Abstract
Introduction:
Endometrial carcinoma (EC) is the most common gynecologic carcinoma in developed countries and accounts for nearly 5% of carcinoma cases and more than 2% of deaths due to female carcinomas worldwide. Because of this reported risk, it is very important to diagnose and stage it accurately. Therefore, we investigated the staging accuracy of EC with contrast-enhanced ultrasonography (CEUS). Due to a lack of studies on the use of CEUS in staging EC, we performed a systematic review and meta-analysis.
Method:
We searched PubMed, EMBASE, Cochrane Library, Scopus, Web of science, China National Knowledge Infrastructure (CNKI), and CBM for studies on CEUS in EC diagnosis. Our search keywords were “ultrasonic angiography,” “endometrial neoplasms,” and their synonyms. The studies were screened according to the inclusion and exclusion criteria, and 4 tabular data were extracted. Quality evaluation was performed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) scale. Statistical analysis was done with Stata version 15.1. A random effect model was selected to calculate the pooled sensitivity and specificity. The summary receiver operating characteristic (SROC) curve was obtained, and the area under the curve was calculated.
Result:
Fifteen studies with 685 patients were included in this quantitative synthesis. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (OR) of CEUS in the diagnosis of EC was 0.81 (95% confidence interval, .76–.85), .90 (.87–.92), 8 (5.8–11.1), .21 (.16–.28), and 38 (22–67), respectively. The area under the curve was 0.93 (.90–.95).
Conclusion:
CEUS has a high sensitivity and specificity in the diagnosis of EC. It can be considered as an effective and feasible method for EC staging.
Keywords: contrast-enhanced ultrasonography, diagnoses, endometrial carcinoma, meta-analysis
1. Introduction
Endometrial carcinoma (EC) is the most common female pelvic malignancy in the United States.[1] The incidence rate of EC is increasing globally, but the 5-year survival rate is decreasing.[2] Although EC is staged according to the International Federation of Gynecology and Obstetrics surgical system, an early and accurate diagnostic assessment of the disease status of gynecologic malignancies is important for optimal treatment planning and outcome prediction. Preoperative imaging can be used in the evaluation of the local extent and detection of distant metastatic disease, and therefore guiding the optimal treatment course.[1] Currently, diagnostic methods for staging patients with EC preoperatively include magnetic resonance imaging,[3–5] 3-dimensional transvaginal ultrasonography,[3] and positron emission tomography/computed tomography[5–9]; these have their advantages and disadvantages. According to Drudi et al[10] and Li et al,[11] contrast-enhanced ultrasonography (CEUS) has improved the diagnostic and staging accuracy of bladder tumors in the last 10 years. Many studies have shown that CEUS improves the accuracy of liver carcinoma diagnosis and is conducive for preoperative staging.[12–14] CEUS is also used in the diagnosis of gynecological tumors.[15–18] Meng et al[19] showed that CEUS increased the diagnosis rate of ovarian carcinoma.
There are reports on the effectiveness of CEUS in the staging of EC; however, they are few. The purpose of this meta-analysis was to evaluate the value of preoperative tumor staging in patients with EC using CEUS.
2. Methods
2.1. Search strategy
This study is a meta-analysis of previously published studies, thus no ethical approval or patient consent was necessary. We searched PubMed, EMBASE, The Cochrane Library, Scopus, and Web of science using the Cochrane Search Strategy for the identification of eligible studies. Our search keywords included, “ultrasonic angiography,” “endometrial neoplasms,” and their synonyms.(full electronic search strategy in PubMed: (((((((((((((((((((((((Endometrial Neoplasm [Title/Abstract]) OR Neoplasm, Endometrial [Title/Abstract]) OR Neoplasms, Endometrial [Title/Abstract]) OR EC [Title/Abstract]) OR Carcinoma, Endometrial [Title/Abstract]) OR Carcinomas, Endometrial [Title/Abstract]) OR ECs [Title/Abstract]) OR Endometrial Cancer[Title/Abstract]) OR Cancer, Endometrial [Title/Abstract]) OR Cancers, Endometrial [Title/Abstract]) OR Endometrial Cancers [Title/Abstract]) OR Endometrium Cancer [Title/Abstract]) OR Cancer, Endometrium [Title/Abstract]) OR Cancers, Endometrium [Title/Abstract]) OR Cancer of the Endometrium [Title/Abstract]) OR Carcinoma of Endometrium [Title/Abstract]) OR Endometrium Carcinoma [Title/Abstract]) OR Endometrium Carcinomas [Title/Abstract]) OR Cancer of Endometrium [Title/Abstract]) OR Endometrium Cancers [Title/Abstract])) OR “Endometrial Neoplasms” [Mesh])) AND (((((Ultrasonic angiography [Title/Abstract]) OR Contrast enhanced ultrasound [Title/Abstract]) OR Contrast-enhanced ultrasound [Title/Abstract]) OR phlebography [Title/Abstract]) OR Venography [Title/Abstract])) We also searched the China Biological Medicine Database (CBM-disc) and China National Knowledge Infrastructure (CNKI) Whole Article Database. We searched for articles published from inception till November 20, 2020.
2.2. Study eligibility
The eligibility criteria included studies in which:
-
1.
the accuracy (sensitivity and specificity) of CEUS in the diagnosis of EC was evaluated;
-
2.
a gold standard was adopted to treat and confirm EC, such as surgery and histopathology;
-
3.
the data allowed for construction of a 2 × 2 table for true-positives, false-positives, true-negatives, and false-negatives;
-
4.
the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)[20] score was ≥11; and
-
5.
no language restriction was used.
