Abstract
Context
There is no consensus on the effect of recombinant human GH (rhGH) therapy on skeletal maturation in children despite the current practice of annual monitoring of skeletal maturation with bone age in children on rhGH therapy.
Aims
To investigate the effects of long-term rhGH therapy on skeletal age in children and explore the accuracy of bone age-predicted adult height (BAPAH) at different ages based on 13 years of longitudinal data.
Methods
A retrospective longitudinal study of 71 subjects aged 2 to 16 years, mean 9.9 ± 3.8 years, treated with rhGH for nonsyndromic short stature for a duration of 2 to 14 years, mean, 5.5 ± 2.6 years. Subjects with syndromic short stature and systemic illnesses such as renal failure were excluded.
Results
Bone age minus chronological age (BA-CA) did not differ significantly between baseline and the end of rhGH therapy (-1.05 ± 1.42 vs -0.69 ± 1.63, P = 0.09). Piecewise regression, however, showed a quantifiable catch-up phenomenon in BA of 1.5 months per year of rhGH therapy in the first 6.5 years (P = 0.017) that plateaued thereafter (P = 0.88). BAPAH overestimated near-adult height in younger subjects but became more accurate in older subjects (P < 0.0001). IGF-I levels correlated significantly with increases in child’s height and BA-CA.
Conclusion
Long-term rhGH therapy demonstrated an initial catch-up phenomenon in skeletal maturation in the first 6.5 years that plateaued thereafter with no overall significant advancement in bone age. These findings are reassuring and support strategic, but not the insurance company mandated reflexive annual monitoring of skeletal maturation with bone age in children receiving rhGH therapy.
Keywords: growth hormone deficiency, short stature, bone age, height velocity
The practice of monitoring GH therapy with annual bone age (BA) assessment in children and adolescents with GH deficiency (GHD) is controversial [1-7]. The 2007 Consensus Statement on the Diagnosis and Treatment of Children with Idiopathic Short Stature recommended only periodic BA monitoring [7], whereas the 2019 Growth Hormone Research Society International Perspective on Diagnosis, Genetics, and Therapy of Short Stature in Children [8] did not address the issue of monitoring bone age during rhGH therapy. Yet, this practice of annual bone age monitoring persists in some pediatric endocrinology clinics, driven mainly by insurance company mandates. GHD arises from the failure of the pituitary gland to produce GH from either congenital cause such as hypopituitarism or acquired causes such as head trauma or brain surgery. GHD is defined clinically as a peak stimulated GH level of <10 ng/mL following a GH stimulation testing using 2 GH secretagogues in a child with suggestive auxologic, anatomic, and laboratory parameters for a dysfunction of the GH/IGF-1 axis [7-9]. Children with GHD are treated with recombinant human GH (rhGH). GH stimulates the production of IGF-1 in the liver, which acts on chondrocytes to promote linear growth in children and adolescents with open long-bone epiphyses [10].
Physiologically, skeletal maturation is mediated by sex hormones, principally estrogens, resulting from the increased sensitivity of growth plates to sex steroids [11]. However, some studies have reported that rhGH increases the rate of both skeletal and sexual maturation [6, 12, 13], leading to the recommendation to monitor rhGH therapy with annual BA assessment [7, 14]. Despite the practice of annual monitoring of skeletal maturation with BA in children on rhGH therapy [1-6], there is no consensus on the effect of rhGH therapy on skeletal maturation and puberty in children and adolescents [1, 5]. Furthermore, the evidence for increased skeletal and sexual maturation by rhGH therapy is controversial because some studies reported proof of this phenomenon [6, 13, 15-17], whereas others did not [3, 18, 19]. A 5-year randomized control trial of 18 controls and 17 subjects who received high-dose rhGH reported advanced skeletal maturation [6], whereas another 6-year randomized control trial of 25 controls and 8 subjects who received rhGH found no evidence for advanced skeletal maturation [20]. For studies reporting evidence of advanced skeletal maturation, there is no agreement on the timing of the occurrence of the skeletal maturation; some investigators suggest that BA advancement occurs in the first year of rhGH therapy [5, 6] or midway through the span of therapy [6], whereas others propose that the BA acceleration seen in rhGH therapy is usually not apparent in the early phases of treatment, but toward the end of therapy [1].
Currently, there is a dearth of long-term studies [3] evaluating the effect of GH therapy on BA advancement in children receiving rhGH, and no study has examined the changes in BA in these children for >6 years. Therefore, it is unclear whether the reported advancement in BA seen in the first year of treatment or midway through therapy is a transient phenomenon that does not progress to the end of therapy. The timing of the cessation of rhGH therapy for purposes of increasing height is defined by the Pediatric Endocrine Society as the attainment of bone age of >16 years in boys or >14 years in girls, or the slowing of growth velocity to <2 cm/year [7, 14].
Therefore, to clarify whether rhGH therapy advances BA, it is imperative to conduct a long-term study of >6 years’ duration that compares the differences in BA and chronological age (CA) in subjects treated with rhGH for a shorter duration (ie, <5 years to those treated for a longer duration of >5 years). It is also important to determine the relationships between bone age–predicted adult height (BAPAH) z score minus child’s concurrent height z score (BAPAH z – height z) and either the duration of rhGH therapy or bone age. Such an investigation will determine whether the reported skeletal maturation in these subjects is a transient phenomenon that does not warrant monitoring with annual bone age assessments or whether BA advancement continues throughout the duration of therapy and should be addressed proactively through the introduction of interventions to limit bone age advancement during rhGH therapy.
To address these pressing questions, we designed a real-world clinical study to explore the long-term effect of rhGH on bone age. This was not a registry study in which patients who are receiving a particular brand of rhGH product, based on prespecified inclusion criteria, are followed for several years and data collected for the purposes of investigating product efficacy and safety. Our primary aim was to investigate the effect of long-term rhGH therapy on BA in children and adolescents by comparing the degree of skeletal maturation in children treated for a shorter duration with those treated for a longer period. Our secondary aim was to determine the relationships between bone age-predicted adult height (BAPAH) z score minus child’s concurrent height z score versus either the bone age or the duration of rhGH therapy. We hypothesized that long-term therapy with rhGH would result in advanced skeletal maturation in children and adolescents. We further hypothesized that BAPAH z – height z will have no relationship with either the bone age or the duration of rhGH therapy.
Subjects and Methods
Ethics statement
The institutional review board of the University of Massachusetts Medical School approved the study protocol and the waiver of authorization to review subjects’ retrospective records under Docket #H00018755. Subjects’ data were deidentified and anonymized before analysis in compliance with the Declaration of Helsinki.
