Abstract
This report describes activity in Europe for the years 2016 and 2017 in the area of cellular and tissue-engineered therapies, excluding hematopoietic stem cell treatments for the reconstitution of hematopoiesis. It is the eighth of its kind and is supported by five established scientific organizations. In 2016 and 2017, a combined 234 teams from 29 countries responded to the cellular and engineered tissue therapy survey; 227 teams reported treating 8236 patients in these 2 years. Indications were categorized in hematology/oncology (40%; predominantly prevention or treatment of graft vs. host disease and hematopoietic graft enhancement), musculoskeletal/rheumatological disorders (29%), cardiovascular disorders (6%), neurological disorders (4%), gastrointestinal disorders (<1%), as well as miscellaneous disorders (20%), which were not assigned to the previous indications. The predominantly used cells were autologous (61%). The majority of autologous cells were used to treat musculoskeletal/rheumatological (44%) disorders, whereas allogeneic cells were mainly used for hematology/oncology (78%). The reported cell types were mesenchymal stem/stromal cells (MSCs) (56%), hematopoietic cells (21%), keratinocytes (7%), chondrocytes (6%) dermal fibroblasts (4%), dendritic cells (2%), and other cell types (4%). Cells were expanded in vitro in 62% of the treatments, sorted in 11% of the cases, and rarely transduced (2%). The processing of cells was outsourced to external facilities in 30% of the cases. Cells were delivered predominantly intravenously or intra-arterially [47%], as suspension [36%], or using a membrane/scaffold (16%). The data are compared with those from previous years to identify trends in a rapidly evolving field. In this edition, the report includes a critical discussion of data collected in the space of orthopedics and the use of MSCs.
Impact statement
The surveying of cell and tissue-engineered therapies is a relevant instrument to capture developing trends and monitor advances in the field, based on effective treatments performed.
Keywords: cellular therapy, clinical trial, regenerative medicine, tissue engineering
Introduction
Delivery of cell and tissue-engineered therapies continues to increase steadily, both in terms of the number of patients receiving transplants and the range of diseases treated. The methodology used in these therapies is also progressively becoming more varied and complex, with a growing number of possible tissue sources, methods of cell preparation and expansion, and transplant delivery procedures. Several aspects of the current therapeutic environment for transplant therapy warrant real-world documentation of the interventions being performed in transplant centers.
First, a large proportion of cell and tissue-engineering treatments is currently administered outside the clinical trial or peer-reviewed study setting. In some cases, these interventions have not yet been approved for the particular indication by corresponding regulatory bodies. The published literature, therefore, does not provide an accurate representation of the current trends associated with cell and tissue-engineered therapies. Furthermore, analysis of peer-reviewed studies indicates that patients treated with cells (stem or otherwise) from the same tissue source may not be receiving the same treatment across centers. This is due to center-specific differences in cell product definition criteria, as well as wide variability in cell/tissue production techniques.
Given this present therapeutic landscape, it is imperative to provide transparent reporting of a representative cross-section of all procedures performed across transplant centers to document treatment patterns and the methodological details of delivered interventions. These data will not only provide documentation of the prevalence of specific procedures relative to supporting data and approval from regulatory bodies but also will help direct and prioritize future research.
Since its first appearance in 2008, the aim of this survey has been to report an unbiased update on the voluntarily provided number of patients treated using cellular and tissue-engineered therapies in Europe and Eurasian countries associated with the European Group for Blood and Marrow Transplantation (EBMT).1–7 The International Society for Cellular Therapy (ISCT), of the European Chapter of the Tissue Engineering and Regenerative Medicine International Society (TERMIS-EU), of the International Federation for Adipose Therapeutics (IFATS), of the International Cartilage Repair Society (ICRS), and the EBMT have made this possible by their continued support.
The survey comprises data of treated patients sorted by specific therapeutic indications, cell/tissue donor types, together with the processing and delivery modes, without reference to the clinical outcome and thus avoiding any infringement of the publication rights for the clinical teams themselves.
Data generated in the surveys of previous years have already been published, along with assessments on the development of treatment numbers and modalities of cell delivery for the target indications.1–7 The combined treatments for 2016 and 2017, as well as distinguished recent trends, are reported in this article as determined by the data provided from the 9th and 10th activity survey, with a description of some recent trends. In addition to presentation of the collected data, the report includes an in-depth analysis of cell therapy development for musculoskeletal/rheumatological indications and discusses the importance of transparency in the processing of cells as well as their clinical use.
Patients and Methods
Definitions
For the purpose of this survey, cellular and tissue-engineered therapy is any clinical treatment based on living cells, excluding donor lymphocyte infusions (DLIs) and nonmanipulated hematopoietic cells, for hematological reconstitution. Data regarding DLIs and nonmanipulated hematopoietic cells for hematological reconstitution are collected and reported independently by the EBMT.8,9
Data collection and validation
Participating teams were, as in previous years, requested to report their data for 2016 and 2017 by indication, cell type and source, donor type, processing method, and delivery mode. In addition, for these two years, the survey was extended to include the type of clinical procedure and the site of processing. The survey followed the traditional principles of the EBMT transplant activity survey, which concentrates on numbers of patients with a first cellular therapy.
For the 2016 and 2017 survey, >500 teams known to be actively transplanting in 49 countries (39 European and 10 EBMT-affiliated countries) were contacted. The non-European countries affiliated with the EBMT activity survey are Algeria, Azerbaijan, Iran, Israel, Jordan, Lebanon, Nigeria, Saudi Arabia, South Africa, and Tunisia.
Aside from past contributors to the survey, newly identified teams were contacted and invited to report their data. These teams were identified either through their contribution to published clinical trials or their reports on the platform https://clinicaltrials.gov (using the search terms “Tissue-engineer” and “Cell” associated with either “Transplant” or “Treatment” in the relevant countries). In addition, the supporting societies distributed the survey directly to their members in Europe by email and/or published the survey and documents on their websites. Extended questionnaires (Supplementary Table S1), were received electronically.
Treatment rates
Treatment rates, defined as the reported numbers of patients receiving cellular or tissue-engineered therapies and the number of teams reporting treatments per 10 million inhabitants, were computed for each country, without adjustments for patients who crossed borders or received treatment in a foreign country. Population numbers were obtained from the 2016 and 2017 Eurostat database (ec.europa.eu/eurostat) and then averaged for the 2 years.
Results
This report discloses the data of the surveys for the years 2016 and 2017 as a sum of both years, unless otherwise specified. For the purpose of comparison, previous data are also displayed in graphs as the sum for two adjacent years (2010/2011, 2012/2013, and 2014/2015).1–5
Participating teams and transplant rates
A total of 234 teams from 25 European countries and 4 EBMT-affiliated countries (i.e., Azerbaijan, Iran, Israel, and Saudia Arabia) responded to either or both the 2016 and 2017 survey editions. A total of 227 teams from these countries reported patient treatments by cellular or tissue-engineered therapies. Details of the indication, source and type of cells used, donor origin, processing, and delivery mode were provided by all the teams. No activity was reported by seven teams in either year. The Appendix A1 lists in alphabetical order of country, all the teams who reported their activity, in addition to the total number of treatments and the split between allogeneic and autologous donors. Group information about EBMT members was anonymized without the groups' consent for disclosure; otherwise, consenting EBMT groups are marked with EBMT CIC code.
