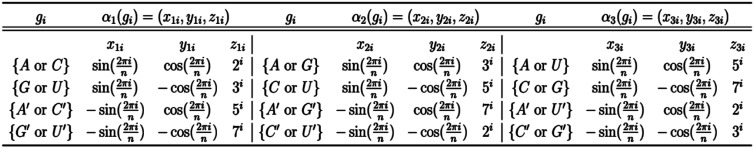Abstract
Different types of noncoding RNAs like microRNAs (miRNAs) and circular RNAs (circRNAs) have been shown to take part in various cellular processes including post-transcriptional gene regulation during infection. MiRNAs are expressed by more than 200 organisms ranging from viruses to higher eukaryotes. Since miRNAs seem to be involved in host–pathogen interactions, many studies attempted to identify whether human miRNAs could target severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNAs as an antiviral defence mechanism. In this work, a machine learning based miRNA analysis workflow was developed to predict differential expression patterns of human miRNAs during SARS-CoV-2 infection. In order to obtain the graphical representation of miRNA hairpins, 36 features were defined based on the secondary structures. Moreover, potential targeting interactions between human circRNAs and miRNAs as well as human miRNAs and viral mRNAs were investigated.
Keywords: circRNA, gene regulation, machine learning, miRNA, SARS-CoV-2
1. Introduction
MicroRNAs (miRNAs) are noncoding RNAs involved in post-transcriptional gene regulation. The precursor miRNAs (pre-miRNAs) fold into characteristic hairpin structures that are used as the primary feature source in many bioinformatics approaches [1]. Another class of noncoding and endogenous RNAs is circular RNAs (circRNAs) that are generated by a unique splicing reaction known as back-splicing [2]. CircRNAs seem to be expressed in a widespread manner and they have important functions in regulation especially as sponges providing binding sites for miRNAs and RNA binding proteins [3] and a player in the regulation of alternative splicing [4].
According to the competitive endogenous RNA (ceRNA) hypothesis, RNA transcripts such as circRNAs, messenger RNAs (mRNAs), and long non-coding RNAs, include miRNA response elements and these are in competition among themselves for miRNA binding to be able to regulate the expression of each other [5]. Previous studies showed that not only miRNA but also circRNA expressions were changed during infections of both DNA and RNA viruses [6]. Although there is not much information about circRNAs’ roles during infection of emerging Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), another member of coronaviruses, Middle East respiratory syndrome coronavirus (MERS-CoV) infection resulted in expression changes of host circRNAs [3].
In this study, we used available differentially expressed miRNA information of SARS-CoV-2 infected cells to build a machine learning based model for prediction. In addition, a comprehensive circRNA-miRNA-mRNA targeting network analysis is performed to identify biologically significant processes in SARS-CoV-2 infection. Our results show that various cellular processes including apoptosis might be affected by the competition of cellular and viral RNAs. These findings could increase the perceptions of infection through RNA-mediated host–virus interactions and lead to development of new strategies for antiviral agents.
2. Related works
Various studies attempted to identify human miRNAs that could target viruses [7], [8], [9], [10]. Although there are not many experimentally validated examples of miRNAs encoded by RNA viruses, computational predictions show that SARS-CoV-2 genome could produce miRNAs that could target human mRNAs [11].
Currently there is not much information about the differences in expression levels of miRNAs during SARS-CoV-2 infection. It has been shown that, highly pathogenic MERS-CoV infection causes substantial changes in the expression of many host cell circRNAs, miRNAs, and mRNAs [3].
3. Architecture/implementation/workflow
All data analysis, machine learning and prediction workflows were generated by using the Konstanz information miner (KNIME) platform [12]. MiRNA – target predictions were performed by using psRNATarget tool [13].
3.1. Graphical representation of RNA secondary structures
An RNA sequence could include four bases (A, G, C, and U) that can form base pairs such as A–U, G–C, and G–U. RNAfold software from the Vienna package was used with default setting to create secondary structures [14]. For better representation, the nucleotides involved in base pairs are shown as A, G, C, and U in Figure 1, while non-base paired ones are shown as A′, G′, C′, and U′, respectively. The workflow generated in KNIME uses RNA sequence and dot-bracket representations of secondary structure to modify bases of the sequence as uppercase and lowercase characters [15].
Figure 1:
The definition of three maps.
Zhang et al. created a dynamic 3D graphical representation for RNA structure based on the chemical properties of the bases [16]
-
–
amino group M = {A, C} and keto group K = {G, U},
-
–
purine group R = {A, G} and pyrimidine group Y = {C, U}
-
–
weak group H-bonds W = {A, U} and strong H-bonds group S = {C, G}.
