Abstract
Background:
Several studies indicate the level of pretreatment lactate dehydrogenase (LDH) may be associated with the prognosis of patients receiving immune checkpoint inhibitors targeting programmed death receptor-1 (PD-1)/programmed death ligand 1 (PD-L1) which had been reported to dramatically improve the survival of patients with advanced or metastatic melanoma; however, no consensus has been reached because the presence of controversial conclusions. This study was to perform a meta-analysis to comprehensively explore the prognostic values of LDH for melanoma patients receiving anti-PD1/PD-L1 monotherapy.
Methods:
A systematic electronic search in the databases of PubMed, EMBASE and the Cochrane library was performed to identify all related articles up to April, 2020. The pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained to assess the prognostic values of pretreatment LDH in blood for overall survival (OS) and progression-free survival (PFS).
Results:
A total of 22 eligible studies involving 2745 patients were included. Of them, 19 studies with 20 results assessed the OS and the pooled analysis showed that an elevated pretreatment LDH level was significantly associated with a worse OS (HR = 2.44; 95% CI: 1.95–3.04, P < .001). Thirteen studies reported PFS and meta-analysis also revealed that a higher pretreatment LDH level predicted a significantly shorter PFS (HR, 1.61; 95% CI, 1.34–1.92; P < .001). Although heterogeneity existed among these studies, the same results were acquired in subgroup analyses based on sample size, country, study design, cut-off of LDH, type of PD-1/PD-L1 inhibitors and statistics for HRs (all HRs > 1 and P < .05).
Conclusion:
This meta-analysis suggests LDH may serve as a potential biomarker to identify patients who can benefit from anti-PD-1/PD-L1 and then schedule treatments.
Keywords: lactate dehydrogenase, melanoma, prognosis, programmed death ligand 1, programmed death receptor-1
1. Introduction
Melanoma is the third most common type of skin cancer (after squamous cell carcinoma and basal cell carcinoma).[1,2] Nevertheless, it represents the leading cause of skin cancer-related death,[2,3] which may be partially attributed to its capacity to metastasize to distant organs. Although there were no curative options for unresectable or metastatic melanoma, the advent of agents targeting the immune system has been reported to dramatically improve the prognosis of patients.[4–6] Clinically used immune checkpoint inhibitors (ICIs) include monoclonal antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA4) (i.e. ipilimumab), programmed death 1 (PD-1) (i.e. nivolumab and pembrolizumab)[4–6] and programmed death ligand 1 (PD-L1) (i.e. atezolizumab, durvalumab and avelumab).[7,8] Compared with ipilimumab, the anti-PD-1/PD-L1, as single agents, had higher clinical activity to improve the overall survival (OS), progression-free survival (PFS) and induce less adverse events.[9,10] Also, the toxicity was increased in the combination group compared to either single agent anti-CTLA4 or anti-PD-1 antibodies.[9,11] Therefore, single agent PD-1/PD-L1 antibodies may be more cost-effective [12] and acceptable [13] for patients with advanced or metastatic melanoma. Unfortunately, there were still approximately 65% of patients who could not benefit from the anti-PD-1/PD-L1 monotherapy.[8,14] Therefore, identifying predictive biomarkers for response to PD-1/PD-L1 inhibitors may be beneficial in guiding treatment selections.
Enhanced aerobic glycolysis (known as the Warburg effect) is the major pathway to provide the metabolic energy for cancer cells to achieve fast proliferation and metastasis. Lactate dehydrogenase (LDH) is an essential metabolic enzyme during the Warburg effect, which catalyzes the reversible conversion of pyruvate into lactate. Also, the accumulated lactate was proved to promote tumor immune escape by reducing the survival and cytolytic capacity of CD8+ T cells and natural killer cells.[15,16] Accordingly, we speculate that a high level of LDH in cancer patients may antagonize the effects of anti-PD-1/PD-L1 antibodies (which can prevent T-cell exhaustion by inhibiting the expression of PD-1/PD-L1)[17] and lead to a poor prognosis.[18] This potential prognostic value of LDH was verified in several studies on melanoma. For example, Chasseuil et al, reported that an increased pretreatment LDH level was significantly associated with a decreased OS [hazard ratios (HR) = 1.31; 95% confidence interval (CI) = 1.18–1.45; P = .01] and PFS (HR = 1.25; 95% CI: 1.13–1.38; P = .01) in patients with advanced melanoma after treatment with nivolumab.[19] The similar conclusion was also confirmed in the studies of Capone et al,[20] Ridolfi et al,[21] Ascierto et al,[22] Suo et al,[23] Cowey et al[24] and Seremet et al[25] who investigated the association between LDH level and outcomes of nivolumab or pembrolizumab treatment. However, whether LDH can serve as a prognostic biomarker for melanoma patients treated with anti-PD-1/PD-L1 antibodies, remains uncertain because some evidence suggested no significant correlations between pretreatment LDH and OS/PFS.[26–28] These conflicting results may be associated with small sample sizes in each individual study or their heterogeneity in study designs.
The goal of this study was to perform a meta-analysis to re-assess the prognostic value of LDH for melanoma patients treated with anti-PD-1/PD-L1 antibodies. Meta-analysis of all evidence may overcome the limitation from the small sample sizes in individual studies and increase the statistical power and hereby, the resultant conclusion may be believable. Furthermore, the subgroup analysis was also performed for studies with consistent designs to further confirm the conclusion.
2. Materials and methods
2.1. Search strategies
A systematic electronic search in the databases of PubMed, EMBASE and the Cochrane library was performed to identify all related articles published up to April, 2020, in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis. The combined search terms were as follows: (“immunotherapy” OR “immune checkpoint inhibitors” OR “programmed death ligand-1” OR “programmed death-1 receptor” OR “PD-1 inhibitor” OR “PD-L1 inhibitor” OR “anti-PD-1 antibodies” OR “nivolumab” OR “pembrolizumab” OR “atezolizumab” “avelumab” OR “durvalumab”) AND (“lactate dehydrogenase” OR “LDH”). The reference lists of original studies and reviews were also manually searched for potential eligible publications. The need of ethical approval and patient consent is waived because of a meta-analysis of the published studies.
