SARS-CoV-2 could enter the brain by infecting endothelial cells and compromising the integrity of the BBB. Once in the brain, SARS-CoV-2 may directly infect resident brain cells (microglia, neurons, and astrocytes) through cell-free neuroinvasion and/or cell-to-cell transmission of the virus (e.g., from infected immune cells to resident brain target cells). Whether the infection is productive or not in the brain, inflammation may result through heightened expression of inflammatory cytokines/chemokines that further attract more immune cells into the CNS. The consequence of which is neuronal necrosis or synaptodendritic damage as well as potentially inflammation of astrocytes (astrogliosis) and/or microglia (microgliosis). BBB, blood–brain barrier; CNS, central nervous system; SARS, severe acute respiratory syndrome.
There have been three case reports of COVID-19 patients with Guillain-Barré syndrome (GBS). The reports are from Wuhan, China, Mazandaran Province, Iran, and Pittsburgh, PA. The only case series of which we are aware, from three hospitals in northern Italy, was conducted from February 28, 2020 to March 21, 2020.38 There were five patients out of an estimated 1,000–1,200 hospitalized patients. One patient was negative at the time of diagnosis but later turned positive. The time between COVID-19 symptom onset and symptom onset for GBS was 5–10 days. The prevalence or lack thereof of SARS-CoV-2 detection remains to be determined, although if not confirmed to present in CSF, it would be in contrast to SARS-CoV, which was detected in human CSF.39,40 Several case studies described neurological complications among COVID-19 patients. In one study, a SARS-CoV-2 confirmed positive patient in her 50s with fever, cough, and altered mental status was diagnosed with acute necrotizing hemorrhagic encephalopathy, determined by MRI and noncontrast head CT scans showing symmetric multifocal lesions in brain stem, cerebral white matter, and cerebellum.41 Another 74-year-old COVID-19 patient with a pre-existing neurological condition (Parkinson disease among other comorbidities) lost the ability to speak42 and another 66-year-old patient developed acute myelitis.43 A 60-year-old patient in Italy had mild respiratory abnormalities yet developed severe progressive encephalopathy, which was resolved with steroid treatment.44 A 24-year-old had meningitis/encephalitis associated with COVID-19 infections.29 Finally, a 5-year-old girl in Detroit developed meningitis and encephalitis and died of COVID-19 (https://www.cnn.com/2020/04/21/us/detroit-girl-dies-coronavirus/index.html). In these cases of COVID-19 the lack of viral confirmation in brain tissue or pathological analysis makes it unclear whether these brain complications represent direct or indirect effects of the virus or are related to other systemic complications.
Coagulopathy leading to both brain infarcts and hemorrhage, as well as clots and emboli in other organs and extremities is associated with severe COVID-19 cases.45–49 Specifically, plasma levels of fibrin degradation D-dimers, fibrinogen, and C-reactive proteins are markers of poor COVID-19 prognosis,45,49 as patients who go on to develop severe COVID-19 demonstrate progressive increase in these coagulation factors over time, whereas those who do not progress exhibit stabilization of these plasma markers. This is similar to pathways thought to drive SARS-CoV pathogenesis.50 A Dutch study of 184 patients evaluated the prevalence of pulmonary embolism (PE), deep-vein thrombosis, ischemic stroke, myocardial infarction, and systemic arterial embolism among hospitalized COVID-19 patients.46 PE was the most reported complication, occurring at a frequency of 81%. Venous thromboembolism occurred at 27% and arterial thrombotic events at 3.7%. The overall frequency of composite thrombotic complications (e.g., any of those measured) was 31% of hospitalized patients. Additional reports have described coagulopathy in COVID-19 case studies.47,51,52 Finally, two patients, 84 and 44 years old, developed acute necrotizing encephalopathy and diffuse hemorrhagic disease, respectively, due to widespread intravascular coagulation.53 These studies reveal an alarmingly high prevalence of thrombotic complications and heterogeneric neurological manifestation of COVID-19 in patients that remain poorly understood. Finally, it has been suggested that SARS-CoV-2-associated cardiopulmonary arrest could in some cases be the result of SARS-CoV-2 neuroinvasion. According to this postulate SARS-CoV-2 infection of the lung mediates neuroinvasion through synapses of the mechanoreceptor and chemoreceptor in the lung and lower respiratory tract that connect to the brain and results in specific necrosis of neurons that regulate the medullary cardiorespiratory center.54 Neuroimaging or ideally brain tissue specimens would be needed to support this hypothesis.