The exclusion criteria were:
-
1.
review articles, letters, comments, case reports, and articles that did not include raw data; and
-
2.
studies with the same sample.
2.3. Data extraction
Two investigators independently evaluated the potential studies, and a checklist was used to determine final eligibility. Any disagreement on study inclusion or exclusion was resolved by consensus. We collected the following features for eligible studies:
-
1.
first author's name,
-
2.
year of publication,
-
3.
age,
-
4.
number of subjects,
-
5.
country of origin,
-
6.
model of the equipment used,
-
7.
study design [prospective, retrospective, or unclear],
-
8.
contrast agent, and
-
9.
staging criteria.
2.4. Study quality
Quality assessment was performed using an assessment system with 14 items proposed by QUADAS.20 The items, phrased as questions, were scored as “yes,” “no,” or “unclear.” The quality assessment score ranged from 0 (observed minimum) to 14 (observed maximum). If the score was greater than or equal to 11, the quality of the study was considered relatively high. All disagreements were resolved by consensus.
2.5. Statistical analysis
The meta-analysis was performed using Stata 15 software. We calculated the pooled sensitivity and specificity, positive and negative likelihood ratios, and the diagnostic odds ratio (OR) of CEUS, along with the respective 95% confidence intervals (CIs), using a meta-analysis model. We constructed a hierarchical summary receiver operating characteristic (SROC) curve plotting sensitivity vs specificity and calculated the area under the curve. These data were pooled using a random effect model.
Cochran Q was used to determine the probability coefficients and the OR. A χ2 test for heterogeneity was conducted for each clinical feature. A P value ≤.05 was considered to denote statistically significant heterogeneity. Forrest plots were drawn to illustrate the distribution of the data points in each study in association with the summary pooled estimate. The heterogeneity of the sensitivities and specificities were tested using the likelihood ratio test. Publication bias was assessed using Deeks funnel plot asymmetry test. P > .05 suggested no significant publication bias.
3. Results
3.1. Study characteristics
We found 417 studies after our systematic review of literature. After duplicate records were removed, 311 studies remained. We then excluded 220 studies after screening titles and abstracts because the content was not relevant to the study question. Full-text versions of the remaining 91 articles were obtained and assessed for eligibility, after which 76 studies were excluded. Among the 76 excluded studies, 70 did not meet the inclusion criteria due to insufficient sensitivity, specificity, accuracy, or correlation values. Six studies with duplicated data were excluded. Thus, 15 studies with 685 patients were included in this meta–analysis. Their data were extracted.[21–35]A flow chart depicting the selection process is presented in Figure 1. After removing studies that were duplicates and did not meet the eligibility criteria, 15 studies with 685 patients were included in this study.
Figure 1.
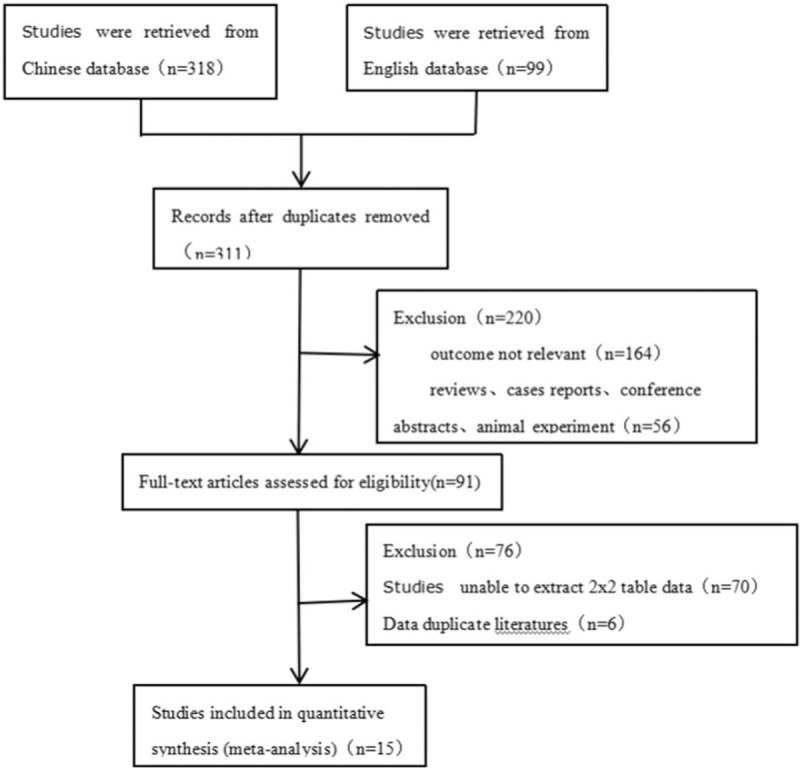
Flowchart. A flowchart of study screening and selection process.
All the studies were carried out in China. All the studies were case series (6 retrospective, 9 prospective). The microbubble contrast agent used in all the studies was SonoVue. In all the studies, EC was pathologically confirmed in addition to the diagnosis by CEUS. Characteristics of the included studies are presented in Table 1. An assessment of quality of the included studies using the QUADAS-2 scale is shown in Figure 2.
Table 1.
