Abstract
Significance: Streptococcus pneumoniae (Spn), a facultative anaerobic Gram-positive human pathogen with increasing rates of penicillin and macrolide resistance, is a major cause of lower respiratory tract infections worldwide. Pneumococci are a primary agent of severe pneumonia in children younger than 5 years and of community-acquired pneumonia in adults. A major defense mechanism toward Spn is the generation of reactive oxygen species, including hydrogen peroxide (H2O2), during the oxidative burst of neutrophils and macrophages. Paradoxically, Spn produces high endogenous levels of H2O2 as a strategy to promote colonization.
Recent Advances: Pneumococci, which express neither catalase nor common regulators of peroxide stress resistance, have developed unique mechanisms to protect themselves from H2O2. Spn generates high levels of H2O2 as a strategy to promote colonization. Production of H2O2 moreover constitutes an important virulence phenotype and its cellular activities overlap and complement those of other virulence factors, such as pneumolysin, in modulating host immune responses and promoting organ injury.
Critical Issues: This review examines the dual role of H2O2 in pneumococcal pneumonia, from the viewpoint of both the pathogen (defense mechanisms, lytic activity toward competing pathogens, and virulence) and the resulting host–response (inflammasome activation, endoplasmic reticulum stress, and damage to the alveolar–capillary barrier in the lungs).
Future Directions: An understanding of the complexity of H2O2-mediated host–pathogen interactions is necessary to develop novel strategies that target these processes to enhance lung function during severe pneumonia.
Keywords: hydrogen peroxide, pneumococci, pneumonia, pyruvate oxidase, virulence factor, ARDS
Introduction
Infections of the lower respiratory tract represent the main cause of infectious disease mortality and represent the fifth highest cause of death overall. In 2015, pneumonia accounted for 2.7 million deaths worldwide (47). Streptococcus pneumoniae (Spn) is a major etiologic agent of community-acquired pneumonia and the leading cause of death in children younger than 5 years, worldwide (50, 51). It is a facultative anaerobe gram-positive bacterium that can colonize the upper respiratory tract as a commensal bacterium in healthy individuals. This asymptomatic transitory phase can lead to severe pneumonia upon migration of bacteria to the lower respiratory tract (119). Infections with Spn can lead to meningitis after crossing the blood–brain barrier and the following entry into the blood stream can contribute to heart disease by causing microlesions in the myocardium (14).
Currently, two main types of vaccines have been developed to reduce or eliminate the burden of infections, that is, the unconjugated 23-valent polysaccharide vaccine and a 10- or 13-valent-conjugated polysaccharide vaccine. However, their serotype coverage is not universal and they are limited with respect to noncapsulated Spn (12, 97). Once symptoms of pneumonia are diagnosed, antibiotic therapy with amoxicillin for ages under five, and azithromycin or clarithromycin for ages over five is indicated (38, 71). Antibiotic therapy, however, needs to be initiated before breakdown of the alveolar–capillary barrier and systemic inflammation in pneumococcal pneumonia (8). Unfortunately, in view of emerging pathogens, demographic shifts toward older populations, and increasing antibiotic resistance, the occurrence of pneumonia is poised to worsen rather than improve in the coming years (30, 119).
Significance
Bacterial pneumonia represents one of the major comorbidities that occur with the acute respiratory distress syndrome (ARDS) (23, 48, 81). One of the major lethal complications of ARDS is the development of pulmonary edema. Indeed, pathological specimens from ARDS patients reveal diffuse alveolar damage, and animal studies of bacterial pneumonia-associated ARDS have demonstrated both alveolar epithelial and lung endothelial injury with accumulation of protein-rich fluid in the alveolar space (27, 55, 78, 88). The ability of Spn to promote lung disease in the human host depends not only on microbial virulence factors, such as the pore-forming toxin pneumolysin (Ply) (77, 79, 111, 137), but also on variables in the host (age, genetic, and environmental factors). These affect the capacity of the immune system to clear bacteria and the susceptibility to develop tissue damage (30, 61).
While there has been focus on the pathogens causing pneumonia in recent years, for example, on Spn, there is a high and urgent need for research from the perspective of the host. The absence of alveolar neutrophilia was shown to be deleterious in murine pneumonia models (8, 101) and has a high negative predictive value for bacterial pneumonia in critically ill patients with suspected infection (129). Reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), play an important but highly complex role in pneumococcal pneumonia-associated ARDS. On the one hand, they are involved in neutrophil- and alveolar macrophage-mediated antibacterial defense, but on the other hand, they can contribute to the dysfunction of the alveolar–endothelial barrier and impair alveolar liquid clearance mechanisms. The following chapters discuss the actions of H2O2 from the viewpoint of the pathogen as well as in the host and outline its roles in driving the unfolded protein response (UPR), in blunting immune defense mechanisms, and in promoting acute lung injury.
Recent Advances: Actions of H2O2 on Spn
Spn generates and exploits H2O2
Spn, which grows predominantly in an aerobic environment of the oxygen-rich airway surface, can endogenously generate millimolar levels of H2O2 as a by-product during aerobic growth (90, 104). Here we describe those enzymes involved in pneumococcal H2O2 generation and examine how the pathogen exploits these high H2O2 levels to promote its colonization and enhance its virulence.
Enzymes involved in H2O2 generation in Spn
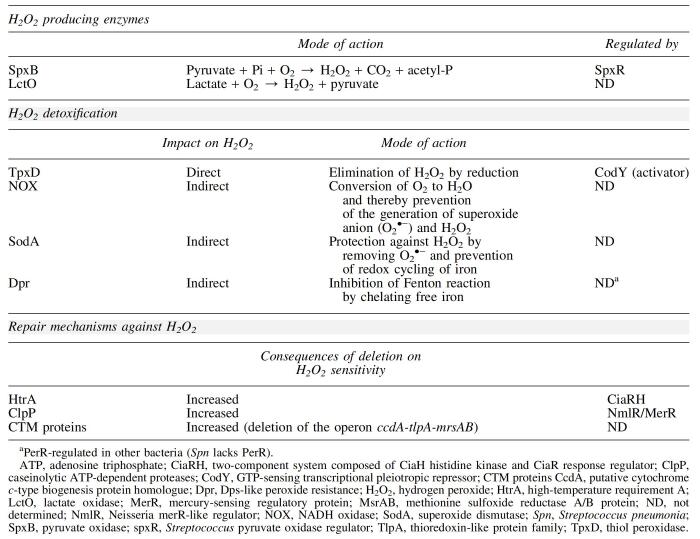
Pyruvate oxidase (SpxB) is considered the main source of H2O2 in Spn, as strains lacking this enzyme produce 87% less H2O2 (74). SpxB catalyzes the conversion of pyruvate to the phosphoryl donor, acetyl phosphate, and releases CO2 and H2O2 as by-products (104). Lactate oxidase (LctO), which converts lactate to pyruvate, positively impacts pyruvate flux through SpxB. Spn mutants lacking lactate oxidase also produce significantly lower levels of H2O2 (38% of the total H2O2) (74, 90, 104, 122) (Overview in Table 1).
Table 1.
Overview of Pneumococcal Enzymes Involved in the Generation of and Protection from Hydrogen Peroxide
Deletion of spxB and lctO significantly reduces bacterial H2O2 production. The absence of these enzymes renders Spn highly susceptible to the presence of exogenous H2O2 stress generated by host phagocytic cells to facilitate clearance of bacteria at infection sites (74, 104). This suggests a link between endogenous bacterial H2O2 generation and resistance to exogenous H2O2 produced by cells of the immune system. Levels of adenosine triphosphate (ATP) decreased more rapidly in ΔspxB pneumococcal mutants than in the wild-type strain during H2O2 exposure, suggesting that the increased killing of these mutants is due to their inability to limit depletion of ATP during H2O2 stress (104).
High levels of secreted H2O2 promote Spn colonization
Endogenously generated H2O2 diffuses rapidly through cell membranes and accumulates in the extracellular milieu of Spn cultures in concentrations high enough to kill or inhibit other common inhabitants of the respiratory tract, such as Haemophilus influenzae, Staphylococcus aureus, Neisseria meningitidis, and Moraxella catarrhalis (103). Using an in vitro model mimicking Spn/S. aureus contact during colonization of the nasopharynx, pneumococcal SpxB/LctO-produced H2O2 was shown to be converted into the more potent oxidant hydroxyl radical (•OH) that rapidly kills S. aureus (139). Thus, high levels of H2O2 provide a competitive advantage to Spn over other pathogens and commensal bacteria. A similar strategy is also applied by lactic acid bacteria at other epithelial interfaces, such as in the gut or the vagina. Thus, excretion of high levels of H2O2 by vaginal lactobacilli (e.g., L. gasseri, L. acidophilus, and L. johnsonii) represents one of the most important defense mechanisms against vaginal colonization by undesirable microorganisms (58, 85).
Adaptation to endogenously generated H2O2 in Spn
Lisher et al. (74) revealed that SpxB/LctO-generated H2O2 functions as an intracellular signaling molecule in Spn that modulates glycolytic-, nucleotide-, and capsule-biosynthesis via protein sulfenylation. The authors demonstrated a clear correlation between sulfenylation levels and endogenous H2O2 production, with SpxB and LctO being among the identified targets of protein sulfenylation. Sulfenylation of SpxB was proposed to allow it to function as an H2O2 “sink,” which is consistent with the fact that ΔspxB strains are more sensitive to exogenous H2O2 (74). Similarly, the thiol peroxidase (TpxD), which limits sulfenylation, was also shown to play a critical role in the adaptation to endogenous H2O2 in Spn (74).
A serine/threonine kinase StkP phosphorylates the response regulator ComE to control different cellular processes, including H2O2 production (107). Deletion of comE and stkP reduced the expression of spxB and tpxD and significantly decreased H2O2 generation (107).
A role for pneumococcal flavin reductase in resistance to oxidative stress has also been suggested, since an inactive mutant of the enzyme significantly increased susceptibility to H2O2, compared with wild-type Spn. Flavin reductase activity in Spn might also to be involved in bacterial virulence, as its absence does not affect Spn phagocytosis by primary mouse peritoneal macrophages, but it blunts adhesion to the type II alveolar-like cell line A549 (94).
