Abstract
Significance: The incidence of chronic wounds is increasing due to our aging population and the augment of people afflicted with diabetes. With the extended knowledge on the biological mechanisms underlying these diseases, there is a novel influx of medical technologies into the conventional wound care market.
Recent Advances: Several nanotechnologies have been developed demonstrating unique characteristics that address specific problems related to wound repair mechanisms. In this review, we focus on the most recently developed nanotechnology-based therapeutic agents and evaluate the efficacy of each treatment in in vivo diabetic models of chronic wound healing.
Critical Issues: Despite the development of potential biomaterials and nanotechnology-based applications for wound healing, this scientific knowledge is not translated into an increase of commercially available wound healing products containing nanomaterials.
Future Directions: Further studies are critical to provide insights into how scientific evidences from nanotechnology-based therapies can be applied in the clinical setting.
Keywords: diabetes, chronic, wound healing, nanoparticles, nanofibers, liposomes

Soledad Pérez-Amodio, PhD
Scope and Significance
This review highlights new nanoplatforms created for the treatment of chronic wounds, specifically diabetic wounds. We briefly introduce the despaired wound healing of chronic wounds and drugs/biomolecules that are being used and particularly discuss the use of nanoparticles (NPs), nanofibers, and liposomes in the treatment of diabetic wounds, emphasizing their mechanisms of action.
TRANSLATIONAL RELEVANCE
Nanotechnology driven therapeutics can influence a specific biochemical event within the impaired healing process, being able to change one or more wound-healing phases.
This offers unique opportunities compared to dressing-based conventional wound care products.
A major advantage of these nanoplatforms is their adaptability and tunability. For instance, nanotherapeutics can be used in controlled and sustained released of the active ingredient over a period of days or weeks, while conventional delivery systems such as dressing films or gels can sustain the release of the therapeutic agent over 1 to 2 days.
CLINICAL RELEVANCE
Chronic wounds have an important economic impact in developed countries, and it is expected to increase as the population ages. Current therapies cannot fully address the impaired healing, provoking wound complications like infections and poor wound closure. Thus, new biomolecules and therapies that promote wound healing, prevent wound infections, or inflammation, among others, are needed. Several nanotechnological approaches with multiple functions and different mechanisms have proved their potential in wound animal models and could be the next generation of wound nanotherapies.
BACKGROUND
Chronic wounds
Chronic wounds exhibit a disturbed repair process, provoking that wounds would not heal within 3 months.1 Among them, nonhealing pressure ulcers (NHPUs), venous ulcers (VUs), and diabetic foot ulcers (DFUs) are the most common ones. VUs are caused by dysfunctional blood valves or obstructed veins, mainly in legs. In contrast, NHPUs are skin and underlying tissue injuries caused by prolonged skin pressure in people confined to bed or with limited mobility for long time periods. DFUs often start from several diabetes complications such as foot deformity, peripheral arterial diseases, and peripheral neuropathy. Diabetic neuropathy is the result of nerve damage caused by uncontrolled glucose blood levels, and it reduces the skin sensitivity. Foot deformation leads to the formation of keratosis and callus, resulting in wound aggravation and even gangrene. Diabetic patients have also alterations in the capillary system (thickening of basement membrane, reduced capillary size, etc.). Over time, alterations in the glucose levels contribute to vasoconstriction and plasma hypercoagulability, developing occlusive arterial disease, ischemia, and ulcer formation (peripheral arterial disease).
Nonhealing ulcers have a considerable impact for patients and their families. These types of wounds cause loss of function, morbidity, severe pain, infections, hospitalization, and in some cases amputations.
Chronic wounds mostly arise associated to population aging, obesity, and diabetes, increasing the health costs.2 This disease is often not regarded as a high priority compared to other conditions because it is not considered to be life-threatening.3 Chronic wounds prevail as a silent epidemy affecting the well-being of over 40 million people in the world.4
Most of the common features of chronic wounds include an extended inflammatory phase, existence of persistent infections, formation of bacterial biofilms, as well as higher levels of proteases and reactive oxygen species (ROS).5 Furthermore, dermal and/or epidermal cells residing in chronic wounds fail to respond to reparative stimuli. These cells present phenotypic abnormalities such as lower expression of growth factor (GF) receptors, as well as lower mitogenic potential, preventing their response to external environmental cues.6 The higher levels of proteases in chronic wounds promote the destruction of extracellular matrix (ECM), GF receptors and GF like platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF), and transforming growth factor-beta (TGF-β).6
The higher ROS levels observed in chronic wounds also stimulate the ECM destruction and cell damage. All these cellular abnormalities do not allow the formation of granulation tissue and ECM deposition, resulting in the formation of nonhealing wounds. Another feature of chronic wounds is impaired angiogenesis. Angiogenesis is a physiological process required for wound healing. In normal wound healing this process depends on the balance between the growth and proliferation of vessels and their maturation and quiescence. In diabetic patients, this balance is disrupted. Endothelial cells exposed to elevated glucose levels have been reported to become senescent leading to integrity loss and eventually apoptotsis. This decreased angiogenesis is also due to macrophage deficit present in these wounds, since these cells produce high levels of VEGF and other pro-angiogenic mediators.7
Current treatments of chronic wounds
Current treatment of chronic wounds depends on the wound etiology. In all cases, an adequate wound cleaning and debridement, a control of possible infections, and the use of wound dressings are needed. NHPU treatments include dressings that accelerate the wound healing and relieve the tissue pressure. However, DFUs and VU focus on dressing that maintains the moist environment conducive to wound healing and on compression.8 Specifically, compression leg bandages are used in VUs to improve the vein circulation. Conventional dressings, such as gauze or gauze-woven cotton composite dressings, provide wound protection from bacterial contamination and allow gaseous/fluid exchange. They are used as secondary dressings to cover complex dressings or for the treatment of superficial and noninfected wounds. Specifically, compression leg bandages are used in VUs to improve the vein circulation.9
These materials are characterized for their easy use and low cost. However, disadvantages such as frequent change needs, lack of control of moisture levels, and adherence to the wound bed have restricted their usage in wound management.10
Nowadays, new synthetic dressings, capable of providing a suitable moist environment, are available.
These dressings are semiocclusive or occlusive. They can be in the form of film, foam, hydrogel, or hydrocolloids. They are fabricated using a variety of synthetic materials such as poly(vinyl alcohol) (PVA), poly(lactide-co-glycolide) (PLGA), polyurethanes, polyethylene glycol (PEG), polycaprolactone (PCL), nylon, or silicone. Among them, hydrogels are seen as promising biomaterials suitable for wound dressings due to their high-water content. Hydrogels can maintain the moist environment at the wound interface while absorbing excessive exudate, allow gaseous exchange, act as barrier to microorganisms, and do not adhere to the wound bed.
Generally, hydrogels are made of natural polymers such as collagen and chitosan and synthetic polymers (PVA, PEG, etc.) to achieve optimal mechanical properties.
New therapeutics in the treatment of chronic wounds
Within the last decade, significant improvements in the development of new therapies have been done, like integrating additives such as antimicrobial molecules, immunomodulatory cytokines, GF, microRNA (miRNA), or exosomes.11 Advanced devices releasing antimicrobial agents, such as iodine or silver, are effective in reducing the bacterial load in the wound bed.
Some commercially available examples in Europe and United States are Actisorb™ Silver220 and Iodosorb®.
Antimicrobial peptides are able to control both inflammation and bacterial infection, acting as wound-healing peptides. These properties are highly desired in novel topical formulations for treatment of chronic wounds.12
Regarding GFs, several studies have demonstrated that their use improves all aspects of tissue repair in animal models.13 The success of topically administered GFs in chronic wounds is limited. Due to their short in vivo half-life, low absorption rate through the outermost skin later around the wound, as well as rapid elimination by exudation before reaching the wound bed, might limit the efficacy of GF topical application. Current strategies using GF may not provide the needed time for GFs in the wound area to interact with the target cells, due to their high degradation rate.14
Conventional medications containing GFs need to be applied in high doses and/or be repeatedly administrated over a long period, leading to important side effects and increasing the cost of the therapy. GF delivery systems that improve their stability in the wound area and control their release provide more effective and secure treatment alternatives. Presently, PDGF, fibroblast growth factor (FGF), and epidermal growth factor (EGF) are widely studied for their application in GF-mediated wound repair.15
Several approved products that include GFs are supplied as medications for external use in the form of solutions, gels, creams, and ointments. Clinical trials of topically administered GF have reported contradictory evidence for therapeutic outcomes.16 There are some commercially available formulations containing GFs such as recombinant human platelet-derived growth factor (rhPDGF; Regranex® Gel), recombinant human basic fibroblast growth factor (bFGF; Fiblast® Spray), and recombinant human EGF (Heberprot-P®, Regen-D™ 150, and Easyef®). Regranex Gel is an aqueous-based sodium carboxymethylcellulose gel containing 0.01% becaplermin (rhPDGF) approved for topical use by the Food and Drug Administration (FDA). Fiblast Spray is a recombinant human bFGF product commercialized in Japan.
Another characteristic of biological dressings is their ability to interact with cells or matrix proteins in the wound bed to promote healing. The ECM is a combination of structural and functional proteins. These proteins are produced by skin cells and arranged into specific patterns, which are responsive for the physiologic and biomechanical requirements of skin. The three-dimensional (3D) ultrastructure of ECM also provides a scaffold that promotes cell organization, proliferation, and differentiation during the process of wound healing. Today, commercially available acellular ECM scaffolds include porcine-derived small intestinal submucosa (e.g., Oasis® wound matrix), porcine urinary bladder matrix (e.g., MatriStem UBMTM), bovine dermis (e.g., PriMatrix® and MatriDerm®), and equine pericardium (Matrix Patch™). Devitalized ECM scaffolds are also commercially available (EpiFix®).
These acellular biological products function as temporary substrates into which cells can migrate and proliferate in a well-organized and controlled manner. In this way, they promote granulation tissue formation and tissue regeneration.
Since the use of ECM-based scaffolds alone seems to be limited for chronic wound care due to an absence of interaction with cells and tissues, autologous cellular elements have been included in these scaffolds.17
These living skin equivalents address the damaged ECM by adding a collagen matrix and also provide immune-privileged living cells that proliferate and actively synthesize GFs, cytokines, and ECM components, creating an optimal wound healing environment.6
Some examples of skin equivalent substitutes commercially available are: Apligraf®, Dermagraft®, and Alloderm™. Apligraf is an FDA product containing an epidermal keratinocyte layer and a dermal layer of fibroblast-seeded collagen. Dermagraft, also FDA approved, is formed by a polymeric scaffold seeded with neonatal allogeneic fibroblasts. These cell-based biological dressings offer an enormous potential in the field of chronic wound management, acting in several ways to improve wound healing.18
miRNAs are highly conserved endogenous small noncoding RNA molecules participating in various biological processes, including diabetic wound healing. miRNAs regulate post-transcriptional gene expression by binding to their target messenger RNAs (mRNAs), leading to mRNA degradation or translation suppression.19 miRNAs are known to be altered in chronic wounds indicating that these molecules are also implicated in impaired angiogenesis.20 In vivo studies using diabetic mice reported that miRNAs were differently expressed in skin cells and that their levels of expression changed during the wound healing process.21
Other studies have demonstrated the in vivo upregulation of miR-129 and -335 enhanced wound closure through the inhibition of Sp1-mediated matrix metalloproteinase-9 (MMP-9) expression in a diabetic wound model.22
RNA delivery techniques have improved in the last years, leading to the creation of functionalized wound dressings carrying stable miRNA or anti-miRNA molecules for skin wound healing applications.23
This has led to an increase in available anti-miRNA-based strategies parallel to that of the functional miRNA transfection approach. For example, light-inducible synthetic antimiR-92a and Locked Nucleic Acid-antimiR-26a show progress made in this respect.24
In another study, synthetic miRNA-92a inhibitor 25 reported increased angiogenesis and wound healing in different animal models such as diabetic mice and normal pig.25
Although their effect in chronic wounds has not been reported yet, exosomal miRNAs obtained from umbilical cord mesenchymal stem cells (MSCs), human amniotic epithelial cells, and human umbilical cord blood plasma have demonstrated to be useful in wound healing.26
DISCUSSION OF FINDINGS AND RELEVANT LITERATURE
Needs of new delivery systems in chronic wound: nanotechnology
Current therapies to treat chronic wounds are intended to cover the wound, protect against bacterial infection, remove dead tissue, provide moistening, and absorb excess of fluid.27 Nanotechnology platforms, due to their characteristics, have demonstrated new promises and benefits in the field.
