Abstract
Many proteins select from a small repertoire of 3-dimensional folds retained over evolutional timescales and recruited for different functions, with changes in local structure and sequence to enable specificity. Recent studies have revealed the evolutionary constraints on protein dynamics to achieve function. The significance of protein dynamics in simultaneously satisfying conformational flexibility/malleability and stability/precision requirements becomes clear upon dissecting the spectrum of equilibrium motions accessible to fold families. Accessibility to highly conserved global modes of motions shared by family members, to low-to-intermediate-frequency modes that distinguish subfamilies and confer specificity, and to conserved high-frequency modes ensuring chemical precision and core stability underlies functional specialization while exploiting highly versatile folds. These design principles are illustrated for the family of PDZ domains.
Keywords: Protein Signature Dynamics; Elastic Network Models; normal modes; PDZ domain, global motions, conserved motions, functional differentiation; protein design and evolution
Introduction
The functions and interactions of proteins, complexes, or assemblies are predominantly affected by their intrinsic structural dynamics, which is defined by the 3-dimensional (3D) structure. The structure, in turn, is predominantly encoded by the amino acid sequence, which selectively evolves to ensure function, thus closing the loop. Intrinsic dynamics thus play a central role in the sequence → structure → dynamics → function → sequence cyclical dependency (Fig 1). It refers to the conformational flexibility, collective fluctuations, and allosteric domain movements that are intrinsically accessible to, or favored by, the 3D architecture. During functional interactions, most proteins perform movements in line with their intrinsic dynamics, to adapt to specific changes in their environment (such as ligand binding, changes in pH, multimerization, or complexation) while retaining to a large extent their fold. There are, of course, exceptions such as intrinsically disordered proteins/regions, which occupy unfolded states and can adopt different folds in different situations, such as binding to different partners [1]. Adaptability of the fold to different environments and intermolecular interactions is an evolutionary requirement for stability; but specificity is equally required for function. How do proteins fulfill such apparently conflicting requirements of flexibility/adaptability, on the one hand, and precision/specificity, on the other?
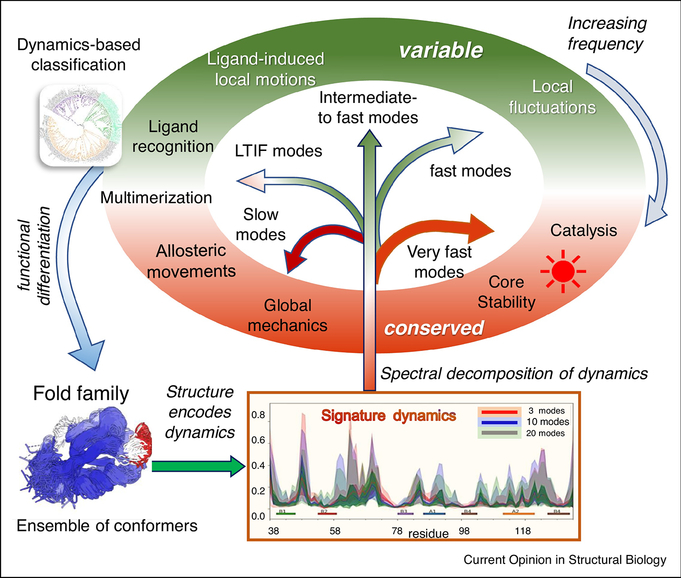
Figure 1: Significance of protein dynamics in enabling functional adaptation and specialization within protein families.
Various events, such as gene duplication and random mutagenesis, give rise to ensembles of homologous protein/domain structures, also called fold families (structurally aligned at the bottom left for PDZ domains). Each family has its unique structure-encoded dynamics under equilibrium conditions, represented by a spectrum of normal modes of motion, and a family-specific signature dynamics (middle bottom for PDZ domains). The proteins whose structures and dynamics are well adapted to do biological functions (in the elliptical shell) are evolutionarily retained/selected and vice versa. Hence, evolution leads to an iterative refinement of protein structure and dynamics to enable fold adaptation and functional specialization (curled blue arrow on the left). An ensemble normal mode analysis allows us to identify and compare the different frequency modes encoded by each structure in the ensemble. Notably, motions at two ends of the spectrum, shortly called global/slowest and very fast modes, are conserved (highlighted in red/brick in the elliptical shell) for diametrically opposed reasons: the former underlies the global mechanics/rearrangements required for adaptation to intermolecular interactions, including ligand binding and allosteric responses; the latter reflects key interactions that cannot tolerate sequence/structure perturbations, such as the highly stable packing at the core of the fold, or specific interactions at chemically active (e.g. catalytic) sites that are precisely retained as the fundamental characteristic feature of the family. LTIF modes usually define subfamilies, that tolerate variations within subfamilies, and drive fold adaptation and functional differentiation. They are conserved within, but vary between, subfamilies. Fast/intermediate modes show maximal variance between members within and across subfamilies (green portions of the ellipse).
