Abstract
Simple Summary
Pineal neoplasms are tumors with different and variable morphological, histological, and radiological characteristics and, consequently different diagnosis and management. Due to their rarity, pineal tumors may be misdiagnosed. Pineal tumors, are divided into germ cell tumors, pineal parenchymal tumors and tumors that derive from adjacent structures. In this review, we report the clinical relevance of the main pineal gland tumors, underlining the importance of studying the triggering causes of pineal region carcinogenesis, to realize appropriate diagnosis and, consequently, better clinical management.
Abstract
The pineal gland is a small, pinecone-shaped endocrine gland that participates in the biological rhythm regulation of vertebrates. The recognized major product of the pineal gland is melatonin—a multifunctional endogenous indoleamine. Accumulating evidence suggests that the pineal gland is important for preserving ideal health conditions in vertebrate. Tumors of the pineal region account for approximately 3–11% of pediatric brain neoplasms but fewer than 1% of brain neoplasms in adults. It is fundamental to expand advanced imaging techniques together with both clinical and laboratory knowledge, to help to differentiate among pineal neoplasms and thus facilitate accurate primary diagnoses and proper therapeutic interventions. In this review, we report the gross anatomy of the pineal gland and its functional significance and discuss the clinical relevance of pineal gland tumors, underlining the importance of identifying the leading causes of pineal region masses.
Keywords: pineal gland, brain neoplasms, pineal germ cell tumors, pineal parenchymal tumor, pineal metastasis
1. Introduction
The Pineal Gland
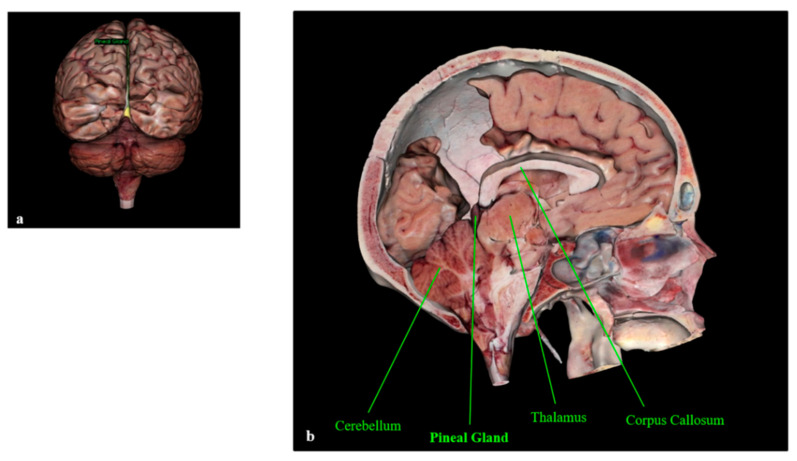
The pineal gland is a pinecone-shaped neuroendocrine gland located in the epithalamus that participates in the biological rhythm regulation of vertebrates. The gland projects posteriorly and inferiorly into the quadrigeminal cistern, and the anatomic boundaries include the backside of the third ventricle wall forming the gland’s base, the splenium of the corpus callosum superiorly, and the thalamus surrounding both sides [1,2,3,4]. Figure 1 shows the location of the pineal gland in the human brain.
Figure 1.
Human pineal gland (a) and its anatomic boundaries (b). The pineal gland is visible in yellow (a). Anatomage Inc.—Anatomage Table EDU. The 3D rendering of the cadaver data is from Anatomage Table.
The pineal gland’s primary purpose is the production and immediate release of melatonin, a pleiotropic and multifunctional indoleamine [5,6,7,8,9,10], into the blood [11].
The pineal gland possesses two populations of cells: about 95% are pinealocytes with dendritic processes, and the other 5% are neuroglial supporting cells that resemble astrocytes. Both these types of cells can form neoplasms, as well as residual germ cells from primordial neural crest cell migration and cells derived from nearby structures [1,2,12,13,14,15,16]. The pineal gland may, unfortunately, harbor a variety of neurosurgical diseases such as pineal cysts, different pineal tumors, and vascular malformations, including cavernous, arteriovenous malformations and aneurysms.
2. Pineal Gland Tumors
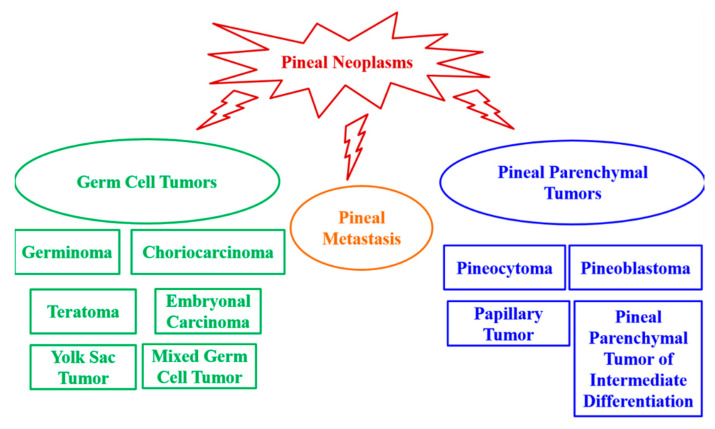
Pineal neoplasms are fairly uncommon tumors, and they are predominantly childhood malignancies representing 3–11% of all pediatric brain tumors compared to <1% of brain tumors in adults [1,14,17,18,19,20]. Age, sex, and ethnicity may modulate the relative incidence of pineal neoplasms [21]. Pineal tumors are classified as: germ cell tumors, pineal parenchymal tumors and tumors that derive from adjacent anatomical structures. Germinoma is the most common pineal tumor, representing up to 50% of pineal tumors in Europe, the United States and Japan [1,22,23]. In a series of 370 pineal tumors in patients aged 3–73 years, it was observed that 27% were germinomas; 26% were astrocytomas; 12% were pineoblastomas; 12% were pineocytomas; 4.3% were ependymomas; 4.3% were teratomas; 2.7% were ganglioglioneuromas, lymphomas, meningiomas, metastases, and pineal cysts; 1.6% were mixed embryonal cell tumors (embryonal carcinomas)/malignant teratomas; 1.1% were choriocarcinomas; and 0.54% were oligodendrogliomas [24] (Figure 2).
Figure 2.
Pineal tumor classification.
Pineal region masses may produce nonspecific signs and symptoms, and they usually cause syndromes of mass effect, including headaches, aqueductal stenosis, and hydrocephalus, or compressive hypothalamic syndromes, such as diabetes insipidus and slowed growth [13,16,25]. A mass in the pineal area may also interfere with the normal function of the pineal gland. Pineal tumors associated with acute and rapidly progressive hydrocephalus may be clinically managed via external ventriculostomy, endoscopic third ventriculostomy, ventriculoperitoneal/ventriculoatrial shunts, or direct removal [26].
It is fundamental to expand advanced imaging techniques, together with both clinical and laboratory knowledge to help to differentiate among the pineal neoplasms and thus realize accurate primary diagnoses and correct treatment and patient management plans. Open surgical resection and stereotactic or endoscopic biopsy are needed for pineal tissue diagnosis [27,28]. However, stereotactic biopsy seems to be associated with a higher risk of hemorrhage in pineal region tumors [18,29]. In practice, the diagnosis of pineal region neoplasms is based on clinical presentation, imaging, and pathology results. Serum and cerebrospinal fluid (CSF) biomarkers complement these standard diagnostic techniques by providing additional data before invasive procedures are performed [1,12,30]. Therefore, research into novel diagnostic markers, therapeutic approaches and follow-up guidelines is fundamental.
In this review, we report the features and clinical relevance of the main pineal gland tumors, underlining the importance of studying the triggering causes of pineal region masses, to enable effective primary diagnosis and, consequently, correct treatment and clinical management.
2.1. Germ Cell Tumors
Germ cell neoplasms are derived from primordial germ cells that develop primarily in the gonads but also in the anterior mediastinum, pineal gland, and brain [31]. Germ cell neoplasms account for 0.5–3.2% of primary intracranial tumors in adults and 11.8% of the same in children. Germ cell tumors are predominantly found in male patients. Pineal germ cell tumors account for about 50% of intracranial germ cell tumors [3] and seem to be more common in Asian populations [3,32]. Germ cell tumor can be classified into six types: germinomas, choriocarcinomas, teratomas, embryonal carcinomas, yolk sac tumors and mixed germ cell tumors (characterized by the features of at least two of the above-cited tumor types) [3,13,33].
2.1.1. Germinomas
Germinomas are the most common pineal tumor type, representing up to 50% of pineal tumors in Europe, the United States, and Japan [22,23]. Only 8% of central nervous system germinoma cases show the simultaneous involvement of pineal and suprasellar regions, and these are called bifocal germinomas [26,34,35]. Germinomas are not encapsulated tumors and thus may invade adjacent brain structures and, through the CSF, disseminate along the brain surface. Germinomas present cellular sheets or lobules of uniform germinoma cells with large round nuclei, prominent nucleoli, and clear cytoplasm with connective tissue septal bands and full of capillaries, lymphocytes, and, occasionally, granulomas [3,36]. Furthermore, germinomas present significant amounts of lipids and macromolecules compared to other pineal gland tumors [37,38].
Germinomas are malignant tumors characterized by a mix of large multipotential primitive germ cells and smaller cells that resemble lymphocytes. Germinomas often present severe inflammatory infiltrates [20]. Furthermore, corticosteroid treatment seems to be able to modify the patient’s immunological defense, enabling the immune system to suppress the tumor [20,39].
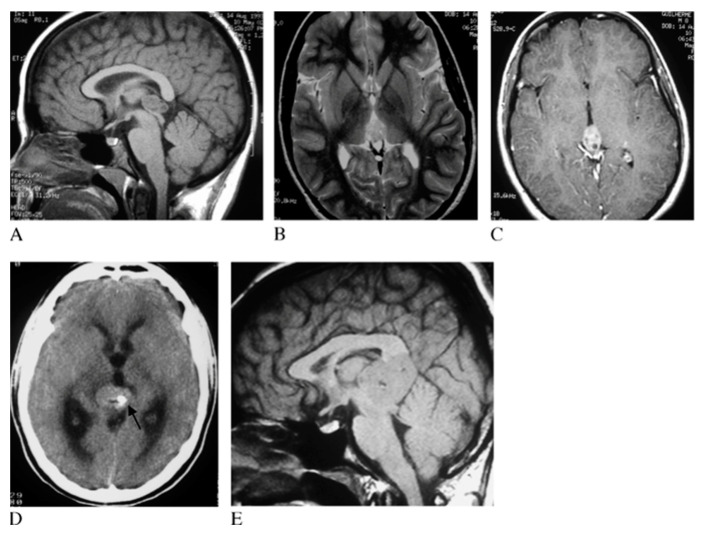
On imaging, germinomas show heterogeneous features, often presenting as solid or solid/cystic masses with engulfed calcifications (Figure 3), different to pineal parenchymal tumors, which exhibit prominent calcifications [14,31,40].
