Abstract
Objective.
Aromatase inhibitors (AI) are frequently prescribed in gynecologic oncology. We sought to define the frequency and duration of AI use, characterize AI side effects and determine the reasons for discontinuation in these patients.
Methods.
Uterine and ovarian cancer patients with AI use for gynecologic cancer therapy were identified retrospectively. Data were abstracted from the electronic medical record, including cancer type, stage, prior cancer treatments, body mass index, concurrent medications, prevalence of AI side effects before and during AI therapy, length of AI treatment and reason for AI discontinuation.
Results.
146 women received AI therapy, with 68 for ovarian cancer (46.6%) and 78 for uterine cancer (53.4%). The majority (71.9%) had advanced stage disease at diagnosis. 54.1% noted AI-associated side effects within the first three visits after starting AI therapy. The most common side effects were arthralgias (29.5%), hot flashes (25.3%), new/worsening fatigue (16.4%), muscle or joint stiffness (8.2%) and myalgias (6.8%). The mean duration of therapy was 14.7 months. Gabapentin or selective serotonin reuptake inhibitor (SSRI) use was associated with decreased musculoskeletal side effects (gabapentin: p < .001, OR 0.88, 95% CI 0.83–0.94; SSRI: p < .001, OR 0.82, 95% CI 0.77–0.89). The most common reason for AI discontinuation was disease progression (87.9%), with 5.0% discontinuing due to side effects and 7.1% for other reasons.
Conclusion.
AI therapy for gynecologic cancers is frequently associated with musculoskeletal side effects, but rarely leads to treatment discontinuation. Thus, AI side effects should be assessed in gynecologic cancer patients to allow potential mitigation of symptoms through adjunct therapies.
1. Introduction
Estrogen signaling is critical to normal cellular growth and homeostasis in tissues of the female breast, ovary and uterus, and aberrant signaling has been implicated in cancer development and progression. The well-characterized role of estrogen signaling in breast cancer tumorigenesis has led to the successful use of antiestrogen therapy for breast cancer prevention and treatment of estrogen receptor alpha (ERα)-positive tumors [1,2]. Aromatase inhibitors (AI), including anastrozole, letrozole and exemestane, function through the inhibition of estrogen production and have demonstrated particular efficacy in preventing recurrence of breast cancer in multiple studies [3].
Extensive preclinical and clinical studies have investigated the role of estrogen and its blockade in gynecologic cancers of the ovary and uterus [4,5]. Both ovarian and uterine cancers are a heterogeneous collection of histologic subtypes of disease—ovarian cancers can be subclassified as epithelial (carcinoma), sex cord stromal or germ cell tumors; uterine cancers can be subclassified as epithelial or mesenchymal (sarcoma) tumors. Many of these tumors express ERα [6]. Furthermore, some ER-negative gynecologic tumors have been shown to respond to antiestrogen therapy [7,8]. Given its potential efficacy, oral administration and unique side effect profile as compared to cytotoxic chemotherapy, there has been continued interest in the use of AI therapy for gynecologic cancers.
Despite the potential clinical efficacy of AIs, their use is associated with a risk of side effects that can be debilitating. These side effects can be broad and diverse, including musculoskeletal symptoms, hot flashes and insomnia [9–12]. The constellation of musculoskeletal symptoms associated with AI therapy has been termed aromatase inhibitor musculoskeletal syndrome (AIMSS) or aromatase inhibitor-related arthralgias (AIA) [13]. The most commonly cited musculoskeletal side effects of AI therapy are arthralgias, myalgias, tendinopathies and stiffness [14], with musculoskeletal stiffness and pain having been reported in 46% of patients taking AI therapy [15]. In patients with early stage breast cancer, AIMSS typically manifests within the first 6–8 weeks of starting an AI, but has been documented to begin up to a year after the initiation of AI therapy [16]. Due to side effects associated with AI therapy, many breast cancer patients take drug holidays or discontinue the medication altogether [17]. Studies have shown a discontinuation rate ranging from 31% to 73% within five years of starting an AI for breast cancer adjuvant therapy [18], with musculoskeletal symptoms cited as the primary reason for early discontinuation [19].
