Supplemental Digital Content is available in the text
Keywords: antihypertensive medications, biochemical adherence testing, nonadherence, uncontrolled hypertension
Abstract
Medication nonadherence represents a modifiable risk factor for patients with hypertension. Identification of nonadherent patients could have significant clinical and economic implications in the management of uncontrolled hypertension.
We analysed the results of 174 urinary adherence screens from patients referred to Addenbrooke's Hospital, Cambridge, for uncontrolled hypertension. Cases were identified for evaluation by results of liquid chromatography-tandem mass spectrometry of urine samples (males: 91; females: 83; age range: 17–87). We performed a binary logistic regression analysis for nonadherence using age, sex, and number of medications prescribed (both antihypertensives and non-antihypertensives separately) as independent predictors. Rates of nonadherence for individual antihypertensive drugs were calculated if prescribed to ≥10 patients.
The overall rate of nonadherence to one or more prescribed antihypertensive medications was 40.3%. 14.4% of all patients were nonadherent to all prescribed antihypertensive medications (complete nonadherence), whereas 25.9% of all patients were nonadherent to at least 1, (but not all) prescribed antihypertensive medications (partial nonadherence). 72% of patients were prescribed ≥3 antihypertensives And for every increase in the number of antihypertensive medications prescribed, nonadherence increased with adjusted odds ratios of 2.9 (P < .001). Logistic regression showed that women were 3.3 times more likely to be nonadherent (P = .004). Polypharmacy (≥6 medications prescribed for hypertension and/or concomitant comorbidities) was prevalent in 52%. Bendroflumethiazide and chlortalidone demonstrated the highest and lowest nonadherences respectively (45.5% and 11.8%).
Rate of nonadherence in patients with hypertension was significantly impacted by sex and number of antihypertensive medications prescribed. Understanding these factors is crucial in identifying and managing nonadherence.
1. Introduction
In 2010 the worldwide prevalence of hypertension (HTN) was estimated at 1.39 billion.[1] This figure continues to rise, explained partly by increasing prevalence in low- and middle-income countries.[2] Pharmacological agents remain the primary treatment option, in order to prevent end-organ damage and reduce the frequency of cardiovascular events. Despite a wide range of effective antihypertensive (AHT) medications, blood pressure (BP) targets are only achieved in 40% to 50% of patients.[3,4] As such, HTN remains the greatest risk factor for morbidity and mortality globally.[5]
Amongst patients treated with AHT medication, two factors contribute to BP control: a) optimal prescribing of medications, and b) pharmacotherapy adherence.[2] Adherence after one year of AHT therapy is reported as <50%,[6,7] contributing to the low proportion of controlled BP amongst ‘treated’ patients (20%–50%).[8] Several qualitative and quantitative methods exist to measure adherence, including questionnaires, pill counts, refill data and directly observed therapy. Electronic pill counter monitoring is an emerging method.[9]
Biochemical methods are considered more objective. Gupta et al. showed that nonadherent hypertensive patients respond to biochemical urine analysis testing with improved adherence and subsequent reduction in BP.[10] Using biochemical analyses may therefore be beneficial in identifying and increasing adherence among patients with ‘treatment-resistant’ HTN. Understanding adherence is crucial to ensure that strategies are developed to optimise adherence whilst ensuring approaches are tailored to meet individual needs. This will uphold the patient-provider relationship, which is the key for successful treatment, as highlighted in the recent review by Poulter et al.[9]
This study aimed to evaluate pharmacotherapy nonadherence using urine screens amongst patients referred to Cambridge University Hospitals (CUH) NHS Foundation Trust, United Kingdom, with uncontrolled HTN, with analyses of demographics, polypharmacy, medication type and comorbidities.
2. Methods
2.1. Study population
A total of 174 urinary adherence screen tests, undertaken between September 2014 and April 2018 in the tertiary specialist hypertension clinic at CUH, were included in this study. The study was approved by the clinical audit committee at CUH (Clinical Project ID number 1241). For the purpose of analysis, clinical information was collected from the existing patient electronic health records retrospectively.