These various reports underscore the diversity in COVID-19 neurological manifestation, which was captured in a recent report from the United Kingdom where 43 COVID-19 patients were evaluated (only 29 were PCR-confirmed cases, the rest were categorized as probable or possible).55 Among this cohort, a spectrum of neurological manifestations was revealed that included encephalopathies with delirium/psychosis, inflammatory CNS syndromes, including encephalitis, acute disseminated encephalomyelitis, hemorrhage, necrosis, myelitis, and peripheral neurological disorders (GBS and brachial plexopathy).55 What drives this diversity in manifestation, whether it is due to an underlying condition, extent of peripheral immune activation that can impact brain, or viral effects (not necessarily infection but responses to virus in brain) is not unclear. Likewise, why a subset of persons with SARS-CoV-2 have neurological manifestations associated with COVID-19 and others do not is also not clear. Given evidence of direct SARS-CoV-2 infection, what is the route or routes of SARS-CoV-2 neuroinvasion (e.g., olfactory bulb, lung/brain axis, and infiltrating lymphocytes) and infectious inoculum? (e.g., some patients having higher degree of exposure through contaminated droplets such as health care workers)? Are the neurological manifestations a secondary reaction to the variation in mucosal response that may be either tightly regulated to control the infection without hyperactivity or a hyperactive immunity? What is the role of systemic illness and the sequelae of the ICU? These and other questions are vitally important to inform therapeutic interventions for SARS-CoV-2 pathogenicity. We think that there are lessons to be learned from our experience with a different virus, a neurovirulent retrovirus with serious nervous system sequelae.56
HIV-Mediated Mechanisms of CNS Dysregulation to Inform Possible Mechanisms of SARS-CoV-2 Neurological Effects: What Can We Learn from Decades of Studying HIV and Associated Neurological Disease?
Before the introduction of combination antiretroviral therapy (cART), 25% of HIV-infected individuals developed HIV-associated dementia (HAD). HIV invades the brain within 7–14 days of infection, as demonstrated through human studies and nonhuman primate animal studies.57–59 HIV neuroinvasion is mediated through trafficking of immune cells and subsequent dissemination of HIV into the brain, mostly infection of perivascular macrophages, microglia, and to a lesser extent astrocytes.60,61 Although neurons are not directly infected by HIV, significant neurodegeneration was demonstrated in HAD/pre-cART, presumed to be driven through the release of neurotoxic viral proteins such as gp120, Tat, Nef, and Vpr that are capable of inducing significant glial inflammation. The development of cART has significantly reduced the development of HAD in infected individuals, through reduction of HIV in the brain and associated inflammation, despite suboptimal penetration of some cART components into the CNS. However, HIV-1-infected patients on cART still experience numerous neurological sequelae, collectively known as HIV-1-associated neurocognitive disorders (HAND). Clinically, 30%–50% of people living with HIV have HIV-associated neurocognitive impairment. HAND symptoms are generally driven by neuroinflammation and synaptodendritic damage, rather than the neuronal loss that defined HAD. It remains unclear if HAND pathology is a legacy effect of residual CNS viral replication after cART initiation or systemic inflammation that impacts the CNS. Lessons drawn from HIV neuroinvasion point to three potential pathways by which HIV can cause damage to the CNS: (1) direct infection of resident target cells, (2) indirect effects of virus on resident brain cells, and/or (3) increased trafficking of hyperactivated immune cells into the brain (the so-called neuroimmune axis). These three pathways can potentially inform how SARS-CoV-2 may have an effect on the CNS (Fig. 1).
Can SARS-CoV-2 Directly Infect Resident Brain Cells: A Direct Viral Effect Hypothesis?
