Abstract
Persistent disease activity is associated with a poor prognosis in patients with inflammatory bowel disease (IBD). This study aims to explore the accuracy of the highly sensitive C-reactive protein/albumin ratio (CAR) in determining IBD activity.
The clinical data of 231 IBD patients treated at Peking Union Medical College Hospital from 2012 to 2018 were analyzed retrospectively. The patients were classified as having active disease or remission according to the Crohn disease activity index scores for patients with Crohn disease (CD) and partial Mayo scores for patients with ulcerative colitis (UC).
This study included 231 IBD patients (137 CD and 94 UC). From these groups, 182 patients had active disease, while 49 patients were in remission. The platelet counts, erythrocyte sedimentation rates, high-sensitivity C-reactive protein levels, and CAR scores were significantly higher, while hemoglobin levels, ALB, and body mass indexes were significantly lower in patients with active disease (P < 0.01). The hsCRP, CAR, and ALB significantly correlated with disease activity for both CD and UC (P < 0.001). The area under the curve (AUC) of CAR was highest among the laboratory indexes at 0.829, and the AUC of CAR in the UC patients was larger than that of the CD patients. Also, CAR with cutoff value of 0.06 displayed the highest sensitivity among the indexes for IBD activity at 83.05%.
CAR is a useful biomarker for identifying disease activity in patients with CD and UC. Higher CAR levels are indicative of increased IBD activity. CAR may be more valuable in UC than that in CD for assessing the degree of IBD activity.
Keywords: albumin, C-reactive protein, inflammatory bowel disease, protein/albumin ratio, ulcerative colitis
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease affecting the gastrointestinal tract that can be classified as Crohn disease (CD) or ulcerative colitis (UC). IBD has a disease course that is characterized by periods of remission, followed by disease relapses. However, the poor control of ongoing inflammation and IBD has been associated with poor outcomes in patients with CD and UC.[1] Several biomarkers and endoscopic procedures have been used in the clinic to monitor IBD disease activity. Compared with biomarker assays, endoscopy is considered as the gold standard for assessing IBD activity. However, endoscopy is an invasive technique that is limited by low patient compliance. Therefore, some other options have been investigated. For example, positive fecal calprotectin has been shown to strongly correlate with active IBD and may be useful in assessing IBD disease activity.[2] Currently, fecal calprotectin is uncommonly used in routine practice due to the high cost and time commitments. Currently, the most commonly used biomarkers for assessing IBD are the erythrocyte sedimentation rate (ESR) and C-reaction protein (CRP), yet the levels of these two biomarkers are affected by other biological factors, such as bacterial and viral infections and other diseases. Therefore, there is an urgent need for novel non-invasive and effective biological biomarkers for the accurate assessment of IBD activity in the clinic.
The C-reactive protein/albumin ratio (CAR) is indicative of the balance between inflammation and nutritional status, making it an excellent marker for assessing disease activity in patients. Recently, Wu et al[3] conducted a meta-analysis to explore the association between CAR and survival outcomes in patients with solid tumors. They found that elevated serum CAR levels were predictive of decreased overall survival in patients with cancer. In another study, Qin et al[4] studied the laboratory indexes of 100 patients with CD, and identified CAR as a useful biomarker for assessing CD activity. To the best of our knowledge, there have been no reports on the accuracy of CAR for the assessment of UC activity, specifically in Chinese patients. This study aimed to explore the role of CAR in the assessment of disease activity in patients with CD and UC. In addition, we compared CAR with other potential biomarkers to determine if CAR was the most effective biomarker for the assessment of IBD activity.
2. Methods
2.1. Study population
This is a single-center retrospective study of patients with IBD treated from January 2012 to May 2018 at Peking Union Medical College Hospital in Beijing, China. The diagnosis of UC and CD was based on the standard clinical, endoscopic, and histologic criteria. The exclusion criteria were concurrent infections, liver cirrhosis, hematological diseases, heart failure, malignancies, autoimmune diseases, or congenital or acquired immunodeficiencies.
The study protocol was approved by the Ethical Committee of Peking Union Medical College Hospital (Date of Approval: Date of Approval: 14-June-2019). All patients provided written informed consent and the study conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Data collection
Demographic data and laboratory test results were collected from a prospectively maintained database. Demographics were recorded, included sex, age, smoking status, duration, and body mass index (BMI) during the admission process. Laboratory tests were also performed upon admission and included the high-sensitivity C-reactive protein (hsCRP), ESR, platelet count (PLT), serum albumin (ALB), and hemoglobin (HGB). The CD activity indexes (CDAI) and partial Mayo scores were also recorded during admission.