The majority (n = 151, 87%) of adherence screening tests were requested on the basis of suboptimal BP control with ≥ 3 AHT medications, as per standard clinical practice (clinic BP ≥140/90 mmHg or home BP ≥ 135/85 mm Hg) in patients ≥18 years of age. Tests were also ordered as part of clinic assessments for secondary causes of hypertension, requiring physicians to order a urine test as part of work-up. Patients were not pre-informed regarding the possibility of spot urine sample testing before clinic appointment, this was mainly to avoid influencing short-term adherence behaviours. During the clinic appointment, patients were consented for spot urine samples to be sent to the National Centre for Drug Adherence testing (NCAT) (University Hospitals of Leicester NHS trust), United Kingdom, for assessment of AHT medication adherence.
The clinical team were aware of the patients’ last drug dosing time, based on their scheduled medication regime as confirmed by patients only. Patients who reported nonadherence during their clinic visit did not undergo urine adherence testing.
2.2. Biochemistry
Adherence was assessed by high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS), which provides a sensitive and specific detection of AHT medications or their metabolites, as previously described by NCAT[10–12] The assay generates a binary qualitative result for the detection of AHT medications in urine samples. Medications that were each prescribed in fewer than 10 patients were excluded from analysis (supplementary Table 1, http://links.lww.com/MD/F708).
Discrepancy between prescribed oral AHT medications and detected AHT medications was defined as nonadherence. Nonadherence was subcategorised into partial and complete nonadherence. Complete nonadherence was defined by the absence of all prescribed AHT medications. Partial nonadherence was defined by the absence of one or more, but not all, AHT medications. Patients whose urine analysis confirmed the presence of all AHT medications were described as adherent.
2.3. Statistical analysis
Descriptive analyses were completed in order to evaluate demographic, clinical and pharmacological characteristics amongst the patient cohort.
Student t test (continuous variables) or chi squared test (proportions) each when appropriate was undertaken to evaluate differences in demographic, clinical and prescriptions between adherent and nonadherent (partial and complete combined) groups. Binary logistic regression analysis was performed for nonadherence using the following variables as independent predictors: age, sex, and number of prescribed AHT medications. Statistical significance was set at P < .05 and all statistical analyses were conducted using IBM SPSS Statistics 26.
3. Results
Table 1 summarises the population baseline characteristics. The mean age of the cohort was 56 years range (17–87) with no significant difference between the adherent and nonadherent groups (P = .9). 70 patients (40%) were either partially or completely non-adherent.
Table 1.
Population baseline characteristics.
| Total (n = 174) | Adherent (n = 104) | Nonadherent (n = 70) | P value1 | |
| Gender: Male/Females (N) | 91/83 | 60/44 | 31/39 | .08 |
| SBP (mm Hg) | 170 ± 27 | 164 ± 25 | 179 ± 25 | <.001 |
| DBP (mm Hg) | 97 ± 18 | 92 ± 17 | 103 ± 18 | <.001 |
| Age (yr) | 56 ± 14 | 56 ± 16 | 56 ± 13 | .9 |
| Number of AHT medications | 4 (1–9) | 4 (1–6) | 5 (1–9) | <.001 |
| Number of non-AHT medications | 3 (0–20) | 3 (0–12) | 4 (0–20) | <.001 |
| Total number of medications | 7 (1–24) | 6 (1–15) | 9 (2–24) | <.001 |
| Comorbidities | n (%) | |||
| Obesity | 93 (53.4) | 59 (56.7) | 34 (48.6) | .27 |
| Cardiovascular disease2 | 82 (47.1) | 44 (42.3) | 38 (54.3) | .16 |
| Dyslipidaemia | 67 (38.5) | 39 (37.5) | 28 (40.0) | .39 |
| Diabetes | 34 (19.5) | 17 (16.3) | 17 (24.3) | .23 |
| Depression, depression or MHD | 33 (19.0) | 19 (18.3) | 14 (20.0) | .23 |
| CKD or other renal pathology | 27 (15.5) | 21 (20.2) | 6 (8.6) | .08 |
| Cancer | 8 (4.6) | 6 (5.8) | 2 (3.0) | .23 |
| Liver disease | 4 (2.3) | 1 (1.0) | 3 (4.3) | .16 |
Data are means ± SD for SBP, DBP and age. Data are mean (range) for number of medications. SBP indicates systolic blood pressure; AHT = antihypertensive, CKD = chronic kidney disease, DBP = diastolic blood pressure, MHD = mental health disorder.
1t-test or chi square
2Cardiovascular disease refers to any pathology of the heart or blood vessels. This includes stroke, heart failure, arrhythmia, valvular heart disease and peripheral artery disease.
