Graphical abstract
Abbreviations: CCL, CC–chemokine ligand; CCR, Cc chemokine receptor; CD, Cluster of differentiation; COPD, Chronic obstructive lung disorder; CXCL, Chemokine (C-X-C motif) ligand; EGFR, Epidermal growth factor receptor; GMCSF, Granulocyte-macrophage colony-stimulating factor; GWAS, Genome-wide association studies GWAS; IFN, Interferon; IL, Interleukin; IRF, Interferon regulatory factor; LPS, Lipopolysaccharides; MCP, Monocyte chemoattractant protein; MHC, Major histocompatibility complex; MIF, Macrophage migration inhibitory factor; MRC, Mannose receptor C-type; MՓ, Macrophage; NFκB, Nuclear factor kappa-light-chain-enhancer of activated B cells; PAMP, Pathogen-associated molecular pattern; PPAR, Peroxisome proliferator-activated receptor; PTX, Pentraxin-related protein; RNA, Ribonucleic acid; SNP, Single nucleotide polymorphisms; STAT, Signal transducer and activator of transcription; TF, Transcription factor; TGFβ, Transforming growth factor beta; TLR, Toll-like receptor; TNFα, Tumor necrosis factor alpha
Keywords: Pneumonia, Macrophages, Cytokines, Inflammation, Single nucleotide polymorphisms
Abstract
Macrophages represent the first line of anti-pathogen defense - they encounter invading pathogens to perform the phagocytic activity, to deliver the plethora of pro- and anti-inflammatory cytokines, and to shape the tissue microenvironment. Throughout pneumonia course, alveolar macrophages and infiltrated blood monocytes produce increasing cytokine amounts, which activates the antiviral/antibacterial immunity but can also provoke the risk of the so-called cytokine “storm” and normal tissue damage. Subsequently, the question of how the cytokine spectrum is shaped and balanced in the pneumonia context remains a hot topic in medical immunology, particularly in the COVID19 pandemic era. The diversity in cytokine profiles, involved in pneumonia pathogenesis, is determined by the variations in cytokine-receptor interactions, which may lead to severe cytokine storm and functional decline of particular tissues and organs, for example, cardiovascular and respiratory systems. Cytokines and their receptors form unique profiles in individual patients, depending on the (a) microenvironmental context (comorbidities and associated treatment), (b) lung monocyte heterogeneity, and (c) genetic variations. These multidisciplinary strategies can be proactively considered beforehand and during the pneumonia course and potentially allow the new age of personalized immunotherapy.
1. Introduction
Monocytes and macrophages (MՓs) are among the first responders against any type of invading pathogens, primarily of viral and bacterial origin. Monocytes/MՓs are the components of the innate immune system with the essential ability for phagocytosis, cytokine production and release, and antigen presentation. Monocytes are normally present in the blood, while MՓs are found in all the tissues, including so-called immune-privileged zones (microglia of the central nervous system, MՓs of eyes, testis, and placenta). The ubiquitous location of monocytes/MՓs makes them one of the first cell populations, which encounter the invading pathogens.
Both monocytes and MՓs express Toll-like receptors (TLRs), which recognize pathogen-associated molecular patterns, such as bacterial lipopolysaccharides (LPS) (TLR 2,4), bacterial or viral DNA and RNA (TLR3, 7–9) [1,2]. The ligand-receptor engagement leads to the monocyte/MՓ proinflammatory activation and cytokine release, which results in increased cellular phagocytic and cytotoxic activity and further regulation of innate and adaptive immune systems together with the surrounding tissues. Thus, cytokines, which include interleukins, interferons, chemokines, colony-stimulating and growth factors, are essential communication molecules involved in cellular cross-talk and signaling. Cytokines shape pro- or anti-inflammatory microenvironment and are involved in a broad number of physiological processes - cell attraction and differentiation, - and pathological events - bacterial and viral infections, autoimmunity, metabolic disorders, and cancer [3].
In pneumonia the cytokine signaling network is formed by multiple cell populations, including airway epithelium, fibroblasts, and MՓs. Here, we address the roles of lung resident MՓs and monocytes in cytokine network in the context of cell microenvironment, disease history, and genetics.
2. Diversity of lung monocytic subsets and cytokine profiles
Host response to viral or bacterial pathogens, which generally penetrate the lungs via inhalation or swallowing, requires the activation of local and systemic components of inborn (monocytes/MՓs, neutrophils, natural killer cells) and adaptive (T- and B-lymphocytes) immunity together with nonimmune resident cells (fibroblasts, airway epithelium) to counteract the pathogen and promote tissue recovery. While all of the cell populations are essential for proper antiviral and antibacterial responses, lung MՓs and infiltrated blood-derived monocytes represent an important cytokine source and remain in focus of attention for understanding the lung homeostasis in health and disease [1].
In the physiological conditions, only tissue-resident MՓs - alveolar and interstitial - populate lungs (Fig. 1 ). Although the precise origin of alveolar MՓs is yet to be established, the developmental studies suggest that they migrate from two independent sources, yolk sac and fetal liver, and populate the alveolar and airway lumen [2,3]. Interstitial MՓs are also essentially present in the lung tissue and comprise around 5–10 % of all lung monocyte cells [4]. Various genomic and single-cell studies in mice and humans distinguish from 2 to 3 various subsets of interstitial MՓs basing on major histocompatibility complex (MHC) II and CD11c expression levels, antigen presentation and phagocytic activities [[5], [6], [7]]. When compared with alveolar MՓs, interstitial MՓs show higher mRNA levels of cytokine (interleukin (IL) 4, IL6, IL10) and interferon (IFN) (IFN A, G) receptors and chemokines (CC-chemokine ligand (CCL) 3,4,6−9; chemokine (C-X-C motif) ligand (CXCL) 1314; CC chemokine receptor (CCR) 1,2) in a non-activated state and increased cytokine (CXCL 1, 2, 9−11, IL11, IL33) expression upon LPS stimulation [5,6,8]. IL10-producing MՓs, predominantly represented by interstitial MՓs, are reduced in asthma patients, and are, thus, believed to play an essential role in physiological and pathological immunoregulation [9,10].
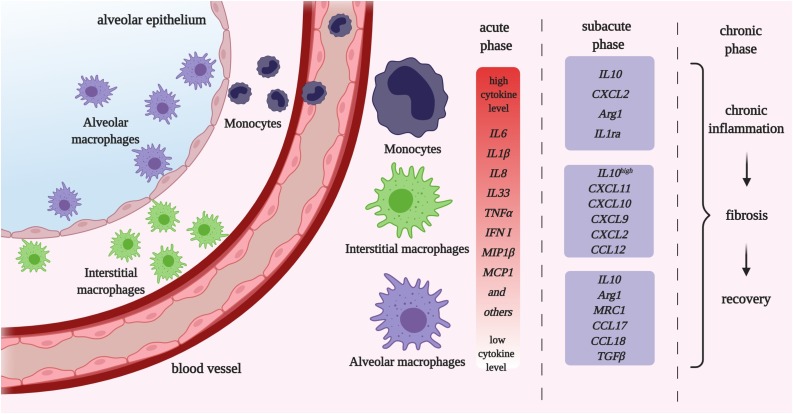
Fig. 1.
The monocyte / macrophage (MՓ) activity throughout the pneumonia course. Under physiological conditions lung monocytic populations include resident alveolar and interstitial MՓs, located in the alveolar and airway lumen and interstitial space, respectively. During infection, the blood derived monocytes penetrate the lung tissue. During the early, or acute, stage monocytes / MՓs develop proinflammatory phenotype and produce proinflammatory cytokines essential for attraction of other immune cell subsets. Among monocytic cells, infiltrated monocytes are the major source of pro-inflammatory cytokines. Later during subacute phase macrophages switch towards anti-inflammatory profiles, which support the lung tissue reorganization (chronic phase) and/or recovery.
Created with BioRender.com
While pathological inflammation arises resident MՓ subsets are supplemented with the peripheral monocytes infiltrated from the blood (Fig. 1). Lymphocytes and eosinophils are also recruited to the lungs, and their amounts gradually decrease with time, while monocytes can remain in the lung tissue for longer periods and convert into MՓs. To address the functional activity monocytes / MՓs can be roughly classified into unprimed (non-stimulated), pro-inflammatory (M1-like) or anti-inflammatory (M2-like) cells (Fig. 2 ).
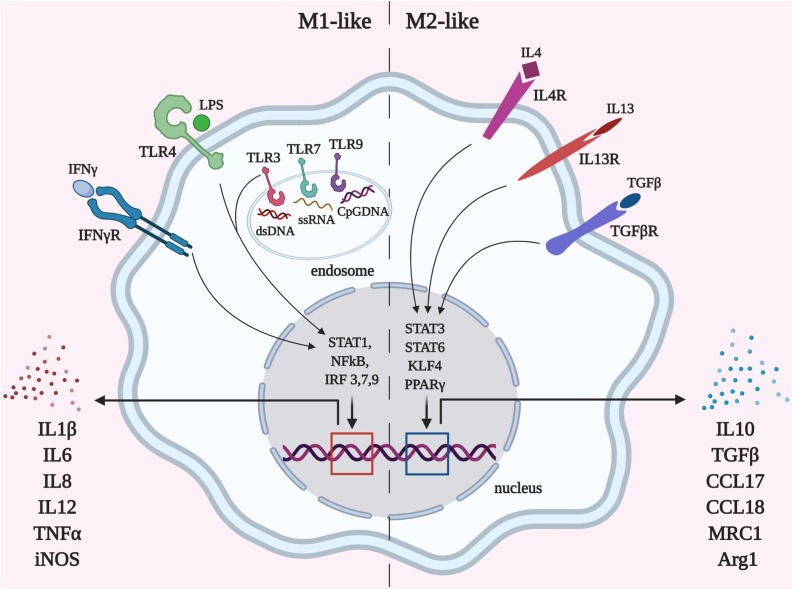
Fig. 2.
Functional polarization of monocytic/macrophage cell subsets. Macrophages can obtain the distinctive phenotype depending on the microenvironment. Polarization towards pro-inflammatory (M1-like) macrophages is triggered by pathogen-associated molecular patterns (PAMPs) such as LPS, bacterial or viral DNA, and some cytokines (IFNγ) via STAT1, NFκB and interferon regulatory factor (IRF) transcription factor signaling, which leads to high pro-inflammatory cytokine production. M1-like monocytic cells are responsible for anti-pathogen defense, acute inflammation, other immune subset attraction and can provoke cytokine storm. Anti-inflammatory (M2-like) polarization of macrophages is elicited by cytokines IL4, IL13, and TGFβ and leads to the resolution of inflammation, tissue reorganization, and regeneration. The balance between M1/M2 states is required for proper pathogen elimination and efficient structural and functional recovery.
Created with BioRender.com
Recent works show that monocytic cell roles throughout pneumonia course significantly depend on the phenotypic subset and origin together with the microenvironment, as different activating stimuli show similar outcomes within one tissue/organic location, but not throughout the whole organism [11]. Alveolar and interstitial MՓs vs. monocytes have different potency for cytokine production in healthy lungs and during the early disease stages (Table 1 , Fig. 1). Blood-derived monocytes produce the highest levels of proinflammatory cytokines (Fig. 1). Of note, younger patients exhibit higher levels of peripheral monocytes and inflammatory cytokines in the nasal lavage than adults, and these parameters are not associated with disease severity and outcome. However, the presence of proinflammatory monocytes in the systemic circulation is a risk factor of uncontrolled cytokine storm and sepsis in all cohorts of patients [12]. During later stages of the disease lung-resident myeloid cells become a predominant source of immunosuppressive cytokine IL10 and effectively control T helper 2 cell activity [13]. Further, lung-resident MՓs, but not monocytes, exhibit reduced capability for phagocytosis long-term after recovery from infectious pneumonia, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcriptional regulation seems to be among the major mechanisms of this dysfunction [14]. This can be one of the key reasons of chronic lung inflammation and fibrosis, when alveolar MՓs become dysfunctional and fail to remove the damaged cells and debris and perform physiological surfactant turnover. MՓs originated from the infiltrated monocytes can unlikely replace them since blood-derived MՓs more intensively undergo Fas-dependent apoptosis and are thus eliminated from the lung microenvironment [15]. Initially, Fas signaling cascade is required for IL1β production via caspase-mediated inflammasome formation in monocytes exclusively and is associated with strong antiviral activity [16,17]. CD44-expressing blood-derived MՓs are more resistant towards Fas-dependent apoptosis, and MՓ migration inhibitory factor (MIF)/CD44 signaling axis can thus be used to expand this cell population and to eliminate the viral/bacterial pathogens more efficiently if needed [18]. Less is known about the distinctive features of interstitial MՓs. Considering their location and predisposition for high IL10 production in the resting state, it may be suggested that this subset prevents systemic monocyte activation, as anti-inflammatory IL10 can be released by interstitial MՓs both into alveolar space and blood flow to restrict cytokine storm on both local and systemic levels [19].
Table 1.
General Characteristics of Lung Monocytic Cell Subsets.
| Mononuclear cell subset | Transcription factor | Secretory profile | Functional role |
|---|---|---|---|
| Alveolar macrophages (SiglecF + CD11c + CD11b − CD71+) | |||
| Unprimed alveolar macrophages | PPARγ, STAT6, STAT3, FOXP3, SOCS3 [20] | Immunosuppressive prostaglandins, TGFβ, GMCSF, retinoic acid, IL10 | Lung microenvironment maintenance; Debris phagocytosis, surfactant turnover. Low antigen presenting activity, suppression of T cell activation [1,21] |
| CD206+CD14+CD169+ | |||
| M1-like alveolar macrophages | STAT1, NFκB, IRF3,7 9 [22] | IFN I, IL6, TNFα, IL1β, IL8, MCP1, MIP1β, IP10, CCL5,CXCL1 | Anti-pathogen defense, acute inflammation and immunoregulation; Attraction of cytotoxic T cells, T helper cells, B-lymphocytes [1,23] |
| CD40+CD80+CD86+ | |||
| M2-like alveolar macrophages | PPARγ, STAT6, STAT3, KLF4, c-MYC, IRF4 [22,24,25] | Arg1, MRC1, CCL17, CCL18, IL10, TGFβ | Alveolar formation in embryogenesis |
| CD71+ CD206+ RELMα + CD163 | Regulatory T cell infiltration, resolution of inflammation; lung tissue reorganization and regeneration [26] | ||
| Interstitial macrophages (SiglecF − CD11b+HLADR + CD71low) | |||
| Unprimed interstitial macrophages | PPARγ, Maf, Maf B, HIF1 [4,5] | IL7, IL10low, IL6, IL4, TNFα, CCL3, CCL4, CCL6−9, CXCL13−14, CCR1, CCR2, IFNA, IFNG | Lung immune homeostasis |
| CD11b + CD11c + CD14+ | Relatively high antigen presenting activity [4,9,10] | ||
| M1-like interstitial macrophages CD206- | STAT1, NFκB, IRF3 | PTX3, IL-12, CXCL13, CCL5, CXCL1,2,9−11, IL11, IL33 | Th1 cell activation |
| T and B lymphocyte chemoattraction | |||
| Phagocytosis [6,27] | |||
| M2-like interstitial macrophages CD206+ | STAT6 | IL10 high, IL1-Ra, CXCL11, CXCL10, CXCL9, CXCL2, CCL12 [6] | Immunoregulation,lung tissue reorganization and regeneration [6,28] |
| STAT3 | |||
| KLF4 | |||
| Blood-derived monocytes CD14+ CD16+/- CCR + CCR5+ CD62L+ | |||
| Unprimed monocytes CD80+CD163+(CD14++CD16-CCR2+ classical, CD14dimCD16++CX3CR1+ | Irf8, Klf2, Klf4, C/EBPβ, Nur77 [31,32] | IL1β low, IL6, TNFα, CCL2, CCR2, CCL24 [33] | Blood homeostasis |
| Non-classical, CD14++CD16 + C × 3CR1+(intermediate) [29][30] | Maintenance of macrophage and dendritic cell populations [34] | ||
| M1-like monocytes/macrophages | STAT1, STAT2,NFκB, IRF1,3,5 [37] | IL6, IL1β, IL8, TNFα, MCP1, MCP3, MIP1β, IP10, GMCSF, CXCL10, GBP1 [38] | Acute inflammation |
| CD14+ CD16+/- HLADR + CD80+ CD163- (predominantly from classical monocytes) [29,35,36] | CD8+ T cell attraction | ||
| Reactive oxygen species production | |||
| Pro-inflammatory activity during late stages of pneumonia | |||
| Maintenance of dendritic cell pool [30,39,40,41] | |||
| M2-like monocytes/macrophages | STAT6, STAT3, KLF4, IRF4 [44] | IL10, CXCL2 (MIP2), Arg1, IL1ra [45] | Immunoregulatory activity |
| CD14DIM CD16- CD80- CD163+(predominantly from non-classical monocytes) [29,42,43] | Alveolar epithelium restoration | ||
| Lung tissue reorganization and regeneration | |||
| Fibrosis [28,45,46] | |||
Importantly, the cytokine contribution to the disease pathogenesis is completely rearranged if a joint bacterial infection develops. For instance, cytokines, such as IL33, which are considered as negative factors and are associated with the cytokine storm in viral infections, become essential for bacterial clearance and further recovery after associated bacterial pneumonia [47]. Moreover, while IL33 is considered as highly proinflammatory, as it promotes γδT cells via IL9 axis in COVID19 disease, can also act as immunosuppressor long-term during and after sepsis [[48], [49], [50]].
