Abstract
Significance: Red blood cell (RBC)-mediated vasodilation plays an important role in oxygen delivery. This occurs through hemoglobin actions, at least in significant part, to convert heme-bound nitric oxide (NO) (in tense [T]/deoxygenated-state hemoglobin) into vasodilator S-nitrosothiol (SNO) (in relaxed [R]/oxygenated-state hemoglobin), convey SNO through the bloodstream, and release it into tissues to increase blood flow. The coupling of hemoglobin R/T state allostery, both to NO conversion into SNO and to SNO release (along with oxygen), under hypoxia supports the model of a three-gas respiratory cycle (O2/NO/CO2).
Recent Advances: Oxygenation of tissues is dependent on a single, strictly conserved Cys residue in hemoglobin (βCys93). Hemoglobin couples SNO formation/release at βCys93 to O2 binding/release at hemes (“thermodynamic linkage”). Mice bearing βCys93Ala hemoglobin that is unable to generate SNO-βCys93 establish that SNO-hemoglobin is important for R/T allostery-regulated vasodilation by RBCs that couple blood flow to tissue oxygenation.
Critical Issues: The model for RBC-mediated vasodilation originally proposed by Stamler et al. in 1996 has been largely validated: SNO-βCys93 forms in vivo, dilates blood vessels, and is hypoxia-regulated, and RBCs actuate vasodilation proportionate to hypoxia. Numerous compensations in βCys93Ala animals to alleviate tissue hypoxia (discussed herein) are predicted to preserve vasodilatory responses of RBCs but impair linkage to R/T transition in hemoglobin. This is borne out by loss of responsivity of mutant RBCs to oxygen, impaired blood flow responses to hypoxia, and tissue ischemia in βCys93-mutant animals.
Future Directions: SNO-hemoglobin mediates hypoxic vasodilation in the respiratory cycle. This fundamental physiology promises new insights in vascular diseases and blood disorders.
Keywords: S-nitrosylation, S-nitrosothiol, autoregulation, hypoxic vasodilation, S-nitrosohemoglobin
Hypoxic Vasodilation
Three-gas respiratory cycle of hemoglobin
The most immediate function of the cardiovascular and pulmonary systems is to ensure an adequate supply of oxygen to all cells, in addition to its roles in metabolic waste removal, nutrient provision, and immune cell circulation. Oxygen taken up in the lungs is carried primarily as a ligand bound to iron in heme groups in hemoglobin (Hb) within red blood cells (RBCs). Hb is by far the most abundant protein in each RBC, and RBCs are the most numerous cell type in the body, attesting to how fundamental this oxygen-carrying function of Hb is to multicellular life.
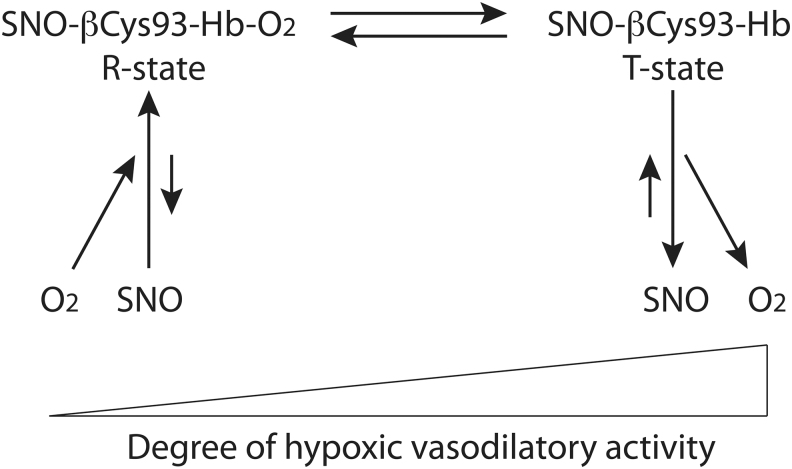
Hb is one of, if not, the most intensively studied of all proteins, both because of its ready availability in nearly pure form and also for its remarkable molecular properties (84). Hb is a tetramer composed of two highly related globin subunits; in adults, the major form of Hb is HbA, composed of two α-globin and two β-globin subunits. Embryonic and fetal globins (ɛ-globin and γ-globin) are utilized in embryonic and fetal Hb forms with shifted oxygen binding curves (the embryo and fetus are hypoxic with only indirect access to oxygen); these developmental Hb forms (HbE, HbF), as well as others of unknown function (HbD), also persist at low abundance (∼3% in total) in adult RBCs. All globins contain a small set of conserved residues (84): three of these serve to keep the heme group in place and correctly oriented (His63, His92, Phe42 in β-globin), whereas one acts as a site for nitric oxide (NO) modification (Cys93 in β-type globins), which will be the major focus of this review. It is important to keep in mind that although mammalian Hb has six cysteines (one in each α-globin and two in each β-globin), all of which may bind NO (134), β-Cys93 has two unique properties: it is the most reactive Hb Cys residue (43) and, more importantly, its reactivity is linked to O2 binding/release at the heme site (“thermodynamic linkage”) and thus to conformation of Hb (Fig. 1) (115). All β-type globins (embryonic ɛ, fetal γ, and adult δ- and β-globins) share this conserved cysteine and likely its essential function (26, 64, 85, 134), so all Hb tetramers contain two such sites. In this review, “Hb” refers to the Hb mixture as found in an adult RBC, unless otherwise noted, and “C93A” refers to mice in which Cys93 is mutated to Ala in the β-globin gene so that this site cannot be modified by S-nitrosothiol (SNO; but in which the remaining Cys93 in embryonic and fetal globin chains is preserved and SNO remains detectable in significant amounts at fetal Hb Cys93).
FIG. 1.
Thermodynamic linkage of βCys93 reactivity to Hb oxygenation state and conformation. Hb βCys93 is highly reactive when Hb is oxygenated and in the R state, and consequently, SNO at this site forms when oxygen binds, whereas βCys93 is unreactive (and SNO is disfavored) when Hb is deoxygenated and in the T state (13, 14, 63, 66). Thus, Hb can carry SNO in the R state and release it along with oxygen upon transition to the T state in hypoxic tissue in a graded manner. Hb, hemoglobin; SNO, S-nitrosothiol.
One of the remarkable functions of Hb is its highly regulated oxygen affinity, which allows efficient oxygen loading (one diatomic oxygen ligand bound to each of the four hemes) in the lungs and also effective release of all oxygen under hypoxia. First, the iron atom being coordinated by four nitrogen atoms within the heme ring and by the two globin His residues keeps the iron predominantly in the Fe(II) state when unliganded but in a state closer to Fe(III) when liganded by O2 (54). This property of the oxidation state of the heme-bound iron atom allows each individual heme iron to bind oxygen and also release it, rather than simply forming stable iron oxide (54). Second, the pioneering work of Bohr and of Haldane over a century ago demonstrated that the affinity of oxygen for Hb is regulated by pH (when CO2 gas is converted to carbonic acid in the blood in tissue capillaries, the lowered pH leads to increased oxygen offloading from Hb: the Bohr effect) and also that conversely, oxygen levels regulate the proton- and bicarbonate-carrying capacity of Hb (the Haldane effect) (124). Third, Hb is well known to display highly cooperative allosteric conformation changes depending on how many of the four heme groups are liganded with diatomic oxygen (84, 133). The molecular explanation for this effect was provided by the X-ray crystal structures of oxygenated and deoxygenated Hb solved by Perutz in the 1960s: Hb undergoes a dramatic conformational shift between the deoxygenated (called tense, or T) state and the oxygenated (called relaxed, or R) state (83, 86). Each oxygen bound to Hb increases the affinity of the remaining hemes for oxygen, and two to three oxygen molecules bound to Hb promote the change from T- to R-conformation to ensure full oxygen loading; conversely, the release of each oxygen molecule decreases the binding affinity of the remaining oxygen, so that release of two oxygen molecules from oxygenated Hb promotes the R- to T-state transition to greatly reduce oxygen affinity to the remaining hemes that facilitate full oxygen offloading and delivery to tissue (84, 133). Notably, about 1% of hemoglobins do not conform to all-or-nothing behavior that is expected of oxy (Hb[(FeII)O2]4) and deoxy (Hb[(FeII)]4) tetramers, but rather represent a micropopulation that includes Fe(II)NO or Fe(III)NO (e.g., Hb[Fe(III)NO][Fe(II)O2]3) (113). These heme-NO species are in equilibrium with SNO-hemoglobin, with FeNO favored in the T state and S-nitrosylated hemoglobin (SNO-Hb) favored in the R state (1, 46, 63, 87, 102, 113). Thus, NO binds heme in deoxygenated blood, whereas SNO forms in oxygenated blood. Within tissues, the release of SNO that is coupled to the release of oxygen results in regeneration of heme-NO, but some SNO escapes the RBCs to dilate blood vessels (63, 88, 113).
It is important to realize that in a healthy human at rest, the difference between fully oxygenated Hb leaving the lungs (nearly 100% O2 saturation) and deoxygenated Hb in mixed venous blood arriving to the lungs (∼70% O2 saturation) corresponds to oxygen delivery/tissue oxygen extraction of only about one of the four oxygen molecules on each Hb during the entire circulatory cycle (e.g., oxygen extraction of ∼25%) (18, 104). This is true despite the fact that tissue pO2 and corresponding Hb-O2 saturation are substantially lower than this within metabolically active tissues. This is explained in part by countercurrents (and the like) between arterioles and venules (which pick up unused oxygen) and to basal blood flow through all tissues being averaged together in mixed venous blood. Despite local oxygen extraction increasing with elevated metabolic activity, such as in muscles during exercise, total body oxygen extraction rarely exceeds 50% except in extreme circumstances (18, 104). During increased metabolic demand, blood flow undergoes large-scale shifts at the level of large tissue-feeding arteries that direct blood away from metabolically inactive tissues toward metabolically active tissues (such as after a meal when gut blood flow increases, or a fight-or-flight response when blood flow increases to the heart and muscle). It has been a great paradox as to why humans cannot tolerate oxygen saturation of less than 50% when half the oxygen remains. However, as detailed elsewhere (88, 89, 113), SNO-mediated vasodilation through R/T transition in hemoglobin is impaired at low oxygen saturation.
Concept of the integrated vascular unit
While the mechanisms for bulk oxygen carriage and delivery by Hb are well understood, less appreciated is the importance of Hb being packaged within RBCs (rather than free-floating) and the constraints that this packaging places on the function of the system overall. Because human RBCs are 6–8 μM in diameter (33) and the finest capillaries are of similar size (70), RBCs must pass through capillaries in single-file (plug flow mode). Indeed, flow through these smallest capillaries requires that both the endothelial tube and RBC deform during passage, so that reduced RBC rheology (such as by sickled RBCs, as an extreme case) can block flow (vaso-occlusion) and lead to tissue hypoxia (70). The capillary endothelium is in intimate contact with the RBC membrane through such passage, allowing direct communication from RBC to capillary, as described in the Autoregulation of blood flow section, and defining the integrated vascular unit (IVU) (88). Oxygen delivery to tissues cannot be understood without considering the role of the IVU that centers around RBCs, as blood flow is often more important to oxygen delivery than is oxygen carriage (88), since flow can vary greatly while carriage is essentially fixed.
Autoregulation of blood flow
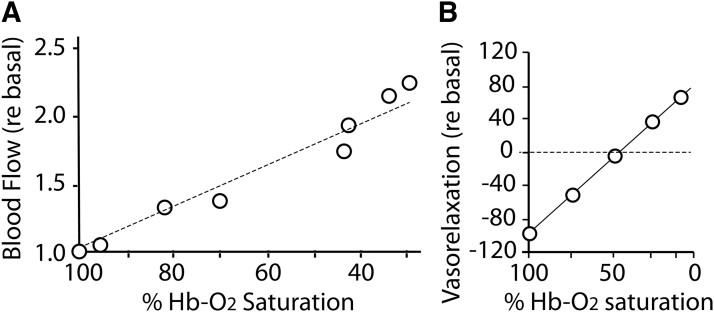
Tissues are able to regulate their blood flow in response to local metabolic demand. Tissue autoregulation of blood flow is most often studied in recent years with regard to cerebral vasculature [e.g., Ogoh and Tarumi (74)] but is a well-known effect in all tissues that was perhaps best demonstrated by classic studies in skeletal muscle by Guyton in the 1960s (100). In the Guyton experiments, one canine hind limb was supplied with oxygenated blood directly from one pulmonary vein through a bypass pump at constant pressure, and the fraction of inspired oxygen (FiO2) to the lung supplying that limb was reduced in stages to evoke tissue hypoxia. The amount of blood flowing through the hind limb and the Hb-O2 saturation of the incoming blood were measured at multiple FiO2 levels. Importantly, the amount of blood flow was shown to increase linearly (>2.5-fold) as Hb-O2 saturation of blood entering the muscle decreased from the normal near 100% at 20% O2 in room air, to ∼25% in the near-absence of inspired O2 (Fig. 2A) (100). This has the effect of increasing tissue blood flow in proportion to tissue hypoxia, maintaining oxygen delivery as consistently high as possible. For small tubes, flow is proportional to Radius4 according to Poiseuille's law. Increasing flow 2.5-fold (the maximal effect observed in Fig. 2A) corresponds to only a 26% increase in diameter. In practice, diameter change in a small upstream vessel is likely far less because the increased flow is a system-wide value that is distributed among many individual downstream vessels, so most physiological changes in flow in the microcirculation may not be readily detectable in terms of vessel diameter.
FIG. 2.
Autoregulation of tissue blood flow under hypoxia. (A) Blood flow through the canine hind limb increases linearly as Hb O2-saturation decreases. Data replotted from Ross et al. (100). (B) Allosteric modulation of vasodilation. Vasodilatory activity of RBCs varies with oxygenation state and effector thiol. Organ bath assay of phenylephrine-preconstricted aorta for vasorelaxation activity of human RBCs was performed in the presence of 10 μM glutathione at various O2 levels. Oxygenated RBCs/SNO-Hb is vasoconstrictive, due to scavenging of endogenous NO, but increasing vasorelaxation occurs as O2 level is dropped, reflecting SNO release. Data replotted from McMahon et al. (66) with permission. NO, nitric oxide; RBCs, red blood cells; SNO-Hb, S-nitrosylated hemoglobin.
Although capillaries abound in tissues, not all capillaries are open to RBC flow at any given time, and it is by recruiting inactive capillaries and increasing the rate of flow through countless individual capillaries that tissue blood flow—and thus tissue oxygen delivery—can be effectively increased [e.g., Erdener and Dalkara (21), Joseph et al. (47), Nortley et al. (73), and Schmid et al. (106)]. Multiple mechanisms operate to regulate microvascular blood flow, including precapillary smooth muscle “sphincters” regulated by vasoactive peptides and metabolites and by electrical signals (12, 82, 118). Importantly, hypoxia-driven blood flow through small arterioles and capillaries appears sensitive to small changes in the hypoxic state, rather than inducing an all-or-none hypoxic response at some specific oxygen threshold sensed by the tissue (such as the P50 for binding to heme in Hb or other metabolite sensor that would produce a nonlinear relationship with Hb-O2 saturation) (100). Instead, this suggests that tissue hypoxia-driven blood flow is somehow related to the amount (or fraction) of Hb in the T state, which is in some way detected by the tissue microvasculature (note that sensing the Hb P50 for O2 or the R/T transition would also produce a nonlinear relationship) (Fig. 2B). But how could tissue microvasculature cells (primarily endothelia) sense Hb T state inside nearby RBCs?