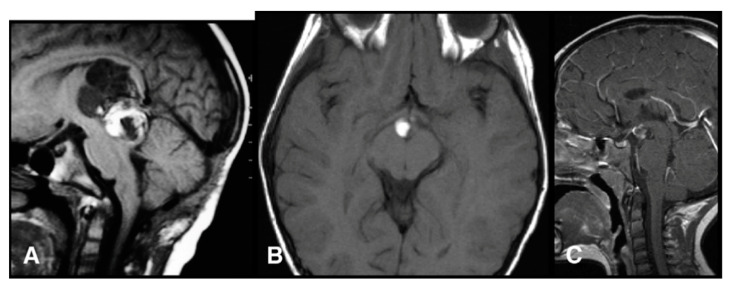
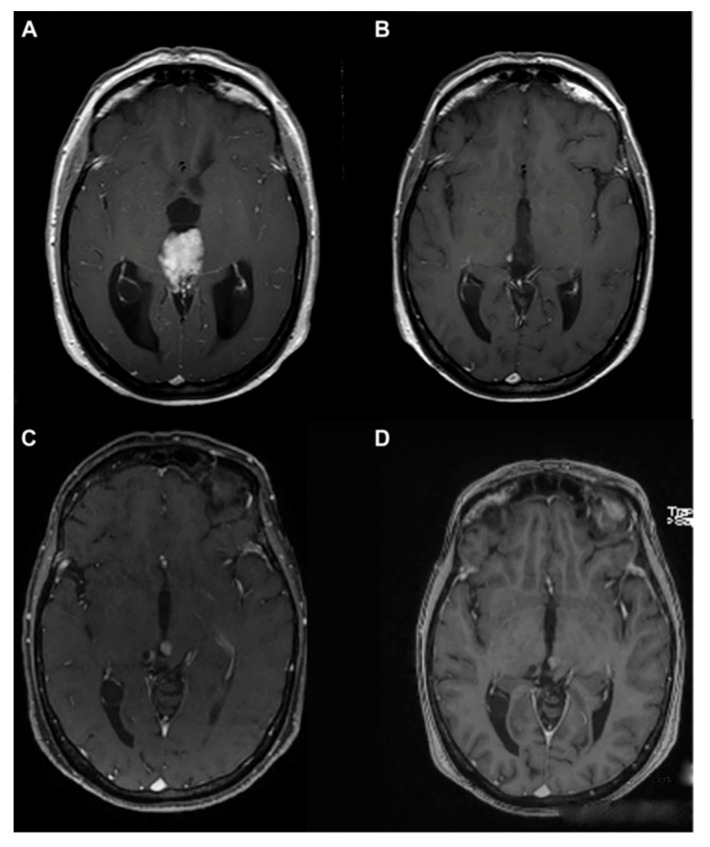
Figure 3.
Pineal germinoma magnetic resonance imaging (MRI). Pineal tumor hypointense on T1 weighted image (T1WI) (A), hyperintense on T2WI (B) and with homogeneous contrast enhancement (C). The arrow identifies hyperdense mass with calcification at computed tomography (D). In another patient, a larger germinoma, isointense on T1WI (E). Reprinted with permission from Reis et al. (2006) [41]. John Wiley and Sons—2021 (License Number 5034711197310).
Imaging alone does not allow us to distinguish among germinomas, nongerminomatous germ cell tumors and pineal parenchymal tumors. Therefore, a complete evaluation is fundamental. In fact, germinomas are diagnosed using imaging together with serum and CSF markers. These tumors present high serum and CSF expression of oncoproteins such as alpha-fetoprotein, beta human chorionic gonadotropin, lactate dehydrogenase, and placental alkaline phosphatase [6].
The treatment regimens used against germinomas include chemo- and radio-therapy or a combination of both, resulting in a positive prognosis and five-year survival of at least 90% [14]. Germinomas are very radiosensitive neoplasms and respond well to specific chemotherapy [13,14,20,31,42,43,44,45]. Furthermore, for germinoma, stereotactic radiosurgery seems to be effective in improving standard adjuvant treatment or in the case of recurrence [21].
2.1.2. Choriocarcinomas
Pineal choriocarcinomas are uncommon malignant nongerminomatous germ cell neoplasms (accounting for fewer than 5% of all pineal masses) [13] and the most aggressive form of gestational trophoblastic disease. Choriocarcinoma shows a poor survival rate with respect to other germ cell tumors [46,47]. Median overall survival of primary intracranial pure choriocarcinoma was 22 months and the three- and five-year survival rate was 45.8% [48]. Primary intracranial choriocarcinoma mainly affects young men (3–22 years of age), who present precocious puberty. These tumors do not show distinctive symptoms, but patients affected by choriocarcinoma have mainly reported headaches, vomiting, nausea, visual impairment, polydipsia, polyuria and endocrinologic alterations [46,49]. Choriocarcinomas present stromal vascular ducts that form blood lakes and intratumoral hemorrhagic necrosis, all factors strictly correlated with a poor prognosis [2,46,50,51]. On imaging, choriocarcinomas appear as ovoid, heterogeneous, and slightly hyperdense masses (Figure 4).
Figure 4.
Pineal choriocarcinoma MRI. Susceptibility-weighted imaging (A), T2WI (B) and pineal heterogeneous mass on T1WI (C,D). The arrows identify the faint peripheral enhancement after the intravenous administration of gadolinium (E). Reprinted with permission from Causil et al. (2016) [46]. Elsevier—2021 (License Number: 5034730033295).
Qi et al. [50] observed sinusoids that expressed laminin and not CD34, thus identifying tumor vasculogenic mimicry. Blood may flow from tumor vessels that express CD34 to sinusoids, leading to blood clotting, the extension of blood lakes and sinusoids, and, sometimes, hemorrhagic necrosis. Choriocarcinoma is also linked with elevated levels of both CSF and plasma human chorionic gonadotropin [50]. Choriocarcinomas and germinomas are both associated with elevated beta human chorionic gonadotropin expression [1,52,53].
Unfortunately, classic treatments frequently fail due to choriocarcinomas being extremely resistant tumors. The first clinical choice is total resection (even if the patient does not present hydrocephalus). However, to treat choriocarcinoma, a mix of total tumor removal, chemotherapy, and radiotherapy is frequently used as a therapeutic combination and seems to show positive outcomes [46,47,48,49].
2.1.3. Teratomas
Intracranial teratomas account for up to 50% of fetal brain neoplasms; in neonates, they comprise 33% of intracranial tumors, but they comprise only 2%–4% of intracranial tumors in patients aged <15 years [54,55]. Intracranial teratomas typically arise from the pineal gland and involve the third ventricle [54]. Pineal teratomas have a male predominance that varies from 2:1 to 8:1 and an overall survival of 90–100% [26]. Histologically, teratomas are classified into: (1) mature tumors, which show completely differentiated tissue; (2) immature tumors, which present a combination of fetal- and mature-type tissue elements and elements from all three germ layers and (3) teratomas with malignant transformation, which involves the malignant degeneration of mature tissue [14,56]. Teratomas are neoplasms characterized by multipotential cells that revert to normal organogenesis, usually producing tissues representing a combination of two or more of the embryological layers of ectoderm, mesoderm, and endoderm [13,14]. Pineal teratomas can be partially or totally encapsulated, but can also be unencapsulated and locally invasive [13]. On imaging, these pineal tumors present foci of fat, calcification and cystic regions [13,54]. On MRI, teratomas appear as lobular, multiloculated, and heterogeneously wide masses [14] (Figure 5).
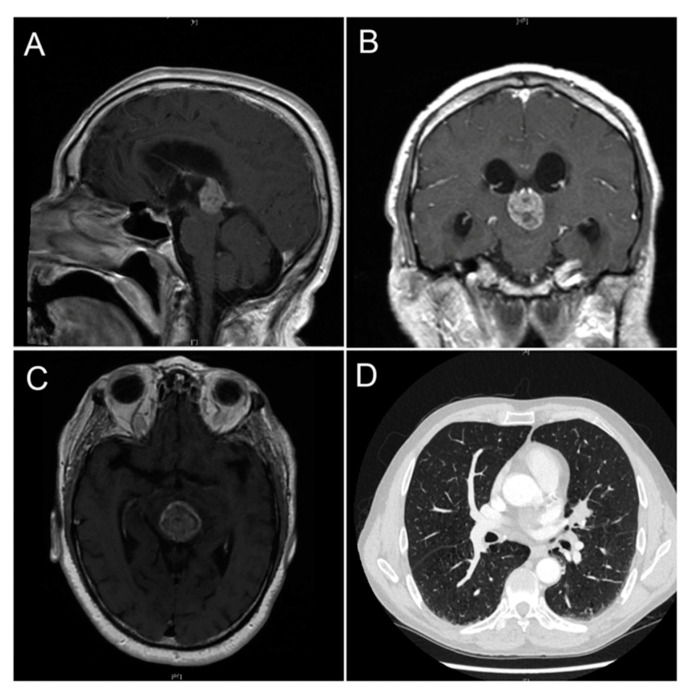
Figure 5.
Pineal teratoma MRI. Sagittal T1 image (A), axial T1 (B) and sagittal contrast-enhanced T1 (C) images. Reprinted with permission from Peterson et al. (2012) [54]. Elsevier—2021 (License Number: 5034851378505).
2.2. Pineal Parenchymal Tumors
Pineal parenchymal tumors are neuroepithelial neoplasms arising from pineocytes. These tumors are uncommon accounting for fewer than 1% of all primitive central nervous system tumors and constituting 15% to 30% of pineal gland tumors [57,58]. Pineal parenchymal tumors present different features, grades, and levels of aggressiveness [33]. The World Health Organization (WHO) recognizes pineal parenchymal tumors in four distinct categories: pineocytomas, pineoblastomas, papillary pineal tumors, and pineal parenchymal tumors of intermediate differentiation [14,57,59,60].
