Abstract
The integrin αIIbβ3 is the most abundant integrin on platelets. Upon platelet activation, the integrin changes its conformation (inside-out signalling) and outside-in signalling takes place leading to platelet spreading, platelet aggregation and thrombus formation. Bloodsucking parasites such as mosquitoes, leeches and ticks express anticoagulant and antiplatelet proteins, which represent major sources of lead compounds for the development of useful therapeutic agents for the treatment of haemostatic disorders or cardiovascular diseases. In addition to hematophagous parasites, snakes also possess anticoagulant and antiplatelet proteins in their salivary glands. Two snake venom proteins have been developed into two antiplatelet drugs that are currently used in the clinic. The group of proteins discussed in this review are disintegrins, low molecular weight integrin-binding cysteine-rich proteins, found in snakes, ticks, leeches, worms and horseflies. Finally, we highlight various oral antagonists, which have been tested in clinical trials but were discontinued due to an increase in mortality. No new αIIbβ3 inhibitors are developed since the approval of current platelet antagonists, and structure-function analysis of exogenous disintegrins could help find platelet antagonists with fewer adverse side effects.
Keywords: platelets, integrin αIIbβ3, antagonists, hematophagous parasites
1. Introduction
Integrins are an important class of transmembrane glycoprotein (GP) receptors consisting of an alpha- and a beta subunit, both of which have an extracellular domain, a transmembrane domain and a cytoplasmic domain. In addition to mediating communication between cells and the extracellular matrix (ECM), integrins enable cell-cell interactions. Integrins, therefore, play a major role in cell adhesion, survival and differentiation [1,2].
The integrin family members can be assembled from 18 α- and 8 β-subunits, which together can form 24 integrins with different effects on the function of cells. The α-subunits can be divided into two groups: subunits with an inserted domain (αI) and subunits without an inserted domain. This domain has a metal ion-dependent adhesion site (MIDAS), which allows ligands to bind the receptor [3]. Integrins are often expressed in the inactive state, although the binding affinity for ligands can be increased by the addition of Mn2+. The presence of Ca2+ in the blood has an essential role in keeping integrins in their inactive state [4].
The 9 α-subunits that contain the inserted domain are part of the collagen receptors (α1, α2, α10, α11) and leukocyte receptors (αE, αL, αM, αD, αX) [5,6]. The 9 subunits without the inserted domain can be classified into four categories based on evolution and specificity: (A) Arg-Gly-Asp (RGD) sequence receptors (αIIb, αv, α5, α8), (B) laminin receptors (α3, α6, α7), (C) PS3 (only found in insects), and (D) α4, α9 subunits [7].
Of the 8 different β-subunits that are found in integrins (β1-β8), two, β1 and β3, are found on platelets. The β1 integrins (α2β1, α5β1, and α6β1) support platelet adhesion to collagen, fibronectin and laminin (1000 copies per platelet). The αvβ3 and αIIbβ3 are β3 integrins, where αIIbβ3 (GPIIb/IIIa, CD41/CD61) is the most abundant integrin on platelets [1,8,9]. αvβ3 is found on smooth muscle cells and endothelial cells but is less abundant on platelets (50–100 copies per platelet) [10].
In this review, we will focus on integrin αIIbβ3, integrin αIIbβ3 inhibitors found in hematophagous parasites and snakes, the current inhibitors used in the clinic and those failed in clinical trials.
2. Integrin αIIbβ3GPIIb/IIIa
Integrin αIIbβ3–GPIIb/IIIa has approximately 80,000 copies per platelet and is crucial for haemostasis. Patients with Glanzmann thrombasthenia, a rare autosomal recessive disorder caused by mutations in genes encoding GPIIb and IIIa, suffer from bleeding episodes [11,12]. On the other hand, malfunction of integrin αIIbβ3 may contribute to thrombotic complications, such as ischemic stroke and myocardial infarction [13]. A partial deletion of the β3 in a megakaryocyte will lead to abnormally large platelets, (macro)thrombocytopenia (platelets < 150 · 109/L blood) and bleeding tendencies [14].
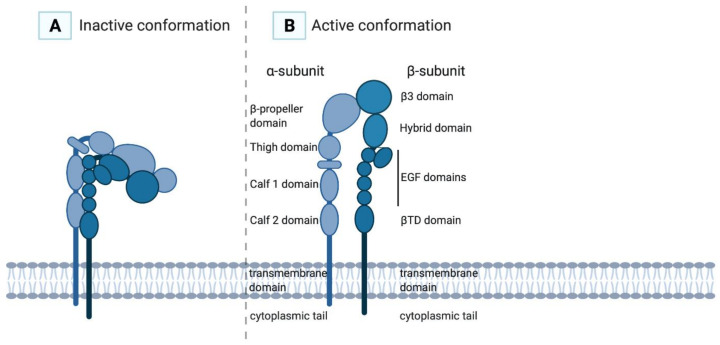
The α-subunit of αIIbβ3 consists of 1008 amino acids and contains a cytoplasmic tail, a transmembrane domain, two calf domains, a thigh domain and a head (a β-propeller domain), which is the main ligand-binding domain (Figure 1) [2,15]. The β-subunit of αIIbβ3 consists of 772 amino acids and contains a cytoplasmic tail, a transmembrane domain, a membrane-proximal β-tail domain (βTD domain), four epidermal growth factor (EGF) domains, a hybrid domain and a β3A domain [15]. The β3 subunit consists of one polypeptide chain stabilised by disulphide bonds, which upon activation will rearrange through the formation of free thiols with a disulphide exchange reaction [16,17]. The integrin has multiple divalent cation-binding sites, necessary for binding of ligands. The cytoplasmic domains of both subunits form a ligand-binding domain for intracellular cytoskeletal molecules. Interaction of the α-subunit with the β-subunit is governed by the β-propeller domain and the β3A domain.
Figure 1.
In the inactive conformation of the αIIbβ3 integrin (A), the ligand-binding site is poorly accessible for its ligands, while in the active conformation (B) the ligand-binding site is exhibited and has high affinity for its ligands.
Nitric oxide and prostacyclin (PGI2), produced by the intact endothelium, inhibit the activation of discoid-shaped resting platelets [18,19]. During haemostasis, integrin αIIbβ3 plays a crucial role in platelet adhesion and platelet aggregation. Activation of platelets occurs when blood vessels are damaged. Platelets interact with collagen-bound von Willebrand factor (VWF) via the GPIb-V-IX receptor. Next to promoting platelet adhesion to the damaged vessel wall, VWF also enhances platelet aggregation under high shear conditions [20,21]. Following platelet activation, the content of alpha- and dense granules is secreted and by released adenosine diphosphate (ADP), platelet activation is further enhanced [22]. Upon platelet activation, conformational changes of the integrin take place, exposing the ligand-binding site of the fibrinogen receptor, which is unavailable in resting platelets, and as a consequence, fibrinogen crosslinks multiple platelets into aggregates, forming a platelet plug [23]
3. Ligands
The integrin αIIbβ3 has multiple ligands, categorised in ligands containing an RGD-motif (fibrinogen, fibrin, VWF, fibronectin) and other ligand-binding motifs, such as the KQAGDV-sequence from the fibrinogen γ-chain C-terminus, which is the primary binding site of fibrinogen [24,25].
4. Inside-out Signalling
Upon platelet activation and subsequent intracellular signalling, so-called “inside-out” signalling, integrin αIIbβ3 changes conformation (Figure 2). The integrin’s inactive state, in which the extracellular domain is folded with the ligand-binding site facing the membrane, transforms into an active state when the extracellular domain is extended (opened), thereby exposing the RGD ligand-binding domain with a high affinity for its ligands (Figure 1). In this high-affinity state, the cytoplasmic tails of the αIIb- and β3-subunits unclasp, separating the transmembrane domains [26].
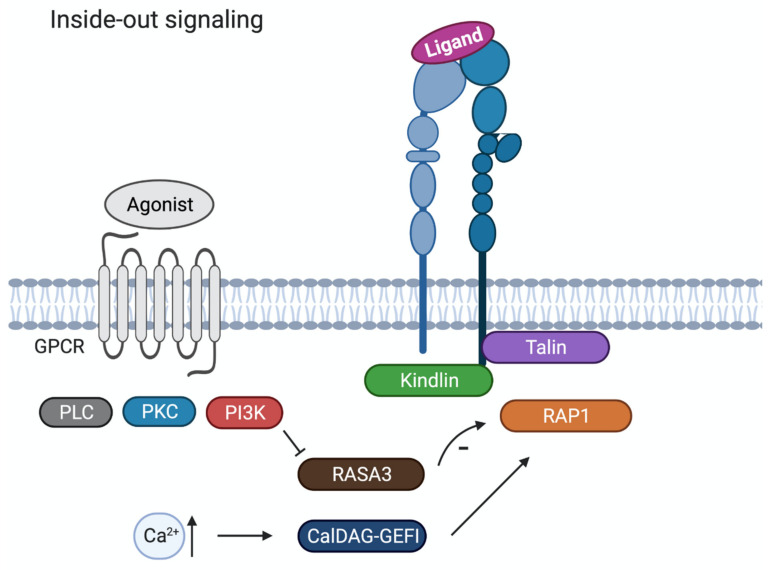
Figure 2.
Schematic overview of inside-out signalling in platelets. Agonist activation of G protein-coupled receptor triggers signalling pathways with key signalling proteins like phospholipase C (PLC), protein kinase C (PKC) and phosphatidylinositide-3-kinase (PI3K). Increased Ca2+ will lead to activation of CalDAG-GEFI that will activate Ras-related protein 1 (RAP1). RASA3 acts as a negative regulator of RAP1 activation, and RASA3 activity is restricted by PI3K activation. Through the separation of the talin head and tail domain, the talin head domain associates with the cytoplasmic tail of the β3 subunit, converting the integrin αIIbβ3 from the inactive to the active form.
Platelet activation induced by the binding of soluble agonists (e.g., ADP, thrombin, thromboxane A2) or adhesive ligands (e.g., collagen, VWF, fibrinogen) to their respective receptors, triggers signalling pathways involving key signalling proteins like phospholipase C (PLC), protein kinase C (PKC) and phosphatidylinositide-3-kinase (PI3K) [27]. These signalling events culminate in the activation of the small GTPase RAP1 (Ras-related protein 1), an important modulator of integrin αIIbβ3 activation. RAP1 is regulated by its activator CalDEG-GEFI, which responds to cytoplasmic Ca2+ rises, and RASA3, acting as a negative regulator of RAP1 activation. For sustained integrin activation, RASA3 activity needs to be restricted and this is accomplished by PI3K activation [28]. The effectors of RAP in platelets remain to be determined.
