Structured Abstract
Background
Many hematopoietic cell transplant (HCT) recipients require rehabilitation due to deconditioning following intensive conditioning regimens and immune reconstitution. HCT recipients are preferentially discharged to home to avoid risk of exposure to healthcare-associated infection in a rehabilitation facility (RF) and with a caregiver who was been provided specific education about the complexity of post-HCT care
Objectives:
Calculate incidence of discharge to RF following HCT, identify pre-HCT and during HCT risk factors for discharge to RF, and estimate effect of discharge disposition on overall survival (OS).
Study Design:
Retrospective, matched 1:4 case-control study including 56 cases over a 10-year period from a single institutions. Controls were matched by transplant type (autologous v. allogeneic) and date of transplant
Results:
The incidence of discharge to RF was 2.2%. Controlling for disease, increasing age (odds ratio [OR]: 1.09, 95% confidence interval [CI]: 1.04 – 1.15, p < 0.001), female sex (OR: 3.11, 95% CI: 1.32 – 7.32, p = 0.01), high risk HCT co-morbidity index score (≥3), OR: 3.44, 95% CI: 1.39 – 8.52, p = 0.008), decreasing pre-HCT serum albumin (OR: 2.60, 95% CI: 1.07 – 6.38, p = 0.037), and development of acute kidney injury during HCT (OR: 4.10, 95% CI: 1.36 – 12.40, p = 0.012) were associated with discharge to RF. Discharge to RF was associated with worse OS and higher non-relapse mortality (NRM) than discharge to home (1-year OS: 70.5% (95% CI: 55.8%−81.1%) vs. 88.8% (95% CI: 83.6%−92.4%), p < 0.001; 100-day NRM: 9.5 % (95% CI: 3.5%−19.2%) v. 1.8% (95% CI: 0.6%−4.3%), p = 0.03)
Conclusions:
Discharge to RF following HCT is a rare event but associated with poor OS. Modifiable risk factors for discharge to RF, including serum albumin as a measure of nutrition and reversible HCT-CI components, should be prospectively studied to determine the effect of mitigation on discharge disposition and survival.
Introduction
The discharge needs of patients following autologous or allogeneic HCT are unique. Incompetent immune systems are common to all HCT recipients and allogeneic HCT recipients also carry the risk of graft versus host disease (GVHD)1,2. Both autologous and allogeneic HCT recipients often suffer from functional decline and may require rehabilitation. The need for assistance could potentially be met by discharge to a rehabilitation facility (RF; i.e.: skilled nursing [SNF] or inpatient rehabilitation facilities [IRF]). However, healthcare associated infections are a major concern for residents of RFs with several thousand infectious outbreaks occurring annually in United States facilities 3. RF discharge is associated with increased rates of readmission and mortality compared to home discharge in general medicine populations 4–6. Approximately 20% of Medicare beneficiaries discharged to RF require hospital readmission from the facility and 10% will die during RF admission or within 30 days of discharge from RF 7. These data likely underestimate morbidity and mortality in the post-HCT population discharged to RF given the heightened susceptibility to infection and deconditioning common to these patients. Discharge to RF following HCT has been reported to occur in 1.6% of allogeneic and 3% of autologous recipients but in 20% of patients with GVHD and 15% with non-GVHD complications during HCT 8.
To our knowledge, outcomes for HCT recipients discharged to RF (SNF or IRF) have not been reported or compared to those who are discharged to home. Here, we determined the incidence of discharge to RF from a single, high-volume HCT center. We have also sought to identify potential risk factors predicting discharge to RF following HCT, including pre-HCT factors and during HCT complications. Finally, we characterize the impact of discharge to RF on rates of readmission, non-relapse mortality (NRM), and overall survival (OS).
Materials and Methods
Study Design
This retrospective study describes patients undergoing HCT at a single institution during a 10 year period from September 2007 through September 2017. Cases were discharged to SNF or IRF as confirmed by chart review. Among 2489 transplants performed, a total of 56 cases were identified with 34 discharging to SNF and 22 to IRF. Controls were discharged to home with or without home healthcare. Patients enrolled in hospice at discharge were excluded. Four controls were matched to each case due to low frequency of RF discharge. Controls were matched by type of HCT (allogeneic or autologous) and by date of transplant. Primary reason for discharge was recorded as physical deconditioning with recommendation from physical therapy, lack of a caregiver, or ongoing medical needs such as intravenous antibiotic therapy.
Predictors
The primary predictor of interest was HCT-CI score 9. Presence or absence of each individual component of the HCT-CI score was documented for all patients. Other predictors of interest included pre-HCT characteristics as well as complications arising during HCT. For autologous HCT recipients, melphalan 200 mg/m2 or BEAM (carmustine, etoposide, cytarabine, and melphalan) regimens were considered myeloablative while lower doses of melphalan were considered reduced intensity. For allogeneic HCT recipients, myeloablative regimens included fludarabine 160mg/m2 with busulfan AUC-targeted dose of 20,000 and cyclophosphamide 120mg/kg with >1000 CGy total body irradiation. The last available serum albumin measurement prior to initiation of conditioning regimen was recorded for all patients. When available, documentation of ability to complete Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs) was recorded. Any pre-HCT referrals to physical therapy were recorded, including whether they were made during a hospitalization due to deficits or as part of a pre-habilitation strategy in the outpatient setting.