To date, the majority of studies on AIMSS have focused on breast cancer patients with early stage disease; little is known about AI-associated symptoms and discontinuation rates in gynecologic cancer patients. Therefore, we sought to determine the frequency and duration of AI use among gynecologic oncology patients, the frequency of side effects and the reasons for discontinuation. We hypothesized that despite frequent side effects in these patients, discontinuation rates would be low given the frequent utilization of AI therapy in the setting of advanced stage or active disease.
2. Materials and methods
A retrospective chart review of all uterine and ovarian gynecologic cancer patients at the University of Michigan from January 1, 1998 to September 1, 2018 was performed under Institutional Review Board approval (protocol #HUM00152085). Following identification of the initial patient cohort, charts were screened with the electronic medical record search engine EMERSE for the generic and trade names for three AIs: anastrazole/Arimidex, letrozole/Femara and exemestane/ Aromasin [20]. Patient charts with an AI identified were then individually reviewed to determine if the AI was prescribed for the treatment of a gynecologic malignancy. Inclusion criteria included age ≥ 18 years and diagnosis of uterine or ovarian cancer. Women who used AI therapy for a non-gynecologic cancer were excluded from the study.
Once the cohort was defined, detailed data were abstracted and entered into a REDCap database, including patient demographic data and cancer-specific information consisting of gynecologic cancer type and stage, prior cancer treatments, prevalence of AI side effects before and during AI therapy, length of AI treatment, medications used in conjunction with AI treatment and reason for AI discontinuation. Age and body mass index (BMI) at diagnosis were recorded. For this study, race/ethnicity was categorized as white versus non-white and Hispanic versus non-Hispanic. The primary tumor site was recorded as uterine or ovarian. Disease histology was categorized as carcinoma or other. Start date of AI therapy, specific AI and prior use of a selective estrogen receptor modulator (SERM) were recorded.
Clinic documentation was reviewed for each office visit following AI therapy initiation. BMI, pain score and the presence or absence of measurable disease were recorded. The presence or absence of pain medication use while on AI therapy was recorded as a categorical yes/no variable. Additionally, the specific pain medications were noted in the categories of opiates, nonsteroidal anti-inflammatory drugs (NSAIDs), gabapentin or other. If there was discrepancy between medications in the clinic notes and the electronic medical record medication list, the clinic note information was used. Presence of new AIMSS symptoms was noted at each visit. Symptoms that fell into the AIMSS criteria were recorded, including arthralgia/joint pain, muscle/joint stiffness, myalgia/muscle pain, tendonitis/tendinopathy, numbness/tingling, carpal tunnel, morning stiffness, fatigue, hot flashes, deep venous thrombosis (DVT)/pulmonary embolism (PE) or other. Treatment plan to continue or discontinue AI therapy was noted. If the AI was discontinued, the reason for discontinuation was categorized as disease progression, side effects or other. If a patienťs treatment was changed to a different AI due to side effects, this was noted.
Data management and analysis were conducted using Statistical Analysis System (SAS) statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were calculated to summarize the distribution and frequencies of demographic information, cancer history, aromatase inhibitor use and other medication use. To determine associations of factors with musculoskeletal symptoms and hot flashes, marginal models with a logit link were estimated using a generalized estimating equation (GEE) approach to regress the occurrence of side effects on each factor of interest one at a time for patients on AI therapy. Given the candidate covariates selected from the univariate analysis, we built a multivariable model using backward elimination to jointly model predictors of these side effects.
3. Results
A total of 3294 patients with ovarian or uterine malignancy diagnosed over the specified interval were identified. Among these patients, 312 had an AI listed in their chart; 146 were on AI therapy specifically for a gynecologic cancer. The remaining patients were either taking AI therapy for breast cancer or had an AI listed in their chart after it was discussed as a treatment option, but were never started on the medication.
