Abstract
The systemic immune-inflammation index (SII) is an independent prognostic predictor of hepatocellular carcinoma (HCC). The present investigation examined whether an association exists between preoperative SII value and postoperative acute kidney injury (pAKI) in HCC patients.
The study included 479 hepatitis B virus (HBV)-associated HCC patients undergoing hepatectomy. The SII was calculated as P × N/L, where P, N, and L represent the counts of platelets, neutrophils, and lymphocytes in routine blood test, respectively. After propensity score matching, logistic regression analysis was used to explore independent predictors of pAKI in HCC patients.
pAKI was confirmed in 51 patients (10.8%). The average SII value was higher in patients with pAKI than patients without pAKI. After multivariate logistic regression analysis, SII, history of hypertension, and tumor size, among others, were found to be predictors of pAKI. The optimal threshold value of SII for predicting pAKI was found to be 547.84 × 109/L. Multivariate analysis performed after propensity score matching confirmed that SII ≥ 547.84 × 109/L was an independent predictor of pAKI.
The preoperative SII qualifies as a novel, independent predictor of pAKI in HCC patients with HBV infection who underwent hepatectomy.
Keywords: hepatocellular carcinoma, postoperative acute kidney injury, propensity score matching, systemic immune-inflammation index
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequent type of carcinoma worldwide, and its mortality rate ranks third globally.[1,2] At present, surgery-based comprehensive treatment is still the primary therapeutic option for HCC patients. Postoperative acute renal injury (pAKI) is one of the main complications after abdominal surgery and its incidence increases continuously,[3,4] affecting approximately 15% of patients undergoing hepatectomy.[3] pAKI is associated with prolonged hospitalization, increased medical costs, higher mortality, and lower long-term survival rate.[5–9] It was estimated that pAKI causes approximately 10-fold increase in in-hospital mortality, reduces postoperative survival by up to 15 years, and potentiates the risk of chronic renal diseases.[6,8,10,11] The extent and burden of pAKI are particularly evident in developing countries, including China, which has the largest number of patients with hepatitis B virus (HBV) infection.[4] Given the close link between HBV infection and HCC,[12] it is critical to explore new methods to identify the predictors of high risk of pAKI in HCC patients after hepatectomy and to design personalized approaches to the prevention and treatment of pAKI.
The etiology of pAKI is complex and multifactorial. Although ischemia is a major cause of this condition, growing evidence indicates that pAKI frequently occurs in the absence of clear signs of hypoperfusion, and may be related to immune and inflammatory responses.[13] Several studies have reported that the systemic immune-inflammation index (SII), based on a composite index of platelet (PLT), neutrophil, and lymphocyte counts in peripheral blood, has a prognostic value in a variety of malignancies, including HCC,[14,15] pancreatic cancer,[16,17] breast cancer,[18] gastric cancer,[19] esophageal squamous cell carcinoma,[20] oral cell carcinoma,[21] and cervical squamous cell carcinoma.[22] Elevated SII in patients with malignant tumor indicates worse prognosis.[14] However, the relationship between preoperative SII and pAKI after hepatectomy in HCC patients was not studied in detail. Therefore, the predictive value of preoperative SII for acute renal injury after hepatectomy in these patients needs to be determined.
The present study addressed the relationship between the novel SII score and postoperative acute renal injury in patients with HCC using retrospective data from a single-center cohort. The analysis demonstrated that the preoperative SII is an independent predictor of AKI in HCC patients undergoing hepatectomy.
2. Patients and methods
2.1. Patients
A total of 759 patients admitted to the Union Hospital of Tongji Medical College from September 2012 to April 2019 were enrolled in this study. All patients were diagnosed with HCC by liver dynamic enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Prior to diagnosis, none of the participants were on treatment for HCC, and on preoperative steroids, aspirin, chemotherapy or other treatments that could have affect the variables used to calculate the SII.
As detailed in Figure 1, 280 of admitted patients were excluded from the analysis. The remaining 479 patients had the diagnosis of HCC confirmed by pathologic examination. All patients signed informed consent for the surgery before the procedure, and agreed that their clinical data and specimens could be used for scientific research. The protocol of the study was approved by the Human Experimental Ethics Committee of Tongji Medical College.
Figure 1.
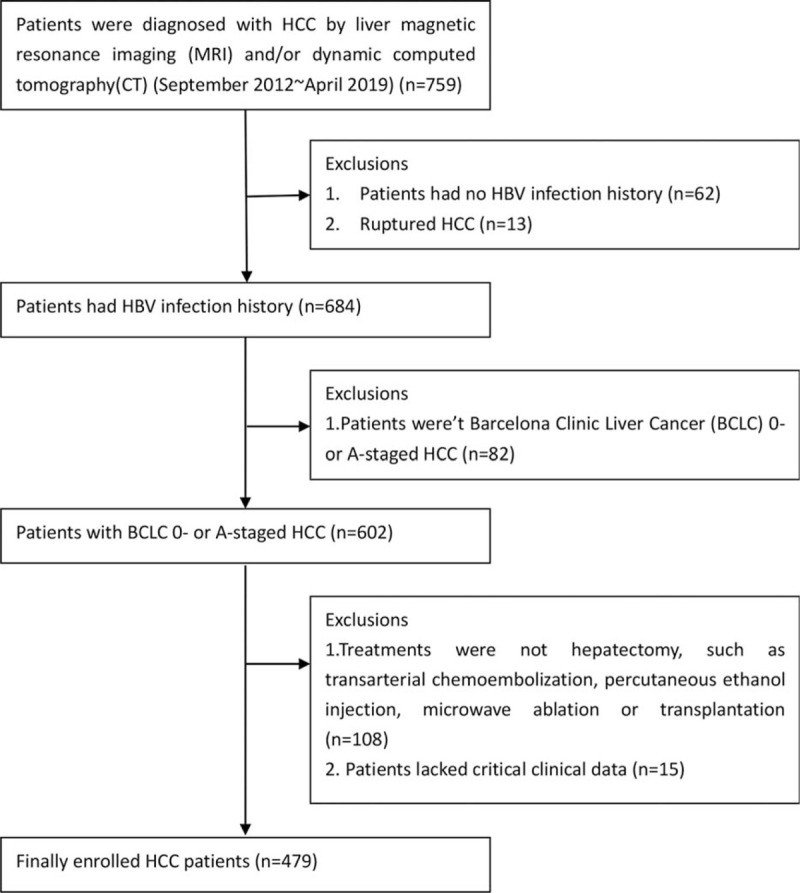
A total of 479 HCC patients was enrolled in this study. BCLC = Barcelona Clinic Liver Cancer, HBV = hepatitis B virus, HCC = hepatocellular carcinoma.