Whether the cellular source of IL33 in the listed situations is diversified remains unclear. Conclusively, the functional outcomes of cytokine signaling have to be considered when cytokine profile is shaped in pneumonia therapeutics. With that, it is particularly important to address the monocyte cell origin together with the pathological context for targeted subset re-polarization and controllable cytokine regulation in personalized and stage-dependent modes for further clinical implementations.
3. Proinflammatory cytokine network in pneumonia
The overstimulation and prolonged activation by proinflammatory stimuli lead to overproduction of cytokines and emerged inflammation, which can also impact surrounding tissues and provoke lung and cardiovascular damage, and even septic-like conditions. An anti-inflammatory cytokine network is on hand for restriction of M1-like polarization at the later stages of pneumonia. Certain cytokines, such as granulocyte-monocyte colony stimulating factor (GMCSF), can be used to redirect MՓs towards less inflammatory and more protective phenotype, and suppression of cytokine storm remains a major therapeutic strategy against pneumonia [51,52].
In general, monocytes/MՓs release a broad spectrum of proinflammatory cytokines (Table 1). The major contributors to the disease course during bacterial or viral-induced pneumonia are IL6, IL1β, tumor necrosis factor alpha (TNFα), IL8, IFNI, and others, which are produced under control of signal transducer and activator of transcription 1 (STAT1) and NFκB transcription factors (TFs). Another important group of TFs is an IFN regulatory factor (IRF) family, primarily, IRF 3, 7, and 9, which positively regulate of viral-induced IFN transcription. Altogether, activation of these proinflammatory TFs is a double-edged sword, as they promote proinflammatory M1-like polarization, cytokine release, and attraction of other immune cells for antibacterial or antiviral activity but may also lead to poor recovery and damage of lung tissue, vasculature, heart, and even more distant organs if sepsis arises. For instance, the high levels of antiviral interferons α and β increase the disease severity, lung damage, and mortality in animal models [53].
It is known that viral components actively modify the cytokine network and can shape the immune microenvironment [54,55]. For instance, numerous works report that viral proteins, such as ORF and NSP families (severe acute respiratory syndrome CoV, SARS-CoV-2) and H5N1, can suppress STAT1 phosphorylation and promote antagonistic STAT3 signaling, which results in impaired IFNI production and signaling in airway epithelium and dendritic cells during pneumonia [[55], [56], [57]]. Of note, STAT3 signaling is also related to apoptotic escape in H5N1 (avian influenza)-loaded bronchial and alveolar epithelial cells. Interestingly, STAT3 signaling is more IL6-dependent and proinflammatory in peripheral monocytes, while STAT3 of MՓs is associated with IL10-mediated response and results in immunoregulatory profile, so that viral STAT3 manipulation may lead to infiltrated monocyte survival and excessive inflammation or development of immunosuppressive microenvironment, which has to be further investigated [58,59]. Moreover, the dysregulated STAT signaling is a hallmark feature of MՓs during various viral infections, other than respiratory: hepatitis B, hepatitis C, human cytomegalovirus, oncolytic vesicular stomatitis [[60], [61], [62], [63]]. However, the details of the interaction between viral proteins and monocytes/MՓ transcriptional machinery in pneumonia remain poorly investigated, so that the viral impact onto M1/M2 polarization is not fully understood.
3.1. Cytokine network in monocyte-to-macrophage transition
As it has been mentioned, alveolar MՓ origin from the fetal liver and yolk sac monocytic precursors during the development, while in adulthood majority of alveolar MՓs are maintained without bone marrow cell contribution unless lung pathology develops (Fig. 3 ) [64,65]. An embryonic monocyte-to-MՓ switch is not passive but occurs in cytokine (transforming growth factor beta (TGFβ), GMCSF)-dependent mode and requires activation of a specific transcriptional program, which relies on key TFs peroxisome proliferator-activated receptor γ (PPARγ) and STAT6, which, in their turn, form a MՓ cytokine profile, distinct from those of monocytes [[64], [65], [66], [67]].
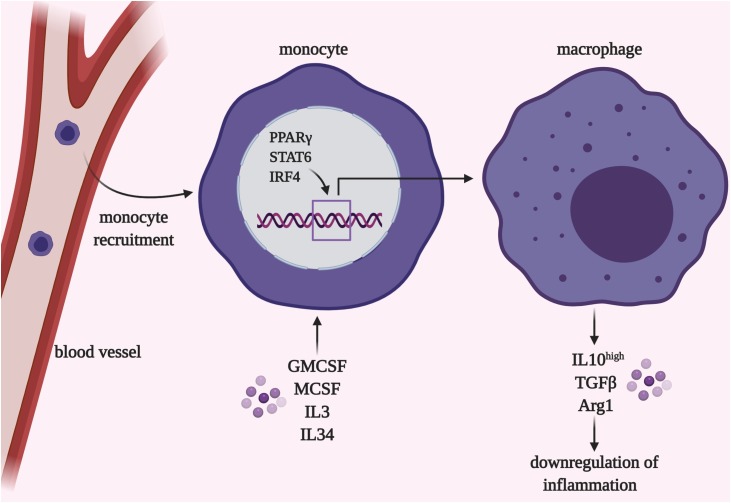
Fig. 3.
Monocyte-to-macrophage differentiation within lung tissue. Switch from monocytes to macrophages occurs during embryonic development or upon acute inflammation or lung damage. This process is governed by locally produced GMCSF, MCSF, IL3, IL34, and others under control of the transcription factors PPARγ, STAT6, and IRF4. In long-term periods cells of peripheral origin become phenotypically similar to the lung-resident macrophages.
Created with BioRender.com
TFs PPARγ and STAT6 are highly expressed in alveolar MՓs of healthy subjects; PPARγ and STAT6 constitutively orchestrate autophagic activity and cytokine production and are thus required for normal MՓ activities [68]. TFs PPARγ, STAT6, and others downregulate proinflammatory cytokine transcription via direct DNA binding or suppression of M1-related TFs STAT1 and NFκB [69] (Fig. 3). Disruption of PPARγ or STAT6 machinery could lead to certain pathologies. For instance, the PPARγ deficiency was found in patients with pulmonary alveolar proteinosis, a condition when lung surfactant deposits within alveoli likely due to MՓ insufficient phagocytic activity [65]. In pneumonia pathogenesis, resident MՓ activation and peripheral monocyte infiltration also require the transcriptional program switch and improve antipathogen response during the early stages and restrict tissue regeneration in later stages [70].
Indeed, mouse research models show that the factors, involved in monocyte-to- MՓ differentiation, can be connected to pneumonia severity. While infiltrated monocytes are major contributors of IL6 during pneumonia, monocytes can become one of the risk factors when the recirculation from the blood to lung tissue is prolonged or monocyte-to- MՓ differentiation is suppressed [70]. The decreased activities of PPARγ and STAT6 are associated with the prolonged inflammation, higher levels of proinflammatory cytokines IL6, IL1β, IL12, CCl2, TNFα, and reduced pathogen burden at the same time complicated with the extensive lung tissue damage during viral infections; therefore, it likely impacts the destiny of resident and infiltrated cells [25,[71], [72], [73]]. Moreover, infiltrated monocytes are exposed to local cytokines such as granulocyte-macrophage colony-stimulating factor (GMCSF) within the lung tissue, undergo transcriptional reprogramming, and become functionally indistinguishable from resident cell populations, once inflammation is completely resolved [51,74]. Indeed, increased levels or externally (intranasal) delivered GMCSF are protective against viral and bacterial pneumonia, first of all during the most severe pneumonia cases, including COVID19 [51,[75], [76], [77], [78], [79]]. Interestingly, Ly6Clo lung MՓs exhibit even higher proinflammatory activities in the absence of type I interferons deactivating stimulus, than newly infiltrated Ly6Chi monocytes during influenza A and SARS-CoV-2 [80,81]. Moreover, certain viruses, such as middle east respiratory syndrome coronavirus, but not SARS-CoV-2, utilize PPARγ activity to stimulate the production of anti-inflammatory cytokines (primarily IL10) and suppress the host immune system [[82], [83], [84]]. The alterations in the cytokine spectrum itself may also participate in various antipathogen responses. TGFβ-TGFR is a cytokine axis, which regulates the monocyte repopulation of lung tissue exclusively [64,85]. TGFβ promotes immune cell infiltration to the lung during bacterial and viral infections, while also worsening the lung injury [86,87].
4. Personalized look onto cytokine profiles in pneumonia
The emerging amount of data shows the substantial roles of genetic signatures, expression levels, and functional activity of cytokines and their producing machinery during pneumonia pathogenesis. While the majority of patients survive pneumonia and completely restore the normal lifestyle, the substantial cohort undergoes undesired complications such as cytokine storm, excessive fibrotic tissue formation, and chronic lung dysfunction, which may be due to individual genetic variations and preliminary history.
In general, the severity and negative outcomes of viral-induced pneumonia are associated with the high cytokine levels, primarily, IL33, IL6, TNFα, IL10, monocyte chemoattractant protein (MCP) 3, which can be detected in plasma, bronchoalveolar fluid, and nasal lavage of patients [12,[88], [89], [90], [91]]. At the moment, the plasma cytokine patterns, which reflect systemic events and risk of cytokine storm and sepsis, are considered more suitable for evaluation of disease course and hospitalization pre-requisite. Moreover, transcriptional profiles of the peripheral blood mononuclear cells are also reflective for disease severity and outcome [92]. Bronchoalveolar fluid and nasal lavage can also be of use, while some patients with high local levels of proinflammatory cytokines show fast viral removal and efficient recovery after infection [12].
4.1. Altered cytokine network within risk groups
Many bacterial and viral infections, including COVID-19, showed that certain comorbidities - chronic pulmonary and cardiovascular disorders, diabetes, autoimmune conditions - are increased risk factors of cytokine disbalance and severe pneumonia [93]. Additionally, the patients undergoing regular therapy such as in cancer are also at risk and have to be considered with particular attention. The substantial cohort of patients (around 60 %) hospitalized with pneumonia undergo medical interventions for other reasons [94]. Here, we address the most common examples of correlations between chronic conditions and cytokine signaling networks involved in pneumonia.
4.2. Systemic disorders
Diabetes. Current studies on COVID-19-related pneumonia show that diabetic patients comprise 5 to more than 50 % of total cases [95]. Such a high disease prevalence can be explained by altered immune status, as well as applied therapeutic interventions against diabetes. Patients with diabetes using PPAR-γ agonists have decreased levels of proinflammatory cytokines during lung infections; however, develop severe forms of bacterial pneumonia with high bacterial burden [96]. Elevated IL6 levels are often found in diabetic patients, suggesting the increased risk for cytokine storm [95]. Statins are commonly used to control hypercholesterolemia and may inhibit NFκB signaling preventing excessive inflammation; however, most studies show no impact of statins in pneumonia prevalence or severity [95,97,98].
Autoimmune conditions require the life-long intake of immunosuppressive medications, such as corticosteroids or hydroxychloroquine [99,100]. In general, autoimmunity is associated with impaired IFN signaling and reduced production of cytokines, such as IL1α and IL6 [101,102]. Indeed, patients with inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis are more susceptible to pneumonia [[103], [104], [105]]. At the same time, the therapeutic interventions used to control autoimmunity relapses may be on hand to restrict cytokine storm severity in pneumonia, which is becoming particularly prominent in COVID-19 treatment [99]. For instance, corticosteroid treatment, which reduces the systemic levels of IL6, IL1RA, and MCP, is widely used in pneumonia management [106].
Cancer. In general, oncological conditions are strongly associated with an immunosuppressive status of the patients due to cancer-related processes and relevant radio- or chemotherapeutic treatment. Lung tumors are among the most prevalent cancer types found in COVID-19 patients. Interestingly, patients after several pneumonia episodes have a lower risk of lung cancer development, which is probably due to their prolonged hyperactivated immune responses within lung tissue [107]. For patients already diagnosed with cancer-specific treatment approaches significantly impact cytokine profiles, as well as other parameters. Anti-epidermal growth factor receptor (EGFR) therapy, for example, is one of the most common approaches in lung cancer patients. However, EGFR signaling is protective against TNFα-induced airway epithelium apoptosis, and anti-EGFR treatment leads to pneumonitis development, the major death cause in lung cancer patients [108]. On the other hand, the excessive activity of the EGF/EGFR axis, found in patients with severe pneumonia course, leads to the risk of lung tissue fibrosis, chronic pulmonary obstruction, and poor recovery prognosis [109,110]. Anti-programmed cell death protein 1 (PD1) immunotherapy is also found to cause pneumonia with subsequent cytokine storm and risk of lung fibrosis and organ failure in various forms of cancer [[111], [112], [113]]. This side effect, which can be corrected by anti-IL6 treatment, is a matter of concern and has to be considered as a dramatic risk factor for prospective patients. Cytokine-based therapies, which implement the antitumor activities of IL2, IL15, IL21, GMCSF or suppress tumorigenic properties of CCL2, 3 and 5 chemokines, are under development and applied in combination with other approaches in clinical trials [114,115].
4.3. Chronic lung pathologies
Chronic obstructive lung disorder (COPD) is associated with an increased predisposition and a less favorable outcome of pneumonia. COPD patients exhibit elevated CXCL1 levels in response to external proinflammatory stimuli, while serum levels of TNFα, IL1β, and IL6 are reduced when compared with patients with pneumonia alone [116,117]. In accordance with these data, peripheral monocytes of COPD patients have reduced cytokine release following ex vivo total bacterial extract or LPS stimulation [118]. Suggesting that COPD is associated with functional deficiency of peripheral monocytes, this cell subset has to be a primary therapeutic target for these patients.