Summary of studies included in the present meta-analysis.
| Author | Year | Country | Mean age | Cases | Predesign | Model | Contrast agent | Blinded | Staging criteria | Quadas score | Refs | Final diagnosis | Symptoms |
| Sun et al | 2008 | China | 55.4 | 30 | Retro | IU22 | SonoVue | Single | FIGO | 11 | (21) | pathology | Irregular vaginal bleeding, discharge, Irregular menstruation |
| Song et al | 2009 | China | none | 35 | Prosp | IU22 | SonoVue | Single | FIGO | 11 | (22) | pathology | Irregular vaginal bleeding |
| Zhang et al | 2011 | China | none | 28 | Retro | IU22 | SonoVue | Single | FIGO 2009 | 11 | (23) | pathology | Irregular vaginal bleeding, discharge |
| Liu et al | 2011 | China | 56 | 37 | Retro | Aloka α10 | SonoVue | Double | FIGO 1988 | 12 | (24) | pathology | Irregular vaginal bleeding |
| Chen et al | 2011 | China | 55.7 | 28 | Prosp | Mylab90 | SonoVue | Single | FIGO 2000 | 11 | (25) | pathology | Irregular vaginal bleeding, Postmenopausal hemorrhage |
| Pei et al | 2011 | China | 52.6 | 48 | Prosp | Acuson Sequoia 512 | SonoVue | Single | FIGO 2009 | 11 | (26) | pathology | Irregular vaginal bleeding |
| Xie et al | 2012 | China | 56 | 37 | Prosp | Aloka α-10 | SonoVue | Single | FIGO 1988 | 11 | (27) | pathology | Irregular vaginal bleeding |
| Wang et al | 2013 | China | 56 | 31 | Prosp | GE Logiq 9 | SonoVue | Single | FIGO 2009 | 11 | (28) | pathology | Irregular vaginal bleeding |
| Xu et al | 2013 | China | 42 | 47 | Prosp | Acuson Sequoia 512 | SonoVue | Single | FIGO 2009 | 11 | (29) | pathology | Irregular vaginal bleeding |
| Ding et al | 2013 | China | 55 | 40 | Retro | MyLabTwice | SonoVue | Double | FIGO 2009 | 12 | (30) | pathology | Irregular vaginal bleeding, discharge, Irregular menstruation |
| Zhou et al | 2013 | China | 52 | 40 | Prosp | Technos MPX DU8 | SonoVue | Single | FIGO 1988 | 11 | (31) | pathology | Irregular vaginal bleeding, discharge |
| Huang et al | 2016 | China | 49.8 | 80 | Prosp | Aloka SSD-ALPHA10 | SonoVue | Single | FIGO 2009 | 11 | (32) | pathology | Irregular vaginal bleeding, discharge |
| Yi et al | 2015 | China | 55.4 | 60 | Retro | GE Logiq E9 | SonoVue | Single | FIGO 2009 | 11 | (33) | pathology | Irregular vaginal bleeding, Irregular menstruation |
| Zhang et al | 2018 | China | 54.58 | 82 | Retro | Acuson Sequoia 512 | SonoVue | Single | FIGO 2013 | 11 | (34) | pathology | Irregular vaginal bleeding, Irregular menstruation |
| Mao et al | 2019 | China | 55 | 62 | Prosp | GE Logiq E9 | SonoVue | Single | FIGO 2009 | 11 | (35) | pathology | Irregular vaginal bleeding |
Retro = retrospective study, Prosp = prospective study.
Figure 2.
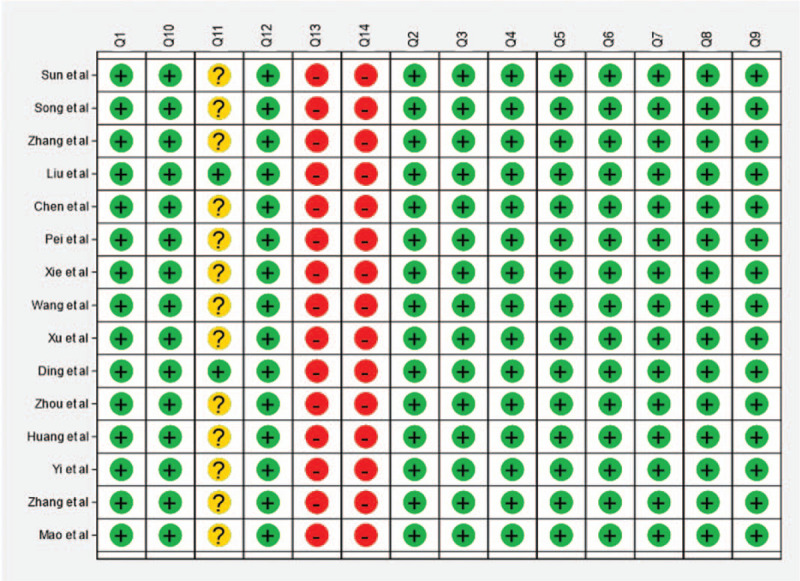
Quality assessment. Quality assessment by STATA 15.1 for the 15 studies included in the meta-analysis.
3.2. Data synthesis
As presented in Figure 3A and 3B, the pooled sensitivity of CEUS in the diagnosis of EC was .81 (95% CI, .76–.85), and the pooled specificity was .90 (95% CI, .87–.92). The positive likelihood ratio of CEUS was 8.0 (95% CI, 5.8–11.1) and the negative likelihood ratio was .21 (95% CI, .16–.28). The diagnostic OR was 38 (95% CI, 22–67). The area under the curve was .93 (Fig. 4).
Figure 3.
