Supplemental Digital Content is available in the text
Keywords: breast neoplasms, core needle biopsy, immunohistochemistry
Abstract
Ultrasound (US)-guided core needle biopsy (CNB) has been recognized as a crucial diagnostic tool for breast cancer. However, there is a lack of guidance for hospitals that are not equipped with adjunctive US. The aim of this study was to assess the sensitivity, specificity, and experience of freehanded CNB in the outpatient department, and to determine the minimum number of tissue strips required to obtain concordance for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and tumor grade with the excised specimen.
A prospective study was performed on 95 patients undergoing CNB and subsequent surgical procedures. The reliability of immunohistochemical assessments of the pathological type, tumor grade, ER, PR, and HER2 status in CNBs was compared with that of surgical specimens. Concordance between the CNBs and surgical samples was estimated as a percentage agreement, and analyzed using the chi-square test. A P < .05 was considered significant.
The concordance rates of ER, PR, and HER2 status and tumor grade status between CNBs and surgically excised specimens were 97.9%, 91.6%, 82.1%, and 84.2%, respectively. The reliability of taking 2 tissue strips was similar to that of taking six tissue strips in distinguishing malignancy from benignancy, and determining the pathological type without the aid of US. Four tissue strips obtained by CNB showed good accuracy comparable to those obtained by surgical specimens in assessing ER, PR, and HER2 status and tumor grade.
Two tissue strips obtained by CNB showed good accuracy in differentiating malignancy from benignancy, while at least 4 strips are recommended to obtain overall conformity of pathological biomarkers.
1. Introduction
Breast cancer is a leading cause of death in women worldwide. In China, it is 1 of the 5 most commonly diagnosed cancers in women, and about 268,600 new breast cancer cases were predicted in 2015.[1] The key point to tailor individualized treatment is to confirm pathological biomarkers of suspicious lesions, such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and ki67. These markers help to classify subtypes of breast cancer, predict response to treatment and long-term prognosis. However, there is no consensus on the best technique for histological sampling. Fine needle aspiration cytology has been widely used initially, as it is minimally invasive and well-tolerated. However, its applications are also restricted by its shortcomings, including insufficient samplings, difficulty in discriminating carcinoma in situ from invasive carcinoma as the material was aspirated, experience requirements for both the operator and the pathologist, and the low rate of definitive diagnoses. In light of this, core needle biopsy (CNB) has been established as a valid tool to assess radiologically and clinically-detected breast lesions.[2] CNB provides larger samples[3] with preserved architecture. The concordance rate of CNBs and surgical specimens in distinguishing benign from malignant lesions[4] ranges from 96% to 100%.[2] In addition, CNB provides predictive information such as tumor grade, ER, PR, and HER2 status,[5,6] which is useful for surgery planning and adjuvant/neoadjuvant treatment.
The accuracy of CNBs is known to increase when a higher number of CNB tissue strips are collected. One of the concerns is the dissemination of tumor cells into the adjacent tissue through the transgression and withdrawal of the biopsy needle,[7,8] but this is extremely rare and has little direct effect on patient outcomes.[9–11] Previous studies[4,12–14] have shown that 4 to 5 specimens are required to obtain a definitive diagnosis, but few studies have focused on the optimal number of biopsy cores required to obtain a reliable pathological diagnosis. In addition, achieving high accuracy usually requires the assistance of ultrasonography. However, not all hospitals are equipped to perform this technique, and additionally, there in an increase in cost and delay in referral to the radiological center with this technique.
Therefore, we conducted a prospective study to assess the number of biopsy strips collected by freehand CNB that are required to obtain a result consistent with the postoperative pathological diagnosis, in an outpatient department. Tumor grade and ER, PR, and HER2 status were evaluated, as they are critical parameters to guide the selection of neoadjuvant therapy and postoperative care.
2. Methods
2.1. Patients and samples
Breast cancer patients attending the Department of Breast and Thyroid Surgery, Jinan Central Hospital (affiliated with Shandong University) from 2013 to 2015 were considered for the prospective study. A total of 95 patients who underwent CNB and subsequent surgical excision were recruited in this prospective study. Patients who had previously received neoadjuvant chemotherapy and/or endocrine therapy were excluded due to potential treatment effects on the receptor status. Patients who had their histology processed in other hospitals or those with an equivocal or unavailable receptor status were also excluded. The period between CNB and the final surgery ranged from 2 to 3 days. The study (Register No.: KYLL-2016–353) was approved by the Ethics Committee of Scientific Research of Shandong University Qilu Hospital, and was conducted in accordance with the principles of the Declaration of Helsinki. Oral and written informed consent for participation in the study was obtained from all participants.