We first determined demographic and cancer-related information for our cohort (Table 1). Among the 146 women in our study, 129 (88.4%) identified as white and the remaining 17 (11.6%) identified as nonwhite. Seventy-eight patients (53.4%) took an AI for uterine or endometrial cancer and 68 patients (46.6%) for ovarian cancer. In our cohort, 130 patients (89.0%) had a carcinoma of the uterus, endometrium or ovary, 15 (10.3%) had a uterine sarcoma, and one (0.7%) had a granulosa cell tumor of the ovary. The majority of patients were diagnosed with advanced stage (stage III or IV) disease (n = 105, 71.9%). The majority (n = 98, 67.1%) of patients had high grade disease, of which approximately half were uterine cancer (n = 48) and half were ovarian cancer (n = 50). Almost one-third (29.5%) of patients had low grade disease; for five patients (3.1%), grade was not documented in the pathology report. Estrogen receptor alpha (ERα) expression status was determined for approximately two-thirds of patients; overall, 61.6% of tumors were ER-positive, 6.2% were ER-negative, and in 32.2% of patient tumors, the ER expression status was unknown. At the initiation of AI therapy, 74.0% of patients had measurable disease and 30.1% of patients had received prior radiation therapy. The majority of patients (n = 124, 85.0%) were treated with at least one line of chemotherapy before AI therapy; these patients received an average of 2.6 lines of treatment (SD 2.0) prior to starting AI therapy. For the overall cohort, patients had been treated with an average of 2.2 chemotherapy regimens (SD 1.89) prior to AI therapy.
Table 1.
Demographics and Cancer History.
| Characteristic | Total (N = 146) |
|---|---|
| Age at Diagnosis, years | 59.5 (11.73)a |
| Body Mass Index, kg/m2 | 31.0 (9.86)a |
| Site of Origin | |
| Uterus | 78 (53.4) |
| Ovary | 68 (46.6) |
| Histology | |
| Carcinoma | 127 (87.0) |
| Sarcoma/Other | 19 (13.0) |
| Stage | |
| I | 32 (21.9) |
| II | 7 (4.8) |
| III | 68 (46.6) |
| IV | 37 (25.3) |
| Unknown | 2 (1.4) |
| Grade | |
| Low | 43 (29.5) |
| High | 98 (67.1) |
| Unknown | 5 (3.1) |
| Estrogen Receptor (ER) Expression | |
| ER-positive | 90 (61.6) |
| ER-negative | 9 (6.2) |
| Unknown | 47 (32.2) |
| Measurable Disease at AI Initiation | |
| Yes | 108 (74.0) |
| No | 32 (21.9) |
| Not documented | 6 (4.1) |
| Prior Radiation Therapy | |
| Yes | 44 (30.1) |
| No | 102 (69.9) |
| Number of Prior Lines of Chemotherapy | 2.2 (1.9)a |
Data presented as n (%) unless otherwise noted.
AI = aromatase inhibitor.
Mean (SD).
We next assessed AI prescribing patterns, as well as patients' concomitant medication use (Table 2). The initially prescribed AIs were letrozole for 74 patients (50.7%), anastrozole for 65 patients (44.5%) and exemestane for seven patients (4.8%). Standard AI dosing was used for all patients: letrozole 2.5 mg daily; anastrozole 1 mg daily; exemestane 25 mg daily. Medical records were reviewed for use of duloxetine, other serotonin-norepinephrine reuptake inhibitors (SNRIs), and SSRIs as well as opiates, NSAIDs, gabapentin or other pain medications. Other reported medications included acetaminophen (n = 19) and pregabalin (n = 2), as well as one patient each taking the following: cyclobenzaprine, methocarbamol, acetominophen/butalbitol/caffeine and lidocaine patch.
Table 2.
