Abstract
Endothelial-to-mesenchymal transition (EndMT) is a process that encompasses extensive transcriptional reprogramming of activated endothelial cells leading to a shift toward mesenchymal cellular phenotypes and functional responses. Initially observed in the context of embryonic development, in the last few decades EndMT is increasingly recognized as a process that contributes to a variety of pathologies in the adult organism. Within the settings of cardiovascular biology, EndMT plays a role in various diseases, including atherosclerosis, heart valvular disease, cardiac fibrosis, and myocardial infarction. EndMT is also being progressively implicated in development and progression of pulmonary hypertension (PH) and pulmonary arterial hypertension (PAH). This review covers the current knowledge about EndMT in PH and PAH, and provides comprehensive overview of seminal discoveries. Topics covered include evidence linking EndMT to factors associated with PAH development, including hypoxia responses, inflammation, dysregulation of bone-morphogenetic protein receptor 2 (BMPR2), and redox signaling. This review amalgamates these discoveries into potential insights for the identification of underlying mechanisms driving EndMT in PH and PAH, and discusses future directions for EndMT-based therapeutic strategies in disease management.
Keywords: pulmonary arterial hypertension, pulmonary hypertension, endothelial-to-mesenchymal transition, EndMT, EndoMT, pulmonary circulation
Introduction
Pulmonary arterial hypertension (PAH) is a cardiopulmonary disorder classified as a subgroup (Group I) of pulmonary hypertension (PH) and defined clinically as an elevation of pulmonary arterial pressure associated with maladaptive right heart dysfunction and hypertrophy (97). Whereas PAH is considered a primary disease (97), PH is an umbrella term that, in addition to PAH, encompasses diseases of heart and lung circulation of various etiologies, where elevated pulmonary pressure and pulmonary vascular and cardiac remodeling present as secondary symptoms to other underlying conditions. These are grouped as follows: PH due to left heart disease (Group II PH), PH due to lung diseases and/or hypoxia (Group III), PH due to pulmonary artery obstructions (Group IV), and PH with unclear and/or multifactorial mechanisms (Group V) (162). PAH is more prevalent in females (71), and, while often idiopathic, multiple risk factors, such as mutations in bone-morphogenetic protein (BMP) receptor 2 (BMPR2) as well as genes of transforming growth factor beta (TGF-β) family (i.e., activin receptor-like kinase 1, ALK1, and endoglin, ENG) and others, viral and parasitic infections (HIV and Schistosomiasis), autoimmune connective tissue disorders (scleroderma), as well as drug use (weight loss and appetite suppressant drugs, exposure to amphetamines and toxic rapeseed oil) predispose to PAH development (161). An update of the hemodynamic definition of PAH proposed at the 2018 World Symposium on Pulmonary Hypertension (WSPH) suggested a change of the cutoff diagnostic value for the mean pulmonary arterial pressure from >25 mmHg to >20 mmHg, paired with an inclusion of pulmonary vascular resistance ≥3 Wood Units and pulmonary arterial wedge pressure <15 mmHg in the clinical diagnosis of PAH (9, 70, 162) (Fig. 1). At the tissue level, dysregulated vascular homeostasis ultimately leads to extensive pulmonary vascular remodeling characterized by perivascular inflammation, vessel narrowing, vascular wall thickening, and vaso-occlusion (160, 179). In the efforts to identify triggering events for pulmonary vascular remodeling, attention of many research groups has been focused on the role of endothelium in PAH (23, 77). Interestingly, in the recent years, an event termed “endothelial-to-mesenchymal” transition (EndMT), also sometimes referred to as endothelial-to-mesenchymal transdifferentiation, emerged as one such potentiating mechanism (168).
FIG. 1.
Definition (left) and vascular manifestations (right) of PAH. Extensive vascular remodeling in PAH is manifested in neointimal and medial thickening resulting from endothelial and smooth muscle cell proliferation, fibrosis, and inflammatory cell infiltration. mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance. Color images are available online.
While EndMT primarily occurs during embryonic development and was first described in the context of endocardial cell differentiation during heart cushion morphogenesis (177), EndMT has also been implicated in the processes of wound healing (121, 137) and inflammation (140, 150) in adult organisms and was shown to contribute to the development of a variety of cardiovascular diseases, including atherosclerosis (46, 67), valvular disease (37, 69, 111), fibroelastosis (195), and most recently, PH (61, 147). A highly dynamic process controlled by a set of developmental signaling pathways, EndMT, is somewhat loosely defined as a process by which endothelial cells (ECs) undergo a shift toward a mesenchymal-like cellular state (141). Nonetheless, EndMT is a complex process whose progression encompasses a broad range of intermediate phenotypes and multiple end-points, and the sequence of signaling events occurring during its progression has been henceforth difficult to fully elucidate and concisely define. Akin to epithelial-to-mesenchymal transition (EMT) (131, 166), induction of EndMT invokes activation of multiple transcription factors, most notably zinc finger transcription factors Snail (SNAI1) (119), Slug (SNAI2) (35), Zeb1 (26), and Zeb2 (30), and a basic helix–loop–helix (bHLH) transcription factor Twist-1 (113), which were first identified as transcriptional repressors of E-cadherin during EMT (12, 19, 34, 44, 181). Acting in concert, these transcription factors function as repressors and/or activators of endothelial and mesenchymal gene expression, commanding the loss of expression of characteristic endothelial marker proteins such as Von Willebrand Factor (vWF, also known as Factor VIII-related antigen), platelet endothelial cell adhesion molecule (PECAM-1, also known as cluster of differentiation 31, CD31), vascular endothelial cadherin (VE-cadherin, also known as type 2 cadherin 5 and CD144), vascular endothelial growth factor receptor (VEGFR), and angiopoietin receptor (Tie-2), and gain of expression of mesenchymal proteins, including S100 calcium binding protein A4 (S100A4, also known as fibroblast-specific protein 1, FSP-1), alpha-smooth muscle actin (α-SMA), transgelin (SM22α), fibronectin (FBN), and vimentin (31) (Fig. 2). Ultimately, genetic cell reprogramming and phenotypic switching seen in EndMT result in a change in cell morphology from compact cobblestone to elongated and spindle-like due to cytoskeletal remodeling and alterations in extracellular matrix composition (7, 163).
FIG. 2.
Sequence of events in the course of EndMT progression. Induction of EndMT-associated transcription factors Snai1, Snai2, Twist-1, Zeb1, and Zeb2 results in progressive loss of endothelial markers PECAM-1 and VE-cadherin, and gain of mesenchymal markers vimentin, fibronectin, SM22α, and α-SMA. α-SMA, alpha-smooth muscle actin; EndMT, endothelial-to-mesenchymal transition; PECAM-1, platelet endothelial cell adhesion molecule; SM22α, transgelin; Snai1, Snail; Snai2, Slug; VE-cadherin, vascular endothelial cadherin. Color images are available online.
In this review, we focus on discussing seminal findings and review some of the newest advances in our understanding of the role of EndMT in PH and PAH pathogenesis. We elucidate some of the key findings in the PH-EndMT field, especially with relation of EndMT to the PAH-related processes of hypoxia, inflammation, and dysregulation of BMP receptor and redox signaling. In addition, we highlight potentially druggable EndMT-relevant targets holding promise for development into future therapies for clinical management of PAH. While the focus of this review is on PAH, where appropriate, we indicate examples of relevance to the parent disease, PH. In that vein, throughout the review, we use the term PH to refer to broad symptoms attributed to the parent disease as a whole. In instances where studies were focused on PAH specifically, we clarify the findings as such. In addition, some of the animal models used for in vivo studies of PAH [extensively reviewed in Gomez-Arroyo et al. (60)] are sometimes referred to as PH models, as they may reflect elements of the parent disease. In such cases, we resorted to conform to the authors' definition of the model.
EndMT in PAH: Groundwork Discoveries and Seminal Studies
As defined earlier, EndMT is a process by which ECs lose their cobblestone morphology and characteristic gene expression, and gain phenotypic features and expression of genes commonly associated with smooth muscle cells (SMCs) and fibroblasts/myofibroblasts. Despite its early discovery in the late 1970s in the settings of embryonic heart development (115), the relevance of EndMT to adult physiology and pathophysiology in the vasculature was uncovered much later (7, 52), and the notion of EndMT in PAH development only began to emerge in the first decade of the 21st century (61, 132, 145, 147, 211). In this section of the review, we highlight pioneering studies of EndMT in PH that had a major impact on this field of research for years to come (Fig. 3).
FIG. 3.
Timeline of seminal discoveries of EndMT with relevance to PH research. Following the early studies of EndMT in cardiac development [Trelstad et al. (177) and Markwald et al. (115)] and isolated endothelial cells in vitro responding to TGF-β [Arciniegas et al. (7) and Frid et al. (52)] or hypoxia [Zhu et al. (211)] treatment, relevance of EndMT to PH was highlighted in the works of Qiao et al. (145), Ranchoux et al. (147), and Good et al. (61). PH, pulmonary hypertension; TGF-β, transforming growth factor beta. Color images are available online.
Identified as a critical process by which endocardial cells of embryonic heart cushions undergo structural and functional changes giving rise to cardiac valves (115, 177), EndMT was initially thought to occur exclusively during cardiac and pulmonary vascular (6) development. However, between the early 1990s and 2000s, EndMT was observed in isolated mature pulmonary and systemic ECs responding to proinflammatory cytokine TGF-β1 challenge (7, 52). Setting the stage for studying EndMT beyond embryogenesis and further in the context of cardiovascular disease, Arciniegas et al. were first to report a potential of mature bovine aortic ECs to reversibly transform into mesenchymal-like cells in vitro, manifested in a gain of expression of α-SMA paired with a loss of vWF expression (7). In a later study, to alleviate concerns of potential contamination of primary ECs with mesenchymal cells upon isolation and potentially influencing their transition in culture (5), Frid et al. utilized fluorescence-activated cell sorting (FACS) to selectively isolate ECs from bovine aortas and pulmonary arteries based on PECAM-1 expression and low-density lipoproteins (LDLs) uptake (52). The authors showed that TGF-β1 challenge could drive EndMT in extensively purified endothelial cultures, as assessed by the emergence of elongated mesenchymal-like cells among the compact cobblestone monolayer and a progressive loss of cellular VE-cadherin and vWF, paired with a simultaneous gain of smooth muscle α-SMA, SM22α, and calponin expression. In addition, authors correlated the loss of LDL uptake capability to a change in cell shape and progressive gain of smooth muscle markers, indicating a connection between EndMT and endothelial dysfunction.
Building on these findings, the first report attempting to link EndMT to PH-associated vascular remodeling identified upregulation of myocardin, a transcription factor critical for SMC differentiation (29, 188, 211), as a common denominator between hypoxia-induced EndMT in porcine pulmonary artery ECs in vitro and chronic hypoxia-induced vascular remodeling and increased α-SMA expression in rats in vivo (211). However, direct evidence of the connection between EndMT and PH-associated vascular remodeling did not come until nearly a decade later, perhaps spurred by advances in genetic recombination-based techniques for cell lineage tracing in living animals (94). Whereas the most recent lineage-tracing studies in mice are starting to utilize newly developed Crispr technology (87), cell marking via genetic recombination has been around for nearly two decades and still remains widely used [reviewed in Kretzschmar and Watt (94)]. Cre-loxP, a mouse-adapted site-specific recombination system originally derived from bacteriophage P1, is used to stably label the progeny of cells expressing a Cre recombinase, usually by marking them with a fluorescent reporter, such as GPF, YFP, or mTomato. To generate lineage-tracing mice, a mouse line constitutively expressing Cre recombinase under the promoter of interest (in case of endothelial lineage tracing, VE-cadherin (3) and Tie2 (35) promoters are used most often) is crossed with another mouse line that ubiquitously expresses a reporter construct (GFP, YFP, or mTomato) flanked by two Cre recognition loxP sites surrounding a STOP codon. In animals expressing both constructs, loxP sites are excised, and cell-specific labeling of Cre-expressing cells is achieved. Lineage-tracing studies in PH revealed the SMC lineage of distal arteriole muscularization and hinted at a possible role of EndMT in the process of alveolar muscularization by confirming the non-SMC origins of alveolar myofibroblasts (158). Another recent study illustrated a pivotal role of pericyte recruitment to the pulmonary vasculature in the early response to hypoxia, and showed that pericytes can differentiate into contractile α-SMA-positive cells and contribute to distal arteriole muscularization in response to hypoxia (20).
Vascular remodeling and neointimal thickening of pulmonary vessels in PAH and the parent syndrome, PH, are complex pathophysiological adaptations that involve vascular and nonvascular cell types across all layers of the vessel wall. Proliferation and migration of adventitial fibroblasts and medial SMCs, dedifferentiation of SMCs from contractile to synthetic state (152), and a generalized promigratory, proinflammatory, and metabolically impaired state of the intimal ECs have all been implicated (160). Infiltration of immune cells also contributes to the overall pathology and remodeling during the disease (180). With the emergence of EndMT as an additional potential contributor to PH-associated vascular remodeling, Qiao et al. utilized a mouse-adapted monocrotaline (MCT) experimental PH model in fate-mapping studies for cells of endothelial origins to identify the cellular source of pathological neointimal remodeling. The authors employed EC-reporter mice generated by crossing dual fluorescent mTomato/mGFP flox mice with endothelial VE-cadherin Cre recombinase mouse line (145). While MCT administration is widely used in rats to induce severe pulmonary vascular remodeling, inflammation, and PAH (98, 192), mice are largely resistant to MCT, and do not develop severe PH symptoms even after chronic MCT administration (126). To adapt this model to mice and achieve severe PH and pulmonary vascular remodeling resulting in neointima formation, authors subjected EC-reporter mice to left pneumonectomy followed by injection with active metabolite monocrotaline pyrrole (MCTP) (145). Immunostaining for α-SMA showed colocalization with a small percentage of GFP-labeled cells of endothelial lineage, suggesting that cells of pulmonary vascular endothelial origin can undergo EndMT and potentially contribute to neointimal formation under stresses associated with PH in vivo (145). Qiao group was also first to report a colocalization of endothelial antigens PECAM-1 and vWF with α-SMA within neointimal cells in human PAH patients, highlighting the translational relevance of EndMT to the human disease. In a later study, Ranchoux et al. focused on immunofluorescent labeling of ECs within intimal and plexiform pulmonary vascular lesions of human PAH patient lungs to show coexpression of endothelial marker CD31 with smooth muscle marker α-SMA, corroborating findings by Qiao et al. (147). In addition, using a BMPR2-deficient rat line that exhibits hypertrophy of muscular pulmonary arteries characteristic of PH, the study was first to loosely associate EndMT to impairment in BMPR2 signaling, as evidenced by upregulation of EndMT transcription factor Twist-1 and mesenchymal marker phosphovimentin in whole lung tissue of BMPR2-mutant rats (147).
