Abstract
Ju, Jia-Der, Cristian Zhang, Francis P. Sgambati, Lidia M. Lopez, Luu V. Pham, Alan R. Schwartz, and Roberto A. Accinelli. Acute altitude acclimatization in young healthy volunteers: nocturnal oxygenation increases over time whereas periodic breathing persists. High Alt Med Biol. 22:14–23, 2021.
Study Objectives: This study aimed to examine the acute effects of high altitude (HA) on sleep disordered breathing (sleep apnea and nocturnal hypoxemia) and acute mountain sickness and to characterize acclimatization over time.
Methods: Ten native lowlanders residing at sea level (SL) completed the Lake Louise Score (LLS) and underwent nocturnal polygraphy (ApneaLink Plus) for nine consecutive nights (N1–N9) at HA (2,761 m) and two nights before and after HA. Nocturnal oxygen profiles were assessed by measuring the mean nocturnal oxyhemoglobin saturation (SpO2) during sleep, and sleep apnea severity as assessed by measuring the Apnea–Hypopnea Index (AHI). Mixed-effects linear regression was used to model responses in outcomes (mean nocturnal SpO2, logAHI, and LLS) between HA and SL. Changes in SpO2 and AHI were examined in subgroups with mild versus marked nocturnal SpO2 and low versus high AHI during exposure to HA and compared between subgroups.
Results: Compared with SL, the mean nocturnal SpO2 was lower (p < 0.0001) and AHI was higher (p < 0.0001) at HA. The mean nocturnal SpO2 increased progressively (p < 0.001), whereas AHI remained high (p < 0.978) and relatively unchanged over nine successive nights at HA. Those with markedly reduced SpO2 upon arrival at HA exhibited progressive increases in the mean nocturnal SpO2 over time at HA compared with those with mild nocturnal desaturation. LLS rose at HA, but no differences were observed between subgroups.
Conclusions: In healthy HA sojourners, the mean nocturnal SpO2 increased progressively over time, whereas AHI remained elevated, suggesting distinctive phenotypes and acclimatization responses to HA.
Keywords: high altitude, sleep, sleep disordered breathing
Introduction
In an increasingly global society, millions of people travel to higher altitude for a variety of reasons. Most are acutely exposed to high altitude (HA) for several days or weeks at a time as is the case with tourists, hikers, miners, transport drivers, and office workers. The prevalence of acute mountain sickness (AMS) varies from 25% to 90% in lowlanders who are acutely exposed to HA, depending on the rate of ascent and the altitude reached (Gonggalanzi et al., 2016; Luks et al., 2017), with symptoms of AMS varying from mild to life-threatening, sometimes requiring immediate descent. For many years, scientists have tried to elucidate factors predisposing to AMS, although relatively little is known about the severity and time course for adaptation to HA.
HA illness can be classified as acute or chronic, depending on the time of exposure to the hypoxic environment. AMS is the most common acute HA illness, and it occurs in unacclimatized individuals. It is characterized by a combination of symptoms including headache, gastrointestinal symptoms, fatigue, and sleep disturbances (Roach et al., 1993). The latter leads to poor quality of sleep, which is considered a risk factor for AMS (Tang et al., 2014). Heinrich et al. (2019) provided evidence that treating sleep disordered breathing (SDB) at HA lessens AMS, suggesting that oxygen desaturation and sleep disruption play a role in the pathogenesis of this syndrome. It has been suggested that progesterone and estrogen can stabilize ventilatory control by increasing cerebral blood flow, making it easier for women to acclimatize compared with men (Lombardi et al., 2013).
Susceptibility to AMS may be related to differences in ventilatory drive, with studies demonstrating both increases and decreases in hypoxic/hypercapnic sensitivity (Erba et al., 2004). Nevertheless, acclimatization to acute HA exposure is characterized by a steady increase in PaO2 (partial pressure of oxygen, arterial) and decrease in PaCO2 (partial pressure of carbon dioxide, arterial), reflecting an increase in peripheral chemosensitivity and hyperventilation. Alterations in gas exchange may last from days to weeks, and the degree of response will depend on the altitude reached (Schoene, 1997; Ainslie et al., 2013; San et al., 2013).
Sleep is associated with decreased ventilatory drive with nocturnal hypoventilation and a reduction of the mean nocturnal oxyhemoglobin saturation (SpO2), which might also predispose to AMS. Under isocapnic conditions, the hypoxic ventilatory response (HVR) is blunted during all sleep stages compared with wakefulness, especially during rapid-eye movement (REM) sleep (White et al., 1987). Hypoxemia is also known to destabilize breathing patterns during sleep, leading to central apneas and hypopneas with intermittent desaturation and arousals from sleep at HA (Reite et al., 1975; Weil, 2004). HA is independently associated with an increase in the prevalence and severity of SDB consisting of sustained nocturnal hypoxemia and/or recurrent central apneas and hypopneas (Pham et al., 2017a). Nonetheless, the severity and time course of HA acclimatization in SDB have not been well characterized.
The major goal of the present study was to examine the effects of HA on SDB (sleep apnea and nocturnal hypoxemia) and to examine the extent to which the study participants acclimatized over time. We hypothesized that participants would develop SDB at HA and would acclimatize progressively during their sojourn. We also explored whether SDB predicted symptoms of AMS. To minimize potential confounding factors, we designed a time course study of SDB parameters to compare differences within participants between baseline sea level (SL) and HA exposure over 9 days.
Methods
Participants
A prospective observational study was conducted in 10 young healthy lowlanders. We enrolled volunteers in Lima, Peru, who agreed to participate in a Cook Stove Mitigation Project at HA (Ayacucho, Peru). Inclusion criteria included age >18 years, born and residing at SL, and without any history of sleep disorders, pulmonary or cardiovascular disease. Ten participants met the inclusion and exclusion criteria, based on medical history and general physical examination conducted by the investigators. Informed consent was obtained for a protocol that was approved by the Universidad Peruana Cayetano Heredia Institutional Ethics Review Board in Lima, Peru.
Study setting and design
The present study was an observational study of acute HA exposure bracketed by measurements at SL. Ten healthy lowlanders aged 19–27 years were evaluated before, during, and after a 9-day journey from SL 154 m (Lima, Peru) to an altitude of 2,761 m (Ayacucho, Peru). Subjects were studied in their homes at SL in the Lima metropolitan area. Participants traveled to Ayacucho in a short flight of <1 hour in duration. Participants followed the same daily routine and ate a normocaloric balanced diet. Participants did not consume any prophylactic medication for HA illness, sleep aids, caffeinated or alcoholic beverages or products.