2.2. Inclusion and exclusion criteria
Studies were included if they fulfilled the following inclusion criteria:
-
(1)
patients were diagnosed as melanoma by histology;
-
(2)
patients were treated with anti-PD-1/PD-L1 antibodies as a single agent;
-
(3)
the associations of pretreatment LDH in blood with prognosis (including OS and PFS) were reported; and
-
(4)
the data of HRs and 95% CIs could be directly extracted or indirectly estimated from Kaplan–Meier curves.
The studies were excluded if they:
-
(1)
belonged to duplicated articles from different databases;
-
(2)
were case reports, reviews, cell or animal studies;
-
(3)
did not provide sufficient data to estimate HRs and 95%CIs;
-
(4)
were unrelated to the topic of interest;
-
(5)
had the patients who received combined treatment with other ICIs or chemotherapy simultaneously or after anti-PD-1/PD-L1 cycle; and
-
(6)
were unpublished or published in non-English language.
2.3. Data extraction
Two researchers independently extracted the relevant data from all eligible studies, and disagreements regarding the definition on the cut-off of LDH were resolved by careful reading the articles and discussion to reach consensus. The extracted information included the name of the first author, publication year, country, median age of patients, case number, study design, follow-up, type of PD-1/PD-L1 inhibitors, cut-off value of LDH, HRs with 95% CIs for OS/PFS and the method for HR collection. HRs and 95%CIs were extracted preferentially from the multivariable analysis where available. If HRs and 95% CIs were not reported directly, they could be estimated from Kaplan-Meier curves using a digitizing software-Engauge Digitizer (version 4.1; http://digitizer.sourceforge.net/).
2.4. Quality assessment
Two reviewers independently assessed the methodological quality of all eligible studies according to Newcastle-Ottawa Scale (NOS).[29] Studies with a NOS score ≥7 stars were defined as having a high quality.[30]
2.5. Statistical analysis
The meta-analysis was performed using the STATA 13.0 software (STATA Corporation, College Station, TX). The pooled HRs and 95% CIs were used to assess the effects of elevated LDH levels on the prognosis in melanoma patients treated with PD-1/PD-L1 inhibitors. A pooled HR of > 1 indicated a poorer OS and PFS in patients with a higher pretreatment LDH level. The association between the level of LDH and the prognosis was thought to be statistically significant if the 95%CI did not overlap 1 and P-value determined by Z-test < 0.05. The statistical heterogeneity was evaluated by Cochrane Q test and I2 statistic. A random-effects model was used for pooled estimates if a significant heterogeneity was identified (P < .10 and I2 > 50%); if not, a fixed-effects model was applied. To explore the source of heterogeneity, a subgroup analysis was also conducted according to the sample size, country, study design, cut-off of LDH, type of PD-1/PD-L1 inhibitors, statistics for HR and HR source. Publication bias was examined by Egger's linear regression test.[31] If publication bias was present (P < .05), the “trim and fill” algorithm was employed for adjustment.[32] Sensitivity analysis was performed to further evaluate the influence of each study on pooled HR via removing 1 study in turn.
3. Results
3.1. Literature search
Figure 1 illustrates the process of literature selection. A total of 2116 records were initially retrieved by searching the online databases with keywords; 618 of them were excluded due to duplicate reports. After reading titles and abstracts, 1471 articles were eliminated because they were either review (n = 15), case reports (n = 19), cell studies (n = 330), animal studies (n = 507), studies without prognostic outcomes (n = 168), unrelated to the current topics (n = 420) or with patients undergoing anti-PD-1/PD-L1 + other ICIs combined treatments (n = 12). The remaining 27 articles were screened by full-text reviewing. As a result, 5 of them were discarded for the following reasons: patients in 3 studies received adjuvant chemotherapy or other ICIs treatment after the use of anti-PD-1/PD-L1; two failed to provide the data to estimate HRs and 95%CIs. Eventually, 22 eligible studies involving 2745 patients were enrolled in our meta-analysis.[19–28,33–44]
Figure 1.
Flowchart of the study selection.
3.2. Study characteristics
The characteristics of 22 included studies are shown in Table 1. These included studies were conducted in 11 countries [including Australia (n = 1), Belgium (n = 1), Canada (n = 1), France (n = 3), Germany (n = 2), Italy (n = 3), Japan (n = 3), Spain (n = 1), Sweden (n = 1), UK (n = 2), USA (n = 4)] and published from 2016 to 2020. The ICI agent for the treatment of these patients with advanced or metastatic melanoma was nivolumab in 7 studies; pembrolizumab in 8 studies; pembrolizumab or nivolumab in 7 studies. The data of most patients were retrospectively (19/22) collected from a single medical center (14/22). The study of Wagner et al[33] used 2 multivariate models to analyze the associations of LDH in blood samples with OS; thus, 19 studies with 20 results were used for OS meta-analysis. Thirteen studies reported the impact of LDH in blood samples on PFS. The cut-off of LDH was upper limit of normal (ULN) (although the value may also be different) in most studies; while some used the 1.5 ULN, 2 ULN or 2.5ULN as the threshold. HRs and 95% CIs were directly obtained from 17 studies (although some only used the univariate analysis), while indirectly estimated from the Kaplan-Meier curve in 5 studies. All studies had the NOS score ≥ 7, suggesting they were of high quality (Table 1).
Table 1.
Characteristics of included studies.