Endogenous H2O2 promotes release of the pore-forming toxin Ply
The 53-kDa cholesterol-dependent pore-forming cytolysin (cholesterol-dependent cytolysin [CDC]) Ply (77, 79, 111, 137) can be released in the lungs by autolysis or upon antibiotic-mediated lysis (2). Although Ply lacks the N-terminal signal sequence found in other CDCs, which allows for extracellular release via the Sec-dependent pathway (128), the toxin's release has also been suggested in the absence of autolysis (4). Interestingly, Spn mutants that lack the pyruvate oxidase gene (spxB) are defective in Ply release. However, whereas complementation of spxB restored Ply release, exogenous addition of H2O2 failed to induce it. Since catalase supplementation prevented Ply release in some strains, these results indicate that intracellularly generated rather than secreted H2O2 promotes Ply release in some Spn strains (17).
Another link between SpxB and Ply release has been recently described. Deoxycholate-induced autolysis of Spn was significantly reduced in an SpxB-deficient strain, indicating a possible weakening of the cell membrane when SpxB is expressed (17). Endogenous H2O2 was found to control membrane fatty acid composition, by specific oxidation of the active site cysteine thiol residue of FabF (7). Nevertheless, it remains to be shown as to whether H2O2 generated by SpxB affects the Ply release directly or rather indirectly, by affecting the expression of yet unknown pneumococcal activators or repressors.
Protective pneumococcal mechanisms against H2O2
Generation of ROS is a conserved strategy of host phagocytic cells, primarily neutrophils, monocytes, and macrophages, to facilitate clearance of bacteria at the infection site. Bacteria can be engulfed and enclosed in phagosomes, into which superoxide is released by activated NADPH oxidase 2 (Nox-2). The resulting superoxide O2−• can then be dismutated to H2O2 by superoxide dismutase (SOD) in macrophages, and H2O2 can further be converted by myeloperoxidase (MPO) in neutrophils to generate the highly microbicidal species, hypochlorous acid (64, 136). Combating endogenous and host innate immune cell-mediated oxidative stress is an integral aspect of colonization and virulence of the facultative anaerobe Spn.
Pneumococcal enzymes involved in defense from high H2O2 levels
Common proteins known to protect against oxidative stress in other bacterial species, such as the H2O2 scavengers catalase and NADPH-peroxidase, are absent in Spn (62, 123). However, previous studies have identified other pneumococcal enzymes implicated in the defense against oxidative stress (Table 1; Fig. 1), such as TpxD (53), Nox (3), SOD (143), and alkyl hydroperoxidase (alkyl hydroperoxide reductase [AhpD]) (100). The pneumococcal TpxD plays an important role in H2O2 elimination by catalyzing its reduction (53, 54).
FIG. 1.
Mechanisms of pneumococcal defense against H2O2. Schematic overview of the three defense mechanisms involved in the resistance against H2O2: (i) defense enzymes that directly or indirectly degrade H2O2, (ii) repair mechanisms, and (iii) regulators linked to H2O2 stress response. H2O2, hydrogen peroxide. Color images are available online.
Exposure to exogenous H2O2 (10–1000 μM) significantly upregulated the expression of tpxD in Spn, whereas deletion of tpxD decreased the growth rate and survival of pneumococci in the presence of exogenously added H2O2. Addition of the H2O2 scavenger catalase in the culture medium reversed the attenuated growth of the tpxD deletion mutant (53, 54). However, Spn lacks the transcription factors OxyR and PerR found in other bacteria such as Escherichia coli, which are described to regulate the expression of tpxD in response to H2O2 (18, 21, 62, 92). Instead, the transcription factor CodY was recently identified to be an activator of tpxD expression, triggering its upregulation in Spn under H2O2 stress (54).
The pneumococcal Nox is suggested to be indirectly involved in H2O2 scavenging, as it converts O2 to H2O and thereby prevents the generation of superoxide anion (O2•−) and H2O2, which occurs when O2 is not fully reduced. Pneumococcal Nox is necessary for Spn virulence in the murine respiratory tract and in Mongolian gerbil otitis media infection models (146). Unexpectedly, Nox mutations are linked to changes in virulence after intraperitoneal infection, where oxygen tension is low (3). However, Nox has also been associated with other functions such as Spn adhesion to A549 epithelial cells (96).
SODs protect cells from the toxic effects of O2•− by converting it into the more diffusible and less reactive H2O2 and O2 (73). In prokaryotes, SODs are distinguished by their metal cofactors: Fe3+, Mn2+, Cu2+/Zn2+, and Ni2+ (31, 37, 140). Previous studies demonstrated that E. coli mutants deficient in both the manganese- and iron-containing SODs are more sensitive to H2O2 (21). Furthermore, the expression of MnSOD (SodA) from S. thermophiles in bacteria not expressing SODs, such as L. gasseri and L. acidophilus, provided protection against H2O2 stress (16). It seems that SOD protects cells against H2O2 by removing O2•− and preventing the redox cycling of iron.
Although Spn contains two types of SODs—MnSOD and FeSOD—only MnSOD (SodA) levels were increased during aerobic growth, and deletion of SodA lowered the growth of the bacterium in aerobic conditions. Virulence studies revealed attenuated growth of the sodA deletion mutant in intranasally infected mice, with growth rates in the lung and bloodstream comparable with wild type (143). In conclusion, the mechanism by which SOD provides protection of Spn against H2O2 is indirectly targeted at removing O2•− and preventing the redox cycling of iron.
A putative gene for ahpD, a group of enzymes known to degrade H2O2, was detected in Spn (92, 100). However, AhpD does not seem to provide H2O2 resistance in Spn, as its depletion rather increased the resistance of Spn against H2O2 (100).
Pneumococcal proteins involved in repair of H2O2-induced damage
Cellular damage caused by oxidative stress is multilayered and rapid. Therefore, in addition to enzymatic degradation of H2O2, immediate repair of damaged proteins and lipids is essential for the survival of the bacterium. Spn is equipped with a number of proteins known to be involved in the repair of H2O2-induced damage. These proteins include the following: (i) high-temperature requirement A, a serine protease, and chaperone protein, (ii) Clp ATP-dependent proteases, and (iii) CTM proteins consisting of CcdA (putative cytochrome c-type biogenesis protein homologue), thioredoxin-like protein family (TlpA), and methionine sulfoxide reductase A/B protein (MsrAB). All of these proteins participate in the recovery from H2O2-induced oxidative stress, as their progressive removal leads to an increased susceptibility of Spn to H2O2 [reviewed in ref. (142)]. Table 1 and Figure 1 summarize the mode of action and the regulators of proteins involved in pneumococcal defense against H2O2.
Impact of H2O2 on iron homeostasis
Transition of Spn from the nasopharynx to the lungs requires adjustment to a variety of environmental conditions, including availability of transition metal ions (98). Iron (Fe2+) potentiates oxidative stress. Expression of Fe2+ transport systems and proteins that protect against oxidative stress is regulated by the orphan response regulator RitR. Deletion of ritR impaired Spn growth in high-Fe2+ medium, which could be reversed upon addition of manganese (Mn2+), the latter of which caused a reduction in the amount of H2O2 produced by Spn.
In Spn, excess levels of Zn2+ prevent the uptake of Mn2+ by irreversibly binding to the extracellular Mn2+-binding protein pneumococcal surface adhesin A (89, 124). As a consequence, Spn becomes hypersensitive to oxidative stress, due to a decrease in the activity of MnSOD, which plays an important role in protecting the pathogen against high exogenous H2O2 levels (125). It is likely that many of the enzymes involved in resistance to ROS in Spn metallate with manganese rather than with iron.
H2O2 can interact with ferrous ions (Fe2+) and form a highly reactive hydroxyl radical (•OH) through the Fenton reaction, causing DNA damage and increased toxicity to the cells. Spn contains only a small number of iron-containing proteins, in contrast to most other bacteria, such as E. coli, and thereby avoid poisoning themselves during radical attack. This is consistent with findings showing that H2O2-mediated killing of Spn is unaffected by iron chelators and appears to be independent of the Fenton reaction (104).
A homologue of DNA-binding protein from starved cells (Dps) and of Dps-like peroxide resistance (Dpr) is present in the pneumococcal genome. Unlike Dps, which protects DNA through direct association, Dpr was found to be an important factor for mediating resistance of oxidative stress caused by H2O2 through chelation of free iron, thereby inhibiting the Fenton reaction. Furthermore, a dpr mutant has a reduced ability to colonize and is more rapidly cleared from the nasopharynx in a mouse model (65). In S. suis, Dpr can bind ferrous ions as well as other divalent cations, such as Cu2+, Mn2+, and Zn2+ (52).
Critical Issues: Actions of H2O2 on the Host's Lung Cells
Spn-derived H2O2 induces the UPR in host cells
Endoplasmic reticulum (ER) stress, resulting from the accumulation of unfolded proteins, increased protein load, or calcium gradient dysregulation, can trigger the UPR. This fundamental stress response is used by eukaryotic cells to match demand for protein synthesis with the capability to fold proteins within the ER, to maintain cellular homeostasis (148). In lower organisms, such as Caenorhabditis elegans, the UPR was demonstrated to participate in the defense against pathogens, since loss of this pathway was shown to induce hypersensitivity to certain pore-forming toxins, but not to other toxic insults (10).