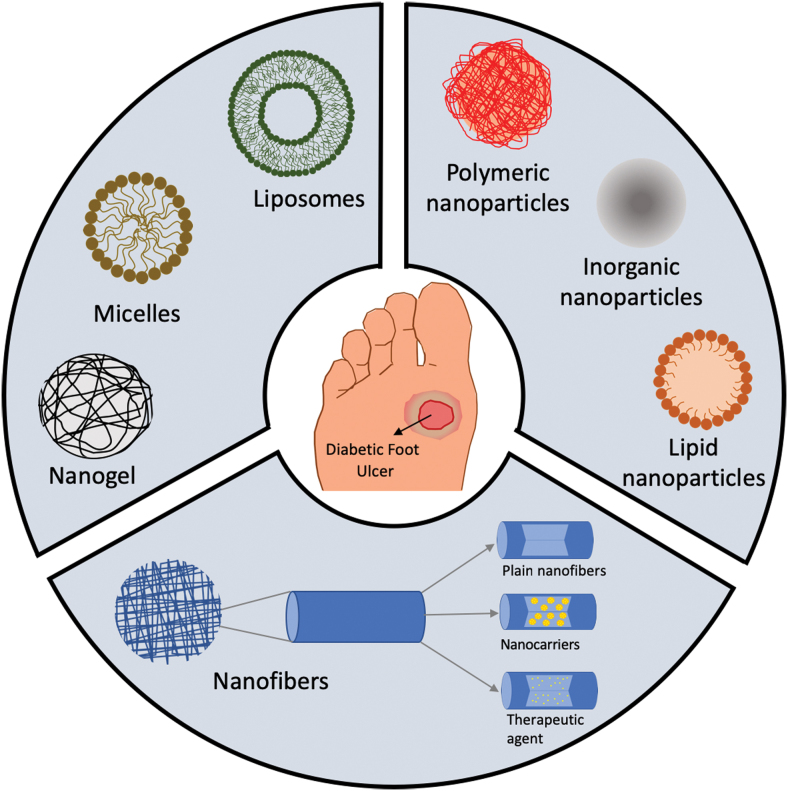
Recent progress in nanotechnology have opened new areas in the field of drug delivery applications allowing the delivery of biomolecules such as DNA/RNA or GFs that can be applied in chronic wound healing. Their small size and physicochemical properties allow the intracellular delivery of these biomolecules or drugs, protect these agents from degradation, and enhance the drug penetration into the wound. All together allow the topical administration and increase the half-life of these agents, lowering the number of applications and costs. In addition, the encapsulation of drugs and biomolecules inside nanocarriers enables different drug release profiles that can match the wound healing requirements. In the next sections, we will review the NPs, nanofibers, and self-assembled nanocarriers used for the treatment of chronic wounds (Fig. 1), specifically those that have proved positive results in wound healing in diabetic animal models.
Figure 1.
Schematic representation of nanocarriers used for chronic wound healing: self-assembled nanocarriers (liposomes, micelles, nanogels), NPs (polymeric, inorganic, lipid), and nanofibers (plain and encapsulating nanocarriers or therapeutic agents). NP, nanoparticle. Color images are available online.
Nanoparticles
NPs, with a diameter of 1–100 nm, are highly explored in the field of biomedicine and tissue engineering. In wound healing, they can be subdivided in two main categories: NPs with intrinsic properties positive for wound healing and NPs as drug delivery systems. Their main advantages are the controlled and sustained release, increase in drug half-life, and bioavailability.
NPs with intrinsic activity for wound healing
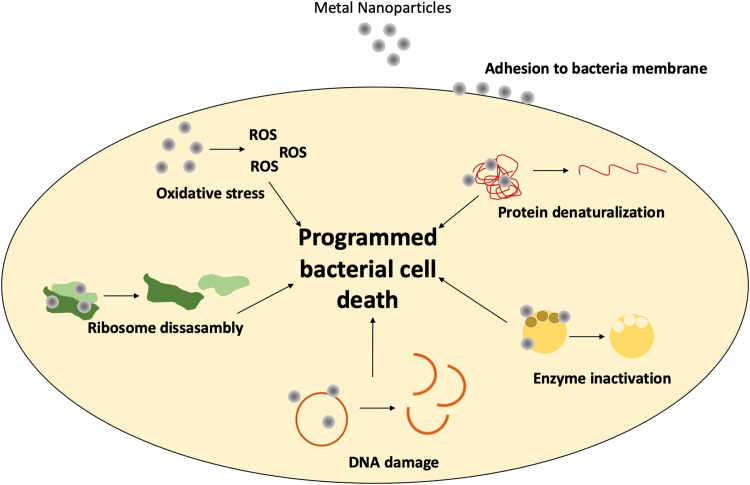
When developing strategies to address the healing of chronic wounds, technologies not using drugs or biologics are attractive to lower product fabrication costs and reduced time to market. Metallic NPs made of silver, copper oxide, gold, iron oxide, zinc oxide (ZnO), aluminum oxide, titanium dioxide, and gallium have proved their antibacterial properties.28–32 Its activity is caused by the production of ROS33 and the interaction with RNA, DNA, and enzymes (inhibitory), which all together provoke bacterial death (Fig. 2). Other materials with intrinsic activity are cerium, bioactive glass (BG), and carbon-based and -bearing nitric oxide (NO) NPs.
Figure 2.
Antibacterial mechanism of action of metallic NPs. Metal NPs provoke protein denaturalization, enzyme inactivation, DNA damage, and the disassembly of ribosomes, as well as ROS generation. Altogether, promote the programmed bacterial cell death. ROS, reactive oxygen species. Color images are available online.
Although these NPs have useful properties, therapies that use metals are limited because excessive levels of metals, especially heavy metals, may damage human cells. Table 1 summarizes the NP formulations that have been tested in diabetic wound healing in animal models and have proved its activity in wound healing and as antibacterial agents. Among heavy metals, silver has been used as an antimicrobial agent due of its relatively low toxicity to human.34 Recently, in response to issues concerning antibiotic resistance, topical application of medicals containing silver has become popular.35
Table 1.
Nanoparticles with intrinsic activity for wound healing for the treatment of diabetic wounds in animal models
| Formulation | Animal Model | Area (cm2) | Time*(Days) | References | |
|---|---|---|---|---|---|
| AgNP | In a chitosan-PEG hydrogel | Rabbit+diabetes 1 | 4 | 8 | 40 |
| Bilayer of AgNP-loaded gelatine cryogel and PDGF-BB gelatine scaffold | Mice+diabetes 2 (C57BL/6JNju DIO) | 0.5 | 9 | 109 | |
| In a chitosan/dextran hydrogel | Mice+diabetes 1 | 1.8 | 10 | 43 | |
| With nicotinamice and impregnated in nonwoven viscose fabrics | Mice+burn+diabetes 1 | 4 | 10 | 147 | |
| In a chitosan/starch gel | Rat+diabetes 1 | 0.8 | 12 | 148 | |
| In a hyaluronic gel | Rat+old+diabetes 1 | 2 | 14 | 99 | |
| In a poly(sulfobetaine acrylamide) hydrogel | Rat+infected+diabetes 1 | 1.8 | 15 | 149 | |
| With an insulin coat | Rat+diabetes 1 | 1.8 | 15 | 150 | |
| ɛ-polylysine | Rat+infected+diabetes 1 | 1.8 | 18 | 151 | |
| In poly(acrylic acid) nanogels | Mice+diabetes 2 (db/db) | — | 18 | 41 | |
| In cellulose nanocrystals | Mice+diabetes 1 | 0.5 | 18 | 45,46 | |
| In polydopamine hydrogels decorated with polyaniline and PVA | Rat+infected+diabetes 1 | 0.2 | 20 | 44 | |
| With recombinant human EGF in PU foams | Mice+diabetes 1 | 0.3 | 20 | 42 | |
| In polyelectrolyte multilayers of poly(allylaminehydrochloride) and poly(acrylic acid) | Mice+splints+diabetes 2 (db/db) | 0.3 | 21 | 152 | |
| Stabilized with bacteria supernatant | Mice+infected+diabetes 1 | 4 | 14–28 | 153,154 | |
| AuNP | With epigallocatechin gallate dispersed in N2 topically administered | Mice+diabetes 1 | 1 cm excision | 7 | 155 |
| With receptor for advanced glycation end products, epigallocatechin gallate and a-lipoic acid | Mice+diabetes 1 | 1 cm excision | 7 | 52 | |
| With antimicrobial peptide LL37 and VEGF plasmid | Mice+infected+diabetes 1 | 0.1 | 10 | 33 | |
| With spherical nucleic acid of ganglioside-monosialic acid 3 synthase dispersed in Aquaphor | Mice+splinted+diabetes 2 (T2D) | 0.3 | 12 | 55 | |
| With calreticulin and chitosan | Mice+diabetes 1 | 0.3 | 16 | 53 | |
| CuNPs | Stabilized with bovine serum albumin and Photothermal therapy | Mice+infected+diabetes 1 | 0.6 | 7 | 56 |
| With yeast extract dispersed in carbon nanofibers | Rat+diabetes 1 | 0.8 | 14 | 58 | |
| Modified with folic acid in an organic framework | Mice+splinted+diabetes 2 (db/db) | 0.3 | 30 | 62 | |
| In an organic framework dispersed in a polydiolcitrate hydrogel | Mice+splinted+diabetes 2 (db/db) | 0.3 | 30 | 63 | |
| Cerium oxide NPs | With miR-146a | Mice+diabetes 2 (db/db) | 0.5 | 14 | 69 |
| In a poly(PHBV) membrane | Rat+diabetes 1 | 2.3 | 30 | 68 | |
| BG | Vaseline | Rat+diabetes 1 | 2.5 | 16 | 72 |
| Si | PDLLA/PCL nanofibers | Mice+diabetes 1 | 0.5 | 13 | 73 |
| NO | PLGA NPs with polyethylenimine/diazeniumdiolate | Mice+infection+diabetes 1 | 0.5 | 12 | 76 |
| Chitosan/PEG hydrogel | Mice+diabetes 1 | 0.12 | 14 | 75 |
Diabetes type 1 was inducted with aloxan (rabbits) or streptozocin (mice and rats) (*times for complete wound closure or more than 90% of full thickness wounds).
AgNPs, silver nanoparticles; AuNPs, gold nanoparticles; BG, bioactive glass; EGF, epidermal growth factor; NP, nanoparticle; PCL, polycaprolactone; PDGF, platelet-derived growth factor; PEG, polyethylene glycol; PLGA, poly(lactide-co-glycolide); PU, polyurethane; PVA, poly(vinyl alcohol); VEGF, vascular endothelial growth factor.
Silver nanoparticles (AgNPs) are probably the most used NPs in wound healing due to its antimicrobial, anti-inflammatory, and wound healing properties (inducing myofibroblast differentiation from fibroblasts and stimulating keratinocyte proliferation/relocation).36 Moreover, no bacterial resistance and toxicity were observed. Nevertheless, silver ions released from the NPs can lead to toxicity,37 through oxidative stress by the generation of ROS.This problem strongly depends on different NP features such as size, shape, concentration, agglomeration, or aggregation.38
Thus, its inclusion in other formulations to control ion release is needed to reduce its cytotoxicity. AgNPs have been incorporated in gels, such as hyaluronic,39 PEG-chitosan,40 polyacrylic acid,41 and foams,42 among others, that had proved their efficiency in diabetic wound healing and in the reduction in bacteria count. For example, Shi et al. prepared hydrogels made of maleic acid-grafted dextran and thiolated chitosan impregnated with AgNPs.43 This hydrogel behaves as antifouling materials, improving the performance of AgNPs. They provided a slow Ag+ release that promoted the wound healing due to its antibacterial activity, inhibition of inflammation, and modulation of the immune response. Zhao et al., fabricated conductive hydrogels mimicking skin made of polydopamine decorated with AgNPs, polyaniline, and PVA with potential as epidermal sensors and wound healing agents.44
The resulting hydrogels had adequate self-healing properties, repeatable adhesiveness, antibacterial activity and promoted angiogenesis and collagen deposition. The impregnation of cellulose nanocrystal matrix with AgNO3 NPs has also shown interesting results in diabetic wound healing.45,46
A reduction in inflammation and an increase in collagen deposition, reepithelialization, and angiogenesis were observed, promoting wound healing.46
In terms of clinical trials, however, there are not enough evidences to establish whether AgNP-containing dressings or topical agents promote wound healing or prevent wound infection.47–49
For DFUs, as well, no randomized trials or controlled clinical trials exist that analyze their clinical effectiveness,50 although new devices are developed that suggest that more clinical trials are needed. Gold nanoparticles (AuNPs) have inherent antibacterial activity and promote the process of wound healing through hemostasis and inflammatory phases.51
AuNPs have recently been used by various research groups for their wound-healing applications. AuNPs have been combined with biomolecules like antioxidants52 to enhance the wound healing activity of the formulation. Martinez et al. fabricated a nanocomposite made of Au NPs functionalized with chitosan and calreticulin,53 a calcium-binding protein of the endoplasmic reticulum that has shown wound healing activity.54
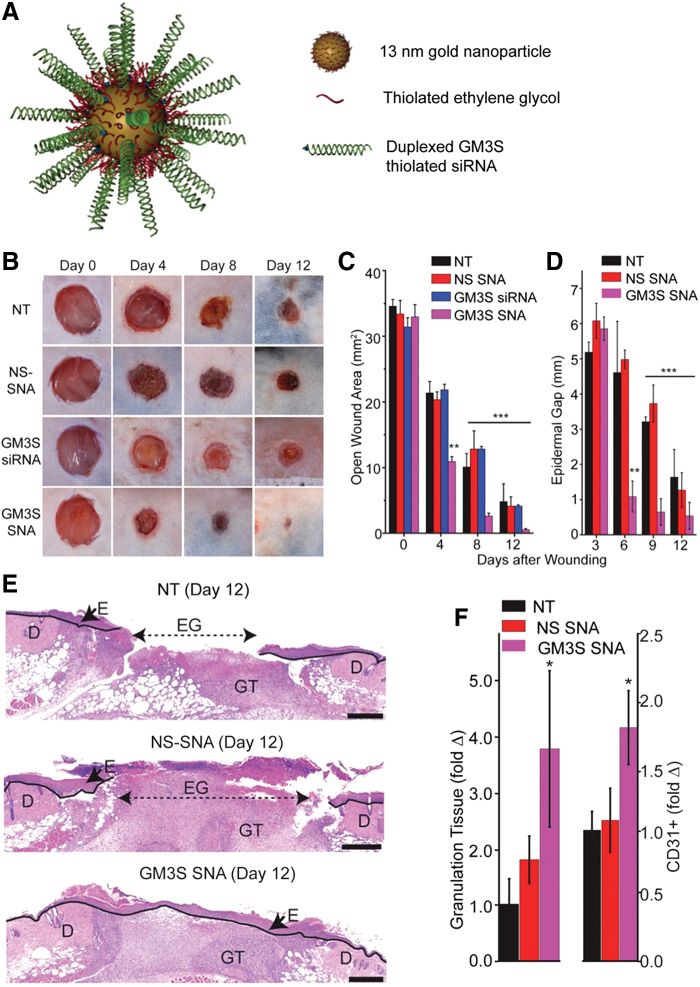
When administered into diabetic mice wounds, improved healing was observed compared to untreated mice. Randeira et al. made AuNP conjugates with a spherical nucleic acid of ganglioside-monosialic acid 3 synthase (GM3S) dispersed in Aquaphor (Fig. 3). This enzyme is overexpressed in diabetic mice and impedes wound healing. Treated wounds in diabetic mice decreased the local enzyme expression and fully healed in 12 days, with an increase in granulation tissue formation and vascularity (Fig. 3).55
Figure 3.