The screened patient population consisted of 91 males (52%) and 83 females (48%). Females (n = 40, 56%) outnumbered males (n = 30, 44%) in the nonadherent cohort, however did not reach statistical significance (P = .08).
The mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) at the time of test in the adherent group were 164 ± 25 mmHg and 92 ± 17 mm Hg respectively. The mean SBP and mean DBP in the nonadherent group were significantly higher at 179 ± 25 mm Hg and 103 ± 18 mm Hg, respectively (P < .001).
The three most common comorbidities in this patient population were obesity (53.4%), cardiovascular disease (stroke, heart failure, arrhythmia, valvular heart disease and peripheral artery disease) (47.1%) and dyslipidaemia (38.5%). Anxiety and/or depression were documented in 19% of patients. The distributions of co-morbidities were not significantly different between the 2 groups.
3.1. AHT prescription evaluation
174 urinary adherence screens were included in the study. 151 patients were prescribed three or more AHT medications. (Fig. 1). Polypharmacy (≥6 total medications, AHT plus non-AHT medications) was prevalent in 52% of the population. The five most commonly prescribed agents were amlodipine (76 patients), doxazosin (74 patients), bisoprolol (67 patients), spironolactone (66 patients) and candesartan (50 patients) (Table 2).
Figure 1.
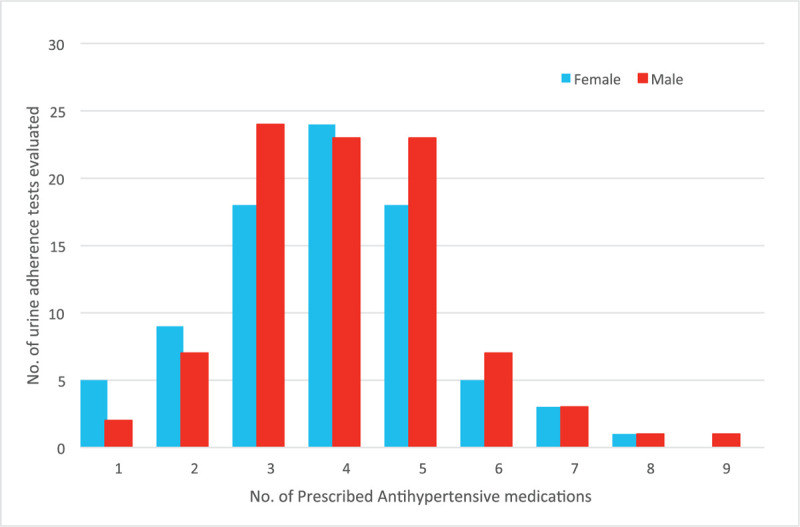
Distribution of number of prescribed antihypertensive medications among males and females.
Table 2.
Antihypertensive medication nonadherence percentages, half-lives, and prescription frequencies.
| Drug∗ | Class | Number of patients prescribed | Nonadherence (%) | t1/2 (h) | References for t1/2 |
| Bendroflumethiazide | Thiazide | 22 | 45.5 | 3–8.5 | [42] |
| Ramipril | ACEi | 18 | 44.4 | 13–17 | [43] |
| Doxazosin | α-blocker | 74 | 41.9 | 22 | [44] |
| Atenolol | β-blocker | 10 | 40.0 | 6–9 | [45] |
| Olmesartan | ARB | 10 | 40.0 | 10–15 | [46] |
| Spironolactone | PSD | 66 | 37.9 | 2.8–11.2 | [47] |
| Lisinopril | ACEi | 28 | 35.7 | 12.6 | [48] |
| Indapamide‡ | Thiazide | 41 | 34.1 | 14–18 | [49] |
| Losartan | ARB | 36 | 30.6 | 6–9 | [50] |
| Amlodipine | CCB | 76 | 30.3 | 35–50 | [51] |
| Candesartan | ARB | 50 | 24.0 | 9 | [52] |
| Bisoprolol | β-blocker | 67 | 20.9 | 10–12 | [53] |
| Amiloride | PSD | 16 | 18.8 | 6 | [54] |
| Nifedipine† | CCB | 27 | 18.5 | 6–11 | [55] |
| Felodipine | CCB | 12 | 16.7 | 25 | [56] |
| Lercanidipine | CCB | 18 | 16.7 | 8–10 | [57] |
| Chlortalidone‡ | Thiazide | 17 | 11.8 | 40–50 | [58] |
ACEi indicates angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker, BB = beta-blocker, CCB = calcium channel blocker, PSD = potassium sparing diuretic.