Strengthening the experimental evidence for the role of EndMT in animal models of PH, Good et al. demonstrated classic characteristics of EndMT in another in vivo PAH model (61) driven by a combination of vascular endothelial growth factor (VEGF) receptor antagonist Sugen 5416 (SU5416) and chronic hypoxia in mice (182). Colocalization of vWF and α-SMA was seen in 6% of lung vessels from Sugen-hypoxia mice compared with 1% in control animals (61), whereas supporting the findings by Qiao et al. (145) and Ranchoux et al. (147) of relevance to human disease, colocalization between vWF and α-SMA was seen in 4% of the pulmonary arterioles from patients with systemic sclerosis-associated PAH (SSc-PAH) and was absent in non-PAH controls (61).
In another study that offered a glimpse into the mechanistic role of EndMT in PH development, Nikitopoulou et al. made a connection between increased pulmonary endothelin-1 (ET-1) expression, activation of endothelial nitric oxide synthase (eNOS), and EndMT (132). ET-1 is a vasoreactive compound that regulates production of endothelial-derived vasodilator nitric oxide (NO, discussed in detail in Impact of Oxidative Stress and Redox Signaling on PAH-Associated EndMT section of this review) (68) by modulating eNOS activity (105). However, ET-1 is also a potent vasoconstrictor (18), excessive production of which is observed in pathological conditions, including PH (59). Nikitopoulou et al. noted that although expression of VE-cadherin in total lung homogenates of MCT-treated rats was downregulated, EndMT transcription factors functioning as transcriptional repressors of VE-cadherin, Snail and Slug were upregulated, and that these changes were associated with increased expression of ET-1 and phosphorylation of eNOS (132). Supportive of in vivo associations, cultured rat ECs treated with synthetic ET-1 responded with an induction of Snail and a decrease in VE-cadherin expression, along with an upregulation of mesenchymal markers vimentin and α-SMA. Notably, cotreatment with eNOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) blocked pro-EndMT actions of ET-1, indicating that eNOS and ET-1 are mechanistically linked in driving EndMT under PAH-related conditions. While the ET-1 pathway is a common target of current PAH standard therapies (100), Nikitopoulou's findings might indicate that clinical efficacy of ET-1 antagonists might, at least in part, be driven by EndMT inhibition. Usage of ET-1 antagonists for treatment of PAH and additional indications of their protective actions against EndMT will be further discussed in Emerging Therapeutic Strategies Targeting EndMT in the Treatment of PAH section.
At this juncture, it is important to note that quantifying EndMT in vivo by purely relying on counting the instances of perivascular endothelial and smooth muscle marker colocalization or measuring their total lung expression or expression of EndMT transcription factors suffers from significant limitations. First, the expression of many classic endothelial and smooth muscle markers is not exclusive to endothelial or SMCs. For example, PECAM-1 is present on the surface of many immune cells, including T- and B-lymphocytes (114), vWF is expressed by cancer cells of nonendothelial origins (125) as well as megakaryocytes (165), and vimentin can be found in focal adhesions of ECs in addition to its presence on mesenchymal cells (178). Thus, costaining can be misleading, and relying on total lung quantification of endothelial and smooth muscle marker expression can present similar problems. Second, EndMT transcription markers are also involved in EMT, and, given a high prevalence of airway epithelial cells versus vascular ECs in the lung parenchyma, quantifying total lung expression of these markers in relation to PAH needs to be interpreted with caution. Studies, therefore, need to follow the call made by prominent researchers in the field to standardize and unify methods of EndMT assessment for both in vitro and in vivo studies (93), and employ multiple methods for EndMT quantification in combination with robust mechanistic approaches on pure cultures in vitro. Whenever possible, a more widespread use of endothelial lineage-tracing mice is highly encouraged to improve the degree of confidence of EndMT detection in vivo. Moreover, direct isolation of pulmonary arterial endothelial cells (PAECs) in various experimental PH models and comparing their expression profiles and responses to various PAH-associated pro-EndMT stimuli are highly encouraged. Furthermore, given the complex dynamics of EndMT and longitudinal nature of phenotypic transition during disease progression, continuous evaluation of EndMT at different time points in animal models may be necessary. Finally, for evaluation of EndMT in human tissues, assessment of multiple markers of ECs and mesenchymal cells is necessary but may still not be sufficient to capture a comprehensive framework of the process, as transitioning cells only will show costaining with EC and mesenchymal markers. With the advent of deep sequencing transcriptomics, this limitation may be partially overcome through the added use of RNAseq data in addition to immunofluorescence staining. Also, super-resolution microscopy may help in morphometric evaluation of cells with endothelial marker staining to assess for mesenchymal features such as contractile fiber alignment and changes in cell shape.
To gain insight into the role of EndMT on phenotypic modulation in PAH, multiple research groups assessed functional read-outs of PAH and PH-associated endothelial dysfunction and reprogramming as they relate to EndMT. Good et al. were among the first to draw an association between EndMT and increased EC migration (61), an observation further corroborated by subsequent studies (169, 201, 202). The authors induced EndMT in human PAECs using a combination of tumor necrosis factor alpha (TNFα), TGF-β, and interleukin-1 beta (IL-1β), and reported that transitioning cells exhibited a significant increase in motility to a level approximating the rate of migration of human lung fibroblasts isolated from patients with SSc-PAH (61). Consistent with these observations, increased migration of smooth muscle-like cells of endothelial origins was later observed in response to hypoxia (201), in transitioning ECs driven by the silencing of bone-morphogenetic protein-7 (202), as well as in completely transitioned ECs isolated from lungs of mice exposed to Sugen-hypoxia (169).
On the contrary, the links between EndMT and cell proliferation are not as obvious. While some studies link EndMT to increased cell proliferation (61, 201), others report contrasting findings (169). Arguing for the negative connection between EndMT and proliferation, Good et al. demonstrated that unstimulated human PAECs exhibited a greater increase in cell number compared with PAECs treated with pro-EndMT cytokines (61), whereas Zhang et al. reported an EndMT-associated increase in cell proliferation in pulmonary microvascular endothelial cells (PMVECs) derived smooth muscle-like cells compared with controls PMVECs (201). However, in a study utilizing cells isolated from lungs of endothelial lineage-tracing mice treated with Sugen-hypoxia, Suzuki et al. reported that cells undergoing EndMT appeared to proliferate faster than mesenchymal cells of nonendothelial origin (169). The inconsistencies in proliferation studies' findings possibly highlight the complexities of potential EndMT–proliferation pathways crosstalk, but also may have a technical explanation. For example, the absence of unified methods for quantifying cellular proliferation poses a challenge to compare observations made by different experimental groups utilizing different assays (e.g., cell count vs. cell metabolism-based assays).
Irrespective of read-out inconsistencies, studies are in universal agreement that EndMT is an important contributor to the endothelial dysfunction that is hallmark of PH, and plays a negative functional role impacting vascular homeostatic function of healthy endothelium. This can be appreciated by the direct links of increased EndMT in PH and specifically within remodeled vessels (145, 147, 182) as well as association between EndMT and PH-related EC pathophysiological phenotypes such as migration and impaired barrier function.
Despite the limitations outlined above, many of the early studies of PAH-associated EndMT established a strong connection between disease development, EndMT, and processes known to contribute to endothelial dysfunction in PAH. Because of the importance of endothelial injury as a priming event in PAH development (175), studies of EndMT in PAH are instrumental to our understanding of disease pathobiology and invaluable for the future development of therapeutic interventions. A substantial body of data collected over the past four decades supports the association between EndMT and PH in animal models (61, 132, 145) and human patients with PAH (61, 147). Several studies dissected this relationship further, connecting EndMT with changes in LDL uptake (52), migration, and proliferation, thereby linking EndMT to endothelial dysfunction (61, 169). To further qualify these relationships, it is crucial to recognize that, within the pulmonary vascular milieu, a number of organ- and systems-level pathophysiological responses are associated with a disease state and can contribute to disease progression. These include hypoxia-induced vascular remodeling (167), a general proinflammatory state (143), impairment of BMP pathways homeostasis (8), and an overall elevation in oxidative stress and dysfunction of redox pathways (191). Interestingly, there are evident links between EndMT and these disease-related states, and these associations are detailed in the sections to follow.
The Role of Hypoxia in PH-Associated EndMT
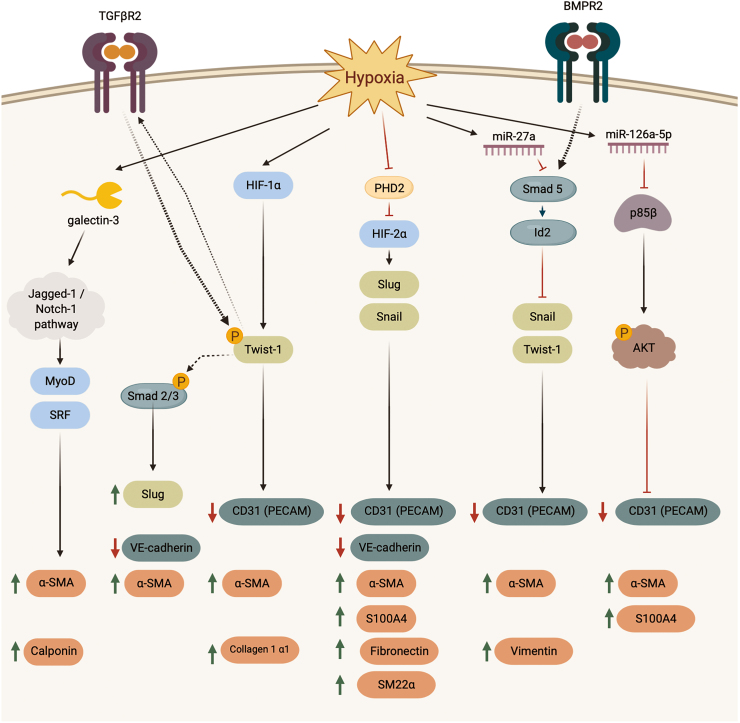
Whereas chronic hypoxia exposure can induce PH in the absence of additional pathological complications (62), a generalized hypoxic environment in the lung often accompanies severe vaso-occlusion and pulmonary remodeling in various forms of PH (primarily Group III PH) (162). However, although hypoxia has been linked to the induction of EndMT in pulmonary arterioral (211), microvascular (201), and arterial (202) ECs, studies of mechanistic relationships between hypoxia and PH-associated EndMT remain scarce. The effects of hypoxia on surrounding tissues are typically mediated through the oxygen-sensitive transcription factors hypoxia-inducible factors 1 alpha and 2 alpha (HIF-1α and HIF-2α), structurally similar homologs with overlapping but nonredundant functions (75). Under baseline conditions of atmospheric concentration of oxygen (normoxia; 20.9% of sea-level air; O2 partial pressure = 21.136 kPa), HIF proteins are targeted for proteasomal degradation by hydroxylation of proline residues by prolyl hydroxylase domain-containing (PHD) proteins (4, 80, 81). In contrast, under hypoxia, commonly defined in vitro as O2 partial pressure = 1%–2% and in vivo as pO2 = 10% (106, 113, 201), the activity of PHD proteins is suppressed, allowing HIFs to escape ubiquitination and undergo nuclear translocation (13), where they bind to hypoxia response elements (HREs) of hypoxia-inducible genes and transactivate their expression (41).
Several recent studies established a causal connection between hypoxic exposure, induction of individual HIF isoforms, and EndMT (106, 113, 174, 196, 201, 204) (Fig. 4). Illustrating the role of HIF-1α in the upregulation of EndMT transcription factor Twist-1, Zhang et al. demonstrated that HIF-1α can directly bind to Twist-1 promoter in rat PMVECs, and showed that HIF-1α knockdown partially reversed a hypoxia-induced loss of CD31 and upregulation of α-SMA and collagen type 1 alpha 1 (Col1a1) expression (201). Importantly, HIF-1α knockdown reversed hypoxia-induced upregulation of Twist-1 expression in PMVECs, whereas the effects of HIF-1α knockdown were recapitulated by Twist-1 silencing, suggesting a PH-relevant mechanistic link between HIF-1α signaling and EndMT. Additional mechanistic insights came from a study by Tang et al. (174) who observed a high expression of HIF-2α in pulmonary vascular endothelial cells (PVECs, a mixed population of PAECs and PMVECs) isolated from the lungs of patients with idiopathic PAH (iPAH). Assessment of messenger RNA (mRNA) expression profiles of iPAH PVECs compared with non-PAH control PVECs showed an increase in EndMT transcription factors Snail and Slug and mesenchymal markers α-SMA, S100A4, and fibronectin, as well as a decrease in endothelial markers PECAM-1 and VE-cadherin, indicative of EndMT progression. Interestingly, arguing for the mechanistic link between HIF-2α and EndMT, the silencing of HIF-2α, but not HIF-1α, in isolated iPAH PVECs decreased Snail and Slug expression. Moreover, endothelial-specific deletion of HIF-2α prevented hypoxia-induced PH in mice, a response that was not observed in endothelial-specific HIF-1α deletion mice. It still remains to be determined whether HIF-2α deletion is sufficient to rescue hypoxia-induced EndMT in vivo and whether reported contradictions on the role of endothelial HIF-1α in PH-associated EndMT are indicative of complex regulatory mechanisms (with HIF-1α control over Twist-1 and HIF-2α control over Snail and Slug), or technical or species-related differences between studies.