Asthma is also reported as a susceptibility factor for pneumonia by numerous studies, while the underlying mechanisms of this connection are not fully understood [119]. One possible factor is IL17 production by Th17 cells, and high levels of IL4 and TNFα, which leads to MՓ / monocytes and neutrophil recruitment with subsequent excessive inflammation [[120], [121], [122]]. Second, corticosteroid-based therapy is often used in asthma management and can be relevant to insufficient immune responses, including cytokine production, and increased bacterial/viral burden in infectious pneumonia [123,124].
The major challenge of cytokine profiling in respect to comorbidity-pneumonia correlations is that the exact cellular cytokine source cannot be precisely determined in the patients. The subset contribution can only be accessed by 1) isolation of peripheral blood monocytes and myeloid cells of bronchoalveolar fluid and their further ex vivo stimulation with bacterial/viral pathogens, 2) by translational research derived from animal model studies, or 3) computational modeling of the cell behavior in microenvironmental and genetical contexts. The first two approaches are not universal as in vitro cellular responses significantly differ from those in the organism, while cytokine profiles and monocyte/MՓ subsets are not uniformed in humans and animals in health and disease. Genetic analysis may assist this issue implying a side-by-side comparison of individual genetic variations and linking them with the functionality of desired cell populations. Moreover, the same genetic variations may overlay pneumonia predisposition and comorbidities, as it will be further discussed (Table 2 ).
Table 2.
Genetics of cytokine network and viral pneumonia pathogenesis.
| Gene | Genetic background | Pneumonia and comorbidity states / prognosis (+/-) |
|---|---|---|
| Cytokines and their receptors | ||
| IL1A | A114S (rs17561) | H1N1 influenza A pneumonia predisposition / - [132] |
| Cancer (lung, ovarian, breast) predisposition / - [133,134,135]; Asthma prevalence / - [136] | ||
| IL1B | rs1143627 | Influenza A pneumonia / - [137] |
| rs16944 (511*C/T) | Cancer (lung, cervical) / + [138,139]; Autoimmunity / - [140] | |
| Systemic inflammatory response syndrome / - [141] | ||
| Diabetes / + [142]; Asthma / - [143] | ||
| IL1R1 | rs3917254; rs2160227 | Invasive pneumococcal disease / - [144,145] |
| IL1RA (secreted inhibitor for IL1) | A1A1 genotype | Community-acquired pneumonia / + [146] |
| A2A2 genotype | Asthma / - [147]; Diabetes / - [148] | |
| Community-acquired pneumonia / - [146] | ||
| Sepsis / - [149] | ||
| IL4 | C−590 T (rs 2,243,250) | Respiratory syncytial virus / - [150] |
| rs2070874 | Respiratory infection predisposition / - [151] | |
| Asthma / - [152]; Autoimmunity (rheumatoid arthritis) / - [153]; Cancer / - [154] | ||
| IL4RA | Q551R (rs1801275) | Respiratory syncytial virus / - [151] Asthma / - [155] |
| IL6 | GG genotype, G allele of IL6−174 G/C SNP (rs1800795) | Community-acquired pneumonia / - [156] |
| Immunodeficiency / - [157] | ||
| Pneumonia-induced sepsis /- [158] | ||
| Sepsis / + [159]; Cancer (various) / - [160]; Asthma / + [161] PMCID: PMC4612856 | ||
| IL9 | rs2069885 | Respiratory syncytial virus / - [162] Asthma / - [163]; COPD / - [164]; Lung inflammation (cystic fibrosis) / - [165] |
| IL10 | rs1800896-A | Community-acquired pneumonia / + [156] |
| rs1800871 (−819 T/T genotype) | Diabetes / - [166,167]; Asthma / - [168]; Breast cancer /- [169] | |
| Postoperative pneumonia / - [170] | ||
| IL12B | rs2195940, rs919766 | Invasive pneumococcal disease / - [145] Acute chest syndrome / - [171]; Inflammatory cardiomyopathy / - [172] |
| CCL5 | rs2107538*CT | Respiratory syncytial virus / - [173] |
| Cancer (breast, prostate) / - [174,175] | ||
| CCL2 | rs1024611 (G-2518A) | SARS-CoV / - [176] |
| Autoimmunity (multiple sclerosis) / - [177]; Cancer / - [178,179] | ||
| CCR5 | CCR5-Δ32 allele | Influenza A / - [180] |
| Diabetes / - [181,182]; Breast cancer / - [183,184] | ||
| TNFα | rs361525 | Influenza A / - [185] |
| 308*G/A (rs1800629) | Systemic inflammatory response syndrome / - [185]; Pneumonia-induced sepsis /- [158] | |
| −238A allele (rs361525) | Diabetic nephropathy / - [185,186]; Pneumonia in patients with systemic lupus erythematosus / - [187] | |
| TNFRSF1B | TNFRSF1B + 676 (rs1061622) | Community-acquired pneumonia / + [188] |
| Autoimmunity (systemic lupus erythematosus, rheumatoid arthritis) / - [189,190] | ||
| Lung cancer / + [191] | ||
| MIF | C allele at −173 G/C (rs 755,622); rs5844572 | Pneumonia-induced sepsis / + [89] |
| Meningitis and bacterial pneumonia / - [192] | ||
| Autoimmunity (systemic lupus erythematosus, rheumatoid arthritis) / + [193,194] | ||
| Transcription factors | ||
| NFκB cREL | rs842647*G | sepsis / - [195,196] |
| NFκB RelA (p65) | −94delATTG (rs28362491) | autoimmune (Behcet’s Disease) / - [197] |
| acute respiratory distress syndrome / - [198] | ||
| cancer / - or + [199,200]; diabetes / - [201] | ||
| STAT1 | L706S, Q463H, E320Q, P293L Complete Stat-1 deficiency |
mycobacterial disease / - [202,203] pneumonia / - [204] autoimmunity / - [204]; viral infections / - [205,206] |
| IRF5 | rs77571059, rs2004640, haplotype GTAA | community-acquired pneumonia / - |
| rs77571059 | autoimmunity (systemic lupus erythematosus, systemic sclerosis) / - [207,208,209,210,211] | |
| diabetes / - [212]; melanoma / - [213] | ||
| IRF7 | F410 V (rs 786,205,223) | influenza A / - [214] |
| rs375323253; Q421X | ||
| IRF9 | Loss-of-function IRF9 allele | Influenza A, parainfluenza virus, respiratory syncytial virus / - [215] |
| loss-of-function c.991 G > A | Influenza A, respiratory syncytial virus / - [216] | |
4.4. Genetic predisposition of pneumonia risks
The growing numbers of evidence suggest that genetic background including variations in viral/bacteria-host interactome and the host immune profile is an important factor that impacts disease predisposition and progression. While adaptive immunity is pathogen-dependent, factors of the innate immune system are more universal and can be used for a generalized prediction of inflammatory processes. The genetic component of the infectious conditions, such as pneumonia, can be detected via single nucleotide polymorphisms (SNPs) of the receptors, which form the first line of anti-pathogen defense (TLRs, pathogen-associated molecular patterns (PAMPs), and cytokine networks, which are responsible for correct pathogen elimination and tissue repair and are described in the current review [125,126].
The genetic predisposition to pneumonia can be associated with the dysfunction in both pro- and anti-inflammatory cytokine systems and lead to excessive (cytokine storm) or insufficient (increased bacterial/viral burden) immune responses. The major gene polymorphisms found in cytokine network genes and associated with pneumonia are summarized in Table 2. IL6 and TNFα can be listed among the major proinflammatory cytokines, and the positive correlation between the severity of illness and the IL6/TNFα allele frequency was demonstrated in the cases of community-acquired pneumonia [127]. In progressive pneumonia and sepsis, anti-inflammatory cytokines such as IL10 are produced to control excessive inflammation. IL10 SNP, which is located in the ETS-like transcription factor recognition site for ETS-like TF, can be used as diagnostic criteria since the increase in its level is also closely related to the severity of disease symptoms. The IL10 level is also higher in patients with sepsis [128]. Pro-inflammatory cytokines including IL1α and β, IL6, IL8, and TNFα can also bear SNPs in the promoter regions. For instance, the presence of SNP in IL1β, IL10, IL17, and IL28 genes determines the outcome of the H3N2 (influenza A) virus-driven pneumonia, and similar results are shown for other viral strains [129]. Interestingly, the same polymorphisms are linked to the predisposition to cancer, asthma, autoimmunity, diabetes, as well as other chronic conditions (Table 2) [130,131]. This connection has to be considered in personalized medicine, as the same genetic background can link together acute (pneumonia or other infections) and chronic immune-related disorders.
The study of SNP contribution has been demonstrated via implementation in-silico studies of pro- and anti-inflammatory cytokine genes as well as of transcription factors. In particular, rs1800795 in IL6 genes can aggravate the course of the disease, leading to sepsis and septic shock due to the cytokine storm. Oppositely, certain polymorphisms can be protective against pneumonia. For instance, SNP rs1800896 in IL10 protects the body from weighting the symptoms of these diseases [217]. Interestingly, some genetic factors can be either harmful or protective throughout the disease course. For instance, GG genotype and G allele of IL6−174 G/C SNP are associated with higher pneumonia rates, while the risk of sepsis is significantly reduced (Table 2). This may be explained by the increased IL6 activity with the suppressed initial antipathogen response and negative prognosis during the early stages of pneumonia, while later the reduced pro-inflammatory activity lowers the risks of cytokine storm [218]. Accordingly, the genetic background contributes to the development of infectious diseases and their phenotypic manifestations. The activity of TFs relies on their interaction with the relevant DNA binding sites and TF-encoding genes. SNPs in the DNA binding sites or target gene promoters can affect TF-DNA interactions, thus impacting transcriptional regulation. These alterations can be predicted by bioinformatics approaches. In particular, it has been identified that out of 80 polymorphisms found in STAT1 or IRF1 motifs, about 34 SNPs impact the TF-DNA interactions [219]. The in-silico experiments predicted that IRF1 can bind T rs9260102 allele, located in the HLA-A promoter, but TF is unable to interact with another allele (G) and fails to perform its transcriptional activity. Later, similar results were obtained by in vitro experimentations [220]. In this way, in-silico methods allow highly efficient and time- and resource-saving prediction of SNP effects on cytokine transcriptional machinery and cytokine functionality [156].
We can also conclude about the impact of SNP on the development of concomitant diseases, which was demonstrated by the example of pneumonia. However, it has to be specified one more time that contribution of each particular protein and corresponding gene polymorphisms is a matter of spatiotemporal factor and disease origin. With that, the additional computational analysis of the SNP association with pneumonia origin (viral, bacterial, or mixed) and its stage (acute, subacute, or chronic) is also essential.
5. Cytokine network in COVID-19 lung pathology
Cytokine storm is a key feature of COVID-19 pathology associated with local lung injury and systemic organ failure if inflammation goes to the systemic level. Anti-cytokine therapy, for instance targeting the IL6-IL6R axis, improves survival and milds symptoms and adverse events throughout the disease course [221]. Cytokine network during COVID-19 course shows some distinctive features when compared with other pneumonia types. For example, the peripheral monocytes from COVID-19 patients are enlarged in size, comprised of mixed M1/M2 polarization with higher, than in influenza, levels of cytokines and their receptors (TNF, IL6R, IL10R) and certain TFs (STAT1, IRF3) [222,223]. At the same time, other researchers report the presence of peripheral myeloid-derived monocyte-like cells, which exhibit signs of immunosuppression with impaired antigen presentation and cytokine production [224,225]. Alveolar MՓs of all Covid19 patients are highly pro-inflammatory, while levels of anti-inflammatory cytokines are elevated only in severe disease cases [224,226].
Transcriptional profiles of SARS-CoV-2-infected human cells and tissue samples reveal the dysregulated chemokine and cytokine (primarily, various interleukins and TNFα) networks, and this dysregulation - at least partially – is mediated by viral protein impact onto host TFs (STAT1, STAT3, IKKβ – NFkB inhibiting protein) [56,[227], [228], [229]]. For instance, the most severe COVID-19 patients exhibit de-mono-ADP-ribosylation of STAT1 by viral nsp3 protein [229]. Additionally, alveolar monocytes and MՓs show the repressed activity of PPARγ TF complex, which is required for maintenance of physiological cytokine levels and resolution of inflammation [230]. Current studies suggest that although monocytes express Ace2 receptor, the SARS-CoV-2 replication does not occur within monocyte/MՓ subsets, and transcriptional alterations are expected to fade gradually once the viral particle number is lowered in the organism [231].
Similar to other viral infections, chronically dysregulated transcriptional factors can be risk factors for increased cytokine production, as it is observed for increased NFkB activity and IL1, IL6, and TNFα cytokine production in the elderly and people with metabolic disorders [232]. Particularly, sensitized IFNα and IL6 signaling pathways of monocytic cells can be associated with the higher predisposition for severe disease course in aged patients [233]. The lung microenvironment is altered in pneumonia higher glycolytic activity alterations triggered during infection lead to metabolic switches in alveolar MՓs with higher glycolytic activity and reactive oxygen species generation, thus, directly connecting the glucose levels – and diabetes – with disease pathogenesis [234]. While the risk factors, such as age, cardiovascular and metabolic disorders, have to be considered for therapeutic design in individual patients, the therapies applied for immunomodulation in a general situation also have a potential for new coronavirus disease management. For instance, tocilizumab (anti-IL6R monoclonal antibody applied in rheumatoid arthritis), metformin, fenretinide (used in type 2 diabetes and metabolic syndrome), and other drugs have been suggested as promising adjuvant therapies in COVID-19 disease [[235], [236], [237]].
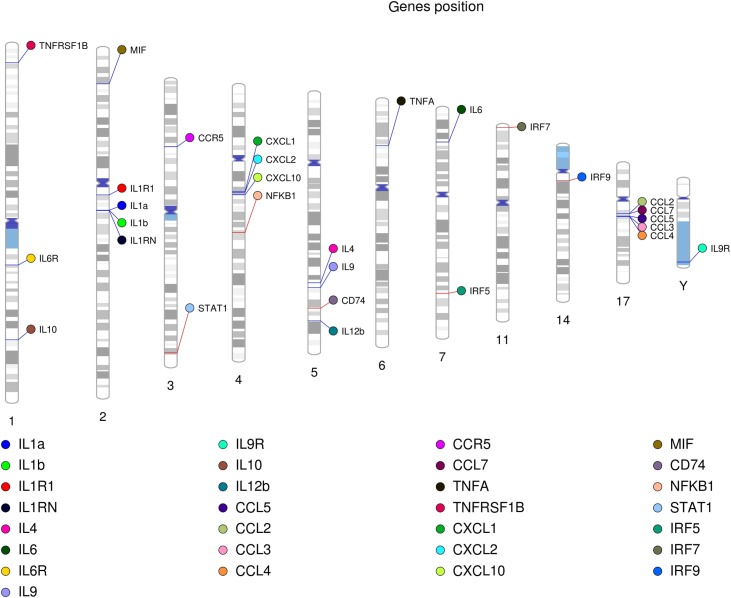
At the moment the major attention is attracted to the studies of the genetic variations and expression patterns of proteins responsible for SARS-CoV-2 intracellular entrance to follow disease predisposition and clinical picture [238]. At the same time, the SNPs within the cytokine network are potential predictive markers of cytokine storm accidents and multiorganic failure in individual patients. As for today, the SNPs in chemokines CCR9, CXCR6, in TMEM189–BE2V1 and TEMEM189–UBE2V1 gene loci (involved in IL1 signaling) have been connected to increased COVID-19 risks [[238], [239], [240]]. It is worth noting that no links between SNPs in TFs STAT1, NFkB, and IRFs have been reported so far, and the search on individual predispositions for COVID-19 predisposition and severity has to be continued. Of note, the genes and SNPs mentioned in the review reflect the distinctive features of cytokine network and can appear to be universal clinical markers for viral-induced pneumonias (Table 2, Fig. 4 ).