We used the same base grouping scheme and defined three maps α1, α2 and α3 (Figure 1), where n is the length of the hairpin sequence and i is the index of base in the sequence.
In order to represent miRNA hairpin secondary structure as vectors, based on the definitions from Figure 1, 36-dimensional vector was calculated as shown in Figure 2.
Figure 2:
The components of 36-dimensional vector.
3.2. Data sets
Human miRNA sequences were obtained from MiRBase (Release 22.1) [17], human circRNA data set was downloaded from circAtlas 2.0 [18], SARS-CoV-2 CDS were based on RefSeq_NC_045512.2 from NCBI. Differentially expressed miRNA list was based on the results of Chow and Salmena [19] with some changes, since their list is composed of mature miRNAs, we used the hairpin sequences of those available (Table 1).
Table 1:
The list of miRNAs used for training of differential expression prediction.
| Regulation type | MiRNAs |
|---|---|
| Upregulated | hsa-mir-4485, hsa-mir-483, hsa-mir-6891, hsa-mir-4284, hsa-mir-4463, |
| hsa-mir-155, hsa-mir-107, hsa-mir-29b-2, hsa-mir-139, hsa-mir-299, | |
| hsa-mir-501, hsa-mir-4745, hsa-mir-12136 | |
| Downregulated | hsa-let-7a-1, hsa-let-7a-2, hsa-let-7a-3, hsa-mir-374a, hsa-mir-194-1,hsa-mir-194-2, |
| hsa-mir-4454, hsa-mir-135b, hsa-mir-16-2, hsa-mir-23b, hsa-mir-21, hsa-let-7f-1, | |
| hsa-mir-429, hsa-mir-5701-1, hsa-mir-5701-2, hsa-mir-5701-3, hsa-mir-450b, | |
| hsa-mir-7-1, hsa-mir-26b, hsa-mir-23c, hsa-mir-374c, hsa-mir-374b, | |
| hsa-mir-26a-1, hsa-mir-365a, hsa-mir-365b, hsa-mir-940, hsa-mir-362, | |
| hsa-mir-1275, hsa-mir-1296, hsa-mir-126, hsa-mir-548d-2 |
4. Results
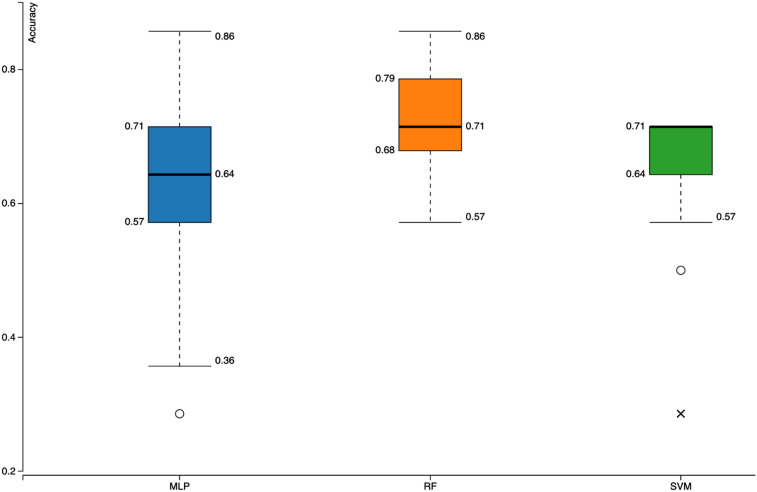
The differential expression prediction workflow was created by using 70% learning and 30% testing ratios and three different classifiers; random forest (RF), support vector machine (SVM) and multilayer perceptron (MLP) were trained with 100-fold MCCV [20] (Figure 3).
Figure 3:
Box-plots of classi-fiers’ accuracy values.
Among 2654 mature human miRNAs available in miRBase, 2498 were involved in 272,822 total targeting events with 18,950 human genes; 2498 were involved in 393,877 total targeting events with 208,642 circRNAs and 484 miRNAs targeted 11 SARS-CoV-2 genes. Some of the miRNAs reported as differentially expressed in Calu3 cells infected with SARS-CoV-2 or mock from GSE148729 did not have any predicted targets (Table 2).
