Abstract
Background:
Ropivacaine is considered the most commonly used for epidural anesthesia. We compared the efficiency and safety of ropivacaine alone (R group) and ropivacaine combined with dexmedetomidine (RD group).
Method:
PubMed, the Cochrane Library, Google Scholar, Ovid Medline, the Web of Science, Scopus, Embase, and ScienceDirect were searched. We considered sensory and motor block, duration of anesthesia, time to rescue, hemodynamics, and adverse effects as the primary endpoints.
Results:
Eleven randomized controlled trials were included with 337 patients in the R group and 336 patients in the RD group. The RD group had a shorter time to onset of sensory (mean difference [MD]: 3.97 [1.90–6.04] minutes; P = .0002) and motor (MD: 2.43 [0.70–4.16] minutes; P = .006) block and a longer duration of anesthesia (MD: -164.17 [-294.43 to -33.91]; P = .01) than the R group. Comparison of the time to rescue between the groups showed no significant difference (MD: -119.01[-254.47–16.46] minutes; P = 0.09). The R group showed more stable hemodynamics than the RD group in heart rate and arterial pressure at 10 minutes. The R group had a lower incidence of bradycardia and a higher incidence of shivering than the RD group.
Conclusion:
RD may be a more suitable choice for epidural anesthesia with better anesthetic outcomes than R alone. However, the safety of the combination must be carefully assessed.
Keywords: dexmedetomidine, epidural anesthesia, meta-analysis, ropivacaine
1. Introduction
Epidural anesthesia (EA) is the most common technique for intraoperative surgical anesthesia and postoperative analgesia for many surgeries, such as lung surgeries, orthopedic surgery, and labor.[1] The alpha-2 adrenoreceptor agonist ropivacaine is the most common medicine used for epidural anesthesia.[2,3] However, when used alone, it has many disadvantages, including hypotension, oversedation, bradycardia, and prolongation of the second stage of labor.[4]
Dexmedetomidine is combined with local anesthetics to enhance the effect of anesthesia, reduce the dose of local anesthetics and decrease the incidence of adverse effects (AEs).[5,6] Many studies have also shown that dexmedetomidine is a highly selective alpha-2 adrenoreceptor agonist and a very effective adjuvant, with stable hemodynamics and sympathoadrenal function.[7,8] Used of the combination of ropivacaine and dexmedetomidine (RD) for EA is a current trend, but some scholars reported that the efficiency and safety of RD are not clear.[9,10] Attri et al reported that epidural dexmedetomidine as an adjuvant to ropivacaine prolonged the time of sensory and motor block and postoperative analgesia, reduced demand for rescue analgesics, and maintained hemodynamic stability.[11] However, Zhao et al suggested that epidural ropivacaine (0.125%) combined with dexmedetomidine (0.5 mg/kg) alleviated pain sensations but showed no significant difference in prolonging the time of motor blockage.[12] Soni et al also reported that a ropivacaine (R group) had a lower incidence of AEs than an RD group.[13]
We performed a meta-analysis to answer this dispute by comparing the efficiency and safety of R and RD for EA.
2. Materials and methods
This meta-analysis and systemic review were performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (Registration information: PROSPERO CRD42020177850).
2.1. Search strategy
The search date was 17 March 2020. Related articles were searched in PubMed, Scopus, Google Scholar, Web of Science, Ovid Medline, Embase, ScienceDirect, and the Cochrane Library to retrieve articles published before 17 March 2020. The following medical subject heading (MeSH) terms were used: “epidural anesthesia,” “ropivacaine,” and “dexmedetomidine.” Table 1 shows the search strategy. No language restrictions were used in the identification of eligible articles.
Table 1.
Search strategy.
| PubMed The database was searched on March 17, 2020, n = 36. Search Strategy: (Epidural analgesia [Title/Abstract]) and (Ropivacaine [Title/Abstract] OR 1-Propyl-2’,6’-pipecoloxylidide [Title/Abstract] OR 1 Propyl 2’,6’ pipecoloxylidide [Title/Abstract] OR Naropin [Title/Abstract] OR Ropivacaine Monohydrochloride [Title/Abstract] OR Ropivacaine Hydrochloride [Title/Abstract] OR AL 381 [Title/Abstract] OR AL-381 [Title/Abstract] OR AL381 [Title/Abstract] OR Naropeine [Title/Abstract] OR LEA 103 [Title/Abstract] OR LEA-103 [Title/Abstract] OR LEA103 [Title/Abstract]) and (Dexmedetomidine [Title/Abstract] OR MPV-1440 [Title/Abstract] OR MPV 1440 [Title/Abstract] OR MPV1440 [Title/Abstract] OR Precedex [Title/Abstract] OR Dexmedetomidine Hydrochloride [Title/Abstract] OR Hydrochloride, Dexmedetomidine [Title/Abstract]) |
| Web of Science The database was searched on March 17, 2020, n = 137. Search Strategy: 1.TOPIC: (“Epidural analgesia”) (136432) 2.TOPIC: (“Ropivacaine” OR “1-Propyl-2’,6’-pipecoloxylidide” OR “1 Propyl 2’,6’ pipecoloxylidide” OR “Naropin” OR “Ropivacaine Monohydrochloride” OR “Ropivacaine Hydrochloride” OR “AL 381” OR “AL-381”OR “AL381” OR “Naropeine” OR “LEA 103” OR “LEA-103” OR “LEA103”) (6798) 3.TOPIC: (“Dexmedetomidine” OR “MPV-1440” OR “MPV 1440” OR “MPV1440” OR “Precedex” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine”) (6179) 4.#1 AND #2 AND #3 (137) |
| EMBASE The database was searched on March 17, 2020, n = 193. Search Strategy: (’Epidural analgesia’:ti,ab,kw) AND (’Ropivacaine’:ti,ab,kw OR ’1-Propyl-2’,6’-pipecoloxylidide’:ti,ab,kw OR ’1 Propyl 2’,6’ pipecoloxylidide’:ti,ab,kw OR ’Naropin’:ti,ab,kw OR ’Ropivacaine Monohydrochloride’:ti,ab,kw OR ’Ropivacaine Hydrochloride’:ti,ab,kw OR ’AL 381’:ti,ab,kw OR ’AL-381’:ti,ab,kw OR ’AL381’:ti,ab,kw OR ’Naropeine’:ti,ab,kw OR ’LEA 103’:ti,ab,kw OR ’LEA-103’:ti,ab,kw OR ’LEA103’:ti,ab,kw) AND (’Dexmedetomidine’:ti,ab,kw OR ’MPV-1440’:ti,ab,kw OR ’MPV 1440’:ti,ab,kw OR ’MPV1440’:ti,ab,kw OR ’Precedex’:ti,ab,kw OR ’Dexmedetomidine Hydrochloride’:ti,ab,kw OR ’Hydrochloride, Dexmedetomidine’:ti,ab,kw) |
| Cochrane Library The database was searched on March 17, 2020, n = 60. Search Strategy: (“Epidural analgesia”): ti,ab,kw AND (“Ropivacaine” OR “1-Propyl-2’,6’-pipecoloxylidide” OR “1 Propyl 2’,6’ pipecoloxylidide” OR “Naropin” OR “Ropivacaine Monohydrochloride” OR “Ropivacaine Hydrochloride” OR “AL 381” OR “AL-381”OR “AL381” OR “Naropeine” OR “LEA 103” OR “LEA-103” OR “LEA103”): ti,ab,kw AND (“Dexmedetomidine” OR “MPV-1440” OR “MPV 1440” OR “MPV1440” OR “Precedex” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine”): ti,ab,kw - (Word variations have been searched) |