Fig. 4.
Arrangement of cytokines and relevant transcription factors in the human genome. Certain genes are grouped in several genomic loci positioned on chromosome 2 (IL1α, IL1β, and their receptor), 4 (CXCL 1, 2, and 10), and 17 (CCL2-7). Transcription factors STAT1 and NFκB have been mapped to chromosome 3 (STAT1) and 4 in the proximity to the CXCL cytokine gene family (NFκB). The IRF transcription factors do not form a single group and are distributed between different chromosomes. Other cytokines and their receptors highlighted in the review also do not show any spatial correlations.
6. Conclusions and future directions
Modulations of cytokine levels remain one of the most important strategies in pneumonia treatment [241]. First, cytokines are required for proper antiviral responses (proinflammatory) and further tissue repair (anti-inflammatory). Second, dysregulated cytokine profiles are risk factors for pneumonia predisposition and severity. Improper cytokine signaling may arise from hereditary factors, chronic metabolic and immune disorders, and therapeutic interventions, and consideration of all the listed factors is essential for pneumonia prognosis and successful treatment. Moreover, the associations between a growing number of newly discovered SNPs for cytokines, their receptors and TFs have not been found; however, these genetic variations can still be connected to certain forms of viral or bacterial pneumonias, and have to be considered in case of further epidemics. Moreover, the cytokine profiles are not uniformed within monocyte/MՓ subsets and other lung cell populations, and this diversity can serve as an important and more sensitive mechanism of immunomodulation. Phenotypic and genetic screening of individual patients may establish the most efficient cellular and molecular targets to prevent and overcome pneumonia and link the genetic variations found in comorbidity conditions and pneumonia.
Funding
This work was financially supported by the Government of the Russian Federation through the ITMO Fellowship and Professorship Program, by the Russian Science Foundation (Grant N0 20-75-10112).
CRediT authorship contribution statement
Marina Dukhinova: Conceptualization, Writing - original draft, Writing - review & editing. Elena Kokinos: Visualization, Writing - original draft. Polina Kuchur: Visualization, Writing - original draft. Alexey Komissarov: Writing - original draft, Writing - review & editing. Anna Shtro: Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest, financial or otherwise.
Biographies

Marina Dukhinova has graduated from Lomonosov Moscow State University, where she studied the mechanisms and screened for markers of functional degeneration. Marina received her PhD degree in biomedical sciences in the Chinese University of Hong Kong, where she investigated local immunity in the context of the central nervous system pathologies. She then continued her studies on the interactions between immunity and local microenvironment of tumors in Naples, Italy. Marina is now a leading researcher in ITMO University (Saint-Petersburg, Russia), and her scientific interests are concentrated on the regulation of macrophage subsets for immunotherapy of viral infections, tumors and chronic inflammatory disorders.

Kokinos Elena received her bachelor degree in biology from National Research Tomsk State University, Department of Genetics and Cell biology. She worked in the National Research Medical genetics Institute in Tomsk, where she studied the association between X- chromosome epigenetics and X-linked mutations and aneuploidy cases in spontaneous abortions. Elena is now a Master student at ITMO University, Saint-Petersburg and she is focused on how transcriptional mechanisms, particularly, governed by the cyclin-dependent kinases 8 and 19 can be implemented into the viral pneumonia development and pathogenesis. Her scientific interests are applied aspects of immunology, oncology, and genetics.

Polina Kuchur graduated from Saint Petersburg State University with a bachelor’s degree in Biology. She is a second-year Master student of ITMO University, SCAMT. Her main interests come mainly from bioinformatics, especially from studying the causes of immune reaction development in response to bacterial pathogens penetration. She is currently studying the structural and genetic composition of somatic antigens of bacterial lipopolysaccharides as part of her Master's degree.

Aleksey Komissarov received his PhD in molecular biology at the Institute of Cytology of the Russian Academy of Sciences in 2012. In 2013, he became a postdoctoral fellow at the Theodosius Dobzhansky Center for Genome Bioinformatics at Saint Petersburg State University, where he gained extensive interdisciplinary experience in molecular biology, genomics, bioinformatics, natural language processing, software development, machine learning, and artificial intelligence. Since 2019, he is a leading researcher at the Advanced Materials and Technology Solution Chemistry Institute at ITMO. Aleksey Komissarov research interests include the development of a genome graph for working with human and animal genomic data, working with the non-coding part of the genome, especially with satellite DNA and Alu-repeats.

Anna Shtro has received her MSc. degree in Dept.of Genetics, St.Petersburg State University, Russia in 2008. Since then, Anna is working in the field of virology with particular interests in the virus strains involved in pneumonia-related disorders. Anna is a head of laboratory of Chemotherapy for viral infections (Smorodintsev Research Institute of Influenza, Saint-Petersburg, Russia). The laboratory participates in the maintainance of one of the largest viral collections in Russia and performs annual analysis of local and global epidemiological situations. Anna is also involved in development and screening of antiviral drugs and therapeutic approaches.
References
- 1.Duan M., Hibbs M.L., Chen W. The contributions of lung macrophage and monocyte heterogeneity to influenza pathogenesis. Immunol. Cell Biol. 2017;95:225–235. doi: 10.1038/icb.2016.97. [DOI] [PubMed] [Google Scholar]
- 2.Cohen M., Giladi A., Gorki A.-D., Solodkin D.G., Zada M., Hladik A., Miklosi A., Salame T.-M., Halpern K.B., David E., Itzkovitz S., Harkany T., Knapp S., Amit I. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell. 2018;175:1031–1044. doi: 10.1016/j.cell.2018.09.009. e18. [DOI] [PubMed] [Google Scholar]
- 3.Ginhoux F., PPAR-titioning Fate. PPAR-γ “instructs” alveolar macrophage development. Nat. Immunol. 2014;15:1005–1007. doi: 10.1038/ni.3011. [DOI] [PubMed] [Google Scholar]
- 4.Liegeois M., Legrand C., Desmet C.J., Marichal T., Bureau F. The interstitial macrophage: a long-neglected piece in the puzzle of lung immunity. Cell. Immunol. 2018;330:91–96. doi: 10.1016/j.cellimm.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Gibbings S.L., Thomas S.M., Atif S.M., McCubbrey A.L., Desch A.N., Danhorn T., Leach S.M., Bratton D.L., Henson P.M., Janssen W.J., Jakubzick C.V. Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cell Mol. Biol. 2017;57:66–76. doi: 10.1165/rcmb.2016-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schyns J., Bai Q., Ruscitti C., Radermecker C., De Schepper S., Chakarov S., Farnir F., Pirottin D., Ginhoux F., Boeckxstaens G., Bureau F., Marichal T. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun. 2019;10:3964. doi: 10.1038/s41467-019-11843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakarov S., Lim H.Y., Tan L., Lim S.Y., See P., Lum J., Zhang X.-M., Foo S., Nakamizo S., Duan K., Kong W.T., Gentek R., Balachander A., Carbajo D., Bleriot C., Malleret B., Tam J.K.C., Baig S., Shabeer M., Toh S.-A.E.S., Schlitzer A., Larbi A., Marichal T., Malissen B., Chen J., Poidinger M., Kabashima K., Bajenoff M., Ng L.G., Angeli V., Ginhoux F. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363 doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 8.Hoppstädter J., Diesel B., Zarbock R., Breinig T., Monz D., Koch M., Meyerhans A., Gortner L., Lehr C.-M., Huwer H., Kiemer A.K. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010;11:124. doi: 10.1186/1465-9921-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draijer C., Boorsma C.E., Robbe P., Timens W., Hylkema M.N., Ten Hacken N.H., van den Berge M., Postma D.S., Melgert B.N. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J. Allergy Clin. Immunol. 2017;140:280–283. doi: 10.1016/j.jaci.2016.11.020. e3. [DOI] [PubMed] [Google Scholar]
- 10.Misharin A.V., Morales-Nebreda L., Mutlu G.M., Budinger G.R.S., Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer F.F., Naxerova K., Schloss M.J., Hulsmans M., Nair A.V., Dutta P., Calcagno D.M., Herisson F., Anzai A., Sun Y., Wojtkiewicz G., Rohde D., Frodermann V., Vandoorne K., Courties G., Iwamoto Y., Garris C.S., Williams D.L., Breton S., Brown D., Whalen M., Libby P., Pittet M.J., King K.R., Weissleder R., Swirski F.K., Nahrendorf M. Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity. 2019;51:899–914. doi: 10.1016/j.immuni.2019.10.010. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshansky C.M., Gartland A.J., Wong S.-S., Jeevan T., Wang D., Roddam P.L., Caniza M.A., Hertz T., Devincenzo J.P., Webby R.J., Thomas P.G. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care Med. 2014;189:449–462. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poe S.L., Arora M., Oriss T.B., Yarlagadda M., Isse K., Khare A., Levy D.E., Lee J.S., Mallampalli R.K., Chan Y.R., Ray A., Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013;6:189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roquilly A., Jacqueline C., Davieau M., Mollé A., Sadek A., Fourgeux C., Rooze P., Broquet A., Misme-Aucouturier B., Chaumette T., Vourc’h M., Cinotti R., Marec N., Gauttier V., McWilliam H.E.G., Altare F., Poschmann J., Villadangos J.A., Asehnoune K. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 2020;21:636–648. doi: 10.1038/s41590-020-0673-x. [DOI] [PubMed] [Google Scholar]
- 15.Janssen W.J., Barthel L., Muldrow A., Oberley-Deegan R.E., Kearns M.T., Jakubzick C., Henson P.M. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidt M.M., Ebert T.S., Chauhan D., Schmidt T., Schmid-Burgk J.L., Rapino F., Robertson A.A.B., Cooper M.A., Graf T., Hornung V. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Eltom S., Belvisi M.G., Yew-Booth L., Dekkak B., Maher S.A., Dubuis E.D., Jones V., Fitzgerald K.A., Birrell M.A. TLR4 activation induces IL-1β release via an IPAF dependent but caspase 1/11/8 independent pathway in the lung. Respir. Res. 2014;15:87. doi: 10.1186/s12931-014-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y., Poon G.F.T., Arif A.A., Lee-Sayer S.S.M., Dosanjh M., Johnson P. The survival of fetal and bone marrow monocyte-derived alveolar macrophages is promoted by CD44 and its interaction with hyaluronan. Mucosal Immunol. 2018;11:601–614. doi: 10.1038/mi.2017.83. [DOI] [PubMed] [Google Scholar]
- 19.Schyns J., Bureau F., Marichal T. Lung interstitial macrophages: past, present, and future. J. Immunol. Res. 2018;2018:5160794. doi: 10.1155/2018/5160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 21.Trapnell B.C., Whitsett J.A. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 22.Hume D.A., Freeman T.C. Transcriptomic analysis of mononuclear phagocyte differentiation and activation. Immunol. Rev. 2014;262:74–84. doi: 10.1111/imr.12211. [DOI] [PubMed] [Google Scholar]
- 23.Schneider C., Nobs S.P., Heer A.K., Kurrer M., Klinke G., van Rooijen N., Vogel J., Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004053. e1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pello O.M., De Pizzol M., Mirolo M., Soucek L., Zammataro L., Amabile A., Doni A., Nebuloni M., Swigart L.B., Evan G.I., Mantovani A., Locati M. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 25.Huang S., Zhu B., Cheon I.S., Goplen N.P., Jiang L., Zhang R., Peebles R.S., Mack M., Kaplan M.H., Limper A.H., Sun J. PPAR-γ in macrophages limits pulmonary inflammation and promotes host recovery following respiratory viral infection. J. Virol. 2019;93 doi: 10.1128/JVI.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones C.V., Williams T.M., Walker K.A., Dickinson H., Sakkal S., Rumballe B.A., Little M.H., Jenkin G., Ricardo S.D. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir. Res. 2013;14:41. doi: 10.1186/1465-9921-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston L.K., Rims C.R., Gill S.E., McGuire J.K., Manicone A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillon A., Arafa E.I., Barker K.A., Belkina A.C., Martin I., Shenoy A.T., Wooten A.K., Lyon De Ana C., Dai A., Labadorf A., Hernandez Escalante J., Dooms H., Blasco H., Traber K.E., Jones M.R., Quinton L.J., Mizgerd J.P. Pneumonia recovery reprograms the alveolar macrophage pool. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costantini A., Viola N., Berretta A., Galeazzi R., Matacchione G., Sabbatinelli J., Storci G., De Matteis S., Butini L., Rippo M.R., Procopio A.D., Caraceni D., Antonicelli R., Olivieri F., Bonafè M. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging (Albany. NY) 2018;10:1268–1280. doi: 10.18632/aging.101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyette L.B., Macedo C., Hadi K., Elinoff B.D., Walters J.T., Ramaswami B., Chalasani G., Taboas J.M., Lakkis F.G., Metes D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Neriah Y., Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 32.Kurotaki D., Sasaki H., Tamura T. Transcriptional control of monocyte and macrophage development. Int. Immunol. 2017;29:97–107. doi: 10.1093/intimm/dxx016. [DOI] [PubMed] [Google Scholar]
