Abstract
Metabolic syndrome (MetS) is a constellation of factors including hypertension, abdominal obesity, dyslipidemia, and insulin resistance that separately and together significantly increase risk for cardiovascular disease (CVD) and diabetes. In sub-Saharan Africa, with a substantial burden of human immunodeficiency virus (HIV) and increasing prevalence of CVD and diabetes, there is a paucity of epidemiological data on demographic, laboratory, and clinical characteristics associated with MetS among people with HIV (people with human [PWH]). Therefore, this study aimed to determine the burden and factors influencing MetS in antiretroviral therapy (ART)-experienced individuals in Zambia.
We collected cross-sectional demographic, lifestyle, anthropometric, clinical, and laboratory data in a cohort of ART-experienced (on ART for ≥6 months) adults in 24 urban HIV treatment clinics of Zambia between August, 2016 and May, 2020. MetS was defined as having ≥3 of the following characteristics: low high density lipoprotein cholesterol (HDL-c) (<1.0 mmol/L for men, <1.3 for women), elevated waist circumference (≥94 cm for men, ≥80 cm for women), elevated triglycerides (≥1.7 mmol/L), elevated fasting blood glucose (≥5.6 mmol/L), and elevated blood pressure (BP) (systolic BP ≥130 or diastolic BP ≥85 mm Hg). Virological failure (VF) was defined as HIV viral load ≥1000 copies/mL. The following statistical methods were used: Chi-square test, Wilcoxon rank-sum test, and multivariable logistic regression.
Among 1108 participants, the median age (interquartile range [IQR]) was 41 years (34, 49); 666 (60.1%) were females. The prevalence of MetS was 26.3% (95% confidence interval [CI] 23.9–29.1). Age (adjusted odds ratio [OR] 1.07; 95% CI 1.04–1.11), female sex (OR 3.02; 95% CI 1.55–5.91), VF (OR 1.98; 95% CI 1.01–3.87), dolutegravir (DTG)-based regimen (OR 2.10; 95% CI 1.05–4.20), hip-circumference (OR 1.03; 95% CI 1.01–1.05), T-lymphocyte count (OR 2.23; 95% CI 1.44–3.43), high-sensitivity C-reactive protein (hsCRP) (OR 1.14; 95% CI 1.01–1.29), and fasting insulin (OR 1.02; 95% CI 1.01–1.04) were significantly associated with MetS.
Metabolic syndrome was highly prevalent among HIV+ adults receiving ART in Zambia and associated with demographic, clinical, anthropometric, and inflammatory characteristics. The association between MetS and dolutegravir requires further investigation, as does elucidation of the impact of MetS on ART outcomes in sub-Saharan African PWH.
Keywords: adult, antiretroviral therapy, cardiovascular disease, dolutegravir, metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) is a health condition that comprises abdominal obesity, hypertension, dyslipidemia, and insulin resistance.[1] It is a major public health concern in many parts of the world[2] due to its strong association with cardiovascular disease (CVD) morbidity and mortality in the general population.[3] Similarly, people with human immunodeficiency virus (HIV) (people with human [PWH]) have an elevated risk of CVD[4] as well as of metabolic abnormalities that increase the probability of CVD events as compared with the general population.[5] Some antiretroviral therapy (ART) agents, such as protease inhibitors and thymidine analogues, have also been implicated in the development of MetS.[4,6,7]
Substantial variations in the prevalence of MetS in PWH have been reported, largely due to limitations in the diagnostic criteria for MetS,[8] as well as differences in underlying population characteristics. In Europe and America, observational studies have reported a wide range of estimates, from 7% to 52%,[9–14] while in Africa, a meta-analysis by Nguyen et al[15] reported a range of 13% to 58%. The differences in the definition of MetS employed make it difficult to conclude whether the prevalence of MetS is comparable in PWH and the general population. Hence, there is a need for regional and country-specific data on the burden of MetS.
The magnitude of and risk factors for MetS are well documented in developed countries, but there are limited data in sub-Saharan Africa (SSA), particularly among PWH, and many ART programs in the region do not routinely address MetS and its components, despite the fact that Africa is disproportionately affected by CVD in HIV.[16] A successful program to reduce CVD will depend on reliable epidemiological data on its risk factors. Here, we assess the prevalence of and factors associated with MetS among adults with HIV receiving ART in Zambia.
2. Materials and methods
2.1. Study design and setting
This was a cross-sectional study of patients on ART for ≥6 months conducted in 24 health facilities located in 19 districts of Zambia (Chibombo, Chipata, Choma, Kasenengwa, Kapiri Mposhi, Kalulushi, Kitwe, Lusaka, Luwingu, Livingstone, Limulunga, Monze, Mumbwa, Mufulira, Ndola, Namwala, Petauke, Samfya, and Serenje). Health facilities were selected using systematic sampling with probability proportionate to size sample. The facilities provide ART services (laboratory, pharmacy, clinical evaluation, and counseling).
2.2. Study participants
The study recruited 1108 participants who were coming for routine HIV care, treatment, and management. Eligible participants were aged 18 years and above and had been receiving ART for ≥6 months. The study was based on a combined dataset arising from 2 studies that collected data on MetS. The first study was examining HIV drug resistance among adult patients on ART for 12 months or more in 20 health facilities across the 19 districts. Due to the nature of the study, patients with documented HIV-2 or HIV1/HIV-2 coinfection were excluded. The second study recruited participants in Livingstone, Zambia between April, 2019 and May, 2020 and focused on MetS and virologic failure in 5 health facilities (Livingstone Central Hospital, Maramba Clinic, St Joseph's Hospice Clinic, Libuyu Clinic, and Mahatma Ghandhi). In this study, pregnant women, participants with a history of ART exposure for prevention of mother to child transmission or for post-exposure prophylaxis, and individuals with known active opportunistic infection or neoplasm were excluded. The data were combined because of the consistency in the parameters of MetS collected in both studies.
2.3. Data collection
Sociodemographic variables (age, sex, marital status [No, Yes], educational status [no formal, primary, secondary, tertiary] and work status [formal employment, self-employed, unemployed], physical measurements [height, weight, waist circumference, and hip circumference], clinical factors [ART regimen, duration on ART, blood pressure {BP}, and diabetes status]), and laboratory parameters were collected from participants and from health records (SmartCare and patient files) using a structured questionnaire and a data collection form. Trained research assistants administered the questionnaire and collected blood samples from the participants. If clinically abnormal findings were discovered during the research data collection, the participant and the physician on duty were notified before the participant left the clinic.