2.2. Procedure
Biopsies were performed by an experienced surgeon using a 14-gauge needle and a spring-loaded biopsy gun (Bard Magnum). Three to 6 cores containing specimens from different parts of each lesion were obtained without the aid of US. Each core was labeled with the pass number in accordance with the order of the puncture. All specimens were formalin-fixed and paraffin-embedded, and the tissue sections were stained with hematoxylin and eosin. The samples were assessed by an experienced pathologist who was blinded to the identity of the final surgical samples, to exclude potential bias.
2.3. ER, PR, and HER2 determination
The status of the ER, PR, and HER2 biomarkers was assessed using standard immunohistochemical methods in paraffin-embedded, formation-fixed tissue stained with hematoxylin and eosin, and with antibodies to the ER, PR, and HER2 proteins (Dako, Glostrup, Denmark). ER positivity (ER+) and PR positivity (PR+) were defined as the presence of more than 1% positively stained invasive tumor cells with nuclear staining. The level of HER2 expression was scored according to the American Society of clinical Oncology/College of American Pathologists 2013 guidelines: score 0 for no staining or membrane staining in < 10% of the tumor cells; score 1 + for faint/barely perceptible partial membrane staining in ≥ 10% of the tumor cells; score 2 + for weak to moderate complete membrane staining in ≥ 10% of the tumor cells; and score 3 + for strong complete membrane staining in ≥ 10% of the tumor cells. HER2 status was categorized as negative (0 or 1 +), inconclusive (2 +), or positive (3 +) according to the membrane staining. Immunohistochemistry (IHC) of the surgical specimens was defined as the gold standard, and CNB was defined as the test technique.
2.4. Statistical analysis
Statistical calculations were performed using the Statistical Package for Social Sciences (SPSS) version 19.0 software. The concordance, sensitivity, and specificity were calculated using CNBs as the test assessment and the surgical specimens as the gold standard; the Fisher's exact test and the chi-square test were used for performing comparisons. The exact 95% confidence intervals were calculated based on the binomial distribution. A 2-tailed P value < .05 was considered statistically significant. The kappa coefficient showed the proportion of agreement.
3. Results
Ninety-five patients meeting the inclusion criteria were enrolled in this study. All patients were diagnosed with malignancies according to the pathology results of the surgical specimens. Except for 1 missed case, all the other patients were also diagnosed as malignant according to the analysis of CNBs. The clinicopathological characteristics are summarized in Table 1. The patients’ mean age was 52 years (range: 34 to 80 years). The mean tumor diameter was 3.3 cm (range: 1 to 10 cm). One patient presented with T0 stage, 22 patients presented with T1 stage, 59 patients presented with T2 stage, 10 patients presented with T3 stage, and 3 patients presented with T4 stage disease. Invasive ductal carcinoma was the most common type of breast cancer (97.9%).
Table 1.
Clinicopathological characteristics of the patients.
| n | |
| Mean Age (yr) (mean ± SD) | 52 (34–80) |
| Pathological T Stage | |
| T0 | 1 |
| T1 | 22 |
| T2 | 59 |
| T3 | 10 |
| T4 | 3 |
| Histology | |
| invasive lobular carcinoma | 1 |
| ductal carcinoma in situ | 1 |
| invasive ductal carcinoma | 93 |
| ER status | |
| Negative | 30 |
| Positive | 64 |
| PR status | |
| Negative | 47 |
| positive | 47 |
| HER2 status | |
| Score0/1+ | 39 |
| Score2+ | 34 |
| Score3+ | 21 |
ER = estrogen receptor, HER2 = human epidermal growth factor receptor-2, PR = progesterone receptor, SD = standard deviation.
3.1. Hormonal status, HER2 status and tumor grade concordance
Among the CNB specimens, the ER status was negative in 31 and positive in 63 specimens. Among the surgical specimens, 30 cases were negative and 64 cases were positive for ER. The concordance rate of ER assessment between the CNB and surgical specimens was 98.9% (kappa value: .975; P < .001), with a discrepancy in 1 case (Table 2).
Table 2.
Concordance between CNB and surgical specimen for ER and PR status.
| Surgical specimen | |||||
| Biopsy | Negative/low | Positive/high | Concordance rate, % | Kappa | P value |
| ER status | |||||
| Negative | 30 | 1 | 98.9 | 0.975 | P < .001 |
| Positive | 0 | 63 | |||
| PR status | |||||
| Negative | 46 | 6 | 92.6 | 0.851 | P < .001 |
| Positive | 1 | 41 | |||
CNB = core needle biopsy, ER = estrogen receptor, PR = progesterone receptor.