- 33.Patel V.K., Williams H., Li S.C.H., Fletcher J.P., Medbury H.J. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis. 2017;263:15–23. doi: 10.1016/j.atherosclerosis.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Yona S., Jung S. Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 2010;17:53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 35.Florentin J., Coppin E., Vasamsetti S.B., Zhao J., Tai Y.-Y., Tang Y., Zhang Y., Watson A., Sembrat J., Rojas M., Vargas S.O., Chan S.Y., Dutta P. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J. Immunol. 2018;200:3612–3625. doi: 10.4049/jimmunol.1701287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong S.-M., Hadadi E., Dang T.-M., Yeap W.-H., Tan C.T.-Y., Ng T.-P., Larbi A., Wong S.-C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9:266. doi: 10.1038/s41419-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emam M., Cánovas A., Islas-Trejo A.D., Fonseca P.A.S., Medrano J.F., Mallard B. Transcriptomic Profiles of Monocyte-Derived Macrophages in Response to Escherichia coli is Associated with the Host Genetics. Sci. Rep. 2020;10:271. doi: 10.1038/s41598-019-57089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole S.L., Dunning J., Kok W.L., Benam K.H., Benlahrech A., Repapi E., Martinez F.O., Drumright L., Powell T.J., Bennett M., Elderfield R., Thomas C., Dong T., McCauley J., Liew F.Y., Taylor S., Zambon M., Barclay W., Cerundolo V., Openshaw P.J., McMichael A.J., Ho L.-P. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight. 2017;2:e91868. doi: 10.1172/jci.insight.91868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu S., Bharat A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ Transplant. 2016;21:239–245. doi: 10.1097/MOT.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva C.O., Gicquel T., Daniel Y., Bártholo T., Vène E., Loyer P., Pôrto L.C., Lagente V., Victoni T. Alteration of immunophenotype of human macrophages and monocytes after exposure to cigarette smoke. Sci. Rep. 2020;10:12796. doi: 10.1038/s41598-020-68753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heung L.J., Hohl T.M. Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog. 2019;15:e1007627. doi: 10.1371/journal.ppat.1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornwell W.D., Kim V., Fan X., Vega M.E., Ramsey F.V., Criner G.J., Rogers T.J. Activation and polarization of circulating monocytes in severe chronic obstructive pulmonary disease. BMC Pulm. Med. 2018;18:101. doi: 10.1186/s12890-018-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 44.Herold S., Mayer K., Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herold S., Tabar T.S., Janssen H., Hoegner K., Cabanski M., Lewe-Schlosser P., Albrecht J., Driever F., Vadasz I., Seeger W., Steinmueller M., Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am. J. Respir. Crit. Care Med. 2011;183:1380–1390. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 46.Groves A.M., Johnston C.J., Williams J.P., Finkelstein J.N. Role of infiltrating monocytes in the development of radiation-induced pulmonary fibrosis. Radiat. Res. 2018;189:300–311. doi: 10.1667/RR14874.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson K.M., Ramanan K., Clay M.E., McHugh K.J., Rich H.E., Alcorn J.F. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol. 2018;11:199–208. doi: 10.1038/mi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zizzo G., Cohen P.L. Imperfect storm: is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow K.N., Coopersmith C.M., Ford M.L. IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Front. Immunol. 2019;10:1982. doi: 10.3389/fimmu.2019.01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nascimento D.C., Melo P.H., Piñeros A.R., Ferreira R.G., Colón D.F., Donate P.B., Castanheira F.V., Gozzi A., Czaikoski P.G., Niedbala W., Borges M.C., Zamboni D.S., Liew F.Y., Cunha F.Q., Alves-Filho J.C. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat. Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halstead E.S., Umstead T.M., Davies M.L., Kawasawa Y.I., Silveyra P., Howyrlak J., Yang L., Guo W., Hu S., Hewage E.K., Chroneos Z.C. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization. Respir. Res. 2018;19:3. doi: 10.1186/s12931-017-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckley L.F., Wohlford G.F., Ting C., Alahmed A., Van Tassell B.W., Abbate A., Devlin J.W., Libby P. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit. Care Explor. 2020;2:e0178. doi: 10.1097/CCE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson S., Crotta S., McCabe T.M., Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth K.M., Gunn J.S., Lafuse W., Satoskar A.R. Francisella inhibits STAT1-mediated signaling in macrophages and prevents activation of antigen-specific T cells. Int. Immunol. 2009;21:19–28. doi: 10.1093/intimm/dxn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C.-Y., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T.-T., Cai J.-P., Zhou J., Yuan S., Zhang A.J., Chan J.F.-W., Yuen K.-Y. Attenuated interferon and proinflammatory response in SARS-CoV-2-Infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222:734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuyama T., Kubli S.P., Yoshinaga S.K., Pfeffer K., Mak T.W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020:1–17. doi: 10.1038/s41418-020-00633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hui K.P.Y., Li H.S., Cheung M.C., Chan R.W.Y., Yuen K.M., Mok C.K.P., Nicholls J.M., Peiris J.S.M., Chan M.C.W. Highly pathogenic avian influenza H5N1 virus delays apoptotic responses via activation of STAT3. Sci. Rep. 2016;6:28593. doi: 10.1038/srep28593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsukawa A., Kudo S., Maeda T., Numata K., Watanabe H., Takeda K., Akira S., Ito T. Stat3 in resident macrophages as a repressor protein of inflammatory response. J. Immunol. 2005;175:3354–3359. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 59.Roca Suarez A.A., Van Renne N., Baumert T.F., Lupberger J. Viral manipulation of STAT3: evade, exploit, and injure. PLoS Pathog. 2018;14:e1006839. doi: 10.1371/journal.ppat.1006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H., Tan G., Yang Y., Cui A., Li H., Li T., Wu Z., Yang M., Lv G., Chi X., Niu J., Zhu K., Crispe I.N., Su L., Tu Z. Hepatitis B Virus-Induced Imbalance of Inflammatory and Antiviral Signaling by Differential Phosphorylation of STAT1 in Human Monocytes. J. Immunol. 2019;202:2266–2275. doi: 10.4049/jimmunol.1800848. [DOI] [PubMed] [Google Scholar]
- 61.Kwon Y.-C., Meyer K., Peng G., Chatterjee S., Hoft D.F., Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology. 2019;69:1873–1884. doi: 10.1002/hep.29843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins-McMillen D., Stevenson E.V., Kim J.H., Lee B.-J., Cieply S.J., Nogalski M.T., Chan G.C., Frost R.W., 3rd, Spohn C.R., Yurochko A.D. Human cytomegalovirus utilizes a nontraditional signal transducer and activator of transcription 1 activation cascade via signaling through epidermal growth factor receptor and integrins to efficiently promote the motility. Differentiation, and Polarizat, J. Virol. 2017;91 doi: 10.1128/JVI.00622-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polzin M., McCanless J., Owen S., Sizemore D., Lucero E., Fuller R., Neufeld H.S., Seals D.F., Ahmed M. Oncolytic vesicular stomatitis viruses selectively target M2 macrophages. Virus Res. 2020;284:197991. doi: 10.1016/j.virusres.2020.197991. [DOI] [PubMed] [Google Scholar]
- 64.Yu X., Buttgereit A., Lelios I., Utz S.G., Cansever D., Becher B., Greter M. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–912. doi: 10.1016/j.immuni.2017.10.007. e4. [DOI] [PubMed] [Google Scholar]
- 65.Bonfield T.L., Farver C.F., Barna B.P., Malur A., Abraham S., Raychaudhuri B., Kavuru M.S., Thomassen M.J. Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell Mol. Biol. 2003;29:677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 66.Schneider C., Nobs S.P., Kurrer M., Rehrauer H., Thiele C., Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 67.Szanto A., Balint B.L., Nagy Z.S., Barta E., Dezso B., Pap A., Szeles L., Poliska S., Oros M., Evans R.M., Barak Y., Schwabe J., Nagy L. STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieto C., Bragado R., Municio C., Sierra-Filardi E., Alonso B., Escribese M.M., Domínguez-Andrés J., Ardavín C., Castrillo A., Vega M.A., Puig-Kröger A., Corbí A.L. The activin A-Peroxisome proliferator-activated receptor gamma Axis Contributes to the transcriptome of GM-CSF-Conditioned human macrophages. Front. Immunol. 2018;9:31. doi: 10.3389/fimmu.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duan S.Z., Usher M.G., Mortensen R.M. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ. Res. 2008;102:283–294. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 70.Cai Y., Sugimoto C., Liu D.X., Midkiff C.C., Alvarez X., Lackner A.A., Kim W.-K., Didier E.S., Kuroda M.J. Increased monocyte turnover is associated with interstitial macrophage accumulation and pulmonary tissue damage in SIV-infected rhesus macaques. J. Leukoc. Biol. 2015;97:1147–1153. doi: 10.1189/jlb.4A0914-441R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heming M., Gran S., Jauch S.-L., Fischer-Riepe L., Russo A., Klotz L., Hermann S., Schäfers M., Roth J., Barczyk-Kahlert K. Peroxisome proliferator-activated Receptor-γ modulates the response of macrophages to lipopolysaccharide and glucocorticoids. Front. Immunol. 2018;9:893. doi: 10.3389/fimmu.2018.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guirado E., Rajaram M.V., Chawla A., Daigle J., La Perle K.M., Arnett E., Turner J., Schlesinger L.S. Deletion of PPARγ in lung macrophages provides an immunoprotective response against M. Tuberculosis infection in mice. Tuberculosis Edinb. (Edinb) 2018;111:170–177. doi: 10.1016/j.tube.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reddy A.T., Lakshmi S.P., Zhang Y., Reddy R.C. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages., FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014;28:5299–5310. doi: 10.1096/fj.14-256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landsman L., Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J. Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 75.Subramaniam R., Hillberry Z., Chen H., Feng Y., Fletcher K., Neuenschwander P., Shams H. Delivery of GM-CSF to protect against influenza pneumonia. PLoS One. 2015;10:e0124593. doi: 10.1371/journal.pone.0124593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umstead T.M., Hewage E.K., Mathewson M., Beaudoin S., Chroneos Z.C., Wang M., Halstead E.S. Lower respiratory tract delivery, airway clearance, and preclinical efficacy of inhaled GM-CSF in a postinfluenza pneumococcal pneumonia model. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L571–L579. doi: 10.1152/ajplung.00296.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonaventura A., Vecchié A., Wang T.S., Lee E., Cremer P.C., Carey B., Rajendram P., Hudock K.M., Korbee L., Van Tassell B.W., Dagna L., Abbate A. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front. Immunol. 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lang F.M., Lee K.M.-C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehta P., Porter J.C., Manson J.J., Isaacs J.D., Openshaw P.J.M., McInnes I.B., Summers C., Chambers R.C. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir. Med. 2020;8:822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stifter S.A., Bhattacharyya N., Pillay R., Flórido M., Triccas J.A., Britton W.J., Feng C.G. Functional interplay between type I and II interferons is essential to limit influenza a virus-induced tissue inflammation. PLoS Pathog. 2016;12:e1005378. doi: 10.1371/journal.ppat.1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-Infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Qahtani A.A., Lyroni K., Aznaourova M., Tseliou M., Al-Anazi M.R., Al-Ahdal M.N., Alkahtani S., Sourvinos G., Tsatsanis C. Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4-mediated induction of IRAK-M and PPARγ. Oncotarget. 2017;8:9053–9066. doi: 10.18632/oncotarget.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H., Chen K., Liu M., Xu H., Xu Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J. Infect. 2020;81:115–120. doi: 10.1016/j.jinf.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J., Chu H., Li C., Wong B.H.-Y., Cheng Z.-S., Poon V.K.-M., Sun T., Lau C.C.-Y., Wong K.K.-Y., Chan J.Y.-W., Chan J.F.-W., To K.K.-W., Chan K.-H., Zheng B.-J., Yuen K.-Y. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S.W., Forsberg E.C., Tanaka M., van Rooijen N., García-Sastre A., Stanley E.R., Ginhoux F., Frenette P.S., Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui X., Zeni F., Vodovitz Y., Correa-de-Araujo R., Quezado M., Roberts A., Wahl S., Danner R.L., Banks S.M., Gerstenberger E., Fitz Y., Natanson C., Eichacker P.Q. TGF-beta1 increases microbial clearance but worsens lung injury during Escherichia coli pneumonia in rats. Cytokine. 2003;24:115–127. doi: 10.1016/j.cyto.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Pokharel S.M., Shil N.K., Bose S. Autophagy, TGF-β, and SMAD-2/3 Signaling Regulates Interferon-β Response in Respiratory Syncytial Virus Infected Macrophages. Front. Cell. Infect. Microbiol. 2016;6:174. doi: 10.3389/fcimb.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao J., Zhao Y. Interleukin-33 and its receptor in pulmonary inflammatory diseases. Crit. Rev. Immunol. 2015;35:451–461. doi: 10.1615/CritRevImmunol.2016015865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yende S., Tuomanen E.I., Wunderink R., Kanaya A., Newman A.B., Harris T., de Rekeneire N., Kritchevsky S.B. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am. J. Respir. Crit. Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kellum J.A., Kong L., Fink M.P., Weissfeld L.A., Yealy D.M., Pinsky M.R., Fine J., Krichevsky A., Delude R.L., Angus D.C. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antunes G., Evans S.A., Lordan J.L., Frew A.J. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 2002;20:990–995. doi: 10.1183/09031936.02.00295102. [DOI] [PubMed] [Google Scholar]
- 92.Ioannidis I., McNally B., Willette M., Peeples M.E., Chaussabel D., Durbin J.E., Ramilo O., Mejias A., Flaño E. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J. Virol. 2012;86:5422–5436. doi: 10.1128/JVI.06757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yousufuddin M., Shultz J., Doyle T., Rehman H., Murad M.H. Incremental risk of long-term mortality with increased burden of comorbidity in hospitalized patients with pneumonia. Eur. J. Intern. Med. 2018;55:23–27. doi: 10.1016/j.ejim.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Han X., Zhou F., Li H., Xing X., Chen L., Wang Y., Zhang C., Liu X., Suo L., Wang J., Yu G., Wang G., Yao X., Yu H., Wang L., Liu M., Xue C., Liu B., Zhu X., Li Y., Xiao Y., Cui X., Li L., Purdy J.E., Cao B. Effects of age, comorbidity and adherence to current antimicrobial guidelines on mortality in hospitalized elderly patients with community-acquired pneumonia. BMC Infect. Dis. 2018;18:192. doi: 10.1186/s12879-018-3098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gopal R., Mendy A., Marinelli M.A., Richwalls L.J., Seger P.J., Patel S., McHugh K.J., Rich H.E., Grousd J.A., Forno E., Alcorn J.F. Peroxisome Proliferator-Activated Receptor Gamma (PPAR□) Suppresses Inflammation and Bacterial Clearance during Influenza-Bacterial Super-Infection. Viruses. 2019;11 doi: 10.3390/v11060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Batais M.A., Khan A.R., Bin Abdulhak A.A. The use of statins and risk of community-acquired pneumonia. Curr. Infect. Dis. Rep. 2017;19:26. doi: 10.1007/s11908-017-0581-x. [DOI] [PubMed] [Google Scholar]