Study procedures and protocols
Anthropometrics
Measurements of age, weight, height, neck, waist and hip circumference, and Mallampati score were performed by the same examiner. Body mass index (BMI) and waist-to-hip ratio were calculated (Table 1).
Table 1.
General Characteristics of the Study Participants
| Characteristic | Entire group | Female | Male |
|---|---|---|---|
| Participants | 10 | 6 | 4 |
| Age (years) | 22.5 (21.0–24.5) | 22.0 (21.0–25.0) | 21.0 (20.0–22.0) |
| Anthropometrics | |||
| Height (m) | 1.66 (1.60–1.78) | 1.60 (1.58–1.64) | 1.78 (1.69–1.87) |
| Weight (kg) | 60.0 (57.0–76.5) | 57.0 (53.0–58.0) | 75.0 (62.0–78.0) |
| BMI (kg/m2) | 21.71 (20.95–24.62) | 20.95 (20.44–23.23) | 21.71 (21.45–24.62) |
| Neck circumference (cm) | 33.0 (31.0–38.5) | 31.0 (30.0–32.0) | 38.0 (33.0–42.0) |
| Waist circumference (cm) | 80.5 (70.0–88.5) | 70.0 (70.0–79.0) | 86.0 (84.0–91.0) |
| Hip circumference (cm) | 89.0 (85.0–94.5) | 84.0 (79.0–91.0) | 89.0 (86.0–94.0) |
| Waist-to-hip | 0.90 (0.85–0.97) | 0.88 (0.83–0.89) | 0.96 (0.96–0.97) |
| Mallampati score | 2.0 (1.5–3.0) | 1.5 (1.0–3.0) | 2.0 (2.0–3.0) |
| Wakefulness SpO2 | |||
| SL | 98.5 (98.0–98.5) | 98.5 (98.0–98.5) | 98.5 (98.0–98.5) |
| HA | 94.0 (92.5–95.0) | 94.0 (93.0–95.0) | 93.3 (92.0–95.0) |
| Sleep parameters | |||
| Mean nocturnal saturation (%) | |||
| SL | 95.7 (95.1–96.2) | 95.9 (95.5–96.2) | 94.9 (94.8–95.7) |
| HA | 86.9 (84.7–88.9) | 88.9 (87.6–90.3) | 88.3 (85.6–90.0) |
| AHI (events per hour) | |||
| SL 3% + arousals | 1.4 (0.8–3.0) | 1.6 (0.8–2.7) | 1.1 (0.8–4.6) |
| SL 4% | 0.7 (0.4–1.5) | 1.3 (0.4–1.6) | 0.5 (0.4–0.7) |
| HA 3% + arousals | 10.3 (5.7–15.4) | 6.3 (3.8–11.4) | 8.4 (3.7–14.2) |
| HA 4% | 4.7 (2.2–7.8) | 2.7 (1.6–5.6) | 3.3 (1.7–5.8) |
Data are presented as n or median (interquartile range). The mean nocturnal saturation: mean nocturnal SpO2.
AHI, Apnea–Hypopnea Index; BMI, body mass index; HA, high altitude; SL, sea level; SpO2, oxyhemoglobin saturation.
Questionnaires
Participants completed the Lake Louise Score (LLS) survey each day, and the overall score was computed.
SDB assessment
Participants underwent unattended nocturnal recordings of nasal airflow, thoracic excursion, and pulse oximetry (ApneaLink Plus; ResMed, Ltd., San Diego, CA) for two nights at SL, followed by nine nights at HA and two nights following descent to SL. Recordings were conducted between the hours of ∼23:00 until 06:00 hours. Before their first night of study, participants attended a practice session with an instructional video demonstrating how to deploy the device. These devices were initialized on a single computer the morning before each nocturnal recording. Participants were instructed to wear and activate the devices at bedtime and to remove their devices after final awakening. A minimum of 3 hours of recording time was required for the recording to be included in the study. A total of 116 sleep recordings were obtained between SL and HA, of which 98 had an adequate recording time.
Recordings were scored and reviewed at the Johns Hopkins Sleep Disorders Center. Sleep–wake state was estimated in 30-second epochs. Each epoch was considered to be asleep unless movement artifact in physiological signals was present for ≥50% of the epoch.
Recording analysis and definitions
The mean nocturnal saturation was defined as the average SpO2, excluding periods of movement artifact, likely indicating wakefulness.
The Apnea–Hypopnea Index (AHI) was defined as the number of apneas and hypopneas per hour of recording time, which were scored and classified in accordance with American Academy of Sleep Medicine (AASM) guidelines. Apneas were defined as the absence of flow for ≥10 seconds and were further classified as obstructive, central, or mixed in accordance with standard criteria. Hypopnea was defined by a 30% reduction in the nasal pressure signal that was accompanied either by 3% (2016 AASM guidelines) or arousal, or by a 4% desaturation with or without an arousal. We further subclassified hypopneas into obstructive and central events in accordance with the 2016 AASM guidelines. Hypopneas were classified as obstructive if they exhibited any of the following: (1) snoring during the event, (2) increased inspiratory flattening of the nasal pressure compared to baseline, or (3) paradoxical thoracoabdominal movements that coincided with event onset. The remaining hypopneas were designated as central rather than obstructive (Berry et al., 2016).
Arousals were defined by movement or swallowing artifact in at least two channels (airflow, SpO2, and respiratory effort) for at least 3 seconds (Kushida et al., 2001; Pham et al., 2017a, 2017b). Of note, the sixth night at HA (8th day of the overall study) was not included in the data analysis because participants slept at a higher altitude on that night (2,970 m rather than 2,750 m).
The LLS
The LLS was used to determine the severity of AMS. This condition is defined as a total score of 3 or more points in the LLS, including at least 1 point from headache. Gastrointestinal symptoms, dizziness, sleep disturbances, and fatigue are also components of the score. The severity of these symptoms ranged from absent (score equivalent to 0) to severe (score of 3).