To date, few autopsy studies of COVID-19 brains found viral RNA in the brain of 4 (33%) of 12 brains,62 8 (36%) of 22 brains,63 and 5 (28%%) of 18 brains.64 The level of virus detected by quantitative reverse transcription-PCR (qRT-PCR) in one study ranged from <5 to 59 copies per cubic millimeter64 and the virus was mostly found in medulla, frontal lobes, and olfactory nerves64; however, immunohistochemistry verification did not detect the virus in the brain in this particular study, due to lack of specificity of antibody.64 The other autopsy studies did not report on brain regions of virus detection, associated pathological changes, or information to inform the route of entry.62,63 It thus remains unclear if SARS CoV-2 can spread in the brain and undergo productive infection of resident brain cells or alternatively it may infect cells and the infection is aborted. However, an aborted infection can still trigger adverse signal transduction cascade through S protein or other viral TLR agonists that can mediate inflammation and/or CNS damage. Notably, atherosclerosis was evident in 78% of the brains and acute hypoxic injury in the cerebrum and cerebellum in 100% of the patients.64 Neuronal loss in cerebral cortex, hippocampus, and cerebellar Purkinje cell layer was also noted.64 MRI scans performed on COVID-19 patients within 24 h of death revealed parenchymal brain abnormalities, which included white matter alterations, posterior reversible encephalopathy syndrome, and hemorrhage in 4/19 patients.65
Other β-coronaviruses are neuroinvasive, as demonstrated in human, animal, and in vitro studies.19,66–73 SARS-CoV was found in thalamus, brain stem, and cerebrum of autopsied confirmed cases of SARS but not in the cerebellum (Table 2).19,74,75 It was also detected within neurons of the thalamus and cortex and in GFAP+ astrocytes.19,75 In one study with SARS-CoV patient with neurological complication, the virus was amplified by RT-PCR and visualized by electron micrography from the brain of this patients.75 Pathological manifestation included neuronal necrosis, hyperplasia of glial cells, and infiltration of CD68+ monocytes/macrophages and CD3+ T lymphocytes in the brain mesenchyma.75 There is only one postmortem study of MERS-CoV and it did not report any significant changes in the brain.76 However, a number of transgenic mice, expressing the MERS-CoV receptor (DDP4) demonstrated its neuroinvasion, as early as 2–4 days postinfection.72,77,78 SARS-CoV-2 tropism in the brain may be broader and it remains to be determined if SARS-CoV-2 shares this tropism with SARS-CoV and MERS-CoV. Furthermore, although ACE-2 (receptor for SARS-CoV-2) expression is well described on vascular endothelium, including blood vessels of BBB, its expression on neurons and astrocytes is somewhat controversial.79 Animal models demonstrate its expression in neurons and it is unclear if same is true for human neurons.79 ACE2 is also expressed on astrocytes both in culture and in postmortem human astrocytes.79,80 Expression of ACE2 is only one requirement as proteases are also required to cleave S protein for viral fusion. Furthermore, alternative receptors may be used for viral entry, including sialic acid and lectin CD209L, which are used by SARS-CoV1, and emerging data suggest that they may also be used by SARS-CoV-2.81–83
Table 2.
Summary of Published Studies on Epidemic/Pandemic Causing β-Coronavirus and the Brain
| β-Coronavirus | Brain region detected | Viral RNA in CSF or brain | References |
|---|---|---|---|
| SARS-CoV | Thalamus, cerebellum | + | 19,39,40,75,113 |
| MERS-CoV | Thalamus, brain stem; midbrain, deep cerebral cortex, and CA2 region of the hippocampus | ND | 72,77,78 |
| SARS-CoV-2 | Medulla, frontal lobes, olfactory nerves | + | 29,63,64,85,86,114 |
CSF, cerebrospinal fluid; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
Three studies to date, using either cerebral brain organoids (CBOs) or brain spheres to model SARS-CoV-2 infection all demonstrated that SARS-CoV-2 infects cortical neurons.84–86 Most strikingly, neurons in CBOs that were infected were also hypermetabolic and did not undergo cell death, whereas bystander neurons that were uninfected were hypoxic and underwent cell death.84 SARS-CoV-2 infection of cortical neurons was associated with hyperphosphorylated Tau indicative of neurodegeneration.85 Furthermore, despite low-level mRNA expression of ACE2 in neurons within CBOs, blocking ACE2 blocked infection as did IgG from CSF of COVID-19 patients.84 It is still unclear, however, if ACE2 expression in human brain is at a level that would support virus entry or if virus can use alternative receptors in human neurons to gain entry. Furthermore, human endothelial cells are infected by SARS-CoV-2, as shown for kidney endothelial cells in COVID-19.87 It is important to note that spike protein binding to ACE2 on brain endothelial cells may also trigger inflammatory responses of endothelial cells, independent of productive infection, as these cells can secrete cytokines upon injury/activation88,89 In fact, S protein of SARS-CoV-2 mediated a compromise in BBB in a 3D microfluid model of human BBB and also mediated inflammatory responses reminiscent of HIV verotoxin effects on BBB integrity.90 Case studies have documented microvascular injury and hypoxia, but is unclear if this is secondary to an underlying condition or mediated by SARS-CoV-2 either directly or indirectly by infecting brain endothelial cells or even pericytes.91 It remains to be determined, however, if SARS-CoV-2 infects endothelial and/or glial cells in vivo and/or mediates their dysfunction in postmortem studies. This potential indirect effect is discussed later on this review.