| Ovid MEDLINE The database was searched on March 17, 2020, n = 184. Search Strategy: 1. Epidural analgesia.ab. (6784) 2. or/1 [Epidural analgesia] (6784) 3. Ropivacaine.ab. (16489) 4. 1-Propyl-2’,6’-pipecoloxylidide.ab. (2430) 5. 1 Propyl 2’,6’ pipecoloxylidide.ab.(2234) 6. Naropin.ab. (1345) 7. Ropivacaine Monohydrochloride.ab. (321) 8. LEA 103.ab. (124) 9. LEA-103.ab.(145) 10. LEA103.ab. (311) 11. or/3-10 [Ropivacaine] (9784) 12. Dexmedetomidine.ab. (9657) 13. MPV-1440.ab. (145) 14. MPV 1440.ab. (112) 15. MPV1440.ab. (113) 16. Precedex.ab. (231) 17. Dexmedetomidine Hydrochloride.ab. (451) 18. Hydrochloride, Dexmedetomidine.ab. (531) 19. or/12-18 [Dexmedetomidine] (6371) 20. 2 and 11 and 19 (245) limit 20 to humans (184) |
| ScienceDirect The database was searched on March 17, 2020, n = 101. Search Strategy: Title, abstract, keywords ((“Epidural analgesia”) and (“Ropivacaine” OR “1-Propyl-2’,6’-pipecoloxylidide” OR “1 Propyl 2’,6’ pipecoloxylidide” OR “Naropin” OR “Ropivacaine Monohydrochloride” OR “Ropivacaine Hydrochloride” OR “AL 381” OR “AL-381”OR “AL381” OR “Naropeine” OR “LEA 103” OR “LEA-103” OR “LEA103”) and (“Dexmedetomidine” OR “MPV-1440” OR “MPV 1440” OR “MPV1440” OR “Precedex” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine”)) |
| Scopus The database was searched on March 17, 2020, n = 99. Search Strategy: TITLE-ABS-KEY ((“Epidural analgesia”) and (“Ropivacaine” OR “1-Propyl-2’,6’-pipecoloxylidide” OR “1 Propyl 2’,6’ pipecoloxylidide” OR “Naropin” OR “Ropivacaine Monohydrochloride” OR “Ropivacaine Hydrochloride” OR “AL 381” OR “AL-381”OR “AL381” OR “Naropeine” OR “LEA 103” OR “LEA-103” OR “LEA103”) and (“Dexmedetomidine” OR “MPV-1440” OR “MPV 1440” OR “MPV1440” OR “Precedex” OR “Dexmedetomidine Hydrochloride” OR “Hydrochloride, Dexmedetomidine”)) |
The combined text and medical subject heading (MeSH) terms used were: “Epidural analgesia,” “Ropivacaine,” and “Dexmedetomidine.”
2.2. Selection criteria
The following inclusion criteria were used:
-
(1)
P (patients): patients who underwent EA;
-
(2)
I (intervention) and C (comparison): R group vs RD group;
-
(3)
O (outcomes): duration of anesthesia; time to rescue; sensory and motor block (time to onset of sensory block and time to onset of motor block); hemodynamics (heart rate [HR]; blood pressure [BP]: mean arterial pressure, systolic blood pressure [SBP], and diastolic blood pressure [DBP]); and AEs.
-
(4)
S (studies): randomized controlled trials (RCTs)
The exclusion criteria included
-
(1)
articles lacking original data,
-
(2)
meta-articles, animal experiments or meeting articles,
-
(3)
articles with abstracts only or duplicated data, and
-
(4)
articles comparing dexmedetomidine combined with other drugs or in which dexmedetomidine was used for purposes other than EA.
2.3. Data extraction
The extracted data included the article name, first author, publication year, type of study, nation, participant number, characteristics of participants (age, sex, weight, height, type of surgery and American Society of Anesthesiologists physical status), duration of anesthesia, time to rescue, time to onset of sensory and motor block, HR, BP (MAP, SBP, and DBP) at 10 and 45 minutes and AEs. Two investigators extracted the data. The HR and BP (MAP, SBP, and DBP) at 10 and 45 minutes were used to indicate the onset and peak times of the anesthesia, respectively, which are the most critical moments during surgery. A third investigator assessed the outcomes when disagreement arose between the 2 investigators.
2.4. Quality assessment
Two investigators used the Cochrane Collaboration's “Risk of Bias” tool, which includes randomization (allocation concealment and sequence generation), selection of outcomes reported, blinding (personnel, participants, and outcome assessors), and completeness of outcome data, to assess the quality of the RCTs.[14] We used the five-point Jadad scale to assess the quality of the RCTs. The randomization, masking, and accountability of all patients were the 3 items of the scale, with scores ≥3 points indicating high-quality studies.[15] We used the Grades of Recommendations Assessment, Development and Evaluation (GRADE) system to evaluate the level of evidence, which includes five evaluation items: inconsistency, risk of bias, imprecision, indirectness, and publication bias. High, moderate, low, and very low were the 4 types of evidence.[16]
2.5. Statistical analysis
We used STATA 12.0 (Stata Corp, TX) and Review Manager 5.3 (Nordic Cochrane Center, Oxford, UK) to evaluate the pooled data. We used the mean difference (MD) to analyze the duration of anesthesia, time to rescue, sensory and motor block, HR, MAP, SBP, and DBP. An MD > 0 (with 95% confidence intervals [CIs]) indicated that the results favored the R group in the analysis of the duration of anesthesia, time to rescue, HR, MAP, SBP, and DBP at 10 and 45 minutes and favored the RD group in the times to onset of sensory and motor block and the decreases in the HR, MAP, SBP, and DBP amplitudes at 10 and 45 minutes. Otherwise, the results supported the RD or R group. Risk ratios (RRs) were used to analyze the dichotomous variables (AEs). An RR > 1 (with 95% CI) indicated that the results favored the RD group in the analysis of AEs. To determine whether nationality, age, type of surgery, or dose of dexmedetomidine changed the results, we performed subgroup analysis of the duration of anesthesia, time to rescue, and HR at 10 minutes. We used the I2 statistic and χ2 test to evaluate heterogeneity. When I2 < 50% or P > 0.1, we used the fixed-effects model to reflect the lack of significant heterogeneity. Otherwise, we used the random effects model. Publication bias and sensitivity analysis were performed using STATA. We used Egger linear regression tests[17] and Begg rank correlation[18] to evaluate the publication bias. Statistical significance was assumed when P < .05.
3. Results
3.1. RCT selection
We retrieved 845 related articles after the first search, and 651 articles after were removed due to duplicate data. After titles and abstracts were read, 79 reports were included for full-text review. Sixty-eight articles were excluded because they were noncomparative studies, did not compare the RD and R groups, were meeting articles, or included different patient types in the 2 groups. Eleven studies, with 337 patients in the R group and 336 patients in the RD group, were ultimately included[11–13,19–26] (Fig. 1).
Figure 1.