FIG. 4.
EndMT signaling cascades activated in response to hypoxia. Hypoxia exposure stimulates a variety of signaling pathways converging on EndMT. BMPR2, bone-morphogenetic protein receptor 2; HIF-1α, hypoxia-inducible factor 1 alpha; HIF-2α, hypoxia-inducible factor 2 alpha; miR, microRNA; MyoD, myoblast determination protein 1; PHD2, hypoxia-inducible factor prolyl hydroxylase 2; SRF, serum response factor; TGFβR2, transforming growth factor-beta receptor 2. Color images are available online.
Even though Twist-1 upregulation was implicated in EndMT (120, 201) and detected in PH and PAH lung samples (61, 74, 145, 147), the mechanism of Twist-1 control over EndMT progression in PH remained largely unknown. The study by Mammoto et al. revealed that hypoxia-induced upregulation of Twist-1 expression and Twist-1 phosphorylation on Ser42 residue increased expression of transforming growth factor-beta receptor 2 (TGFβR2) and potentiated its signaling (113). In vitro, an overexpression of full-length Twist-1 in human PAECs recapitulated effects of hypoxia, leading to an increase in Slug expression, loss of VE-cadherin, and gain of α-SMA, along with increased phosphorylation (indicative of activation) of the molecular transducers of TGFβR signaling Smads 2/3. Importantly, overexpression of a dominant-negative mutant of Twist-1 (Ser-42-Ala) failed to induce similar changes and prevented hypoxia-induced downregulation of VE-cadherin and upregulation of α-SMA, compared with cells transfected with full-length Twist-1. Highlighting the role of Twist-1 in hypoxia-induced PH in vivo, endothelial-specific conditional knockout of Twist-1 partially alleviated vascular remodeling and hemodynamic manifestations of the disease in mice subjected to chronic hypoxia. By mechanistically connecting Twist-1 signaling to the TGFβR2 pathway, this study revealed an important connection between hypoxia, EndMT, and critical pathways in PAH pathobiology (extensively discussed in The Impact of Inflammation and Dysregulated TGF-β-BMP Signaling on PH and PAH-Associated EndMT section).
Several reports suggest that, in addition to modulating EndMT-related transcription factors, hypoxia may control EndMT progression via microRNAs (miRNAs) (106, 196). miRNAs, highly conserved noncoding short RNA fragments, can regulate gene expression at the post-transcriptional level by binding to nucleotide sequences on the 3′-untranslated regions (3′-UTRs) of target gene mRNA transcripts (184). miRNAs are increasingly studied in the context of PAH and PH; although the discussion of the involvement of miRNAs in PAH and PH pathobiology is outside the scope of this review, we refer the reader to excellent reviews by Boucherat et al. (21), Zhou et al. (210), and Negi and Chan (129). In a recent study, Liu et al. demonstrated that miR-27a contributes to hypoxic PAH-associated EndMT in the pulmonary vasculature through the transcriptional effector Inhibitor of DNA Binding 2 (Id2) (106). In addition to its upregulation in pulmonary arteries of chronic hypoxia-exposed rats, miR-27a was upregulated in human PAECs subjected to hypoxia in vitro. Interestingly, cell culture experiments revealed that treatment with miR-27a inhibitor rescued hypoxia-induced EndMT, and that miR-27a mimic induced EndMT in normoxia, evidenced by changes in expression of CD31, α-SMA, and vimentin. In addition, the group demonstrated that miR-27a suppressed Smad5, a mediator of BMP signaling, and suggested that miR-27a-induced downregulation of Smad5 can potentially downregulate Id2, a suppressor of Snail and Twist-1, thereby inducing EndMT. More evidence for miRNAs came from another study, where, rather than homing in on a specific miRNA isoform, Xu et al. performed an unbiased microarray screen to identify specific miRNAs relevant to hypoxia-induced PH of newborns (196). Expression of miR-126a-5p, in particular, was significantly upregulated in the lungs of PH neonatal rats as well as in plasma of human PH neonates compared with healthy neonates. Importantly, the study demonstrated that hypoxia-induced upregulation of miR-126a-5p in newborn rats in vivo and rat PMVECs in vitro contributes to EndMT likely through inhibition of p85-β/Akt pathway, a signaling cascade previously demonstrated to inhibit EndMT in endothelial progenitor cells (203).
Finally, most recent evidence on hypoxia control of EndMT implicates yet another set of processes involving β-galactoside-binding lectins (galectins), a family of secretory proteins with intracellular and extracellular functions (204). Galectins have been increasingly linked to chronic and acute inflammatory responses in cardiovascular pathologies (72), and used clinically as serological prognostic markers in patients with chronic heart failure (107). A member of this family, galectin-3, was shown to mediate platelet-derived growth factor (PDGF) signaling in PAH (65), and to promote pulmonary vascular remodeling in PH (10). Moreover, global galectin-3 gene deletion was shown to be protective against hypoxia-induced PH in mice (66). Advancing our understanding of the role of galectins in PAH and EndMT, Zhang et al. demonstrated that hypoxia-induced upregulation of galectin-3 contributes to the gain of α-SMA expression by pulmonary ECs through Jagged-1/Notch-1 signaling cascade (204), an evolutionarily conserved pathway critical for embryonic development and previously implicated in EMT (200). The authors demonstrated increased galectin-3 expression in the intima and adventitia of chronic hypoxia-exposed rats in vivo and reversal of hypoxia-induced upregulation of α-SMA in PAECs upon galectin-3 gene silencing in vitro. Moreover, treatment of PAECs with recombinant galectin-3 contributed to a gain of α-SMA expression, whereas this effect was abolished in cells preincubated with the Notch signaling pathway inhibitor 3,5-difluorophenylacety-lL-alanyl-L-2-phenylglycinetert-butyl ester (DAPT).
Collectively, the studies described above shed mechanistic insight on hypoxia-mediated control of EndMT in the context of PH and PAH, highlighting the central role of HIF signaling with implications for the potential involvement of miRNAs as well as galectins. EndMT factors Twist-1 (113, 201), Snail (106, 174), and Slug (113, 174) appear to be downstream targets, and future studies are expected to delineate the precise molecular pathways controlling hypoxia-induced EndMT in the context of PAH-associated endothelial pathologies. In addition to galectin-3, a growing body of work implicates galectin-8 in vascular angiogenesis (38) and cell adhesion and growth (212), warranting its further study in the context of PH pathologies and EndMT. Finally, while reports causally linking miRNAs to PAH and PH-associated EndMT are still scarce (106, 196), they persuasively highlight the importance of this relationship and warrant further exploration.
Many hypoxia-induced signaling pathways described above are mediated through pathways associated with inflammatory signaling [i.e., TGFβR signaling (113)] that have been implicated in PAH pathologies [i.e., BMPs (106)]. The following section of the review aims to discuss connections between proinflammatory environment and signaling in PH and disease-relevant potentiation of EndMT.
The Impact of Inflammation and Dysregulated TGF-β-BMP Signaling on PH and PAH-Associated EndMT
The impact of inflammation on PH and PAH development has been extensively studied over the years. First reports hinting at the role of inflammation in PAH described an infiltration of T- and B-cells and macrophages in plexiform lesions (180), and a dramatic increase in the concentration of inflammatory cytokines IL-1β and interleukin 6 (IL-6) in the serum of patients with severe PAH (78). Evidence for a well-established inflammatory disease component in human PAH (143) and animal models of PH and PAH, including MCT (183), Sugen-hypoxia (136), chronic hypoxia (155), and Schistosomiasis-associated PAH (95), was comprehensively reviewed by El Chami and Hassoun (27) and Rabinovitch et al. (146).
ECs can be critically affected by the proinflammatory environment, and the impact of inflammation on endothelial function has been thoroughly described and reviewed in great detail elsewhere (1, 142). In brief, inflammatory cytokines, such as IL-1β, TNFα, TGF-β1, and TGF-β2, activate widespread signaling cascade networks in ECs, increasing endothelial permeability (56) and expression of adhesion molecules aiding immune cell recruitment and extravasation (156, 171). Of note, a large body of data collectively reveals connections between inflammation, EndMT, and endothelial dysfunction in the systemic circulation (31, 140). However, unlike systemic ECs, such as umbilical vein ECs and aortic ECs, that undergo EndMT in response to IL-1β, TGF-β1, and TGF-β2, and their combination (112, 153), response of PAECs to TGF-β2 in relation to EndMT progression has not been sufficiently explored. Interestingly, treatment with an isoform-specific inhibitor of TGF-β1 and TGF-β3, but not TGF-β2, appeared to effectively prevent MCT PAH in rats and partially reversed sustained PAH in a Sugen-hypoxia rat model, suggesting that TGF-β2 signaling may not be essential in PAH pathogenesis (199).
The first indirect evidence suggesting such a link in the pulmonary circulation and PH pathology came from a study by Li et al., in which the authors demonstrated that inhibition of the inflammatory NFκB signaling pathway in a MCT mouse model of PH alleviated disease manifestations, and speculated the involvement of EndMT reversal in the observed phenomena (103). To study the role of inflammation in PH and EndMT in vivo, the authors used mice constitutively expressing inactive mutated IκBα driven by the Club (Clara) cell-10 (CC-10) promoter. In addition to being protected from MCT-induced rise in right ventricular (RV) pressure, IκBα mutant mice were partially protected from the upregulation of total lung expression of α-SMA and downregulation of CD31 and VE-cadherin, compared with wild-type (WT) mice. While the experimental evidence for the involvement of EndMT in this study was mostly associative and subject to other interpretations, this study was among the first to draw parallels between inflammatory signaling and EndMT in PH.
One key pathway whose dysregulation is strongly linked to inflammation is BMPR2 signaling, which also has strong connections to PH and PAH development. The family of BMP receptors is comprised of two subtypes: type 1, which includes Activin receptor-like kinases (ALK) 1/2 and 3/6, and type 2, which includes BMPR2, Activin A Receptor Type 2A (ACVR2A), and Activin A Receptor Type 2B (ACVR2B) (197). The BMPR ligands, BMPs, belong to the TGF-β superfamily of secreted protein ligands (28) and activate downstream mediators SMADs 1/5/8, while TGF-β primarily signals through the recruitment of SMADs 2 and 3 (122). The crosstalk on the level of common signaling mediators, including SMADs and others, adds extra layers of complexity and interdependency between the two signaling cascades (123), and can be relevant to vascular remodeling in PH and PAH by affecting differential regulation of various cellular responses, including EndMT.
Dysregulation of BMPR signaling has been long associated with predisposition to PAH (40, 99). Nonsense and frameshift mutations, as well as gene rearrangements and splice-site defects of the BMPR2 gene, have been predicted to occur in 50%–70% of patients with hereditable PAH (33, 110), and in 10%–40% of patients with iPAH (42, 92, 128, 176). Additional in-depth insights from animal studies demonstrated that a loss of BMPR2 in the endothelium, as well as an overexpression of mutated BMPR2 in vascular SMCs, predispose mice to spontaneous development of PAH (73, 189). Moreover, BMPR2 deficiency has been shown to augment inflammatory cell infiltration to the pulmonary vasculature (164), corroborating reports of anti-inflammatory effects of BMPR2 signaling in the pulmonary endothelium, as opposed to in systemic ECs (36). Reduced BMPR2 signaling has been demonstrated to facilitate the proinflammatory state in PAECs by inducing expression of proinflammatory cytokines IL-6 and IL-8 (43), further connecting BMP signaling deficiency to development of the inflammatory environment in PAH. Collectively, and considering the evidence for links between inflammation and EndMT in multiple pathologies including PAH, these observations support the existence of a link between PAH-associated inflammation, dysregulation of BMPR2 signaling, and EndMT.
One of the earliest reports connecting restoration of BMPR2 signaling in pulmonary ECs to improvement of PAH-associated hemodynamic impairment also indirectly implicated inhibition of EndMT as a potential mechanism (148). Targeted EC delivery of the BMPR2 gene via a vector-harboring adenovirus conjugated to an antibody against angiotensin-converting enzyme (ACE; a membrane-bound protease highly expressed on pulmonary ECs) restored BMPR2 signaling and reversed elevation in RVSP, PVRI, and RV hypertrophy and a reduction in cardiac output in MCT-treated rats. Moreover, BMPR2 gene delivery reversed MCT-induced TGF-β1 expression in the lungs. In vitro, using TGF-β1 as a stimulus in apparent phenocopy experiments and supportive of a role for BMPR2 signaling in the control of PAH-associated EndMT, characteristic changes in cell shape of human PMVECs from cobblestone to spindle like, along with upregulation of mesenchymal markers fibronectin and S100A4, were partially reversed in cells treated with recombinant human BMP-2 or BMP-7. Pieced together, this study primarily argued for a causal relationship between impaired BMPR signaling and EndMT in vitro, leaving room for additional studies of EndMT-associated changes in the vasculature in vivo.