Subjects
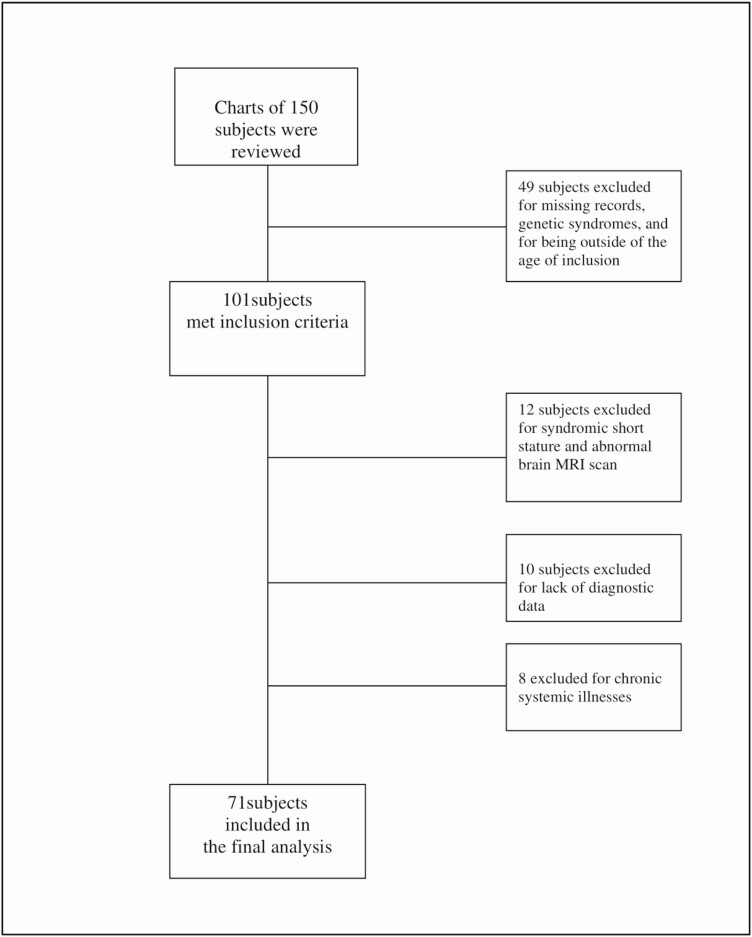
The clinical records of children (n = 71; 50 male subjects and 21 female subjects) of mean age 9.9 ± 3.8 years, were included if they received rhGH from 2004 to 2016 (Fig. 1). Patients’ records were extracted from the electronic medical records of the Children’s Medical Center of the UMass Memorial Medical Center. Male and female subjects ages 2 through 16 years who received rhGH therapy and had none of the exclusion criteria detailed later were included in this real-world study. Rigorous approaches to prevent the introduction of bias included the exclusion of subjects with diseases, therapies, or syndromes that could either affect the GH/IGF-I system or confound the subjects’ response to GH therapy. Three subjects had bone ages of 14 to 16 years, but showed an increased height velocity response to rhGH therapy by improved height velocity and were thus included in the study. Ten subjects were excluded because they either had no baseline information because of transfer of care from another institution or did not have a final bone age study. Subjects were further excluded if they had abnormal results of magnetic resonance imaging of the pituitary gland suggesting neoplastic lesions such as medulloblastoma, pituitary adenoma, or any systemic illness or syndrome affecting growth such as renal failure or Turner syndrome, respectively. Twelve subjects were excluded based on magnetic resonance imaging criteria and the presence of syndromic short stature. Subjects with malnutrition, undernutrition, and chronic system illnesses such as liver or kidney disease were excluded. Fourteen subjects had hypopituitarism. Twelve subjects received hydrocortisone at a dose of 8 to 10 mg/kg/day; 6 subjects had hypothyroidism and were euthyroid on levothyroxine therapy. No subject was diagnosed with hypogonadism and no subject received sex hormone therapy with either testosterone or estradiol, or puberty modulators, such as GnRH analogs [7].
Figure 1.
CONSORT flow diagram.
Anthropometry
The methodology for anthropometry has been previously described in detail [21-24]. Briefly, height and weight were measured in the clinic by standard techniques, and body mass index (BMI) was calculated from the formula: weight/height2 (kg/m2). Height, weight, and BMI were expressed as z scores for age and sex, based on National Center for Health Statistics data [25, 26]. Overweight was defined as BMI of ≥85th but <95th percentile, and obesity was defined as BMI of ≥95th percentile for age and sex. Sexual maturity rating was determined by pediatric endocrinologists using the method of Tanner and Marshall [27, 28], with Tanner I designated as prepubertal status and Tanner II-V as pubertal status. Prepubertal status was marked in boys by a testicular volume of <4 mL as measured with a Prader orchidometer and in girls by Tanner stage 1 breasts, as indicated by the absence of breast buds or breast tissue [29]. For our analysis, we classified the Tanner staging into 2 groups: Tanner I-II as barely mature and Tanner III-V as moderate to completely mature. At baseline, 55 subjects were at Tanner stage I-II, 13 subjects at Tanner III-IV, and 1 subject was at Tanner V at a bone age of 14.5 years. The subject denoted as being at Tanner V stage had an increased height velocity response to rhGH therapy and was thus included in the study.
Baseline height velocity data were obtained from at least 2 height measurements of at least 6- to 12-month intervals, while making certain that all measurements occurred before the institution of GH therapy. Final height velocity was assessed as the difference between the height at the time of conclusion of therapy and the previous height at -12 months from the conclusion of rhGH therapy. Height velocity data were expressed as z scores based on established norms [30-32]. The protocol for rhGH therapy was based on the Consensus Statements that growth hormone dosage be selected and adjusted by weight while using IGF-I concentration to monitor safety and compliance [7, 8].
Midparental target height was calculated by the Tanner method for each subject as follows: for boys, 13 cm was added to the mother’s height and then averaged with the father’s height. For girls, 13 cm was subtracted from the father’s height and then averaged with the mother’s height [7, 29].
Bone age was assessed using the Greulich and Pyle method [33]. Serial BA minus CA data were obtained by subtracting a patient’s chronological age from his or her skeletal age [34] for each time point. All radiographs were coded and validated in a blinded manner by a board-certified pediatric radiologist (E.C.W.).
Biochemical studies
Subjects underwent sequential GH stimulation tests without sex-steroid priming in a single day using clonidine (0.125 mg/m2 by mouth) and arginine (0.5 g/kg IV) as GH secretagogues. Samples were obtained for GH determination at times 0, 15, 30, 60, 90, and 120 minutes according to established procedure [35]. GHD was defined as a peak stimulated GH level of <10 ng/mL [7-9].
Assays
Both serum GH and IGF-I concentrations were measured using a solid-phase, 2-site chemiluminescent immunometric assay, Immulite 2000 (Siemens Healthcare Diagnostics, Deerfield, IL). The GH assay had an analytical sensitivity of 0.01 µg/L, intraassay coefficient of variation (CV) of 2.9% to 4.6% and interassay CV of 4.2% to 6.6%, whereas the IGF-I assay had an analytical sensitivity of 20 µg/L, intraassay CV of 2.3% to 3.9% and interassay CV of 3.7% to 8.1%.
Statistical analysis
Subjects’ anthropometric, biochemical, and radiological parameters were summarized and compared between baseline and final visits using pairwise t test for means or χ 2 test for proportions. The power analysis showed that, for correlation = 0.5 and alpha = 0.05, the power of the pairwise t test of means with N = 71 (pairs) is 0.81 to detect an effect size of 0.34. Because multiple tests were performed, P values for statistical significance were provided for explanatory purposes only. Subjects were then stratified into 2 roughly equal-size groups by ≤5 and >5 years of duration of rhGH therapy and compared for age at the start of rhGH therapy, sex, height, weight, BMI, Tanner stage, IGF-1, height velocity, and BA-CA using 2-sample t test of means or χ 2 test for contingency table. The decision to use 5 years as the cutoff point for short-term therapy was based on the establishment of no differences in BA-CA between the short-term versus long-term treatment groups at baseline for mean ages 4 years through 7 years. We then chose the year that represents the greatest equity in the distribution of patient numbers between the 2 groups.