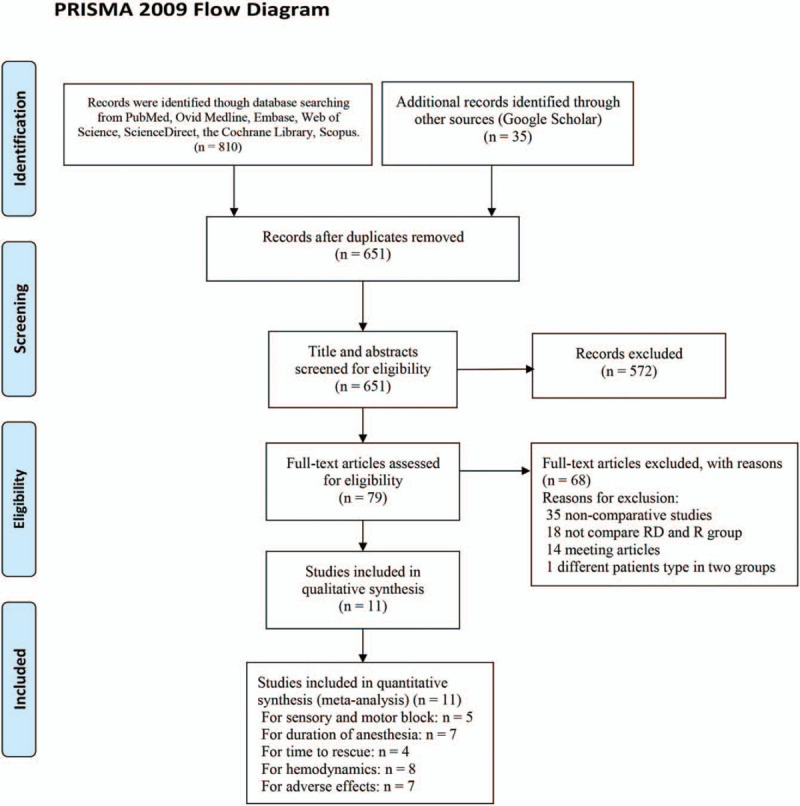
Flow chart of the study selection.
3.2. Study characteristics
Across the included studies, all of the trials were published between 2014 and 2020, and the average age of the enrolled patients varied from 3.93 to 48.13 years. Patients had undergone thoracotomy, labor, cesarean section, lumbosacral spine surgery, infra-umbilical surgery, lower abdominal surgery, or lower limb surgery. Table 2 lists the baseline characteristics of the studies, and the quality of the articles is listed in Figure 2 and Table 3. The level of evidence of the results is presented in Table 4.
Table 2.
Characteristics of the included studies.
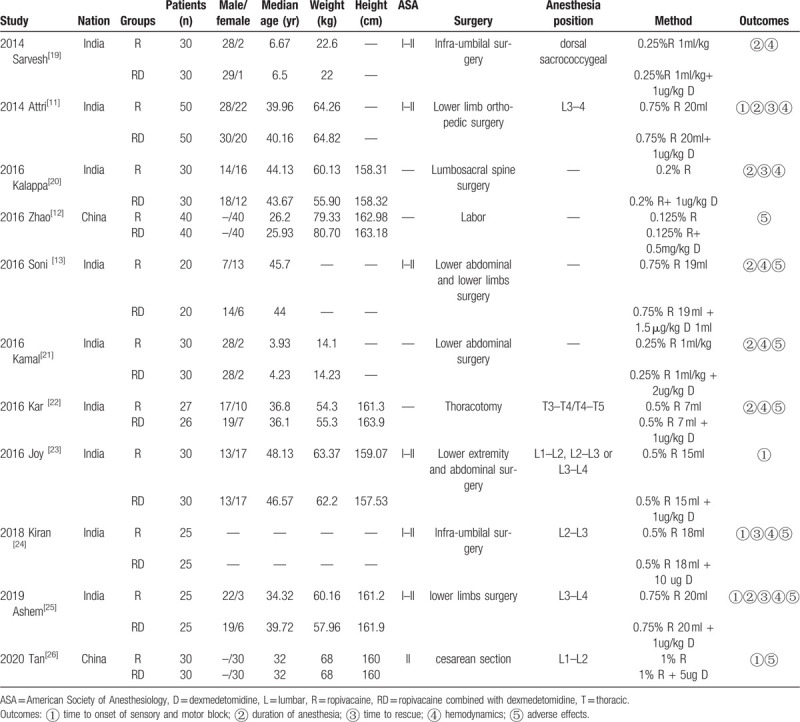
Figure 2.

Risk of bias assessments for studies in a Cochrane review.
Table 3.
Quality assessment of all included studies.
| Study | Randomization | Masking | Accountability of all patients | Quality (scores) | |
| 2014 | Sarvesh [19] | ∗∗ | ∗∗ | ∗ | 5 |
| 2014 | Attri[11] | ∗∗ | ∗ | ∗ | 4 |
| 2016 | Kalappa[20] | ∗∗ | ∗∗ | ∗ | 5 |
| 2016 | Zhao[12] | ∗∗ | ∗ | ∗ | 4 |
| 2016 | Soni[13] | ∗ | ∗ | ∗ | 3 |
| 2016 | Kamal[21] | ∗∗ | ∗ | ∗ | 4 |
| 2016 | Kar[22] | ∗∗ | ∗∗ | ∗ | 5 |
| 2016 | Joy[23] | ∗ | ∗∗ | ∗ | 4 |
| 2018 | Kiran[24] | ∗∗ | ∗∗ | ∗ | 5 |
| 2019 | Ashem[25] | ∗ | ∗∗ | ∗ | 4 |
| 2020 | Tan[26] | ∗∗ | ∗∗ | ∗ | 5 |
Table 4.
Evidence assessment with the GRADE system.