- 98.Di Yacovo S., Garcia-Vidal C., Viasus D., Adamuz J., Oriol I., Gili F., Vilarrasa N., García-Somoza M.D., Dorca J., Carratalà J. Clinical features, etiology, and outcomes of community-acquired pneumonia in patients with diabetes mellitus. Medicine (Baltimore). 2013;92:42–50. doi: 10.1097/MD.0b013e31827f602a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lipworth B., Chan R., Lipworth S., RuiWen Kuo C. Weathering the cytokine storm in susceptible patients with severe SARS-CoV-2 infection. J. Allergy Clin. Immunol. Pract. 2020;8:1798–1801. doi: 10.1016/j.jaip.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rice J.B., White A.G., Scarpati L.M., Wan G., Nelson W.W. Long-term systemic corticosteroid exposure: a systematic literature review. Clin. Ther. 2017;39:2216–2229. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 101.Singanayagam A., Glanville N., Girkin J.L., Ching Y.M., Marcellini A., Porter J.D., Toussaint M., Walton R.P., Finney L.J., Aniscenko J., Zhu J., Trujillo-Torralbo M.-B., Calderazzo M.A., Grainge C., Loo S.-L., Veerati P.C., Pathinayake P.S., Nichol K.S., Reid A.T., James P.L., Solari R., Wark P.A.B., Knight D.A., Moffatt M.F., Cookson W.O., Edwards M.R., Mallia P., Bartlett N.W., Johnston S.L. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat. Commun. 2018;9:2229. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sperber K., Quraishi H., Kalb T.H., Panja A., Stecher V., Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J. Rheumatol. 1993;20:803–808. [PubMed] [Google Scholar]
- 103.Kageyama T., Furuta S., Ikeda K., Kagami S.-I., Kashiwakuma D., Sugiyama T., Umibe T., Watanabe N., Yamagata M., Nakajima H. Prognostic factors of Pneumocystis pneumonia in patients with systemic autoimmune diseases. PLoS One. 2019;14:e0214324. doi: 10.1371/journal.pone.0214324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chew L.-C., Maceda-Galang L.M., Tan Y.K., Chakraborty B., Thumboo J. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J. Clin. Rheumatol. Pract. Reports Rheum. Musculoskelet. Dis. 2015;21:72–75. doi: 10.1097/RHU.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 105.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., Alijotas-Reig J., Zinserling V., Semenova N., Amital H., Shoenfeld Y. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Endeman H., Meijvis S.C.A., Rijkers G.T., van Velzen-Blad H., van Moorsel C.H.M., Grutters J.C., Biesma D.H. Systemic cytokine response in patients with community-acquired pneumonia. Eur. Respir. J. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
- 107.Koshiol J., Rotunno M., Consonni D., Pesatori A.C., De Matteis S., Goldstein A.M., Chaturvedi A.K., Wacholder S., Landi M.T., Lubin J.H., Caporaso N.E. Lower risk of lung cancer after multiple pneumonia diagnoses. Cancer Epidemiol. Biomarkers Prev. a Publ. Am. Assoc. Cancer Res. Cosponsored by Am. Soc. Prev. Oncol. 2010;19:716–721. doi: 10.1158/1055-9965.EPI-09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamaoka T., Arata S., Homma M., Homma T., Kusumoto S., Ando K., Manabe R., Kishino Y., Ohba M., Tsurutani J., Takimoto M., Ohmori T., Sagara H. Blockade of EGFR activation promotes TNF-Induced lung epithelial cell apoptosis and pulmonary injury. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20164021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tzouvelekis A., Ntolios P., Karameris A., Vilaras G., Boglou P., Koulelidis A., Archontogeorgis K., Kaltsas K., Zacharis G., Sarikloglou E., Steiropoulos P., Mikroulis D., Koutsopoulos A., Froudarakis M., Bouros D. Increased expression of epidermal growth factor receptor (EGF-R) in patients with different forms of lung fibrosis. Biomed Res. Int. 2013;2013:654354. doi: 10.1155/2013/654354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khalfaoui S., Eichhorn V., Karagiannidis C., Bayh I., Brockmann M., Pieper M., Windisch W., Schildgen O., Schildgen V. Lung infection by human bocavirus induces the release of profibrotic mediator cytokines in vivo and in vitro. PLoS One. 2016;11:e0147010. doi: 10.1371/journal.pone.0147010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rotz S.J., Leino D., Szabo S., Mangino J.L., Turpin B.K., Pressey J.G. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr. Blood Cancer. 2017;64 doi: 10.1002/pbc.26642. [DOI] [PubMed] [Google Scholar]
- 112.Schoenfeld J.D., Nishino M., Severgnini M., Manos M., Mak R.H., Hodi F.S. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J. Immunother. Cancer. 2019;7:112. doi: 10.1186/s40425-019-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jodai T., Yoshida C., Sato R., Kakiuchi Y., Sato N., Iyama S., Kimura T., Saruwatari K., Saeki S., Ichiyasu H., Fujii K., Tomita Y. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-PD-1 immune checkpoint antibody in a lung cancer patient. Immunity, Inflamm. Dis. 2019;7:3–6. doi: 10.1002/iid3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Pérez-Gracia J.L., Rodríguez-Ruiz M.E., Ponz-Sarvise M., Castañón E., Melero I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Waldmann T.A. Cytokines in Cancer immunotherapy. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inui T., Watanabe M., Nakamoto K., Sada M., Hirata A., Nakamura M., Honda K., Ogawa Y., Takata S., Yokoyama T., Saraya T., Kurai D., Wada H., Ishii H., Takizawa H. Bronchial epithelial cells produce CXCL1 in response to LPS and TNFα: A potential role in the pathogenesis of COPD. Exp. Lung Res. 2018;44:323–331. doi: 10.1080/01902148.2018.1520936. [DOI] [PubMed] [Google Scholar]
- 117.Crisafulli E., Menéndez R., Huerta A., Martinez R., Montull B., Clini E., Torres A. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest. 2013;143:1009–1017. doi: 10.1378/chest.12-1684. [DOI] [PubMed] [Google Scholar]
- 118.Knobloch J., Panek S., Yanik S.D., Jamal Jameel K., Bendella Z., Jungck D., Bürger P., Bülthoff E., Struck B., Giannakis N., Rupp J., Kronsbein J., Peters M., Koch A. The monocyte-dependent immune response to bacteria is suppressed in smoking-induced COPD. J. Mol. Med. 2019;97:817–828. doi: 10.1007/s00109-019-01778-w. [DOI] [PubMed] [Google Scholar]
- 119.Zaidi S.R., Blakey J.D. Why are people with asthma susceptible to pneumonia? A review of factors related to upper airway bacteria. Respirology. 2019;24:423–430. doi: 10.1111/resp.13528. [DOI] [PubMed] [Google Scholar]
- 120.Sergejeva S., Ivanov S., Lötvall J., Lindén A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2005;33:248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 121.Cai T., Qiu J., Ji Y., Li W., Ding Z., Suo C., Chang J., Wang J., He R., Qian Y., Guo X., Zhou L., Sheng H., Shen L., Qiu J. IL-17-producing ST2(+) group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J. Allergy Clin. Immunol. 2019;143:229–244. doi: 10.1016/j.jaci.2018.03.007. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanania N.A. Targeting airway inflammation in asthma: current and future therapies. Chest. 2008;133:989–998. doi: 10.1378/chest.07-0829. [DOI] [PubMed] [Google Scholar]
- 123.Kim M.H., Rhee C.K., Shim J.S., Park S.Y., Yoo K.H., Kim B.Y., Bae H.W., Sim Y.S., Chang J.H., Cho Y.J., Lee J.H. Inhaled corticosteroids in asthma and the risk of pneumonia. Allergy Asthma Immunol. Res. 2019;11:795–805. doi: 10.4168/aair.2019.11.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian C.J., Coulombe J., Suissa S., Ernst P. Pneumonia risk in asthma patients using inhaled corticosteroids: a quasi-cohort study. Br. J. Clin. Pharmacol. 2017;83:2077–2086. doi: 10.1111/bcp.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int. Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kloch A., Wenzel M.A., Laetsch D.R., Michalski O., Bajer A., Behnke J.M., Welc-Falęciak R., Piertney S.B. Signatures of balancing selection in toll-like receptor (TLRs) genes - novel insights from a free-living rodent. Sci. Rep. 2018;8:8361. doi: 10.1038/s41598-018-26672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gallagher P.M., Lowe G., Fitzgerald T., Bella A., Greene C.M., McElvaney N.G., O’Neill S.J. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–156. doi: 10.1136/thorax.58.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arnalich F., López J., Codoceo R., Jim nez M., Madero R., Montiel C. Relationship of plasma leptin to plasma cytokines and human survivalin sepsis and septic shock. J. Infect. Dis. 1999;180:908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- 129.Keshavarz M., Namdari H., Farahmand M., Mehrbod P., Mokhtari-Azad T., Rezaei F. Association of polymorphisms in inflammatory cytokines encoding genes with severe cases of influenza A/H1N1 and B in an Iranian population. Virol. J. 2019;16:79. doi: 10.1186/s12985-019-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khateeb J., Fuchs E., Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev. Diabet. Stud. 2019;15:1–15. doi: 10.1900/RDS.2019.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kankaanranta H., Kauppi P., Tuomisto L.E., Ilmarinen P. Emerging comorbidities in adult asthma: risks, clinical associations, and mechanisms. Mediators Inflamm. 2016;2016:3690628. doi: 10.1155/2016/3690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Y., Li S., Zhang G., Nie G., Meng Z., Mao D., Chen C., Chen X., Zhou B., Zeng G. Genetic variants in IL1A and IL1B contribute to the susceptibility to 2009 pandemic H1N1 influenza A virus. BMC Immunol. 2013;14:37. doi: 10.1186/1471-2172-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woods N.T., Monteiro A.N., Thompson Z.J., Amankwah E.K., Naas N., Haura E.B., Beg A.A., Schabath M.B. Interleukin polymorphisms associated with overall survival, disease-free survival, and recurrence in non-small cell lung cancer patients. Mol. Carcinog. 2015;54(Suppl 1):172–184. doi: 10.1002/mc.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Charbonneau B., Block M.S., Bamlet W.R., Vierkant R.A., Kalli K.R., Fogarty Z., Rider D.N., Sellers T.A., Tworoger S.S., Poole E., Risch H.A., Salvesen H.B., Kiemeney L.A., Baglietto L., Giles G.G., Severi G., Trabert B., Wentzensen N., Chenevix-Trench G., Whittemore A.S., Sieh W., Chang-Claude J., Bandera E.V., Orlow I., Terry K., Goodman M.T., Thompson P.J., Cook L.S., Rossing M.A., Ness R.B., Narod S.A., Kupryjanczyk J., Lu K., Butzow R., Dörk T., Pejovic T., Campbell I., Le N.D., Bunker C.H., Bogdanova N., Runnebaum I.B., Eccles D., Paul J., Wu A.H., Gayther S.A., Hogdall E., Heitz F., Kaye S.B., Karlan B.Y., Anton-Culver H., Gronwald J., Hogdall C.K., Lambrechts D., Fasching P.A., Menon U., Schildkraut J., Pearce C.L., Levine D.A., Kjaer S.K., Cramer D., Flanagan J.M., Phelan C.M., Brown R., Massuger L.F.A.G., Song H., Doherty J.A., Krakstad C., Liang D., Odunsi K., Berchuck A., Jensen A., Lubinski J., Nevanlinna H., Bean Y.T., Lurie G., Ziogas A., Walsh C., Despierre E., Brinton L., Hein A., Rudolph A., Dansonka-Mieszkowska A., Olson S.H., Harter P., Tyrer J., Vitonis A.F., Brooks-Wilson A., Aben K.K., Pike M.C., Ramus S.J., Wik E., Cybulski C., Lin J., Sucheston L., Edwards R., McGuire V., Lester J., du Bois A., Lundvall L., Wang-Gohrke S., Szafron L.M., Lambrechts S., Yang H., Beckmann M.W., Pelttari L.M., Van Altena A.M., van den Berg D., Halle M.K., Gentry-Maharaj A., Schwaab I., Chandran U., Menkiszak J., Ekici A.B., Wilkens L.R., Leminen A., Modugno F., Friel G., Rothstein J.H., Vergote I., Garcia-Closas M., Hildebrandt M.A.T., Sobiczewski P., Kelemen L.E., Pharoah P.D.P., Moysich K., Knutson K.L., Cunningham J.M., Fridley B.L., Goode E.L. Risk of ovarian cancer and the NF-κB pathway: genetic association with IL1A and TNFSF10. Cancer Res. 2014;74:852–861. doi: 10.1158/0008-5472.CAN-13-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sigurdson A.J., Bhatti P., Doody M.M., Hauptmann M., Bowen L., Simon S.L., Weinstock R.M., Linet M.S., Rosenstein M., Stovall M., Alexander B.H., Preston D.L., Struewing J.P., Rajaraman P. Polymorphisms in apoptosis- and proliferation-related genes, ionizing radiation exposure, and risk of breast cancer among U.S. Radiologic Technologists. Cancer Epidemiol. Biomarkers Prev. a Publ. Am. Assoc. Cancer Res. Cosponsored by Am. Soc. Prev. Oncol. 2007;16:2000–2007. doi: 10.1158/1055-9965.EPI-07-0282. [DOI] [PubMed] [Google Scholar]
- 136.Leal V.N.C., Genov I.R., Mallozi M.C., Solé D., Pontillo A. Polymorphisms in inflammasome genes and risk of asthma in Brazilian children. Mol. Immunol. 2018;93:64–67. doi: 10.1016/j.molimm.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 137.Lind H., Haugen A., Zienolddiny S. Differential binding of proteins to the IL1B -31 T/C polymorphism in lung epithelial cells. Cytokine. 2007;38:43–48. doi: 10.1016/j.cyto.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 138.Eaton K.D., Romine P.E., Goodman G.E., Thornquist M.D., Barnett M.J., Petersdorf E.W. Inflammatory gene polymorphisms in lung Cancer susceptibility. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2018;13:649–659. doi: 10.1016/j.jtho.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L., Zhao W., Hong J., Niu F., Li J., Zhang S., Jin T. Association between IL1B gene and cervical cancer susceptibility in Chinese Uygur Population: a Case-Control study. Mol. Genet. Genomic Med. 2019;7:e779. doi: 10.1002/mgg3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ponomarenko M.P., Arkova O., Rasskazov D., Ponomarenko P., Savinkova L., Kolchanov N. Candidate SNP Markers of Gender-Biased Autoimmune Complications of Monogenic Diseases Are Predicted by a Significant Change in the Affinity of TATA-Binding Protein for Human Gene Promoters. Front. Immunol. 2016;7:130. doi: 10.3389/fimmu.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Watanabe E., Hirasawa H., Oda S., Shiga H., Matsuda K., Nakamura M., Abe R., Nakada T. Cytokine-related genotypic differences in peak interleukin-6 blood levels of patients with SIRS and septic complications. J. Trauma. 2005;59:1181–1190. doi: 10.1097/00005373-200511000-00025. [DOI] [PubMed] [Google Scholar]
- 142.Iglesias Molli A.E., Bergonzi M.F., Spalvieri M.P., Linari M.A., Frechtel G.D., Cerrone G.E. Relationship between the IL-1β serum concentration, mRNA levels and rs16944 genotype in the hyperglycemic normalization of T2D patients. Sci. Rep. 2020;10:9985. doi: 10.1038/s41598-020-66751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Falfán-Valencia R., Pavón-Romero G.F., Camarena A., de la M., García L., Galicia-Negrete G., Negrete-García M.C., Teran L.M. The IL1B-511 polymorphism (rs16944 AA genotype) is increased in aspirin-exacerbated respiratory disease in mexican population. J. Allergy (Cairo) 2012;2012:741313. doi: 10.1155/2012/741313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sangil A., Arranz M.J., Güerri-Fernández R., Pérez M., Monzón H., Payeras A., Andrés M., Torviso J., Ibañez L., Garau J., Calbo E. Genetic susceptibility to invasive pneumococcal disease. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018;59:126–131. doi: 10.1016/j.meegid.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 145.Lingappa J.R., Dumitrescu L., Zimmer S.M., Lynfield R., McNicholl J.M., Messonnier N.E., Whitney C.G., Crawford D.C. Identifying host genetic risk factors in the context of public health surveillance for invasive pneumococcal disease. PLoS One. 2011;6:e23413. doi: 10.1371/journal.pone.0023413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Awasthi S., Yadav K.K., Pandey M., Mahdi A.A., Awasthi N. Interleukin 1 receptor antagonist (IL1RA) gene polymorphism and levels associated with adverse outcome in severe community-acquired pneumonia in children: a hospital-based study in India. Pediatr. Pulmonol. 2018;53:1276–1283. doi: 10.1002/ppul.24090. [DOI] [PubMed] [Google Scholar]