Blood pressure was measured using an automated cuff (Omron-HEM-7120, USA) and the average calculated from 3 readings: reading one at 2 minutes of rest, reading 2 at 5 minutes of rest, and reading 3 at 10 minutes of rest. Height (cm), weight (kg), and waist circumference (WC) were measured using a height measurement chart, digital scale, and tape measure, respectively. Visceral fat was estimated using bioimpedance analysis (TANITA BC-418 Segmental Body Composition Analyzer).
2.4. Blood samples and measurements
Fasting and non-fasting blood samples were collected from the study participants. All participants who arrived for their visit having eaten something within 8 hours were asked to return to the clinic the following day after fasting for 8 hours for blood collection. However, for those who could not return, random blood glucose and lipid profile were measured. After collection of the blood samples, vacutainer tubes were clearly labeled and packaged in a cooler box for transportation to the laboratory for analysis. We collected samples for total cholesterol (TC) (mmol/L), high density lipoprotein cholesterol (HDL-c) (mmol/L), low density lipoprotein cholesterol (LDL-c) (mmol/L), and triglycerides (mmol/L) tests in a lithium heparin tube. Pre-analysis phase involved centrifuging samples for 10 minutes at 10,000 relative centrifugal force. Thereafter, the lipid tests were analyzed using a HumaStar 600 machine. Glucose was measured with an Accu-chek point of care machine in which samples were collected using a finger prick. Total lymphocyte and CD4 lymphocyte count (cells/μL) samples were collected in ethylenediaminetetraacetic acid (EDTA) containers and assayed using a Becton Dickson flow cytometer. Viral load (copies/mL) samples were collected in EDTA containers and analyzed using an Ampliprep/Taqman 96 PCR analyzer. For insulin measurements, samples were collected in a plain container and assayed using the human insulin (Hu Insulin TM) Enzyme-Linked Immunosorbent Assay (ELISA) and insulin resistance (IR) was estimated with the homeostasis model assessment index (HOMA), calculated as fasting glucose (in mmol/L) times fasting insulin divided by 22.5. Samples for tumor necrosis factor alpha (TNF-α) were collected in plain blood specimen containers and analyzed using the Biosource Sensitivity Immunoassay (EASIA) TM. Samples for high-sensitivity C-reactive protein (hsCRP) were collected in plain blood specimen containers and assayed using Abcam's hsCRP Human ELISA kit.
2.5. ART regimens
-
Non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens contained efavirenz (EFV) or nevirapine (NVP) with one of the following nucleoside reverse transcriptase inhibitors (NRTIs):
-
∘
Abacavir and lamivudine/emtricitabine (ABC/XTC) or tenofovir disoproxil fumarate and lamivudine/emtricitabine (TDF/XTC)
-
∘
-
Protease inhibitor (PI) regimens contained either lopinavir/ritonavir (LPV/r) or atazanavir/ritonavir (ATV/r) with one of the following NRTI combinations:
-
∘
ABC/XTC or zidovudine/XTC (AZT/XTC) or TDF/XTC
-
∘
An integrase strand transfer inhibitor (INSTI) regimen contained dolutegravir (DTG) with TDF/lamivudine (TDF/3TC).
2.6. Operational definitions
2.6.1. Primary outcome
MetS was defined as the presence of ≥3 of the following factors[17]: waist circumference >94 cm in men and >80 cm in women, triglycerides ≥1.7 mmol/L, HDL-c < 1.0 mmol/L in men and <1.3 mmol/L in women, systolic BP ≥130 or diastolic BP ≥85 mm Hg, or fasting glucose ≥5.6 mmol/L.
Abnormal TC (≥5.1 mmol/L) and LDL-c (≥3.4 mmol/L) were defined according to 2018 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.[18] Detectable viral load (VL) was defined as plasma HIV-1 RNA VL of ≥200 copies/mL[19] and virological failure (VF) was defined as having one HIV-1 plasma VL of ≥1000 copies/mL after 6 months or more of ART.
Body mass index (BMI) was classified as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2).[20]
3. Sample size calculation
We assumed 90% power, 5% significance level, 3% margin of error, associated metabolic syndrome prevalence rate of 32%, accounting for design effect of 1.5, and adjusting for 10% error rate to obtain a sufficient sample of 1047. We achieved a sample of 1108.
3.1. Statistical analysis
All analyses were performed using STATA software, version 15.0/IC (Stata Corporation, College Station, TX). Categorical data were summarized using frequencies and proportions. Continuous variables were summarized using medians and percentiles. Q-Q plots and Shapiro Wilk test were used to determine the normality of the data. All the continuous variables were found not to be normally distributed and hence we used medians and interquartile ranges (IQR). The Pearson chi-square test was used to test for statistically significant associations between categorical variables. For continuous variables, Wilcoxon rank sum test was used to ascertain statistical difference between 2 medians for metabolic syndrome and non-metabolic syndrome groups. Logistic regression was used to estimate the odds ratios and 95% confidence intervals for associations between potential risk factors and MetS with and without adjustment for sociodemographic, immuno-virologic, clinical, and laboratory factors. Covariates included in the final model were selected based on published evidence and variables which were statistically significant at bivariate analysis. Hosmer-Lemeshow goodness-of-fit was used to test for model fitness. No interactions were included in the models. Table 1 was based on complete data collected during a study examining HIV drug resistance among adult patients. Statistical significance was defined as P < .05.
Table 1.
Visceral fat, hsCRP, TNF-α, and insulin as factors associated with MetS.
| Adjusted analysis | |||
| Characteristic | AOR | 95% CI | P-value |
| Visceral fat levels, IU | 1.04 | 0.99–1.09 | .070 |
| hsCRP, mg/L | 1.14 | 1.01–1.30 | .037 |
| TNF-α, pg/mL | 1.01 | 0.99–1.02 | .288 |
| Insulin, mIU/L | 1.02 | 1.01–1.04 | .001 |
CI = confidence interval, hsCRP = high-sensitivity C-reactive protein, OR = odds ratio, TNF-α = tumor necrosis factor alpha, adjusted OR for age, sex, and BMI.
Bold P-values shows variables statistically significant <.05.
3.2. Ethical considerations
Ethical approval for the study was obtained from University of Zambia Biomedical Research Ethics Committee (UNZABREC) and Zambia National Health Research Ethics Board (ZNHREB). The purpose of the study was explained to all the participants in a language familiar to them, and they provided written informed consent.