Generalized linear regression model (GENMOD) was used in subsequent analysis to investigate the association between changes in BA-CA and the duration of rhGH from the time of initiation of therapy based on our longitudinal data. Interestingly, our exploratory analysis of the scatterplots for BA-CA versus the duration of rhGH therapy revealed a potential change of slope of the regression line before and after a certain time point suggesting a piecewise regression. To search for the optimal time point where the change of slope occurred, several piecewise GENMODs were performed by selecting a few time points where the change might occur. Quasi-likelihood under the Independence Model Criterion (QIC) model was used to aid this search. QIC is a measure of goodness-of-fit for GENMOD models where a smaller QIC value suggests a better fit [36]. All piecewise GENMODs were adjusted for age and sex, the basic pair of factors that affects growth in children. GENMOD provided estimates of slope (β) before and after the change, their confidence intervals (CIs), and P values for significance. We also examined the relationship between BAPAH z score minus child’s concurrent height z score (BAPAH z – height z) and either the duration of rhGH therapy or the bone age. To accomplish this, we used GENMOD models to regress BAPAH z – height z score on logarithmic transformation of duration or bone age, adjusted for age and sex.
Results
General clinical parameters
This retrospective longitudinal study reports the results of the anthropometric, biochemical, and radiological parameters of 71 subjects age 2 through 18 years who were treated with rhGH for nonsyndromic short stature (Table 1). Their mean age at the start of therapy was 9.9 ± 3.8 years, and the median age was 9.8 years. There were 50 male subjects of mean age 10.1 ± 4 years, and 21 female subjects of mean age 9.4 ± 3.0 years. The mean age of the subjects at the final visit was 15.1 ± 3.1 years. The mean duration of rhGH therapy was 5.5 ± 2.6 years (Table 2), with a range of 2 to 14 years, and a median duration of 4.9 years.
Table 1.
Anthropometric and biochemical characteristics of subjects
| Parameters | Baseline | Final | P valuea | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age: all, y | 9.9 | 3.8 | 15.1 | 3.1 | <0.0001 |
| Age: males (n = 50) | 10.1 | 4.0 | 14.8 | 3.1 | <0.0001 |
| Age: females (n = 21) | 9.4 | 3.0 | 15.6 | 3.4 | <0.0001 |
| Height z score | -2.07 | 2.27 | -0.78 | 1.05 | <0.0001 |
| Weight z score | -0.95 | 1.74 | -0.10 | 1.23 | <0.0001 |
| BMI z score | 0.30 | 1.19 | 0.64 | 1.44 | 0.005 |
| Height velocity, cm/y | 5.18 | 2.30 | 4.85 | 3.43 | 0.33 |
| IGF-1, ng/mL | 194.1 | 144.3 | 402.7 | 200.1 | <0.0001 |
| IGF-1 z score | -0.92 | 1.40 | 0.36 | 1.47 | 0.0004 |
| GH dose, mg/kg/d | 0.03 | 0.02 | 0.04 | 0.02 | 0.08 |
| BA, y | 8.57 | 3.83 | 13.41 | 2.83 | <0.0001 |
| CC, y | 9.67 | 3.73 | 14.12 | 2.90 | <0.0001 |
| BA-CA | -1.05 | 1.42 | -0.69 | 1.63 | 0.09 |
| Proportions | n | % | n | % | P valueb |
| Pubertal status | |||||
| Tanner I-II | 52 | 78.8 | 13 | 25.5 | 0.0002 |
| Tanner III-V | 14 | 21.2 | 38 | 74.5 | |
| BMI percentiles | |||||
| Normal weight (<85th percentile) | 48 | 69.6 | 43 | 60.6 | 0.004 |
| Overweight/obese (≥85th percentile) | 21 | 30.4 | 28 | 39.4 | |
| Age range (y) | Min | Max | Min | Max | |
| All | 2.5 | 16.8 | 6.7 | 21.7 | |
| Males (n = 50) | 2.5 | 16.8 | 6.7 | 20.2 | |
| Females (n = 21) | 4.8 | 15.0 | 11.0 | 21.7 | |
| BA vs CA | |||||
| Proportions | n | % | n | % | P valueb |
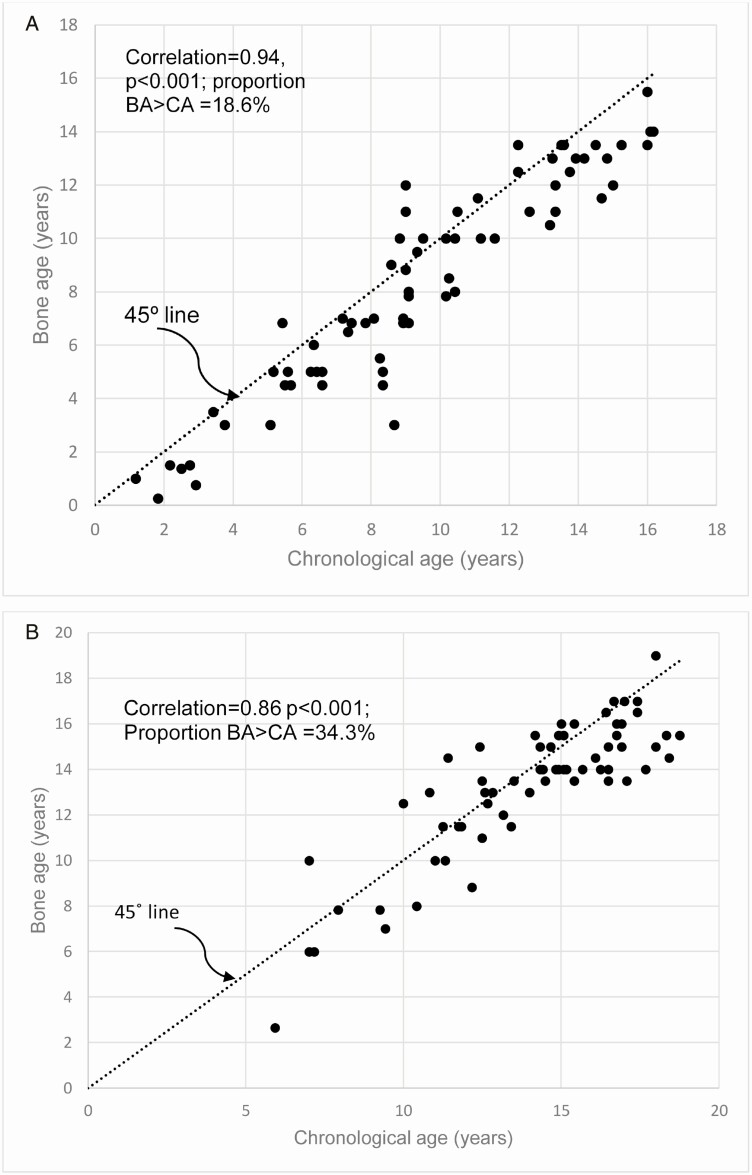
| BA ≤ CA | 57 | 81.4 | 44 | 65.7 | 0.036 |
| BA > CA | 13 | 18.6 | 23 | 34.3 | |
Abbreviations: BA, bone age; BMI, body mass index; CA, chronological age.
a Pairwise t test.
b Test of 2 proportions.
Table 2.