Pineal parenchymal tumors seem to have no sexual predominance and occur most frequently in pediatric patients [13,14,58]. Patients mainly report headaches, vomiting (correlated with increased intracranial pressure due to the blockade of the ventricular system) and gait ataxia [1,26,57,61,62]. Pineal parenchymal cell tumors consistently produce a hypomelatoninemic or hypermelatoninemic state [63]. However, exogenous melatonin supplementation after pinealectomy may mitigate the ensuing syndrome [1,64,65]. Pineal parenchymal tumors are negative for the three tumor markers alpha-fetoprotein, beta human chorionic gonadotropin, and placental alkaline phosphatase [1]. However, synaptophysin is expressed in pineal parenchymal tumors of intermediate differentiation, and neuronal marker positivity is not related to histological grade, mitosis, proliferation index, or prognosis [66]. The standard treatment for pineal parenchymal tumors is radiation. Surgery is another possible treatment option; however, it has a mortality rate of 5%–10% [1,24], and even after complete tumor removal, many patients present recurrence. Hence, adjuvant radiation or chemotherapy or a mix of both is often suggested with the aim of improving survival [1,61,62]. Thus, appropriate and close follow-up is also fundamental.
2.2.1. Pineocytomas
Pineocytomas are slow-growing grade I/II pineal parenchymal neoplasms derived from the pineal epithelium [1,15,51,60,67]. They are tumors characterized by well-differentiated mature cells arranged in sheets that are virtually indiscernible from the healthy pineal parenchyma [13,14,68]. They are circumscribed, unencapsulated tumors that may remain locally confined. Pineocytomas may arise at all ages but are more frequent in adults aged 30 to 60 years old [14,69].
On MRI, pineocytomas appear as hyperintense, round or lobular masses [2,14,15,56,70] (Figure 6).
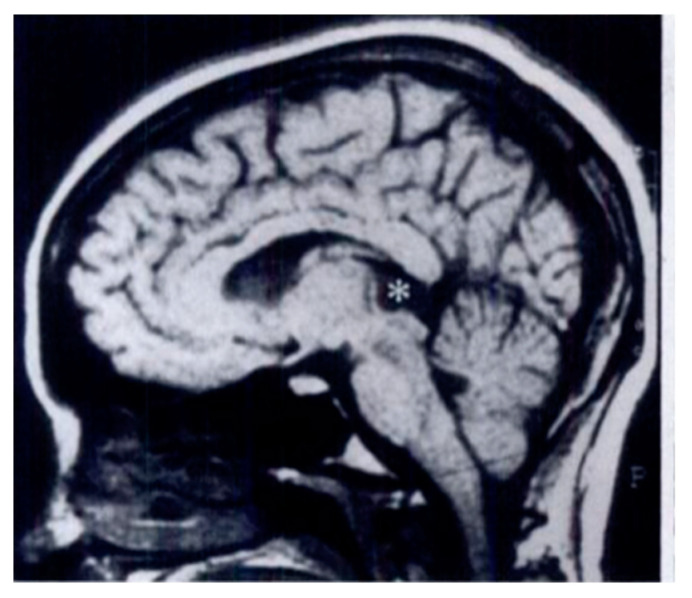
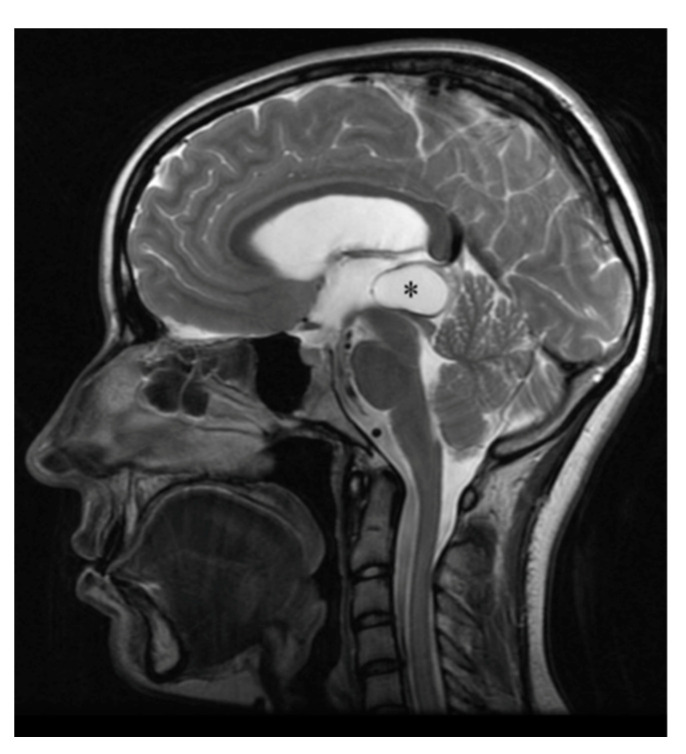
Figure 6.
Pineocytoma MRI. The asterisk indicates the homogeneous mass in the pineal gland. Reprinted with permission from Smirniotopoulos et al. (1992) [13]. Radiological Society North America–2021. Small pineocytomas often do not induce symptoms, but if they are of large dimensions, they may induce obstructive hydrocephalus and Parinaud syndrome, defined as upward gaze palsy, pupillary light-near dissociation, and convergence retraction nystagmus [15]. The five- and twenty-year survival rates are, respectively, 100% and 76% [21,26]. For pineocytomas, stereotactic radiosurgery seems to be highly effective as a primary treatment, so stereotactic radiosurgery alone may be considered appropriate clinical management for pineocytomas [21,28,71,72].
2.2.2. Pineoblastomas
As previously reported, pineal parenchymal cell tumors also include pineoblastomas, aggressive, grade IV neoplasms derived from primitive neuroectoderm [1,17]. They account for 40% of parenchymal pineal cancers. Pineoblastomas are undifferentiated, embryonal tumors categorized as primitive neuroectodermal tumors. Similar to other primitive neuroectodermal tumors, pineoblastomas are extremely aggressive and present a poor prognosis and aggressive clinical behavior due to the frequent invasion of adjacent structure and CSF dissemination, with reported five-year survival rates of <60% [23,57,73,74]. The highest incidence is in childhood, particularly in children less than 2 years old, where pineoblastomas can occur in combination with retinoblastomas [13,14,26,59,75]. Adult cases are very rare, and for this reason, limited data are currently available for the effort to develop a standard management course for the diseases. Pineoblastomas are malignant, unencapsulated tumors that, unfortunately, may often recur and also disseminate throughout the craniospinal axis, as well as presenting metastasis all over the body, such as in the calvarial bones, vertebrae, lungs, peritoneum, mandible, and pelvis [59,76,77,78] (Figure 7).
Figure 7.
Pineoblastoma MRI. Preoperative MRI (A–C), MRI 1.5 months after surgery showing spinal metastasis (D). Reprinted with permission from Huo et al. (2020) [79]. Dove Medical Press—2021.
Pineoblastomas are composed of undifferentiated or immature pineal cells [13,74]. Synaptophysin and chromogranin are markers of primitive neuroendocrine tumors that may be expressed in pineoblastomas and detectable in the serum or CSF [1,80]. The molecular background of pineoblastoma is not clearly known. However, recent studies showed that the DICER1 and DROSHA genes, involved in microRNA dysregulation, are fundamental to pineoblastoma carcinogenesis [81,82].
Aggressive surgical resection is the first-line therapy against pineoblastoma. Notably, radiotherapy after resection helps in increasing patient survival. As also reported for other pineal gland tumors, combined therapy seems to be an effective approach [59]. A report from the International Gamma Knife Research Foundation described actuarial local control and survival rates following the stereotactic radiosurgery of pineoblastomas of 27% and 48% at 5 years [29]. Recently, Jing et al. [59] evaluated 213 adult patients with pineoblastomas and observed that young patients, especially those not receiving radiotherapy during their initial treatment, often had extremely poor outcomes [59,83]. This study suggested that beam radiotherapy does not improve overall patient survival. Furthermore, tumors that invade tissue near the pineal gland are more aggressive than tumors confined to the pineal gland, increasing the risk of patient death about threefold. Jing et al. [59] also reported that, for every 1 mm increase in tumor size, the risk of death is reduced by 4%, so tumor size may not be considered a prognostic factor.
2.2.3. Papillary Tumors
Pineal papillary tumors are uncommon WHO grade II or III neuroepithelial neoplasms [14,15,56]. Among them, 68% have been found to recur at a mean follow-up of 4.2 years (with overall survival rates of 73% at 5 years and 58% at 10 years), and in a pediatric population, 47% of papillary tumors of the pineal gland recurred at a mean follow-up of 6.5 years [83,84]. The age of patients presenting pineal papillary tumors is 15 months to 67 years and there is a mild female prevalence. The main symptom reported by patients with pineal papillary tumors is headaches, which are related to obstructive hydrocephalus [85,86,87,88,89].
Pineal papillary tumors at MRI appear as partially cystic masses with obstructive hydrocephalus (Figure 8).
Figure 8.
Pineal papillary tumor MRI before (A) and after 15 years of treatments (B). Reprinted with permission from Fernández-Mateos et al. (2018) [90]. Elsevier—2021 (License Number: 5034870401490).
Pineal papillary tumors show some papillary morphological features forming ependymal rosettes and pseudorosettes. The rosetted cells have thick processes resting on collagen from adjacent blood vessels. Vascular connective tissue present various layers of cells with a large cuboidal or columnar epithelial-like growth pattern. These cells show small round/oval nuclei, stippled chromatin, small nucleoli, and eosinophilic cytoplasm with distinct cell borders [61,83,85,90,91]. Mitotic figures are uncommon, whereas necrosis is often present [83,91]. It is important to underline that pineal papillary tumors present wide morphological variability [82,92,93,94,95]. Apart from the characteristic papillary structures, papillary tumors have morphological features in common with other papillary-like tumors that occur in the pineal region, including pineal parenchymal neoplasms, choroid plexus papillomas, papillary ependymomas, metastatic papillary carcinomas, papillary meningiomas, and germ cell tumors, which complicates the clinical diagnosis [60,83,87,92,96,97]. These pineal tumors often present increased proliferative activity (Ki67/MIB1 proliferation index) [90], which is correlated with worse prognosis. Immunohistochemically, papillary tumors are also positive for S100, CAM 5.2, and prealbumin. Pineal papillary tumors usually show neuron-specific enolase reactivity but do not present neurofilament proteins—interesting papillary tumor features that may help in differentiating them from pineal parenchymal tumors with intermediate differentiation, whereas—MAP-2 is used for differentiating them from choroid plexus papillomas in diagnosis [17,60,98,99].