2.2. Data collection
Complete clinical data for all patients were obtained by retrieving the electronic data records for each case, including sex, age, the presence of hypertension, diabetes, and liver cirrhosis, length of postoperative hospital stay, serum levels of hemoglobin (Hb), albumin, total bilirubin (TB), γ-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, blood urea nitrogen, α-fetoprotein (AFP). Additional information included serum HBV DNA levels (HBV DNA), The Cancer of the Liver Italian Group Score (CLIP), Child-Turcotte-Pugh classification (CTP), surgery type, tumor size, number of tumors, and their differentiation. Preoperative SII was calculated based on the results of routine blood test, according to the formula: SII = P × N/L, where P, N, and L represent the count of platelets, neutrophils, and lymphocytes, respectively. All the data obtained by the test were the latest data before the operation. The Milan criteria for HCC, that is, a single lesion < 5 cm, or no more than 3 lesions ≤3 cm were used.[23] Clinical stages of HCC were defined according to the Barcelona Clinical Liver Cancer (BCLC) staging.[24]
2.3. Surgical treatment
All HCC patients with HCC were subjected to hepatectomy. Hepatectomy was considered minor if less than 4 liver segments were removed and major if 4 or more segments were removed.[25] Pathologic examination was performed for all surgically removed specimens. The Edmondson grading system was used to define the degree of carcinoma differentiation.[26]
2.4. Definition of postoperative AKI
The diagnostic classification of pAKI was based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria. The KDIGO criteria are as follows:
-
1.
Increase in blood creatinine within 48 hours after surgery greater than or equal to 0.3 mg/dL (26.5 mmol/L); and
-
2.
Increase in blood creatinine within 7 days after surgery to more than 1.5 times of the preoperative value.
Patients meeting one of these criteria are diagnosed with pAKI.[4]
2.5. Propensity score matching
All patients were divided into 2 groups based on the best threshold value of SII predicting pAKI: the high SII (≥547.84 × 109/L) group and the low SII (<547.84 × 109/L) group. The propensity score matching (PSM) introduced by Rubin and Rosenbaum was used to pair the patients.[27] The propensity score was calculated using logistic regression model in which sex, age, hypertension, diabetes, cirrhosis, Hb, albumin, TB, GGT, ALT, creatinine, AFP, CLIP, CTP, surgery type, tumor size, number of tumors, BCLC stage, fulfillment of Milan criteria, and differentiation of carcinoma were all considered. To minimize conditional bias, 1:1 matching was adopted. By this approach, patients in the high SII group were matched with patients in the low SII group having the closest propensity score. Subsequently, both subjects were eliminated from the next round of matching. Patients selected by PSM were enrolled in a new cohort and subjected to further analysis.
2.6. Statistical analyses
Continuous variables and categorical variables are described by mean (range), and frequency (percentage), respectively. If the variance in the 2 groups was the same, the continuous variables were compared by the t test, otherwise, the Welch's t test was used. Categorical variables were compared by the χ2 test or Fisher's exact test. After univariate logistic regression analysis, variables with P-value less than .05 were selected for multivariate analysis to obtain predictors of pAKI. The best threshold value of SII was obtained using the Youden index. The two-sided P-value < .05 was considered to indicate a statistically significant difference. The computing process of PSM was performed using the Empower Stats software. SPSS24.0 software was used for statistical analysis.
3. Results
3.1. Patient characteristics
A total of 479 HCC patients with HBV infection were enrolled in this study (Fig. 1); their clinical data are listed in Table 1. There were 424 (88.5%) males and the mean age of all patients was 54.3 years (15–84 years). The population under study included 155 (32.4%) patients with hypertension, 54 (11.3%) patients with a history of diabetes, and 309 (73.2%) patients with liver cirrhosis. The average length of hospital stay after hepatectomy was 15.1 days (3–50 days). In routine blood tests, the average values of Hb, PLT, neutrophils, lymphocytes, white blood cells, and SII were 132.3 (69–181) g/L, 151.6 (25–375) × 109/L, 3.43 (0.76–13.45) × 109/L, 1.41 (0.28–3.70) × 109/L, 5.49 (1.49–16.03) × 109/L, and 438.3 (38.2–3461.0) × 109/L, respectively. In biochemical tests, the mean levels of albumin, TB, GGT, ALT, AST, creatinine, and BUN were 39.3 (24.8–64.3) g/L, 16.6 (3.4–143.8) μmol/L, 115.3 (10–2777) IU/L, 48.6 (1–477) IU/L, 48.3 (15–489) IU/L, 70.7 (37.1–234.1) mmol/L, and 5.69 (1.01–81.50) mmol/L. Low CLIP score was diagnosed in 424 (88.5%) patients, and CTP class A liver function in 461 (96.2%) patients. Major liver resection was performed in 116 (24.2%) patients. The mean carcinoma size was 6.1 cm (1–26 cm). A small amount of ascites was present in 37 (7.7%) participants. The BCLC stage A HCC was identified in 450 (93.9%) participants, and the Milan criteria were met in 272 (56.8%) cases. According to the Edmondson grading system, HCC differentiation I–II and III–IV were confirmed in 324 (67.6%) and 155 (32.4%) patients, respectively.