Data analysis
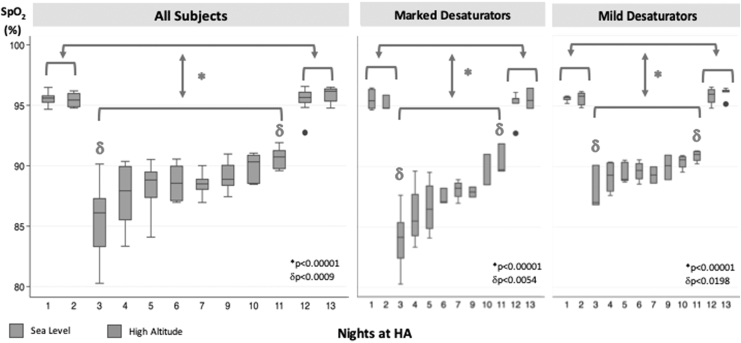
Statistical analyses were designed to test our primary hypotheses examining the effect of altitude and acclimatization (days at HA) on nocturnal oxygenation (mean nocturnal SpO2), sleep apnea, and AMS symptoms (LLS). AHI was log-transformed to normalize the distribution and minimize undue influence of outliers on linear regression analysis. The term AHI is used to indicate logAHI for simplicity in the Results and Discussion sections. Mixed-effects linear regression was used to examine responses in outcome variables (mean nocturnal SpO2, logAHI) to fixed factors (HA vs. SL, and time spent at HA over nine consecutive nights), while accounting for random variation in these outcomes among the participants (XTMixed; Stata, Inc., StataCorp LP, College Station, TX). This procedure allowed us to model changes in outcomes by altitude and by the number of nights spent at HA in sequential models. As noted, mixed-effects linear regression was utilized to test a priori hypotheses that the SDB parameters, mean nocturnal SpO2, and logAHI (1) changed at HA compared with SL and that (2) these parameters changed progressively over time while at altitude. The Spearman rank correlation was also used to characterize the association between the mean nocturnal SpO2 and logAHI in the entire group and in subgroups of marked and mild desaturators (see Fig. 2 for more details).
FIG. 2.
Mean nocturnal SpO2 over the study time course. In each panel, the mean nocturnal SpO2 versus study night before, during, and after high altitude exposure for the entire group (all subjects, left panel) and for subgroups of marked (middle panel) and mild desaturators (right panel), based on median split of the mean nocturnal SpO2 values during the first two nights at HA. Night 8 was not considered for the analysis because subjects slept at a higher altitude during that night (from 2,750 to 2,970 m). *, Mean nocturnal SpO2 sea level versus high altitude; δ, mean nocturnal SpO2, first versus last night at HA. See results section, “Effect of altitude and acclimatization on nocturnal oxyhemoglobin saturation profile.” SpO2, oxyhemoglobin saturation.
In post hoc analyses, we examined potential sources of variability in the mean nocturnal SpO2 and logAHI at HA. In these analyses, subjects were divided into groups based on median values for: (1) the mean nocturnal SpO2 and (2) logAHI on night 1 and 2 at HA as follows. First, we studied acclimatization responses at HA by characterizing the time course in outcome variables (mean nocturnal SpO2 and logAHI) separately within the marked and mild desaturation subgroups. Second, we compared differences in the mean nocturnal SpO2 response between subgroups with low and high logAHI over the time of exposure at HA. Third, we examined anthropometric and demographic differences between subgroups that could account for differences observed in outcome variables. Nonparametric Mann–Whitney rank sum tests were used to compare parameters between subgroups with marked/mild nocturnal desaturation and low/high logAHI.
Respiratory events and sleep parameters for the entire group and post hoc subgroups are presented as median (interquartile range) (Table 2). Analyses were conducted using STATA (version 14.1; Universidad Peruana Cayetano Heredia, Lima, Peru). Significance was inferred for p < 0.05.
Table 2.
Respiratory and Sleep Parameters in Study Participants
| Entire group | Nocturnal saturation subgroups |
AHI subgroups |
|||||
|---|---|---|---|---|---|---|---|
| Marked | Mild | p | Low | High | p | ||
| Participants | 10 | 5 | 5 | 5 | 5 | ||
| Wakefulness SpO2 | |||||||
| SL | 98.5 (98.0–98.5) | 98.5 (98.0–98.5) | 98.5 (98.0–98.5) | 0.78992 | 98.5 (98.0–98.5) | 98.5 (98.0–98.5) | 0.1253 |
| HA | 94.0 (92.5–95.0) | 94.0 (92.5–95.0) | 94.0 (93.0–95.0) | 0.7316 | 93.5 (92.0–94.5) | 95.0 (93.0–95.0) | 0.0933 |
| Sleep parameters | |||||||
| Mean nocturnal saturation (%) | |||||||
| SL | 95.7 (95.1–96.2) | 95.5 (94.8–96.0) | 95.8 (95.2–96.2) | 0.1 | 95.8 (95.3–96.4) | 95.7 (94.8–96.1) | 0.14 |
| HA | 86.9 (84.7–88.9) | 85.2 (83.3–85.8) | 88.3 (87.0–90.3) | 0.0095 | 84.7 (82.9–88.6) | 87.3 (86.3–89.2) | 0.14 |
| AHI (events per hour) | |||||||
| SL 3% + arousals | 1.40 (0.80–3.00) | 0.85 (0.65–1.30) | 2.40 (1.10–3.30) | 0.0275 | 0.90 (0.65–2.25) | 2.30 (1.10–3.30) | 0.0973 |
| SL 4% | 0.70 (0.40–1.50) | 0.45 (0.25–0.65) | 1.50 (0.70–2.30) | 0.0002 | 0.50 (0.40–0.75) | 1.50 (0.40–2.30) | 0.02 |
| HA 3% + arousals | 10.30 (5.70–15.40) | 5.90 (2.80–13.20) | 14.90 (8.20–15.40) | 0.24 | 5.70 (2.60–8.15) | 15.15 (11.70–16.15) | 0.0207 |
| HA 4% | 4.65 (2.20–7.80) | 2.70 (2.00–4.30) | 9.80 (5.00–12.60) | 0.0172 | 2.20 (1.55–2.95) | 7.80 (5.65–11.45) | 0.0008 |
| Hypopnea index | |||||||
| SL | 0.54 (0.17–1.17) | 0.34 (0.08–0.73) | 0.58 (0.33–1.71) | 0.44 | 0.34 (0.08–1.13) | 0.68 (0.33–1.17) | 0.29 |
| HA | 5.68 (4.06–9.30) | 5.39 (2.54–10.11) | 5.98 (4.51–7.72) | 0.79 | 5.08 (2.47–7.75) | 7.57 (5.24–9.30) | 0.25 |
| % Central hypopneaa | |||||||
| SL | 50% (25%–88%) | 25% (0%–50%) | 81% (50%–100%) | 0.0001 | 25% (0%–50%) | 72% (50%–97%) | 0.01 |
| HA | 86% (72%–96%) | 79% (60%–88%) | 90% (80%–97%) | 0.071 | 86% (73%–95%) | 82% (60%–96%) | 0.426 |
| Apnea indexb | |||||||
| SL | 0.70 (0.40–1.50) | 0.40 (0.25–0.50) | 1.30 (0.70–2.00) | 0.0002 | 0.45 (0.30–0.75) | 1.20 (0.40–2.00) | 0.0167 |
| HA | 1.75 (0.40–5.00) | 0.50 (0.30–1.40) | 6.80 (2.20–11.30) | 0.005 | 0.40 (0.30–0.65) | 5.00 (2.45–9.50) | 0.0011 |
Data are presented as n or median (interquartile range). The mean nocturnal saturation: mean nocturnal SpO2.