- 147.Settin A., Zedan M., Farag M., Ezz El M., Regal E. Osman, Gene polymorphisms of IL-6(-174) G/C and IL-1Ra VNTR in asthmatic children. Indian J. Pediatr. 2008;75:1019–1023. doi: 10.1007/s12098-008-0161-z. [DOI] [PubMed] [Google Scholar]
- 148.Settin A., Ismail A., El-Magd M.A., El-Baz R., Kazamel A. Gene polymorphisms of TNF-alpha-308 (G/A), IL-10(-1082) (G/A), IL-6(-174) (G/C) and IL-1Ra (VNTR) in Egyptian cases with type 1 diabetes mellitus. Autoimmunity. 2009;42:50–55. doi: 10.1080/08916930802292510. [DOI] [PubMed] [Google Scholar]
- 149.Waterer G.W., Wunderink R.G. Science review: genetic variability in the systemic inflammatory response. Crit. Care. 2003;7:308–314. doi: 10.1186/cc2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patarčić I., Gelemanović A., Kirin M., Kolčić I., Theodoratou E., Baillie K.J., de Jong M.D., Rudan I., Campbell H., Polašek O. The role of host genetic factors in respiratory tract infectious diseases: systematic review, meta-analyses and field synopsis. Sci. Rep. 2015;5:16119. doi: 10.1038/srep16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoebee B., Rietveld E., Bont L., van Oosten M., Hodemaekers H.M., Nagelkerke N.J.D., Neijens H.J., Kimpen J.L.L., Kimman T.G. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J. Infect. Dis. 2003;187:2–11. doi: 10.1086/345859. [DOI] [PubMed] [Google Scholar]
- 152.Hussein I.A., Jaber S.H. Genotyping of IL-4 -590 (C&T) gene in iraqi asthma patients. Dis. Markers. 2017;2017:5806236. doi: 10.1155/2017/5806236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park H.K., Kim S.K., Kweon H.Y., Lee K.G., Arasu M.V., Kim Y.O. Promoter polymorphism (-590, T/C) of interleukin 4 (IL4) gene is associated with rheumatoid arthritis: an updated meta-analysis. Saudi J. Biol. Sci. 2017;24:444–449. doi: 10.1016/j.sjbs.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jia Y., Xie X., Shi X., Li S. Associations of common IL-4 gene polymorphisms with cancer risk: a meta-analysis. Mol. Med. Rep. 2017;16:1927–1945. doi: 10.3892/mmr.2017.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Loza M.J., Chang B.-L. Association between Q551R IL4R genetic variants and atopic asthma risk demonstrated by meta-analysis. J. Allergy Clin. Immunol. 2007;120:578–585. doi: 10.1016/j.jaci.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 156.Smelaya T.V., Belopolskaya O.B., Smirnova S.V., Kuzovlev A.N., Moroz V.V., Golubev A.M., Pabalan N.A., Salnikova L.E. Genetic dissection of host immune response in pneumonia development and progression. Sci. Rep. 2016;6:35021. doi: 10.1038/srep35021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perovic D., Perovic V., Pravica V., Bonaci-Nikolic B., Mijanovic R., Bunjevacki V. Evaluation of cytokine genetic polymorphisms in adult patients with common variable immunodeficiency: a single-center study. Immunol. Lett. 2016;176:97–104. doi: 10.1016/j.imlet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 158.Feng B., Mao Z., Pang K., Zhang S., Li L. Association of tumor necrosis factor α -308G/A and interleukin-6-174G/C gene polymorphism with pneumonia-induced sepsis. J. Crit. Care. 2015;30:920–923. doi: 10.1016/j.jcrc.2015.04.123. [DOI] [PubMed] [Google Scholar]
- 159.Zidan H.E., Elbehedy R.M., Azab S.F. IL6-174 G/C gene polymorphism and its relation to serum IL6 in Egyptian children with community-acquired pneumonia. Cytokine. 2014;67:60–64. doi: 10.1016/j.cyto.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 160.Peng X., Shi J., Sun W., Ruan X., Guo Y., Zhao L., Wang J., Li B. Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget. 2018;9:12351–12364. doi: 10.18632/oncotarget.24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li F., Xie X., Li S., Ke R., Zhu B., Yang L., Li M. Interleukin-6 gene -174G/C polymorphism and bronchial asthma risk: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8:12601–12608. [PMC free article] [PubMed] [Google Scholar]
- 162.Schuurhof A., Bont L., Siezen C.L.E., Hodemaekers H., van Houwelingen H.C., Kimman T.G., Hoebee B., Kimpen J.L.L., Janssen R. Interleukin-9 polymorphism in infants with respiratory syncytial virus infection: an opposite effect in boys and girls. Pediatr. Pulmonol. 2010;45:608–613. doi: 10.1002/ppul.21229. [DOI] [PubMed] [Google Scholar]
- 163.Sordillo J.E., Kelly R., Bunyavanich S., McGeachie M., Qiu W., Croteau-Chonka D.C., Soto-Quiros M., Avila L., Celedón J.C., Brehm J.M., Weiss S.T., Gold D.R., Litonjua A.A. Genome-wide expression profiles identify potential targets for gene-environment interactions in asthma severity. J. Allergy Clin. Immunol. 2015;136:885–892. doi: 10.1016/j.jaci.2015.02.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McNamara P.S., Smyth R.L. Interleukin-9 as a possible therapeutic target in both asthma and chronic obstructive airways disease. Drug News Perspect. 2005;18:615–621. doi: 10.1358/dnp.2005.18.10.959575. [DOI] [PubMed] [Google Scholar]
- 165.Moretti S., Renga G., Oikonomou V., Galosi C., Pariano M., Iannitti R.G., Borghi M., Puccetti M., De Zuani M., Pucillo C.E., Paolicelli G., Zelante T., Renauld J.-C., Bereshchenko O., Sportoletti P., Lucidi V., Russo M.C., Colombo C., Fiscarelli E., Lass-Flörl C., Majo F., Ricciotti G., Ellemunter H., Ratclif L., Talesa V.N., Napolioni V., Romani L. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat. Commun. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kang J., Liu C.-H., Lee C.-N., Li H.-Y., Yang C.-W., Huang S.-C., Lin S.-Y., Jou T.-S. Novel Interleukin-10 gene polymorphism is linked to gestational diabetes in taiwanese population. Front. Genet. 2019;10:89. doi: 10.3389/fgene.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bai H., Jing D., Guo A., Yin S. Association between interleukin 10 gene polymorphisms and risk of type 2 diabetes mellitus in a Chinese population. J. Int. Med. Res. 2014;42:702–710. doi: 10.1177/0300060513505813. [DOI] [PubMed] [Google Scholar]
- 168.Amirian E., Liu Y., Scheurer M.E., El-Zein R., Gilbert M.R., Bondy M.L. Genetic variants in inflammation pathway genes and asthma in glioma susceptibility. Neuro. Oncol. 2010;12:444–452. doi: 10.1093/neuonc/nop057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu Z., Liu J.-B., Liu X., Qian L. Association of interleukin 10 rs1800896 polymorphism with susceptibility to breast cancer: a meta-analysis. J. Int. Med. Res. 2020;48 doi: 10.1177/0300060520904863. 300060520904863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sakamoto K., Oka M., Yoshino S., Hazama S., Takeda S., Yoshimura K., Okayama N., Hinoda Y. Relationship between cytokine gene polymorphisms and risk of postoperative pneumonia with esophageal cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2014;18:1247–1253. doi: 10.1007/s11605-014-2531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barber L.A., Soldano K., Garrett M., Orringer E.P., Eckman J.R., Telen M.J., Ashley-Koch A.E. Inflammatory polymorphisms link the risk of acute chest syndrome with asthma in adults with sickle cell disease. Blood. 2011;118:1072. doi: 10.1182/blood.V118.21.1072.1072. [DOI] [Google Scholar]
- 172.Frade-Barros A.F., Ianni B.M., Cabantous S., Pissetti C.W., Saba B., Lin-Wang H.T., Buck P., Marin-Neto J.A., Schmidt A., Dias F., Hirata M.H., Sampaio M., Fragata A., Pereira A.C., Donadi E., Rodrigues V., Kalil J., Chevillard C., Cunha-Neto E. Polymorphisms in genes affecting Interferon-γ production and Th1 t cell differentiation are associated with progression to chagas disease cardiomyopathy. Front. Immunol. 2020;11:1386. doi: 10.3389/fimmu.2020.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alvarez A.E., Marson F.A.L., Bertuzzo C.S., Bastos J.C.S., Baracat E.C.E., Brandão M.B., Tresoldi A.T., das Neves Romaneli M.T., Almeida C.C.B., de Oliveira T., Schlodtmann P.G., Corrêa E., de Miranda M.L.F., Dos Reis M.C., De Pieri J.V., Arns C.W., Ribeiro J.D. Association between single nucleotide polymorphisms in TLR4, TLR2, TLR9, VDR, NOS2 and CCL5 genes with acute viral bronchiolitis. Gene. 2018;645:7–17. doi: 10.1016/j.gene.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kidd L.R., Jones D.Z., Rogers E.N., Kidd N.C., Beache S., Rudd J.E., Ragin C., Jackson M., McFarlane-Anderson N., Tulloch-Reid M., Morrison S., Brock G.N., Barve S.S., Kimbro K.S. Chemokine Ligand 5 (CCL5) and chemokine receptor (CCR5) genetic variants and prostate cancer risk among men of African Descent: a case-control study. Hered. Cancer Clin. Pract. 2012;10:16. doi: 10.1186/1897-4287-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shan J., Chouchane A., Mokrab Y., Saad M., Boujassoum S., Sayaman R.W., Ziv E., Bouaouina N., Remadi Y., Gabbouj S., Roelands J., Ma X., Bedognetti D., Chouchane L. Genetic variation in CCL5 signaling genes and triple negative breast Cancer: susceptibility and prognosis implications. Front. Oncol. 2019;9:1328.. doi: 10.3389/fonc.2019.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tu X., Chong W.P., Zhai Y., Zhang H., Zhang F., Wang S., Liu W., Wei M., Siu N.H.O., Yang H., Yang W., Cao W., Lau Y.L., He F., Zhou G. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J. Infect. 2015;71:101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fu Z., Jiang Y., Liu J., Lin Z., Jin Y. Study on plasma CC chemokine ligand 2 level and its promoter region 2518A/G polymorphism in MS patients. Eur. J. Inflamm. 2020;18 doi: 10.1177/2058739220959913. 2058739220959913. [DOI] [Google Scholar]
- 178.He S., Zhang X. The rs1024611 in the CCL2 gene and risk of gynecological cancer in Asians: a meta-analysis. World J. Surg. Oncol. 2018;16:34. doi: 10.1186/s12957-018-1335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun T., Mary L.G.-S., Oh W.K., Freedman M.L., Pomerantz M., Pienta K.J., Kantoff P.W. Inherited variants in the chemokine CCL2 gene and prostate cancer aggressiveness in a Caucasian cohort. Clin. Cancer Res. an Off. J. Am. Assoc. Cancer Res. 2011;17:1546–1552. doi: 10.1158/1078-0432.CCR-10-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Keynan Y., Juno J., Meyers A., Ball T.B., Kumar A., Rubinstein E., Fowke K.R. Chemokine receptor 5 △32 allele in patients with severe pandemic (H1N1) 2009. Emerg. Infect. Dis. 2010;16:1621–1622. doi: 10.3201/eid1610.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mlynarski W.M., Placha G.P., Wolkow P.P., Bochenski J.P., Warram J.H., Krolewski A.S. Risk of diabetic nephropathy in type 1 diabetes is associated with functional polymorphisms in RANTES receptor gene (CCR5): a sex-specific effect. Diabetes. 2005;54:3331–3335. doi: 10.2337/diabetes.54.11.3331. [DOI] [PubMed] [Google Scholar]
- 182.Muntinghe F.L.H., Gross S., Bakker S.J.L., Landman G.W.D., van der Harst P., Bilo H.J.G., Navis G., Zuurman M.W. CCR5Delta32 genotype is associated with outcome in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2009;86:140–145. doi: 10.1016/j.diabres.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 183.Mañes S., Mira E., Colomer R., Montero S., Real L.M., Gómez-Moutón C., Jiménez-Baranda S., Garzón A., Lacalle R.A., Harshman K., Ruíz A., Martínez-A C. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J. Exp. Med. 2003;198:1381–1389. doi: 10.1084/jem.20030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Azhar A., Fatima F., Hameed A., Saleem S. Delta 32 mutation in CCR5 gene and its association with breast cancer. J. Clin. Oncol. 2015;33:17. doi: 10.1200/jco.2015.33.28_suppl.17. [DOI] [Google Scholar]
- 185.García-Ramírez R.A., Ramírez-Venegas A., Quintana-Carrillo R., Camarena Á.E., Falfán-Valencia R., Mejía-Aranguré J.M. TNF, IL6, and IL1B Polymorphisms Are Associated with Severe Influenza A (H1N1) Virus Infection in the Mexican Population. PLoS One. 2015;10:e0144832. doi: 10.1371/journal.pone.0144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hameed I., Masoodi S.R., Malik P.A., Mir S.A., Ghazanfar K., Ganai B.A. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018;661:51–59. doi: 10.1016/j.gene.2018.03.095. [DOI] [PubMed] [Google Scholar]
- 187.Kinder B.W., Freemer M.M., King T.E.J., Lum R.F., Nititham J., Taylor K., Edberg J.C., Bridges S.L.J., Criswell L.A. Clinical and genetic risk factors for pneumonia in systemic lupus erythematosus. Arthritis Rheum. 2007;56:2679–2686. doi: 10.1002/art.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Solé-Violán J., v de Castro F., García-Laorden M.I., Blanquer J., Aspa J., Borderías L., Briones M.L., Rajas O., Carrondo I.M.-L., Marcos-Ramos J.A., Ferrer Agüero J.M., Garcia-Saavedra A., Fiuza M.D., Caballero-Hidalgo A., Rodriguez-Gallego C. Genetic variability in the severity and outcome of community-acquired pneumonia. Respir. Med. 2010;104:440–447. doi: 10.1016/j.rmed.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 189.Komata T., Tsuchiya N., Matsushita M., Hagiwara K., Tokunaga K. Association of tumor necrosis factor receptor 2 (TNFR2) polymorphism with susceptibility to systemic lupus erythematosus. Tissue Antigens. 1999;53:527–533. doi: 10.1034/j.1399-0039.1999.530602.x. [DOI] [PubMed] [Google Scholar]
- 190.Dieudé P., Petit E., Cailleau-Moindrault S., Osorio J., Pierlot C., Martinez M., Fauré S., Alibert O., Lasbleiz S., De Toma C., Bardin T., Prum B., Cornélis F. Association between tumor necrosis factor receptor II and familial, but not sporadic, rheumatoid arthritis: evidence for genetic heterogeneity. Arthritis Rheum. 2002;46:2039–2044. doi: 10.1002/art.10101. [DOI] [PubMed] [Google Scholar]
- 191.Guan X., Liao Z., Ma H., Qian J., Liu Z., Yuan X., Gomez D., Komaki R., Wang L.-E., Wei Q. TNFRSF1B +676 T&G polymorphism predicts survival of non-small cell lung cancer patients treated with chemoradiotherapy. BMC Cancer. 2011;11:447. doi: 10.1186/1471-2407-11-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Savva A., Brouwer M.C., Roger T., Valls Serón M., Le Roy D., Ferwerda B., van der Ende A., Bochud P.-Y., van de Beek D., Calandra T. Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:3597–3602. doi: 10.1073/pnas.1520727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kang I., Bucala R. The immunobiology of MIF: function, genetics and prospects for precision medicine. Nat. Rev. Rheumatol. 2019;15:427–437. doi: 10.1038/s41584-019-0238-2. [DOI] [PubMed] [Google Scholar]