Drugs were only included in the analysis when prescribed for >10 patients.
None of the patients were on short acting nifedipine.
Chlortalidone and indapamide are both thiazide-like diuretics.
3.2. Nonadherence to AHT medication
The overall prevalence of nonadherence was 40%, including 26% who were partially nonadherent, (at least one of the prescribed AHT medication was detected by the urinary assay), and 14% who were completely nonadherent, (all of the prescribed AHT medications were undetected) (Fig. 2).
Figure 2.
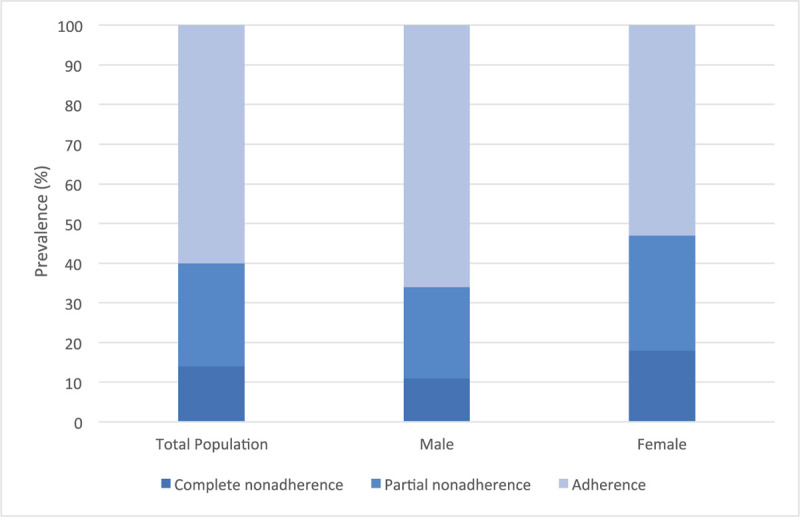
Prevalence of nonadherence in total, male and female patient populations.
Nonadherence was highly variable between individual drugs of the same class (Fig. 3A and 3B). For example, bendroflumethiazide and chlortalidone exhibited the highest (45.5%) and lowest (11.8%) levels of nonadherence respectively, despite both being thiazide diuretics (Table 2).[13–15]
Figure 3.
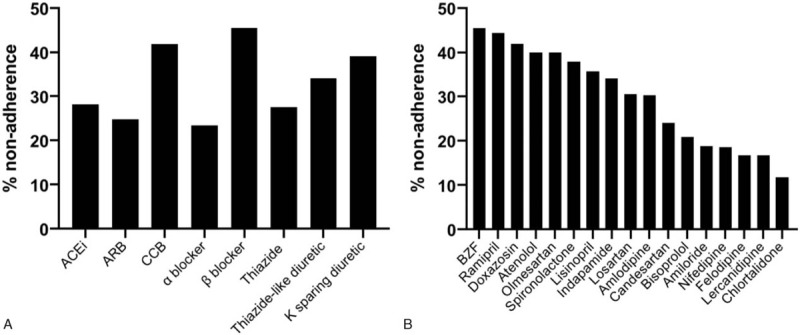
a Percentage nonadherence by antihypertensive medication class. 3b–Percentage nonadherence by individual antihypertensive medication.
A logistic regression was performed to ascertain the effects of age, gender and number of AHT medications on the likelihood of nonadherence (Table 3). 15 patients did not have information on non-AHT medications and they were excluded from regression analysis. The logistic regression model was significant, χ2 = 42.3, P < .001. The model explained 36.2% (Nagelkerke R2) of the variance in nonadherence and correctly classified 75.5% of cases. Women were 3.31 times more likely to be nonadherent compared to men (Odds ratio (OR) = 3.31, P = .004, 95% CI (1.45–7.55)). Number of AHT medications was a positive independent predictor of nonadherence (OR = 2.90, P < .001, 95% CI (1.96–4.30)) but number of non-AHT medications was not (OR = 1.08, P = .16, 95% CI (0.97–1.21)). Overall, age trended toward inverse prediction of nonadherence, however this did not reach statistical significance (OR 0.97, P = .063, 95% CI (0.94–1.00).