Remaining hypopneas were all obstructive.
All the apneas at SL and HA were central.
Results
Patient characteristics
All subjects were healthy lowlanders (4 males, 6 females). Six were Hispanic, three were Asian, and one was Caucasian. Anthropometric, demographic, and sleep parameters (HA and SL) for the entire group are presented as medians (interquartile range) (Table 1). Subjects were young adults of normal BMI, and BMI and waist-to-hip ratio were slightly higher in men compared with women. These participants did not have significant sleep apnea or nocturnal oxyhemoglobin desaturation at SL, but both metrics worsened at HA for the entire group and for subgroups of men and women.
Nocturnal oximetry profiles at HA
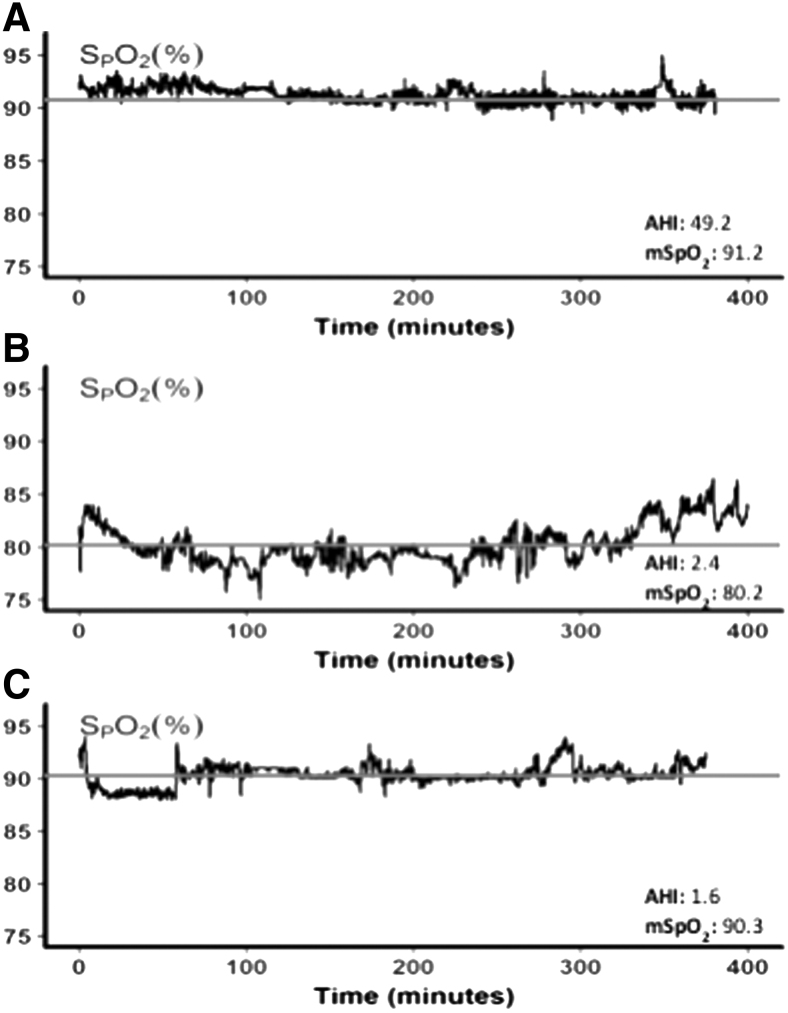
In Figure 1, representative nocturnal oximetry profiles illustrate three distinct patterns observed in normal subjects at HA. In top panel (A), a subject demonstrated a relatively high mean SpO2 with frequent small intermittent desaturations. In middle panel (B), the subject exhibited an overall reduction in SpO2 and low AHI. Conversely, the subject in bottom panel (C) had a relatively high mean nocturnal SpO2 but no significant sleep apnea. Thus, various combinations of nocturnal hypoxemia and sleep apnea severity were observed at HA.
FIG. 1.
Illustrative nocturnal oxygen profiles at high altitude. SpO2 versus time (hours) for illustrative nocturnal sleep recordings during the first night at HA. Top panel (A) illustrates the trace of a representative subject with intermittent hypoxemia with relatively high mean SpO2. Middle and bottom panels (B, C) exhibit different nocturnal SpO2 tracings of representative subjects from subgroups with marked and mild reductions in mean SpO2 and no significant sleep apnea, respectively. Red line shows the mean nocturnal SpO2 during the night. AHI values are reported for each tracing. AHI, Apnea–Hypopnea Index; HA, high altitude; SpO2, oxyhemoglobin saturation.
Sleep parameters
Estimates of total sleep time (defined as total recording time minus movement time) were not significantly different between SL and HA (316 vs. 310 minutes, respectively) (p = 0.88); nor did total sleep time differ between subgroups with marked versus mild mean nocturnal SpO2 (303 vs. 319 minutes, respectively) (p = 0.22) and high versus low AHI (319 vs. 304 minutes, respectively) (p = 0.37). Sleep parameters and respiratory events are presented for the entire group by sex and by subgroups (interquartile range) (Tables 1 and 2). The mean nocturnal saturation for the entire group was 95.7% (95.1%–96.2%) and 86.9% (84.7%–88.9%) at SL and HA, respectively. The AHI (defined by SpO2 drop of ≥4% or movement arousals) for the entire group at SL and HA was 0.7 (0.4–1.5) and 4.7 (2.2–7.8) events per hour, respectively, which mainly consisted of central apneas. No significant differences were found when comparing sleep parameters by sex, except for a greater mean nocturnal SpO2 at SL in women than in men (Table 1).
AHI event duration was longer in males than in females at SL (19.0 vs. 15.5 seconds, p < 0.05), but this difference did not persist at HA (15.9 vs. 15.9 seconds, p = n.s.). The males exhibited significant reductions in AHI duration when they ascended from SL to HA (19.0 vs. 15.9 seconds, p = 0.006), but not the females.
Effect of altitude and acclimatization on nocturnal oxyhemoglobin saturation profile
The mean nocturnal SpO2 did not differ between SL nights before and after the HA sojourn. Compared with SL, the mean nocturnal SpO2 for the entire group was significantly lower during the first night at HA (−8.8 ± 0.6%, p < 0.0001) but increased progressively over time at HA (1.5 ± 0.2% per night at HA, p < 0.0001) (Fig. 2, left panel).