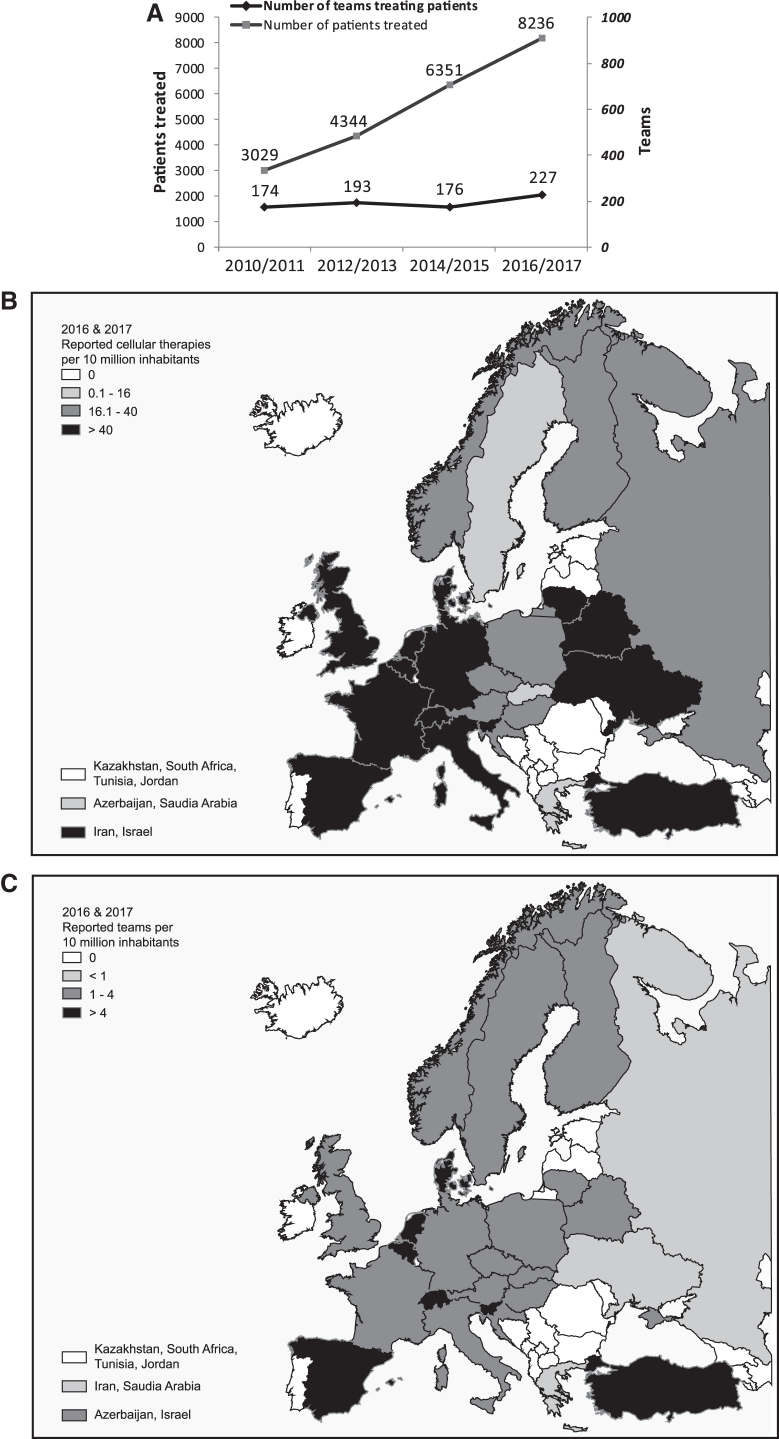
The reported number of treated patients was higher in the 2016 and 2017 survey editions compared to previous years. Also, the number of reporting teams was increased in 2016/2017, after the previous decrease in 2014/2015. Over the last 8 years, the number of reporting teams thus increased by 30.4% (from 174 in 2010/2011), and the number of treated patients increased by 171.9% (from 3029 in 2010/2011) (Fig. 1A).
FIG. 1.
(A) Number of reporting teams and patients treated using cell and tissue-engineered therapies in two consecutive years from 2010 to 2017. Data used for this chart were derived from this study and previous reports (1–5). Number of cell and tissue-engineered therapies (B), and teams (C) per 10 million inhabitants reported in Europe for the combined years of 2016 and 2017.
Figure 1B displays the average of transplants reported for 2016 and 2017 per 10 million inhabitants in the investigated countries. The highest transplant rates (i.e., >40 per 10 million population) were reported in (in decreasing order) Slovenia, Denmark, Spain, Turkey, Belgium, France, Iran, Italy, Lithuania, the Netherlands, Switzerland, Belarus, Israel, Germany, United Kingdom, and Ukraine.
Figure 1C displays the number of reporting teams in the investigated countries, normalized to the inhabitant numbers (Fig. 1C). The number of reporting teams per 10 million inhabitants, which was higher than 4 (in decreasing order), were in Slovenia, Switzerland, Denmark, Spain, Belgium, and the Netherlands. Data reported by the top 10 countries accounted for 88% of all treated patients.
Number of cellular or tissue-engineered therapies, disease indications, and donor type
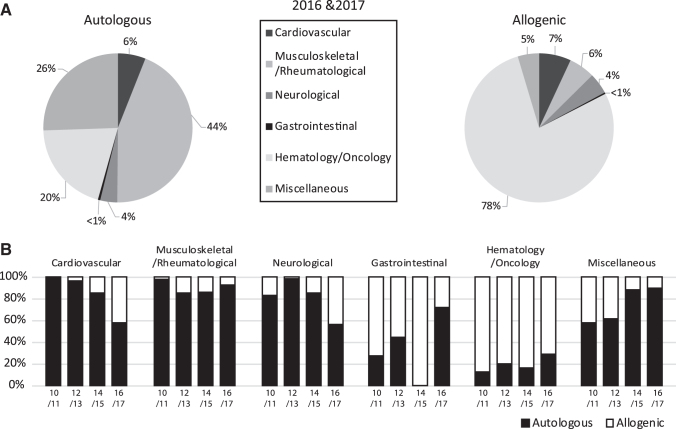
Eight thousand two hundred thirty-six patients were reported to have been treated with cellular or tissue-engineered therapies in 2016 (4501) and 2017 (3735). Of these patients, 5033 (61%) were treated with autologous and 3203 (39%) with allogeneic cells (Table 1). Out of all treatments, musculoskeletal/rheumatological disorders (44%) were the most representative indications using autologous cells, followed by miscellaneous (26%) and hematology/oncology (20%) indications. Therapies within hematology/oncology predominantly used allogeneic cells (78%) (Fig. 2A).
Table 1.
Number of Reported Cell and Tissue-Engineered Therapy Treatments in Europe in 2017 and [2016] Sorted by Indication, Cell Source, and Donor Type
| Cell type and source | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication | Autologous |
Allogeneic |
|
||||||||||||||
| HPC | MSC | Chondrocyte | Keratinocyte | Dendritic cell | Dermal fibroblast | Other | HPC | MSC | Chondrocyte | Keratinocyte | Dendritic cell | Dermal fibroblast | Other | Autologous | Allogeneic | Total | |
| Cardiovascular | |||||||||||||||||
| Peripheral artery disease | 6 [7] | 0 [6] | 6 [13] | 6 [13] | |||||||||||||
| Cardiomyopathy | 0 [14] | 4 [2] | 4 [16] | 4 [16] | |||||||||||||
| Heart failure | 4 [26] | 10 [11] | 0 [1] | 0 [75] | 1 [3] | 14 [38] | 1 [78] | 15 [116] | |||||||||
| Myocardial ischemia | 2 [2] | 7 [0] | 54 [0] | 9 [2] | 54 [0] | 63 [2] | |||||||||||
| Decubitus and leg ulcers | 10 [79] | 10 [79] | 10 [79] | ||||||||||||||
| Other | 2 [0] | 3 [196] | 5 [196] | 5 [196] | |||||||||||||
| Musculoskeletal/rheumatological | |||||||||||||||||
| Bone repair (maxillofacial) | 1 [7] | 0 [4] | 0 [2] | 1 [11] | 0 [2] | 1 [13] | |||||||||||
| Bone repair (orthopedics) | 6 [10] | 9 [58] | 15 [68] | 15 [68] | |||||||||||||
| Osteogenesis imperfecta | 2 [0] | 2 [0] | 2 [0] | ||||||||||||||
| Cartilage repair (maxillofacial) | 0 [3] | 0 [10] | 0 [10] | 0 [2] | 0 [23] | 0 [12] | 0 [35] | ||||||||||
| Cartilage repair (orthopedics) | 843 [43] | 217 [225] | 52 [0] | 0 [6] | 1060 [268] | 52 [6] | 1112 [274] | ||||||||||
| Muscle repair | 12 [0] | 0 [29] | 12 [0] | 0 [29] | 12 [29] | ||||||||||||
| Tendon/ligament | 0 [13] | 36 [4] | 0 [18] | 0 [2] | 36 [35] | 0 [2] | 36 [37] | ||||||||||
| Reconstructive surgery/tissue enhancement | 11 [19] | 0 [1] | 0 [1] | 0 [27] | 11 [21] | 0 [27] | 11 [48] | ||||||||||
| Scleroderma | 1 [1] | 1 [0] | 6 [6] | 2 [1] | 6 [6] | 8 [7] | |||||||||||
| Arthritis | 0 [50] | 10 [485] | 12 [0] | 0 [14] | 22 [535] | 0 [14] | 22 [549] | ||||||||||
| Other | 0 [6] | 28 [40] | 2 [14] | 0 [10] | 2 [21] | 30 [70] | 2 [21] | 32 [91] | |||||||||
| Neurological | |||||||||||||||||
| Multiple sclerosis | 13 [9] | 5 [35] | 0 [4] | 26 [15] | 18 [48] | 26 [15] | 44 [63] | ||||||||||
| Amyotrophic lateral sclerosis | 28 [10] | 6 [10] | 18 [0] | 28 [10] | 24 [10] | 52 [20] | |||||||||||
| Parkinson's | 0 [1] | 0 [1] | 0 [1] | ||||||||||||||
| Peripheral nerve regeneration (trauma) | 0 [1] | 0 [8] | 0 [9] | 0 [9] | |||||||||||||
| Other | 10 [45] | 0 [17] | 69 [2] | 10 [62] | 69 [2] | 79 [64] | |||||||||||
| Gastrointestinal | |||||||||||||||||
| Crohn's disease | 0 [17] | 0 [17] | 0 [17] | ||||||||||||||