A number of coronaviruses invade the brain through retrograde peripheral nerves from nasal mucosa to the brain. The infected neurons undergo necrosis compromising their key function, whether peristaltic control, respiratory control, and/or cardiac function control.54 SARS-CoV and MERS-CoV route of transmission into the brain, based on animal studies, point to intranasal transmission through the olfactory nerves and eventually to the thalamus and brainstem, from there the virus may spread more widely to other reported areas such as the cerebellum.71,92 Of interest, in mice that were given low doses of MERS-CoV nasally the virus was found in the brain and not the lung and these animals also died despite this low viral inoculum.72 This study suggests that neuroinvasion of coronaviruses, especially into the brain stem, may dysregulate the command center for the autonomic nervous system, leading to multiorgan failure in severe COVID-19 cases. Although this may be true for MERS-CoV (which has high fatality rate), to date, it is unclear if SARS-CoV-2 behaves similarly.
Can SARS-CoV-2 Cause Indirect Deleterious Effects on Resident Brain Cells: An Indirect Viral Effect Hypothesis?
SARS-CoV-2 may not need to productively infect resident brain cells to elicit an inflammatory response. It is possible that the effects of abortive infection, such as endocytosis into nonpermissive cells, can induce inflammatory reactions in some cell types. In this scenario, SARS-CoV-2 virion is captured within the phagosomes of microglia/macrophage/astrocytes, it then fuses with the endosomes then lysosomes. As the viral envelope is degraded by proteases and lipases, the SARS-CoV-2 ssRNA genome is released, which can be recognized by TLR7/8 in the phagolysosome. This recognition may lead to robust activation and cytokine production. In the brain, glial cells express a number of TLRs, including TLR7 and respond to its agonists through robust cytokine expression.93 In HIV neuropathogenesis, the mere binding of its envelope (gp120) in a nonspecific manner to neurons, astrocytes, and brain endothelial cells elicit a signal transduction cascade that leads to inflammation and/or death of target cells.94–97 As mentioned previously, the spike protein of SARS-CoV-2, as it is less thermostable than SARS-CoV spike protein, may be more easily shed and in context of brain this shedding may mediate neuroinflammation. There is also a growing body of evidence demonstrating the ability of viruses to be released through exosomes/endosomes, such as flavivirus and/or its proteins packaged into these vesicles.98 Microvesicles can travel long distances and if their cargo carries viral proteins, they can also elicit a response as they unload their cargo into a receipt cells.99,100 Activation of microglia, especially through release of TNFα, complement receptor 1q, and IL-1α can also initiate a cascade of events that skew astrocyte to an inflammatory phenotype, disrupting their prototypical functions of maintaining BBB, pruning of damaged neurons, and scavenging of excess neurotransmitters.101–103 These sequalae can mediate indirect effects of neurodegeneration and their potential role in SARS-CoV-2-mediated neuropathogenesis remains to be elucidated.
Is There Heightened Trafficking of Inflammatory Cells into the CNS in COVID-19: A Neuroimmune Axis Effect Hypothesis?
Although the brain has classically been considered to be an immune privileged site, it is increasingly appreciated that immune cells migrate to the CNS in the absence of infection or a breakdown in the BBB, although to a slower rate and in fewer numbers than other tissues.104,105 If there is an inflammatory response in the brain, immune cells home in greater numbers to the brain and a balanced response is essential to control an infection without mediating extensive inflammation in the brain. Furthermore, a functional lymphatic system in the meninges exists, which drains its products from the CSF to the deep cervical lymph nodes.106,107 Monocytes, in particular, are often referred to as Trojan horse for virus dissemination into a number of organs,108 as these migratory cells can infiltrate a number of tissues, including the brain, differentiate into resident tissue macrophages, and disseminate the virus to the target tissue. In the case of HIV monocytes are not productively infected, but as they differentiate into macrophages they can support productive infection.109 In human autopsy cases of SARS-CoV infected CD68 (macrophages/microglia) and CD3+ T cells were detected in the brain.75 CD3+ T cell detection in autopsied SARS-CoV brain was much lower than that of macrophages, and this may be because T cells have a shorter life span than macrophages and infected T cells undergo rapid cell death in the CNS, complicating their detection.59 As such, it is unlikely that T cells are involved in any potential SARS-CoV-2 neuroinvasion. Nonetheless, if SARS-CoV-2 infects leukocytes (monocytes and/or granulocytes) or another immune cell, these immune cells may release virus into the CNS to mediate neuroinflammation as described earlier (direct or indirect viral effect). The immune infected cells are likely to be inflammatory and their release of inflammatory cytokines/chemokines in the brain can facilitate further neuroinflammation.
