Abstract
Objectives
The present study analyzed surgical outcomes of laryngotracheal separation (LTS) in children with neurological disorders. The purpose of this study was to investigate respiratory impairment and severe complications after LTS in children, and identify the possibility of permanent tracheostomy without a tracheostomy tube as the safest respiratory management method.
Methods
Twenty‐eight patients (male:female = 16:12) with neurological disorders (6 months to 32 years) who underwent LTS between January 2012 and April 2018 were reviewed. Tracheal diameter, Cobb angle, and sternocervical spine distance (SCD) were measured to assess the potential risk and possibility of removing tracheostomy tube management.
Results
Tracheostomy tube could be removed shortly after LTS in 57% (16/28). However, nine of these patients developed respiratory problems that required tracheostomy tube placement 2 years after LTS. New requirements for a tracheostomy tube as a stent were strongly correlated with SCD (P < .05, odds ratio > 1) as well as tracheal deformity.
Conclusions
Respiratory management in neurologically impaired children after LTS without a tracheostomy tube is challenging because thoracic deformity during physical growth affects tracheal disfiguration. Thoracic deformities and progression of scoliosis should be considered in respiratory management approaches in children with neurological disorders, and long‐term follow‐up by computed tomography is necessary.
LEVEL OF EVIDENCE
IV
Keywords: mechanical ventilation, obstructive respiratory distress, scoliosis, tracheal deformity, tracheo‐innominate artery fistula

1. INTRODUCTION
Laryngotracheal separation (LTS) is the most effective surgical procedure when other conservative treatments such as medications and posture control are ineffective for the prevention of recurrent aspiration pneumonia. The advantages of LTS for caregivers include a reduced need for suctioning of saliva at home 1 and the possibility of managing respiration without placing a tracheostomy tube. However, several complications have been reported in children, of which one major complication is the formation of a trachea‐innominate artery fistula (TIF), an uncommon but life‐threatening condition caused by long‐term tracheostomies with an incidence of 0.1% to 1%. 2 , 3 , 4 Children with neurological disorders often have thoracic deformities with severe scoliosis. As a result, the innominate artery may be compressed against the trachea, which gradually increases the risk of TIF bleeding. 5 If the tracheostomy tube does not fit properly, it can damage the tracheal mucosa and cause inflamed granulation and ulcers. 6 , 7 For this reason, one of the best ways to prevent the formation of a TIF is to manage breathing without a tracheostomy tube. In most adults' cases, an LTS allows for the management of respiratory problems without a tracheostomy tube 8 ; however, there are only a few reports in children cases. 5 , 9 The reason is that children with neurological disorders develop respiratory problems, requiring the replacement of a tracheostomy tube due to the development of central apnea or lower airway obstruction associated with scoliosis. 9 Lee et al reported that chest wall deformities and scoliosis may increase the risk of TIF development secondary to the association of the typical tracheostomy tube angle with the atypical anatomic position of the trachea. 10 Sato et al investigated the chest computed tomography (CT) images of patients who underwent LTS and had bleeding due to a TIF, and found that a sternocervical spine distance (SCD) of <2 cm is predictive of TIF development. 5
The long‐term prognosis and short‐term outcomes of LTS may differ between adults and children because children are in the process of physical development. Despite the low risk of aspiration pneumonia after LTS, scoliosis and chest deformities result in the need for continuous respiratory management in some children. It is important to identify the advantages and disadvantages of LTS in children with neurological disorders for proper respiratory management in this population. Therefore, in this study, we aimed to investigate the long‐term prognosis of respiratory management in children with neurological disorders who underwent LTS at our institution.
2. MATERIALS AND METHODS
We conducted a retrospective review of the medical records of 29 children with refractory aspiration pneumonia who underwent LTS surgery at our hospital between January 2012 and April 2018. The LTS procedure followed was the tracheal flap method that does not involve transection of the trachea—it achieves separation of the larynx from the trachea by using the tracheal, mucoperichondrial, and sternohyoid muscles, together with anterior cervical skin flaps. 11 All patients were followed up for at least 2 years. One patient was lost to follow‐up and was excluded; consequently, 28 children were included in this study.