| Liver insufficiency | 0 [8] | 2 [8] | 0 [8] | 2 [8] | 2 [16] | ||||||||||||
| Hematology/oncology | |||||||||||||||||
| Hematological malignancies | 367 [284] | 37 [59] | 4 [3] | 8 [58] | 565 [169] | 669 [923] | 11 [2] | 57 [86] | 416 [404] | 1302 [1180] | 1718 [1584] | ||||||
| Solid tumor | 22 [31] | 12 [0] | 42 [86] | 4 [0] | 1 [0] | 76 [117] | 14 [0] | 90 [117] | |||||||||
| Miscellaneous | |||||||||||||||||
| Skin reconstruction—burns | 1 [325] | 7 [3] | 0 [1] | 0 [5] | 36 [0] | 8 [329] | 36 [5] | 44 [334] | |||||||||
| Cornea repair | 8 [18] | 3 [2] | 8 [0] | 8 [18] | 11 [2] | 19 [20] | |||||||||||
| Diabetes | 9 [3] | 2 [0] | 0 [8] | 10 [3] | 11 [3] | 10 [11] | 21 [14] | ||||||||||
| Other | 7 [4] | 4 [37] | 234 [273] | 1 [0] | 51 [291] | 0 [6] | 7 [3] | 8 [50] | 0 [5] | 297 [611] | 15 [58] | 312 [669] | |||||
| Total | 440 [507] | 1071 [1375] | 231 [250] | 241 [276] | 47 [89] | 51 [293] | 18 [144] | 576 [182] | 906 [1276] | 0 [6] | 39 [2] | 21 [2] | 0 [5] | 94 [94] | 2099 [2934] | 1636 [1567] | 3735 [4501] |
HPC, hematopoietic progenitor cells; MSC, mesenchymal stromal/stem cells.
FIG. 2.
(A) Percentage of indications for cell and engineered tissue therapies in Europe for the combined years of 2016 and 2017, sorted by donor type. (B) Comparative analysis of indications for cell and tissue-engineered therapies in Europe in two consecutive years from 2010 to 2017, sorted by donor type. Data used for this chart were derived from this study, and five previous reports (1–5).
Reported indications were hematology/oncology (predominantly prevention or treatment of Graft vs. Host Disease [GvHD], and hematopoietic graft enhancement) (40% of all reported patients; 25% of these treatments were based on autologous cells), musculoskeletal/rheumatological disorders (29%; 92% autologous), cardiovascular disorders (6%; 58% autologous), neurological disorders (4%; 56% autologous), gastrointestinal disorders (<1%; 71% autologous), and miscellaneous indications (20%; 90% autologous) (Fig. 2B). Subtype indications classified as “other” with corresponding patient numbers can be found in Supplementary Table S2.
The use of allogeneic cells clearly increased for the treatment of cardiovascular and neurological indications, compared to the previous double years. Instead, a larger number of patients were treated with autologous cells for the miscellaneous indications, as well as gastrointestinal indications, despite a strong deviation for the years 2014/2015. For musculoskeletal/rheumatological and hematology/oncology indications, the percentages were relatively stable over the years (Fig. 2B).
Cell type and source
In descending order of frequency, used cell types were mesenchymal stem/stromal cells (MSC) (56%), hematopoietic stem and progenitors (HPC) (21%), keratinocytes (7%), chondrocytes (6%), dermal fibroblasts (4%), dendritic cells (2%), and others (4%) (Table 1). Source of MSCs was bone marrow (55%), fat tissue (20%), placenta/amniotic membrane (12%), cord blood (10%), and others (3%), which included Wharton's jelly and endometrium (Supplementary Fig. S1A). Furthermore, 68% of autologous MSCs were used for musculoskeletal/rheumatological indications and 73% of allogenic MSCs for hematological/oncological malignancies. The source of HPC was mainly confined to peripheral blood (82%), followed by bone marrow (13%), cord blood (< 1%), and other sources (5%), which were not explicitly declared (Supplementary Fig. S1B).
The types of “other” cells in the received forms are reported as cardiac stem cells, cardiovascular progenitors, endothelial cells, gingival fibroblasts, limbal epithelial stem cells, melanocytes, muscle cells, neural crest stem/progenitor cells, embryonic neural stem cells, and pancreatic islet-derived cells. Indications and patient numbers treated with the “other” cells can be found in “A” in Supplementary Table S3. Future surveys will be adapted accordingly if specific “other” cell sources are regularly reported over the years.
In some instances, patients were treated with more than one cell source. These patients were categorized in the survey under the provided primary cell source. Combined cell types were MSCs with chondrocytes, muscle cells, or osteoprogenitor and endothelial cells. In other instances, melanocytes, fibroblasts (autologous as well as allogenic), or limbal epithelial stem cells were additional cell sources in combination with keratinocytes (“B” in Supplementary Table S3).
Cell processing
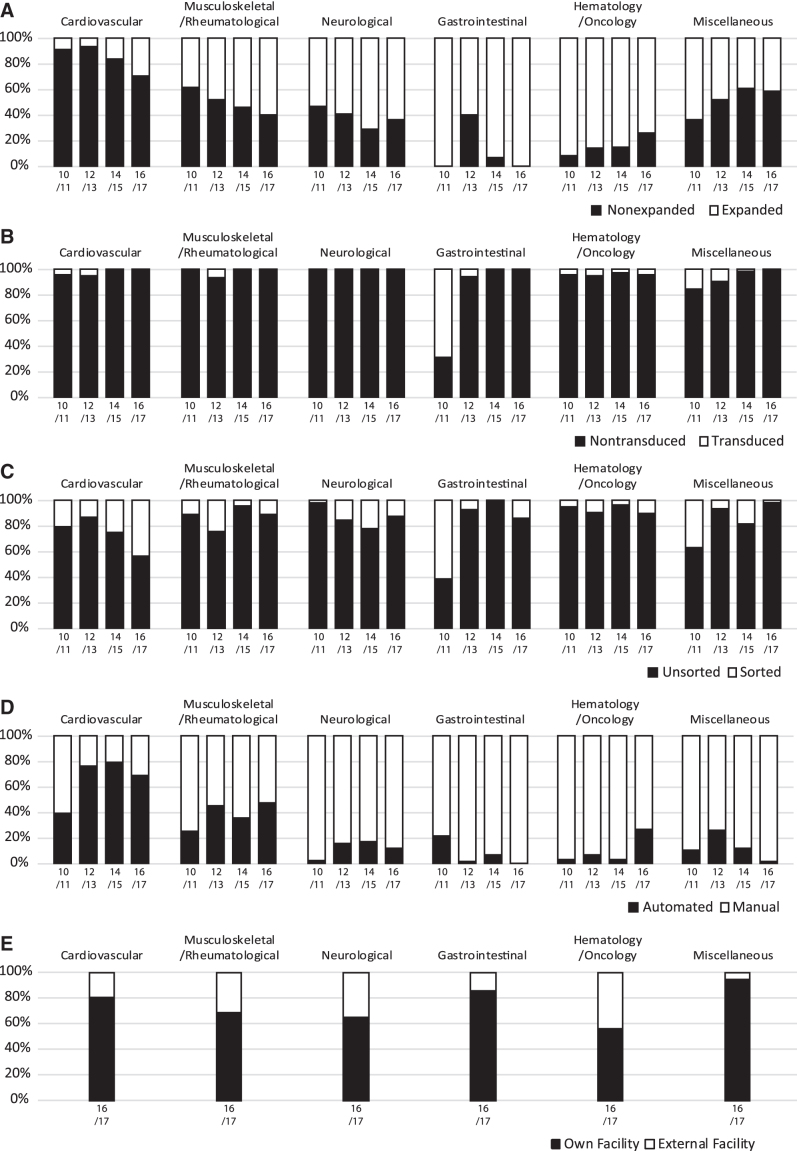
Cells were expanded in 62% of the overall reported treatments (Supplementary Table S4). When comparing with previous years, cells were more frequently expanded for the treatment of cardiovascular, musculoskeletal/rheumatological, and gastrointestinal indications. Conversely, the usage of expanded cells decreased for hematology/oncology and miscellaneous indications (Fig. 3A).