Concluding Remarks
Additional postmortem studies of COVID-19 patients will be critical to determine extent of SARS-CoV-2 neuroinvasiveness and its direct and/or indirect viral effects on the CNS. If this virus behaves similarly to SARS-CoV, which it has ∼80% genomic homology, it is likely to be neuroinvasive as already demonstrated by few studies and brain organoid cultures (Table 2). Detailed studies that would include histopathological assessment of cytotoxicity, inflammation, morphological changes, neuronal activity, as well as evaluation of glial cell phenotypes, preferably complemented by functional studies are warranted to fully comprehend the impact of SARS-CoV-2 on the brain. The primary obstacle in moving forward quickly with these important studies is the concern in harvesting COVID-19 brains in the midst of a pandemic in a way that does not compromise the safety of the pathologists and laboratory workers handling the tissue. To date, the center for disease control has published its guidelines for safely performing and handling of COVID-19 autopsies (https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html) as did others.110 The danger of aerosolizing the virus from an oscillating bone saw during brain removal creates the need for special equipment, high-level protective personal equipment (PPE) and facilities with negative pressure rooms, including the appropriate number of air cycle exchanges exhausted to the outside. These primary obstacles to rapid autopsies are compounded by the uncertainties surrounding the infectivity and risks associated with this new virus. Furthermore, most recommendations are to formalin fix all tissue to kill the virus; however, this limits studies using postmortem brain from COVID-19 patients to histopathological studies. Freezing and storing frozen tissue, which will be important for advanced technologies, will likely require implementing extra safety precautions. In the meantime, additional research will be essential. Animal models for SARS-CoV-2 may emerge that recapitulate aspects of the disease in the CNS, which is quickly evolving to facilitate such studies.
The considerable success against the HIV pandemic in the 1980s was achieved through understanding virus/host interactions for drug design. The lessons that were learned over a decade were critical to turn HIV from a deadly disease to a chronic disease and to dramatically reduce its effects on the brain. Regrettably, given the transmission rate and respiratory route of SARS-CoV-2 infection as well as its disastrous impact on global economies we do not have the luxury of time. There are several anti-SARS-CoV-2 drugs under clinical investigation, which target SARS-CoV-2 life cycle such as Remdesivir and Baricitinb https://www.nih.gov/news-events/news-releases/nih-clinical-trial-testing-antiviral-remdesivir-plus-anti-inflammatory-drug-baricitinib-covid-19-begins). There are also immune-based therapies (e.g., anti-IL-6 and plasmapheresis) https://clinicaltrials.gov/ct2/show/NCT04322773; https://clinicaltrials.gov/ct2/show/NCT04321421). As we learn more about the short- and possibly long-term consequence of SARS-CoV-2 on the brain and define mechanism (s) that mediate these effects, therapeutic strategies should not ignore the impact of therapy on the brain and most importantly whether the therapy will be effective in crossing the BBB to provide benefit and/or protection to the brain.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study is supported by R01NS060632 (L.A.-H.), RO1NS108796 (L.A.-H.), RO1 MH113425 (L.A.-H.), RO1 MH100628 (L.A.-H.), and R01 AG58639 (D.A.B.)