Outcomes
The outcomes of interest included rates of hospital readmission as well as NRM and OS. Date of readmission was documented and time to first readmission calculated from the date of hospital discharge following HCT. Date of last follow-up and mortality status were recorded. Cause of death was classified as relapse-related if relapse was confirmed or suspected at the time of death. Cause of death data was missing for one patient who underwent autologous HCT and transitioned to local follow-up after day +20 post-HCT. Patients undergoing subsequent HCT after the admission of interest for study were censored at the time of admission for next HCT.
Statistical Analysis
Descriptive statistics were calculated for predictors under study including median and range values for continuous variables and frequency and percentage values for categorical variables. Potential predictors of discharge disposition were analyzed by univariable and multivariable conditional logistic regression. HCT-CI raw score was treated as a continuous variable but also evaluated as categorical by risk stratification group as initially proposed by Sorror et al 9. Multivariable models predicting discharge disposition were ascertained by backwards selection. Time to first readmission was calculated from the date of discharge to the date of first readmission, treating death as a competing risk. Time to NRM was calculated from the date of discharge to date of death, treating death due to disease relapse as a competing risk. Cumulative incidence of readmission and NRM were estimated and compared between subgroups using the Gray’s test. OS was calculated from date of discharge to date of death censoring those alive at the last date of contact. OS was analyzed using Kaplan-Meier method and comparison made between cases and controls utilizing log-rank test. All tests were two-sided and all statistical analyses utilized an alpha value of 0.05.
Results
The incidence of discharge to RF following any HCT was 2.2%, with 1.3% discharged to SNF and 0.9% discharged to IRF. Among 975 allogeneic HCT recipients, the incidence of RF discharge was 1.7% and among 1514 autologous HCT recipients, it was 2.6%. Odds of discharge to RF for pre-transplant characteristics are presented in table 1. Patients discharged to RF were older, had lower pre-transplant albumin, were more likely to be female, had higher HCT-CI score, and less likely to receive myeloablative conditioning. The most common primary reason for discharge to RF was physical deconditioning in 70%, followed by 21% with lack of caregiver, and 9% who had ongoing medical needs.
Table 1.
Pre-transplant characteristics of case and control groups
| Variable | RF (N = 56) | Home (N = 224) | p-value |
|---|---|---|---|
| Age at HCT (years), median (range) | 65 (49 – 77) | 57 (20 – 74) | <0.001 |
| Female | 32 (57.1%) | 94 (42.0%) | 0.036 |
| Disease ALL AML/MDS Amyloidosis Hodgkin Disease Multiple Myeloma MPN (MF, CML, CMML) NHL, B-cell NHL, T-cell Other |
1 (1.8%) 8 (14.3%) 6 (10.7%) 2 (3.6%) 20 (35.7%) 1 (1.8%) 13 (23.2%) 3 (5.4%) 2 (3.6%) |
13 (5.8%) 32 (14.3%) 4 (1.8%) 20 (8.9%) 93 (41.5%) 7 (3.1%) 45 (20.1%) 7 (3.1%) 3 (1.3%) |
0.049 |
| Disease Status at HCT CR1 CR2 or more VGPR PR/Stable Disease Refractory/PD |
14 (25.0%) 6 (10.7%) 8 (14.3%) 21 (37.5%) 7 (12.5%) |
59 (26.3%) 30 (13.4%) 29 (12.9%) 84 (37.5%) 22 (9.8%) |
0.953 |
| Prior HCT First Auto Second Auto Allo after Auto First Allo Second Allo |
34 (60.7%) 5 (8.9%) 4 (7.1%) 13 (23.2%) 0 |
143 (63.8%) 13 (5.8%) 7 (3.1%) 59 (26.3%) 2 (1.0%) |
0.279 |
| Conditioning Dose Myeloablative Non-Myeloablative |
32 (57.1%) 24 (42.9%) |
174 (77.7%) 50 (22.3%) |
< 0.001 |
| HCT-CI, median (range) | 3 (0–10) | 2 (0–9) | <0.001 |
| HCT-CI Risk Group Low (0) Intermediate (1–2) High (3+) |
4 (7.1%) 10 (17.9%) 42 (75.0%) |
44 (19.6%) 73 (32.6%) 107 (47.8%) |
<0.001 |
| Albumin (mg/dL), median (range)* | 3.85 (2.1 – 4.9) | 4 (2.3 – 5.1) | <0.001 |
| ADL Deficits No deficit At least 1 deficit Missing |
47 (83.9%) 3 (5.4%) 6 |
193 (86.2%) 3 (1.3%) 28 |
0.244 |
| Physical Therapy Referral Referred in hospital Referred from clinic No referral |
10 (17.9%) 6 (10.7%) 40 (71.4%) |
18 (8.0%) 18 (8.0%) 188 (83.9%) |
0.076 |
ALL: Acute lymphoblastic leukemia, AML: Acute myeloid leukemia, MDS: Myelodysplastic syndrome, MPN: Myeloproliferative neoplasm, MF: Myelofibrosis, CML: Chronic myeloid leukemia, CMML: Chronic myelomonocytic leukemia, NHL: Non-Hodgkin lymphoma, Other includes plasma cell leukemia, Waldenstrom’s macroglobulinemia, and blastic plasmacytoid dendritic cell neoplasm
Albumin followed a non-normal distribution.