In addition to RAP1 activation, inside-out signalling leads to the recruitment and binding of actin cytoskeletal proteins talin and kindlin to the cytoplasmic tails of αIIbβ3. Through the separation of the talin head and tail domain, the talin head domain can associate with the β3-subunit cytoplasmic tail, which converts the integrin αIIbβ3 from the inactive to the active form [25]. The importance of kindlins in these integrin regulation processes is well established. Kindlin-1 is found in epithelial cells, kindlin-2 in solid mesenchymal tissue and kindlin-3 is found in hematopoietic cells. Cell studies revealed that the kindlins can only activate integrins together with talin [29].
5. Outside-in Signalling
Ligand binding to integrin αIIbβ3 triggers multiple complex signal transduction events in the cell, known as outside-in signalling, which promote actin polymerization and cytoskeletal reorganisation. Clustering of integrins by multivalent ligands further promotes outside-in signalling. Outside-in signalling drives important haemostatic processes as platelet spreading, stable thrombus formation and clot retraction (Figure 3). One of the earliest events occurring is tyrosine phosphorylation of different proteins by the Src family of kinases (SFK), c-Src, Lyn and Fyn. Following platelet activation, c-Src associates with the β3-integrin tail and becomes activated [25]. The work of Gong et al. showed that binding of the G protein subunit Gα13 to the β3-integrin tail promotes the activation of c-Src [30]. SFK activation then results in the phosphorylation and activation of the tyrosine kinase Syk and its interaction with the cytoplasmic tail of β3 [31]. Furthermore, SFK-induced phosphorylation of the β3 tail promotes the recruitment of intracellular adaptor proteins. Downstream of SFKs and Syk many signalling and cytoskeletal proteins become phosphorylated and/or activated, such as PKC, PLCγ2, focal adhesion kinase (FAK), PI3Kβ and RhoA [25]. PI3Kβ was shown to be of importance for thrombus stability [32,33], while RhoA regulates platelet spreading and clot retraction. Talin and kindlin-3 also appear to have a role in outside-in signalling by directly linking the β3 tail to the actin cytoskeleton [25,34].
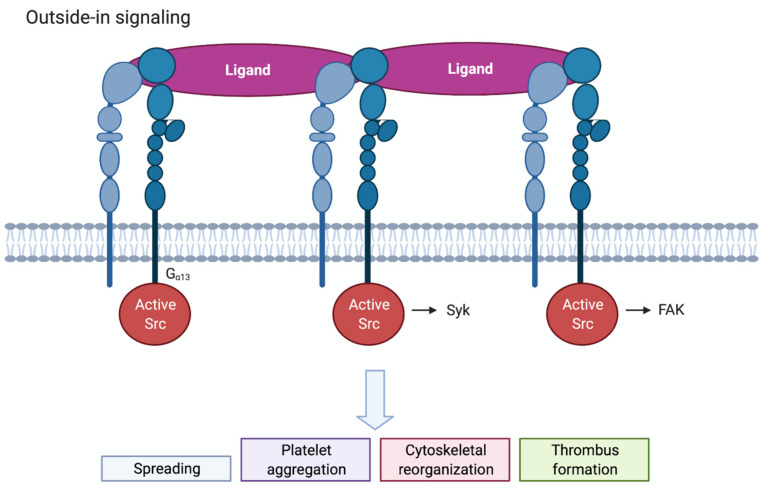
Figure 3.
Schematic overview of outside-in signalling in platelets. Clustered integrins will initiate outside-in signalling. c-Src associates with the β3-integrin tail and becomes activated. Src, Syk and FAK will regulate downstream signalling via tyrosine phosphorylation. Outside-in signalling leads to spreading, platelet aggregation, cytoskeletal reorganisation and thrombus formation.
6. Antithrombotic Agents from Nature
Abnormal platelet activation and aggregation can lead to thrombotic vessel occlusion [35]. Arterial thrombosis comprises the formation of platelet-rich thrombi under high shear flow as a result of eroded or ruptured atherosclerotic plaques, resulting in ischemic injuries. Arterial thromboembolism usually results in myocardial infarction or stroke, while venous thrombosis occurs under low shear flow around an intact endothelial wall and can lead to venous thromboembolism (VTE) and pulmonary embolism (PE) [36]. A difference between arterial and venous thrombosis is that venous thrombi constitute fibrin and red blood cells, with a few platelets, while arterial thrombi are platelet-rich [37]. Treatment of venous thrombosis usually consists of drugs directed against proteins involved in the coagulation cascade, while the treatment of arterial thrombosis is based on antiplatelet therapy [36]. However, several clinical studies have shown some beneficial effects on the combination of antiplatelet and anticoagulant therapies in patients with coronary and peripheral artery disease [38]. Some antiplatelet strategies are derived from nature.
Bloodsucking parasites such as mosquitoes, ticks, bugs, leeches, sandflies, hookworms and bats, express a vast variety of anticoagulant and antiplatelet proteins that counteract the host’s haemostatic system, allowing them to feed for extended periods of time. Therefore, these organisms represent major sources of lead compounds for the development of pharmacological tools and potentially useful therapeutic agents for the treatment of haemostatic disorders or cardiovascular disease. Feeding occurs through cannulation of venules or via haemorrhagic pools that accumulate in tissues because of a deep cut in the skin. One example of an anticoagulant peptide from a hematophagous parasite is hirudin. Hirudin (MW: 7 kDa) is isolated from the salivary glands of leeches and inhibits thrombin, by the formation of a complex of hirudin and thrombin, thereby inhibiting the activity of thrombin [39].
Snakes, especially vipers (venomous snakes), have venom in their glands that is rich in proteins that modulate blood clotting and thereby cause organ degeneration and generalised tissue damage. The function of these hemotoxic proteins is not only to immobilise the prey but also to aid in its digestion. Snake venoms have become a valuable source for drug development. One interesting group of peptides found in snake venom are the disintegrins.
Disintegrins are low molecular weight integrin-binding cysteine-rich peptides that have different functions, such as the inhibition of cell adhesion and proliferation. Most disintegrins have one of the following motifs: RGD or KGD, however, some have an MVD or RED motif. Most disintegrins display antagonistic activity towards integrins such as αIIbβ3, αvβ3, and α5β1, but also other integrins are inhibited. Disintegrins are found not only in snake venom but also in the excretion glands of hematophagous parasites. Three αIIbβ3-antagonising drugs are currently used in the clinic and two of them, eptifibatide and tirofiban, are derived from snake venom disintegrins. In contrast to eptifibatide and tirofiban which are low molecular weight compounds (see below), abciximab, the other FDA approved drug, is a chimeric monoclonal antibody binding to αIIbβ3.
In Table 1 an overview of αIIbβ3-targeting disintegrins from different species is given. Subsequently, we elaborate on specific examples per species, based on the number of hits on Pubmed.
Table 1.
Overview of the disintegrins in different species that target the αIIbβ3 integrin.
| Name | Species | Target | Integrin | Sequence | Ref. |
|---|---|---|---|---|---|
| Accutin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [40,41] |
| Acostatin 1 | Snake | platelets | αIIbβ3 | RGD | [42] |
| Acostatin 2 | Snake | platelets | αIIbβ3 | RGD | [42] |
| Albolabrin | Snake | platelets | αIIbβ3 | RGD | [43] |
| Albolatin | Snake | platelets | αIIbβ3/α2β1 | KGD | [44] |
| Applagin | Snake | platelets | αIIbβ3 | RGD | [45] |
| Arietin | Snake | platelets | αIIbβ3 | RGD | [46] |
| Atrolysin E | Snake | platelets | αIIbβ3?/others? | MVD | [47] |
| Atropoimin | Snake | platelets | αvβ3 | RGD | [48] |
| Atroxatin | Snake | platelets | αIIbβ3 | RGD | [49] |
| Barbourin | Snake | platelets | αIIbβ3 | KGD | [50] |
| Basicilin | Snake | platelets | αIIbβ3/αvβ3/α5β1 | RGD | [51] |
| Batroxostatin | Snake | platelets | αIIbβ3 | RGD | [52] |
| Bitistatin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [53] |
| Bothrasperin | Snake | platelets | αIIbβ3?/αvβ3 | RGD | [54] |
| Cerastin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [51] |
| Cereberin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [51] |
| Colombistatin | Snake | platelets/human urinary and skin melanoma cancer cells | αIIbβ3/α5β1 | RGD | [55] |
| Contortrostatin | Snake | platelets/cancer cells | αIIbβ3/αvβ3/ α5β1/αvβ5 | RGD | [56,57] |
| Cotiarin | Snake | platelets | αIIbβ3/α5β1 | RGD | [51] |
| Crotavirin | Snake | platelets | αIIbβ3 | RGD? | [58] |
| Crotratroxin | Snake | platelets | αIIbβ3/α5β1 | RGD | [51] |
| Cumanastatin 1 | Snake | platelets | αIIbβ3 | RGD? | [59] |
| Dendroaspin | Snake | platelets/endothelial cells | αIIbβ3/αvβ3/α5β1 | RGD | [60,61] |
| Echistatin | Snake | platelets/osteoclasts | αIIbβ3/αvβ3/α5β1 | RGD | [62,63] |
| Elegantin | Snake | platelets | αIIbβ3 | RGD | [64] |
| Flavoridin | Snake | platelets | αIIbβ3 | RGD | [65] |
| Flavostatin | Snake | platelets | αIIbβ3 | RGD | [66] |
| Gabonin | Snake | platelets | αIIbβ3 | RGD | [67] |
| Halysin | Snake | platelets | αIIbβ3 | RGD | [68] |
| Halystatin | Snake | platelets | αIIbβ3? | RGD | [69] |
