Abstract
Context
Pregnancy- and lactation-associated osteoporosis (PLO) is a rare condition characterized by fragility fractures, mostly vertebral, during the third trimester of pregnancy or the early postpartum period.
Objective
The aim of this study was to evaluate bone microarchitecture in women with PLO to better understand the pathophysiology of this disease.
Methods
In this retrospective study, we included women with PLO referred to our bone center between November 2007 and July 2012. We assessed bone mineral density (BMD) by dual-energy x-ray absorptiometry, bone turnover markers, and bone microarchitecture by high-resolution peripheral quantitative computed tomography. Results were compared with a control group of healthy lactating women.
Results
Of the 7 primiparous patients with PLO, 6 suffered vertebral fractures and 1 developed a hip fracture during the seventh month of gestation. Fractures occurred within the eighth month of pregnancy and the fourth month post partum; vertebral fractures were multiple in 85.7%. Major or minor risk factors for osteoporosis were present in 86% of our patients. Trabecular density, number, and thickness were 34%, 20% and 22% lower than controls (P < .01, P = .01, and P = .01, respectively). Cortical parameters were also deteriorated but to a lesser extent.
Conclusion
In comparison with healthy lactating women, patients with PLO presented severe deterioration of bone trabecular and cortical microarchitecture. This significant compromise may explain the occurrence of multiple fractures in these otherwise healthy young women. Further prospective studies are needed to determine whether bone microarchitecture might be able to be restored in the future.
Keywords: pregnancy and lactation osteoporosis, bone microarchitecture, premenopausal osteoporosis, HR-pQCT
Pregnancy- and lactation-associated (PLO) osteoporosis is a rare condition. It was first described by Nordin and Roper in 1955 [1]. This disorder is characterized by fragility fractures, most commonly vertebral, occurring during late pregnancy or early postpartum. The incidence is calculated to be around 0.4 per 100 000 women [2]. It usually affects primiparous women in their fourth decade of life. The most common clinical presentation is severe back pain with potential lifelong consequences: chronic pain and irreversible static disorders of the spine in women of childbearing age [3-5].
During pregnancy and lactation, the female physiology adapts to meet the added nutritional demands of fetuses and neonates. An average full-term fetus contains 30 g calcium, 20 g phosphorus, and 0.8 g magnesium. About 80% of these minerals are obtained during the third trimester of gestation. During pregnancy, there is an upregulation of α-1-hydroxilase in the kidney, leading to an increase in the level of dihydroxycolecalciferol, the active form of vitamin D. Thus, the efficacy of calcium intestinal absorption doubles from week 12 of gestation, and maternal calcium intake can meet the nutritional demand from the fetus [6].
The neonate requires 200 mg calcium daily from milk during the first 6 months, and 120 mg during the second 6 months [6]. A temporary demineralization of the skeleton appears to be the main mechanism by which humans meet these calcium requirements. The breast, as a fundamental part of the “brain-breast-bone” circuit, is a central regulator of skeletal metabolism during lactation. Suckling and prolactin inhibit hypothalamic gonadotropin-releasing hormone, which in turn suppresses the gonadotropins, leading to low levels of the ovarian sex steroids, estradiol and progesterone. The decrease in estrogen levels leads to an “upregulation” of RANKL (receptor activator of nuclear factor κB ligand), boosting osteoclastogenesis and bone resorption. Upregulated bone resorption, mediated by high levels of breast-produced parathyroid hormone–related peptide in this setting of low estrogen levels, releases calcium into the bloodstream so it can reach the breast ducts [6, 7].
There is limited information regarding changes in bone mineral density (BMD) measured by dual-energy x-ray absorptiometry (DXA) during pregnancy. Whereas one study found no change in lumbar spine BMD measurements, other studies reported a 4% to 5% decrease in lumbar spine. So, most women have either no change or a very modest decrease in BMD by term [6]. Regarding lactation, according to DXA studies, during the first 2 to 6 months there is a 3% to 10% loss of BMD mostly in the trabecular lumbar spine compartment, with a less important loss in the mostly cortical region of the hip. The rate of bone loss during this period is 1% to 3% per month. After weaning, there is a substantial increase in bone mass and mineralization, reversing the loss that occurs during lactation [6, 8]. The available DXA data suggest that lactational loss of bone density is completely reversed 12 months after weaning in most women [8-29].
The vast majority of epidemiologic studies of premenopausal and postmenopausal women have found no adverse effect of a history of pregnancy and lactation on peak bone mass, bone density, or hip fracture risk [6, 8]. Although pregnancy and lactation are major challenges for female bone metabolism, the maternal skeleton resistance does not seem to be affected in most cases. So, why do some women suffer fragility fractures during pregnancy and lactation? To better understand the pathophysiology of this rare event, we aimed to assess bone microarchitecture in women with PLO using high-resolution peripheral quantitative computed tomography (HR-pQCT). To better comprehend the relevance of the results, we compared them with a group of healthy lactating women undergoing the same physiologic changes as our patients.