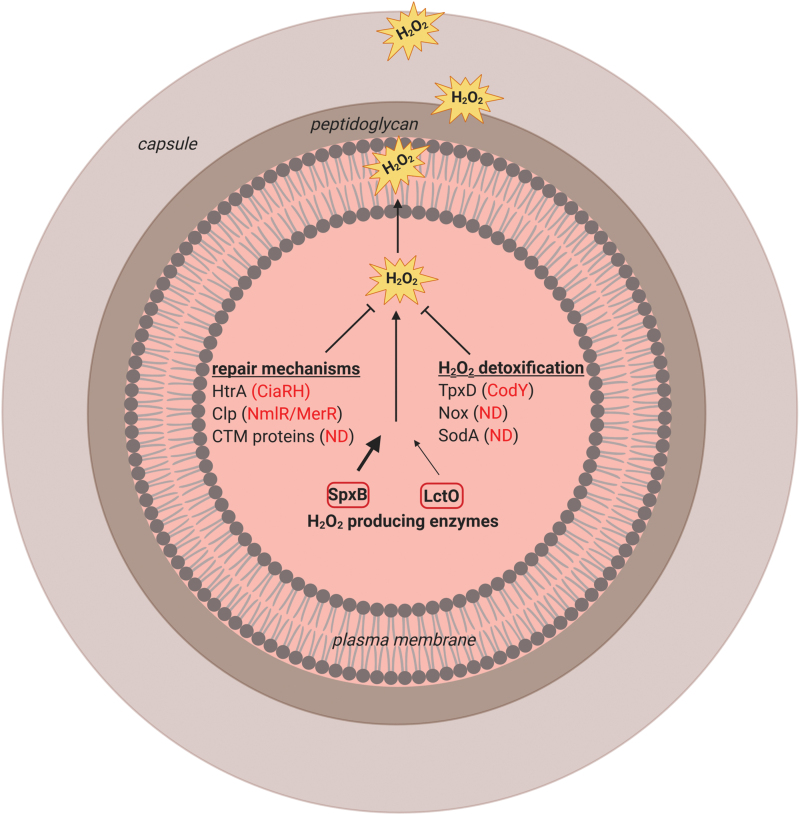
UPR signaling is mediated by three transducers that are inserted into the ER membrane: activating transcription factor 6 (ATF6), protein kinase R (PKR)-like ER kinase (PERK), and inositol-requiring enzyme 1 (IRE1). The ER-resident chaperone immunoglobulin heavy chain-binding protein (BiP) binds to the luminal domain of the ER stress sensors and keeps them in a monomeric inactive state. Accumulation of unfolded proteins leads to release of BiP from the ER stress sensors and induces its subsequent binding to the unfolded proteins (20, 24). PERK and IRE1 become activated by dimerization (or oligomerization) and subsequent phosphorylation (19, 26). Phosphorylated IRE1 activates its own endoribonuclease, which mediates splicing from X-box-binding protein 1 (XBP1) mRNA. Translation of the spliced transcript produces active XBP1 protein that translocates to the nucleus, where it induces the transcription of several ER-resident chaperones and protein-folding enzymes (57, 70).
PERK activation induces phosphorylation of serine 51 of eukaryotic translation initiation factor 2α (eIF2α). As a consequence, translation is inhibited and protein folding stress in the ER is decreased. However, PERK activation also increases the translation of the transcription factor activating transcription factor 4 (ATF4). ATF4 mRNA possesses internal ribosome entry site sequences in its 5′-untranslated regions and as such escapes translational suppression. Upon entering the nucleus, ATF4 regulates the expression of UPR target genes (20). Dissociation of BiP from ATF6 initiates its translocation to the Golgi compartment where it can be cleaved by two proteases. The activated transcription factor ATF6 then migrates to the nucleus and activates the expression of genes encoding proteins, which enhance ER capacity and function (including BiP) (138, 145).
Although UPR signaling can alleviate ER stress, it can also—when sustained—activate deleterious cell death pathways if homeostasis cannot be restored (99). Recently, activation of the UPR was even suggested to mediate vascular disease (105, 112). Indeed, the p22phox subunit, an essential component of most Noxs, was shown to be a novel target of the UPR transcription factor ATF4 under ER stress conditions, thereby increasing ROS generation from Noxs and promoting pathogenesis of cardiovascular disease. Hence, the UPR plays an important role in physiology, but can also contribute to development of cardiovascular disease. Infection with Spn can induce the UPR (6, 70, 93). This was demonstrated by the expansion of ER membranes in the H441 cell line (human lung adenocarcinoma similar to club-like lung epithelial cells) upon infection (75), a common feature observed during the UPR (106).
Secretion of Spn-derived H2O2 leads to activation of PERK, ATF-6, and IRE1 (Fig. 2). Dimerization and phosphorylation of activated PERK induce phosphorylation of eIF2α leading to inhibition of protein translation and ATF4 modulating expression of target genes (Fig. 2). Activated ATF-6 translocates to the Golgi, where it is cleaved by site-1 protease and site-2 protease. The processed ATF-6 enters the nucleus acting as a transcription factor of target genes. Activation of IRE1 leads to splicing of xbp1 mRNA, which acts as transcription factor of target genes. IRE1 activation can also lead to regulated Ire1-dependent decay (RIDD of mRNA) or c-Jun N-terminal kinase (JNK) signaling activation. Induction of the UPR slows down ongoing protein synthesis and increases the folding capacity of the ER.
FIG. 2.
Actions of Spn-derived H2O2 on the UPR in host cells. Infection with Spn induces the activation of the UPR. Secretion of Spn-derived H2O2 leads to activation of PERK, ATF-6, and IRE1. Dimerization and phosphorylation of activated PERK induce phosphorylation of eIF2α leading to inhibition of protein translation and ATF4 modulating expression of target genes. Activated ATF-6 translocates to the Golgi, where it is cleaved by S1P and S2P. The processed ATF-6 enters the nucleus acting as a transcription factor of target genes. Activation of IRE1 leads to splicing of xbp1 mRNA, which acts as transcription factor of target genes. IRE1 activation can also lead to RIDD of mRNA or JNK signaling activation. ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; eIF2α, eukaryotic translation initiation factor 2α; ER, endoplasmic reticulum; IRE1, inositol-requiring enzyme 1; JNK, c-Jun N-terminal kinase; PERK, protein kinase R (PKR)-like ER kinase; RIDD, regulated IRE1-dependend decay; S1P, site-1-protease; S2P, site-1-protease; Spn, Streptococcus pneumonia; UPR, unfolded protein response; xbp1, X-box-binding protein 1. Color images are available online.
Although a number of bacterial virulence factors, including lipopolysaccharide (LPS) and some pore-forming toxins, such as listeriolysin-O, were shown to induce the UPR (10, 24, 75), infections with Spn in H441 cells revealed that H2O2, rather than the pore-forming toxin Ply, is the main trigger of UPR, mainly by means of activating the PERK pathway (Fig. 2). The important role of H2O2 in the induction of the UPR during pneumococcal infection is documented by the observation that a mutant Spn strain lacking pyruvate oxidase (SpxB), which secretes a very low level of H2O2, does not induce UPR in H441 cells (75).
Induction of DNA damage by pneumococcal-secreted H2O2
Spn not only generates large amounts of H2O2 endogenously (34), but also releases large quantities of H2O2 in vitro and in vivo (42). Host cells typically trigger an antioxidant stress response during Spn infection, to prevent oxidative injury. This protective response occurs by inducing the production of the transcription factor nuclear factor erythroid 2-related factor 2, which plays a crucial role in the transcriptional activation of antioxidant enzymes and ROS scavengers. These include heme oxygenase 1, NADPH dehydrogenase, and SOD (73, 83).
At excessive concentrations, H2O2 can, however, induce toxic DNA double-strand breaks, which precede apoptosis, as was shown using cultures of the human A549 cell line (a model for type II pneumocytes) exposed to three serotypes of Spn. Catalase reduced the frequency of DNA damage-positive cells in these studies by 50% or more (109).
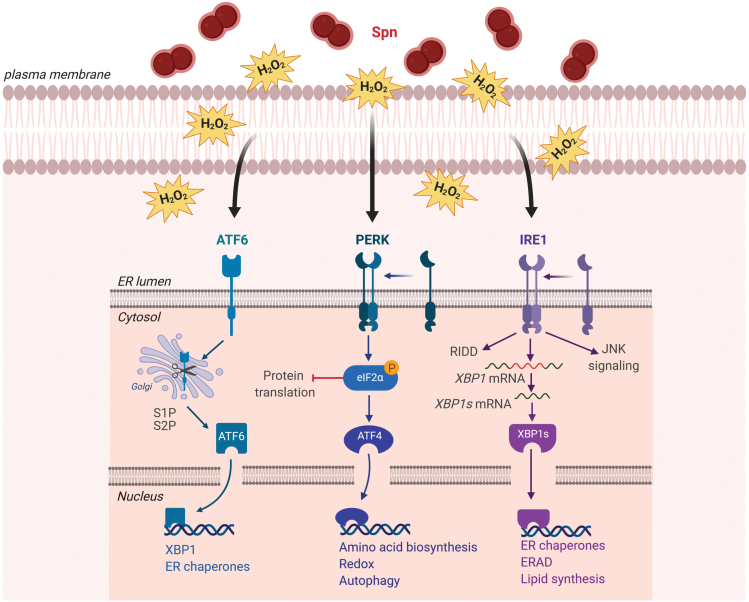
Another consequence of bacterial H2O2 release is an increased production of mitochondrial ROS in the host cells, as observed during infection with wild-type Spn, with a Ply-negative mutant strain (Δply), but not with a SpxB-negative Spn strain (63, 75) (Fig. 3). Spn-secreted H2O2 can also cause oxidative damage to mitochondrial DNA (mtDNA) and lead to the release of mtDNA into the cytoplasm, which in turn induces type I interferon expression in the A549 cell line, involving the stimulator of interferon gene signaling pathway (46) (Fig. 3).
FIG. 3.
H2O2-induced responses in the host cell. Spn produces large amounts of H2O2, which induce a plethora of host cell responses. This includes oxidative and ER stress (red), activation of all three MAPK subfamilies (green), translocation of NF-κB-complex into the nucleus (violet), and inflammasome inhibition (blue). Oxidative stress leads to production of mtROS and release of mtDNA and subsequent STING-dependent type I IFN (IFN-β) expression. Translocation of NF-κB-complex into the nucleus leads to expression of proinflammatory chemokines and cytokines (such as IL-8 and IL23a) and H2O2-dependent production of Nrf-2, which induces expression of HO-1. HO-1, heme oxygenase 1; IFN, interferon; IL, interleukin; MAPK, mitogen-activated protein kinase; mtDNA, mitochondrial DNA; mtROS, mitochondrial reactive oxygen species; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; STING, stimulator of interferon genes. Color images are available online.