Subjects’ characteristics stratified by short-term and long-term recombinant human GH therapy
| Parameters | All (N = 71) | Duration of recombinant human GH therapy | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤5 y (n = 37) | >5 y (n = 34) | |||||||||
| N | Mean | SD | n | Mean | SD | N | Mean | SD | ||
| Age at start of treatment, y | 71 | 9.9 | 3.8 | 37 | 12.0 | 3.2 | 34 | 7.6 | 2.9 | <0.0001 |
| Mean age of subjects ≤10 y | 36 | 6.9 | 2.2 | 9 | 7.7 | 2.5 | 27 | 6.6 | 2.1 | 0.22 |
| Mean age of subjects > 10 y | 35 | 13.1 | 2.0 | 28 | 13.4 | 1.9 | 7 | 11.5 | 1.6 | 0.021 |
| Age at final visit, y | 71 | 15.1 | 3.1 | 37 | 15.4 | 3.1 | 34 | 14.7 | 3.2 | 0.39 |
| Height z score | ||||||||||
| Baseline | 70 | -2.07 | 2.27 | 36 | -2.16 | 0.88 | 34 | -1.98 | 3.15 | 0.75 |
| Final | 71 | -0.78 | 1.05 | 37 | -0.87 | 0.91 | 34 | -0.68 | 1.18 | 0.45 |
| Weight z score | ||||||||||
| Baseline | 70 | -0.95 | 1.74 | 37 | -1.22 | 1.39 | 33 | -0.65 | 2.05 | 0.19 |
| Final | 71 | -0.10 | 1.23 | 37 | -0.17 | 1.27 | 34 | -0.02 | 1.20 | 0.59 |
| BMI z score | ||||||||||
| Baseline | 69 | 0.30 | 1.19 | 36 | 0.12 | 1.15 | 33 | 0.50 | 1.23 | 0.19 |
| Final | 71 | 0.64 | 1.44 | 37 | 0.50 | 1.21 | 34 | 0.80 | 1.67 | 0.34 |
| BMI percent | ||||||||||
| Baseline | 69 | 56.7 | 32.0 | 36 | 53.2 | 31.9 | 33 | 60.5 | 32.1 | 0.34 |
| Final | 71 | 64.4 | 31.7 | 37 | 62.5 | 32.5 | 34 | 66.4 | 31.3 | 0.61 |
| IGF-1, ng/mL | ||||||||||
| Baseline | 70 | 194 | 144 | 36 | 229 | 153 | 34 | 158 | 127 | 0.039 |
| Final | 68 | 403 | 200 | 35 | 452 | 208 | 33 | 351 | 180 | 0.037 |
| Ln (IGF-1) | ||||||||||
| Baseline | 70 | 4.97 | 0.86 | 36 | 5.20 | 0.74 | 34 | 4.72 | 0.91 | 0.016 |
| Final | 68 | 5.87 | 0.54 | 35 | 6.03 | 0.41 | 33 | 5.70 | 0.61 | 0.014 |
| IGF-1 z score | ||||||||||
| Baseline | 20 | -0.92 | 1.40 | 16 | -1.13 | 1.35 | 4 | -0.10 | 1.46 | 0.20 |
| Final | 57 | 0.36 | 1.47 | 27 | 0.64 | 1.07 | 30 | 0.10 | 1.74 | 0.16 |
| Peak GH, ng/mL | 61 | 6.06 | 3.29 | 37 | 6.08 | 2.38 | 24 | 6.02 | 4.40 | 0.95 |
| LH, IU/L | 35 | 1.76 | 1.72 | 20 | 1.67 | 1.50 | 15 | 1.87 | 2.03 | 0.74 |
| FSH, IU/L | 35 | 3.05 | 3.18 | 20 | 3.63 | 3.75 | 15 | 2.29 | 2.10 | 0.19 |
| Estradiol, pg/mL | 16 | 24.5 | 9.2 | 7 | 20.80 | 2.12 | 9 | 27.34 | 11.56 | 0.13 |
| Testosterone, ng/dL | 25 | 102.4 | 131.6 | 15 | 105.1 | 120.8 | 10 | 98.5 | 153.1 | 0.91 |
| Height velocity, cm/y | ||||||||||
| Baseline | 58 | 5.18 | 2.30 | 30 | 5.53 | 2.45 | 28 | 4.80 | 2.11 | 0.23 |
| 1 y | 69 | 7.81 | 3.48 | 35 | 8.62 | 3.02 | 34 | 6.97 | 3.77 | 0.049 |
| Final | 71 | 4.85 | 3.43 | 37 | 5.71 | 3.40 | 34 | 3.92 | 3.25 | 0.026 |
| Height velocity z score | ||||||||||
| Baseline | 53 | -0.50 | 2.00 | 29 | 0.06 | 1.86 | 24 | -1.16 | 2.00 | 0.026 |
| 1 y | 65 | 1.33 | 2.75 | 34 | 1.91 | 1.59 | 31 | 0.70 | 3.54 | 0.086 |
| Final | 58 | 1.02 | 1.49 | 29 | 1.74 | 1.38 | 29 | 0.29 | 1.24 | <0.0001 |
| BA-CA, y | ||||||||||
| Baseline | 70 | -1.05 | 1.42 | 37 | -1.23 | 1.12 | 33 | -0.84 | 1.69 | 0.26 |
| Final | 67 | -0.69 | 1.63 | 34 | -1.37 | 1.33 | 33 | 0.00 | 1.63 | 0.0003 |
| Duration of rhGH therapy, y | 71 | 5.5 | 2.6 | 37 | 3.6 | 0.8 | 34 | 7.6 | 2.4 | <0.0001 |
| N | n | % | N | n | % | N | n | % | ||
| Sex | ||||||||||
| Male | 71 | 50 | 70.4 | 37 | 27 | 73.0 | 34 | 23 | 67.7 | 0.62 |
| Female | 71 | 21 | 29.6 | 37 | 10 | 27.0 | 34 | 11 | 32.4 | |
| Prepubertal (Tanner stage I) | ||||||||||
| Baseline | 66 | 42 | 63.6 | 35 | 14 | 40.0 | 31 | 28 | 90.3 | <0.0001 |
| Final | 51 | 9 | 17.7 | 30 | 2 | 6.7 | 21 | 7 | 33.3 | 0.023 |
| Near-adult height, cm | 26 | 163.8 | 8.1 | 10 | 165.5 | 7.4 | 16 | 162.8 | 8.6 | 0.41 |
| Female | 9 | 160.9 | 9.9 | 2 | 156.8 | 7.6 | 7 | 162.1 | 10.7 | 0.54 |
| Male | 17 | 165.3 | 6.8 | 8 | 167.7 | 5.9 | 9 | 163.2 | 7.2 | 0.19 |
| Bone age–predicted adult height at final visit, cm | 65 | 169.0 | 9.9 | 31 | 167.4 | 8.9 | 34 | 170.5 | 10.6 | 0.22 |
| Female | 20 | 164.3 | 10.0 | 10 | 159.3 | 4.9 | 10 | 169.2 | 11.5 | 0.03 |
| Male | 45 | 171.1 | 9.2 | 21 | 171.3 | 7.8 | 24 | 171.0 | 10.4 | 0.92 |
| Midparental target height, cm: all males | 10 | 171.0 | 5.6 | 7 | 172.1 | 5.5 | 3 | 168.4 | 6.1 | 0.37 |
Treatment duration is 2.1-13.7 years; baseline age, age at the start of rhGH therapy; P values were obtained by 2-sample t test of means, χ 2, or Fisher exact test of proportions, whichever is appropriate; sexual maturity rating was determined by endocrinologist using the Tanner method. For percentage, % = 100 × (n/N).
Abbreviations: BA-CA, bone age minus chronological age; BMI, body mass index; rhGH, recombinant human growth hormone.