Nonetheless, marked variability in the mean nocturnal SpO2 was observed among subjects on the first two nights at HA, but this variability decreased over time. To examine the source of this variability, we noted in post hoc analyses that the mean nocturnal SpO2 increased progressively in both marked and mild desaturation subgroups but rose faster in the marked versus mild desaturators (2.3 ± 0.3 vs. 0.8 ± 0.2% per night at HA, p < 0.0001) (Fig. 2, middle and right panel). The average mean nocturnal SpO2 at HA in marked and mild desaturators was 85.2% and 88.3%, respectively (p < 0.01). Compared with the mild desaturators, marked desaturators were predominantly Asian (n = 3) and male (n = 3) but did not differ in BMI or age (p < n.s.).
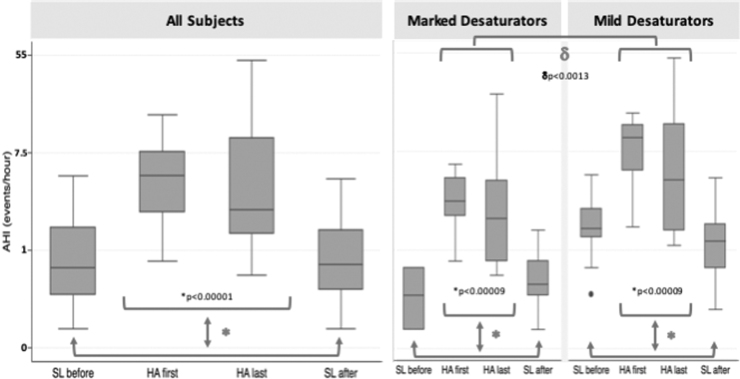
Effect of altitude and acclimatization on sleep apnea
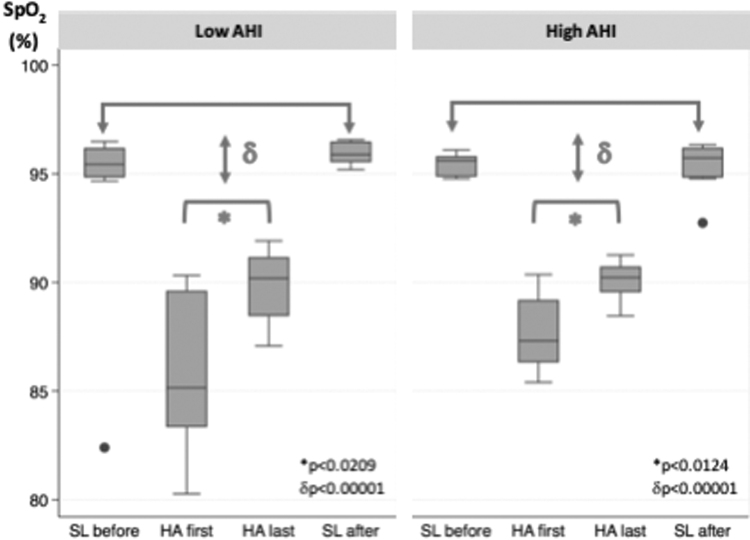
Compared with SL, AHI was significantly higher at HA for the entire group (p < 0.00001). Unlike the mean nocturnal SpO2, AHI did not change at HA over time (Fig. 3, left panel). Of note, marked variability in AHI was also observed among subjects, which prompted a post hoc analysis comparing characteristics of subgroups with low versus high AHI on nights 1 and 2 at HA (Fig. 3, middle and right panel). In both subgroups, AHI did not change significantly over the entire duration of exposure to HA; nor did the mean nocturnal SpO2 differ between AHI subgroups at the end of the HA exposure period (Fig. 4). Subjects with low AHI were predominantly female (M:F 1:3) and did not differ in BMI or age from those with a high AHI (M:F 3:3) at HA (p = n.s.) (Table 2).
FIG. 3.
AHI over the study time course. In each panel, AHI versus study night before, during, and after high altitude exposure for the entire group (all subjects, left panel) and for subgroups of marked (middle panel) and mild desaturators (right panel), based on median split of AHI values during the first two nights at HA. *, AHI sea level versus AHI high altitude; δ, AHI high altitude first night (HA first) versus high altitude last night (HA last); μ, AHI high altitude deep desaturators versus high altitude mild desaturators. See results section, “Effect of altitude and acclimatization on sleep apnea.”
FIG. 4.
Differences in the mean nocturnal SpO2 in AHI subgroups. In each panel, the mean nocturnal SpO2 versus study night before, during and after high altitude exposure for the subgroups of low AHI (left panel) and high AHI (right panel), based on median split of AHI values during the first two nights at HA. *, AHI high altitude first night (HA first) versus high altitude last night (HA last); δ, high altitude versus low altitude. See results section, “Effect of altitude and acclimatization on sleep apnea.” SpO2, oxyhemoglobin saturation.
Interrelationship between the mean nocturnal SpO2 and AHI at HA
The AHI of the mild and marked desaturators were 09:80 (05:00–12:60) and 02:70 (02:00–04:30) events per hour, respectively. AHI was significantly greater in mild compared with marked desaturator subgroups (p = 0.0013; Fig. 3, right panel).
The mean nocturnal SpO2 between subgroups of low and high AHI was 84.7% versus 87.3%, respectively (p = 0.14). In contrast with the difference found in AHI between mild and marked subgroups, the mean nocturnal SpO2 at the end of the HA exposure period did not differ significantly between subgroups with low AHI versus high AHI.
Effect of altitude and acclimatization on AMS
LLS rose modestly at HA versus SL (1.38 vs. 0.17, p < 0.001), possibly reflecting exposure to moderate rather than high altitude (HA) in our young healthy cohort, and did not change over time at HA (p = 0.76) (Supplementary Fig. S1). Moreover, no differences in LLS were observed between subgroups with high or low AHI and between subgroups with mild or marked nocturnal desaturations.