FIG. 3.
Comparative analysis of indications for cell and tissue-engineered therapies in Europe for the combined years of 2016 and 2017, sorted by mode of processing (A) expansion, (B) transduction, (C) sorting, (D) process handling, and (E) facility of processing. Data used for this chart were derived from this study, and five previous reports (1–5).
Most treatments were based on nontransduced (98%) and unsorted (89%) cells (Supplementary Table S4). Although transduced cells were employed for various indications between 2010–2013, they have been reduced to only being used for hematology/oncology indications in following years (Fig. 3B). On the other hand, the sorting of cells has been a part of cell processing in a subset of all indication categories. Cell sorting for cardiovascular indications appears to be establishing itself as it has steadily increased over the last 6 years. However, no other apparent trend could be determined, as the percentage of patients treated with sorted cells fluctuated over the years in the other indication groups (Fig. 3C).
Cells were manually processed for 89% of the treatments. The majority of therapies with automated cell processing are found for cardiovascular (373 patients), musculoskeletal/rheumatological (1143 patients), and hematology/oncology (928 patients) indications (Supplementary Table S4). In the instance of cardiovascular and musculoskeletal indications, the percentages of the patients who were treated with automated processed cells in the last 6 years were higher than 60% and 35%, respectively. Otherwise, <30% of the remaining indications were treated with manually processed cells (Fig. 3D).
As the sector of cellular and tissue-engineered therapies evolves and prospers, it is also becoming commercialized. There are >230 companies providing services for regenerative medicine and advanced therapies in Europe and Israel.10 As we consider this outsourcing of cell processing an important development in the field, we have expanded the survey to include whether cells were processed in the team's own facility or an external facility. External facilities performed 30% of the cell processing for the combined years 2016/2017 (Supplementary Table S4). The processing of cells for musculoskeletal/rheumatological and hematology/oncology indications were the primary contributors, as they not only have the most patients (2402 and 2509, respectively) but also have the highest percentage of external facility processing (31%, and 43%, respectively) (Fig. 3E). As this is the first time that we have followed up on the production facility, neither trends nor predictions could be determined.
Delivery mode
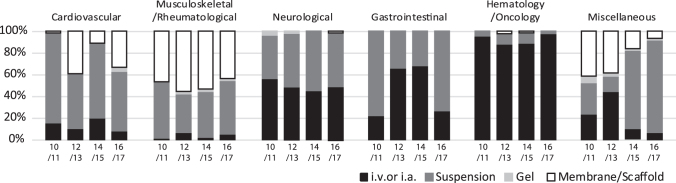
In 2016/2017, 47% of the treatments were based on intravenous (i.v.) or intra-arterial (i.a.) delivery. The remainder 53%, delivered intraorgan, were based on injection of cell suspension (36%), use of a membrane/scaffold (16%), and use of a gel (<1%) (Supplementary Table S5).
Therapies for cardiovascular, musculoskeletal/rheumatological, and miscellaneous indications used all four modes of delivery, whereas those for neurological, gastrointestinal, and hematology/oncology indications administered cells either by i.v./i.a. or in suspension intraorgan (Fig. 4). Interestingly, in the last 4 years, the intraorgan injection of cell suspensions has become the dominant means of cell delivery for the miscellaneous indications. When comparing with the previous years, indications for musculoskeletal/rheumatological, neurological, and hematology/oncology have maintained similar percentages of the different modalities of cell delivery. In contrast, cardiovascular indications have been treated with cells delivered in variable ways and—since 2016/2017 for the first time—also in combination with a gel (Fig. 4).
FIG. 4.
Comparative analysis of indications for cell and tissue-engineered therapies in Europe for the combined years of 2016 and 2017, sorted by delivery mode. Data used for this chart were derived from this study, and five previous reports (1–5). i.v. or i.a., intravenous or intra-arterial.
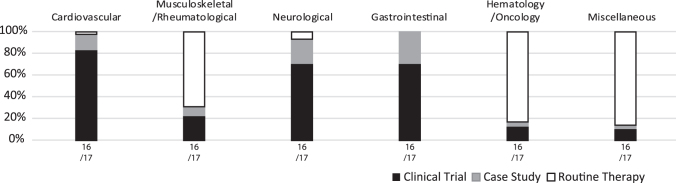
Clinical procedure
As cellular and tissue-engineered therapies advance, they may move from single case studies and clinical trials to routine therapies. For the surveys of 2016 and 2017, a query to the practitioners was included about the context in which the therapies were offered to the patients. In 2016/2017, cellular and tissue-engineered therapies for cardiovascular, neurological, and gastrointestinal indications were predominantly proposed as clinical trials and case studies. Nonetheless, few procedures for cardiovascular (peripheral artery disease) and neurological (multiple sclerosis and stiff person syndrome) indications were reported as routine therapies.
Surprisingly, the majority of musculoskeletal/rheumatological (orthopedic cartilage repair, arthritis, intervertebral disc degeneration, tendon/ligament, bone repair, scleroderma, periodontal tissue repair, pump syndrome, and femoroacetabular impingement, fibromyalgia, as well as reconstructive surgery/tissue enhancement), hematology/oncology (GvHD and HSC graft enhancement), and miscellaneous (adrenoleukodystrophy, autoimmune disease, bone marrow aplasia, Epstein–Barr virus, Histiocytosis X, infection, necrobiotic xanthogranuloma, primary immune deficiency, relapsing cytomegalovirus reactivation, sickle cell disease, vasculitis, and skin—aging, facial deformities, and vitiligo) indications were reported as routine therapy offers (Fig. 5 and Supplementary Table S6).
FIG. 5.
Comparative analysis of indications for cell and tissue-engineered therapies in Europe for the combined years of 2016 and 2017, sorted by clinical procedure.
Detailed analysis of treatments for musculoskeletal/rheumatological disorders
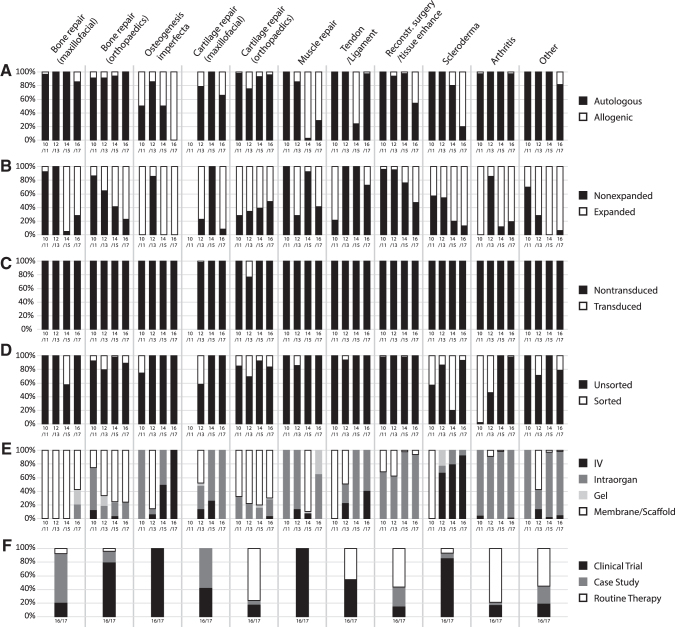
This edition of the survey reports more detailed data in the specific context of musculoskeletal/rheumatological disorders. The most commonly used cell sources for these applications were autologous cells (92%), predominantly derived from either bone marrow aspirate or fat tissue. Allogenic cells were used in >20% of the treatments for osteogenesis imperfecta, maxillofacial cartilage or muscle repair, reconstructive surgery and tissue enhancement, or scleroderma (Fig. 6A).