Aromatase Inhibitor and Other Medication Use.
| Medication | Total (N = 146) |
|---|---|
| First Aromatase Inhibitor | |
| Letrozole | 74 (50.7) |
| Anastrozole | 65 (44.5) |
| Exemestane | 7 (4.8) |
| Letrozole Use Ever | |
| Yes | 76 (52.1) |
| No | 70 (47.9) |
| Anastrozole Use Ever | |
| Yes | 68 (46.6) |
| No | 78 (53.4) |
| Exemestane Use Ever | |
| Yes | 13 (8.9) |
| No | 133 (91.1) |
| Tamoxifen Use Ever | |
| Yes | 1 (0.7) |
| No | 145 (99.3) |
| Tamoxifen Prior to AI | |
| Yes | 14 (9.6) |
| No | 131 (89.7) |
| Unknown | 1 (0.7) |
| Tamoxifen After AI | |
| Yes | 10 (6.8) |
| No | 134 (91.8) |
| Unknown | 2 (1.4) |
| AIMSS-like symptoms prior to AI Initiation | |
| Yes | 44 (30.1) |
| No | 102 (69.9) |
| Musculoskeletal Symptoms during AI Use | |
| Yes | 52 (35.6) |
| No | 94 (64.4) |
| Hot Flashes during AI Use | |
| Yes | 37 (25.3) |
| No | 109 (74.7) |
| Duloxetine Use during AI Use | |
| Yes | 8 (5.5) |
| No | 138 (94.5) |
| Other SNRI (Venlafaxine) Use during AI Use | |
| Yes | 6 (4.1) |
| No | 140 (95.9) |
| SSRI Use during AI Use | |
| Yes | 17 (11.6) |
| No | 129 (88.4) |
| NSAID Use during AI Use | |
| Yes | 54 (37.0) |
| No | 92 (63.0) |
| Narcotic Use during AI Use | |
| Yes | 54 (37.0) |
| No | 92 (63.0) |
| Gabapentin Use during AI Use | |
| Yes | 28 (19.2) |
| No | 118 (80.8) |
| Other Pain Medication Use during AI Use | |
| Yes | 39 (26.7) |
| No | 107 (73.3) |
Data presented as n (%).
AI = aromatase inhibitor; AIMSS = aromatase inhibitor musculoskeletal syndrome; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepi-nephrine reuptake inhibitor; NSAID = nonsteroidal anti-inflammatory drug.
To allow assessment of possible attribution of symptoms specifically to AI use, we first queried patient medical records to determine if the women had symptoms commonly associated with AI use prior to starting the medication (Table 2). Specifically, we determined whether or not a patient reported any of the following prior to initiation of therapy: hot flashes, arthralgias, myalgias, joint stiffness, tendonitis, carpal tunnel symptoms, morning stiffness, DVT/PE or numbness and tingling. To then determine the impact of AI therapy on symptoms, we assessed whether or not each of these side effects was specifically recorded for the first three visits after the patient started AI therapy (Fig. 1A). The average interval from AI start to first follow-up was 10 weeks and the average interval from AI start to third follow-up was 30 weeks. A total of 79 patients (54.1%) noted significant side effects within these first three visits. The most common side effects were arthralgias or joint pain (29.5%), hot flashes (25.3%), new/worsening fatigue (16.4%), muscle or joint stiffness (8.2%) and myalgias (6.8%). Other reported symptoms included new diagnosis of osteopenia or osteoporosis, heel spurs, sciatic pain, lower extremity edema and upper extremity edema.
Fig. 1.