Among the CNB specimens, the PR status was negative in 52 and positive in 42 specimens. Among the surgical specimens, 47 cases were negative and 47 cases were positive for PR. The concordance rate of PR assessment between the CNB and surgical specimens was 92.6% (kappa value: 0.851; P < .001), with a discrepancy in 7 cases (Table 2). Out of the 7 discrepancies, six positive specimens certified by postoperative histology were underestimated as negative in the CNB analysis, and only 1 negative specimen was overestimated as positive by the CNB analysis.
Among the CNB samples, the HER2 scores were as follows: score 0/1+, 43 cases; score 2+, 29 cases; and score 3+, 22 cases. Among the surgical specimens, the HER2 scores were as follows: score 0/1+, 39 cases; score 2+, 34 cases; and score 3+, 21 cases. The concordance rate of HER2 status between the CNB and surgical specimens was 83% (kappa value: 0.737; P < .001), with a discrepancy in 16 cases (Table 3).
Table 3.
Concordance between CNB and surgical specimen for HER2 status.
| Surgical specimen | ||||||
| CNB | Score1+ | Score2+ | Score3+ | concordance rate, % | Kappa | P value |
| Score0/1+ | 37 | 5 | 1 | 83 | 0.737 | P < .001 |
| Score2+ | 2 | 24 | 3 | |||
| Score3+ | 0 | 5 | 17 | |||
CNB = core needle biopsy, HER2 = human epidermal growth factor receptor-2.
The concordance rate of tumor grade between the CNB and surgical specimens was 84.2%. Consistent with previous studies,[15] our results showed that the tumor grade was more likely to be underestimated by preoperative CNBs. Nine cases were underestimated and six were overestimated when 5 and 6 cores were collected, respectively. Detailed information is shown in Table 4.
Table 4.
Discordance in the tumor grade between CNBs and excised specimens.
| First | Second | Third | Fourth | Fifth | Sixth | |
| Underestimated | 21 | 13 | 12 | 10 | 9 | 9 |
| Overestimated | 14 | 8 | 6 | 6 | 6 | 6 |
| Total | 35 | 21 | 18 | 16 | 15 | 15 |
CNB = core needle biopsy.
3.2. The optimal number of CNB specimens to detect malignancy and achieve overall concordance
It is not difficult to understand that the diagnostic accuracy of CNB increases with an increase in the number (one core, 77.9%; 2 cores, 93.7%, 3 cores, 96.8%; four cores, 98.9%; five cores, 98.9%; and six cores, 98.9%) of the CNB specimens collected (Table 5, Fig. 1). The above results demonstrated good concordance between the CNB and surgical specimens in evaluating IHC-assessed ER, PR, and HER2 status, and tumor grade, based on CNB with six passes, in each patient. There is a lack of studies assessing the minimal and optimal number of CNB specimens required to obtain a reliable result. Thus, we next sought to determine the minimum number of CNB samples needed to achieve the best concordance with surgical specimens with reference to detecting malignancy and gaining overall concordance. We performed a chi-square test to investigate the difference between the contiguous numbers of core biopsies in the diagnosis of malignancy, ER, PR, and HER2 status, and tumor grade. We observed that for the diagnosis of a malignancy, 2 cores are significantly more reliable than 1 (P < .001), while there is no significant increase in reliability upon further increasing the number of tissue strips (P > .05). For this reason, at least 2 specimens are required to confirm the diagnosis of malignancy. In terms of overall concordance (ER, PR, and HER2 status, and tumor grade), little difference was observed between obtaining 4 and 5 cores (P = .558), 5 and 6 cores (P = .656), and between obtaining 4 and 6 cores (P = .656). Detailed data explaining how the core numbers affected the concordance between surgical specimens and CNBs are listed in Supplementary Table 1, http://links.lww.com/MD2/A44. A comparison of immunohistochemical staining for the hormonal receptors and HER2 between CNBs and surgical specimens is shown in Figure 2.
Table 5.
Concordance (positive and negative) between each pass of the CNB and surgical specimen for histological and biomarker status.
| Core biopsy (n = 95) | |||||||
| First | Second | Third | Fourth | Fifth | Sixth | Surgical specimen (n = 95) | |
| Malignancy found | 74 | 89 | 92 | 94 | 94 | 94 | 95 |
| Tumor size | |||||||
| TIS | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T1 | 3 | 7 | 11 | 13 | 15 | 16 | 22 |
| T2 | 15 | 18 | 29 | 30 | 35 | 36 | 59 |
| T3 | 2 | 5 | 5 | 5 | 5 | 5 | 10 |
| T4 | 1 | 2 | 2 | 2 | 2 | 2 | 3 |
| Pathological type | 62 | 84 | 89 | 91 | 91 | 91 | 95 |
| ER | 70 | 85 | 90 | 92 | 93 | 93 | 95 |
| PR | 62 | 78 | 83 | 86 | 86 | 87 | 95 |
| Her2 | 43 | 53 | 60 | 70 | 76 | 78 | 95 |
| Grade | 60 | 74 | 77 | 79 | 80 | 80 | 95 |
| Overall concordance | 24 | 34 | 44 | 52 | 56 | 59 | 95 |
CNB = core needle biopsy, ER = estrogen receptor, HER2 = human epidermal growth factor receptor-2, PR = progesterone receptor.