| Insularin | Snake | platelets/endothelial cells | αIIbβ3/αvβ3 | RGD | [70] |
| Jararacin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [51,71] |
| Jarastatin | Snake | platelets | αIIbβ3 | RGD | [71] |
| Jerdonatin | Snake | platelets | αIIbβ3 | RGD | [72] |
| Lachesin | Snake | platelets | αIIbβ3/αvβ3/α5β1 | RGD | [51] |
| Leucogastin A | Snake | platelets | αIIbβ3? | RGD | [73] |
| Leucogastin B | Snake | platelets | αIIbβ3? | RGD | [73] |
| Lutosin | Snake | platelets | αIIbβ3/αvβ3/α5β1 | RGD | [51] |
| Mojastin 1 | Snake | platelets/human urinary bladder cell adhesion to fibronectin | αIIbβ3/α5β1 | RGD | [74] |
| Mojastin 2 | Snake | platelets/human urinary bladder cell adhesion to fibronectin | αIIbβ3/α5β1 | RGD | [74] |
| Molossin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [51] |
| Morulustatin | Snake | platelets | αIIbβ3? | ? | [75] |
| Multisquamatin | Snake | platelets | αIIbβ3 | RGD | [56,73] |
| Ocelatin | Snake | platelets | αIIbβ3? | RGD | [73] |
| PAIEM | Snake | platelets | αIIbβ3 | RGD | [76] |
| Piscivostatin | Snake | platelets | αIIbβ3 | RGD/KGD | [77] |
| Pyramidin A | Snake | platelets | αIIbβ3? | RGD | [73] |
| Pyramidin B | Snake | platelets | αIIbβ3? | RGD | [73] |
| Rhodostomin/Kistrin | Snake | platelets/endothelial cells | αIIbβ3/αvβ3/α5β1 | RGD | [61] |
| Rubistatin | Snake | platelets/SK-Mel-28 cells | αIIbβ3? | MVD | [78] |
| Salmosin 1 | Snake | platelets/endothelial cells | αIIbβ3/αvβ3 | RGD | [79] |
| Salmosin 2 | Snake | platelets | αIIbβ3 | KGD | [80] |
| Saxatilin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [81] |
| Schistatin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [82] |
| Tergeminin | Snake | platelets | αIIbβ3 | RGD | [50] |
| Triflavin | Snake | platelets | αIIbβ3 | RGD | [83] |
| Trigramin | Snake | platelets/human melanoma cells | αIIbβ3 | RGD | [84,85] |
| Trimucrin | Snake | platelets/endothelial cells | αIIbβ3/αvβ3 | RGD | [86] |
| Ussuristatin-1 | Snake | platelets | αIIbβ3 | RGD | [87] |
| Ussuristatin-2 | Snake | platelets | αIIbβ3/α5β1 | KGD | [87] |
| Vicrostatin | Snake | platelets | αIIbβ3/α5β1 | RGD | [88] |
| Viridin | Snake | platelets | αIIbβ3/αvβ3 | RGD | [51] |
| Disagregin | Tick | platelets | αIIbβ3 | RED | [89] |
| Ixodegrin | Tick | platelets? | αIIbβ3? | RGD | [90] |
| Monogrin | Tick | platelets | αIIbβ3 | RGD | [91] |
| Savignygrin | Tick | platelets | αIIbβ3 | RGD/RED | [92] |
| Variabilin | Tick | platelets | αIIbβ3/αvβ3 | RGD | [93] |
| Decorsin | Leech | platelets | αIIbβ3 | RGD | [94] |
| Ornatin E | Leech | platelets | αIIbβ3 | RGD | [95] |
| Hookworm platelet inhibitor (HPI) | Worm | platelets | αIIbβ3/α2β1 | KGD | [96,97] |
| Tabinhibitin | Horsefly | platelets | αIIbβ3 | RGD | [98] |
| Tablysin-15 | Horsefly | platelets/endothelial cells | αIIbβ3/αvβ3/α5β1 | RGD | [99] |
7. Disintegrins from Snakes
7.1. Echistatin
Echistatin (5.4 kDa), first isolated from snake venom in 1988, is a polypeptide (49 amino acids) stabilised by 4 disulphide bonds [63]. Echistatin contains an RGD sequence and the half-maximal binding concentrations to several integrins were tested in solid-phase binding assays; integrin αvβ3 (0.46 nM), α5β1 (0.57 nM) and αIIbβ3 (0.90 nM) [63]. Echistatin is therefore not specific for the αIIbβ3 integrin. It is proposed that echistatin binds to the αIIbβ3 integrin to the same binding pocket on the β3 subunit as abciximab [100]. The clinically approved tirofiban has been developed from Echistatin and is discussed below.
7.2. Rhodostomin/Kistrin
Rhodostomin (8.4 kDa) consists of 68 amino acids and is stabilised by 6 disulphide bonds. Rhodostomin was expressed in Pichia pastoris and it inhibited cell adhesion of CHO cells (which express αIIbβ3 and αvβ3, IC50 21 nM and 13 nM, respectively) to fibrinogen [61]. Rhodostomin also inhibited the adhesion of K562 cells to fibronectin (α5β1, IC50 256 nM) [61].
8. Disintegrins from Ticks
8.1. Disagregin
Disagregin (6.9 kDa) consists of 60 amino acids stabilised by 3 disulphide bonds. Disagregin was isolated from the tick Ornithodoros moubata and inhibited ADP-stimulated platelet aggregation. Disagregin blocked platelet adhesion to fibrinogen, suggesting it is a fibrinogen antagonist [89]. Recently disagregin was obtained by total chemical synthesis and was shown to inhibit platelet adhesion, thrombus formation and fibrin formation [101].
8.2. Variabilin
Variabilin (5 kDa) consists of 47 amino acids and contains 5 cysteines. Variabilin was isolated from the tick Dermacentor variabilis, and inhibited ADP-induced platelet aggregation. Variabilin also blocked the interaction of αIIbβ3 with fibrinogen as well as the interaction of αvβ3 with vitronectin [93].
9. Disintegrins from Leeches
9.1. Decorsin
Decorsin (4.4 kDa) consists of 39 amino acids stabilised by 3 disulphide bonds. Decorsin was isolated from the leech Macrobdella decora and blocked the interaction of αIIbβ3 with fibrinogen and ADP-induced platelet aggregation [94]. Since leeches store blood for a long time, the antiplatelet aggregation function of this protein is important.
9.2. Ornatin E
Ornatin E (5.7 kDa) consists of 50 amino acids stabilised by 3 disulphide bonds. It is similar to decorsin. It was isolated from the leech Placobdella ornate and blocked the interaction of αIIbβ3 with fibrinogen (IC50 4.4 nM) and platelet aggregation activated by ADP (IC50 438 nM) [95]. Ornatin E was also recombinantly expressed, showing similar results as the isolated ornatin E, except showing a lower IC50 of ADP-induced platelet aggregation (284 nM) [102].
10. Disintegrins from Worms
Hookworm Platelet Inhibitor (HPI)
Hookworm platelet inhibitor (15 kDa) was isolated from the worm Ancylostoma caninum. It inhibits platelet aggregation and platelet adhesion to fibrinogen (mediated via αIIbβ3) and collagen (mediated via α2β1) [97].
11. Disintegrins from Horseflies
11.1. Tabinhibitin
Tabinhibitin (25 kDa) was purified from the horsefly Tabanus yao Macquart salivary glands. It blocks platelet aggregation, suggested via the αIIbβ3 integrin, but it is not yet known whether tabinhibitin acts on other receptors [98].
11.2. Tablysin-15
Tablysin-15 (26 kDa) was purified from the horsefly Tabanus yao Macquart salivary glands. The sequence of Tablysin-15 is homologous to tabinhibitin. It blocks platelet aggregation and inhibits platelet adhesion to fibrinogen (αIIbβ3), endothelial cell adhesion to vitronectin (αvβ3) and endothelial cell adhesion to fibronectin (α5β1) [99].
12. Antagonist Binding to the αIIbβ3 Receptor; Used in (Pre)Clinical Setting
12.1. β3. Chain Binding
Abciximab
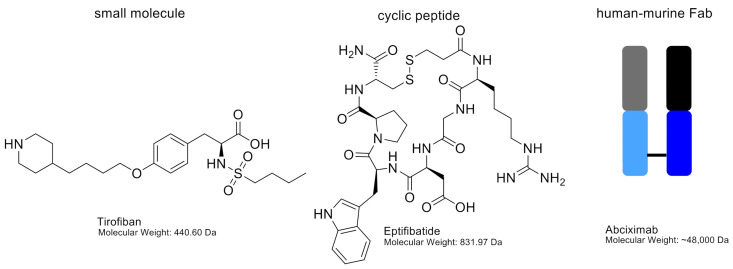
Abciximab (47.6 kDa) is the Fab fragment from a mouse/human chimeric antibody. When abciximab binds the β3 chain of the integrin receptor, the access of ligands to the binding pocket is impaired (Figure 4). Abciximab is not specific for the αIIbβ3 integrin, it can also block the vitronectin receptor αvβ3 with the same affinity (Kd 5 nM) and the MAC-1 receptor on leukocytes, albeit at lower affinity (Kd 160 nM) [103]. After administration, platelet function will restore within 24 to 36 h, however, Abciximab remains bound to platelets for 2 weeks after drug administration [104]. Although the main binding site of abciximab to αIIbβ3 is the ligand-binding pocket of the β3 subunit, it can also bind to KQAGDV, which is part of the αIIb subunit (Figure 4). Abciximab prevents access of ligands to binding pockets by steric hindrance or conformational effects [103]. Abciximab binds to both non-stimulated and stimulated platelets [105]. Abciximab is indicated for use in patients undergoing primary percutaneous coronary intervention and myocardial ischemia, together with heparin and acetylsalicylic acid [106]. Side effects of abciximab use are thrombocytopenia, bleeding, abdominal pain, nausea and vomiting [107]. Currently, abciximab is in shortage, as listed by the FDA, because of an interruption in manufacturing at a third-party site [108]. A schematic structure of abciximab is shown in Figure 5.
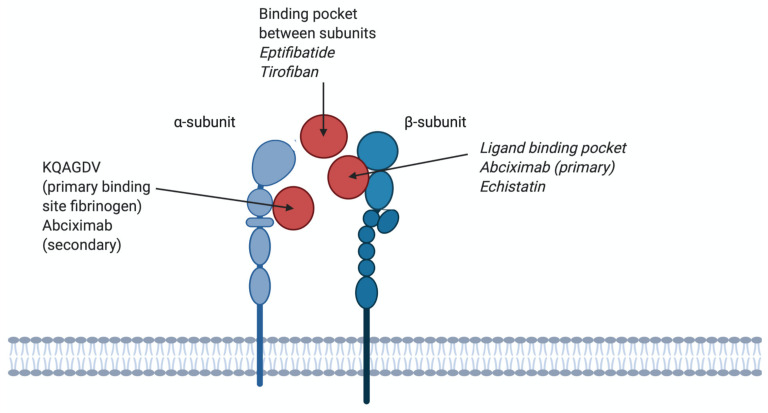
Figure 4.
Different binding sites of antagonists on αIIbβ3 integrins. KQAGDV; the primary binding site for fibrinogen and a secondary binding site for abciximab. The binding pocket between subunits; binding site for eptifibatide and tirofiban, therefore competing with fibrinogen. Binding pocket β3; primary binding site for abciximab and echistatin, therefore not competing with fibrinogen.
Figure 5.