Materials and Methods
Women with PLO that were referred to our center for bone metabolism assessment were included in this retrospective study. We recorded data about relevant clinical and demographic characteristics as well as the presence of any risk factors for osteoporosis: age at menarche, menstrual cycle regularity, parity, body mass index (BMI), previous fragility fractures, low calcium intake, tobacco and/or alcohol use, hypercalciuria, primary hyperparathyroidism, hyperprolactinemia, eating disorders, drugs that may affect bone metabolism, and a family history of osteoporosis. Fracture date, type, and characteristics were recorded.
Bone Microarchitecture
Bone microarchitecture was evaluated by HR-pQCT (XtremeCT, Scanco Medical AG) of the nondominant distal tibia and distal radius. The scan builds a 3-dimensional representation by obtaining 110 slices with a resolution of 82 µm at 22.5 and 9.5 mm from a reference line at the end plate of the distal tibia and radius, respectively. In both sites, we evaluated total volumetric BMD (vBMD), trabecular density, cortical density, trabecular number, thickness and spacing, heterogeneity, and cortical density. The first 3 are known as densitometric parameters, whereas the rest are known as structural parameters. In premenopausal women, the reproducibility of vBMD measurements ranges from 0.5% to 0.8%, whereas the reproducibility of the structural parameters is slightly lower, ranging from 0.4% to 3.1%, as was previously published by our group [30].
Bone Mineral Density
Areal bone mineral density (aBMD) was assessed by DXA scans of the lumbar spine and femoral neck (LUNAR) and was reported as grams divided by centimeters squared (g/cm2) and z score as recommended by the International Society for Clinical Densitometry for premenopausal women [31]. A negative z score of less than 2 or lower was defined as below the expected range for age.
Biochemical Parameters
Serum calcium was measured by ion-selective electrode (normal range, 8.8-10.5 mg/dL). Urinary calcium was measured by ion-selective electrode chromophore 5-nitro-5′-methyl-(1,2-bis[o-aminophenoxy] ethan-N, N, N′, N′-tetraacetic acid; NM-BAPTA, normal range, 33-229 mg/24 h). Serum phosphorus was measured by colorimetric assay (normal range, 2.7-4.5 mg/dL). Parathyroid hormone (PTH) and beta-cross laps (CTX) were measured by electrochemiluminescence (normal range, 10-64 pg/mL and 74-550 pg/mL, respectively). Vitamin D levels were measured by radioimmunoassay (normal range, 20-45 ng/mL). Serum osteocalcin was measured by electrochemiluminescence (normal range, 11-43 ng/mL).
Control Group
Eight healthy lactating women among acquaintances of the research team were asked to participate as a control group. Bone microarchitecture by HR-pQCT and BMD by DXA were assessed. Data were collected regarding relevant clinical and demographic characteristics as well as the presence of any risk factors for osteoporosis: age at menarche, menstrual cycle regularity, parity, BMI, previous fragility fractures, low calcium intake, tobacco and/or alcohol use, hypercalciuria, primary hyperparathyroidism, hyperprolactinemia, eating disorders, drugs that may affect bone metabolism, and family history of osteoporosis.
Statistical Analysis
Based on distribution, descriptive data were reported either as mean ± SD or median and range. Comparisons between groups were performed using unpaired t test or Mann-Whitney according to distribution of data. The statistical level of significance was .05. Data were analyzed using MedCalc version 11.2.1.0 (MedCalc Software bvba).
Results
Clinical Characteristics
Seven women with PLO evaluated between November 2007 and July 2012 were included. Mean age was 30.6 ± 3.3 years and mean BMI was 20.9 ± 1.9 kg/m2 (Table 1). All women in the PLO group were primiparous and reported regular menstrual cycles before pregnancy. None of them had a previous personal history of fragility fractures. The mean time between the appearance of the fracture and the assessment was 18 months (range, 2-52 months). None of the patients were receiving any pharmacological treatment at the time of evaluation.
Table 1.
