Abstract
Programmed cell death-ligand 1 (PD-L1) has emerged as a potential biomarker for selection of patients more likely to respond to immunotherapy and as a prognostic factor in non-small cell lung cancer (NSCLC). In this network meta-analysis, we aimed to evaluate the efficacy of first-line anti-PD-(L)1 monotherapy in advanced NSCLC patients with high PD-L1 expression (≥50%) compared to platinum-based chemotherapy. We also evaluated efficacy outcomes according to tumor mutational burden (TMB). To that end, we conducted a systematic review. Six clinical trials with 2111 patients were included. In head-to-head comparisons, immunotherapy showed a significant improvement in progression-free survival (PFS: HRpooled = 0.69, 95% CI: 0.52–0.90, p = 0.007), overall survival (OS: HRpooled = 0.69, 95% CI: 0.61–0.78; p < 0.001) and overall response rate (ORR) (Risk ratio (RR)pooled = 1.354, 95% CI: 1.04–1.762, p = 0.024). In the assessment of relative efficacy for PFS through indirect comparisons, pembrolizumab (results from KEYNOTE-024) ranked highest followed by cemiplimab and atezolizumab, with statistical significance determined for some of the drugs. In terms of OS, cemiplimab ranked highest followed by atezolizumab and pembrolizumab, although non-significant OS was determined for these drugs. In conclusion, PD-(L)1 inhibitor monotherapy improves efficacy outcomes in the first line setting of advanced NSCLC patients with high PD-L1 expression. Evaluations with longer follow up are still needed to determine the superiority of any specific drug.
Keywords: non-small cell lung cancer, network meta-analysis, immunotherapy, first-line treatment, PD-(L)1 inhibitors, efficacy
1. Introduction
Lung cancer is the leading cause of cancer death among men, and the second among women worldwide [1]. Non-small cell lung cancer (NSCLC), the most frequent lung carcinoma, accounts for 85% of all diagnosed cases [2] and is frequently detected in the advanced stages [3]. Its prognosis is poor, with five-year survival rates of 0–5% with the use of chemotherapy [4], which has been the only systemic therapeutic strategy available for decades [3]. Since then, the understanding of the biology of this cancer has rapidly increased and the use of targeted therapy with tyrosine kinase inhibitors (TKIs) improve the management of patients with oncogenic driven cancers and their survival rates. Major progress was made with the emergence of the immunotherapy with reduced overall toxicity and almost complete absence of non-specific side effects compared to chemotherapy and other classic cancer therapies, but with specific toxicity profiles depending on the mechanisms of action [5,6,7]. In this regard, immunotherapy targeting programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) has markedly improved the overall survival (OS) of patients, not only in those with metastatic NSCLC, but also in patients with locally advanced disease and extensive-stage small-cell lung cancer [8,9,10,11,12,13].
PD-L1 is expressed on tumor cells (TCs) and tumor-infiltrating immune cells (ICs) [14]. The binding of PD-L1 to its receptor PD-1 on activated T cells can lower the T-cell immune responses and prevent elimination of tumor cells [15,16]. In addition to its central role as a key element of current immunotherapy strategies, PD-L1 has emerged as a potential prognostic factor and biomarker to predict which patients are more likely to respond to immunotherapy in NSCLC [17,18,19,20,21,22,23,24,25,26]. The success of the anti-PD-1 antibodies, nivolumab and pembrolizumab, and the PD-L1 antibody, atezolizumab, approved so far in patients with previously treated NSCLC [27,28,29] understandably aroused considerable interest in extending these therapies to the first-line setting, both in combination with chemotherapy regardless of PD-L1 expression [30], and in monotherapy in PD-L1-positive patients. In this context, different cut-off values for PD-L1 expression were used in clinical trials evaluating PD-(L)1 inhibitors as monotherapy vs. chemotherapy in patients with no targetable mutations. In the phase III open-label KEYNOTE-024 trial [31], metastatic NSCLC tumors with a PD-L1 tumor proportion score (TPS) ≥50% showed improved progression-free survival (PFS) (Hazard ratio (HR) 0.50; 95% confidence interval (CI) 0.37–0.68), p < 0.001), and overall response rate (ORR) (44.8% vs. 27.8%) with pembrolizumab. Furthermore, at the most recent follow-up analysis (median time from randomization to data cut-off was 59.9 (55.1–68.4) months), median OS also improved: 26.3 months with pembrolizumab vs. 13.4 months with chemotherapy (HR 0.62; 95% CI 0.48‒0.81) [32]. These results were confirmed in a subsequent evaluation of pembrolizumab in the phase III open-label KEYNOTE-042 study [33], in which OS improved with the PD-1 antibody compared with chemotherapy (HR 0.69; 95% CI 0.56–0.85, p = 0.0003); this was also observed at other PD-L1 TPS cut-offs (TPS ≥ 20% and TPS ≥ 1%). Median PFS was 7.1 months (95% CI 5.9–9.0) in the pembrolizumab group and 6.4 months (95% CI 6.1–6.9) in the chemotherapy group. In the case of atezolizumab, a recent interim analysis of the phase III IMpower110 trial [34] has recently shown a statistically significant and clinically meaningful improvement in OS vs. platinum-based chemotherapy in a PD-L1–high population (20.2 months vs.13.1 months; HR, 0.59; 95% CI: 0.40, 0.89, p = 0.0106), as well as longer PFS (8.1 months vs. 5 months; HR, 0.63; 95% CI: 0.45, 0.88, p = 0.0007 [34]. Unlike atezolizumab and pembrolizumab, neither nivolumab nor durvalumab demonstrated statistically significant survival benefits in previously untreated PD-L1-positive mNSCLC (CheckMate 026 [35] and MYSTIC [36] trials, respectively). Finally, cemiplimab, a highly potent anti-PD-1 already approved for the treatment of advanced cutaneous squamous cell carcinoma (CSCC), is being evaluated in monotherapy vs. investigator’s choice platinum-doublet chemotherapy in patients with advanced NSCLC and PD-L1 TPS ≥50% (EMPOWER Lung-01 trial [37]). Interim results (median follow-up: 10 months) have shown that cemiplimab monotherapy significantly improves PFS and OS vs. chemotherapy in patients with high PD-L1 expression (PFS: 8.2 months vs. 5.7 months; HR, 0.54; 95% CI: 0.43, 0.68, p < 0.0001). Median OS was not reached for the cemiplimab arm vs. 14.2 months for the control arm; HR, 0.57; 95% CI: 0.42, 0.77, p = 0.0002).
The literature suggests that the first-line immunotherapy monotherapy strategy has become the new standard of care in locally advanced and metastatic NSCLC patients with high PD-L1 expression levels and no targetable mutations. Nevertheless, the because of the lack of direct cross-comparison studies or comparisons between trials, choosing the best treatment is still challenging. Apart from PD-L1, the tumor mutational burden (TMB) has recently emerged as a promising biomarker for immune checkpoint inhibitor (ICI) patient stratification [38]. TMB is defined as the total number of non-synonymous mutations per coding area of a tumor genome and is an indirect measure of tumor-derived neoantigens [39,40]. Several TMB testing panels are currently available, and their variability needs to be fully understood. Additionally, optimal TMB cut-offs for treatment decisions may need to be specified across different cancer types [41]. In NSCLC, preliminary results support this potential predictive role for TMB [38,42], but more evidence is needed. Thus, several clinical trials have assessed the predictive value of TMB in different studies with combined ICI regimens, such as nivolumab plus ipilimumab [43,44,45,46], or ICI monotherapy, such as with atezolizumab [47,48,49] and pembrolizumab [50].