Building on these findings, a more recent report underscored the impact of endothelial BMP signaling restoration on reversal of PH-associated EndMT (202). In this study, Zhang et al. demonstrated that expression and endothelial staining of BMP-7 were decreased in lungs of rats subjected to chronic hypoxia in vivo, as well as after hypoxia exposure of PAECs in vitro (202). Treatment with recombinant BMP-7 (rhBMP-7) improved hemodynamic parameters in experimental animals, and partially reduced a colocalization of VE-cadherin and vimentin in the lining of small pulmonary vessels, suggestive of EndMT reversal. In total lung tissue of animals treated with rhBMP-7, the authors observed a decrease in hypoxia-induced upregulation of EndMT-associated transcription factors Snail, Slug, and Twist-1, as well as a downregulation of α-SMA, vimentin, and fibronectin. Confirming and extending these findings in vitro and echoing findings of Reynolds et al. (148), upregulation of EndMT transcription factors and mesenchymal markers, and colocalization of α-SMA with VE-cadherin after hypoxia exposure, was reduced with rhBMP-7 treatment. The effects of BMP-7 appeared to occur in a mechanism that involves the mammalian target of rapamycin (mTOR) pathway via mTORC1 signaling since hypoxia-induced phosphorylation of S6 protein was attenuated by rhBMP-7 and the rhBMP-7-dependent reversal of hypoxia-induced mesenchymal markers expression was inhibited by rapamycin. Functionally, the addition of exogenous BMP-7 abolished hypoxia-induced migration, a phenotype associated with EndMT (61, 169, 201), while BMP-7 small interfering RNA (siRNA) by itself induced an increase in migration.
Another group later explored the connection between TGF-β1, PDGF, and a membrane-bound zinc metallopeptidase, neprilysin, in their relevance to PH-associated EndMT (163) (Fig. 5). Total lung expression of PDGF and PDGFR was upregulated in hypoxia-treated rats that also exhibited signs of EndMT in the endothelium of pulmonary arteries, manifested in the upregulation of mesenchymal markers fibronectin and vimentin. Interestingly, total lung expression of neprilysin, or neutral endopeptidase (NEP), a negative regulator of hypoxia-induced pulmonary vascular remodeling (39) with a known connection to PDGF signaling (90), was downregulated. In vitro, treatment of calf PAECs with TGF-β1 and PDGF subunits PDGF-AA and PDGF-BB induced EndMT, manifested in the loss of endothelial VE-cadherin and gain of mesenchymal markers fibronectin, vimentin, and α-SMA, whereas PDGF-A/B silencing prevented hypoxia-induced EndMT. Indicative of a mechanistic connection between NEP and EndMT, transfection of PAECs with NEP siRNA downregulated the expression of VE-cadherin and upregulated expression of fibronectin and vimentin, whereas addition of exogenous recombinant NEP reversed hypoxia-induced EndMT in vitro. Interestingly, expression of TGF-β1 and PDGF-B was increased by siNEP while hypoxia-induced upregulation of PDGF-B and TGF-β1 was ablated by exogenous NEP, tying together the connection between the TGF-β and BMPR pathway, and their role in controlling PAH-mediated EndMT.
FIG. 5.
Crosstalk between BMP/TGF-β and PDGF signaling in EndMT progression. TGF-β1, PDGF-AA, and PDGF-BB induced EndMT in a dose-dependent manner. Transitioning cells exhibited loss of VE-cadherin and CD31 (PECAM), induction of EndMT transcription factors Snail and Slug, and gain of alpha smooth muscle actin (α-SMA), vimentin, fibronectin, and S100A4 expression, along with a shift of cell morphology from cobblestone to spindle like. BMPs BMP2 and BMP7 inhibit pro-EndMT effects of TGF-β1, and dysregulated BMPR2 releases inhibitory effects on activity of HMGA1, potentiating EndMT. BMP, bone-morphogenetic protein; CD31, cluster of differentiation 31; HMGA1, High Mobility Group AT-hook 1; PDGF, platelet-derived growth factor; S100A4, S100 calcium binding protein A4. Color images are available online.
Further insight into molecular links between the loss of BMPR2 in ECs and an increase in EndMT in PAH revealed a role in this process for the chromatin architecture factor High Mobility Group AT-hook 1 (HMGA1) (74) as outlined in Figure 5. HMGA1 is a protein that can bind to AT-rich motifs on DNA and alter chromatin structure, thereby influencing gene transcription. While the expression of HMGA1 is nearly undetectable in mature tissues, its expression is upregulated in a subset of cancers (53) and had been linked to EMT (139). Intriguingly, Hopper et al. reported an increase in HMGA1 expression in isolated pulmonary ECs and the neointima of pulmonary vessels from PAH patients (74). HMGA1-positive cells within the neointima also coexpressed the endothelial marker vWF and the smooth muscle marker SM22α, suggesting an association between HMGA1 and the process of EndMT. Mechanistic studies in cell culture demonstrated that BMPR2 knockdown upregulated HMGA1 expression and induced EndMT, where expression of EndMT-associated transcription factors Snail and Slug was upregulated, expression of endothelial marker PECAM-1 was downregulated, and expression of smooth muscle markers α-SMA and phosphovimentin was upregulated. In addition, utilizing HMGA1 gene silencing alone and in combination with BMPR2 gene silencing by siRNA, the authors were able to demonstrate that upregulation of Slug and α-SMA driven by BMPR2 silencing was abrogated in HMGA1 siRNA-transfected PAECs, thus suggesting that HMGA1 acts as an effector of dysregulated BMPR2 signaling. Considering these findings, it would be valuable to explore the changes in total lung HMGA1 expression in response to BMPR2 gene delivery (148) or rhBMP-7 treatment (202), which would not only further strengthen the in vivo link between BMPR2 impairment and EndMT, but might also provide additional mechanistic insights for the development of future PAH therapeutics. It is interesting to note that the BMP antagonist, Gremlin-1, which has been implicated in vascular remodeling in PH (24, 58), was recently shown to induce EndMT in PAECs (205), further supporting the links between impaired BMP signaling and EndMT in the disease. It is intriguing to speculate whether the use of Gremlin-1 neutralizing antibodies, which have been demonstrated to reduce PH in the Sugen-hypoxia mouse model (32), can effectively ameliorate EndMT and serve as viable candidates for future therapeutic development.
Notably, 3 years after their original study (103), Li et al. mechanistically connected PH-associated activation of proinflammatory NFκB signaling to an upregulation of miR-130a, loss of BMPR2 and TGF-β1-induced EndMT (102) (Fig. 6). In vitro, in addition to a decrease of BMPR2 expression and an upregulation of miR-130a, which is predicted to directly bind to the 3′-UTR of BMPR2 mRNA, treatment of isolated pulmonary microvascular ECs with TGF-β1 induced gain of α-SMA and loss of CD31 expression, suggestive of EndMT progression. Moreover, changes in endothelial and mesenchymal gene expression were recapitulated by BMPR2 siRNA transfection, and treatment with a miR-130a inhibitor restored TGF-β1-induced downregulation of BMPR2 and protected ECs from EndMT-associated loss of CD31 and gain of α-SMA. This study provided evidence of a causal connection between inflammation, TGF-β and BMPR signaling, and brought to the forefront the role of miRNAs, which are increasingly being recognized as key messengers in propagating PH-related vascular dysfunction (14, 106, 154, 190, 196). Considering the known role of Hippo pathway downstream coregulators Yes-associated protein (YAP) and Tafazzin (TAZ) in modulating the miR-130/301 family in PH-linked vascular stiffness (14, 15), it would be intriguing to test associations between mechanosensing endothelial pathways, EndMT modulation, and the role of miRNAs in PH pathogenesis.
FIG. 6.
Mechanism of inflammatory NFκB control over miR-130a-associated EndMT. Inflammatory cell infiltration characteristic to PH-associated vascular remodeling increases local concentration of proinflammatory cytokines IL-6 and IL-1β. In vivo, inhibition of inflammatory NFκB signaling in monocrotaline-treated rats decreases total lung levels of inflammatory cytokines IL-6, IL-1β, and TNFα and prevents induction of microRNA miR-130a, downregulation of CD31 and VE-cadherin, and upregulation of α-SMA. In vitro, inhibition of NFκB signaling prevents TGF-β1-induced EndMT manifested in a gain of α-SMA and loss of CD31. miR-130a was shown to negatively regulate BMPR2 signaling that, in the absence of inhibition, prevents spontaneous EndMT. TNFα, tumor necrosis factor alpha. Color images are available online.
Together, the studies highlighted in this section emphasize complex interconnections between hypoxia, dysregulated BMPR2 signaling, inflammation, and EndMT in PAH. It is important to note that additional work is still needed to fully map out the relationship between BMP signaling and EndMT. In many cases, molecular mechanisms and intermediate players connecting the two processes have not been detailed, and their elucidation would be beneficial for the field at large.
Nonetheless, with that need in mind, by presenting compelling data arguing for utilization of BMP signaling restoration strategies targeting EndMT in the treatment of PH (148, 202), and offering a number of mechanistic insights into the BMPR-mediated control over EndMT progression (74, 102, 163, 202), the works discussed above do make a persuasive argument for the development of therapeutics aimed at the reversal of PAH-associated EndMT and pave a way for future explorations (198). Finally, the embryonic origin and developmental importance of both BMP signaling [reviewed in Wang et al. (186)], EndMT (115, 177), and their connections (109, 118) suggest an early deep-rooted interconnection between the two processes that may be subject to temporal regulation and perhaps also suggest a critical coregulation in cell fate determination. As far as PH is concerned, studying this interaction could reveal valuable insights into early events in disease development versus disease progression and could help identify causal triggering events for early vascular remodeling in PH.
Impact of Oxidative Stress and Redox Signaling on PAH-Associated EndMT
In addition to hypoxic and proinflammatory environments discussed above, pulmonary vasculature undergoing PH-associated remodeling experiences an upregulation of reactive oxygen and nitrogen species and enhanced susceptibility to oxidative stress. Oxidative stress has been implicated in PH either as a consequence of excessive pathological production of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS), imbalance in antioxidant capacity, or both [extensively reviewed in Fulton et al. (55) and Wong et al. (191)]. Some of the major enzymes involved in ROS and RNS production in physiological and pathophysiological conditions include iron and iron derivatives (such as heme or iron–sulfur clusters), NADPH oxidases (Noxs), mitochondrial electron transport chain complexes I, II, and III, NO synthases (NOS, uncoupled), and xanthine oxidase (XO) (16). Antioxidant systems include catalase, superoxide dismutase (SOD), thioredoxin system, glutathione peroxidases, and peroxiredoxins, as well as antioxidant molecules such as Vitamin C and E (16). The parent ROS compound, superoxide anion radical (O2−•), and its dismutation product hydrogen peroxide (H2O2) have both been shown to increase in various vascular cells of the pulmonary circulation as well as the right ventricle under PAH conditions and related stimuli, and to contribute to the observed pathophysiology (58, 187). Similarly, products of NO oxidation, such as the RNS peroxynitrite (ONOO−), have also been implicated in PH (55, 173), whereas the levels of vasodilatory NO and its reaction products NO2−, NO3−, and S-nitrosothiols are decreased in lungs of PAH patients (89). It is speculated that elevated vascular ROS contribute to PH by attenuating vasodilatory effects of NO (85), and induce proproliferative phenotypes in SMCs by activating the Erk signaling pathway [reviewed in Wong et al. (191)].
Increased superoxide production by the endothelial Nox2 (gp91phox) in PH has been reported by multiple research groups. Observed protective effect of Nox2 deletion on pathological changes associated with PH and improvement of NO-mediated vasorelaxation demonstrated a critical role of Nox2-derived ROS in hypoxia-induced PH models (51, 104). Other studies reported an elevation of Nox1 expression in total lung tissue samples of PAH patients, linking Nox1-derived ROS to regulation of the BMP antagonist Gremlin1 (58). More evidence implicates an increase in adventitial, endothelial, and smooth muscle expression of Nox4 in vitro in response to hypoxia and in animal model of PH (11, 108, 133). Mounting signs of the role of ROS in PH spurred efforts to develop antioxidant-based therapies for the management and treatment of PH [extensively reviewed by Suzuki et al. (172)]. Both pharmacological mimetics of SOD (45) and targeted gene delivery of extracellular SOD (88) were shown to be protective against hypoxia-induced and MCT PH, as were the glutathione precursor N-acetylcysteine (96), the XO inhibitor allopurinol (82), and a pharmacological inhibition of Nox4 (11). However, while antioxidant treatments produced highly effective results in preclinical animal studies, clinical trials continue to yield disappointing outcomes. For example, dietary supplementation of mitochondria-targeting antioxidant Coenzyme Q10 did not improve hemodynamic performance in PAH patients [ClinicalTrials.gov Identifier: NCT01148836 (157)]. Interestingly, some animal studies argue for the importance of ROS in the adaptation to PH (151), and might offer an explanation for the discrepancy between effects of antioxidants for PH treatment in animals and human subjects.
While oxidative stress remains one of the hallmark events in PH vasculature (22) and upregulation of ROS has been causally connected to PAH development (83), relevance of oxidative stress to PAH-associated EndMT has not been sufficiently explored until recently. Even though endothelial dysfunction has long been connected to excessive ROS production (25) and ROS were known to induce EMT via stimulation of TGF-β1 signaling (54), mechanistic insights into the role of oxidative stress in mediating EndMT were first explored only in mid-2010s in the work by Montorfano et al. (127). In their study, the group demonstrated that exposure of ECs to exogenous H2O2 induces EndMT in a mechanism dependent on the synthesis and secretion of TGF-β1 and -2, as well as activation of TGF-β receptor subunit ALK5 and its downstream signal transduction modulator Smad3. In addition to inducing expression of TGF-β1 and -2, potent activators of EMT in keratinocytes and renal tubular epithelial cells (54, 149) as well as of EndMT in systemic ECs (7, 52, 102, 111, 119, 163), H2O2 concentrations as low as 0.1 μM and as high as 10 μM induced loss of expression of endothelial markers VE-cadherin and CD31, gain in expression of mesenchymal markers α-SMA and FSP-1 (S100A4), loss of cell–cell contacts and change in cellular morphology from cobblestone-like to the elongated spindle-like shape characteristic of transitioning ECs. This indicative shift from an endothelial to a mesenchymal-like cellular phenotype was associated with an H2O2-dependent increase in biosynthesis of collagen III and fibronectin, two extracellular matrix proteins associated with fibrotic responses. This study was among the first to establish a link between redox signaling and EndMT in vascular ECs, but did not explore whether enzymatic production of H2O2, for example via Nox4 activation, is sufficient to induce a similar phenotype.