4. Results
4.1. Descriptive characteristics
The study comprised 1108 ART participants; 666 (60.1%) were women and the median (interquartile range [IQR]) age and duration on ART were 41 years (34, 49) and 108 months (60, 144), respectively. The majority of participants had secondary education (347 [54.7%]) and normal BMI (637 [57.5%]), while 41.5% (459) were not employed. Detectable VL and VF was present in 284 (25.7%; 95% CI 23.1–28.3) and 163 (14.7%; 95% CI 12.7–16.9) participants, respectively. The median (IQR) CD4 count and HOMA-IR were 475 cells/μL (301, 697) and 1.66 (0.83, 3.68), respectively. Most patients were on NNRTI (EFV and NVP)-based regimens, (898, 81.1%), followed by INSTI (DTG)-based regimen (133, 12%) and TDF/3TC NRTI-backbone regimen (1034, 93.3%).
4.2. Prevalence of MetS and its components
Metabolic syndrome was observed in 293/1108 (26.4% [95% CI 23.9–29.1]) participants. The most common components of MetS in all participants were elevated BP (471 [42.5%; 95% CI 39.6–45.5]) and WC (452 [40.8%; 95% CI 37.9–43.7]), see Fig. 1. Similarly, among individuals with MetS the most prevalent components of MetS were elevated WC (225 [76.8%; 71.5–81.5]) and BP (222 [75.8%; 95% CI 70.4–80.6]), followed by reduced HDL-c (203 [69.3%; 95% CI 63.6–74.5]).
Figure 1.
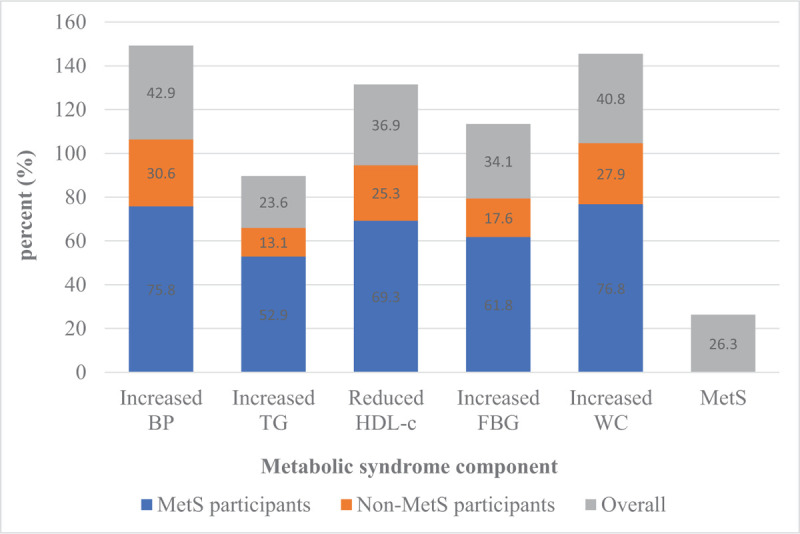
Prevalence of MetS and its components among the study participants. MeTS = metabolic syndrome.
4.3. Relationships of MetS with sociodemographic, behavioral, physical, and clinical factors
Table 1 shows the relationships between MetS and sociodemographic, behavioral, physical, and clinical characteristics. As compared with participants without MetS, those with MetS were older (median 43 vs 41 years) and larger proportions were women (73.4% vs 55.3%) and were unemployed (49.5% vs 38.6%). Individuals with MetS had been on ART longer (120 vs 102 months). MetS was associated with ART regimen, with slightly more participants with versus without MetS being on the DTG-based (13.7% vs 11.4%) or NNRTI (EFV and NVP) regimen (82.9% vs 80.4%). Individuals with MetS had significantly higher BMI, TC, LDL-c, hip circumference, monocytes, neutrophils, T-lymphocytes, white blood cells (WBC), platelets, visceral fat levels, hsCRP, TNF-α, and insulin levels.
4.4. Factors associated with MetS
Table 2 shows results of unadjusted and adjusted multivariable analyses. We observed that a 1-year increment in age was associated with a significant 7% increase in the odds of having MetS. Women had 3-fold higher odds of having MetS relative to men. Participants with viral loads ≥1000 copies/mL had nearly twice the odds of MetS as compared with those with lower viral loads. Individuals on the DTG-based regimen had 2-fold greater odds of MetS as compared with those on an NNRTI-based regimen. A 1-cm increment in hip circumference was associated with 3% increased odds of MetS. A 109 cells/L increase in T-lymphocyte absolute count was associated with 2-fold increased odds of MetS.
Table 2.
Sociodemographic, behavioral, physical, and clinical factors according to MetS status.