The aim of this study was to conduct a network meta-analysis (NMA) to evaluate the efficacy of the available PD-(L)1-containing immunotherapy strategies in monotherapy for the first-line treatment of patients with high PD-L1 expression (≥50%) and locally advanced or metastatic NSCLC. We also evaluated efficacy outcomes according to TMB.
2. Materials and Methods
2.1. Search Strategies and Study Selection
We conducted a systematic search in PubMed to identify all eligible trials from inception until 1 November 2020, with no start date limit applied. Literature search terms used were “non-small cell lung cancer” (or “NSCLC”), “PD-L1”, “PD-1”, “pembrolizumab”, “nivolumab”, “atezolizumab”, “durvalumab”, “cemiplimab”, and all terms related to clinical trial registration (ClinicalTrials.gov, EU Clinical Trials Register, ISRCTN and ANZCTR. Accessed: 10. Dec. 2020). We also performed an additional search for abstracts presented at meetings or conferences held by the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), American Association for Cancer Research for Medical Oncology (AACR) and World Conference on Lung Cancer (WCLC).
2.2. Selection Criteria
Only phase III trials conducted in patients with locally advanced/advanced NSCLC selected according to their PD-L1 expression status, not previously treated for their metastatic disease and receiving first-line PD-(L)1 monotherapy were eligible for inclusion. In order to compare homogenous populations, only subjects with PD-L1 ≥50% were considered for this NMA, and only studies reporting efficacy outcomes for PD-(L)1 monotherapy expressed as PFS or OS were included. Observational studies, editorials, reviews and commentaries were excluded. Studies conducted in subsets of patients already included in their corresponding pivotal trials were also excluded.
2.3. Statistical Analysis
We performed a NMA to indirectly compare all monotherapy treatments against the common comparator, chemotherapy, an NMA was conducted. A specific application of the generalized pairwise modelling (GPM) framework [51] was applied. The Bucher method [52] was used for adjusted indirect comparisons. Cox proportional HRs along with their corresponding 95% CIs were used as the summary estimates of relative treatment effects. Summary league tables were generated for all comparisons (OS and PFS). Agents with higher efficacy appear in the first column and agents with lower efficacy compared to the first agent are presented in rows in descending order of efficacy.
For direct comparisons, the DerSimonian–Laird random effects model for main and subgroup analyses was implemented, assessing heterogeneity of effect-size estimates from the individual studies with Cochran’s Q test and the I2 statistic. Additionally, a meta-analysis (MA) corresponding to analysis of binary data of proportions was also performed using a DerSimonian–Laird random effects model without transformed proportion. A high level of heterogeneity was considered if I2 was greater than 50%. Statistical significance was reached for p-values less than 0.05. Analyses were not controlled for multiplicity; no alpha was assigned to the different analyses. HRs and 95% CI for OS and PFS from the overall population and subgroups from each individual trial of advanced NSCLC were calculated (only OS subgroup analysis was performed). For dichotomous data, odds ratios (OR) were estimated. The NMA was performed using Open Meta Analyst v. 10 (Center for Evidence Synthesis in Health, Brown University). Heterogeneity between studies must be considered as guidance only due to the relatively low number of trials included in this NMA [53]. Recommendations of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for this MA [54].
Sensitivity analyses did not quantitatively alter the results or conclusions of the main analyses.
3. Results
3.1. Studies Included in the Meta-Analysis
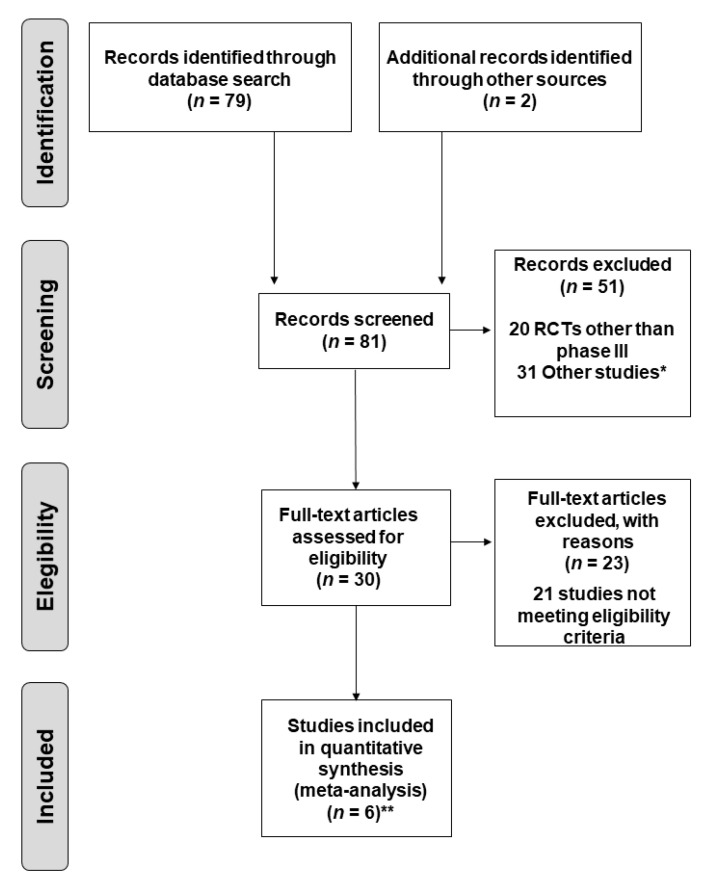
A total of 79 records from PubMed were screened. Two additional studies presented at ASCO [13] and ESMO [37] were also included. Study selection and exclusion criteria are summarized in Figure 1. Finally, six clinical trials carried out with 2111 patients met the inclusion criteria and were included in the MA [13,31,33,34,35,36,37].
Figure 1.
Flow chart of study selection (up to 1 November 2020). RCTs, randomized controlled trials * Other studies included pooled analyses, post-marketing studies, clinical trial protocols, patient-reported outcome assessments and any study on biomarkers/gene profiling. ** Two publications (one of them presented at the ASCO congress) were included for one of the trials (KEYNOTE-024 [10,33]).
3.2. Study Characteristics
The specific characteristics of the studies included in the MA are summarized in Table 1. The control arm in all studies was platinum-based chemotherapy. Two key methodological differences in cemiplimab clinical trials should be noted. First, in EMPOWER Lung-01 [37], patients in the cemiplimab arm who responded to cemiplimab monotherapy were allowed to continue the drug plus treatment with four cycles of chemotherapy in the event of progressive disease. In KEYNOTE-024 [13,31], EMPOWER Lung-01 [37], and CheckMate-026 [35] crossover was permitted. Second, studies on cemiplimab did not include a never-smoker population.
Table 1.