Pineal papillary tumors often recur, and radiotherapy is frequently effective [14,60,92,99,100]. Radiotherapy and chemotherapy are both used in initial tumor management and in cases of recurrences after gross total resection; however, the optimal adjuvant therapy is actually not known [99]. Stereotactic radiosurgery seems to be effective against pineal papillary tumors, but a high rate of local recurrence is also observed after this treatment. Recurrence can be safely and successfully managed by repeat stereotactic radiosurgery [21].
2.2.4. Pineal Parenchymal Tumors of Intermediate Differentiation
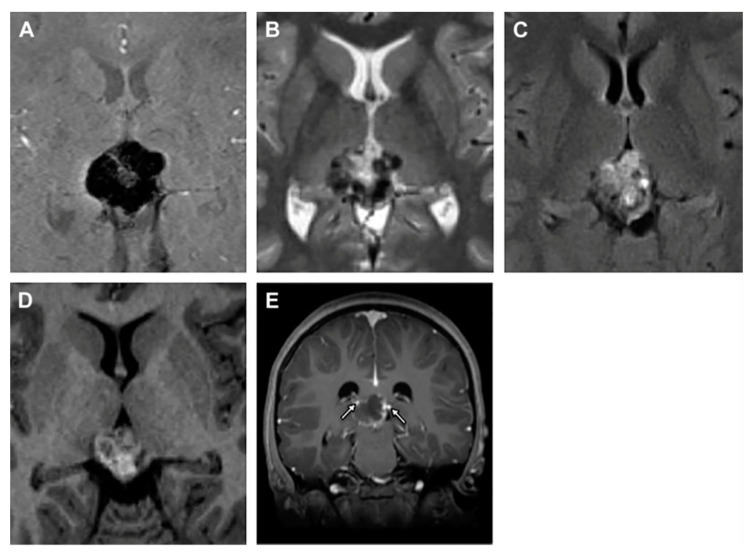
Pineal parenchymal tumors of intermediate differentiation are uncommon tumors arising from the pineal parenchyma that present features between those of pineocytomas and pineoblastomas [62,101,102,103]. Pineal parenchymal tumors of intermediate differentiation can occur at all ages. They appear more commonly among women, teenagers, and middle-aged patients [51,58,62,101]. Pineal parenchymal tumors of intermediate differentiation are often considered to be not a single disease, but a spectrum of grade II and III pineal parenchymal tumors, thus explaining the huge variation in the behavior of these tumors [101,104]. Although grading criteria for differentiating pineal parenchymal tumors of intermediate differentiation of grades II and III have not actually been established, the 2007 WHO Classification of Central Nervous System Tumors considers as outcome predictors both proliferative activity and immunoreactivity for neurofilament protein [102,105]. Thus, WHO grade II pineal parenchymal tumors of intermediate differentiation may present as lobulated or diffuse with a higher expression of neurofilaments, 0–5 mitoses per 10 high-power fields and moderate MIB1 labeling indices (ranging from 3% to 10% [101,106,107,108]). Pineal parenchymal tumors of intermediate differentiation show moderate cellularity, mild-to-moderate atypical nuclei, and low-to-moderate mitoses [101]. Choque-Velasquez et al. [102] reported that, on MRI, pineal parenchymal tumors of intermediate differentiation tend to appear as isointense lesions on T1WI and hypointense masses on T2WI, with some cystic components, and a complete contrast enhancement (Figure 9). Multiple cystic elements may also be observed. Pineal calcifications may be engulfed or peripherally displaced by the mass [15]. These tumors present positive expression for synaptophysin, neurofilament, chromogranin A, and renal S antigen.
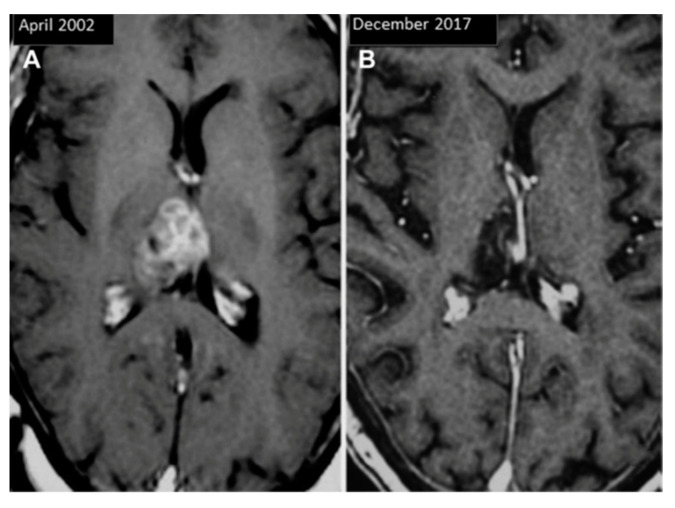
Figure 9.
Pineal parenchymal tumors of intermediate differentiation MRI. Preoperative MRI (A), postoperative MRI showing a very small residual pineal lesion (B). MRI before (C) and after (D) radiation therapy. Reprinted with permission from Choque- Velasquez et al. (2019) [102]. Elsevier—2021 (License Number: 5035440109275). Pineal parenchymal tumors of intermediate differentiation may be morphologically classified into (1) lobulated endocrine-like and highly vascular lesions; (2) those with diffuse growth patterns, similar to oligodendrogliomaneurocytomas; and (3) those of transitional type, with areas of lobulated and diffuse growth patterns, correlated with areas of pineocytomatous rosettes [82,102,109]. Recently, Wu et al. [103] reported that CD24 and PRAME may be novel markers useful for the grading and prognostic evaluation of pineal parenchymal tumors of intermediate differentiation and, thus, useful in therapeutic decision-making. The best treatment for pineal parenchymal tumors of intermediate differentiation has not yet been found, partly due to the low numbers of reported cases. The maximal surgical removal of the tumor is the optimal treatment in practice for pineal parenchymal tumors of intermediate differentiation. However, even after complete surgical tumor removal, many patients experience recurrence. Hence, adjuvant radio- or chemo-therapy, or a mix of both, is often recommended to improve patient survival. A report from the International Gamma Knife Research Foundation described actuarial local control and survival rates following stereotactic radiosurgery on pineal parenchymal tumors of intermediate differentiation of 50% and 56%, respectively, at 5 years [19,26].
3. A Brief Overview of Other Neoplastic and Non-Neoplastic Pineal Masses
3.1. Pineal Metastasis
Metastatic cancer spreading to the pineal gland is exceedingly uncommon and originates from lung carcinoma in most cases (Figure 10). Other tumors that have documented cases of metastases to the pineal gland are pancreatic, esophageal and bladder neoplasms [110,111,112,113].
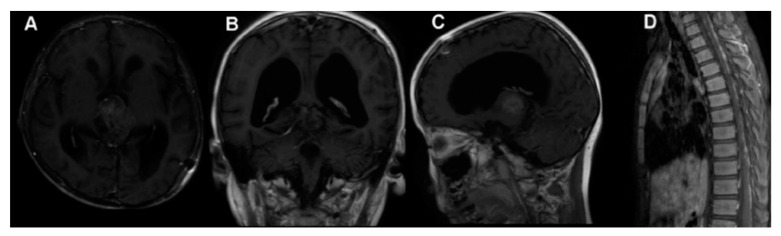
Figure 10.
Pineal metastasis from lung adenocarcinoma. Brain MRI sagittal (A), coronal (B) and transverse (C) sections showing the pineal gland metastatic mass. Computed tomography showing a lung nodule over the left hilar region in the lower right corner (D). Image from Abdallah et al. (2020) [110]—Ochsner Journal.
Metastasis to the central nervous system is usually an end-stage disease features and the treatment of pineal region metastases varies due to systemic conditions and neurological symptoms [111,112]. However, Hogan et al. [111] described, for the first time, a successful treatment of metastatic prostate cancer that had spread to the pineal gland. The pineal metastasis was treated with stereotactic radiosurgery, and, after 9 months, the pineal metastatic mass was significantly smaller.
Blas John et al. [114] discussed an uncommon case of an adult woman with an esophageal primitive neuroectodermal tumor that presented pineal gland metastasis. The head computed tomography of the patient showed tumor invasion of the third ventricle, with incipient hydrocephalus. The patient underwent a craniotomy of the posterior fossa with a supracerebellar infratentorial approach to the pineal region and an external ventricle drainage. Histopathologically, the tumor appeared rich in cellular density, forming lobes. The metastatic cells showed scant cytoplasm, elongated atypical nuclei, and abundant mitotic figures with up to 10 mitoses in one high-power field. Foci of necrosis and areas with abundant electrocautery artifacts were also observed. These areas had a lower cellular density and peripheral calcification, suggestive of a pineoblastoma. In terms of imunohistochemistry the pineal tumor showed a patchy expression of synaptophysin, a diffuse and strong expression of CD99 and weak expression of the cytokeratins AE1/AE3 and CAM 5.2. Partial resection of the pineal metastasis was performed. The patient was given chemotherapy; however, tumor progression was observed.
3.2. Pineal Cysts
Non-neoplastic pineal cysts are frequently observed during both MRI scans and autopsy studies, and the prevalence of benign pineal cysts ranges between 0.6% and 23% in the general population [115,116,117,118,119,120,121]. Pineal cysts, usually considered normal anatomical variations, are present at all ages but mainly in adult women in the fourth decade of life. Symptomatic cysts are more frequent among young women [116,119,122].
It is, in our opinion, important to at least introduce pineal cysts because they are pineal non-neoplastic masses that are relatively common in the general population, and a heterogeneous group of tumors, such as pineoblastomas, astrocytomas, meningiomas and pineocytomas, may feature a cystic nature on MRI, without showing hemorrhage within the masses [119,123]. Pineal cysts present an inner glial layer, a middle layer of pineal tissue and an outer fibrous capsule. On MRI scans, benign pineal cysts appear as round or ovoid areas of signal abnormality in the pineal region [120,124] (Figure 11).
Figure 11.
Pineal cyst MRI. The asterisk indicates the pineal cyst. Reprinted with permission from Májovský et al. (2017) [124]—Elsevier—2021 (License Number 5020240575766).