Figure 1.
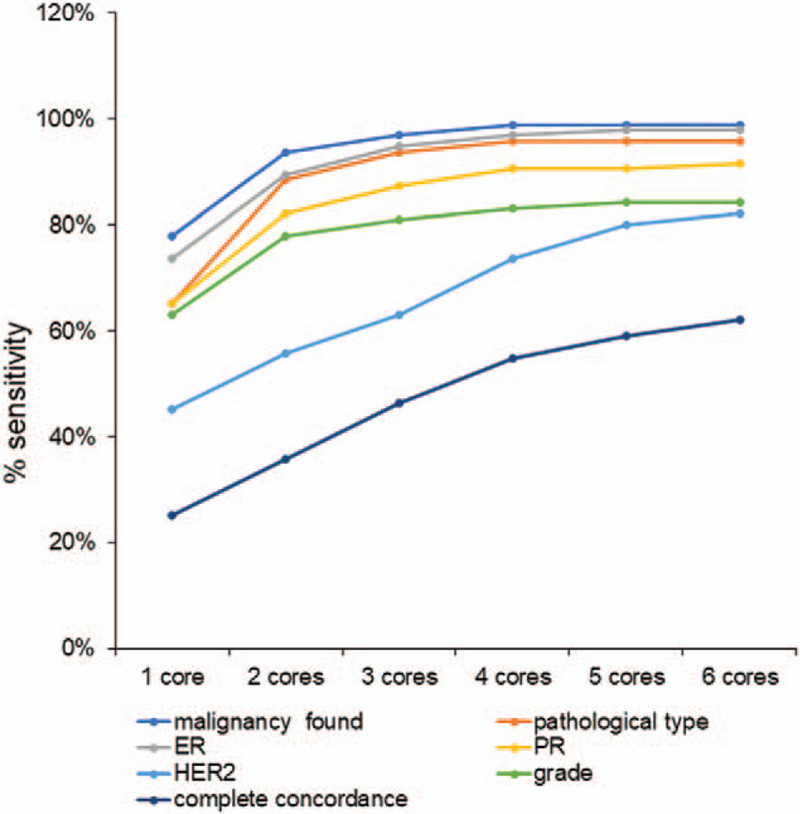
Cumulative sensitivity of the core needle biopsies (CNBs).
Figure 2.
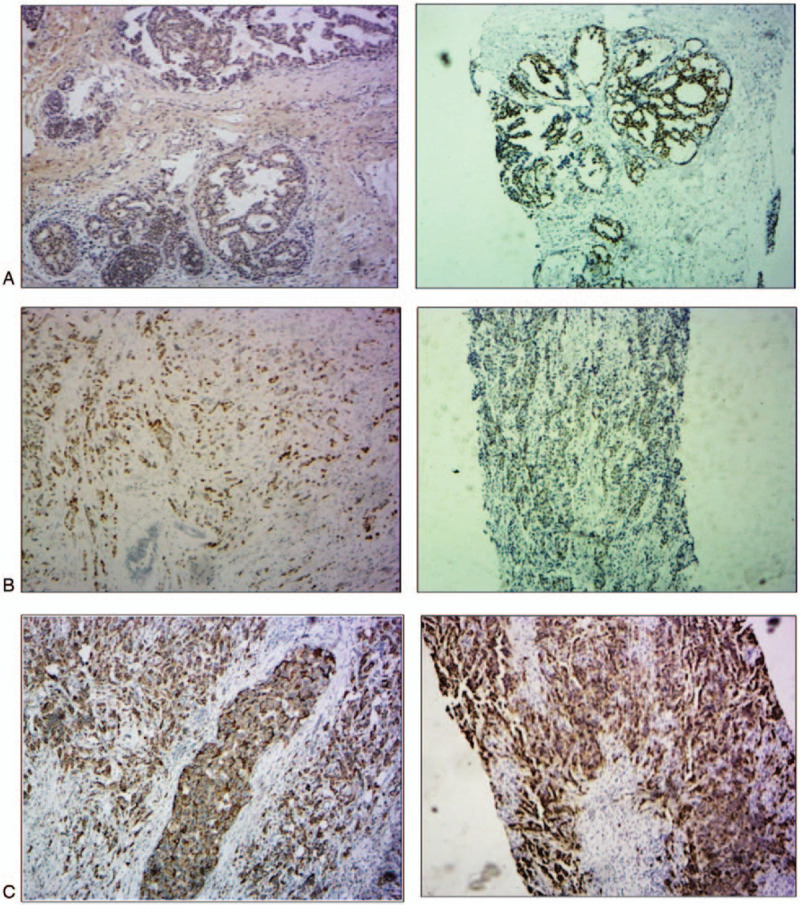
Comparison of the immunohistochemical staining between the CNBs and surgical specimens. A. Estrogen receptor, (ER) staining (left, immunohistochemistry (IHC) staining of surgical specimens; right, IHC staining of 2 passes of CNBs showing concordance). B. Progesterone receptor (PR) staining (left, IHC staining of surgical specimens; right, IHC staining of four passes of CNB showing concordance). C. Human epidermal growth factor receptor-2 (HER2) staining (left, IHC staining of surgical specimens; right, IHC staining of one pass of CNB showing concordance).