Changes in BA minus CA during rhGH therapy
There was an increase in BA-CA from baseline to the end of study; however, this increase did not reach statistical significance: -1.05 ± 1.42 versus -0.69 ± 1.63, P = 0.09, (Table 1). The proportion of subjects with BA > CA was significantly higher at the end of study compared with the baseline: 34.3% versus 18.6%, P = 0.036 (Table 1, Fig. 2A and 2B). The percentage of prepubertal subjects decreased from 63.6% at baseline or the start of the rhGH therapy to 19.2% at the final visit (P < 0.0001). There were statistically significant increases in height z score in the first year of rhGH therapy, -2.07 ± 2.27 vs. -0.78 ± 1.05, P < 0.0001; and IGF-1 z score, -0.92 ± 1.40 vs. 0.36 ± 1.47, P = 0.0004, but no significant change in the weight-adjusted rhGH dose (P = 0.08) throughout the duration of therapy. Interestingly, IGF-I was associated with increases in the value of BA-CA, β = 0.003, P = 0.04 (95% CI, 0.00-0.005), whereas rhGH dose was not, β = -6.70, P = 0.57 (95% CI, -29.72 to 16.32). Additionally, IGF-I was associated with increases in child height compared with the corresponding bone age predicated adult height, β = 0.001, P = 0.0022, (95% CI, -0.001 to 0.00), whereas rhGH dose was not, P = 0.09.
Figure 2.
(A) Scatterplot of the relationship between bone age (BA) and chronological age (CA) at baseline, before recombinant human GH (rhGH) therapy, showing a strong correlation between BA and CA. Additionally, 81.4% of the subjects had BA values that were less than their CA. (B) Scatterplot of the relationship between BA and CA at the final visit showing a significant but reduced correlation between BA and CA when compared with the baseline relationship in Fig. 2A. Interestingly, 65.7% of the subjects still had BA values that were less than their CA, and such patients have the potential for further growth in height for a few more years even after the cessation of rhGH therapy.
We explored the effect of BMI on BA-CA in a GENMOD by dichotomizing BMI status into BMI of <85th percentile (normal weight, assigned a value of 0) versus BMI of ≥85th percentile (overweight/obese, assigned a value of 1) and found no effect of BMI on BA-CA (β = 0.11; 95 CI, -0.56 to 0.78; P = 0.75,). There was equally no significant difference in BA-CA between the male and female subjects (P = 0.68, Table 3).
Table 3.
Piecewise generalized regression model for bone age minus chronological age
| Parameter | Estimate | SE | 95% confidence interval | P value | |
|---|---|---|---|---|---|
| Intercept | -0.546 | 0.534 | -1.593 | 0.500 | 0.31 |
| t (treatment duration in y) | 0.126 | 0.053 | 0.023 | 0.229 | 0.017 |
| t2 | -0.110 | 0.130 | -0.366 | 0.145 | 0.040 |
| Age at the start of therapy | -0.082 | 0.042 | -0.164 | 0.000 | 0.049 |
| Male (vs female) | 0.156 | 0.381 | -0.591 | 0.903 | 0.68 |
| t + t2 | 0.015 | 0.105 | -0.191 | 0.221 | 0.88 |
Note: For GENMOD, we set distribution=normal, link=identity, repeated measure=subject identification, covariance structure=compound symmetry; time point of slope change=6.5 (years). Parameter estimate for t is the slope before the change point (6.5 years); that for t+t2 is the slope after the change point. The mathematical model for the piece-wise GENMOD is:
Abbreviation: GENMOD, generalized linear regression model.
Assessment of the correlation between BA and CA in the younger and older subjects
Using a cutoff age of 10 years to dichotomize the subjects into younger versus older participants, the correlation between BA and CA for subjects of ≤10 years was 0.89 (P < 0.0001) from 200 observations over time. The correlation between BA and CA for subjects of >10 years was 0.84 (P < 0.0001) from 126 observations over time. Thus, there was no significant difference in the correlations between BA and CA for the younger and older subjects (P = 0.08 by z test of 2 correlations).
Comparison of short-term and long-term rhGH therapy
When the duration of rhGH therapy was stratified into shorter-term therapy (≤5 years) and longer term therapy (>5 years), there were significant differences in height velocity z scores between the shorter and longer term groups at baseline and at final visit (P = 0.026 and <0.0001, respectively), but not at the end of 1 year of rhGH (Table 2). There was no difference in BA-CA between the 2 groups at baseline, -1.23 ± 1.12 versus -0.84 ± 1.69, P = 0.26 (Table 2). In contrast, BA-CA was significantly lower in the ≤5-year group compared with the >5-year group at the final visit (-1.37 ± 1.33 vs. 0.00 ± 1.63, P = 0.0003). Further validation analysis showed that when the duration of rhGH therapy was stratified into ≤6 years and >6 years, there was equally no difference in BA-CA between the 2 groups at baseline (-1.17 ± 1.17 vs. -0.83 ± 1.8, P = 0.39), and similar to the result of the 5-year cutoff, BA-CA was significantly lower in the ≤6-year group at the final visit (-1.05 ± 1.48 vs. -0.09 ± 1.72, P = 0.019). Table 2 shows no differences in near-adult heights, bone age predicted adult heights at final visit, and midparental target heights between the shorter term versus longer term treatment groups.
Characterization of skeletal age catch-up phenomenon during rhGH therapy
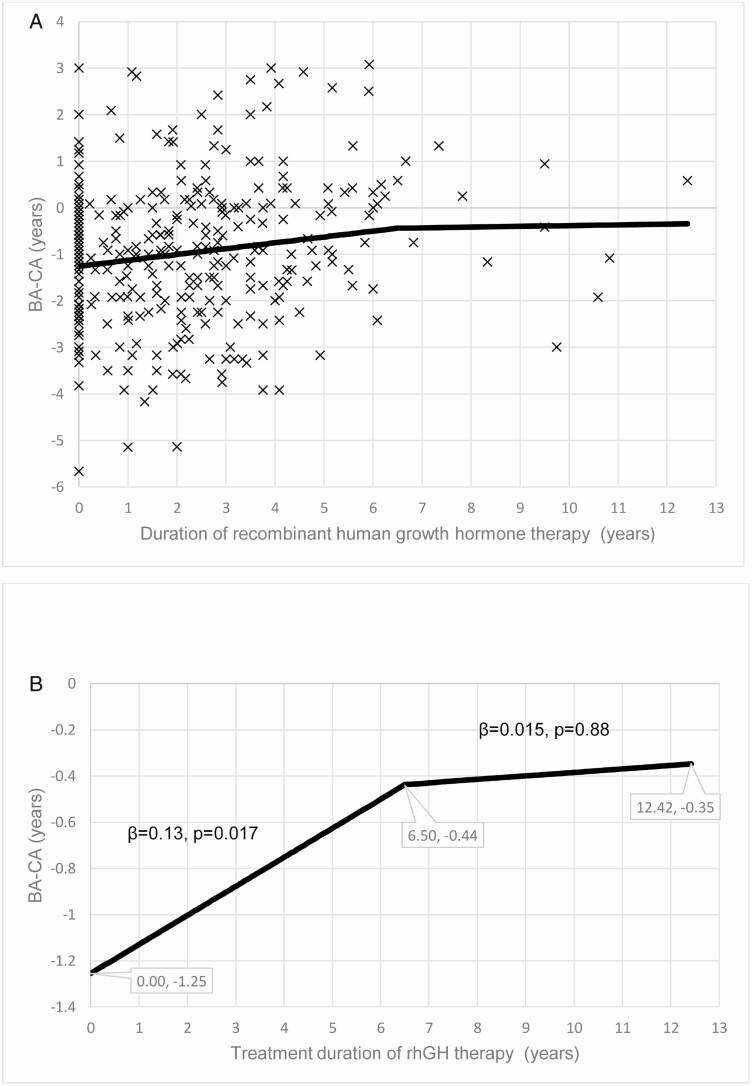
For the longitudinal analysis, model QIC depicted 6.5 years as the optimal cutoff point for slope change for the regression line between BA-CA and the duration of rhGH therapy (Fig. 3). Using this cutoff of 6.5 years, the piecewise GENMOD showed that before 6.5 years, each additional year of treatment increased BA-CA by 1.5 months (β = 0.126 in years; 95% CI, 0.023-0.229; P = 0.017, Table 3, Fig. 4A and 4B). Noting that Fig. 4A and 4B are outputs for the same results, Fig. 4A shows all individual scatter points on a wide vertical scale (y-axis), whereas Fig. 4B is a focused output without the scatter points and on a smaller range for the vertical scale to enable the characterization of the regression line in a greater detail. Because the regression line for BA-CA was in the negative territory, the increase in BA-CA during rhGH therapy indicates that the erstwhile delayed BA underwent a catchup phenomenon that brought it in line with the CA, but did not significantly advance beyond CA. Specifically, the model predicted that BA-CA at the start of therapy was -1.25 years, and then advanced to -0.44 years at the 6.5-year mark (Fig. 4B). Beyond 6.5 years of rhGH, BA showed minimal advancement toward CA (β = 0.015; 95% CI, -0.191 to 0.221; P = 0.88) because the final predicted BA-CA was -0.35 years.