Characteristics and main outcomes of the studies included in the meta-analysis (the most up-to-date data have been used for this network meta-analysis).
| Study | PD-L1 Expression |
Primary Endpoint | Experimental Arm *** | Control Arm *** | Experimental Arm # | Control Arm # | Analysis Timing |
|---|---|---|---|---|---|---|---|
| KEYNOTE-024 [13,31,32] |
|
PFS (ITT-WT *) | Pembrolizumab (n = 154) |
Platinum-based chemotherapy (n = 151) |
Pembrolizumab (n = 154) |
Platinum-based chemotherapy (n = 151) |
PFS: Final |
| EMPOWER Lung-01 [37] |
|
PFS (ITT-WT **) OS (ITT-WT **) |
Cemiplimab (n = 283) |
Platinum-based chemotherapy a (n = 280) |
Cemiplimab (n = 283) |
Platinum-based chemotherapy (n = 280) |
PFS: Interim OS: Interim |
| IMpower110 [34] |
|
OS (ITT *) | Atezolizumab (n = 107) |
Platinum-based chemotherapy b (n = 98) |
Atezolizumab (n = 285) |
Platinum-based chemotherapy (n = 287) |
OS: Interim |
| KEYNOTE-042 [33,55] |
|
OS (ITT-WT *) | Pembrolizumab (n = 299) |
Platinum-based chemotherapy (n = 300) |
Pembrolizumab (n = 637) |
Platinum-based chemotherapy (n = 637) |
OS: Final |
| MYSTIC [36] |
|
PFS (ITT-WT *) OS (ITT-WT *) |
Durvalumab ± tremelimumab c (n = 118) |
Platinum-based chemotherapy b (n = 107) |
Durvalumab ± tremelimumab (n = 369) |
Platinum-based chemotherapy (n = 352) |
PFS: Final OS: Final |
| CheckMate-026 [35] |
|
PFS (ITT-WT *) | Nivolumab (n = 88) |
Platinum-based chemotherapy (n = 126) |
Nivolumab (n = 271) |
Platinum-based chemotherapy (n = 270) |
PFS: Final |
* Patients with EGFR or ALK mutations excluded ** Patients with EGFR, ALK, or ROS1 mutations excluded *** Patients included in this network meta-analysis (high PD-L1 expression (≥50%)) # Total of patients randomized in each study a Only the subgroup of patients with PD-L1 ≥50% were included b Only the durvalumab monotherapy arm was considered for the study PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; OS, overall survival; ITT, intention-to-treat; TCs, tumor cells; ICs, tumor-infiltrating immune cells; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TPS, tumor proportion score. All studies enriched their populations by selecting patients according to their PD-L1 expression status. In two of them (KEYNOTE-024 [13,35] and EMPOWER Lung-01 [37]), only patients with PD-L1 expression levels ≥50% were included. In the IMpower-110 [28], KEYNOTE-042 [33], and CheckMate-026 [35] studies, patients with PD-L1 expression on at least 1% of TCs or at least 1% of tumor-infiltrating ICs were included and further classified into different groups according to PD-L1 expression level. Finally, in the MYSTIC trial [36], patients were selected regardless of their PD-L1 expression status and subsequently stratified into patients with PD-L1 < 25% and PD-L1 ≥ 25%. In all cases, and in order to compare homogenous populations, only subjects with PD-L1 ≥ 50% were considered for this network meta-analysis (NMA).
In terms of primary endpoints, PFS and OS were co-primary endpoints in both the MYSTIC [36] and EMPOWER Lung-01 [37] studies; two studies (KEYNOTE-024 and CheckMate-026) had PFS as the primary endpoint [13,31,35] while two others (IMpower-110 and KEYNOTE-042) used OS [33,37]. Final PFS data were reported in three studies included in this NMA (KEYNOTE-024 [13,31], MYSTIC [36], and CheckMate-026 [35]), while final data for OS was available for only one of them [36]. Interim analyses were provided for the other four [33,34,35,37]. Both endpoints were evaluated in the wild-type intention-to-treat (ITT) population (patients with EGFR or ALK mutations were excluded from all of the studies according to the eligibility criteria).
All the studies included patients with squamous and non-squamous disease, stratified according to their histology [13,31,33,34,35,36,37]. Additionally, all studies included metastatic patients, except for KEYNOTE-042 [33], which also included locally advanced NSCLC patients. Patient population characteristics of all the studies included in the MA are shown in Supplementary Table S1.
3.3. Efficacy Endpoints in the Overall Population
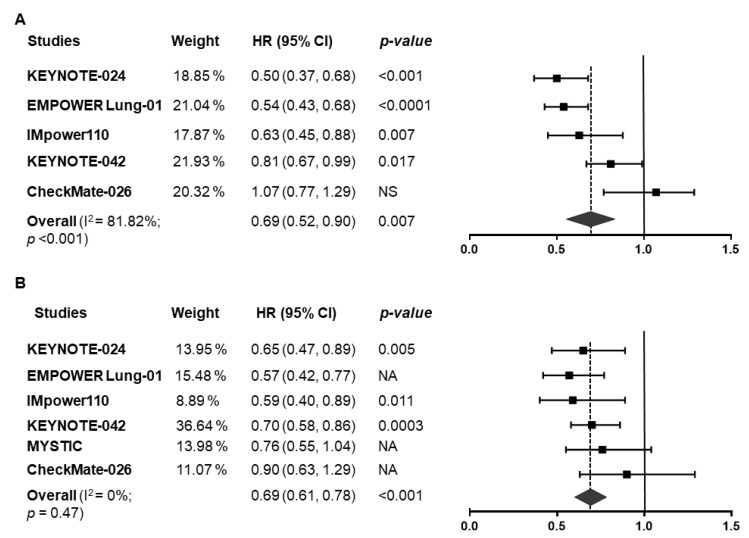
The evidence formed a connected star-shaped network (Supplementary Figure S1). Median PFS ranged from 5 to 6.4 months in the control arms, and from 5.4 to 10.3 months in the treatment arms. Median OS ranged from 12.2 to 15.9 months in the control arms, and from 13.9 to 26.3 months in the treatment arms. Monotherapy with three drugs (pembrolizumab, cemiplimab and atezolizumab) showed a significant improvement in PFS compared to chemotherapy in head-to-head comparisons (PFS: HRpooled = 0.69, 95% CI: 0.52–0.90, p = 0.007, Figure 2A). The same drugs also showed improvements in OS (OS: HRpooled = 0.69, 95% CI: 0.61–0.78; p < 0.001, Figure 2B). The ORR also significantly improved with PD-(L)1 inhibitor monotherapy (Risk ratio (RR)pooled = 1.354, 95% CI: 1.04–1.762, p = 0.024, Supplementary Figure S2).
Figure 2.
Forest plot of pooled hazard ratios for (A) progression-free survival (PFS) and (B) overall survival (OS) in patients who received PD-1/PD-L1 inhibitors vs. chemotherapy alone. HR, hazard ratio; CI, confidence interval.