Baseline characteristics and dual-energy x-ray absorptiometry values of pregnancy- and lactation-associated osteoporosis women and controls
| Baseline characteristics | PLO (n = 7) | Controls (n = 8) |
|---|---|---|
| Mean age ± SD, ya | 30.6 ± 3.3 | 38.4 ± 2.9 |
| Mean age at menarche, yb | 14.1 | 12.5 |
| Eumenorrhea | 7/7 | 7/8 |
| Primiparous | 7/7 | 4/8 |
| History of fractures | None | None |
| BMI, kg/m2, mean ± SDc | 20.9 ± 1.9 | 22.3 ± 2.6 |
| DXA assessments | ||
| Lumbar spine, n = 6 | ||
| BMD, g/cm2 ± SDa | 0.772 ± 0.115 | 1.136 ± 0.133 |
| Z score ± SDa | –3.2 ± 0.7 | –0.3 ± 0.9 |
| Femoral neck, n = 6 | ||
| BMD, g/cm2 ± SDa | 0.672 ± 0.111 | 0.908 ± 0.121 |
| Z score ± SDa | –2.0 ± 0.9 | –0.4 ± 0.9 |
Abbreviations: BMD, bone mineral density; BMI, body mass index; DXA, dual-energy x-ray absorptiometry; PLO, pregnancy- and lactation-associated osteoporosis.
a P less than .01.
b P less than .6.
c P less than .27.
At least one risk factor for osteoporosis was present in 85.7%: treatment with high doses of glucocorticoids (1 of 7), hyperprolactinemia and kidney stones with hypercalciuria (1 of 7), low calcium intake (3 of 7), smoking (1 of 7), and a family history of osteoporosis (3 of 7). Celiac disease and thyroid disorders were ruled out in all participants.
Six patients suffered vertebral fractures. Spinal fractures occurred during the first months of postpartum in 4 patients (30-120 days post partum), while the other had compatible symptoms during the eighth month of pregnancy. Most of them (85.7%) had multiple vertebral fractures that were localized between D7 and L5. The mean number of vertebral fractures per patient was 4.33 ± 1.5 with a median of 3 (range, 1-11). One patient who was receiving very high doses of glucocorticoids for thrombocytopenia suffered 11 fractures. The patient with a personal history of kidney stones and hypercalciuria had a hip fracture during the seventh month of pregnancy. Detailed fracture history is shown in Table 2.
Table 2.
Age, detailed fracture history, dual-energy x-ray absorptiometry values, and risk factors in each patient with pregnancy- and lactation-associated osteoporosis
| Patient | Age, y | Risk factors | Z score | Type of FC | No. of FC | Time of FC | CTX, pg/mL | |
|---|---|---|---|---|---|---|---|---|
| Spine | FN | |||||||
| .1 | 27 | FMH of osteoporosis | –3 | –1.6 | D7, D9, L1 | 3 | 30 d PP | 1274.0 |
| Mild | ||||||||
| .2 | 29 | Smoking | –3.3 | –2.1 | D4, D7, D10, L1 | 3 | 45 d PP | ND |
| Low calcium intake | Mild | |||||||
| .3 | 33 | FMH of osteoporosis | –3.7 | –2.2 | ND | 1 | 60 d PP | 495.0 |
| .4 | 36 | Hyperprolactinemia, kidney stones, hypercalciuria | –2.2 | –2.0 | Left hip | 1 | 7th mo of PR | ND |
| .5 | 30 | FMH of osteoporosis | –3.8 | –3.7 | D11, D12, L1, L2 | 4 | 120 d PP | 1323.0 |
| Low calcium intake | Mild | |||||||
| .6 | 32 | None | –3.4 | –1.5 | L1, L3, L4, L5 | 4 | 8th mo of PR | ND |
| Mild | ||||||||
| .7 | 27 | Glucocorticoids | –1.9 | –1.1 | D7-L5 | 11 | 8th mo of PR | 839.0 |
| Low calcium intake | Mild, moderate, and severe |
Abbreviations: CTX, beta cross-laps; D, dorsal; FC, fractures; FN, femoral neck; FMH, familial medical history; L, lumbar; ND, data not available; PP, postpartum; PR, pregnancy.
There were no significant differences regarding age at menarche or BMI between the PLO patients and the control group. Women in the PLO group were slightly younger than controls (mean age 30.6 ± 3.3 vs 38.4 ± 2.9 years, respectively, P < .01) and had no risk factors regarding bone metabolism (see Table 1).