Table 1.
Distribution of patients according to AKI based on the “KDIGO” criteria.
| Variables | Total | AKI (−) | AKI (+) | P |
| Total | 479 (100) | 428 (89.4) | 51 (1.6) | |
| Sex (male) | 424 (88.5) | 376 (87.9) | 48 (94.1) | .184 |
| Age (yr) | 54.3 (15–84) | 53.7 (15–79) | 59.5 (41–84) | .001∗ |
| Hypertension | 155 (32.4) | 128 (29.9) | 27 (52.9) | .001∗ |
| Diabetes | 54 (11.3) | 42 (9.8) | 12 (23.5) | .003∗ |
| Liver cirrhosis | 358 (74.7) | 319 (74.5) | 39 (76.5) | .763 |
| Splenomegaly | 103 (21.5) | 90 (21.0) | 13 (25.5) | .463 |
| Postoperative hospital stay (d) | 15.1 (3–50) | 14.9 (3–50) | 17.2 (9–26) | .001∗ |
| Hb (g/L) | 132.3 (69–181) | 133.6 (73–181) | 121.7 (69–151) | .001∗ |
| PLT (G/L) | 151.6 (25–375) | 148.8 (25–375) | 175.6 (87–323) | .008∗ |
| Neutrophil (G/L) | 3.43 (.76–13.45) | 3.33 (.76–13.45) | 4.32 (2.44–8.20) | .001∗ |
| Lymphocyte (G/L) | 1.41 (.28–3.70) | 1.47 (.32–3.70) | .86 (.28–2.06) | .001∗ |
| SII (G/L) | 438.3 (38.2–3461.0) | 383.4 (38,2–3461.0) | 899.2 (283.7–1488.9) | .001∗ |
| WBC (G/L) | 5.49 (1.49–16.03) | 5.38 (1.49–16.03) | 6.40 (3.24–1.70) | .001∗ |
| Albumin (g/L) | 39.3 (24.8–64.3) | 39.4 (24.8–5.4) | 38.6 (29.8–64.3) | .451 |
| TB (μmol/L) | 16.6 (3.4–143.8) | 15.8 (3.4–143.8) | 23.0 (7.2–66.3) | .001∗ |
| GGT (IU/L) | 115.3 (10–2777) | 105.2 (10–2777) | 20.1 (24–820) | .001∗ |
| ALT (IU/L) | 48.6 (1–477) | 5.8 (11–477) | 29.5 (1–66) | .005∗ |
| AST (IU/L) | 48.3 (15–489) | 48.5 (15–489) | 46.3 (21–94) | .568 |
| Baseline Cr, (mmol/L) | 7.7 (37.1–234.1) | 7.2 (37.1–234.1) | 75.3 (44.2–175.1) | .106 |
| BUN (mmol/L) | 5.69 (1.01–81.50) | 5.62 (1.01–81.50) | 6.22 (2.70–9.44) | .073 |
| AFP (ng/mL) | 4765.5 (1.4–80005.1) | 4968.5 (1.4–80005.1) | 3085.0 (1.5–22331.0) | .366 |
| HBV DNA, ≥2000/<2000 | 97/131 (42.4/57.5) | 85/116 (42.3/57.7) | 12/15 (44.4/55.6) | 0.832 |
| CLIP score, 0–1/2–3 | 424/55 (88.5/11.5) | 391/37 (91.4/8.6) | 33/18 (64.7/35.3) | .001∗ |
| CTP, A/B | 461/18 (96.2/3.8) | 416/12 (97.2/2.8) | 45/6 (88.2/11.8) | .001∗ |
| Surgery type, major/minor | 116/363 (24.2/75.8) | 92/336 (21.5/78.5) | 24/27 (47.1/52.9) | .001∗ |
| Tumor size (cm) | 6.1 (1.0–26.0) | 5.9 (1–22) | 8.2 (3–26) | .001∗ |
| Tumor number, 1/2–3 | 440/39 (91.1/8.9) | 389/39 (9.9/9.1) | 51/0 (100/0) | 0.999 |
| Ascites | 37 (7.7) | 31 (7.2) | 6 (11.8) | .253 |
| BCLC stage, 0/A | 29/450 (6.1/93.9) | 29/399 (6.8/93.2) | 0/51 (0/100) | 0.999 |
| Within Milan criteria, n (%) | 272 (56.8) | 254 (59.3) | 18 (35.3) | .001∗ |
| Resection, R0/R1 | 389/90 (81.2/18.8) | 356 (83.2) | 33 (64.7) | .001∗ |
| Differentiation, I–II/III–IV, n (%) | 324/155 (67.6/32.4) | 285/143 (66.6/33.4) | 39/12 (76.5/23.5) | .154 |
| Microwave | 281 (58.7) | 256 (59.8) | 25 (49.0) | .139 |
AFP = α-fetoprotein, AKI = acute kidney injury, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinical Liver Cancer, BUN = blood urea nitrogen, CLIP = The Cancer of the Liver Italian Group Score, Cr = creatinine, CTP = Child-Turcotte-Pugh classification, GGT = γ-glutamyltransferase, Hb = hemoglobin, HBV DNA = serum HBV DNA levels, HBV = hepatitis B virus, KDIGO = Kidney Disease Improving Global Outcomes, PLT = platelet, SII = systemic immune-inflammation index, TB = total bilirubin, WBC = white blood cell.
P values < .05 were considered statistically significant.