4. Discussion
The demand for an accurate preoperative assessment of biomarker status has been growing in recent years. The determination of ER and PR[16] status plays a particularly important role in predicting patients’ responses to endocrine therapy and long-term outcomes, and the HER2 status helps to identify candidates for trastuzumab therapy.[5] Both gene expression assays and molecular subtype classification are valid tools to help stratify patients who may benefit from neoadjuvant chemotherapy or more conservative surgical procedures, and provide potential candidate targets for new therapies; pertuzumab and everlorimus are examples of such therapies. Since gene expression assays are not routinely available, IHC-based CNB can provide a reliable diagnosis,[17–23] supply histological information, and assist in individual treatment planning.[24]
However, most studies have reported its application with the aid of ultrasonography guidance, which is not available in many developing countries. Here, we report our experience and accuracy of freehand CNB in comparison with the results of postoperative pathology.
The diagnosis made based on CNB was concordant with that made based on the surgical specimens in 98.9% of patients, which is consistent with the results of previous studies (92%-100%).[5] A concordance between the assessment of ER and PR status in the CNB and surgical specimens was found in 93 (98.9%) and 87 (92.6%) cases, respectively. PR status in CNB should be treated cautiously because of the reported heterogeneous distribution of PR within the tumor.[25] Out of the discrepancies found in ER and PR determination, most were underestimated by CNB, which may be attributed to sampling error and tumor heterogeneity (without the aid of ultrasonography, the core biopsies were not able to sample the worst area to reflect the whole tumor status).[6] While under the ultrasonography guidance, there is a higher tendency of upscoring in CNB than in surgical specimens.[26] This is probably related to the freshness of the histopathological specimens, shorter interval from sampling to fixation, and better fixation in formalin, leading to better preservation and exposure of the antigen.[27] Therefore, some studies have suggested that the hormone receptor status in CNB specimens is more reliable than that in surgical specimens [26,28] and both surgical specimens and CNBs should be considered when planning therapeutic strategies. Other studies recommend repeating these assessments in CNB, especially when the results of surgical specimens are negative; this ensures that eligible patients do no miss out on endocrine treatment.[25]
HER2 amplification is related to worse outcomes, which is also an indicator for trastuzumab treatment. The concordance rate of HER2 status was only 83%, as concordance was found in 78 cases; this was much lower than that for ER and PR in the corresponding cores, consequently affecting the therapeutic strategy. Based on our results, the sensitivity of inconclusive samples (2 +) was obviously lower than that of the negative (0 or 1 +) and positive (3 +) samples (68.6% vs. 94.9% and 80.9%). One study implied that the sensitivity of CNB is influenced by the definition of HER2 positivity. The sensitivity increases from 80% to 97.7% after altering the definition of HER2 positivity from IHC 2+ or 3+ or FISH (fluorescence in situ hybridization) +, to IHC 3+ or FISH+. Borderline tumor properties may contribute more to the differences in HER2 determination. The aim of this study was to compare IHC-based concordance between the CNB and surgical specimens. We did not include FISH as an adjunctive method to discriminate HER2 positivity from HER2 negativity. The relatively small sample size was also responsible for the differences.