References
- 1. Ksiazek TG, Erdman D, Goldsmith CS, et al. : A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953–1966 [DOI] [PubMed] [Google Scholar]
- 2. de Wit E, van Doremalen N, Falzarano D, Munster VJ: SARS and MERS: Recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14:523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zumla A, Hui DS, Perlman S: Middle East respiratory syndrome. Lancet 2015;386:995–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM: Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814–1820 [DOI] [PubMed] [Google Scholar]
- 5. Gossner C, Danielson N, Gervelmeyer A, et al. : Human-dromedary camel interactions and the risk of acquiring zoonotic middle east respiratory syndrome coronavirus infection. Zoonoses Public Health 2016;63:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haagmans BL, Al Dhahiry SH, Reusken CB, et al. : Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. Lancet Infect Dis 2014;14:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Yang XL, Wang XG, et al. : A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lam TT, Shum MH, Zhu HC, et al. : Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 2020;583:282–285 [DOI] [PubMed] [Google Scholar]
- 10. Li X, Zai J, Zhao Q, et al. : Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol 2020;92:602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan Y, Shang J, Graham R, Baric RS, Li F: Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020;94:e00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine-Weber H, Schroeder S, et al. : SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ou X, Liu Y, Lei X, et al. : Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D: Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281–292.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ: COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen SF, Ho YC: SARS-CoV-2: A storm is raging. J Clin Invest 2020;130:2202–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye Q, Wang B, Mao J: The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 2020;80:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo J, Wei X, Li Q, et al. : Single-cell RNA analysis on ACE2 expression provides insight into SARS-CoV-2 blood entry and heart injury. medRxiv 2020:2020.2003.2031.20047621. J Cell Physiol 2020. DOI: 10.1002/jcp.29802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu J, Gong E, Zhang B, et al. : Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H: Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Millet JK, Whittaker GR: Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res 2015;202:120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ziegler CJK, Allon SJ, Nyquist SK, et al. : SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020. [Epub ahead of print]; DOI: 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Channappanavar R, Fehr AR, Vijay R, et al. : Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Totura AL, Baric RS: SARS coronavirus pathogenesis: Host innate immune responses and viral antagonism of interferon. Curr Opin Virol 2012;2:264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang X, Wu C, Li X, et al. : On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev 2020;7:1012–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korber B, Fischer WM, Gnanakaran S, et al. : Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv 2020:2020.2004.2029.069054 [Google Scholar]
- 27. Mao L, Jin H, Wang M, et al. : Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helms J, Kremer S, Merdji H, et al. : Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020;382:2268–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moriguchi T, Harii N, Goto J, et al. : A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020;94:55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Destras G, Bal A, Escuret V, et al. : Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe 2020. 10.1016/S2666-5247(20)30066-5 [DOI] [PMC free article] [PubMed]
- 31. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. : Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giacomelli A, Pezzati L, Conti F, et al. : Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin Infect Dis 2020;ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spinato G, Fabbris C, Polesel J, et al. : Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020;323:2089–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beltran-Corbellini A, Chico-Garcia JL, Martinez-Poles J, et al. : Acute-onset smell and taste disorders in the context of Covid-19: A pilot multicenter PCR-based case-control study. Eur J Neurol 2020. [Epub ahead of print]; DOI: 10.1111/ene.14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS: Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol 2020;10:821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL: Smell dysfunction: A biomarker for COVID-19. Int Forum Allergy Rhinol 2020. [Epub ahead of print]; DOI: 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mori I, Nishiyama Y, Yokochi T, Kimura Y: Olfactory transmission of neurotropic viruses. J Neurovirol 2005;11:129–137 [DOI] [PubMed] [Google Scholar]
- 38. Toscano G, Palmerini F, Ravaglia S, et al. : Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hung EC, Chim SS, Chan PK, et al. : Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 2003;49:2108–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y: Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 2004;10:342–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B: COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020:201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Filatov A, Sharma P, Hindi F, Espinosa PS: Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus 2020;12:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S: Acute myelitis after SARS-CoV-2 infection: A case report. medRxiv 2020:2020.2003.2016.20035105 [Google Scholar]