3.2. Comparison of clinical characteristics with regard to pAKI
A total of 51 (10.6%) HCC patients suffered from pAKI (Table 1). Table 1 indicates also that the patients with pAKI were 5.8 years older than in participants not affected by pAKI (59.5 vs 53.7 years, P = .001). HCC patients with pAKI had a higher rate of concurrent hypertension (52.9% vs 29.9%, P = .001) and diabetes (23.5% vs 9.8%, P = .003), a lower level of Hb (121.7 vs 133.6 g/L, P = .001), and stayed in hospital longer after the surgery (17.2 days vs 14.9 days, P = .001). They had also higher counts of PLT (175.6 vs 148.8 × 109/L, P = .008) and neutrophils (4.32 vs 3.33 × 109/L, P = .001), lower count of lymphocytes (0.86 vs 1.47 × 109/L, P = .001), and higher of SII values (899.2 vs 383.4 × 109/L, P = .001). Participants with pAKI had higher levels of TB (23.0 vs 15.8 μmol/L, P = .001)and GGT (200.1 vs 105.2 IU/L, P = .001), and lower levels of ALT (29.5 vs 50.8 μmol/L, P = .005). The CLIP score was higher in HCC patients with pAKI (35.3% vs 8.6%, P = .001), and higher fraction of them underwent major hepatectomy (47.1% vs 21.5%, P = .001). The mean tumor diameter of HCC patients with pAKI was larger (8.2 cm vs 5.9 cm, P = .001), lower fraction of them fulfilled the Milan criteria (35.3% vs 59.3%, P = .001), or underwent the R0 resection (64.7% vs 83.2%, P = .001).
3.3. Predictive factors of pAKI
To explore predictors of pAKI, the logistic regression model was used for univariate analysis to evaluate multiple clinical parameters (Table 2). The univariate analysis identified the following variables with P < .05: age (odds ratio, OR = 1.054, P = .001), hypertension (OR = 2.637, P = .001), diabetes (OR = 2.828, P = .005), Hb (OR = 0.970, P = .001), PLT (OR = 1.006, P = .007), neutrophil (OR = 1.228, P = .001), lymphocyte (OR = 0.025, P = .001), SII (OR = 1.002, P = .001), WBC (OR = 1.193, P = .002), TB (OR = 1.047, P = .001), GGT (OR = 1.001, P = .022), ALT (OR = 0.967, P = .001), CLIP score (OR = 5.764, P = .001), CTP (OR = 4.622, P = .003), surgery type (OR = 3.246, P = .010), carcinoma size (OR = 1.103, P = .001), within Milan criteria (OR 2.676, P = .001), and surgical margin (OR 2.697, P = .002). On this basis, these variables were included in multivariate regression analysis which documented that SII, history of hypertension, Hb, PLT, neutrophil, lymphocyte, WBC, GGT, ALT, surgery type and tumor size were independent predictors of pAKI after hepatectomy in HCC patients.
Table 2.
Univariate and multivariate analysis of postoperative AKI based on the “KDIGO” criteria.
| Univariate analysis | Multivariate analysis | |||||
| Patient characteristics | OR | 95%CI | P | OR | 95%CI | P |
| Sex | .452 | .136–1.503 | .195 | |||
| Age | 1.054 | 1.023–1.086 | .001 | .990 | .927–1.057 | .762 |
| Hypertension | 2.637 | 1.465–4.745 | .001 | 9.366 | 2.260–38.805 | .002∗ |
| Diabetes | 2.828 | 1.375–5.816 | .005 | .395 | .066–2.352 | .307 |
| Liver cirrhosis | 1.111 | .561–2.198 | .763 | |||
| Hb | .970 | .957–.984 | .001 | .974 | .946–1.002 | .067 |
| PLT | 1.006 | 1.001–1.010 | .007 | 1.025 | 1.005–1.045 | .012∗ |
| Neutrophil | 1.228 | 1.089–1.386 | .001 | .062 | .009–427 | .005∗ |
| Lymphocyte | .025 | .009–.069 | .001 | .001 | .001–001 | .001∗ |
| SII | 1.002 | 1.002–1.003 | .001 | .997 | .994–1.001 | .110 |
| WBC | 1.193 | 1.066–1.335 | .002 | 3.099 | 5.190–174.537 | .001∗ |
| Albumin | .969 | .913–1.028 | .291 | |||
| TB | 1.047 | 1.021–1.074 | .001 | .995 | .955–1.036 | .793 |
| GGT | 1.001 | 1.000–1.002 | .022 | 1.010 | 1.005–1.015 | .001∗ |
| ALT | .967 | .949–.985 | .001 | .928 | .895–962 | .001∗ |
| AST | .999 | .992–1.006 | .750 | |||
| Baseline Cr | 1.009 | .998–1.020 | .114 | |||
| BUN | 1.024 | .974–1.077 | .345 | |||
| AFP | 1.000 | 1.000–1.000 | .374 | |||
| HBV DNA, ≥2000/<2000 | 1.000 | 1.000–1.000 | .411 | |||
| CLIP score, 0–1/2–3 | 5.764 | 2.962–11.219 | .001 | 1.495 | .108–19.638 | .776 |
| CTP, A/B | 4.622 | 1.655–12.910 | .003 | 1.401 | .107–18.316 | .797 |
| Surgery type, major/minor | 3.246 | 1.788–5.893 | .001 | 31.585 | 5.654–176.435 | .001∗ |
| Tumor size | 1.103 | 1.044–1.165 | .001 | .855 | .734–997 | .045∗ |
| Ascites | 1.599 | .655–3.899 | .302 | |||
| Within Milan criteria | 2.676 | 1.460–4.905 | .001 | 2.146 | .242–19.040 | .493 |
| Resection, R0/R1 | 2.697 | 1.440–5.052 | .002 | .787 | .147–4.200 | .779 |
| Differentiation, I–II/III–IV | .613 | .311–1.207 | .157 | |||
| Microwave | 1.548 | .865–2.770 | .141 | |||
AFP = α-fetoprotein, AKI = acute kidney injury, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BUN = blood urea nitrogen, CLIP = The Cancer of the Liver Italian Group Score, Cr = creatinine, CTP = Child-Turcotte-Pugh classification, GGT = γ-glutamyltransferase, Hb = hemoglobin, HBV DNA = serum HBV DNA levels, HBV = hepatitis B virus, KDIGO = Kidney Disease Improving Global Outcomes, PLT = platelet, SII = systemic immune-inflammation index, TB = total bilirubin, WBC = white blood cell.