HCT-CI as pre-transplant predictor of discharge disposition
HCT score was predictive of RF discharge as both continuous variable (OR: 1.34, 95% CI: 1.15–1.56, p<0.01) and categorically with high risk compared to low and intermediate risk groups (HCT-CI 3+ v. 0–2, OR: 3.59, 95% CI: 1.76–7.31, p<0.01) in the univariable modeling, while the intermediate risk (HCT-CI 1 or 2) was not predictive of RF discharge compared to low risk (HCT-CI 0; OR: 1.69; 95% CI 0.49 – 5.84; p = 0.41). Table 2 shows the total number of patients for each HCT-CI component as well as the OR associated with discharge to RF. Some HCT-CI components were exceedingly rare in the study population and were excluded from analysis (valvular heart disease [1], inflammatory bowel disease [0], moderate-severe hepatic impairment [1], and peptic ulcer disease [2]). Psychosocial disorder such as depression and anxiety requiring treatment and renal impairment were predictive of RF discharge in univariable conditional logistic regression.
Table 2.
Effect of individual HCT-CI component on odds of discharge to rehabilitation facility
| Co-Morbidity Component | Total (%) | RF (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Arrhythmia | 20 (7.1) | 5 (8.9) | 1.31 | 0.42 – 4.05 | 0.639 |
| CAD/CHF | 38 (13.6) | 12 (21.4) | 1.66 | 0.77 – 3.58 | 0.196 |
| Diabetes | 41 (14.6) | 13 (23.2) | 1.82 | 0.88 – 3.74 | 0.1133 |
| Cerebrovascular accident | 5 (1.8) | 3 (5.4) | 6.00 | 1.00 – 35.91 | 0.05 |
| Depression/Anxiety | 76 (27.1) | 24 (42.9) | 2.28 | 1.14 – 4.56 | 0.0189 |
| Mild hepatic | 34 (12.1) | 5 (8.9) | 0.55 | 0.18 – 1.67 | 0.292 |
| Obesity | 57 (20.3) | 13 (23.2) | 1.09 | 0.54 – 2.22 | 0.8121 |
| Active infection | 6 (2.1) | 1 (1.8) | 0.59 | 0.07 – 5.09 | 0.628 |
| Rheumatologic disease | 5 (1.8) | 1 (1.8) | 0.89 | 0.10 – 7.96 | 0.914 |
| Renal | 11 (3.9) | 6 (10.7) | 4.61 | 1.26 – 16.83 | 0.021 |
| Moderate pulmonary | 98 (35.0) | 23 (41.1) | 1.06 | 0.56 – 2.00 | 0.8501 |
| Severe pulmonary | 56 (20.0) | 13 (23.2) | 1.11 | 0.53 – 2.32 | 0.7769 |
| Prior solid malignancy | 27 (9.6) | 10 (17.9) | 1.68 | 0.71 – 3.98 | 0.2459 |
Nutritional and functional status pre-transplant
Pre-transplant albumin was a predictor of discharge to RF (table 1). Odds of discharge to RF increased with each one g/dL decrease in albumin (OR: 3.74, 95% CI: 1.85–7.53, p<0.001) in the univariable model, and it remained statistical significance in the multivariable model (OR: 2.60, 95% CI: 1.06 – 6.38, p = 0.037). Data regarding functional status were incomplete with missing ADL data in 34 patients and IADL data in 242 patients. Fifty two patients were referred to physical therapy prior to HCT (16 or 28.6% cases, 36 or 16.1% controls). When referral was made during a hospitalization, all patients underwent physical therapy evaluation. Of the 24 patients with referral made in the outpatient setting, only 20 underwent physical therapy evaluation. There were 2 patients with documented deficits in ADLs who were not referred to physical therapy and 5 with IADL deficits without referral. There were 6 patients with at least one ADL deficit. Bathing (4), toileting (3), and transferring (3) were the most common. Among 38 patients with known IADL status, 14 had at least one deficit. Shopping (8), housekeeping (5), and laundry (5) were the most common.
Complications during transplant as predictors of discharge disposition
During transplant complications were encountered by all patients discharged to RF and by the majority of controls; only 13 or 5.8% of controls experienced no complications during their hospital stay. Complications encountered during transplant admission with frequency and effect on disposition by univariable modeling are found in table 3. Neutropenic fever occurred commonly and was predictive of disposition when the source of infection could be identified. Severe complications such as respiratory failure requiring mechanical ventilation, renal failure requiring hemodialysis, and nutrition complications such as requiring enteral or parenteral nutrition were not frequent events but were predictive of discharge disposition. Resolution of complications prior to hospital discharge was predictive of discharge to home (OR for discharge home: 2.84; 95% CI: 1.52 – 5.32; p = 0.001). Among complications requiring ongoing treatment or observation at discharge, acute GVHD (OR: 4.79; 95% CI: 1.31 – 17.59; p = 0.018), new onset or uncontrolled arrhythmia (OR: 2.93; 95% CI: 1.39 – 6.16; p = 0005), and ongoing acute kidney injury (OR: 8.50; 95% CI: 3.30 – 21.91; p < 0.001) were predictive of discharge to RF while controlled infection requiring ongoing antibiotic therapy was not.
Table 3.