We evaluated the clinical features of these patients, including their clinical history, complications, and respiratory condition. To evaluate the severity of scoliosis, the median value of the Cobb angle was calculated from radiographs (Figure 1A), and the lateral and vertical diameters of the trachea were measured on axial images of chest CT scans. Scoliosis is defined as a Cobb angle of >10°, whereas moderate to severe scoliosis are defined as an angle of >25°. The SCD, defined as the distance between the posterior surface of the sternum and the anterior surface of the cervical spine, was measured on the sagittal image of a perioperative chest CT scan (Figure 1B). An SCD of <20 mm is defined as short and is considered a risk factor for innominate artery decompression. 12 Tracheal deformity (TD) is defined as the vertical diameter of the trachea being <60% of the lateral diameter (Figure 1C).
FIGURE 1.
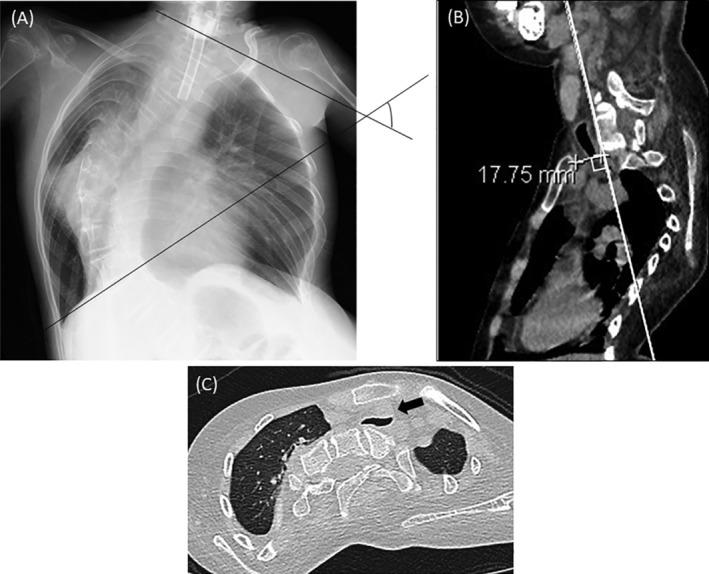
Assessment of computed tomography (CT) and chest X‐ray image; A, Cobb angle: Cobb angle was measured at the image of chest X‐ray; B, SCD: Sternocervical spine distance was measured between the posterior surface of the sternum and anterior surface of the cervical spine at sagittal image of cervical CT scan. C, Tracheal deformity: Trachea was strongly decompressed by juxtaposed innominate artery. Arrow: innominate artery was juxtaposed to tracheal wall
This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the National Center for Child Health and Development (NCCHD2020‐523). Informed consent was obtained from all patients who participated in the study.
3. RESULTS
3.1. Description
Patient demographics and underlying conditions are summarized in Table 1. Twenty‐eight patients (male:female = 16:12) aged 0 to 32 years (median, 7 years) had undergone LTS. Four patients presented with airway anomalies, including craniofacial anomalies (cases #14 and 18), agnathia (case #2), and multiple anomalies (case #1). These patients had moderate to severe neurological problems and several gastrointestinal system anomalies, which led to feeding difficulties and recurrent infections. The remaining 24 patients had severe motor and intellectual disabilities due to fatal asphyxia, fetal infection, and chromosomal aberration. Seventeen of the 28 patients had an existing tracheostomy and 5 patients were mechanical ventilator dependent before LTS surgery. Nissen fundoplication was performed before LTS in 16 of the 28 patients (57%). All cases were failed to control recurrent aspiration pneumonia with conservative treatment therefore required LTS. For all cases, aspiration pneumonia was successfully managed after LTS, and the chance of hospitalization was decreased.
TABLE 1.