Bone Microarchitecture Assessment
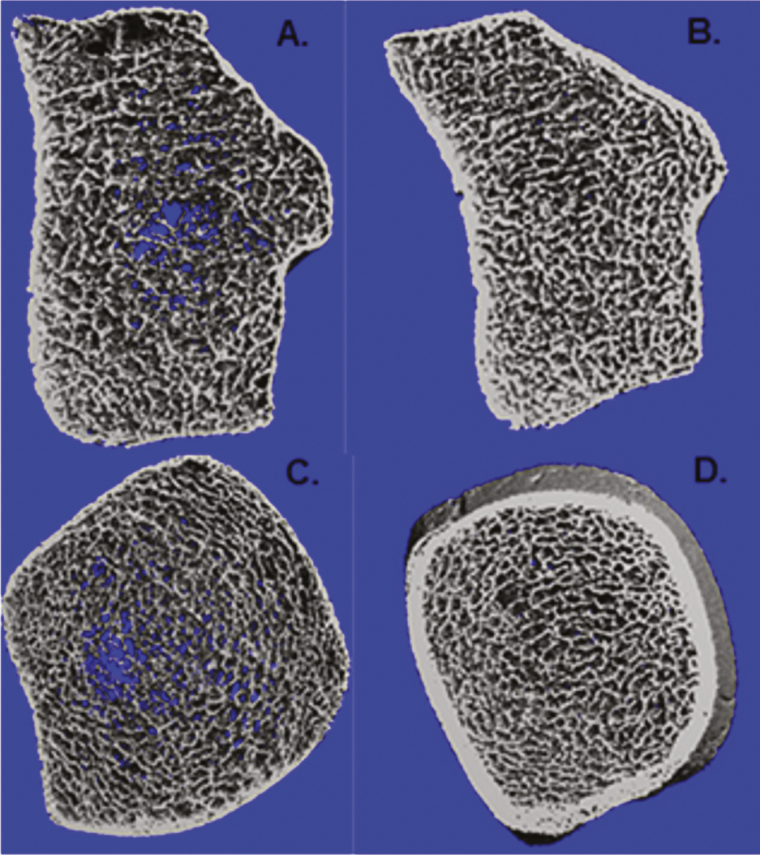
At the distal radius, women with PLO had lower vBMD in comparison with the controls (–25%, P < .02). The trabecular compartment was the most affected; trabecular density was 34% lower that the control group because of lower trabecular number and thickness (–20.4% and –22%, respectively, both with P = .01), more heterogeneity, and trabecular separation (+59%, P = .02 and +34%, P < .01, respectively). In the cortical compartment, women with PLO showed lower cortical density and thickness than the controls (–6% and –20%, respectively). These differences were not statistically significant. Bone microarchitecture parameters are shown in detail in Table 3 and Fig. 1.
Table 3.
Comparison of volumetric bone mineral density values and bone microarchitecture parameters measured by high-resolution peripheral quantitative computed tomography in the distal radius and tibia between women with pregnancy- and lactation-associated osteoporosis and the control group
| PLO | Control | P | |
|---|---|---|---|
| Distal radius | |||
| Total density, mg HA/cm3 | 240 ± 60.7 | 319.4 ± 53.5 | < .02 |
| Dtrab, mg HA/cm3 | 94.6 ± 25.8 | 143.4 ± 31.1 | < .01 |
| Dcomp, mg HA/cm3 | 852 ± 69 | 905 ± 44 | .12b |
| BV/TV, % | 7.9 ± 2.1 | 12 ± 2.6 | < .01 |
| TrabNo., 1/mm | 1.48 ± 0.23 | 1.86 ± 0.18 | .01 |
| TrabTh, mm | 0.053 ± 0.011 | 0.067 ± 0.009 | .01 |
| TrabSp, mm | 0.634 ± 0.100 | 0.473 ± 0.047a | < .01 b |
| Tb.I/N.SD, mm | 0.303 ± 0.088 | 0.191 ± 0.020a | .02c |
| Ct.Th, mm | 0.60 ± 0.14 | 0.75 ± 0.14 | .05 |
| Distal tibia | |||
| D100, mg HA/cm3 | 240 ± 66 | 285 ± 42 | .16a |
| Dtrab, mg HA/cm3 | 116 ± 31 | 142 ± 32 | .15 |
| Dcomp, mg HA/cm3 | 891 ± 61 | 919 ± 32 | .30a |
| BV/TV, % | 9.7 ± 2.6 | 11.8 ± 2.6 | .15 |
| TrabNo., 1/mm | 1.47 ± 0.20 | 1.75 ± 0.17 | .01 |
| TrabTh, mm | 0.065 ± 0.012 | 0.067 ± 0.013 | .78 |
| TrabSp, mm | 0.628 ± 0.105 | 0.508 ± 0.060 | .03a |
| Tb.I/N.SD, mm | 0.343 ± 0.116 | 0.234 ± 0.048 | .02b |
| Ct.Th, mm | 0.95 ± 0.24 | 1.11 ± 0.14 | .12b |
Nonparametric test: Wilcoxon rank sum test.
Abbreviations: BV/TV, trabecular bone volume; Ct.Th, cortical thickness; D100, total volumetric bone mineral density; Dcomp, cortical volumetric bone mineral density; Dtrab, trabecular volumetric bone mineral density; HA, hydroxyapatite; PLO, pregnancy- and lactation-associated osteoporosis; Tb.I/N.SD, bone microarchitecture heterogeneity; TrabNo., trabecular number; TrabSp, trabecular separation; TrabTh, trabecular thickness.
P = t test.
an = 7.
bDifferent variances.
cVariables with no normal distribution.
Figure 1.