Indispensable NOS cofactor tetrahydrobiopterin (BH4) and sepiapterin reductase, an enzyme that transforms exogenous sepiapterin into BH4 in an alternative “salvage pathway,” are critical for the maintenance of vascular homeostasis (50, 91), and were demonstrated to have protective antioxidant and antifibrotic properties in myocardial infarction and PH (124, 159). In the article by Almudéver et al., the authors examined the role of BH4 system in pulmonary vascular remodeling associated with idiopathic pulmonary fibrosis (IPF). IPF is a disease of the lung parenchyma characterized by excessive scarring and myofibroblast activation, which can often be complicated by the development of associated PH (48). Almudéver et al. demonstrated that rescue of BH4 levels by stimulation of de novo biosynthesis using sepiapretin administration reduced pathological vascular remodeling in the bleomycin animal model of IPF (2). In addition to reducing the number of α-SMA and VE-cadherin coexpressing cells in pulmonary arteries, sepiapterin alleviated bleomycin-induced upregulation of iNOS and rescued expression of eNOS (2). In vitro, treatment of HPAECs with exogenous sepiapterin reversed TGF-β1- and ET-1-induced upregulation of Snail and Slug, upregulation of α-SMA and SM22α, and downregulation of VE-cadherin and VEGFR. Importantly, these sepiapterin effects were attributed to downstream downregulation of ROS and phosphorylation of Smad3. This study gave further evidence supporting the role of BH4 in alleviating bleomycin-induced EndMT, and aligns with previous findings on the impact of chronic inhibition of NOS on induction of EndMT in the kidney (134). On the other hand, and in contrast with the decrease in eNOS expression in IPF-associated PH (group 3 PH) observed in this study (2), other studies reported increases in levels of eNOS expression and phosphorylation in plexiform lesions of PAH patients, and associated dysfunctional eNOS activity with enzyme uncoupling and increased ROS production (57, 116). Indeed, in the report by Nikitopoulou et al. (132) discussed earlier, the authors observe an increase in eNOS phosphorylation as causal of EndMT. Moreover, it has been demonstrated that increased eNOS activity in caveolin-1-deficient mice induces PH through increased nitration of cGMP-dependent protein kinase, also known as Protein Kinase G (PKG) (207). These discrepancies may reflect differences in disease severity, underlying mechanisms behind the group 1 versus group 3 PH subtypes, and/or activation of distinct upstream signal cascades controlling the observed eNOS pathophysiology (e.g., uncoupling vs. increased expression and/or phosphorylation). Despite the outlined inconsistencies, convergence on ROS appears to play a central part. Moreover, the role of NO signaling dysregulation in PAH is unambiguous, and further studies of the therapeutic potential of modulating eNOS or iNOS expression and restoration of enzyme uncoupling, especially in its relation to EndMT and pulmonary vascular remodeling, are warranted.
Highlighting a potentially protective role for ROS as signaling mediators, a recent study by Rudyk et al. (151) identified oxidation of Protein kinase G Iα (PKGIα) as an adaptive mechanism limiting PH, likely through a disruption of EndMT. PKGIα, an isoform of PKGI mainly expressed in the heart and lungs, is a redox-sensitive Serine/Threonine kinase whose oxidation leads to a formation of an active disulfide homodimer that is associated with endothelium-derived hyperpolarizing factor (EDHF)-dependent vasodilation (144). Previous studies have identified an upregulation of the pulmonary PKGI expression after chronic hypoxia exposure and hypoxia-associated ROS production (84), and reported that PKGI knockout mice develop spontaneous PAH in normoxia (208). However, up until Rudyk's study, the functional significance of post-translational modifications of PKGI in PAH remained largely unexplored, and its connection to EndMT signaling was unknown. Using a whole lung transcriptomic analysis in Cys42Ser PKGIα knock-in (KI) mice that are resistant to PKGIα oxidation, the authors demonstrated an upregulation in expression of EndMT-related genes compared with WT mice after 3 days of hypoxia exposure (i.e., before development of structural remodeling in the pulmonary vasculature of WTs but at the beginning of development of remodeling in KIs). The authors also noted increased vascular muscularization in the lung as well as an increased number of cells coexpressing CD31 and α-SMA in hypoxic KI mice compared with WTs. Consistent with the EndMT paradigm, expression of Twist-1 was upregulated in the whole lung tissue, along with increased α-SMA, desmin, and phosphovimentin. It must be noted, however, that gleaning insight into EndMT using whole lung tissue expression rather than specific pulmonary vascular endothelial expression suffers from significant limitations and needs to be interpreted with caution, as outlined earlier in the review. Therefore, building on the current findings, additional mechanistic studies implementing robust experimental techniques are needed to disentangle the role of oxidative stress and PKGIα oxidation in the development of PAH-associated EndMT in vitro and in vivo.
Over the last 5 years, several reports implicated a direct link between oxidative stress and EndMT in PH-relevant context (Fig. 7). A connection between oxidative stress, TGF-β, and EndMT signaling was delineated in a proof-of-principle in vitro study (127), and the roles of specific ROS- and RNS-producing enzymes and cofactors (2), and oxidation-sensitive signaling mediators (151) and their association with PH-associated EndMT were later explored in translational animal studies. Despite the evidence of a causative role of ROS in EndMT, there are multiple redox signaling pathways that could be activated and mediate an intrinsic adaptation to PH by off-setting some of the pathological changes. Redox-regulated PKGIα is just one known example, and there are probably others that are yet to be discovered. Finally, studies showing a connection between BMPR2 dysfunction and oxidative stress in the context of PH (58, 86) highlight the tight interconnectedness between signaling pathways implicated in endothelial dysfunction and disease pathogenesis, and make a compelling argument for further exploring these relationships and their possible links to EndMT.
FIG. 7.
A role of ROS in the induction of PH-associated EndMT. Increased production of ROS due to an imbalanced redox state in the pulmonary vasculature in PH leads to an induction of EndMT via an upregulation of TGF-β1/TGF-β2/ALK5/Smad3 signaling axis as well as a yet to be defined mechanism likely involving eNOS uncoupling. On the contrary, oxidation of PKGIα serves as a protective mechanism to limit PH-associated EndMT. ALK, activin receptor-like kinase; eNOS, endothelial nitric oxide synthase; PKGIα, protein kinase G Iα; ROS, reactive oxygen species. Color images are available online.
Despite strong advances in our understanding of the interplay between oxidative stress, EndMT, and PH, more studies are needed to determine oxidative stress mechanisms controlling EndMT in PH and PAH, and their links to PH-associated hypoxia, inflammation, and dysregulated BMPR signaling. In addition, collected over the last decade, a vast body of data demonstrating the presence of PH- and PAH-associated EndMT of multifactorial origin in the pulmonary vasculature makes a compelling case for developing EndMT-targeting therapies for the treatment of the disease beyond symptoms management.
Emerging Therapeutic Strategies Targeting EndMT in the Treatment of PAH
Following accumulating evidence for the impact of EndMT in PH progression and PAH-associated pulmonary vascular remodeling, and, given the urgent need for development of novel therapeutic approaches for this devastating disease, strategies aimed toward EndMT reversal could present a viable option for disease management. While evidence for this remains limited, a number of recent studies presented EndMT targeting as a viable protection strategy against PAH in animal models via the usage of mesenchymal stem cells (MSCs) (76), as well as by inhibiting dipeptidyl peptidase-4 (DPP-4) (193) and adhesion molecule CD44 (79). Related to the former, the latest developments in the field of cell-based regenerative therapies galvanized research into the utility of stem cells as a treatment strategy for PH (49). Despite the known benefits of stem cell transplantation for improvement of RV pressure, hypertrophy, and inflammation in animal models of PH, and even though EndMT has been reported as a feature of endothelial dysfunction in human PAH (147) and animal models of the disease [MCT (132, 145) and Sugen-hypoxia (61)], only a few studies explored the potential for targeting PH-associated EndMT using stem cell therapies. Promising early evidence came from the recent work by Huang et al. (76). The authors showed that intravenous injection of MSCs reduced RV systolic pressure, RV hypertrophy and pulmonary vascular muscularization, attenuated increases in collagen deposition, and normalized MMP2 and MMP9 tissue expression in Sugen-hypoxia rats. Pointing to the potential effect of MSC therapy on EndMT reversal, increased colocalization of α-SMA and vWF in cells within the pulmonary vascular wall of Sugen-hypoxia rats was attenuated in MSC-injected rats. These findings were supported by in vitro studies, indicating that hypoxia-induced accumulation of HIF-2α, whose association to EndMT was discussed in The Role of Hypoxia in PH-Associated EndMT section of the review (174), and EndMT in PMECs was reversed in PMECs exposed to MSCs-conditioned cell culture medium. However, despite presenting a number of promising findings, this study assessed the effects of MSC therapy on EndMT as an association, rather than a causative mechanistic connection, and has significant flaws concerning experimental design lacking appropriate non-MSC-injected control animals.
Inhibition of DPP-4 (CD26), a serine protease that regulates activity of secreted polypeptides such as chemokines, cytokines, and vasoactive peptides, is another potential strategy for PH treatment targeting EndMT inhibition (193). DPP-4 is widely expressed on the plasma membrane of a variety of cell types, including ECs of pulmonary capillaries (130). At present, DPP-4 inhibitors are being used in clinic for the management of type 2 diabetes mellitus, as they lower plasma glucose levels by inhibiting the breakdown of incretin hormone glucagon-like peptide-1 (GLP-1) (138). Interestingly, in addition to their antihyperglycemic activity, DPP-4 inhibitors were recently shown to have protective effects on the cardiovascular system in vitro and in vivo (47, 209) and, importantly, to reverse pulmonary EndMT following endotoxin-induced acute lung injury models (170). To examine the role of DPP-4 in PH and pulmonary vascular remodeling, Xu et al. utilized a DPP-4 inhibitor, sitagliptin, in the rat MCT model of PH (193). MCT treatment increased serum levels of DPP-4, whereas, alluding to the association between DPP-4 and EndMT (170), the number of α-SMA/CD31 double-positive cells in pulmonary artery sections of MCT rats was reduced with sitagliptin. In addition, total lung protein expression of α-SMA, vimentin, and fibronectin was reduced, while expression of VE-cadherin and vWF was restored to normal with sitagliptin treatment in MCT rats.
A follow-up study providing additional mechanistic insights into the protective effects of DPP-4 inhibition on EndMT in PH demonstrated that DPP-4 inhibition restored GLP-1 signaling (185). Administration of GLP-1 analog, liraglutide, also decreased the number of α-SMA/CD31+ double-positive cells in the pulmonary vasculature of MCT rats. In addition, liraglutide partially reversed MCT-induced downregulation of total lung mRNA expression of VE-cadherin and vWF, and upregulation of vimentin and fibronectin. Beyond associative in vivo observations, liraglutide treatment dose dependently rescued TGF-β-IL-1β-induced loss of VE-cadherin and gain of α-SMA and vimentin expression in cultured systemic ECs. This observation supports the hypothesis that the therapeutic action of GLP-1 on PH reversal is at least partially due to its role as an EndMT inhibitor, especially since the therapeutic effect of liraglutide on reversal of EndMT phenotype was blocked in cells treated with GLP-1R antagonist.
Finally, a recently published study explored the parallels between PH pathophysiology and cancer (63), and connected the upregulation of cancer-associated isoform of adhesion molecule variant CD44v8-10 to EndMT and PH. Similar to cancer cells, ECs in remodeled pulmonary vessels exhibit increased proliferation and resistance to apoptosis (117), and metabolic shift from oxidative phosphorylation to glycolysis (194). Beyond its functionality in normal physiological conditions, CD44 was shown to promote cancer metastasis (64), and increased expression of v8-10 isoform of CD44 protein, which results from the alternative splicing of CD44 gene, has been found on the surface of cancer stem cells (101). Interestingly, Isobe et al. detected expression of CD44v in cells undergoing EndMT in the neointimal lesions in lung vasculature of patients with PAH as well as in lungs of mice subjected to chronic Sugen-hypoxia treatment (79). Supporting the involvement of CD44 in EndMT, stimulation of EndMT in cultured HPAECs with a combination of TGF-β, TNFα, and IL-1β resulted in an increased expression of preprocessed CD44 and its isoform CD44v8-10, along with an induction of EndMT transcriptional factors Snail and Slug, loss of endothelial CD31, and a gain of mesenchymal markers α-SMA, SM22α, MMP2, and MMP9. In addition, a small subset of transitioned CD44v8-10-positive HPAECs exhibited high expression of cysteine-glutamate antiporter subunit xCT, upregulation of which serves as a protective mechanism against oxidative stress in cancer cells by modulating levels of intracellular antioxidant GSH. These observations thus strongly support the involvement of CD44v in PH-associated EndMT, and open doors for potential therapeutics targeting CD44 and xCL for the treatment of PH in clinic.
Interestingly, some of the current PH therapies may have an indirect effect on EndMT inhibition in addition to its intended targets. For example, given the connection between ET-1 signaling and EndMT in PAECs (132), widely used inhibition of ET-1 signaling by way of ET-1 antagonists (such as bosentan) might have an additional beneficial effect for PAH treatment by inhibiting EndMT (17). Further, given extensive evidence for the link between PAH, dysregulated BMPR2 signaling, and EndMT, continuing development of BMPR2-targeting therapies (135) will likely be beneficial against the onset of EndMT in the pulmonary vasculature. However, some therapeutic approaches, such as the use of antioxidant therapies, need to be considered with caution due to their potentially EndMT-promoting effects (151). Finally, given the known stimulatory effects of chronic hypoxia on the expression of zinc transporter ZIP12 in the pulmonary endothelium in PH (206), it is curious to elucidate whether changes in intracellular levels of zinc are linked to PH-associated EndMT and vascular remodeling.