S-Nitrosylation and the Importance of Hemoglobin βCys93
Microcirculatory NO bioactivity
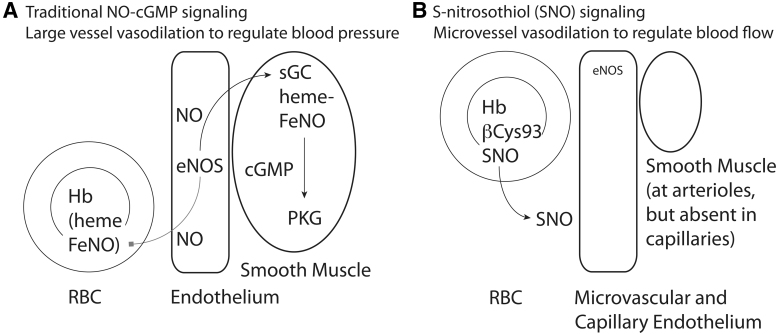
The classic means to control vessel diameter in blood vessels is via the gasotransmitter NO (8). In the classic paradigm (Fig. 3A), vasoactive mediators in blood activate receptors on vascular endothelial cells that stimulate the conversion of arginine to citrulline plus NO by endothelial nitric oxide synthase (eNOS). The NO gas diffuses from the endothelia into surrounding smooth muscle cells, where it binds to the heme cofactor in the enzyme soluble guanylyl cyclase (sGC) to stimulate the conversion of GTP to cyclic GMP (cGMP), and the cGMP-mediated activation of the cGMP-activated protein kinase, also called protein kinase G (PKG). PKG then phosphorylates substrate proteins and enzymes to cause the smooth muscle cell to relax, increasing vessel diameter and reducing vascular resistance, which increases bulk flow while reducing systemic blood pressure (8). However, eNOS is absent in distal arterioles (51), and capillaries lack smooth muscle and sGC-mediated vasorelaxation (110). Also, NO-generating enzymes require O2 as a substrate, so this mechanism is ill-suited to control tissue microvascular hypoxic autoregulation. Maximal “resistance” blood vessel vasorelaxation reduces resistance to blood flow only by about half, indicating that the tissue capillaries also account for about half of the total systemic resistance. Thus, small changes in microvascular resistance to flow (effective capillary diameter) have the potential to have dramatic effects on tissue perfusion by blood, since by Poiseuille's law, flow through a narrow vessel is proportional to the fourth power of the vessel radius.
FIG. 3.
Mechanisms for NO bioactivity. (A) Traditional signaling by NO gas. Vasodilatory substances in blood activate eNOS to generate NO gas. Gaseous diffusion into adjacent vascular smooth muscle cells allows NO to bind to heme in sGC to generate cGMP, which activates cGMP-activated PKG to promote muscle relaxation and vasodilation. NO gas diffusion into the bloodstream leads to NO binding to heme in Hb, where it is unable to release in the free form; this removal of free NO is thus vasoconstrictive. (B) Signaling by SNO. NO gas can be redox-activated to form nitrosonium that can react with free cysteine residues to create protein SNO. Hb can utilize the Fe in heme to generate SNO at βCys93, that is, Hb acts as an SNO synthase using NO, SNO, or nitrite. Released SNO is vasodilatory at endothelial cells and arteriole smooth muscle cells. cGMP, cyclic GMP; eNOS, endothelial nitric oxide synthase; PKG, protein kinase G; sGC, soluble guanylyl cyclase.
One major limitation on signaling by NO itself is that while NO binds tightly to the iron center in heme in sGC leading to activation of this enzyme and signaling (69), NO binding to any other heme group leads to NO inactivation, so that the vast amount of heme within Hb in blood acts as a tremendous sink that inactivates NO gas that diffuses into the bloodstream from the endothelium (24, 78). Thus, NO gas, itself, cannot readily act as a vasodilator, and RBCs will act to constrict blood vessels (which at face value makes little sense if RBCs are to supply tissues with oxygen). However, if NO is oxidized to nitrosonium ion (NO+) equivalents, it can now react with free sulfhydryl groups (R-SH)—such as those in free cysteine residues in proteins or in metabolic thiols such as cysteine, glutathione, or coenzyme A (CoA)—to form an SNO (Fig. 3B) (37). This conversion of NO to SNO is essential for preserving and spreading NO bioactivity, because SNOs are no longer inhibited by heme yet remain endowed with NO bioactivity (116). More broadly, an SNO group attached to a specific cysteine residue in a target protein can alter the function of that target protein, by acting as a regulatory post-translational modification, akin to phosphorylation or ubiquitination (37, 120). SNO-bound proteins can be inhibited (particularly for enzymes with active-site cysteine residues), activated, stabilized, degraded, bound to or prevented from binding to specific partners, or translocated from one cellular site to another, compared with the same protein lacking SNO modification (37). This broad regulatory ability of SNO groups has expanded the reach of NO biology well beyond traditional sGC–cGMP–PKG-mediated events (25). In particular, however, SNO can act as a vasodilator both by being attached to target proteins involved in vasodilation responses (e.g., K+ or Ca2+ channels) (9) as well as by being released from protein to bind to the heme in sGC to activate traditional cGMP/PKG pathways (19, 41).
While early concepts of SNO formation and action posited purely random chemical actions in forming SNO from NO and in the location of SNO groups on cysteine residues in target proteins, current evidence indicates that a tightly regulated enzymatic system in fact regulates SNO function, allowing SNO to act as an integrated redox signaling system. In particular, four distinct classes of SNO-regulatory enzymes have been identified: NO synthases (NOSs), SNO synthases, SNO transferases, and SNO reductases (120). NOS enzymes (endothelial, neuronal, and inducible) generate NO in cells in response to diverse cellular signals (68). SNO synthases utilize transition metals such as iron to redox-activate bound NO to NO+, and heme Fe3+ is particularly well suited to this reaction since Fe3+-NO (in equilibrium with Fe2+-NO+) can directly react with appropriately oriented sulfhydryl (SH) groups to generate thiol-bound SNO (113, 120). When this thiol is in a cysteine residue of a protein, protein SNO is formed; other cellular thiols (glutathione, CoA, etc.) are similarly S-nitrosylated. SNO transferases transfer their thiol-bound SNO to specific free (sulfhydryl) cysteine residues on target proteins, based on direct protein–protein interaction or potentially using SNO-thiol cofactors (120). Because SNO groups can be transferred from protein to protein in a specific manner via transnitrosylases, this allows dissemination of SNO signals and provides specificity to SNO signaling and also allows SNO levels in general to be in an overall equilibrium (including with metabolic thiol-SNOs such as SNO-CoA and SNO-glutathione [GSNO]). SNO reductases eliminate SNO through its reduction to inactive forms (113, 120). Currently, known SNO reductases act via metabolic nitrosothiols (SNO-CoA or GSNO) thus indirectly reducing protein SNO levels (120) or directly on SNO proteins themselves (7).
S-nitrosylated hemoglobin
NO that makes its way into the blood is produced not only by endothelial NOS in blood vessel walls and within RBCs (52) but also by neuronal NOS and inducible NOS (46), as well as from the reduction of nitrite ions by metal-containing enzymes [including by Hb itself (1, 66)], and perhaps even from microbes in the gut (111). Nearly all NO that enters the bloodstream (rather than into surrounding muscle) ends up bound to heme in Hb, and this NO was originally thought to be inactive (24, 78). Inactivation of NO would result in vasoconstriction by RBCs. Fortunately, this model is incorrect. NO bioactivity depends on whether the NO resides on α- or β-globin hemes, and while αFeNO is trapped, βFe-NO provides the NO substrate for S-nitrosylation of a fraction of Hb (60). Although the extent of S-nitrosylation appears relatively low (about 1 in 1000 Hb molecules carries SNO) (20, 63, 113), the vast quantity of Hb in the blood makes SNO-Hb a major store of NO bioactivity in the body. Experiments in isolated Hb and with RBCs have demonstrated that Hb can act essentially enzymatically (as an SNO synthase) to convert NO bound to β-heme iron (βFeNO) to SNO-Hb at βCys93 (1, 60). In order for this to occur, FeNO must formally lose an electron (to generate NO+), a reaction favored in the oxygenated R state of hemoglobin due to lower R-state redox potential (113). This implies that the transition of FeNO in the deoxygenated T state to the oxygenated R state couples electron loss to βCys93 binding by the resulting NO+ (113). Thus, oxygen binding to the hemes, which induces the T to R transition, is necessary for FeNO to be converted to SNO (113). Conversely, deoxygenation of hemes, which induces the R to T transition in Hb, is necessary for SNO to be released, generating both vasodilatory NO bioactivity (66) and a reservoir of recyclable βFeNO substrate (66, 87).
With all that said, recent data show that multiple Cys thiols in Hb are in fact S-nitrosylated under native conditions (134), but the primary site of S-nitrosylation is β-globin cysteine 93 (βCys93) (14, 46). NO binding to βCys93 is allosterically coupled uniquely to oxygen binding in Hb (Fig. 1). Thus, Hb S-nitrosylation at βCys93 is favored in the R state (oxygenated Hb) and disfavored in the T state (deoxygenated Hb) (115). Mutant mice deficient in βCys93 (C93A mice) compensate by increasing SNO at alternative sites in Hb (134) (including within the remaining ∼3% of Hbs that still have this essential Cys [see the Hypoxic Vasodilation (Three-gas respiratory cycle of hemoglobin) section]), and within low-molecular-weight thiols (43), but lose allosteric regulation of SNO formation/release (134).
Although less well appreciated, Hbs can attain an R-like state through oxidation of hemes (Fe2+ to Fe3+) (85). In fact, metHb (Fe3+) can assume either R- or T-like properties (a function of “spin state”) and is also an excellent electron acceptor (NO+ generator) (113). For these reasons, Hbs that act as SNO synthases contain Fe3+ (112, 113). For example, β-heme Fe3+NO in the T state (i.e., Hb[βFe+3NO][Fe2+]3) will convert to SNO-Hb[FeO2]4 in the R state (i.e., upon binding oxygen) and vice versa (113). Alternatively stated, Hb tetramers that carry Fe3+NO and FeO2 (e.g.., Hb[βFe+3NO][Fe2+O2]3) are primed to make SNO-Hb. Because the concentration of FeNO and Fe3+ are low relative to Fe2+ or FeO2, these Hbs are referred to as “micropopulations”; Hb micropopulations that carry and process NO behave differently from the other Hbs (113).
SNO-Hb in the R state then can carry NO bioactivity around the body since R-state Hb carries this SNO in a relatively unreactive, protected pocket (115). Conversely, when Hb reaches hypoxic tissues and transitions to the T state, SNO at βCys93 is rotated into an orientation more favorable for reactivity, where it becomes available for transfer to other thiols through a transnitrosylase reaction (115) (Fig. 1). Experiments mixing SNO-Hb with glutathione have demonstrated the rapid deoxygenation-dependent production of authentic GSNO as identified by mass spectroscopy, but this transfer does not occur in the presence of high oxygen (57). SNO release is thus proportional to the fraction of R-state SNO-Hb transitioning to the T state (20, 57).
Autoregulation of blood flow
The hallmark of hypoxic autoregulation of blood flow is a linear relationship between Hb-deoxygenated state and increased blood flow (Fig. 2) (100). At the time of this observation by Guyton, there was no oxygen sensor known that had the required properties to account for this finding. Because SNO/NO bioactivity can be liberated from SNO-Hb βCys93 in direct linear proportion to Hb deoxygenation, and because SNO has vasodilatory actions, SNO-Hb βCys93 was proposed as the mediator of hypoxic autoregulation of blood flow in tissue microcirculation (46). Evidence for this hypothesis comes from multiple studies using isolated RBCs and Hb, and as detailed further below, from more recent physiological studies in Hb βCys93Ala mice that cannot generate, carry, or release SNO on Hb normally.
Evidence for SNO-Hb in RBCs as the mediator of autoregulation includes:
-
(i)
Microvessel blood flow in vivo is increased by isolated SNO-Hb (115) and by SNO-replete RBCs (95), but only under hypoxia.
-
(i
i) Microvessel blood flow in tissue increases proportionally with decreasing Hb oxygen saturation, with isolated SNO-Hb (115) and with SNO-replete RBCs (94).
-
(i
ii) Aortic vasodilation in vitro by isolated SNO-Hb (66) and by freshly isolated (46) or SNO-replete RBCs (94) under hypoxic conditions is lost when SNO is removed from Hb or from RBCs (94), does not require intact vessel endothelium (19), is not reduced by inhibitors of eNOS (endothelial NO-independent) (19), but is inhibited by sGC inhibitor (cGMP/PKG-dependent) (19).
-
(i
v) SNO-Hb and SNO-RBCs do not cause vasodilation under normoxic (oxygen-bound; R state) conditions (19).
-
(v)
Acute injection of a bolus of SNO-Hb into rats produces a transient decrease in mean blood pressure (46), consistent with direct SNO-mediated vasodilation that is impervious to Hb inactivation.
-
(v
i) SNO-Hb gradients between arterial and venous blood in animals (46, 95) and in humans (63) are consistent with SNO formation upon oxygenation and SNO discharge from Hb into hypoxic tissues upon deoxygenation.
-
(v
ii) Endogenous SNO is liberated from human RBCs in direct proportion to degree of deoxygenation (20).
-
(v
iii) Autoregulation is lost in mice with mutant βCys93 hemoglobin: blood flow, hypoxic vasodilation, and tissue oxygenation are markedly impaired, and flow and oxygenation deficits are exacerbated by reducing oxygen content of inspired air (90).
However, controversies have arisen contesting this interpretation as some groups have reported failure to observe particular aspects of this function (particularly impaired vasodilation by SNO-mutant C93A RBCs in vitro under certain conditions, Table 1), misinterpret results, or misapply the fundamentals of this physiology (Table 2). Confusingly, the objections have changed over time, as more and more of the above observations are replicated and accepted. For example, whether SNO-Hb exists in vivo [(30, 92) vs. (71, 113, 129)], whether NO bioactivity can escape heme and RBCs [(3, 48) vs. (29, 36, 55, 71, 107)], whether RBCs can produce vasodilation [(38, 130) vs. (107, 122)], or whether RBCs can release NO bioactivity [(3, 28) vs. (55, 107, 122)], or whether hemoglobin oxygenation-dependent allostery has any role in RBC/Hb-mediated NO bioactivity [(132) vs. (17, 40)]—these issues are largely resolved; that is, no one would reasonably dispute that NO can form SNO in Hb, that SNO can escape heme, that SNO-Hb forms in vivo, that SNO-Hb dilates blood vessels, and that vasodilation by SNO-Hb is controlled allosterically by PO2. Where controversy persists, major methodological differences or unsuitable physiology are usually the reason. For example, the most widely referenced physiological data against SNO-Hb were based on lack of blood pressure changes in Cys93-mutant animals (43), but Cys93 is implicated in moment-to-moment blood flow, not systemic blood pressure set point. Blood flow is in fact dramatically altered (134), as described in detail below.
Table 1.