| Study | Year | Country | Case No. | Median age (range) | Disease status | Study design | Median follow-up | Type of Anti-PD-1/PD-L1 | Cut-off of LDH | Survival endpoint | Statistical method for HR | HR source | NOS |
| Failing JJ[26] | 2017 | USA | 133 | 61 (18–90) | Metastatic | Single-center, retrospective | 12 mo | Pembrolizumab | ULN | PFS, OS (both non-significant | PFS (UV), OS (MV) | Reported | 8 |
| Chasseuil E[19] | 2018 | France | 87 | 71 (27–92) | Advanced | Single-center, retrospective | 227 d | Nivolumab | ULN | PFS, OS (both significant) | UV | Reported | 9 |
| Wagner NB[33] | 2018 | Germany | 152 | – | Advanced | Single-center, retrospective | 9.9 mo | Pembrolizumab | 1.5ULN | OS (two MV models, one significant; one non-significant) | MV | Reported | 8 |
| Diem S[34] | 2016 | UK | 66 | 56 (49–68) | Metastatic | Single-center, retrospective | 9 mo | Pembrolizumab or nivolumab | ULN | OS (significant) | UV | Estimated from K-M | 9 |
| Heidelberger V[35] | 2017 | France | 63 | 65 (22–90) | Metastatic | Single-center, retrospective | 7 mo | Pembrolizumab or nivolumab | ULN | PFS (significant) | MV | Reported | 8 |
| Arheden A[36] | 2019 | Sweden | 116 | 66 (27–98) | Metastatic | Single-center, retrospective | 17 mo | Pembrolizumab or nivolumab | ULN | OS (significant) | UV | Reported | 8 |
| Capone M[20] | 2018 | Italy | 97 | 61 (21–85) | Advanced | Single-center, retrospective | – | Nivolumab | ULN | OS, PFS (both significant) | MV | Reported | 7 |
| Ridolfi L[21] | 2020 | Italy | 174 | 79 (75–93) | Metastatic | Multi-center, retrospective | 8.97 mo | Pembrolizumab or nivolumab | ULN | OS, PFS (both significant) | UV | Reported | 8 |
| Liu FX[37] | 2019 | USA | 359 | – | Advanced | Multi-center, retrospective | – | Pembrolizumab | ULN | OS (significant) | UV | Estimated from K-M | 7 |
| Ascierto PA[22] | 2019 | Italy | 71 | 61 (28–86) | Metastatic | Single-center, retrospective | – | Pembrolizumab or nivolumab | 2ULN | OS, PFS (both significant) | MV | Reported | 7 |
| Weide B[38] | 2016 | Germany | 512 | – | Advanced | Multi-center, retrospective | – | Pembrolizumab | 2.5ULN | OS (significant) | MV | Reported | 7 |
| Suo A[23] | 2020 | Canada | 143 | – | Advanced | Multi-center, retrospective | 24 mo | Pembrolizumab or nivolumab | ULN | OS, PFS (both significant) | MV | Reported | 8 |
| Cowey CL[24] | 2018 | USA | 168 | 66 (26–90) | Advanced | Multi-center, retrospective | 10.5 mo | Pembrolizumab | ULN | OS, PFS (both significant) | MV | Reported | 8 |
| Bocquet-Tremoureux S [39] | 2019 | France | 87 | – | Metastatic | Single-center, retrospective | 31 | Nivolumab | ULN | PFS | MV | Reported | 8 |
| Namikawa K [27] | 2020 | Japan | 14 | 60 (42–74) | Metastatic | Single-center, retrospective | 15 mo | Nivolumab | 2ULN | OS, PFS (both non-significant) | UV | Estimated from K-M | 8 |
| Nakamura Y[40] | 2016 | Japan | 93 | 67 (17–93) | Advanced | Multi-center, retrospective | – | Nivolumab | ULN | OS (significant) | MV | Reported | 7 |
| Gide TN[28] | 2019 | Australia | 27 | 67 | Metastatic | Single-center, retrospective | – | Pembrolizumab or nivolumab | ULN | PFS (non-significant) | UV | Reported | 7 |
| Seremet T[25] | 2019 | Belgium | 85 | 57 (27–82) | Metastatic | Single-center, prospective | 84 wk | Pembrolizumab | ULN | OS, PFS (both significant) | UV | Reported | 8 |
| Wang X[41] | 2016 | USA | 221 | 59.2 | Advanced | Single-center, prospective | – | Nivolumab | ULN | OS (significant) | MV | Reported | 7 |
| Yamazaki N[42] | 2017 | Japan | 23 | – | Advanced | Multi-center, prospective | – | Nivolumab | ULN | OS, PFS (both non-significant) | UV | Estimated from K-M | 8 |
| Karydis I[43] | 2016 | UK | 25 | 58 (32–83) | Metastatic | Single-center, retrospective | 225 d | Pembrolizumab | ULN | OS (significant) | UV | Estimated from K-M | 7 |
| Gonza’lez-Cao M[44] | 2016 | Spain | 29 | – | Advanced | Multi-center, retrospective | – | Pembrolizumab | ULN | OS (significant) | MV | Reported | 7 |
HR = hazard ratio, K-M = Kaplan-Meier curve, LDH = lactate dehydrogenase, MV = multivariate analysis, NOS = Newcastle-Ottawa Scale, OS = overall survival, PD-1 = programmed death receptor-1, PD-L1 = programmed death ligand 1, PFS = progression-free survival, ULN = upper limit of normal, UV = univariate analysis, w = week.
3.3. Correlation between pretreatment LDH level in blood samples and OS
The heterogeneity existed among the 19 studies with 20 results (I2 = 71.3%, P < .001), so a random-effect model was used to calculate pooled HRs. As shown in Figure 2, the pooled analysis suggested that an elevated pretreatment LDH level was significantly associated with a worse OS in patients treated with PD-1/PD-L1 inhibitors (HR = 2.44; 95% CI: 1.95–3.04, P < .001).
Figure 2.
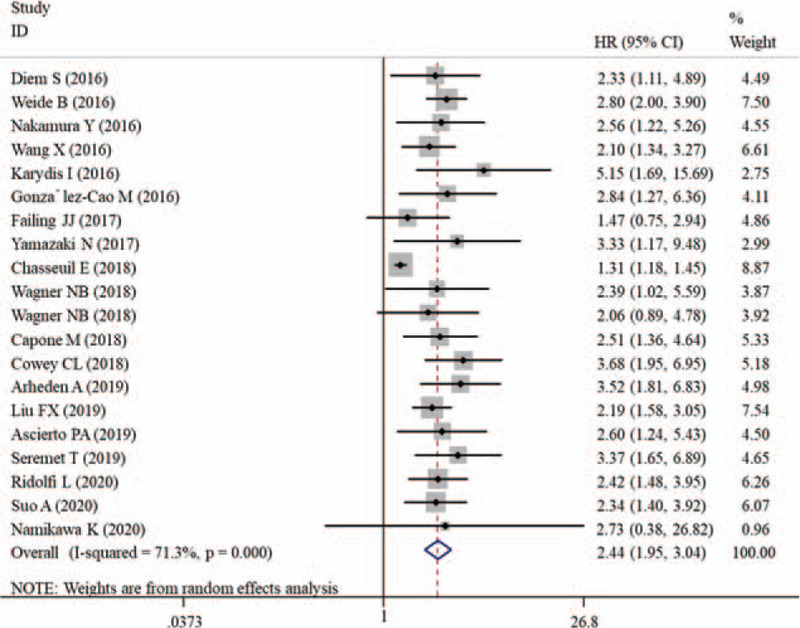
Forest plot of HR for the association between pretreatment lactate dehydrogenase and overall survival in melanoma patients receiving PD-1/PD-L1 inhibitors. CIs = confidence intervals, HR = hazard ratio, PD-1 = programmed death receptor-1, PD-L1 = programmed death ligand 1.