FIG. 6.
Comparative analysis for cell and tissue-engineered therapies in Europe of musculoskeletal/rheumatological subindications from 2010 to 2017 for two consecutive years, sorted by mode of processing (A) donor type, (B) expansion, (C) transduction, (D) Sorting, (E) delivery mode, and (F) clinical procedure. Data used for this chart were derived from this study and five previous reports (1–5).
Cells were expanded in vitro in some applications, which would likely lead to a higher proportion of progenitor cells. This report documents an extensive use (>50%) of expanded cells for the treatment of bone, cartilage or muscle repair, osteogenesis imperfecta, arthritis, reconstructive surgery and tissue enhancement, and scleroderma, as well as the other indications not explicitly listed (Fig. 6B).
Transduction of cells was only reported in rare cases for cartilage repair in the last 8 years (Fig. 6C). Sorting for a specific population was only performed in ∼10% of orthopedic bone and cartilage repair, as well as the other categories (Fig. 6D). However, even in these applications using expanded cells, cell sorting to isolate and concentrate progenitor/stem cells was rarely used.
The vast majority of cell therapy applications for orthopedic indications involved either local injection or direct cell implantation at the time of surgery, with systemic administration used uncommonly in most indications, with the exception of osteogenesis imperfecta and scleroderma (Fig. 6E). Local cell delivery was sometimes done with a “carrier vehicle,” such as a collagen membrane scaffold or injectable materials (gels), mainly used in bone repair, orthopedic cartilage repair, and muscle repair (Fig. 6E). These carrier vehicles may well affect cell behavior, so further studies are required to understand the effect of such materials on different cell types. Based on cell- and material-specific findings, the manufactured grafts could be classified from a regulatory standpoint as possible “combination products.”
Interestingly, >40% of treatments in the areas of cartilage repair, tendon/ligament, reconstructive surgery, arthritis, and other were offered as a routine therapy (Fig. 6F).
Discussion
Since 2010, the collected data have continuously increased with regard to both participating teams and patient numbers. As more clinical groups and treatments are collected, the report may provide useful data in capturing trends and innovations in the field. In this report, data of two consecutive year surveys were summed together to allow a more compact presentation, as well as to try and make trends and predictions more apparent, by reducing the impact of transient fluctuations.
Cell-based and tissue-engineered therapies for applications in the areas listed in this survey hold a great deal of promise, but these procedures are not without risk.11,12 As noted in this survey, the majority of procedures utilized autologous cells, but the use of allogeneic cells appears to be increasing in certain areas (Fig. 2B), despite the fact that they present the risk of transmission of disease from a donor to a recipient. However, this change seems to reflect the perception that use of autologous cells is often not an achievable business model in comparison with allogeneic cells, which could be “used off the shelf.”13,14
In a perfect world, it would be most expeditious to be able to isolate autologous cells from an appropriate tissue source for a specific treatment based on their cell surface phenotype or other sentinel marker of cell identity or function, and to use these cells directly without ex vivo manipulation. However, cell products for tissue regeneration other than for bone marrow transplantation generally require ex vivo expansion due to the rarity of stem/progenitor cells within a given tissue, and the need for large numbers of cells to regenerate 2D and 3D tissues.
It is well known that ex vivo manipulation can change the character of a given cell population, and maintaining their initial biological activity is imperative. Furthermore, ex vivo expansion and prolonged periods of tissue construction provide the opportunity for microbial and viral contamination of the cell product or tissue, or even unintended expansion of unwanted cell types.12 Beyond the risks of cell processing and tissue construction, how a cell population or a reconstructed tissue is to be delivered is a major consideration, given the importance of cell and tissue interactions with a potential carrier. Furthermore, the in vivo environment, can be very hostile and may not contain the necessary nutritional and cell-signaling factors required for optimal cell differentiation and function. Biocompatibility and level of toxicity have a high impact on potential success.
Following are some remarks related to specific findings in the area of musculoskeletal and rheumatological treatments, as a starting point for more general observations. Most musculoskeletal tissues, including cartilage, ligament, tendon, meniscus, muscle, and even bone, in case of certain critical conditions, have limited healing potential. Cell therapy and tissue engineering approaches, therefore, hold great promise for the treatment of various musculoskeletal tissues, as evidenced by the finding that 29% of the indications in this survey were for musculoskeletal/rheumatological disorders.
The most commonly used cell sources for these applications were of autologous origin, delivered without being transduced or sorted, and thus bypassing several regulatory challenges associated with extensive manipulation. Interestingly, for bone repair, there was a clear trend over time toward using expanded cells (Fig. 6B), possibly due to the recognition that the amount of tissue-resident cells available intraoperatively (e.g., within bone marrow or fat tissue) may not be sufficient to enhance processes of compromised bone regeneration. Conversely, the use of nonexpanded cells has gained popularity for cartilage repair, likely associated with the trend of combining intraoperatively available chondrocytes with an accessory fraction of stromal cells.15
The combination of the perceived safety of autologous cells, the inherently poor healing capacity of various musculoskeletal tissues, and the current lack of effective therapies for treatment of difficult clinical problems such as osteoarthritis, chronic tendinopathy, and meniscus injury has provided the “perfect storm” leading to the often indiscriminate use of unproven cellular therapies.
It is notable that the majority of treatments (69%) reported for musculoskeletal/rheumatological disorders and diseases were offered outside the context of a clinical study and considered to constitute of “routine” clinical care. This was particularly dominant in cartilage repair, reconstructive surgery and tissue enhancement, arthritis, and the other group (Fig. 6F). While these treatments were performed within the context of legitimate treatment centers, it has to be highlighted that in none of these areas, we can find scientific evidences of verified clinical effectiveness, which would justify their broad adoption outside the framework of clinical study cohorts.
More generally, the use of cell therapies without proper scientific rationale, reasonable preclinical data, and approval by appropriate regulatory bodies has contributed to the explosion of unauthorized clinics around the world, including in Europe. It is now recognized by many that such clinics are not providing treatments approved by regulatory bodies and present real risks to unsuspecting patients who pay considerable sums of money for such unauthorized treatments.
There are several examples in the United States, where patients were treated directly with poorly characterized fat aspirates and developed blindness.16 In another report, at least a dozen patients received contaminated umbilical cord “stem cells” for treatment of a variety of diseases and disorders, leading to their hospitalization, some for extended periods of time,17 and it is very likely that such adverse effects have occurred worldwide.
In conclusion, it is clear that cell therapy holds tremendous potential for healing and regeneration, but realizing this potential will require carefully planned clinical trials. Progress in this field will only be achieved by the initiation of multicenter studies, possibly employing clinical registries and biospecimen repositories, which would allow the ability to correlate the composition and biologic activity of the cell formulation with the clinical effectiveness. Rigorous basic and preclinical studies, along with well-controlled and well-designed clinical trials that measure appropriate outcomes, will pave the way to making cell-based therapy and tissue engineering an effective clinical reality. In this perspective, the here described survey represents a relevant instrument to gain transparency and capture trends in a variety of diverse clinical areas.
Supplementary Material
Acknowledgments
We would like to acknowledge contribution by all teams participating in the survey, and their staff (listed in the Appendix A1). We are grateful to the Orthoregeneration Network (ON) for offering visibility to the program through their website (www.on-foundation.org). We thank Silvia Lopa for her assistance in the head hunting of treating groups.
Appendix A1
Appendix A1. List of Centers Reporting the Use of Cellular and Engineered Tissue Therapy in Europe in 2016 and 2017
Group information about EBMT members was anonymized; otherwise, consenting EBMT groups are marked with EBMT CIC code.
EBMT data for teams treating GvHD or HSC enhancement in 2016 and 2017 are not explicitly named in the appendix without the groups' consent for disclosure provided by the EBMT. Consenting EBMT groups are marked with EBMT CIC code. The anonymized EBMT group data are provided as an overview of participating teams and their combined activity per country. Contributing EBMT teams are listed in the appendix of the Bone Marrow Transplant surveys (8, 9).