| No. of participants | Quality assessment | |||||||||
| Primary outcomes | No. of Studies | R | RD | Differences∗ (95%CI) | Risk of Bias† | Inconsistency | Indirectness | Imprecision | PublicationBias‡ | Quality |
| Sensory and motor block | ||||||||||
| Time to onset of sensory block | 4 | 130 | 130 | 3.97 [1.90, 6.04] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Time to onset of motor block | 5 | 160 | 160 | 3.12 [1.85, 4.40] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Duration of anesthesia | 7 | 212 | 211 | −164.17 [−294.43,−33.91] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| Time to rescue | 4 | 130 | 130 | −119.01 [−254.47, 16.46] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| HR | ||||||||||
| At 10 min | 3 | 95 | 95 | 5.45 [0.01,10.89] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| At 45 min | 3 | 95 | 95 | 4.72 [2.65,6.79] | Low | Serious (−1) | No indirectness | No imprecision | Unlikely | Medium |
| Decreasing amplitude at 10 min | 3 | 95 | 95 | 7.44 [4.85,10.03] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Decreasing amplitude at 45 min | 3 | 95 | 95 | 5.34 [3.07,7.62] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| BP | ||||||||||
| MAP | ||||||||||
| At 10 min | 2 | 55 | 55 | 7.77 [5.13,10.41] | Low | Very serious (−2) | No indirectness | Serious (−1) | Unlikely | Very low |
| At 45 min | 2 | 55 | 55 | 3.45 [0.83,6.06] | Low | No inconsistency | No indirectness | Serious (−1) | Unlikely | Medium |
| Decreasing amplitude at 10 min | 2 | 55 | 55 | 7.01 [4.03,9.99] | Low | Very serious (−2) | No indirectness | Serious (−1) | Unlikely | Very low |
| Decreasing amplitude at 45 min | 2 | 55 | 55 | 2.28 [−0.61,5.18] | Low | No inconsistency | No indirectness | Serious (−1) | Unlikely | Medium |
| SBP | ||||||||||
| At 10 min | 2 | 70 | 70 | 7.21 [3.17,11.24] | Low | Serious (−1) | No indirectness | No imprecision | Unlikely | Medium |
| At 45 min | 2 | 70 | 70 | 5.38 [2.03,8.74] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Decreasing amplitude at 10 min | 2 | 70 | 70 | 8.05 [−0.4,16.49] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| Decreasing amplitude at 45 min | 2 | 70 | 70 | 7.39 [3.18,11.61] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| DBP | ||||||||||
| At 10 min | 2 | 70 | 70 | 3.26 [0.44,6.09] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| At 45 min | 2 | 70 | 70 | 4.20 [1.55,6.85] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Decreasing amplitude at 10 min | 2 | 70 | 70 | 5.90 [2.83,8.96] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Decreasing amplitude at 45 min | 2 | 70 | 70 | 6.75 [3.75,9.74] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| Adverse effects | ||||||||||
| Hypotension | 5 | 130 | 130 | 0.36 [0.12,1.10] | Low | Very serious (−2) | No indirectness | No imprecision | Unlikely | Low |
| Nausea or vomiting | 5 | 145 | 145 | 1.17 [0.65,2.09] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Shivering | 3 | 80 | 80 | 2.82 [1.13,7.01] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Pruritus | 1 | 30 | 30 | 1.33 [0.33,5.45] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Bradycardia | 5 | 127 | 127 | 0.29 [0.13,0.65] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Dry mouth | 2 | 45 | 45 | 0.33 [0.07,1.56] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
| Oversedation | 1 | 30 | 30 | 1.50 [0.27,8.34] | Low | No inconsistency | No indirectness | No imprecision | Unlikely | High |
DBP = diastolic blood pressure, HR = heart rate, MAP = mean arterial pressure, R = ropivacaine, RD = ropivacaine combined with dexmedetomidine, SBP = systolic blood pressure.
Differences: Mean difference (MD) for sensory and motor block, duration of anesthesia, time to rescue, HR, MAP, SBP, DBP; risk ratios (RR) for adverse effects.
Risk of bias assessed using the Jada for randomized studies.
Publication bias was assessed by Egger and Begg tests.
3.3. Efficiency of anesthesia
Five articles were included to assess the time to onset of sensory and motor block. The results showed that the RD group had a shorter time to onset of sensory block (MD: 3.97 minutes; 95% CI: 1.90–6.04; P = .0002) and motor block (MD: 2.43 minutes; 95% CI: 0.70–4.16; P = .006) (Fig. 3) than the R group.
Figure 3.
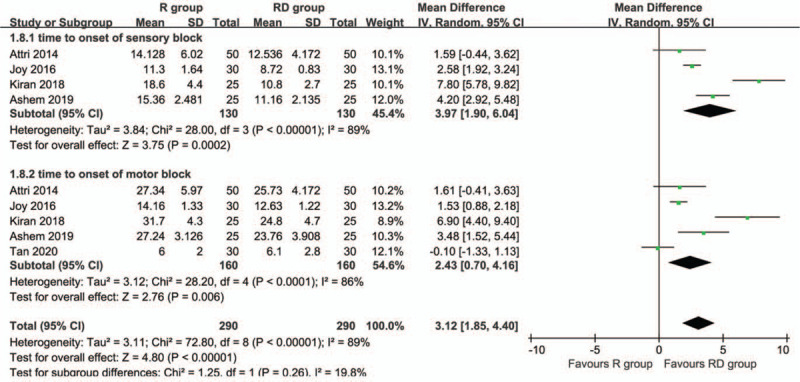
Forest plot of the time to onset of sensory and motor block associated with the ropivacaine group and ropivacaine combined with dexmedetomidine.
Seven articles that included 212 patients in the R group and 211 in the RD group to assess the duration of the anesthesia between the groups. The RD group had a longer duration of anesthesia than the R group (MD: -164.17 minutes; 95% CI: -294.43 to -33.91; P = .01) (Fig. 4A).
Figure 4.
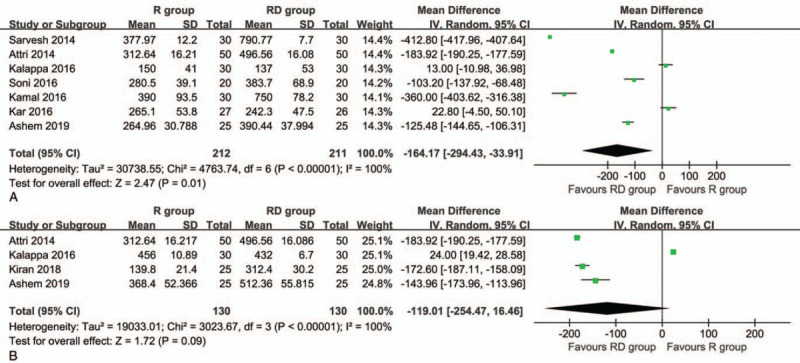
Forest plot of the duration of anesthesia (A) and time to rescue (B) associated with the ropivacaine group and ropivacaine combined with dexmedetomidine.
Four articles that included 260 patients were used to compare the time to rescue between the groups. The comparison between the groups showed no significant difference (MD: -119.01 minutes; 95% CI: -254.47 to 16.46; P = .09) (Fig. 4B).
3.4. Hemodynamics
Three articles were used to evaluate whether the administration of dexmedetomidine was associated with a lower HR. The results showed that the R group had a higher HR than the RD group at 10 minutes (MD: 8.73 beats/min; 95% CI: 2.37–15.08; P = .007) and 45 minutes (MD: 4.72 beats/min; 95% CI: 2.65–6.79; P < .00001) (Table 5). We also used the decrease in the HR amplitude at 10 and 45 minutes to compare the efficiency between the groups. The results showed that the R group had a smaller decrease in the HR amplitude than the RD group at 10 minutes (MD: -6.03 beats/min; 95% CI: -10.97 to -1.09; P = .02) and 45 minutes (MD: -4.46 beats/min; 95% CI: -8.52 to -0.40; P = .03) (Table 6).
Table 5.
Comparison of the HR and BP at 10 and 45 minutes in the R and RD groups.
| Heterogeneity | ||||||
| Hemodynamics | R group (n) | RD group (n) | MD (95% CI) | P value | I2 (%) | P value |
| At 10 min | ||||||
| HR[12,20,24] | 95 | 95 | 8.73 [2.37, 15.08] | .007 | 81 | .005 |
| MAP[12,20] | 55 | 55 | 7.77 [5.13,10.41] | <.00001 | 0 | .4 |
| SBP[12,20] | 70 | 70 | 7.21 [3.17,11.24] | .0005 | 38 | .2 |
| DBP[12,20] | 70 | 70 | 3.26 [0.44,6.09] | .02 | 34 | .22 |
| At 45 min | ||||||
| HR[12,20,24] | 95 | 95 | 4.72 [2.65,6.79] | <.00001 | 47 | .15 |
| MAP[12,20] | 55 | 55 | 3.45 [0.83,6.06] | .01 | 0 | .7 |
| SBP[12,20] | 70 | 70 | 5.38 [2.03,8.74] | .002 | 0 | .99 |
| DBP[12,20] | 70 | 70 | 4.20 [1.55,6.85] | .002 | 0 | .55 |
DBP = diastolic blood pressure, HR = heart rate, MAP = mean arterial pressure, MD = mean difference, R group = ropivacaine alone, RD group = ropivacaine combined with dexmedetomidine, SBP = systolic blood pressure.