A, Bone microarchitecture high-resolution peripheral quantitative computed tomography (HR-pQCT) image of the distal radius in a patient with pregnancy- and lactation-associated osteoporosis (PLO). B, Bone microarchitecture HRp-QCT image of the distal radius in a healthy lactating woman. C, Bone microarchitecture HRp-QCT image of the distal tibia in a patient with PLO. D, Bone microarchitecture HRp-QCT image of distal tibia in a healthy lactating woman.
At the distal tibia, HR-pQCT showed deterioration of total volumetric density and of most parameters in the trabecular compartment. Total and trabecular vBMD were 16% and 18% lower than in the control group, respectively, with slightly lower trabecular thickness (–3%, P = .78) and significant lower trabecular number (–16%, P = .01), greater separation (+24%, P = .01), and network heterogeneity (+47%, P = .02). In the cortical compartment, women with PLO had lower density and thickness (–3% and –14.4%, respectively), although these differences were not statistically significant (see Table 3 and Fig. 1).
Bone Mineral Density
At the lumbar spine, mean aBMD was 0.772 ± 0.115 g/cm2, and mean z score was –3.2 ± 0.7 SD. At the femoral neck, mean aBMD was 0.683 ± 0.133 g/cm2 and z score was –2.0 ± 0.9 SD. In comparison with the control group, the aBMD in women with PLO was decreased by 32% and 24.7% at the lumbar spine and femoral neck, respectively (P < .01) (see Table 1).
Biochemical Parameters
Among the women in the PLO group, mean serum calcium level was 9.8 ± 0.3 mg/dL, and serum phosphorus, 4.32 ± 0.62 mg/dL, both within the normal range. Mean PTH level was 16.14 ± 5.04 pg/mL. Vitamin D was measured in 6 patients and its mean value was 28.17 ± 6.03 ng/dL; 85.7% of patients had values above 20 ng/mL. CTX were measured in 4 patients and their mean value was 1082.20 ± 405.27 pg/mL (above the mean reference value) (see Table 2). Osteocalcin was measured in 3 patients; its mean value was 31.4 ± 11.8 ng/mL (within range), and 1 of the patients had a value in the lower tertile of the normal range (14.7 ng/mL). Average urinary calcium was 202 ± 118.2 mg/day (median, 120 mg/d), with one patient who had hypocalciuria and another one, hypercalciuria (n = 5) (see Table 2). Mean biochemical parameters values are shown in Table 4.
Table 4.
Mean values of bone metabolism biochemical parameters in patients with pregnancy- and lactation-associated osteoporosis
| Mean ± SD | |
|---|---|
| Calcium, mg/dL | 9.8 ± 0.3 |
| Phosphorus, mg/dL | 4.32 ± 0.62 |
| PTH, pg/mL | 16.14 ± 5.04 |
| 25(OH)D, n = 6; ng/dL | 28.17 ± 6.03 |
| Beta-cross laps, n = 4; pg/mL | 1082.20 ± 405.27 |
| Osteocalcin, n = 3; ng/mL | 31.4 ± 11.8 |
| Urine calcium, n = 5, mg/24 h | 202 ± 118.2 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
Discussion
According to our results, women with PLO had a severe impairment of bone microarchitecture compared to a control group of healthy lactating women. This deterioration was more severe in the trabecular compartment, with significantly lower trabecular density, number, and thickness, which led to greater trabecular separation and network heterogeneity. Trabecular bone is the area closest to the bone marrow and intimately related to blood vessels, therefore resulting in the most metabolically active bone compartment. It is specially designed to rapidly liberate calcium to maintain normal calcium serum levels [32]. Recently, Bjørnerem et al found a significant deterioration in the trabecular compartment in healthy lactating women, with lower trabecular density and number as well as greater trabecular separation [33]. These findings confirm that the response to the extra calcium demand during lactation comes, indeed, from trabecular bone. Thus, we can better picture the magnitude of bone microarchitecture impairment in our patients with PLO, who presented with a more severe deterioration than the control group, composed of healthy women going through an intense trabecular bone resorption process related to lactation. We could hypothesize that most of our patients might have had an impaired bone quality before pregnancy and that this is the reason why they were not able to successfully meet the increased calcium demand.
As for cortical density and thickness, they showed a clear deterioration, but to a lesser extent. Cortical bone is harder and more compact than trabecular bone, with less proximity to the vessels and lower metabolic activity. Thus, when bone turnover increases, as in pregnancy and lactation, it is not as easily and rapidly affected. Recently, Ó Breasail et al observed that there seems to be a physiological decrease in cortical thickness with an increase in cortical perimeter during pregnancy, perhaps as a compensatory adaptation [34]. Therefore, although to a lesser extent, cortical bone does suffer minor physiological changes during pregnancy and lactation. Again, we could hypothesize that our patients might have had an impaired bone quality before pregnancy because in comparison with a group of women also undergoing physiological changes in cortical bone, their cortical density and thickness were lower.