Vasodilation of Blood Vessels In Vitro by Red Blood Cells
| System and components | Measured response | Results | Interpretation | Reference |
|---|---|---|---|---|
| Rat RBCs loaded with excess SNO-Cys (10:1 Hb) under oxygenated conditions; rabbit aorta (endothelium denuded) Intact rat infusion of RBCs vs. SNO-loaded rat RBCs |
Hb- and RBC-mediated vasodilation (under normoxia) Blood pressure |
SNO-Hb- and SNO-loaded RBCs vasodilate aorta under normoxic conditions where nonloaded Hb or RBCs are vasoconstrictive. Infused Hb or RBCs cause increased blood pressure, but SNO-loaded Hb or RBCs cause a transient pressure drop. |
First demonstration that SNO-Hb and SNO-RBCs are vasodilatory in vitro, a heretical concept at the time since Hb heme was well known as an effective vasoconstrictor due to NO scavenging. SNO-Hb and SNO-RBCs are vasoactive in vivo as well. No hypoxic component in these assays as Hb/RBCs were SNO overloaded. | (46) |
| Human RBCs and human Hb, loaded with excess SNO-Cys (10:1 Hb) under oxygenated conditions; rabbit aorta (endothelium denuded) | Hb-mediated vasodilation | In room air, R-state Hb and SNO-Hb are equally potent at vasoconstriction, while at <1% O2, T-state Hb remains vasoconstrictive while T-state SNO-Hb loses this activity. Addition of glutathione augments vasorelaxation by SNO-Hb (but not Hb) under hypoxia, whereas SNO-glutathione alone relaxes aorta independent of O2 level. | R/T-state allostery controls the reactivity of Hb βCys93 to facilitate SNO release at low oxygen. Vasoactivity of released SNO is augmented by the addition of carrier thiol (glutathione). Less SNO loading in human vs. rat RBCs (39) preserves hypoxic vasodilator response. Recognition that excessive loading overrides allostery in SNO-Hb. |
(115) |
| Human RBCs loaded with physiological amounts of NO gas (1 μM; NO/Hb <1:100). Nitrosylated via deoxygenation/oxygenation | RBC-mediated vasodilation | RBCs are vasoconstrictive under room air but vasodilatory under 1% O2. Blocked by inhibitor of RBC membrane AE1/Band III anion channel. Hypoxic vasodilation was potentiated by loading RBCs with 1 μM NO to form SNO-Hb. | First demonstration that native RBCs dilate blood vessels; RBC-and SNO-Hb-mediated hypoxic vasodilation was measured and potentiated by 1 μM NO. SNO release requires RBC membranes and AE1/Band III function. Physiological NO enhances R/T-regulated vasodilation by RBCs. |
(80) |
| Human RBCs; rabbit aorta (and pulmonary artery) | RBC-mediated vasodilation | RBCs with native amounts of SNO are vasoconstrictive under room air but vasodilatory under 1% O2, with a graded response to intermediate O2 levels (21%, ∼10%, ∼5%, ∼1%). | SNO-Hb-mediated hypoxic vasodilation was measured. Consistent with allosteric regulation by R/T transition in hemoglobin. | (63) |
| Human RBCs loaded with NO gas under deoxygenation then oxygenation (1 μM NO; NO/Hb: 1:100); isolated perfused mouse lung | RBC-mediated vasodilation (includes intact microvasculature) | RBCs lacking SNO vasoconstrict to increase pulmonary artery pressure in the isolated lungs under normoxia and under hypoxia, and this hypoxia response is reduced with SNO-loaded RBCs (e.g., flow is maintained with SNO-RBCs). | SNO-Hb-mediated hypoxic vasodilation was measured in an isolated organ. | (20) |
| Human RBCs from normal and PAH patients; rabbit aorta Human RBCs from PAH patients breathing air vs. ENO (70 ppm 10 min); rabbit aorta Human RBCs, SNO-depleted vs. SNO-repleted (using NO/Hb <1:100; NO 1 μM); isolated perfused rabbit lungs Porcine RBCs; perfused pig lung (in situ); breathing air vs. ENO (100 ppm 15 min), or infused SNO-depleted vs. SNO-repleted |
RBC-mediated vasodilation RBC-mediated vasodilation RBC effect on pulmonary artery pressure (intact microvasculature) RBC effect on PVR A-a O2 difference |
Vasodilation response by fresh native RBCs from normal controls is significantly diminished compared with native RBCs from patients with PAH. Reduced vasodilation response by PAH patient RBCs is restored to normal levels after breathing ENO. RBC-mediated increase in pulmonary pressure under hypoxia is reduced using SNO-repleted RBCs. Breathing ENO leads to reduced PVR and increased A-a O2 difference (improved blood oxygenation); SNO-repleted RBCs also increase A-a O2 difference compared with depleted RBCs. |
Chronic hypoxemia in PAH patients results in reduced RBC C93-SNO content and in reduced aorta vasodilatory activity. Breathing ENO increases SNO-Hb in situ in physiological amounts and increases RBC hypoxic vasodilation activity to normal levels. Increasing RBC C93-SNO content increases lung blood flow to improve ventilation–perfusion matching. Breathing ENO or repleting isolated RBCs with SNO then reinfusion improves ventilation–perfusion matching via lung blood flow to increase arterial blood O2 content. |
(62) |
| Human RBCs (normal vs. sickle cell disease patients, fresh or SNO-repleted using NO/Hb <1:100; NO 1 μM); Rabbit aorta | RBC-mediated vasodilation | RBCs from SCD patients with severe disease lose hypoxic vasodilation ability compared with control RBCs or mild-SCD RBCs. Severe-SCD RBCs contained less SNO than control and mild-SCD RBCs. SNO repletion of severe-SCD RBCs restored hypoxic vasodilation. | Severe SCD impairs the ability of RBCs to process NO into SNO at Cys93, contributing to vaso-occlusive behavior through loss of RBC-mediated hypoxic vasodilation. | (81) |
| Human RBCs; mouse and rabbit aorta | RBC-mediated vasodilation | Vasodilatory activity of RBCs with native SNO levels under 1% O2 is potentiated by thiol (cysteine) and blocked by inhibition of soluble guanylyl cyclase. Vasodilation is preserved in eNOS knockout and denuded blood vessels. | SNO-Hb-mediated hypoxic vasodilation was measured and is independent of endothelium and NOS. | (19) |
| Mouse RBCs (C93 vs. C93A); rabbit pulmonary artery. Mouse RBCs (C93 vs. C93A); rabbit pulmonary artery (denuded of endothelium). |
RBC-mediated vasodilation | RBC-mediated vasodilation under 0% oxygen is equivalent for C93 and C93A SNO levels in C93 = C93A. RBC-mediated vasodilation under 0% hypoxia, partially inhibited by theophylline (itself a vasodilator). |
SNO-Hb-mediated vasodilation was not being measured. ∼50% of response is endothelial ATP; Adenosine? Non-C93 SNO? No other report of denuded vessel responding to ATP (even indirectly). Response likely includes SNO, but allosteric activity was not tested. |
(43) |
| Sheep RBCs, aged to deplete SNO vs. aged then renitrosylated via ENO (50 ppm), introduced in vivo by transfusion (2 units). Sheep intact in vivo | Organ blood flow by microsphere dilution; arterial/mixed venous O2 saturation | Kidney blood flow and glomerular filtration rate, and systemic oxygen delivery, are elevated more following transfusion with renitrosylated RBCs compared with normally stored (SNO-depleted) RBCs. | Deficits in organ blood flow and oxygenation due to transfusion of stored, SNO-depleted RBCs can be corrected by SNO repletion to physiological levels (<1 SNO:100 Hb) immediately before transfusion. | (95) |
| Human vs. mouse RBCs. Mouse, rat, rabbit thoracic aorta, or rat, rabbit pulmonary artery | RBC-mediated vasodilation (measured at 0% oxygen) | RBC-mediated vasodilation for human (but absent in mouse RBCs on mouse aorta) Extent and pattern/time course varies (particularly, rat pulmonary artery pattern is very different using human vs. mouse RBCs) |
Vasodilation mechanisms vary among species and vessels. | (59) |
| Human vs. mouse RBCs (C93 and C93A); rat pulmonary artery | RBC-mediated vasodilation (measured at 0% oxygen) | RBC-mediated vasodilation extent is equivalent for all RBCs. Mouse C93 vs. C93A RBC responses inhibited by apyrase, partially inhibited by L-NAME, theophylline, CSC, MRS1754, and adenosine deaminase (adenosine A2a, A2b receptors) Human RBC responses partially inhibited by L-NAME, CSC. |
Adenosine-mediated response; SNO-mediated allosteric activity not tested or excluded. |
(59) |
| Mouse RBCs (C93 vs. C93A). mouse aorta (eNOS−/−) | RBC-mediated vasodilation (measured under 1% hypoxia) | RBC-mediated vasodilation greater (by 25%) for C93 vs. C93A. SNO levels are equivalent between C93 and C93A | Mouse C93 vs. C93A RBCs are predicted to have approximately equivalent SNO-based response. Small role for SNO-Hb can be detected at steady state. | (134) |
| Mouse RBCs (C93 vs. C93A); mouse aorta (eNOS−/− mouse); reduced GSH | RBC-mediated vasodilation (measured under 1% hypoxia) | RBC-mediated vasodilation under hypoxia is increased by GSH for C93 but not for C93A. | Mouse RBCs have SNO-Hb-mediated response regulated by R/T- and SNO-Hb-independent responses, which do not exclude other SNO or fetal SNO-Hb. | (134) |
| Mouse RBCs (C93 vs. C93A); mouse aorta (WT mouse) | RBC-mediated vasodilation in vivo (following brief hypoxia) | RBC-mediated vasodilation under hypoxia is increased for C93 but not for C93A. | Mouse RBCs have major SNO-Hb-mediated responses in situ. | (134) |
| Human RBCs (normal vs. sickle cell patients), stored to deplete SNO, then renitrosylated via deoxygenation/oxygenation (<1 μM NO; NO/Hb: 1:250), and fluorescently labeled; mouse dorsal skin fold window for direct microvascular imaging | RBC adhesion and vaso-occlusion | EPI-stimulated sickle cell (ss)RBCs adhere strongly to microvessels leading to vaso-occlusion, while SNO repletion reduces ssRBC adhesion and vaso-occlusion. Mixing normal RBCs with EPI-treated ssRBCs (1:4) prevents vaso-occlusion when normal RBCs are fresh (SNO-replete) but does not when normal RBCs are stored/banked for 30 days (SNO-deficient). SNO repletion of 30-day stored normal RBCs restores ability to inhibit vaso-occlusion by EPI-treated ssRBCs. | ssRBCs are deficient in SNO as are stored/banked normal RBCs. EPI treatment induces ssRBC adherence to microvessels and vaso-occlusion. Increasing ssRBC SNO level improves hypoxic vasodilation and reduces ssRBC adherence to microvessels, as does native SNO on fresh, normal RBCs or added SNO on stored RBCs (even when present in the same microvessels as ssRBCs). | (64) |
| Mouse RBCs (C93 vs. C93A) loaded with 400 μM Cys-NO (∼4:1 NO:Hb = 4:1) in vitro; rat aorta | RBC-mediated vasodilation | RBC-mediated vasodilation is equivalent for C93 and C93A; vasodilation in 21% O2 and insensitive to hypoxia (21% O2vs. ∼0% O2). | Hypoxic vasodilation was not being measured. Experiments replicate Jia (1996): Massive SNO loading at nonphysiological sites, oxidation of hemoglobin overwhelms R/T allostery (46). | (122) |
Human and mouse RBCs exhibit distinct differences in vasodilation responses. SNO-Hb-mediated effects can be readily revealed under basal conditions and with physiological loading. RBC-mediated hypoxic vasodilation is overwhelmed by nonphysiological SNO loading and oxidation of Hb. All SNO in RBCs is potentially vasodilatory; only βCys93 SNO release is linked allosterically to Hb oxygenation (and oxidation) state.
A-a, alveolar-arterial; ENO, ethyl nitrite; eNOS, endothelial nitric oxide synthase; GSH, glutathione; Hb, hemoglobin; NO, nitric oxide; NOS, NO synthase; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RBCs, red blood cells; SCD, sickle cell disease; SNO, S-nitrosothiol; SNO-Hb, S-nitrosylated hemoglobin; ssRBC, sickle RBC; WT, wild type.
Table 2.
Common Misunderstandings of Vascular Physiology with Regard to Hypoxic Vasodilation and SNO-βCys93-Hb
| Physiology | Misunderstanding | Actuality |
|---|---|---|
| Blood pressure (baseline) | C93A mice should have elevated blood pressure (because vasodilation will decrease systemic blood pressure). C93A mice should have elevated pulmonary blood pressure, reflected in histological changes in the lungs. |
Blood pressure should not change in C93A mouse. Systemic blood pressure is effectively independent of hypoxic vasodilation, which affects blood flow primarily. Microvascular flow regulating oxygen delivery is not a primary contributor to systemic blood pressure (109). Classic autoregulation as described by Guyton is independent of blood pressure (100). Histological changes are not a useful measure of blood pressure in the lungs or response to hypoxia (31, 123). |
| Blood pressure (under systemic hypoxia) | Elevated systemic blood pressure in C93A mice will be worsened in systemic hypoxia. | There is no predicted relationship between blood pressure and hypoxic vasodilation governing tissue blood flow (117). |
| Pulmonary hypertension | C93A mice will have elevated pulmonary artery blood pressure and develop lung disease. | There is no relationship between systemic hypoxic vasodilation and pulmonary pressure (117). Lack of histological change does not inform pulmonary pressure (31, 123). Pulmonary pressure was not measured in C93A mice. |
| SNO-mediated vasodilation | C93A mouse RBCs will lack SNO-mediated effects on vessels in vitro at 0% O2. | On the contrary, C93A mouse RBCs compensate to contain normal total amounts of SNO, at non-Cys93 sites on HbA and elsewhere (43, 134). These SNOs will cause vasodilation in bioassays at 0% O2 (100% T state) but will lack R/T allosteric control by O2 (96). Also, C93A mouse RBCs contain fetal Hb that carries C93-SNO (97, 98), which is subject to allosteric regulation. |
| SNO-mediated vasodilation | Artificially SNO-loaded C93 and C93A mouse RBCs are informative for hypoxia-driven SNO-mediated effects on vessels in vitro. | The literature includes two sets of data: physiological loading (NO <1 μM; NO/Hb <1:100) vs. artificial overloading (NO ∼1 mM; NO:Hb >1:10). SNO-overloaded RBCs (and C93A RBCs with SNO at non-C93 sites) will produce nonspecific vasodilation lacking allosteric/O2-dependent control (Table 1). High pharmacological SNO-loading of C93A and C93 RBCs artifactually oxidizes Hb and eliminates all oxygen responsivity even in normal C93 cells (113, 117). |
| Hb Cys93Ala mutation | Elimination of Cys93 will prevent RBC-mediated SNO-based vasodilatory activity. | Mutation of Cys93 does not change amount of SNO in RBCs due to compensations: SNO binds other sites on Hb and the amounts of SNO on other RBC thiols increase (43, 134). Therefore, total SNO-mediated vasodilation should be comparable in C93- vs. C93A-mutant RBCs under low PO2. What should change is sensitivity to PO2 and/or thiol at intermediate range of Hb oxygen-saturation values (R/T transition points). Also, effects of γC93-SNO should be accounted for in βC93A. |
| Ischemia–perfusion injury in an isolated mouse heart model system | Normal RBCs injected in coronary artery at the start of an acute ischemic insult will be protective of heart function during later reperfusion due to SNO-mediated effects, but C93A RBCs will be deficient. | Model is intended as a surrogate of reactive hyperemia (which is markedly abnormal in C93A mice), but is not: reperfusion/injury models are critically dependent on duration of hypoxia/reperfusion and species (90); cardiac function after injury is not a surrogate for blood flow. Model is also complicated by ROS (which eliminates SNO) that is generated by ischemia/reperfusion (11). |
RBC-mediated vasodilation responses are linked to blood flow responses, not blood pressure. Blood flow responses linked to tissue oxygenation are disrupted by excessive/nonphysiological loading of RBCs, which disrupts physiological allosteric (Hb O2-saturation-dependent) control of flow responses. Physiological SNO loading of RBCs entails SNO/Hb <10/1000 and met Hb <5%.