The immunity transmission turbidity method was used to measure serum hsCRP levels. ESR was determined by the ESR Westergren method. Serum ALB was determined by the Bromocresol Green method. PLT was measured by the laser scattering method, while HGB was measured using the cyanmethemoglobin colorimetry method.
For CD patients, disease activity was defined according to the CDAI score. Patients were further divided into the active CD (CDAI ≥150) and remission CD (CDAI <150) groups. For the UC patients, those with partial Mayo score ≤2 with no individual sub-scores of <1 were included in the remission group, while the others were included in the active disease group.
2.3. Statistical analysis
Results were presented as the medians and interquartile ranges (IQR) for non-parametric data, and means ± standard deviations (SD) for parametric data. Categorical data were shown as percentages or proportions. For nonparametric comparisons, the Wilcoxon matched-pairs signed-rank test and the Mann–Whitney test were used to analyze paired and non-paired data, respectively. The Chi-square test and Fisher exact test were applied to test for differences among categorical variables. The Spearman correlation coefficient was used to assess the association between biomarkers and disease activity. The receiver operating characteristic (ROC) curve analysis was performed to identify optimal cutoff values for biomarkers. All statistical analyses were performed using the software Statistical Package for Social Sciences (SPSS) Version 22.0 (IBM, Chicago, IL, USA). ROC curve plotting was performed with the Medcalc software, and the Delong method was used to compare AUCs between the two markers. P-values <0.05 were considered as statistically significant.
3. Results
3.1. Patient demographics and laboratory tests
This study included 231 patients with IBD, which included 137 CD and 94 UC patients. There were 139 men and 92 women with a male-to-female ratio of 1.7:1. The median patient age was 38 years old (IQR 26.00, 57.00) with a range of 15 to 85 years. The median duration of disease illness was 5.04 years (IQR 1.67, 9.79).
There were 182 patients in the active disease group and 49 patients in the remission group. Among the patients with CD, 109 had active disease and 28 were in remission. Among the patients with UC, 73 had active disease and 21 were in remission. There were no significant differences in age, male-to-female ratio, the proportion of smokers, or the duration of illness between the active disease and remission groups (P > 0.05).
3.2. Association between biomarkers and CD and UC disease activity
The PLT count, ESR, hsCRP levels, and CAR were significantly higher in patients with active disease when compared with patients in the remission group (P < 0.01), while HGB, ALB, and BMI levels were significantly lower in patients with active disease when compared with those in remission (P < 0.01), as shown in Tables 1–3.
Table 1.
The differences between active and remission group in IBD patients.
| Remission group (n = 49) | Active group (n = 182) | P-value | |
| Age, yr | 35.00 (25.50, 56.50) | 38.50 (26.75, 57.00) | 0.767 |
| Males, n (%) | 30 (61.22) | 109 (59.89) | 0.866 |
| Smoking, n (%) | 10 (20.4) | 53 (29.1) | 0.224 |
| Duration, yr | 5.44 (2.58, 10.38) | 4.71 (1.50, 9.75) | 0.342 |
| BMI (kg/m2) | 23.30 (19.50, 24.80) | 19.95 (17.50, 22.61) | <0.001 |
| PLT (×109/L) | 211.00 (153.00, 242.00) | 285.00 (228.50, 344.50) | <0.001 |
| HGB (g/L) | 138.00 (126.50, 159.00) | 114.50 (100.75, 130.25) | <0.001 |
| ALB (g/L) | 42.00 (39.25, 45.00) | 36.50 (32.00, 41.00) | <0.001 |
| ESR (mm/h) | 6.00 (2.00, 11.00) | 19.00 (10.25, 33.75) | <0.001 |
| hsCRP (mg/L) | 1.07 (0.60, 5.32) | 8.99 (3.37, 21.55) | <0.001 |
| CAR | 0.02 (0.01, 0.15) | 0.25 (0.09, 0.63) | <0.001 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, IBD = inflammatory bowel disease, PLT = platelet.
a Reported as median (interquartile range) unless noted otherwise.
Table 3.