P values < .05 were considered statistically significant.
The receiver operating curve analysis indicated that the optimal threshold value of preoperative SII for predicting pAKI in HCC patients was 547.84 × 109/L. The area under the curve, reflecting the accuracy of predicting pAKI by SII was 0.907 ((95%CI: 0.877–0.937) (Fig. 2)). Sensitivity, specificity, positive predictive value, and negative predictive value were 0.96 (95%CI: 0.85–0.99), 0.84 (95%CI: 0.80–0.87), 0.41 (95%CI: 0.32–0.51), and 0.99 (95%CI: 0.98–0.99), respectively.
Figure 2.
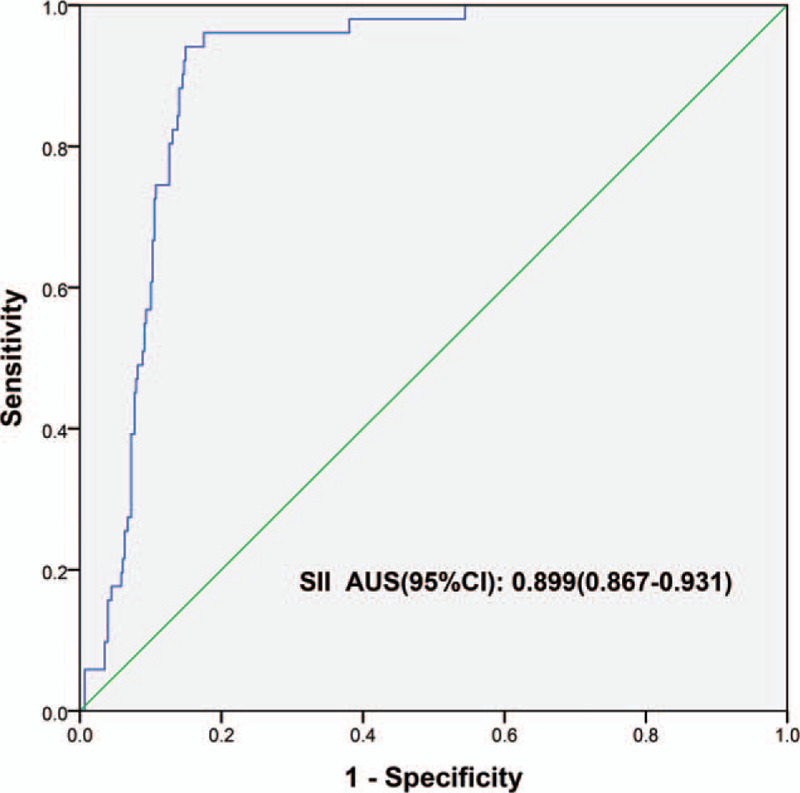
Receiver operating curve (ROC) of predicting postoperative AKI with SII. AKI = acute kidney injury, SII = systemic immune-inflammation index.
According to the optimal cut-off value of SII, all patients enrolled in the study were divided into 2 groups: the high SII (≥547.84 × 109/L) group (n = 119) and the low SII (<547.84 × 109/L) group (n = 360). After univariate analysis, the SII grouping variables with P < .05 were included in the multivariate regression analysis, which indicated that the SII ≥547.84 × 109/L was still an independent predictor of pAKI after hepatectomy in HCC patients.
3.4. Predictive factors of pAKI after propensity score matching
The variables that were statistically different between the high SII and low SII groups, included gender, history of diabetes, Hb, PLT, neutrophil, lymphocyte, WBC, TB, GGT, AST, CLIP score, surgery type, tumor size, BCLC stage, and Milan criteria (Table 3). Ninety-three propensity score-matched pairs of HCC patients pairs with HCC were selected using the Empower Stats software. Their clinical data were compared, and no significant differences in the matched variables between the 2 groups were found (P > .05). After univariate analysis, the variables with P < .05, including age (OR = 1.070, P = .001), history of hypertension (OR = 2.644, P = .008), lymphocyte count (OR = 0.119, P = .001), SII group (OR = 18.94, P = .001), GGT (OR = 1.003, P = .043), ALT (OR = 0.962, P = .001), carcinoma size (OR = 0.903, P = .043), surgical margin (OR = 2.525, P = .026), and carcinoma differentiation (OR = 0.375, P = .040), were included in the multivariate analysis (Table 4). Multivariate regression analysis confirmed that SII ≥ 547.84 × 109/L was an independent predictor of pAKI in HCC patients (Table 4).
Table 3.