Measurements and methodologies
SNO-Hb measurement and activity have also been a source of controversy, but again, the objections have changed as key observations are replicated. For example, it has been shown by multiple groups that effective formation of SNO-Hb requires low ratios of NO/Hb (<1:100) and low NO (<1 μM) to recapitulate physiological conditions (32, 36, 64, 80, 113), whereas controversy has resulted from using high pharmacological amounts (millimolar) of NO (113, 122, 132). Similarly, whether SNO-Hb is detectable in vivo [(30, 92) vs. (129)] is simply not in dispute; early claims that it was too unstable to exist in vivo (30) and could not be detected in human RBCs (91) or represent a source of NO bioactivity released from RBCs [(30, 48) vs. (29, 55, 122)] are long forgotten. Even vasodilation by isolated SNO-Hb (46) was viewed originally with great skepticism, as the identification of NO as a vasodilator came about in significant part due to the ability of Hb to scavenge NO gas to block vasodilation (42). Our proposed resolution to this paradox, namely, that NO could convert through redox activation to SNO (1, 60), was challenged on thermodynamic and kinetic grounds (48), whereas there is now agreement that SNOs can readily form and elicit vasodilation even in the presence of Hb (122). Furthermore, while former critics agree that SNO in Hb (SNO-Hb) can serve as a donor of SNO/NO bioactivity to dilate blood vessels, it has been fairly questioned whether SNO-βCys93 does so physiologically (vs. other mediators/mechanisms such as nitrite or other SNO). Vasodilation by SNO-Hb entails allosteric regulation by R/T transition (88, 113), which is linearly coupled to oxygen saturation, and thus faithfully replicates classic autoregulation (88). It specifically requires selective S-nitrosylation of βCys93 versus other Cys in Hb, or SNO at alternate, unregulated Cys sites, which may be potentiated by hypoxia (38, 66) but are not regulated by R/T transition (66, 80, 113). Likewise, nitrite vasodilation is not coupled linearly to R/T transition nor replicates any known blood flow physiology (i.e., autoregulation, shear-induced, mental activity-based, fight-or-flight).
An additional complication for Hb measurements is that cycling between deoxygenated and oxygenated conditions can promote the movement of NO between heme-FeNO and βCys93-bound SNO. Therefore, care must be taken to maintain the native oxygenation state of blood samples (high oxygen for arterial samples vs. low oxygen for venous samples) during processing before assay for NO content. Only methods that do so (46, 63, 95, 113) will detect arterial–venous SNO-Hb gradients, consistent with SNO release as RBCs transit the microcirculation. Also, NO-bound Hbs exist as multivalency subpopulations (e.g., membrane-associated Hb[Fe(III)/FeNO/FeO2]) whose behavior is distinct from fully oxygenated or deoxygenated cytosolic forms, and assays are often assumed to work equivalently and quantitatively on all Hb populations, but there is strong evidence this is not true (34, 113). Ultimately, no criticism has been found with the method of Hg-coupled photolysis for SNO quantification (34, 63, 65), which has proven useful in detecting SNO-Hb deficiency states in sickle cell disease (SCD), pulmonary hypertension, and transfusion, and simultaneously detects FeNO. Similarly, the 3C method for SNO detection is effective at quantifying total SNO in RBCs (20) as a function of oxygenation and allows for complete human blood gas measurements of O2/NO/CO2. This methodology has shown direct correlation between O2 saturation and SNO levels in human RBCs strongly supporting the notion of a three-gas respiratory cycle.
Finally, SNO is rather labile when studied in RBCs, in part due to lack of knowledge of enzymes involved in SNO synthesis, movement, and removal. That is, in contrast to phosphorylation studies where phosphatases are well understood and effective phosphatase inhibitors are a standard part of phosphorylation analysis, current understanding has not yet led to the standard use of SNO reductase or transnitrosylase inhibitors as SNO stabilizers during sample collection, processing, and assay. While protecting samples from light is a standard procedure, some chemical SNO stabilizers have been proposed but are not uniformly accepted as effective (4, 30, 34, 61, 99, 129); novel SNO reductases and transnitrosylases are still being identified [e.g., Stomberski et al. (119)] and not all the known enzymes have effective inhibitors yet.
What allosteric regulation means in terms of vasodilation by βCys93-SNO (why C93A RBC mice are expected to elicit vasodilation)
βCys93-SNO-Hb in the oxygenated or R state cannot dilate blood vessels (46, 115). The molecule is inactive because the SNO is buried and inaccessible to solvent. Vasodilation requires transition to the T state whereby the SNO can be released (115). The fraction of molecules in the T state is inversely proportional to Hb saturation (i.e., 100% T state at 0% sat). SNO-Hb in the T state will transfer its SNO to other Cys such as glutathione that directly mediates vasodilation (113). What this means, effectively, is that SNO-Hb will be equivalent, at best, in potency to GSNO (at 0% oxygen saturation [100% T state]), but always less potent than GSNO at higher oxygen saturation.
Compensation in C93A-mutant mice leads to SNO binding to alternative Cys in Hb and to glutathione, forming GSNO (43, 89, 96, 134). Amounts of SNO in RBCs, including SNO-Hb and GSNO, are unchanged or elevated in C93A mice. Therefore, RBC-mediated vasodilation should not be less in C93A cells and may well exceed wild-type (WT) cells under less than 0% oxygen saturation. What will change, however, is the responsivity of cells to oxygen; that is, the relationship between vasodilation and oxygen saturation, and thus between blood flow and tissue oxygenation, will be disrupted. This is precisely what is observed (88). C93A-mutant RBCs show impairment of allosteric regulation of vasodilation under hypoxia (e.g., responsivity to thiol) (66, 134) yet elicit effective vasodilation at extreme hypoxia (43) and in room air (after NO loading) (122), exemplifying loss of hypoxic control. Mutant mice show dramatic consequence, as measured by deficits in blood flow and tissue oxygenation (134).
Strengths and Limitations of the βC93A Knock-In Mouse Model
Hemoglobin genetics: mouse versus human
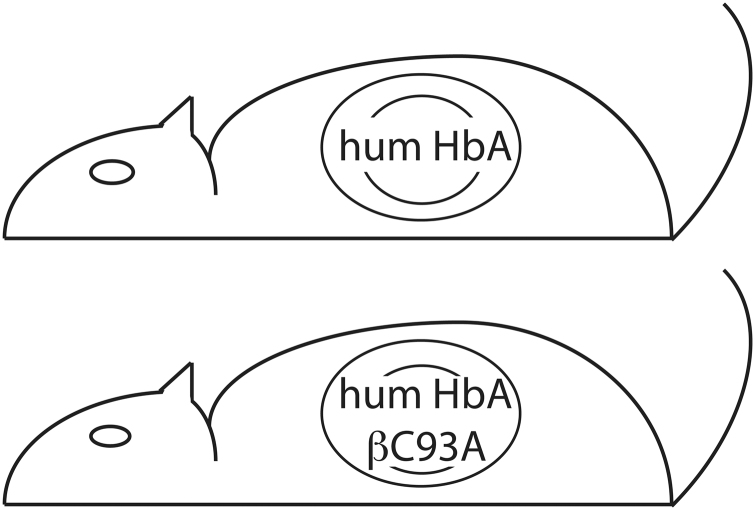
Because mouse Hb does not accurately replicate human physiology, a humanized mouse model expressing human instead of mouse Hb was created in the Townes laboratory to better explore human-like physiology of Hb (Fig. 4) (131). Human α-globin and human β-globin genes were replaced into the corresponding loci in the mouse, and in their initial report using this system, β-globin was mutated to Val6Ser to model human SCD (131). Using this humanized WT α/β-globin mouse, human β-globin was mutated Cys93Ala (C93A) so that adult Hb would be unable to carry SNO at Cys93 of β-globin (43). This C93A mouse therefore should model the effect of human Hb unable to synthesize, bind to, or transfer SNO, and has been utilized in several studies by multiple research groups. However, this model system has several caveats.
FIG. 4.
Schematic of humanized mouse model. The C93 mouse carries normal human adult Hb (HbA) in its mouse RBCs, because it has the human α-globin and β-globin genes in place of their mouse counterparts. The C93A mouse additionally has a point mutation of the human β-globin gene to replace Cys93 with Ala that is unable to carry SNO. Fetal Hb C93 is always present.
The human and mouse β-globin loci are complex, with multiple developmentally expressed genes that are not precisely equivalent (10). In mouse, the β-globin locus on chromosome 7 contains Ey-globin (embryonic ɛ-globin homolog), βH1-globin (the fetal γ-globin homolog), and two distinct β-globin genes, named βmaj-globin (major isoform) and βmin-globin (minor isoform) (10, 16, 22). In contrast, the human β-globin locus on chromosome 11 contains embryonic ɛ-globin, two fetal γ-globins named γG-globin and γA-globin that differ by one amino acid (i.e., either Gly136 or Ala136), and two adult genes named δ-globin (a minor β-type isoform) and β-globin itself that accounts for ∼97% of adult β-type globin expression (10, 16). In the humanized mouse model, the human γA-globin and β-globin coding regions replace the βmaj-globin and βmin-globin coding regions (43, 131). One consequence of replacing these human coding regions into the mouse genome is a slight imbalance of α-globin over β-globin expression, leading to minor β-thalassemia (43, 131). Furthermore, the mouse βH1-globin will also still be present at minor levels in adults, along with human γ-globin. Since both these mouse and human γ-globins also contain Cys residues cognate with βCys93, this small fraction of fetal Hbs [∼1% of β-type globin in C93 and C93A mice (26)] remains able to carry SNO normally. Also, this remaining fraction of Hb is plentiful from an NO-carrying standpoint (100–1000 × excess). Thus, C93A mice still contain significant fetal Hb with reactive Cys93 (both mouse and human), representing ∼100 μM Cys thiol (vs. ∼100 nM–1 μM NO). Furthermore, measurement of Hb-SNO levels indicates that C93A Hb carries SNO at additional sites, basally, at apparently higher levels than normal (134), and also particularly after SNO overloading using NO donors in vitro (43, 122). The point being that C93A mutation does not eliminate SNO in RBCs or in Hb. On the contrary, total SNO in RBCs is unchanged or increased (43, 134) in C93A mice and residual SNO-C93 is undoubtedly present in ɛ, γ Hbs to account for the fact that survival is reduced but still possible, whereas no known animal or bird is homozygous for C93 mutation.
Hb βC93 is the most highly reactive cysteine residue on Hb and can be modified by a variety of redox events, including formation of sulfenic acid, S-glutathione conjugate, or S-cysteinyl disulfide (93, 103). Only a small fraction of Hb is modified in this way normally, with no consequence for overall Hb function. In the C93A mouse, reactive Cys content of Hb is greatly reduced (43, 125), and none of these modifications of β-globin residue 93 are possible. However, absent any established role or connection to the essential allosteric function of hemoglobin, which defines βCys93 (reactivity is uniquely linked to oxygen binding), these modifications affect no known physiology. Note also that the mouse β-globin gene varies among strains of mice, with some strains carrying the βS variant where βCys93 is the only reactive cysteine in β-globin (as in human) but other strains carrying the βD or βP variants where a second highly reactive cysteine, βCys13, is also present (35, 121). How mouse RBCs are adapted, with regard to SNO, to utilizing this distinct reactive cysteine remains unknown.
While C93A Hb is unable to generate or carry SNO at βCys93, it remains able to bind NO via heme and alternative Cys. In fact, levels of Hb FeNO should be elevated in this mouse since FeNO generated from NO diffusion into the blood would be stabilized by the inability of C93A-mutant Hb to process it into SNO. Indeed, levels of total Hb NO species have been reported to be elevated in the C93A mouse (43), consistent with FeNO elevation.
Mouse versus human RBCs
Mouse RBCs exhibit properties that are notably distinct from human RBCs. Mouse RBCs are much smaller in diameter than human RBCs and have less than half the volume (33, 72). While human RBCs produce hypoxic relaxation in aortic ring bioassays that is eNOS-independent and endothelium-independent, mouse RBCs exhibit a high degree of ATP-mediated, eNOS- and endothelium-dependent vasorelaxation (19, 43). Such functional differences between human and mouse RBCs complicate interpretation of experiments using these humanized mice (they contain mouse RBCs with human Hb, unlike any other system ever tested), or indeed, comparing studies using RBCs from different species. As an example, one group has reported that hypoxic vasodilation of several types of large vessels in vitro by human RBCs versus mouse RBCs differs fundamentally, with mouse RBCs producing essentially no vasodilation of mouse thoracic aorta in side-by-side experiments under their conditions (59). In our hands, about 25% of humanized mouse RBC-mediated vasodilation can be attributed directly to endogenous SNO-βCys93-Hb in endothelium-intact aortic ring bioassays (presumably the remainder is ATP dependent), whereas about 50% is SNO-βCys93 mediated in the absence of endothelium (134); others have reported no difference using mouse RBCs artificially loaded with approximately millimolar SNO-cysteine donor (122). However, under these artifactual conditions, Hb is oxidized (39), SNO approaches 50–100 μM (∼100-fold above physiological) (105), and RBCs exhibit complete loss of hypoxic responsivity; that is, these conditions override the allosteric role of βCys93. Human Hb expressed in mouse has also been noted to be relatively deficient in membrane association within mouse RBCs compared with human Hb in human RBCs (15), and since physiological SNO export from RBCs requires transfer of SNO from Hb to membrane proteins [e.g., band 3/AE1 or protein disulfide isomerase (PDI) (50, 80)] directly or via low-molecular-weight thiols such as glutathione (58) or H2S (45), mouse RBCs expressing WT human HbA (i.e., control humanized mice) may already exhibit reduced SNO export compared with normal human RBCs. Since it has been proposed that RBC membrane-associated Hb is most likely to function as SNO synthase, this effect may reduce the apparent function of C93 in this mouse model. Overall, mechanisms within mouse and human RBCs differ in ways that complicate direct comparison of experiments utilizing native human RBCs versus humanized mouse βC93 RBCs.
HbβC93 and allosteric control by oxygen (understanding of the expected role of HbβC93 in vasodilation)
Human Hb C93A has been characterized as recombinant protein, and exhibits properties that are largely normal (32). In particular, Hb C93A binds and releases oxygen with affinities comparable to WT Hb, suggesting normal R/T state transitions (32, 43). However, the behavior of the Cys93 mutant vis-a-vis NO binding and release is altered (32, 88). Specifically, binding of oxygen to WT HbNO under physiological conditions [NO <1 μM; NO/Hb <1:100 (80, 113)] induces the transfer of NO from β heme-Fe to βCys93, whereas NO is not released from heme of Cys93-mutant proteins (32). Thus, thermodynamic linkage between R/T transition and SNO formation/release is lost in Hb-βC93A.
The Hb C93A mouse model was originally premised on the idea that loss of βCys93 activity should be informative for the roles of SNO synthesis, carriage, and release from this site in Hb in vivo under physiological conditions; that is, SNO release would be expected to be proportionate to drop in oxygen saturation. And indeed, SNO content per heme is reduced in the Hb-containing >10 kDa filtration fraction of RBCs isolated from C93A mice (43) or in these RBCs after treatment with an SNO donor (122). Note, however, that overall SNO levels do not differ between normal and C93A RBCs (43, 122). Furthermore, it needs to be recognized that normal physiological processes also produce SNO levels within RBCs (on both proteins and low-molecular-weight thiols) that can appear normal even in the absence of βC93 and that these additional SNOs are equally endowed with potential vasodilatory activity (66, 130), although these are not hypoxia-dependent. This alternate SNO can also be loaded artificially, by treatment of RBCs or Hb with NO donors to induce SNO-Hb formation at other Hb cysteine residues, and on cysteines on other RBC proteins or on low-molecular-weight thiols. What is specifically missing in the C93A Hb is allosterically based oxygen saturation-dependent release of SNO (66, 134). The C93A mice will lack this one very specific essential mechanism for SNO release from RBCs and its attendant vasodilation.