Subgroup analyses were then performed to explore the potential source of the heterogeneity. From the results in Table 2, we could see that melanoma patients with a higher LDH level had a poorer prognosis regardless of different sample sizes, countries, study designs, cut-offs of LDH, types of PD-1/PD-L1 inhibitors, statistical methods for HR and HR sourced, all with HRs > 1 and P < .05. But compared with overall estimates, the absence of a significant heterogeneity was seen in the analysis of studies with sample size > 100, non-European population, prospective-multi-center design, cut-off > ULN, pembrolizumab/mixed treatment and multivariate results (I2 < 50%, P > .1) which meant the influence of heterogeneity had been partially excluded.
Table 2.
Subgroup analysis on the association between LDH and OS.
| Comparison | Studies | HR (95%CI) | PA-value | I 2 | PH-value | Model |
| Sample size | ||||||
| <100 | 10 | 2.51 (1.72,3.66) | < .001 | 68.8 | .001 | R |
| >100 | 10 | 2.43 (2.08,2.84) | < .001 | 0.0 | .681 | F |
| Country | ||||||
| European | 13 | 2.46 (1.84,3.28) | < .001 | 76.5 | <.001 | R |
| Non-European | 7 | 2.34 (1.88,2.92) | < .001 | 0.0 | .623 | F |
| Study design | ||||||
| Retrospective | 17 | 2.40 (1.88,3.06) | < .001 | 73.1 | <.001 | R |
| Prospective | 3 | 2.49 (1.75,3.56) | < .001 | 0.0 | .462 | F |
| Single center | 12 | 2.28 (1.69,3.07) | < .001 | 66.5 | .001 | R |
| Multi-center | 8 | 2.57 (2.16,3.06) | < .001 | 0.0 | .897 | F |
| Cut-off of LDH | ||||||
| ULN | 15 | 2.42 (1.88,3.12) | < .001 | 73.7 | <.001 | R |
| 1.5ULN | 2 | 2.22 (1.22,4.03) | .009 | 0.0 | .808 | F |
| 2ULN | 2 | 2.61 (1.30,5.25) | .007 | 0.0 | .966 | F |
| 2.5ULN | 1 | 2.80 (2.01,3.91) | <.001 | – | – | F |
| Type of PD-1/PD-L1 inhibitors | ||||||
| Nivolumab | 6 | 2.02 (1.38,2.96) | <.001 | 64.0 | .016 | R |
| Pembrolizumab | 9 | 2.55 (2.13,3.07) | < .001 | 0.0 | .499 | F |
| Mixed | 5 | 2.56 (1.96,3.35) | < .001 | 0.0 | .892 | F |
| HR source | ||||||
| Reported | 15 | 2.38 (1.84,3.07) | <.001 | 75.1 | <.001 | R |
| Estimated | 5 | 2.41 (1.83,3.18) | < .001 | 0.0 | .645 | F |
| Statistics for HR | ||||||
| Multivariate | 11 | 2.48 (2.08,2.95) | <.001 | 0.0 | .881 | F |
| Univariate | 9 | 2.48 (1.72,3.59) | <.001 | 77.3 | < .001 | R |
CI = confidence interval, F = fixed-effects model, HR = hazard ratios, I2 = the degree of heterogeneity by I2 statistic, LDH = lactate dehydrogenase, OS = overall survival, PD-1 = programmed death receptor-1, PD-L1 = programmed death ligand 1, PH = P-value for heterogeneity measured by Q-test, PZ = P-value for association determined by Z-test, R = random-effects model, ULN = upper limit of normal.
3.4. Correlation between pretreatment LDH level in blood samples and PFS
There was also evidence of a significant heterogeneity among the 13 studies (I2 = 57.3%, P = .005) assessing the relationship between the LDH level and PFS, so a random-effect model was utilized to estimate the pooled effect size. Similar to the results of OS, our meta-analysis revealed that a higher pretreatment LDH level of patients treated with PD-1/PD-L1 inhibitors predicted a significantly shorter PFS (HR, 1.61; 95% CI, 1.34–1.92; P < .001) (Fig. 3).
Figure 3.
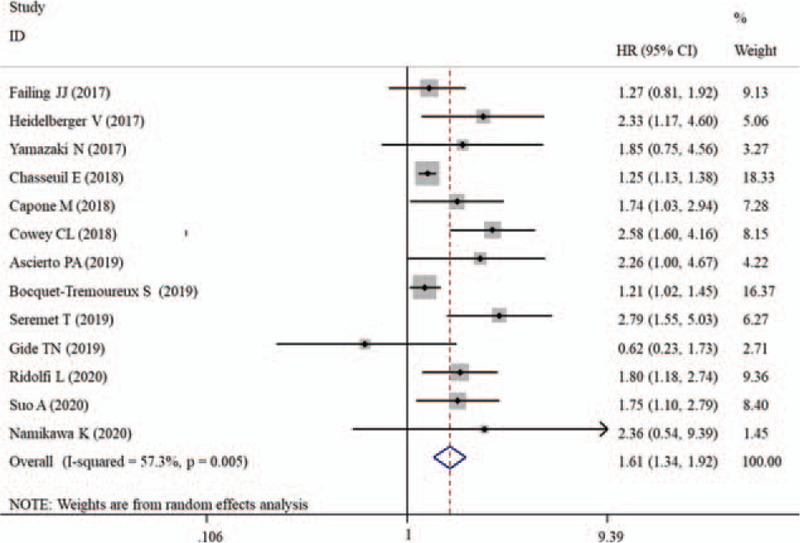
Forest plot of HR for the association between pretreatment lactate dehydrogenase and progression-free survival in melanoma patients receiving PD-1/PD-L1 inhibitors. CIs = confidence intervals, HR = hazard ratio, PD-1 = programmed death receptor-1, PD-L1 = programmed death ligand 1.