A different severity of impairment in bone microarchitecture was evidenced between the distal radius and tibia in our study. This difference—with the radius being the most affected—might be because the tibia is a weight-bearing bone. Body weight gain during pregnancy might protect weight-bearing bones from density loss, mediated by bone’s mechanostat. Since Frost’s investigations in 1996, it has been known that there is continuing feedback between muscle and bone tissues, with the osteocytes being the “sensors” that translate the stress and strains provoked by higher loads in increased bone formation [35]. Bones that bear higher weight are the ones that need higher BMD, and this is achieved through the mechanostat mechanism [36]. Similarly to the findings in the present study, in our previous work comparing bone microarchitecture in women with celiac disease vs premenopausal healthy women, we observed a more severe deterioration at the radius than at the tibia, as well as at the trabecular than at the cortical bone [30].
Regarding aBMD, DXA scans of women with PLO showed lower values than expected for their age at the lumbar spine and femoral neck, in agreement with previous reports [37, 38]. As expected, the deterioration was more severe at the lumbar spine, composed of 66% trabecular bone, in comparison with the mostly cortical femoral neck.
Our patients with PLO were primiparous, eumenorrheic, and in their third or four decade of life. They had no history of previous fragility fractures. Fractures occurred within the third trimester of pregnancy or the early postpartum period. In concurrence with the international literature, most of our patients suffered multiple vertebral fractures, with a mean number of 4.33 ± 1.5 and a median of 3. Previously, Laroche et al had reported a mean fracture number of 3.8 ± 2.0 per patient [39].
The pathophysiology of PLO remains unclear. However, several risk factors have been described, such as low peak bone mass; genetic factors, namely inactivating mutations in low-density lipoprotein receptor-related protein 5 (LRP5); impaired calcium absorption; inadequate calcium intake; vitamin D insufficiency; inadequate high release of parathyroid hormone–related peptide; diverse other conditions such as anorexia, oligomenorrhea, or relative estradiol deficiency; premature ovarian failure; low BMI; smoking; hypercalciuria; bed rest; and pharmacotherapy that may induce bone loss such as heparin, oral glucocorticoids, hypothalamic gonadotropin-releasing hormone analogues, and anticonvulsants [40]. In our study, major risk factors were present in 2 patients: One had a history of hyperprolactinemia and kidney stones with hypercalciuria, and the other had received very high doses of glucocorticoids during pregnancy for severe thrombocytopenia. The latter suffered 11 vertebral fractures starting during the eighth month of pregnancy. The other 5 patients presented with at least 1 minor risk factor for osteoporosis: low calcium intake, family history of osteoporosis, smoking, and/or low BMI. In our clinical view these risk factors would not explain per se the occurrence of vertebral fractures. Patients with PLO might have had low bone density prior to pregnancy without even knowing it, given that there is no indication for BMD assessment in healthy premenopausal women [6]. Cohen et al evaluated a group of women with PLO and observed that they had lower tissue mineral apposition rates and bone formation rates assessed by transiliac bone biopsy in comparison with women with premenopausal osteoporosis. Based both on serum remodeling markers and micro-CT findings, they reported that women with PLO had evidence of low bone formation, in absence of lower osteoblast numbers, suggesting the possibility of an underlying defect in osteoblast function [41].
Regarding biochemical parameters of bone metabolism, our patients showed increased levels of CTX, reflecting an exacerbated bone resorption state at the time of evaluation. Osteocalcin levels were within the normal range, although one patient presented with levels in the lower tertile. Cohen et al reported that, when compared with women with idiopathic premenopausal osteoporosis, patients with PLO presented with lower levels of serum CTX and P1NP (procollagen type 1 N-terminal propeptide) [41]. In that study, women were evaluated 12 months or later after delivery, whereas in our patients, bone turnover markers were measured closer to the time of delivery and fracture occurrence. This might explain why they had higher levels of CTX, reflecting the high bone-turnover process they were undergoing after suffering fragility fractures.
Our study has several limitations; first, the sample size is small. The fact that this is a retrospective study implies that some data are missing and that we were unable to evaluate all patients at the same time after the fracture occurred. Another limitation is that HR-pQCT can assess microarchitecture only of the peripheral skeleton. However, a previous study by Liu et al has suggested that HR-pQCT determinations in the peripheral skeleton may also reflect the mechanical competence and risk of fractures in the central skeleton, where many of the most clinically serious fractures can occur [42]. The women in the control group were older than our patients with PLO. However, considering that they were all premenopausal women, this difference should not affect BMD or microarchitecture. Even more, older women should have shown lower BMD and worse bone microarchitecture parameters and not the opposite way around, as we observed. Finally, an additional control group for the PLO patients might have been healthy nonlactating premenopausal women, and we will take this into consideration for future studies.