Figure 3.
This figure shows that the initial catchup phase of bone age (BA) advancement following the institution of rhGH therapy spans the first 6.5 years and then slows down. This is represented in this graph of the Quasi-likelihood under the Independence Model Criterion (QIC) for generalized linear regression model (GENMOD) at various cutoff points in years. The QIC shows that the optimal time point for the change of slope of the scatter plots for BA minus chronological age (BA-CA) versus the duration of recombinant human GH (rhGH) occurred at the 6.5-year mark. QIC was used because of its precision as a measure of goodness-of-fit for generalized estimating equation (GEE) models, whereas a smaller QIC value denotes a better fit [36].
Figure 4.
(A) Scatterplots of the relationship between serial bone age minus chronological age (BA-CA) assessments and the duration of recombinant human GH (rhGH) therapy. (B) Graph of the relationship between serial BA-CA assessments and the duration of rhGH therapy. This graph shows that the delayed BA increases during the first 6.5 years of rhGH to approximate the CA but does not progress to BA advancement in this long-term study.
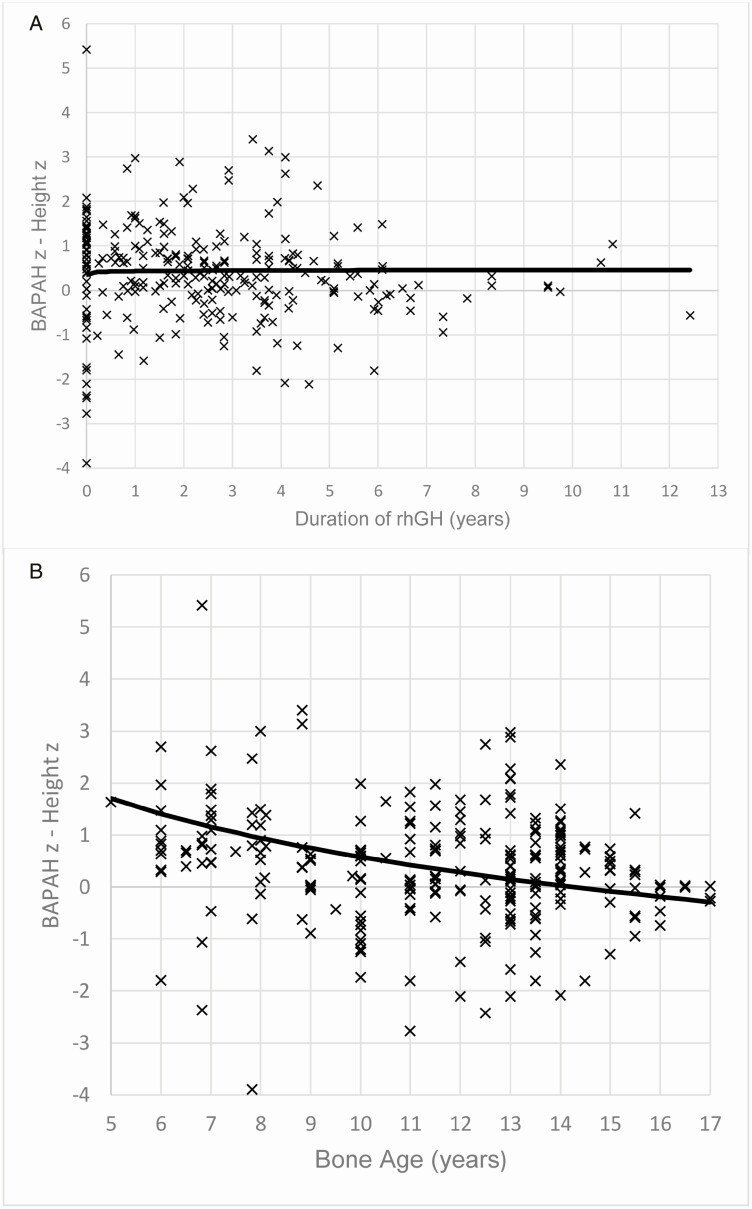
Determination of the relationship between BAPAH z score minus child’s concurrent height z score versus either the duration of rhGH therapy or bone age
Subsequent investigation examined the relationship between BAPAH z score minus child’s concurrent height z score (BAPAH z – height z) and either the duration of rhGH therapy or bone age. There was no relationship between BAPAH z – height z and the duration of rhGH therapy (P = 0.68) (Fig. 5A). In contrast, the difference between BAPAH z score and height z score was greater at younger bone age, but became negligible with advancing bone age, signifying a more accurate prediction of adult height with advancing years (P < 0.0001) (Fig. 5B). Thus, in line with previous findings, the Bayley-Pinneau prediction model [37] may be inaccurate in the early years when delayed bone age is more pronounced [7] compared with later adolescence [38].
Figure 5.
(A) Graph of the relationship of bone age–predicted adult height (BAPAH) z score minus child’s concurrent height z score versus the duration of recombinant human GH (rhGH) therapy. The duration of rhGH therapy did not affect the difference between BAPAH z score and concurrent height z score (P = 0.68). (B) Graph of the relationship of BAPAH z score minus child’s concurrent height z score versus bone age. The difference between BAPAH z score and concurrent height z score was greater at a younger bone age but became negligible with advancing bone age (P < 0.0001), signifying a more accurate prediction of final adult height with advancing years. Thus, the Bayley-Pinneau prediction model may be inaccurate in the early years compared with the later years.
Discussion
This long-term study found no evidence for sustained skeletal advancement in children and adolescents who received rhGH therapy. There was a small initial increase in BA-CA that was not sustained over the longer term course of rhGH therapy. The subjects who received long-term rhGH therapy showed a normalization of BA at parity with their CA. Final BA-CA was significantly lower in the group that received shorter term rhGH therapy compared with those that received longer term therapy. Given that those who received shorter term rhGH therapy were older than those in the longer term group, this finding suggests the absence of any synergistic effect of rhGH with sex steroids on skeletal maturation in this group with a greater proportion of pubertal subjects.
The regression analysis showed a significant association between BA-CA and the duration of rhGH therapy for the first 6.5 years of treatment but not in the subsequent years. Thus, the early increases in BA-CA noted in the first 6.5 years of rhGH therapy was not maintained in the later years (ie, 7-14 years). Hence, the initial increase in BA most likely represents a skeletal catchup phenomenon that is commensurate with increasing statural growth because it plateaued with continued rhGH therapy such that BA was at parity with CA at the end of therapy. This is crucial as the initial increase in BA did not exceed the CA.