In indirect comparisons of PFS (Table 2; Results were considered separately for pembrolizumab comparisons since significant heterogeneity (I2 = 80.65%, p = 0.0064) was determined between KEYNOTE studies), cemiplimab was superior to pembrolizumab, although this superiority was statistically significant only when KEYNOTE-042 results were considered (KEYNOTE-042 [33] (HR 0.67; 95% CI 0.49–0.90; p = 0.008); KEYNOTE-024 [13,31] (HR 0.93; 95% CI 0.63–0.36; p = 0.621)). Additionally, nivolumab was inferior to pembrolizumab (KEYNOTE-024 [13,31]; (HR 0.47; 95% CI 0.31–0.70; p = 0.000); KEYNOTE-042 [33] results; (HR 0.76; 95% CI 0.58–0.99; p = 0.04), atezolizumab (HR 0.59; 95% CI 0.39–0.90; p = 0.014) and cemiplimab (HR 0.50; 95% CI 0.36–0.71; p = 0.001). In the assessment of relative efficacy for PFS, pembrolizumab (KEYNOTE-024 [13,31]) ranked highest followed by cemiplimab and atezolizumab. KEYNOTE-042 [33] results did not confirm pembrolizumab superiority.
Table 2.
Network meta-analysis: PFS (HR, 95% CI and p-values are shown).
| Pembrolizumab (KEYNOTE-024) | Cemiplimab | Atezolizumab | Pembrolizumab (KEYNOTE-042) | |
|---|---|---|---|---|
| Cemiplimab | 0.93 (0.63–1.36) p = 0.621 |
|||
| Atezolizumab | 0.79 (0.50–1.26) p = 0.317 |
0.86 (0.57–1.29) p = 0.457 |
||
|
Pembrolizumab
KEYNOTE-042 |
0.62 (0.43–0.89) p = 0.009 |
0.67 (0.49–0.90) p = 0.008 |
0.78 (0.53–1.15) p = 0.204 |
|
| Nivolumab | 0.47 (0.31–0.70) p = 0.000 |
0.50 (0.36–0.71) p = 0.001 |
0.59 (0.39–0.90) p = 0.014 |
0.76 (0.58–0.99) p = 0.040 |
Note: The table must be read as the drug in the column against the drug in the row. For example, the PFS HR of pembrolizumab (KEYNOTE-024) against cemiplimab is 0.93 (95% CI 0.63, 1.36). No results available for durvalumab.
In terms of OS, no statistically significant results were determined by indirect comparisons (Table 3). Results from both KEYNOTE studies were grouped for pembrolizumab comparisons since there was no significant heterogeneity between the studies (I2 = 0.0%, p = 0.6978). In the assessment of relative efficacy for OS, cemiplimab ranked highest followed by atezolizumab, pembrolizumab, durvalumab, and nivolumab.
Table 3.
Network meta-analysis: OS (HR, 95% CI and p-values are shown).
| Cemiplimab | Atezolizumab | Pembrolizumab (KN-024/KN-042) |
Durvalumab | |
|---|---|---|---|---|
| Atezolizumab | 0.97 (0.58–1.60) p = 0.893 |
|||
|
Pembrolizumab
(KN-024/KN-042) |
0.84 (0.59–1.19) p = 0.319 |
0.87 (0.58–1.29) p = 0.483 |
||
| Durvalumab | 0.75 (0.48–1.16) p = 0.199 |
0.78 (0.47–1.29) p = 0.331 |
0.90 (0.63–1.30) p = 0.578 |
|
| Nivolumab | 0.63 (0.40–1.01) p = 0.056 |
0.66 (0.38–1.12) p = 0.123 |
0.76 (0.51–1.12) p = 0.166 |
0.84 (0.52–1.36) p = 0.484 |
Note: The table must be read as the drug in the column against the drug in the row. For example, the OS HR of cemiplimab against atezolizumab is 0.97 (95% CI 0.58, 1.60). KN, KEYNOTE.
3.4. Subgroup Analysis
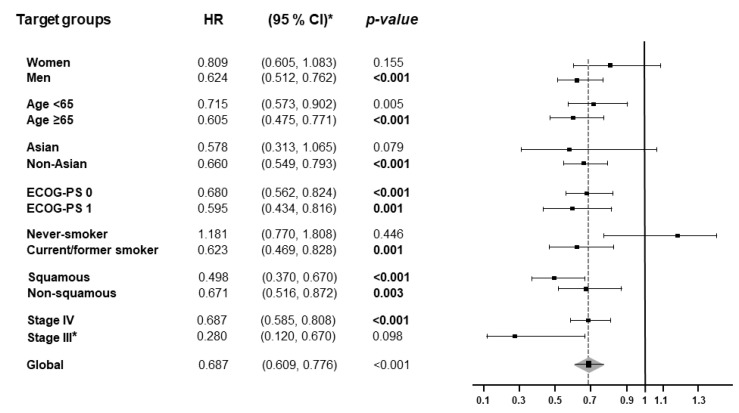
OS subgroup analyses were carried out according to sex (women vs. men), age (<65 years vs. ≥65 years), race (Asian vs. non-Asian), Eastern Cooperative Oncology Group performance status (ECOG-PS = 0 vs. ECOG-PS = 1), smoking status (never-smoker vs. current/former smoker), and histology (squamous vs. non-squamous). As shown in Figure 3 and Supplementary Figure S3, overall, first-line PD-(L)1 monotherapy improved OS in almost all subgroups, reaching statistical significance in men (HRpooled = 0.624, 95% CI: 0.51–0.72, p < 0.001), non-Asian patients (HRpooled = 0.66, 95% CI: 0.55–0.79, p < 0.001), all patients regardless of age (<65 years: HRpooled = 0.72, 95% CI: 0.57–0.90, p = 0.005; ≥65 years: HRpooled = 0.61, 95% CI: 0.48–0.77, p < 0.001), ECOG PS status (ECOG PS = 0, HRpooled = 0.68, 95% CI: 0.56–0.82, p < 0.001; ECOG PS = 1, HRpooled = 0.59, 95% CI: 0.43–0.82, p = 0.001), and tumor histological type (Squamous, HRpooled = 0.49, 95% CI: 0.37–0.67, p < 0.001; Non-squamous, HRpooled = 0.67, 95% CI: 0.52–0.87, p = 0.003). In the case of smokers and NSCLC stage, only current/former smokers (HRpooled = 0.623, 95% CI: 0.47–0.83, p = 0.001) benefited from single PD-(L)1 monotherapy over chemotherapy.
Figure 3.
Forest plot of hazard ratios for overall survival (OS) in the subgroup analysis. HR, hazard ratio; CI, confidence interval. * Only KEYNOTE-042 included patients with stage III NSCLC.
3.5. Efficacy Results According to Tumor Mutational Burden
OS and PFS were also analyzed according to TMB. A cut-off value of 16 mutations per megabase was established. The number of patients with available TMB results in each study were: 104 patients in KEYNOTE-024; 345 patients in KEYNOTE-042; 107 patients in CheckMate-026 and 87 patients in IMpower110. As shown in Supplementary Figure S4A, in terms of PFS, a benefit with PD-(L)1 monotherapy was observed in patients with TMB ≥16, but not in those with lower cut-off values in all the studies included in the analysis. A similar trend was observed for OS (Supplementary Figure S4B), except for nivolumab (CheckMate-026), for which no benefits were observed in any case.