Description of 28 cases
| Diagnosis | Age at LTS (years) | Sex | Previous tracheostomy | Epilepsy | Previous Nissen fundoplication | Feeding | |
|---|---|---|---|---|---|---|---|
| 1 | Multiple anomaly | 0 | M | Yes | No | Yes | G‐tube |
| 2 | Agnathia | 0 | F | Yes | No | Yes | G‐tube |
| 3 | Chromosome aberration | 1 | M | Yes | Yes | No | NG‐tube |
| 4 | Fetal asphyxia | 1 | M | No | Yes | No | NG‐tube |
| 5 | CFC syndrome | 1 | F | Yes | Yes | No | NG‐tube |
| 6 | Intracranial bleed | 2 | M | No | Yes | Yes | G‐tube |
| 7 | Robin sequence | 2 | M | Yes | Yes | Yes | G‐tube |
| 8 | Lissencephaly | 3 | F | No | Yes | Yes | G‐tube |
| 9 | Freeman‐Sheldon syndrome | 3 | M | Yes | Yes | Yes | G‐tube |
| 10 | Niemann‐Pick disease | 3 | M | No | Yes | No | NG‐tube |
| 11 | Chromosome aberration | 3 | M | Yes | No | No | NG‐tube |
| 12 | WEST syndrome | 4 | F | No | Yes | No | NG‐tube |
| 13 | WEST syndrome | 4 | F | Yes | Yes | No | NG‐tube |
| 14 | Pfeiffer syndrome | 5 | F | Yes | No | No | NG‐tube |
| 15 | Fetal asphyxia | 5 | M | No | Yes | Yes | G‐tube |
| 16 | Congenital CMV | 6 | M | Yes | Yes | Yes | G‐tube |
| 17 | Chromosome aberration | 6 | F | Yes | Yes | No | NG‐tube |
| 18 | Pfeiffer syndrome | 7 | M | Yes | No | Yes | G‐tube |
| 19 | Post meningitis | 8 | M | Yes | Yes | Yes | G‐tube |
| 20 | Fetal asphyxia | 8 | M | No | Yes | Yes | G‐tube |
| 21 | Fetal asphyxia | 9 | F | No | Yes | Yes | G‐tube |
| 22 | Fetal asphyxia | 11 | F | Yes | Yes | Yes | G‐tube |
| 23 | Fetal asphyxia | 12 | M | Yes | Yes | No | NG‐tube |
| 24 | Dystrophy | 13 | F | No | No | Yes | G‐tube |
| 25 | SSPE | 14 | M | No | Yes | No | G‐tube |
| 26 | Fetal asphyxia | 19 | F | No | No | No | NG‐tube |
| 27 | Encepharitis | 21 | M | Yes | Yes | Yes | G‐tube |
| 28 | Fetal asphyxia | 32 | F | Yes | Yes | Yes | G‐tube |
Abbreviations: CFC, cardiofaciocutaneous; CMV, cytomegalovirus; F, female; G‐tube, gastrostomy tube feeding; M, male; NG‐tube, nasopharyngeal tube feeding; SSPE, subacute sclerosingpanencephalitis.
3.2. Tracheostomy tube removal and mechanical ventilation
Two of five patients who required mechanical ventilation supports prior to LTS were succeeded in avoiding ventilation postoperatively. Ten patients (35.7%) developed hypoventilation and hypoxemia, eventually requiring mechanical ventilation (Table 2). Of these 10 patients, 4 required positive‐pressure ventilation exclusively during sleep, and the remaining 6 required continuous mechanical ventilation. Five of 17 patients who had existing tracheostomy could remove tracheostomy tube post operatively. Overall, 16 of the 28 patients (57.1%) achieved permanent tracheostomy management without a tracheostomy tube immediately after LTS surgery. However, due to developing hypoxia and respiratory distress over the course of >2 years, 14 of the 28 cases (50%) required mechanical ventilation, and another 6 cases (21.4%) required a tracheostomy tube as a stent, whereas 1 case with tracheomalacia could subsequently avoid both mechanical ventilation and a tracheostomy tube. Therefore, the management of a permanent tracheostomy without a tracheostomy tube was possible in seven cases (25%). Some of these patients required a tracheostomy tube with an inflated cuff for positive‐pressure ventilation.
TABLE 2.