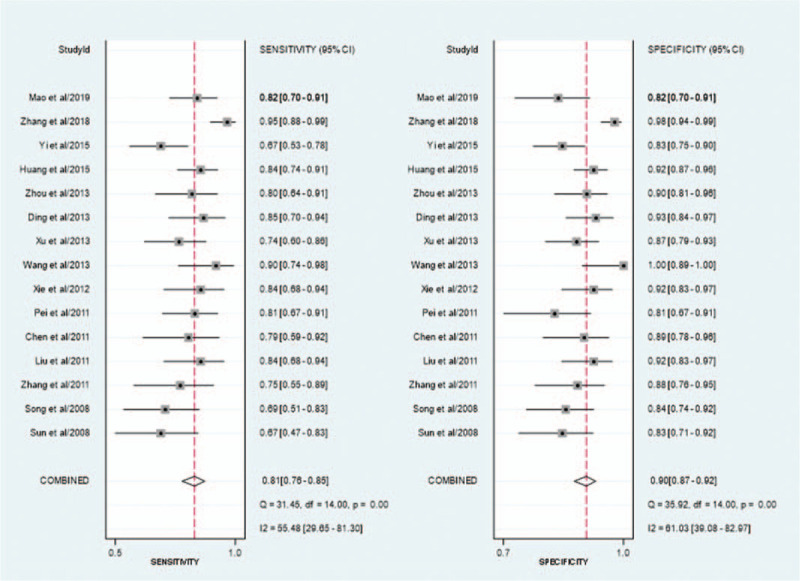
Forest plot. (A) Forest plot of study–specific estimates of sensitivity (ratio with 95% confidence interval) of CEUS in the diagnosis of EC; (B) Forest plot of study–specific estimates of specificity (ratio with 95% confidence interval) of CEUS in the diagnosis of EC, every plot represents a related study, global results (pooled sensitivity/specificity) are presented at the bottom. The horizontal bars are the CI 95% ranges, and the squares within the bars are the estimate values. CEUS, contrast– enhanced ultrasound; EC, endometrial carcinoma; CI, confidence interval.
Figure 4.
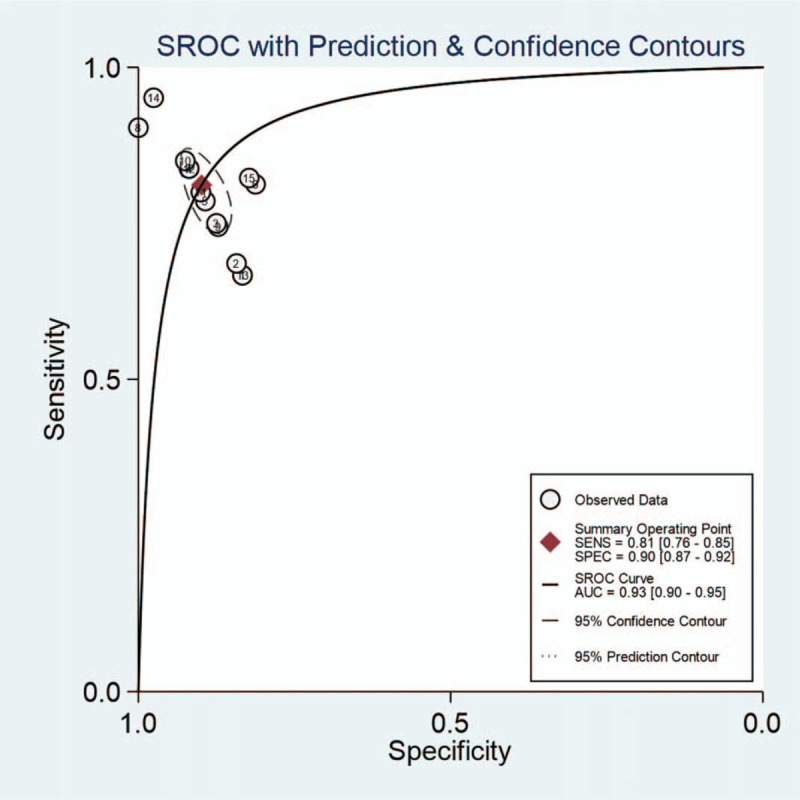
SROC curve. SROC curve of contrast-enhanced ultrasound in the diagnosis of endometrial carcinoma. Numbers represent the studies in Table I in the order that they are presented. SROC, summary receiver-operating characteristics; AUC, area under curve; SENS, sensitivity; SPEC, specificity.
The Deeks funnel plot in Figure 5 shows that the studies were distributed symmetrically (P value =.21).
Figure 5.
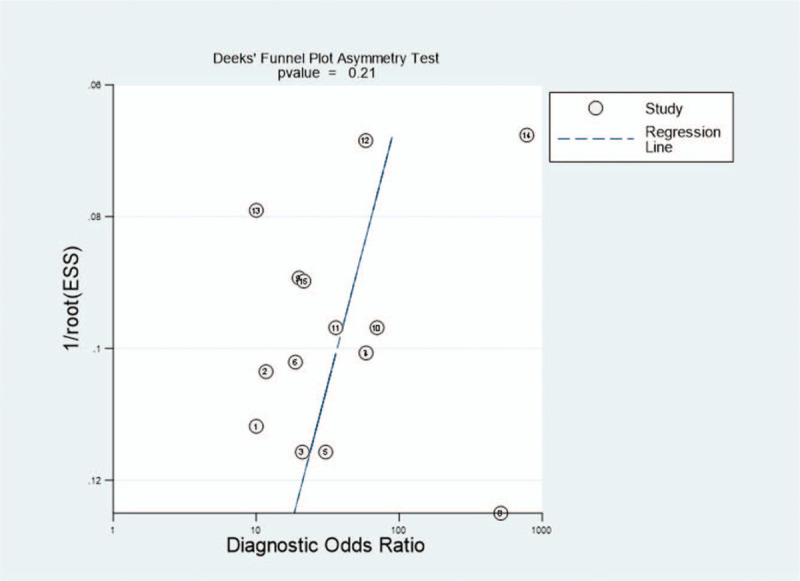
Deek funnel plot asymmetry test. Deek funnel plot asymmetry test for publication bias for the 15 studies included in the meta-analysis. Numbers represent the studies in Table I in the order that they are presented. ESS, effective sample size.