New advances in our understanding of the role of EndMT in PH and PAH pathobiology offer rich material for targeting this process in the development of early disease interventions. In addition to inhibition of DPP-4 (193) and CD44 (79), and utilizing EndMT-targeting MSC therapies (76) described in this section, targeting other mediators of PH-associated EndMT as detailed above, such as microRNAs (102, 106, 196), galectin-3 (204), and HMGA1 (74), as well as aiding the restoration of BMPR signaling (148, 202) and redox balance (2) in the pulmonary vasculature, present new promising avenues to reverse EndMT and alleviate PH-induced vascular remodeling. Indeed, accumulating evidence for EndMT in PAH and the increasing understanding of its underlying mechanisms in disease pathophysiology make it an appealing target to seek in future treatment development.
Conclusion
As described above, mounting evidence points to a contribution of EndMT to PAH development and progression through associations with a variety of environmental or signaling responses implicated in PAH. Hypoxia, dysregulated BMPR2 signaling, inflammation, and oxidative stress were all reported to contribute to EndMT, and all are important for PAH pathophysiology. However, despite gaining recognition, this process remains understudied, and more extensive investigations are needed to elucidate the mechanistic role of EndMT in PAH.
The early findings, supportive of the association between EndMT and PH in animal models (61, 132, 145) and human patients with PAH (61, 147), paved way for further explorations and expanded our understanding of the complex interconnections between hypoxia-associated pulmonary remodeling, inflammation, oxidative stress and dysregulated BMP signaling, and their role in disease-associated EndMT. Several studies have offered mechanistic insights into hypoxia-mediated control of EndMT (106, 113, 174, 196, 201), links between BMPR signaling EndMT progression (74, 102, 163, 202) and relations between oxidative stress and EndMT-relevant signaling mediators (2, 127, 151). Others offered compelling strategies for the development of EndMT-targeting PH therapies (76, 79, 193).
It has to be noted that while many of the studies described in this review were fundamental in establishing the concept of EndMT in PAH-associated vascular remodeling and other PAH pathologies, many of the experimental techniques used in the field remain to suffer from considerable limitations. For example, many of the studies that have connected EndMT progression to changes in endothelial and SMC marker expression and expression of EndMT-associated transcription factors did so in total lung tissue rather than isolated intimal or neointimal cells. Although this association can be informative, it needs to be recognized that such changes can be indicative of underlying fibrotic response or EMT, which shares signaling pathways with EndMT. Moreover, markers of ECs most commonly used for endothelial lineage identification, such as CD31, are expressed by other cell types, and using a single marker can therefore lead to false-positive results. In addition, because of the complexity of EndMT and its associated temporal patterns, as well as an absence of unified and clear EndMT read-outs on the basis of mesenchymal and endothelial phenotypes, cross-comparison between different studies remains difficult. Data sharing and cross-collaboration, along with a more widespread use of endothelial lineage-tracing mouse models and genetic studies, will be essential to overcome these hurdles. New technologies, such as single-cell RNA sequencing and super-resolution microscopy, will allow for detailed profiling of transitioning pulmonary ECs in human patients, which will enrich our understanding of potential druggable targets in PH.
Together, the insights into EC biology and EndMT provide us valuable information about the potential nature of vascular remodeling associated with PAH and point to a potential direction on the development of therapeutics targeting the root cause of endothelial activation and reprogramming, which can potentially stop the disease before its onset.
Acknowledgment
The figures in this article were created with BioRender.
Abbreviations Used
- α-SMA
alpha-smooth muscle actin
- ALK
activin receptor-like kinases
- BH4
tetrahydrobiopterin
- BMP
bone-morphogenetic protein
- BMPR2
bone-morphogenetic protein receptor 2
- CD31
cluster of differentiation 31
- DPP-4
dipeptidyl peptidase-4
- EC
endothelial cell
- EMT
epithelial-to-mesenchymal transition
- EndMT
Endothelial-to-mesenchymal transition
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- FSP-1
fibroblast-specific protein 1
- GLP-1
glucagon-like peptide-1
- H2O2
hydrogen peroxide
- HIF
hypoxia-inducible factor
- HIF-1α
hypoxia-inducible factor 1 alpha
- HIF-2α
hypoxia-inducible factor 2 alpha
- HMGA1
High Mobility Group AT-hook 1
- Id2
inhibitor of DNA binding 2IL-1β: interleukin-1 beta
- IL-6
interleukin 6
- iPAH
idiopathic pulmonary arterial hypertension
- IPF
idiopathic pulmonary fibrosis
- KI
knock-in
- LDL
low-density lipoprotein
- MCT
monocrotaline
- miRNA
microRNA
- mPAP
mean pulmonary arterial pressure
- mRNA
messenger RNA
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- NEP
neutral endopeptidase
- NO
nitric oxide
- NOS
NO synthase
- Nox
NADPH oxidase
- PAEC
pulmonary arterial endothelial cell
- PAH
pulmonary arterial hypertension
- PAWP
pulmonary arterial wedge pressure
- PDGF
platelet-derived growth factor
- PECAM-1
platelet endothelial cell adhesion molecule
- PH
pulmonary hypertension
- PHD
prolyl hydroxylase domain containing
- PKGIα
protein kinase G Iα
- PMVEC
pulmonary microvascular endothelial cell
- PVEC
pulmonary vascular endothelial cell
- PVR
pulmonary vascular resistance
- rhBMP-7
recombinant BMP-7
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- S100A4
S100 calcium binding protein A4
- siRNA
small interfering RNA
- SM22α
transgelin
- SMC
smooth muscle cell
- SNAI1
Snail
- SNAI2
Slug
- SOD
superoxide dismutase
- SSc-PAH
systemic sclerosis-associated pulmonary arterial hypertension
- TGF-β
transforming growth factor beta
- TGFβR2
transforming growth factor-beta receptor 2
- TNFα
tumor necrosis factor alpha
- UTR
untranslated region
- VE-cadherin
vascular endothelial cadherin
- VEGFR
vascular endothelial growth factor receptor
- vWF
von Willebrand factor
- WT
wild type
- XO
xanthine oxidase
Funding Information
Dr. Al Ghouleh received research support from the National Institutes of Health (R01HL148712-01), American Heart Association (AHA Scientist Development Grant, grant No. 15SDG24910003), American Lung Association (American Lung Association Biomedical Research Grant, grant No. RG-515656), and Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension. VMI researchers receive support from Vitalant and the Hemophilia Center of Western Pennsylvania.
References
- 1. Al-Soudi A, Kaaij MH, and Tas SW. Endothelial cells: from innocent bystanders to active participants in immune responses. Autoimmun Rev 16: 951–962, 2017 [DOI] [PubMed] [Google Scholar]
- 2. Almudéver P, Milara J, De Diego A, Serrano-Mollar A, Xaubet A, Perez-Vizcaino F, Cogolludo A, and Cortijo J. Role of tetrahydrobiopterin in pulmonary vascular remodelling associated with pulmonary fibrosis. Thorax 68: 938–948, 2013 [DOI] [PubMed] [Google Scholar]
- 3. Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, and Iruela-Arispe ML. VE-cadherin-cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235: 759–767, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Appelhoffl RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, and Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Arciniegas E, Frid MG, Douglas IS, and Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Cell Mol Physiol 293: L1–L8, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Arciniegas E, Neves CY, Carrillo LM, Zambrano EA, and Ramírez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium 12: 193–200, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Arciniegas E, Sutton AB, Allen TD, and Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci 103(Pt 2): 521–529, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, and Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Badesch DB, Champion HC, Gomez Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, and Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54: S55–S66, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Barman SA, Chen F, Li X, Haigh S, Stepp DW, Kondrikov D, Mahboubi K, Bordan Z, Traber P, Su Y, and Fulton DJR. Galectin-3 promotes vascular remodeling and contributes to pulmonary hypertension. Am J Respir Crit Care Med 197: 1488–1492, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJR. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batlle M, Campos B, Farrero M, Cardona M, González B, Castel MA, Ortiz J, Roig E, Pulgarín MJ, Ramírez J, Bedini JL, Sabaté M, García de Frutos P, and Pérez-Villa F. Use of serum levels of high sensitivity troponin T, galectin-3 and C-terminal propeptide of type I procollagen at long term follow-up in heart failure patients with reduced ejection fraction: comparison with soluble AXL and BNP. Int J Cardiol 225: 113–119, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, and Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, and Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep 13: 1016–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, and Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birben E, Sahiner UM, Sackesen C, Erzurum S, and Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J 5: 9–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bochenek ML, Leidinger C, Rosinus NS, Gogiraju R, Guth S, Hobohm L, Jurk K, Mayer E, Münzel T, Lankeit M, Bosmann M, Konstantinides S, and Schäfer K. Activated endothelial TGFβ1 signaling promotes venous thrombus nonresolution in mice via endothelin-1: potential role for chronic thromboembolic pulmonary hypertension. Circ Res 126: 162–181, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohm F and Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, and Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 116: 499–511, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Bordenave J, Tu L, Berrebeh N, Thuillet R, Cumont A, Le Vely B, Fadel E, Nadaud S, Savale L, Humbert M, Huertas A, and Guignabert C. Lineage tracing reveals the dynamic contribution of pericytes to the blood vessel remodeling in pulmonary hypertension. Arterioscler Thromb Vasc Biol 40: 766–782, 2020 [DOI] [PubMed] [Google Scholar]
- 21. Boucherat O, Potus F, and Bonnet S. microRNA and pulmonary hypertension. Adv Exp Med Biol 888: 237–252, 2015 [DOI] [PubMed] [Google Scholar]
- 22. Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, and Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 169: 764–769, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Budhiraja R, Tuder RM, and Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Cahill E, Costello CM, Rowan SC, Harkin S, Howell K, Leonard MO, Southwood M, Cummins EP, Fitzpatrick SF, Taylor CT, Morrell NW, Martin F, and McLoughlin P. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 125: 920–930, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Cai H and Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Chakraborty S, Zawieja DC, Davis MJ, and Muthuchamy M. MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. Am J Physiol Cell Physiol 309: 680–692, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Chami H and Hassoun PM.. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis 55: 218–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen D, Zhao M, and Mundy G. Bone morphogenetic proteins. Growth Factors 22: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Kitchen CM, Streb JW, and Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Chen P-Y, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, and Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125: 4514–4528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho JG, Lee A, Chang W, Lee M-S, and Kim J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol 9: 294, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ciuclan L, Sheppard K, Dong L, Sutton D, Duggan N, Hussey M, Simmons J, Morrell NW, Jarai G, Edwards M, Dubois G, Thomas M, Van Heeke G, and England K. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol 183: 1461–1473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, Loyd JE, and Nichols WC. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am J Respir Crit Care Med 174: 590–598, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, and van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7: 1267–1278, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Cooley BC, Nevado J, Mellad J, Yang D, St. Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, and Boehm M. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 6: 227ra34, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Csiszar A, Labinskyy N, Jo H, Ballabh P, and Ungvari Z. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am J Physiol Circ Physiol 295: H569–H577, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dahal S, Huang P, Murray BT, and Mahler GJ. Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J Biomed Mater Res A 105: 2729–2741, 2017 [DOI] [PubMed] [Google Scholar]
- 38. Delgado VMC, Nugnes LG, Colombo LL, Troncoso MF, Fernández MM, Malchiodi EL, Frahm I, Croci DO, Compagno D, Rabinovich GA, Wolfenstein-Todel C, and Elola MT. Modulation of endothelial cell migration and angiogenesis: a novel function for the “tandem-repeat” lectin galectin-8. FASEB J 25: 242–254, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Dempsey EC, Wick MJ, Karoor V, Barr EJ, Tallman DW, Wehling CA, Walchak SJ, Laudi S, Le M, Oka M, Majka S, Cool CD, Fagan KA, Klemm DJ, Hersh LB, Gerard NP, Gerard C, and Miller YE. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol 174: 782–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, and Knowles JA. Familial primary pulmonary hypertension (Gene PPH1) is caused by mutations in the bone morphogenetic protein receptor–ii gene. Am J Hum Genet 67: 737–744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dengler VL, Galbraith MD, and Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol 49: 1–15, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, and Eddahibi S. Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Respir J 34: 1100–1110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MAA, Hopper RK, Ji L, Feldman BJ, and Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, and Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24: 2375–2385, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Elmedal B, De Dam MY, Mulvany MJ, and Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. Br J Pharmacol 141: 105–113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman K, D'Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, and Kovacic JC. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 7: 11853, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fadini GP and Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 55: 10–16, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Farkas L, Gauldie J, Voelkel NF, and Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1–15, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Foster WS, Suen CM, and Stewart DJ. Regenerative cell and tissue-based therapies for pulmonary arterial hypertension. Can J Cardiol 30: 1350–1360, 2014 [DOI] [PubMed] [Google Scholar]