Pneumococcal H2O2 affects inflammation in host cells
Apart from activating mtROS generation, DNA damage, and UPR in host cells, H2O2 secreted by Spn can also induce activation of all three mitogen-associated protein kinase subfamilies, that is, p38, JNK, and ERK, and cause nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) translocation and transcription of proinflammatory chemokines and cytokines (interleukin [IL]-8 and IL-23a) (Fig. 3) (9, 75). Canonical activation of the transcriptional regulatory factor NF-κB protein complex involves phosphorylation-induced proteolysis of the inhibitory protein IκBα, which is bound to NF-κB subunits RelA and p50 by IκB kinase. Proteolysis of IκBα leads to subsequent translocation of RelA/p50 protein complex to the nucleus (Fig. 3).
The ability of lung parenchymal cells to contribute to proinflammatory cytokine production has also been demonstrated in response to other pathogens, including Legionella pneumophila, Pseudomonas aeruginosa, Klebsiella pneumoniae, and H. influenza (22, 69, 110, 114). A mutation in pyruvate oxidase (SpxB), causing reduced H2O2 secretion, was shown to diminish adhesive properties of Spn to type II pneumocytes and capillary endothelial cells, which may contribute to diminished virulence in vivo (118).
Despite activating certain inflammatory pathways, pneumococcal-secreted H2O2 does not necessarily activate the host's immune defense. By contrast, recent results indicate that H2O2 released by Spn inhibits inflammasomes, key components of the innate immune system, as such further contributing to virulence and to colonization of the host (36). Thereby, H2O2, which is secreted in millimolar quantities by some Spn strains, has the capacity to counteract the NLRP3 inflammasome-activating effect of Ply, the main virulence factor of Spn (91). On the contrary, pneumococcus-derived H2O2 was shown to directly promote aggregation and activation of platelets, cells orchestrating the systemic inflammatory response (1).
Dose-dependent effects of H2O2 on vectorial Na+ transport in alveolar epithelial cells
The level of fluid in alveoli represents a critical balance between the rate of fluid movement from pulmonary capillaries and lung interstitium across the alveolar barrier into the alveolar space and the transport of salt and water out of the alveoli. Vectorial Na+ transport out of the alveoli by the apically expressed epithelial sodium channel (ENaC) and the basolaterally expressed Na+–K+ pump in flat type I (cover 90% of alveolar surface) and cuboidal type II alveolar epithelial cells (cover 5% of alveolar surface) is the major mechanism to maintain an optimal level of airway and alveolar surface liquid (35, 86, 87, 116, 126, 127).
ENaC, whose activity is defined by its surface expression (N) and its open probability time (Po), is critically involved in alveolar fluid clearance (AFC) (35, 87). Indeed, genetic deletion of the crucial α subunit in neonatal mice prevents them from clearing fluid from their lungs, leading to respiratory distress and death shortly after birth (66). The observation that single-nucleotide polymorphisms rs4149570 and rs7956915 of ENaC-α are associated with neonatal respiratory distress syndrome and lung fluid absorption disorders indicates that ENaC-α (SCNN1A) also has a significant role in fluid clearance in man (72). When these mechanisms become impaired, as is the case in severe pneumonia, alveolar flooding occurs, which can precipitate a lethal hypoxemia by impairing gas exchange.
Concentrations of H2O2 in the alveolar space in healthy individuals were estimated to be in the micromolar to tens of micromolar range (25). Physiological concentrations of endogenous, dual oxidases 1/2-mediated and exogenous H2O2 were shown to increase ENaC activity (39, 60, 82). This occurs at least partially through a reduction in ubiquitination of the ENaC-α subunit, which blunts the subunit's degradation and thus increases its surface expression N (33). At physiological concentrations, H2O2 also activates PI3-kinases that produce the anionic phospholipids, phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate, which were shown to increase the amount of time that ENaC spends in an open state, transporting Na+ (Po) (60, 67, 108, 147). Physiologic levels of H2O2 also stimulate the Na+–K+ pump in alveolar epithelial cells (29, 49, 127).
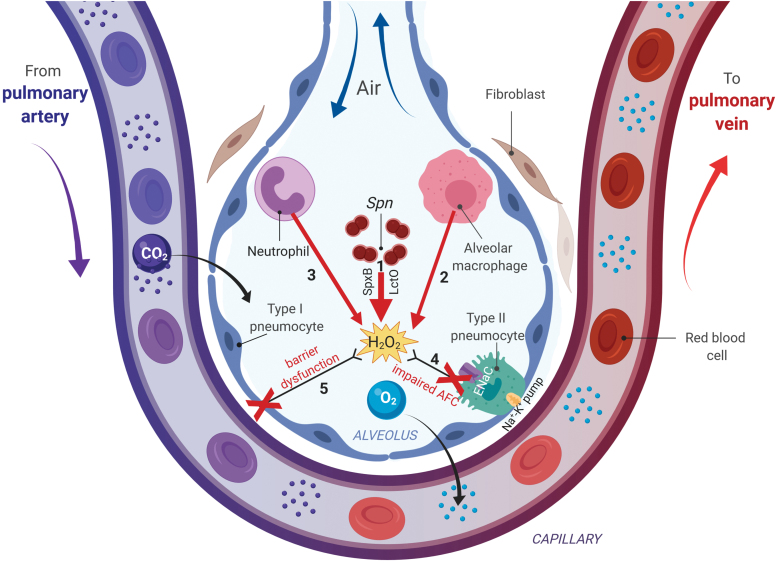
However, during pneumococcal pneumonia, near millimolar concentrations of H2O2 can be secreted by Spn in the alveolar space, in addition to the H2O2 generated by activated neutrophils and alveolar macrophages (Fig. 4). These high levels suppress ENaC-α transcription, in an ERK-dependent manner, and can inhibit the Na+–K+ pump (29, 49, 127, 131, 141). As a consequence, vectorial Na+ transport is impaired and alveolar flooding can occur, which can precipitate a lethal hypoxemia by impairing gas exchange (Fig. 4). Spn release of Ply can aggravate this further, since this toxin has also been shown to impair ENaC activity (77).
FIG. 4.
The complex actions of H2O2 in alveoli during pneumococcal pneumonia. Spn will migrate into the lower respiratory tract, and since it lacks catalase will generate millimolar levels of H2O2, through the actions of pyruvate oxidase (SpxB) and lactate oxidase (LctO), which will diffuse into the alveolar space (1). Moreover, an early neutrophil-mediated and a later macrophage-derived generation and secretion of μmolar levels of H2O2 will occur (2, 3). High ROS levels in the alveolar space will promote alveolar endothelial barrier function (4) and will impair AFC (5), which is mainly mediated through vectorial sodium transport, involving the apically expressed ENaC and the basolateral Na+-K+ pump in type II pneumocytes. AFC, alveolar fluid clearance; ENaC, epithelial sodium channel; ROS, reactive oxygen species. Color images are available online.
Impact of H2O2 on alveolar–capillary barrier function
A tight barrier structure of only 0.3 μm thickness separates capillary blood from alveolar gas and is a crucial interface for efficient gas exchange (133, 135). A disruption of the alveolar–capillary barrier, if not resolved, can result in alveolar flooding and poor alveolar gas exchange, both of which lead to hypoxia and hypercapnia, hallmarks of ARDS (127). During pneumococcal pneumonia, high H2O2 levels accumulate in the alveolar space, originating mainly from Spn secretion, and to a lesser extent also from inflammatory neutrophils and alveolar macrophages, which first generate superoxide from Nox-2, which is then further converted into H2O2 by SOD.
An important role of H2O2 in the pathogenesis of acute lung injury was first recognized by the observation that both catalase and SOD protected sheep lungs from air emboli-induced increased vascular permeability (42). Moreover, intravascular H2O2 challenge in intact lungs was shown to cause both capillary endothelial and alveolar epithelial barrier dysfunction, characterized by increased actin stress fiber formation, intercellular gaps, and intracellular Ca2+ levels. These effects on barrier function could be partially inhibited by increasing cAMP or cGMP levels (102, 115, 117).
H2O2 can mediate alveolar epithelial–endothelial paracrine signaling. As such, increased H2O2 levels in the alveolar space and pneumocytes can contribute to capillary endothelial barrier dysfunction (63). Recently, an important role for transient receptor potential (TRP) channels was suggested in the actions of ROS. This family of cation channels depolarizes the membrane potential and regulates intracellular concentrations of cations such as Ca2+, the latter of which is known to regulate barrier function (113). Several TRP channels, including transient receptor potential melastatin 2 (TRPM2), transient receptor potential vanilloid-4 (TRPV4), and transient receptor potential channel 6 (TRPC6), are redox sensors that can be activated by H2O2 (5, 32, 84, 95). Micromolar levels of H2O2 were shown to stimulate ADP ribose formation in human pulmonary artery endothelial cells and to activate the Ca2+-permeable and oxidant-activated TRPM2 channel. H2O2-mediated Ca2+ entry and diminished transendothelial electrical resistance in these cells were blunted by TRPM2 siRNA depletion or by a neutralizing antibody (59). TRPM2 is also expressed in alveolar epithelial cells, and TRPM2 knockout mice were shown to be less susceptible to bleomycin-induced lung inflammation, which is known to be accompanied by increased H2O2 levels (144).
Phosphorylation of TRPV4 by the Src family kinase Fyn, which is tethered to the cell membrane by the fatty acid transporter CD36, was recently shown to significantly contribute to H2O2-induced Ca2+ influx in lung microvascular endothelial cells (120). Proposed molecular mechanisms of TRPV4-mediated edema formation include Ca2+-induced activation of myosin light-chain kinase in endothelial cells (11) and the opening of Ca2+-activated K+ channels (KCa3.1) in epithelial cells, which induce hyperpolarization (130).
Finally, TRPC6 was shown to be critically involved in the regulation of pulmonary vascular permeability and lung edema formation during LPS or ischemia/reperfusion-induced acute lung injury (134).
Clinical significance of endogenous H2O2 generation during pneumococcal pneumonia
The SpxB gene found in nearly all Spn strains encodes pyruvate oxidase, an enzyme that converts pyruvate to acetyl phosphate and generates near suicidal levels of H2O2. These high levels of endogenous H2O2 would normally cause bacterial death in the absence of catalase, yet Spn is able to resist them and to even use them as a factor to outcompete S. aureus in the nose and to foster colonization in the host. As indicated above, a general strategy used by Spn and lactic-acid bacteria to avoid the toxic effects of the Fenton reaction is replacing iron with manganese. Also, the production of high levels of ferritin-binding proteins keeps free-iron levels low enough to suppress damage to DNA.