Then, stratified analyses were conducted. The results also demonstrated that this significant prognostic potential of pretreatment LDH for PFS was not changed after subgroup analyses according to sample size, country, study design, cut-off of LDH, type of PD-1/PD-L1 inhibitors and statistics for HR (Table 3). However, no significant association was observed any more between pretreatment LDH and PFS in studies with the HR estimated from the Kaplan-Meier curve (HR, 1.98; 95% CI, 0.93–4.25; P = .079). The significant heterogeneity also disappeared in the subgroup analysis of studies with non-European population, prospective-multi-center design, cut-off of 2ULN and nivolumab/mixed treatment multivariate results (I2 < 50%, P > .1), suggesting the results for them may be especially robust.
Table 3.
Subgroup analysis on the association between LDH and PFS.
| Comparison | Studies | HR (95%CI) | PZ-value | I 2 | PH-value | Model |
| Sample size | ||||||
| <100 | 11 | 1.55 (1.29,1.86) | < .001 | 51.7 | .023 | R |
| >100 | 2 | 1.80 (0.90,3.60) | .098 | 78.5 | .031 | R |
| Country | ||||||
| European | 7 | 1.56 (1.27,1.92) | <.001 | 62.3 | .014 | R |
| Non-European | 6 | 1.64 (1.15,2.33) | .006 | 42.9 | .119 | F |
| Study design | ||||||
| Retrospective | 11 | 1.52 (1.28,1.81) | < .001 | 53.6 | .018 | R |
| Prospective | 2 | 2.47 (1.51,4.04) | < .001 | 0.0 | .455 | F |
| Single center | 9 | 1.44 (1.20,1.74) | < .001 | 51.1 | .038 | R |
| Multi-center | 4 | 1.98 (1.54,2.54) | < .001 | 0.0 | .644 | F |
| Cut-off of LDH | ||||||
| ULN | 11 | 1.57 (1.31,1.89) | < .001 | 61.1 | .004 | R |
| 2ULN | 2 | 2.28 (1.16,4.50) | .007 | 0.0 | .958 | F |
| Type of PD-1/PD-L1 inhibitors | ||||||
| Nivolumab | 5 | 1.26 (1.16,1.37) | < .001 | 0.0 | .538 | F |
| Pembrolizumab | 3 | 2.04 (1.22,3.43) | .007 | 69.4 | .038 | R |
| Mixed | 5 | 1.76 (1.29,2.39) | <.001 | 22.6 | .271 | F |
| HR source | ||||||
| Reported | 11 | 1.60 (1.32,1.93) | <.001 | 63.0 | .003 | R |
| Estimated | 2 | 1.98 (0.93,4.25) | .079 | 0.0 | .778 | F |
| Statistics for HR | ||||||
| Multivariate | 6 | 1.80 (1.32,2.47) | <.001 | 63.9 | .016 | R |
| Univariate | 7 | 1.51 (1.14,1.98) | .004 | 52.7 | .048 | R |
CI = confidence interval, F = fixed-effects model, HR = hazard ratios, I2 = the degree of heterogeneity by I2 statistic, LDH = lactate dehydrogenase, PD-1 = programmed death receptor-1, PD-L1 = programmed death ligand 1, PFS = progression-free survival, PH = P-value for heterogeneity measured by Q-test, PZ = P-value for association determined by Z-test, R = random-effects model, ULN = upper limit of normal.
3.5. Sensitivity analysis and publication bias
Although Egger's linear regression test showed there was potential publication bias in the analysis of OS (p < 0.001) and PFS (p = 0.014), the adjusted results by the trim and fill method (Fig. 4) showed that the significant associations between an elevated LDH level and unfavorable OS (HR = 1.69; 95% CI: 1.37–2.08, P < .001) and PFS (HR = 1.38; 95% CI: 1.14–1.67, P < .001) were still present. Thus, the impact of publication bias on the pooled results may be weak.
Figure 4.
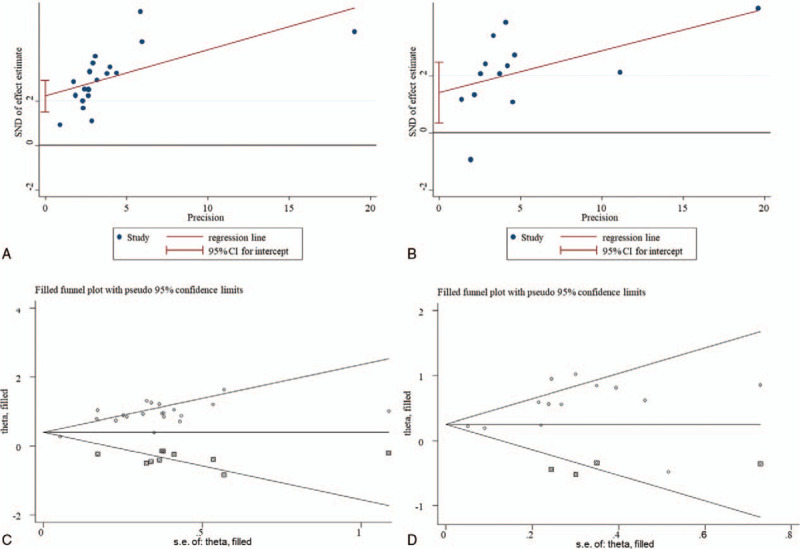
Funnel plot for the assessment of publication bias. A, Egger's funnel plot for overall survival; B, Egger's funnel plot for progression-free survival; C, trim and fill-adjusted funnel plot for overall survival; D, trim and fill-adjusted funnel plot for progression-free survival. CI = confidence intervals, SND = standard normal deviation, SE = standard error.
Sensitivity analysis was performed to further assess the robustness of the pooled HR assessing the association between LDH and OS/PFS by omitting single study in turn. The results showed that no single study significantly influenced the summary HRs (Fig. 5), indicating the consequence of this meta-analysis was stable and reliable.
Figure 5.