Chemical structures of tirofiban and eptifibatide, including molecular weights. Abciximab is shown as a schematic figure. The murine variable chain region is shown in grey (light chain) and black (heavy chain). The human constant regions are shown in light blue (light chain) and dark blue (heavy chain).
12.2. Binding Pocket between αIIb and β3 Subunits
12.2.1. Eptifibatide
Eptifibatide (Integrilin; 832 Da) is a cyclic heptapeptide, containing a homo-arginine–glycine–aspartic acid (hArg–Gly–Asp) sequence, derived from the disintegrin barbourin (P22827), found in the venom of the southeastern pygmy rattlesnake (Sistrurus miliarius barbouri) [109,110]. The plasma half-life of eptifibatide is 3 h [111]. Cyclisation is usually performed to constrain the confirmation of a peptide, and it reduces the susceptibility to be degraded by proteases [112,113]. Eptifibatide is not specific for αIIbβ3 as it also binds to αvβ3, although with a lower affinity [114]. Eptifibatide is based on the KGD motif of barbourin, however, to enhance affinity the lysine was replaced by a homoarginine residue [50,110]. Eptifibatide can bind to both non-stimulated and stimulated platelets [110].
The binding site of eptifibatide is the binding pocket between the αIIb and β3 subunits, thereby blocking the binding domain for fibrinogen and thus inhibiting the formation of platelet thrombi (Figure 4). Because the binding site is in the ligand-binding pocket of αIIbβ3, eptifibatide competes with fibrinogen and VWF for the binding site, but not the binding site of abciximab [115]. Eptifibatide is used in patients with angina pectoris to prevent myocardial infarction and in patients undergoing primary percutaneous coronary intervention, often in combination with unfractionated heparin and acetylsalicylic acid [116]. Side effects of eptifibatide are bleeding, thrombocytopenia and hypotension [117]. The chemical structure of eptifibatide is shown in Figure 5.
12.2.2. Tirofiban
Tirofiban (Aggrastat; 440 Da) is a synthetic non-peptide reversible antagonist of the αIIbβ3 platelet integrin. Tirofiban was designed to mimic the RGD recognition motif within disintegrin echistatin, isolated from the venom of the saw-scaled viper Echis carinatrus [62,110]. It can be cleared from the plasma by renal excretion, and its half-life is 2 h. Tirofiban has a higher affinity to the αIIbβ3 receptor compared to eptifibatide (Kd 15 nM) [103]. Tirofiban recognises the RGD-binding sequence and binds within the ligand-binding pocket of αIIbβ3 receptors to compete with fibrinogen and VWF (Figure 4). Tirofiban can bind to both non-stimulated and stimulated platelets [110]. Tirofiban is indicated in patients with acute coronary syndrome and in patients undergoing primary percutaneous coronary intervention, where its use is often combined with heparin [118]. Side effects of tirofiban are headaches, nausea, thrombocytopenia and bleeding [119]. The chemical structure of tirofiban is shown in Figure 5.
13. Oral Antagonists
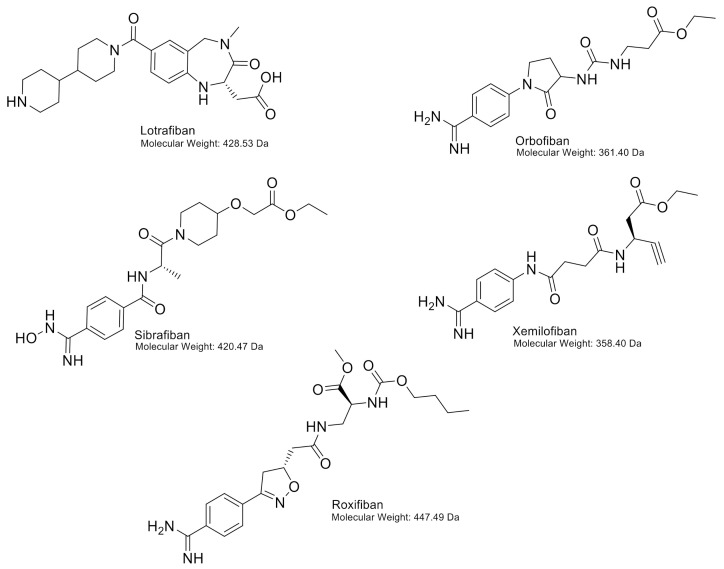
In the past, various oral antagonists have been evaluated, such as orbofiban, sibrafiban, xemilofiban, lotrafiban and roxifiban, the use of which has been associated with a prolonged bleeding time, an increase in the incidence of thrombocytopenia and a 30–35% increase in mortality (including cardiovascular mortality) [120]. All the following oral antagonists are discontinued in clinical trials for arterial thrombosis, myocardial infarction and unstable angina pectoris. The chemical structures of these antagonists are shown in Figure 6.
Figure 6.
Chemical structures of lotrafiban, orbofiban, sibrafiban, xemilofiban and roxifiban, including molecular weights.
13.1. Orbofiban
Orbofiban is a prodrug from SC-57101, a non-peptide antagonist, that blocks the binding of fibrinogen to αIIbβ3 thereby preventing platelet aggregation. After hydrolysis of the ethyl ester by esterases, the active SC-57101 prevents fibrinogen to bind to the receptor with an IC50 of 47 nM. SC-57101 binds to both resting and activated platelets (Kd; 70 nM and 109 nM, respectively). It can also reverse thrombus formation by binding to platelets even when fibrinogen is already bound [121]. In the OPUS-TIMI 16 trial, it was investigated whether orbofiban was effective. The trial arms were 50 mg of orbofiban twice daily, 50 mg of orbofiban twice daily for 30 days and thereafter 30 mg of orbofiban twice daily, or placebo (all groups received 150–162 mg of acetylsalicylic acid). However, after 30 days, the 50/30 treatment arm showed increased mortality [122]. Several groups performed research to investigate the mortality in the treatment arm, showing increased platelet reactivity [123] and neutrophil activation [124].
13.2. Sibrafiban
Sibrafiban is a prodrug from Ro 44-3888, a non-peptide selective antagonist of αIIbβ3 [125]. Platelet aggregation is blocked with IC50 15 µg/L (~35 nM) [125]. In the SYMPHONY trial, it was investigated whether sibrafiban prevents more ischemic events than acetylsalicylic acid after acute coronary syndrome. The trial included three arms; acetylsalicylic acid (80 mg every 12 h), low-dose sibrafiban (3.0–4.5 mg every 12 h) and high-dose sibrafiban (4.5–6.0 mg every 12 h). Bodyweight and serum creatinine concentration were used to assign patients to the low- or high-dose group [126]. The result of the trial was that sibrafiban was as effective as acetylsalicylic acid for the prevention of ischemic events, however, it showed more bleeding in trial participants [127].
13.3. Xemilofiban
Xemilofiban is a prodrug from SC-54701, a non-peptide mimetic of RGDF, blocking the binding of fibrinogen to αIIbβ3. The active SC-54701 prevents fibrinogen to bind to the receptor with IC50 of 10 nM, showing increased potency when compared to SC-57101 [128]. In the EXCITE trial, it was investigated if long-term administration of xemilofiban prevents ischemic events and stabilises plaques. The trial included three arms; placebo, xemilofiban (10 mg, three times daily) and xemilofiban (20 mg, three times daily) [129]. The result of the trial was that xemilofiban (administration before PCI up until 6 months thereafter) did not decrease the incidence of death and nonfatal myocardial infarction. As a consequence of the lacking beneficial effect of xemilofiban, the development was discontinued.
13.4. Lotrafiban
Lotrafiban is a non-peptide prodrug from SB-214857 that blocks fibrinogen binding to αIIbβ3 [130]. Platelet aggregation is inhibited by lotrafiban with IC50 40–80 nM [130]. In the BRAVO trial, it was investigated if lotrafiban therapy is effective in patients with recent unstable angina, stroke or myocardial infarction. The trial included two arms; placebo including acetylsalicylic acid, one time per day) or lotrafiban (30 mg or 50 mg, two times daily including acetylsalicylic acid, one time per day). The dose of lotrafiban was dependent on age and creatinine clearance. The phase III clinical trial was stopped because of increased mortality and increased thrombocytopenia and bleeding [131].
13.5. Roxifiban
Roxifiban (DMP754) is a prodrug of XV459, binds specifically to the αIIbβ3 receptor with IC50 0.03–0.05 µM [132]. It binds to both non-stimulated and stimulated platelets [133]. In the ROCKET trial, it was investigated if roxifiban administration in patients with coronary artery disease affected platelet aggregation and receptor expression. The trial included three arms; acetylsalicylic acid (325 mg + placebo, one time per day), roxifiban (1.0 mg + placebo, one time per day) or the combination of acetylsalicylic acid and roxifiban (325 mg and 1.5 mg, one time per day). In clinical trials, roxifiban showed enhanced expression of P-selectin and PECAM-1. Changes in effectivity suggest that young platelets adjust their pathways to aggregate as roxifiban is still present and thus blocking the αIIbβ3 receptor [134].
14. Conclusions
For the prevention of thrombotic complications in patients suffering from acute coronary syndrome or unstable angina pectoris, the integrin αIIbβ3 antagonists abciximab, eptifibatide and tirofiban (all administered intravenously) are occasionally used in the clinical setting. Eptifibatide and tirofiban, based on the ligand (RGD)-mimetics, bind to the integrin with low affinity and induce a conformational change, which results in outside-in signalling and paradoxical platelet activation [135]. The use of all three inhibitors is complicated by the increased risk of bleeding and less frequently, thrombocytopenia. Thrombocytopenia is the result of an immunological reaction caused by antibodies directed against ligand-induced binding sites (LIBS), that become exposed after integrin/inhibitor interaction [135]. Typically, thrombocytopenia is more frequent with the use of abciximab, since more epitopes are exposed which can be recognised by antibodies already present in the bloodstream [136].
These ligand-mimetic effects might not be induced by disintegrins, since hematophagous parasites and snakes often have shielding surface components at the moment they become opsonized by the immune system of the host [137]. They can also change their surface to escape recognition by the host [137]. We can speculate that disintegrins have evolved into forms that escape the human immune system. A previous study revealed a disintegrin that did not cause a conformational change of integrin αIIbβ3, resulting in antiplatelet activity without causing bleeding [138]. More research into disintegrins, including structure-activity relationships, may provide more insight into their biological activity and mechanism of platelet inhibition.