| Metabolic syndrome | ||||
| Characteristic | N = 1108 | Yes, 293 (26.4%) | No, 815 (73.6%) | P-value |
| Sociodemographic | ||||
| Age, y, m(IQR) | 1108 | 43 (35, 51) | 41 (33, 48) | .003 † |
| Sex, n (%) | <.001 | |||
| Male | 442 | 78 (26.6) | 364 (44.7) | |
| Female | 666 | 215 (73.4) | 451 (55.3) | |
| Current smokers∗, n (%) | .123‡ | |||
| No | 609 | 116 (93.6) | 493 (96.7) | |
| Yes | 25 | 8 (6.4) | 17 (3.3) | |
| Currently married∗, n (%) | .938 | |||
| No | 307 | 128 (44.3) | 360 (44.6) | |
| Yes | 328 | 161 (55.7) | 448 (55.7) | |
| Education level∗, n (%) | .591‡ | |||
| No formal | 10 | 3 (2.4) | 7 (1.4) | |
| Primary | 185 | 32 (25.8) | 153 (29.9) | |
| Secondary | 347 | 72 (58.1) | 275 (53.8) | |
| Tertiary | 93 | 17 (13.7) | 76 (14.9) | |
| Work status∗, n (%) | <.001 | |||
| Formal employment | 345 | 94 (32.1) | 251 (30.8) | |
| Self-employed | 303 | 54 (18.4) | 249 (30.6) | |
| Unemployed | 459 | 145 (49.5) | 314 (38.6) | |
| Income above K1500∗, n (%) | .743 | |||
| No | 391 | 75 (60.5) | 316 (62.1) | |
| Yes | 242 | 49 (39.5) | 193 (37.9) | |
| Ever consumed alcohol∗, n (%) | .923 | |||
| No | 299 | 58 (46.8) | 241 (47.2) | |
| Yes | 335 | 66 (53.2) | 269 (52.8) | |
| Physical exercise∗, n (%) | .679 | |||
| No | 491 | 96 (79.3) | 395 (77.6) | |
| Yes | 139 | 25 (20.7) | 114 (22.4) | |
| Clinical characteristics | ||||
| BMI, kg/m2, m(IQR) | 1108 | 23.1 (20.4, 25.9) | 22.0 (19.4, 25.2) | <.001 |
| CD4 absolute count (cells/μL)∗, m(IQR) | 1095 | 469 (298, 693) | 475 (304, 705) | .910 |
| Viral load (copies/μL)∗, n (%) | .721 | |||
| <1000 | 944 | 248 (84.6) | 696 (85.5) | |
| ≥1000 | 163 | 45 (15.4) | 118 (14.5) | |
| Duration on ART, mo∗, m(IQR) | 635 | 120 (84, 160) | 102 (60, 144) | .001 |
| ART-based regimen, n (%) | .016 | |||
| NNRTI (EFV & NVP) | 898 | 243 (82.9) | 655 (80.4) | |
| PI (LPV/r & ATV/r) | 77 | 10 (3.4) | 67 (8.2) | |
| INSTI (DTG) | 133 | 40 (13.7) | 93 (11.4) | |
| NRTI-backbone ART regimen, n (%) | .185 | |||
| ABC/3TC | 18 | 5 (1.7) | 13 (1.6) | |
| AZT/3TC | 56 | 9 (3.1) | 47 (5.8) | |
| TDF/3TC | 1034 | 279 (95.2) | 451 (92.6) | |
| TC, mmol/L∗, m(IQR) | 1106 | 4.52 (3.75, 5.31) | 4.3 (3.54, 5.10) | .003 † |
| LDL, mmo/L∗, m(IQR) | 1102 | 2.2 (1.5, 2.8) | 2.0 (1.38, 2.6) | .016 † |
| Hip circumference, m(IQR) | 1108 | 97 (88, 105) | 92 (85, 98) | <.001 † |
| HB, g/dL∗, m(IQR) | 966 | 12.8 (11.8, 13.9) | 12.9 (11.6, 14.2) | .476† |
| Monocytes (109 cells/L)∗, m(IQR) | 600 | 0.40 (0.32, 0.49) | 0.36 (0.28, 0.48) | .021 † |
| Neutrophils (109 cells/L)∗, m(IQR) | 600 | 2.64 (2.12, 3.36) | 2.27 (1.78, 3.03) | <.001 † |
| T-Lymphocytes (109 cells/L), m(IQR) | 603 | 2.28 (1.94, 2.75) | 2.01 (1.58, 2.42) | <.001 † |
| WBC (109 cells/L)∗, m(IQR) | 629 | 5.4 (4.7, 6.6) | 4.8 (3.9, 5.8) | <.001 † |
| Platelets (109 cells/L)∗, m(IQR) | 604 | 280 (224, 319) | 249 (207, 305) | .045 † |
| Visceral fat levels (IU)∗, m(IQR) | 470 | 8 (5, 13) | 5 (3, 8) | <.001 † |
| hsCRP, mg/L∗, m(IQR) | 473 | 1.88 (0.79, 3.18) | 0.85 (0.48, 1.72) | <.001 † |
| TNF-α, pg/mL∗, m(IQR) | 472 | 29.75 (22.26, 37.52) | 22.02 (15.58, 31.43) | <.001 † |
| Insulin, mIU/L∗, m(IQR) | 473 | 12.94 (6.42, 26) | 5.04 (2.95, 8.39) | <.001 † |
ART = antiretroviral therapy.
Variables with missing values, m(IQR) = median (interquartile, n [%]—frequency and percentage).
Wilcoxon rank sum test, BMI = body mass index, kg/m2 = kilogram per meter squared, NNRTI = non-nucleoside/nucleotide reverse transcriptase inhibitor (EFV = efavirenz and NVP = Nevirapine), PI = protease inhibitor (LPV/r = lopinavir/ritonavir and ATV/r = atazanavir/ritonavir), INSTI = integrase strand transfer inhibitor (DTG = dolutegravir), NRTI = nucleotide reverse transcriptase inhibitor, ABC/3TC = abacavir/lamivudine, AZT/3TC = zidovudine/lamivudine, TDF/3TC = tenofovir/lamivudine, TC = total cholesterol, LDL = low density lipoprotein cholesterol, HB = hemoglobin, T-lymphocytes = total-lymphocytes, WBC = white blood cells, hsCRP = high-sensitivity C-reactive protein, TNF-α = tumor necrosis factor alpha.
Bold P-values shows variables statistically significant <.05.
4.5. Factors associated with MetS in a model with complete data on inflammatory markers (hsCRP and TNF-alpha), visceral fat, and insulin levels
In Table 3, we analyzed complete data collected during a study focusing on HIV drug resistance among adult patients. After adjusting for age, sex, and BMI, it a unit increase in hsCRP or insulin was associated with 14% and 2% increased odds of having MetS, respectively, but the univariate associations with visceral fat and TNF-α were not significant.
Table 3.
Multivariable analysis of factors associated with MetS.