The answer perhaps lies in two important facts. First, a ‘measured’ conformational flexibility entropically adds up to support stability, as long as the interactions that maintain the fold or assembly are not disrupted. Excessive conformational flexibility, on the other hand, would disrupt the favorable intra- and intermolecular interactions/energetics, thereby adversely affecting stability or functional associations. For example, the existence of a flexible/disordered loop at the binding epitope is usually required by design to optimize the interfacial interactions and ensure stable binding; but complete lack of a structure or scaffold supporting this loop’s rearrangements may easily lead to loss of function (inability to bind). Second, regarding precision or specificity, the flexible (loop) regions actually offer excellent frameworks to accommodate varieties of amino acids, the specific sequence of which would also drive specificity. Not surprisingly, recognition loops such as the complementarity determining regions (CDRs or hypervariable loops) of antibodies enjoy high structural variability or dynamics that goes hand-in-hand with their sequence variability. In a sense, flexibility assists in specificity, by allowing for the evolutionary selection of the amino acids that would ensure specificity.
A closer look at dynamics as a means of reconciling flexibility and specificity
A deeper understanding of the subtle balance between flexibility/adaptability and precision/specificity using both first physical principles as well as evolutionary perspectives is of crucial importance in many research areas, from synthetic biology to protein engineering to pharmacology. Attention has been directed to assessing protein dynamics as a means of reconciling the need for flexibility and specificity [2–4]. A growing field centers around protein dynamics for analyzing protein evolution, both ancestral [2–11] as well as synthetic/directed [2,5,12–16]. Recent advances in cryo-electron microscopy and computational methods are facilitating the study of large and dynamic systems [17,18], including whole viral capsids in different conformational states [19].
A key step for these goals is dissection of the dynamics of the protein of interest. In physical terms, this amounts to decomposing the intrinsic dynamics into a spectrum of modes and evaluating the significance of the modes of different types/frequencies in fulfilling different roles or enabling different types of function, as will be elaborated below. Such studies are facilitated by elastic network models (ENMs) [20–23], especially the Gaussian network model (GNM) [21] and the anisotropic network model (ANM) [20], which provide robust analytical solutions to this problem [15,24,25].
While computational tools and hardware for all-atom molecular dynamics (MD) simulations are constantly improving, evaluation of intrinsic dynamics using ENMs remains orders of magnitude faster, and ENM-predicted global modes show strong correlations with the essential motions observed in sufficiently long MD simulations [26,27]. As such, ENMs have provided efficient frameworks for analyzing biomolecular systems conformational dynamics over the last two decades [20–23]. They have also provided new insights into the role of intrinsic dynamics in achieving the balance between evolutionary adaptability and functional specificity as described next.
Comparative studies of intrinsic dynamics among family members reveal how dynamics evolved to ensure specialization or differentiation
The high level of redundancy in the Protein Data Bank (PDB) [28] has recently enabled a number of comparative studies, showing that just as protein families have conserved sequence and structural motifs, they also have distinct conserved patterns of signature dynamics [2–4,8,29,30]. This is particularly evident when the dynamic fluctuations are decomposed into different modes of motion [2,3,8,29]. SignDy [3] was recently introduced as a pipeline for characterizing the signature dynamics of protein families, using the ProDy [31] framework for sequence, structure, and dynamics analyses. Briefly, SignDy workflow consists of: (1) Alignment of an ensemble of proteins structures that share the same fold; (2) Normal mode calculation for each ensemble member using ENMs; (3) Evaluation of the signature dynamics by determining the shared global modes; and (4) Classification of family members based on the most discriminative modes that drive functional specificity or evolutionary differentiation. The Reuter, Echave and Micheletti groups have also made significant contributions in this area [2,32,33] and developed servers such as WEBnm@ [34] and ALADYN [35]. The Jernigan lab also showed the importance of evolving dynamics for the identification of functional sites [36–38].