Different oral integrin αIIbβ3 antagonists have been tested, but clinical trials were stopped due to undesired side effects, including excess mortality or the absence of improvements over existing therapy. Various arguments are described in the literature as to why these antagonists failed in clinical trials: (1) plasma concentrations vary over the treatment period, resulting in variation in levels of integrin inhibition and eventual thrombotic events [128]; (2) the bioavailability of oral antagonists results in differences in platelet inhibition in and between patients [139]; (3) the difference between early effects and chronic effects, suggesting that for chronic use, different therapeutic approaches are required. However, oral antagonists have some beneficial effects: the administration of drugs is easier, and patients can administer drugs themselves during chronic therapy. In addition, oral antagonists can be used for prophylaxis [140].
Antiplatelet therapy is the standard treatment of atherothrombotic disease in the heart, peripheral arteries and brain. Either monotherapy or dual antiplatelet therapy with acetylsalicylic acid (aspirin) and/or a P2Y12 receptor blocker (clopidogrel, prasugrel, ticagrelor or cangrelor) is recommended for the secondary prevention of arterial thrombotic events [141]. Acetylsalicylic acid is also used as prophylaxis, however, there is a controversy between international guidelines and research that has shown less benefit of prophylactic acetylsalicylic acid [142]. At this time point, αIIbβ3 inhibitors are only used in patients who undergo percutaneous coronary intervention (without pretreatment with P2Y12 receptor blockers) or in high-risk patients, because less bleeding complications occur with P2Y12 receptor blockers [143].
No new αIIbβ3 inhibitors are developed since the approval of current platelet antagonists. Research has shown already promising effects of αIIbβ3 inhibitors for the prevention of ischemia/reperfusion injury to preserve cardiac function [144]. Future directions could be based on structure-function analysis of exogenous disintegrins, which might aid in finding platelet antagonists that maintain haemostasis and have fewer adverse side effects.
Acknowledgments
Figures are created using BioRender.com.
Author Contributions
D.L.v.d.K. analysed the literature and wrote the manuscript; P.E.J.v.d.M. wrote and edited the manuscript; T.M.H. edited the manuscript; I.D. wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the Netherlands Organization for Scientific Research (NWO; VIDI 723.013.009 and Aspasia 0.15.010.005) and the Van de Laar Foundation on Biochemistry of Cardiovascular Disease for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang J., Li X., Shi X., Zhu M., Wang J., Huang S., Huang X., Wang H., Li L., Deng H., et al. Platelet integrin alphaIIbbeta3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019;12:26. doi: 10.1186/s13045-019-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L.R., Owens T.W., Naylor M.J. Structural and mechanical functions of integrins. Biophys. Rev. 2014;6:203–213. doi: 10.1007/s12551-013-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang K., Chen J. The regulation of integrin function by divalent cations. Cell Adhes. Migr. 2012;6:20–29. doi: 10.4161/cam.18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazzoni G., Shih D.T., Buck C.A., Hemler M.E. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 5.Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- 6.Rose D.M., Alon R., Ginsberg M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 7.Takada Y., Ye X., Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett J.S. Structure and function of the platelet integrin alphaIIbbeta3. J. Clin. Investig. 2005;115:3363–3369. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coller B.S., Shattil S.J. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: A technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rupp P.A., Czirok A., Little C.D. alphavbeta3 integrin-dependent endothelial cell dynamics in vivo. Development. 2004;131:2887–2897. doi: 10.1242/dev.01160. [DOI] [PubMed] [Google Scholar]
- 11.Bury L., Zetterberg E., Leinoe E.B., Falcinelli E., Marturano A., Manni G., Nurden A.T., Gresele P. A novel variant Glanzmann thrombasthenia due to co-inheritance of a loss- and a gain-of-function mutation of ITGB3: Evidence of a dominant effect of gain-of-function mutations. Haematologica. 2018;103:e259–e263. doi: 10.3324/haematol.2017.180927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurden A.T. Glanzmann thrombasthenia. Orphanet J. Rare Dis. 2006;1:10. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrbod M., Trisno S., Mofrad M.R. On the activation of integrin alphaIIbbeta3: Outside-in and inside-out pathways. Biophys. J. 2013;105:1304–1315. doi: 10.1016/j.bpj.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bury L., Malara A., Gresele P., Balduini A. Outside-in signalling generated by a constitutively activated integrin alphaIIbbeta3 impairs proplatelet formation in human megakaryocytes. PLoS ONE. 2012;7:e34449. doi: 10.1371/journal.pone.0034449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y.Q., Qin J., Plow E.F. Platelet integrin alpha(IIb)beta: Activation mechanisms. J. Thromb. Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 16.Jordan P.A., Gibbins J.M. Extracellular disulfide exchange and the regulation of cellular function. Antioxid. Redox Signal. 2006;8:312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 17.Levin L., Zelzion E., Nachliel E., Gutman M., Tsfadia Y., Einav Y. A single disulfide bond disruption in the beta3 integrin subunit promotes thiol/disulfide exchange, a molecular dynamics study. PLoS ONE. 2013;8:e59175. doi: 10.1371/annotation/b4e96e4b-3106-4040-a63c-a3f018f0e5c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorris S.L., Peebles R.S., Jr. PGI2 as a regulator of inflammatory diseases. Mediators Inflamm. 2012;2012:926968. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radomski M.W., Palmer R.M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Versteeg H.H., Heemskerk J.W., Levi M., Reitsma P.H. New fundamentals in hemostasis. Physiol. Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 21.Kanaji S., Fahs S.A., Shi Q., Haberichter S.L., Montgomery R.R. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J. Thromb Haemost. 2012;10:1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharda A., Flaumenhaft R. The life cycle of platelet granules. F1000Res. 2018;7:236. doi: 10.12688/f1000research.13283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filkova A.A., Martyanov A.A., Garzon Dasgupta A.K., Panteleev M.A., Sveshnikova A.N. Quantitative dynamics of reversible platelet aggregation: Mathematical modelling and experiments. Sci. Rep. 2019;9:6217. doi: 10.1038/s41598-019-42701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu D.D., White C.A., Panzer-Knodle S., Page J.D., Nicholson N., Smith J.W. A new model of dual interacting ligand binding sites on integrin alphaIIbbeta3. J. Biol. Chem. 1999;274:4633–4639. doi: 10.1074/jbc.274.8.4633. [DOI] [PubMed] [Google Scholar]
- 25.Durrant T.N., van den Bosch M.T., Hers I. Integrin alphaIIbbeta3 outside-in signaling. Blood. 2017;130:1607–1619. doi: 10.1182/blood-2017-03-773614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye F., Kim C., Ginsberg M.H. Reconstruction of integrin activation. Blood. 2012;119:26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bye A.P., Unsworth A.J., Gibbins J.M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. 2016;14:918–930. doi: 10.1111/jth.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanini L., Bergmeier W. RAP1-GTPase signaling and platelet function. J. Mol. Med. 2016;94:13–19. doi: 10.1007/s00109-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harburger D.S., Bouaouina M., Calderwood D.A. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong H., Shen B., Flevaris P., Chow C., Lam S.C., Voyno-Yasenetskaya T.A., Kozasa T., Du X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buensuceso C.S., Arias-Salgado E.G., Shattil S.J. Protein-protein interactions in platelet alphaIIbbeta3 signaling. Semin. Thromb. Hemost. 2004;30:427–439. doi: 10.1055/s-2004-833478. [DOI] [PubMed] [Google Scholar]
- 32.Martin V., Guillermet-Guibert J., Chicanne G., Cabou C., Jandrot-Perrus M., Plantavid M., Vanhaesebroeck B., Payrastre B., Gratacap M.-P. Deletion of the p110beta isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood. 2010;115:2008–2013. doi: 10.1182/blood-2009-04-217224. [DOI] [PubMed] [Google Scholar]
- 33.Canobbio I., Stefanini L., Cipolla L., Ciraolo E., Gruppi C., Balduini C., Hirsch E., Torti M. Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood. 2009;114:2193–2196. doi: 10.1182/blood-2009-03-208074. [DOI] [PubMed] [Google Scholar]
- 34.Das M., Ithychanda S., Qin J., Plow E.F. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim. Biophys. Acta. 2014;1838:579–588. doi: 10.1016/j.bbamem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggeri Z.M. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 36.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017;38:785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Previtali E., Bucciarelli P., Passamonti S.M., Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120–138. doi: 10.2450/2010.0066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurbel P.A., Fox K.A.A., Tantry U.S., Ten Cate H., Weitz J.I. Combination Antiplatelet and Oral Anticoagulant Therapy in Patients With Coronary and Peripheral Artery Disease. Circulation. 2019;139:2170–2185. doi: 10.1161/CIRCULATIONAHA.118.033580. [DOI] [PubMed] [Google Scholar]
- 39.Nowak G. Pharmacology of recombinant hirudin. Semin. Thromb. Hemost. 2002;28:415–424. doi: 10.1055/s-2002-35293. [DOI] [PubMed] [Google Scholar]