Discussion
This study examined the effects of HA on SDB (sleep apnea and nocturnal hypoxemia) and the extent to which the study subjects acclimatized over time. We found that the mean nocturnal SpO2 decreased sharply upon ascent to HA but increased progressively over time. In contrast, AHI increased as expected at HA but did not fall over time. We also observed marked variability among subjects in the mean nocturnal SpO2 and AHI, which prompted post hoc analyses comparing high with low HA responders in these outcomes. Distinct respiratory patterns (phenotypes) were discerned at HA between those with or without central apneas and hypopneas at HA. Specifically, the mean nocturnal SpO2 was better maintained in the subgroup with high compared with low levels of AHI. Moreover, AHI persisted for the entire time at HA, whereas marked nocturnal desaturation improved over time at HA. The findings suggest that individual factors, possibly genetically determined predicate responses in nocturnal hypoxemia and AHI to HA, and that acclimatization only mitigates the former over time. These responses could also account for observed increases in LLS (i.e., headache and fatigue), which we observed initially at HA.
Several mechanisms can account for reductions in the mean nocturnal SpO2 when our subjects slept in hypoxic conditions at HA. Both sleep and recumbency can decrease lung volume and increase V/Q mismatch and/or shunt (Orem et al., 1974; Douglas et al., 1982a; White et al., 1983; Remmers et al., 1978; Hakala et al., 1995, 2000). These factors were unlikely to account for the severity of nocturnal hypoxemia since they should have remained constant over the duration of HA exposure while nocturnal desaturation improved. Moreover, our young healthy subjects were relatively lean, suggesting that significant venous admixture was not likely to have contributed substantially to nocturnal hypoxemia (Hakala et al., 1995, 2000; Parameswaran et al., 2006; Mokhlesi and Tulaimat, 2007; Littleton, 2012; Piper, 2016). Alternatively, the mean SpO2 fell at sleep onset from wakeful levels initially, and the mean nocturnal SpO2 improved gradually over time at HA. Although we did not quantify ventilation in our study, sleep-related hypoventilation most likely accounts for this fall in nocturnal SpO2 (Douglas et al., 1982a, 1982b; Casey et al., 2007), particularly in those with marked nocturnal desaturations. Thus, the improvement in the mean nocturnal SpO2, particularly in those with the greatest reductions in nocturnal SpO2, was consistent with sleep-related hypoventilation that improved over time (Zouboules et al., 2018).
In contrast to alterations in the mean nocturnal SpO2, AHI remained elevated at HA and did not change significantly over time at altitude. Nonetheless, AHI varied widely among our subjects and was associated with the level of mean nocturnal SpO2 during the first two nights at altitude. This variability may be due to sex-related differences in responses to altitude, with greater increases in AHI in men than in women (Lombardi et al., 2013). Only at the highest altitude did females start to display increases in central apneic events and greater length compared with males. This variability may also be due to differences in nocturnal saturation since our subjects with marked nocturnal desaturation exhibited lower levels of AHI than those who desaturated only mildly during sleep.
These findings suggest that two distinct SDB patterns emerge when normal subjects go to HA: (1) steady-state hypoventilation during sleep with few central apneas and hypopneas, leading to marked nocturnal desaturation, and (2) central respiratory events with periodic arousals that prevent sustained nocturnal desaturation, consistent with higher overall levels of nocturnal ventilation (Fig. 5). It is also possible that participants with high HVR were more likely to arouse, leading to greater ventilatory instability during sleep and an increased AHI (San et al., 2013). The subgroup with high nocturnal ventilation is likely to arouse more readily in response to small disturbances in gas exchange, thereby triggering repeated cycles of central respiratory events. In contrast, we speculated that those with marked nocturnal desaturation slept more soundly and tolerated nocturnal desaturation without arousing from sleep. These observations lead us to postulate two nocturnal phenotypes in HA sojourners who either (1) sleep soundly but exhibit sustained oxyhemoglobin desaturation or (2) maintain ventilation and oxygenation by arousing periodically from sleep. We speculate that inherent arousal thresholds rather than acclimatization responses to respiratory stimuli could account for the persistence of SDB throughout the HA sojourn. Nevertheless, we could not confirm excess arousals at HA in the high versus low AHI subgroup, possibly due to small sample size.
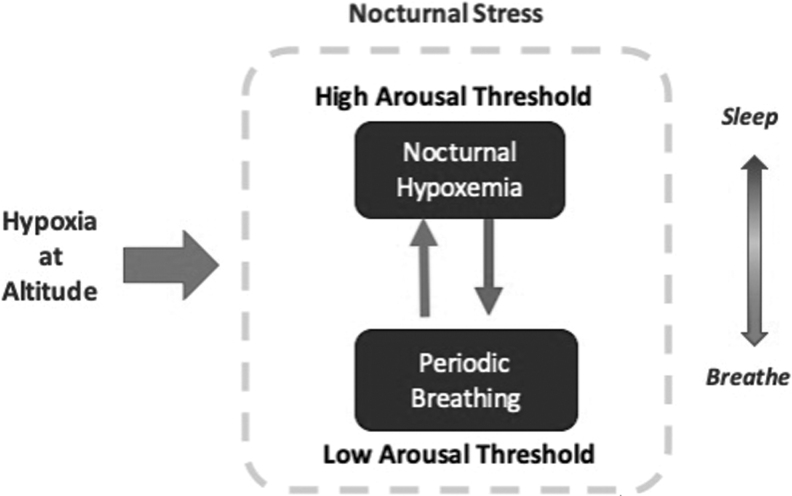
FIG. 5.
Nocturnal ventilatory phenotypes during sleep at high altitude. Diagram illustrates the mechanism by which nocturnal ventilatory phenotypes during sleep at HA develop. Sleep can be considered a physiological stress for the respiratory system, which aggravates with altitude hypobaric hypoxia. This increased burden during sleep at HA can trigger compensatory mechanisms for the maintenance of nocturnal SpO2. All subjects experience nocturnal hypoxemia during sleep, but some experience sharp decreases in the mean nocturnal SpO2 with few central apneas (if they have a high arousal threshold), whereas others will maintain the mean nocturnal SpO2 but demonstrate high frequencies of central apneas (low arousal threshold). These two phenotypes are characterized by either sleep continuity at expense of hypoventilation and desaturation, or frequent arousals from nocturnal alterations in gas exchange, thereby maintaining ventilation and oxygenation at expense of sleep continuity.
Alternatively, increases in AHI at HA may have resulted from ambient hypoxemia and/or elevated HVR, which can lower PaCO2 toward the apneic threshold. Variability in HVR and hypocapnia can explain differences in AHI at HA among subjects and observed reductions in prior studies when supplemental oxygen is administered (Reite et al., 1975; Weil, 2004). Alternatively, White et al. (1987) suggested that elevations in hypercapnic responses could account for the persistent SDB in our subjects over their entire stay at HA.