Table 3.
Predictors of nonadherence to antihypertensive treatment.
| Step wise Logistic regression | ||||||
| B | S.E | Sig. (p value) | OR | Lower | Upper | |
| Sex (1) | 1.19 | 0.42 | 0.004 | 3.31 | 1.30 | 7.55 |
| Age (yr) at the time of test | –0.028 | 0.155 | 0.063 | 0.97 | 0.94 | 1.00 |
| No. of prescribed AHT medications | 1.07 | 0.20 | <0.001 | 2.9 | 1.96 | 4.30 |
| No. of other (non AHT) prescribed medications | 0.08 | 0.06 | 0.16 | 1.08 | 0.97 | 1.21 |
| Constant | –3.94 | 1.030 | <0.001 | 0.02 | ||
Sex (code, 0=male, 1=female).
B = unstandardized regression co-efficient, CI = confidence interval, OR = odds ratio, S.E. = standard error.
4. Discussion
This study was intended to investigate medication non-adherence in the context of uncontrolled hypertension.
The present study offers valuable insights into nonadherence in patients referred to a tertiary care hypertension clinic. Published AHT medication adherence prevalence in other studies based on biochemical testing (urine or plasma) using LC-MS/MS technique on range from 41.6% to 60%.[12,16–19] We detected a 40.3% rate of nonadherence to AHT medications which is strikingly similar to the most recently published large study (676 patients with HTN) which reported 41.6% prevalence of biochemically detected nonadherence.[19]
The data demonstrate statistically significant associations between nonadherence and sex, and between nonadherence and number of AHT medications prescribed. Overall women were more likely to be nonadherent compared to men, although existing literature indicates no clear association between adherence and sex.[19,20]
The data shows an early inverse relationship between age and nonadherence. The majority of studies demonstrate that AHT nonadherence either decreases with age[19–23] or fails to show a significant association.[24] Several studies in chronic patient populations (including chronic myeloid leukaemia, renal transplant and gout) have demonstrated an inverse relationship between age and nonadherence,[25–30] with suggested reasons including interference with education or work, differences in accommodation of side effects, increased prevalence of co-morbidity and resultant polypharmacy, variability in impact of disease on activities of daily living and negative beliefs on pharmacological intervention. Demographic factors like level of education, socioeconomic factors and marital status may have significant impact on medication taking behaviours. Unfortunately, this could not be assessed systematically in this study. Side effects to medications, difficulty in managing medications and financial constraints can directly affect adherence. However, these barriers to adherence were discussed during clinic consultations as part of standard practice and patients who admitted to factors such as side effects to medications impacting their adherence did not proceed to undergo urinary testing and hence excluded from the study.
Among patients included in the study, 72% of patients were prescribed three or more AHT medications and 52% of patients were taking six or more medications in total (AHT plus non-AHT). In this study nonadherence was significantly associated with number of prescribed AHT but not number of non-AHT medications. Increased risk of nonadherence with number of prescribed AHT medications has been identified in previous studies.[21,31,32] Amongst 1348 hypertensive patients in the United Kingdom and Czech Republic, Gupta et al. (2017) found that each increase in the number of prescribed AHT medications led to 85% and 77% increases in nonadherence, respectively (P < .001).[19] However, as most of these studies are retrospective by design, so causality cannot be established.