What does this mean physiologically? It implies that changes in the amount of vasodilation as a function of pO2 (i.e., responsivity of RBCs to oxygen) will be different between WT and C93A, but the maximal amount of vasodilation by RBCs may be unchanged or even greater. In fact, C93A-mutant RBCs containing GSNO instead of SNO-Hb will vasodilate independent of pO2 (46, 122) (Table 1) and thus to a greater extent at high pO2 (vs. WT native cell), and no differently at very low pO2. The predicted ramifications are borne out by profound disruption in relationship between hypoxic blood flow and hypoxia in C93A mice (134). But like all global genetically modified animals, these mice have developed and survived lacking this activity in part by adapting and compensating to make up for the missing activity, and what we observe physiologically is the activity loss that the animal has been unable to compensate for by other means. The adult mice available for study are those that have survived, but it is clear that about half of C93A pups do not survive development and birth (134), so those mice that did not compensate adequately enough to survive are of course not studied at all.
Finally, while hemoglobin is expressed at extraordinarily high levels in RBCs, low-level expression of Hb can be detected in numerous tissues throughout the body (101). Physiological effects of Hb-C93A are attributed to Hb circulating in RBCs, but it remains possible that nonerythrocyte Hb may also have important physiological functions that include βCys93.
Expected Phenotypes Confirmed in C93A Knock-In Mice
SNO-Hb levels
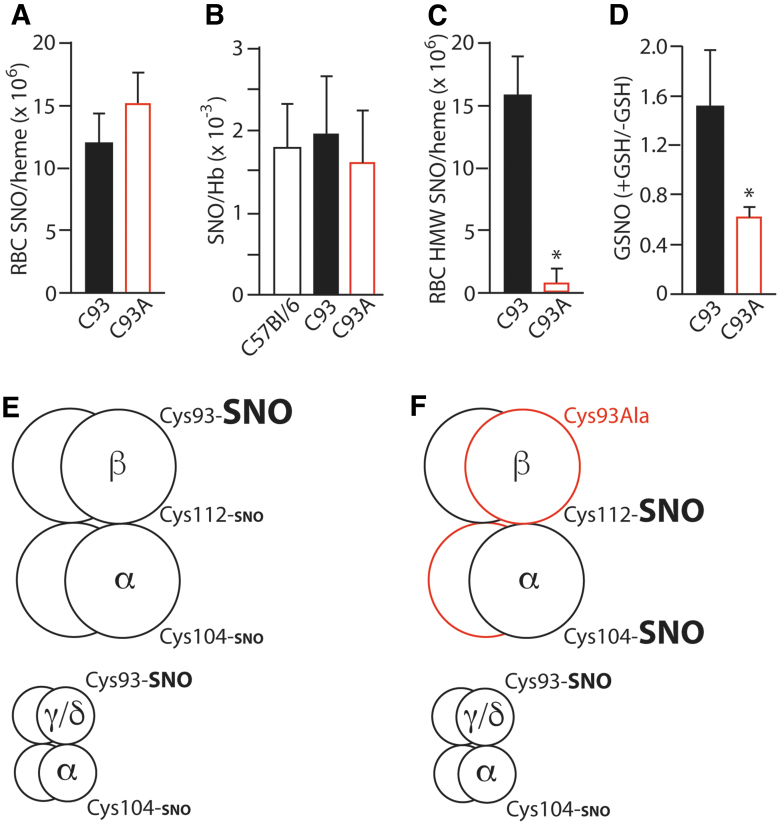
Loss of βCys93 as a site for S-nitrosylation of Hb should decrease total SNO-Hb level to the extent that no other HbCys is modified and concordantly increase heme FeNO levels due to inability of Hb to convert FeNO to SNO on βCys93 (or elevated non-Hb SNOs). Although RBCs from C93A mice contain total SNO levels that are unchanged or increased compared with control RBCs (43, 134), the Hb-containing RBC lysate gel filtration fraction >10 kDa shows a greatly reduced protein-SNO level (43), consistent with reduced SNO-Hb and a compensatory shift to low-molecular-weight SNOs (Fig. 5A–D). Furthermore, deoxygenation-dependent transfer of SNO from isolated C93A Hb to glutathione is diminished (134), indicating that βCys93 is responsible for most of the SNO liberated from Hb upon deoxygenation-induced R/T transition. However, we have observed increased SNO at alternative Cys sites in Hb (Cys104 in α-globin and Cys112 in β-globin) (134) consistent with compensation (Fig. 5E). Altogether, the data point to βCys93-SNO as a major site in normal conditions (as verified by multiple groups (23, 32, 43), but total SNO in C93A RBCs is unchanged reflecting both increased low-molecular-weight SNO carriers and Hb bearing SNO at alternative Cys within both the α- and β-chains.
FIG. 5.
SNO in humanized C93A RBCs. (A) RBC total SNO content is unaltered by C93A mutation. (B) Isolated Hb total SNO content is unaltered by C93A mutation. (C) SNO content of C93A RBC lysate, separated into a >10 kDa fraction (containing Hb but not low-molecular-weight thiols), is significantly reduced (43) but includes new SNO in α- and β-globin chains (134). (D) GSNO content of C93A RBCs is significantly increased. (E, F) Schematic diagrams of SNO carried by HbA (α2β2) and smaller Hb populations of HbF (α2γ2) and HbD (α2δ2) in C93 mice (E), versus compensatory changes in SNO-Hbs in C93A mice (F), based on data in (134). *p < 0.05 for C93 versus C93A. (A, C) Replotted from Isbell et al. (43) with permission, (B, D) replotted from Zhang et al. (134) with permission. GSNO, SNO-glutathione.
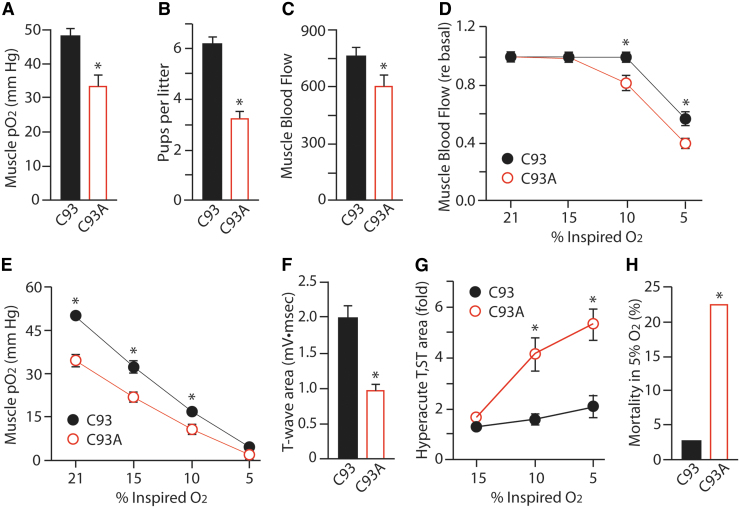
One expectation from these results, therefore, is that total SNO-based RBC vasoactivity in C93A mice would be relatively unchanged, but its regulation by deoxygenation (“thermodynamic linkage”) would be lost. Blood flow as a function of pO2 would be impaired and tissue hypoxia would ensue. This is indeed the case as β-globin-mutated C93A mice are hypoxemic even under normal conditions (Fig. 6A) (134). Resultant chronic tissue oxygen insufficiency should be detrimental to life, especially as fetal Hbs (bearing normal C93) are replaced with adult HbA including β-globin-mutated C93A. Indeed, C93A pups appear to have substantial prenatal mortality, as evidenced by reduced live litter size for C93A homozygotes than for C93 homozygotes (Fig. 6B) (134). The effect of C93A on development leading to this lethality of approximately half of developing pups has yet to be assessed directly, however. Similarly, chronically hypoxic C93A mice should be more sensitive to hypoxia or other insults, and such phenotypes are observed (see the Myocardial infarction and vessel growth section).
FIG. 6.
Active and reactive hyperemia are reduced in C93A mice. (A) Oxygenation of the gastrocnemius muscle is significantly lower in C93A mice at baseline. (B) Decreased liter size in C93A pairs compared with C93 pairs, suggesting poor survival during development. (C) Basal blood flow through the gastrocnemius muscle (in Doppler units) is significantly lower in C93A mice. (D) Blood flow through the gastrocnemius muscle is significantly lower in C93A mice as the oxygen content of inspired air (FiO2) is reduced from the normal 21% to 5%. (E) Oxygenation of the gastrocnemius muscle is significantly lower in C93A mice as the oxygen content of inspired air (FiO2) is reduced from the normal 21% to 5%. (F) Electrocardiogram T-wave area, which is correlated with cardiac tissue oxygenation, is significantly decreased in hypoxic C93A mice breathing room air, evidence of basal heart muscle oxygen deficiency. (G) Electrocardiogram hyperacute T, ST-wave area is significantly increased in hypoxic C93A mice breathing air with reduced oxygen content, reflective of ischemia and injury. (H) Mortality is significantly increased in C93A mice after 5 min exposure to 5% oxygen environment. *p < 0.05 for C93 versus C93A. Replotted from Zhang et al. (134) with permission. FiO2, fraction of inspired oxygen.
Hypoxic vasodilation
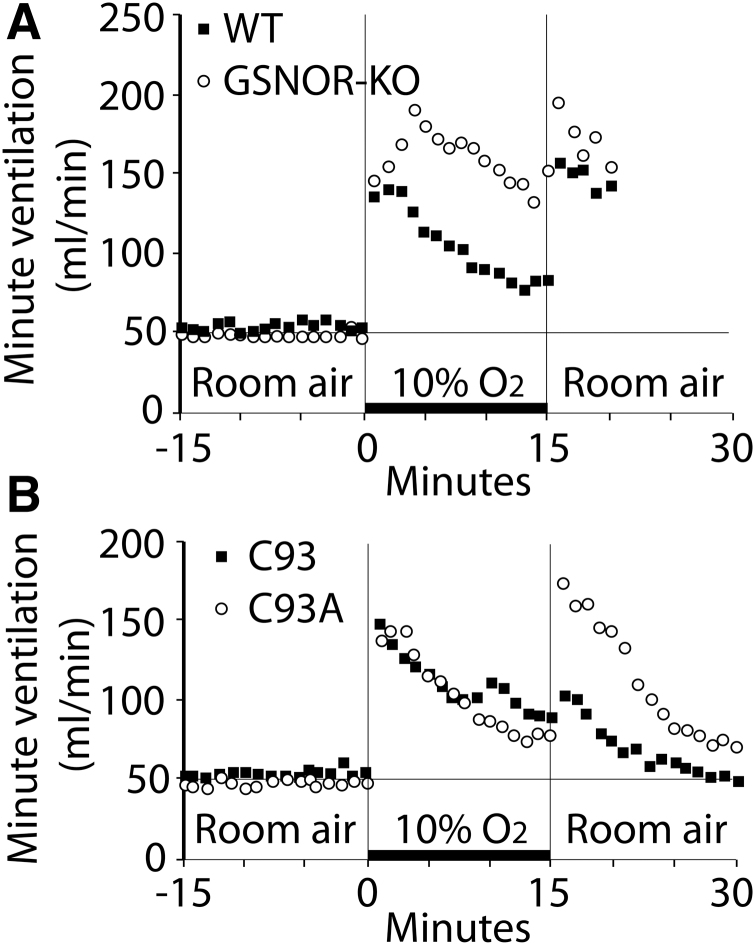
Another expectation is that C93A RBCs are predicted to be unable to produce deoxygenation-linked SNO-based vasodilation of large arteries in an organ bath bioassay (Table 1). More specifically, RBC-mediated vasodilation by SNO should still occur due to the excess compensatory SNO in C93A RBCs, but the dependence on Hb desaturation (R/T allostery) of SNO transfer to acceptor thiols should be impaired (66). Indeed, RBC-mediated hypoxic relaxation of mouse aorta was observed, although reduced, in the presence of C93A RBCs compared with C93 RBCs (134). Moreover, control βC93 RBC-induced vasorelaxation was potentiated by added glutathione (an allosteric effector of SNO bioactivity), but vasorelaxation produced by C93A RBCs was not potentiated by added glutathione (134). However, Isbell et al. (43) reported equivalent vasodilation of rabbit pulmonary artery by both C93 control and C93A RBCs under full deoxygenation. Tellingly, their vasodilatory effect was completely inhibited by the NO synthase inhibitor L-NMMA for both C93 and C93A RBCs (43), suggesting that SNO-mediated NOS-independent (i.e., SNO-βCys93-mediated) RBC vasodilation was simply not being measured under their test conditions—instead, they appear to have measured predominantly ATP-dependent vasodilation. More importantly, under conditions of 0% O2 saturation (R/T = 0), as in Ref. (43), one would not a priori predict differences in vasorelaxation as allosteric mechanisms have been overridden (recall, SNO levels do not change in mutant cells, only SNO disposition).
It is important to recognize that when our laboratory discovered RBC-mediated hypoxic vasodilation of blood vessels and developed this into a large vessel bioassay for SNO-Hb (46, 80), this in vitro bioassay was optimized to illuminate the concept of deoxygenation-mediated thermodynamic linkage of Cys93 reactivity (63, 85, 115) as a mechanistic explanation for a specific known physiology: the hypoxic autoregulation of blood flow (100). There is now no question that RBCs have both vasodilatory and vasoconstrictive effects on blood vessels through various distinct mechanisms, and these are each more or less prominent depending on precise assay conditions. From our perspective, it is important to use the physiology (e.g., autoregulation) as a guide when designing such in vitro assays and interpreting the results. That is, experiments should be designed to recognize alternative mediators of vasodilatory responses, not mask the role of SNO. In C93A versus C93 RBCs, the major shift in SNO forms makes this particularly important.
Autoregulation of microvascular blood flow
SNO-βCys93 Hb has been implicated in multiple physiological events related to cardiovascular function. These include autoregulation of blood flow (hypoxic vasodilation of tissue microcirculation to regulate tissue perfusion) and reactive hyperemia following transient obstruction of blood flow.
Autoregulation of blood flow results from acute release of SNO from SNO-C93A Hb in response to acute reduction in oxygenation in a tissue to increase perfusion by oxygenated blood through the tissue microcirculation, to match oxygen delivery to tissue oxygen demand. This mechanism normally acts under local conditions where high tissue metabolic activity induces lower-than-normal tissue oxygen levels, prompting increased SNO release from RBCs to vasodilate that specific region of the microcirculation. Loss of this effect should result in reduced basal blood flow, and this is observed in resting gastrocnemius muscle (Fig. 6C) (134). This effect can also be observed globally by progressively reducing blood oxygen carrying capacity through reduced inspired oxygen, and measuring acute blood flow increases to maintain delivery of oxygen near normal levels. Reducing the FiO2 from the normal 21% to 5% in stages results in stable blood flow through the gastrocnemius muscle in control C93 mice until the very lowest oxygen level (5%) was tested, when flow dropped (Fig. 6D) (134). This was accompanied by sustained mean blood pressure, cardiac output, and cardiac ejection fraction, and by normal electrocardiogram patterns (134). In the C93A mice, however, muscle blood flow (Fig. 6C, D) and muscle pO2 (Fig. 6A, E) were low at baseline and lower still at every decrement in O2, and this was accompanied by abnormal electrocardiogram patterns (134). Indeed, even at baseline and mild hypoxemia, C93A mice show electrocardiogram responses (S- and T-waves) consistent with cardiac oxygen insufficiency despite entirely normal cardiac function and blood pressure (Fig. 6F, G). Furthermore, a significantly higher number of C93A mice died during the 5 min exposure to 5% O2 due to cardiac injury (Fig. 6H) (134).
One group has suggested that vasodilation within 40–60 micron blood vessels at 10% O2 changing equivalently in C93A and C93 mice is evidence against a physiological role for βCys93 (122). This reflects a misunderstanding of the predicted role of βCys93 (Table 2). First, time is factor. Allosteric effects cannot be appreciated at steady state. Vessel relaxation under these conditions proceeds through multiple mechanisms that change with duration of vasodilatory stimuli (67). Autoregulation is only one component of this response and is measured as acute changes in tissue blood flow relative to HbO2 saturation, not as a single static measurement of diameter. Second, autoregulation is not measured at one site in this way; it is a composite effect including much smaller vessels that dictate tissue oxygenation. Third, blood flow in small vessels is a function of radius to fourth power, and relevant changes in vessel diameter, which are distributed across the entire microcirculation, are easily missed. Finally, not all hypoxic vasodilation is designed for autoregulation of flow, and mechanisms differ for different physiological effects. Consequently, measurement of βCys93-dependent hypoxic autoregulation (under systemic hypoxia) entails blood flow, not vessel diameter.