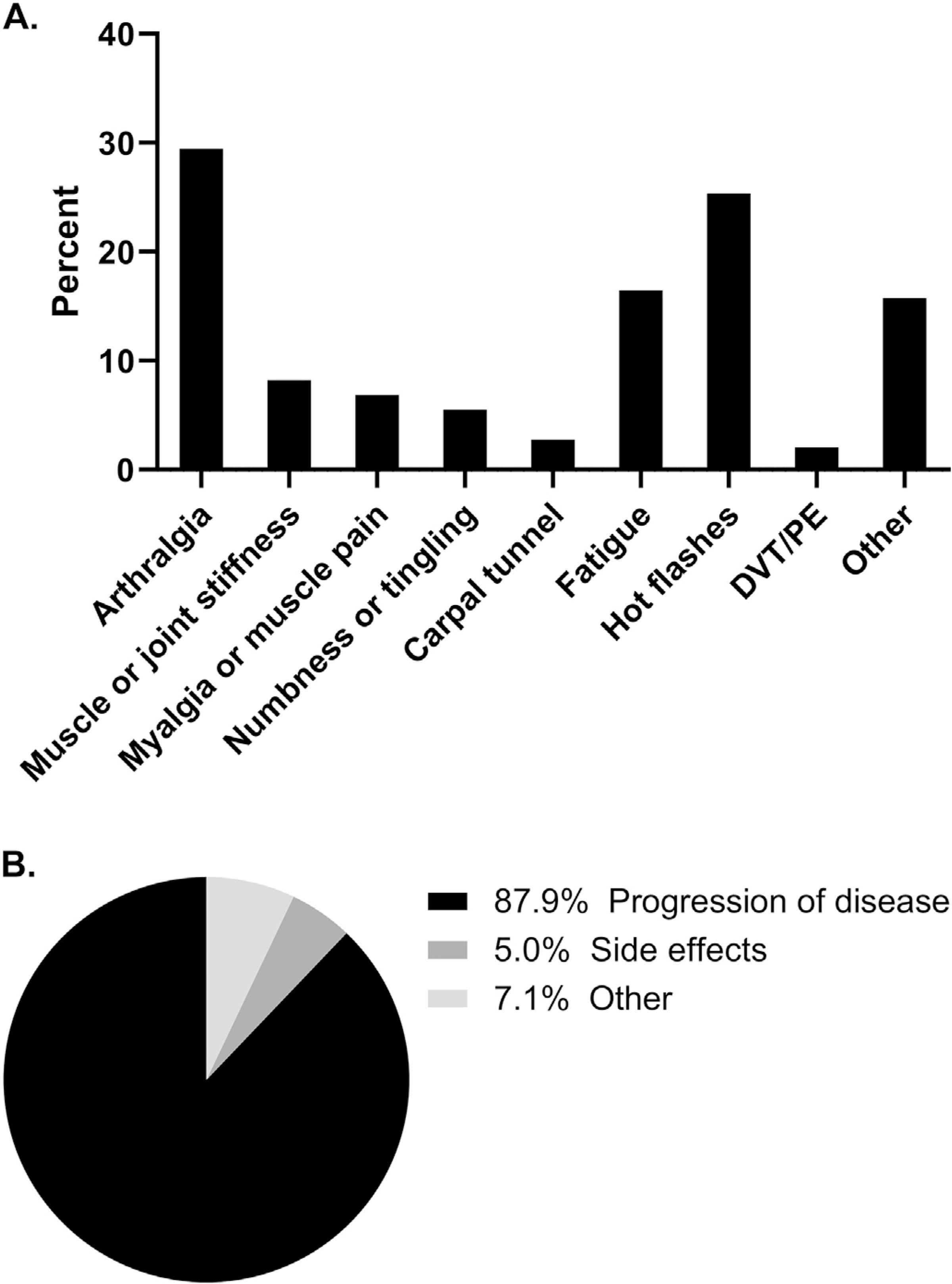
Aromatase Inhibitor Side Effects and Reasons for Discontinuation. (A) Side effect rates. Medical record chart review revealed that over half of patients (54.1%, 79 patients) reported at least one AI-associated side effect within the first three visits of starting an aromatase inhibitor. (B) Reasons for AI discontinuation. A total of 99 patients discontinued aromatase inhibitor therapy during the reviewed treatment period. Rates and reasons for discontinuation are shown.