Format: City, Hospital, Department, Center Identification Code (CIC—as used for EBMT teams in the EBMT standard survey); Physicians 2016, (Total treatments: allogeneic/autologous in 2016); Physicians 2017, [Total treatments: allogeneic/autologous in 2017]
-
I
f group is in italic, the group reported not performing therapies in 2016 and 2010
Austria
Krems, University Hospital Krems, Orthopedic Department; I. Hackl, T. Luksch, S. Nehrer, (0/6); I. Hackl, T. Luksch, S. Nehrer, [0/9]
Salzburg, Paracelsus Medical University of Salzburg, Spinal Cord Injury and Tissue Regeneration Center Salzburg, (SCI-TReCS); K.Schallmoser, E. Rohde, M. Gimona, D. Strunk, (0/2)
One EBMT team, (3/0); [2/0]
Azerbaijan
One EBMT team, [1/0]
Belarus
Two EBMT teams, (27/0); [48]
Beligium
Brussel, Queen Astrid Military Hospital, Burn Wound Centre; G. Verbeken, J.P. Pirnay, N. Delmotte, [36/0]
Edegem, University Hospital Antwerp, Center for Cellular Therapy and Regenerative Medicine; N. Zakaria, M.J. Tassignon, S. Ní Dhubhghaill, C. Koppen, (0/7); J. Behaegel, B. Ballet, S. Ní Dhubhghaill, C. Koppen, [3/2]
Edegem, University Hospital Antwerp, Department of Hematology, CIC 996; A. Devos, Z. Berneman, W. Schroyens, (0/25); Z.Berneman, W. Schroyens, A. Van de Velde, S. Anguille, [46/46]
Liege, University of Liege, Department of Hematology; Y. Beguin, C. Lechanteur, M. Pereira, L. Weekers, (9/0); Y. Beguin, C. Lechanteur, M. Pereira, O. Detry, [5/0]
Three groups 2016, (8/36); Two groups 2017, [4/0]
Croatia
Zagreb, University Hospital Sveti Duh, Department of Orthopeadic Surgery; A. Ivković, F. Vuletić, D. Hudetz, S. Janković, [0/7]
Czech Republic
Four EBMT teams, (11/0); [28/0]
Denmark
Herlev, Copenhagen University Hospital, Center for Cancer Immune Therapy; I. M. Svane, O. Met, (0/11); I. M. Svane, O. Met, [0/8]
Copenhagen, Rigshospitalet University of Copenhagen, Cardiac Stem Cell Centre, The Heart Centre; J. Kastrup, (75/2); J. Kastrup, [54/0]
Copenhagen, Rigshospitalet University of Copenhagen, Cell Therapy Facility, Department of Clinical Immunology; A. Fischer-Nielsen, E. Haastrup, L. M. Fog, (0/17); A. Fischer-Nielsen, E. Haastrup, L. M. Fog, [0/3]
Odense, Odense University Hospital, Department of Urology; M. Haahr, (0/0); [0/0]
Finland
Two EBMT teams 2016, (9/0); Three EBMT teams 2017, [12/0]
France
Grenoble, CHU Grenoble Alpes, Unité Neuro-Vasculaire, Pôle Psychiatrie-Neurologie-Réducation; O. Detante, (0/0); [0/0]
Lille, CHU LILLE Hôpital Claude Huriez; M.C. Vantyghem, K. le Mapihan, G. Lavergne, [7/2]
Marseille, Clinique Vert Coteau, Arthrosport Center; M. Assor, [0/570]
Nantes, CHU Nantes, Unité de thérapie cellulaire et génique; B. Dreno, S. Saiagh, M. Benjelloun-Zahar, F. Legrand, (0/11)
Paris, Hôpital Saint Louis, service de médecine Interne, Paris, France, CIC 461; D. Farge, A. Cras, D. Michonneau, P. Lansiaux, (4/0); D. Farge, A. Cras, D. Michonneau, M. Chaouat, [3/1]
Paris, Saint-Louis Hospital, (Hôpitaux Universitaires Saint-Louis, Laboisière, Fernand-Widal) Cell Therapy Unit; J. Larghero, (8/0); J. Larghero, [202/203]
Strasbourg, Hôpital de Hautepierre—service d'Onco-Hématologie Pédiatrique, CIC 672; C. Paillard, O. Rick, [9/9]
Toulouse, Hopital de Rangueil CHU Toulouse; J. Roncalli, [0/13]
Five EBMT teams 2016, (9/1); Three EBMT teams 2017, [5/0]
Germany
Darmstadt, Akademisches Lehrkrankenhaus, Agaplesion Elisabethenstift gemeinnützige GmbH; Th. Schreyer, M. Schneider, (0/16); Th. Schreyer, M. Schneider, [0/18]
Dinslaken, St. Vinzenz-Hospital; W. Zinser, K. Szember, (0/76); W. Zinser, K. Szember, [0/89]
Dresden, University Hospital Dresden; M. Bornhäuser, J. Schetelig, C.Theuser, (20/0)
Halle, BG Klinikum Bergmannstrost Halle, Halle, Germany; H.J. Meisel, Y. Minkus, (0/12); H.J. Meisel, Y. Minkus, [0/11]
Hannover, Hannover Medical School, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, CIC 295; A. Ganser, M. Eder, G. Beutel, M. Stadler, [3/0]
Kiel, University Medical Center Scheswig-Holstein, Campus Kiel Department of Pediatrics, Kiel, Germany, CIC 256:2; M. Helweg, M. Schrappe, A. Claviez, G. Cario, D. Schewe, (1/0)
Homburg, Universität des Saarlandes, Zentrum für Experimentelle, Orthopädie Universitätsklinikum des Saarlandes, Homburg, Germany; H. Madry, (0/7)
Tübingen, Klinik für Kinder- und Jugendmedizin Tübingen, Stammzelllabor der Kinderklinik und Medizinischen Klinik und des Immunologischen Labors der Kinderklinik Abteilung für Allgemeine Pädiatrie, Hämatologie/Onkologie, CIC 535; B. Walter, P. Lang, R. Fischer, R. Handgretinger, J. Böhringer, [5/2]
Tübingen, Medizinische Universitätsklinik, Departement of Onkologie, Hämatologie, Rheumatologie, and Immunologie; J. Henes, W. Vogel, S. Wirths (0/1)
Twentythree EBMT teams 2016, (94/18); Twentytwo EBMT teams 2017, [83/8]
Greece
Two EBMT teams 2016, (5/0); One EBMT teams 2017, [3/0]
Hungary
Budapest, United St. István and St. László Hospital Pediatric Hematology and Stem Cell Transplantation Unit, CIC 824; G. Kirván, K. Kállay, C. Kassa, G. Kertész, (4/0); G. Kirván, K. Kállay, C. Kassa, G. Kertész, [6/0]
Two EBMT teams, (14/0); [2/0]
Iran
Shiraz, Nemazee Hospital, Shiraz University of Medical Sciences, Haematology-Oncology & Bone Marrow Transplant Department, Shiraz, Iran; M. Ramzi, (0/42)
Tehran, Royan Institute for Stem Cell Biology and Technology, Tehran, Iran; H. Baharvand, M. Vosough, (28/603); H. Baharvand, M. Vosough, [5/389]
One EBMT team (0/0); [18/0]
Israel
Two EBMT teams 2016, (57/0); One EBMT teams 2017, [11/0]
Italy
Aviano, National Cancer Institute Aviano, Centro di Riferimento Oncologico, CIC 162; D. Gussetti, M. Michieli, C. Durante, M. Mazzucato, (0/3)
Bergamo, ASST-Papa Giovanni XXIII; G. Remuzzi, M. Zambelli, E. Gotti, M. Introna, (5/0); G. Remuzzi, N. Perico, E. Gotti, M. Introna, [2/0]
Bergamo, ASST Papa Giovanni XXIII, Hematology and Bone Marrow Transplant Unit; A. Rambaldi, A. Grassi, M. Introna, J. Goaly, (9/1);
Bologna, Azienda Ospedaliera Policlinico Sant'Orsola-Malpighi; G. Remuzzi, A. Pinna, M. Buzzi, M. Introna, (1/0);
Milano, IRCCS Istituto Ortopedico Rizzoli, Laboratorio di Fisiopatologia Ortopedica e Medicina Rigenerativa, and SC Clinica Ortopedica e Traumatologica III a prevalente indirizzo oncologico; N. Baldini, G. Ciapetti, D. Granchi, D. M. Donati, (0/0); [0/0]
Milano, IRCCS Istituto Ortopedico Galeazzi; L. de Girolamo, S. Criniti, H. Schoenhuber, M. Ulivi, (0/35)
Milano, OASI Bioresearch foundation; A. Gobbi, G.P. Whyte, A.B. Desale, (0/105)
Milano, Ospedale San Giuseppe, Plastic Surgery; F. Casabona, (0/312)
Pescara, Ospedale Civile, Bone Marror Transplant Unit, Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie, CIC 248; P. Olioso, M. Di Ianni, P. Di Bartolomeo, S. Santarone, (1/0);
Rome, University Sapienza of Rome; C. Marchese, S. Ceccarelli, E. Vescarelli, (0/6)