The topical administration of AuNPs conjugated to spherical nucleic acid for GM3S shows a reduction in local GM3S expression and heals the wound in 12 days in diabetic mice wounds. An increase in granulation tissue, new blood vessel formation, and IGF-1 and EGF receptor phosphorylation is observed. (A) SNA are 13-nm gold cores functionalized with thiolated siRNA duplexes (targeted to) and oligoethylene glycol for colloidal stability. (B–E) Topical application of GM3S SNA prevents the delayed wound healing in the DIO mouse. (B) Representative clinical images of wounds. (C) Computerized measurements of the open wound area. (D) Epidermal gap (the maximum distance between KCs at the leading wound edges) was measured by computerized morphometry. (E) Representative histologic images of NT and NS SNA- and GM3S SNA-treated wounds at day 12. D, dermis; E, epidermis; EG, epidermal gap; GT, granulation tissue (Scale bar: 500 μm.). (F) Granulation tissue area and vascularity (CD31+ staining) of the diabetic wounds. Adapted from 55 with permission. *p < 0.05, **p < 0.01, ***p < 0.001. AuNPs, gold nanoparticles; EGF, epidermal growth factor; GM3S, ganglioside-monosialic acid 3 synthase; IGF-1, insulin-like growth factor-1. Color images are available online.
Copper ions (Cu2+) also promote wound healing in diabetic mice56–58 due to its pro-angiogenic properties.59 Cu2+ stabilize the expression of hypoxia-inducible factor and promote the secretion of VEGF, mimicking hypoxia, that plays a role in cell recruitment, cell differentiation, and blood vessel formation.60
In addition, Cu2+ have antimicrobial activity.28 However, multiple applications are necessary,61 provoking copper toxicity. Slower ion release can reduce its toxicity. Metal−organic frameworks (MOFs) are a type of crystalline porous coordination polymers composed by inorganic metal ions and organic ligands that interact to form clusters with tunable release rates, being also efficient in diabetic wound healing.62,63 Xiao et al. prepared MOFs modified with folic acid that released slowly Cu2+ and improved the wound closure by inducing angiogenesis, collagen synthesis, and reepithelialization.62
Copper-based MOFs, however, might be unstable in physiological protein containing solutions, making difficult their direct use in wound healing.62
ZnO NPs also exhibit activity in wound healing.64 So far, zinc promotes reepithelialization, pro-angiogenesis, and it is anti-inflammatory.65 Other metallic NPs such as graphene oxide,66 iron oxides, or titanium have been also included in wound healing alternatives with positive results.67 However, further studies need to be performed to ensure their efficacy in diabetic wound healing. Cerium oxide nanoparticles (CeONPs) have been proven to have antioxidant and pro-angiogenic activity, enhancing diabetic wound healing,67,68 being even effective in the treatment of DFUs.69 BGs have also been reported to be successful in wound healing applications due to their high biocompatibility and positive biological responses of their ionic products.70
Several reports indicate that BG NPs can efficiently enhance diabetic wound healing. Lin and coworkers reported an accelerated wound healing in diabetic rats probably due to an increase in angiogenesis related factors such as VEGF and FGF-2.71
Silicon (Si) ions have also been used for the treatment of diabetic wounds. Jiang et al. prepared a spaced-oriented scaffold for Si ion release. The scaffolds were coated with silicon-doped amorphous calcium phosphate NPs coating its surface to promote angiogenesis.72 The Si ions released promoted wound healing by enhancing angiogenesis, collagen deposition, and reepithelialization of the diabetic wound. Porous Si NPs loaded with Flightless I neutralizing antibodies showed a significant improvement in healing compared to controls and the antibody alone in diabetic wounds.73
NO is a potent anti-biofilm in wound healing and it has been proved that diabetic wounds have a low NO level in the wound bed.74
NO has been formulated in NPs to improve wound healing in diabetic animal models.75 NO-releasing NPs prepared by doping PLGA NPs with polyethylenimine/diazeniumdiolate have been reported to accelerate healing of methicillin-resistant Staphylococcus aureus biofilm-infected wounds in diabetic mice together with biofilm clearance and reduced bacterial count.76
Overall, we must highlight that mostly bioactivity and mechanism proposed in the literature are related to the ion species rather the metal or oxide. That means that features that affect degradability and dissolution such as particle size, crystallinity, and solubility should be strongly controlled.
NPs as drug delivery systems
NPs can also be used in wound healing as encapsulation platforms (Table 2). Polymers such as polyesthers, polysaccharides, peptides, and lipids can be used for their preparation. PLGA can release lactate into the wound bed to promote neovascularization and wound healing.77 PLGA has been used to encapsulate a variety of molecules, such as insulin,78 ferulic acid,79 and GFs,77,80,81 showing accelerated wound healing in diabetic rodent models. For example, wounds treated with NPs encapsulating VEGF have an increase in collagen deposition, reepithelialization, and angiogenesis in diabetic wounds.77 Compared with free VEGF, wounds required only 19 days to be completely closed in the case of VEGF encapsulated in the PLGA NPs compared with the 28 days for free GF.77
Table 2.
Nanoparticles for drug delivery of wound healing drugs or biomolecules for the treatment of diabetic wounds in animal models
| NP Composition | Formulation | Biomolecule | Animal Model | Area (cm2) | Time* (Days) | References |
|---|---|---|---|---|---|---|
| PLGA | In (PVA-borate) hydrogel | Insulin | Rat+diabetes 1 | 0.3 | 16 | 78 |
| In poly(ether)urethane– polydimethylsiloxane/fibrin-based scaffolds | VEGF and bFGF | Mice+diabetes 2 (db/db) | 0.5 | 15 | 80 | |
| — | VEGF | Mice+diabetes 2 (db/db) | 0.5 | 19 | 77 | |
| — | EGF | Rat+diabetes 1 | 2.5 | 21 | 81 | |
| In carbopol 980 | Ferulic acid (FA) | Rat+diabetes 1 | 2.5 cm excision | 14 | 79 | |
| Lipid | — | TNFα | Mice+diabetes 2 (db/db) | 0.5 | 16 | 82 |
| Lecithin | In pluronic gel | Deferoxamine | Rat+diabetes 1 | 4 | 11 | 83 |
| Protamine | In calcium alginate hydrogel/hyaluronan oligosaccharide | — | Rat+diabetes 1 | 3 | 16 | 87 |
| ELP | In fibrin gel | KGF | Mice+diabetes 2 (db/db) | 1 | 14 | 84 |
| In fibrin gel | SDF-1 | Mice+diabetes 2 (db/db) | 1 | 28 | 86 | |
| In fibrin gel | SDF-1 | Mice+diabetes 2 (db/db) | 1 | 28 | 86 | |
| Chitosan | Collagen/alginate | Curcumin | Rat+diabetes 1 | 4 | 15 | 156 |
Diabetes type 1 was inducted with aloxan (rabbits) or streptozocin (mice and rats) (*times for complete wound closure or more than 90% of full thickness wounds).
bFGF, basic fibroblast growth factor; ELP, elastin-like protein; KGF, keratinocyte growth factor; STF-1, stromal cell-derived factor-1; TNFα, tumor necrosis factor alpha.
Lipid nanoparticles (LNPs) are generally prepared with physiological lipids or lipid molecules in processes that do not require any potentially toxic organic solvents. Generally, for wound healing applications, LNPs are loaded with specific siRNAs. Topical application of LNPs formulated with an ionizable and degradable lipid and a siRNA specific for tumor necrosis factor alpha (TNFα) have shown a decreased TNFα mRNA expression in the wound bed by 40–55% in diabetic mice compared to untreated wounds.82 Another example of LNP application in chronic wounds is lecithin-based NPs. Topical application of these NPs complexed with deferoxamine in a model of wound healing in diabetic rats showed an increased wound closure along with an improved collagen deposition and neovascularization compared with free deferoxamine. This suggests that the NPs encapsulating the therapeutic have a better healing outcome than the free therapeutic.83
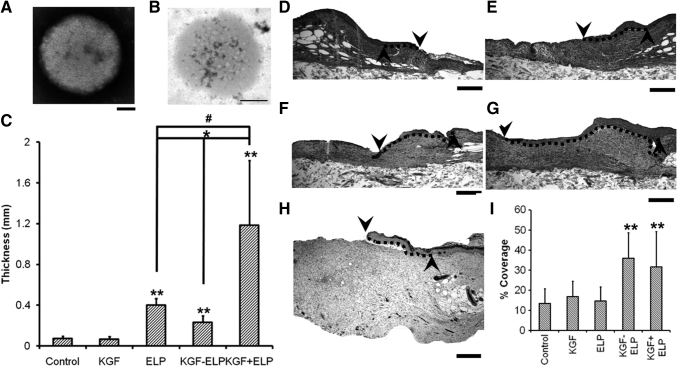
NPs made of peptides have also shown their utility in the field of wound healing. Elastin-like protein (ELP)-based fusion proteins, by forming NPs, have been shown to protect biomolecules from proteolysis and can act as “drug depots” that supply the biomolecules over an extended period. Recently, self-assembling elastin-like peptide GF chimeric NPs have been used to treat chronic wounds. These NPs of ELP and KGF84 or ELP and stromal cell-derived factor-1 (SDF-1)85,86 were applied to excisional wounds on the back of diabetic mice. These treatments showed accelerated wound closure and increased vascularization compared to free GF, ELP alone, or vehicle (Fig. 4). Other peptide, such as the cationic antimicrobial peptide protamine, has been reported to be suitable for the development of treatments for chronic wounds. Wang et al. developed protamine NPs and hyaluronan oligosaccharide loaded in alginate hydrogels. Using a diabetic mouse-skin defect model, these authors demonstrated that this composite reduced bacterial-induced chronic inflammation at the wound site and accelerated the wound-healing process by promoting angiogenesis.87
Figure 4.
NPs of fusion protein of ELP and KGF enhance wound healing in diabetic mice by promoting reepithelialization and granulation tissue formation. (A, B) TEM images of KGF-ELP (A) and ELP (B) NPs; scale bar = 100 nm. (C) Quantification of granulation tissue formation in diabetic mice wounds, after the treatment with a fibrin gel, KGF-fibrin gel, ELP-NPs in fibrin gel, ELP-KGF-NPs in fibrin gel, and KGF and ELP-NPs in fibrin gel for 14 days. (D–I) Reepithelialization enhancement of wounds of diabetic mice after treatment. Hematoxylin–eosin staining of wounds after 14 days of treatment with fibrin gel (D), KGF-fibrin gel (E), ELP-NPs in fibrin gel (F), ELP-KGF-NPs in fibrin gel (G), and KGF and ELP-NPs in fibrin gel (H). Dotted line represents the reepithelialization tissue (scale bar of 400 μm). (F) Reepithelialization quantification, normalized to the initial wound gap. Each value represents the mean thickness from 7 mice (n = 7). ** denotes p < 0.05 when compared to control or KGF. # denotes p = 0.043 when compared to ELP particles. * denotes p < 0.01 when KGF-ELP particles are compared with either ELP particles treatment or free KGF + ELP particles treatment. The up arrow indicates the edge of the created wound and the down arrow indicates the tip of the migrating tongue of the wound. Dotted line represents the extent of reepithlialization. Adapted from 84 with permission. ELP, elastin-like protein; KGF, keratinocyte growth factor.
Nanofibers
Nanofibers are filaments with diameters within the nanoscale. They are usually produced by the electrospinning technique due to its low cost and simplicity. This technique uses a high electric force between a needle point capillary tip and a collector to spin the polymeric solutions and obtain fiber meshes. Nanofiber meshes are a promising tool in the field of chronic wounds as they can partially reproduce the ECM due to its organization and random alignment. Indeed, its high surface area-to-volume ratio and tunable porous morphology can promote the wound homeostasis, as well as allow the gas/nutrient interchange.88
Moreover, nanofiber meshes should also provide moisture to avoid the wound dehydration and bacteria contamination. They can also encapsulate biological molecules, drugs, or nanocarriers encapsulating those, which consequently allow their topical administration with the subsequent advantages (lower dose and less side effects). In contrast, nanofiber meshes have also been tested as artificial skin. When MSCs are used they can differentiate into endothelial cells or release GFs to trigger the wound healing process.89
Nanofibers can be categorized depending on their composition (natural polymers, artificial polymers, polymer blends). Table 3 describes the strategies followed for diabetic wound healing.
Table 3.