With that said, C93A-mutant RBCs show abnormal vasodilatory activity when examined appropriately. An in vitro correlate of in vivo RBC deoxygenation-regulated hypoxic vasodilation of microvasculature is the RBC-based vasodilatory action on large vessels. This assay measures vasomodulatory action of RBCs: in oxygenated conditions, RBCs induce vasoconstriction of large vessels in organ bath assay in part through scavenging of endothelially produced NO that would otherwise increase vasodilatory tone, while upon transition to deoxygenated conditions, RBCs induce transient vasorelaxation, at least in significant part, through release of SNO from Hb βCys93, export of SNO from RBCs, and SNO transfer to vessels (20, 46, 57, 66). To unmask allostery, assays can be performed by adding an SNO carrier, such as glutathione, N-acetylcysteine, or cysteine (19, 57, 66, 113, 134). RBCs from C93A mice produce vasorelaxation of aorta (from eNOS-deficient mice to exclude endogenous NO mechanisms) that is not augmented by the addition of glutathione as SNO carrier, compared with C93 mice where glutathione potentiates vasorelaxation (134). This indicates that the mouse RBCs produce vasorelaxation predominantly via a non-SNO-Cys93-based mechanism [other exported SNO including HSNO, GSNO, or PDI-SNO remain possibilities (50)], but that the C93-bearing RBCs also exhibit additional vasorelaxing activity that depends on SNO release from βCys93, as this is lost in the C93A RBCs. Similar assays using endothelium-intact aorta with normal eNOS levels also show loss of vasorelaxing activity of RBCs from C93A mice, although the percentage difference is smaller because mouse RBCs produce vasorelaxant activities via multiple mechanisms that involve endothelial NO production (such as ATP release) (134). It should be noted that physiological corollaries of this in vitro activity have only been well described for βCys93.
Others have reported that C93A RBCs induce vasorelaxation of rabbit or rat pulmonary artery that did not differ from C93 RBCs at 0% oxygen (43, 59), but in light of above discussions on allostery [see the HbβC93 and Allosteric Control by Oxygen (Understanding of the Expected Role of HbβC93 in Vasodilation) section] and of physiological mechanisms (above), this should no longer be a surprising finding. In one study, the hypoxic vasodilation responses of both C93 and C93A RBCs on rabbit pulmonary artery were completely blocked by an inhibitor of NOS, indicating that SNO-dependent mechanisms were not being assessed under their test conditions; so unsurprisingly, inhibiting SNO at βCys93 had no consequence (43). Closer examination of measured vasorelaxation by C93 and C93A RBCs acting on rat pulmonary artery indicates that these responses are clearly composed of multiple components with distinct mechanisms sensitive or not to various inhibitors and surprisingly unaffected by denudation of the endothelia (43, 59). The latter effect may be best interpreted in terms of an SNO-based activity (see the Hypoxic Vasodilation section above). Interestingly, direct comparison of thoracic aorta versus pulmonary artery from various species demonstrated remarkable diversity of RBC-induced vasomodulatory responses between species of RBC, species of vessel, and vessel type (59), suggesting that comparing such vasodilation bioassays across studies utilizing different species and type of vessel is fraught with complications.
Reactive hyperemia
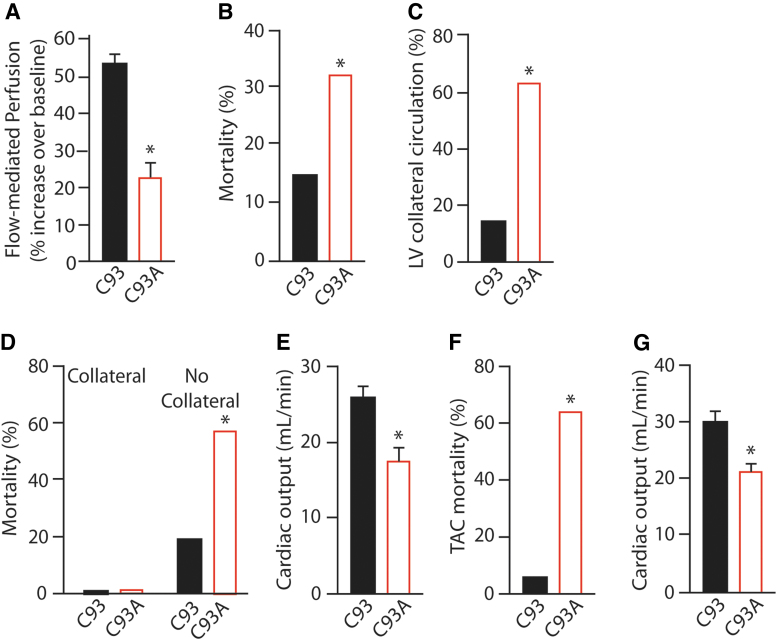
An in vivo corollary and physiological consequence of direct vasodilation by SNO-βC93-Hb is reactive hyperemia, where an episode of oxygen lack in a tissue (such as due to blood flow disruption by ligature or external pressure) leads to transient hyperperfusion through vasodilation once the blockade is removed. In the C93A mouse, reactive hyperemia after transient blood flow blockade to the gastrocnemius muscle is reduced by about half compared with that in C93 control mice (134), demonstrating a major role for SNO-Hb in promoting this increased muscle blood flow (Fig. 7A) Presumably, high amounts of SNO are released from Hb into excessively deoxygenated tissue immediately after blood flow is restored, which then subside to normal levels as tissue oxygenation is raised. The remaining hyperemia is due to canonical mechanisms, including flow-mediated activation of eNOS that then promotes smooth muscle relaxation via PKG to increase blood supply reaching the affected area (49, 108).
FIG. 7.
Impaired blood flow as cause of cardiac injury and heart failure in C93A mice. (A) Reactive hyperemia is significantly blunted in C93A mice, measured as elevated blood flow over basal after release of 5 min blockade of blood flow to the gastrocnemius muscle. (B) Increased mortality in C93A mice following cardiac ischemia–reperfusion. (C) Increased presence of collateral vessels from the left or right coronary artery supplying the left ventricle in C93A mice. (D) Mortality following cardiac ischemia–reperfusion occurs only in mice lacking collateral vessels. (E) Reduced cardiac output in C93A mice after acute (2 day) TAC pressure overload-induced heart failure. (F) Increased mortality in C93A mice following chronic (28 day) TAC. (G) Reduced cardiac output in C93A mice after chronic TAC. *p < 0.05 for C93 versus C93A. Replotted from Zhang et al. (135) with permission. TAC, transverse aortic constriction.
Myocardial infarction and vessel growth
The importance of microvascular blood flow as a causal factor in heart attacks is increasingly appreciated, particularly in women (2, 53). C93A mice were subjected to an acute myocardial infarction injury model by occluding the left coronary artery for 30 min, releasing the ligature, and allowing recovery for 24 h (135). While 85% of control C93 mice survived the recovery period after ischemia–reperfusion injury, only 68% of C93A mice survived (Fig. 7B), indicating a cardioprotective activity of Hb-βCys93 in hypoxic injury (135). Comparing mice that died versus those that survived, it was observed that surviving mice more frequently contained arterial collaterals from the left or right coronary arteries that perfused the left ventricle (135). Indeed, C93A mice more often had such collaterals than did control C93 mice (Fig. 7C), and no C93A mice with such a collateral artery died after ischemia–reperfusion, whereas 60% of C93A mice without such a collateral artery did die (Fig. 7D) (135). C93A mice with an arterial collateral fared better than those without by all measures (the % of left ventricular area recovered at 24 h, the % area of necrosis, and the % area at risk), and C93A had worse injury than C93 controls (decreased area of recovery and increased area of necrosis) (135). Echocardiography of survivors 24 h after ischemia–reperfusion injury demonstrated similar results: C93A mice had significantly reduced cardiac function compared with C93 controls, measured as ejection fraction and fractional shortening, and C93A mice lacking a collateral were worse-off than those with a compensatory collateral artery (135). The presence of collateral arteries is also indirect evidence for chronic basal hypoxia in C93A mice, confirming electrocardiographic changes (134), and with or without such collateral arteries, C93A mice are less able to withstand ischemic injury to the heart, suggesting reduced functional oxygenation reserve.
Isolated heart reperfusion
The effect of C93A versus C93 RBCs on recovery of the isolated perfused heart from temporary ischemia has also been assessed (122). At the onset of ischemia, RBCs were injected into the coronary circulation to be present through the ischemia period (i.e., no RBC flow). As a measure of heart function, left ventricular developed pressure (LVDP) was monitored after restoration of oxygenated flow. Hearts with C93A RBCs in their coronary circulation fared significantly less well than those with C93 RBCs, with reduced rate of recovery of LVDP over 1 h of oxygenated reperfusion. This suggests a protective effect of SNO-Hb βCys93 during transient cardiac ischemia, although the effect after a longer recovery period was not assessed. Altogether, βCys93 has been shown to be protective at baseline and in four different in vivo models of ischemia (Table 3).
Table 3.
Protective Effects of βCys93 in Ischemia Models
| Ischemic model | Evidence | Reference |
|---|---|---|
| Acute hypoxic insult (breathing 10% O2 for 15 min) | Rapid increase in respiration upon return to normoxia (short-term potentiation of ventilation) is lost in C93A mice. | (26) |
| Acute progressive hypoxic insult (breathing 15%, 10% then 5% O2 for 5 min each) | Progressive failure to maintain cardiac function in C93A mice. Reduced survival in 5% O2 by C93A mice (cardiogenic shock). Reduced hind limb muscle PO2 (ischemia) and blood flow in C93A mice at baseline and worsened by progressive hypoxia. |
(134) |
| Basal cardiac hypoxia | Cardiac ischemia at baseline in C93A. Increased development of collateral vessels serving left ventricle in C93A mice. | (135) |
| Left coronary artery occlusion (30 min), then reperfusion (24 h) | Increased size of infarct, cardiac dysfunction, and death in C93A mice lacking compensatory cardiac collateral vessel. | (135) |
| Transverse aortic constriction (pressure overload, at 2 and 4 weeks) | Increased heart failure and death in C93A mice. | (135) |
| Isolated mouse heart, coronary arteries filled with oxygenated RBCs and flow stopped at 0% O2 (40 min), then reperfused and reoxygenated (60 min). | Reduced recovery of postischemic left ventricular pressure (as a measure of muscle function) in the hearts treated with C93A RBCs. | (122) |
Native SNO-Hb operates across integrated cardiovascular and respiratory system to protect against ischemic insult. RBCs endowed with SNO-C93-Hb are protective against ischemia through R/T-transition-regulated vasodilation. Multiple compensations are found in C93A-mutant animals and RBCs, including growth of new blood vessels.
Heart failure
Microvascular blood flow plays a critical role in evolution of heart failure. In a model of pressure overload cardiomyopathy using transverse aortic constriction (TAC) to limit cardiac outflow, C93A mice were assessed for both acute effects at 2 days and chronic effects at 4 weeks post-TAC (135). After 2 days of constricted blood flow, C93A mice exhibited worsened cardiac function compared with C93 mice, including significantly reduced ejection fraction, fractional shortening, and cardiac output, and significantly elevated end-systolic diameter and heart and lung weight due to cardiac hypertrophy and pulmonary edema (Fig. 7E) (135). In mice undergoing chronic TAC, more than 60% of C93A mice died within the first 5 days compared with only 5% of C93 mice (Fig. 7F) (135). Surviving C93A mice also exhibited worsened cardiac function compared with C93 mice, including significantly reduced ejection fraction, fractional shortening, and cardiac output, and significantly elevated end-systolic diameter and heart and lung weight, consistent with heart failure (Fig. 7G) (135). Thus, Hb Cys93 has protective functions in heart failure models, and loss of Cys93-SNO vasodilation exacerbates heart failure due to reduced SNO and O2 delivery to the heart.
Surprises from C93A Mice
Physiological studies of genetically modified mice allow studies of expected physiological phenotypes and also present the opportunity for unappreciated physiological roles of target genes to become manifest.
Ventilatory drive
The mechanisms controlling ventilatory drive are complex, involving intrinsic neuronal pacemaker-type activities in the brainstem overlaid by chemical sensing control mechanisms (27, 56). Direct chemical sensing of blood gasses (primarily carbon dioxide as bicarbonate/pH, but also oxygen) occurs both in the brainstem and at the carotid body (27, 56). One aspect of ventilatory drive to changing oxygen environments, however, appears to be regulated by S-nitrosylation via SNO-Hb. Mice lacking glutathione-SNO reductase (GSNOR), an enzyme that removes SNO from GSNO to indirectly regulate protein SNO levels, were noted to exhibit a loss of ventilatory roll-off following brief hypoxia, measured as rate of breathing, tidal volume, and minute ventilation (Fig. 8A) (77). Roll-off, also known as hypoxic ventilatory decline or hypoxic ventilatory depression, is the adaptive phenomenon where the increased breathing due to acute hypoxia or hypercapnia (elevated CO2) fails to be sustained but instead subsides quickly to a more normal rate despite continued low O2/high CO2 (27). Conversely, short-term potentiation of ventilation is the increased breathing observed after return to normoxia following a period of hypoxia, which also quickly returns to normal levels. The C93A mice exhibit near-normal ventilatory roll-off in response to breathing low O2 but exhibit significantly augmented short-term potentiation of ventilation upon return to room air, measured as breathing rate, tidal volume, or minute ventilation (Fig. 8B) (26). Basal ventilation parameters under normoxia did not differ in these mice. Bilateral transection of the carotid sinus nerves to prevent signaling by the carotid body to the brainstem resulted in deficiencies in both hypoxia-induced hyperventilation and posthypoxia normoxia-induced hyperventilation (26), suggesting that the carotid body may contain the sensor for these responses and thus be the locus where SNO-βCys93-Hb appears to act to drive the short-term potentiation response following brief hypoxia. The precise molecular mechanisms and protein targets through which SNO-Hb can alter carotid body regulation of ventilatory drive remain to be determined.
FIG. 8.
SNO-mediated regulation of ventilatory physiology. (A) Male mice lacking GSNOR (Adh5), an enzyme that reduces tissue SNO levels, lack respiratory roll-off during exposure to hypoxia (10% oxygen) since their minute ventilation fails to return to baseline like that of WT control mice. (B) C93A mice have augmented short-term potentiation of ventilation (measured as minute ventilation) following return to room air (21% oxygen) after brief hypoxia (10% oxygen). Replotted from Palmer et al. (77) (A) and Gaston et al. (26) (B), with permissions. GSNOR, glutathione-SNO reductase; WT, wild type.
Compensation in SNO
Given that Hb is by far the major protein in RBCs and βCys93 is the major site of SNO on native Hb (23, 113), one could reasonably predict that the total SNO content of C93A RBCs would be greatly reduced. However, RBC total SNO content appears roughly normal in C93A RBCs, even as high-molecular-weight SNOs (primarily Hb) can be significantly reduced (43, 95, 122, 134). This was proposed to result from compensatory elevation of low-molecular-weight (<10 kDa) SNO species (43, 122), although the specific species involved (e.g., GSNO, Cys-NO) has not been identified, nor the mechanisms responsible for this compensatory upregulation of low-molecular-weight SNOs. Furthermore, compensatory increases in SNO-Hb, including α-globin CysNO, β-globin Cys112-SNO, and γ-globin Cys93, have been observed (134). In any case, one clear consequence of this shift from SNO(βCys93)-Hb to alternative SNOs in C93A RBCs is that SNO carriage by RBCs is not actually prevented by the C93A mutation as one would have predicted beforehand. Instead, it appears likely that these compensatory low-molecular-weight (and other) SNOs are able to support SNO export from RBCs, although likely less efficiently than native C93 Hb and not in a strictly deoxygenation-dependent manner, to potentially alleviate hypoxia in tissues.