4. Discussion
The development of ICIs, and specifically of antibodies against programmed death-1 (PD-1) and its ligand (PD-L1), have dramatically altered the therapeutic scenario in NSCLC. The optimal treatment strategy for advanced disease has been the focus of several randomized clinical trials with promising findings that have resulted in the approval of some combined strategies containing PD (L)-1 inhibitors in the first or subsequent lines of treatment [10,56,57,58,59,60,61,62,63]. Despite this success, there are still some patients who do not respond to immune-checkpoint blockade, turning predictive biomarkers have become a useful tool to guide the selection of individuals for these therapies [64]. In this sense, PD-L1 has been identified as a potential good predictive biomarker to select those treatment naïve and refractory patients more likely to respond to immunotherapy [65]. To date, pembrolizumab, and more recently atezolizumab, have received FDA approval as first-line monotherapy in patients with high PD-L1 expression based on the KEYNOTE-024/042 [13,31,32,33] and Impower-110 [34] trial results, respectively. Additionally, cemiplimab, supported by the results of the EMPOWER Lung-01 [37], has been accepted for FDA priority review, and a final decision is expected by February 2021. In this NMA, we evaluated these trials along with others assessing the efficacy of first-line PD (L)-1 monotherapy.
Our results demonstrate an overall benefit in terms of both PFS and OS of PD-(L)1 monotherapy over chemotherapy in advanced NSCLC patients showing high PD-L1 expression. While other MAs have evaluated the efficacy and/or safety of PD-(L)1-containing strategies according to PD-L1 status [66,67,68], this is the first MA to date to include studies evaluating front-line single immunotherapy agents with a PD-L1 enriched design. The latest data available were considered for this NMA, including trials such as the EMPOWER Lung-01, the results of which were recently presented at the ESMO 2020 virtual congress [37]. Although the selection of single-agent immunotherapy or combination immunotherapy for first-line treatment of advanced NSCLC remains controversial among medical oncologists, our results support the evidence that single PD-(L)1 inhibitor monotherapy is beneficial compared to chemotherapy alone in patients with high PD-L1 expression (≥50%). Further studies are required to assess the potential benefit/risk ratio of monotherapy vs. immunotherapy combination strategies.
It is notable, however, that KEYNOTE-042 was the only study among those analyzed in this NMA that included locally advanced NSCLC patients. These patients usually show better efficacy outcomes given their less advanced stage, which may explain the superiority of pembrolizumab.
For PFS, cemiplimab performed better than pembrolizumab in indirect comparisons, but results were only significant when KEYNOTE-042 [33] data were considered. In addition, nivolumab was inferior to pembrolizumab in both KEYNOTE-024 [13,31] and KEYNOTE-042 [33], and to atezolizumab and cemiplimab. In the assessment of relative efficacy for PFS, pembrolizumab (KEYNOTE-024 [13,31]) again ranked highest, followed by cemiplimab and atezolizumab. Nevertheless, the study population of KEYNOTE-024 was highly selective [13,31] and more importantly, the results were not further replicated in subsequent analyses, such as that included in KEYNOTE-042 [33,55]. This could explain why in our indirect comparisons showed that pembrolizumab results from KEYNOTE-042 [33] ranked lowest compared to the other PD (L)-1 inhibitors. The fact that KEYNOTE-042 was the only trial including locally advanced and advanced NSCLC patients must also be considered when interpreting the results, given the better efficacy outcomes in the locally advanced population (HR 0.28, 95% CI 0.12–0.72 vs. HR 0.75, 95% CI 0.60–0.94). Finally, although cemiplimab results are promising, the median follow-up period was short (10.8 months for cemiplimab) [37], and further studies are required to confirm their efficacy and safety outcomes.
In the assessment of relative efficacy regarding OS, cemiplimab ranked highest followed by atezolizumab, pembrolizumab, durvalumab, and nivolumab. In terms of OS, no significant results were determined by indirect comparisons. Importantly, it should be noted that OS was not a primary endpoint for either KEYNOTE-024 [13,31,32] or CheckMate-026 [35]. The same considerations previously mentioned for PFS must be taken into account when interpreting these results.
With respect to subgroup analyses, benefits in OS were reported across the different categories. Patients benefited from first-line anti-PD-(L)1 monotherapy regardless of cancer histology (squamous or non-squamous) or age. Regarding cancer histology no indirect comparisons of efficacy of single anti-PD-L1 agents were performed in squamous versus non squamous lung cancer patients as subgroup analyses were only exploratory in nature. Specifically, the impact of advanced age on the effectiveness of ICIs has not been strongly established so far [69], but older patients are usually more frail, a fact that strengthens the importance of our results. In line with this, OS values according to ECOG PS also showed overall benefits for immunotherapy over chemotherapy. The impact of performance status on the efficacy of immunotherapy is already well known [70]. A recent NMA of real-world data suggests that performance status at treatment initiation retains prognostic significance in patients with immunotherapy, with worse outcomes determined for patients with poorer clinical conditions [71]. However, our study supports the notion that this group of patients with worse conditions may also benefit from first-line immunotherapy monotherapy. Regarding smoking status, the results of our NMA are in line with those obtained in previous studies. Thus, in general, a better response to PD-(L)1 inhibitors was observed in current or former smokers than in non-smokers [72,73,74,75]. Although smoking status is frequently considered an important factor, it should be mentioned that the cemiplimab studies did not include the never-smoker subpopulation. As expected, both histological types benefited equally from treatment. Additionally, it is important to note that, apart from the overall benefits shown by PD-(L)1 inhibitor monotherapy in terms of efficacy, immunotherapy has been found to be associated with lower toxicity compared to chemotherapy [76], which clearly represents an improvement for patients. Additionally, the putative differences between PD-L1 and PD-1 inhibitors should be considered. Thus, as demonstrated by an NMA published in 2019 [7], and according to the available evidence, PD-L1 may have the best safety profile in terms of both treatment-related and immune-related adverse events compared to PD-1 inhibitors. Finally, in the real-world setting, some studies have demonstrated that patients with high PD-L1 expression levels show better response to first-line immunotherapy monotherapy [77,78]. In this regard, EMPOWER Lung-01 is the only study to date to show a correlation between the efficacy improvements achieved in the experimental arm and baseline PD-L1 expression levels [37].
Other clinical factors should be considered when assessing the efficacy of the immunotherapy, such as the presence of liver or brain metastases and the TMB. As demonstrated in our NMA, the latter showed a remarkable predictive value for efficacy, with clear improvements with immunotherapy monotherapy showing improvements in terms of PFS for patients with cut-off values above 16 mutations per megabase, and a similar trend was observed in terms of OS. This is in line with the results obtained with anti-PD-(L)1 regimens in different lines of treatment, which showed better efficacy outcomes for those patients with higher TMB values [27,44,79,80,81,82,83,84].
This NMA has also some limitations. First, the PD-L1 assay methods were not consistent across all studies. Thus, in IM110 [28] Ventana PD-L1 (SP142), an immunohistochemistry assay was used in both TCs and ICs, while in the MYSTIC trial [36], CheckMate-026 [35], and EMPOWER Lung-01, only TCs were considered and differently assessed by Ventana PD-L1 (SP263), Dako PD-L1 22C3, and Dako 28–8, respectively. Moreover, for the KEYNOTE studies [13,31,32,33,55], Dako PD-L1 immunohistochemistry assay 22C3 for TPS was used. Second, the final analysis for both PFS and OS was not available for two studies (EMPOWER Lung-01 [37] and IMpower110 [28]), which may change the overall efficacy in the future. Third, some data were not available, such as PFS results in the MYSTIC trial [36]. Finally, the subgroup analysis relied on limited available information, and results must therefore be interpreted with caution. In this regard, certain limitations were also found in the available EMPOWER Lung-01 data [37], the results of which have thus far only been published as personal communications at conferences. Last, due to the relatively low number of trials involved in this NMA, the results of heterogeneity between studies must be considered as guidance only. Despite these limitations, our results confirm those obtained in individual studies.