Table 6.
Comparison of the decreasing amplitudes of HR and BP at 10 and 45 minutes in the R and RD groups.
| Heterogeneity | ||||||
| Hemodynamics | R group (n) | RD group (n) | MD (95% CI) | P value | I2 (%) | P value |
| The decreasing amplitude at 10 min | ||||||
| HR [12,20,24] | 95 | 95 | −6.03 [−10.97, −1.09] | .02 | 68 | .04 |
| MAP[12,20] | 55 | 55 | −7.01 [−9.99, −4.03] | <.00001 | 31 | .23 |
| SBP[12,20] | 70 | 70 | −7.50 [−14.56, −0.43] | .04 | 54 | .14 |
| DBP[12,20] | 70 | 70 | −3.29 [−6.36, −0.23] | .04 | 100 | 0 |
| The decreasing amplitude at 45 min | ||||||
| HR[12,20,24] | 95 | 95 | −4.46 [−8.52, −0.40] | .03 | 62 | .07 |
| MAP[12,20] | 55 | 55 | −2.28 [−5.18, 0.61] | .12 | 0 | .98 |
| SBP[12,20] | 70 | 70 | −7.39 [−11.61, −3.18] | .0006 | 0 | .54 |
| DBP[12,20] | 70 | 70 | −5.25 [−8.24, −2.25] | .0006 | 100 | 0 |
DBP = diastolic blood pressure, HR = heart rate, MAP = mean arterial pressure, MD = mean difference, R = ropivacaine, RD = ropivacaine combined with dexmedetomidine, SBP = systolic blood pressure.
Two articles that included 110 patients were included to compare the MAP between the groups. The R group had a higher MAP than the RD group at 10 minutes (MD: 7.77 mm Hg; 95% CI: 5.13–10.41; P < .00001) and 45 minutes (MD: 3.45 mm Hg; 95% CI: 0.83–6.06; P = .01) (Table 5). The results also showed that the R group had a smaller decrease in the MAP amplitude than the RD group at 10 minutes (MD: -7.01 mm Hg; 95% CI: -9.99 to -4.03; P < .00001). However, no significant difference was found at 45 minutes (MD: -2.28 mm Hg; 95% CI: -5.18 to 0.61; P = .12) (Table 6).
Two articles that included 140 patients were used to compare the SBP and DBP between the groups. The R group had a higher SBP and DBP than the RD group at 10 minutes (SBP: MD: 7.21 mm Hg; 95% CI: 3.17–11.24; P = .0005), (DBP: MD: 3.26 mm Hg; 95% CI: 0.44–6.09; P = .02) and 45 minutes (SBP: MD: 5.38 mm Hg; 95% CI: 2.03–8.74; P = .002), (DBP: MD: 4.20 mm Hg; 95% CI: 1.55–6.85; P = .002) (Table 5). The decrease in the SBP amplitude in the R group at 10 and 45 minutes was smaller than that in the RD group (10 minutes: MD: -7.50 mm Hg; 95% CI: -14.56 to -0.43; P = .04), (45 minutes: MD: -7.39 mm Hg; 95% CI: -11.61 to -3.18; P = .0006). The decrease in the DBP amplitude at 10 and 45 minutes in the R group was smaller than that in the RD group (10 minutes: MD: -3.29 mm Hg; 95% CI: -6.36 to -0.23; P = .04) (45 minutes: MD: -5.25 mm Hg; 95% CI: -8.24 to -2.25; P = .0006) (Table 6). The curves of the MDs in HR (A), MAP (B), SBP (C), and DBP (D) between the R and RD group are presented in Figure 5.
Figure 5.
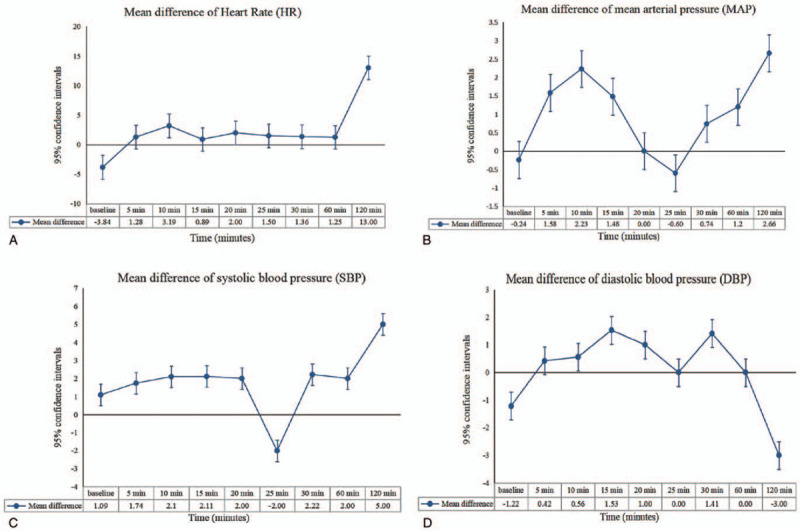
Curves of the mean differences in heart rate (A), mean arterial pressure (B), systolic blood pressure (C), and diastolic blood pressure (D) between the ropivacaine group and ropivacaine combined with dexmedetomidine.
3.5. Adverse effects
No significant difference in the total AEs was found between the R and RD groups (RR: 0.67; 95% CI: 0.22–2.05; P = .48) (Table 7). The following main AEs were reported: hypotension, nausea or vomiting, shivering, pruritus, bradycardia, dry mouth, and oversedation. The results showed that the R group had a higher incidence of shivering (RR: 2.82; 95% CI: 1.13–7.01; P = .03), and lower incidence of bradycardia (RR: 0.29; 95% CI: 0.13–0.65; P = .003) than the RD group. No significant differences in other AEs were found (Table 7).
Table 7.
Adverse effects associated in the R and RD groups.
| Heterogeneity | |||||||
| Adverse effects | R group (event/total) | RD group (event/total) | Total incidence rate (%) | RR (95% CI) | P value | I2 (%) | P value |
| Total adverse effects[12,13,21,22,24,25,26] | 60/197 | 93/196 | 38.9 | 0.67 [0.22,2.05] | .48 | 95 | <.00001 |
| Hypotension[13,21,24,25,26] | 16/130 | 43/130 | 22.7 | 0.36 [0.12,1.10] | .07 | 64 | .02 |
| Nausea or vomiting[12,13,21,24,26] | 21/145 | 18/145 | 13.4 | 1.17 [0.65,2.09] | .60 | 0 | .61 |
| Shivering[24,25,26] | 15/80 | 5/80 | 12.5 | 2.82 [1.13,7.01] | .03 | 0 | .69 |
| Pruritus[26] | 4/30 | 3/30 | 12.0 | 1.33 [0.33,5.45] | .69 | – | – |
| Bradycardia[13,22,24,25,26] | 6/127 | 23/126 | 11.5 | 0.29 [0.13,0.65] | .003 | 22 | .28 |
| Dry mouth[13,25] | 2/45 | 6/45 | 8.9 | 0.33 [0.07,1.56] | .16 | 0 | .53 |
| Oversedation[26] | 3/30 | 2/30 | 8.3 | 1.50 [0.27,8.34] | .64 | – | – |
R = ropivacaine, RD group = ropivacaine combined with dexmedetomidine group, RR = risk ratios.