- 194.Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J. Clin. Immunol. 2013;33(Suppl 1):S72–S78. doi: 10.1007/s10875-012-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Toubiana J., Courtine E., Tores F., Asfar P., Daubin C., Rousseau C., Ouaaz F., Marin N., Cariou A., Chiche J.-D., Mira J.-P. Association of REL polymorphisms and outcome of patients with septic shock. Ann. Intensive Care. 2016;6:28. doi: 10.1186/s13613-016-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pan W., Zhang A.Q., Gu W., Gao J.W., Du D.Y., Zhang L.Y., Zeng L., Du J., Wang H.Y., Jiang J.X. Identification of haplotype tag single nucleotide polymorphisms within the nuclear factor-κB family genes and their clinical relevance in patients with major trauma. Crit. Care. 2015;19:95. doi: 10.1186/s13054-015-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen F., Xu L., Zhao T., Xiao X., Pan Y., Hou S. Genetic variation in the REL gene increases risk of behcet’s disease in a chinese han population but that of PRKCQ does not. PLoS One. 2016;11:e0147350. doi: 10.1371/journal.pone.0147350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bajwa E.K., Cremer P.C., Gong M.N., Zhai R., Su L., Thompson B.T., Christiani D.C. An NFKB1 promoter insertion/deletion polymorphism influences risk and outcome in acute respiratory distress syndrome among Caucasians. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019469. e19469–e19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Oltulu Y.M., Coskunpinar E., Ozkan G., Aynaci E., Yildiz P., Isbir T., Yaylim I. Investigation of NF-κB1 and NF-κBIA gene polymorphism in non-small cell lung Cancer. Biomed Res. Int. 2014;2014:530381. doi: 10.1155/2014/530381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang D., Xie T., Xu J., Wang H., Zeng W., Rao S., Zhou K., Pei F., Zhou Z. Genetic association between NFKB1 −94 ins/del ATTG Promoter Polymorphism and cancer risk: a meta-analysis of 42 case-control studies. Sci. Rep. 2016;6:30220. doi: 10.1038/srep30220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gautam A., Gupta S., Mehndiratta M., Sharma M., Singh K., Kalra O.P., Agarwal S., Gambhir J.K. Association of NFKB1 gene polymorphism (rs28362491) with levels of inflammatory biomarkers and susceptibility to diabetic nephropathy in Asian Indians. World J. Diabetes. 2017;8:66–73. doi: 10.4239/wjd.v8.i2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chapgier A., Boisson-Dupuis S., Jouanguy E., Vogt G., Feinberg J., Prochnicka-Chalufour A., Casrouge A., Yang K., Soudais C., Fieschi C., Santos O.F., Bustamante J., Picard C., de Beaucoudrey L., Emile J.-F., Arkwright P.D., Schreiber R.D., Rolinck-Werninghaus C., Rösen-Wolff A., Magdorf K., Roesler J., Casanova J.-L. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2006;2:e131. doi: 10.1371/journal.pgen.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boisson-Dupuis S., Kong X.-F., Okada S., Cypowyj S., Puel A., Abel L., Casanova J.-L. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr. Opin. Immunol. 2012;24:364–378. doi: 10.1016/j.coi.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Aldave Becerra J.C., Cachay Rojas E. A 3-Year-Old Girl with Recurrent Infections and Autoimmunity due to a STAT1 Gain-of-Function Mutation: The Expanding Clinical Presentation of Primary Immunodeficiencies. Front. Pediatr. 2017;5:55. doi: 10.3389/fped.2017.00055. https://www.frontiersin.org/article/10.3389/fped.2017.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chapgier A., Wynn R.F., Jouanguy E., Filipe-Santos O., Zhang S., Feinberg J., Hawkins K., Casanova J.-L., Arkwright P.D. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J. Immunol. 2006;176:5078–5083. doi: 10.4049/jimmunol.176.8.5078. [DOI] [PubMed] [Google Scholar]
- 206.Dupuis S., Dargemont C., Fieschi C., Thomassin N., Rosenzweig S., Harris J., Holland S.M., Schreiber R.D., Casanova J.L. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–303. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 207.Li D., Matta B., Song S., Nelson V., Diggins K., Simpfendorfer K.R., Gregersen P.K., Linsley P., Barnes B.J. IRF5 genetic risk variants drive myeloid-specific IRF5 hyperactivation and presymptomatic SLE. JCI Insight. 2020;5 doi: 10.1172/jci.insight.124020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Clark D., Read R., Mayhew V., Petersen S., Argueta L., Stutz L., Till R., Bergsten S., Robinson B., Baumann D., Heap J., Poole B. Four promoters of IRF5 respond distinctly to stimuli and are affected by autoimmune-risk polymorphisms. Front. Immunol. 2013;4:360. doi: 10.3389/fimmu.2013.00360. https://www.frontiersin.org/article/10.3389/fimmu.2013.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Carmona F.D., Martin J.-E., Beretta L., Simeón C.P., Carreira P.E., Callejas J.L., Fernández-Castro M., Sáez-Comet L., Beltrán E., Camps M.T., Egurbide M.V., the S.S. Group, Airó P., Scorza R., Lunardi C., Hunzelmann N., Riemekasten G., Witte T., Kreuter A., Distler J.H.W., Madhok R., Shiels P., van Laar J.M., Fonseca C., Denton C., Herrick A., Worthington J., Schuerwegh A.J., Vonk M.C., Voskuyl A.E., Radstake T.R.D.J., Martín J. The systemic lupus erythematosus IRF5 risk haplotype is associated with systemic sclerosis. PLoS One. 2013;8:e54419. doi: 10.1371/journal.pone.0054419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee Y.H., Song G.G. Association between the rs2004640 functional polymorphism of interferon regulatory factor 5 and systemic lupus erythematosus: a meta-analysis. Rheumatol. Int. 2009;29:1137–1142. doi: 10.1007/s00296-008-0801-7. [DOI] [PubMed] [Google Scholar]
- 211.Jia X., Hu M., Lin Q., Ren H. Association of the IRF5 rs2004640 polymorphism with rheumatoid arthritis: a meta-analysis. Rheumatol. Int. 2013;33:2757–2761. doi: 10.1007/s00296-013-2806-0. [DOI] [PubMed] [Google Scholar]
- 212.Qu H.-Q., Marchand L., Grabs R., Polychronakos C. The IRF5 polymorphism in type 1 diabetes. J. Med. Genet. 2007;44:670–672. doi: 10.1136/jmg.2007.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Uccellini L., De Giorgi V., Zhao Y., Tumaini B., Erdenebileg N., Dudley M.E., Tomei S., Bedognetti D., Ascierto M.L., Liu Q., Simon R., Kottyan L., Kaufman K.M., Harley J.B., Wang E., Rosenberg S.A., Marincola F.M. IRF5 gene polymorphisms in melanoma. J. Transl. Med. 2012;10:170. doi: 10.1186/1479-5876-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ciancanelli M.J., Huang S.X.L., Luthra P., Garner H., Itan Y., Volpi S., Lafaille F.G., Trouillet C., Schmolke M., Albrecht R.A., Israelsson E., Lim H.K., Casadio M., Hermesh T., Lorenzo L., Leung L.W., Pedergnana V., Boisson B., Okada S., Picard C., Ringuier B., Troussier F., Chaussabel D., Abel L., Pellier I., Notarangelo L.D., García-Sastre A., Basler C.F., Geissmann F., Zhang S.-Y., Snoeck H.-W., Casanova J.-L. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hernandez N., Melki I., Jing H., Habib T., Huang S.S.Y., Danielson J., Kula T., Drutman S., Belkaya S., Rattina V., Lorenzo-Diaz L., Boulai A., Rose Y., Kitabayashi N., Rodero M.P., Dumaine C., Blanche S., Lebras M.-N., Leung M.C., Mathew L.S., Boisson B., Zhang S.-Y., Boisson-Dupuis S., Giliani S., Chaussabel D., Notarangelo L.D., Elledge S.J., Ciancanelli M.J., Abel L., Zhang Q., Marr N., Crow Y.J., Su H.C., Casanova J.-L. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J. Exp. Med. 2018;215:2567–2585. doi: 10.1084/jem.20180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bravo García-Morato M., Calvo Apalategi A., Bravo-Gallego L.Y., Blázquez Moreno A., Simón-Fuentes M., Garmendia J.V., Méndez Echevarría A., Del Rosal Rabes T., Domínguez-Soto Á., López-Granados E., Reyburn H.T., Rodríguez Pena R. Impaired control of multiple viral infections in a family with complete IRF9 deficiency. J. Allergy Clin. Immunol. 2019;144:309–312. doi: 10.1016/j.jaci.2019.02.019. e10. [DOI] [PubMed] [Google Scholar]
- 217.Jing J.-S., Wang Z.-Q., Jiang Y.-K., Zhang X.-Y., Jiang W.-M. Association of cytokine gene polymorphisms with chronic hepatitis C virus genotype 1b infection in Chinese Han population: an observational study. Bull. Sch. Med. Md. 2020;99:e22362. doi: 10.1097/MD.0000000000022362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vyas D., Javadi P., Dipasco P.J., Buchman T.G., Hotchkiss R.S., Coopersmith C.M. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:1048–1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ponomarenko J.V., Orlova G.V., Merkulova T.I., Gorshkova E.V., Fokin O.N., Vasiliev G.V., Frolov A.S., Ponomarenko M.P. rSNP_Guide: an integrated database-tools system for studying SNPs and site-directed mutations in transcription factor binding sites. Hum. Mutat. 2002;20:239–248. doi: 10.1002/humu.10116. [DOI] [PubMed] [Google Scholar]
- 220.Abou El Hassan M., Huang K., Eswara M.B.K., Xu Z., Yu T., Aubry A., Ni Z., Livne-Bar I., Sangwan M., Ahmad M., Bremner R. Properties of STAT1 and IRF1 enhancers and the influence of SNPs. BMC Mol. Biol. 2017;18:6. doi: 10.1186/s12867-017-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Roumier M., Paule R., Vallée A., Rohmer J., Ballester M., Brun A.-L., Cerf C., Chabi M.-L., Chinet T., Colombier M.-A., Farfour E., Fourn E., Géri G., Khau D., Marroun I., Ponsoye M., Roux A., Salvator H., Schoindre Y., Si Larbi A.-G., Tchérakian C., Vasse M., Verrat A., Zuber B., Couderc L.-J., Kahn J.-E., Groh M., Ackermann F. Tocilizumab for severe worsening COVID-19 pneumonia: a propensity score analysis. J. Clin. Immunol. 2020 doi: 10.1007/s10875-020-00911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhu L., Yang P., Zhao Y., Zhuang Z., Wang Z., Song R., Zhang J., Liu C., Gao Q., Xu Q., Wei X., Sun H.-X., Ye B., Wu Y., Zhang N., Lei G., Yu L., Yan J., Diao G., Meng F., Bai C., Mao P., Yu Y., Wang M., Yuan Y., Deng Q., Li Z., Huang Y., Hu G., Liu Y., Wang X., Xu Z., Liu P., Bi Y., Shi Y., Zhang S., Chen Z., Wang J., Xu X., Wu G., Wang F.-S., Gao G.F., Liu L., Liu W.J. Single-cell sequencing of peripheral mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. Immunity. 2020;53:685–696. doi: 10.1016/j.immuni.2020.07.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., Qian H., Dai T., Zhang T., Lai Y., Wang J., Liu Z., Chen T., He A., O’Dwyer M., Hu J. COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. n/a. 2020 doi: 10.1002/JLB.4HI0720-470R. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu G., Qi F., Li H., Yang Q., Wang H., Wang X., Liu X., Zhao J., Liao X., Liu Y., Liu L., Zhang S., Zhang Z. The differential immune responses to COVID-19 in peripheral and lung revealed by single-cell RNA sequencing. Cell Discov. 2020;6:73. doi: 10.1038/s41421-020-00225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., Voillet V., Duvvuri V.R., Scherler K., Troisch P., Baloni P., Qin G., Smith B., Kornilov S.A., Rostomily C., Xu A., Li J., Dong S., Rothchild A., Zhou J., Murray K., Edmark R., Hong S., Heath J.E., Earls J., Zhang R., Xie J., Li S., Roper R., Jones L., Zhou Y., Rowen L., Liu R., Mackay S., O’Mahony D.S., Dale C.R., Wallick J.A., Algren H.A., Zager M.A., Wei W., Price N.D., Huang S., Subramanian N., Wang K., Magis A.T., Hadlock J.J., Hood L., Aderem A., Bluestone J.A., Lanier L.L., Greenberg P.D., Gottardo R., Davis M.M., Goldman J.D., Heath J.R. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nienhold R., Ciani Y., Koelzer V.H., Tzankov A., Haslbauer J.D., Menter T., Schwab N., Henkel M., Frank A., Zsikla V., Willi N., Kempf W., Hoyler T., Barbareschi M., Moch H., Tolnay M., Cathomas G., Demichelis F., Junt T., Mertz K.D. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat. Commun. 2020;11:5086. doi: 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Islam A.B.M.M.K., Khan M.A.-A.-K. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-76404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Puray-Chavez M., Tenneti K., Vuong H.R., Lee N., Liu Y., Horani A., Huang T., Case J.B., Yang W., Diamond M.S., Brody S.L., Dougherty J., Kutluay S.B. The translational landscape of SARS-CoV-2 and infected cells. BioRxiv. 2020 doi: 10.1101/2020.11.03.367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Claverie J.-M. A putative role of de-Mono-ADP-Ribosylation of STAT1 by the SARS-CoV-2 Nsp3 protein in the cytokine storm syndrome of COVID-19. Viruses. 2020;12 doi: 10.3390/v12060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Desterke C., Turhan A.G., Bennaceur-Griscelli A., Griscelli F. PPARγ cistrome repression during activation of lung monocyte-macrophages in severe COVID-19. IScience. 2020;23:101611. doi: 10.1016/j.isci.2020.101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dalskov L., Møhlenberg M., Thyrsted J., Blay-Cadanet J., Poulsen E.T., Folkersen B.H., Skaarup S.H., Olagnier D., Reinert L., Enghild J.J., Hoffmann H.J., Holm C.K., Hartmann R. SARS-CoV-2 evades immune detection in alveolar macrophages. EMBO Rep. 2020:e51252. doi: 10.15252/embr.202051252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 Patients. Inflammopharmacology. 2020:1–10. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Filbin M.R., Mehta A., Schneider A.M., Kays K.R., Guess J.R., Gentili M., Fenyves B.G., Charland N.C., Gonye A.L.K., Gushterova I., Khanna H.K., LaSalle T.J., Lavin-Parsons K.M., Lilly B.M., Lodenstein C.L., Manakongtreecheep K., Margolin J.D., McKaig B.N., Rojas-Lopez M., Russo B.C., Sharma N., Tantivit J., Thomas M.F., Gerszten R.E., Heimberg G.S., Hoover P.J., Lieb D.J., Lin B., Ngo D., Pelka K., Reyes M., Smillie C.S., Waghray A., Wood T.E., Zajac A.S., Jennings L.L., Grundberg I., Bhattacharyya R.P., Parry B.A., Villani A.-C., Sade-Feldman M., Hacohen N., Goldberg M.B. Plasma proteomics reveals tissue-specific cell death and mediators of cell-cell interactions in severe COVID-19 patients. BioRxiv. 2020 doi: 10.1101/2020.11.02.365536. [DOI] [Google Scholar]
- 234.Codo A.C., Davanzo G.G., de L., Monteiro B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo Dos Santos K., Toledo-Teixeira D.A., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Vinolo M.A.R., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen X., Guo H., Qiu L., Zhang C., Deng Q., Leng Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury. Front. Immunol. 2020;11:2056. doi: 10.3389/fimmu.2020.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Orienti I., Gentilomi G.A., Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID-19. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21113812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hernández-Mora M.G., Cabello Úbeda A., Pérez L.P., Álvarez F.V., Álvarez B.Á., Rodríguez Nieto M.J., Acosta I.C., Ormaechea I.F., Al-Hayani A.W.M., Carballosa P., Martínez S.C., Ezzine F., González M.C., Naya A., de Las Heras M.L., Rodríguez Guzmán M.J., Guijarro A.C., Lavado A.B., Valcayo A.M., García M.M., Martínez J.B., Roblas R.F., Piris Pinilla M.Á., Alen J.F., Pernaute O.S., Bueno F.R., Frades S.H., Romero G.P.B. Compassionate use of tocilizumab in severe SARS-CoV2 pneumonia. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.10.045. [DOI] [Google Scholar]
- 238.Chen L., Zheng S. Understand variability of COVID-19 through population and tissue variations in expression of SARS-CoV-2 host genes. Informatics Med. Unlocked. 2020;21:100443. doi: 10.1016/j.imu.2020.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang F., Huang S., Gao R., Zhou Y., Lai C., Li Z., Xian W., Qian X., Li Z., Huang Y., Tang Q., Liu P., Chen R., Liu R., Li X., Tong X., Zhou X., Bai Y., Duan G., Zhang T., Xu X., Wang J., Yang H., Liu S., He Q., Jin X., Liu L. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. 2020;6:83. doi: 10.1038/s41421-020-00231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burrage D.R., Koushesh S., Sofat N. Immunomodulatory drugs in the management of SARS-CoV-2. Front. Immunol. 2020;11:1844. doi: 10.3389/fimmu.2020.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]