Another aspect that needs special attention is the false-negative rate of CNB,[20,29–33] which can be attributed primarily to histological interpretation.[34] In terms of the comparison of pathological type in our study, four (4.2%) cases of infiltrating ductal carcinoma were underestimated as ductal carcinoma in situ by CNB. The reported false negative rate of atypical ductal hyperplasia diagnosis ranges from 11.6% to 48%.[35–39] The reasons for the above can be separated into 2 aspects: one is the heterogeneity of carcinoma,[40] which is a mixture of intraductal and infiltrating components; the other is the use of either an inappropriate image guiding technique (stereotactic guidance instead of US), or an inappropriate biopsy system. In our study, this phenomenon is more likely to have been a result of the use of a freehand non-monitored CNB technique. Some studies suggested that additional core biopsies and other IHC markers (CD44, CK5/6, calponin, and p63[41–43]) are required.[44]
Although US-guided automated CNB has become a widely practiced method for investigating suspicious lesions,[45] few studies have investigated the correlation between the number of CNBs and the accuracy of hormonal and biomarker status determination.[13–15,46] To our knowledge, no universal standard has been established regarding the number of specimens that are most effective and economical. Concerns over potential cell displacement and neoplastic seeding of the needle tract remain, although this phenomenon is rare and may not translate to neoplastic seeding. This is not only because the rates of cell displacement vary from 2 to 63%, but also because the reported local recurrence rate obtained by percutaneous biopsy is higher than that of surgical specimens (1.1%–3.7% vs 0.3%–2.1%), especially in triple negative breast cancer with invasive ductal carcinoma, or grade 3 breast cancer. Most studies reported that the mean number of CNB specimens obtained ranges from 3 to 5. Tamaki[47] reported that 4 cores obtained by CNB can achieve a diagnostic accuracy of 100% in terms of ER and PR. In our study, we propose that 2 strips are the minimum number of specimens required to determine a diagnosis of malignancy. In order to obtain a reliable diagnosis from the perspective of the concordance of parameters (ER, PR, and HER2 status and tumor grade) between CNBs and surgically excised specimens, at least 4 strips obtained by CNB are required.
5. Conclusions
In summary, the purpose of this study was to determine the minimum number of CNBs required to achieve concordance with the postoperative pathology. A minimum of 2 strips are required to determine a diagnosis of malignancy, while 4 or more specimens are recommended to achieve complete concordance of the pathology parameters (ER, PR, HER2 status, and tumor grade) between CNBs and surgically excised specimens.
Author contributions
TS performed the core needle biopsies, WG made the pathological diagnosis, and both TS and WG collected and interpreted the data. HZ performed the statistical analyses of the data, literature search, and wrote the paper. QY and TS conceived and designed the study, and reviewed and modified the paper. All authors have read and approved the final manuscript.
Conceptualization: Tao Sun, Qifeng Yang.
Data curation: Tao Sun, Wei Gao.
Formal analysis: Tao Sun, Hanwen Zhang.
Funding acquisition: QIFENG YANG.
Investigation: Hanwen Zhang, Wei Gao.
Project administration: Tao Sun, Qifeng Yang.
Resources: Wei Gao, Qifeng Yang.
Software: Hanwen Zhang.
Supervision: Tao Sun, Qifeng Yang.
Writing – original draft: Hanwen Zhang.
Writing – review & editing: Qifeng Yang.
Footnotes
Abbreviations: CNB = core needle biopsy, ER = estrogen receptor, FISH = fluorescence in situ hybridization, HER2 = human epidermal growth factor receptor-2, IHC = immunohistochemistry, PR = progesterone receptor, US = ultrasound.
How to cite this article: Sun T, Zhang H, Gao W, Yang Q. The appropriate number of preoperative core needle biopsy specimens for analysis in breast cancer. Medicine. 2021;100:14(e25400).
All data generated and analyzed during this study are included in this published article (and its Supplementary Information files), and are available from the corresponding author upon reasonable request.