Application to CATH superfamilies and specific case studies for transporters, triosephosphate isomerase (TIM) and related α/β barrel-containing enzymes, type-1 periplasmic binding protein clamshell domains, and lipoxygenases [2,3,8,39] have revealed how proteins select from the available repertoire of normal modes to perform various types of activities (Fig. 1), ranging from global cleft opening/closing and allosteric transitions, to high frequency fluctuations reflective of the energy localization at the core of the folds. As illustrated in Figure 1, we can approximately divide the spectrum of modes into four regimes: slowest or global modes, which account for the large, collective rearrangements of the overall structure, low-to-intermediate frequency (LTIF) modes, intermediate-to-fast modes, and very fast modes; the respective mode numbers vary with protein size. The emerging features are: (i) The global modes are conserved across protein fold family members [2–4,8,9,29,30,40]; (ii) subfamilies and specific family members exhibit differentiated motions in the LTIF regime, which influence functional specificity as revealed by recent studies [3,8]; (iii) The fastest modes are also highly conserved [3,8], their conservation being required for chemical precision, reactivity (e.g. catalysis), or structural stability [41]. The first two groups and their variance across family members define the signature dynamics of the family.
A case study of PDZ domains provides insights into mechanisms of adaptation and specialization
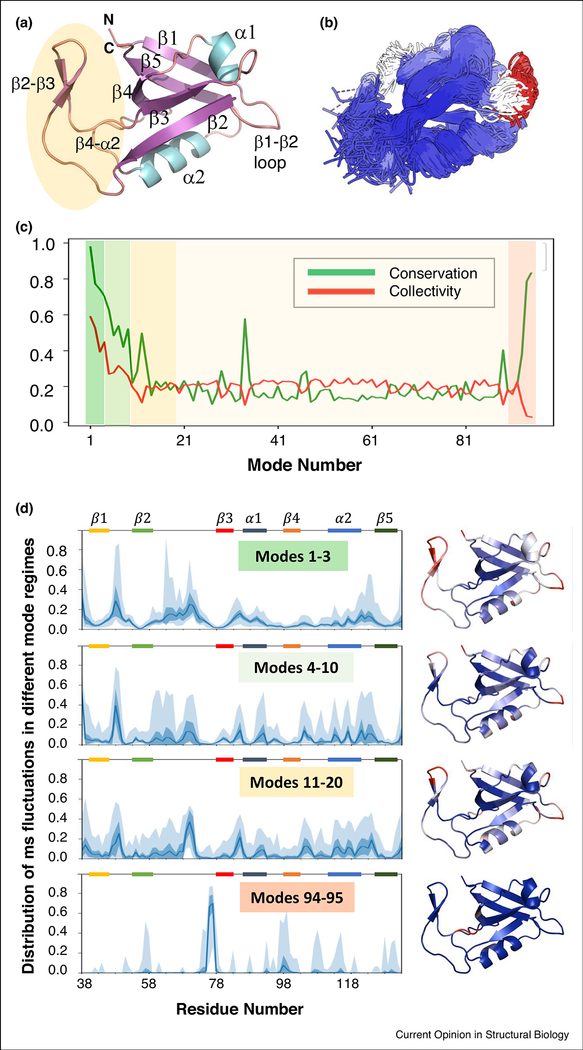
PDZ domains are found in a variety of signaling and scaffolding proteins [42–46], especially at cell junctions such as synapses [47–50]. PDZ domains consists of five β-strands and two α-helices (Fig. 2A). They exhibit a wide range of conformational plasticity and specificity [43–46]. Their extensive functional range renders them critical drug targets [42]. The β-sheet core and close juxtaposition of α2, decorated by flexible loops especially the β2-β3 loop (yellow), provide a highly versatile architecture for efficiently recognizing and binding ligands.
Figure 2: An example signature dynamics analysis of the PDZ domain family illustrates the roles of different mode regimes.