Nanofiber formulations used for the treatment of diabetic wounds in animal models
| Composition | Biomolecule | Animal Model | Area (cm2) | Time*(Days) | References |
|---|---|---|---|---|---|
| Chitosan+PVA | Desferrioxamine | Rat+diabetes 1 | 1.8 | 18 | 96 |
| — | 1 | 10 | 95 | ||
| ZnO | Rabbit+diabetes 1 | 0.28 | 12 | 97 | |
| Hyaluronic acid | AgNPs | Rat+diabetes 1 | 6.25 | 15 | 99 |
| Hyaluronic acid+collagen | VEGF, PDGF, and EGF and bFGF-loaded gelatin NPs | Rat+diabetes 1 | 1.8 | 28 | 15 |
| HPMC+PEO | Beta glucan | Mice+diabetes 2 (BKS db) | 1 | 14 | 100 |
| SF | — | Rabbit+diabetes 1 | 1.1 | 12 | 105 |
| Insulin loaded MPs | Rat+diabetes 1 | 0.8 | 14 | 104 | |
| EGF and ciprofloxacin | Rabbit+diabetes 1 | 0.3 | 14 | 102 | |
| Adipose MSC or decellularized | Mice+diabetes 2 (db/db) | 0.2 | 10 | 106 | |
| SF+PLGA | — | Rat+diabetes 1 | 3.14 | 15 (80%) | 101 |
| SF+PVA | rhEGF | Rabbit+diabetes 1 | 1.1 | 18 | 103 |
| Collagen+PLGA | rhPDGF | Rat+diabetes 1 | 0.5 | 14 | 114 |
| Glucophage | 0.5 | 14 | 115 | ||
| Collagen+PCL | Dimethyloxalyl-glycine | Rat+diabetes 1 | 2.6 | 14 | 112 |
| — | 2.6 | 14 | 111 | ||
| BG NPs | Rat+diabetes 1 | 3.1 | 21 | 113 | |
| Gelatin+PCL | Silicate-based bioceramics | Mice+diabetes 1 | 0.5 | 13 | 110 |
| PLA | Dimethyloxalylglycine-loaded MSNPs | Mice+diabetes 1 | 0.5 | 15 | 116 |
| PCL | Curcumin | Mice+diabetes 1 | 0.3 | 10 | 117 |
| Aloe vera+MSC | Mice+diabetes 2 | 0.5 | 28 | 157 | |
| Bixin | Mice+diabetes 1 | 0.2 | 14 | 119 | |
| PCL+PEG | EGF+bFGF | Mice+burn+diabetes 1 | 0.5 | 7 | 120 |
| PCL+Gum tragacanth | Curcumin+MSC | Rat+diabetes 1 | 0.8 | 15 | 118 |
| PLGA | Aloe vera+rhEGF | Mice+splinted+diabetes 2 (db/db) | 0.8 | 8 (30%) | 123 |
| rhPDGF | Rat+diabetes 1 | 0.5 | 14 | 121 | |
| Neurotensin+cellulose nanocrystals | Mice+diabetes 2 (BKS.CgDock7m./.Leprdb/JNju) | 0.3 | 14 | 122 | |
| Metformin | Rat+diabetes 1 | 0.5 | 14 | 158 | |
| PVA+curdlan | Silver nitrate | Rat+diabetes 1 | 4 | 14 | 124 |
| PLA-PEG | bFGF | Rat+diabetes 1 | 2.5 | 21 | 126 |
| RAD | — | Mice+diabetes 2 (db/db) | 0.3 | 28 | 128 |
| Heparin-mimic amphiphilic peptides | — | Mice+diabetes 2 (db/db) | 0.3 | 21 | 130 |
| Multidomain peptide (k2(SL)6k2) | — | Mice+diabetes 2 (BKS.Cg-Dock7 < m> +/+ Lepr < db>/J) | 0.5 | 14 | 129 |
Diabetes type 1 was inducted with aloxan (rabbits) or streptozocin (mice and rats) (*times for complete wound closure or more than 90% of full thickness wounds).
HPMC, hydroxypropyl methylcellulose; MP, microparticles; MSC, mesenchymal stem cell; MSNP, mesoporous silica nanoparticles; PEO, polyethylene oxide; PLA, poly(lactide); rhPDGF, recombinant human platelet-derived growth factor; SF, silk fibroin; ZnO, zinc oxide.
Natural polymers
Natural polymers offer an excellent biocompatibility and biodegradability, low antigenicity, and even, some of them have innate properties for wound healing (antibacterial and hemostatic activity).
Among them, polysaccharides and proteins are often used for nanofiber preparation. The main limitation for its use is its innate variability and the poor mechanical properties, normally requiring their combination with synthetic polymers.
Polysaccharides
Polysaccharides such as chitosan, alginate, and hyaluronic acid have emerged as good candidates in wound dressings due to their innate properties, similarities with the skin ECM, and abundance. However, its use in chronic wounds is still limited. The fabrication of polysaccharide nanofibers through electrospinning is challenging, due to the critical chain entanglement concentration (CEC),90 that it might be too high (hyaluronic) provoking high or insufficient viscosity (alginate).
Alginate is one of the most used biomaterials for the developing of wound dressings,91 being even some commercially available options (Tegagen™). It is generally combined with synthetic polymers like polyethylene oxide (PEO) to be electrospun.92 Its use in chronic wounds has not been tested yet.
Chitosan has innate antibacterial and hemostatic activity.93 However, its chemical characteristics (hydrogen bonds, amino groups) reduce its chain flexibility, limiting the fabrication of nanofibers.93 Chitosan has been electrospun using strong acids to break chemical interactions or combining it with other polymers, such as PEO, PVA, or PCL.94 Xie et al. created a scaffold made of chitosan and PEO, loaded with VEGF and NPs encapsulating platelet derived growth factor-BB (PDGF-BB). The scaffold had antibacterial activity due to chitosan and released VEGF in the early stage to promote blood vessel formation.94
In addition, NPs were able to slow the release of PDGF-BB over time promoting the formation of new blood vessels and cell proliferation during the wound healing process.
Chitosan/PVA nanofibers have a good wound healing profile by itself,95 and they were also combined with other therapeutics like desferrioxamine.96 These nanofibers sustained desferrioxamine release for 3 days and were able to recruit cells to promote angiogenesis in chronic wounds through the hypoxia-inducible factor 1-alpha (HIF-1a) expression and other pro-angiogenic GFs.
ZnO was also encapsulated into chitosan/PVA fibers to implement its antimicrobial and antioxidant activities.91 Chitosan/gelatin nanofibers containing BG were also tested in chronic wound treatment, proving their antibacterial activity and healing properties.92
Hyaluronic acid is a natural constituent of the ECM, having a relevant physiological role in inflammation and wound healing.93 Its molecular weight (MW) determines its activity; MWs greater than 15 kDa render polymers recognized by the CD44 receptor, which induces fibroblast migration and proliferation and cell growth,93 and are beneficial for wound healing.93 Hyaluronic electrospinning is challenging due to its high viscosity at the CEC, forcing the combination with other materials.93 Nevertheless, some authors have reported the fabrication of nanofibers when it is dissolved in strong acids or bases and dimethylformamide.93 Collagen/hyaluronic nanofibers encapsulating angiogenic GFs (VEGF, PDGF) and GF-loaded gelatin NPs (EGF and bFGF) were assayed as skin substitutes. The nanofibrous membrane possessed similar mechanical properties to human skin and allowed a faster wound regeneration than the control. The formulation was able to release EGF and bFGF in the early stage of the wound (to promote epithelialization and angiogenesis), and PDGF and VEGF in the late stage (to aid vasculature maturation), promoting enhanced wound healing.15
Abdel-Mohsen et al. prepared hyaluronan nanofibers containing AgNPs. The nanofibers had antibacterial activity against gram negative bacteria and higher wound repair efficacy compared to controls, proving its efficacy in wound and chronic ulcers.99
Cellulose derivatives are also used for the preparation of nanofibers through electrospinning. Grip et al. used hydroxypropyl methylcellulose combined with PEO to form nanofibers encapsulating beta-glucan. Nanofibers were prepared by the Nanospider™ technology. When used in vivo in diabetic mice, they showed an improved wound healing capacity.100
Proteins
The most used proteins for the fabrication of nanofibers for wound healing are silk fibroin (SF), gelatin, and collagen. Insects and arachnids naturally produce SF, which is a very common protein applied in the electrospinning process to produce strong fibers due to its mechanical properties. These nanofibers have demonstrated to accelerate wound dressing progress.102 Chouhan et al. showed that SF nanofibers loaded with antibiotic and EGF had a faster wound healing in diabetic rabbits than other commercially available bandages.103
These authors also fabricated fibers combining SF with PVA and encapsulating EGF together with an antimicrobial peptide. They observed a remarkably faster healing, progress of the granulation tissue formation, angiogenesis, and reepithelialization of the wounds in diabetic rabbits.104
Li et al. prepared nanofiber dressing of SF encapsulating microparticles loaded with insulin.105 Insulin stimulates keratinocyte and endothelial cell proliferation and migration, promoting wound reepithelialization and vascularization. These authors observed that the dressing improved the wound healing in diabetic rats due to a sustained release of the insulin during the wound healing progress.
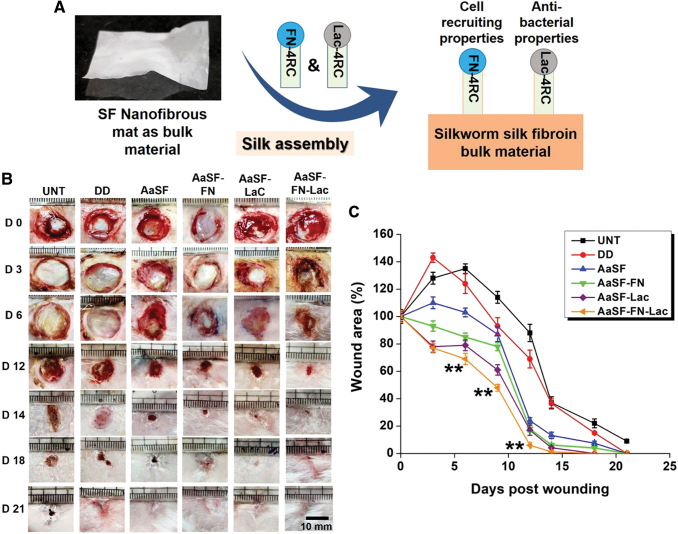
Chouhan and coworkers developed nanofibrous meshes of SF coated with two classes of recombinant silk fusion proteins from arachnids: FN-4RepCT (contains fibronectin-derived binding motifs) and Lac-4RepCT (includes a cationic antibacterial peptide) (Fig. 5). When mats were coated with both spider silk proteins, they had a faster wound healing, improved granulation, angiogenesis, collagen deposition, and reepithelialization than the uncoated or one single coat in diabetic wounds.105 SF scaffolds were also used as skin substitutes, by seeding human adipose-MSCs on them.106 Interestingly, when the scaffolds were decellularized they were almost as effective as the seeded scaffolds in the treatment of diabetic wounds.
Figure 5.
SF nanofiber meshes coated with spider silk fusion proteins FN-4RC (contains motifs of fibronectin for cell adhesion) and/or Lac-4RC (contains lactoferrin, an antimicrobial peptide). When both fusion proteins are used, a faster wound healing in a diabetic model is observed, provoked by a better granulation tissue formation and reepithelialization compared to a positive control. (A) Scheme representing the fabrication and morphology of the dressing. (B, C) Wound evolution after no treatment (UNT) and treatment with Duoderm wound dressing (positive control, DD), uncoated mat (AaSF), mat coated with FN-4RC (AaSF-FN), mat coated with Lac-4RC (AaSF-Lac), and mat coated with both (AaSF-FN-Lac). **p ≤ 0.01. Scale bar 100 mm. Adapted from 102 with permission. SF, silk fibroin. Color images are available online.
Gelatin is a polyaminoacid obtained by the partial acid or alkaline hydrolysis of natural collagen.
It has been widely applied in the area of biomaterials for tissue engineering because of its hemostatic activity, high number of Arg-Gly-Asp (RGD) sequences to promote cell adhesion, low antigenicity, and easy chemical modification.