This work was supported by National Key Research and Development Program (No. 2020YFA0712400), Special Foundation for Taishan Scholars (No. ts20190971), National Natural Science Foundation of China ( No. 81874119; No. 82072912), Special Support Plan for National High Level Talents (Ten Thousand Talents Program W01020103), National Key Research and Development Program (No. 2018YFC0114705), Foundation from Clinical Research Center of Shandong University (No.2020SDUCRCA015), Qilu Hospital Clinical New Technology Developing Foundation (No. 2018-7; No. 2019-3) to Qifeng Yang.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Harris GC, Denley HE, Pinder SE, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol 2003;27:11–5. [DOI] [PubMed] [Google Scholar]
- [3].Schoonjans JM, Brem RF. Fourteen-gauge ultrasonographically guided large-core needle biopsy of breast masses. J Ultrasound Med 2001;20:967–72. [DOI] [PubMed] [Google Scholar]
- [4].Liberman L, Dershaw DD, Rosen PP, et al. Stereotaxic 14-gauge breast biopsy: how many core biopsy specimens are needed? Radiology 1994;192:793–5. [DOI] [PubMed] [Google Scholar]
- [5].Arnedos M, Nerurkar A, Osin P, et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol 2009;20:1948–52. [DOI] [PubMed] [Google Scholar]
- [6].Mann GB, Fahey VD, Feleppa F, et al. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol 2005;23:5148–54. [DOI] [PubMed] [Google Scholar]
- [7].Chao C, Torosian MH, Boraas MC, et al. Local recurrence of breast cancer in the stereotactic core needle biopsy site: case reports and review of the literature. Breast J 2001;7:124–7. [DOI] [PubMed] [Google Scholar]
- [8].Santiago L, Adrada BE, Huang ML, et al. Breast cancer neoplastic seeding in the setting of image-guided needle biopsies of the breast. Breast Cancer Res Treat 2017;166:29–39. [DOI] [PubMed] [Google Scholar]
- [9].Loughran CF, Keeling CR. Seeding of tumour cells following breast biopsy: a literature review. Br J Radiol 1006;84:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mathenge EG, Dean CA, Clements D, et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia (New York, NY) 2014;16:950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liebens F, Carly B, Cusumano P, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 2009;62:113–23. [DOI] [PubMed] [Google Scholar]
- [12].Dennison G, Anand R, Makar SH, et al. A prospective study of the use of fine-needle aspiration cytology and core biopsy in the diagnosis of breast cancer. Breast J 2003;9:491–3. [DOI] [PubMed] [Google Scholar]
- [13].Fishman JE, Milikowski C, Ramsinghani R, et al. US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology 2003;226:779–82. [DOI] [PubMed] [Google Scholar]
- [14].Brenner RJ, Fajardo L, Fisher PR, et al. Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol 1996;166:341–6. [DOI] [PubMed] [Google Scholar]
- [15].O’Leary R. Agreement between preoperative core needle biopsy and postoperative invasive breast cancer histopathology is not dependent on the amount of clinical material obtained. J Clin Pathol 2004;57:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pal A, Milevcic D, Bosilj D, et al. The value of hormone receptor assessment in ultrasound guided core needle biopsy of the breast. Collegium antropologicum 2015;39:923–5. [PubMed] [Google Scholar]
- [17].Jackman RJ, Burbank F, Parker SH, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology 1997;204:485–8. [DOI] [PubMed] [Google Scholar]
- [18].Jackman RJ, Nowels KW, Shepard MJ, et al. Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology 1994;193:91–5. [DOI] [PubMed] [Google Scholar]
- [19].Acheson MB, Patton RG, Howisey RL, et al. Histologic correlation of image-guided core biopsy with excisional biopsy of nonpalpable breast lesions. Arch Surg 1997;132:815–8. [DOI] [PubMed] [Google Scholar]
- [20].Boba M, Koltun U, Bobek-Billewicz B, et al. False-negative results of breast core needle biopsies - retrospective analysis of 988 biopsies. Pol J Radiol 2011;76:25–9. [PMC free article] [PubMed] [Google Scholar]
- [21].Hao S, Liu ZB, Ling H, et al. Changing attitudes toward needle biopsies of breast cancer in Shanghai: experience and current status over the past 8 years. Oncotargets Ther 2015;8:2865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hatada T, Ishii H, Ichii S, et al. Diagnostic value of ultrasound-guided fine-needle aspiration biopsy, core-needle biopsy, and evaluation of combined use in the diagnosis of breast lesions. J Am Coll Surgeons 2000;190:299–303. [DOI] [PubMed] [Google Scholar]
- [23].Osanai T, Gomi N, Wakita T, et al. Ultrasound-guided core needle biopsy for breast cancer: preliminary report. Jpn J Clin Oncol 2000;30:65–7. [PubMed] [Google Scholar]
- [24].Houssami N, Cuzick J, Dixon JM. The prevention, detection, and management of breast cancer. Med J Aust 2006;184:230–4. [DOI] [PubMed] [Google Scholar]
- [25].Chen X, Yuan Y, Gu Z, et al. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat 2012;134:957–67. [DOI] [PubMed] [Google Scholar]