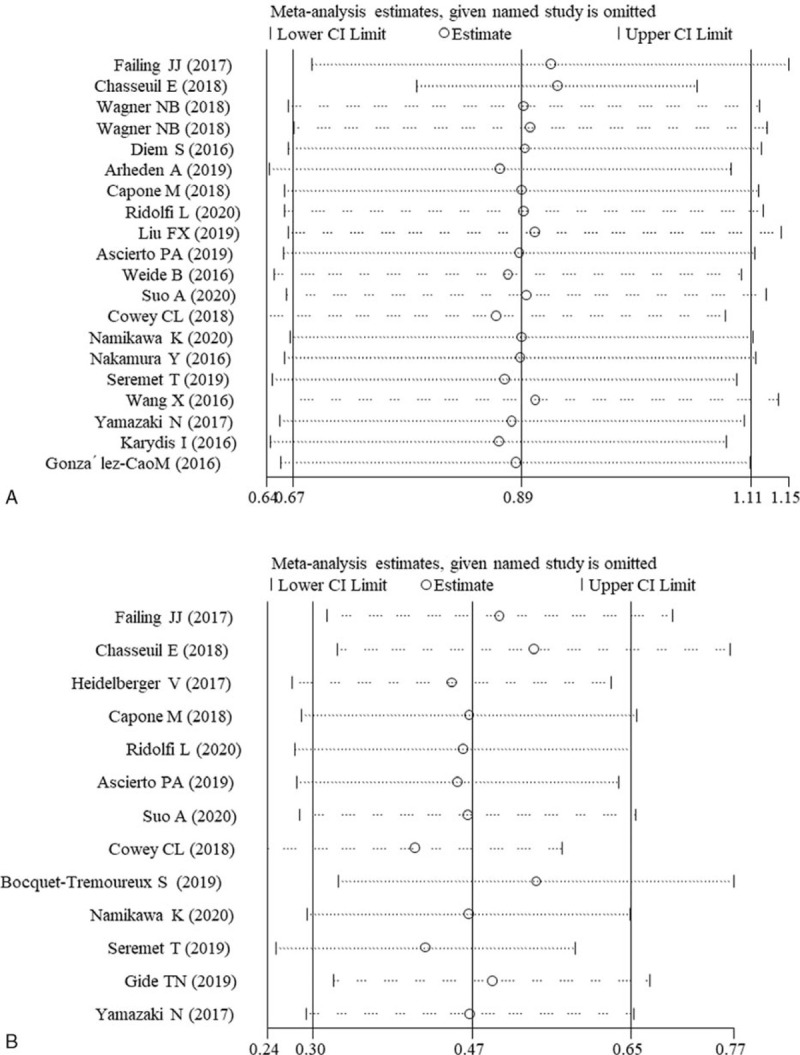
Sensitivity analysis. A, overall survival; B, progression-free survival. CI = confidence interval.
4. Discussion
Although there were several meta-analyses to investigate the prognostic values of pretreatment LDH level for cancer patients,[45–48] only 2 focused on the patients treated with ICIs: one was for non-small-cell lung cancer [18] and the other was for melanoma.[49] Also, in the study of Petrelli et al,[49] the literatures involving all ICIs (including anti-PD-1/PD-L1 monotherapy, anti-CTLA4 monotherapy and combined therapy) were integrated together for meta-analyses. In the present study, we, for the first time, attempted to confirm the prognostic significance of pretreatment LDH for melanoma patients undergoing anti-PD-1/PD-L1 monotherapy. Compared with the study of Petrelli et al[49] which searched the articles until 28 January 2018, our study newly enrolled some papers published in 2019 and 2020 to further increase the statistical power; thus, our conclusion may be more credible. This hypothesis has been confirmed in our analysis: overall estimate analysis using 19 and 13 publications showed that an elevated pretreatment LDH level was significantly associated with a poor OS (HR = 2.44) and PFS (HR = 1.61) in melanoma patients treated with anti-PD-1/PD-L1 single agents. The similar conclusions were also achieved in the subgroup analyses based on sample size, country, study design, cut-off of LDH, type of PD-1/PD-L1 inhibitors and statistics for HR. Sensitivity analysis and publication bias analyses also demonstrated the combined HR was stable. Accordingly, we got the conclusion that pretreatment LDH may serve as a potential prognostic biomarker for anti-PD-1/PD-L1 in patients with melanoma.
The potential mechanism to explain the inferior survival in the presence of elevated LDH levels is the activation of the Warburg effect (increased aerobic glycolysis) in advanced or metastatic melanoma. LDH is a key glycolytic enzyme responsible for pyruvate-to-lactate conversion, accompanied by the reproduction of oxidized nicotinamide adenine dinucleotide from reduced NADH for continued glycolysis. It had been widely observed that melanoma cells with suppressed proliferation, invasion and metastasis ability usually exhibited the characteristics of a decreased glucose uptake, lactate production, ATP generation, extracellular acidification rate, and an increased oxygen consumption rate.[50,51] The expression of genes encode for LDH was also increased in malignant melanoma compared with controls.[52–55] Knockout of LDH genes strongly reduced the LDH activity and lactate secretion as well as proliferation rates of melanoma in vitro and in vivo compared with their counterparts.[56] High serum LDH was found to be associated with a significant increase in LDH isoenzymes.[55] Hereby, a high concentration of LDH in blood may indirectly reflect the content and metabolism of LDH in the melanoma cells and predict the tumor progression and patient's prognosis.
In addition, the prognostic potential of LDH for patients receiving PD-1/PD-L1 inhibitors may be attributed to the correlation between LDH/lactate and PD-1/PD-L1-mediated immune response. PD-1 is a surface receptor expressed on various activated immune cells, such as T cells, macrophages and dendritic cells. It could interact with its ligand PD-L1 and then reduce the survival of CD8+ T cells and macrophages (M1 type) and their cytotoxicity on tumor cells, thus evading the immune surveillance and promoting the progression of cancers.[17,57,58] PD-1 expression on dendritic cells supported tumor growth by suppressing CD8 + T cell function.[59] Lactate was demonstrated to upregulate vascular endothelial growth factor and arginase 1 via the transcription factor hypoxia-inducible factor 1a and then induce macrophages skewed to M2-polarizated macrophages which represented a tumor-promoting state.[60] LDH-associated lactate accumulation in melanomas also inhibited tumor surveillance by diminishing the production of interferon-γ in T and natural killer cells.[15] Blood dendritic cells were dramatically depleted in melanomas, particularly in patients with a high LDH level. Exposure of lactic acidosis to dendritic cells impaired both the viability and functions of dendritic cells.[61] Based on these findings, we speculate LDH may exert similar functions to the activation of PD-1/PD-L1 pathway and therefore, the presence of a high LDH level in melanomas may antagonize the therapeutic effects of PD-1/PD-L1 inhibitors and lead to a poor prognosis.