Effect of during transplant complications on discharge to rehabilitation facility
| Total (%) | RF (%) | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| All Transplant Complications | |||||
| Neutropenic fever (culture-negative) | 165 (58.9) | 28 (50.0) | 0.64 | 0.36 – 1.15 | 0.135 |
| Neutropenic fever (source identified) | 61 (21.8) | 18 (32.1) | 1.89 | 1.01 – 3.54 | 0.045 |
| Mucositis | 111 (39.6) | 22 (39.3) | 0.98 | 0.53 – 1.81 | 0.95 |
| Encephalopathy | 34 (12.1) | 25 (44.6) | 28.58 | 8.58 – 95.20 | <0.001 |
| Ileus | 5 (1.8) | 1 (1.8) | 1.00 | 0.11 – 8.95 | 0.99 |
| Respiratory failure | 14 (5.0) | 12 (21.4) | 45.58 | 5.91 – 351.39 | <0.001 |
| Acute coronary syndrome | 2 (1.0) | 1 (1.8) | 4.00 | 0.25 – 63.95 | 0.327 |
| New or uncontrolled arrhythmia | 41 (14.6) | 15 (26.8) | 2.93 | 1.39 – 6.16 | 0.005 |
| Acute kidney injury | 31 (11.1) | 17 (30.4) | 8.50 | 3.30 – 21.91 | <0.001 |
| AKI requiring hemodialysis | 9 (3.2) | 7 (12.5) | 14.00 | 2.91 – 67.39 | 0.001 |
| Gastrointestinal bleed | 4 (1.4) | 3 (5.4) | 12.00 | 1.25 – 115.36 | 0.031 |
| Nutrition Complications | 19 (6.8) | 16 (28.6) | 30.81 | 7.07 – 134.23 | <0.001 |
| All resolved prior to discharge | 141 (50.4) | 18 (32.1) | 0.35 | 0.19 – 0.66 | 0.001 |
| Allogeneic HCT Complications | |||||
| Acute GVHD | 13 (15.3) | 6 (35.3) | 4.79 | 1.31 – 17.59 | 0.018 |
| CMV reactivation | 7 (8.2) | 5 (29.4) | 10.00 | 1.94 – 51.54 | 0.006 |
| Vaso-occlusive disease | 2 (2.4) | 1 (5.9) | 4.00 | 0.25 – 63.95 | 0.327 |
Multivariable model of discharge disposition
Pre-transplant factors as well as during transplant complications significant in the univariable model and with sufficient frequencies were further included in a multivariable model to be evaluated for their independent risk for discharge disposition. From HCT-CI component analysis, psychosocial disorder and renal impairment were individually predictive of discharge to RF, but were not included in multivariable modeling because they are not independent of total HCT-CI risk score. The final multivariable model is presented in table 4. Of the during transplant complications, only acute kidney injury (AKI) remained significant in the multivariable model.
Table 4.
Final multivariable model of factors predicting RF discharge
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age at HCT | 1.09 | 1.04 – 1.15 | 0.001 |
| Female sex | 3.11 | 1.32 – 7.32 | 0.01 |
| RIC preparative regimen | 3.06 | 0.97 – 9.69 | 0.057 |