The differences between active and remission group in UC patients.
| Remission group (n = 21) | Active group (n = 73) | P-value | |
| Age, yr | 35.00 (29.50, 53.00) | 40.00 (29.00, 57.00) | 0.446 |
| Males, n (%) | 12 (57.1) | 40 (54.8) | 0.849 |
| Smoking, n (%) | 6 (28.6) | 20 (27.4) | 0.916 |
| Duration, yr | 4.74 (2.33, 7.09) | 2.72 (1.26, 6.05) | 0.058 |
| BMI (kg/m2) | 23.40 (20.50, 24.90) | 20.70 (18.00, 22.90) | 0.005 |
| PLT (×109/L) | 191.50 (145.50, 238.00) | 308.00 (258.75, 423.25) | <0.001 |
| HGB (g/L) | 152.00 (130.00, 198.50) | 114.00 (95.50, 128.50) | <0.001 |
| ALB (g/L) | 43.50 (41.00, 47.00) | 36.00 (32.00, 40.00) | <0.001 |
| ESR (mm/h) | 4.00 (2.00, 10.00) | 16.00 (10.00, 31.50) | <0.001 |
| hsCRP (mg/L) | 0.76 (0.34, 1.15) | 7.74 (3.13, 22.7) | <0.001 |
| CAR | 0.02 (0.01, 0.02) | 0.21 (0.09, 0.71) | <0.001 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, PLT = platelet, UC = ulcerative colitis.
a Reported as median (interquartile range) unless noted otherwise.
Table 2.
The differences between active and remission group in CD patients.
| Remission group (n = 28) | Active group (n = 109) | P-value | |
| Age, yr | 33.66 (23.40, 65.77) | 37.28 (25.69, 56.58) | 0.879 |
| Males, n (%) | 18 (64.3) | 69 (63.3) | 0.923 |
| Smoking, n (%) | 4 (14.3) | 33 (30.3) | 0.089 |
| Duration, yr | 6.19 (2.65, 10.70) | 6.46 (2.40, 10.83) | 0.913 |
| BMI (kg/m2) | 22.82 (18.83, 24.98) | 19.10 (16.90, 22.26) | 0.002 |
| PLT (×109/L) | 220.00 (166.00, 245.00) | 272.00 (207.50, 318.00) | 0.006 |
| HGB (g/L) | 133.50 (123.25, 153.00) | 115.00 (102.00, 131.50) | <0.001 |
| ALB (g/L) | 41.00 (37.25, 43.00) | 37.00 (32.00, 41.00) | <0.001 |
| ESR (mm/h) | 8.00 (3.00, 19.00) | 21.00 (12.00, 34.00) | <0.001 |
| hsCRP (mg/L) | 3.08 (0.69, 7.93) | 9.53 (3.84, 21.04) | <0.001 |
| CAR | 0.08 (0.02, 0.19) | 0.29 (0.1, 0.61) | <0.001 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, CD = Crohn disease, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, PLT = platelet.
a Reported as median (interquartile range) unless noted otherwise.
The Spearman correlation analysis suggested a significant association between the biomarker levels and CD/UC activity (Table 4). In patients with CD, the hsCRP, CAR, PLT, and ESR positively correlated with disease activity, while ALB, HGB, and BMI levels negatively correlated with disease activity (P < 0.01). In patients with UC, the hsCRP, CAR, PLT, and ESR positively correlated with disease activity, while ALB and HGB levels negatively correlated with disease activity (P < 0.001). Additionally, the associations between hsCRP, ALB, CAR, ESR, and PLT levels and disease activity were significantly higher in patients with UC in comparison to CD patients. However, the association between HGB levels and disease activity was higher in patients with CD (Table 4).
Table 4.
Spearman correlations between biomarkers and CD/UC activity.
| Biomarkers | CD (n = 137) | UC (n = 94) | ||
| R | P-value | R | P-value | |
| hsCRP | 0.391 | <0.001 | 0.770 | <0.001 |
| ALB | –0.539 | <0.001 | –0.577 | <0.001 |
| CAR | 0.453 | <0.001 | 0.767 | <0.001 |
| BMI | –0.424 | <0.001 | –0.177 | 0.087 |
| PLT | 0.221 | 0.009 | 0.503 | <0.001 |
| HGB | –0.548 | <0.001 | –0.537 | <0.001 |
| ESR | 0.477 | <0.001 | 0.670 | <0.001 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, CD = Crohn disease, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, PLT = platelet, UC = ulcerative colitis.
3.3. Diagnostic accuracy of specific biomarkers for assessing disease activity
From the ROC analysis of all IBD patients, the AUC of CAR was the highest among the biomarkers [AUC of CAR (0.829) vs CRP (0.808) (Z = 3.57, P < 0.001); AUC of CAR (0.829) vs ESR (0.811) (Z = 0.51, P = 0.61)]. The optimal cutoff level for CAR was 0.06 based on Youden index (sensitivity: 83.05%, specificity: 68.09%) for identifying IBD patients with active disease (Table 5 and Fig. 1).