Several limitations should be considered when reviewing our results. First, our inferences were based on a small sample of healthy young adults. The small sample size impeded our ability to detect small changes in SDB over time at HA and limited the generalizability of the study. Nevertheless, most previous HA studies also reported findings from relatively small sample sizes (Burgess et al., 2004; Horiuchi et al., 2017; Steier et al., 2017; Tannheimer et al., 2017), mainly due to the complexity of the study design and costs. Furthermore, the majority of prior studies did not repeat sleep studies over the entire course of HA exposure (Eichenberger et al., 1996; Lombardi et al., 2013; Insalaco et al., 2016), whereas we obtained nightly nocturnal recordings to understand how normal untrained lowlanders acclimatize to HA. We further subjected our participants to a strict daily routine and meal schedule to reduce the potential confounds of concomitant nicotine, alcohol and caffeine consumption, as well as minimizing the impact of comorbid conditions often found in older participants. Second, we derived inferences about acclimatization responses from post hoc comparisons of subgroups that could be discerned based on initial AHI and mean nocturnal SpO2. Although limited by the number of subjects in each subgroup, repeated measurements of these parameters allowed us to examine trajectories of SDB characteristics over time at HA. These trajectories instilled confidence that a pronounced upward trend was detected in the mean nocturnal SpO2 in those with initial marked nocturnal desaturation, but not in those with a high AHI, indicating acclimatization in gas exchange but not AHI.
Third, since we used a respiratory polygraph, we recognize that our nocturnal recording devices did not provide a definitive assessment of sleep–wake stage or arousals and limited our ability to stratify our analyses by hypopneas terminated by desaturations or movement arousals. Furthermore, ambient hypoxia at HA leads to significant desaturations during hypopneas, making it more difficult to discern independent effects of desaturations and arousals. Nonetheless, we excluded epochs of putative wakefulness based on the presence of movement artifacts, thereby enhancing our ability to estimate sleep apnea severity (AHI) at baseline and at altitude. Fourth, we lacked objective measurements of carbon dioxide (bicarbonate), which could have mediated ventilatory acclimatization responses in nocturnal ventilation and mean nocturnal SpO2 at HA. Such measurements would help establish the underlying mechanism for acclimatization at HA. Fifth, sojourn to moderate altitude did not appear to stress our normal young, healthy subjects unduly, as evidenced by only modest elevations in LLS. Our subjects demonstrated persistent mild nonspecific symptoms throughout their HA stay, which could arise from various environmental stressors rather than AMS per se (Supplementary Fig. S1). Moreover, our small sample size made it difficult to detect associations between multiple SDB parameters (e.g., AHI, oxygen desaturation index [ODI], event duration) and specific sleep-related and nonsleep-related components of LLS.
There are several important implications of our study. First, our findings demonstrate that sleep is a vulnerable period for acute HA sojourners in which normal individuals exhibit pronounced disturbances in sleep continuity and/or gas exchange. Second, our findings emphasize that nocturnal respiratory “phenotypes” can be mutually exclusive with some individuals who sleep soundly (but desaturate markedly) and others who maintain ventilation and oxygenation by arousing frequently from sleep. The genetic determinants of these responses, however, remain largely unknown. Nevertheless, it is quite likely that these respiratory phenotypes are implicated in the pathogenesis of AMS symptoms, including headache, fatigue, light-headedness, gastrointestinal symptoms (nausea, vomiting, anorexia), and dyspnea. Our data showed an increase in AMS symptoms at HA, even at a relatively moderate altitude, although our sample size lacked power to detect significant decreases over time. Future research involving larger cohorts will be required to elucidate interactions between nocturnal phenotypes, AMS symptoms, and daytime neurocognitive function at HA, which could compromise the safety and productivity of sojourners such as miners, transport workers, athletes, and tourists.
Conclusions
As expected, nocturnal oxygenation deteriorated at HA acutely but improves progressively over a week's exposure, particularly in the men. The mean nocturnal SpO2 in the subgroup with marked desaturation decreased sharply at acute HA exposure but rose progressively over time. AHI also increased acutely at HA and remained elevated over a week's exposure. Furthermore, AHI predicted elevations in LLS and can account for mild persistent elevations at HA over time. AHI and nocturnal hypoxemia may be mutually exclusive HA phenotypes with manifestations of AMS symptoms, which can compromise daytime function markedly in miners, transport workers, athletes, and tourists.
Authors' Contributions
J.-D.J. and C.Z.: Participated in data recollection in field work, organized and analyzed data from sleep recordings, statistical analysis, and article preparation. F.P.S.: Participated in data management and analysis. L.M.L.: Participated in data recollection in field work. L.V.P., A.R.S., and R.A.A.: Conceptual development, data analysis, and article preparation.
Supplementary Material
Acknowledgments
We thank the Center of Interdisciplinary Sleep Research and Education (CISRE) team at Johns Hopkins for providing technical assistance and REMLogic software to review nocturnal recordings. We also wish to acknowledge the invaluable contribution of our research assistants: Mayra Cerna Rodríguez, Seungseo Choi, Mariana Jaramillo de la Riva Agüero, Allisson Dávila Cruz, María Claudia Mauricio Lévano, Ángel Pérez Rojas, Erick Phocco Núñez, and Dr. Angelo Jurado Crispin.
Author Disclosure Statement
None of the authors have any relevant conflicts of interest to disclose. All the authors of this article have properly seen, reviewed, and approved the article before the submission.
Funding Information
This study was supported by NIH R34 HL135360 and R01 HL144859.