Table 2:
Number of targets of differentially expressed miRNAs on human genes (Gene), human circRNAs (CircRNA) and SARS-CoV-2 coding sequences.
| MiRNA | Gene | CircRNA | SARS-CoV-2 | Regulation |
|---|---|---|---|---|
| hsa-miR-6891-5p | 197 | 200 | 1 (ORF3a) | Up |
| hsa-miR-4284 | – | – | – | Up |
| hsa-miR-4463 | – | – | – | Up |
| hsa-miR-12136 | – | – | – | Up |
| hsa-miR-181-5p | – | – | – | Up |
| hsa-miR-126-5p | 130 | 193 | 1 (ORF1ab) | Down |
| hsa-miR-194-5p | 76 | 132 | 1 (ORF1ab) | Down |
| hsa-miR-374a-3p | 100 | 155 | 2 (ORF1ab, S) | Down |
| hsa-miR-181-3p | – | – | – | Down |
| hsa-miR-1275 | – | – | – | Down |
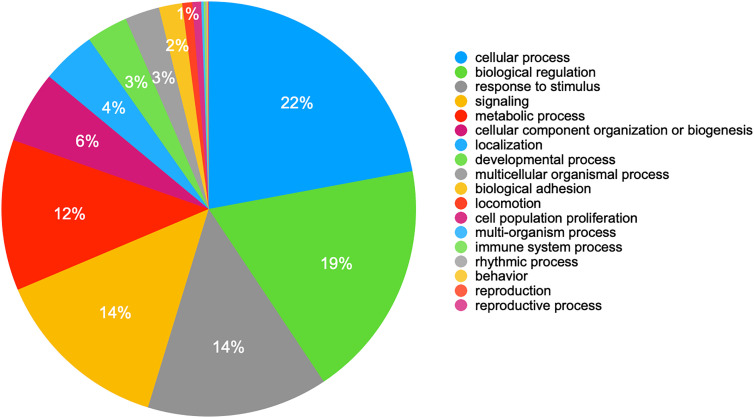
Upregulated human miRNA hsa-miR-6891-5p might target not only human genes and circRNAs but also ORF3a gene of SARS-CoV-2 (Table 2). PANTHER Gene Ontology analysis [21] of human gene targets showed that various biological processes could potentially be affected by the actions of this miRNA (Figure 4).
Figure 4:
Pie-chart for the biological processes of human genes that could be targeted by hsa-miR-6891-5p. Labels on the right part are sorted in descending order making the chart direction in a clockwise order.
5. Discussion
Inter-kingdom communication mechanisms mediated by RNAs have been investigated for several organisms including a variety of viruses, Toxoplasma gondii (protozoan eukaryotic parasite) [22], Histoplasma capsulatum (infectious fungus) [23]. Viruses are parasites that depend on their host for many of their processes. Usually viral infections result in alterations of cellular pathways to modulate viral gene expression and/or accommodate virus in a favourable environment. In some cases, e.g. SARS-CoV-2 infection, host post-transcriptional gene regulation elements like miRNAs might also show differential expression levels during infection [19]. In this study, we analysed such human miRNAs (Table 1) to build a machine learning based workflow that might be used for prediction of expression changes of miRNAs during SARS-CoV-2 infection. Among the 300 models generated, the highest accuracy value was observed with RF classifier (Figure 3). While applying machine learning approaches to miRNA datasets, there are various elements that would affect the overall performance [24]. Among them, feature sets [25], [26] and the quality of data [27] might be the most important parts. When there are more datasets available, the workflow can be easily updated to include them and it is also possible to use this workflow for any kind of differentially expressed miRNAs.
There is not much known about the individual functions of circRNAs but they are acknowledged as sponges providing binding sites for miRNAs and some RNA-binding proteins [28]. The activities of host circRNAs have been investigated in Hepatitis C virus-infected cells [6] and MERS-CoV infection [3]. We performed a comprehensive target prediction analysis for human miRNAs to measure their capacity to bind human mRNAs, human circRNAs and SARS-CoV-2 genes. Based on the results represented in Table 2, SARS-CoV-2 ORF3a is the only viral target for upregulated human miRNAs. Since ORF3a protein is associated with apoptosis which is an essential mechanism for host antiviral defence to control viral infection [29], upregulation of hsa-miR-6891-5p might be crucial to decrease ORF3a expression during certain stages of infection.
Out of 2498 miRNAs that have predicted targets, 2448 had more targets in circRNAs, 27 had more in mRNAs and 23 miRNAs had equal number of targets in both groups. If the mRNA and circRNA targets of specific miRNAs are coexpressed there might be a competition for miRNA binding and considering the wide range of biological processes of a single miRNA’s targets (Figure 4) circRNA-miRNA-mRNA network could play important roles in overall gene expression especially when there is a new set of genes as target candidates during viral infections.
Footnotes
Author contribution: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Research funding: This work was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK, Grant No: 120E042).
Conflict of interest statement: Authors state no conflict of interest.