(A) The representative PDZ domain from CATH [55]: sorting nexin-27 (SNX27) bound to PTHR PDZ-binding motif (PDB: 4Z8J) [56], which is critical for PTHR signaling and bone development [56,57]. (B) Overlay of the ensemble of PDZ domain family structures, colored by the extent of structural variation with the most variable regions in red, structurally conserved parts in blue, and regions with intermediate variability in white. The ensemble was aligned using mappings from DALI [58] and refined using RMSD filters of 1.0 Å to reduce redundancy and 10.0 Å to exclude outliers. (C-D) SignDy analysis of this ensemble. (C) Conservation of GNM modes (green curve, based on pairwise overlaps between matched modes of family members) shows high conservation of global modes (dark green shaded; 1 ≤ k ≤ 3), less conservation for intermediate/fast modes (light yellow), and conservation again for the very fast modes (dark orange shaded). LTIF modes show a mixed behavior: the slow portion (light green shaded) shows a moderate conservation and intermediate portion (yellow shaded) is less conserved like the fast modes. The red curve shows the degree of collectivity, which shows anticorrelation with the green curve throughout the spectrum except for global and slow modes. (D) Mean-square (ms) fluctuations (MSFs) of residues for different frequency windows (left) and corresponding color-coded structures (right). In the left panels, the dark blue curves represent the average MSF profile of the ensemble, blue shades represent the standard deviation, and the light blue area displays the range. Global and LTIF modes are highly collective and activate the β2-β3 loop, followed by β1-β2 and α2-β5 loops; the highest frequency modes 94–95 point to the core region (at β3 N-terminal end) as a center of energy localization.
Not surprisingly, the PDZ domain has been the focus of several studies exploring its dynamics and allostery, as well as thermodynamics vis-à-vis its ligand-binding properties [46,51–53]. We present in Figures 2 and 3 results from SignDy analysis of a PDZ domain structure ensemble. The GNM mode spectra for family members yielded several features consistent with those observed in other fold families: First, the global modes are highly conserved among family members (shaded green in Fig 2C). The level of conservation levels off at about mode 15, after which it remains flat (with fluctuations), except for the highest frequency end of the spectrum (orange), where we observe a sharp peak. This high conservation at the two ends of the spectrum has been noticed previously and attributed to function and stability requirements (see Fig 1). Second, while the degree of collectivity of the modes (red curve in Fig 2C) decreases with increasing mode number for global modes, a heterogeneous behavior is observed in the LTIF regime: the slower portion (4 ≤ k ≤ 10) follows the same pattern as the global modes, but modes 11 ≤ k ≤ 20 show an anticorrelation between collectivity and conservation, i.e. the more localized motions tend to be more conserved.
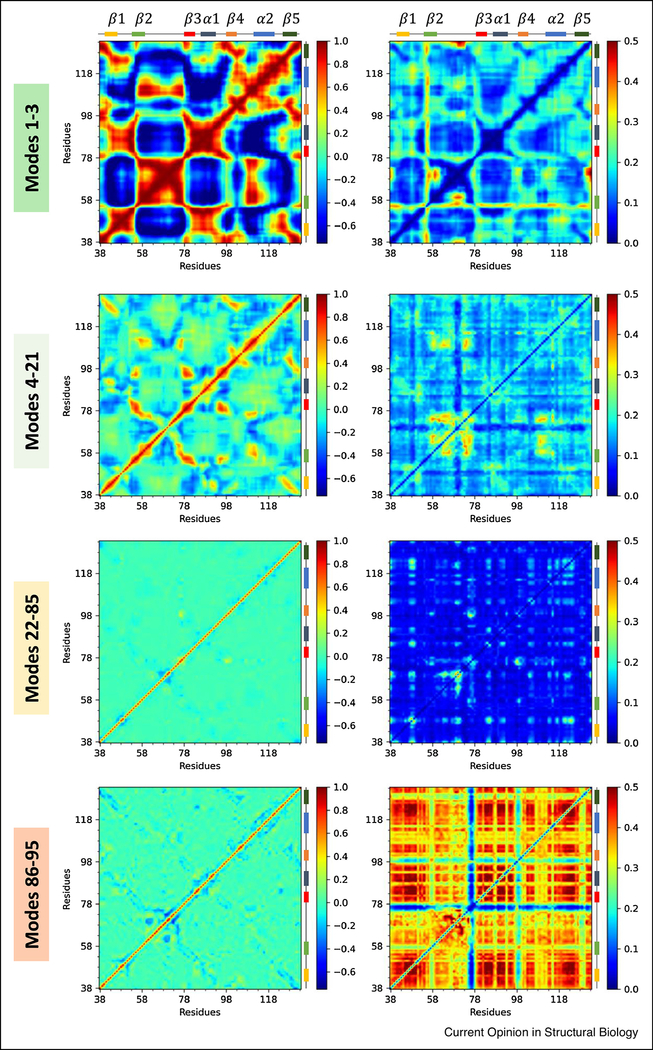
Figure 3: Cross-correlations between residue movements and their variance, in different frequency regimes, illustrated for the PDZ domain family.