There was no relationship between the duration of rhGH therapy and the difference between BAPAH z score and child’s concurrent height z score. This analysis confirms our earlier result that long-term rhGH does not advance skeletal maturation. In contrast, the difference between BAPAH z score and concurrent height z score was greater at younger bone age, but became negligible with advancing bone age, signifying that BAPAH is more variable in younger subjects, usually with greater bone age delay, compared with later adolescence. This is consistent with observations from the most commonly used height prediction tool, the Bayley-Pinneau method [37], stating that near-adult height predictions may be less accurate, especially at younger ages [37, 38]. Thus, annual BA assessment in younger children on rhGH therapy could overestimate the child’s near-adult height, which would initially be a welcome news to the family. However, this often turns to disappointment in the later years of treatment when BAPAH becomes more accurate and is better aligned with the child’s near-adult height, which often is less than the earlier BAPAH [7, 39].
The results of this study could be reassuring for children and adolescents who receive longer term rhGH therapy because despite reports of early- [5, 6] or later onset [1, 6] BA advancement from prior short-term studies, there is no overall advancement in skeletal maturation when these subjects are monitored over the long term. This finding is critical for clinical practice because these yearly BA assessments are often mandated by the child’s health insurance carriers as a key requirement for the renewal of treatment authorization, despite the recommendations by the 2007 Consensus statement for periodic, but not annual BA monitoring [7], whereas the 2019 Consensus statement [8] did not specify a schedule for BA monitoring.
Our study builds on earlier short-term and medium-term studies that reported that the rate of BA progression in children and adolescents on treatment with rhGH is variable and can either be within the normal range [3, 18, 19] or accelerated [16, 17]. These studies further suggested that the rate of BA progression could be affected by factors such as age, sex, pubertal status, GH dose, IGF-I level, duration of rhGH therapy, BMI, and underlying medical conditions [5]. This suggests that definitive investigations on the effect of rhGH therapy on skeletal maturation should be of longer term duration and should be adjusted for these variables to ensure the validity of the results. Our long-term study showed that after controlling for these confounders, rhGH therapy (dose) was not associated with an overall significant BA advancement. This conclusion is consistent with earlier reports that found no significant effect of rhGH on skeletal maturation [3, 18, 19], but differs from studies that reported evidence for increased skeletal maturation [6, 13, 15-17]. Taken together, our findings support strategic, but not reflexive annual bone age assessment during rhGH therapy. This is consistent with earlier suggestions to discontinue the practice of routine BA monitoring during rhGH therapy because BA does not provide meaningful data or guidance that could prompt corrective measures to therapeutic approaches during treatment [1]. Though our study is of long duration, the results may not be generalizable because of the limitations of our sample size and the retrospective nature of the study.
These results compare favorably with both the 2007 and 2019 Consensus Statements [7, 8], which did not mandate annual bone age assessments for monitoring of children and adolescents receiving rhGH therapy. Neither of the Consensus Statements categorically stated that rhGH advances BA. The 2007 Consensus Statement further called for longer term studies of >6 years to determine the role of baseline and treatment-related IGF-I levels, whereas both Consensus Statements [7, 8] emphasized the limitations of using bone age to predict adult height in children because of the inaccuracies deriving from factors such as delayed bone age, BMI-related bone age advancement, and the limitations of the Greulich and Pyle method to accurately predict near-adult height in children of non-European descent.
Our study found that IGF-I was associated with increases in the value of BA-CA, as well as increases in child’s height compared with the corresponding bone age predicated adult height. This is a response to the 2007 Consensus Statement [7] that called for studies to validate the role of baseline and treatment-related biochemical data including IGF-I in long-term studies, while recognizing that short-term studies of 2-year duration suggest that treatment-related rise in IGF-I correlates with short-term height gain [6].
The procedures and findings from our long-term study agree with the stipulations of both Consensus Statements [7, 8], as shown by the selection and adjustment of GH dosage by weight, the demonstration of a robust response to GH therapy in the first year of treatment, the characterization of the limitations of bone age prediction by delayed bone age and other factors, and the confirmation that IGF-I concentrations correlate with height gain and BA-CA.
This is the first long-term study to present a detailed analysis of the longitudinal changes in bone age in children receiving rhGH therapy. It demonstrates that the initial increases in bone age at the start of rhGH therapy were principally a catchup phenomenon that slows down once the BA reaches parity with the chronological age (ie, target-seeking, similar to the height, because height often crosses major percentile lines to settle on the height percentile of the midparental target height). It is reassuring that the initial bone age advancement was not greater than 2 SDs, which would have met the clinical definition of bone age advancement and thus, herald accelerated epiphyseal fusion.
There are, however, limitations that should be considered in the interpretation of these results. The retrospective nature of the study design precludes any inference to causality among the parameters studied. The inclusion of only subjects with serial anthropometric and radiologic data may represent a compliant population that may not accurately reflect the traditional pattern of rhGH adherence in children and adolescents. The lack of a control group of healthy children and adolescents who were not receiving rhGH limited our ability to determine whether the catchup phenomenon noted during the early phase of rhGH therapy was only applicable to GH-treated children, or was rather a phenomenon that occurs in all children. The key strengths of this study include its adequate statistical power to detect any significant differences between the baseline and final BA-CA if such a difference were present. Further strengths include its longitudinal design, the representative sample of subjects, and the long duration of therapy that enabled a comprehensive assessment of the short-, medium-, and long-term effects of rhGH therapy on BA. The long-term duration of repeated measures enabled the detection of the novel inflection point of 6.5 years as the end of the period of catchup phenomenon in skeletal maturation during rhGH therapy. All bone ages were read from a research point of view as they were coded and validated in a blinded manner and read by the same board-certified pediatric radiologist.
Conclusions
Long-term treatment with recombinant human GH in growth hormone-deficient children and adolescents does not result in advanced skeletal maturation. IGF-I concentrations correlate with increases in both height and BA. BA assessments in younger children are less accurate and often overestimate their BAPAH. These findings support strategic, but not reflexive, annual monitoring of skeletal maturation with bone age determination in children receiving rhGH therapy. These data are crucial to address the reflexive mandates from some insurance companies, who in their bid to prevent rhGH treatment in patients with closed epiphyses, often start to require yearly bone age assessments at a surprisingly young age. Our data suggest that obtaining a bone age before mid-to-late puberty is very unlikely to identify a bone age that is more mature than expected. This will be reassuring to pediatric endocrinologists who may resort to reflexive annual bone age in a bid to fulfill insurance companies’ mandate for continuation of authorization for rhGH for their patients. These results, which agree with the stipulations of both the 2007 and 2019 Consensus Statements for rhGH therapy in children from the Pediatric Endocrine Society, the European Society for Paediatric Endocrinology, and the Growth Hormone Research Society, call for a revision of the current rhGH treatment and monitoring guidelines to reduce the frequency of BA assessments in pediatric subjects receiving rhGH therapy.
Acknowledgments
Financial Support: No funding received.
Author Contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Glossary
Abbreviations
- BA
bone age
- BAPAH
bone age–predicted adult height
- BMI
body mass index
- CA
chronological age
- CV
coefficient of variation
- GENMOD
generalized linear regression model
- GHD
GH deficiency
- QIC
quasi-likelihood under the Independence Model Criterion
- rhGH
recombinant human GH
Additional Information
Disclosures: The authors have no conflict of interest to disclose.
Data Availability
All data generated or analyzed during this study are included in this article.