| Unadjusted analysis | Adjusted analysis | |||||
| Characteristic | OR | 95%CI | P-value | aOR | 95%CI | P-value |
| Age (per year) | 1.02 | 1.01–1.03 | .002 | 1.07 | 1.04–1.11 | <.001 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 2.22 | 1.66–2.98 | <.001 | 3.02 | 1.55–5.91 | .001 |
| Physical exercise | ||||||
| No | Ref | Ref | ||||
| Yes | 1.01 | 0.77–1.32 | .938 | 0.83 | 0.42–1.63 | .583 |
| BMI, kg/m2 | 1.04 | 1.01–1.06 | .007 | 0.95 | 0.90–1.01 | .098 |
| Viral load, copies/μL | ||||||
| <1000 | Ref | Ref | ||||
| ≥1000 | 1.07 | 0.74–1.55 | .721 | 1.98 | 1.01–3.87 | .047 |
| Duration on ART, mo | 1.00 | 0.99–1.00 | .089 | 1.00 | 0.99–1.00 | .807 |
| ART regimen | ||||||
| NNRTI (EFV & NVP) | ref | Ref | ||||
| PI (LPV/r & ATV/r) | 0.40 | 0.20–0.79 | .009 | 0.30 | 0.03–3.15 | .318 |
| INSTI (DTG) | 1.16 | 0.78–1.73 | .467 | 2.10 | 1.05–4.20 | .036 |
| NRTI regimen | ||||||
| ABC/3TC | ref | Ref | ||||
| AZT/3TC | 0.50 | 0.14–1.74 | .276 | 1.55 | 0.13–18.88 | .732 |
| TDF/3TC | 0.96 | 0.34–2.72 | .940 | 0.53 | 0.14–2.01 | .351 |
| TC | 1.16 | 1.05–1.29 | .005 | 0.95 | 0.72–1.26 | .722 |
| LDL | 1.13 | 1.01–1.28 | .037 | 1.15 | 0.82–1.60 | .423 |
| Hip circumference | 1.03 | 1.01–1.04 | <.001 | 1.03 | 1.01–1.05 | .013 |
| Lymphocytes (109 cells/L) | 2.05 | 1.54–2.73 | <.001 | 2.23 | 1.44–3.43 | <.001 |
| White blood cells (109 cells/L) | 1.11 | 1.00–1.22 | .041 | 1.07 | 0.96–1.18 | .213 |
ABC/3TC = abacavir/lamivudine, AZT/3TC = zidovudine/lamivudine, ART = antiretroviral therapy, BMI = body mass index, CI = confidence interval, INSTI = integrase strand transfer inhibitor (DTG = dolutegravir), kg/m2 = kilogram per meter squared, LDL = low density lipoprotein cholesterol, NRTI = nucleotide reverse transcriptase inhibitor, NNRTI = non-nucleoside/nucleotide reverse transcriptase inhibitor (EFV = efavirenz and NVP = Nevirapine), OR = odds ratio, PI = protease inhibitor (LPV/r = lopinavir/ritonavir and ATV/r = atazanavir/ritonavir), TDF/3TC = tenofovir/lamivudine, TC = total cholesterol, WBC = white blood cells, model was adjusted for all variables in the table, bold P-values shows variables statistically significant <.05.
5. Discussion
Metabolic syndrome was common among ART-experienced adults in Zambia, with a prevalence of 26.3% (95% CI 23.9–29.1), and it appears to be associated with inflammatory markers, sociodemographic, anthropometric, clinical, and laboratory characteristics. Other investigators have also observed high prevalences of MetS in similar groups in Ethiopia,[21] Cameroon[22] India,[23,24] Thailand,[25] and Portugal.[26] However, those studies reported a range of prevalences which could be partly explained by differences in sociodemographics, MetS definitions, and ART regimens. It has been shown that different definitions of MetS lead to varied results.[27] In the current study, we observed a higher prevalence of MetS than previously reported in Kenya (16.9%),[28] Lesotho (16.7%),[29] and Ethiopia (18.1% using Adult Treatment Panel [ATP] criteria and 25% using International Diabetes Federation [IDF] criteria).[30]
The most prevalent components of MetS were elevated BP and WC, which are measured easily and with low cost. Prior studies have similarly shown that hypertension is the most prevalent feature of the MetS in ART-experienced patients; hypertension is perhaps the most important CVD risk factor among ART patients, and it is straightforwardly treatable.[31–33] Some of the factors that have been implicated in hypertension are aging, metabolic abnormalities, endothelial dysfunction, inflammation and antiretroviral drugs.[31,32] These factors might have been the major drivers of abnormal BP in our study. Abdominal obesity, measured by WC, has also been strongly associated with CVD risk and mortality.[34] It is therefore critical that national guidelines and ART programs include efficient and cost-effective methods to detect and institute appropriate interventions for individuals with abdominal obesity and hypertension, with a view to preventing CVD-related deaths.
Age was significantly positively associated with MetS. Earlier reports have shown similar results.[25,26,30,35] Although similar findings are observed in the general population, the odds in PWH remains increased.[15] We also noted that females were more likely to have MetS than males. Similar findings were observed in Ethiopia,[30] Kenya,[28] Thailand,[25] and the United States,[36] whereas studies in Poland[35] and China[37] found a higher prevalence of MetS among males. In Brazil, the burden of MetS was comparable in men and women.[38] The discrepancies could have arisen from differences in the criteria used to define MetS. For instance, Rogalska-Płońska et al[35] used IDF to define MetS, which stipulates that definitions of MetS use ethnicity-specific criteria. For accurate estimation of the burden of MetS and appropriate policy development, there is need for pragmatic data on region- and country-specific cutoff points for metabolic characteristics.
Participants on a DTG (INSTI)-based regimen were slightly more likely to have MetS. INSTI have only recently been instituted in Zambia, so experience with them was short at the time the study was conducted, but they have been associated with weight gain, especially DTG.[34,39,40] The expansion of body fat associated with some INSTI could be an influencing factor for MetS, as some molecules of INSTI affect lipid, glucose, and adipose tissue metabolism.[34] In addition, weight gain from INSTI has been associated with insulin resistance[41] and diabetes[42]; DTG has particularly been associated with hyperglycemia in PWH in Uganda.[43] Presently the mechanisms through which INSTI leads to adiposity and MetS are unknown. Other studies have also implicated PIs in the development of MetS,[44,45] an association that warrants further investigation.
We also found that VF was associated with increased odds of MetS, consistent with previous studies in the United States[46] and Italy.[47] A possible explanation is that HIV affects the metabolism of lipids,[48] especially HDL-c, one of the components of MetS. HIV-1 lowers plasma HDL by damaging the cholesterol-dependent efflux transporter ATP-binding cassette protein A1 in macrophages, and this condition also increases risk of atherogenesis.[49–51] As observed previously, HIV viral loads, higher in persons with VF, could be influencing MetS through low HDL-c.[25,52] It is also possible that persons with MetS are at higher risk of VF; our cross-sectional design prevents us from inferring causality, or the direction of influence if the association is causal. If VF increases risk for MetS, optimizing viral suppression could be a way to reduce MetS; if the association is causal in the opposite direction, addressing the components of MetS could be a way to optimize viral suppression.
Our finding was consistent with some previous studies on the association of T-lymphocyte counts with MetS.[37,53,54] This could be partly explained by the role of HIV-associated chronic inflammation on the production and clonal proliferation of T-lymphocytes.[55] Earlier reports have suggested that insulin and insulin growth factors (I and II), which are elevated in persons with insulin resistance, promote the proliferation of WBC.[52,53,56,57] In sub-Saharan Africa there is still limited information on MetS and hematological parameters.