Respiratory condition and impact of tracheal deformity
| Perioperative X‐ray and CT | 2nd X‐ray and CT | Tracheal tube required | Mechanical ventilation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age at LTS (years) | Cobb angle (°) | SCD (mm) | Tracheal deformity | 2nd data obtained after LTS (years) | Cobb angle (°) | SCD (mm) | Tracheal deformity | After LTS | Newly required | After LTS | Newly required during night | Newly required all day | Latest statement | Complications |
| 1 | 0 | 4.1 | 27.45 | No | NA | NA | NA | Free | ― | NR | ― | ― | NR | ||
| 2 | 0 | 4 | 26.7 | No | 7.9 | NA | NA | Tube | Free | Required | ― | ― | NR | ||
| 3 | 1 | 7.3 | 28.1 | No | 14.5 | NA | NA | Free | ― | NR | ― | ― | NR | ||
| 4 | 1 | 7.8 | 23 | Yes | 4 | 8.3 | NA | Yes | Free | ― | NR | ― | ― | NR | |
| 5 | 1 | 4 | 21.3 | No | 4 | 3.8 | 32.8 | No | Tube | ― | Required | ― | ― | Required | |
| 6 | 2 | 0.5 | 23.03 | No | NA | NA | NA | Tube | ― | NR | Required | Required | Required | ||
| 7 | 2 | 8.6 | 32.7 | Yes | NA | NA | Yes | Tube | ― | NR | ― | ― | NR | ||
| 8 | 3 | 6.8 | 20.3 | Yes | 4 | 12.7 | 17.3 | Progress | Free | Tube | NR | ― | ― | NR | Granulation |
| 9 | 3 | 12.8 | 33.3 | No | 6 | 47.9 | NA | NA | Tube | ― | NR | ― | ― | NR | |
| 10 | 3 | 3.9 | 29.6 | No | NA | NA | NA | Tube | ― | NR | Required | Required | Required | ||
| 11 | 3 | 5.3 | 24.41 | Yes | 2 | 10.8 | 25.92 | Progress | Tube | ― | Required | ― | NR | Required | |
| 12 | 4 | 4.8 | 21.3 | Yes | 5 | 16.6 | 19.5 | Progress | Free | Tube | NR | ― | ― | NR | |
| 13 | 4 | 11 | 25.4 | No | 6 | 52.7 | NA | NA | Tube | ― | NR | ― | ― | NR | |
| 14 | 5 | 3.2 | 22.7 | No | 2 | 8.3 | NA | NA | Tube | ― | Required | ― | ― | Required | |
| 15 | 5 | 35.2 | 35.6 | No | NA | NA | NA | Free | ― | NR | ― | ― | NR | ||
| 16 | 6 | 5.3 | 25.6 | No | 5 | 16.5 | NA | NA | Tube | ― | NR | Required | NR | Required | |
| 17 | 6 | 3.9 | 26.7 | No | 2 | 5.2 | 27.45 | No | Free | Tube | NR | Required | NR | Required | TIF before LTS surgery |
| 18 | 7 | 7.9 | 21.5 | Yes | 3 | 22.1 | 18.1 | Progress | Tube | ― | NR | Required | Required | Required | Granulation |
| 19 | 8 | 35.9 | 36.4 | NA | 4 | 160.7 | 37.7 | NA | Free | ― | NR | ― | ― | NR | |
| 20 | 8 | 41.1 | 21 | Yes | NA | NA | Yes | Free | ― | NR | ― | ― | NR | Granulation | |
| 21 | 9 | 10.2 | 31.3 | Yes | 8 | 40.7 | 14.2 | Progress | Free | Tube | NR | Required | NR | Required | |
| 22 | 11 | 47.3 | 21.8 | Yes | 3 | 59.7 | NA | Yes | Free | Tube | NR | Required | Required | Required | |
| 23 | 12 | 62 | 26.6 | No | 2 | 61.2 | 27.5 | Progress | Free | ― | NR | ― | ― | NR | |
| 24 | 13 | 2.5 | 31.6 | No | 2 | 26.4 | 18.3 | Yes | Free | Tube | NR | Required | Required | Required | |
| 25 | 14 | 23.4 | 15.97 | No | 5 | 56.1 | 13.3 | Progress | Free | Tube | NR | Required | NR | Required | TIF after 6 years of LTS |
| 26 | 19 | 59 | 8.5 | Yes | 4 | 69.7 | 13.03 | Yes | Free | Tube | NR | Required | Required | Required | TIF after 5 years of LTS |
| 27 | 21 | 42.4 | NA | NA | NA | NA | NA | Tube | ― | NR | ― | ― | NR | TIF in 2 weeks of LTS | |
| 28 | 32 | 36.3 | 19.59 | Yes | NA | NA | Yes | Free | Tube | Required | ― | ― | Required | ||
Notes: Seven patients had progressive tracheal deformity at the second CT data obtained. Tracheostomy tubes were successfully removed in 16 of 28 cases after LTS, but only 8 cases remained tracheostomy tube free status several years later. Mechanical ventilation was required after LTS in 5 of 28 cases, and another 10 cases needed mechanical ventilation several years later.
Abbreviations: CT, computed tomography; LTS, laryngotrachealseparation; NA, not assessed; NR, not required mechanical ventilation; TIF, trachea‐innominate artery fistula; Required, mechanical ventilation required.