Terni, AOSP Santa Maria, Laboratorio Cellule Staminali, Cell Factory e Biobanca, and AOSP Terni in cooperation with University of Novara and Padova; A.L. Vescovi, M. Gelati, G. Muzi, I. Bicchi, [18/0]
Verona & Vicenza, AOUI Policlinico G.B. Rossi & University of Verona, Department of Medicine; Vicenza Hospital, Department of Hematology; Vicenza Hospital, Laboratorio di Terapie Cellulari Avanzate, Cell Factory, CIC 797; R. Ciccocioppo, M. Ruggeri, G. Astori, [0/0]
Three EBMT teams 2016, (39/2); Nine EBMT teams 2017, [94/4]
Lithuania
One EBMT team (10/0); [17/0]
Netherlands
Amsterdam, The Netherlands Cancer Institute, Amsterdam, Netherlands; J. Haanen, S. Wilgenhof, S. Vliek, C. Voermans, (0/8); J. Haanen, S. Wilgenhof, S. Vliek, S.C. Linn, [0/23]
Amsterdam, VU University Medical Center, Department of Dermatology; S. Gibbs, M. Thon, (0/0); [0/0]
Amsterdam, VU University Medical Center, Dept. of Oral & Maxillofacial Surgery/Oral Pathology, Amsterdam Movement Sciences; Vrije Universiteit and University of Amsterdam, Academic Centre for Dentistry Amsterdam; E.A.J.M Schulten, T. Forouzanfar, M.N. Helder, J. Klein-Nulend, (0/0); [0/0]
Nijmegen, Radboud University, Nijmegen Medical Centre, Department of Hematology, CIC 237; M. Schaap, J. Oomen, (2/0); M. Schaap, J. Oomen, [3/0]
Utrecht, UMC Utrecht, Department of Orthopedics; D.B.F. Saris, R.J.H. Custers, J.W.M. Kouwenhoven, E. Kester, (2/59)
Utrecht, University Medical Center Utrecht, Dept of Nephrology and Hypertension of the UMC Utrecht; M. C. Verhaar, [0/0]
Three EBMT teams, (14/0); [25/18]
Norway
Oslo, Oslo university hospital Rikshospit, Ex vivo cell laboratory; J. E. Brinchmann, (0/6); J. E. Brinchmann, [0/7]
One EBMT team, (1/0)
Poland
Czestochowa, Klara Medical Center; D. Slugocka, B. Świątkowska-Flis, A. Borkowska-Kuczkowska, M. Halkiewicz, [78/0]
Warsaw, Carolina Medical Center; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology; R. Smigielski, Z. Pojda, (0/4); R. Smigielski, Z. Pojda, U. Zdanowicz, K. Siennicka, [0/26]
Warsaw, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Department of Neurology, Military Institute of Medicine; A. Stepien, Z. Pojda, (0/2)
Warsaw, Medical University of Warsaw, Laboratory for Cell Research and Application; Timeless Plastic Surgery; Melitus; M. Lewandowska-Szumiel, J. Jaworowski, M. Noszczyk, [0/0]
Wroclaw, Dolnośląskie Centrum Transplantacji Komórkowych, CIC 538; A. Lange, S. Danuta, L. Joanna, W. Natalia, (0/10);, [0/2]
Two EBMT teams 2016, (2/2); Three EBMT teams 2017, [3/0]
Russian Federation
Moscow, Central Research Institute of Dental and Maxillofacial Surger; A. Nerobeev, S. Zharkova; A. Zorina, V. Zorin (Human Stem Cell Institute, Department of Regenerative Medicine, (0/1)
Moscow, Clinic of cosmetology and plastic surgery “Beauty Trend”; L. Kogan; A. Zorina, V. Zorin (Human Stem Cell Institute, Department of Regenerative Medicine), (0/1)
Moscow, “European Medical Center”, Dental clinic; N. Romanenko; E. Gusarina (St. Petersburg, “MEDI clinic”, Stomatology); A. Zorina, V. Zorin (Human Stem Cell Institute, Department of Regenerative Medicine), (0/5)
Moscow, Medical Center of Cosmetology “RayLife”; S. Shokolova; A. Zorina, V. Zorin (Human Stem Cell Institute, Department of Regenerative Medicine), (0/1)
Moscow, Scientific Center of Children's Health, Dermatology; N. Murashkin; A. Zorina, V. Zorin (Human Stem Cell Institute, Department of Regenerative Medicine), (0/1)
Moscow, Blokhin Russian Cancer Research Centre, Laboratory of Cytogenetics; P. Kopnin; A. Isaev, V. Zorin, A. Zorina (Human Stem Cell Institute, Department of Regenerative Medicine), (0/250)
Moscow, Moscow Regional Clinical and Research Institute n.a. Vladimirsky M.F.; A. E. Mashkov, A. V. Sigachev, J. N. Filjushkin, D. A. Kulikov, (5/0); A. E. Mashkov, A. V. Sigachev, J. N. Filjushkin, D. A. Kulikov, [0/12]
St. Petersburg, Russian Inst. Of Hematology, Dept. Clin. Hematology; S.V.Voloshin, A. Kuzyaeva, (0/0); [0/0]
Yekaterinburg, Ural Institute of Cardiology; J. L. Gabinsky, A. N. Kharlamov, (0/196)
Yekaterinburg, The Ural state medical University; O. G. Makeyev, A. V. Korotkov, D. A. Sichkar, V. V. Melekhin, (0/0); [0/0]
Three EBMT teams 2016, (51/0); One EBMT teams 2017, [33/0]
Saudi Arabia
One EBMT team, (1/0); [0/0]
Slovakia
One EBMT, (1/0); [0/0]
Slovenia
Trzin, Educell Ltd; A. Barlič, M. Knežević, L. Girandon, M. Veber, (2/55); A. Barlič, M. Knežević, L. Girandon, M. Veber, [5/38]
Ljubljana, University Medical centre Ljubljana; B. Vrtovec, M. Jaklič, (0/26)
One EBMT, (2/0); [0/0]
Spain
Seville, Andalusian Initiative for Advanced Therapies; L. Rafael Solana, F. Lora Ulgar, R.C.Mata Alcazar-Caballero, G. Carmona Sanchez, (14/30); L. Rafael Solana, F. Lora Ulgar, R.C.Mata Alcazar-Caballero, G. Carmona Sanchez, [24/42]
Badalona, Germans Trias i Pujol Hospital, ICOR Heart Institute; A. Bayes-Genis, C. Galvez-Monton, S. Roura, P. Gastelurrutia, [0/7]
Barcelona, Banc de Sang i Teixits; R. Coll, L. Rodríguez, M. Codinach, J. Garcia, (11/32)
Barcelona, Centro Médico Teknon, Institut de Terapia Regenerativa Tissular; L. Orozco, R.S. Rich, X. Peirau, A. Munar, (11/227); L.Orozco, R.S. Rich, X. Peirau, J. Rius, [13/182]
Barcelona, University Hospital Vall d'Hebron, Pediatric Hematology and Oncology Department and Pediatric Infectious Diseases and Inmunodeficiencies Unit; C. Díaz-de-Heredia, P. Soler, L. Alonso, R. Coll Bonet, (9/0)
Barakaldo, Cruces University Hospital, Biocruces Bizkaia Health Research Institute; C. I. Rodríguez, B. Gener, I. Astigarraga, [2/0]
Madrid, Amplicel-Clinica CEMTRO, Traumatology Unit; P. Guillen-Garcia, I. Guillen-Vicente, E. Rodriguez-Iñigo, J.M. Lopez-Alcorocho, (0/37); P. Guillen-Garcia, I. Guillen-Vicente, M. Guillen-Vicente, T. F. Fernandez-Jaen, [0/44]
Madrid, Hospital General Universitario Gregorio Marañón, Hematology Dpt., CIC 819; J. Anguita, C. Pascual, A M. Pérez-Corral, J. L. Díez-Martín, (0/19)
Madrid, Hospital Infantil Universitario Niño Jesús; M.A. Díaz Pérez, M. González Vicent, J. Ruiz Pato, (6/0)
Madrid, Hospital La Paz-University Autónoma of Madrid, Dept. of Orthopaedic Surgery and Traumatology; E. Gómez-Barrena, J.C. Rubio, E. García-Rey, (0/2); E. Gómez-Barrena, J.C. Rubio, E. García-Rey, [0/5]
Madrid, University Hospital 12 de Octubre; J. Martinez Lopez, M Liz Paciello, [0/0]
Malaga, Regional University Hospital of Malaga, Cell Manufacturing Unit; L. Leyva Fernández, A. Rodríguez Acosta, [0/12]
Murica, Hospital Clínico Universitario Virgen de la Arrixaca; J. M. Moraleda Jiménez, A. M. García-Hernández, M. Blanquer Blanquer, (65/8); J. M. Moraleda Jiménez, A. M. García-Hernández, M. Blanquer Blanquer, [13/13]
Palma de Mallorca, Hospital Son Espases; A. Sampol, (0/4)
Pamplona, Clinica Universidad de Navarra; F. Prosper, E. J. Andreu, S. Inoges, A. Lopez, (16/63)
-