Comparison of clinical parameters between the low and high SII groups.
| Clinical parameters | SII ≥ 547.84 | SII < 547.84 | P |
| Total | 119 (24.8) | 360 (75.2) | |
| AKI | 49 (41.2) | 2 (.6) | .001∗ |
| Gender (male) | 113 (95.0) | 311 (86.4) | .011∗ |
| Age (y) | 53.6 (15.0–84.0) | 54.6 (21.0–79.0) | .415 |
| Hypertension | 44 (37.0) | 111 (3.8) | .214 |
| Diabetes | 21 (17.6) | 33 (9.2) | .011∗ |
| Liver cirrhosis | 86 (72.3) | 272 (75.6) | .474 |
| Hb (g/L) | 129.0 (69.0–162.0) | 133.4 (73.0–181.0) | .043∗ |
| PLT (G/L) | 195.1 (6.0–375.0) | 137.3 (25.0–33.0) | .001∗ |
| Neutrophil (G/L) | 5.5 (2.4–13.5) | 2.8 (.8–8.9) | .001∗ |
| Lymphocyte (G/L) | 1.1 (.3–2.1) | 1.5 (.5–3.7) | .001∗ |
| WBC (G/L) | 7.5 (3.2–16.0) | 4.8 (1.5–11.7) | .001∗ |
| Albumin (g/L) | 38.9 (24.8–64.3) | 39.4 (27.9–5.4) | .405 |
| TB (μmol/L) | 19.4 (3.4–143.8) | 15.6 (4.3–58.9) | .013∗ |
| GGT (IU/L) | 189.0 (12.0–2777.0) | 91.0 (1.0–962.0) | .005∗ |
| ALT (IU/L) | 56.4 (1.0–477.0) | 46.0 (11.0–19.0) | .192 |
| AST (IU/L) | 63.0 (15.0–489.0) | 43.5 (15.0–258.0) | .009∗ |
| Baseline Cr, (mmol/L) | 7.8 (4.4–175.1) | 7.7 (37.1–234.1) | .953 |
| CLIP score, 0–1/2–3 | 94/25 (79.0/21.0) | 330/30 (91.7/8.3) | .001∗ |
| CTP, A/B | 112/7 (94.1/5.9) | 349/11 (96.9/3.1) | .160 |
| Surgery type, major/minor | 51/68 (42.9/57.1) | 65/295 (18.1/81.9) | .001∗ |
| Tumor size (cm) | 8.2 (1.5–26.0) | 5.4 (1.0–21.0) | .001∗ |
| Tumor number, 1/2–3 | 110/9 (92.4/7.6) | 330/30 (91.7/8.3) | .790 |
| Ascites | 9 (7.6) | 28 (7.8) | .939 |
| BCLC stage, 0/A | 1/118 (.8/99.2) | 28/332 (7.8/92.2) | .006∗ |
| Within Milan criteria | 41 (34.5) | 231 (64.2) | .001∗ |
| Resection, R0/R1 | 91/28 (76.5/23.5) | 298/62 (82.8/17.2) | .127 |
| Differentiation, I–II/III–IV | 82/37 (68.9/31.1) | 242/118 (67.2/32.8) | .733 |
AKI = acute kidney injury, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinical Liver Cancer, CLIP = The Cancer of the Liver Italian Group Score, Cr = creatinine, CTP = Child-Turcotte-Pugh classification, GGT = γ-glutamyltransferase, Hb = hemoglobin, PLT = platelet, SII = systemic immune-inflammation index, TB = total bilirubin, WBC = white blood cell.
P values < .05 were considered statistically significant.
Table 4.
Univariate and multivariate analysis of predictors of postoperative AKI after propensity score matching.
| Univariate analysis | Multivariate analysis | |||||
| Patient data | OR | 95%CI | P | OR | 95%CI | P |
| Age | 1.070 | 1.032–1.108 | .001 | 1.014 | .962–1.068 | .612 |
| Hypertension | 2.644 | 1.286–5.436 | .008 | 2.773 | 1.019–7.550 | .046∗ |
| Diabetes | 2.294 | .933–5.644 | .071 | |||
| Liver cirrhosis | 1.424 | .624–3.247 | .401 | |||
| Hb | .991 | .974–1.009 | .338 | |||
| PLT | 1.003 | .998–1.008 | .213 | |||
| Neutrophil | 1.064 | .928–1.220 | .375 | |||
| Lymphocyte | .119 | .042–.335 | .001 | .241 | .060–.974 | .046∗ |
| High SII vs low SII | 18.947 | 5.574–64.412 | .001 | 15.723 | 3.595–68.766 | .001∗ |
| WBC | 1.080 | .949–1.229 | .245 | |||
| Albumin | .991 | .931–1.056 | .781 | |||
| TB | 1.022 | .981–1.064 | .301 | |||
| GGT | 1.003 | 1.000–1.006 | .043 | 1.010 | 1.005–1.016 | .001∗ |
| ALT | .962 | .940–.985 | .001 | .943 | .904–.983 | .005∗ |
| AST | .987 | .972–1.002 | .081 | |||
| Baseline Cr | 1.008 | .997–1.020 | .159 | |||
| AFP | 1.000 | 1.000–1.000 | .220 | |||
| CLIP score, 0–1/2–3 | 1.390 | .508–3.802 | .521 | |||
| CTP, A/B | 2.367 | .540–1.369 | .253 | |||
| Surgery type, major/minor | .987 | .478–2.038 | .971 | |||
| Tumor size | .903 | .818–.997 | .043 | .873 | .763–.999 | .048∗ |
| Ascites | .945 | .275–3.246 | .929 | |||
| Within Milan criteria | 1.043 | .505–2.153 | .910 | |||
| Resection, R0/R1 | 2.525 | 1.115–5.717 | .026 | 1.145 | .342–3.834 | .826 |
| Differentiation, I–II/III–IV | .375 | .147–.956 | .040 | .480 | .143–1.610 | .235 |
AFP = α-fetoprotein, AKI = acute kidney injury, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CLIP = The Cancer of the Liver Italian Group Score, Cr = creatinine, CTP = Child-Turcotte-Pugh classification, GGT = γ-glutamyltransferase, Hb = hemoglobin; PLT = platelet, SII = systemic immune-inflammation index, TB = total bilirubin, WBC = white blood cell.
P values < .05 were considered statistically significant.