Supplementary Material
References
- Ainslie PN, Lucas SJ, and Burgess KR. (2013). Breathing and sleep at high altitude. Respir Physiol Neurobiol 188:233–256 [DOI] [PubMed] [Google Scholar]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, and Vaughn BV. (2016). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine [Google Scholar]
- Burgess KR, Johnson P, Edwards N, and Cooper J. (2004). Acute mountain sickness is associated with sleep desaturation at high altitude. Respirology 9:485–492 [DOI] [PubMed] [Google Scholar]
- Casey KR, Cantillo KO, and Brown LK. (2007). Sleep-related hypoventilation/hypoxemic syndromes. Chest 131:1936–1948 [DOI] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Pickett CK, Martin RJ, and Hudgel DW. (1982a). Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis 125:286–289 [DOI] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Pickett CK, and Zwillich CW. (1982b). Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 126:758–762 [DOI] [PubMed] [Google Scholar]
- Eichenberger U, Weiss E, Riemann D, Oelz O, and Bärtsch P. (1996). Nocturnal periodic breathing and the development of acute high-altitude illness. Am J Respir Crit Care Med 154:1748–1754 [DOI] [PubMed] [Google Scholar]
- Erba P, Anastasi S, Senn O, Maggiorirni M, and Bloch KE. (2004). Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur Respir J 24:303–308 [DOI] [PubMed] [Google Scholar]
- Gonggalanzi, Labasangzhu, Nafstad P, Stigum H, Wu T, Haldorsen ØD, Ommundsen K, and Bjertness E. (2016). Acute mountain sickness among tourists visiting the high-altitude city of Lhasa at 3658 m above sea level: A cross-sectional study. Arch Public Health 74:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakala K, Maasilta P, and Sovijärvi AR. (2000). Upright body position and weight loss improve respiratory mechanics and daytime oxygenation in obese patients with obstructive sleep apnoea. Clin Physiol 20:50–55 [DOI] [PubMed] [Google Scholar]
- Hakala K, Mustajoki P, Aittomäki J, and Sovijärvi AR. (1995). Effect of weight loss and body position on pulmonary function and gas exchange abnormalities in morbid obesity. Int J Obes Relat Metab Disord 19:343–346 [PubMed] [Google Scholar]
- Heinrich EC, Djokic MA, Gilbertson D, DeYoung PN, Bosompra N-O, Wu L, Anza-Ramirez C, Orr JE, Powell FL, Malhotra A, and Simonson TS. (2019). Cognitive function and mood at high altitude following acclimatization and use of supplemental oxygen and adaptive servoventilation sleep treatments. PLoS One 14:e0217089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Oda S, Uno T, Endo J, Handa Y, and Fukuoka Y. (2017). Effects of Short-Term Acclimatization at the Summit of Mt. Fuji (3776 m) on Sleep Efficacy, Cardiovascular Responses, and Ventilatory Responses. High Alt Med Biol 18:171–178 [DOI] [PubMed] [Google Scholar]
- Insalaco G, Salvaggio A, Pomidori L, Cogo A, and Romano S. (2016). Heart rate variability during sleep at high altitude: Effect of periodic breathing. Sleep Breath 20:197–204 [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, and Dement WC. (2001). Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med 2:389–396 [DOI] [PubMed] [Google Scholar]
- Littleton SW. (2012). Impact of obesity on respiratory function. Respirology 17:43–49 [DOI] [PubMed] [Google Scholar]
- Lombardi C, Meriggi P, Agostoni P, Faini A, Bilo G, Revera M, Caldara G, Di Rienzo M, Castiglioni P, Maurizio B, Gregorini F, Mancia G, Parati G, and HIGHCARE Investigators. (2013). High-altitude hypoxia and periodic breathing during sleep: Gender-related differences. J Sleep Res 22:322–330 [DOI] [PubMed] [Google Scholar]
- Luks AM, Swenson ER, and Bärtsch P. (2017). Acute high-altitude sickness. Eur Respir Rev 26:160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi B, and Tulaimat A. (2007). Recent advances in obesity hypoventilation syndrome. Chest 132:1322–1336 [DOI] [PubMed] [Google Scholar]
- Orem J, Montplaisir J, and Dement WC. (1974). Changes in the activity of respiratory neurons during sleep. Brain Res 82:309–315 [DOI] [PubMed] [Google Scholar]
- Parameswaran K, Todd DC, and Soth M. (2006). Altered respiratory physiology in obesity. Canad Respir J 13:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Meinzen C, Arias RS, Schwartz NG, Rattner A, Miele CH, Smith PL, Schneider H, Miranda JJ, Gilman RH, Polotsky VY, Checkley W, and Schwartz AR. (2017a). Cross-Sectional Comparison of sleep-disordered breathing in native Peruvian highlanders and lowlanders. High Alt Med Biol 18:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Miele CH, Schwartz NG, Arias RS, Rattner A, Gilman RH, Miranda JJ, Polotsky VY, Checkley W, Schwartz AR. (2017b). Cardiometabolic correlates of sleep disordered breathing in Andean highlanders. Eur Respir J 49:1601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper A. (2016). Obesity hypoventilation syndrome: Weighing in on therapy options. Chest 149:856–868 [DOI] [PubMed] [Google Scholar]
- Reite M, Jackson D, Cahoon RL, and Weil JV. (1975). Sleep physiology at high altitude. Electroencephalogr Clin Neurophysiol 38:463–471 [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, and Anch AM. (1978). Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44:931–938 [DOI] [PubMed] [Google Scholar]
- Roach RC, Bärtsch P, Oelz O, and Hackett PH. (1993). The Lake Louise acute mountain sickness scoring system. In: Hypoxia and Molecular Medicine. Sutton JR, Houston CS, and Coates G, eds. Burlington, VT: Queen City Press. pp. 272–274 [Google Scholar]
- San T, Polat S, Cingi C, Eskiizmir G, Oghan F, and Cakir B. (2013). Effects of high altitude on sleep and respiratory system and theirs adaptations. ScientificWorldJournal 2013:241569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene RB. (1997). Control of breathing at high altitude. Respiration 64:407–415 [DOI] [PubMed] [Google Scholar]
- Steier J, Cade N, Walker B, Moxham J, and Jolley C. (2017). Observational study of neural respiratory drive during sleep at high altitude. High Alt Med Biol 18:242–248 [DOI] [PubMed] [Google Scholar]
- Tang X-G, Zhang J-H, Gao X-B, Li Q-N, Li J-B, Yu J, Qin J, and Huang L. (2014). Sleep quality changes in insomniacs and non-insomniacs after acute altitude exposure and its relationship with acute mountain sickness. Neuropsychiatr Dis Treat 10:1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannheimer M, van der Spek R, Brenner F, Lechner R, Steinacker JM, and Treff G. (2017). Oxygen saturation increases over the course of the night in mountaineers at high altitude (3050–6354 m). J Travel Med 24:tax041. [DOI] [PubMed] [Google Scholar]
- Weil JV. (2004). Sleep at high altitude. High Alt Med Biol 5:180–189 [DOI] [PubMed] [Google Scholar]
- White DP, Douglas NJ, Pickett CK, Weil JV, and Zwillich CW. (1983). Sexual influence on the control of breathing. J Appl Physiol 54:874–879 [DOI] [PubMed] [Google Scholar]
- White DP, Gleeson K, Pickett CK, Rannels AM, Cymerman A, and Weil JV. (1987). Altitude acclimatization: influence on periodic breathing and chemoresponsiveness during sleep. J Appl Physiol 63:401–412 [DOI] [PubMed] [Google Scholar]
- Zouboules SM, Lafave HC, O'Halloran KD, Brutsaert TD, Nysten HE, Nysten CE, Steinback CD, Sherpa MT, and Day TA. (2018). Renal reactivity: Acid-base compensation during incremental ascent to high altitude. J Physiol 596:6191–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.