In conclusion, first-line anti-PD-(L)1 monotherapy resulted in significantly longer OS and PFS in advanced NSCLC patients with high PD(L)1 expression compared to chemotherapy alone. This supports the potential of this therapeutic option as a first-line strategy for this subgroup of patients. However, efficacy should be further evaluated in comparison with anti-PD-(L)-1-chemotherapy combinations. Additionally, although some drugs yielded significant results in indirect comparisons, the heterogeneity of results support the requirement for further evaluations to determine the superiority of any specific PD-(L)1 inhibitor.
Acknowledgments
The authors would like to thank Almudena Fuster-Matanzo from Medical Statistics Consulting S.L. (Valencia) for providing scientific support and medical writing services.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10071365/s1.
Author Contributions
M.M., M.C., L.P.-A., and D.P.-P. were responsible for data analyses and manuscript preparation and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Roche, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Conflicts of Interest
M.M. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Amgen, Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Bayer, Amgen and Boehringer; and travel/accommodation expenses from Roche, Merck and Lilly. M.C. reports advisory and consultancy honoraria from Roche, Bristol-Myers Squibb, AstraZeneca and Pfizer; and travel/accommodation expenses from Roche, Bristol-Myers Squibb and AstraZeneca. D.I. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Bayer, Abbvie, Amgen and Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Bayer, Amgen, Boehringer and Sanofi; grant funding from Roche, Bristol-Myers Squibb, AstraZeneca and Lilly; and travel/accommodation expenses from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, Amgen and Boehringer. D.M.-M. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer and Boehringer; and speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer and Boehringer. D.R.-A. reports advisory and consultancy honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Pfizer, Boehringer and Takeda; speaker honoraria from Roche, Merck, Bristol-Myers Squibb, AstraZeneca, Boehringer and Takeda; and travel/accommodation expenses from Roche, Merck, Bristol-Myers Squibb and AstraZeneca. J.C.-R. reports advisory and consultancy honoraria from Roche and AstraZeneca; and travel/accommodation expenses from Roche. T.M.-B. reports advisory and consultancy honoraria from Roche, Bristol-Myers Squibb, AstraZeneca and Boehringer; speaker honoraria from Merck, Bristol-Myers Squibb and AstraZeneca; and research grants from Kyowa Kirin. R.B.-C. reports advisory honoraria from Roche, Bristol-Myers Squibb and AstraZeneca; and speaker honoraria from Roche, Bristol-Myers Squibb and Amgen. L.P.-A. reports advisory honoraria from Roche, Merck (MSD), Merck Serono, Bristol-Myers Squibb, AstraZeneca, Lilly, Pfizer, PharmaMar, Bayer, Abbvie, Amgen, Janssen, GSK, Novartis, Ipsen, Boehringer, Takeda, Sanofi, Tesaro, Blueprint, Mirati; and research grants from Bristol-Myers Squibb, AstraZeneca and PharmaMar. Additionally, he is a co-founder and board member of Altum Sequencing, as well as an external board member of Genomica. D.P.-P., P.R.-G. and M.M.A. were full-time employees of Roche Farma S.A. at the time the study was conducted.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., Van Schil P.E., Hellmann M.D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L.G., de Marinis F., Dediu M., Thomas M., Pujol J.L., Bidoli P., Molinier O., Sahoo T.P., Laack E., Reck M., et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 5.Liang J., Ming L., Qihai S., Zhengyang H., Yunyi B., Yiwei H., Cheng Z., Wei J., Qun W., Lijie T. Compare the efficacy and safety of programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors for advanced non-small cell lung cancer: A Bayesian analysis. Transl. Lung Cancer Res. 2020;9:1302–1323. doi: 10.21037/tlcr-20-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khunger M., Jain P., Rakshit S., Pasupuleti V., Hernandez A.V., Stevenson J., Pennell N.A., Velcheti V. Safety and Efficacy of PD-1/PD-L1 Inhibitors in Treatment-Naive and Chemotherapy-Refractory Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin. Lung Cancer. 2018;19:e335–e348. doi: 10.1016/j.cllc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y.F., Xie W.J., Fan H.Y., Du J. Comparative Safety of PD-1/PD-L1 Inhibitors for Cancer Patients: Systematic Review and Network Meta-Analysis. Front. Oncol. 2019;9:972. doi: 10.3389/fonc.2019.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Yokoi T., Chiappori A., Lee K.H., de Wit M., et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 9.Horn L., Mansfield A.S., Szczesna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M., et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L., Dvorkin M., Chen Y., Reinmuth N., Hotta K., Trukhin D., Statsenko G., Hochmair M.J., Ozguroglu M., Ji J.H., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 11.Garon E.B., Hellmann M.D., Rizvi N.A., Carcereny E., Leighl N.B., Ahn M.J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gettinger S., Borghaei H., Brahmer J., Chow L., Burgio M., De Castro Carpeno J., Pluzanski A., Arrieta O., Frontera O.A., Chiari R., et al. OA14.04 Five-Year Outcomes From the Randomized, Phase 3 Trials CheckMate 017/057: Nivolumab vs. Docetaxel in Previously Treated NSCLC. J. Thorac. Oncol. 2019;14:S244–S245. doi: 10.1016/j.jtho.2019.08.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 14.Chen D.S., Irving B.A., Hodi F.S. Molecular pathways: Next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 15.Soria J.C., Marabelle A., Brahmer J.R., Gettinger S. Immune checkpoint modulation for non-small cell lung cancer. Clin. Cancer Res. 2015;21:2256–2262. doi: 10.1158/1078-0432.CCR-14-2959. [DOI] [PubMed] [Google Scholar]
- 16.Buchbinder E.I., Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha Y.J., Kim H.R., Lee C.Y., Cho B.C., Shim H.S. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016;97:73–80. doi: 10.1016/j.lungcan.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.B., Mu C.Y., Huang J.A. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: A 5-year-follow-up study. Tumori J. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 19.Cooper W.A., Tran T., Vilain R.E., Madore J., Selinger C.I., Kohonen-Corish M., Yip P., Yu B., O’Toole S.A., McCaughan B.C., et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 20.D’Incecco A., Andreozzi M., Ludovini V., Rossi E., Capodanno A., Landi L., Tibaldi C., Minuti G., Salvini J., Coppi E., et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br. J. Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur M.K., Gadgeel S.M. Predictive and Prognostic Biomarkers in Non-Small Cell Lung Cancer. Semin. Respir. Crit. Care Med. 2016;37:760–770. doi: 10.1055/s-0036-1592337. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi T., Teramoto K., Ishida M., Hanaoka J., Daigo Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open. 2016;1:e000083. doi: 10.1136/esmoopen-2016-000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huijuan L., Xu Y., Wan B., Song Y., Zhan P., Hu Y., Zhang Q., Zhang F., Liu H., Li T., et al. The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: A meta-analysis of 50 studies with 11,383 patients. Transl. Lung Cancer Res. 2019;8:429–449. doi: 10.21037/tlcr.2019.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh J., Go H., Keam B., Kim M.Y., Nam S.J., Kim T.M., Lee S.H., Min H.S., Kim Y.T., Kim D.W., et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: Comparison with histology and driver oncogenic alteration status. Mod. Pathol. 2015;28:1154–1166. doi: 10.1038/modpathol.2015.63. [DOI] [PubMed] [Google Scholar]