| High risk HCT-CI score | 3.44 | 1.39 – 8.52 | 0.008 |
| Albumin | 0.38 | 0.16 – 0.94 | 0.037 |
| Acute kidney injury during HCT | 4.10 | 1.36 – 12.40 | 0.012 |
Readmission rates and time to first readmission
Readmission within 100 days of HCT discharge occurred in 40.0% (95% CI: 27.1% – 52.6%) of patients discharged to RF and in 27.5% (95% CI: 21.8% – 33.5%) of patients discharged to home. Readmissions in the first year after HCT discharge occurred in 50.2% (95% CI: 36.1% – 62.8%) of RF discharge and in 34.5% (95% CI: 28.3% – 40.9%) of home discharge patients. Through the first year of follow-up, the cumulative incidence of first readmission was higher for patients discharged to RF (figure 1).
Figure 1. Cumulative incidence of readmission after HCT.
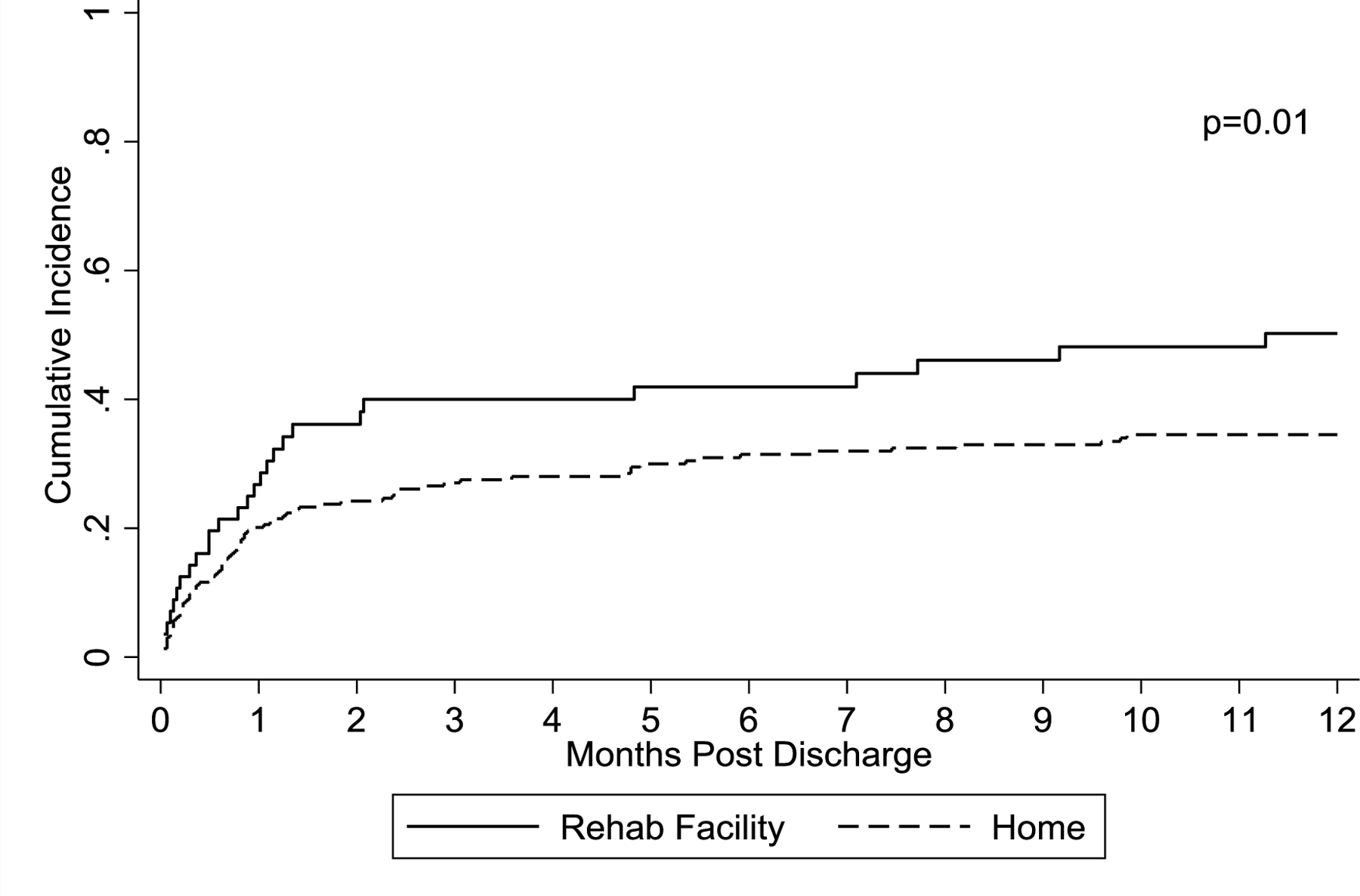
Cumulative incidence of first readmission in first year after transplant censored at 12 months.
Overall Survival and non-relapse mortality by transplant type and discharge disposition
One-year and five-year OS for patients discharged to RF was 70.5% (95%CI: 55.8% – 81.1%) and 53.0% (95% CI: 37.1 – 66.6%) compared to 88.8% (95% CI: 83.6% – 92.4%) and 63.5% (53.7% – 71.9%), respectively among patients discharged to home. At 100 days post-HCT discharge, NRM was 9.5% (95% CI: 3.5% – 19.2%) among patients discharged to RF and 1.8% (95% CI: 0.6% – 4.3%) for those discharged to home. One-year NRM was similarly higher in patients discharged to RF at 13.5% (95% CI: 5.9% – 24.2%) compared to 4.8% (95% CI: 2.4% – 8.3%) for discharge to home. With stratification by transplant type and discharge disposition, allogeneic HCT recipients discharged to RF emerge as a very high risk group with 100-day NRM of 23.5% (95% CI: 7.3% – 44.9%) and 1-year OS of 39.2% (95% CI: 16.6% – 61.4%). Allogeneic HCT recipients discharged to home, autologous HCT recipients discharged to RF, and autologous HCT recipients discharged to home experienced 100-day NRM of 4.4% (95% CI: 1.2% – 11.2%), 2.9% (95% CI: 0.2% – 12.7%), and 0.6% (95% CI: 0.1% – 3.3%) and 1-year OS of 79.2% (95% CI: 67.4% – 87.1%), 85.4% (95% CI: 68.4% – 93.7%), and 92.6% (95% CI: 86.7% – 96.0%), respectively. Kaplan-Meier curves are shown in Figure 2 including OS from HCT discharge by disposition (2A) and OS by disposition stratified by transplant type (2B) as well as NRM by disposition (2C) and NRM by disposition stratified by HCT type (2D).
Figure 2. Overall survival and non-relapse morality by discharge disposition and transplant type.