Compensation in Hb
An additional level of compensation by C93A RBCs involves the increased expression of Hb. Although RBC numbers appear equivalent in C93A and C93 mice, total Hb level is significantly elevated in the C93A animals, with ∼10% more Hb per RBC (43). As noted previously, total fetal Hb expression in C93 and C93A mice appears roughly equivalent (26), although this was tested only using individual animals of varying ages, so statistical tests are not possible to have confidence in any differences. In any case, elevated Hb levels in C93A mice also suggest that this might be the result of compensation to chronic tissue hypoxia through mechanisms such as those that lead to increased Hb levels in humans living at high altitude (5, 6).
Compensation in blood flow
As noted in the Myocardial infarction and vessel growth section, the hearts from C93A mice more often contain arterial collaterals from the left or right coronary arteries to provide extra perfusion to the left ventricle (135). This is also evidence that these mice with chronic SNO-Hb deficiency exhibit chronic cardiac hypoxia that drives compensatory blood vessel development (134). A role for SNO-Hb-βCys93 in blood vessel development was not previously appreciated, and the age at which this cardiac collateral vessel develops is unknown as is the reason that only some animals develop this compensatory vessel.
Nonphenotypes in the C93A Knock-In
As noted previously, the initial report of the characterization of the C93A mouse claimed that these mice had no systemic or pulmonary phenotype (43). In this study, mice were assessed for several macrohemodynamic parameters, including mean arterial pressure, and pulmonary vascular histology as a marker of severe pulmonary hypertension (43), which are not predicted to be mediated in significant part (or at all) by βCys93 SNO-Hb (76, 117). Unsurprisingly, these measures were unchanged in the C93A mice (43).
In this initial study, C93A mice were not subjected to hypoxia challenge; instead, run-time to exhaustion in two mice was used as a surrogate for muscle blood flow and oxygen delivery, and this did not differ (43). However, run-time to exhaustion is a complex behavior that involves regulatory mechanisms from the central nervous system (CNS) down to individual muscle fibers and is more a reflection of muscle calcium handling than oxygen delivery, since muscles readily switch from respiration to glycolysis to maintain function when activity outstrips oxygen delivery, but cannot replenish calcium stores beyond a maximal rate (128). Also, C93A mice develop collaterals (134) and blood flow was not controlled for in these animals. In light of this, Hb βCys93 may have no role in run-time to exhaustion (43), with the caveat that this should be assessed more rigorously in more than two mice. Moreover, while gross organ histopathology (by hematoxylin and eosin staining at low power) is a poor surrogate for hypoxia (43), PO2 was not actually measured in tissues. One might expect evidence of pulmonary hypertension due to chronic oxygen insufficiency; however, the mice tested were unchallenged with hypoxia, and pressure was not measured (43).
A recent study concluded that RBCs from the C93A mouse failed to show evidence for this Cys residue in an assortment of gross pharmacological functions (122). For example, dilute solutions of RBCs from C93A or C93 mice were exposed to enormous amounts of SNO-cysteine (NO/Hb 4:1 = 10,000-fold > physiological; note: RBC Hb is ∼5 mM) to load Hb with SNO and then tested for ability to vasorelax rat aorta in an in vitro bioassay (122). However, at such high loading with SNO, which creates very high amounts of SNO (39, 79), even WT RBCs elicit vasodilation in room air, that is, oxygen dependence is lost; C93A only comes into play under more physiological conditions (NO/Hb <1:100; NO ∼1 μM) (80) (Table 1). Indeed, with extreme loading C93A and C93, RBCs showed more vasodilation at 21% O2 than at 1% O2 (122) in stark contrast to native and physiologically loaded cells (80). This can be understood by appreciating that SNO that binds to non-Cys93 sites (including in Hb, proteins in the lysates or membranes, or to low-molecular-weight thiols), will all then act as nonspecific SNO donors to produce vasorelaxation irrespective of the oxygenation state or conformation of Hb. Furthermore, presumably much of the Hb was oxidized under these conditions (39), and SNO-metHb is an oxygen-independent vasodilator (i.e., R/T allostery is eliminated) (46). Therefore, these results do not reflect on the activity of βCys93-SNO to produce specific oxygen saturation-linked vasorelaxation.
The ability of platelets to undergo activation has been reported to slightly inhibited by nitrite in the presence of deoxygenated RBCs, due to reduction of nitrite to NO by Hb and/or generation of SNO (114). C93A RBCs did not differ from C93 RBCs in ability to inhibit platelet activation (122), which is unsurprising as there has been no suggestion that platelets provide a surrogate for hypoxic vasodilation (126, 127) or that inhibition of platelet function by nitrite is physiologically relevant.
Conclusions and Future Directions
Physiological evidence from the humanized Hb C93A mice clearly demonstrates that these mice exhibit fundamental differences from their C93 controls. These include functional differences in Hb deoxygenation-dependent SNO release and SNO-dependent vasorelaxation during autoregulation of blood flow, reactive hyperemia after transient blockade of blood flow, regulation of ventilatory responses to hypoxia and normoxia, structural developmental differences in cardiac vasculature, electrocardiographic evidence for chronic oxygen deficit in heart muscle, basal cardiovascular oxygenation, reduced cardiac function and viability during an acute ischemic insult and worse recovery after this injury, and reduced viability in a chronic heart failure model with worse consequences for cardiac function. All these factors are either known to be mediated by SNO or can be modeled as being mediated by SNO. Thus, the evidence that Hb βCys93 carries and releases SNO and plays a fundamental role in cardiovascular and respiratory physiology is strong.
As importantly, the few studies that have reported a lack of effect of this mutation in mice measure irrelevant functions (such as blood pressure or platelet inhibition), misattribute roles and functions of SNO-Hb and βCys93 in particular with other functions of RBCs or endothelium due to distinct mediators (e.g., ATP), or utilize methodologies that are not suited to accurate measurement of SNO-Hb in vivo (e.g., expose blood gases to room air). It is also important to keep in mind that this mouse model has significant compensations as well as caveats.
Additional evidence for a role of SNO-Hb and βCys93 comes from studies using human subjects and patient-derived samples. Briefly, these include arterial–venous gradients in SNO-Hb (63) and findings that blood SNO levels follow blood O2 saturation (20), findings of deficits in SNO-Hb in SCD (81), pulmonary hypertension (62), and stored blood used for transfusion leading to transfusion-associated tissue hypoxia (94, 95), as well as in normal humans adapting to low-oxygen environment at high altitude (44). Conversely, elevated SNO-Hb in sepsis has been associated with poor tissue oxygenation due to blood shunting (58). These and other clinically relevant manifestations of SNO-Hb dysfunctions, and potential therapeutics, are discussed in detail in a recent review (88).
Future work needs to be focused on additional physiological systems where βCys93 may have consequences for health in the C93A animal model and human patients. These include in particular CNS function in basal hypoxia-driven oxygen delivery and in neurodegeneration, and pulmonary functions such as ventilation–perfusion matching, adaptation to hypoxia, and pulmonary hypertension. The role of SNO-Hb and βCys93 in β-thalassemia (mutated or reduced/absent β-globin) and in diabetes (elevated glycated HbA1c) (75) remains to be fully characterized. Finally, therapeutics based on altering SNO-Hb will continue to be tested in clinical trials to determine if this treatment is effective in particular disease states.
Abbreviations Used
- βCys93
cysteine-93 residue of β-globin
- C93
mice bearing human Hb (α-globin and β-globin) in place of these mouse genes
- C93A
mice bearing human Hb (α-globin and β-globin), with β-globin Cys93 mutated to Ala
- cGMP
cyclic GMP
- CoA
coenzyme A
- CysNO
S-nitroso-cysteine
- ENO
ethyl nitrite
- eNOS
the NOS3 isoform, endothelial nitric oxide synthase
- EPI
epinephrine
- FeNO
NO bound to heme iron on Hb, either on α-globin or on β-globin chain heme
- FiO2
fraction of inspired oxygen (normally 21% in room air)
- GSH
glutathione (in reduced form with free thiol)
- GSNO
SNO-glutathione
- GSNOR
glutathione-SNO reductase enzyme
- Hb
hemoglobin, in general used to denote the mixture of Hb isoforms found in adult RBCs
- HbA
hemoglobin A (adult), composed of two α-globin and two β-globin chains
- HbD
hemoglobin D (adult minor form), composed of two α-globin and two δ-globin chains
- HbE
hemoglobin E (embryonic), composed of two α-globin and two ɛ-globin chains
- HbF
hemoglobin F (fetal), composed of two α-globin and two γ-globin chains
- IVU
integrated vascular unit
- LVDP
left ventricular developed pressure
- NO
nitric oxide
- NO+
nitrosonium ion or its functional equivalent of NO bound to a metal ion
- NOS
NO synthase
- P50
the oxygen gas pressure at which 50% of Hb is in the R state and 50% in the T state
- PDI
protein disulfide isomerase
- PKG
protein kinase G, the cyclic-GMP-activated protein kinase
- RBC
red blood cell or erythrocyte
- R-SH
free sulfhydryl group on a molecule or protein R
- R state
“relaxed,” the hemoglobin conformation where four heme ligands (oxygen) are bound
- SCD
sickle cell disease
- sGC
soluble guanylyl cyclase
- SH
sulfhydryl (reduced or “free”)
- SNO
S-nitrosothiol
- SNO-Hb
S-nitrosylated hemoglobin
- ssRBC
sickle RBC
- TAC
transverse aortic constriction
- T state
“tense,” the hemoglobin conformation where no heme ligand (oxygen) is bound
- WT
wild type
Author Disclosure Statement
Drs. Stamler and Reynolds hold patents related to renitrosylation of hemoglobin and erythrocytes. Case Western Reserve University and University Hospitals have appropriate conflict management plans in place.
Funding Information
Supported by National Institutes of Health grants P01 HL075443, P01 HL126900, R01 HL128192, and R01 DK119506, and American Heart Association-Allen Brain Health Initiative grant 19PABHI34580006 (to J.S.S.).
References
- 1. Angelo M, Singel DJ, and Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A 103: 8366–8371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arthur HM, Campbell P, Harvey PJ, McGillion M, Oh P, Woodburn E, and Hodgson C. Women, cardiac syndrome X, and microvascular heart disease. Can J Cardiol 28: S42–S49, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Azizi F, Kielbasa JE, Adeyiga AM, Maree RD, Frazier M, Yakubu M, Shields H, King SB, and Kim–Shapiro DB. Rates of nitric oxide dissociation from hemoglobin. Free Radic Biol Med 39: 145–151, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Basu S, Wang X, Gladwin MT, and Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods Enzymol 440: 137–156, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A 104 Suppl 1: 8655–8660, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beall CM, Laskowski D, and Erzurum SC. Nitric oxide in adaptation to altitude. Free Radic Biol Med 52: 1123–1134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benhar M, Forrester MT, and Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10: 721–732, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Bian K and Murad F. What is next in nitric oxide research? From cardiovascular system to cancer biology. Nitric Oxide 43: 3–7, 2014 [DOI] [PubMed] [Google Scholar]
- 9. Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, and Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850–853, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Bulger M, van Doorninck JH, Saitoh N, Telling A, Farrell C, Bender MA, Felsenfeld G, Axel R, and Groudine M. Conservation of sequence and structure flanking the mouse and human beta-globin loci: the beta-globin genes are embedded within an array of odorant receptor genes. Proc Natl Acad Sci U S A 96: 5129–5134, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 117: 76–89, 2018 [DOI] [PubMed] [Google Scholar]
- 12. Cai C, Fordsmann JC, Jensen SH, Gesslein B, Lonstrup M, Hald BO, Zambach SA, Brodin B, and Lauritzen MJ. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proc Natl Acad Sci U S A 115: E5796–E5804, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan NL, Kavanaugh JS, Rogers PH, and Arnone A. Crystallographic analysis of the interaction of nitric oxide with quaternary-T human hemoglobin. Biochemistry 43: 118–132, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Chan NL, Rogers PH, and Arnone A. Crystal structure of the S-nitroso form of liganded human hemoglobin. Biochemistry 37: 16459–16464, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Chen Q, Balazs TC, Nagel RL, and Hirsch RE. Human and mouse hemoglobin association with the transgenic mouse erythrocyte membrane. FEBS Lett 580: 4485–4490, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Collins FS and Weissman SM. The molecular genetics of human hemoglobin. In: Progress in Nucleic Acid Research and Molecular Biology, edited by Cohn WE and Moldave K. Orlando, FL: Academic Press, 1984, pp. 315–465 [DOI] [PubMed] [Google Scholar]
- 17. Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, and Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Dantzker DR, Foresman B, and Gutierrez G. Oxygen supply and utilization relationships. A reevaluation. Am Rev Respir Dis 143: 675–679, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Diesen DL, Hess DT, and Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal. Circ Res 103: 545–553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, and Gaston B. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A 102: 5709–5714, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erdener ŞE and Dalkara T. Small vessels are a big problem in neurodegeneration and neuroprotection. Front Neurol 10: 889, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erhart MA, Simons KS, and Weaver S. Evolution of the mouse beta-globin genes: a recent gene conversion in the Hbbs haplotype. Mol Biol Evol 2: 304–320, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Ferranti P, Malorni A, Mamone G, Sannolo N, and Marino G. Characterisation of S-nitrosohaemoglobin by mass spectrometry. FEBS Lett 400: 19–24, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Furchgott RF and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Furuta S. Basal S-Nitrosylation is the guardian of tissue homeostasis. Trends Cancer 3: 744–748, 2017 [DOI] [PubMed] [Google Scholar]
- 26. Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, and Lewis SJ. Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice. J Appl Physiol (1985) 116: 1290–1299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gauda ES and Martin RJ. Control of breathing. In: Avery's Diseases of the Newborn, edited by Gleason C and Devaskar S. Philadelphia, PA: Saunders, 2012, pp. 584–597 [Google Scholar]
- 28. Gladwin MT, Ognibene FP, Pannell LK, Nichols JS, Pease-Fye ME, Shelhamer JH, and Schechter AN. Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc Natl Acad Sci U S A 97: 9943–9948, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gladwin MT and Schechter AN. NO contest: nitrite versus S-nitroso-hemoglobin. Circ Res 94: 851–855, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, and Schechter AN. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J Biol Chem 277: 27818–27828, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, and Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gow AJ and Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391: 169–173, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Gregory TR. Nucleotypic effects without nuclei: genome size and erythrocyte size in mammals. Genome 43: 895–901, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Hausladen A, Rafikov R, Angelo M, Singel DJ, Nudler E, and Stamler JS. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci U S A 104: 2157–2162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hempe JM, Ory-Ascani J, and Hsia D. Genetic variation in mouse beta globin cysteine content modifies glutathione metabolism: implications for the use of mouse models. Exp Biol Med (Maywood) 232: 437–444, 2007 [PubMed] [Google Scholar]
- 36. Herold S and Rock G. Reactions of deoxy-, oxy-, and methemoglobin with nitrogen monoxide. Mechanistic studies of the S-nitrosothiol formation under different mixing conditions. J Biol Chem 278: 6623–6634, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Hess DT, Matsumoto A, Kim SO, Marshall HE, and Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Hobbs AJ, Gladwin MT, Patel RP, Williams DL, and Butler AR. Haemoglobin: NO transporter, NO inactivator or NOne of the above? Trends Pharmacol Sci 23: 406–411, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Hrinczenko BW, Schechter AN, Wojtkowski TL, Pannell LK, Cashon RE, and Alayash AI. Nitric oxide-mediated heme oxidation and selective beta-globin nitrosation of hemoglobin from normal and sickle erythrocytes. Biochem Biophys Res Commun 275: 962–967, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, and Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ignarro LJ, Barry BK, Gruetter DY, Edwards JC, Ohlstein EH, Gruetter CA, and Baricos WH. Guanylate cyclase activation by nitroprusside and nitrosoguanidine is related to formation of S-nitrosothiol intermediates. Biochem Biophys Res Commun 94: 93–100, 1980 [DOI] [PubMed] [Google Scholar]