- 44. Pilotto A, Odolini S, Masciocchi S, et al. : Steroid-responsive severe encephalopathy in SARS-CoV-2 infection. medRxiv 2020:2020.2004.2012.20062646 [Google Scholar]
- 45. Fogarty H, Townsend L, Ni Cheallaigh C, et al. : COVID-19 Coagulopathy in Caucasian patients. Br J Haematol 2020;189:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klok FA, Kruip M, van der Meer NJM, et al. : Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lushina N, Kuo JS, Shaikh HA: Pulmonary, cerebral, and renal thromboembolic disease associated with COVID-19 infection. Radiology 2020:201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang D, Hu B, Hu C, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang N, Li D, Wang X, Sun Z: Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gralinski LE, Bankhead A, 3rd, Jeng S, et al. : Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio 2013;4:e00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Danzi GB, Loffi M, Galeazzi G, Gherbesi E: Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur Heart J 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Cao W, Xiao M, et al. : [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia]. Zhonghua Xue Ye Xue Za Zhi 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 53. Radmanesh F, Rodriguez-Pla A, Pincus MD, Burns JD: Severe cerebral involvement in adult-onset hemophagocytic lymphohistiocytosis. J Clin Neurosci 2020;76:236–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li YC, Bai WZ, Hashikawa T: The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020;92:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paterson RW, Brown RL, Benjamin L, et al. : The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020;awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Musarrat F, Chouljenko V, Dahal A, et al. : The anti-HIV drug Nelfinavir Mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARS-CoV-2 Spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J Med Virol 2020. [Epub ahead of print]; DOI: 10.1002/jmv.25985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Valcour V, Chalermchai T, Sailasuta N, et al. : Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012;206:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chakrabarti L, Hurtrel M, Maire MA, et al. : Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol 1991;139:1273–1280 [PMC free article] [PubMed] [Google Scholar]
- 59. Richards MH, Narasipura SD, Seaton MS, Lutgen V, Al-Harthi L: Migration of CD8+ T cells into the central nervous system gives rise to highly potent anti-HIV CD4dimCD8bright T cells in a Wnt signaling-dependent manner. J Immunol 2016;196:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Albright AV, Soldan SS, Gonzalez-Scarano F: Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol 2003;9:222–227 [DOI] [PubMed] [Google Scholar]
- 61. Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS: The neuropathogenesis of HIV-1 infection. J Leukoc Biol 1994;56:389–398 [DOI] [PubMed] [Google Scholar]
- 62. D W, J-P S, M L, et al.: Autopsy findings and venous thromboembolism in patients With COVID-19: A prospective cohort study. Ann Intern Med 2020. [Epub ahead of print]; DOI: 10.7326/M20-M2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. : Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Solomon IH, Normandin E, Bhattacharyya S, et al. : Neuropathological features of Covid-19. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coolen T, Lolli V, Sadeghi N, et al. : Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 2020. DOI: 10.1212/WNL.0000000000010116 [DOI] [PubMed] [Google Scholar]
- 66. Arbour N, Day R, Newcombe J, Talbot PJ: Neuroinvasion by human respiratory coronaviruses. J Virol 2000;74:8913–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arbour N, Ekande S, Cote G, et al. : Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol 1999;73:3326–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Arbour N, Talbot PJ: Persistent infection of neural cell lines by human coronaviruses. Adv Exp Med Biol 1998;440:575–581 [DOI] [PubMed] [Google Scholar]
- 69. Lachance C, Arbour N, Cashman NR, Talbot PJ: Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J Virol 1998;72:6511–6519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Talbot PJ, Ekande S, Cashman NR, Mounir S, Stewart JN: Neurotropism of human coronavirus 229E. Adv Exp Med Biol 1993;342:339–346 [DOI] [PubMed] [Google Scholar]
- 71. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S: Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li K, Wohlford-Lenane C, Perlman S, et al. : Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2016;213:712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jacomy H, Fragoso G, Almazan G, Mushynski WE, Talbot PJ: Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006;349:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ding Y, He L, Zhang Q, et al. : Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol 2004;203:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu J, Zhong S, Liu J, et al. : Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005;41:1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alsaad KO, Hajeer AH, Al Balwi M, et al. : Histopathology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection—Clinicopathological and ultrastructural study. Histopathology 2018;72:516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Agrawal AS, Garron T, Tao X, et al. : Generation of a transgenic mouse model of middle east respiratory syndrome coronavirus infection and disease. J Virol 2015;89:3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhao G, Jiang Y, Qiu H, et al. : Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with middle east respiratory syndrome-coronavirus. PLoS One 2015;10:e0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xia H, Lazartigues E: Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J Neurochem 2008;107:1482–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS: Angiotensin-converting enzyme 2 is reduced in Alzheimer's disease in association with increasing amyloid-β and tau pathology. Alzheimers Res Ther 2016;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Amraie R, Napoleon MA, Yin W, et al. : CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv 2020. [Epub ahead of print]; DOI: 10.1101/2020.06.22.165803 [DOI] [Google Scholar]