- [26].Asogan AB, Hong GS, Arni Prabhakaran SK. Concordance between core needle biopsy and surgical specimen for ER, PgR and Her2neu receptor status in breast cancer and study of difference in reliability between the two groups with the change in ASCO/CAP guidelines. Singapore Med J 2017;58:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meattini I, Bicchierai G, Saieva C, et al. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: single-institution experience and review of published literature. Eur J Surg Oncol 2017;43:642–8. [DOI] [PubMed] [Google Scholar]
- [28].Park YJ, Youk JH, Son EJ, et al. Comparison of hormonal receptor and HER2 status between ultrasound-guided 14-gauge core needle biopsy and surgery in breast cancer patients. Ultrasonography (Seoul, Korea) 2014;33:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Houssami N, Ambrogetti D, Marinovich ML, et al. Accuracy of a preoperative model for predicting invasive breast cancer in women with ductal carcinoma-in-situ on vacuum-assisted core needle biopsy. Ann Surg Oncol 2011;18:1364–71. [DOI] [PubMed] [Google Scholar]
- [30].Kim H, Youk JH, Kim JA, et al. US-guided 14G core needle biopsy: comparison between underestimated and correctly diagnosed breast cancers. Asian Pac J Cancer Prev 2014;15:3179–83. [DOI] [PubMed] [Google Scholar]
- [31].Park AY, Gweon HM, Son EJ, et al. Ductal carcinoma in situ diagnosed at US-guided 14-gauge core-needle biopsy for breast mass: preoperative predictors of invasive breast cancer. Eur J Radiol 2014;83:654–9. [DOI] [PubMed] [Google Scholar]
- [32].Wiratkapun C, Treesit T, Wibulpolprasert B, et al. Diagnostic accuracy of ultrasonography-guided core needle biopsy for breast lesions. Singapore Med J 2012;53:40–5. [PubMed] [Google Scholar]
- [33].Asogan AB, Hong GS, Arni Prabhakaran SK. Concordance between core needle biopsy and surgical specimen for oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 status in breast cancer. Singapore Med J 2017;58:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chang WC, Hsu HH, Yu JC, et al. Underestimation of invasive lesions in patients with ductal carcinoma in situ of the breast diagnosed by ultrasound-guided biopsy: a comparison between patients with and without HER2/neu overexpression. Eur J Radiol 2014;83:935–41. [DOI] [PubMed] [Google Scholar]
- [35].Tokiniwa H, Horiguchi J, Takata D, et al. Papillary lesions of the breast diagnosed using core needle biopsies. Exp Ther Med 2011;2:1069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Houssami N, Ciatto S, Bilous M, et al. Borderline breast core needle histology: predictive values for malignancy in lesions of uncertain malignant potential (B3). Br J Cancer 2007;96:1253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harvey JM, Sterrett GF, Frost FA. Atypical ductal hyperplasia and atypia of uncertain significance in core biopsies from mammographically detected lesions. Pathology 2002;34:410–6. [DOI] [PubMed] [Google Scholar]
- [38].Pena A, Shah SS, Fazzio RT, et al. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat 2017;164:295–304. [DOI] [PubMed] [Google Scholar]
- [39].Polom K, Murawa D, Kurzawa P, et al. Underestimation of cancer in case of diagnosis of atypical ductal hyperplasia (ADH) by vacuum assisted core needle biopsy. Rep Pract Oncol Radiother 2012;17:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shiino S, Tsuda H, Yoshida M, et al. Intraductal papillomas on core biopsy can be upgraded to malignancy on subsequent excisional biopsy regardless of the presence of atypical features. Pathol Int 2015;65:293–300. [DOI] [PubMed] [Google Scholar]
- [41].Shah VI, Flowers CI, Douglas-Jones AG, et al. Immunohistochemistry increases the accuracy of diagnosis of benign papillary lesions in breast core needle biopsy specimens. Histopathology 2006;48:683–91. [DOI] [PubMed] [Google Scholar]
- [42].Saddik M, Lai R. CD44 s as a surrogate marker for distinguishing intraductal papilloma from papillary carcinoma of the breast. J Clin Pathol 1999;52:862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan PH, Aw MY, Yip G, et al. Cytokeratins in papillary lesions of the breast: is there a role in distinguishing intraductal papilloma from papillary ductal carcinoma in situ? Am J Surg Pathol 2005;29:625–32. [DOI] [PubMed] [Google Scholar]
- [44].Polat AK, Soran A, Kanbour-Shakir A, et al. The role of molecular biomarkers for predicting adjacent breast cancer of Atypical Ductal Hyperplasia diagnosed on core biopsy. Cancer Biomark 2016;17:293–300. [DOI] [PubMed] [Google Scholar]
- [45].Parker SH, Lovin JD, Jobe WE, et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology 1991;180:403–7. [DOI] [PubMed] [Google Scholar]
- [46].Sauer G, Deissler H, Strunz K, et al. Ultrasound-guided large-core needle biopsies of breast lesions: analysis of 962 cases to determine the number of samples for reliable tumour classification. Br J Cancer 2005;92:231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tamaki K, Sasano H, Ishida T, et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci 2010;101:2074. [DOI] [PMC free article] [PubMed] [Google Scholar]