3.3. Complications of LTS
Severe complications, such as a TIF, occurred in four cases (14.2%). One patient developed emergent tracheal bleeding due to a TIF, 10 days after surgery, because the tracheostomy tube did not fit the shape of the trachea; this patient required emergency innominate artery ligation. Two other patients have remained tracheostomy tube‐free for 5 and 6 years postoperatively. However, due to respiratory problems, they required placement of a tracheostomy tube for mechanical ventilation, and developed TIFs that required innominate artery ligation 6 months later. Another patient that was managed with a tracheostomy presented with tracheal bleeding before LTS; therefore, prophylactic innominate artery ligation and LTS were performed simultaneously. Granulation tissue developed in three cases—the trachea was deformed due to scoliosis, leading to the tracheostomy tube scratching the mucosa of the tracheal wall and forming granulation. No patients developed stoma stenosis required tracheostomy tube.
3.4. Evaluation of scoliosis and tracheal compression
Scoliosis was identified in 12 of the 28 patients (42.9%), of which 7 (58.3%) were moderate or severe. The SCD measured 8.5 to 36.4 mm (average 25.3 mm); in three patients, it measured <20 mm. Lateral and vertical tracheal diameters measured 7.4 to 20.6 mm (average 12.4 mm) and 5.6 to 15.9 mm (average 9.4 mm), respectively. On comparing lateral and vertical tracheal diameters, the trachea collapsed in the anterior‐posterior direction in 11 of the 28 cases (39.3%).
Following >2 years of observation, 12 (42.9%) and 20 (71.4%) of the 28 patients underwent a chest CT and chest X‐ray, respectively. The chest radiograph revealed advanced scoliosis in 15 of the 20 cases (75%) (Figure 2A), with 9 (60%) of these classified as moderate or severe. Therefore, 13 of the 28 patients (46.4%) had moderate to severe scoliosis 2 years after the LTS procedure. The SCD was reduced to <2 cm in 7 of 12 cases, meaning that an SCD <2 cm was found in a total of 8 of the 28 cases (28.6%) (Figure 2B). The trachea displayed progressed deformation in 7 of 12 cases (58.3%), and a total of 14 of the 28 patients (50%) showed TD.
FIGURE 2.

Cobb angle and sterno‐cervical spine distance after laryngo‐tracheal separation. A, Cobb angle increased 2 to 7 years after LTS. Cobb angle >25° indicates moderate to severe scoliosis (n = 20). B, SCD decreased 2 to 7 years after LTS. SCD <20 mm was reported highly predictive of TIF (n = 11). LTS, laryngotracheal separation; SCD, sternocervical spine distance; TIF, trachea‐innominate fistula
3.5. Clinical symptoms of airway compression
Correlations and odds ratios (ORs) among the Cobb angle, SCD, TD, and respiratory condition and complications are shown in Table 3. A newly required tracheostomy tube was strongly correlated with the SCD and TD (Fisher's exact test, OR > 1, P < .05). The requirement for mechanical ventilation was not correlated with anatomical tracheal deformities.
TABLE 3.
Correlation between chest deformity and intervention
| Cobb angle >25° (13) | SCD <20 mm (8) | Tracheal decompression (13) | ||||
|---|---|---|---|---|---|---|
| P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | |
| Tracheostomy tube required (20) | 1.000 | 0.818 (0.170‐3.934) | .063 | NA | .678 | 2.037 (0.407‐10.004) |
| Newly required (9) | .637 | 2.000 (0.305‐13.114) | <.05 a | 133.000 (9.252‐1968.920) | <.05 a | 13.333 (1.294‐120.412) |
| Mechanical ventilation (14) | 1.000 | 0.750 (0.175‐3.218) | .209 | 4.500 (0.790‐24.432) | .706 | 1.778 (0.411‐7.686) |
| During night (5) | 1.000 | 0.727 (0.121‐4.479) | .606 | 1.889 (0.302‐12.406) | .639 | 1.950 (0.316‐11.743) |
| All day (13) | .686 | 0.600 (0.123‐3.000) | .172 | 4.000 (0.744‐22.041) | 1.000 | 1.222 (0.254‐5.898) |
| Newly required (10) | 1.000 | 1.000 (0.207‐4.840) | .085 | 6.000 (0.948‐36.578) | .689 | 1.333 (0.274‐6.508) |
| Severe complications (6) | .670 | 1.788 (0.344‐9.089) | .142 | 5.667 (0.978‐33.489) | .198 | 4.063 (0.700‐22.571) |
Notes: Nine cases developed respiratory distress and required management with a tracheostomy tube. These symptoms were strongly correlated with chest deformity (SCD < 20 mm) and tracheal deformity, which revealed progressively narrowed trachea causes obstructive respiratory problems required tracheostomy tube as stent. Numbers in parentheses indicate number of patients.