The present study had some limitations. First, considerable heterogeneity was identified among studies. However, the similar results were obtained in the subgroup and sensitivity analysis, suggesting our results are stable and credible. Without doubt, whether there were other potential factors (i.e. gender) that influence the heterogeneity may be still uncertain due to limited information in the articles. Second, most of included articles were retrospective cohort studies which may introduce some unavoidable bias (such as selection bias). Third, although the results were significant using all the cut-off value of LDH, which is the optimal, remains uncertain because the number of studies with cut-off of LDH > 1.5, 2 and 2.5 ULN was relatively small. Fourth, the HRs extracted from the survival curve may introduce potential errors, which may be a potential reason to explain the non-significant association between LDH and PFS in the estimated HR subgroup. Fifth, the associations between LDH and some therapeutic outcomes (such as the response rate and adverse effect events) were not investigated due to lack of sufficient data and controversial conclusions. Sixth, all the included studies explored the effects for anti-PD-1 antibodies and no studies of anti-PD-L1 antibodies were enrolled. Seventh, the relationship between the mRNA expression status of LDH gene and the prognosis of melanoma patients was not explored like other genes.[62–66] Therefore, the significance of LDH for predicting the therapeutic effects in melanoma patients treated with anti-PD-1/PD-L1 needs to be validated and updated in the future.
5. Conclusion
Our meta-analysis shows that a high pretreatment LDH level is significantly associated with poor OS and PFS of melanoma patients treated with anti-PD-1/PD-L1. LDH may serve as a potential biomarker to identify patients who can benefit from anti-PD-1/PD-L1 and then schedule treatments.
Author contributions
Conceptualization: Jun Xu, Xiaoling Zhu.
Data curation: Jun Xu, Jianguo Zhao.
Formal analysis: Jun Xu, Jianguo Zhao.
Investigation: Jianguo Zhao.
Methodology: Jianfang Wang, Caiping Sun.
Project administration: Caiping Sun.
Resources: Jianfang Wang.
Software: Jianfang Wang, Caiping Sun.
Supervision: Xiaoling Zhu.
Writing – original draft: Jun Xu.
Writing – review & editing: Xiaoling Zhu.
Footnotes
Abbreviations: CI = confidence interval, CTLA4 = cytotoxic T-lymphocyte antigen 4, HR = hazard ratios, ICIs = immune checkpoint inhibitors, LDH = lactate dehydrogenase, NOS = Newcastle-Ottawa Scale, OS = overall survival, PD-1 = programmed death 1, PD-L1 = programmed death ligand 1, PFS = progression-free survival, ULN = upper limit of normal.
How to cite this article: Xu J, Zhao J, Wang J, Sun C, Zhu X. Prognostic value of lactate dehydrogenase for melanoma patients receiving anti-PD-1/PD-L1 therapy: a meta-analysis. Medicine. 2021;100:14(e25318).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Oh CM, Cho H, Won YJ, et al. Nationwide trends in the incidence of melanoma and non-melanoma skin cancers from 1999 to 2014 in South Korea. Cancer Res Treat 2018;50:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tejera-Vaquerizo A, Descalzo-Gallego MA, Otero-Rivas MM, et al. Skin cancer incidence and mortality in spain: a systematic review and meta-analysis. Actas Dermosifiliogr 2016;107:318–28. [DOI] [PubMed] [Google Scholar]
- [3].Garrett GL, Lowenstein SE, Singer JP, et al. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J Am Acad Dermatol 2016;75:106–12. [DOI] [PubMed] [Google Scholar]
- [4].Yun S, Vincelette ND, Green MR, et al. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med 2016;5:1481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pyo JS, Kang G. Immunotherapy in advanced melanoma: a network meta-analysis. Immunotherapy 2017;9:471–9. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Gu J. Efficacy and safety of PD-1 inhibitors for treating advanced melanoma: a systematic review and meta-analysis. Immunotherapy 2018;10:1293–302. [DOI] [PubMed] [Google Scholar]
- [7].Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med 2019;25:929–35. [DOI] [PubMed] [Google Scholar]
- [8].Keilholz U, Mehnert JM, Bauer S, et al. Avelumab in patients with previously treated metastatic melanoma: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 2019;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pasquali S, Chiarion-Sileni V, Rossi CR, et al. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: A network meta-analysis. Cancer Treat Rev 2017;54:34–42. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Gu J. Efficacy and safety of ipilimumab for treating advanced melanoma: a systematic review and meta-analysis. J Clin Pharm Ther 2019;44:420–9. [DOI] [PubMed] [Google Scholar]
- [11].Almutairi AR, McBride A, Slack M, et al. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol 2020;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang J, Chmielowski B, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naïve patients with advanced melanoma in the United States. J Manag Care Spec Pharm 2017;23:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khunger M, Patil PD, Khunger A, et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS One 2018;13:e0197743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health Syst Pharm 2016;73:193–201. [DOI] [PubMed] [Google Scholar]
- [15].Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 2016;24:657–71. [DOI] [PubMed] [Google Scholar]
- [16].Pucino V, Bombardieri M, Pitzalis C, et al. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur J Immunol 2017;47:14–21. [DOI] [PubMed] [Google Scholar]
- [17].Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007;56:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Z, Li Y. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med 2019;8:1467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chasseuil E, Saint-Jean M, Chasseuil H, et al. Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venereol 2018;98:406–10. [DOI] [PubMed] [Google Scholar]
- [20].Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ridolfi L, De Rosa F, Petracci E, et al. Anti-PD1 antibodies in patients aged ≥ 75 years with metastatic melanoma: a retrospective multicentre study. J Geriatr Oncol 2020;11:515–22. [DOI] [PubMed] [Google Scholar]