- 50. Francis BN, Hale A, Channon KM, Wilkins MR, and Zhao L. Effects of tetrahydrobiopterin oral treatment in hypoxia-induced pulmonary hypertension in rat. Pulm Circ 4: 462–470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, and Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frid MG, Kale VA, and Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation. Circ Res 90: 1189–1196, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Fu F, Wang T, Wu Z, Feng Y, Wang W, Zhou S, Ma X, and Wang S. HMGA1 exacerbates tumor growth through regulating the cell cycle and accelerates migration/invasion via targeting miR-221/222 in cervical cancer. Cell Death Dis 9: 594, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fukawa T, Kajiya H, Ozeki S, Ikebe T, and Okabe K. Reactive oxygen species stimulates epithelial mesenchymal transition in normal human epidermal keratinocytes via TGF-beta secretion. Exp Cell Res 318: 1926–1932, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Fulton D, Li X, Bordan Z, Haigh S, Bentley A, Chen F, and Barman S. Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Antioxidants 6: 54, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, and Pittet JF. Interleukin-1β causes acute lung injury via αvβ5 and αvβ6 integrin-dependent mechanisms. Circ Res 102: 804–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ghosh S, Gupta M, Xu W, Mavrakis DA, Janocha AJ, Comhair SAA, Haque MM, Stuehr DJ, Yu J, Polgar P, Naga Prasad SV, and Erzurum SC. Phosphorylation inactivation of endothelial nitric oxide synthesis in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 310: L1199–L1205, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghouleh I Al, Sahoo S, Meijles DN, Amaral JH, de Jesus DS, Sembrat J, Rojas M, Goncharov DA, Goncharova EA, and Pagano PJ. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin Sci (Lond) 131: 2019–2035, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, and Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Gomez-Arroyo J, Nikolic I, and Yu PB. Animal models of pulmonary hypertension. In: Pulmonary Hypertension: Basic Science to Clinical Medicine. Springer International Publishing, 2015, pp. 161–172 [Google Scholar]
- 61. Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, and Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 185: 1850–1858, 2015 [DOI] [PubMed] [Google Scholar]
- 62. Grimminger J, Richter M, Tello K, Sommer N, Gall H, and Ghofrani HA. Thin air resulting in high pressure: mountain sickness and hypoxia-induced pulmonary hypertension. Can Respir J 2017: 1–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, and Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev 22: 543–551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haußmann I, Matzku S, Wenzel A, Ponta H, and Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65: 13–24, 1991 [DOI] [PubMed] [Google Scholar]
- 65. Guo S and Feng Z. Galectin-3 mediates the effect of PDGF on pulmonary arterial hypertension. Int J Clin Exp Med 8: 15302–15307, 2015 [PMC free article] [PubMed] [Google Scholar]
- 66. Hao M, Li M, and Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep 15: 160–168, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Helmke A, Casper J, Nordlohne J, David S, Haller H, Zeisberg EM, and von Vietinghoff S. Endothelial-to-mesenchymal transition shapes the atherosclerotic plaque and modulates macrophage function. FASEB J 33: 2278–2289, 2019 [DOI] [PubMed] [Google Scholar]
- 68. Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, and Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest 91: 1367–1373, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hjortnaes J, Shapero K, Goettsch C, Hutcheson JD, Keegan J, Kluin J, Mayer JE, Bischoff J, and Aikawa E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 242: 251–260, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, and Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 62: D42–D50, 2013 [DOI] [PubMed] [Google Scholar]
- 71. Hoeper MM, Simon R, and Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 23: 450–457, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Hoeven NW, Hollander MR, Yildirim C, Jansen MF, Teunissen PF, Horrevoets AJ, van der Pouw Kraan TCTM, and van Royen N. The emerging role of galectins in cardiovascular disease. Vascul Pharmacol 81: 31–41, 2016 [DOI] [PubMed] [Google Scholar]
- 73. Hong K-H, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, and Oh SP. Genetic ablation of the Bmpr2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hopper RK, Moonen J-RAJ, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, and Rabinovitch M. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation 133: 1783–1794, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hu C-J, Wang L-Y, Chodosh LA, Keith B, and Simon MC. Differential roles of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huang J, Lu W, Ouyang H, Chen Y, Zhang C, Luo X, Li M, Shu J, Zheng Q, Chen H, Chen J, Tang H, Sun D, Yuan JX-J, Yang K, and Wang J. Transplantation of mesenchymal stem cells attenuates pulmonary hypertension by normalizing the EndMT. Am J Respir Cell Mol Biol 62: 49–60, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, and Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, and Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 151: 1628–1631, 1995 [DOI] [PubMed] [Google Scholar]
- 79. Isobe S, Kataoka M, Endo J, Moriyama H, Okazaki S, Tsuchihashi K, Katsumata Y, Yamamoto T, Shirakawa K, Yoshida N, Shimoda M, Chiba T, Masuko T, Hakamata Y, Kobayashi E, Saya H, Fukuda K, and Sano M. Endothelial–mesenchymal transition drives expression of CD44 variant and xCT in pulmonary hypertension. Am J Respir Cell Mol Biol 61: 367–379, 2019 [DOI] [PubMed] [Google Scholar]
- 80. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, and Kaelin WG. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, and Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Jankov RP, Kantores C, Pan J, and Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008 [DOI] [PubMed] [Google Scholar]
- 83. Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, and Resta TC. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One 12: e0180455, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jernigan NL, Walker BR, and Resta TC. Pulmonary PKG-1 is upregulated following chronic hypoxia. Am J Physiol Cell Mol Physiol 285: L634–L642, 2003 [DOI] [PubMed] [Google Scholar]
- 85. Jernigan NL, Walker BR, and Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004 [DOI] [PubMed] [Google Scholar]
- 86. de Jesus DS, DeVallance E, Li Y, Falabella M, Guimaraes D, Shiva S, Kaufman BA, Gladwin MT, and Pagano PJ. Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration. Redox Biol 22: 101138, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kalhor R, Kalhor K, Mejia L, Leeper K, Graveline A, Mali P, and Church GM. Developmental barcoding of whole mouse via homing CRISPR. Science 361: eaat9804, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, and Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, and Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158: 917–923, 1998 [DOI] [PubMed] [Google Scholar]
- 90. Karoor V, Oka M, Walchak SJ, Hersh LB, Miller YE, and Dempsey EC. Neprilysin regulates pulmonary artery smooth muscle cell phenotype through a platelet-derived growth factor receptor–dependent mechanism. Hypertension 61: 921–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, and Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation 111: 2126–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 92. Koehler R, Grünig E, Pauciulo MW, Hoeper MM, Olschewski H, Wilkens H, Halank M, Winkler J, Ewert R, Bremer H, Kreuscher S, Janssen B, and Nichols WC. Low frequency of BMPR2 mutations in a German cohort of patients with sporadic idiopathic pulmonary arterial hypertension. J Med Genet 41: e127, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, and Baker AH. Endothelial to mesenchymal transition in cardiovascular disease. J Am Coll Cardiol 73: 190–209, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kretzschmar K and Watt FM. Lineage tracing. Cell 148: 33–45, 2012 [DOI] [PubMed] [Google Scholar]
- 95. Kumar R, Mickael C, Chabon J, Gebreab L, Rutebemberwa A, Garcia AR, Koyanagi DE, Sanders L, Gandjeva A, Kearns MT, Barthel L, Janssen WJ, Mauad T, Bandeira A, Schmidt E, Tuder RM, and Graham BB. The causal role of IL-4 and IL-13 in Schistosoma mansoni pulmonary hypertension. Am J Respir Crit Care Med 192: 998–1008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lachmanová V, Hnliličková O, Povýšilová V, Hampl V, and Herget J. N-acetylcysteine inhibits hypoxic pulmonary hypertension most effectively in the initial phase of chronic hypoxia. Life Sci 77: 175–182, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Lai YC, Potoka KC, Champion HC, Mora AL, and Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115: 115–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lalich JJ and Ehrhart LA. Monocrotaline-induced pulmonary arteritis in rats. J Atheroscler Res 2: 482–492, 1962 [DOI] [PubMed] [Google Scholar]
- 99. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, Loyd JE, Nichols WC, Trembath RC, and Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84, 2000 [DOI] [PubMed] [Google Scholar]
- 100. Lau EMT, Giannoulatou E, Celermajer DS, and Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol 14: 603–614, 2017 [DOI] [PubMed] [Google Scholar]
- 101. Lau WM, Teng E, Chong HS, Lopez KAP, Tay AYL, Salto-Tellez M, Shabbir A, So JBY, and Chan SL. CD44v8–10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res 74: 2630–2641, 2014 [DOI] [PubMed] [Google Scholar]
- 102. Li L, Kim I-K, Chiasson V, Chatterjee P, and Gupta S. NF-κB mediated miR-130a modulation in lung microvascular cell remodeling: implication in pulmonary hypertension. Exp Cell Res 359: 235–242, 2017 [DOI] [PubMed] [Google Scholar]
- 103. Li L, Wei C, Kim I-K, Janssen-Heininger Y, and Gupta S. Inhibition of nuclear factor-κB in the lungs prevents monocrotaline-induced pulmonary hypertension in mice. Hypertension 63: 1260–1269, 2014 [DOI] [PubMed] [Google Scholar]
- 104. Liu JQ, Zelko IN, Erbynn EM, Sham JS, and Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Liu S, Premont RT, Kontos CD, Huang J, and Rockey DC. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein βγ subunit signaling to protein kinase B/Akt. J Biol Chem 278: 49929–49935, 2003 [DOI] [PubMed] [Google Scholar]
- 106. Liu T, Zou X-Z, Huang N, Ge X-Y, Yao M-Z, Liu H, Zhang Z, and Hu C-P. miR-27a promotes endothelial-mesenchymal transition in hypoxia-induced pulmonary arterial hypertension by suppressing BMP signaling. Life Sci 227: 64–73, 2019 [DOI] [PubMed] [Google Scholar]
- 107. Lok DJA, Van Der Meer P, De La Porte PWBA, Lipsic E, Van Wijngaarden J, Hillege HL, and Van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99: 323–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lu X, Murphy TC, Nanes MS, and Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ma L, Lu MF, Schwartz RJ, and Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611, 2005 [DOI] [PubMed] [Google Scholar]
- 110. Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, Eickelberg O, Olschewski H, Gregory Elliott C, Glissmeyer E, Carlquist J, Kim M, Torbicki A, Fijalkowska A, Szewczyk G, Parma J, Abramowicz MJ, Galie N, Morisaki H, Kyotani S, Nakanishi N, Morisaki T, Humbert M, Simonneau G, Sitbon O, Soubrier F, Coulet F, Morrell NW, and Trembath RC. Mutations of the TGF-β type II receptorBMPR2 in pulmonary arterial hypertension. Hum Mutat 27: 121–132, 2006 [DOI] [PubMed] [Google Scholar]
- 111. Mahler GJ, Farrar EJ, and Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol 33: 121–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Maleszewska M, Moonen JRAJ, Huijkman N, van de Sluis B, Krenning G, and Harmsen MC. IL-1β and TGFβ2 synergistically induce endothelial to mesenchymal transition in an NFκB-dependent manner. Immunobiology 218: 443–454, 2013 [DOI] [PubMed] [Google Scholar]
- 113. Mammoto T, Muyleart M, Konduri GG, and Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension Through TGFβ-Smad signaling. Am J Respir Cell Mol Biol 58: 194–207, 2018 [DOI] [PubMed] [Google Scholar]
- 114. Marelli-Berg FM, Clement M, Mauro C, and Caligiuri G. An immunologist's guide to CD31 function in T-cells. J Cell Sci 126: 2343–2352, 2013 [DOI] [PubMed] [Google Scholar]
- 115. Markwald RR, Fitzharris TP, and Smith WNA. Structural analysis of endocardial cytodifferentiation. Dev Biol 42: 160–180, 1975 [DOI] [PubMed] [Google Scholar]
- 116. Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, and Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 185: 313–318, 1998 [DOI] [PubMed] [Google Scholar]
- 117. Masri FA, Xu W, Comhair SAA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, and Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Cell Mol Physiol 293: L548–L554, 2007 [DOI] [PubMed] [Google Scholar]
- 118. McCulley DJ, Kang JO, Martin JF, and Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn 237: 3200–3209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Medici D, Potenta S, and Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial–mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J 437: 515–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mendoza FA, Piera-Velazquez S, Farber JL, Feghali-Bostwick C, and Jiménez SA. Endothelial cells expressing endothelial and mesenchymal cell gene products in lung tissue from patients with systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 68: 210–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miscianinov V, Martello A, Rose L, Parish E, Cathcart B, Mitić T, Gray GA, Meloni M, Al Haj Zen A, and Caporali A. MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther 26: 1996–2007, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Miyazono K, Kusanagi K, and Inoue H. Divergence and convergence of TGF-β/BMP signaling. J Cell Physiol 187: 265–276, 2001 [DOI] [PubMed] [Google Scholar]
- 123. Miyazono K, Maeda S, and Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 16: 251–263, 2005 [DOI] [PubMed] [Google Scholar]
- 124. Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, and Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation 117: 2626–2636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mojiri A, Stoletov K, Carrillo MAL, Willetts L, Jain S, Godbout R, Jurasz P, Sergi CM, Eisenstat DD, Lewis JD, and Jahroudi N. Functional assessment of von Willebrand factor expression by cancer cells of non-endothelial origin. Oncotarget 8: 13015–13029, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Molteni A, Ward WF, Ts'ao CH, and Solliday NH. Monocrotaline pneumotoxicity in mice. Virchows Arch B Cell Pathol Incl Mol Pathol 57: 149–155, 1989 [DOI] [PubMed] [Google Scholar]
- 127. Montorfano I, Becerra A, Cerro R, Echeverría C, Sáez E, Morales MG, Fernández R, Cabello-Verrugio C, and Simon F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-β1 and TGF-β2-dependent pathway. Lab Investig 94: 1068–1082, 2014 [DOI] [PubMed] [Google Scholar]
- 128. Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, and Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Hum Mutat 23: 632, 2004 [DOI] [PubMed] [Google Scholar]
- 129. Negi V and Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2: e91327, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nieto-Fontarigo JJ, González-Barcala FJ, San José E, Arias P, Nogueira M, and Salgado FJ. CD26 and asthma: a comprehensive review. Clin Rev Allergy Immunol 56: 139–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nieto MA, Huang RYJ, Jackson RA, and Thiery JP. EMT: 2016. Cell 166: 21–45, 2016 [DOI] [PubMed] [Google Scholar]