- 82. Jeffers SA, Tusell SM, Gillim-Ross L, et al. : CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 2004;101:15748–15753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim CH: SARS-CoV-2 evolutionary adaptation toward host entry and recognition of receptor O-acetyl sialylation in virus-host interaction. Int J Mol Sci 2020;21:4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Song E, Zhang C, Israelow B, et al. : Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. bioRxiv 2020:2020.2006.2025.169946 [Google Scholar]
- 85. Ramani A, Müller L, Ostermann PN, et al. : SARS-CoV-2 targets cortical neurons of 3D human brain organoids and shows neurodegeneration-like effects. bioRxiv 2020:2020.2005.2020.106575 [Google Scholar]
- 86. Bullen C, Hogberg H, Bahadirli-Talbott A, et al. : Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2.” ALTEX—Alternatives to animal experimentation 2020. [Epub ahead of print]; DOI: 10.14573/altex.2006111 [DOI] [PubMed] [Google Scholar]
- 87. Varga Z, Flammer AJ, Steiger P, et al. : Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Johnson RH, Kho DT, O’ Carroll SJ, Angel CE, Graham ES: The functional and inflammatory response of brain endothelial cells to Toll-Like Receptor agonists. Sci Rep 2018;8:10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Verma S, Nakaoke R, Dohgu S, Banks WA: Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun 2006;20:449–455 [DOI] [PubMed] [Google Scholar]
- 90. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, et al. : The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood–brain barrier. bioRxiv 2020:2020.2006.2015.150912. DOI: 10.1101/2020.06.15.150912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jaunmuktane Z, Mahadeva U, Green A, et al. : Microvascular injury and hypoxic damage: Emerging neuropathological signatures in COVID-19. Acta Neuropathol 2020:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McCray PB Jr., Pewe L, Wohlford-Lenane C, et al. : Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 2007;81:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kielian T: Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res 2006;83:711–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brenneman DE, Westbrook GL, Fitzgerald SP, et al. : Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 1988;335:639–642 [DOI] [PubMed] [Google Scholar]
- 95. Lannuzel A, Barnier JV, Hery C, et al. : Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol 1997;42:847–856 [DOI] [PubMed] [Google Scholar]
- 96. Yang B, Akhter S, Chaudhuri A, Kanmogne GD: HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: Modulatory effects of STAT1 signaling. Microvasc Res 2009;77:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li J, Bentsman G, Potash MJ, Volsky DJ: Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC neuroscience 2007;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhou W, Woodson M, Neupane B, et al. : Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog 2018;14:e1006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pérez PS, Romaniuk MA, Duette GA, et al. : Extracellular vesicles and chronic inflammation during HIV infection. J Extracellular Vesic 2019;8:1687275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meckes DG, Raab-Traub N: Microvesicles and viral infection. J Virol 2011;85:12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liddelow S, Barres B: SnapShot: Astrocytes in health and disease. Cell 2015;162:1170–1170.e1. [DOI] [PubMed] [Google Scholar]
- 102. Liddelow SA, Barres BA: Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017;46:957–967 [DOI] [PubMed] [Google Scholar]
- 103. Liddelow SA, Guttenplan KA, Clarke LE, et al. : Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Louveau A, Harris TH, Kipnis J: Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015;36:569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hickey WF: Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol 1991;1:97–105 [DOI] [PubMed] [Google Scholar]
- 106. Sandrone S, Moreno-Zambrano D, Kipnis J, van Gijn J: A (delayed) history of the brain lymphatic system. Nat Med 2019;25:538–540 [DOI] [PubMed] [Google Scholar]
- 107. Louveau A, Smirnov I, Keyes TJ, et al. : Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Peluso R, Haase A, Stowring L, Edwards M, Ventura P: A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 1985;147:231–236 [DOI] [PubMed] [Google Scholar]
- 109. Aljawai Y, Richards MH, Seaton MS, Narasipura SD, Al-Harthi L: beta-Catenin/TCF-4 signaling regulates susceptibility of macrophages and resistance of monocytes to HIV-1 productive infection. Curr HIV Res 2014;12:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hanley B, Lucas SB, Youd E, Swift B, Osborn M: Autopsy in suspected COVID-19 cases. J Clin Pathol 2020;73:239–242 [DOI] [PubMed] [Google Scholar]
- 111. Virani A, Rabold E, Hanson T, et al. : Guillain-Barre Syndrome associated with SARS-CoV-2 infection. IDCases 2020:e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao H, Shen D, Zhou H, Liu J, Chen S: Guillain-Barre syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ding Y, Wang H, Shen H, et al. : The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J Pathol 2003;200:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wichmann D, Sperhake JP, Lütgehetmann M, et al. : Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020;M20-2003 [DOI] [PubMed] [Google Scholar]