The complex role of H2O2 in pneumococcal-infected patients was clearly documented by recent observations with clinical isolates of serotype 1 belonging to 2 major clonal complexes (CCs), that is, CC228, associated with low mortality, and CC217, associated with high mortality (15, 121). Mutations in SpxB resulted in larger colony variants unable to produce endogenous H2O2. Intravenous challenge of mice with these mutants revealed that early macrophage-mediated clearance was lower than that of wild-type bacteria, and resulted in a higher bacterial load (121). Nevertheless these mutants were less efficient in the colonization process when compared with the wild-type strain. Thus, a requirement for H2O2 in the infection process varies as the bacteria moves from niches that are oxygen rich to those that have low oxygen tension.
Alcoholics are at increased risk for developing pneumococcal pneumonia and a role for pneumococcal H2O2 for this predilection has also been postulated. The Spn type 2 D39 strain, which is ethanol-tolerant due to increased alcohol dehydrogenase E (adhE) expression, exhibits increased H2O2 generation. Thus, ethanol-fed mice were more susceptible to infection with the D39 wild-type bacteria than with the ΔadhE strain (80).
Future Directions
Pulmonary permeability edema as a consequence of the ARDS is a main cause of morbidity and mortality of pneumococcal pneumonia. In the absence of proven pharmacologic treatments, clinicians have to rely on ventilation strategies to deliver oxygen to patients, which can sometimes make the situation worse if the ventilation pressure is too high and causes ventilator-induced lung injury. Permeability edema is characterized by hyperpermeability of the alveolar–capillary barrier, combined with a deficiency to clear liquid from the alveolar space. Vectorial Na+ transport through the apically expressed ENaC and the basolateral Na+–K+ pump is crucial for AFC. ARDS patients with an impaired capacity to clear alveolar fluid were shown to be at significantly higher risk to die (132).
During pneumococcal pneumonia, the pathogen can generate millimolar amounts of H2O2 that can rapidly diffuse through cell membranes and accumulate in the alveolar space. Whereas endogenous H2O2 clearly represents a colonization advantage to the pathogen, exogenous H2O2 in the ∼10 μM range generated by the Nox-2/MPO or Nox2/SOD pathways in neutrophils and alveolar macrophages promotes bacterial clearance. Although the detailed mechanism by which the catalase-negative pneumococci can withstand high concentrations of endogenous and phagocyte-derived exogenous H2O2 is incompletely understood, several well-defined enzymatic pathways in the pathogen and in phagocytic cells of the host are involved, as summarized in Figure 5.
FIG. 5.
Overview of positive (green) and negative (red) actions of endogenous and secreted H2O2 in the pathogen and in the host lung during pneumococcal pneumonia. In Spn, endogenous H2O2 promotes nucleotide, glycolytic, and capsule biosynthesis and increases Ply secretion. Secreted H2O2 kills competing pathogens in the respiratory tract and serves as a virulence factor, since it reduces NLRP3 inflammasome activation in the host. In the alveolar space, Spn-secreted H2O2 can induce activation of the UPR and cause DNA damage, which can precede apoptosis. Moreover, H2O2 can impair the alveolar–capillary barrier, induce inflammation, and blunt vectorial Na+ transport, crucial for AFC in pneumocytes. All of these actions of H2O2 on the host lung cells can promote the development of pulmonary edema during pneumococcal pneumonia. Ply, pneumolysin. Color images are available online.
Excessively high levels of exogenous H2O2 in the alveolar space during pneumococcal pneumonia can be damaging to the pathogen, they also have effects on host cells of the alveolar space. Because H2O2 is membrane permeant, it can promote DNA damage and apoptosis, induce the UPR and blunt inflammasome-mediated host defense, and increase inflammation. In particular, type 1 and 2 alveolar epithelial cells and the microvascular endothelial cells make up the alveolar–capillary barrier in the lungs affected by high H2O2 concentrations. As a consequence of reduced subunit expression, impaired Na+-transport by ENaC and the Na+–K+ pump in AT1/2 cells will lead to a dysfunctional AFC. Moreover, impaired barrier function in both the epithelial and endothelial compartments will promote fluid, protein, and cell accumulation in the alveolar space.
Future therapeutic approaches for pneumococcal pneumonia should potentially aim at reducing the harmful effects of ROS in lung cells, without impairing host defense. As findings with SpxB mutants have indicated, strategies to blunt endogenous H2O2 generation in the pathogen seem challenging. However, agents able to impair endogenous H2O2 generation or promote their downstream effects on iron homeostasis could decrease the harmful effects of the pathogen and improve macrophage-mediated host defense.
In view of their role in barrier function, TRP channel inhibitors could represent attractive therapeutic candidates to counteract the actions of high levels of H2O2 during pneumococcal pneumonia. However, their rather ubiquitous expression and the multitude of their functions (regulation of the inflammatory response, pulmonary vasomotor control, and systemic blood pressure) may render also this approach challenging.
As such, therapeutic candidates that reduce the deleterious effects of oxidative stress on barrier function and AFC specifically in the host cells, without impairing antipneumococcal host defense, might hold the key to developing a successful pharmacological approach to the potentially lethal permeability edema associated with severe pneumonia.
The alpha-tocopherol form of vitamin E was shown to boost elastase activity of human neutrophils as well as their ability to kill Spn (13). Especially noteworthy are promising preclinical data with the potent antioxidant ascorbic acid (vitamin C), which in preclinical studies was shown to attenuate systemic inflammation and blunt sepsis-induced coagulopathy and vascular injury (40, 41, 43). In a recent phase 2 clinical trial in severely ill patients with sepsis and ARDS, high-dose intravenous administration of vitamin C did not significantly improve the primary endpoint, that is, modified Sequential Organ Failure Assessment (mSOFA) score at 96 h (44). Yet, taking into account mSOFA scores from patients who died during the trial, the mSOFA score at 96 h was significantly lower in patients on the test drug than in the placebo group (45).
A tumor necrosis factor (TNF)-derived peptide—the TIP peptide (a.k.a. AP301, solnatide), which mimics the lectin-like domain of the cytokine and directly binds to the α subunit of ENaC, the latter of which is expressed in both epithelial and endothelial lung cells (27, 28), was shown to significantly reduce oxidative stress in pulmonary artery endothelial cells under hypoxia/reoxygenation and in transplanted rat lungs (55, 56). The peptide significantly blunted bacterial toxin-induced capillary leak and pulmonary edema in mice, rats, rabbits, and pigs and was moreover shown in a phase 2 clinical trial to significantly reduce extravascular lung water index in acute lung injury patients (a measure for edema) (68). Interestingly the lectin-like domain of TNF was shown not to interfere with antibacterial activities of the cytokine in a murine model of septic peritonitis (76).
In conclusion, H2O2 plays a complex role during pneumococcal pneumonia. On the one hand, its generation by the host's phagocytes can reduce bacterial burden. On the other hand, excessive endogenous generation of H2O2 by the pathogen provides a means to outcompete other pathogens inhabiting the respiratory tract and to increase resistance to exogenous levels of H2O2. Moreover, excessive levels of ROS can significantly impair host defense and foster the formation of permeability edema in the lungs, through the impairment of alveolar–capillary barrier function and AFC capacity.
Strategies that specifically blunt H2O2's deleterious actions on the host and promote pathogen susceptibility could represent a promising approach to reduce the high mortality associated with pneumococcal pneumonia.
Acknowledgments
Figures 1–4 were prepared using BioRender. The authors acknowledge funding institutions.
Abbreviations Used
- Ac-P
acetyl phosphate
- AdhE
aldehyde-alcohol dehydrogenase E
- ADP
adenosine diphosphate
- AFC
alveolar fluid clearance
- AhpD
alkyl hydroperoxide reductase
- ARDS
acute respiratory distress syndrome
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- ATP
adenosine triphosphate
- BiP
immunglobulin heavy-chain-binding protein
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- CAP
community-acquired pneumonia
- CC
clonal complex
- CcdA
cytochrome c-type biogenesis protein homologue
- CD36
cluster of differentiation 36
- CDC
cholesterol-dependent cytolysin
- cGMP
cyclic guanosine monophosphate
- CiaRH
ciaH = histidine kinase gene
- ClpP
ATP-dependent Clp protease proteolytic subunit
- CO2
carbon dioxide
- CodY
GTP-sensing transcriptional pleiotropic repressor
- ComE
response regulator ComE
- CTM
CcdA, TlpA, MsrAB
- Dpr
Dps-like peroxide resistance
- Dps
DNA-binding protein from starved cells
- DUOX1/2
dual oxidase 1/2
- eiF2α
eukaryotic translation initiation factor 2α
- ENaC
epithelial sodium channel
- ER
endoplasmic reticulum
- ERAD
endoplasmic-reticulum-associated protein degradation
- ERK
extracellular signal-regulated kinases
- FabF
3-oxoacyl-[acyl-carrier-protein] synthase 2
- Fyn
proto-oncogene tyrosine-protein kinase Fyn
- H2O2
hydrogen peroxide
- HO-1
heme oxygenase 1
- HtrA
high-temperature requirement A
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor, alpha
- IFN-I
type I interferon
- IL
interleukin
- IRE1
inositol-requiring enzyme 1
- JNK
c-Jun N-terminal kinase
- LctO
lactate oxidase
- LPS
lipopolysaccharide
- MAPK
mitogen-associated protein kinase
- MerR
mercury-sensing regulatory protein
- MLCK
myosin light-chain kinase
- MPO
myeloperoxidase
- mSOFA
modified sequential organ failure assessment
- MsrAB
methionine sulfoxide reductase A/B protein
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- Na+–K+ pump
sodium–potassium pump
- ND
not determined
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nox-2
NADPH oxidase 2
- Nrf-2
nuclear factor erythroid 2-related factor 2
- O2
oxygen
- OxyR
hydrogen peroxide-inducible genes activator
- PERK
protein kinase R (PKR)-like ER kinase
- PerR
ferric uptake regulator
- PI3-kinase
phosphoinositide 3-kinase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- Ply
pneumolysin
- PsaA
pneumococcal surface adhesin A
- RelA
nuclear factor NF-kappa-B p65 subunit
- RIDD
regulated Ire1-dependent decay
- RitR
orphan response regulator
- ROS
reactive oxygen species
- S1P
site-1-protease
- S2P
site-2-protease
- siRNA
silencing RNA
- SOD
superoxide dismutase
- Spn
Streptococcus pneumoniae
- SpxB
pyruvate oxidase
- STING
stimulator of interferon genes
- StkP
serine/threonine kinase
- TCS04
two-component system 4
- TlpA
thioredoxin-like protein family
- TNF
tumor necrosis factor
- TpxD
thiol peroxidase
- TRP
transient receptor potential
- TRPC6
transient receptor potential channel 6
- TRPM2
transient receptor potential melastatin 2
- TRPV2
transient receptor potential vanilloid
- UPR
unfolded protein response
- VILI
ventilator-induced lung injury
- XBP1
X-box-binding protein 1
Author Disclosure Statement
R.L. is inventor on patents related to the use of the TIP peptide in ARDS. The other authors have no conflict of interest.