We also observed that hip circumference was significantly associated with MetS. A similar finding was observed in China among HIV-negative men and women.[58] However, some studies have shown that larger hip circumference is associated with lower insulin resistance,[55,59] glucose, and triglycerides and higher levels of HDL-c.[60] Of note also is that visceral fat levels, measured by bioimpedance analysis, were significantly higher among MetS participants. This is supported by previous studies in Korea,[61] and a review paper.[62] Accumulation of visceral fat has been attributed to development of metabolic risk factors in both HIV-negative[62,63] and PWH.[64] Generally, altered anthropometric[65] and adipose tissue (such as visceral fat)[66] measurements have been associated with MetS in persons with and without HIV.[67,68]
We found an association between MetS and hsCRP, a marker of systemic inflammation, in keeping with previous studies.[69,70] A univariate association of TNF-alpha with MetS did not remain statistically significant in multivariable logistic regression. Earlier it has been shown that activation of 11-beta-hydroxysteroid dehydrogenase type-1 by TNF-alpha resulted in increased lipid accumulation in adipocytes and led to IR.[71] This could explain the mechanism through which TNF-alpha influences MetS. In addition, increased production of inflammatory cytokines has been linked to impaired insulin signaling, leading to hyperglycemia.[70]
The parent datasets did not include all possible behavioral factors associated with MetS, so our analysis may have missed some factors. Because the study was cross-sectional, we could not determine the temporal relationships between MetS and the explanatory variables. To do so will require prospective studies, but our findings provide evidence of a need to identify and target interventions toward PWH at high risk of MetS.
6. Conclusions
Our findings indicate that MetS is prevalent among adult patients receiving ART in our setting; it is well documented that individuals with MetS have about 75% higher cardiovascular risk compared with those without.[72] Screening for MetS is not routinely done in Zambia, so there is a possibility that metabolic CVD-related morbidities and mortalities are missed. Metabolic syndrome was associated with age, sex, VF, ART-regimen, hip circumference, T-lymphocytes, BMI, hsCRP, and fasting insulin. Our findings suggest need for routine screening for MetS throughout the course of ART. In sub-Saharan Africa, little is known about the metabolic effects of INSTI regimens, as they are relatively new to the region. Therefore, studies should be undertaken to confirm our findings and to understand the mechanisms involved in the INSTI effects on components of MetS, as it is currently the most recommended ART class in both ART-naïve and ART-experienced patients. Finally, there is need for more robust designs to assess MetS as patients start ART and observe metabolic related outcomes among PWH in our setting.
Acknowledgments
The authors thank the research assistants, Susan Banda, Royd Zulu, Choolwe Chilembo, and Namukulo Kayaka.
Author contributions
Conceptualization: Benson Malambo Hamooya, John R. Koethe, Loren Lipworth, Douglas C. Heimburger.
Data curation: Benson Malambo Hamooya, Lloyd B. Mulenga, Lameck Chirwa.
Formal analysis: Benson Malambo Hamooya, Isaac Fwemba, Loren Lipworth, Douglas C. Heimburger, Patrick Musonda, Wilbroad Mutale.
Funding acquisition: Douglas C. Heimburger.
Investigation: Benson Malambo Hamooya, Lloyd B. Mulenga, Sepiso K. Masenga, Lameck Chirwa, Mpanji Siwingwa.
Methodology: Benson Malambo Hamooya, Lloyd B. Mulenga, Isaac Fwemba, Hikabasa Halwiindi, John R. Koethe, Loren Lipworth, Douglas C. Heimburger, Patrick Musonda, Wilbroad Mutale.
Project administration: Benson Malambo Hamooya, Lloyd B. Mulenga.
Resources: Benson Malambo Hamooya, Lloyd B. Mulenga, Douglas C. Heimburger, Wilbroad Mutale.
Supervision: Benson Malambo Hamooya, Lloyd B. Mulenga, Hikabasa Halwiindi, John R. Koethe, Loren Lipworth, Douglas C. Heimburger, Patrick Musonda, Wilbroad Mutale.
Validation: Benson Malambo Hamooya, Lloyd B. Mulenga, Hikabasa Halwiindi.
Visualization: Benson Malambo Hamooya, Lloyd B. Mulenga.
Writing – original draft: Benson Malambo Hamooya.
Writing – review & editing: Benson Malambo Hamooya, Lloyd B. Mulenga, Sepiso K. Masenga, Isaac Fwemba, Lameck Chirwa, Mpanji Siwingwa, Hikabasa Halwiindi, John R. Koethe, Loren Lipworth, Douglas C. Heimburger, Patrick Musonda, Wilbroad Mutale.
Footnotes
Abbreviations: 3TC = Lamivudine, ABC/XTC = abacavir and lamivudine/emtricitabine, ART = antiretroviral therapy, ATV/r = atazanavir/ritonavir, AZT = zidovudine, BMI = body mass index, BP = blood pressure, CVD = cardiovascular disease, DTG = dolutegravir, EDTA = ethylenediaminetetraacetic acid, EFV = efavirenz, ELISA = Enzyme-Linked Immunosorbent Assay, HDL-c = high density lipoprotein cholesterol, HIV = human immunodeficiency virus, HOMA = homeostasis model assessment index, hsCRP = high-sensitivity C-reactive protein, INSTI = integrase strand transfer inhibitor, IQR = interquartile range, IR = insulin resistance, LPV/r = lopinavir/ritonavir, MeTS = metabolic syndrome, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTIs = nucleoside reverse transcriptase inhibitors, NVP = nevirapine, OR = odds ratio, PI = protease inhibitor, PWH = people with human, SSA = Sub-Saharan Africa, TC = total cholesterol, TDF/XTC = tenofovir disoproxil fumarate and lamivudine/emtricitabine, TNF-α = tumor necrosis factor alpha, UNZABREC = Zambia Biomedical Research Ethics Committee, VF = virological failure, VL = viral load, WBC = white blood cell, WC = waist circumference, ZNHREB = Zambia National Health Research Ethics Board.
How to cite this article: Hamooya BM, Mulenga LB, Masenga SK, Fwemba I, Chirwa L, Siwingwa M, Halwiindi H, Koethe JR, Lipworth L, Heimburger DC, Musonda P, Mutale W. Metabolic syndrome in Zambian adults with human immunodeficiency virus on antiretroviral therapy: Prevalence and associated factors. Medicine. 2021;100:14(e25236).