- 25.Tuminello S., Sikavi D., Velusw R., Gamarra C., Lieberman-Cribbin W., Flores R., Taioli E. PD-L1 as a prognostic biomarker in surgically resectable non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2020;9:1343–1360. doi: 10.21037/tlcr-19-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada K., Okamoto T., Shoji F., Shimokawa M., Akamine T., Takamori S., Katsura M., Suzuki Y., Fujishita T., Toyokawa G., et al. Clinical Significance of PD-L1 Protein Expression in Surgically Resected Primary Lung Adenocarcinoma. J. Thorac. Oncol. 2016;11:1879–1890. doi: 10.1016/j.jtho.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Fehrenbacher L., von Pawel J., Park K., Rittmeyer A., Gandara D.R., Ponce Aix S., Han J.Y., Gadgeel S.M., Hida T., Cortinovis D.L., et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018;13:1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Herbst R., Baas P., Kim D.-S., Felip E., Perez-Gracia J.L., Han J.-Y., Molina J., Kim J.-P., Arvis C., Ahn M.-J., et al. Factors associated with better overall survival (OS) in patients with previously treated, PD-L1–expressing, advanced NSCLC: Multivariate analysis of KEYNOTE-010. J. Clin. Oncol. 2017;35:9090. doi: 10.1200/JCO.2017.35.15_suppl.9090. [DOI] [Google Scholar]
- 29.Antonia S.J., Borghaei H., Ramalingam S.S., Horn L., De Castro Carpeño J., Pluzanski A., Burgio M.A., Garassino M., Chow L.Q.M., Gettinger S., et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol. 2019;20:1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-González J., Ruiz-Bañobre J., Afonso-Afonso F.J., Amenedo-Gancedo M., Areses-Manrique M.D.C., Campos-Balea B., Casal-Rubio J., Fernández-Núñez N., Fírvida Pérez J.L., Lázaro-Quintela M., et al. PD-(L)1 Inhibitors in Combination with Chemotherapy as First-Line Treatment for Non-Small-Cell Lung Cancer: A Pairwise Meta-Analysis. J. Clin. Med. 2020;9:2093. doi: 10.3390/jcm9072093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 32.Brahmer J.R., Rodriguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. LBA51 KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs. platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50% Ann. Oncol. 2020;31:S1181–S1182. doi: 10.1016/j.annonc.2020.08.2284. [DOI] [Google Scholar]
- 33.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 34.Herbst R.S., Giaccone G., de Marinis F., Reinmuth N., Vergnenegre A., Barrios C.H., Morise M., Felip E., Andric Z., Geater S., et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 35.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., Felip E., van den Heuvel M.M., Ciuleanu T.E., Badin F., et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizvi N.A., Cho B.C., Reinmuth N., Lee K.H., Luft A., Ahn M.-J., van den Heuvel M.M., Cobo M., Vicente D., Smolin A., et al. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sezer A., Saadettin K., Gümüş M., Bondarenko I., Özgüroğlu M., Gogishvili M., Turk H.M., Cicin I., Bentsion D., Gladkov O., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 38.Alborelli I., Leonards K., Rothschild S.I., Leuenberger L.P., Savic Prince S., Mertz K.D., Poechtrager S., Buess M., Zippelius A., Läubli H., et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J. Pathol. 2020;250:19–29. doi: 10.1002/path.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong A., Zhao Y., Li Z., Hu H. PD-L1 versus tumor mutation burden: Which is the better immunotherapy biomarker in advanced non-small cell lung cancer? J. Gene Med. 2020;23:e3294. doi: 10.1002/jgm.3294. [DOI] [PubMed] [Google Scholar]
- 42.Greillier L., Tomasini P., Barlesi F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl. Lung Cancer Res. 2018;7:639–646. doi: 10.21037/tlcr.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gettinger S., Beck T., Yang X., Telivala B., Morgensztern D., Velcheti V., Ramalingam S.S., Schalper K., Dajee M., Ranck A., et al. CheckMate 592: A phase II exploratory study of biomarkers associated with the efficacy of first-line nivolumab (nivo) plus ipilimumab (ipi) in patients (pts) with stage IV or recurrent NSCLC. Ann. Oncol. 2018;29:viii544–viii545. doi: 10.1093/annonc/mdy292.124. [DOI] [Google Scholar]
- 44.Hellmann M.D., Ciuleanu T.E., Pluzanski A., Lee J.S., Otterson G.A., Audigier-Valette C., Minenza E., Linardou H., Burgers S., Salman P., et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellmann M.D., Nathanson T., Rizvi H., Creelan B.C., Sanchez-Vega F., Ahuja A., Ni A., Novik J.B., Mangarin L.M.B., Abu-Akeel M., et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell. 2018;33:843–852.e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ready N., Hellmann M.D., Awad M.M., Otterson G.A., Gutierrez M., Gainor J.F., Borghaei H., Jolivet J., Horn L., Mates M., et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019;37:992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Socinski M., Velcheti V., Mekhail T., Chae Y.K., Leal T.A., Dowell J.E., Tsai M.L., Dakhil C.S., Stella P., Shen V., et al. LBA83-Final efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC) Ann. Oncol. 2019;30:v919–v920. doi: 10.1093/annonc/mdz394.081. [DOI] [Google Scholar]
- 48.Gandara D.R., Paul S.M., Kowanetz M., Schleifman E., Zou W., Li Y., Rittmeyer A., Fehrenbacher L., Otto G., Malboeuf C., et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 49.Gandara D.R., Kowanetz M., Mok T.S.K., Rittmeyer A., Fehrenbacher L., Fabrizio D., Otto G., Malboeuf C., Lieber D., Paul S.M., et al. 1295O-Blood-based biomarkers for cancer immunotherapy: Tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK) Ann. Oncol. 2017;28:v460. doi: 10.1093/annonc/mdx380. [DOI] [Google Scholar]
- 50.Garassino M., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., Speranza G., Reck M., Hui R., Boyer M., Cristescu R., et al. OA04.06 Evaluation of TMB in KEYNOTE-189: Pembrolizumab Plus Chemotherapy vs. Placebo Plus Chemotherapy for Nonsquamous NSCLC. J. Thorac. Oncol. 2019;14:S216–S217. doi: 10.1016/j.jtho.2019.08.427. [DOI] [Google Scholar]
- 51.Doi S.A.R., Barendregt J.J. A generalized pairwise modelling framework for network meta-analysis. Int. J. Evid. Based Healthc. 2018;16:187–194. doi: 10.1097/XEB.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 52.Bucher H.C., Guyatt G.H., Griffith L.E., Walter S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997;50:683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 53.Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions: Chichester, West Sussex. John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 54.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., de Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Final analysis of the phase III KEYNOTE-042 study: Pembrolizumab (Pembro) versus platinum-based chemotherapy (Chemo) as first-line therapy for patients (Pts) with PD-L1-positive locally advanced/metastatic NSCLC. Ann. Oncol. 2019;30:i38. doi: 10.1093/annonc/mdz063. [DOI] [Google Scholar]