We next assessed reasons for AI discontinuation within our cohort (Fig. 1B). The mean duration of therapy was 14.7 months (range 0.09–130.0, SD 19.3). Progression of disease was the most common reason for AI discontinuation, occurring in 87.9% of women. Side effects were the cause of discontinuation in 5.0% of women, while 7.1% discontinued for other reasons. Among the four women who discontinued due to side effects, one experienced numbness, tingling and joint pain in her hands. The second patient initially stopped AI therapy due to arthralgias; she then initiated megestrol acetate, but upon progression of disease resumed exemestane, which she continued on for several years. The third patient took letrozole for three years and then discontinued due to severe osteopenia that led to multiple rib fractures and a compression fracture. The final patient who discontinued AI therapy due to side effects experienced arthralgias on exemestane and therefore switched to tamoxifen therapy.
Univariate analysis was performed to determine factors associated with the development of musculoskeletal symptoms while patients were on AI therapy (Table 3). Women with measurable disease at the start of therapy had a lower likelihood of having musculoskeletal side effects (p = .019, OR 0.90, 95% CI 0.829–0.983). There was a statistically significant decrease in musculoskeletal symptoms on AI therapy for patients who had undergone radiation therapy (p = .01, OR = 0.91, 95% CI 0.85–0.98). Anastrozole or letrozole therapy was associated with decreased odds of musculoskeletal side effects, as compared to exemestane use (p = .012, OR 0.82, 95% CI 0.70–0.95 and OR 0.79, 95% CI 0.67–0.92, respectively). Finally, increased age at the time of diagnosis trended toward a lower frequency of musculoskeletal symptoms (p = .069, OR 1.00, 95% CI 0.99–1.00).
Table 3.
Univariate Results of Factors of Interest with Musculoskeletal Symptoms.
| Characteristic | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age at Diagnosis, per 10 years | 0.97 (0.94–1.00) | 0.069 |
| Body Mass Index, kg/m2 | 1.00 (0.99–1.00) | 0.002 |
| Site of Origin – ovarian vs. uterine | 1.01 (0.94–1.10) | 0.767 |
| Histology – carcinoma vs. other | 0.90 (0.80–1.03) | 0.120 |
| Stage | 0.358 | |
| II vs. I | 1.03 (0.94–1.14) | 0.547 |
| III vs. I | 1.00 (0.90–1.10) | 0.931 |
| IV vs. I | 1.10 (0.96–1.27) | 0.152 |
| Prior Radiation Therapy – yes vs. no | 0.91 (0.85–0.98) | 0.010 |
| Number of Prior Lines of Chemotherapy | 1.00 (0.98–1.02) | 0.947 |
| Aromatase Inhibitor | 0.012 | |
| Anastrozole vs. Exemestane | 0.82 (0.70–0.95) | 0.010 |
| Letrozole vs. Exemestane | 0.79 (0.67–0.92) | 0.003 |
| Patient Previously on a SERM – yes vs.no | 1.01 (0.90–1.14) | 0.814 |
| AIMSS-like Symptoms Prior to Start Date – yes vs. no | 1.01 (0.93–1.11) | 0.789 |
| Medications During AI Use | ||
| Duloxetine: yes vs. no | 1.06 (0.86–1.32) | 0.577 |
| Other SNRI (Venlafaxine): yes vs. no | 0.98 (0.79–1.21) | 0.852 |
| SSRI: yes vs. no | 0.86 (0.81–0.92) | <0.001 |
| NSAIDS: yes vs. no | 1.03 (0.95–1.10) | 0.51 |
| Narcotics: yes vs. no | 1.00 (0.94–1.08) | 0.894 |
| Gabapentin: yes vs. no | 0.86 (0.82–0.91) | <0.001 |
| Measurable Disease at AI Initiation – yes vs. no | 0.90 (0.83–0.98) | 0.019 |
Odds ratio is calculated using generalized estimating equations approach to explore associations between musculoskeletal symptoms and factors of interest.
SERM = selective estrogen receptor modulator; AI = aromatase inhibitor; AIMSS = aromatase inhibitor musculoskeletal syndrome; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor; NSAID = nonsteroidal anti-inflammatory drug.