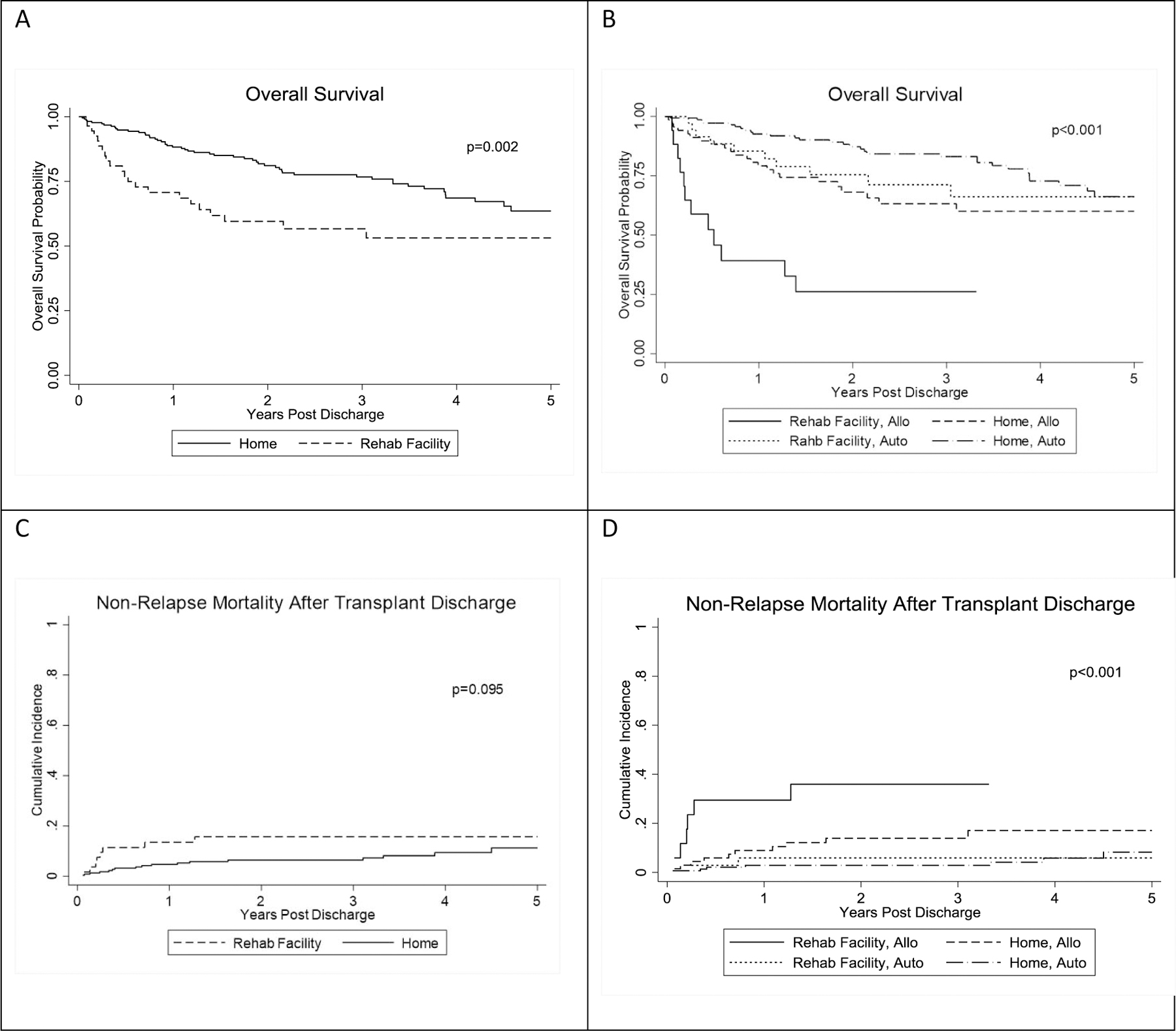
Overall survival is presented as a function of discharge disposition (A) and as a function of both disposition and transplant type (B). Non relapse mortality is presented as a function of discharge disposition (C) and as a function of both disposition and transplant type (D).
Discussion
Discharge to RF following HCT is a rare event, but we have demonstrated that it is associated with substantially greater odds of all-cause and non-relapse mortality than discharge to home. We have also identified risk factors for discharge to RF including advanced age, female sex, high risk HCT-CI score, pretransplant albumin, and during transplant AKI. Of these predictors, HCT-CI, pre-transplant albumin, and AKI represent potentially modifiable factors. Correction of risk factors present prior to admission for HCT may help to mitigate the risk of discharge to RF and thus improve OS and NRM. In addition, the most common reason for discharge to RF was physical deconditioning, highlighting the importance of physical activity and early consultation with physical and occupational therapy for patients with complicated transplant course.
Concerns related to discharge to RF following HCT, such as risk of infection, have been inferred based on patient-specific factors, like profound immune suppression, and facility-specific factors, as in reports of infectious disease outbreaks. Here, for the first time, we have demonstrated poorer OS and higher NRM among patients who are discharged to RF. Having demonstrated the association, it is important to understand the relationship between mortality and RF discharge. This correlation may reflect an increased risk of complications by virtue of residing in a RF or it may be a reflection of a sicker population of patients who had already developed multiple transplant-related complications necessitating additional nursing or rehabilitative care. Irrespective of the mechanism for inferior outcomes, identification and mitigation of risk factors for discharge to RF, particularly those present prior to HCT, is critical.
High risk HCT-CI score is associated with discharge to RF but not all components of the HCT-CI are amenable to intervention. Some components are awarded points if they require treatment but the severity and response to treatment are not accounted for. Importantly, this is true of the depression/anxiety component, which does not account for disease control and is not scored for patients with undiagnosed psychological disease. The impact on post-HCT survival of better control of chronic disease through treatment is unclear. While we did not demonstrate a specific link between hepatic or pulmonary co-morbidity and discharge disposition, abnormal hepatic or pulmonary function may be addressed through evaluation and treatment of underlying disease prior to HCT for some patients. Our finding that depression or anxiety is associated with discharge to RF aligns with previous work from our institution showing higher readmission rates in patients with high Transplant Evaluation Rating Scale (TERS) scores during pre-HCT psychosocial assessment 10. Further study of current state of psychological stress and coping mechanisms at time of HCT is warranted.