Funding Information
The study was funded by the National Institutes of Health HL134934 (Y.S.), HL125926 (D.F.), DK110409 (D.C.E.), and HL138410 (R.L.), by the Lungen- und Atmungsstiftung Bern, Switzerland (J.H., R.L.), and by the German Research Foundation SFB-TR 84 “Innate Immunity of the Lung: Mechanisms of Pathogen Attack and Host Defense in Pneumonia” (M.A.M. and T.C.).
References
- 1. Anderson R and Feldman C. Review manuscript: mechanisms of platelet activation by the pneumococcus and the role of platelets in community-acquired pneumonia. J Infect 75: 473–485, 2017 [DOI] [PubMed] [Google Scholar]
- 2. Anderson R, Steel HC, Cockeran R, von Gottberg A, de Gouveia L, Klugman KP, Mitchell TJ, and Feldman C. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J Antimicrob Chemother 60: 1155–1158, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi AD, Le Thomas I, Garel JR, Paton JC, and Trombe MC. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol 34: 1018–1028, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Balachandran P, Hollingshead SK, Paton JC, and Briles DE. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J Bacteriol 183: 3108–3116, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, and Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baruch M, Hertzog BB, Ravins M, Anand A, Cheng CY, Biswas D, Tirosh B, and Hanski E. Induction of endoplasmic reticulum stress and unfolded protein response constitutes a pathogenic strategy of group A Streptococcus. Front Cell Infect Microbiol 4: 105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benisty R, Cohen AY, Feldman A, Cohen Z, and Porat N. Endogenous H2O2 produced by Streptococcus pneumoniae controls FabF activity. Biochim Biophys Acta 1801: 1098–1104, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Berger S, Goekeri C, Gupta SK, Vera J, Dietert K, Behrendt U, Lienau J, Wienhold SM, Gruber AD, Suttorp N, Witzenrath M, and Nouailles G. Delay in antibiotic therapy results in fatal disease outcome in murine pneumococcal pneumonia. Crit Care 22: 287, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatia M, Zemans RL, and Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol 46: 566–572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, and Aroian RV. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 4: e1000176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bogatcheva NV, Zemskova MA, Poirier C, Mirzapoiazova T, Kolosova I, Bresnick AR, and Verin AD. The suppression of myosin light chain (MLC) phosphorylation during the response to lipopolysaccharide (LPS): beneficial or detrimental to endothelial barrier? J Cell Physiol 226: 3132–3146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser N, Caspers E, Smorenburg A, Emini EA, Gruber WC, and Grobbee DE. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 372: 1114–1125, 2015 [DOI] [PubMed] [Google Scholar]
- 13. Bou Ghanem EN, Lee JN, Joma BH, Meydani SN, Leong JM, and Panda A. The alpha-tocopherol form of vitamin E boosts elastase activity of human PMNs and their ability to kill Streptococcus pneumoniae. Front Cell Infect Microbiol 7: 161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown AO, Millett ER, Quint JK, and Orihuela CJ. Cardiotoxicity during invasive pneumococcal disease. Am J Respir Crit Care Med 191: 739–745, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brueggemann AB and Spratt BG. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J Clin Microbiol 41: 4966–4970, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruno-Bárcena JM, Andrus JM, Libby SL, Klaenhammer TR, and Hassan HM. Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl Environ Microbiol 70: 4702–4710, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bryant JC, Dabbs RC, Oswalt KL, Brown LR, Rosch JW, Seo KS, Donaldson JR, McDaniel LS, and Thornton JA. Pyruvate oxidase of Streptococcus pneumoniae contributes to pneumolysin release. BMC Microbiol 16: 271, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlioz A and Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J 5: 623–630, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carrara M, Prischi F, Nowak PR, and Ali MM. Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. EMBO J 34: 1589–1600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaudhari N, Talwar P, Parimisetty A, Lefebvre d‘Hellencourt C, and Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8: 213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiang SM and Schellhorn HE. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys 525: 161–169, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Clemans DL, Bauer RJ, Hanson JA, Hobbs MV, St Geme, JW, 3rd, Marrs CF, and Gilsdorfv JR. Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by non-typeable Haemophilus influenzae. Infect Immun 68: 4430–4440, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cochi SE, Kempker JA, Annangi S, Kramer MR, and Martin GS. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc 13: 1742–1751, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coope A, Milanski M, Arruda AP, Ignacio-Souza LM, Saad MJ, Anhê GF, and Velloso LA. Chaperone insufficiency links TLR4 protein signaling to endoplasmic reticulum stress. J Biol Chem 287: 15580–15589, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Corradi M, Pignatti P, Brunetti G, Goldoni M, Caglieri A, Nava S, Moscato G, and Balbi B. Comparison between exhaled and bronchoalveolar lavage levels of hydrogen peroxide in patients with diffuse interstitial lung diseases. Acta Biomed 79: 73–78, 2008 [PubMed] [Google Scholar]
- 26. Cui W, Li J, Ron D, and Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr 67: 423–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Czikora I, Alli A, Bao HF, Kaftan D, Sridhar S, Apell HJ, Gorshkov B, White R, Zimmermann A, Wendel A, Pauly-Evers M, Hamacher J, Garcia-Gabay I, Fischer B, Verin A, Bagi Z, Pittet JF, Shabbir W, Lemmens-Gruber R, Chakraborty T, Lazrak A, Matthay MA, Eaton DC, and Lucas R. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am J Respir Crit Care Med 190: 522–553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Czikora I, Alli AA, Sridhar S, Matthay MA, Pillich H, Hudel M, Berisha B, Gorshkov B, Romero MJ, Gonzales J, Wu G, Huo Y, Su Y, Verin AD, Fulton D, Chakraborty T, Eaton DC, and Lucas R. Epithelial sodium channel-α mediates the protective effect of the TNF-derived TIP peptide in pneumolysin-induced endothelial barrier dysfunction. Front Immunol 8: 842, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dazy AC, Auger F, Bailbé D, Blouquit S, Lombet A, and Marano F. The toxicity of H2O2 on the ionic homeostasis of airway epithelial cells in vitro. Toxicol In Vitro 17: 575–580, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Dela Cruz CS, Wunderink RG, Christiani DC, Cormier SA, Crothers K, Doerschuk CM, Evans SE, Goldstein DR, Khatri P, Kobzik L, Kolls JK, Levy BD, Metersky ML, Niederman MS, Nusrat R, OrihuelaCJ, Peyrani P, Prince AS, Ramírez JA, Ridge KM, Sethi S, Suratt BT, Sznajder JI, Tsalik EL, Walkey AJ, Yende S, Aggarwal NR, Caler EV, and Mizgerd JP. Future research directions in pneumonia. NHLBI working group report. Am J Respir Crit Care Med 198: 256–263, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desideri A and Falconi M. Prokaryotic Cu,Zn superoxide dismutases. Biochem Soc Trans 31: 1322–1325, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Dietrich A, Steinritz D, and Gudermann T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 67: 123–137, 2017 [DOI] [PubMed] [Google Scholar]
- 33. Downs CA, Kumar A, Kreiner LH, Johnson NM, and Helms MN. H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8. J Biol Chem 288: 8136–8145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duane P, Rubins JB, Weisel H, and Janoff E. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun 61: 4392–4397, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eaton DC, Helms MN, Koval M, Bao HF, and Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Erttmann SF and Gekara NO. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat Commun 10: 3493, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fee JA. Regulation of sod genes in Escherichia coli: relevance to superoxide dismutase function. Mol Microbiol 5: 2599–2610, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Feldman C and Richards G. Appropriate antibiotic management of bacterial lower respiratory tract infections. F1000Res 7: F1000, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA, 3rd, and Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 303:L20–L32, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA, 3rd, and Natarajan R. Ascorbic acid attenuates lipopolysaccharide induced acute lung injury. Crit Care Med. 39: 1454–1460, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Flick MR, Milligan SA, Hoeffel JM, and Goldstein IM. Catalase prevents increased lung vascular permeability during air emboli in unanesthetized sheep. J Appl Physiol 64: 929–935, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Fowler AA, 3rd, Fisher BJ and Kashiouris MG. Vitamin C for sepsis and acute respiratory failure-reply. JAMA 25;323: 792–793, 2020 [DOI] [PubMed] [Google Scholar]
- 44. Fowler AA, 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Fisher BJ, and Natarajan R. Medical respiratory intensive care unit nursing. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 12: 32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fowler AA, 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR, 2nd, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, and Halquist M. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 322: 1261–1270, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao Y, Xu W, Dou X, Wang H, Zhang X, Yang S, Liao H, Hu X, and Wang H. Mitochondrial DNA leakage caused by Streptococcus pneumoniae hydrogen peroxide promotes type I IFN expression in lung cells. Front Microbiol 10: 630, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and etiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17: 1133–1161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gonzales JN, Lucas R, and Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med 2: pii: , 2015 [PMC free article] [PubMed] [Google Scholar]
- 49. González-Flecha B, Evelson P, Ridge K, and Sznajder JI. Hydrogen peroxide increases Na+/K(+)-ATPase function in alveolar type II cells. Biochim Biophys Acta 1290: 46–52, 1996 [PubMed] [Google Scholar]