4. Discussion
The retrospective analysis performed in the present investigation demonstrated that HCC patients infected by HBV with pAKI, defined according to the KDIGO criteria, had a higher preoperative value of blood SII value than patients without pAKI. Moreover, multivariate regression documented that SII was an independent predictor of pAKI after hepatectomy in these patients. Based on the receiver operating curve analysis, the best threshold truncation value of SII predicting pAKI was found to be 547.84 × 109/L. Multivariate analysis performed following the PSM confirmed that the SII ≥547.84 × 109/L was an independent predictor of pAKI after hepatectomy in HCC patients. This value of SII correctly predicted pAKI in 96.1% (95% CI: 85.4–99.3%) of participants with pAKI, and absence of pAKI in 83.6% (95% CI: 79.7–87.0%) of patients not affected by this condition. The positive predictive value of SII was slightly lower.
These results indicate that preoperative SII may be a novel, low-cost, and simple biomarker for predicting pAKI. Among HCC patients with concurrent HBV infection, preoperative SII may serve as a new prognostic tool to assess the risk of pAKI. Thus, preoperative SII may help to identify HCC patients that are at high risk of pAKI, enabling timely prevention and treatment of pAKI in high-risk patients and improving their prognosis.
The incidence of pAKI in patients undergoing hepatectomy is approximately15%.[3] pAKI causes a 10-fold increase in in-hospital mortality and reduces long-term survival.[6,8] In agreement with a previous report,[3] the current analysis showed that HCC patients with pAKI had a longer postsurgical hospital stay than patients without pAKI. A worse prognosis for surgically treated HCC patients with elevated preoperative value of SII was also reported.[14] This present investigation found that SII is an independent predictor of pAKI in HCC patients, suggesting that HCC patients with elevated SII value may have a worse prognosis due to the occurrence of pAKI. In this study, the presence of cirrhosis and ascites was not an independent predictor of pAKI. The reasonable explanations are as follows. According to the inclusion and exclusion criteria, the patients who were eventually included in the study had BCLC stage 0/A and Child-Turcotte-Pugh classification A/B. Therefore, the degree of cirrhosis and ascites in the vast majority of patients is relatively mild.
Immune and inflammatory responses, reflected by the SII index, have a significant role in the development of pAKI and may be related to the presence of HBV.[13] HBV infection is associated with the occurrence of kidney disease.[28] In addition, the presence of HBV DNA and HBV antigen in renal tubular epithelial cells has been well-documented,[28,29] and immune complexes released from the kidney may participate in the pathogenesis of HBV-associated nephropathy.[28] Experimentally, HBV replication in infected tubular cells may induce apoptosis of tubular cells.[30] In fact, serum from patients infected with HBV promotes apoptosis of renal tubular epithelial cells by a mechanism involving upregulation of Fas.[30] Apoptosis might promote cell loss and aggravate organ damage in acute kidney injury.[30] Tissue damage may be also promoted by excessive and unresolved inflammation.[31] Recent studies indicated that T cells are also involved in the early AKI reaction.[32] Activated dendritic cells and renal parenchymal cells secrete several chemokines, including CXCL8, CXCL1, CCL5, and CCL2, which promote acute neutrophil-dependent inflammatory responses in AKI[33,34] by recruiting cells to the site of injury. Consistent with these findings, experimental blockade of chemokines and cytokines can abrogate the AKI response.[35]
Oxidative stress may represent another mechanism that might participate in renal tubular cells injury because renal tubular epithelial cells are very susceptible to reactive oxygen species.[36] Some experimental studies suggested that exposure of cells to superoxide anion leads to an increase in intracellular calcium concentration, resulting in renal vasoconstriction, and decreased renal blood flow and glomerular filtration rate, ultimately generating kidney damage.[37,38] The role of reactive oxygen in renal injury is supported by the demonstration that antioxidant therapy can prevent kidney damage and improve renal dysfunction.[39]
The majority of thus far performed clinical trials on the prevention or treatment of AKI was negative. The unfavorable outcomes may be related to the multifactorial nature and dynamic progression of AKI, as well as the difficulty in establishing extent of tissue damage that has occurred.[40,41] This present work demonstrates that SII is an independent predictor of pAKI in HCC patients, and HCC patients with elevated SII value are at high risk of pAKI. The identification of a predictor of pAKI creates an opportunity to provide individualized treatment for high-risk patients, including measures to prevent pAKI and treat a subclinical condition. The availability of these options might improve the prognosis of patients.
It should also be recognized that the current study has some limitations. First, this work is a single-center retrospective study with a low incidence of pAKI (10.8%), and relatively few pAKI cases were included in the study. Therefore, large-scale multicenter prospective cohort studies are necessary to strengthen the results. Second, it should be noted that most HCC patients in China are infected by HBV, which is not the case in patient populations in the United States, Japan, and Europe. Therefore, the predictive significance of the SII needs to be validated in HCC patients with HCC from those geographic regions. Third, the best threshold value of SII identified here may not be suitable for other studies. Prospective validation or a meta-analysis of various studies focused on SII is required to confirm the optimal cut-off value of SII. Despite these limitations, to the best of our knowledge, this study provides the first demonstration of the predictive value of SII for pAKI in HCC patients who underwent hepatectomy.
5. Conclusions
The results of this study indicate that SII qualifies as a novel, independent predictor of pAKI in HCC patients with HBV infection who underwent hepatectomy.
Author contributions
Jianjun Xu, Shaobo Hu, Suzhen Li, Xiang Cheng, and Qichang Zheng researched literature and conceived the study. Weimin Wang, Yuzhe Wu, Zhe Su, Xing Zhou, and Yang Gao were involved in protocol development, gaining ethical approval, patient recruitment, and data analysis. Jianjun Xu, Shaobo Hu, and Suzhen Li wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Conceptualization: Jianjun Xu, Shaobo Hu, Xiang Cheng, Qichang Zheng.
Data curation: Jianjun Xu, Suzhen Li, Weimin Wang, Yuzhe Wu, Zhe Su, Xing Zhou, Yang Gao.