Four panels show the mean (left) and standard deviation (right) values for the cross-correlations between residue movements, corresponding to global, LTIF, intermediate-to fast and very fast regimes, from top to bottom. The left matrices are colored from anti-correlated in dark blue to positively correlated in dark red via uncorrelated in green. The right matrices are colored from less variable in dark blue to more variable in dark red. Note the strong correlations in the global modes (top left) and the strongly coupled variations in cross-correlations in the fastest modes (bottom right), which show the concerted movements in the soft modes and the necessity for co-varying residue motions in the fastest/stiffest modes.
The distributions of residue mean-square fluctuations (MSFs) in different regimes (Fig 2D) show that the global hinge regions (minima in top panel) exhibit minimal variations among family members. The fastest modes (lowest panel and ribbon diagram) are dominated by fluctuations of L76-Q77 at the N-terminal end of β3, a critical hotspot that bridges binding site element β2 with the rest of the structure. This is in striking contrast to the slow and LTIF regimes where the MSFs are distributed across the domain (Fig 2D top three panels). We also notice a relatively greater degree of variability for the LTIF modes (middle two panels) in line with previous studies [3,8]. The modes in this regime are typically conserved within subfamilies but differentiated across subfamilies, thus defining subfamily specificities. Notably, high variances in LTIF modes occurs at the β2-β3 and α2-β5 loops. The β2-β3 loop and the crevice between β2 andα2 have been identified as ligand binding sites [54].
Residue-residue cross-correlations in different regimes can be seen in Fig 3. The left panels display the average cross-correlations in different regimes, and right panels, their variance. As expected, the global modes (top panel) exhibit robust cross-correlations between en bloc movements of pairs of structural elements moving in the same (red) or opposite (blue) directions. Strong cross-correlations can be detected in the LTIF regime as well, although concertedly moving regions become smaller, and the couplings weaker. Again, the largest variations across family members in this regime take place within the β2-β3 loop, and between that loop and the spatially neighboring β4-α2 loop, pointing to the dominant role of this region in functional differentiation consistent with experimental data. The remaining modes (k > 21) lack any shared feature that stands out, but the variations in pairwise correlations in the fastest mode regime (bottom, right panel) are far from random, reflecting the necessity of coupled rearrangements in the densely packed (core) substructures. These constraints are consistent with the decrease in the rate of evolution with increasing packing density, noted by Echave [32].
Conclusions and Future Directions
In this review, we emphasized how proteins select from a repertoire of modes to enable their functional evolution, and reconcile the need for flexibility/malleability, on the one hand, and precision/specificity, on the other. The slowest modes of protein domains, or small proteins, are recruited and conserved by a variety of sequences that share the same fold, despite their low sequence similarity, because these define highly versatile mechanisms (e.g. cleft opening/closing) that can be ‘plugged in’ within the complex machinery of biomolecular systems. On the other hand, the LTIF regime is where much of the specificity in intrinsic dynamics of family members is found. The focus on dynamics brings into consideration a new way of classifying proteins, based on their shared or differentiated dynamics. The latter could be equally (if not more) informative compared to sequence- or structure-based classifications, as it relates to functional mechanisms and could provide a new dimension in protein design and engineering.
Acknowledgment
We gratefully acknowledge support from NIH grant P41 GM103712 (IB) and a MolSSI/NSF COVID-19 Seed Software Fellowship (JK). Useful discussions with Dr. She (John) Zhang are gratefully acknowledged.
Footnotes
Conflict of interest statement
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Berlow RB, Dyson HJ, Wright PE: Expanding the Paradigm: Intrinsically Disordered Proteins and Allosteric Regulation. J Mol Biol 2018, 430:2309–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari SP, Reuter N: Conservation of intrinsic dynamics in proteins-what have computational models taught us? Curr Opin Struct Biol 2018, 50:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Li H, Krieger JM, Bahar I: Shared Signature Dynamics Tempered by Local Fluctuations Enables Fold Adaptability and Specificity. Mol Biol Evol 2019, 36:2053–2068.** This paper introduces the SignDy pipeline for identifying and comparing signature dynamics of protein families and uses it to perform a systematic analysis of 116 CATH superfamilies. It reveals several important features in the evolution of protein dynamics for adaptability and specificity, including the roles of motions in different frequency regimes.