- 50. Grousd JA, Rich HE, and Alcorn JF. Host-pathogen interactions in gram-positive bacterial pneumonia. Clin Microbiol Rev 32: e00107-18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grudzinska FS, Brodlie M, Scholefield BR, Jackson T, Scott A, Thickett DR, and Sapey E. Neutrophils in community-acquired pneumonia: parallels in dysfunction at the extremes of age. Thorax 75 [Epub ahead of print]; DOI: 10.1136/thoraxjnl-2018-212826, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haikarainen T, Thanassoulas A, Stavros P, Nounesis G, Haataja S, and Papageorgiou AC. Structural and thermodynamic characterization of metal ion binding in Streptococcus suis Dpr. J Mol Biol 405: 448–460, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Hajaj B, Yesilkaya H, Benisty R, David M, Andrew PW, and Porat N. Thiol peroxidase is an important component of Streptococcus pneumoniae in oxygenated environments. Infect Immun 80: 4333–4343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hajaj B, Yesilkaya H, Shafeeq S, Zhi X, Benisty R, Tchalah S, Kuipers OP, and Porat N. CodY regulates thiol peroxidase expression as part of the pneumococcal defense mechanism against H2O2 stress. Front Cell Infect Microbiol 7: 210, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamacher J, Hadizamani Y, Borgmann M, Mohaupt M, Männel DN, Moehrlen U, Lucas R, and Stammberger U. Cytokine-ion channel interactions in pulmonary inflammation. Front Immunol 8: 1644, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hamacher J, Stammberger U, Roux J, Kumar S, Yang G, Xiong C, Schmid RA, Fakin RM, Chakraborty T, Hossain HM, Pittet JF, Wendel A, Black SM, and Lucas R. The lectin-like domain of tumor necrosis factor improves lung function after rat lung transplantation-potential role for a reduction in reactive oxygen species generation. Crit Care Med 38: 871–878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hassler R, Scheuner DL, Wang S, Han J, Kodali VK, Li P, Nguyen J, George JS, Davis C, Wu SP, Bai Y, Sartor M, Cavalcoli J, Malhi H, Baudouin G, Zhang Y, Yates JR III, Itkin-Ansari P, Volkmann N, and Kaufman RJ. The IRE1α/XBP1s pathway is essential for the glucose response and protection of β cells. PLoS Biol 13: e1002277, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, and Holmes KK. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 174: 1058–1063, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Hecquet CM, Ahmmed GU, Vogel SM, and Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Helms MN, Jain L, Self JF, and Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Henriques-Normark B and Normark S. Commensal pathogens, with a focus on Streptococcus pneumoniae, and interactions with the human host. Exp Cell Res 316: 1408–1414, 2010 [DOI] [PubMed] [Google Scholar]
- 62. Hoskins J, Alborn WE Jr., Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matsushima P, McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH, O'Gara M, Peery RB, Robertson GT, Rockey P, Sun PM, Winkler ME, Yang Y, Young-Bellido M, Zhao G, Zook CA, Baltz RH, Jaskunas SR, Rosteck PR Jr., Skatrud PL, and Glass JI.. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol 183: 5709–5717, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hough RF, Islam MN, Gusarova GA, Jin G, Das S, and Bhattacharya J. Endothelial mitochondria determine rapid barrier failure in chemical lung injury. JCI Insight 4: pii: , 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu L, Zachariae ED, Larsen UG, Vilhardt F, and Petersen SV. The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response. Redox Biol 26: 101268, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hua CZ, Howard A, Malley R, and Lu YJ. Effect of nonheme iron-containing ferritin Dpr in the stress response and virulence of pneumococci. Infect Immun 82: 3939–3947, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, and Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 67. Kooijman EE, Kuzenko SR, Gong D, Best MD, and Folkesson HG. Phosphatidylinositol 4,5-bisphosphate stimulates alveolar epithelial fluid clearance in male and female adult rats. Am J Physiol Lung Cell Mol Physiol 301: L804–L811, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Krenn K, Lucas R, Croizé A, Boehme S, Klein KU, Hermann R, Markstaller K, and Ullrich R. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit Care 21: 194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kube D, Sontich U, Fletcher D, and Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Cell Mol Phyiol 280: L493–L502, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Lee AH, Iwakoshi NN, and Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leung AKC, Wong AHC, and Hon KL. Community-acquired pneumonia in children. Recent Pat Inflamm Allergy Drug Discov 12: 136–144, 2018 [DOI] [PubMed] [Google Scholar]
- 72. Li W, Long C, Renjun L, Zhangxue H, Yin H, Wanwei L, Juan M, and Yuan S. Association of SCNN1A single nucleotide polymorphisms with neonatal respiratory distress syndrome. Sci Rep 5: 17317, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liochev SI and Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med 42: 1465–1469, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Lisher JP, Tsui HT, Ramos-Montañez S, Hentchel KL, Martin JE, Trinidad JC, Winkler ME, and Giedroc DP. Biological and chemical adaptation to endogenous hydrogen peroxide production in Streptococcus pneumoniae D39. mSphere 2: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loose M, Hudel M, Zimmer KP, Garcia E, Hammerschmidt S, Lucas R, Chakraborty T, and Pillich H. Pneumococcal hydrogen peroxide-induced stress signaling regulates inflammatory genes. J Infect Dis 211: 306–316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lucas R, Echtenacher B, Sablon E, Juillard P, Magez S, Lou J, Donati Y, Bosman F, Van de Voorde A, Fransen L, Männel DN, Grau GE, and de Baetselier P. Generation of a mouse tumor necrosis factor mutant with anti-peritonitis and desensitization activities comparable to those of the wild type but with reduced systemic toxicity. Infect Immun 65: 2006–2010, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, Oseghale A, Verin AD, Chakraborty T, Matthay MA, Zemskov EA, White R, Block NL, and Schally AV. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci U S A 109: 2084–2089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lucas R, Verin AD, Black SM, and Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol 77: 1763–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lucas R, Yang G, Gorshkov BA, Zemskov EA, Sridhar S, Umapathy NS, Jezierska-Drutel A, Alieva IB, Leustik M, Hossain H, Fischer B, Catravas JD, Verin AD, Pittet JF, Caldwell RB, Mitchell TJ, Cederbaum SD, Fulton DJ, Matthay MA, Caldwell RW, Romero MJ, and Chakraborty T. Protein kinase C-α and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am J Respir Cell Mol Biol 47: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Luong TT, Kim EH, Bak JP, Nguyen CT, Choi S, Briles DE, Pyo S, and Rhee DK. Ethanol-induced alcohol dehydrogenase E (AdhE) potentiates pneumolysin in Streptococcus pneumoniae. Infect Immun 83: 108–119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lynn H, Sun X, Casanova N, Gonzales-Garay M, Bime C, and Garcia JGN. Genomic and genetic approaches to deciphering acute respiratory distress syndrome risk and mortality. Antioxid Redox Signal 31: 1027–1052, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malczyk M, Erb A, Veith C, Ghofrani HA, Schermuly RT, Gudermann T, Dietrich A, Weissmann N, and Sydykov A. The role of transient receptor potential channel 6 channels in the pulmonary vasculature. Front Immunol 8: 707, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Martín R and Suárez JE. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl Environ Microbiol 76: 400–405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matalon S, Bartoszewski R, and Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Matthay MA, Fukuda N, Frank J, Kallet R, Daniel B, and Sakuma T. Alveolar epithelial barrier. Role in lung fluid balance in clinical lung injury. Clin Chest Med 21: 477–490, 2000 [DOI] [PubMed] [Google Scholar]
- 88. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, and Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, and Paton JC. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7: e1002357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McLeod JW and Gordon J. Production of hydrogen peroxide by bacteria. Biochem J 16: 499–506, 1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Pétrilli V, Andrew PW, Kadioglu A, and Lavelle EC. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 6: e1001191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mishra S and Imlay J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch Biochem Biophys 525: 145–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mitzel DN, Lowry V, Shirali AC, Liu Y, and Stout-Delgado HW. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN-β production during Streptococcus pneumoniae infection. J Immunol 192: 4273–4283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Morozov GI, Porat N, Kushnir T, Najmuldeen H, Adawi A, Chalifa-Caspi V, Benisty R, Ohayon A, Liron O, Azriel S, Malka I, Dotan S, Portnoi M, Piotrowski AA, Kafka D, Hajaj B, Fishilevich T, Shagan M, Tal M, Ellis R, Morrison DA, Mitchell AM, Mitchell TJ, Dagan R, Yesilkaya H, and Nebenzahl YM. Flavin reductase contributes to pneumococcal virulence by protecting from oxidative stress and mediating adhesion and elicits protection against pneumococcal challenge. Sci Rep 8: 314, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Morty RE and Kuebler WM. TRPV4: an exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol 307: L817–L821, 2014 [DOI] [PubMed] [Google Scholar]
- 96. Muchnik L, Adawi A, Ohayon A, Dotan S, Malka I, Azriel S, Shagan M, Portnoi M, Kafka D, Nahmani H, Porgador A, Gershoni JM, Morrison DA, Mitchell A, Tal M, Ellis R, Dagan R, and Nebenzahl YM. NADH oxidase functions as an adhesin in Streptococcus pneumoniae and elicits a protective immune response in mice. PLoS One 13: e61128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ngocho JS, Magoma B, Olomi GA, Mahande MJ, Msuya SE, de Jonge MI, and Mmbaga BT. Effectiveness of pneumococcal conjugate vaccines against invasive pneumococcal disease among children under five years of age in Africa: a systematic review. PLoS One 14: e0212295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ong CL, Potter AJ, Trappetti C, Walker MJ, Jennings MP, Paton JC, and McEwan AG. Interplay between manganese and iron in pneumococcal pathogenesis: role of the orphan response regulator RitR. Infect Immun 81: 421–429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, and Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paterson GK, Blue CE, and Mitchell TJ. An operon in Streptococcus pneumoniae containing a putative alkylhydroperoxidase D homologue contributes to virulence and the response to oxidative stress. Microb Pathog 40: 152–160, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Paudel S, Baral P, Ghimire L, Bergeron S, Jin L, DeCorte JA, Le JT, Cai S, and Jeyaseelan S. CXCL1 regulates neutrophil homeostasis in pneumonia-derived sepsis caused by Streptococcus pneumoniae serotype 3. Blood 133: 1335–1345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pearse DB, Shimoda LA, Verin AD, Bogatcheva N, Moon C, Ronnett GV, Welsh LE, and Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium 10: 309–317, 2003 [DOI] [PubMed] [Google Scholar]