- 40.Yeh C.H., Peng H.C., Huang T.F. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta3 antagonist and inducing apoptosis. Blood. 1998;92:3268–3276. doi: 10.1182/blood.V92.9.3268. [DOI] [PubMed] [Google Scholar]
- 41.Yeh C.H., Peng H.C., Yih J.B., Huang T.F. A new short chain RGD-containing disintegrin, accutin, inhibits the common pathway of human platelet aggregation. Biochim. Biophys. Acta. 1998;1425:493–504. doi: 10.1016/S0304-4165(98)00104-4. [DOI] [PubMed] [Google Scholar]
- 42.Okuda D., Koike H., Morita T. A new gene structure of the disintegrin family: A subunit of dimeric disintegrin has a short coding region. Biochemistry. 2002;41:14248–14254. doi: 10.1021/bi025876s. [DOI] [PubMed] [Google Scholar]
- 43.Calvete J.J., Schäfer W., Soszka T., Lu W.Q., Cook J.J., Jameson B.A., Niewiarowski S. Identification of the disulfide bond pattern in albolabrin, an RGD-containing peptide from the venom of Trimeresurus albolabris: Significance for the expression of platelet aggregation inhibitory activity. Biochemistry. 1991;30:5225–5229. doi: 10.1021/bi00235a016. [DOI] [PubMed] [Google Scholar]
- 44.Singhamatr P., Rojnuckarin P. Molecular cloning of albolatin, a novel snake venom metalloprotease from green pit viper (Trimeresurus albolabris), and expression of its disintegrin domain. Toxicon. 2007;50:1192–1200. doi: 10.1016/j.toxicon.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Chao B.H., Jakubowski J.A., Savage B., Chow E.P., Marzec U.M., Harker L.A., Maraganore J.M. Agkistrodon piscivorus piscivorus platelet aggregation inhibitor: A potent inhibitor of platelet activation. Proc. Natl. Acad. Sci. USA. 1989;86:8050–8054. doi: 10.1073/pnas.86.20.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang T.F., Wang W.J., Teng C.M., Ouyang C. Mechanism of action of the antiplatelet peptide, arietin, from Bitis arietans venom. Biochim. Biophys. Acta. 1991;1074:144–150. doi: 10.1016/0304-4165(91)90053-J. [DOI] [PubMed] [Google Scholar]
- 47.Shimokawa K., Jia L.G., Shannon J.D., Fox J.W. Isolation, sequence analysis, and biological activity of atrolysin E/D, the non-RGD disintegrin domain from Crotalus atrox venom. Arch. Biochem. Biophys. 1998;354:239–246. doi: 10.1006/abbi.1998.0698. [DOI] [PubMed] [Google Scholar]
- 48.Angulo Y., Castro A., Lomonte B., Rucavado A., Fernandez J., Calvete J.J., Gutiérrez J.M. Isolation and characterization of four medium-size disintegrins from the venoms of Central American viperid snakes of the genera Atropoides, Bothrops, Cerrophidion and Crotalus. Biochimie. 2014;107:376–384. doi: 10.1016/j.biochi.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Soto J.G., White S.A., Reyes S.R., Regalado R., Sanchez E.E., Perez J.C. Molecular evolution of PIII-SVMP and RGD disintegrin genes from the genus Crotalus. Gene. 2007;389:66–72. doi: 10.1016/j.gene.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Scarborough R., Rose J., Hsu M., Phillips D., Fried V., Campbell A., Nannizzi L., Charo I. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J. Biol. Chem. 1991;266:9359–9362. doi: 10.1016/S0021-9258(18)92826-7. [DOI] [PubMed] [Google Scholar]
- 51.Scarborough R., Rose J., Naughton M., Phillips D., Nannizzi L., Arfsten A., Campbell A., Charo I. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J. Biol. Chem. 1993;268:1058–1065. doi: 10.1016/S0021-9258(18)54041-2. [DOI] [PubMed] [Google Scholar]
- 52.Rucinski B., Niewiarowski S., Holt J.C., Soszka T., Knudsen K.A. Batroxostatin, an Arg-Gly-Asp-containing peptide from Bothrops atrox, is a potent inhibitor of platelet aggregation and cell interaction with fibronectin. Biochim. Biophys. Acta. 1990;1054:257–262. doi: 10.1016/0167-4889(90)90096-V. [DOI] [PubMed] [Google Scholar]
- 53.Shebuski R.J., Ramjit D.R., Bencen G.H., Polokoff M.A. Characterization and platelet inhibitory activity of bitistatin, a potent arginine-glycine-aspartic acid-containing peptide from the venom of the viper Bitis arietans. J. Biol. Chem. 1989;264:21550–21556. doi: 10.1016/S0021-9258(20)88220-9. [DOI] [PubMed] [Google Scholar]
- 54.Pinto A., Angulo Y., Jiménez R., Lomonte B. Isolation of bothrasperin, a disintegrin with potent platelet aggregation inhibitory activity, from the venom of the snake Bothrops asper. Rev. Biol. Trop. 2003;51:253–259. [PubMed] [Google Scholar]
- 55.Sánchez E.E., Rodríguez-Acosta A., Palomar R., Lucena S.E., Bashir S., Soto J.G., Pérez J.C. Colombistatin: A disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Arch. Toxicol. 2008;83:271–279. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- 56.Trikha M., Rote W.E., Manley P.J., Lucchesi B.R., Markland F.S. Purification and characterization of platelet aggregation inhibitors from snake venoms. Thromb. Res. 1994;73:39–52. doi: 10.1016/0049-3848(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Q., Hu P., Ritter M.R., Swenson S.D., Argounova S., Epstein A.L., Markland F.S. Molecular Cloning and Functional Expression of Contortrostatin, a Homodimeric Disintegrin from Southern Copperhead Snake Venom. Arch. Biochem. Biophys. 2000;375:278–288. doi: 10.1006/abbi.1999.1682. [DOI] [PubMed] [Google Scholar]
- 58.Liu C.Z., Peng H.C., Huang T.F. Crotavirin, a potent platelet aggregation inhibitor purified from the venom of the snake Crotalus viridis. Toxicon. 1995;33:1289–1298. doi: 10.1016/0041-0101(95)00074-V. [DOI] [PubMed] [Google Scholar]
- 59.Da Silva M., Lucena S., Aguilar I., Rodríguez-Acosta A., Salazar A.M., Sánchez E.E., Girón M.E., Carvajal Z., Arocha-Piñango C.L., Guerrero B. Anti-platelet effect of cumanastatin 1, a disintegrin isolated from venom of South American Crotalus rattlesnake. Thromb. Res. 2009;123:731–739. doi: 10.1016/j.thromres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Williams J.A., Lu X., Rahman S., Keating C., Kakkar V. Dendroaspin: A potent integrin receptor inhibitor from the venoms of Dendroaspis viridis and D. jamesonii. Biochem. Soc. Trans. 1993;21:73. doi: 10.1042/bst021073s. [DOI] [PubMed] [Google Scholar]
- 61.Cheng C.H., Chen Y.C., Shiu J.H., Chang Y.T., Chang Y.S., Huang C.H., Chen C.H., Chuang W.J. Dynamics and functional differences between dendroaspin and rhodostomin: Insights into protein scaffolds in integrin recognition. Protein Sci. 2012;21:1872–1884. doi: 10.1002/pro.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gan Z.R., Gould R.J., Jacobs J.W., Friedman P.A., Polokoff M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J. Biol. Chem. 1988;263:19827–19832. doi: 10.1016/S0021-9258(19)77710-2. [DOI] [PubMed] [Google Scholar]
- 63.Kapp T.G., Rechenmacher F., Neubauer S., Maltsev O.V., Cavalcanti-Adam E.A., Zarka R., Reuning U., Notni J., Wester H.-J., Mas-Moruno C., et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017;7:39805. doi: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams J.A., Ashby B., Daniel J.L. Ligands to the platelet fibrinogen receptor glycoprotein IIb-IIIa do not affect agonist-induced second messengers Ca2+ or cyclic AMP. Biochem. J. 1990;270:149–155. doi: 10.1042/bj2700149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senn H., Klaus W. The nuclear magnetic resonance solution structure of flavoridin, an antagonist of the platelet GP IIb-IIIa receptor. J. Mol. Biol. 1993;232:907–925. doi: 10.1006/jmbi.1993.1439. [DOI] [PubMed] [Google Scholar]
- 66.Kawasaki T., Sakai Y., Taniuchi Y., Sato K., Maruyama K., Shimizu M., Kaku S., Yano S., Inagaki O., Tomioka K., et al. Biochemical characterization of a new disintegrin, flavostatin, isolated from Trimeresurus flavoviridis venom. Biochimie. 1996;78:245–252. doi: 10.1016/0300-9084(96)82187-0. [DOI] [PubMed] [Google Scholar]
- 67.Huang T.F., Peng H.C., Peng I.S., Teng C.M., Ouyang C. An antiplatelet peptide, gabonin, from Bitis gabonica snake venom. Arch. Biochem. Biophys. 1992;298:13–20. doi: 10.1016/0003-9861(92)90087-D. [DOI] [PubMed] [Google Scholar]
- 68.Huang T.F., Liu C.Z., Ouyang C.H., Teng C.M. Halysin, an antiplatelet Arg-Gly-Asp-containing snake venom peptide, as fibrinogen receptor antagonist. Biochem. Pharmacol. 1991;42:1209–1219. doi: 10.1016/0006-2952(91)90256-5. [DOI] [PubMed] [Google Scholar]
- 69.Bazaa A., Juarez P., Marrakchi N., Lasfer Z.B., El Ayeb M., Harrison R.A., Calvete J.J., Sanz L. Loss of Introns Along the Evolutionary Diversification Pathway of Snake Venom Disintegrins Evidenced by Sequence Analysis of Genomic DNA from Macrovipera lebetina transmediterranea and Echis ocellatus. J. Mol. Evol. 2006;64:261–271. doi: 10.1007/s00239-006-0161-4. [DOI] [PubMed] [Google Scholar]
- 70.Della-Casa M.S., Junqueira-De-Azevedo I., Butera D., Clissa P.B., Lopes D.S., Serrano S.M., Pimenta D.C., Magalhães G.S., Ho P.L., Moura-Da-Silva A.M. Insularin, a disintegrin from Bothrops insularis venom: Inhibition of platelet aggregation and endothelial cell adhesion by the native and recombinant GST-insularin proteins. Toxicon. 2011;57:125–133. doi: 10.1016/j.toxicon.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Wermelinger L.S., Geraldo R.B., Frattani F.S., Rodrigues C.R., Juliano M.A., Castro H.C., Zingali R.B. Integrin inhibitors from snake venom: Exploring the relationship between the structure and activity of RGD-peptides. Arch. Biochem. Biophys. 2009;482:25–32. doi: 10.1016/j.abb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X.-D., Ding C.-H., Tai H., Jin Y., Chen R.-Q., Lu Q.-M., Wang W.-Y., Xiong Y.-L. A novel disintegrin, jerdonatin, inhibits platelet aggregation and sperm–egg binding. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004;139:117–122. doi: 10.1016/j.cbpc.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Okuda D., Nozaki C., Sekiya F., Morita T. Comparative biochemistry of disintegrins isolated from snake venom: Consideration of the taxonomy and geographical distribution of snakes in the genus Echis. J. Biochem. 2001;129:615–620. doi: 10.1093/oxfordjournals.jbchem.a002898. [DOI] [PubMed] [Google Scholar]