In our univariate analysis, we also assessed the impact of concomitant medications on the likelihood of developing AI-associated musculoskeletal symptoms (Table 3). Gabapentin and SSRI use were both associated with decreased musculoskeletal side effects (gabapentin: p < .001, OR 0.86, 95% CI 0.82–0.91; SSRI: p < .001, OR 0.86, 95% CI 0.81–0.92). In contrast, there were no significant associations between duloxetine, other SNRI (venlafaxine), NSAID or narcotic use and musculoskeletal side effects. Of note, there was no statistically significant difference in musculoskeletal symptoms related to type of cancer, stage of cancer or number of prior lines of chemotherapy.
Multivariable analysis was next performed using backward elimination to assess the effects of multiple predictors on their joint association with musculoskeletal side effects (Table 4). Candidate variables were those with a p-value <.2 in the univariate analysis and included age at diagnosis, anastrozole use versus exemestane use, letrozole use versus exemestane use, SSRI use, gabapentin use and measurable disease at time of initiation of AI therapy. BMI and prior radiation therapy were no longer statistically significant with multivariable analysis. Controlling for the other variables, exemestane use continued to be associated with a higher odds ratio of musculoskeletal side effects in the multivariable model (p = .031). The presence of measurable disease at the start of AI therapy was also independently associated with a lower odds ratio for musculoskeletal side effects (p = .033, OR 0.91, 95% CI 0.84–0.99). Finally, the findings remained that patients taking gabapentin or SSRIs were less likely to report musculoskeletal side effects (gabapentin: p < .001, OR 0.88, 95% CI 0.831–0.89; SSRIs: p < .001, OR 0.82, 95% CI 0.77–0.89) after adjusting for the other predictors in the model.
Table 4.
Multivariable Model of Musculoskeletal Symptoms.
| Characteristic | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age at Diagnosis, per 10 years | 0.97 (0.94,1.01) | 0.092 |
| Aromatase Inhibitor | 0.031 | |
| Anastrozole vs. Exemestane | 0.85 (0.76, 0.96) | 0.008 |
| Letrozole vs. Exemestane | 0.87 (0.76, 0.99) | 0.036 |
| SSRI Use – yes vs. no | 0.82 (0.77, 0.89) | <0.001 |
| Gabapentin Use – yes vs. no | 0.88 (0.83, 0.94) | <0.001 |
| Measurable Disease at AI Initiation – yes vs. no | 0.91 (0.84, 0.99) | 0.033 |
Odds ratio is calculated using generalized estimating equations approach after backward elimination.
SSRI = selective serotonin reuptake inhibitor; AI = aromatase inhibitor.
Similarly, we assessed for factors associated with hot flashes. Univariate analyses (Table 5) revealed that increased age at diagnosis was the only variable that was associated with decreased rates of hot flashes (p = .027, OR 0.98, 95% CI 0.96–1.00), so multivariable analysis was not performed.
Table 5.
Univariate Model of Factors Associated with Hot Flashes.