Abbreviations: SCD, sternocervical spine distance; OR, odds ratio; CI, confidence interval.
Indicates statistical significance is <.05.
Statistical analyses revealed an association between tracheal compression and the SCD (P < .001), but not between TD and the Cobb angle (P = .46). In patients with scoliosis, the trachea was decompressed because the mediastinum was deformed and narrowed due to the scoliosis.
4. DISCUSSION
Chronic aspiration is a serious problem in many children with neurological disorders. They suffer repeated episodes of aspiration pneumonia, which induces pulmonary fibrosis and bronchiectasis as a result of frequent infections. Upon failure of conservative treatment methods—such as swallowing rehabilitation, placement of a feeding tube, head position adjustment, and medication—surgical treatment including tracheostomy or LTS should be considered. Tracheostomy is a simple procedure, although it requires frequent suction for neurologically impaired patients, and using a tracheostomy tube with an inflated cuff leads to laryngeal desensitization of the cough reflex. An LTS can reduce the need for tracheal suctioning and aspiration, decrease the number of hospitalizations for aspiration pneumonia, 13 , 14 , 15 , 16 and improve respiratory status, sometimes without the need for tracheostomy tubes. In our study, an LTS facilitated the respiratory management for all the patients, which led to a decreased need for hospitalization due to pneumonia. However, some patients are more prone to major complications, such as development of a TIF, than others. Chida et al reported that 6 of 15 children who had undergone LTS required therapeutic intervention for a TIF, 13 whereas Takano et al reported that 2 of 40 patients who had undergone LTS or tracheostomy were associated with TIF. 15 In our study, four patients had a TIF. Chest wall deformities and scoliosis increase the risk of TIF development, secondary to the association of the typical tracheostomy tube angle, 10 , 17 low tracheostomy, overinflated cuffs, 18 narrow thoracic cage, and scoliosis due to an imbalance between muscular strength and gravitational effects. 5 , 17 , 18 , 19 , 20 Patients with scoliosis or a thorax deformity might always sustained the pressure on the tracheal wall, leading to inflammation and granulation. 18 , 21 Pathological findings show that a TIF is surrounded by epithelialized granulation and squamous metaplasia caused by chronic and repetitive irritation, which indicates the loss of tracheal cartilage and the arterial wall. 7 Therefore, to decrease the risk of TIF development, a tracheostomy tube that fits the tracheal shape properly should be selected to prevent granulation. However, it is sometimes difficult to conform an ordinary tracheostomy tube to a TD in neurologically impaired children. Sato et al revealed that the average age of onset of bleeding due to a TIF is 10.3 years (range, 4‐16 years), 5 which coincides with the time when a thoracic deformity becomes apparent. Patients should also be recommended LTS to allow them the possibility of avoiding a tracheostomy tube. 22
In our study, the tracheostomy tube could be removed shortly after LTS in 16 of the 28 patients; however, 9 of these patients developed respiratory problems that required tracheostomy tube placement 2 years after LTS. Several authors have reported that, in adults, LTS enabled removal of a tracheostomy tube in all patients. 22 , 23 In contrast, only 2 of 24 children achieved tracheostomy tube‐free status, 9 because progressive respiratory conditions in children require ventilator management due to the weakening of the tracheal cartilage framework, central apnea, and deformation of the stoma. 24 Compared with adults, children with neurological disorders are much more likely to develop compression of the main stem of the bronchus against the vertebra and mediastinal structures due to scoliosis. Li et al found that obstructive respiratory dysfunction associated with neurological disorders is caused by a narrow trachea and bronchus, which reduces expiratory flow and increases airway resistance. 24 , 25 In our study, deterioration of respiratory problems requiring a tracheostomy tube was correlated with the SCD and TD, although the need for mechanical ventilation was not correlated with the SCD or TD. Respiratory symptoms are aggravated by TD due to twisting and deformation of the rib cage, requiring respiratory management with a tracheostomy tube. One hypothesis is that a decrease in end‐expiratory pressure without tracheostomy tube management may lead to alveolar collapse, inhibiting expansion of the chest, which may result in a respiratory disorder.