Table 5.
Discriminatory power of biomarkers for disease activity by the ROC curves in IBD patients.
| Biomarkers | Cutoff value | Youden index | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | P-value | AUC | AUC 95% Cl |
| CAR | 0.06 | 0.51 | 83.05 | 68.09 | 90.74 | 51.61 | <0.001 | 0.829 | 0.763–0.896 |
| hsCRP (mg/L) | 2.14 | 0.51 | 82.32 | 68.75 | 90.85 | 50.77 | <0.001 | 0.808 | 0.738–0.877 |
| ALB (g/L) | 39.50 | 0.44 | 68.89 | 75.00 | 91.18 | 39.13 | <0.001 | 0.791 | 0.726–0.857 |
| HGB (g/L) | 120.50 | 0.47 | 59.34 | 87.76 | 94.74 | 36.75 | <0.001 | 0.812 | 0.748–0.875 |
| ESR (mm/h) | 11.50 | 0.51 | 73.89 | 77.08 | 92.36 | 44.05 | <0.001 | 0.811 | 0.746–0.875 |
| PLT (×109/L) | 246.50 | 0.47 | 67.40 | 79.17 | 92.42 | 39.18 | <0.001 | 0.750 | 0.672–0.827 |
| BMI (kg/m2) | 23.26 | 0.32 | 80.90 | 51.06 | 86.22 | 41.38 | <0.001 | 0.702 | 0.621–0.782 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, IBD = inflammatory bowel disease, PLT = platelet, ROC = receiver operating characteristics.
Figure 1.
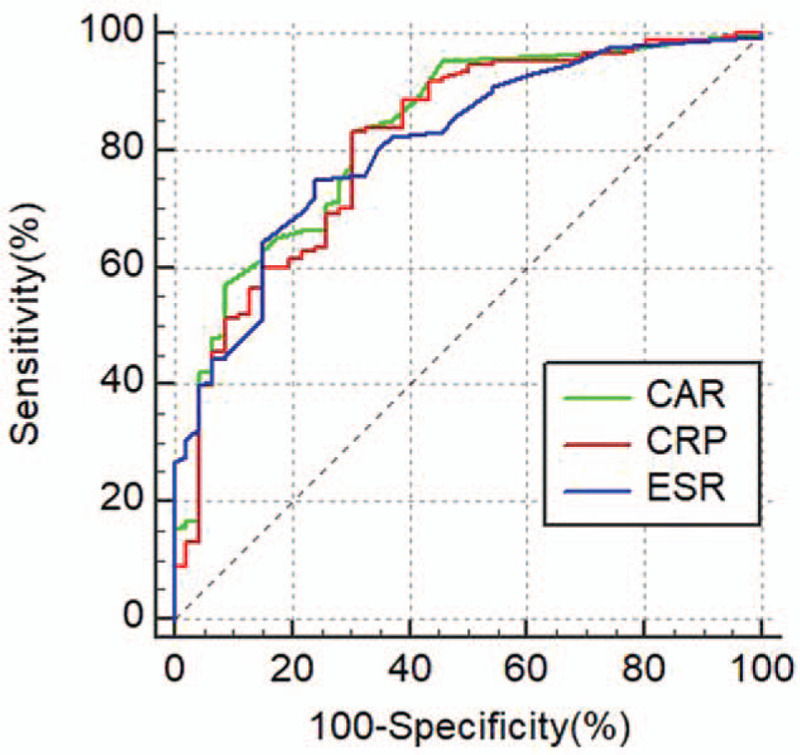
ROC curves for CAR, CRP, and ESR in IBD patients. CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, IBD = inflammatory bowel disease, ROC = receiver operating characteristics.
For patients with CD, the AUC of ESR was the highest among the biomarkers [AUC of ESR (0.769) vs CAR (0.766) (Z = 0.12, P = 0.91); AUC of CAR (0.766) vs CRP (0.734) (Z = 2.77, P = 0.01)]. The optimal cutoff level for CAR was 0.21 based on Youden index (sensitivity: 61.54%, specificity: 85.19%) for identifying CD patients with active disease (Table 6 and Fig. 2).
Table 6.