- 103. Pericone CD, Overweg K, Hermans PW, and Weiser JN. Inhibitory and bactericidal effects of Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 68: 3990–3997, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pericone CD, Park S, Imlay JA, and Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol 185: 6815–6825, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Petry A, Zhang Z, Trautz B, Rieß F, and Görlach A. Cross talk between p22phox and ATF4 in the endothelial unfolded protein response. Antioxid Redox Signal 30: 40–55, 2019 [DOI] [PubMed] [Google Scholar]
- 106. Pillich H, Loose M, Zimmer K, and Chakraborty T. Activation of the unfolded protein response by Listeria monocytogenes. Cell Microbiol 14: 949–964, 2012 [DOI] [PubMed] [Google Scholar]
- 107. Piñas GE, Reinoso-Vizcaino NM, Yandar Barahona NY, Cortes PR, Duran R, Badapanda C, Rathore A, Bichara DR, Cian MB, Olivero NB, Perez DR, and Echenique J. Crosstalk between the serine/threonine kinase StkP and the response regulator ComE controls the stress response and intracellular survival of Streptococcus pneumoniae. PLoS Pathog 14: e1007118, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pochynyuk O, Tong Q, Staruschenko A, Ma HP, and Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006 [DOI] [PubMed] [Google Scholar]
- 109. Rai P, Parrish M, Tay IJ, Li N, Ackerman S, He F, Kwang J, Chow VT, and Engelward BP. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc Natl Acad Sci U S A 112: E3421–E3430, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Raju S, Painter R, Bagby G, Nelson S, and Wang G. Response of differentiated human airway epithelia to alcohol exposure and Klebsiella pneumoniae challenge. Med Sci 1: 2–19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rubins JB, Duane PG, Clawson D, Charboneau D, Young J, and Niewoehner DE. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun 61: 1352–1358, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rzymski T, Petry A, Kračun D, Rieß F, Pike L, Harris AL, and Görlach A. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 31: 3621–3634, 2012 [DOI] [PubMed] [Google Scholar]
- 113. Sakaguchi R and Mori Y. Transient receptor potential (TRP) channels: biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med 19: 30701–30704, 2019 [DOI] [PubMed] [Google Scholar]
- 114. Schmeck B, N'Guessan PD, Ollomang M, Lorenz J, Zahlten J, Opitz B, Flieger A, Suttorp N, and Hippenstiel S. Legionella pneumophila induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. Eur Respir J 29: 25–33, 2006 [DOI] [PubMed] [Google Scholar]
- 115. Seeger W, Hansen T, Rössig R, Schmehl T, Schütte H, Krämer HJ, Walmrath D, Weissmann N, Grimminger F, and Suttorp N. Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability-effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res 50: 1–17, 1995 [DOI] [PubMed] [Google Scholar]
- 116. Shlyonsky V, Boom A, and Mies F. Hydrogen peroxide and sodium transport in the lung and kidney. Biomed Res Int 2016: 9512807, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Simmons S, Erfinanda L, Bartz C, and Kuebler WM. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J Physiol 597: 997–1021, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idanpaan-Heikkila I, Rosenow C, and Masure HR. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol 19: 803–813, 1996 [DOI] [PubMed] [Google Scholar]
- 119. Subramanian K, Henriques-Normark B, and Normark S. Emerging concepts in the pathogenesis of the Streptococcus pneumoniae: from nasopharyngeal colonizer to intracellular pathogen. Cell Microbiol 11: e13077, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Suresh K, Servinsky L, Reyes J, Undem C, Zaldumbide J, Rentsendorj O, Modekurty S, Dodd-O JM, Scott A, Pearse DB, and Shimoda LA. CD36 mediates H2O2-induced calcium influx in lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 312: L143–L153, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Syk A, Norman M, Fernebro J, Gallotta M, Farmand S, Sandgren A, Normark S, and Henriques-Normark B. Emergence of hypervirulent mutants resistant to early clearance during systemic serotype 1 pneumococcal infection in mice and humans. J Infect Dis 210: 4–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Taniai H, Iida K, Seki M, Saito M, Shiota S, Nakayama H, and Yoshida S. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source. J Bacteriol 190: 3572–3579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith H, Venter J, Dougherty BA, Morrison DA, Hollingshead SK, and Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293: 498–506, 2001 [DOI] [PubMed] [Google Scholar]
- 124. Tseng HJ, McEwan AG, Paton JC and Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun 70: 1635–1639, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, and Walker MJ. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 6: e00278-15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vadász I and Lucas R. Editorial: cytokine-ion channel interactions in pulmonary inflammation. Front Immunol 9: 2598, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vadász I and Sznajder JI. Gas exchange disturbances regulate alveolar fluid clearance during acute lung injury. Front Immunol 8: 757, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Walker JA, Allen RL, Falmagne P, Johnson MK, and Boulnois GJ. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun 55: 1184–1189, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Walter JM, Ren Z, Yacoub T, Reyfman PA, Shah RD, Abdala-Valencia H, Nam K, Morgan VK, Anekalla KR, Joshi N, McQuattie-Pimentel AC, Chen CI, Chi M, Han S, Gonzalez-Gonzalez FJ, Soberanes S, Aillon RP, Watanabe S, Williams KJN, Lu Z, Paonessa J, Hountras P, Breganio M, Borkowski N, Donnelly HK, Allen JP, Amaral LA, Bharat A, Misharin AV, Bagheri N, Hauser AR, Budinger GRS, and Wunderink RG. Multidimensional assessment of the host response in mechanically ventilated patients with suspected pneumonia. Am J Respir Crit Care Med 199: 1225–1237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wandall-Frostholm C, Dalsgaard T, Bajoriunas V, Olivan-Viguera A, Sadda V, Beck L, Mogensen S, Stankevicius E, Simonsen U, and Kohler R. Genetic deficit of KCa 3.1 channels protects against pulmonary circulatory collapse induced by TRPV4 channel activation. Br J Pharmacol 172, 4493–4505, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang HC, Zentner MD, Deng HT, Kim KJ, Wu R, Yang PC, and Ann DK. Oxidative stress disrupts glucocorticoid hormone-dependent transcription of the amiloride-sensitive epithelial sodium channel alpha-subunit in lung epithelial cells through ERK-dependent and thioredoxin-sensitive pathways. J Biol Chem 275: 8600–8609, 2000 [DOI] [PubMed] [Google Scholar]
- 132. Ware LB and Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 133. Weibel ER. Lung morphometry: the link between structure and function. Cell Tissue Res 367: 413–426, 2017 [DOI] [PubMed] [Google Scholar]
- 134. Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos Y, Schnitzler M, Brandes RP, Grimminger F, Meissner M, Freichel M, Offermanns S, Veit F, Pak O, Krause KH, Schermuly RT, Brewer AC, Schmidt HH, Seeger W, Shah AM, Gudermann T, Ghofrani HA, and Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun 3: 649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. West JB. Role of the fragility of the pulmonary blood-gas barrier in the evolution of the pulmonary circulation. Am J Physiol Regul Integr Comp Physiol 304: R171–R176, 2013 [DOI] [PubMed] [Google Scholar]
- 136. Winterbourn CC, Kettle AJ, and Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem 85: 765–792, 2016 [DOI] [PubMed] [Google Scholar]
- 137. Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, and Schütte H. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med 34: 1947–1954, 2006 [DOI] [PubMed] [Google Scholar]
- 138. Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, and Kaufman RJ. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13: 351–364, 2007 [DOI] [PubMed] [Google Scholar]
- 139. Wu X, Gordon O, Jiang W, Antezana BS, Angulo-Zamudio UA, Del Rio C, Moller A, Brissac T, Tierney ARP, Warncke K, Orihuela CJ, Read TD, and Vidal JE. Interaction between Streptococcus pneumoniae and Staphylococcus aureus generates •OH radicals that rapidly kill Staphylococcus aureus strains. J Bacteriol 201: e00474-19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wuerges J, Lee JW, Yim YI, Yim HS, Kang SO, and Djinovic Carugo K. Crystal structure of nickel-containing superoxide dismutase reveals another type of active site. Proc Natl Acad Sci U S A 101: 8569–8574, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu H and Chu S. ENaC alpha-subunit variants are expressed in lung epithelial cells and are suppressed by oxidative stress. Am J Physiol Lung Cell Mol Physiol 293: L1454–L1462, 2007 [DOI] [PubMed] [Google Scholar]
- 142. Yesilkaya H, Andisi VF, Andrew PW, and Bijlsma JJ. Streptococcus pneumoniae and reactive oxygen species: an unusual approach to living with radicals. Trends Microbiol 21: 187–195, 2013 [DOI] [PubMed] [Google Scholar]
- 143. Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, and Andrew PW. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun 68: 2819–2826, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yonezawa R, Yamamoto S, Takenaka M, Kage Y, Negoro T, Toda T, Ohbayashi M, Numata T, Nakano Y, Yamamoto T, Mori Y, Ishii M, and Shimizu S. TRPM2 channels in alveolar epithelial cells mediate bleomycin-induced lung inflammation. Free Radic Biol Med 90: 101–113, 2016 [DOI] [PubMed] [Google Scholar]
- 145. Yoshida H, Matsui T, Yamamoto A, Okada T, and Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 146. Yu J, Bryant AP, Marra A, Lonetto MA, Ingraham KA, Chalker AF, Holmes DJ, Holden D, Rosenberg M, and McDevitt D. Characterization of the Streptococcus pneumoniae NADH oxidase that is required for infection. Microbiol 147: 431–438, 2001 [DOI] [PubMed] [Google Scholar]
- 147. Yue G, Malik B, Yue G and Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]
- 148. Zhang K and Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279: 25935–25938, 2004 [DOI] [PubMed] [Google Scholar]