3.6. Subgroup analysis
We performed subgroup analysis to evaluate whether the efficiency of the R and RD groups was consistent across subgroups. Young age (<10 years) (MD: -391.02 minutes; 95% CI: -441.97 to -340.08; P < .00001), 1.5 μg/kg dexmedetomidine (MD: -103.20 minutes; 95% CI: -137.92 to -68.48; P < .00001) and 2.0 μg/kg dexmedetomidine (MD: -360 minutes; 95% CI: -403.62 to -316.38; P < .00001) may prolonged the duration of anesthesia in the RD group (Table 8).
Table 8.
Subgroup analysis of the duration of anesthesia, time to rescue, and HR at 10 minutes.
| Duration of anesthesia | Time to rescue | HR at 10 min | ||||||||||
| Group | No. of studies | MD (95% CI) | P | I2 (%) | No. of studies | MD (95% CI) | P | I2 (%) | No. of studies | MD (95% CI) | P | I2 (%) |
| Total | 7 | −164.17 [−294.43, −33.91] | .01 | 100 | 4 | −119.01 [−254.47, 16.46] | .09 | 100 | 3 | 5.45 [0.01, 10.89] | .05 | 74 |
| Nation | ||||||||||||
| China | – | – | – | – | – | – | – | – | 1 | 8.28 [4.69, 11.87] | <.00001 | – |
| India | 7 | −164.17 [−294.43, −33.91] | .01 | 100 | 4 | −119.01 [−254.47, 16.46] | .09 | 100 | 2 | 3.66 [−5.24, 12.55] | .42 | 81 |
| Age (yr) | ||||||||||||
| ≤ 10 | 2 | −391.02 [−441.97, −340.08] | <.00001 | 82 | – | – | – | – | – | – | – | – |
| 10–30 | – | – | – | – | – | – | – | – | 1 | 8.28 [4.69, 11.87] | <.00001 | – |
| >30 | 5 | −75.71 [−164.47, 13.05] | .09 | 99 | 3 | −101.13 [−264.79, 62.52] | .23 | 100 | 1 | −0.98 [−6.76, 4.80] | .74 | – |
| NR | – | – | – | – | 1 | −172.60 [−187.11, −158.09] | <.00001 | – | 1 | 8.10 [2.94, 13.26] | .002 | – |
| Surgery | ||||||||||||
| Thoracotomy | 1 | 22.80 [−4.50, 50.10] | .1 | – | – | – | – | – | – | – | – | – |
| Lower abdominal and lower limbs surgeries | 4 | −190.49 [−254.60, −126.38] | <.00001 | 97 | 2 | −166.74 [−205.51, −127.97] | <.00001 | – | – | – | – | – |
| Infraumbilical surgeries | 1 | −412.80 [−417.96, −407.64] | <.00001 | – | 1 | −172.60 [−187.11, −158.09] | <.00001 | – | 1 | 8.10 [2.94, 13.26] | .002 | – |
| Lumbosacral spine surgeries | 1 | 13.00 [−10.98, 36.98] | .29 | – | 1 | 24.00 [19.42, 28.58] | <.00001 | – | 1 | −0.98 [−6.76, 4.80] | .74 | – |
| Labor | – | – | – | – | – | – | – | – | 1 | 8.28 [4.69, 11.87] | <.00001 | – |
| Does of dexmedetomidine | ||||||||||||
| 0.5 μg/kg | – | – | – | – | – | – | – | – | 1 | 8.28 [4.69, 11.87] | <.00001 | – |
| 1 μg/kg | 5 | −137.61 [−293.22, 17.99] | .08 | 100 | 3 | −101.13 [−264.79, 62.52] | .23 | 100 | 1 | −0.98 [−6.76, 4.80] | .74 | – |
| 1.5 μg/kg | 1 | −103.20 [−137.92, −68.48] | <.00001 | – | – | – | – | – | – | – | – | – |
| 2 μg/kg | 1 | −360.00 [−403.62, −316.38] | <.00001 | – | – | – | – | – | – | – | – | – |
| NR | – | – | – | – | 1 | −172.60 [−187.11, −158.09] | <.00001 | – | 1 | 8.10 [2.94, 13.26] | .002 | – |
HR = heart rate, MD = mean difference, NR = not reported.
3.7. Sensitivity analysis
The results showed significant heterogeneity in the duration of anesthesia and time to rescue. Sensitivity analysis revealed that each study was stable and reliable (Fig. 6).
Figure 6.
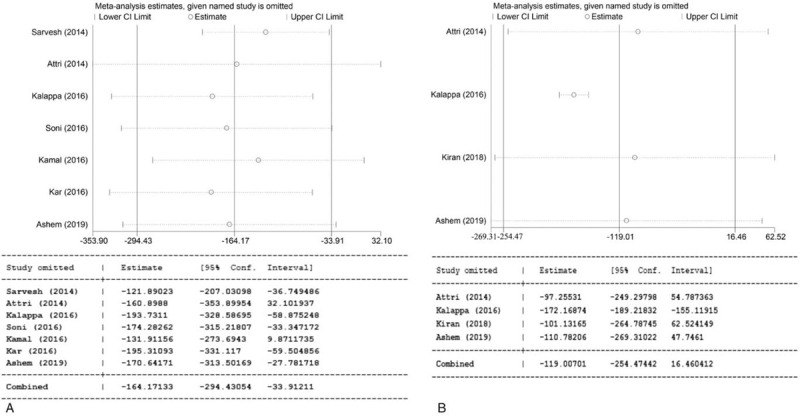
Sensitivity analysis of the duration of anesthesia (A) and time to rescue (B).
3.8. Publication bias
Publication bias analysis revealed the following publication biases: duration of anesthesia (Begg test P = 0.548; Egger test P = 0.204) (Fig. 7A); time to rescue (Begg test P = 1.000; Egger test P = 0.536) (Fig. 7B); HR at 10 minutes (Begg test P = 1.000; Egger test P = .905) (Fig. 7C).
Figure 7.
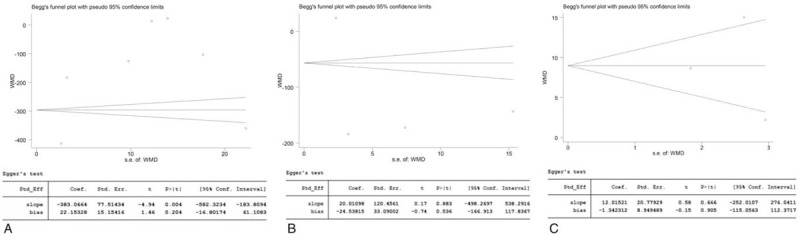
Publication bias of the duration of anesthesia (A), time to rescue (B), and heart rate at 10 minutes (C).
4. Discussion
Epidural anesthesia is a common and unique anesthesia technique that is used for intraoperative surgical anesthesia and postoperative analgesia for many surgeries. However, this technique has some disadvantages when used alone. Many studies reported that RD group was more efficient and associated with fewer AEs. However, comparisons of the efficiency and safety of R and RD for EA are lacking. The present study is the first meta-analysis to evaluate the efficiency and safety of R and RD. The RD group had a shorter time to onset of sensory and motor block and a longer duration of anesthesia than the R group. However, no significant difference in the time to rescue was found between the groups. The R group had more stable hemodynamics than the RD group with stable HR and MAP at 10 min. The R group had a lower incidence of bradycardia and a higher incidence of shivering than the RD group. Subgroup analyses revealed that young age (<10 years) and administration of 1.5 or 2.0 μg/kg dexmedetomidine may prolonged the duration of anesthesia in the RD group.