References
- 1. Saçar Demirci MD, Baumbach J, Allmer J. On the performance of pre-microRNA detection algorithms. Nat Commun 2017;8:330. 10.1038/s41467-017-00403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 2016;17:205–11. 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Chu H, Wen L, Shuai H, Yang D, Wang Y, et al. Competing endogenous RNA network profiling reveals novel host dependency factors required for MERS-CoV propagation. Emerg Microb Infect 2020;9:733–46. 10.1080/22221751.2020.1738277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA 2014;20:1829–42. 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language?. Cell 2011;146:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen TC, Tallo-Parra M, Cao QM, Kadener S, Böttcher R, Pérez-Vilaró G, et al. Host-derived circular RNAs display proviral activities in Hepatitis C virus-infected cells. PLoS Pathog 2020;16:1–23. 10.1371/journal.ppat.1008346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruscella P, Bottini S, Baudesson C, Pawlotsky J-M, Feray C, Trabucchi M. Viruses and miRNAs: more friends than foes. Front Microbiol 2017;8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Girardi E, López P, Pfeffer S. On the importance of host MicroRNAs during viral infection. Front Genet 2018;9:1–17. 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saçar Demirci MD, Toprak M, Allmer J. A machine learning approach for MicroRNA precursor prediction in retro-transcribing virus genomes. J Integr Bioinform 2016;13:1–8. 10.1515/jib-2016-303. [DOI] [PubMed] [Google Scholar]
- 10. Wong RR, Abd-Aziz N, Affendi S, Poh CL. Role of microRNAs in antiviral responses to dengue infection. J Biomed Sci 2020;27:1–11. 10.1186/s12929-019-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saçar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ 2020;2020:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berthold MR, Cebron N, Dill F, Gabriel TR, Kötter T, Meinl T, et al. KNIME: the Konstanz information miner. In: SIGKDD explorations 2008:319–26. [Google Scholar]
- 13. Dai X, Zhao PX. PsRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 2011;39(2):W155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res 2003;31:3429–31. 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saçar Demirci MD.. MicroRNA prediction based on 3D graphical representation of RNA secondary structures. Turkish J Biol 2019;434:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Huang H, Dong X, Fang Y, Wang K, Zhu L, et al. A dynamic 3D graphical representation for RNA structure analysis and its application in non-coding RNA classification. PloS One 2016;11:1–15. 10.1371/journal.pone.0152238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffiths-Jones S.. miRBase: microRNA sequences and annotation. Curr Protoc Bioinformatics 2010;12:12.9.1–10. [DOI] [PubMed] [Google Scholar]
- 18. Wu W, Ji P, Zhao F. CircAtlas: an integrated resource of one million highly accurate circular RNAs from 1070 vertebrate transcriptomes. Genome Biol 2020;21:1–14. 10.1186/s13059-020-02018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chow JT-S, Salmena L. Prediction and analysis of SARS-CoV-2-targeting MicroRNA in human lung epithelium. Genes (Basel) 2020;11:1002. 10.3390/genes11091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Q-S, Liang Y-Z. Monte Carlo cross validation. Chemom Intell Lab Syst 2001;56:1–11. 10.1016/s0169-7439(00)00122-2. [DOI] [Google Scholar]
- 21. Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 2013;41:D377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acar IE, Saçar Demirci MD, Groß U, Allmer J. The expressed MicroRNA-mRNA interactions of Toxoplasma gondii. Front Microbiol 2018;8:1–15. 10.3389/fmicb.2017.02630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saçar Demirci MD. Computational prediction of microRNAs in Histoplasma capsulatum. Microb Pathog 2020;148:104433. [DOI] [PubMed] [Google Scholar]
- 24. Saçar Demirci MD, Allmer J. Delineating the impact of machine learning elements in pre-microRNA detection. PeerJ 2017;5:e3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saçar MD, Allmer J. Comparison of four ab initio MicroRNA prediction tools. Bioinformatics 2013. Spain: Barcelona; 2013. [Google Scholar]
- 26. Khalifa W, Yousef M, Saçar Demirci MD, Allmer J. The impact of feature selection on one and two-class classification performance for plant microRNAs. PeerJ 2016;2016:1–13. 10.7717/peerj.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saçar Demirci MD, Allmer J. Improving the quality of positive datasets for the establishment of machine learning models for pre-microRNA detection. J Integr Bioinform 2017;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panda AC. Circular RNAs act as miRNA sponges, Xiao J, editor. Singapore: Springer Singapore; 2018:67–79. [DOI] [PubMed] [Google Scholar]
- 29. Ren Y, Shu T, Wu D, Mu J, Wang C, Huang M, et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol Immunol 2020;17:881–3. 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]