To prevent TIF development, avoiding a tracheostomy tube is one of the best choices for keeping the tracheal wall free from stress. 8 , 23 Conversely, the development of disfiguration of the chest wall and trachea may require respiratory management with a tracheostomy tube, which, in turn, may lead to frequent local infection of the tracheal wall, resulting in a TIF. Multiple factors may lead to the development of respiratory distress in children with neurological problems, including low end‐expiratory pressure, scoliosis, nonambulatory activity level, and neurological disturbances. Therefore, respiratory management without a tracheostomy tube is challenging because thoracic deformity during physical growth affects tracheal disfiguration. A shorter SCD and TD in the anterior‐posterior direction should be considered high risk factors for respiratory problems. Consequently, respiratory management with a tracheostomy tube will be safe, as long as the SCD is >2 cm, and the lateral and vertical tracheal diameters at the level of the thoracic inlet are approximately equal. However, if the SCD is <2 cm, and the tracheal cross‐section appears to be collapsed in the anterior‐posterior direction, prophylactic innominate arterial transection should be considered.
5. CONCLUSIONS
Despite the lower risk of aspiration pneumonia after LTS in neurologically impaired children, obstructive respiratory distress requires a tracheostomy tube as a stent, which is associated with TD and thoracic disfiguration. In adults, tracheostomy without a tracheostomy tube is recommended as the safest and most effective method, although it is challenging in children due to progressive respiratory distress. Therefore, respiratory management should be considered in cases of thoracic deformity and progression of scoliosis in children with neurological disorders. Under long‐term observation, the respiratory management of neurologically impaired children requires consideration of the potential for complications—long‐term follow‐up with CT is necessary.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
ACKNOWLEDGMENTS
We thank everyone who participated in this study.
Morimoto N, Maekawa T, Kubota M, Kitamura M, Takahashi N, Kubota M. Challenge for management without tracheostomy tube after laryngo‐tracheal separation in children with neurological disorders. Laryngoscope Investigative Otolaryngology. 2021;6:332–339. 10.1002/lio2.534
Funding information Health and Labor Science Reserch Grants (Japan), Grant/Award Number: 20FC1017
BIBLIOGRAPHY
- 1. Cook SP. Candidate's thesis: laryngotracheal separation in neurologically impaired children: long‐term results. Laryngoscope. 2009;119(2):390‐395. [DOI] [PubMed] [Google Scholar]
- 2. Schaefer OP, Irwin RS. Tracheoarterial fistula: an unusual complication of tracheostomy. J Intensive Care Med. 1995;10(2):64‐75. [DOI] [PubMed] [Google Scholar]
- 3. Iodice F, Brancaccio G, Lauri A, Di Donato R. Preventive ligation of the innominate artery in patients with neuromuscular disorders. Eur J Cardiothorac Surg. 2007;31(4):747‐749. [DOI] [PubMed] [Google Scholar]
- 4. Allan JS, Wright CD. Tracheoinnominate fistula: diagnosis and management. Chest Surg Clin N Am. 2003;13(2):331‐341. [DOI] [PubMed] [Google Scholar]
- 5. Sato H, Kawase H, Furuta S, Shima H, Wakisaka M, Kitagawa H. Tracheoinnominate artery fistula after laryngotracheal separation: prevention and management. J Pediatr Surg. 2012;47(2):341‐346. [DOI] [PubMed] [Google Scholar]
- 6. Chauhan JC, Hertzog JH, Viteri S, Slamon NB. Tracheoinnominate artery fistula formation in a child with long‐term tracheostomy dependence. J Pediatr Intensive Care. 2019;8(2):96‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyake N, Ueno H, Kitano H. Pathological consideration of tracheo‐innominate artery fistula with a case report. Int J Pediatr Otorhinolaryngol. 2013;77(8):1322‐1324. [DOI] [PubMed] [Google Scholar]