- 56.Socinski M.A., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodriguez-Abreu D., Moro-Sibilot D., Thomas C.A., Barlesi F., et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 57.Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gumus M., Mazieres J., Hermes B., Cay Senler F., Csoszi T., Fulop A., et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 58.Reck M., Ciuleanu T.-E., Dols M.C., Schenker M., Zurawski B., Menezes J., Richardet E., Bennouna J., Felip E., Juan-Vidal O., et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs. 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J. Clin. Oncol. 2020;38:9501. doi: 10.1200/JCO.2020.38.15_suppl.9501. [DOI] [Google Scholar]
- 59.Conforti F., Pala L., Bagnardi V., De Pas T., Martinetti M., Viale G., Gelber R.D., Goldhirsch A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 60.Tan P.S., Aguiar P., Haaland B., Lopes G. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer—A systematic review and network meta-analysis of 3024 participants. Lung Cancer. 2018;115:84–88. doi: 10.1016/j.lungcan.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 61.You W., Liu M., Miao J.D., Liao Y.Q., Song Y.B., Cai D.K., Gao Y., Peng H. A Network Meta-analysis Comparing the Efficacy and Safety of Anti-PD-1 with Anti-PD-L1 in Non-small Cell Lung Cancer. J. Cancer. 2018;9:1200–1206. doi: 10.7150/jca.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Q., Xie R., Lin S., You X., Weng X. Anti-PD-1/PD-L1 Antibody Therapy for Pretreated Advanced or Metastatic Nonsmall Cell Lung Carcinomas and the Correlation between PD-L1 Expression and Treatment Effectiveness: An Update Meta-Analysis of Randomized Clinical Trials. BioMed Res. Int. 2018;2018:3820956. doi: 10.1155/2018/3820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou G.-W., Xiong Y., Chen S., Xia F., Li Q., Hu J. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials. Medicine. 2016;95:e4611. doi: 10.1097/MD.0000000000004611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tray N., Weber J.S., Adams S. Predictive Biomarkers for Checkpoint Immunotherapy: Current Status and Challenges for Clinical Application. Cancer Immunol. Res. 2018;6:1122–1128. doi: 10.1158/2326-6066.CIR-18-0214. [DOI] [PubMed] [Google Scholar]
- 65.Voong K., Feliciano J., Becker D., Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann. Transl. Med. 2017;5:376. doi: 10.21037/atm.2017.06.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J., Li C., Seery S., Yu J., Meng X. Identifying optimal first-line interventions for advanced non-small cell lung carcinoma according to PD-L1 expression: A systematic review and network meta-analysis. Oncoimmunology. 2020;9:1746112. doi: 10.1080/2162402X.2020.1746112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z.-J., Zhan P., Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2015;4:203–208. doi: 10.1016/j.lungcan.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi A., Noia V.D., Gkountakos A., D’Argento E., Sartori G., Vita E., Monteverdi S., Lombardo F., Iacovelli R., Carbognin L., et al. PD-L1 for selecting non-small-cell lung cancer patients for first-line immuno-chemotherapy combination: A systematic review and meta-analysis. Immunotherapy. 2019;11:921–930. doi: 10.2217/imt-2018-0198. [DOI] [PubMed] [Google Scholar]
- 69.Kanesvaran R., Cordoba R., Maggiore R. Immunotherapy in Older Adults With Advanced Cancers: Implications for Clinical Decision-Making and Future Research. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:400–414. doi: 10.1200/EDBK_201435. [DOI] [PubMed] [Google Scholar]
- 70.Campos-Balea B., de Castro Carpeño J., Massutí B., Vicente-Baz D., Pérez Parente D., Ruiz-Gracia P., Crama L., Cobo Dols M. Prognostic factors for survival in patients with metastatic lung adenocarcinoma: An analysis of the SEER database. Thorac. Cancer. 2020;11:3357–3364. doi: 10.1111/1759-7714.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dall’Olio F.G., Maggio I., Massucci M., Mollica V., Fragomeno B., Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. 2020;145:95–104. doi: 10.1016/j.lungcan.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gainor J.F., Shaw A.T., Sequist L.V., Fu X., Azzoli C.G., Piotrowska Z., Huynh T.G., Zhao L., Fulton L., Schultz K.R., et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 75.Norum J., Nieder C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): A review of the literature. ESMO Open. 2018;3:e000406. doi: 10.1136/esmoopen-2018-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michot J.M., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., Berdelou A., Varga A., Bahleda R., Hollebecque A., et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Aguilar E.J., Ricciuti B., Gainor J.F., Kehl K.L., Kravets S., Dahlberg S., Nishino M., Sholl L.M., Adeni A., Subegdjo S., et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann. Oncol. 2019;30:1653–1659. doi: 10.1093/annonc/mdz288. [DOI] [PubMed] [Google Scholar]
- 78.Takeyasu Y., Yoshida T., Shibaki R., Matsumoto Y., Goto Y., Kanda S., Horinouchi H., Yamamoto N., Motoi N., Ohe Y. Differential Efficacy of Pembrolizumab According to Metastatic Sites in Patients With PD-L1 Strongly Positive (TPS ≥ 50%) NSCLC. Clin. Lung Cancer. 2020;22:127–133.e3. doi: 10.1016/j.cllc.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., Park K., Smith D., Artal-Cortes A., Lewanski C., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 80.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herbst R.S., Lopes G., Kowalski D.M., Nishio M., Wu Y.L., de Castro Junior G., Baas P., Kim D.W., Gubens M.A., Cristescu R., et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann. Oncol. 2019;30:v916–v917. doi: 10.1093/annonc/mdz394.077. [DOI] [Google Scholar]
- 82.Kim E.S., Velcheti V., Mekhail T., Leal T.A., Dowell J.E., Tsai M.L., Dakhil C.S.R., Stella P., Shen V., Hu S., et al. Primary efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC) Ann. Oncol. 2018;29:viii744. doi: 10.1093/annonc/mdy424.067. [DOI] [Google Scholar]
- 83.Paz-Ares L., Langer C., Novello S., Halmos B., Cheng Y., Gadgeel S., Hui R., Sugawara S., Borghaei H., Cristescu R., et al. Abstract LBA80-Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann. Oncol. 2019;30:v917–v918. doi: 10.1093/annonc/mdz394.078. [DOI] [Google Scholar]
- 84.Peters S., Creelan B., Hellmann M.D., Socinski M.A., Reck M., Bhagavatheeswaran P., Chang H., Geese W.J., Paz-Ares L., Carbone D.P. Abstract CT082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage iv or recurrent non-small cell lung cancer: An exploratory analysis of CheckMate 026. Cancer Res. 2017;77 doi: 10.1158/1538-7445.AM2017-CT082. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).