3.3. Subgroup analyses
We conducted a subgroup analysis of the predesign and staging standard of the studies to explore whether they were sources of heterogeneity. Significant differences (I-squared = 82.1%, P < .001) were found in the retrospective studies (Fig. 6). The test for in-group differences (I-squared = 24.4%, P = .23) was not statistically significant in prospective studies. In total, the test for between-group differences (I-squared = 61.7%, P = .001) was statistically significant. There were no differences in the studies with the same staging standard (Fig. 7). However, the test for among-group differences (I-squared = 58.8%, P = .002) were statistically significant in different groups with different staging standards. Figures 8 and 9
Figure 6.
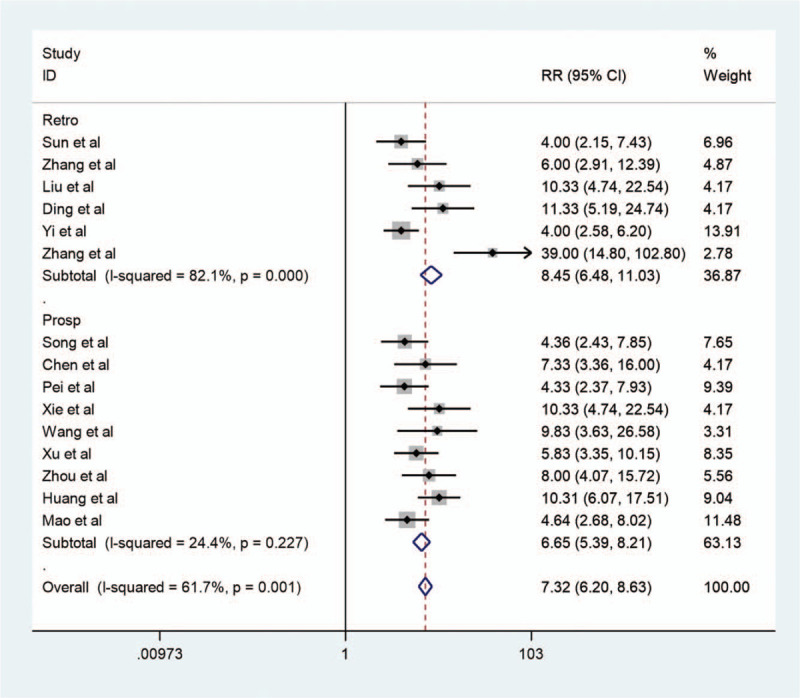
Forest map of subgroup analysis1. Forest map of subgroup analysis for contrast-enhanced ultrasound in the diagnosis of endometrial carcinoma. Retro = retrospective study; Prosp = prospective study. Black diamonds indicate the weight of each study; blue diamonds indicate the overall result.
Figure 7.
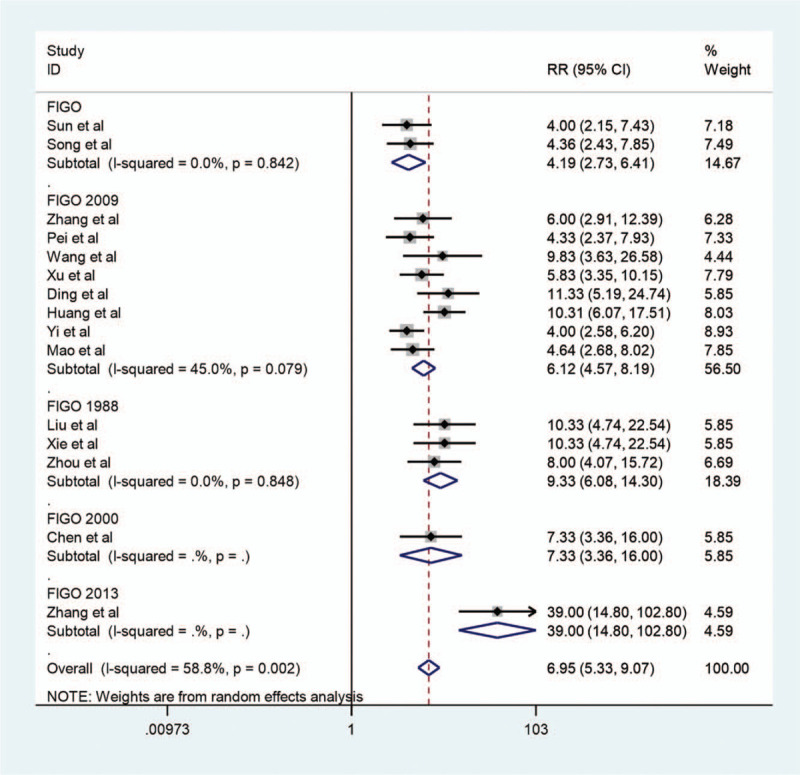
Forest map of subgroup analysis2. Forest map of subgroup analysis for contrast-enhanced ultrasound in the diagnosis of endometrial carcinoma. Black diamonds indicate the weight of each study; blue diamonds indicate the overall result. FIGO, the International Federation of Gynecology and Obstetrics.
Figure 8.