- 132. Nikitopoulou I, Orfanos SE, Kotanidou A, Maltabe V, Manitsopoulos N, Karras P, Kouklis P, Armaganidis A, and Maniatis NA. Vascular endothelial-cadherin downregulation as a feature of endothelial transdifferentiation in monocrotaline-induced pulmonary hypertension. Am J Physiol Cell Mol Physiol 311: L352–L363, 2016 [DOI] [PubMed] [Google Scholar]
- 133. Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, and Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. O'Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, and Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Circ Physiol 292: H285–H294, 2007 [DOI] [PubMed] [Google Scholar]
- 135. Orriols M, Gomez-Puerto MC, and Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci 74: 1–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Otsuki S, Sawada H, Yodoya N, Shinohara T, Kato T, Ohashi H, Zhang E, Imanaka-Yoshida K, Shimpo H, Maruyama K, Komada Y, and Mitani Y. Potential contribution of phenotypically modulated smooth muscle cells and related inflammation in the development of experimental obstructive pulmonary vasculopathy in rats. PLoS One 10: e0118655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Patel J, Baz B, Wong HY, Lee JS, and Khosrotehrani K. Accelerated endothelial to mesenchymal transition increased fibrosis via deleting Notch signaling in wound vasculature. J Invest Dermatol 138: 1166–1175, 2018 [DOI] [PubMed] [Google Scholar]
- 138. Pathak R and Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. P T 35: 509–513, 2010 [PMC free article] [PubMed] [Google Scholar]
- 139. Pegoraro S, Ros G, Piazza S, Sommaggio R, Ciani Y, Rosato A, Sgarra R, Sal G Del, and Manfioletti G. HMGA1 promotes metastatic processes in basal-like breast cancer regulating EMT and stemness. Oncotarget 4: 1293–1308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pérez L, Muñoz-Durango N, Riedel CA, Echeverría C, Kalergis AM, Cabello-Verrugio C, and Simon F. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev 33: 41–54, 2017 [DOI] [PubMed] [Google Scholar]
- 141. Piera-Velazquez S and Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev 99: 1281–1324, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pober JS and Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7: 803–815, 2007 [DOI] [PubMed] [Google Scholar]
- 143. Price LC, Wort SJ, Perros F, Dorfmüller P, Huertas A, Montani D, Cohen-Kaminsky S, and Humbert M. Inflammation in pulmonary arterial hypertension. Chest 141: 210–221, 2012 [DOI] [PubMed] [Google Scholar]
- 144. Prysyazhna O and Eaton P. Redox regulation of cGMP-dependent protein kinase Iα in the cardiovascular system. Front Pharmacol 6: 139, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR, Berry GJ, Pearl RG, and Kao PN. Endothelial fate mapping in mice with pulmonary hypertension. Circulation 129: 692–703, 2014 [DOI] [PubMed] [Google Scholar]
- 146. Rabinovitch M, Guignabert C, Humbert M, and Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, and Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131: 1006–1018, 2015 [DOI] [PubMed] [Google Scholar]
- 148. Reynolds AM, Holmes MD, Danilov SM, and Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 39: 329–343, 2012 [DOI] [PubMed] [Google Scholar]
- 149. Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh S, and Lee HB. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 150. Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, and Fiocchi C. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol 179: 2660–2673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rudyk O, Rowan A, Prysyazhna O, Krasemann S, Hartmann K, Zhang M, Shah AM, Ruppert C, Weiss A, Schermuly RT, Ida T, Akaike T, Zhao L, and Eaton P. Oxidation of PKGIα mediates an endogenous adaptation to pulmonary hypertension. Proc Natl Acad Sci U S A 116: 13016–13025, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rzucidlo EM, Martin KA, and Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 45: A25–A32, 2007 [DOI] [PubMed] [Google Scholar]
- 153. Sabbineni H, Verma A, and Somanath PR. Isoform-specific effects of transforming growth factor β on endothelial-to-mesenchymal transition. J Cell Physiol 233: 8418–8428, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sahoo S, Meijles DN, Al Ghouleh I, Tandon M, Cifuentes-Pagano E, Sembrat J, Rojas M, Goncharova E, and Pagano PJ. MEF2C-MYOCD and leiomodin1 suppression by miRNA-214 promotes smooth muscle cell phenotype switching in pulmonary arterial hypertension. PLoS One 11: e0153780, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, and Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 10: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Scott DW, Vallejo MO, and Patel RP. Heterogenic endothelial responses to inflammation: role for differential N-glycosylation and vascular bed of origin. J Am Heart Assoc 2: e000263, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sharp J, Farha S, Park MM, Comhair SA, Lundgrin EL, Tang WHW, Bongard RD, Merker MP, and Erzurum SC. Coenzyme Q supplementation in pulmonary arterial hypertension. Redox Biol 2: 884–891, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sheikh AQ, Lighthouse JK, and Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep 6: 809–817, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Shimazu T, Otani H, Yoshioka K, Fujita M, Okazaki T, and Iwasaka T. Sepiapterin enhances angiogenesis and functional recovery in mice after myocardial infarction. Am J Physiol Circ Physiol 301: H2061–H2072, 2011 [DOI] [PubMed] [Google Scholar]
- 160. Shimoda LA and Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med 91: 297–309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, and Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62: D34–D41, 2013 [DOI] [PubMed] [Google Scholar]
- 162. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, and Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53: 1801913, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Song S, Zhang M, Yi Z, Zhang H, Shen T, Yu X, Zhang C, Zheng X, Yu L, Ma C, Liu Y, and Zhu D. The role of PDGF-B/TGF-β1/neprilysin network in regulating endothelial-to-mesenchymal transition in pulmonary artery remodeling. Cell Signal 28: 1489–1501, 2016 [DOI] [PubMed] [Google Scholar]
- 164. Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, and Zhang Y-Y. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Circ Physiol 295: H677–H690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sporn LA, Chavin SI, Marder VJ, and Wagner DD. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest 76: 1102–1106, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stemmler MP, Eccles RL, Brabletz S, and Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol 21: 102–112, 2019 [DOI] [PubMed] [Google Scholar]
- 167. Stenmark KR, Fagan KA, and Frid MG. Hypoxia-induced pulmonary vascular remodeling. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 168. Stenmark KR, Frid M, and Perros F. Endothelial-to-mesenchymal transition: an evolving paradigm and a promising therapeutic target in PAH. Circulation 133: 1734–1737, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K, and West J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Cell Mol Physiol 314: L118–L126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Suzuki T, Tada Y, Gladson S, Nishimura R, Shimomura I, Karasawa S, Tatsumi K, and West J. Vildagliptin ameliorates pulmonary fibrosis in lipopolysaccharide-induced lung injury by inhibiting endothelial-to-mesenchymal transition. Respir Res 18: 177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Suzuki Y, Tanigaki T, Heimer D, Wang W, Ross WG, Murphy GA, Sakai A, Sussman HH, Vu TH, and Raffin TA. TGF-β 1 causes increased endothelial ICAM-1 expression and lung injury. J Appl Physiol 77: 1281–1287, 1994 [DOI] [PubMed] [Google Scholar]
- 172. Suzuki YJ, Steinhorn RH, and Gladwin MT. Antioxidant therapy for the treatment of pulmonary hypertension. Antioxidants Redox Signal 18: 1723–1726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tabima DM, Frizzell S, and Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 52: 1970–1986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, Wang Z, Gupta A, Zhou T, Sun X, Dash S, Wang Z, Balistrieri A, Zheng Q, Cordery AG, Desai AA, Rischard F, Khalpey Z, Wang J, Black SM, Garcia JGN, Makino A, and Yuan JXJ. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 314: L256–L275, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, and Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001 [DOI] [PubMed] [Google Scholar]
- 176. Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, and Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 37: 741–745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Trelstad RL, Hay ED, and Revel J-P. Cell contact during early morphogenesis in the chick embryo. Dev Biol 16: 78–106, 1967 [DOI] [PubMed] [Google Scholar]
- 178. Tsuruta D and Jones JCR. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci 116: 4977–4984, 2003 [DOI] [PubMed] [Google Scholar]
- 179. Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tuder RM, Groves B, Badesch DB, and Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 181. Vesuna F, van Diest P, Chen JH, and Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun 367: 235–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA, and Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ 4: 619–629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Voelkel NF, Tuder RM, Bridges J, and Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol 11: 664–675, 1994 [DOI] [PubMed] [Google Scholar]
- 184. Wahid F, Shehzad A, Khan T, and Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta Mol Cell Res 1803: 1231–1243, 2010 [DOI] [PubMed] [Google Scholar]
- 185. Wang J, Yu M, Xu J, Cheng Y, Li X, Wei G, Wang H, Kong H, and Xie W. Glucagon-like peptide-1 (GLP-1) mediates the protective effects of dipeptidyl peptidase IV inhibition on pulmonary hypertension. J Biomed Sci 26: 6, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, and Shi LL. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1: 87–105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wang X, Shults NV, and Suzuki YJ. Oxidative profiling of the failing right heart in rats with pulmonary hypertension. PLoS One 12: e0176887, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wang Z, Wang D-Z, Pipes GCT, and Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A 100: 7129–7134, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, and Fagan K. Mice expressing BMPR2 R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Cell Mol Physiol 295: L744–L755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, Perrella MA, Osorio JC, Haley KJ, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Khan OF, Bader A, Gochuico BR, Matar M, Polach K, Johannessen NM, Prosser HM, Anderson DG, Langer R, Zweier JL, Bindoff LA, Systrom D, Waxman AB, Jin RC, and Chan SY. Genetic and hypoxic alterations of the micro RNA-210-ISCU 1/2 axis promote iron–sulfur deficiency and pulmonary hypertension. EMBO Mol Med 7: 695–713, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wong CM, Bansal G, Pavlickova L, Marcocci L, and Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal 18: 1789–1796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Xiao R, Su Y, Feng T, Sun M, Liu B, Zhang J, Lu Y, Li J, Wang T, Zhu L, and Hu Q. Monocrotaline induces endothelial injury and pulmonary hypertension by targeting the extracellular calcium–sensing receptor. J Am Heart Assoc 6: e004865, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Xu J, Wang J, He M, Han H, Xie W, Wang H, and Kong H. Dipeptidyl peptidase IV (DPP-4) inhibition alleviates pulmonary arterial remodeling in experimental pulmonary hypertension. Lab Invest 98: 1333–1346, 2018 [DOI] [PubMed] [Google Scholar]
- 194. Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, and Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A 104: 1342–1347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Xu X, Friehs I, Hu TZ, Melnychenko I, Tampe B, Alnour F, Iascone M, Kalluri R, Zeisberg M, Del Nido PJ, and Zeisberg EM. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res 116: 857–866, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Xu YP, He Q, Shen Z, Shu XL, Wang CH, Zhu JJ, Shi LP, and Du LZ. MiR-126a-5p is involved in the hypoxia-induced endothelial-to-mesenchymal transition of neonatal pulmonary hypertension. Hypertens Res 40: 552–561, 2017 [DOI] [PubMed] [Google Scholar]
- 197. Yadin D, Knaus P, and Mueller TD. Structural insights into BMP receptors: specificity, activation and inhibition. Cytokine Growth Factor Rev 27: 13–34, 2016 [DOI] [PubMed] [Google Scholar]
- 198. Yu M, Peng L, Liu P, Yang M, Zhou H, Ding Y, Wang J, Huang W, Tan Q, Wang Y, Xie W, Kong H, and Wang H. Paeoniflorin ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition. Drug Des Devel Ther 14: 1191–1202, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yung LM, Nikolic I, Paskin-Flerlage SD, Scott Pearsall R, Kumar R, and Yu PB. A selective transforming growth factor-β ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care Med 194: 1140–1151, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zavadil J, Cermak L, Soto-Nieves N, and Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23: 1155–1165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhang B, Niu W, Dong H-Y, Liu M-L, Luo Y, and Li Z-C. Hypoxia induces endothelial-mesenchymal transition in pulmonary vascular remodeling. Int J Mol Med 42: 270–278, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhang H, Liu Y, Yan L, Du W, Zhang X, Zhang M, Chen H, Zhang Y, Zhou J, Sun H, and Zhu D. Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia. J Cell Physiol 233: 4077–4090, 2018 [DOI] [PubMed] [Google Scholar]
- 203. Zhang J, Zhang Z, Zhang DY, Zhu J, Zhang T, and Wang C. microRNA 126 inhibits the transition of endothelial progenitor cells to mesenchymal cells via the PIK3R2-PI3K/Akt signalling pathway. PLoS One 8: e83294, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhang L, Li YM, Zeng XX, Wang XY, Chen SK, Gui LX, and Lin MJ. Galectin-3-mediated transdifferentiation of pulmonary artery endothelial cells contributes to hypoxic pulmonary vascular remodeling. Cell Physiol Biochem 51: 763–777, 2018 [DOI] [PubMed] [Google Scholar]
- 205. Zhang Y, Zhang M, Xie W, Wan J, Tao X, Liu M, Zhen Y, Lin F, Wu B, Zhai Z, and Wang C. Gremlin-1 is a key regulator of endothelial-to-mesenchymal transition in human pulmonary artery endothelial cells. Exp Cell Res 390:111941, 2020 [DOI] [PubMed] [Google Scholar]
- 206. Zhao L, Oliver E, Maratou K, Atanur SS, Dubois OD, Cotroneo E, Chen CN, Wang L, Arce C, Chabosseau PL, Ponsa-Cobas J, Frid MG, Moyon B, Webster Z, Aldashev A, Ferrer J, Rutter GA, Stenmark KR, Aitman TJ, and Wilkins MR. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature 524: 356–360, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhao Y-Y, Zhao YD, Mirza MK, Huang JH, Potula H-HSK, Vogel SM, Brovkovych V, Yuan JX-J, Wharton J, and Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Zhao YD, Cai L, Mirza MK, Huang X, Geenen DL, Hofmann F, Yuan JX-J, and Zhao Y-Y. Protein kinase G-I deficiency induces pulmonary hypertension through Rho A/Rho kinase activation. Am J Pathol 180: 2268–2275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhong J, Rao X, and Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 226: 305–314, 2013 [DOI] [PubMed] [Google Scholar]
- 210. Zhou G, Chen T, and Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 52: 139–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Zhu P, Huang L, Ge X, Yan F, Wu R, and Ao Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol 87: 463–474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, and Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J 19: 517–526, 2002 [DOI] [PubMed] [Google Scholar]