Discriminatory power of biomarkers for disease activity as analyzed by the ROC curves in CD patients.
| Biomarkers | Cutoff value | Youden index | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | P-value | AUC | AUC 95%Cl |
| CAR | 0.21 | 0.47 | 61.54 | 85.19 | 94.12 | 36.51 | <0.001 | 0.766 | 0.665–0.867 |
| hsCRP (mg/L) | 8.92 | 0.39 | 53.70 | 85.19 | 93.55 | 31.51 | <0.001 | 0.734 | 0.629–0.840 |
| ALB (g/L) | 36.50 | 0.38 | 48.60 | 89.29 | 94.55 | 31.25 | <0.001 | 0.728 | 0.633–0.823 |
| HGB (g/L) | 113.50 | 0.42 | 49.54 | 92.86 | 96.43 | 32.10 | <0.001 | 0.758 | 0.664–0.851 |
| ESR (mm/h) | 11.50 | 0.46 | 75.70 | 70.37 | 91.01 | 42.22 | <0.001 | 0.769 | 0.676–0.863 |
| PLT (×109/L) | 246.50 | 0.38 | 59.63 | 78.57 | 91.55 | 33.33 | <0.001 | 0.670 | 0.560–0.780 |
| BMI (kg/m2) | 23.25 | 0.31 | 81.31 | 50.00 | 87.00 | 39.39 | <0.001 | 0.698 | 0.589–0.806 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, CD = Crohn disease, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, PLT = platelet, ROC = receiver operating characteristics.
Figure 2.
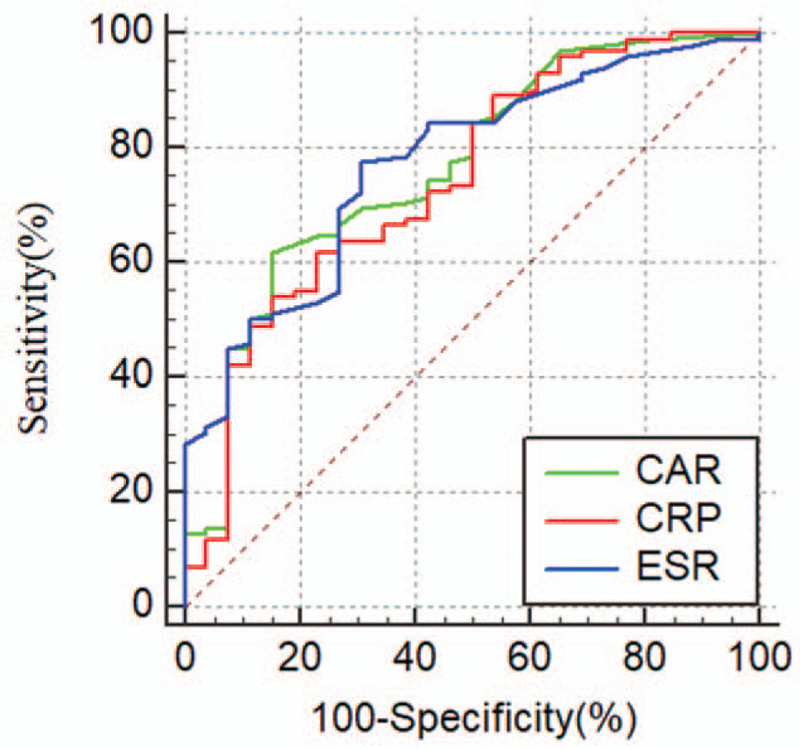
ROC curves for CAR, CRP, and ESR in CD patients. CAR = high-sensitivity C-reactive protein/albumin ratio, CD = Crohn disease, ESR = erythrocyte sedimentation rate, ROC = receiver operating characteristics.
For patients with UC, the AUC of CAR was the highest among the biomarkers [AUC of CAR (0.918) vs CRP (0.912) (Z = 1.33, P = 0.19); AUC of CAR (0.918) vs ESR (0.872) (Z = 1.19, P = 0.23)]. The optimal cutoff level for CAR was 0.06 (sensitivity: 82.19%, specificity: 95.00%) for identifying UC patients with active disease (Table 7 and Fig. 3).
Table 7.