Dongargaonkar et al. were able to produce gelatin nanofibers using water solvents by then increasing its water stability crosslinking them with oxidized sucrose, reducing the toxicity associated to other cross-linkers like glutaraldehyde.107 Aduba et al. electrospun gelatin and arabinoxylan encapsulating silver sulfadiazine, obtaining trifunctional fibers due to its antioxidant and antimicrobial activities and similarities with the ECM. However, its instability in water forced to cross-link the fibers, reducing its mechanical properties.108
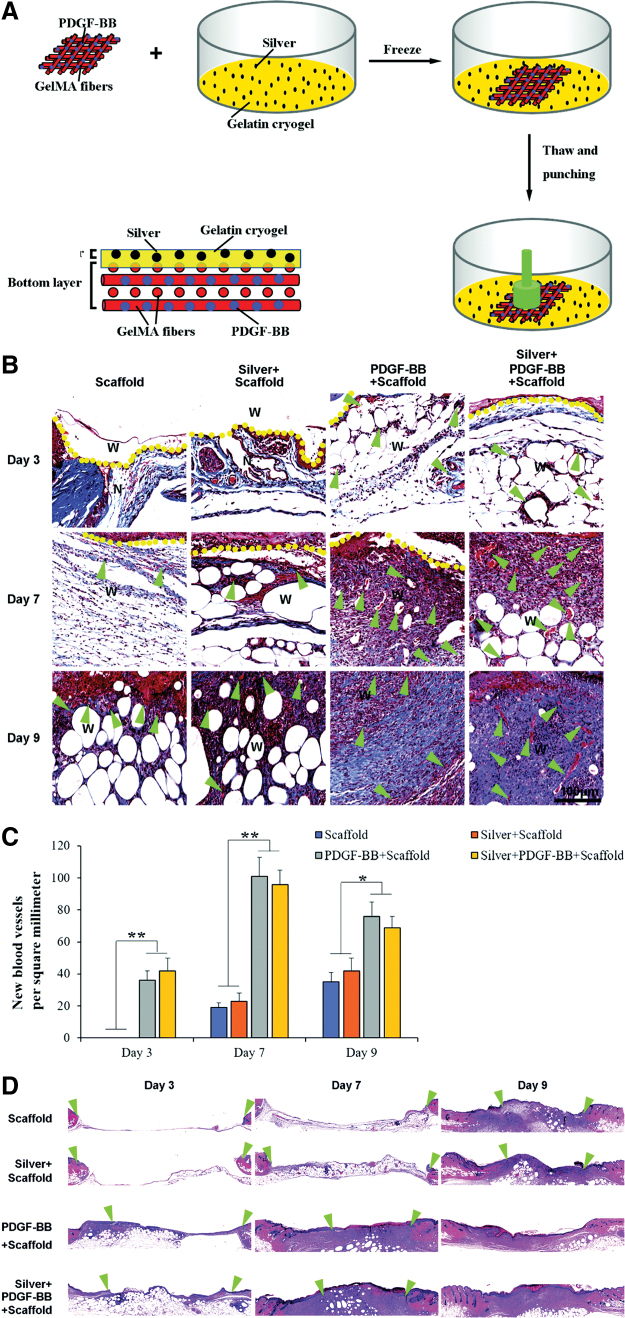
Gelatin nanofibers encapsulating AgNPs and PDGF-BB showed a better antibacterial activity, promotion of angiogenesis, reepithelialization, collagen deposition, and granulation tissue formation in diabetic wounds than the gelatin scaffold (Fig. 6).109 PCL was also used for electrospinning gelatin. Lv et al. prepared gelatin/PCL nanofibers encapsulating silicate-based bioceramic particles with a sustained release of Si ions. In vivo, an enhanced angiogenesis, collagen deposition, reepithelialization, and reduction of inflammation were observed.110
Figure 6.
A skin-inspired 3D bilayer scaffold made of gelatin, AgNPs, and PDGF-BB had a faster wound healing, more collagen deposition, blood vessel formation, and reepithelialization in diabetic wounds. (A) Representation of the preparation process of silver and PDGF-BB coloaded bilayer 3D scaffolds. (B–D) Histological observations of skin wound healing treated with scaffold, silver-loaded scaffold, PDGF-BB-loaded scaffold, and silver and PDGF-BB coloaded scaffold. (B) Masson trichrome staining shows collagen deposition and new blood vessel formation of treated wounds. Arrows indicate newly formed blood vessels. (C) Quantification of new blood vessels. (D) Hematoxylin–eosin staining showing the reepithelialization of the treated wounds. Arrows indicate re-epithelization. Adapted from 109 with permission. *p < 0.05, **p < 0.01. AgNP, silver nanoparticle; PDGF, platelet-derived growth factor. 3D, three-dimensional. Color images are available online.
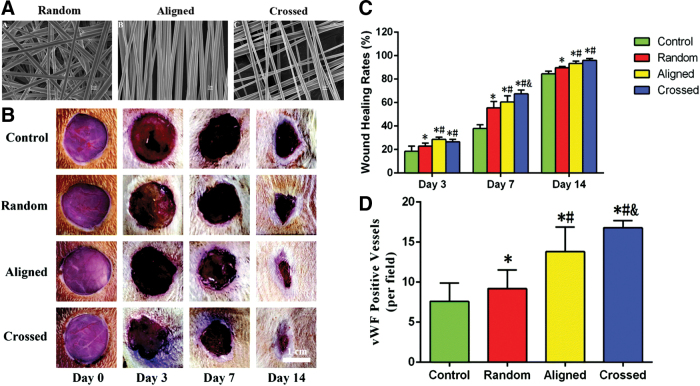
Collagen is the main constituent in the ECM, skin, and connective tissues, and its role in ECM regeneration has been demonstrated. Collagen has been combined with other materials for fabricating dressings by electrospinning. Sun et al. prepared nanofibers of collagen I and PCL with different patterns: mimicking the collagen fibril pattern in vivo (crossed), random, and aligned (Fig. 7). In vitro, fibroblasts responded differently in each nanofiber configuration, having differences in the wound healing related gene expression. Moreover, diabetic rats treated with crossed nanofibers had a faster healing, increased angiogenesis, and lower inflammation compared to the other nanofiber architectures.111
Figure 7.
Nanofiber meshes made of Collagen I and PCL with different fiber organizations have different outputs in wound healing. Crossed nanofibers, which mimic the collagen pattern in skin, have a better wound healing rate and promote angiogenesis in diabetic rats. (A) Scanning Electron Microscopy images of the nanofibers with different organizations (random, aligned, and crossed). (B, C) Wound evolution in diabetic rats after treatment with the scaffolds. (D) Quantification of von Willebrand factor-positive vessels. Adapted from 111 with permission. * denotes statistical significance, p < 0.05 vs. control; # denotes statistical significance, p < 0.05 vs. random; & denotes statistical significance, p < 0.05 vs. aligned. PCL, polycaprolactone. Color images are available online.
Gao et al. also fabricated Collagen 1/PCL nanofibers encapsulating an inhibitor of the prolyl hydroxylases: dimethyloxalylglycine, which is able to avoid HIF-1-alpha degradation.
These fibers were produced by co-axial electrospinning, having a drug/polymer core and a polymer shell, showing sustained biomolecule release for 2 weeks and a stabilization of HIF-1a in vivo.
The nanofibers improved wound healing in diabetic rats.112 They also prepared similar nanofibers encapsulating BG NPs instead. These new nanofibers mimicking the bone ECM improved angiogenesis, granulation tissue formation, ECM remodeling, and epidermis differentiation.113
Lee et al. encapsulated recombinant human PDGF in collagen/PLGA nanofibers to test its suitability in chronic wound healing, and they also observed a faster closure rate of the wounds, combined with a higher reepithelialization and collagen I deposition.114 When Glucophage is loaded in collagen/PLGA nanofibers, a downregulation of MMP-9 is observed in murine diabetic models, promoting faster healing.115
Synthetic polymers have a more defined molecular structure and a better solubility in organic solvents than natural materials, easing its formulation in nanofibers. In addition, most of them have better mechanical properties than natural polymers.
Polyesters
Polyesters are the most used materials for nanofiber formation, owing to their tunable biodegradability, their innate biocompatibility, and their approval by the FDA. For example, poly(lactide) (PLA) fibers embedding mesoporous silica NPs loaded with a pro-angiogenic drug (dimethyloxalylglycine) enhanced new blood vessel formation reepithelialization and collagen deposition, as well as a decrease in inflammation in a diabetic wound model.116
Curcumin-encapsulated PCL nanofibers had also proved its benefits as antioxidants and anti-inflammation in wound healing in diabetic mice.117 When combining PCL with the curcumin and gum tragacanth, the nanofibers were active against bacteria. MSC seeded scaffolds improved the wound closure rates and it enhanced granulation tissue formation, reepithelialization and collagenous synthesis, and even sweat gland and hair follicle formation.118 Bixin encapsulated in PCL nanofibers also proved its efficacy in reducing scar formation and accelerating the wound healing process in diabetic models.119 PCL nanofibers encapsulating GFs have been also synthetized and tested in chronic wound healing. Choi et al. prepared PCL/PCL-PEG copolymer blends for electrospinning, making nanofibers with a core of bFGF, that afterward were chemically modified in their surface with EGF. Animal studies showed that the double encapsulation with biphasic release profiles rendered a higher collagen accumulation and keratin cement formation, which implemented the wound closure and reduced the scar formation.120
PLGA nanofibers have also a great potential in the development of new wound dressings for chronic wound healing. Molecules such as metformin or recombinant human PDGF121 have been encapsulated in the fiber meshes, showing remarkable wound healing. PLGA has also been combined with cellulose nanocrystals or aloe vera to form nanofibers.122,123 Neurotensin encapsulated PLGA/cellulose nanocrystals induced a faster healing than controls, with a decrease in inflammatory cytokines and a higher epidermal/dermal regeneration.122 PLGA/aloe vera nanofibers encapsulating rhEGF had antimicrobial activity and a faster wound closure and reepithelialization.123
Hydrophilic polymers
Hydrophilic polymers like PEG or PVA have also been studied in wound healing due to their hydrophilicity that allows moisture of the wounds and their similarities with the ECM. PVA has been combined with curdlan and silver nitrate to fabricate nanofiber meshes effective against gram positive and gram negative bacteria. The in vivo results showed an anti-inflammatory activity and a faster wound healing.124 Yang et al. tested whether the incorporation of a GF125 into PLGA-PEG copolymer fibers with a core-shell structure could be beneficial in chronic healing. They found out that the scaffolds had a higher neovascularization, faster wound closure, better reepithelialization, and regeneration of skin appendages.126
Self-assembled peptides
Milder fabrication methods for the encapsulation of biomolecules have also been tested to avoid degradation problems of the encapsulated molecules. Self-assembled peptides like the Ac-RADARADARADARADA-CONH2 (RADA16) form 3D nanofibers when deposited into wounds and can be used as skin bioequivalents. Schneider et al. prepared RAD16 nanofiber encapsulating EGF that increased wound closure rate by fivefold compared to controls in an in vitro human skin equivalent wound healing model.127 Balaji et al. proved that the use of angiogenic injectable peptide nanofibers (ACN–RARADADARARADADA–CNH2) in diabetic wounds promoted the angiogenesis and wound closure and reduced the inflammation.128 Multidomain peptide nanofibers were also checked in chronic wounds, and they showed an accelerated wound healing rate, high vascularization, granulation, and hair follicle regeneration129
Nanofibers made of heparin-mimic amphiphilic peptides also accelerated wound healing in vivo in diabetic-induced and diabetic mice.130 Treated diabetic mice showed an accelerated wound closure, better reepithelialization, induction of angiogenesis, and high levels of VEGF during time. TNF-α was elevated only at early stages proving a transition from inflammation to proliferative phases.130 Pro-inflammatory cytokines were elevated only at early stages proving a transition from inflammation to proliferative phases.130
Liposomes and other self-assembled structures
Liposomes are promising topical drug delivery systems, because they allow the reduction of the drug dosage and it increases the transdermal absorption compared with traditional emulsions and ointments. Liposomes are sphere-shaped vesicles having one or more lipid bilayers. Liposomes can be classified according to their size (from nm to mm), number of bilayers, or the method of fabrication. They are generally prepared from phospholipids and cholesterol.
They can encapsulate hydrophilic (in the internal aqueous compartments) and hydrophobic (in the bilayer) molecules in their structure, making them very suitable for drug delivery131 to have a slower and constant release of the drug. Liposomal formulations used in the treatment of diabetic rodent wounds are shown in Table 4.
Table 4.
Liposomal formulations used for the treatment of diabetic wounds in animal models
| Composition | Biomolecule | Animal Model | Area (cm2) | Time*(Days) | References |
|---|---|---|---|---|---|
| DOTAP+sodium cholate | Keap1 siRNA | Mice+stent+diabetes 2 (db/db) | 0.8 | 24 | 132 |
| Unknown | microRNA miR-132 | Mice+diabetes 2 (db/db) | 0.13 | 6 | 23 |
| RGDK-lipopeptide+cholesterol | PDGF-B gen | Rat+diabetes 1 (STZ) | 13.9 | 10 | 133 |
| Phosphatidylcholine+cholesterol+tween80+stearylamine | Bacteriophages | Mice+infected+diabetes 1 | 0.2 | 7 | 134 |
| Phosphatidylcholine+DOTAP | ATP | Rabbit+ischemic+diabetes 1 | 0.3 | 15 | 136 |
| Hyaluronic acid+cholesterol+DOTAP+hydrogenated phosphatidylcholine | EGF, IGF-1 PDGF-A | Mice+diabetes 1 | 0.5 | 11 | 138 |
| Phosphatidylcholine+cholesterol+phosphatidate+phosphoethanolamine-n-(lissamine rhodamine b sulfonyl) | SDF-1 | Mice+diabetes 2 | 1 | 21 | 139 |
| Phosphocholine+phosphoethanolamine+cholesterol+sphingomyelin | Syndecan-4 PDGF-BB | Mice+stent+diabetes 2 (ob/ob) | 0.2 | 14 | 140 |
Diabetes type 1 was inducted with aloxan (rabbits) or streptozocin (mice and rats) (*times for complete wound closure or more than 90% of full thickness wounds).
ATP, adenosine triphosphate; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; IGF-1, insulin-like growth factor-1; RGDK, Arg-Gly-Asp-Lys.
Cationic liposomes can work as gene delivery systems by forming complexes with the negatively charged nucleic acids. They are made of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP). The encapsulation of genes protects them from degradation, increasing the circulation half-life. Moreover, the overall positive charge of the liposome enhances its endocytosis due to interactions with the cell membrane. They can be administered topically or by intradermic/subcutaneous injections. Rabbani et al. prepared cationic liposomes and peptide-based ternary complexes to increase the transfection of Keap1 siRNA. This molecule can activate antioxidant mechanisms, proving tissue regeneration. They observed that wounds regenerate faster in murine diabetic model.132
Li et al. encapsulated miR-132-loaded liposomes in pluronic F127 gels and applied them to diabetic mice on human ex vivo skin wounds. The gels were administered intradermal in the edges of the diabetic wounds, resulting in an accelerated wound healing closure, which repressed the inflammation and increased proliferation of keratinocytes.