Barboriak et al. [125] undertook a follow-up MRI study and, interestingly, observed that pineal cysts usually remain stable. Only a few pineal cyst cases may enlarge, making them of neurological significance. Large cysts typically exert a mass effect on the cerebral aqueduct, encircling venous structures and the dorsal midbrain. The most reported symptoms, correlated to the compression of the surrounding structures, are headaches, vertigo, visual and oculomotor disturbances, and obstructive hydrocephalus [117,119,125]. Symptomatic pineal cysts are divided into three syndromes: (1) paroxysmal headaches and gaze palsy; (2) chronic headaches, papilledema, gaze paresis, and hydrocephalus; and (3) pineal apoplexy with acute hydrocephalus. The last is the least frequent but most dangerous form. In practice, there are no accepted therapeutic indications or criteria for intervention and/or follow-up. However, only a few patients with pineal cysts requires treatment. In fact, as previously reported, pineal cysts usually have no clinical implications and are asymptomatic [119,126,127].
Choque-Velasquez et al. [117] reported in their recent case series that surgically treated pineal cysts represent a progressive disease with acute or progressive hydrocephalus at the final stage. The authors also suggested that young women with active sexual hormone status (aged >10-years) comprise the patient group at the highest risk for pineal cyst progression.
4. Conclusions
As reported in the present review and summarized in Table 1, pineal neoplasms are heterogeneous tumors with different histological, morphological, and radiological features and, consequently, different diagnosis and management.
Table 1.
The table summarizes the main characteristics of pineal gland tumors.
| Histological Subtype |
Morphology/Histology | Incidence, Age and Sex Distribution | |
|---|---|---|---|
| Germ Cell Tumors | Germinomas | Not encapsulated tumors with variable proportions of germinoma cellular sheets or lobules composed of a mixture of large multipotential primitive germ cells and smaller cells that resemble lymphocytes. Often presented inflammatory infiltrates. | Most common pineal tumor (>50% of pineal tumors in Europe, the United States, and Japan). Male predominance. |
| Choriocarcinomas | Tumors with stromal vascular channels that form blood lakes and intratumoral hemorrhagic necrosis. | Rare pineal tumor (<5% of all pineal masses). Young men predominance (3–22 years of age). |
|
| Teratomas | Encapsulated tumors with multipotential cells that recapitulate normal organogenesis. (Teratoma may also be unencapsulated). | Second most common pineal tumors. Male predominance. |
|
| Pineal Parenchymal Tumor | Pineocytomas | Unencapsulate tumors with well-differentiated mature cells arranged in sheets. Pineocytic rosettes. |
About 14–30% of all pineal parenchymal tumors. More common in adults (30–60 years old). |
| Pineoblastomas | Undifferentiated or immature pineal cells. | 40% of all pineal parenchymal tumors. Highest incidence in children less of 2 years old. Slightly female predominance. |
|
| Papillary Tumors | Tumors with partially papillary structures lined by slightly polymorphic cells forming ependymal rosettes and pseudorosettes. The rosetted cells had thick processes resting on collagen surrounding the vessels. | Rare pineal parenchymal tumors. | |
| Pineal Parenchymal Tumors of Intermediate Differentiation | Pseudolobulated architecture with multiple cystic components. | Rare pineal parenchymal tumors which may occur in all ages. Slightly female predominance (teenagers and middle-aged women). |
Furthermore, due to their rarity, pineal tumors may be misdiagnosed. Establishing a means of specific tissue diagnosis for patients with pineal region tumors is needed to distinguish among the variety of possible histomorphological tumor subtypes. In the last decade, specialized surgical and stereotactic techniques have evolved to provide specific, safer, and more effective options for obtaining, at least, correct tissue diagnosis. Advanced microsurgical techniques combined with improved preoperative management and postoperative critical care methods have made surgical resection the optimal therapy for almost all pineal tumors. However, the identification of new therapeutic targets and the development of more effective adjuvant therapies, as well as follow-up guidelines, are needed to improve pineal tumor outcomes.
Author Contributions
Conceptualization, R.R. and G.F.; critically analyzed literature, G.F. and F.B.; wrote the manuscript draft, G.F. and F.B.; drew and acquired figures and table, G.F.; supervised and improved the manuscript content, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carr C., O’Neill B.E., Hochhalter C.B., Strong M.J., Ware M.L. Biomarkers of pineal region tumors: A review. Ochsner J. 2019;19:26–31. doi: 10.31486/toj.18.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fèvre-Montange M., Vasiljevic A., Champier J., Jouvet A. Histopathology of tumors of the pineal region. Future Oncol. 2010;6:791–809. doi: 10.2217/fon.10.28. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa D.T., Lagman C., Sun M., Yew A., Chung L.K., Lee S.J., Bui T.T., Ooi Y.C., Robison R.A., Zada G., et al. Pineal germ cell tumors: Two cases with review of histopathologies and biomarkers. J. Clin. Neurosci. 2017;38:23–31. doi: 10.1016/j.jocn.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Palanca P., Méndez-Blanco C., Fondevila F., Tuñón M.J., Reiter R.J., Mauriz J.L., González-Gallego J. Melatonin as an antitumor agent against liver cancer: An updated systematic review. Antioxidants. 2021;10:103. doi: 10.3390/antiox10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moretti R., Zanin A., Pansiot J., Spiri D., Manganozzi L., Kratzer I., Favero G., Vasiljevic A., Rinaldi V.E., Pic I., et al. Melatonin reduces excitotoxic blood-brain barrier breakdown in neonatal rats. Neuroscience. 2015;311:382–397. doi: 10.1016/j.neuroscience.2015.10.044. Erratum in: Neuroscience2016, 315, 296. [DOI] [PubMed] [Google Scholar]
- 6.Reiter R.J., Tan D.X., Fuentes-Broto L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- 7.Favero G., Moretti E., Bonomini F., Reiter R.J., Rodella L.F., Rezzani R. Promising antineoplastic actions of melatonin. Front. Pharmacol. 2018;9:1086. doi: 10.3389/fphar.2018.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favero G., Lonati C., Giugno L., Castrezzati S., Rodella L.F., Rezzani R. Obesity-related dysfunction of the aorta and prevention by melatonin treatment in ob/ob mice. Acta Histochem. 2013;115:783–788. doi: 10.1016/j.acthis.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Shafabakhsh R., Mirzaei H., Asemi Z. Melatonin: A promising agent targeting leukemia. J. Cell Biochem. 2020;121:2730–2738. doi: 10.1002/jcb.29495. [DOI] [PubMed] [Google Scholar]
- 10.Tan D.X., Manchester L.C., Hardeland R., Lopez-Burillo S., Mayo J.C., Sainz R.M., Reiter R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079X.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 11.Reiter R.J., Rosales-Corral S., Sharma R. Circadian disruption, melatonin rhythm perturbations and their contributions to chaotic physiology. Adv. Med. Sci. 2020;65:394–402. doi: 10.1016/j.advms.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Mayol Del Valle M., De Jesus O. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Pineal gland cancer. [PubMed] [Google Scholar]
- 13.Smirniotopoulos J.G., Rushing E.J., Mena H. Pineal region masses: Differential diagnosis. Radiographics. 1992;12:577–596. doi: 10.1148/radiographics.12.3.1609147. [DOI] [PubMed] [Google Scholar]
- 14.Tamrazi B., Nelson M., Blüml S. Pineal region masses in pediatric patients. Neuroimaging Clin. N. Am. 2017;27:85–97. doi: 10.1016/j.nic.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang K.Y., Chen M.M., Malayil Lincoln C.M. Adult primary brain neoplasm, including 2016 World Health Organization classification. Radiol. Clin. N. Am. 2019;57:1147–1162. doi: 10.1016/j.rcl.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang K.Y., Chen M.M., Malayil Lincoln C.M. Adult primary brain neoplasm, including 2016 World Health Organization classification. Neuroimaging Clin. N. Am. 2021;31:121–138. doi: 10.1016/j.nic.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. Erratum in Acta Neuropathol.2007, 114, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbassy M., Aref K., Farhoud A., Hekal A. Outcome of single-trajectory rigid endoscopic third ventriculostomy and biopsy in the management algorithm of pineal region tumors: A case series and review of the literature. Childs Nerv. Syst. 2018;34:1335–1344. doi: 10.1007/s00381-018-3840-8. [DOI] [PubMed] [Google Scholar]
- 19.Ostrom Q.T., Gittleman H., Xu J., Kromer C., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18:v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schipmann S., Keurhorst D., Köchling M., Schwake M., Heß K., Sundermann B., Stummer W., Brentrup A. Regression of pineal lesions: Spontaneous or iatrogenic? A case report and systematic literature review. World Neurosurg. 2017;108:939–947. doi: 10.1016/j.wneu.2017.08.106. [DOI] [PubMed] [Google Scholar]
- 21.Iorio-Morin C., Kano H., Huang M., Lunsford L.D., Simonová G., Liscak R., Cohen-Inbar O., Sheehan J., Lee C.C., Wu H.M., et al. Histology-stratified tumor control and patient survival after stereotactic radiosurgery for pineal region tumors: A report from the International Gamma Knife Research Foundation. World Neurosurg. 2017;107:974–982. doi: 10.1016/j.wneu.2017.07.097. [DOI] [PubMed] [Google Scholar]