Discriminatory power of biomarkers for disease activity as calculated by the ROC curves in UC patients.
| Cutoff value | Youden index | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | P-value | AUC | AUC 95%Cl | |
| CAR | 0.06 | 0.77 | 82.19 | 95.00 | 98.36 | 59.38 | <0.001 | 0.918 | 0.860–0.977 |
| hsCRP (mg/L) | 2.14 | 0.77 | 82.19 | 95.24 | 98.36 | 60.61 | <0.001 | 0.912 | 0.851–0.973 |
| ALB (g/L) | 39.50 | 0.58 | 68.49 | 90.00 | 96.15 | 43.90 | <0.001 | 0.876 | 0.799–0.952 |
| HGB (g/L) | 136.50 | 0.56 | 84.93 | 71.43 | 91.18 | 57.69 | <0.001 | 0.882 | 0.808–0.955 |
| ESR (mm/h) | 14.50 | 0.58 | 57.53 | 100.00 | 100.00 | 40.38 | <0.001 | 0.872 | 0.799–0.946 |
| PLT (×109/L) | 241.50 | 0.62 | 81.94 | 80.00 | 93.65 | 55.17 | <0.001 | 0.851 | 0.750–0.951 |
| BMI (kg/m2) | 22.00 | 0.38 | 66.20 | 71.43 | 88.68 | 38.46 | 0.005 | 0.701 | 0.578–0.823 |
ALB = albumin, BMI = body mass index, CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, HGB = hemoglobin, hsCRP = high sensitivity C reactive protein, PLT = platelet, ROC = receiver operating characteristics, UC = ulcerative colitis.
Figure 3.
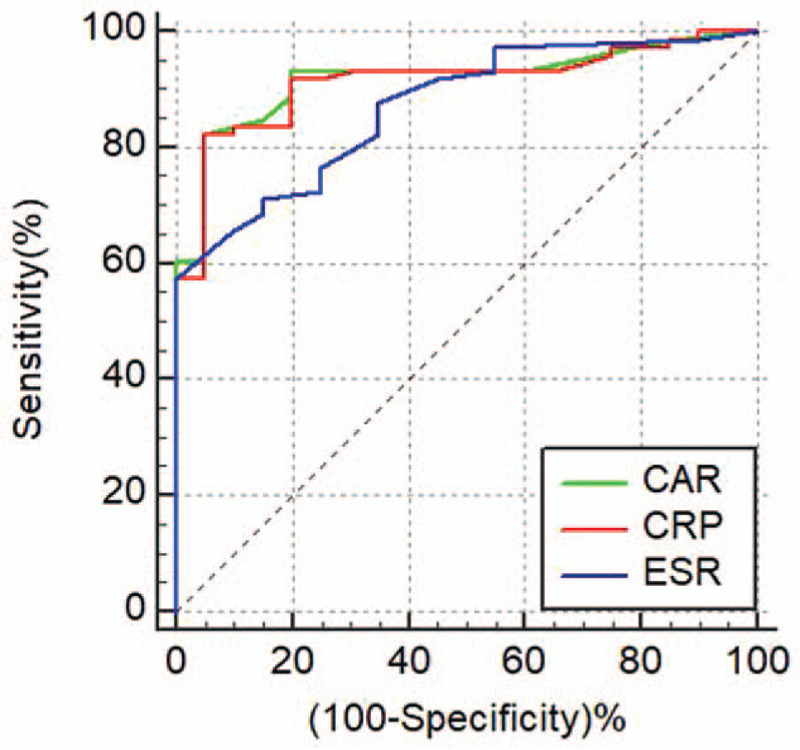
ROC curves for CAR, CRP, and ESR in UC patients. CAR = high-sensitivity C-reactive protein/albumin ratio, ESR = erythrocyte sedimentation rate, ROC = receiver operating characteristics, UC = ulcerative colitis.
4. Discussion
IBD is a disease where patients experience a series of relapses and remissions. There is an urgent need for clinicians to accurately monitor and assess disease activity and severity. Previous studies have suggested that the early detection of disease activity could significantly reduce the mortality of CD.[5,6] In the present study, we found that CAR can be used for identifying disease activity in both UC and CD patients. This study is the first of its kind to demonstrate that CAR may be more valuable for assessing disease activity in UC patients than CD patients.
CAR was first reported to be useful for identifying seriously ill patients in the emergency medical ward.[7] Later, Kim et al[8] reported that CAR was an independent predictor of mortality in patients with severe sepsis or septic shock. It was also reported that CAR could also serve as an effective prognostic factor for solid tumors.[3] However, few studies have investigated the association between CAR and disease activity in UC and CD patients. In the present study, CAR was found to positively correlate with disease activity (for CD, r = 0.453, P < 0.001; for UC, r = 0.767, P < 0.001). The AUC of CAR was the largest among the laboratory indexes. With a cutoff value of 0.06, CAR had the best sensitivity (83.05%) and specificity (68.09%) for the detection of active disease compared with the other biomarkers. We performed a subgroup analysis to detect the accuracy of CAR in determining disease activity of CD and UC separately. Our analysis showed that CAR with a cutoff value of 0.21 had a sensitivity and specificity of 61.54% and 85.19%, respectively, for identifying CD activity. This was different from that reported by Qin et al who found that CAR with a cutoff value of 0.69 had a sensitivity of 67.7% and specificity of 81.6% for CD activity.[4] Further research with larger sample size is required to determine the optimal cutoff value of CAR for judging disease activity. Moreover, CAR was found to be more accurate for identifying the disease activity of UC patients when compared with CD patients (Tables 6–7).