Formal analysis: Jianjun Xu, Shaobo Hu, Suzhen Li.
Funding acquisition: Yang Gao, Qichang Zheng.
Investigation: Jianjun Xu, Xing Zhou, Yang Gao, Xiang Cheng, Qichang Zheng.
Methodology: Jianjun Xu, Shaobo Hu, Suzhen Li, Weimin Wang, Yuzhe Wu, Zhe Su, Xing Zhou, Yang Gao, Xiang Cheng, Qichang Zheng.
Project administration: Qichang Zheng.
Resources: Shaobo Hu, Weimin Wang, Zhe Su, Yang Gao, Xiang Cheng, Qichang Zheng.
Software: Yuzhe Wu.
Supervision: Xiang Cheng, Qichang Zheng.
Validation: Xiang Cheng, Qichang Zheng.
Writing – original draft: Jianjun Xu, Shaobo Hu, Suzhen Li.
Writing – review & editing: Jianjun Xu, Shaobo Hu, Suzhen Li, Xiang Cheng, Qichang Zheng.
Footnotes
Abbreviations: AFP = α-fetoprotein, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinical Liver Cancer, CLIP = The Cancer of the Liver Italian Group Score, CT = computed tomography, CTP = Child-Turcotte-Pugh classification, GGT = γ-glutamyltransferase, Hb = hemoglobin, HBV = hepatitis B virus, HBV DNA = serum HBV DNA levels, HCC = hepatocellular carcinoma, KDIGO = Kidney Disease Improving Global Outcomes, MRI = magnetic resonance imaging, pAKI = postoperative acute renal injury, PLT = platelet, SII = systemic immune-inflammation index, TB = total bilirubin.
How to cite this article: Xu J, Hu S, Li S, Wang W, Wu Y, Su Z, Zhou X, Gao Y, Cheng X, Zheng Q. Systemic immune-inflammation index predicts postoperative acute kidney injury in hepatocellular carcinoma patients after hepatectomy. Medicine. 2021;100:14(e25335).
JX, SH, and SL contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No. 81874231 and No. 81372668).
This retrospective study was approved by the Tongji Medical College research ethics committee, Wuhan, China. (No: IORG0003571).
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002;122:1609–19. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [3].Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg 2015;261:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- [5].Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med 2013;41:2570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009;249:851–8. [DOI] [PubMed] [Google Scholar]
- [7].Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- [8].Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444–53. [DOI] [PubMed] [Google Scholar]
- [9].Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann Surg 2016;263(Jun):1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg 2010;252:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011;171:226–33. [DOI] [PubMed] [Google Scholar]
- [12].Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018;391:1301–14. [DOI] [PubMed] [Google Scholar]
- [13].Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock (Augusta, GA) 2014;41:03–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res: an official journal of the American Association for Cancer Research 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [15].Zhao LY, Yang DD, Ma XK, et al. The prognostic value of aspartate aminotransferase to lymphocyte ratio and systemic immune-inflammation index for overall survival of hepatocellular carcinoma patients treated with palliative treatments. J Cancer 2019;10:2299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang K, Hua YQ, Wang D, et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med 2019;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jomrich G, Gruber ES, Winkler D, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg: official journal of the Society for Surgery of the Alimentary Tract 2020;24:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun Y, Li W, Li AJ, et al. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manage Res 2019;11:3153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer 2019;10:3188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Diao P, Wu Y, Li J, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. J Transl Med 2018;16:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep 2019;9(Mar):3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lackner C, Struber G, Liegl B, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology (Baltimore, MD) 2005;41:1376–82. [DOI] [PubMed] [Google Scholar]
- [24].Myers RP, Tainturier MH, Ratziu V, et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol 2003;39:222–30. [DOI] [PubMed] [Google Scholar]
- [25].Reddy SK, Barbas AS, Turley RS, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB: the official journal of the International Hepato Pancreato Biliary Association 2011;13:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- [27].Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33–8. [Google Scholar]
- [28].Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol 2004;24:198–211. [DOI] [PubMed] [Google Scholar]
- [29].Park MH, Song EY, Ahn C, et al. Two subtypes of hepatitis B virus-associated glomerulonephritis are associated with different HLA-DR2 alleles in Koreans. Tissue antigens 2003;62:505–11. [DOI] [PubMed] [Google Scholar]
- [30].Deng CL, Song XW, Liang HJ, et al. Chronic hepatitis B serum promotes apoptotic damage in human renal tubular cells. World J Gastroenterol 2006;12:1752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010;140:771–6. [DOI] [PubMed] [Google Scholar]
- [32].Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando, FL) 2009;23:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kurts C, Panzer U, Anders HJ, et al. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 2013;13:738–53. [DOI] [PubMed] [Google Scholar]
- [34].Bolisetty S, Agarwal A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int 2009;75:674–6. [DOI] [PubMed] [Google Scholar]
- [35].Yalavarthy R, Edelstein CL. Therapeutic and predictive targets of AKI. Clin Nephrol 2008;70:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010;10:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 2000;279:H7–14. [DOI] [PubMed] [Google Scholar]
- [38].Meng S, Roberts LJ, 2nd, Cason GW, et al. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regulatory, integrative and comparative physiology 2002;283:R732–8. [DOI] [PubMed] [Google Scholar]
- [39].Tian N, Thrasher KD, Gundy PD, et al. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension (Dallas, TX: 1979) 2005;45:934–9. [DOI] [PubMed] [Google Scholar]
- [40].Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2007;2:356–65. [DOI] [PubMed] [Google Scholar]
- [41].Molitoris BA, Okusa MD, Palevsky PM, et al. Design of clinical trials in AKI: a report from an NIDDK workshop. Trials of patients with sepsis and in selected hospital settings. Clin J Am Soc Nephrol 2012;7:856–60. [DOI] [PubMed] [Google Scholar]