- 22.Nomura K. Epidemiology of germ cell tumors in Asia of pineal region tumor. J. Neurooncol. 2001;54:211–217. doi: 10.1023/A:1012771204732. [DOI] [PubMed] [Google Scholar]
- 23.Villano J.L., Propp J.M., Porter K.R., Stewart A.K., Valyi-Nagy T., Li X., Engelhard H.H., McCarthy B.J. Malignant pineal germ-cell tumors: An analysis of cases from three tumor registries. Neuro Oncol. 2008;10:121–130. doi: 10.1215/15228517-2007-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regis J., Bouillot P., Rouby-Volot F., Figarella-Branger D., Dufour H., Peragut J.C. Pineal region tumors and the role of stereotactic biopsy: Review of the mortality, morbidity, and diagnostic rates in 370 cases. Neurosurgery. 1996;39:907–912. doi: 10.1097/00006123-199611000-00003. discussion 912–914. [DOI] [PubMed] [Google Scholar]
- 25.Seilanian Toosi F., Aminzadeh B., Faraji Rad M., Nekooei S., Nahidi M., Keykhosravi E. Pineal and suprasellar germinoma cooccurence with vertebra plana: A case report. Brain Tumor Res. Treat. 2018;6:73–77. doi: 10.14791/btrt.2018.6.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choque-Velasquez J., Resendiz-Nieves J., Jahromi B.R., Colasanti R., Raj R., Vehviläinen J., Tynninen O., Collan J., Niemelä M., Hernesniemi J. Extent of resection and long-term survival of pineal region tumors in Helsinki neurosurgery. World Neurosurg. 2019;131:e379–e391. doi: 10.1016/j.wneu.2019.07.169. [DOI] [PubMed] [Google Scholar]
- 27.Cho A., Cho S.S., Buch V.P., Buch L.Y., Lee J.Y.K. Second Window Indocyanine Green (SWIG) near infrared fluorescent transventricular biopsy of pineal tumor. World Neurosurg. 2020;134:196–200. doi: 10.1016/j.wneu.2019.10.113. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu D., Iorio-Morin C. Stereotactic radiosurgery for pineal region tumors. Prog. Neurol. Surg. 2019;34:173–183. doi: 10.1159/000493062. [DOI] [PubMed] [Google Scholar]
- 29.Field M., Witham T.F., Flickinger J.C., Kondziolka D., Lunsford L.D. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J. Neurosurg. 2001;94:545–551. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 30.Chiba K., Aihara Y., Komori T., Kawamata T. Placental alkaline phosphatase in cerebrospinal fluid as a biomarker for optimizing surgical treatment strategies for pineal region germ cell tumors. Brain Tumor Pathol. 2020;37:60–68. doi: 10.1007/s10014-020-00364-0. [DOI] [PubMed] [Google Scholar]
- 31.Sadiq Q., Khan F.A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Germ cell seminoma. [PubMed] [Google Scholar]
- 32.Takami H., Perry A., Graffeo C.S., Giannini C., Narita Y., Nakazato Y., Saito N., Nishikawa R., Matsutani M., Ichimura K., et al. Comparison on epidemiology, tumor location, histology, and prognosis of intracranial germ cell tumors between Mayo Clinic and Japanese consortium cohorts. J. Neurosurg. 2020;31:1–11. doi: 10.3171/2019.11.JNS191576. [DOI] [PubMed] [Google Scholar]
- 33.Korogi Y., Takahashi M., Ushio Y. MRI of pineal region tumors. J. Neurooncol. 2001;54:251–261. doi: 10.1023/A:1012773727022. [DOI] [PubMed] [Google Scholar]
- 34.Kanamori M., Takami H., Yamaguchi S., Sasayama T., Yoshimoto K., Tominaga T., Inoue A., Ikeda N., Kambe A., Kumabe T., et al. So-called “bifocal tumors” with diabetes insipidus and negative tumor markers: Are they all germinoma? Neuro Oncol. 2020;20:noaa199. doi: 10.1093/neuonc/noaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B., Lv W., Li C., Yang J., Chen J., Feng J., Chen L., Ma Z., Li Y., Wang J., et al. Comparison between craniospinal irradiation and limited-field radiation in patients with non-metastatic bifocal germinoma. Cancer Res. Treat. 2020;52:1050–1058. doi: 10.4143/crt.2020.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan G.C., Sallapan S., Haworth K., Finlay J., Boue D.R., Pierson C.R. CNS germinoma with extensive calcification: An unusual histologic finding. Malays. J. Pathol. 2019;41:71–73. [PubMed] [Google Scholar]
- 37.Tong T., Zhenwei Y., Xiaoyuan F. MRI and 1H-MRS on diagnosis of pineal region tumors. Clin. Imaging. 2012;36:702–709. doi: 10.1016/j.clinimag.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 38.Reiter R.J., Tan D.X., Kim S.J., Cruz M.H. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct Funct. 2014;219:1873–1887. doi: 10.1007/s00429-014-0719-7. [DOI] [PubMed] [Google Scholar]
- 39.Murai Y., Kobayashi S., Mizunari T., Ohaki Y., Adachi K., Teramoto A. Spontaneous regression of a germinoma in the pineal body after placement of a ventriculoperitoneal shunt. J. Neurosurg. 2000;93:884–886. doi: 10.3171/jns.2000.93.5.0884. [DOI] [PubMed] [Google Scholar]
- 40.Awa R., Campos F., Arita K., Sugiyama K., Tominaga A., Kurisu K., Yamasaki F., Karki P., Tokimura H., Fukukura Y., et al. Neuroimaging diagnosis of pineal region tumors-quest for pathognomonic finding of germinoma. Neuroradiology. 2014;56:525–534. doi: 10.1007/s00234-014-1369-4. [DOI] [PubMed] [Google Scholar]
- 41.Reis F., Faria A.V., Zanardi V.A., Menezes J.R., Cendes F., Queiroz L.S. Neuroimaging in pineal tumors. J. Neuroimaging. 2006;16:52–58. doi: 10.1177/1051228405001514. [DOI] [PubMed] [Google Scholar]
- 42.Nagaishi M., Suzuki R., Tanaka Y., Hoya K., Narita Y., Shinomiya A., Shibui S., Hyodo A. Pure germinoma of the pineal gland with synchronous spinal dissemination—Case report. Neurol. Med. Chir. 2010;50:505–508. doi: 10.2176/nmc.50.505. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa K., Shikama N., Toita T., Nakamura K., Uno T., Onishi H., Itami J., Kakinohana Y., Kinjo T., Yoshii Y., et al. Long-term results of radiotherapy for intracranial germinoma: A multi-institutional retrospective review of 126 patients. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:705–713. doi: 10.1016/j.ijrobp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Ronchi A., Cozzolino I., Montella M., Panarese I., Zito Marino F., Rossetti S., Chieffi P., Accardo M., Facchini G., Franco R. Extragonadal germ cell tumors: Not just a matter of location. A review about clinical, molecular and pathological features. Cancer Med. 2019;8:6832–6840. doi: 10.1002/cam4.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoenfeld G.O., Amdur R.J., Schmalfuss I.M., Morris C.G., Keole S.R., Mendenhall W.M., Marcus R.B., Jr. Low-dose prophylactic craniospinal radiotherapy for intracranial germinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:481–485. doi: 10.1016/j.ijrobp.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Causil L.D., Ames R., Puac P., Castillo M. Adult brain tumors and pseudotumors: Interesting (Bizarre) cases. Neuroimaging Clin. N. Am. 2016;26:667–689. doi: 10.1016/j.nic.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Shinoda J., Sakai N., Yano H., Hattori T., Ohkuma A., Sakaguchi H. Prognostic factors and therapeutic problems of primary intracranial choriocarcinoma/germ-cell tumors with high levels of HCG. J. Neurooncol. 2004;66:225–240. doi: 10.1023/B:NEON.0000013499.74404.81. [DOI] [PubMed] [Google Scholar]
- 48.Jiang T., Raynald, Yang H., Zhang W., Li C. Predictive factors of overall survival in primary intracranial pure choriocarcinoma. J. Clin. Neurosci. 2019;61:93–101. doi: 10.1016/j.jocn.2018.10.136. [DOI] [PubMed] [Google Scholar]
- 49.Lv X.F., Qiu Y.W., Zhang X.L., Han L.J., Qiu S.J., Xiong W., Wen G., Zhang Y.Z., Zhang J. Primary intracranial choriocarcinoma: MR imaging findings. AJNR Am. J. Neuroradiol. 2010;31:1994–1998. doi: 10.3174/ajnr.A2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi S.T., Zhang H., Song Y., Zhang J.L. Tumor cells forming sinusoids connected to vasculature are involved in hemorrhage of pineal choriocarcinoma. J. Neurooncol. 2014;119:159–167. doi: 10.1007/s11060-014-1468-4. [DOI] [PubMed] [Google Scholar]
- 51.Sato K., Takeuchi H., Kubota T. Pathology of intracranial germ cell tumors. Prog. Neurol. Surg. 2009;23:59–75. doi: 10.1159/000210053. [DOI] [PubMed] [Google Scholar]
- 52.Patil A.S., Menon G., Easwer H.V., Nair S. Extraventricular neurocytoma, a comprehensive review. Acta Neurochir. 2014;156:349–354. doi: 10.1007/s00701-013-1971-y. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez F.J., Mota R.A., Scheithauer B.W., Giannini C., Blair H., New K.C., Wu K.J., Dickson D.W., Jenkins R.B. Interphase cytogenetics for 1p19q and t(1;19)(q10;p10) may distinguish prognostically relevant subgroups in extraventricular neurocytoma. Brain Pathol. 2009;19:623–629. doi: 10.1111/j.1750-3639.2008.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson C.M., Buckley C., Holley S., Menias C.O. Teratomas: A multimodality review. Curr. Probl. Diagn. Radiol. 2012;41:210–219. doi: 10.1067/j.cpradiol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Sandow B.A., Dory C.E., Aguiar M.A., Abuhamad A.Z. Best cases from the AFIP: Congenital intracranial teratoma. Radiographics. 2004;24:1165–1170. doi: 10.1148/rg.244035164. [DOI] [PubMed] [Google Scholar]
- 56.Smith A.B., Rushing E.J., Smirniotopoulos J.G. From the archives of the AFIP: Lesions of the pineal region: Radiologic-pathologic correlation. Radiographics. 2010;30:2001–2020. doi: 10.1148/rg.307105131. [DOI] [PubMed] [Google Scholar]
- 57.Cuccia F., Mortellaro G., Cespuglio D., Valenti V., DE Gregorio G., Quartuccio E., Blasi L., Francaviglia N., Gallo C., Lo Casto A., et al. A case report of adult pineoblastoma occurring in a pregnant woman. Anticancer Res. 2019;39:2627–2631. doi: 10.21873/anticanres.13386. [DOI] [PubMed] [Google Scholar]