CRP production is primarily accomplished by hepatocytes and its release is stimulated by interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor (TNF-α).[9] CRP is a sensitive indicator of an inflammatory response and one of the most important indicators for judging IBD activity. We found that the concentration of hsCRP to be significantly higher in patients with active disease (1.07 vs 8.99 mg/dL), along with a significant correlation with disease activity (for UC, r = 0.770, P < 0.001; for CD, r = 0.391, P < 0.001). In addition, hsCRP with a cutoff value of 2.14 mg/L had the sensitivity and specificity of 83.33% and 95.24%, respectively, for identifying UC activity. In contrast, Schoepfer et al[10] showed CRP with a cutoff value of 6 mg/L to have lower sensitivity and specificity of 68% and 72%, respectively, for judging UC activity. These findings suggest that compared with CRP, hsCRP with a cutoff value of 2.14 mg/L has higher sensitivity and specificity for identifying UC activity.
ALB is primarily produced in the liver and hypoalbuminemia may be associated with liver dysfunction and malnutrition. However, few studies have assessed the usefulness of ALB in determining CD activity.[11] Our study showed that ALB levels were significantly lower in patients with active disease. Moreover, ALB levels significantly correlated with disease activity in patients with UC and CD (Tables 5–7). Compared with the findings of Qin et al[4] (ALB, cutoff value 34.55 g/L, sensitivity 67.7%, and specificity 73.7%), our results showed that ALB had lower sensitivity and higher specificity (cutoff value 36.5 g/L, sensitivity 51.4%, specificity 89.29%) for determining CD activity. We also found ALB to be more valuable in UC than CD for judging disease activity (Tables 6–7).
The other parameters, such as HGB, PLT, and ESR were also helpful for assessing disease activity. However, their concentrations were easily affected by other biological factors. For example, nutritional deficiency and gastrointestinal bleeding can lead to anemia and thrombotic diseases, which can affect the PLT levels. In the present study, we have demonstrated that HGB levels were significantly lower in patients with active IBD, while PLT and ESR were significantly higher in IBD patients with active disease. Also, HGB, PLT, and ESR were significantly associated with disease activity, yet the sensitivities of these three biomarkers were low for assessing IBD activity (Table 5). Previously, Schoepfer et al[10] demonstrated that the sensitivity and specificity of PLT (cutoff value 298 g/L) for predicting UC activity was 69% and 66%, respectively while HGB (cutoff value 136 g/L) had the sensitivity and specificity of 65% and 64%, respectively. In another study, Liu et al[12] showed that the AUC of ESR was 0.78 with the sensitivity and specificity of 68.9% and 76.0%, respectively, at the cutoff value of 8.5 mm/h for identifying CD disease activity. Different from the above two studies, we enrolled both CD and UC patients. We found that the PLT with a cutoff value of 241.5 × 109/L had the sensitivity and specificity of 81.94% and 80%, respectively, for assessing UC activity, while HGB with a cutoff value of 136.5 g/L had the sensitivity and specificity of 84.93% and 71.43%, respectively, for UC activity; and ESR with a cutoff value of 11.5 mm/h had the sensitivity and specificity of 75.7% and 70.37%, respectively, for determining CD activity. The diagnostic value of these markers was greater for UC than CD.
BMI is an index used for the assessment of nutritional status. Previously, Dong et al[13] conducted meta-analyses about the association between BMI and IBD and found that BMI was lower in patients with CD than in healthy subjects. In the present study, we demonstrated that BMI was significantly lower in patients with active disease and that BMI negatively correlated with CD activity but showed no correlation with UC activity. The AUC of BMI was the lowest when compared with the other indexes in IBD patients, suggesting that BMI has a limited role in assessing disease activity.
This study has some limitations. First, it is a single-center retrospective study, which may lead to a potential selection bias. Secondly, the number of patients with inactive disease (ie, remission) was low. Future prospective studies with larger sample sizes are required to validate the findings of this study.