- 74.Sánchez E.E., Galán J.A., Russell W.K., Soto J.G., Russell D.H., Pérez J.C. Isolation and characterization of two disintegrins inhibiting ADP-induced human platelet aggregation from the venom of Crotalus scutulatus scutulatus (Mohave Rattlesnake) Toxicol. Appl. Pharmacol. 2006;212:59–68. doi: 10.1016/j.taap.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Borja M., Galan J.A., Cantu E., Zugasti-Cruz A., Rodríguez-Acosta A., Lazcano D., Lucena S., Suntravat M., Sánchez Y.E.E. Morulustatin, A. Disintegrin that Inhibits ADP-Induced Platelet Aggregation, Isolated from the Mexican Tamaulipan Rock Rattlesnake (Crotalus lepidus morulus) Rev. Cient. 2016;26:86–94. [PMC free article] [PubMed] [Google Scholar]
- 76.Chernyshenko V., Petruk N., Korolova D., Kasatkina L., Gornytska O., Platonova T., Chernyshenko T., Rebriev A., Dzhus O., Garmanchuk L., et al. Antiplatelet and anti-proliferative action of disintegrin from Echis multisquamatis snake venom. Croat. Med. J. 2017;58:118–127. doi: 10.3325/cmj.2017.58.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okuda D., Morita T. Purification and characterization of a new RGD/KGD-containing dimeric disintegrin, piscivostatin, from the venom of Agkistrodon piscivorus piscivorus: The unique effect of piscivostatin on platelet aggregation. J. Biochem. 2001;130:407–415. doi: 10.1093/oxfordjournals.jbchem.a003000. [DOI] [PubMed] [Google Scholar]
- 78.Carey C.M., Bueno R., Gutierrez D.A., Petro C., Lucena S.E., Sánchez E.E., Soto J.G. Recombinant rubistatin (r-Rub), an MVD disintegrin, inhibits cell migration and proliferation, and is a strong apoptotic inducer of the human melanoma cell line SK-Mel-28. Toxicon. 2012;59:241–248. doi: 10.1016/j.toxicon.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang I.C., Chung K.H., Lee S.J., Yun Y., Moon H.M., Kim D.S. Purification and molecular cloning of a platelet aggregation inhibitor from the snake (Agkistrodon halys brevicaudus) venom. Thromb. Res. 1998;91:65–73. doi: 10.1016/S0049-3848(98)00053-X. [DOI] [PubMed] [Google Scholar]
- 80.Park D., Kang I., Kim H., Chung K., Kim D.S., Yun Y. Cloning and characterization of novel disintegrins from Agkistrodon halys venom. Mol. Cells. 1998;8:578–584. [PubMed] [Google Scholar]
- 81.Hong S.Y., Koh Y.S., Chung K.H., Kim D.S. Snake venom disintegrin, saxatilin, inhibits platelet aggregation, human umbilical vein endothelial cell proliferation, and smooth muscle cell migration. Thromb. Res. 2002;105:79–86. doi: 10.1016/S0049-3848(01)00416-9. [DOI] [PubMed] [Google Scholar]
- 82.Bilgrami S., Tomar S., Yadav S., Kaur P., Kumar J., Jabeen T., Sharma S., Singh T.P. Crystal Structure of Schistatin, a Disintegrin Homodimer from Saw-scaled Viper (Echis carinatus) at 2.5 Å Resolution. J. Mol. Biol. 2004;341:829–837. doi: 10.1016/j.jmb.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 83.Huang T.F., Sheu J.R., Teng C.M., Chen S.W., Liu C.S. Triflavin, an antiplatelet Arg-Gly-Asp-containing peptide, is a specific antagonist of platelet membrane glycoprotein IIb-IIIa complex. J. Biochem. 1991;109:328–334. [PubMed] [Google Scholar]
- 84.Huang T.F., Holt J.C., Lukasiewicz H., Niewiarowski S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J. Biol. Chem. 1987;262:16157–16163. doi: 10.1016/S0021-9258(18)47710-1. [DOI] [PubMed] [Google Scholar]
- 85.Knudsen K.A., Tuszynski G.P., Huang T.F., Niewiarowski S. Trigramin, an RGD-containing peptide from snake venom, inhibits cell-substratum adhesion of human melanoma cells. Exp. Cell Res. 1988;179:42–49. doi: 10.1016/0014-4827(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 86.Hung Y.C., Hsu C.C., Chung C.H., Huang T.F. The disintegrin, trimucrin, suppresses LPS-induced activation of phagocytes primarily through blockade of NF-κB and MAPK activation. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389:723–737. doi: 10.1007/s00210-016-1233-7. [DOI] [PubMed] [Google Scholar]
- 87.Oshikawa K., Terada S. Ussuristatin 2, a novel KGD-bearing disintegrin from Agkistrodon ussuriensis venom. J. Biochem. 1999;125:31–35. doi: 10.1093/oxfordjournals.jbchem.a022264. [DOI] [PubMed] [Google Scholar]
- 88.Minea R.O., Helchowski C.M., Zidovetzki S.J., Costa F.K., Swenson S.D., Markland F.S., Jr. Vicrostatin—An anti-invasive multi-integrin targeting chimeric disintegrin with tumor anti-angiogenic and pro-apoptotic activities. PLoS ONE. 2010;5:e10929. doi: 10.1371/journal.pone.0010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karczewski J., Endris R., Connolly T.M. Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata. J. Biol. Chem. 1994;269:6702–6708. doi: 10.1016/S0021-9258(17)37432-X. [DOI] [PubMed] [Google Scholar]
- 90.Francischetti I.M.B., Pham V.M., Mans B.J., Andersen J.F., Mather T.N., Lane R.S., Ribeiro J.M.C. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae) Insect Biochem. Mol. Biol. 2005;35:1142–1161. doi: 10.1016/j.ibmb.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mans B.J., Andersen J.F., Schwan T.G., Ribeiro J.M. Characterization of anti-hemostatic factors in the argasid, Argas monolakensis: Implications for the evolution of blood-feeding in the soft tick family. Insect Biochem. Mol. Biol. 2008;38:22–41. doi: 10.1016/j.ibmb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mans B.J., Louw A.I., Neitz A.W. Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold. J. Biol. Chem. 2002;277:21371–21378. doi: 10.1074/jbc.M112060200. [DOI] [PubMed] [Google Scholar]
- 93.Wang X., Coons L.B., Taylor D.B., Stevens S.E., Gartner T.K., Jr. Variabilin, a novel RGD-containing antagonist of glycoprotein IIb-IIIa and platelet aggregation inhibitor from the hard tick Dermacentor variabilis. J. Biol. Chem. 1996;271:17785–17790. doi: 10.1074/jbc.271.30.17785. [DOI] [PubMed] [Google Scholar]
- 94.Seymour J.L., Henzel W.J., Nevins B., Stults J.T., Lazarus R.A. Decorsin. A potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor from the leech Macrobdella decora. J. Biol. Chem. 1990;265:10143–10147. doi: 10.1016/S0021-9258(19)38791-5. [DOI] [PubMed] [Google Scholar]
- 95.Mazur P., Henzel W.J., Seymour J.L., Lazarus R.A. Ornatins: Potent glycoprotein IIb-IIIa antagonists and platelet aggregation inhibitors from the leech Placobdella ornata. Eur. J. Biochem. 1991;202:1073–1082. doi: 10.1111/j.1432-1033.1991.tb16472.x. [DOI] [PubMed] [Google Scholar]
- 96.Del Valle A., Jones B.F., Harrison L.M., Chadderdon R.C., Cappello M. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol. Biochem. Parasitol. 2003;129:167–177. doi: 10.1016/S0166-6851(03)00121-X. [DOI] [PubMed] [Google Scholar]
- 97.Chadderdon R.C., Cappello M. The hookworm platelet inhibitor: Functional blockade of integrins GPIIb/IIIa (alphaIIbbeta3) and GPIa/IIa (alpha2beta1) inhibits platelet aggregation and adhesion in vitro. J. Infect. Dis. 1999;179:1235–1241. doi: 10.1086/314724. [DOI] [PubMed] [Google Scholar]
- 98.Ma D., Wang Y., Yang H., Wu J., An S., Gao L., Xu X., Lai R. Anti-thrombosis Repertoire of Blood-feeding Horsefly Salivary Glands. Mol. Cell. Proteom. 2009;8:2071–2079. doi: 10.1074/mcp.M900186-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu H., Yang X., Andersen J.F., Wang Y., Tokumasu F., Ribeiro J.M.C., Ma D., Xu X., An S., Francischetti I.M.B., et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets αIIbβ3 or αVβ3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011;105:1032–1045. doi: 10.1160/TH11-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marcinkiewicz C., Rosenthal L.A., Mosser D.M., Kunicki T.J., Niewiarowski S. Immunological characterization of eristostatin and echistatin binding sites on alpha IIb beta 3 and alpha V beta 3 integrins. Biochem. J. 1996;317:817–825. doi: 10.1042/bj3170817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van den Kerkhof D.L., Nagy M., Wichapong K., Brouns S.L.N., Heemskerk J.W.M., Hackeng T.M., Dijkgraaf I. Inhibition of platelet adhesion, thrombus formation, and fibrin formation by a potent alphaIIbbeta3 integrin inhibitor from ticks. Res. Pract. Thromb. Haemost. 2021;5:231–242. doi: 10.1002/rth2.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mazur P., Dennis M.S., Seymour J.L., Lazarus R.A. Expression, purification, and characterization of recombinant ornatin E, a potent glycoprotein IIb-IIIa antagonist. Protein Expr. Purif. 1993;4:282–289. doi: 10.1006/prep.1993.1036. [DOI] [PubMed] [Google Scholar]
- 103.Schror K., Weber A.A. Comparative pharmacology of GP IIb/IIIa antagonists. J. Thromb. Thrombolysis. 2003;15:71–80. doi: 10.1023/B:THRO.0000003308.63022.8d. [DOI] [PubMed] [Google Scholar]
- 104.Mascelli M.A., Lance E.T., Damaraju L., Wagner C.L., Weisman H.F., Jordan R.E. Pharmacodynamic profile of short-term abciximab treatment demonstrates prolonged platelet inhibition with gradual recovery from GP IIb/IIIa receptor blockade. Circulation. 1998;97:1680–1688. doi: 10.1161/01.CIR.97.17.1680. [DOI] [PubMed] [Google Scholar]
- 105.Schwarz M., Meade G., Stoll P., Ylanne J., Bassler N., Chen Y.C., Hagemeyer C.E., Ahrens I., Moran N., Kenny D., et al. Conformation-specific blockade of the integrin GPIIb/IIIa: A novel antiplatelet strategy that selectively targets activated platelets. Circ. Res. 2006;99:25–33. doi: 10.1161/01.RES.0000232317.84122.0c. [DOI] [PubMed] [Google Scholar]
- 106.Bedjaoui A., Allal K., Lounes M.S., Belhadi C.E., Mekarnia A., Sediki S., Kara M., Azaza A., Monsuez J.-J., Benkhedda S. Intracoronary or intravenous abciximab after aspiration thrombectomy in patients with STEMI undergoing primary percutaneous coronary intervention. Cardiovasc. J. Afr. 2019;30:45–51. doi: 10.5830/CVJA-2018-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stoffer K., Bistas K.G., Reddy V., Shah S. Abciximab. StatPearls; Treasure Island, FL, USA: 2020. [Google Scholar]
- 108.(FDA) US FDA Drug Shortages. [(accessed on 8 February 2021)]; Available online: https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Abciximab%20(ReoPro)%20Injection&st=c&tab=tabs-1.