- 58.Jouvet A., Saint-Pierre G., Fauchon F., Privat K., Bouffet E., Ruchoux M.M., Chauveinc L., Fèvre-Montange M. Pineal parenchymal tumors: A correlation of histological features with prognosis in 66 cases. Brain Pathol. 2000;10:49–60. doi: 10.1111/j.1750-3639.2000.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jing Y., Deng W., Zhang H., Jiang Y., Dong Z., Fan F., Sun P. Development and validation of a prognostic nomogram to predict cancer-specific survival in adult patients with pineoblastoma. Front. Oncol. 2020;10:1021. doi: 10.3389/fonc.2020.01021. Erratum in Front. Oncol.2020, 10, 594049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 61.Lancia A., Becherini C., Detti B., Bottero M., Baki M., Cancelli A., Ferlosio A., Scoccianti S., Sun R., Livi L., et al. Radiotherapy for papillary tumor of the pineal region: A systematic review of the literature. Clin. Neurol. Neurosurg. 2020;190:105646. doi: 10.1016/j.clineuro.2019.105646. [DOI] [PubMed] [Google Scholar]
- 62.Mallick S., Benson R., Rath G.K. Patterns of care and survival outcomes in patients with pineal parenchymal tumor of intermediate differentiation: An individual patient data analysis. Radiother. Oncol. 2016;121:204–208. doi: 10.1016/j.radonc.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 63.Rousselle C., des Portes V., Berlier P., Mottolese C. Pineal region tumors: Clinical symptoms and syndromes. Neurochirurgie. 2015;61:106–112. doi: 10.1016/j.neuchi.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Leston J., Mottolese C., Champier J., Jouvet A., Brun J., Sindou M., Chazot G., Claustrat B., Fèvre-Montange M. Contribution of the daily melatonin profile to diagnosis of tumors of the pineal region. J. Neurooncol. 2009;93:387–394. doi: 10.1007/s11060-008-9792-1. [DOI] [PubMed] [Google Scholar]
- 65.Su S.C., Hsieh M.J., Yang W.E., Chung W.H., Reiter R.J., Yang S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee D., Lath K., Singla N., Kumar N., Radotra B.D. Pathologic prognostic factors of pineal parenchymal tumor of intermediate differentiation. Appl. Immunohistochem. Mol. Morphol. 2019;27:210–215. doi: 10.1097/PAI.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 67.Freeman D., Guillaume D., Bell W.R., Chen C.C. Devascularization of a hemorrhagic pineocytoma by laser thermal ablation followed by endoscopic resection: A proof-of-principle case report. World Neurosurg. 2020;139:583–587. doi: 10.1016/j.wneu.2020.04.125. [DOI] [PubMed] [Google Scholar]
- 68.Almahariq F., Raguz M., Romic D., Dlaka D., Oreskovic D., Sesar P., Chudy D. A biphasic tumor in posterior cranial fossa and the pineal region in young adult. Surg. Neurol. Int. 2020;11:64. doi: 10.25259/SNI_288_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumrongpisutikul N., Intrapiromkul J., Yousem D.M. Distinguishing between germinomas and pineal cell tumors on MR imaging. AJNR Am. J. Neuroradiol. 2012;33:550–555. doi: 10.3174/ajnr.A2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Connell K., Crimmins D., Power S., Ligon K.L., Cryan J., Beausang A. Pineal apoplexy due to pleomorphic variant pineocytoma. Clin. Neuropathol. 2019;38:253–255. doi: 10.5414/NP301196. [DOI] [PubMed] [Google Scholar]
- 71.Blakeley J.O., Grossman S.A. Management of pineal region tumors. Curr. Treat. Options Oncol. 2006;7:505–516. doi: 10.1007/s11864-006-0025-6. [DOI] [PubMed] [Google Scholar]
- 72.Cardenas R., Javalkar V., Haydel J., Wadhwa R., Fowler M., Scheithauer B., Nanda A. Papillary tumor of pineal region: Prolonged control rate after gamma knife radiosurgery—A case report and review of literature. Neurol. India. 2010;58:471–476. doi: 10.4103/0028-3886.66051. [DOI] [PubMed] [Google Scholar]
- 73.Liu A.P.Y., Gudenas B., Lin T., Orr B.A., Klimo P., Jr., Kumar R., Bouffet E., Gururangan S., Crawford J.R., Kellie S.J., et al. Risk-adapted therapy and biological heterogeneity in pineoblastoma: Integrated clinico-pathological analysis from the prospective, multi-center SJMB03 and SJYC07 trials. Acta Neuropathol. 2020;139:259–271. doi: 10.1007/s00401-019-02106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu A.P.Y., Priesterbach-Ackley L.P., Orr B.A., Li B.K., Gudenas B., Reddingius R.E., Suñol M., Lavarino C.E., Olaciregui N.G., Santa-María López V., et al. WNT-activated embryonal tumors of the pineal region: Ectopic medulloblastomas or a novel pineoblastoma subgroup? Acta Neuropathol. 2020;140:595–597. doi: 10.1007/s00401-020-02208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Jong M.C., Kors W.A., de Graaf P., Castelijns J.A., Kivelä T., Moll A.C. Trilateral retinoblastoma: A systematic review and meta-analysis. Lancet Oncol. 2014;15:1157–1167. doi: 10.1016/S1470-2045(14)70336-5. [DOI] [PubMed] [Google Scholar]
- 76.Charafe-Jauffret E., Lehmann G., Fauchon F., Michiels J.F., Paquis P., Maraninchi D., Hassoun J. Vertebral metastases from pineoblastoma. Arch. Pathol. Lab. Med. 2001;125:939–943. doi: 10.5858/2001-125-0939-VMFP. [DOI] [PubMed] [Google Scholar]
- 77.Fraser G., Rampling R., Smith C., Nicoll J., Stephen M. Long-term survival following extra-neural metastasis from a pineoblastoma. J. Neurooncol. 2000;48:141–144. doi: 10.1023/A:1006430022068. [DOI] [PubMed] [Google Scholar]
- 78.Golbin D., Nikitin K.V., Konovalov A.N., Pitskhelauri D.I., Shishkina L.V., Golanov A.V., Cherekaev V.A., Kobiakov G.L., Absalyamova O.V., Lasunin N., et al. Intraosseous metastasizing of pineoblastoma into the anterior skull base, calvarial bones, and vertebrae. Cureus. 2015;7:e437. doi: 10.7759/cureus.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huo X.L., Wang B., Zhang G.J., Ma J.P., Wang L., Zhang L.W., Xu X.Y., Li X.J., Li H., Li D., et al. Adverse factors of treatment response and overall survival in pediatric and adult patients with pineoblastoma. Cancer Manag. Res. 2020;12:7343–7351. doi: 10.2147/CMAR.S258476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian Y., Liu R., Qin J., Wang J., Ma Z., Gong J., Li C. Retrospective analysis of the clinical characteristics, therapeutic aspects, and prognostic factors of 18 cases of childhood pineoblastoma. World Neurosurg. 2018;116:e162–e168. doi: 10.1016/j.wneu.2018.04.135. [DOI] [PubMed] [Google Scholar]
- 81.Blessing M.M., Alexandrescu S. Embryonal tumors of the central nervous system: An update. Surg. Pathol. Clin. 2020;13:235–247. doi: 10.1016/j.path.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Lee J.C., Mazor T., Lao R., Wan E., Diallo A.B., Hill N.S., Thangaraj N., Wendelsdorf K., Samuel D., Kline C.N., et al. Recurrent KBTBD4 small in-frame insertions and absence of DROSHA deletion or DICER1 mutation differentiate pineal parenchymal tumor of intermediate differentiation (PPTID) from pineoblastoma. Acta Neuropathol. 2019;137:851–854. doi: 10.1007/s00401-019-01990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choque-Velasquez J., Raj R., Hernesniemi J. One burr-hole craniotomy: Supracerebellar infratentorial paramedian approach in Helsinki Neurosurgery. Surg. Neurol. Int. 2018;9:162. doi: 10.4103/sni.sni_164_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fèvre Montange M., Vasiljevic A., Champier J., Jouvet A. Papillary tumor of the pineal region: Histopathological characterization and review of the literature. Neurochirurgie. 2015;61:138–142. doi: 10.1016/j.neuchi.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Alkhotani A., Bilbao J.M., Mainprize T.G. A 49 year-old woman with a pineal mass. Brain Pathol. 2014;24:191–192. doi: 10.1111/bpa.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boco T., Aalaei S., Musacchio M., Byrne R., Cochran E. Papillary tumor of the pineal region. Neuropathology. 2008;28:87–92. doi: 10.1111/j.1440-1789.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 87.Hua X., Yang P., Zhang M., Zhao Y., Wang B. Papillary tumor of the pineal region: A case report and review of the literature. Exp. Ther. Med. 2015;10:1375–1379. doi: 10.3892/etm.2015.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaloshi G., Rroji A., Lame A., Leka L., Haxhihyseni E., Vreto G., Petrela M. Natural history of papillary tumor of the pineal region: New insights on biological explanation. J. Neurooncol. 2010;100:487–488. doi: 10.1007/s11060-010-0198-5. [DOI] [PubMed] [Google Scholar]
- 89.Poulgrain K., Gurgo R., Winter C., Ong B., Lau Q. Papillary tumour of the pineal region. J. Clin. Neurosci. 2011;18:1007–1017. doi: 10.1016/j.jocn.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Fernández-Mateos C., Martinez R., Vaquero J. Long-term follow-up after radiosurgery of papillary tumor of pineal region: 2 case reports and review of literature. World Neurosurg. 2018;116:190–193. doi: 10.1016/j.wneu.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 91.Shibahara J., Todo T., Morita A., Mori H., Aoki S., Fukayama M. Papillary neuroepithelial tumor of the pineal region. A case report. Acta Neuropathol. 2004;108:337–340. doi: 10.1007/s00401-004-0898-z. [DOI] [PubMed] [Google Scholar]
- 92.Braun M., Tomasik B., Bieńkowski M., Wiśniewski K., Kupnicka D.J., Jaskólski D., Papierz W., Fijuth J., Kordek R. Recurrent pineocytomalike papillary tumor of the pineal region: A case report and literature review. World Neurosurg. 2018;120:1–14. doi: 10.1016/j.wneu.2018.08.125. [DOI] [PubMed] [Google Scholar]
- 93.Fèvre-Montange M., Hasselblatt M., Figarella-Branger D., Chauveinc L., Champier J., Saint-Pierre G., Taillandier L., Coulon A., Paulus W., Fauchon F., et al. Prognosis and histopathologic features in papillary tumors of the pineal region: A retrospective multicenter study of 31 cases. J. Neuropathol. Exp. Neurol. 2006;65:1004–1011. doi: 10.1097/01.jnen.0000240462.80263.13. [DOI] [PubMed] [Google Scholar]