- 4.Zsolyomi F, Ambrus V, Fuxreiter M: Patterns of Dynamics Comprise a Conserved Evolutionary Trait. J Mol Biol 2020, 432:497–507.* This paper presents a systematic computational analysis of conservation of protein dynamics and reveals that certain patterns of dynamics are highly conserved across Pfam families. Their method predicts overall residue dynamics from sequence.
- 5.Ben-David M, Soskine M, Dubovetskyi A, Cherukuri KP, Dym O, Sussman JL, Liao Q, Szeler K,Kamerlin SCL, Tawfik DS: Enzyme Evolution: An Epistatic Ratchet versus a Smooth Reversible Transition. Mol Biol Evol 2020, 37:1133–1147. [DOI] [PubMed] [Google Scholar]
- 6.Kaltenbach M, Burke JR, Dindo M, Pabis A, Munsberg FS, Rabin A, Kamerlin SCL, Noel JP,Tawfik DS: Evolution of chalcone isomerase from a noncatalytic ancestor. Nat Chem Biol 2018, 14:548–555. [DOI] [PubMed] [Google Scholar]
- 7.Liang Z, Hu J, Yan W, Jiang H, Hu G, Luo C: Deciphering the role of dimer interface in intrinsic dynamics and allosteric pathways underlying the functional transformation of DNMT3A. Biochim Biophys Acta Gen Subj 2018, 1862:1667–1679. [DOI] [PubMed] [Google Scholar]
- 8.Mikulska-Ruminska K, Shrivastava I, Krieger J, Zhang S, Li H, Bayir H, Wenzel SE, VanDemark AP, Kagan VE, Bahar I: Characterization of Differential Dynamics, Specificity, and Allostery of Lipoxygenase Family Members. J Chem Inf Model 2019, 59:2496–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabis A, Risso VA, Sanchez-Ruiz JM, Kamerlin SC: Cooperativity and flexibility in enzyme evolution. Curr Opin Struct Biol 2018, 48:83–92.** The autors review the role of different kinds of conformational dynamics in enzyme evolution using a number of widely studied examples and illustrate drivers of promiscuity and adaptability.
- 10.Tawfik DS, Gruic-Sovulj I: How evolution shapes enzyme selectivity - lessons from aminoacyl-tRNA synthetases and other amino acid utilizing enzymes. FEBS J 2020, 287:1284–1305. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Doruker P, Kaynak B, Zhang S, Krieger J, Li H, Bahar I: Intrinsic dynamics is evolutionarily optimized to enable allosteric behavior. Curr Opin Struct Biol 2019, 62:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z, Liu Q, Qu G, Feng Y, Reetz MT: Utility of B-Factors in Protein Science: Interpreting Rigidity, Flexibility, and Internal Motion and Engineering Thermostability. Chem Rev 2019, 119:1626–1665. [DOI] [PubMed] [Google Scholar]
- 13.Campbell EC, Correy GJ, Mabbitt PD, Buckle AM, Tokuriki N, Jackson CJ: Laboratory evolution of protein conformational dynamics. Curr Opin Struct Biol 2018, 50:49–57. [DOI] [PubMed] [Google Scholar]
- 14.Otten R, Liu L, Kenner LR, Clarkson MW, Mavor D, Tawfik DS, Kern D, Fraser JS: Rescue of conformational dynamics in enzyme catalysis by directed evolution. Nat Commun 2018, 9:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flechsig H, Togashi Y: Designed Elastic Networks: Models of Complex Protein Machinery. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maria-Solano MA, Serrano-Hervas E, Romero-Rivera A, Iglesias-Fernandez J, Osuna S: Role of conformational dynamics in the evolution of novel enzyme function. Chem Commun (Camb) 2018, 54:6622–6634.* The authors provide a good review of recent computational and experimental methods for probing the role of conformational dynamics on the evolution of novel enzyme functions.