- [22].Ascierto PA, Capone M, Grimaldi AM, et al. Proteomic test for anti-PD-1 checkpoint blockade treatment of metastatic melanoma with and without BRAF mutations. J Immunother Cancer 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suo A, Chan Y, Beaulieu C, et al. Anti-PD1-induced immune-related adverse events and survival outcomes in advanced melanoma. Oncologist 2020;25:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cowey CL, Liu FX, Black-Shinn J, et al. Pembrolizumab utilization and outcomes for advanced melanoma in US community oncology practices. J Immunother 2018;41:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Seremet T, Jansen Y, Planken S, et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J Transl Med 2019;17:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Failing JJ, Yan Y, Porrata LF, et al. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res 2017;27:596–600. [DOI] [PubMed] [Google Scholar]
- [27].Namikawa K, Takahashi A, Mori T, et al. Nivolumab for patients with metastatic uveal melanoma previously untreated with ipilimumab: a single-institution retrospective study. Melanoma Res 2020;30:76–84. [DOI] [PubMed] [Google Scholar]
- [28].Gide TN, Silva IP, Quek C, et al. Close proximity of immune and tumor cells underlies response to anti-PD-1 based therapies in metastatic melanoma patients. Oncoimmunology 2020;9:1659093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [30].Liu Z, Cai L, Liu Y, et al. Association between prenatal cadmium exposure and cognitive development of offspring: a systematic review. Environ Pollut 2019;254(Pt B):113081. [DOI] [PubMed] [Google Scholar]
- [31].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- [33].Wagner NB, Forschner A, Leiter U, et al. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br J Cancer 2018;119:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 2016;114:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heidelberger V, Goldwasser F, Kramkimel N, et al. Clinical parameters associated with anti-programmed death-1 (PD-1) inhibitors-induced tumor response in melanoma patients. Invest New Drugs 2017;35:842–7. [DOI] [PubMed] [Google Scholar]
- [36].Arheden A, Skalenius J, Bjursten S, et al. Real-world data on PD-1 inhibitor therapy in metastatic melanoma. Acta Oncol 2019;58:962–6. [DOI] [PubMed] [Google Scholar]
- [37].Liu FX, Ou W, Diede SJ, et al. Real-world experience with pembrolizumab in patients with advanced melanoma: a large retrospective observational study. Medicine (Baltimore) 2019;98:e16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bocquet-Tremoureux S, Scharbarg E, Nguyen JM, et al. Efficacy and safety of nivolumab in metastatic melanoma: real-world practice. Eur J Dermatol 2019;29:315–21. [DOI] [PubMed] [Google Scholar]
- [40].Nakamura Y, Kitano S, Takahashi A, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016;7:77404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang X, Feng Y, Bajaj G, et al. Quantitative characterization of the exposure-response relationship for cancer immunotherapy: a case study of nivolumab in patients with advanced melanoma. CPT Pharmacometrics Syst Pharmacol 2017;6:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci 2017;108:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Karydis I, Chan PY, Wheater M, et al. Clinical activity and safety of Pembrolizumab in Ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 2016;5:e1143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].González-Cao M, Arance A, Piulats JM, et al. Pembrolizumab for advanced melanoma: experience from the Spanish Expanded Access Program. Clin Transl Oncol 2017;19:761–8. [DOI] [PubMed] [Google Scholar]
- [45].Kong W, Zuo X, Liang H, et al. Prognostic value of lactate dehydrogenase in patients with hepatocellular carcinoma: a meta-analysis. Biomed Res Int 2018;2018:1723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu D, Wang D, Wu C, et al. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: a meta-analysis. Cancer Manag Res 2019;11:3611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fu Y, Lan T, Cai H, et al. Meta-analysis of serum lactate dehydrogenase and prognosis for osteosarcoma. Medicine (Baltimore) 2018;97:e0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gan J, Wang W, Yang Z, et al. Prognostic value of pretreatment serum lactate dehydrogenase level in pancreatic cancer patients: a meta-analysis of 18 observational studies. Medicine (Baltimore) 2018;97:e13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Petrelli F, Ardito R, Merelli B, et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res 2019;29:01–12. [DOI] [PubMed] [Google Scholar]
- [50].Yang X, Zhao H, Yang J, et al. MiR-150-5p regulates melanoma proliferation, invasion and metastasis via SIX1-mediated Warburg Effect. Biochem Biophys Res Commun 2019;515:85–91. [DOI] [PubMed] [Google Scholar]
- [51].Yang X, Zhu X, Yan Z, et al. miR-489-3p/SIX1 axis regulates melanoma proliferation and glycolytic potential. Mol Ther Oncolytics 2020;16:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhuang L, Scolyer RA, Murali R, et al. Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod Pathol 2010;23:45–53. [DOI] [PubMed] [Google Scholar]
- [53].Koch A, Ebert EV, Seitz T, et al. Characterization of glycolysis-related gene expression in malignant melanoma. Pathol Res Pract 2020;216:152752. [DOI] [PubMed] [Google Scholar]
- [54].Schwab M, Ahuja MR, Anders F. Elevated levels of lactate dehydrogenase in genetically controlled melanoma of xiphophorin fish. Comp Biochem Physiol B 1976;54:197–9. [DOI] [PubMed] [Google Scholar]
- [55].Ho J, de Moura MB, Lin Y, et al. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol Cancer 2012;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ždralević M, Brand A, Di Ianni L, et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J Biol Chem 2018;293:15947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hartley GP, Chow L. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation 2018;6:1260–73. [DOI] [PubMed] [Google Scholar]
- [59].Lim TS, Chew V, Sieow JL, et al. PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology 2016;5:e1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Monti M, Vescovi R, Consoli F. Plasmacytoid dendritic cell impairment in metastatic melanoma by lactic acidosis. Cancers (Basel) 2020;12:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 2020;47:2509–19. [DOI] [PubMed] [Google Scholar]
- [63].Abdel Ghafar MT, Gharib F, Al-Ashmawy GM, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Reports 2020;20:100706. [Google Scholar]
- [64].Abdel Ghafar MT, Allam AA, Darwish S. Serum HOX transcript antisense RNA expression as a diagnostic marker for chronic myeloid leukemia. Egypt J Haematol 2019;44:91–7. [Google Scholar]
- [65].El-Guindy DM, Wasfy RE, Abdel Ghafar MT, et al. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst 2019;31:03. [DOI] [PubMed] [Google Scholar]
- [66].Habib EM, Nosiar NA, Eid MA, et al. Circulating miR-146a expression predicts early treatment response to imatinib in adult chronic myeloid leukemia. J Investig Med 2021;69:333–7. [DOI] [PubMed] [Google Scholar]