Impaired renal function was identified as both a pre-HCT and during HCT factor associated with discharge to RF. These results support more proactive evaluation and treatment of renal insufficiency before and during HCT, especially important among allogeneic HCT recipients for whom standard of care includes the use of calcineurin inhibitors for prevention of GVHD. New “calcineurin-free” strategies that rely on ex vivo graft manipulation for GVHD prevention may further reduce the impact of pre-existing renal impairment on discharge disposition and lower the incidence of during HCT AKI. Other strategies during transplant to avoid AKI, such as medication dosing adjustments for creatinine clearance, avoidance of nephrotoxic medications, and early identification and treatment of infection to prevent hypoperfusion as can occur in septic shock, should remain best practices.
In addition to AKI, many during transplant complications were predictive of discharge disposition in univariable modeling. Logically, this makes sense that the population needing more assistance at discharge experienced more complications during the transplant. For the multivariable model, we included only variables that were significant in univariable modeling and occurred in at least 10% of cases and 5% of controls. Most complications occurred at very low frequency and were associated with very wide confidence intervals, limiting the ability to draw definitive conclusions about their impact on discharge disposition.
Albumin was another potentially modifiable risk factor for RF discharge. Increasing albumin by 1mg/dL was associated with a nearly 70% reduction in the odds of discharge to RF. In adults, lower biomarkers of nutritional status have been associated with inferior survival 11,12. In recent pediatric studies, lower pre-transplant serum albumin has been associated with early mortality, severe acute GVHD, and utilization of critical care interventions 13,14. There are also data to support the role of multidisciplinary “nutritional support teams” in pre- and post-transplant care 15. In contrast to these studies, at least one group has found that weight loss during transplant and sustained following transplant does not appear to affect survival 16. While we did not review weight change in this study population, we did show that during transplant need for nutritional intervention with enteral or parenteral feeding predicted discharge to RF. Based on our findings and the available literature, we recommend pre-transplant nutrition assessment with intervention directed at improving nutritional status, particularly aimed at increasing protein stores in the case of low albumin. HCT recipients should also be followed throughout their hospital course with early nutrition intervention when anticipating prolonged periods of decreased intake due to mucositis, dysgeusia, or nausea.
Non-modifiable pre-HCT risk factors for discharge disposition included age and female sex. While the factors themselves are not modifiable, there is opportunity for intervention to better support patients who are older or female. Increased odds of RF discharge among females is most likely multifactorial and influenced by a number of social constructs. Traditional gender roles in which women tend to take more responsibility for housekeeping duties may lead to discharge to RF due to inability to perform IADLs. Social isolation among older women, particularly those who may have outlived their husbands based on greater life expectancy for women compared to men, may also contribute. It is possible that there is a physiologic explanation for higher odds of RF discharge among women, though unlikely as significant differences in patient outcomes based on sex have not been consistently reported in the HCT literature.
The finding of increased age predicting RF discharge is not surprising. Aging is a physiologic process that occurs differently in all people regardless of cancer diagnosis, though an active malignancy will modify the aging process through biologic processes like inflammation and immune senescence as well as psychosocial and other extrinsic environmental factors 17. It is very difficult to separate the effect of advanced chronological age from expected, but uniquely individual, decline in organ function. Further contributing to the complex aging process is the role of chemotherapy. Our group has previously shown evidence of T-cell senescence following autologous HCT in multiple myeloma patients that is equivalent to 33.7 years of chronologic aging compared to multiple myeloma patients who were not transplanted 18. It is generally well-accepted that decisions regarding cancer therapies, including HCT, should not be based on chronologic age alone. Guidelines for patient evaluation and tools to predict risk of chemotherapy toxicity have been developed for oncology patients, but have not yet been validated in patients undergoing HCT 19–22. In older HCT recipients, higher HCT-CI and inability to perform IADLs have been associated with inferior survival 23,24. A more robust assessment of function in older HCT recipients is currently under study through the Blood and Marrow Transplantation Clinical Trials Network (ClinicalTrials.gov Identifier: NCT03992352).
Our study is limited due to small group size and the inability to complete all of the intended statistical analyses. Fortunately, this is due to a very small proportion of patients requiring RF care at hospital discharge. We did compare the percentage of each disease in our control group to that in the total population of patients transplanted during this time period and found no difference between groups providing reassurance that the sample models the overall population. We were missing a large amount of IADL data, limiting the assessment of baseline functional status as a factor in predicting discharge disposition. Finally, as a regional referral center for HCT, we see many patients who live a considerable distance from our hospital. We follow all allogeneic HCT recipients through the first 5 years after transplant and many indefinitely, but autologous HCT recipients often complete a single post-discharge office visit and then resume care with their primary hematologist/oncologist. Due to this practice, our follow-up data for autologous HCT recipients were potentially incomplete as it often occurs outside of our system. Despite this concern, the median follow-up for autologous compared to allogeneic HCT recipients was significantly longer and the paired case-control analysis with matching based on type of transplant should limit the effect of this difference in follow-up between groups.
In summary, we have shown poorer OS and NRM in patients discharged to RF after hospitalization for HCT. We have identified albumin and HCT-CI as modifiable risk factors to target for pre-HCT patient optimization efforts to improve post-HCT outcomes. Renal function in particular appears to be an important predictor of discharge to RF and incorporation of nephrology consultation pre-HCT may be beneficial to avoiding discharge to RF and improving survival in candidates with baseline renal impairment. Importantly, advancing chronological age is a factor that is inseparable from many of the risk factors identified for RF discharge, but we caution against its use as a surrogate for assessment of individual functional status. There is growing interest in a more holistic approach to pre-HCT patient evaluation, especially among patients of greater chronological age or those of any age with identified functional deficits. Future research in pre-HCT optimization needs to be comprehensive, including nutrition and social factors not accounted for by traditional organ function-based scoring systems.