This work was supported by the Fogarty International Center of the National Institutes of Health under the Award Number D43 TW009744. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med J Br Diabet Assoc 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- [2].Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mazloomzadeh S, Karami Zarandi F, Shoghli A, et al. Metabolic syndrome, its components and mortality: a population-based study. Med J Islam Re∗pub Iran 2019;33:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alvarez C, et al. Metabolic syndrome in HIV-infected patients receiving antiretroviral therapy in Latin America. Braz J Infect Dis 2010;14:256–63. [PubMed] [Google Scholar]
- [5].Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep 2008;10:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lombo B, et al. Prevalence of metabolic syndrome in patients with HIV in the era of highly active antiretroviral therapy. Conn Med 2015;79:277–81. [PubMed] [Google Scholar]
- [7].Guira O, et al. Features of metabolic syndrome and its associated factors during highly active antiretroviral therapy in Ouagadougou (Burkina Faso). J Int Assoc Provid AIDS Care 2016;15:159–63. [DOI] [PubMed] [Google Scholar]
- [8].Li Vecchi V, Maggi P, Rizzo M, et al. The metabolic syndrome and HIV infection. Curr Pharm Des 2014;20:4975–5003. [DOI] [PubMed] [Google Scholar]
- [9].Biron A, et al. Metabolic syndrome in French HIV-infected patients: prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Res Hum Retroviruses 2012;28:1672–8. [DOI] [PubMed] [Google Scholar]
- [10].Bonfanti P, et al. The feature of Metabolic Syndrome in HIV naive patients is not the same of those treated: Results from a prospective study. Biomed Pharmacother 2012;66:348–53. [DOI] [PubMed] [Google Scholar]
- [11].Jacobson DL, et al. Incidence of metabolic syndrome in a Cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey). JAIDS J Acquir Immune Defic Syndr 2006;43:458. [DOI] [PubMed] [Google Scholar]
- [12].Gazzaruso C, Sacchi P, Garzaniti A, et al. Prevalence of metabolic syndrome among hiv patients. Diabetes Care 2002;25:1253–4. [DOI] [PubMed] [Google Scholar]
- [13].Palacios R, Santos J, González M, et al. Incidence and prevalence of the metabolic syndrome in a cohort of naive HIV-infected patients: prospective analysis at 48 weeks of highly active antiretroviral therapy. Int J STD AIDS 2007;18:184–7. [DOI] [PubMed] [Google Scholar]
- [14].Sobieszczyk ME, et al. Prevalence and predictors of metabolic syndrome among HIV-infected and HIV-uninfected women in the women's interagency HIV study. JAIDS J Acquir Immune Defic Syndr 2008;48:272. [DOI] [PubMed] [Google Scholar]
- [15].Nguyen KA, Peer N, Mills EJ, et al. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One 2016;11:e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Keates AK, Mocumbi AO, Ntsekhe M, et al. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat Rev Cardiol 2017;14:273–93. [DOI] [PubMed] [Google Scholar]
- [17].Alberti KGMM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [18].Grundy SM, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- [19].LeMessurier J, et al. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. CMAJ 2018;190:E1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].WHO. “Body mass index - BMI,”; 2020. Available at: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed Jan. 17, 2020). [Google Scholar]
- [21].Bune GT, Yalew AW, Kumie A. The global magnitude of metabolic syndrome among antiretroviral therapy (ART) exposed and ART-naïve adult HIV-infected patients in gedio-zone, southern Ethiopia: Comparative cross-sectional study, using the Adult Treatment Panel III criteria. Diabetes Metab Syndr 2019;13:2833–41. [DOI] [PubMed] [Google Scholar]
- [22].Mbunkah HA, Meriki HD, Kukwah AT, et al. Prevalence of metabolic syndrome in human immunodeficiency virus - infected patients from the South-West region of Cameroon, using the adult treatment panel III criteria. Diabetol Metab Syndr 2014;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Idiculla J, Ravindra’n GD, D'Souza J, et al. Diabetes mellitus, insulin resistance, and metabolic syndrome in HIV-positive patients in South India. Int J Gen Med 2011;4:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Theengh DP, Yadav P, Jain AK, et al. Assessment of metabolic syndrome in HIV-infected individuals. Indian J Sex Transm Dis AIDS 2017;38:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jantarapakde J, et al. Prevalence of metabolic syndrome among antiretroviral-naive and antiretroviral-experienced HIV-1 infected Thai adults. AIDS Patient Care STDs 2014;28:331–40. [DOI] [PubMed] [Google Scholar]
- [26].Policarpo S, Rodrigues T, Moreira AC, et al. Cardiovascular risk in HIV-infected individuals: a comparison of three risk prediction algorithms. Rev Port Cardiol 2019;38:463–70. [DOI] [PubMed] [Google Scholar]
- [27].Worm SW, et al. High prevalence of the metabolic syndrome in HIV-infected patients: impact of different definitions of the metabolic syndrome. AIDS 2010;24:427. [DOI] [PubMed] [Google Scholar]
- [28].Osoti A, et al. Metabolic syndrome among antiretroviral therapy-naive versus experienced hiv-infected patients without preexisting cardiometabolic disorders in Western Kenya. AIDS Patient Care STDs 2018;32:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Labhardt ND, et al. Metabolic syndrome in patients on first-line antiretroviral therapy containing zidovudine or tenofovir in rural Lesotho, Southern Africa. Trop Med Int Health 2017;22:725–33. [DOI] [PubMed] [Google Scholar]
- [30].Tesfaye DY, et al. Burden of metabolic syndrome among HIV-infected patients in Southern Ethiopia. Diabetes Metab Syndr 2014;8:102–7. [DOI] [PubMed] [Google Scholar]
- [31].Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa-a systematic review and meta-analysis. Syst Rev 2019;8:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pangmekeh PJ, Awolu MM, Gustave S, et al. Association between highly active antiretroviral therapy (HAART) and hypertension in persons living with HIV/AIDS at the Bamenda regional hospital, Cameroon. Pan Afr Med J 2019;33:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Martin-Iguacel R, Negredo E, Peck R, et al. Hypertension is a key feature of the metabolic syndrome in subjects aging with HIV. Curr Hypertens Rep 2016;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lagathu C, et al. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf 2019;18:829–40. [DOI] [PubMed] [Google Scholar]
- [35].Rogalska-Płońska M, Grzeszczuk A, Rogalski P, et al. Metabolic syndrome in HIV infected adults in Poland. Kardiol Pol 2018;76:548–53. [DOI] [PubMed] [Google Scholar]