In conclusion, this retrospective analysis of 231 IBD patients found CAR to be a useful marker to assess disease activity in UC and CD patients. Our findings indicate that higher CAR levels are indicative of higher disease activity. In addition, we found that CAR may be more valuable in patients with UC than in patients with CD for judging disease activity.
Acknowledgments
None.
Author contributions
JQ and HL conceived and designed research; AL and BT collected data and conducted research; AL, HS, HY, and JL analyzed and interpreted data; AL wrote the initial paper; HL and JQ revised the paper; JQ had primary responsibility for final content. All authors read and approved the final manuscript.
Conceptualization: Hong Lv, Jiaming Qian.
Data curation: Ailing Liu, Bei Tan.
Formal analysis: Huijun Shu, Hong Yang.
Investigation: Ailing Liu, Bei Tan.
Methodology: Ailing Liu, Huijun Shu, Hong Yang, Ji Li.
Project administration: Jiaming Qian.
Resources: Hong lv
Software: Ailing Liu, Huijun Shu, Hong Yang, Ji Li.
Supervision: Jiaming Qian.
Validation: Ailing Liu, Hong Lv, Bei Tan, Huijun Shu,Hong Yang, Ji Li, Jiaming Qian.
Visualization: Ailing Liu, Hong Lv, Bei Tan, Huijun Shu, Hong Yang, Ji Li, Jiaming Qian.
Writing – original draft: Ailing Liu.
Writing – review & editing: Hong Lv, Jiaming Qian.
Footnotes
Abbreviations: ALB = serum albumin, CAR = highly sensitive C-reactive protein/albumin ratio (CRP/ALB), CD = Crohn disease, CDAI = Crohn disease activity index, HGB = hemoglobin, hsCRP = high-sensitivity C-reactive protein, IBD = inflammatory bowel disease, IL-1β = interleukin-1β, IL-6 = interleukin-6, IQR = interquartile ranges, PLT = platelet count, ROC = receiver operating characteristics, TNF-α = tumor necrosis factor, UC = ulcerative colitis.
How to cite this article: Liu A, Lv H, Tan B, Shu H, Yang H, Li J, Qian J. Accuracy of the highly sensitive C-reactive protein/albumin ratio to determine disease activity in inflammatory bowel disease. Medicine. 2021;100:14(e25200).
This study protocol was approved by the Ethical Committee of Peking Union Medical College Hospital (Date of Approval: 14-June-2019). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from all individual participants included in the study.
This study was supported by the Health Research & Special Projects Grant of China (201002020), the Chinese Academy of Medical Sciences Medicine and Health Technology Innovation Project (2016-I2M-3-001), the Natural Sciences Foundation of China (81570505), and the National Science and Technology Ministry 973 Plan(2015CB943203).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- [2].Abej E, El-Matary W, Singh H, et al. The utility of fecal calprotectin in the real-world clinical care of patients with inflammatory bowel disease. Can J Gastroenterol Hepatol 2016;2016:2483261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu J, Tan W, Chen L, et al. Clinicopathologic and prognostic significance of C-reactive protein/albumin ratio in patients with solid tumors: an updated systemic review and meta-analysis. Oncotarget 2018;9:13934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qin G, Tu J, Liu L, et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn's disease activity. Med Sci Monit 2016;22:4393–400. [DOI] [PubMed] [Google Scholar]
- [5].Wong A, Bass D. Laboratory evaluation of inflammatory bowel disease. Curr Opin Pediatr 2008;20:566–70. [DOI] [PubMed] [Google Scholar]
- [6].Sandborn WJ, Loftus EV, Jr, Colombel JF, et al. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn's disease. Inflamm Bowel Dis 2001;7:192–201. [DOI] [PubMed] [Google Scholar]
- [7].Fairclough E, Cairns E, Hamilton J, et al. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim MH, Ahn JY, Song JE, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One 2015;10:e0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10:661–5. [DOI] [PubMed] [Google Scholar]
- [10].Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105:162–9. [DOI] [PubMed] [Google Scholar]
- [11].Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res 2012;159:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu S, Ren J, Han G, et al. Mean platelet volume: a controversial marker of disease activity in Crohn's disease. Eur J Med Res 2012;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dong J, Chen Y, Tang Y, et al. Body mass index is associated with inflammatory bowel disease: a systematic review and meta-analysis. PLoS One 2015;10:e0144872. [DOI] [PMC free article] [PubMed] [Google Scholar]