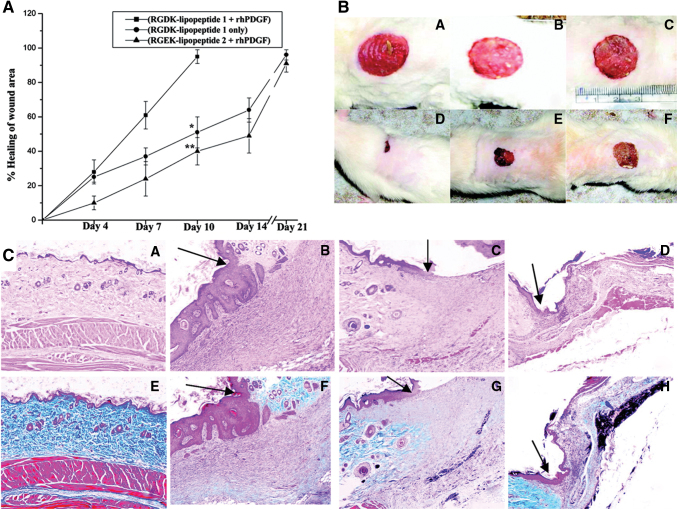
Moreover, in human ex vivo skin wounds, a faster reepithelialization of the wounds was observed.23 They can also encapsulate genes encoding GFs. Bhattacharyya et al. encapsulated the gene encoding for PDGF into liposomes, and with only one subcutaneous administration, wounds were healed with higher degree of epithelialization, keratinization, collagen deposition, and blood vessel formation compared to controls (Fig. 8).133
Figure 8.
Cationic liposomes made of an electrostatic complex between the plasmid of human recombinant PDGF-B, integrin receptor selective RGDK-lipopeptide, and cholesterol are able to improve wound healing in diabetic rats. The administration of RGDK-lipopeptide 1:rhPDGF-B on wound of diabetic animals shows a faster wound healing rate, reepithelialization and fibrocollagen, and keratin and vessel formation. (A) Relative wound healing and (B) wound images before the administration of the treatment (B [A–C]) and after 10 days of treatment (B [D–F]) with RGDK-lipopeptide1:rhPDGF-B complex (B [D]), RGDK-liposome (B [E]), and RGEK-lipopeptide2:rhPDGF-B complex (B [F]) in diabetic rats after the subcutaneous administration of a single dose. (C) Hematoxylin–eosin (C [A–D]) and Masson's trichrome (C [E–H]) tissue staining of wounds before the treatment (C [A, E]) and 10 days after the treatment with RGDK-lipopeptide 1:rhPDGF-B (C [B, F]); liposomes of RGDK-lipopeptide 1 (C [C, G]) and RGEK-lipopeptide 2:rhPDGF-B (C [D, H]). Adapted from 133 with permission. *p < 0.05, **p < 0.01. RGDK, Arg-Gly-Asp-Lys; rhPDGF, recombinant human platelet-derived growth factor. Color images are available online.
Chronic wounds are more likely to develop wound infections, especially with multidrug resistance microorganisms. Thus, new approaches like the phage therapy are appearing. Chhibber et al. encapsulated bacteriophages into cationic liposomes to resolve S. aureus-infected diabetic mice wounds.134 Liposome-treated mice evidenced an accelerated wound closure and healing than the free phages, proving that the reduction of clearance can increase the activity of the phages.
Insulin-loaded liposomes included into a chitosan hydrogel demonstrated its efficacy in chronic wounds in patients, reducing the erythema and the time of wound duration.135 Wang et al. tried the liposomes using a model of diabetic rabbit with ischemic tissue. They topically administered adenosine triphosphate liposomes in the wounds and observed a faster wound closure than the control.136
The encapsulation of GF into liposomes increases its stability and prolongs its release as it protects against degradation in the wound site.137 Choi et al. prepared cationic liposome encapsulating EGF, insulin-like growth factor-1, and PDGF-A. All the GFs were combined with a low-MW protamine, complexed with hyaluronic acid, and then encapsulated in cationic liposomes made of hydrogenated phosphatidylcholine, cholesterol, and DOTAP. Liposomes were able to increase the wound healing ratio and showed reepithelialization and dermal tissue remodeling in diabetic mice ulcers because of the combination of GFs and hyaluronic acid and a sustained release of them.138 SDF-1, known to enhance angiogenesis in ischemic tissues, was encapsulated into liposomes and then included in decellularized dermis scaffolds.139 A higher cell proliferation in the dermis was observed, which resulted in faster wound closure and increased granulation.
Another approach followed for the increase of the activity of GFs was developed by Das et al.140 They studied the delivery of syndecan-4 (a proteoglycan that works as coreceptor of many GFs) into liposomes in combination with PDGF-BB, all embedded in an alginate dressing. They observed an increase in wound closure rate, angiogenesis, and a reduced inflammation.
Micelles are self-assembled structures made of polymers or surfactants that have a hydrophobic and hydrophilic part. They have sizes on the range of 10–100 nm and can encapsulate hydrophobic molecules in its core. Nevertheless, micelles were also poorly explored in wound healing field. Only few authors have reported the use of PLA-PEG,141 PEG-PCL-PEG,142 or pluronic micelles143 for encapsulation of drugs like curcumin. Nanogels144 and nanoemulsions145 have also been poorly explored in the field of wound healing.
Future Directions
The appearance of new biomolecules active in wound healing, such as GFs or nucleic acids, shows the necessity of designing new formulations to protect them from degradation and to deliver them at specific rates. The emergence of nanotechnology, especially the fabrication and characterization of nanoparticulate systems, has increased the number of available interventions for healing of chronic wounds.
The new chronic wound nanotherapeutics are multifunctional platforms that promote wound healing with minimal scar formation, avoid/treat bacteria contamination, and can even release the active biomolecules encapsulated at specific rates that match wound healing necessities. In addition, they need to be highly biocompatible and encapsulate high amounts of the biomolecule. It is also required that they are easy to apply into the wound, which means, for NPs and self-assembling carriers, to be included in another formulation.146
The translation of these technologies into the market and, therefore, in the development of new healing therapies has several hurdles that should be addressed. From a biological perspective, the limited knowledge of the pathophysiology of patients is the major one. Both in the healthy and diseased states, there is a need to understand the in vivo fate of the interactions of NPs with blood, tissue, cellular, and intracellular compartments.147
On the other side, from a technological point of view, the major obstacle is to gain insight into the physicochemical properties of such nanoscale systems, as well as their in vivo behavior and toxicity.67
For nanotechnology systems to have a feasible clinical scalability and market transfer potential, the difficulty in their development requires to be simplified. The goal is to fabricate reproducible, controlled, and monitored nanotechnology platforms. Thus, there is a need to improve the synthesis and characterization of the nanotechnology-based wound healing systems, as well as to introduce site-specificity and targeting ability to decrease the undesirable effects of these nanosystems in the human body. In addition, some aspects like systemic absorption or the polymers/materials used for their fabrication, that in general are no-FDA approved materials, need also to be addressed.
Summary
Chronic wounds present a disrupted repair process, taking several months to show progress. They cause pain, infections, costs, and frequently lead to amputations or sepsis, persisting as a silent epidemic affecting over 40 million people worldwide. Traditional dressings cannot fully address all the wound healing necessities. Thus, new technologies are needed. A high variety of nanoplatforms are being explored for chronic wound treatment, specifically in the case of diabetic wounds. Herein, we focused on NPs, nanofibers, and self-assembling nanocarriers used for the treatment of diabetic wounds. Among NPs, we can categorize them in two groups, active NPs and delivery systems. Metallic (AgNPs, AuNPs, etc.) and ion release NPs have shown their potential as antimicrobial agents and for enhancing the wound closure. On the other side, NPs for drug delivery have been applied for the sustained release of GFs, drugs, or nucleic acids and can be made from different polymers like polyesters, lipids, polysaccharides, or peptides. In general, these systems are included in other formulations (gels, nanofibers) to ease its application. Nanofibers are ideal wound dressings as they can mimic the ECM of the skin and allow the oxygen/nutrient interchange. Moreover, they can load biomolecules in their matrix or even NPs encapsulating biomolecules to render different release profiles of the biomolecules that can match the needs of the wound physiopathology. Polymers like hyaluronic acid, collagen, SF, polyesters, or self-assembled peptides have proved their potential for the fabrication of nanofiber as wound dressing. Liposomes are also interesting approaches for the treatment of wounds, especially for nucleic acids, as they can protect them from degradation and deliver them intracellularly, working as gen delivery systems. Nevertheless, more efforts are required for the obtaining of new nanomaterials that can be used and approved by the regulatory agencies.
Take-Home Messages
Traditional dressings cannot fully address all the chronic wound healing necessities, and new technologies are needed.
Current new nanoplatforms used for chronic wound healing include NPs, nanofibers, and self-assembling nanocarriers.
Metallic and ion release NPs have shown their potential as antimicrobial agents and for improving wound closure. NPs can also be used as delivery systems for therapeutics like GFs, drugs, or nucleic acids.
Nanofibers are ideal wound dressings as they can mimic the skin ECM and allow the oxygen/nutrient interchange. Moreover, they can load biomolecules in their matrix or even nanocarriers encapsulating the active ingredients.
Liposomes have a high potential for the intracellular delivery of genes involved in wound healing.
Bigger efforts are needed to develop new nanomaterials that can be approved by the regulatory agencies. Safety assessment, differences in bioavailability, and scale-up problems, among others, are some of the issues that need to be addressed.
Abbreviations and Acronyms
- 3D
three-dimensional
- AgNPs
silver nanoparticles
- AuNPs
gold nanoparticles
- bFGF
basic fibroblast growth factor
- BG
bioactive glass
- CEC
chain entanglement concentration
- CeONPs
cerium oxide nanoparticles
- Cu2+
copper ions
- DFUs
diabetic foot ulcers
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ELP
elastin-like protein
- FDA
Food and Drug Administration
- GF
growth factor
- GM3S
ganglioside-monosialic acid 3 synthase
- HIF-1a
hypoxia-inducible factor 1-alpha
- HPMC
hydroxypropyl methylcellulose
- IGF-1
insulin-like growth factor-1
- KGF
keratinocyte growth factor
- LNP
lipid nanoparticle
- miRNA
microRNA
- MMP-9
matrix metalloproteinase-9
- MOFs
metal−organic frameworks
- mRNA
messenger RNA
- MSC
mesenchymal stem cell
- MW
molecular weight
- NHPUs
nonhealing pressure ulcers
- NO
nitric oxide
- NPs
nanoparticles
- PCL
polycaprolactone
- PEG
polyethylene glycol
- PEO
polyethylene oxide
- PLA
poly(lactide)
- PLGA
poly(lactide-co-glycolide)
- PVA
poly(vinyl alcohol)
- PU
polyurethane
- rhPDGF
recombinant human platelet-derived growth factor
- ROS
reactive oxygen species
- SDF-1
stromal cell-derived factor-1
- SF
silk fibroin
- Si
silicon
- TNFα
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- VUs
venous ulcers
- ZnO
zinc oxide
Acknowledgments and Funding Sources
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER) through the project MAT2012–38793 and MAT2015-68906-R and the Dermoglass project funded by CaixaImpulse Programme of Obra Social La Caixa (CaixaImpulse CI0015). Barbara Blanco-Fernandez acknowledges the Marie Skłodowska-Curie grant (agreement no. 712754) and the Severo Ochoa grant (SEV-2014-0425). O. Castano acknowledges the support from the Serra Hunter programme.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Barbara Blanco-Fernandez, PhD, is postdoc (Marie Skłodowska-Curie fellow) in the group of Biomaterials for Regenerative therapies at the IBEC. She holds a PhD in Pharmaceutical Science from the University of Santiago de Compostela. Previously, she joined as a postdoctoral research associate to the Molecular and Cellular Imaging Laboratory at Michigan State University. She worked as a nanotechnology scientist for skin delivery at the Pharmaceutical Company Reig Jofre. Oscar Castaño, PhD, is a Serra Hunter Fellow at the Electronics and Biomedical Engineering, Universitat de Barcelona. Miguel Ángel Mateos-Timoneda, PhD, is a Senior Researcher affiliated at the CIBER-BBN in the group Biomaterials for Regenerative Therapies (IBEC). Elisabeth Engel, PhD, is the Group Leader of the Biomaterials for Regenerative Therapies group at IBEC and full professor in the Technical University of Catalonia (UPC). Soledad Pérez-Amodio, PhD, is a Senior Researcher affiliated at the CIBER-BBN in the group of Biomaterials for Regenerative Therapies (IBEC). She is also an associate professor in the UPC. She holds a PhD in Cellular and Molecular Biology from the University of Amsterdam. She worked as a postdoctoral researcher at the Tissue Regeneration Group (University of Enschede), Plastic and Reconstructive Surgery (Erasmus Medical Center, Rotterdam), and the department of Oral Cell Biology (Vrij University).