- 109.Phillips D.R., Scarborough R.M. Clinical pharmacology of eptifibatide. Am. J. Cardiol. 1997;80:11B–20B. doi: 10.1016/S0002-9149(97)00572-9. [DOI] [PubMed] [Google Scholar]
- 110.Hashemzadeh M., Furukawa M., Goldsberry S., Movahed M.R. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review. Exp. Clin. Cardiol. 2008;13:192–197. [PMC free article] [PubMed] [Google Scholar]
- 111.Valgimigli M., Campo G., Tebaldi M., Carletti R., Arcozzi C., Ferrari R., Percoco G. Abciximab: A reappraisal of its use in coronary care. Biologics. 2008;2:29–39. doi: 10.2147/BTT.S1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gang D., Kim D.W., Park H.S. Cyclic Peptides: Promising Scaffolds for Biopharmaceuticals. Genes. 2018;9:557. doi: 10.3390/genes9110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lele M., Sajid M., Wajih N., Stouffer G.A. Eptifibatide and 7E3, but not tirofiban, inhibit alpha(v)beta integrin-mediated binding of smooth muscle cells to thrombospondin and prothrombin. Circulation. 2001;104:582–587. doi: 10.1161/hc3101.092199. [DOI] [PubMed] [Google Scholar]
- 115.Tcheng J.E. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: Bleeding, reversal, thrombocytopenia, and retreatment. Am. Heart J. 2000;139:S38–S45. doi: 10.1067/mhj.2000.103742. [DOI] [PubMed] [Google Scholar]
- 116.Bansal A.B., Sattar Y., Jamil R.T. Eptifibatide. StatPearls; Treasure Island, FL, USA: 2020. [Google Scholar]
- 117.The PURSUIT Trial Investigators Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N. Engl. J. Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 118.Valgimigli M., Percoco G., Barbieri D., Ferrari F., Guardigli G., Parrinello G., Soukhomovskaia O., Ferrari R. The additive value of tirofiban administered with the high-dose bolus in the prevention of ischemic complications during high-risk coronary angioplasty: The ADVANCE Trial. J. Am. Coll. Cardiol. 2004;44:14–19. doi: 10.1016/j.jacc.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 119.Tcheng J.E. Enhancing safety and outcomes with the newer antithrombotic and antiplatelet agents. Am. Heart J. 1995;130:673–679. doi: 10.1016/0002-8703(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 120.Yeh E.T., Khan B.V. The potential role of antiplatelet agents in modulating inflammatory markers in atherothrombosis. J. Thromb. Haemost. 2006;4:2308–2316. doi: 10.1111/j.1538-7836.2006.02202.x. [DOI] [PubMed] [Google Scholar]
- 121.Nicholson N.S., Abood N.A., Panzer-Knodle S.G., Frederick L.G., Page J.D., Salyers A.K., Suleymanov O.D., Szalony J.A., Taite B.B., Anders R.J. Orbofiban: An orally active GPIIb/IIIa platelet receptor antagonist. Med. Res. Rev. 2001;21:211–226. doi: 10.1002/med.1007. [DOI] [PubMed] [Google Scholar]
- 122.Cannon C.P., McCabe C.H., Wilcox R.G., Langer A., Caspi A., Berink P., Lopez-Sendon J., Toman J., Charlesworth A., Anders R.J., et al. Oral Glycoprotein IIb/IIIa Inhibition With Orbofiban in Patients With Unstable Coronary Syndromes (OPUS-TIMI 16) Trial. Circulation. 2000;102:149–156. doi: 10.1161/01.CIR.102.2.149. [DOI] [PubMed] [Google Scholar]
- 123.Holmes M.B., Sobel B.E., Cannon C.P., Schneider D.J. Increased platelet reactivity in patients given orbofiban after an acute coronary syndrome: An OPUS-TIMI 16 substudy. Orbofiban in Patients with Unstable coronary syndromes. Thrombolysis In Myocardial Infarction. Am. J. Cardiol. 2000;85:491–493. doi: 10.1016/S0002-9149(99)00778-X. [DOI] [PubMed] [Google Scholar]
- 124.Serrano C.V., Jr., Nicolau J.C., Venturinelli M., Baracioli L.M., Anders R.J., Cannon C.P., Ramires J.A. Role of oral blockade of platelet glycoprotein IIb/IIIa on neutrophil activation in patients with acute coronary syndromes. Cardiovasc. Drugs Ther. 2003;17:129–132. doi: 10.1023/A:1025335718142. [DOI] [PubMed] [Google Scholar]
- 125.Dooley M., Goa K.L. Sibrafiban. Drugs. 1999;57:225–230. doi: 10.2165/00003495-199957020-00012. [DOI] [PubMed] [Google Scholar]
- 126.Newby L.K. Long-term oral platelet glycoprotein IIb/IIIa receptor antagonism with sibrafiban after acute coronary syndromes: Study design of the sibrafiban versus aspirin to yield maximum protection from ischemic heart events post-acute coronary syndromes (SYMPHONY) trial. Symphony Steering Committee. Am. Heart J. 1999;138:210–218. doi: 10.1016/s0002-8703(99)70104-3. [DOI] [PubMed] [Google Scholar]
- 127.The SYMPHONY Investigators Comparison of sibrafiban with aspirin for prevention of cardiovascular events after acute coronary syndromes: A randomised trial. Lancet. 2000;355:337–345. doi: 10.1016/S0140-6736(99)11179-6. [DOI] [PubMed] [Google Scholar]
- 128.Anders R., Kleiman J., Nicholson N., Wazowicz B., Burns D. Xemilofiban/orbofiban: Insight into drug development. Cardiovasc. Drug Rev. 2001;19:116–132. doi: 10.1111/j.1527-3466.2001.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 129.O’Neill W.W., Serruys P., Knudtson M., Van Es G.-A., Timmis G.C., Van Der Zwaan C., Kleiman J., Gong J., Roecker E.B., Dreiling R., et al. Long-Term Treatment with a Platelet Glycoprotein-Receptor Antagonist after Percutaneous Coronary Revascularization. N. Engl. J. Med. 2000;342:1316–1324. doi: 10.1056/NEJM200005043421803. [DOI] [PubMed] [Google Scholar]
- 130.Liu F., Craft R.M., Morris S.A., Carroll R.C. Lotrafiban: An oral platelet glycoprotein IIb/IIIa blocker. Exp. Opin. Investig. Drugs. 2000;9:2673–2687. doi: 10.1517/13543784.9.11.2673. [DOI] [PubMed] [Google Scholar]
- 131.Topol E.J., Easton D., Harrington R.A., Amarenco P., Califf R.M., Graffagnino C., Davis S., Diener H.-C., Ferguson J., Fitzgerald D., et al. Randomized, Double-Blind, Placebo-Controlled, International Trial of the Oral IIb/IIIa Antagonist Lotrafiban in Coronary and Cerebrovascular Disease. Circulation. 2003;108:399–406. doi: 10.1161/01.CIR.0000084501.48570.F6. [DOI] [PubMed] [Google Scholar]
- 132.Mousa S.A., Kapil R., Mu D.X. Intravenous and oral antithrombotic efficacy of the novel platelet GPIIb/IIIa antagonist roxifiban (DMP754) and its free acid form, XV459. Arterioscler. Thromb. Vasc. Biol. 1999;19:2535–2541. doi: 10.1161/01.ATV.19.10.2535. [DOI] [PubMed] [Google Scholar]
- 133.Mousa S.A., Bozarth J.M., Lorelli W., Forsythe M.S., Thoolen M.J., Slee A.M., Reilly T.M., A Friedman P. Antiplatelet efficacy of XV459, a novel nonpeptide platelet GPIIb/IIIa antagonist: Comparative platelet binding profiles with c7E3. J. Pharmacol. Exp. Ther. 1998;286:1277–1284. [PubMed] [Google Scholar]
- 134.Serebruany V.L., Malinin A.I., O’Connor C.M., Gurbel P.A. Roxifiban Oral Compound Kinetics Evaluation Trial IPS. Effects of roxifiban on platelet aggregation and major receptor expression in patients with coronary artery disease for the Roxifiban Oral Compound Kinetics Evaluation Trial-I (ROCKET-I Platelet Substudy) Am. Heart J. 2003;146:91–98. doi: 10.1016/S0002-8703(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 135.Armstrong P.C., Peter K. GPIIb/IIIa inhibitors: From bench to bedside and back to bench again. Thromb. Haemost. 2012;107:808–814. doi: 10.1160/TH11-10-0727. [DOI] [PubMed] [Google Scholar]
- 136.Merlini P.A., Rossi M., Menozzi A., Buratti S., Brennan D.M., Moliterno D.J., Topol E.J., Ardissino D. Thrombocytopenia Caused by Abciximab or Tirofiban and Its Association With Clinical Outcome in Patients Undergoing Coronary Stenting. Circulation. 2004;109:2203–2206. doi: 10.1161/01.CIR.0000127867.41621.85. [DOI] [PubMed] [Google Scholar]
- 137.Schmid-Hempel P. Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomena’ such as virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:85–98. doi: 10.1098/rstb.2008.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuo Y.J., Chung C.H., Pan T.Y., Chuang W.J., Huang T.F. A Novel alphaIIbbeta3 Antagonist from Snake Venom Prevents Thrombosis without Causing Bleeding. Toxins. 2019;12:11. doi: 10.3390/toxins12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kam P.C., Egan M.K. Platelet glycoprotein IIb/IIIa antagonists: Pharmacology and clinical developments. Anesthesiology. 2002;96:1237–1249. doi: 10.1097/00000542-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 140.Theroux P. Oral inhibitors of platelet membrane receptor glycoprotein IIb/IIIa in clinical cardiology: Issues and opportunities. Am. Heart J. 1998;135:107–112. doi: 10.1016/S0002-8703(98)70238-8. [DOI] [PubMed] [Google Scholar]
- 141.Olie R.H., van der Meijden P.E.J., Ten Cate H. The coagulation system in atherothrombosis: Implications for new therapeutic strategies. Res. Pract. Thromb. Haemost. 2018;2:188–198. doi: 10.1002/rth2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gaziano J.M., Brotons C., Coppolecchia R., Cricelli C., Darius H., Gorelick P.B., Howard G.H., Pearson T.A., Rothwell P.M., Ruilope L.M., et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McFadyen J.D., Schaff M., Peter K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018;15:181–191. doi: 10.1038/nrcardio.2017.206. [DOI] [PubMed] [Google Scholar]
- 144.Ziegler M., Wang X., Peter K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019;115:1178–1188. doi: 10.1093/cvr/cvz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.