The prevalence of obesity, cardiovascular disease and dyslipidaemia was substantial in both adherent and nonadherent patients, though there were no significant differences in prevalence between the 2 groups. A recent meta-analysis of 13,688 hypertensive patients from 28 studies which showed that comorbidity was not significantly associated with nonadherence to AHT.[33]
Similarly, there was no statistical difference between adherent and nonadherent groups in those diagnosed with a mental health disorder (anxiety and/or depression). Previous research has shown depression to be associated with a threefold increased risk of nonadherence to both pharmacological and non-pharmacological treatment strategies.[34] However the relationship with AHT nonadherence is contentious, particularly as a considerable proportion of studies have employed either subjective or self-reported measures to generate adherence data.[35]
Interestingly, the data demonstrate considerable variation in nonadherence between individual AHTs, including medications within the same drug class. Bendroflumethiazide and chlortalidone showed the highest and lowest levels of nonadherence respectively, despite both being diuretics. This is in contrast to previous studies published where diuretic as a class was associated with increased nonadherence.[19] The discrepancies between medications in the same class are likely explained by differences in the pharmacokinetic properties and/or tolerability profiles between the AHT agents. Most AHTs are present in bodily fluids long after pill ingestion by virtue of their half-lives. The half-life of chlortalidone is 40 to 50 hours meaning that, following a single dose of chlortalidone, 6.25% of the original plasma concentration will still be detected after around 6.7 to 8.3 days (160–180 hours) (supplementary Figure 1, http://links.lww.com/MD/F707). Hence, by virtue of their long half-lives, it is possible that some medications, such as chlortalidone, may be detected by the urinary assay even if they have not been taken for a number of days. Similarly for medications such as candesartan the elimination half-life maybe much longer (29 hours in two-compartmental model) than the published ranges (4–9 hours), and this may lead to detection of candesartan for longer duration in urine, thereby having a reduced nonadherence ratio in comparison to another angiotensin receptor blocker (ARB) such as olmesartan.[36] In summary, there is a risk that the long half-lives of some drugs even when associated with intermittent patient nonadherence, maybe confused for adherence and in turn, lead to significant underestimation of nonadherence burden within the patient cohort.
Tolerability profiles of medications can be affected not just by side effects, but also by the ease of drug intake regime. The shape, form, taste, size and single versus multiple regimes may affect tolerability of medications. Nonadherence percentages among various calcium channel blockers were in the descending order as follows: amlodipine>nifedipine>felodipine=lercanidipine. This was despite a reversed order of half-life ranges, with amlodipine having the longest half-life and lercanidipine the shortest. This may indeed reflect better tolerability of lercanidipine in comparison amlodipine, for instance amlodipine tends to cause more pedal oedema in comparison to lercanidipine.[37] As such various factors likely contribute to the nonadherence variability observed between individual AHT drugs. Such limitations, could not be systematically analysed in this study.
A further limitation is the fact that this analysis is susceptible to short-term patient behaviours, most notably the classification of adherence in individuals who have knowingly or coincidentally ingested their prescribed AHTs shortly before their clinic visit, whilst otherwise omitting doses between appointments (also referred to as the ‘toothbrush effect’ or ‘white coat adherence’).[9] To the contrary, some patients may avoid taking their diuretics on the morning of the clinic visit, especially if they have a long journey and it is unknown whether there are any biochemical limitations of the well-established and validated LC-MS/MS analysis. Repeating urine analysis at different time points might help capture intermittent adherence more fully. However this would certainly increase costs. Some patients in the study did have repeat testing in future appointments, especially if BP remained uncontrolled despite further discussions based on adherence results and other factors affecting BP were ruled out. However, this analysis is beyond the scope of the present study.
In this study patients were not informed in advance of their clinic appointments that they would be asked to provide a urine sample to check for medication compliance, to minimise the risk of patients acutely increasing compliance in the days before the appointment. However, patients were told during the consent process that the urine samples would be used to check medication compliance, which could lead non-adherent patients to refuse consent and cause an underestimation of nonadherence. It would be difficult to reduce this selection bias in future studies as patients must be honestly informed of the purpose of the urine samples during the consent process.
4.1. Improving adherence
Urinary screening is a useful, objective and validated test for identifying patients who are adherent. A detailed discussion around methods to improve adherence is beyond the scope of this article. However, patients may benefit from the use of additional/parallel methods of nonadherence assessment alongside urinary screening, including questionnaires (e.g. MMAS-8), pharmacy/primary care refill data and directly observed therapy. The use of fixed-dose combinations (FDC) of AHT may also be a useful method to explore. Previous analysis has reported a significant increase in AHT adherence in patients on fixed-dose pharmacotherapy when compared with those on free-drug regimens.[32] However, FDC therapy does come with its own challenges, especially while medications are being titrated to treat BP to target. More recently, medications with sensors to detect adherence have also been proposed,[38] but evaluating the cost-effectiveness of such strategies will determine whether these methods will be integrated into clinical practice. An alternative to the prescription of a daily oral regime are various strategies of newer and personalised drug delivery[39] and development of newer modulatory therapies, both still in experimental stages as of now. For instance RNAi strategies targeting angiotensin pathway showed efficacy in animal models. The therapeutic effects of which will ideally last for several weeks or months.[40]
5. Conclusion and perspectives
This study reports on the analysis of 174 urinary adherence screens from patients referred to a tertiary clinical pharmacology centre with uncontrolled HTN. Rate of nonadherence was significantly impacted by sex and numbers of AHT medications prescribed.