In conclusion, in comparison with healthy lactating women who were going through a physiological intense bone resorption process, our patients with PLO had severe deterioration of bone microarchitecture assessed by HR-pQCT. This novel, noninvasive tool provided new insight into the pathophysiology of this rare condition. We hypothesize that these women might have had impaired bone microarchitecture before they became pregnant. Therefore, their skeleton was not able to successfully meet the increased calcium requirements of pregnancy and lactation. Our findings open the way for future larger, prospective, controlled studies, which are necessary to determine whether these women might be able to restore their bone microarchitecture in the future.
Acknowledgments
Author Contributions: M.B.Z. collected and analyzed the data, designed the study, and revised the manuscript. M.F.S. analyzed and interpreted the data and drafted the manuscript. S.N., Y.M., A.P., N.E., H.C., A.D., C.A.V., C.B., C.C., G.M., S.G., and J.R.Z. collected the data. M.B.Z. and M.F.S. are responsible for the integrity of the data analysis. All authors read and approved the final manuscript.
Glossary
Abbreviations
- aBMD
areal bone mineral density
- BMD
bone mineral density
- BMI
body mass index
- CTX
beta-cross laps
- DXA
dual-energy x-ray absorptiometry
- HR-pQCT
high-resolution peripheral quantitative computed tomography
- PLO
pregnancy- and lactation-associated osteoporosis
- PTH
parathyroid hormone
- vBMD
volumetric bone mineral density.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Di Gregorio S, Danilowicz K, Rubin Z, Mautalen C. Osteoporosis with vertebral fractures associated with pregnancy and lactation. Nutrition. 2000;16(11-12):1052-1055. [DOI] [PubMed] [Google Scholar]
- 2. Terzi R, Terzi H, Özer T, Kale A. A rare cause of postpartum low back pain: pregnancy- and lactation-associated osteoporosis. Biomed Res Int. 2014;2014:287832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozturk C, Atamaz FC, Akkurt H, Akkoc Y. Pregnancy-associated osteoporosis presenting severe vertebral fractures. J Obstet Gynaecol Res. 2014;40(1):288-292. [DOI] [PubMed] [Google Scholar]
- 4. Saraux A, Bourgeais F, Ehrhart A, Baron D, Le Goff P. Osteoporosis during pregnancy. 4 cases [article in French]. Rev Rhum Ed Fr. 1993;60(9):596-600. [PubMed] [Google Scholar]
- 5. Sarikaya S, Ozdolap S, Açikgöz G, Erdem CZ. Pregnancy-associated osteoporosis with vertebral fractures and scoliosis. Joint Bone Spine. 2004;71(1):84-85. [DOI] [PubMed] [Google Scholar]
- 6. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449-547. [DOI] [PubMed] [Google Scholar]
- 7. Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS. Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology. 2006;147(9):4010-4021. [DOI] [PubMed] [Google Scholar]
- 8. Sowers M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res. 1996;11(8):1052-1060. [DOI] [PubMed] [Google Scholar]
- 9. Caird LE, Reid-Thomas V, Hannan WJ, Gow S, Glasier AF. Oral progestogen-only contraception may protect against loss of bone mass in breast-feeding women. Clin Endocrinol (Oxf). 1994;41(6):739-745. [DOI] [PubMed] [Google Scholar]
- 10. Chan SM, Nelson EA, Leung SS, Cheng JC. Bone mineral density and calcium metabolism of Hong Kong Chinese postpartum women—a 1-y longitudinal study. Eur J Clin Nutr. 2005;59(7):868-876. [DOI] [PubMed] [Google Scholar]
- 11. Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res. 1995;10(9):1312-1320. [DOI] [PubMed] [Google Scholar]
- 12. Sowers M, Randolph J, Shapiro B, Jannausch M. A prospective study of bone density and pregnancy after an extended period of lactation with bone loss. Obstet Gynecol. 1995;85(2):285-289. [DOI] [PubMed] [Google Scholar]
- 13. Henderson PH III, Sowers M, Kutzko KE, Jannausch ML. Bone mineral density in grand multiparous women with extended lactation. Am J Obstet Gynecol. 2000;182(6):1371-1377. [DOI] [PubMed] [Google Scholar]
- 14. Holmberg-Marttila D, Leino A, Sievänen H. Bone turnover markers during lactation, postpartum amenorrhea and resumption of menses. Osteoporos Int. 2003;14(2):103-109. [DOI] [PubMed] [Google Scholar]
- 15. Holmberg-Marttila D, Sievänen H, Laippala P, Tuimala R. Factors underlying changes in bone mineral during postpartum amenorrhea and lactation. Osteoporos Int. 2000;11(7):570-576. [DOI] [PubMed] [Google Scholar]