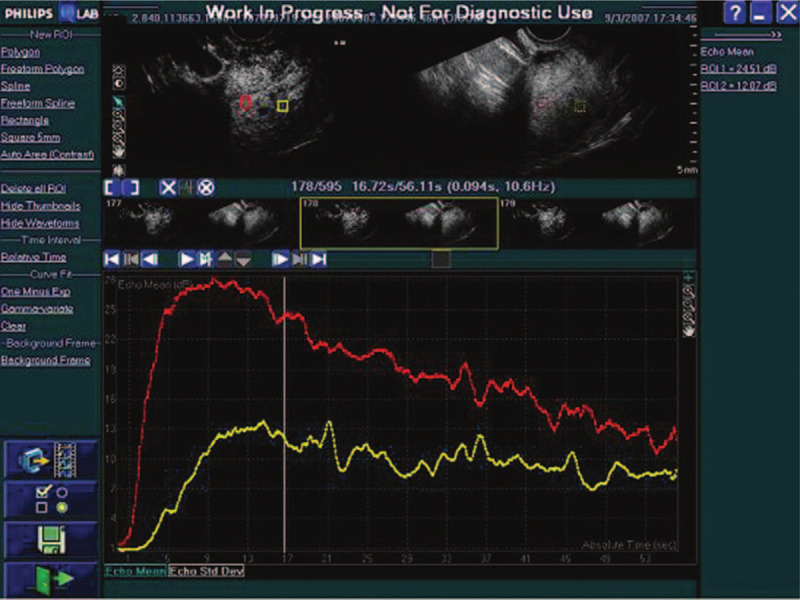
Time–intensity curves. Time–intensity curves derived from contrast-enhanced ultrasonography of tumor and normal myometrium (red: tumor, yellow: normal myometrium).
Figure 9.
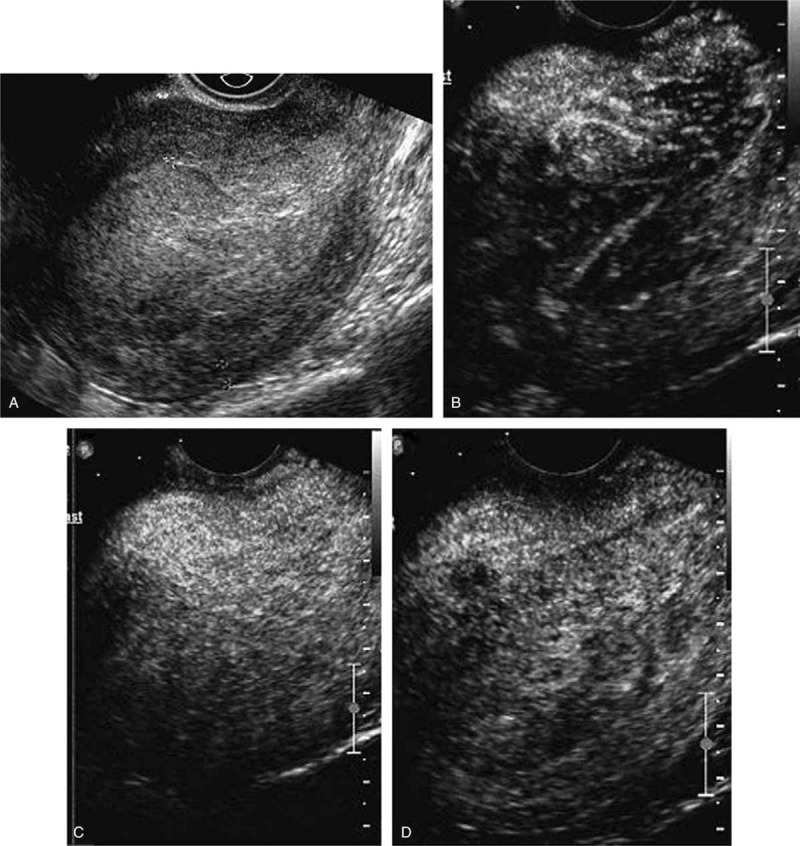
Images of a stage IA endometrial carcinoma with bulky tumor. (A) Image before the injection of the bolus showed marked enlargement of endometrium. (B) Image at 14 seconds after administration showed asymmetrically enhanced tumor. (C) Image at 18 seconds showed the maximal concentration of contrast agent in the tumor. (D) Image at 27 seconds showed that tumor washed out earlier than normal myometrium, thus formed a clear peritumoral halo.
4. Discussion
EC is the most common gynecologic tumor in developed countries, and the incidence rate is increasing annually.[36] Since the introduction of CEUS, it has gained an important role in the diagnosis and management of abdominal and pelvic diseases. Compared to conventional ultrasonography, CEUS can improve lesion detection rates and success rates of interventional procedures. Additionally, CEUS enables the interventionist to assess the dynamic enhancement of different tissues and lesions, without the adverse effects of contrast-enhanced computed tomography, such as exposure to ionizing radiation and nephrotoxicity from iodinated contrast material.[37] Fifteen studies were included in this meta-analysis. The sensitivity and specificity of CEUS were 81% and 90%, respectively. This showed that CEUS has a high accuracy in the diagnosis of EC. We reduced all bias types. We did a comprehensive literature search and detailed data extraction to avoid selection bias. All the included studies had a QUADAS score ≥11, indicating that they had a high methodological quality, and the possibility of bias was low. Literature retrieval, data extraction, and study quality assessment were conducted by 2 researchers independently. Deeks funnel chart showed a P value of .21, indicating that there was no significant publication bias in this study. When the area under the SROC curve is ≤.5, there is no diagnostic value. When the area under the SROC curve is ≤.7, the diagnostic value is low. When the area under the SROC curve is between .7 and .9, the diagnostic value is high. When the area under the SROC curve is >.9, the diagnostic value is very high. In this study, the area under the curve was 0.93, which shows that the diagnostic accuracy of CEUS is very high.