Highlights.
Discharge to rehabilitation facility after hematopoietic cell transplant is rare
Overall survival is worse if discharged to rehabilitation facility after transplant
Pre-transplant albumin, a marker of nutrition, predicted later rehabilitation need
Acknowledgements
SW was supported by a T32 grant from the National Cancer Institute of the National Institutes of Health (award number 5T32CA165998). SW also wishes to acknowledge her faculty mentors in the College of Public Health at The Ohio State University, Drs. Philip Binkley and Randall Harris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement
None of the authors has any financial interest relevant to the subject matter presented in this manuscript.
References
- 1.Lazarus HM, Hamadani M, Hari PN: 130: Autologous Stem Cell Transplantation, in Jr VTD, Lawrence TS, Rosenberg SA (eds): Devita, Hellman, and Rosenberg’s Cancer: principles & practice of oncology (ed 10). Philadelphia, Lippincott Williams & Wilkins, 2015, pp 1909–1915 [Google Scholar]
- 2.Riddell SR, Warren EH: 131: Allogeneic Stem Cell Transplantation, in Jr VTD, Lawrence TS, Rosenberg SA (eds): Devita, Hellman, and Rosenberg’s Cancer: principles & practice of oncology (ed 10). Philadelphia, Lippincott Williams & Wilkins, 2015, pp 1917–1929 [Google Scholar]
- 3.Strausbaugh LJ, Sukumar SR, Joseph CL: Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis 36:870–6, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Karmarkar A, Downer B, et al. : Current Risk Adjustment and Comorbidity Index Underperformance in Predicting Post-Acute Utilization and Hospital Readmissions After Joint Replacements: Implications for Comprehensive Care for Joint Replacement Model. Arthritis Care Res (Hoboken) 69:1668–1675, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acher AW, Squires MH, Fields RC, et al. : Can the risk of non-home discharge after resection of gastric adenocarcinoma be predicted: a seven-institution study of the US Gastric Cancer Collaborative. J Gastrointest Surg 19:207–16, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Na L, Hennessy S, Xie D, et al. : Premorbid Activity Limitation Stages Are Associated With Posthospitalization Discharge Disposition. Am J Phys Med Rehabil 97:440–449, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke RE, Hess E, Baron AE, et al. : Predicting Potential Adverse Events During a Skilled Nursing Facility Stay: A Skilled Nursing Facility Prognosis Score. J Am Geriatr Soc 66:930–936, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guddati AK, Kumar G, Lewis RD, et al. : Comparison of Disposition and Complications in Geriatric (≥ 65 years) and Non-Geriatric (50–64 years) Cancer Patients Receiving Hematopoietic Stem Cell Transplantation. Blood 128:2408–2408, 2016 [Google Scholar]
- 9.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson DR, Huang Y, McGinty HL, et al. : Psychosocial risk predicts high readmission rates for hematopoietic cell transplant recipients. Bone Marrow Transplant 53:1418–1427, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivgin S, Baldane S, Ozenmis T, et al. : The impact of pretransplant hypoalbuminemia on survival in patients with leukemia who underwent allogeneic hematopoietic stem cell transplantation (alloHSCT): a nutritional problem? Transplant Proc 45:3371–4, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Krawczyk J, Kraj L, Korta T, et al. : Nutritional Status of Hematological Patients before Hematopoietic Stem Cell Transplantation and in Early Posttransplantation Period. Nutr Cancer 69:1205–1210, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Kerby EH, Li Y, Getz KD, et al. : Nutritional risk factors predict severe acute graft-versus-host disease and early mortality in pediatric allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 65, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Teagarden AM, Skiles JL, Beardsley AL, et al. : Low serum albumin levels prior to pediatric allogeneic HCT are associated with increased need for critical care interventions and increased 6-month mortality. Pediatr Transplant 21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuji S, Einsele H, Savani BN, et al. : Systematic Nutritional Support in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant 21:1707–13, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Rieger CT, Wischumerski I, Rust C, et al. : Weight Loss and Decrease of Body Mass Index during Allogeneic Stem Cell Transplantation Are Common Events with Limited Clinical Impact. PLoS One 10:e0145445, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Meng X, Chen Y, et al. : The Biology of Aging and Cancer: Frailty, Inflammation, and Immunity. Cancer J 23:201–205, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Rosko A, Hofmeister C, Benson D, et al. : Autologous hematopoietic stem cell transplant induces the molecular aging of T-cells in multiple myeloma. Bone Marrow Transplant 50:1379–81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurria A, Mohile S, Gajra A, et al. : Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 34:2366–71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wildiers H, Heeren P, Puts M, et al. : International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Extermann M, Boler I, Reich RR, et al. : Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118:3377–86, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Hamaker ME, Jonker JM, de Rooij SE, et al. : Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 13:e437–44, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Lin RJ, Elko TA, Devlin SM, et al. : Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplant 55:157–164, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LW, Sheng Y, Andreadis C, et al. : Functional Status as Measured by Geriatric Assessment Predicts Inferior Survival in Older Allogeneic Hematopoietic Cell Transplantation Recipients. Biol Blood Marrow Transplant 26:189–196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