REFERENCES
- 1. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 2017;34:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurd T. Understanding the financial benefits of optimising wellbeing in patients living with a wound. Wounds Int 2013;4:13–17 [Google Scholar]
- 4. Martinengo LOM, Bajpai R, Soljak M, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15 [DOI] [PubMed] [Google Scholar]
- 5. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 2017;26:20–25 [DOI] [PubMed] [Google Scholar]
- 6. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4:560–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurd T, Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 2014;3:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickinson LE, Gerecht S. Engineered biopolymeric scaffolds for chronic wound healing. Front Physiol 2016;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery 2011;29:491–495 [Google Scholar]
- 10. Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther 2013;26:197–206 [DOI] [PubMed] [Google Scholar]
- 11. Goodarzi P, Larijani B, Alavi-Moghadam S, et al. Mesenchymal stem cells-derived exosomes for wound regeneration. Adv Exp Med Biol 2018;1119:119–131 [DOI] [PubMed] [Google Scholar]
- 12. Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol 2016;25:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gainza G, Bonafonte DC, Moreno B, et al. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J Control Release 2015;197:41–47 [DOI] [PubMed] [Google Scholar]
- 14. Losi P, Briganti E, Magera A, et al. Biomaterials Tissue response to poly (ether) urethane polydimethylsiloxane-fi brin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 2010;31:5336–5344 [DOI] [PubMed] [Google Scholar]
- 15. Lai HJ, Kuan CH, Wu HC, et al. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater 2014;10:4156–4166 [DOI] [PubMed] [Google Scholar]
- 16. Robson MC, Steed D, Franz M. Wound healing. Biologic features and approaches to maximize healing trajectories. Curr Probl Surg 2001;38:72–140 [DOI] [PubMed] [Google Scholar]
- 17. Pourmoussa A, Gardner DJ, Johnson MB, Wong AK. An update and review of cell-based wound dressings and their integration into clinical practice. Ann Transl Med 2016;4:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brennecke J, Stark A, Russell RBC, Cohen SM. Principles of microRNA-target recognition. PLoS Biol 2005;3:0404–0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun LL, Li WD, Lei FR, Li XQ. The regulatory role of microRNAs in angiogenesis-related diseases. J Cell Mol Med 2018;22:4568–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 2012;9:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Yang C, Wang XY, et al. MicroRNA-129 and -335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes 2018;67:1627–1638 [DOI] [PubMed] [Google Scholar]
- 23. Li X, Li D, Wang A, et al. MicroRNA-132 with therapeutic potential in chronic wounds. J Invest Dermatol 2017;137:2630–2638 [DOI] [PubMed] [Google Scholar]
- 24. Mu P, Lucas T, Scha F, Eming SA, Heckel A, Dimmeler S. Light-inducible antimiR-92a as a therapeutic strategy to promote skin repair in healing-impaired diabetic mice. Nat Commun 2017;8:15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen 2018;26:311–323 [DOI] [PubMed] [Google Scholar]
- 26. Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018;8:169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care 2014;3:511–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chatterjee AK, Chakraborty R, Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014;25:135101. [DOI] [PubMed] [Google Scholar]
- 29. Shaikh S, Nazam N, Rizvi SMD, et al. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci 2019;20:2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hijazi S, Visaggio D, Pirolo M, Frangipani E, Bernstein L, Visca P. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front Cell Infect Microbiol 2018;8:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh K, Mishra A, Sharma D, Singh K. Antiviral and antimicrobial potentiality of nano drugs. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, eds. Micro and Nano Technologies, Applications of Targeted Nano Drugs and Delivery Systems. Amsterdam, The Netherlands:Elsevier, 2019:343–356 [Google Scholar]
- 32. Gabrielyan L, Hovhannisyan A, Gevorgyan V, Ananyan M, Trchounian A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl Microbiol Biotechnol 2019;103:2773–2782 [DOI] [PubMed] [Google Scholar]
- 33. Wang S, Yan C, Zhang X, et al. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci 2018;6:2757–2772 [DOI] [PubMed] [Google Scholar]
- 34. Drake PL, Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg 2005;49:575–585 [DOI] [PubMed] [Google Scholar]
- 35. Castellano JJ, Shafii SM, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J 2007;4:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 2010;12:1531–1551 [Google Scholar]
- 37. Mihai MM, Dima MB, Dima B, Holban AM. Nanomaterials for wound healing and infection control. Materials 2019;12:2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akter M, Sikder MT, Rahman MM, et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res 2018;9:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fouda MMG, Abdel-Mohsen AM, Ebaid H, et al. Wound healing of different molecular weight of hyaluronan; in-vivo study. Int J Biol Macromol 2016;89:582–591 [DOI] [PubMed] [Google Scholar]
- 40. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm 2019;559:23–36 [DOI] [PubMed] [Google Scholar]
- 41. Choi JB, Park JS, Khil MS, et al. Characterization and antimicrobial property of poly(acrylic acid) nanogel containing silver particle prepared by electron beam. Int J Mol Sci 2013;14:11011–11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choi HJ, Thambi T, Yang YH, et al. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv 2017;7:13714–13725 [Google Scholar]
- 43. Shi G, Chen W, Zhang Y, Dai X, Zhang X, Wu Z. An antifouling hydrogel containing silver nanoparticles for modulating the therapeutic immune response in chronic wound healing. Langmuir 2019;35:1837–1845 [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y, Li Z, Song S, et al. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv Funct Mater 2019;29:1901474 [Google Scholar]
- 45. Singla R, Soni S, Patial V, et al. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int J Biol Macromol 2017;105:45–55 [DOI] [PubMed] [Google Scholar]
- 46. Singla R, Soni S, Patial V, et al. Cytocompatible anti-microbial dressings of syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci Rep 2017;7:10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev 2010;3:CD006478. [DOI] [PubMed] [Google Scholar]
- 48. Dumville JC, Gray TA, Walter CJ, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2016;12:CD003091. [DOI] [PubMed] [Google Scholar]
- 49. Ambrogi V, Donnadio A, Pietrella D, et al. Chitosan films containing mesoporous SBA-15 supported silver nanoparticles for wound dressing. J Mater Chem B 2014;2:6054–6063 [DOI] [PubMed] [Google Scholar]
- 50. Bergin S, Wraight PS. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev 2006;25:CD005082. [DOI] [PubMed] [Google Scholar]
- 51. Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-based dressing: the future of wound treatment? Trends Biotechnol 2017;35:770–784 [DOI] [PubMed] [Google Scholar]
- 52. Chen SA, Chen HM, Yao YD, Hung CF, Tu CS, Liang YJ. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci 2012;47:875–883 [DOI] [PubMed] [Google Scholar]
- 53. Martinez SPH, Gonzalez TIR, Molina MAF, et al. A novel gold calreticulin nanocomposite based on chitosan for wound healing in a diabetic mice model. Nanomaterials 2019;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Greives MR, Samra F, Pavlides SC, et al. Exogenous calreticulin improves diabetic wound healing. Wound Repair Regen 2012;20:715–730 [DOI] [PubMed] [Google Scholar]
- 55. Randeria PS, Seeger MA, Wang XQ, et al. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad Sci U S A 2015;112:5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao Y, Cai Q, Qi W, et al. BSA-CuS nanoparticles for photothermal therapy of diabetic wound infection in vivo. ChemistrySelect 2018;3:9510–9516 [Google Scholar]
- 57. Bhadauriya P, Mamtani H, Ashfaq M, et al. Synthesis of yeast-immobilized and copper nanoparticle-dispersed carbon nanofiber-based diabetic wound dressing material: simultaneous control of glucose and bacterial infections. ACS Appl Bio Mater 2018;1:246–258 [DOI] [PubMed] [Google Scholar]
- 58. Borkow G, Gabbay J, Dardik R, et al. Molecular mechanisms of enhanced wound healing by copperoxide-impregnated dressings. Wound Repair Regen 2010;18:266–275 [DOI] [PubMed] [Google Scholar]
- 59. Gérard C, Bordeleau LJ, Barralet J, Doillon CJ. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010;31:824–831 [DOI] [PubMed] [Google Scholar]
- 60. Wu C, Zhou Y, Xu M, et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013;34:422–433 [DOI] [PubMed] [Google Scholar]
- 61. Sen CK, Khanna S, Venojarvi M, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol 2002;282:1821–1827 [DOI] [PubMed] [Google Scholar]
- 62. Xiao J, Zhu Y, Huddleston S, et al. Copper metal-organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes. ACS Nano 2018;12:1023–1032 [DOI] [PubMed] [Google Scholar]
- 63. Xiao J, Chen S, Yi J, Zhang HF, Ameer GA. A cooperative copper metal-organic framework-hydrogel system improves wound healing in diabetes. Adv Funct Mater 2017;27:1604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical application. Drug Discov Today 2017;22:1825–1834 [DOI] [PubMed] [Google Scholar]
- 65. Castaño O, Pérez-Amodio S, Navarro-Requena C, Mateos-Timoneda MA, Engel E. Instructive microenvironments in skin wound healing: biomaterials as signal releasing platforms. Adv Drug Deliv Rev 2018;129:95–117 [DOI] [PubMed] [Google Scholar]
- 66. Fu JP, Zhang Y, Chu J, et al. Oxide incorporated acellular dermal composite scaffold enables efficient local delivery of mesenchymal stem cells for accelerating diabetic wound healing. ACS Biomater Sci Eng 2019;5:4054–4066 [DOI] [PubMed] [Google Scholar]
- 67. Hamdan S, Pastar I, Drakulich S, et al. Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci 2017;3:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Augustine R, Hasan A, Patan NK, et al. Cerium oxide nanoparticle incorporated electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater Sci Eng 2019. (in press: doi.org/10.1021/acsbiomaterials.1028b01352) [DOI] [PubMed] [Google Scholar]
- 69. Zgheib C, Hilton SA, Dewberry LC, et al. Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg 2019;228:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nazarii K, Ludovico A, Liudmyla K, Dmytro K, Mykola S. Neuropathic diabetic foot ulcers treated with cerium dioxide nanoparticles: a case report. Diabetes Metab Syndr 2019;13:228–234 [DOI] [PubMed] [Google Scholar]
- 71. Miguez-Pacheco V, Hench LL, Boccaccini AR. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater 2015;13:1–15 [DOI] [PubMed] [Google Scholar]
- 72. Lin C, Mao C, Zhang J, Li Y, Chen X. Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed Mater 2012;7:045017. [DOI] [PubMed] [Google Scholar]
- 73. Jiang Y, Han Y, Wang J, et al. Space-oriented nanofibrous scaffold with silicon-doped amorphous calcium phosphate nanocoating for diabetic wound healing. ACS Applied Bio Materials 2019;2:787–795 [DOI] [PubMed] [Google Scholar]
- 74. Turner CT, McInnes SJP, Melville E, Cowin AJ, Voelcker NH. Wound healing: delivery of flightless I neutralizing antibody from porous silicon nanoparticles improves. Wound healing in diabetic mice. Adv Healthc Mater 2017;6:1600707. [DOI] [PubMed] [Google Scholar]
- 75. Samaha R, Othman AI, El-Sherbiny IM, et al. Topical nitric oxide in nanoformulation enhanced wound healing in experimental diabetes in mice. Res J Pharm Biol Chem Sci 2017;8:499–514 [Google Scholar]
- 76. Hasan N, Cao J, Lee J, et al. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mat Sci Eng C-Mater 2019;103:109741. [DOI] [PubMed] [Google Scholar]
- 77. Chereddy KK, Lopes A, Koussoroplis S, et al. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine 2015;11:1975–1984 [DOI] [PubMed] [Google Scholar]
- 78. Abdelkader DH, Tambuwala MM, Mitchell CA, et al. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly(vinyl alcohol)-borate hydrogels. Drug Deliv Transl Res 2018;8:1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bairagi U, Mittal P, Singh J, Mishra B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev Ind Pharm 2018;44:1783–1796 [DOI] [PubMed] [Google Scholar]
- 80. Losi P, Briganti E, Errico C, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 2013;9:7814–7821 [DOI] [PubMed] [Google Scholar]
- 81. Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Rep Reg 2010;18:499–505 [DOI] [PubMed] [Google Scholar]
- 82. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor a to improve wound healing in diabetic mice. Bioeng Transl Med 2019;4:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qayoom A, Aneesha VA, Anagha S, Dar JA, Kumar P, Kumar D. Lecithin-based deferoxamine nanoparticles accelerated cutaneous wound healing in diabetic rats. Eur J Pharm 2019;858:172478. [DOI] [PubMed] [Google Scholar]
- 84. Koria P, Yagi H, Kitagawa Y, et al. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Natl Acad Sci U S A 2011;108:1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yeboah A, Cohen RI, Faulknor R, Schloss R, Yarmush ML, Berthiaume F. The development and characterization of SDF1a-elastin-like-peptide nanoparticles for wound healing. J Control Release 2016;232:238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yeboah A, Maguire T, Schloss R, Berthiaume F, Yarmush ML. Stromal cell-derived growth factor-1 alpha-elastin like peptide fusion protein promotes cell migration and revascularization of experimental wounds in diabetic mice. Adv Wound Care 2017;6:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang T, Zheng Y, Shi Y, Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res 2019;9:227–239 [DOI] [PubMed] [Google Scholar]