This study has begun to guide clinical practice within the CUH specialist HTN service, where urinary adherence screens are now routinely performed for all treatment-resistant hypertensive patients. Urinary screens could also be considered for other chronic conditions (e.g. hyperlipidaemia, rheumatoid arthritis and ischaemic heart disease) where compliance rates are often variable.[41]
Further studies should be designed to compare and correlate the pharmacokinetic profiles of medications detected by this method. Further prospective research is also needed to delineating factors and patient characteristics that affect adherence and thus help identify those at risk of nonadherence. Future prospective trials to assess trends between AHT medication nonadherence and psychiatric diagnoses, including conditions beyond depression and anxiety may help. Efforts should focus on implementing personalised interventions to deal with nonadherence including bringing about awareness and educating patients in a non-judgemental fashion. Improving and maintaining adherence to AHT as well as non-pharmacological therapies will reduce the burden of uncontrolled hypertension.
References 42–58 provided in Supplementary Material, Available at: http://links.lww.com/MD/F709.
Author contributions
Conceptualization: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Data curation: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Formal analysis: Spoorthy Kulkarni, James Delman Harry Goodman, Kathleen Connolly, Kevin M. O'Shaughnessy.
Funding acquisition: Kevin M. O'Shaughnessy.
Investigation: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Methodology: Spoorthy Kulkarni, James Delman Harry Goodman, Kathleen Connolly, Kevin M. O'Shaughnessy.
Project administration: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Resources: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Software: Spoorthy Kulkarni, James Delman Harry Goodman, Kevin M. O'Shaughnessy.
Supervision: Spoorthy Kulkarni, Kevin M. O'Shaughnessy.
Validation: Spoorthy Kulkarni, James Delman Harry Goodman, Kathleen Connolly, Kevin M. O'Shaughnessy.
Visualisation: Spoorthy Kulkarni, James Delman Harry Goodman, Kathleen Connolly, Kevin M. O'Shaughnessy.
Writing – original draft: Spoorthy Kulkarni, Raunak Rao.
Writing – review and editing: Spoorthy Kulkarni, Raunak Rao, James Delman Harry Goodman, Kathleen Connolly, Kevin M. O'Shaughnessy.
Footnotes
Abbreviations: AHT = antihypertensive, BP = blood pressure, CUH = Cambridge University Hospitals, LC-MS/MS = liquid chromatography-tandem mass spectrometry, NCAT = National Centre for Drug Adherence testing.
How to cite this article: Kulkarni S, Rao R, Goodman JDH, Connolly K, O'Shaughnessy KM. Nonadherence to antihypertensive medications amongst patients with uncontrolled hypertension: a retrospective study. Medicine. 2021;100:14(e24654).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burnier M, Egan BM. Adherence in Hypertension. Circ Res 2019;124:1124–40. [DOI] [PubMed] [Google Scholar]
- [3].Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–48. [DOI] [PubMed] [Google Scholar]
- [4].Yoon SSuS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief 2015. 01–8. [PubMed] [Google Scholar]
- [5].Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- [6].Vrijens B, Vincze G, Kristanto P. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hill MN, Miller NH, Degeest S. Adherence and persistence with taking medication to control high blood pressure. J Am Soc Hypertens 2011;5:56–63. [DOI] [PubMed] [Google Scholar]
- [8].Burnier M. Drug adherence in hypertension. Pharmacol Res 2017;125:142–9. [DOI] [PubMed] [Google Scholar]
- [9].Poulter NR, Borghi C, Parati G, et al. Medication adherence in hypertension. J Hypertens 2020;38:579–87. [DOI] [PubMed] [Google Scholar]
- [10].Gupta P, Patel P. Štrauch B et al biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension 2017;70:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HL LC-MS/MS) urine analysis. Heart 2014;100:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Siddiqui M, Judd EK, Dudenbostel T, et al. Antihypertensive medication adherence and confirmation of true refractory hypertension. Hypertens (Dallas, Tex 1979) 2020;75:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Home - electronic medicines compendium (emc) [Internet]. [cited 2020 Feb 19]. Available at: https://www.medicines.org.uk/emc/. [Google Scholar]