There are limitations in our research. First, the editions of the adopted staging standards of the International Union of Gynecology and Obstetrics varied with the research institutes for example, 2009,[28–30] 1988,[24,27] and 2000.[25] This was a source of heterogeneity. Second, the use of different types of machines and operators could lead to measurement bias, resulting in an underestimation or overestimation of the diagnostic accuracy of EC. Third, all the included studies were Chinese studies; this could be because CEUS is widely used in the staging and diagnosis of EC in China, but not in other countries. Therefore, further research is needed to evaluate the value of CEUS in the diagnosis of EC.
5. Conclusion
Our results show that CEUS has a high clinical value in the staging of EC. CEUS provides a simple and low-cost method for the diagnosis of EC. Early and accurate diagnosis and evaluation is very important for the selection of the best treatment plan and outcome prediction. Accurate preoperative staging is helpful for preoperative preparation and selection of the best operation method. Therefore, CEUS is worth popularizing for the staging of EC.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Data curation: Xiao fen Wu, Qiaohong Zhang.
Formal analysis: Xiao fen Wu.
Investigation: Xiaozhen Tong, Qiaohong Zhang.
Methodology: Xiaozhen Tong.
Project administration: Xiaozhen Tong.
Software: Xiao fen Wu, Qiaohong Zhang.
Supervision: Xiaozhen Tong.
Validation: Xiaozhen Tong.
Writing – original draft: Xiaozhen Tong.
Writing – review & editing: Xiaozhen Tong.
Footnotes
Abbreviations: CBM = China Biological Medicine Database, CEUS = contrast-enhanced ultrasonography, CI = confidence interval, CNKI = China National Knowledge Infrastructure, EC = endometrial carcinoma, OR = odds ratio, QUADAS = quality assessment of diagnostic accuracy studies, SROC = summary receiver operating characteristic.
How to cite this article: Tong X, Wu X, Zhang Q. Value of preoperative staging of endometrial carcinoma with contrast-enhanced ultrasonography: a PRISMA compliant meta-analysis. Medicine. 2021;100:14(e25434).
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Faria SC, Devine CE, Rao B, et al. Imaging and staging of endometrial cancer. Semin Ultrasound CT MR 2019;40:287–94. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:05–29. [DOI] [PubMed] [Google Scholar]
- [3].Yang T, Tian S, Li Y, et al. Magnetic resonance imaging (MRI) and three-dimensional transvaginal ultrasonography scanning for preoperative assessment of high risk in women with endometrial cancer. Med Sci Monit 2019;25:2024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coussoou C, Laigle-Quérat V, Loussouarn D, et al. Magnetic resonance imaging for local preoperative staging in endometrial cancer: Nantes local experience. Gynecol Obstet Fertil Senol 2020;48:374–83. [DOI] [PubMed] [Google Scholar]
- [5].Tsuyoshi H, Tsujikawa T, Yamada S, et al. Diagnostic value of 18F-FDG PET/MRI for staging in patients with endometrial cancer. Cancer Imaging 2020;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crivellaro C, Landoni C, Elisei F, et al. Combining positron emission tomography/computed tomography, radiomics, and sentinel lymph node mapping for nodal staging of endometrial cancer patients. Int J Gynecol Cancer 2020;30:378–82. [DOI] [PubMed] [Google Scholar]
- [7].Takagi H, Sasagawa T, Shibata T, et al. Association between 18F-fluorodeoxyglucose-PET/CT and histological grade of uterine endometrial carcinoma. Taiwan J Obstet Gynecol 2018;57:283–8. [DOI] [PubMed] [Google Scholar]
- [8].Kulkarni R, Bhat RA, Dhakharia V, et al. Role of positron emission tomography/computed tomography in preoperative assessment of carcinoma endometrium-a retrospective analysis. Indian J Surg Oncol 2019;10:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mapelli P, Bergamini A, Fallanca F, et al. Prognostic role of FDG PET-derived parameters in preoperative staging of endometrial cancer. Rev Esp Med Nucl Imagen Mol 2019;38:03–9. [DOI] [PubMed] [Google Scholar]
- [10].Drudi FM, Cantisani V, Liberatore M, et al. Role of low-mechanical index CEUS in the differentiation between low and high grade bladder carcinoma: a pilot study. Ultraschall Med 2010;31:589–95. [DOI] [PubMed] [Google Scholar]
- [11].Li Q, Tang J. Role of low-mechanical index CEUS in the differentiation between low and high grade bladder carcinoma: a pilot study. Ultraschall Med 2012;33:87–8. [DOI] [PubMed] [Google Scholar]
- [12].Sporea I, Badea R, Popescu A, et al. Contrast-enhanced ultrasound (CEUS) for the evaluation of focal liver lesions - a prospective multicenter study of its usefulness in clinical practice. Ultraschall Med 2014;35:259–66. [DOI] [PubMed] [Google Scholar]
- [13].Numata K, Fukuda H, Nihonmatsu H, et al. Use of vessel patterns on contrast-enhanced ultrasonography using a perflubutane-based contrast agent for the differential diagnosis of regenerative nodules from early hepatocellular carcinoma or high-grade dysplastic nodules in patients with chronic liver disease. Abdom Imaging 2015;40:2372–83. [DOI] [PubMed] [Google Scholar]