Analysis of the efficiency of anesthesia revealed that the RD group had a shorter time to onset of sensory and motor block and a longer duration of anesthesia than the R group. Attri et al reported that an RD group had a longer duration of sensory and motor block than an R group (sensory block: 375.20 ± 15.97 minutes in the R group; 535.18 ± 19.85 minutes in the RD group [P < .001]), (motor block: 259.80 ± 15.48 minutes in the R group; 385.92 ± 17.71 minutes in the RD group [P < .001]).[11] Kiran et al also suggested that the RD group had a shorter time to onset of sensory block than the R group (RD: 10.8 ± 2.7; R: 18.6 ± 4.4).[24] As mentioned in previous studies, dexmedetomidine inhibits the release of C-fiber neurotransmitters and via the hyperpolarization of postsynaptic dorsal horn neurons.[27–29] The complementary action of local anesthetics and 2 adrenergic agonists explains the profound analgesic properties.[30] This synergistic effect may explain the shorter time to onset of sensory and motor block in the RD group. To determine whether nationality, age, type of surgery, or dose of dexmedetomidine changed the results, we performed subgroup analysis. The advantage of RD was primarily reflected in young patients and at a dose of 1.5 or 2.0 μg/kg dexmedetomidine. These data suggest that the RD group has a longer duration of anesthesia and shorter time to onset of sensory and motor block.
To fully assess the efficiency of the R and RD groups, we also evaluated the HR, MAP, SBP, DBP, and decreases in their amplitudes at 10 and 45 minutes in the 2 groups. Our article found that the R group had more stable hemodynamics than the RD group with higher HR, MAP, SBP, and DBP at 10 and 45 minutes, smaller decreases in the HR, MAP, SBP, and DBP amplitudes at 10 minutes, and smaller decreases in the HR, SBP, and DBP amplitudes at 45 minutes. These results are similar to those of Krian et al[24] who reported that HR and MAP in the RD group were lower than the R group. Some studies reported that the use of dexmedetomidine as an adjuvant to ropivacaine for EA decreased the HR and BP.[31,32] However, the results of Kalappa et al and Kamal et al suggested no clinically significant hemodynamic changes in either groups.[20,21] Although these results were statistically significant, whether these decreases are a major clinical concern is controversial, especially when they can be controlled with atropine administration.
Differences in major AEs between the R and RD groups remain controversial and were assessed in present article. Both techniques were safe with a similar incidence rate of the total AEs. The major AEs were hypotension (22.7%), nausea or vomiting (13.4%), and shivering (12.5%). Kiran et al showed that the R group had milder AEs than the RD group.[24] However, Zhao et al and Kamal et al reported that both therapies were safe.[12,21] Our article suggests that the R group had a lower incidence of bradycardia and a higher incidence of shivering than the RD group, similar to findings in previous studies.[33–35] Dexmedetomidine can be combined with alpha-2 adrenoreceptors in the dorsal horn of the spinal cord to suppress the spontaneous firing rate of neurons and sympathetic tone.[36] These characteristics may explain the lower incidence of bradycardia and a higher incidence of shivering in the R group.
Our article has several limitations that call for caution in the interpretation of the results. First, this meta-analysis only included 11 articles with 673 patients, which limits the reliability of the results. Second, the different clinical centers, different periods, different devices, and different postoperative pain assessments increased the heterogeneity of the results. Although we used the random effects model to analyze data with high heterogeneity, we should interpret the results conservatively. Third, some of the included articles lacked information about the method of randomization and allocation concealment, which may decrease the quality of the articles. Fourth, the sample size in the subgroup analysis was underpowered, and these results should be interpreted cautiously.
Our study is different from that of Qian[37] for the following reasons. First, the purposes of these 2 studies were different: our study solved the problem of the necessity of the addition of dexmedetomidine, and Qian's study compared the efficiency of dexmedetomidine + ropivacaine and fentanyl + ropivacaine. Second, the data were retrieved from different databases. Qian's study also included the Wanfang and China National Knowledge Infrastructure (CNKI) databases. Third, we performed subgroup analysis to evaluate whether the efficiency of the R and RD groups was consistent across the subgroups, while Qian did not.
5. Conclusion
RD may be a more suitable choice for EA with better anesthetic function than R. However, RD showed more obvious interference effects on hemodynamics than the R. A higher incidence of bradycardia and a lower incidence of shivering were found in the RD group. Subgroup analyses suggested that a younger age (<10 years old) and the use of 1.5 or 2.0 μg/kg dexmedetomidine prolonged the duration of anesthesia in the RD group. However, more high-quality studies are needed to evaluate the differences between R and RD.
Acknowledgments
The authors thank professor Yiping, Wei, PHD, MD (The second affiliated hospital of Nanchang University) for his statistical advice and professor Jichun Liu, PhD, MD (The second affiliated hospital of Nanchang University) for his data collection. The authors also thank the support from National Natural Science Foundation of China (NSFC).
Author contributions
Conceptualization: Jiani Zhao, Fumou Deng, Wenxiong Zhang.
Data curation: Jiani Zhao, Chen Liao, Qian Wu, Li Wang, Fumou Deng, Wenxiong Zhang.
Formal analysis: Jiani Zhao, Qian Wu, Li Wang, Wenxiong Zhang.
Funding acquisition: Wenxiong Zhang.
Investigation: Chen Liao, Qian Wu, Fumou Deng, Wenxiong Zhang.
Methodology: Jiani Zhao, Chen Liao, Qian Wu, Li Wang, Fumou Deng, Wenxiong Zhang.
Project administration: Fumou Deng, Wenxiong Zhang.
Resources: Jiani Zhao, Qian Wu, Li Wang, Wenxiong Zhang.
Software: Jiani Zhao, Chen Liao, Qian Wu, Li Wang, Fumou Deng, Wenxiong Zhang.
Supervision: Fumou Deng.
Validation: Fumou Deng.
Visualization: Fumou Deng, Wenxiong Zhang.
Writing – original draft: Jiani Zhao, Wenxiong Zhang.
Writing – review & editing: Jiani Zhao, Fumou Deng, Wenxiong Zhang.
Footnotes
Abbreviations: AEs = adverse effects, BP = blood pressure, C = comparison, CI = confidence intervals, D = dexmedetomidine, DBP = diastolic blood pressure, EA = epidural anesthesia, HR = heart rate, I = Intervention, L = lumbar, MD = mean difference, O = outcomes, P = patients, R = ropivacaine group, RCT = random controlled trial, RD group = ropivacaine combined with dexmedetomidine, RR = risk ratios, SBP = systolic blood pressure.
How to cite this article: Zhao J, Liao C, Wu Q, Wang L, Deng F, Zhang W. Evaluation of ropivacaine combined with dexmedetomidine versus ropivacaine alone for epidural anesthesia: a meta-analysis. Medicine. 2021;100:14(e25272).
FD and WZ authors contributed equally to this work.