- [14].DrugBank [Internet]. [cited 2020 May 2]. Available at: https://www.drugbank.ca/. [Google Scholar]
- [15].Brunton LL, Lazo JS, Parker KL. Goodman & gilman's: the pharmacological basis of therapeutics. 11th Edition. New York: McGraw-Hill Professional; 2006. [Google Scholar]
- [16].Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013;31:766–74. [DOI] [PubMed] [Google Scholar]
- [17].Štrauch B, Petrak O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens 2013;31:2455–61. [DOI] [PubMed] [Google Scholar]
- [18].Lawson AJ, Shipman KE, George S, et al. A Novel “dilute-and-shoot” liquid chromatography-tandem mass spectrometry method for the screening of antihypertensive drugs in urine. J Anal Toxicol 2016;40:17–27. [DOI] [PubMed] [Google Scholar]
- [19].Gupta P, Patel P, Štrauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension 2017;69:1113–20. [DOI] [PubMed] [Google Scholar]
- [20].Esposti LD, Saragoni S, Benemei S, et al. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res 2011;3:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morrison VL, Holmes EAF, Parveen S, et al. Predictors of self-reported adherence to antihypertensive medicines: a multinational, cross-sectional survey. Value Heal 2015;18:206–16. [DOI] [PubMed] [Google Scholar]
- [22].Ross S, Walker A, MacLeod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens 2004;18:607–13. [DOI] [PubMed] [Google Scholar]
- [23].Maguire LK, Hughes CM, McElnay JC. Exploring the impact of depressive symptoms and medication beliefs on medication adherence in hypertension-A primary care study. Patient Educ Couns 2008;73:371–6. [DOI] [PubMed] [Google Scholar]
- [24].Swain S, Hariharan M, Rana S, et al. Doctor-patient communication: impact on adherence and prognosis among patients with primary hypertension. Psychol Stud (Mysore) 2015;60:25–32. [Google Scholar]
- [25].Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28:2381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation 1998;66:1718–26. [DOI] [PubMed] [Google Scholar]
- [27].De Vera MA, Marcotte G, Rai S, et al. Medication adherence in gout: a systematic review. Arthritis Care Res 2014;66:1718–26. [DOI] [PubMed] [Google Scholar]
- [28].StCharles M, Bollu VK, Hornyak E, et al. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. Blood 2009;114:2209.19745073 [Google Scholar]
- [29].Larizza MA, Dooley MJ, Stewart K, Kong DC. Factors influencing adherence to molecular therapies in haematology-oncology outpatients. J Pharm Pract Res 2006;36:115–8. [Google Scholar]
- [30].Geissler J, Sharf G, Bombaci F, et al. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol 2017;143:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev 2004;(2):CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension 2010;55:399–407. [DOI] [PubMed] [Google Scholar]
- [33].Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs a systematic review and meta-analysis. Medicine (United States) 2017;96:e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- [35].Eze-Nliam CM, Thombs BD, Lima BB, et al. The association of depression with adherence to antihypertensive medications: a systematic review. J Hypertens 2010;28:1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gleiter CH, Mörike KE. Clinical pharmacokinetics of candesartan. Clin Pharmacokinet 2002;41:07–17. [DOI] [PubMed] [Google Scholar]
- [37].Messerli FH, Grossman E. Pedal edema - Not all dihydropyridine calcium antagonists are created equal. Am J Hypertens 2002;15:1019–20. [DOI] [PubMed] [Google Scholar]
- [38].Thompson D, Mackay T, Matthews M, et al. Direct adherence measurement using an ingestible sensor compared with self-reporting in high-risk cardiovascular disease patients who knew they were being measured: a prospective intervention. JMIR mHealth uHealth 2017;5:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goyanes A, Wang J, Buanz A, et al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm 2015;12:4077–84. [DOI] [PubMed] [Google Scholar]
- [40].2019;Uijl E, Mirabito Colafella KM, Sun Y, et al. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. hypertens (Dallas, Tex 1979). 73:1249–57. [DOI] [PubMed] [Google Scholar]
- [41].National Centre for Drug Adherence Testing (NCAT). Available at: https://www.leicestershospitals.nhs.uk/aboutus/departments-services/pathology/blood-sciences-chemical-pathology/national-centre-for-drug-adherence-testing-ncat/. Accessed 29 July 2020. [Google Scholar]