- 17.Callaway E: Revolutionary cryo-EM is taking over structural biology. Nature 2020, 578:201. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y: Single-particle cryo-EM-How did it get here and where will it go. Science 2018, 361:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huet A, Duda RL, Boulanger P, Conway JF: Capsid expansion of bacteriophage T5 revealed by high resolution cryoelectron microscopy. Proc Natl Acad Sci U S A 2019, 116:21037–21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atilgan AR, Durell SR, Jernigan RL, Demirel MC, Keskin O, Bahar I: Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys J 2001, 80:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahar I, Atilgan AR, Erman B: Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Folding and Design 1997, 2:173–181. [DOI] [PubMed] [Google Scholar]
- 22.Hinsen K: Analysis of domain motions by approximate normal mode calculations. Proteins 1998, 33:417–429. [DOI] [PubMed] [Google Scholar]
- 23.Tirion MM: Large Amplitude Elastic Motions in Proteins from a Single-Parameter, Atomic Analysis. Phys. Rev. Lett. 1996, 77:1905–1908. [DOI] [PubMed] [Google Scholar]
- 24.Dill KA, Bahar I, Jernigan RL: Protein Actions: Principles and Modeling. New York: Garland Science; 2017. [Google Scholar]
- 25.Leioatts N, Romo TD, Grossfield A: Elastic Network Models are Robust to Variations in Formalism. J Chem Theory Comput 2012, 8:2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekpinar M, Yildirim A: Only a Subset of Normal Modes is Sufficient to Identify Linear Correlations in Proteins. J Chem Inf Model 2018, 58:1947–1961. [DOI] [PubMed] [Google Scholar]
- 27.Gur M, Zomot E, Bahar I: Global motions exhibited by proteins in micro- to milliseconds simulations concur with anisotropic network model predictions. J Chem Phys 2013, 139:121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marino-Buslje C, Monzon AM, Zea DJ, Fornasari MS, Parisi G: On the dynamical incompleteness of the Protein Data Bank. Brief Bioinform 2019, 20:356–359.* This communication provides an interesting perspective on the need for resolving more conformations for each protein in the PDB in order to better understand their dynamics.
- 29.Campitelli P, Modi T, Kumar S, Ozkan SB: The Role of Conformational Dynamics and Allostery in Modulating Protein Evolution. Annu Rev Biophys 2020, 49:267–288.** The authors provide an informative review about the interplay between protein dynamics, allostery and evolution, including mechanisms for creating new or divergent dynamics.
- 30.Narayanan C, Bernard DN, Bafna K, Gagne D, Chennubhotla CS, Doucet N, Agarwal PK: Conservation of Dynamics Associated with Biological Function in an Enzyme Superfamily. Structure 2018, 26:426–436 e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakan A, Meireles LM, Bahar I: ProDy: protein dynamics inferred from theory and experiments. Bioinformatics 2011, 27:1575–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echave J: Beyond Stability Constraints: A Biophysical Model of Enzyme Evolution with Selection on Stability and Activity. Mol Biol Evol 2019, 36:613–620. [DOI] [PubMed] [Google Scholar]
- 33.Micheletti C: Comparing proteins by their internal dynamics: exploring structure-function relationships beyond static structural alignments. Phys Life Rev 2013, 10:1–26. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari SP, Fuglebakk E, Hollup SM, Skjaerven L, Cragnolini T, Grindhaug SH, Tekle KM,Reuter N: WEBnm@ v2.0: Web server and services for comparing protein flexibility. BMC Bioinformatics 2014, 15:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potestio R, Aleksiev T, Pontiggia F, Cozzini S, Micheletti C: ALADYN: a web server for aligning proteins by matching their large-scale motion. Nucleic Acids Res 2010, 38:W41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra SK, Jernigan RL: Protein dynamic communities from elastic network models align closely to the communities defined by molecular dynamics. PLoS One 2018, 13:e0199225.** The authors introduced a method incorporating sequence, structure and dynamics features of proteins to predict active and potential allosteric sites. They show that ENMs can be used for rapid assessment of dynamic communities that are key to functional dynamics and eficient assessment of potentially disruptive mutations.