- [14].El Kaffas A, Sigrist RMS, Fisher G, et al. Quantitative three-dimensional dynamic contrast-enhanced ultrasound imaging: first-in-human pilot study in patients with liver metastases. Theranostics 2017;7:3745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu C, Tang Y, Zhao Y, et al. Use of contrast-enhanced ultrasound in evaluating the efficacy and application value of microwave ablation for adenomyosis. J Cancer Res Ther 2020;16:365–71. [DOI] [PubMed] [Google Scholar]
- [16].Zheng W, Chen K, Peng C, et al. Contrast-enhanced ultrasonography vs MRI for evaluation of local invasion by cervical cancer. Br J Radiol 2018;91:20170858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pálsdóttir K, Epstein E. A pilot study on diagnostic performance of contrast-enhanced ultrasonography for detection of early cervical cancer. Ultrasound Med Biol 2018;44:1664–71. [DOI] [PubMed] [Google Scholar]
- [18].Lahtinen O, Eloranta M, Anttila M, et al. Preoperative sentinel lymph node localization in vulvar cancer: preliminary experience with inguinal intradermal contrast-enhanced ultrasound. Eur Radiol 2018;28:2089–95. [DOI] [PubMed] [Google Scholar]
- [19].Meng W, Ying W, Qichao Z, et al. Clinical value of combining transvaginal contrast-enhanced ultrasonography with serum human epididymisprotein-4 and the resistance index for early-stage epithelial ovarian cancer. Saudi Med J 2017;38:592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [21].Sun ZJ, Yang JX, Shen K, et al. Contrast-enhanced ultrasound in the evaluation of myometrial invasion in endometrial carcinoma. J Reprod Med 2008;17:187–91. [Google Scholar]
- [22].Song Y, Yang J, Liu Z, et al. Preoperative evaluation of endometrial carcinoma by contrast-enhanced ultrasonography. BJOG 2009;116:294–8. [DOI] [PubMed] [Google Scholar]
- [23].Zhang XZ, Zhao HY, Peng M, et al. Application of contrast-enhanced ultrasound in the myometrial invasion of endometrial carcinoma. J Bengbu Med Col 2011;36:285–7. [Google Scholar]
- [24].Liu CY, Wang XF, Xie Q, et al. Diagnostic value of contrast-enhanced ultrasound for myometrial invasion of stage I endometrial carcinoma. Chin J Med Imaging Technol 2011;27:1443–6. [Google Scholar]
- [25].Chen LX, Dai KK, Hu YP, et al. Clinical value of contrast-enhanced TVS in the assessment of invasion depth of endometrial cancer. Chin J Prim Med Pharm 2011;18:1743–4. [Google Scholar]
- [26].Pei XQ, Xie YJ, Li LH, et al. Is contrast enhanced ultrasonography helpful to assessment of myometrial invasion in endometrial carcinoma in stage I. Chin J Ultrasonogr 2011;20:598–601. [Google Scholar]
- [27].Xie Q, Lei XY, Wu XP, et al. The study for myometrial invasion of stage I endometrial carcinoma by contrast-enhanced ultrasound and diffusion-weighted magnetic resonance imaging. Chin J Med Ultrasound 2012;9:232–6. [Google Scholar]
- [28].Wang YL, Yang CX, Zhang HP, et al. Preoperative evaluation of myometrial invasion of endometrial carcinoma by transvaginal contrast-enhanced ultrasound. Chin J Clin 2013;7:11030–2. [Google Scholar]
- [29].Xu Y, Liu Y, Guo CL, et al. Application value of transvaginal contrast-enhanced ultrasound in the diagnosis of the stage I endometrial carcinoma. Chin J Clin 2013;7:5928–32. [Google Scholar]
- [30].Ding Y, Guo YZ, Guan L, et al. Application value of contrast-enhanced ultrasound for stage of endometrial carcinoma. J Chongqing Med 2013;42:2103–6. [Google Scholar]
- [31].Zhou XQ, Zhai YX, Zheng CY, et al. Clinical research of contrast -enhanced ultrasound in the myometrial invasion of endometrial carcinoma. Chin J Med peop Heal 2013;25:05–6. 85. [Google Scholar]
- [32].Huang ZJ, Kan N. Comparative study of the effect of application of contrast enhanced ultrasound and transvaginal color Doppler sonography in diagnosis of stage I endometrial carcinoma. Chin Med equip 2016;31:48–50. 61. [Google Scholar]
- [33].Yilinuer S, Wuliyati S. Application of contrast-enhanced ultrasound in diagnosis of myometrial invasion in endometrial carcinoma. Med Inform 2015;28:271–2. [Google Scholar]
- [34].Zhang W. Effect of CEUS on preoperative diagnostic accuracy of patients with stage I endometrial carcinoma. Mod Med Imaging 2018;27:1619–20. [Google Scholar]
- [35].Mao H, Hong L, Jia ZY, et al. Value of transvaginal sonography contrast-enhanced ultrasonography in preoperative staging for patients with endometrial carcinoma stage I. Chin J Oncol 2019;25:71–4. [Google Scholar]
- [36].Felix AS, Yang HP, Bell DW, et al. Epidemiology of endometrial carcinoma: etiologic importance of hormonal and metabolic influences. Adv Exp Med Biol 2017;943:03–46. [DOI] [PubMed] [Google Scholar]
- [37].Kessner R, Nakamoto DA, Kondray V, et al. Contrast-enhanced ultrasound guidance for interventional procedures. J Ultrasound Med 2019;38:2541–57. [DOI] [PubMed] [Google Scholar]