References
- 1. Wilson DM. Regular monitoring of bone age is not useful in children treated with growth hormone. Pediatrics. 1999;104(4 Pt 2):1036-1039. [PubMed] [Google Scholar]
- 2. Crowe BJ, Rekers-Mombarg LT, Robling K, Wolka AM, Cutler GB Jr, Wit JM; European Idiopathic Short Stature Group . Effect of growth hormone dose on bone maturation and puberty in children with idiopathic short stature. J Clin Endocrinol Metab. 2006;91(1):169-175. [DOI] [PubMed] [Google Scholar]
- 3. Zadik Z, Chalew S, Zung A, et al. Effect of long-term growth hormone therapy on bone age and pubertal maturation in boys with and without classic growth hormone deficiency. J Pediatr. 1994;125(2):189-195. [PubMed] [Google Scholar]
- 4. Darendeliler F, Ranke MB, Bakker B, et al. Bone age progression during the first year of growth hormone therapy in pre-pubertal children with idiopathic growth hormone deficiency, Turner syndrome or idiopathic short stature, and in short children born small for gestational age: analysis of data from KIGS (Pfizer International Growth Database). Horm Res. 2005;63(1):40-47. [DOI] [PubMed] [Google Scholar]
- 5. Kang MJ, Kim EY, Shim YS, et al. Factors affecting bone age maturation during 3 years of growth hormone treatment in patients with idiopathic growth hormone deficiency and idiopathic short stature: Analysis of data from the LG growth study. Medicine (Baltimore). 2019;98(14):e14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamp GA, Waelkens JJ, de Muinck Keizer-Schrama SM, et al. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch Dis Child. 2002;87(3):215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen P, Rogol AD, Deal CL, et al. ; 2007 ISS Consensus Workshop participants . Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210-4217. [DOI] [PubMed] [Google Scholar]
- 8. Collett-Solberg PF, Ambler G, Backeljauw PF, et al. Diagnosis, genetics, and therapy of short stature in children: a growth hormone research society international perspective. Horm Res Paediatr. 2019;92(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett J, Maranda L, Nwosu BU. The relationship between subnormal peak-stimulated growth hormone levels and auxological characteristics in obese children. Front Endocrinol (Lausanne). 2014;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baron J, Sävendahl L, De Luca F, et al. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol. 2015;11(12):735-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res. 1998;49 Suppl 1:2-8. [DOI] [PubMed] [Google Scholar]
- 12. Goodman HG, Grumbach MM, Kaplan SL. Growth and growth hormone. II. A comparison of isolated growth-hormone deficiency and multiple pituitary-hormone deficiencies in 35 patients with idiopathic hypopituitary dwarfism. N Engl J Med. 1968;278(2):57-68. [DOI] [PubMed] [Google Scholar]
- 13. Darendeliler F, Hindmarsh PC, Preece MA, Cox L, Brook CG. Growth hormone increases rate of pubertal maturation. Acta Endocrinol (Copenh). 1990;122(3):414-416. [DOI] [PubMed] [Google Scholar]
- 14. Richmond E, Rogol AD. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best Pract Res Clin Endocrinol Metab. 2016;30(6):749-755. [DOI] [PubMed] [Google Scholar]
- 15. Chung S, Yoo JH, Choi JH, et al. Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study. Ann Pediatr Endocrinol Metab. 2018;23(1):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawai M, Momoi T, Yorifuji T, Yamanaka C, Sasaki H, Furusho K. Unfavorable effects of growth hormone therapy on the final height of boys with short stature not caused by growth hormone deficiency. J Pediatr. 1997;130(2):205-209. [DOI] [PubMed] [Google Scholar]
- 17. Hopwood NJ, Hintz RL, Gertner JM, et al. Growth response of children with non-growth-hormone deficiency and marked short stature during three years of growth hormone therapy. J Pediatr. 1993;123(2):215-222. [DOI] [PubMed] [Google Scholar]
- 18. Lesage C, Walker J, Landier F, Chatelain P, Chaussain JL, Bougnères PF. Near normalization of adolescent height with growth hormone therapy in very short children without growth hormone deficiency. J Pediatr. 1991;119(1 Pt 1):29-34. [DOI] [PubMed] [Google Scholar]
- 19. Frindik JP, Kemp SF, Sy JP. Effects of recombinant human growth hormone on height and skeletal maturation in growth hormone-deficient children with and without severe pretreatment bone age delay. Horm Res. 1999;51(1):15-19. [DOI] [PubMed] [Google Scholar]
- 20. McCaughey ES, Mulligan J, Voss LD, Betts PR. Randomised trial of growth hormone in short normal girls. Lancet. 1998;351(9107):940-944. [DOI] [PubMed] [Google Scholar]
- 21. Veit LE, Maranda L, Fong J, Nwosu BU. The vitamin D status in inflammatory bowel disease. Plos One. 2014;9(7):e101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lundberg RL, Marino KR, Jasrotia A, et al. Partial clinical remission in type 1 diabetes: a comparison of the accuracy of total daily dose of insulin of <0.3 units/kg/day to the gold standard insulin-dose adjusted hemoglobin A1c of ≤9 for the detection of partial clinical remission. J Pediatr Endocrinol Metab. 2017;30(8):823-830. [DOI] [PubMed] [Google Scholar]
- 23. Marino KR, Lundberg RL, Jasrotia A, et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. Plos One. 2017;12(5):e0176860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nwosu BU, Zhang B, Ayyoub SS, et al. Children with type 1 diabetes who experienced a honeymoon phase had significantly lower LDL cholesterol 5 years after diagnosis. Plos One. 2018;13(5):e0196912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 2002;11:1-190. [PubMed] [Google Scholar]
- 26. National Center for Health Statistics. Z-score data files. Accessed March 12, 2021. https://www.cdc.gov/growthcharts/zscore.htm.
- 27. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nwosu BU, Lee MM. Evaluation of short and tall stature in children. Am Fam Physician. 2008;78(5):597-604. [PubMed] [Google Scholar]
- 30. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317-329. [DOI] [PubMed] [Google Scholar]
- 31. Rikken B, Wit JM. Prepubertal height velocity references over a wide age range. Arch Dis Child. 1992;67(10):1277-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Onis M, Siyam A, Borghi E, Onyango AW, Piwoz E, Garza C. Comparison of the World Health Organization growth velocity standards with existing US reference data. Pediatrics. 2011;128(1):e18-e26. [DOI] [PubMed] [Google Scholar]
- 33. Greulich WW, Pyle SI.. Radiographic Atlas of Skeletal Development of the Hands and Wrists. 2nd ed. Stanford: University Press, 1959. [Google Scholar]
- 34. Nwosu BU, Coco M, Jones J, Barnes KM, Yanovski JA, Baron J. Short stature with normal growth hormone stimulation testing: lack of evidence for partial growth hormone deficiency or insensitivity. Horm Res. 2004;62(2):97-102. [DOI] [PubMed] [Google Scholar]
- 35. Ham JN, Ginsberg JP, Hendell CD, Moshang T Jr. Growth hormone releasing hormone plus arginine stimulation testing in young adults treated in childhood with cranio-spinal radiation therapy. Clin Endocrinol (Oxf). 2005;62(5):628-632. [DOI] [PubMed] [Google Scholar]
- 36. Cui J. QIC Program and model selection in GEE analysis. Stata J. 2007; 7:209-220. [Google Scholar]
- 37. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423-441. [DOI] [PubMed] [Google Scholar]
- 38. Tarım O. Height predictions by Bayley-Pinneau method may misguide pediatric endocrinologists. Turk J Pediatr. 2013;55(5):485-492. [PubMed] [Google Scholar]
- 39. Wit JM, Rekers-Mombarg LT; Dutch Growth Hormone Advisory Group . Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab. 2002;87(2):604-611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.