- 37.Mishra SK, Kandoi G, Jernigan RL: Coupling dynamics and evolutionary information with structure to identify protein regulatory and functional binding sites. Proteins 2019, 87:850–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankar K, Mishra SK, Jernigan RL: Comparisons of Protein Dynamics from Experimental Structure Ensembles, Molecular Dynamics Ensembles, and Coarse-Grained Elastic Network Models. J Phys Chem B 2018, 122:5409–5417. [DOI] [PubMed] [Google Scholar]
- 39.Ponzoni L, Zhang S, Cheng MH, Bahar I: Shared dynamics of LeuT superfamily members and allosteric differentiation by structural irregularities and multimerization. Philos Trans R Soc Lond B Biol Sci 2018, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haliloglu T, Bahar I: Adaptability of protein structures to enable functional interactions and evolutionary implications. Curr Opin Struct Biol 2015, 35:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahar I, Atilgan AR, Demirel MC, Erman B: Vibrational Dynamics of Folded Proteins: Significance of Slow and Fast Motions in Relation to Function and Stability. Phys. Rev. Lett. 1998, 80:2733–2736. [Google Scholar]
- 42.Christensen NR, Calyseva J, Fernandes EFA, Luchow S, Clemmensen LS, Haugaard-Kedstrom LM, Stromgaard K: PDZ Domains as Drug Targets. Adv Ther (Weinh) 2019, 2:1800143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Fuentes EJ: Emerging Themes in PDZ Domain Signaling: Structure, Function, and Inhibition. Int Rev Cell Mol Biol 2019, 343:129–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manjunath GP, Ramanujam PL, Galande S: Structure function relations in PDZ-domain-containing proteins: Implications for protein networks in cellular signalling. J Biosci 2018, 43:155–171. [PubMed] [Google Scholar]
- 45.Amacher JF, Brooks L, Hampton TH, Madden DR: Specificity in PDZ-peptide interaction networks: Computational analysis and review. J Struct Biol X 2020, 4:100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murciano-Calles J: The Conformational Plasticity Vista of PDZ Domains. Life (Basel) 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buonarati OR, Hammes EA, Watson JF, Greger IH, Hell JW: Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci Signal 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Won S, Levy JM, Nicoll RA, Roche KW: MAGUKs: multifaceted synaptic organizers. Curr Opin Neurobiol 2017, 43:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye F, Zeng M, Zhang M: Mechanisms of MAGUK-mediated cellular junctional complex organization. Curr Opin Struct Biol 2018, 48:6–15. [DOI] [PubMed] [Google Scholar]
- 50.Erlendsson S, Thorsen TS, Vauquelin G, Ammendrup-Johnsen I, Wirth V, Martinez KL, Teilum K, Gether U, Madsen KL: Mechanisms of PDZ domain scaffold assembly illuminated by use of supported cell membrane sheets. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerek ZN, Ozkan SB: Change in allosteric network affects binding affinities of PDZ domains: analysis through perturbation response scanning. PLoS Comput Biol 2011, 7:e1002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumawat A, Chakrabarty S: Hidden electrostatic basis of dynamic allostery in a PDZ domain. Proc Natl Acad Sci U S A 2017, 114:E5825–E5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Golden LC, Lopez JA, Shepherd TR, Yu L, Fuentes EJ: Conformational Dynamics and Cooperativity Drive the Specificity of a Protein-Ligand Interaction. Biophys J 2019, 116:2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morais Cabral JH, Petosa C, Sutcliffe MJ, Raza S, Byron O, Poy F, Marfatia SM, Chishti AH,Liddington RC: Crystal structure of a PDZ domain. Nature 1996, 382:649–652. [DOI] [PubMed] [Google Scholar]
- 55.Sillitoe I, Dawson N, Lewis TE, Das S, Lees JG, Ashford P, Tolulope A, Scholes HM, Senatorov I, Bujan A, et al. : CATH: expanding the horizons of structure-based functional annotations for genome sequences. Nucleic Acids Res 2019, 47:D280–D284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan AS, Clairfeuille T, Landao-Bassonga E, Kinna G, Ng PY, Loo LS, Cheng TS, Zheng M, Hong W, Teasdale RD, et al. : Sorting nexin 27 couples PTHR trafficking to retromer for signal regulation in osteoblasts during bone growth. Mol Biol Cell 2016, 27:1367–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGarvey JC, Xiao K, Bowman SL, Mamonova T, Zhang Q, Bisello A, Sneddon WB, Ardura JA,Jean-Alphonse F, Vilardaga JP, et al. : Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling. J Biol Chem 2016, 291:10986–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holm L, Laakso LM: Dali server update. Nucleic Acids Res 2016, 44:W351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]