- 8. Nakaya M, Onuki Y, Kida W, Watanabe K, Abe K. New surgical procedure for laryngotracheal separation without a cannula or postoperative treatment. Ann Otol Rhinol Laryngol. 2011;120(8):519‐522. [DOI] [PubMed] [Google Scholar]
- 9. Shino M, Yasuoka Y, Murata T, et al. Improvement of tracheal flap method for laryngotracheal separation. Laryngoscope. 2013;123(2):440‐445. [DOI] [PubMed] [Google Scholar]
- 10. Lee SK, Son JH, Kim YS, Park JM, Kim DH. Tracheo‐innominate artery fistula caused by isolated innominate artery pseudo‐aneurysm rupture. J Thorac Dis. 2018;10(7):E577‐E580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ninomiya H, Yasuoka Y, Inoue Y, et al. Simple and new surgical procedure for laryngotracheal separation in pediatrics. Laryngoscope. 2008;118(6):958‐961. [DOI] [PubMed] [Google Scholar]
- 12. Fujimoto Y, Hirose K, Ota N, et al. Suprasternal approach for impending tracheo‐innominate artery fistula. Gen Thorac Cardiovasc Surg. 2010;58(9):480‐483. discussion 483‐4. [DOI] [PubMed] [Google Scholar]
- 13. Chida I, Tamura K, Nakagawa S, et al. Clinical outcomes of tracheoesophageal diversion and laryngotracheal separation in neurologically impaired children. Auris Nasus Larynx. 2013;40(4):383‐387. [DOI] [PubMed] [Google Scholar]
- 14. Hara H, Hori T, Sugahara K, Ikeda T, Kajimoto M, Yamashita H. Effectiveness of laryngotracheal separation in neurologically impaired pediatric patients. Acta Otolaryngol. 2014;134(6):626‐630. [DOI] [PubMed] [Google Scholar]
- 15. Takano K, Kurose M, Mitsuzawa H, Nagaya T, Himi T. Clinical outcomes of tracheoesophageal diversion and laryngotracheal separation for aspiration in patients with severe motor and intellectual disability. Acta Otolaryngol. 2015;135(12):1304‐1310. [DOI] [PubMed] [Google Scholar]
- 16. Antunes LA, Talini C, Carvalho BCN, et al. Laryngotracheal separation in pediatric patients: 13‐year experience in a reference service. Einstein (Sao Paulo). 2019;17(3):eAO4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hisamatsu C, Okata Y, Zaima A, et al. Innominate artery transection for patients with severe chest deformity: optimal indication and timing. Pediatr Surg Int. 2012;28(9):877‐881. [DOI] [PubMed] [Google Scholar]
- 18. Silva RC, Chi DH. Successful management of a tracheo‐innominate fistula in a 7‐year‐old child. Int J Pediatr Otorhinolaryngol. 2010;74(8):946‐948. [DOI] [PubMed] [Google Scholar]
- 19. Wang XL, Xu ZG, Tang PZ, Yu Y. Tracheo‐innominate artery fistula: diagnosis and surgical management. Head Neck. 2013;35(12):1713‐1718. [DOI] [PubMed] [Google Scholar]
- 20. Grant CA, Dempsey G, Harrison J, Jones T. Tracheo‐innominate artery fistula after percutaneous tracheostomy: three case reports and a clinical review. Br J Anaesth. 2006;96(1):127‐131. [DOI] [PubMed] [Google Scholar]
- 21. Shima H, Kitagawa H, Wakisaka M, Furuta S, Hamano S, Aoba T. The usefulness of laryngotracheal separation in the treatment of severe motor and intellectual disabilities. Pediatr Surg Int. 2010;26(10):1041‐1044. [DOI] [PubMed] [Google Scholar]
- 22. Ise K, Kano M, Yamashita M, et al. Surgical closure of the larynx for intractable aspiration pneumonia: cannula‐free care and minimizing the risk of developing trachea‐innominate artery fistula. Pediatr Surg Int. 2015;31(10):987‐990. [DOI] [PubMed] [Google Scholar]
- 23. Kimura Y, Kishimoto S, Sumi T, et al. Improving the quality of life of patients with severe dysphagia by surgically closing the larynx. Ann Otol Rhinol Laryngol. 2019;128(2):96‐103. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Guo H, Chen C, et al. Does scoliosis affect sleep breathing? World Neurosurg. 2018;118:e946‐e950. [DOI] [PubMed] [Google Scholar]
- 25. Ito K, Kawakami N, Miyasaka K, Tsuji T, Ohara T, Nohara A. Scoliosis associated with airflow obstruction due to endothoracic vertebral hump. Spine (Phila Pa 1976). 2012;37(25):2094‐2098. [DOI] [PubMed] [Google Scholar]