- 42. Ignarro LJ, Buga GM, Wood KS, Byrns RE, and Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, and Townes TM. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med 14: 773–777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janocha AJ, Koch CD, Tiso M, Ponchia A, Doctor A, Gibbons L, Gaston B, Beall CM, and Erzurum SC. Nitric oxide during altitude acclimatization. N Engl J Med 365: 1942–1944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jennings ML. Transport of H2S and HS− across the human red blood cell membrane: rapid H2S diffusion and AE1-mediated Cl−/HS− exchange. Am J Physiol Cell Physiol 305: C941–C950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia L, Bonaventura C, Bonaventura J, and Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Joseph A, Guevara-Torres A, and Schallek J. Imaging single-cell blood flow in the smallest to largest vessels in the living retina. eLife 8: e45077, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joshi MS, Ferguson TB, Han TH, Hyduke DR, Liao JC, Rassaf T, Bryan N, Feelisch M, and Lancaster JR. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A 99: 10341, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Joyner MJ and Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kallakunta VM, Slama-Schwok A, and Mutus B. Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells. Redox Biol 1: 373–380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kashiwagi S, Kajimura M, Yoshimura Y, and Suematsu M. Nonendothelial source of nitric oxide in arterioles but not in venules. Circ Res 91: e55–e64, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, and Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood 107: 2943–2951, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Kothawade K and Bairey Merz CN. Microvascular coronary dysfunction in women—pathophysiology, diagnosis, and management. Curr Probl Cardiol 36: 291–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurtz DM Jr. Oxygen-carrying proteins: three solutions to a common problem. Essays Biochem 34: 85–100, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Lancaster JR Jr. How are nitrosothiols formed de novo in vivo? Arch Biochem Biophys 617: 137–144, 2017 [DOI] [PubMed] [Google Scholar]
- 56. Levitsky MG. Pulmonary Physiology. New York: McGraw-Hill Education, 2018 [Google Scholar]
- 57. Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, and Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, and Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116: 617–628, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Liu Y, Sun CW, Honavar J, Townes T, and Patel RP. Role of the b93cys, ATP and adenosine in red cell dependent hypoxic vasorelaxation. Int J Physiol Pathophysiol Pharmacol 5: 21–31, 2013 [PMC free article] [PubMed] [Google Scholar]
- 60. Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, and Singel DJ. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A 100: 461–466, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. MacArthur PH, Shiva S, and Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci 851: 93–105, 2007 [DOI] [PubMed] [Google Scholar]
- 62. McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, Califf RM, Singel DJ, Piantadosi CA, Tapson VF, and Stamler JS. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A 102: 14801–14806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, and Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med 8: 711–717, 2002 [DOI] [PubMed] [Google Scholar]
- 64. McMahon TJ, Shan S, Riccio DA, Batchvarova M, Zhu H, Telen MJ, and Zennadi R. Nitric oxide loading reduces sickle red cell adhesion and vaso-occlusion in vivo. Blood Adv 3: 2586–2597, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McMahon TJ and Stamler JS. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol 301: 99–114, 1999 [DOI] [PubMed] [Google Scholar]
- 66. McMahon TJ, Stone AE, Bonaventura J, Singel DJ, and Stamler JS. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem 275: 16738–16745, 2000 [DOI] [PubMed] [Google Scholar]
- 67. Meng L, Wang Y, Zhang L, and McDonagh DL. Heterogeneity and variability in pressure autoregulation of organ blood flow: lessons learned over 100+ years. Crit Care Med 47: 436–448, 2019 [DOI] [PubMed] [Google Scholar]
- 68. Moncada S, Higgs A, and Furchgott R. International union of pharmacology nomenclature in nitric oxide research. Pharmacol Rev 49: 137–142, 1997 [PubMed] [Google Scholar]
- 69. Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest 78: 1–5, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nader E, Skinner S, Romana M, Fort R, Lemonne N, Guillot N, Gauthier A, Antoine-Jonville S, Renoux C, Hardy-Dessources M-D, Stauffer E, Joly P, Bertrand Y, and Connes P. Blood rheology: key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front Physiol 10: 1329, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nagababu E, Ramasamy S, Abernethy DR, and Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem 278: 46349–46356, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Namdee K, Carrasco-Teja M, Fish MB, Charoenphol P, and Eniola-Adefeso O. Effect of variation in hemorheology between human and animal blood on the binding efficacy of vascular-targeted carriers. Sci Rep 5: 11631, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, and Attwell D. Amyloid beta oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science 365: eaav9518, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ogoh S and Tarumi T. Cerebral blood flow regulation and cognitive function: a role of arterial baroreflex function. J Physiol Sci 69: 813–823, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Padron J, Peiro C, Cercas E, Llergo JL, and Sanchez-Ferrer CF. Enhancement of S-nitrosylation in glycosylated hemoglobin. Biochem Biophys Res Commun 271: 217–221, 2000 [DOI] [PubMed] [Google Scholar]
- 76. Palmer LA, Doctor A, and Gaston B. SNO-hemoglobin and hypoxic vasodilation. Nat Med 14: 1009; author reply 100 9–1010, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Palmer LA, May WJ, deRonde K, Brown-Steinke K, Bates JN, Gaston B, and Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir Physiol Neurobiol 185: 571–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 79. Palmerini CP, Palombari R, and Arienti G. Electrochemical assay of human haemoglobin S-nitrosylation by nitrosocysteine. Amino Acids 25: 59–62, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Pawloski JR, Hess DT, and Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature 409: 622–626, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Pawloski JR, Hess DT, and Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A 102: 2531–2536, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Peppiatt CM, Howarth C, Mobbs P, and Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perutz MF. Relation between structure and sequence of haemoglobin. Nature 194: 914–917, 1962 [DOI] [PubMed] [Google Scholar]
- 84. Perutz MF. Hemoglobin structure and respiratory transport. Sci Am 239: 92–125, 1978 [DOI] [PubMed] [Google Scholar]
- 85. Perutz MF. Blood. Taking the pressure off. Nature 380: 205–206, 1996 [DOI] [PubMed] [Google Scholar]
- 86. Perutz MF, Kendrew JC, and Watson HC. Structure and function of haemoglobin: II. Some relations between polypeptide chain configuration and amino acid sequence. J Mol Biol 13: 669–678, 1965 [Google Scholar]
- 87. Pezacki JP, Ship NJ, and Kluger R. Release of nitric oxide from S-nitrosohemoglobin. Electron transfer as a response to deoxygenation. J Am Chem Soc 123: 4615–4616, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Premont RT, Reynolds JD, Zhang R, and Stamler JS. Role of nitric oxide carried by hemoglobin in cardiovascular physiology: developments on a three-gas respiratory cycle. Circ Res 126: 129–158, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Premont RT and Stamler JS. Essential role of hemoglobin betaCys93 in cardiovascular physiology. Physiology (Bethesda) 35: 234–243, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rai V, Sharma P, Agrawal S, and Agrawal DK. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem 424: 123–145, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rassaf T, Bryan NS, Maloney RE, Specian V, Kelm M, Kalyanaraman B, Rodriguez J, and Feelisch M. NO adducts in mammalian red blood cells: too much or too little? Nat Med 9: 481–482; author reply 48 2–483, 2003 [DOI] [PubMed] [Google Scholar]
- 92. Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiß C, Strauer B-E, Feelisch M, and Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 109: 1241–1248, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Regazzoni L, Panusa A, Yeum K-J, Carini M, and Aldini G. Hemoglobin glutathionylation can occur through cysteine sulfenic acid intermediate: electrospray ionization LTQ-Orbitrap hybrid mass spectrometry studies. J Chromatogr B 877: 3456–3461, 2009 [DOI] [PubMed] [Google Scholar]
- 94. Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, and Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A 104: 17058–17062, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reynolds JD, Bennett KM, Cina AJ, Diesen DL, Henderson MB, Matto F, Plante A, Williamson RA, Zandinejad K, Demchenko IT, Hess DT, Piantadosi CA, and Stamler JS. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci U S A 110: 11529–11534, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reynolds JD, Premont RT, and Stamler JS. Letter by Reynolds et al. Regarding Article, “Hemoglobin beta93 cysteine is not required for export of nitric oxide bioactivity from the red blood cell”. Circulation 140: e758–e759, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Riccio DA, Malowitz JR, Cotten CM, Murtha AP, and McMahon TJ. S-Nitrosylated fetal hemoglobin in neonatal human blood. Biochem Biophys Res Commun 473: 1084–1089, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rochette J, Craig JE, Thein SL, and Rochette J. Fetal hemoglobin levels in adults. Blood Rev 8: 213–224, 1994 [DOI] [PubMed] [Google Scholar]
- 99. Rogers SC, Khalatbari A, Gapper PW, Frenneaux MP, and James PE. Detection of human red blood cell-bound nitric oxide. J Biol Chem 280: 26720–26728, 2005 [DOI] [PubMed] [Google Scholar]
- 100. Ross JM, Fairchild HM, Weldy J, and Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol 202: 21–24, 1962 [DOI] [PubMed] [Google Scholar]
- 101. Saha D, Patgaonkar M, Shroff A, Ayyar K, Bashir T, and Reddy KVR. Hemoglobin expression in nonerythroid cells: novel or ubiquitous? Int J Inflam 2014: 8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Salgado MT, Ramasamy S, Tsuneshige A, Manoharan PT, and Rifkind JM. A New paramagnetic intermediate formed during the reaction of nitrite with deoxyhemoglobin. J Am Chem Soc 133: 13010–13022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sampathkumar R, Balasubramanyam M, Sudarslal S, Rema M, Mohan V, and Balaram P. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem 38: 892–899, 2005 [DOI] [PubMed] [Google Scholar]
- 104. Samsel RW and Schumacker PT. Oxygen delivery to tissues. Eur Respir J 4: 1258, 1991 [PubMed] [Google Scholar]
- 105. Sandmann J, Schwedhelm KS, and Tsikas D. Specific transport of S-nitrosocysteine in human red blood cells: implications for formation of S-nitrosothiols and transport of NO bioactivity within the vasculature. FEBS Lett 579: 4119–4124, 2005 [DOI] [PubMed] [Google Scholar]
- 106. Schmid F, Barrett MJP, Obrist D, Weber B, and Jenny P. Red blood cells stabilize flow in brain microvascular networks. PLoS Comput Biol 15: e1007231, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schmidt H and Feelisch M. Red blood cell-derived nitric oxide bioactivity and hypoxic vasodilation. Circulation 139: 2664–2667, 2019 [DOI] [PubMed] [Google Scholar]
- 108. Schuler D, Sansone R, Freudenberger T, Rodriguez-Mateos A, Weber G, Momma TY, Goy C, Altschmied J, Haendeler J, Fischer JW, Kelm M, and Heiss C. Measurement of endothelium-dependent vasodilation in mice—brief report. Arterioscler Thromb Vasc Biol 34: 2651–2657, 2014 [DOI] [PubMed] [Google Scholar]
- 109. Secomb TW. Theoretical models for regulation of blood flow. Microcirculation 15: 765–775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sellke FW, Myers PR, Bates JN, and Harrison DG. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol 258: H515–H520, 1990 [DOI] [PubMed] [Google Scholar]
- 111. Seth P, Hsieh PN, Jamal S, Wang L, Gygi SP, Jain MK, Coller J, and Stamler JS. Regulation of MicroRNA machinery and development by interspecies S-nitrosylation. Cell 176: 1014–1025.e12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Singel DJ and Stamler JS. Blood traffic control. Nature 430: 297, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Singel DJ and Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005 [DOI] [PubMed] [Google Scholar]
- 114. Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, and Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One 7: e30380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, and Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997 [DOI] [PubMed] [Google Scholar]
- 116. Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, and Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A 89: 444–448, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stamler JS, Singel DJ, and Piantadosi CA. SNO-hemoglobin and hypoxic vasodilation. Nat Med 14: 1008–1009; author reply 100 9–1010, 2008 [DOI] [PubMed] [Google Scholar]
- 118. Stefansson E, Chan YK, Bek T, Hardarson SH, Wong D, and Wilson DI. Laws of physics help explain capillary non-perfusion in diabetic retinopathy. Eye (Lond) 32: 210–212, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stomberski CT, Anand P, Venetos NM, Hausladen A, Zhou HL, Premont RT, and Stamler JS. AKR1A1 is a novel mammalian S-nitroso-glutathione reductase. J Biol Chem 294: 18285–18293, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stomberski CT, Hess DT, and Stamler JS. Protein S-nitrosylation: determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid Redox Signal 30: 1331–1351, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Storz JF, Weber RE, and Fago A. Oxygenation properties and oxidation rates of mouse hemoglobins that differ in reactive cysteine content. Comp Biochem Physiol A Mol Integr Physiol 161: 265–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sun CW, Yang J, Kleschyov A, Zhuge Z, Carlstrom M, Pernow J, Wajih N, Isbell TS, Oh JY, Cabrales P, Tsai A, Townes T, Kim-Shapiro DB, Patel RP, and Lundberg JO. Hemoglobin beta93 cysteine is not required for export of nitric oxide bioactivity from the red blood cell. Circulation 139: 2654–2663, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tyuma I. The Bohr effect and the Haldane effect in human hemoglobin. Jpn J Physiol 34: 205–216, 1984 [DOI] [PubMed] [Google Scholar]
- 125. Vitturi DA, Sun CW, Harper VM, Thrash-Williams B, Cantu-Medellin N, Chacko BK, Peng N, Dai Y, Wyss JM, Townes T, and Patel RP. Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free Radic Biol Med 55: 119–129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wajih N, Basu S, Jailwala A, Kim HW, Ostrowski D, Perlegas A, Bolden CA, Buechler NL, Gladwin MT, Caudell DL, Rahbar E, Alexander-Miller MA, Vachharajani V, and Kim-Shapiro DB. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol 12: 1026–1039, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wajih N, Liu X, Shetty P, Basu S, Wu H, Hogg N, Patel RP, Furdui CM, Kim-and Shapiro DB. The role of red blood cell S-nitrosation in nitrite bioactivation and its modulation by leucine and glucose. Redox Biol 8: 415–421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wan J-J, Qin Z, Wang P-Y, Sun Y, and Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med 49: e384, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, and Feelisch M. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J Biol Chem 281: 26994–27002, 2006 [DOI] [PubMed] [Google Scholar]
- 130. Wolzt M, MacAllister RJ, Davis D, Feelisch M, Moncada S, Vallance P, and Hobbs AJ. Biochemical characterization of S-nitrosohemoglobin. Mechanisms underlying synthesis, no release, and biological activity. J Biol Chem 274: 28983–28990, 1999 [DOI] [PubMed] [Google Scholar]
- 131. Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, and Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 108: 1183–1188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu X, Cho M, Spencer NY, Patel N, Huang Z, Shields H, King SB, Gladwin MT, Hogg N, and Kim-Shapiro DB. Measurements of nitric oxide on the heme iron and beta-93 thiol of human hemoglobin during cycles of oxygenation and deoxygenation. Proc Natl Acad Sci U S A 100: 11303–11308, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yonetani T and Laberge M. Protein dynamics explain the allosteric behaviors of hemoglobin. Biochim Biophys Acta 1784: 1146–1158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, and Stamler JS. Hemoglobin betaCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci U S A 112: 6425–6430, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang R, Hess DT, Reynolds JD, and Stamler JS. Hemoglobin S-nitrosylation plays an essential role in cardioprotection. J Clin Invest 126: 4654–4658, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]