V
alladolid, University of Valladolid, Institute of Applied Ophthalmobiology and Institute of Molecular Biology and Genetics; Carlos III National Institute of Health, Biomedical Research Networking Centre in Bioengineering, Biomaterials, and Nanomedicine; J. María Herreras, M. López-Paniagua, A. Sánchez, M. Calonge, [3/0]
Twelve EBMT teams 2016, (47/0); Fifteen EBMT teams 2017, [72/0]
Sweden
Solna, Karolinska Institutet, Department of Clinical Science, Intervention & Technology; C. Götherström, [0/0]
One EBMT, (1/0); [0/0]
Switzerland
Basel, University Hospital Basel; I. Martin, M. Jakob, D. Schäfer, (0/4); I. Martin, M. Jakob, D. Schäfer, [0/12]
Geneva, Concept-Clinic; K.U. Schlaudraff, G. Soldati, L. Mariotta, (24/0)
Zurich, Sport Clinic; M. Steinwachs, (0/20)
Zurich, University of Zurich, Tissue Biology Research Unit; Wyss Zurich, ETH; E. Reichmann, C. Sax, (0/1)
Zurich, University of Zurich and University Hospital Zurich, Department of Urology; D. Eberli, D. Mohr, J.A. Prange, (0/0); [0/0]
Four EBMT teams 2016, (6/1); Three EBMT teams 2017, [2/1]
Turkey
Ankara, Lösante Hospital; M. Kantarcioglu, (0/5);
-
I
stanbul, Altunizade Acibadem Hospital, Haematology Dept.; Acibadem University Atakent Hospital, Pediatric BMT Unit, CIC 478 &457; S. Ratip, E. Ovali, N.B. Pelit, M. Kongur, (506/374); S. Ratip, E. Ovali, G. Öztürk, D. Atay, [368/122]
Kayseri, Erciyes Pediatric BMT Center; M. Karakukcu, E. Unal, A. Ozca, T. Patiroglu, (14/0)
Twentyfour EBMT teams 2016, (98/0); Twentysix EBMT teams 2017, [135/0]
Ukraine
Kiev, Medical company ilaya; R. Vasyliev, D. Zubov, V. Grytsyk, V. Oksymets, (101/101)
United Kingdom
Bristol, Bristol Royal Hospital for Children/Bristol Oncology Centre; C. Rice, D. Owen, D. Marks, (0/17); C. Rice, D. Owen, D. Marks, [21/0]
London, Great Ormond Street Hospital; P. Veys, P. Amrolia, K. Rao, R. Chiesa, (5/11)
London, St. Mary's Hospital, Division of Paediatrics, CIC 866; J. de la Fuente, S. Loaizia, F. O'Boyle, (7/0); J. de la Fuente, S. Loaizia, F. O'Boyle, [12/0]
Manchester, University of Manchester, Devision of cell matrix biology and regenerative medicine; Royal Manchester Children Hospital; Manchester Academic Health Science Centre, Faculty of Biology, Medicine and Health; I. Hughes, G. Mc Cullogh, L. Bragg, F. Galli, (0/0); [0/0]
Sheffield, Royal Hallamshire Hospital, CIC 778; J.A. Snowden, L. Scott, (0/11); J.A. Snowden, L. Scott, [37/100]
Shropshire, RJAH Orthopaedic Hospital NHS Foundation Trust; P. Harrison, (0/14)
Fifteen EBMT teams 2016, (68/0); Fourteen EBMT teams 2017, [55/4]
Contributor Information
Collaborators: For the International Society for Cellular Therapy (ISCT), the Tissue Engineering and Regenerative Medicine International Society (TERMIS)-Europe, the International Cartilage Repair Society (ICRS), the International Federation for Adipose Therapeutics (IFATS) and the European Group for Blood and Marrow Transplantation (EBMT)
Disclosure Statement
No competing financial interests exist.
Funding Information
Supported by the different international societies and their highly committed representatives, namely ISCT-Europe (Meagan Pasternak), TERMIS-EU (Sarah Wilburn), ICRS (Stephan Seiler), IFATS (Catherine B. Foss), and EBMT (Helen Baldomero).
Supplementary Material
References
- 1. Ireland, H., Gay, M.H.P., Baldomero, H., et al. The survey on cellular and tissue-engineered therapies in Europe and neighboring Eurasian countries in 2014 and 2015. Cytotherapy 20, 1, 2018 [DOI] [PubMed] [Google Scholar]
- 2. Martin, I., Ireland, H., Baldomero, H., Dominici, M., Saris, D.B., and Passweg, J.. The survey on cellular and engineered tissue therapies in Europe in 2013. Tissue Eng Part A 22, 5, 2016 [DOI] [PubMed] [Google Scholar]
- 3. Martin, I., Ireland, H., Baldomero, H., and Passweg, J.. The survey on cellular and engineered tissue therapies in Europe in 2012. Tissue Eng Part A 21, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin, I., Baldomero, H., Bocelli-Tyndall, C., et al. The survey on cellular and engineered tissue therapies in Europe in 2011. Tissue Eng Part A 20, 842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin, I., Baldomero, H., Bocelli-Tyndall, C., Passweg, J., Saris, D., and Tyndall, A.. The survey on cellular and engineered tissue therapies in Europe in 2010. Tissue Eng Part A 18, 2268, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Martin, I., Baldomero, H., Bocelli-Tyndall, C., Slaper-Cortenbach, I., Passweg, J., and Tyndall, A.. The survey on cellular and engineered tissue therapies in Europe in 2009. Tissue Eng Part A 17, 2221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin, I., Baldomero, H., Tyndall, A., Niederwieser, D., andGratwohl, A.. A survey on cellular and engineered tissue therapies in europe in 2008. Tissue Eng Part A 16, 2419, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Passweg, J.R., Baldomero, H., Bader, P., et al. Is the use of unrelated donor transplantation leveling off in Europe? The 2016 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 53, 1139, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Passweg, J.R., Baldomero, H., Basak, G.W., et al. The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant 54, 1575, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alliance for Regenerative Medicine. Advancing Gene, Cell, and Tissue-Based Therapies: ARM Annual Report & Sector Year in Review, 2019. 2020. https://alliancerm.org/indication-data/rare-disease-2019-pdf
- 11. Baker, H.B., McQuilling, J.P., and King, N.M.P.. Ethical considerations in tissue engineering research: Case studies in translation. Methods 99, 135, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams, D. Benefit and risk in tissue engineering. Mater Today 7, 24, 2004 [Google Scholar]
- 13. Mason, C., and Dunnill, P.. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regen Med 4, 835, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Smith, D.M. Assessing commercial opportunities for autologous and allogeneic cell-based products. Regen Med 7, 721, 2012 [DOI] [PubMed] [Google Scholar]
- 15. de Windt, T.S., Vonk, L.A., Slaper-Cortenbach, I.C., et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 35, 256, 2017 [DOI] [PubMed] [Google Scholar]
- 16. Kuriyan, A.E., Albini, T.A., Townsend, J.H., et al. Vision loss after intravitreal injection of autologous “Stem Cells” for AMD. N Engl J Med 376, 1047, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perkins, K.M., Spoto, S., Rankin, D.A., et al. Notes from the field: Infections after receipt of bacterially contaminated umbilical cord blood-derived stem cell products for other than hematopoietic or immunologic reconstitution—United States, 2018. MMWR Morb Mortal Wkly Rep 67, 1397, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.