- 94.Matyja E., Grajkowska W., Nauman P., Bonicki W. Histopathological patterns of papillary tumour of the pineal region. Folia Neuropathol. 2011;49:181–190. [PubMed] [Google Scholar]
- 95.Nakamura H., Makino K., Kochi M., Nakazato Y., Kuratsu J. Successful treatment of neoadjuvant therapy for papillary tumor of the pineal region. Brain Tumor Pathol. 2009;26:73–77. doi: 10.1007/s10014-009-0250-3. [DOI] [PubMed] [Google Scholar]
- 96.Koziarski A., Grala B., Skrobowska E. Papillary tumor of the pineal region. Report of two cases and literature review. Neurol. Neurochir. Pol. 2014;48:356–362. doi: 10.1016/j.pjnns.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Vandergriff C., Opatowsky M., O’Rourke B., Layton K. Papillary tumor of the pineal region. Proc. Bayl. Univ. Med. Cent. 2012;25:78–79. doi: 10.1080/08998280.2012.11928791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gutenberg A., Brandis A., Hong B., Gunawan B., Enders C., Schaefer I.M., Burger R., Ostertag H., Gaab M., Krauss J.K., et al. Common molecular cytogenetic pathway in papillary tumors of the pineal region (PTPR) Brain Pathol. 2011;21:672–677. doi: 10.1111/j.1750-3639.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamamoto D., Sasaki H., Ohara K., Mizutani K., Yoshida K. A case of papillary tumor of the pineal region with a long clinical history: Molecular characterization and therapeutic consideration with review of the literature. Brain Tumor Pathol. 2016;33:271–275. doi: 10.1007/s10014-016-0270-8. [DOI] [PubMed] [Google Scholar]
- 100.Edson M.A., Fuller G.N., Allen P.K., Levine N.B., Ghia A.J., Mahajan A., Brown P.D., DeMonte F., Li J. Outcomes after surgery and radiotherapy for papillary tumor of the pineal region. World Neurosurg. 2015;84:76–81. doi: 10.1016/j.wneu.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 101.Bando T., Ueno Y., Shinoda N., Imai Y., Ichikawa K., Kuramoto Y., Kuroyama T., Shimo D., Mikami K., Hori S., et al. Therapeutic strategy for pineal parenchymal tumor of intermediate differentiation (PPTID): Case report of PPTID with malignant transformation to pineocytoma with leptomeningeal dissemination 6 years after surgery. J. Neurosurg. 2018:1–7. doi: 10.3171/2018.2.JNS171876. [DOI] [PubMed] [Google Scholar]
- 102.Choque-Velasquez J., Resendiz-Nieves J.C., Jahromi B.R., Colasanti R., Raj R., Tynninen O., Collan J., Hernesniemi J. Pineal parenchymal tumors of intermediate differentiation: A long-term follow-up study in Helsinki Neurosurgery. World Neurosurg. 2019;122:e729–e739. doi: 10.1016/j.wneu.2018.10.128. [DOI] [PubMed] [Google Scholar]
- 103.Wu X., Wang W., Lai X., Zhou Y., Zhou X., Li J., Liang Y., Zhu X., Ren X., Ding Y., et al. CD24 and PRAME are novel grading and prognostic indicators for pineal parenchymal tumors of intermediate differentiation. Am. J. Surg. Pathol. 2020;44:11–20. doi: 10.1097/PAS.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 104.Fomchenko E.I., Erson-Omay E.Z., Kundishora A.J., Hong C.S., Daniel A.A., Allocco A., Duy P.Q., Darbinyan A., Marks A.M., DiLuna M.L., et al. Genomic alterations underlying spinal metastases in pediatric H3K27M-mutant pineal parenchymal tumor of intermediate differentiation: Case report. J. Neurosurg. Pediatr. 2019;25:1–10. doi: 10.3171/2019.8.PEDS18664. [DOI] [PubMed] [Google Scholar]
- 105.Scheithauer B.W., Fuller G.N., VandenBerg S.R. The 2007 WHO classification of tumors of the nervous system: Controversies in surgical neuropathology. Brain Pathol. 2008;18:307–316. doi: 10.1111/j.1750-3639.2008.00179.x. Erratum in Brain Pathol.2008, 18, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fauchon F., Jouvet A., Paquis P., Saint-Pierre G., Mottolese C., Ben Hassel M., Chauveinc L., Sichez J.P., Philippon J., Schlienger M., et al. Parenchymal pineal tumors: A clinicopathological study of 76 cases. Int. J. Radiat. Oncol. Biol. Phys. 2000;46:959–968. doi: 10.1016/S0360-3016(99)00389-2. [DOI] [PubMed] [Google Scholar]
- 107.Tsumanuma I., Tanaka R., Washiyama K. Clinicopathological study of pineal parenchymal tumors: Correlation between histopathological features, proliferative potential, and prognosis. Brain Tumor Pathol. 1999;16:61–68. doi: 10.1007/BF02478904. [DOI] [PubMed] [Google Scholar]
- 108.Nam J.Y., Gilbert A., Cachia D., Mandel J., Fuller G.N., Penas-Prado M., de Groot J., Kamiya-Matsuoka C. Pineal parenchymal tumor of intermediate differentiation: A single-institution experience. Neurooncol. Pract. 2020;7:613–619. doi: 10.1093/nop/npaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amato-Watkins A.C., Lammie A., Hayhurst C., Leach P. Pineal parenchymal tumours of intermediate differentiation—An evidence-based review of a new pathological entity. Br. J. Neurosurg. 2016;30:11–15. doi: 10.3109/02688697.2015.1096912. [DOI] [PubMed] [Google Scholar]
- 110.Abdallah M.A., Shahid M., Ellithi M., Yeddi A., Cunningham A., Askeland R., Dodin J. Pulmonary adenocarcinoma presenting as a pineal gland mass with obstructive hydrocephalus. Ochsner J. 2020;20:232–235. doi: 10.31486/toj.18.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hogan E., Almira-Suarez I., Li S., Collins S.P., Jean W.C. Clinical management of prostate cancer metastasis to pineal gland: Case report and review of literature. World Neurosurg. 2019;122:464–468. doi: 10.1016/j.wneu.2018.11.111. [DOI] [PubMed] [Google Scholar]
- 112.Ji J., Gu C., Zhang M., Zhang H., Wang H., Qu Y., Ren M., Ning W., Yu C. Pineal region metastasis with intraventricular seeding: A case report and literature review. Medicine. 2019;98:e16652. doi: 10.1097/MD.0000000000016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitsumasa A., Shinya N., Motoki O., Hirotaka K., Tadashi K. Diplopia presenting in a case of pineal metastasis of pulmonary sarcomatoid carcinoma refractory to treatment. Asian J. Neurosurg. 2020;15:449–454. doi: 10.4103/ajns.AJNS_60_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blas Jhon L., Sánchez-Fayos P., Martín Relloso M.J., Calero Barón D., Porres Cubero J.C. Primitive neuroectodermal tumor of the esophagus with metastasis in the pineal gland. Endosc. Int. Open. 2019;7:E1163–E1165. doi: 10.1055/a-0977-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Al-Holou W.N., Terman S.W., Kilburg C., Garton H.J., Muraszko K.M., Chandler W.F., Ibrahim M., Maher C.O. Prevalence and natural history of pineal cysts in adults. J. Neurosurg. 2011;115:1106–1114. doi: 10.3171/2011.6.JNS11506. [DOI] [PubMed] [Google Scholar]
- 116.Bosnjak J., Budisić M., Azman D., Strineka M., Crnjaković M., Demarin V. Pineal gland cysts--an overview. Acta Clin. Croat. 2009;48:355–358. [PubMed] [Google Scholar]
- 117.Choque-Velasquez J., Colasanti R., Baluszek S., Resendiz-Nieves J., Muhammad S., Ludtka C., Hernesniemi J. Systematic review of pineal cysts surgery in pediatric patients. Childs Nerv. Syst. 2020;36:2927–2938. doi: 10.1007/s00381-020-04792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gokce E., Beyhan M. Evaluation of pineal cysts with magnetic resonance imaging. World J. Radiol. 2018;10:65–77. doi: 10.4329/wjr.v10.i7.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim E., Kwon S.M. Pineal cyst apoplexy: A rare complication of common entity. Brain Tumor Res. Treat. 2020;8:66–70. doi: 10.14791/btrt.2020.8.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nevins E.J., Das K., Bhojak M., Pinto R.S., Hoque M.N., Jenkinson M.D., Chavredakis E. Incidental pineal cysts: Is surveillance necessary? World Neurosurg. 2016;90:96–102. doi: 10.1016/j.wneu.2016.02.092. [DOI] [PubMed] [Google Scholar]
- 121.Taraszewska A., Matyja E., Koszewski W., Zaczyński A., Bardadin K., Czernicki Z. Asymptomatic and symptomatic glial cysts of the pineal gland. Folia Neuropathol. 2008;46:186–195. [PubMed] [Google Scholar]
- 122.Storey M., Lilimpakis K., Grandal N.S., Rajaraman C., Achawal S., Hussain M. Pineal cyst surveillance in adults—A review of 10 years’ experience. Br. J. Neurosurg. 2020;34:565–568. doi: 10.1080/02688697.2019.1635989. [DOI] [PubMed] [Google Scholar]
- 123.Choy W., Kim W., Spasic M., Voth B., Yew A., Yang I. Pineal cyst: A review of clinical and radiological features. Neurosurg. Clin. N. Am. 2011;22:341–351. doi: 10.1016/j.nec.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Májovský M., Řezáčová L., Sumová A., Pospíšilová L., Netuka D., Bradáč O., Beneš V. Melatonin and cortisol secretion profile in patients with pineal cyst before and after pineal cyst resection. J. Clin. Neurosci. 2017;39:155–163. doi: 10.1016/j.jocn.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 125.Barboriak D.P., Lee L., Provenzale J.M. Serial MR imaging of pineal cysts: Implications for natural history and follow-up. AJR Am. J. Roentgenol. 2001;176:737–743. doi: 10.2214/ajr.176.3.1760737. [DOI] [PubMed] [Google Scholar]
- 126.Asundi A., Tampieri D., Melançon D., Del Maestro R., Petrecca K., Cortes M.D. Pineal apoplexy: Imaging diagnosis and follow-up of three new cases. Can. J. Neurol. Sci. 2011;38:931–933. doi: 10.1017/S0317167100012567. [DOI] [PubMed] [Google Scholar]
- 127.Bruno F., Arrigoni F., Maggialetti N., Natella R., Reginelli A., Di Cesare E., Brunese L., Giovagnoni A., Masciocchi C., Splendiani A., et al. Neuroimaging in emergency: A review of possible role of pineal gland disease. Gland Surg. 2019;8:133–140. doi: 10.21037/gs.2019.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]