This article was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345) and Natural Science Foundation of Jiangxi Province (Grant number: 20181BAB215027).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Wink J, Veering BT, Aarts LPHJ, et al. Effects of thoracic epidural anesthesia on neuronal cardiac regulation and cardiac function. Anesthesiology 2019;130:472–91. [DOI] [PubMed] [Google Scholar]
- [2].Jain K, Sethi SK, Yadav SL, et al. Dexmedetomidine enhances the efficacy of 0.25% ropivacaine for postoperative analgesia in pediatric caudal epidurals. Anaesth Pain Intens Care 2018;22:199–206. [Google Scholar]
- [3].Elfawal SM, Abdelaal WA, Hosny MR. A comparative study of dexmedetomidine and fentanyl as adjuvants to levobupivacaine for caudal analgesia in children undergoing lower limb orthopedic surgery. Saudi J Anaesth 2016;10:423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anim-Somuah M, Smyth RM, Jones L. Epidural versus nonepidural or no analgesia in labour. Cochrane Database Syst Rev 2011;12:CD000331. [DOI] [PubMed] [Google Scholar]
- [5].Cucchiaro G, Adzick SN, Rose JB, et al. A comparison of epidural bupivacaine-fentanyl and bupivacaineclonidine in children undergoing the Nuss procedure. Anesth Analg 2006;103:322–7. [DOI] [PubMed] [Google Scholar]
- [6].Farmery AD, Wilson-MacDonald J. The analgesic effect of epidural clonidine after spinal surgery: a randomized placebo-controlled trial. Anesth Analg 2009;108:631–4. [DOI] [PubMed] [Google Scholar]
- [7].Yuan D, Liu Z, Kaindl J, et al. Activation of the α 2B adrenoceptor by the sedative sympatholytic dexmedetomidine. Nat Chem Biol 2020;16:507–12. [DOI] [PubMed] [Google Scholar]
- [8].Zhao Y, Feng X, Li B, et al. Dexmedetomidine protects against lipopolysaccharide-induced acute kidney injury by enhancing autophagy through inhibition of the PI3K/AKT/mTOR pathway. Front Pharmacol 2020;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kang RA, Jeong JS, Yoo JC, et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: a randomised controlled trial. Eur J Anaesthesiol 2019;36:360–8. [DOI] [PubMed] [Google Scholar]
- [10].Kang R, Jeong JS, Yoo JC, et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med 2018;43:488–95. [DOI] [PubMed] [Google Scholar]
- [11].Attri JP, Kaur S, Kaur G, et al. Comparative evaluation of ropivacaine versus dexmedetomidine and ropivacaine in epidural anesthesia in lower limb orthopedic surgeries. Saudi J Anaesth 2014;8:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao Y, Xin Y, Liu Y, et al. Effect of epidural dexmedetomidine combined with ropivacaine in labor analgesia: a randomized double-blinded controlled study. Clin J Pain 2017;33:319–24. [DOI] [PubMed] [Google Scholar]
- [13].Soni P. Comparative study for better adjuvant with ropivacaine in epidural anesthesia. Anesth Essays Res 2016;10:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shuster JJ. Review: Cochrane Handbook for systematic reviews for interventions, version 5.1.0, published 3/2011. Res Synth Methods 2011;2:126–30. [Google Scholar]
- [15].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:01–12. [DOI] [PubMed] [Google Scholar]
- [16].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Sarvesh B, Raj PG, Soumya MS, et al. Dexmedetomidine as an adjuvant to ropivacaine in ultrasound guided paediatric caudal epidural block: a randomised controlled study. J Clin Diagn Res 2019;13:10–3. [Google Scholar]
- [20].Kalappa S, Sridhara RB, Kumaraswamy S. Dexmedetomidine as an adjuvant to pre-emptive caudal epidural ropivacaine for lumbosacral spine surgeries. J Clin Diagn Res 2016;10:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamal M, Mohammed S, Meena S, et al. Efficacy of dexmedetomidine as an adjuvant to ropivacaine in pediatric caudal epidural block. Saudi J Anaesth 2016;10:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kar P, Durga P, Gopinath R. The effect of epidural dexmedetomidine on oxygenation and shunt fraction in patients undergoing thoracotomy and one lung ventilation: a randomized controlled study. J Anaesthesiol Clin Pharmacol 2016;32:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Joy R, Pujari VS, Chadalawada MV, et al. Epidural ropivacaine with dexmedetomidine reduces propofol requirement based on bispectral index in patients undergoing lower extremity and abdominal surgeries. Anesth Essays Res 2016;10:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kiran S, Jinjil K, Tandon U, et al. Evaluation of dexmedetomidine and fentanyl as additives to ropivacaine for epidural anesthesia and postoperative analgesia. J Anaesthesiol Clin Pharmacol 2018;34:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ashem J, Tikendrajit N, Takhelmayum H, et al. A comparative study of ropiavacine versus ropivacaine plus dexmedetomidine under epidural anesthesia in lower limb surgeries. J Med Soc 2019;33:20–7. [Google Scholar]
- [26].Tang Y, Yang M, Fu F, et al. Comparison of the ED50 of intrathecal hyperbaric ropivacaine coadministered with or without intrathecal dexmedetomidine for cesarean section: a prospective, double-blinded, randomized dose-response trial using up-down sequential allocation method. J Clin Anesth 2020;62:109725. [DOI] [PubMed] [Google Scholar]
- [27].Oxlund J, Clausen AH, Venø S, et al. A randomized trial of automated intermittent ropivacaine administration vs. continuous infusion in an interscalene catheter. Acta Anaesthesiol Scand 2018;62:85–93. [DOI] [PubMed] [Google Scholar]
- [28].Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of lowdose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand 2006;50:222–7. [DOI] [PubMed] [Google Scholar]
- [29].AI-Ghanem SM, Massad IM, AI-Mustafa MM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynaecological procedures: a double blind controlled study. Am J Appl Sci 2009;6:882–7. [Google Scholar]
- [30].Zheng SX, Zheng WW, Zhu TQ, et al. Continuing epidural analgesia during the second stage and ACOG definition of arrest of labor on maternal-fetal outcomes. Acta Anaesthesiol Scand 2020;64:1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Milligan KR, Convery PN, Weir P, et al. The effcacy and safety of epidural infusions of levobupivacaine with and without clonidine for postoperative pain relief in patients undergoing total hip replacement. Anesth Analg 2000;91:393–7. [DOI] [PubMed] [Google Scholar]
- [32].Mato M, Pérez A, Otero J, et al. Dexmedetomidine, a promising drug. Rev Esp Anestesiol Reanim 2002;49:407–20. [PubMed] [Google Scholar]
- [33].Nasseri K, Ghadami N, Nouri B. Effects of intrathecal dexmedetomidine on shivering after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Drug Des Devel Ther 2017;11:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He L, Xu JM, Liu SM, et al. Intrathecal dexmedetomidine alleviates shivering during cesarean delivery under spinal anesthesia. Biol Pharm Bull 2017;40:169–73. [DOI] [PubMed] [Google Scholar]
- [35].Naaz S, Bandey J, Ozair E, et al. Optimal dose of intrathecal dexmedetomidine in lower abdominal surgeries in average Indian adult. J Clin Diagn Res 2016;10:UC09–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang M, Wang L, Chen H. Efficacy of dexmedetomidine as a neuraxial adjuvant for elective cesarean sections: a meta-analysis of randomized trials. Int J Clin Exp Med 2018;11:8855–64. [Google Scholar]
- [37].Qian M, Gao F, Liu J, et al. Dexmedetomidine versus fentanyl as adjuvants to ropivacaine for epidural anaesthesia: a systematic review and meta-analysis. Int J Clin Pract 2020;00:e13772. [DOI] [PubMed] [Google Scholar]