- 88. Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci 2014;14:772–792 [DOI] [PubMed] [Google Scholar]
- 89. Gizaw M, Faglie A, Pieper M, Poudel S, Chou SF. The role of electrospun fiber scaffolds in stem cell therapy for skin tissue regeneration. Med One 2019;4:e190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nie H, He A, Zheng J, Xu S, Li J, Han CC. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromolecules 2008;9:1362–1365 [DOI] [PubMed] [Google Scholar]
- 91. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics 2018;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hajiali H, Summa M, Russo D, et al. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J Mater Chem B 2016;4:1686–1695 [DOI] [PubMed] [Google Scholar]
- 93. Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM, Ferrari F. Hyaluronic acid and chitosan-based nanosystems: a new dressing generation for wound care. Expert Opin Drug Deliv 2019;16:715–740 [DOI] [PubMed] [Google Scholar]
- 94. Xie Z, Paras CB, Weng H, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013;9:9351–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ahmadi Majd S, Rabbani Khorasgani M, Moshtaghian S, Talebi A, Khezri M. Application of Chitosan/PVA Nano fiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int J Biol Macromol 2016;92:1162–1168 [DOI] [PubMed] [Google Scholar]
- 96. Chen H, Jia P, Kang H, et al. Upregulating Hif-1α by hydrogel Nanofi brous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016;5:907–918 [DOI] [PubMed] [Google Scholar]
- 97. Ahmed R, Tariq M, Ali I, et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol 2018;120:385–393 [DOI] [PubMed] [Google Scholar]
- 98. Ma W, Yang X, Ma L, et al. Fabrication of bioactive glass-introduced nanofibrous membranes with multifunctions for potential wound dressing. RSC Adv 2014;4:60114–60122 [Google Scholar]
- 99. Abdel-Mohsen AM, Jancar J, Abdel-Rahmana RM, et al. A novel in situ silver/hyaluronan bio-nanocomposite fabrics for wound and chronic ulcer dressing: in vitro and in vivo evaluations. Int J Pharm 2017;520:241–253 [DOI] [PubMed] [Google Scholar]
- 100. Grip J, Engstad RE, Skjaeveland I, et al. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur J Pharm Sci 2018;121:269–280 [DOI] [PubMed] [Google Scholar]
- 101. Shahverdi S, Hajimiri M, Esfandiari MA, et al. Fabrication and structure analysis of poly(lactide-co-glycolic acid)/silk fibroin hybrid scaffold for wound dressing applications. Int J Pharm 2014;473:345–355 [DOI] [PubMed] [Google Scholar]
- 102. Chouhan D, Chakraborty B, Nandi SK, Mandal BB. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater 2017;48:157–174 [DOI] [PubMed] [Google Scholar]
- 103. Chouhan D, Janani G, Chakraborty B, Nandi SK, Mandal BB. Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J Tissue Eng Regen Med 2018;12:e1559–e1570 [DOI] [PubMed] [Google Scholar]
- 104. Li X, Liu Y, Zhang J, You R, Qua J, Li M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater Sci Eng C 2017;72:394–404 [DOI] [PubMed] [Google Scholar]
- 105. Chouhan D, Das P, Thatikonda N, Nandi SK, Hedhammar M, Mandal BB. Silkworm silk matrices coated with functionalized spider silk accelerate healing of diabetic wounds. ACS Biomater Sci Eng 2019;5:3537–3548 [DOI] [PubMed] [Google Scholar]
- 106. Navone SE, Pascucci L, Dossena M, et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther 2014;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dongargaonkar AA, Bowlin GL, Yang H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound dressing and drug delivery platform. Biomacromolecules 2013;14:4038–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Aduba DC, An SS, Selders GS, et al. Electrospun gelatin–arabinoxylan ferulate composite fibers for diabetic chronic wound dressing application. Int J Polym Mater 2019;68:660–668 [Google Scholar]
- 109. Wan W, Cai F, Huang J, Chen S, Liao Q. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J Mater Chem B 2019;7:2954–2961 [Google Scholar]
- 110. Lv F, Wang J, Xu P, et al. A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater 2017;60:128–143 [DOI] [PubMed] [Google Scholar]
- 111. Sun L, Gao W, Fu X, et al. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater Sci 2018;6:340–349 [DOI] [PubMed] [Google Scholar]
- 112. Gao W, Sun L, Fu X, et al. Enhanced diabetic wound healing by electrospun core–sheath fibers loaded with dimethyloxalylglycine. J Mater Chem B 2018;6:277–288 [DOI] [PubMed] [Google Scholar]
- 113. Gao W, Jin W, Li Y, et al. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J Mater Chem B 2017;5:7285–7296 [DOI] [PubMed] [Google Scholar]
- 114. Lee CH, Chao YK, Chang SH, et al. Nanofibrous rhPDGF-eluting PLGA–collagen hybrid scaffolds enhance healing of diabetic wounds. RSC Adv 2016;6:6276–6284 [Google Scholar]
- 115. Lee CH, Chang SH, Chen WJ, et al. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J Colloid Interface Sci 2015;439:88–97 [DOI] [PubMed] [Google Scholar]
- 116. Ren X, Han Y, Wang J, et al. An aligned porous electrospun fibrous membrane with controlled drug delivery—an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater 2018;70:140–153 [DOI] [PubMed] [Google Scholar]
- 117. Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly(e-caprolactone) nanofibres: diabetic wound dressing with anti-oxidant and antiinflammatory properties. Clin Exp Pharmacol Physiol 2009;36:1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mohammadi MR, Rabbani S, Bahrami SH, Joghataei MT, Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(e-caprolactone) electrospun nanofibers. Mater Sci Eng C Mater Biol Appl 2016;69:1183–1191 [DOI] [PubMed] [Google Scholar]
- 119. Pinzon-Garcia AD, Cassini-Vieira P, Ribeiro CC, et al. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J Biomed Mater Res B 2017;105:1938–1949 [DOI] [PubMed] [Google Scholar]
- 120. Choi JS, Choi SH, Yoo HS. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J Mater Chem 2011;21:5258–5267 [Google Scholar]
- 121. Lee CH, Liu KS, Chang SH, et al. Promoting diabetic wound therapy using biodegradable rhPDGF-loaded nanofibrous membranes: CONSORT-compliant article. Medicine 2015;94:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zheng Z, Liu Y, Huang W, et al. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif Cells Nanomed Biotechnol 2018;46:493–501 [DOI] [PubMed] [Google Scholar]
- 123. Garcia-Orue I, Gainza G, Gutierrez FB, et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm 2017;523:556–566 [DOI] [PubMed] [Google Scholar]
- 124. Basha RY, Kumar TSS, Ramasamy S, Doble M. Silver loaded nanofibrous curdlan mat for diabetic wound healing: an in vitro and in vivo study. Macromol Mater Eng 2018;303:1800234 [Google Scholar]
- 125. Xiang Q, Xiao J, Zhang H, et al. Preparation and characterisation of bFGF-encapsulated liposomes and evaluation of wound-healing activities in the rat. Burns 2011;37:886–895 [DOI] [PubMed] [Google Scholar]
- 126. Yang Y, Xia T, Zhi W, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011;32:4243–4254 [DOI] [PubMed] [Google Scholar]
- 127. Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS One 2008;3:e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Balaji S, Vaikunth SS, Lang SA, et al. Tissue-engineered provisional matrix as a novel approach to enhance diabetic wound healing. Wound Rep Reg 2012;20:15–27 [DOI] [PubMed] [Google Scholar]
- 129. Carrejo NC, Moore AN, Lopez Silva TL, et al. Multidomain peptide hydrogel accelerates healing of full-thickness wounds in diabetic mice. ACS Biomater Sci Eng 2018;4:1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Senturk B, Mercan S, Delibasi T, Guler MO, Tekinay AB. Angiogenic peptide nanofibers improve wound healing in STZ induced diabetic rats. ACS Biomater Sci Eng 2016;2:1180–1189 [DOI] [PubMed] [Google Scholar]
- 131. Ferreira H, Matamá T, Silva R, Silva C, Gomes AC, Cavaco-Paulo A. Functionalization of gauzes with liposomes entrapping an anti-inflammatory drug: a strategy to improve wound healing. React Funct Polym 2013;73:1328–1334 [Google Scholar]
- 132. Rabbani PS, Zhou A, Borab ZM, et al. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials 2017;132:1–15 [DOI] [PubMed] [Google Scholar]
- 133. Bhattacharyya J, Mondal G, Madhusudana K, et al. Single subcutaneous administration of RGDK-lipopeptide: rhPDGF-B gene complex heals wounds in streptozotocin-induced diabetic rats. Mol Pharm 2009;6:918–927 [DOI] [PubMed] [Google Scholar]
- 134. Chhibber S, Kaur J, Kaur S. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front Microbiol 2018;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dawoud MHS, Yassin GE, Ghorab DM, Morsi NM. Insulin mucoadhesive liposomal gel for wound healing: a formulation with sustained release and extended stability using quality by design approach. AAPS PharmSciTech 2019;20:158. [DOI] [PubMed] [Google Scholar]
- 136. Wang J, Wan R, Mo Y, Li M, Zhang Q, Chien S. Intracellular delivery of adenosine triphosphate enhanced healing process in full-thickness skin wounds in diabetic rabbits. Am J Surg 2010;199:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ternullo S, Basnet P, Holsaeter AM, Flaten GE, Weerd L, Škalko-Basnet N. Deformable liposomes for skin therapy with human epidermal growth factor: the effect of liposomal surface charge. Eur J Pharm Sci 2018;125:163–171 [DOI] [PubMed] [Google Scholar]
- 138. Choi JU, Lee WW, Pangeni R, Byun Y, Yoon IS, Park JW. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater 2017;57:197–215 [DOI] [PubMed] [Google Scholar]
- 139. Olekson MAP, Faulknor R, Bandekar A, Sempkowski M, Hsia HC, Berthiaume F. SDF-1 liposomes promote sustained cell proliferation in mouse diabetic wounds. Wound Rep Reg 2015;23:711–723 [DOI] [PubMed] [Google Scholar]
- 140. Das S, Majid M, Bake AB. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomater 2016;42:56–65 [DOI] [PubMed] [Google Scholar]
- 141. Alibolandi M, Mohammadi M, Taghdisi SM, Abnous K, Ramezani M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int J Pharm 2017;532:466–477 [DOI] [PubMed] [Google Scholar]
- 142. Gong C, Wu Q, Wang Y, et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013;34:6377–6387 [DOI] [PubMed] [Google Scholar]
- 143. Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterial 2018;183:185–199 [DOI] [PubMed] [Google Scholar]
- 144. Kobayashi H, Katakura O, Morimoto N, Akiyoshi K, Kasugai S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J Biomed Mater Res B Appl Biomater 2009;91:55–60 [DOI] [PubMed] [Google Scholar]
- 145. Shanmugapriya K, Kim H, Kang H. A new alternative insight of nanoemulsion conjugated with κ-carrageenan for wound healing study in diabetic mice: in vitro and in vivo evaluation. Eur J Pharm Sci 2019;133:236–250 [DOI] [PubMed] [Google Scholar]
- 146. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guthrie KM, Agarwal A, Teixeira LBC, et al. Integration of silver nanoparticle-impregnated polyelectrolyte multilayers into murine-splinted cutaneous wound beds. J Burn Care Res 2013;34:e359–e367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Montaser AS, Abdel-Mohsen AM, Ramadan MA, et al. Preparation and characterization of alginate/silver/nicotinamide nanocomposites for treating diabetic wounds. Int J Biol Macromol 2016;92:739–747 [DOI] [PubMed] [Google Scholar]
- 149. El-Naggar MY, Gohar YM, Sorour MA, Waheeb MG. Hydrogel dressing with a nano-formula against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa diabetic foot bacteria. J Microbiol Biotechn 2016;26:408–420 [DOI] [PubMed] [Google Scholar]
- 150. Huang KT, Fang YL, Hsieh PS, Li CC, Dai NT, Huang CJ. Non-sticky and antimicrobial zwitterionic nanocomposite dressings for infected chronic wounds. Biomater Sci 2017;5:1072–1081 [DOI] [PubMed] [Google Scholar]
- 151. Kaur P, Sharma AK, Nag D, et al. Novel nano-insulin formulation modulates cytokine secretion and remodeling to accelerate diabetic wound healing Nanomedicine 2018;15:47–57 [DOI] [PubMed] [Google Scholar]
- 152. Dai X, Guo Q, Zhao Y, et al. Functional silver nanoparticle as a benign antimicrobial agent that eradicates antibiotic-resistant bacteria and promotes wound healing. ACS Appl Mater Interfaces 2016;8:25798–25807 [DOI] [PubMed] [Google Scholar]
- 153. Krishnan N, Velramar B, Pandiyan R, Velu RK. Anti-pseudomonal and anti-endotoxic effects of surfactin-stabilized biogenic silver nanocubes ameliorated wound repair in streptozotocin-induced diabetic mice. Artif Cells Nanomed Biotechnol 2018;46:488–499 [DOI] [PubMed] [Google Scholar]
- 154. Krishnan N, Velramar B, Ramatchandirin B, et al. Effect of biogenic silver nanocubes on matrix metalloproteinases 2 and 9 expressions in hyperglycemic skin injury and its impact in early wound healing in streptozotocin-induced diabetic mice. Mater Sci Eng C Mater Biol Appl 2018;91:146–152 [DOI] [PubMed] [Google Scholar]
- 155. Huang YH, Chen CY, Chen PJ, et al. Gas-injection of gold nanoparticles and anti-oxidants promotes diabetic wound healing. RSC Adv 2014;4:4656–4662 [Google Scholar]
- 156. Karri VV, Kuppusamy G, Talluri SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol 2016;93:1519–1529 [DOI] [PubMed] [Google Scholar]
- 157. Tam K, Cheyyatraviendran S, Venugopal J, et al. A nanoscaffold impregnated with human Wharton's jelly stem cells or its secretions improves healing of wounds. J Cell Biochem 2014;115:794–803 [DOI] [PubMed] [Google Scholar]
- 158. Lee CH, Hsieh MJ, Chang SH, et al. Enhancement of diabetic wound repair using biodegradable nanofibrous metformin-eluting membranes: in vitro and in vivo. ACS Appl Mater Interfaces 2014;6:3979–3986 [DOI] [PubMed] [Google Scholar]