- 16. Holmberg-Marttila D, Sievänen H, Tuimala R. Changes in bone mineral density during pregnancy and postpartum: prospective data on five women. Osteoporos Int. 1999;10(1):41-46. [DOI] [PubMed] [Google Scholar]
- 17. Hopkinson JM, Butte NF, Ellis K, Smith EO. Lactation delays postpartum bone mineral accretion and temporarily alters its regional distribution in women. J Nutr. 2000;130(4):777-783. [DOI] [PubMed] [Google Scholar]
- 18. Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med. 1997;337(8):523-528. [DOI] [PubMed] [Google Scholar]
- 19. Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12(10):828-834. [DOI] [PubMed] [Google Scholar]
- 20. Kent GN, Price RI, Gutteridge DH, et al. Human lactation: forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Miner Res. 1990;5(4):361-369. [DOI] [PubMed] [Google Scholar]
- 21. Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci (Lond). 1998;94(4):405-412. [DOI] [PubMed] [Google Scholar]
- 22. Krebs NF, Reidinger CJ, Robertson AD, Brenner M. Bone mineral density changes during lactation: maternal, dietary, and biochemical correlates. Am J Clin Nutr. 1997;65(6):1738-1746. [DOI] [PubMed] [Google Scholar]
- 23. Laskey MA, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94(4):608-615. [DOI] [PubMed] [Google Scholar]
- 24. López JM, González G, Reyes V, Campino C, Díaz S. Bone turnover and density in healthy women during breastfeeding and after weaning. Osteoporos Int. 1996;6(2):153-159. [DOI] [PubMed] [Google Scholar]
- 25. More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int. 2001;12(9):732-737. [DOI] [PubMed] [Google Scholar]
- 26. Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34(3):570-578. [DOI] [PubMed] [Google Scholar]
- 27. Ritchie LD, Fung EB, Halloran BP, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693-701. [DOI] [PubMed] [Google Scholar]
- 28. Sowers M, Corton G, Shapiro B, et al. Changes in bone density with lactation. JAMA. 1993;269(24):3130-3135. [PubMed] [Google Scholar]
- 29. Polatti F, Capuzzo E, Viazzo F, Colleoni R, Klersy C. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94(1):52-56. [DOI] [PubMed] [Google Scholar]
- 30. Zanchetta MB, Costa F, Longobardi V, et al. Significant bone microarchitecture impairment in premenopausal women with active celiac disease. Bone. 2015;76:149-157. [DOI] [PubMed] [Google Scholar]
- 31. Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16(4):455-466. [DOI] [PubMed] [Google Scholar]
- 32. Frost HM. Defining osteopenias and osteoporoses: another view (with insights from a new paradigm). Bone. 1997;20(5):385-391. [DOI] [PubMed] [Google Scholar]
- 33. Bjørnerem Å, Ghasem-Zadeh A, Wang X, et al. Irreversible deterioration of cortical and trabecular microstructure associated with breastfeeding. J Bone Miner Res. 2017;32(4):681-687. [DOI] [PubMed] [Google Scholar]
- 34. Ó Breasail M, Prentice A, Ward K. Pregnancy-related bone mineral and microarchitecture changes in women aged 30 to 45 years. J Bone Miner Res. 2020;35(7):1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frost HM. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm). Anat Rec. 1996;244(2):139-147. [DOI] [PubMed] [Google Scholar]
- 36. Ferretti JL, Capozza RF, Mondelo N, Zanchetta JR. Interrelationships between densitometric, geometric, and mechanical properties of rat femora: inferences concerning mechanical regulation of bone modeling. J Bone Miner Res. 1993;8(11):1389-1396. [DOI] [PubMed] [Google Scholar]
- 37. O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17(7):1008-1012. [DOI] [PubMed] [Google Scholar]
- 38. Sarli M, Hakim C, Rey P, Zanchetta J. Osteoporosis during pregnancy and lactation. Report of eight cases [article in Spanish]. Medicina (B Aires). 2005;65(6):489-494. [PubMed] [Google Scholar]
- 39. Laroche M, Talibart M, Cormier C, Roux C, Guggenbuhl P, Degboe Y. Pregnancy-related fractures: a retrospective study of a French cohort of 52 patients and review of the literature. Osteoporos Int. 2017;28(11):3135-3142. [DOI] [PubMed] [Google Scholar]
- 40. Ryan BA, Kovacs CS. The puzzle of lactational bone physiology: osteocytes masquerade as osteoclasts and osteoblasts. J Clin Invest. 2019;129(8):3041-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen A, Kamanda-Kosseh M, Dempster DW, et al. Women with pregnancy and lactation-associated osteoporosis (PLO) have low bone remodeling rates at the tissue level. J Bone Miner Res. 2019;34(9):1552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25(10):2229-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”