- [36].Sears S, et al. Metabolic syndrome among people living with HIV receiving medical care in southern United States: prevalence and risk factors. AIDS Behav 2019;23:2916–25. [DOI] [PubMed] [Google Scholar]
- [37].Su B-Y, Tian C-F, Gao B-L, et al. Correlation of the leucocyte count with traditional and non-traditional components of metabolic syndrome. Postgrad Med 2016;128:805–9. [DOI] [PubMed] [Google Scholar]
- [38].Alencastro PR, Fuchs SC, Wolff FH, et al. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDs 2011;25:627–34. [DOI] [PubMed] [Google Scholar]
- [39].Sax PE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020;71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bourgi K, et al. Greater weight gain in treatment-naive persons starting Dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020;70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Katlama C, et al. Dual therapy combining raltegravir with etravirine maintains a high level of viral suppression over 96 weeks in long-term experienced HIV-infected individuals over 45 years on a PI-based regimen: results from the Phase II ANRS 163 ETRAL study. J Antimicrob Chemother 2019;74:2742–51. [DOI] [PubMed] [Google Scholar]
- [42].Fong PS, Flynn DM, Evans CD, et al. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS 2017;28:626–8. [DOI] [PubMed] [Google Scholar]
- [43].Lamorde M, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV 2020;7:e461–2. [DOI] [PubMed] [Google Scholar]
- [44].Katoto PDMC, et al. Prevalence and risk factors of metabolic syndrome in HIV-infected adults at three urban clinics in a post-conflict setting, eastern Democratic Rep∗ublic of the Congo. Trop Med Int Health 2018;23:795–805. [DOI] [PubMed] [Google Scholar]
- [45].Wohl DA, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis 2006;43:645–53. [DOI] [PubMed] [Google Scholar]
- [46].Krishnan S, et al. “Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naïve HIV-infected individuals”. J Acquir Immune Defic Syndr 1999 2012;61:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Squillace N, et al. Detectable HIV viral load is associated with metabolic syndrome. J Acquir Immune Defic Syndr 1999 2009;52:459–64. [DOI] [PubMed] [Google Scholar]
- [48].Dubé MP, Cadden JJ. Lipid metabolism in treated HIV Infection. Best Pract Res Clin Endocrinol Metab 2011;25:429–42. [DOI] [PubMed] [Google Scholar]
- [49].Hanley TM, Blay Puryear W, Gummuluru S, et al. PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog 2010;6:e1000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hanley TM, Viglianti GA. Nuclear receptor signaling inhibits HIV-1 replication in macrophages through multiple trans-repression mechanisms. J Virol 2011;85:10834–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].da Cunha J, Maselli LMF, Stern ACB, et al. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol 2015;4:56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Raposo MA, et al. Metabolic disorders and cardiovascular risk in people living with HIV/AIDS without the use of antiretroviral therapy. Rev Soc Bras Med Trop 2017;50:598–606. [DOI] [PubMed] [Google Scholar]
- [53].Chen H, et al. Lymphocyte to high-density lipoprotein ratio as a new indicator of inflammation and metabolic syndrome. Diabetes Metab Syndr Obes Targets Ther 2019;12:2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rodríguez CP, González MC, Aguilar-Salinas CA, et al. Peripheral lymphocytes, obesity, and metabolic syndrome in young adults: an immunometabolism study. Metab Syndr Relat Disord 2018;16:342–9. [DOI] [PubMed] [Google Scholar]
- [55].Moro-García MA, Mayo JC, Sainz RM, et al. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol 2018;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pasini E, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol 2010;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bersch N, Groopman JE, Golde DW. Natural and biosynthetic insulin stimulates the growth of human erythroid progenitors in vitro. J Clin Endocrinol Metab 1982;55:1209–11. [DOI] [PubMed] [Google Scholar]
- [58].Katz EG, Stevens J, Truesdale KP, et al. Hip circumference and incident metabolic risk factors in chinese men and women: The People's Rep∗ublic of China Study. Metab Syndr Relat Disord 2011;9:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rocha PM, Barata JT, Teixeira PJ, et al. Independent and opposite associations of hip and waist circumference with metabolic syndrome components and with inflammatory and atherothrombotic risk factors in overweight and obese women. Metabolism 2008;57:1315–22. [DOI] [PubMed] [Google Scholar]
- [60].Snijder MB, Zimmet PZ, Visser M, et al. Independent association of hip circumference with metabolic profile in different ethnic groups. Obes Res 2004;12:1370–4. [DOI] [PubMed] [Google Scholar]
- [61].Cho S-A, et al. Visceral fat area and serum adiponectin level predict the development of metabolic syndrome in a community-based asymptomatic population. PloS One 2017;12:e0169289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011;18:629–39. [DOI] [PubMed] [Google Scholar]
- [63].Nomura K, et al. Visceral fat accumulation and metabolic risk factor clustering in older adults. J Am Geriatr Soc 2010;58:1658–63. [DOI] [PubMed] [Google Scholar]
- [64].Lake JE. The fat of the matter: obesity and visceral adiposity in treated HIV infection. Curr HIV/AIDS Rep 2017;14:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang YJ, et al. Relationship between the optimal cut-off values of anthropometric indices for predicting metabolic syndrome and carotid intima-medial thickness in a Korean population. Medicine (Baltimore) 2019;98:e17620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest 2019;129:3990–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gadekar T, Dudeja P, Basu I, et al. Correlation of visceral body fat with waist-hip ratio, waist circumference and body mass index in healthy adults: a cross sectional study. Med J Armed Forces India 2020;76:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bhagwat P, et al. Changes in abdominal fat following antiretroviral therapy initiation in HIV-infected individuals correlate with waist circumference and self-reported changes. Antivir Ther 2017;22:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Samaras K, Wand H, Law M, et al. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007;30:113–9. [DOI] [PubMed] [Google Scholar]
- [70].Swami A. Metabolic syndrome and HIV infection. J HIV Retro Virus 2016;2: [Google Scholar]
- [71].Barbaro G. Visceral fat as target of highly active antiretroviral therapy-associated metabolic syndrome. Curr Pharm Des 2007;13:2208–13. [DOI] [PubMed] [Google Scholar]
- [72].Qiao Q, Gao W, Zhang L, et al. Metabolic syndrome and cardiovascular disease. Ann Clin Biochem 2007;44(pt 3):232–63. [DOI] [PubMed] [Google Scholar]