| Characteristic | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age at Diagnosis, per 10 years | 0.98 (0.96,1.00) | 0.027 |
| Body Mass Index, kg/m2 | 1.00 (1.00,1.00) | 0.672 |
| Site of Origin – ovarian vs. uterine | 1.02 (0.97,1.08) | 0.390 |
| Histology – carcinoma vs. other | 1.00 (0.94,1.06) | 0.966 |
| Stage | 0.115 | |
| II vs. I | 1.00 (0.92,1.08) | 0.917 |
| III vs. I | 1.07(1.01,1.13) | 0.025 |
| IV vs. I | 1.02 (0.96,1.08) | 0.501 |
| Prior Radiation Therapy – yes vs. no | 0.98 (0.93,1.03) | 0.397 |
| Number of Prior Lines of Chemotherapy | 1.00 (0.99,1.02) | 0.935 |
| Aromatase Inhibitor | 0.843 | |
| Anastrozole vs. Exemestane | 0.93 (0.73,1.19) | 0.560 |
| Letrozole vs. Exemestane | 0.93 (0.73,1.19) | 0.570 |
| Patient Previously on a SERM – yes vs.no | 0.97 (0.91,1.04) | 0.370 |
| AIMSS-like Symptoms Prior to Start Date – yes vs. no | 0.98 (0.94,1.03) | 0.494 |
| Medications During AI Use | ||
| Duloxetine: yes vs. no | 1.10 (0.87,1.40) | 0.423 |
| Other SNRI (Venlafaxine): yes vs. no | 0.96 (0.90,1.03) | 0.280 |
| SSRI: yes vs. no | 1.01 (0.94,1.08) | 0.831 |
| NSAIDS: yes vs. no | 1.01 (0.96,1.07) | 0.719 |
| Narcotics: yes vs. no | 1.00 (0.94,1.06) | 0.965 |
| Gabapentin: yes vs. no | 1.02 (0.93,1.11) | 0.744 |
| Measurable Disease at AI Initiation – yes vs. no | 0.96 (0.90,1.01) | 0.131 |
Odds ratio is calculated using generalized estimation equation approach to explore associations between hot flashes and factors of interest.
SERM = selective estrogen receptor modulator; AI = aromatase inhibitor; AIMSS = aromatase inhibitor musculoskeletal syndrome; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor; NSAID = nonsteroidal anti-inflammatory drug.
4. Discussion
The primary objective of this study was to determine the frequency of AI-associated side effects and therapy discontinuation rates among gynecologic oncology patients. Similar to breast cancer patients taking AI therapy [16], our patients frequently experienced musculoskeletal symptoms including arthralgias, joint pain, hot flashes, fatigue, muscle and joint stiffness and myalgias. Greater than half (54.1%) of our gynecologic cancer patients cited an AIMSS symptom within the first three visits (approximately 4–6 months) after starting an AI. Arthralgias were the most common symptoms, reported by 29.5% of patients within three visits after starting AI therapy. This is similar to reported rates in breast cancer patients on AI therapy of 5.4% to 35.6% [21,22].
Importantly, despite the high frequency of AIMSS symptoms in our gynecologic cancer patients, side effects were rarely the cause of AI discontinuation (5.0% of patients who stopped therapy). We hypothesize that the low rates of discontinuation among gynecologic oncology patients is a result of the severity of their cancer diagnoses. AI therapy was the initially prescribed treatment for only a small percentage of these patients (15.1%, 22 patients). More commonly, AI therapy was used in the recurrent setting, with these patients having received an average of 2.6 lines of chemotherapy prior to starting an AI. Furthermore, 74.0% of patients had measurable disease at the initiation of AI therapy; we found that these patients had a lower odds ratio of musculoskeletal side effects. We postulate that this may be due to patient concerns over limited treatment options and possibly higher rates of baseline discomfort associated with their measurable disease. Gynecologic cancer patients were on AI therapy for an average of 14.7 months before discontinuation. Disease progression was the most common reason for medication discontinuation, which was noted in 87.9% of patients.
Studies have shown that predictors of developing musculoskeletal toxicity in breast cancer patients include younger age, prior taxane-based chemotherapy and the presence of pain at the start of treatment with AI therapy [23]. We found that increasing age trended toward association with a lower odds ratio of musculoskeletal side effects in gynecologic cancer patients. This is consistent with breast cancer literature in which women closer to menopause at the time of AI therapy had more symptoms [24]. In our gynecologic cancer population, we postulate that as women become older, lower basal estrogen levels result in a less dramatic decrease in circulating estrogen levels following the initiation of AI therapy. Our finding that exemestane use is associated with an increased odds of musculoskeletal side effects as compared to astrozole or letrozole therapy aligns with breast cancer trial results reporting that the time to treatment discontinuation from symptoms was shorter in patients taking exemestane than in those taking letrozole [19].
Symptoms typically do not respond well to conventional analgesics [25]. Prior studies have shown a number of approaches to manage AI-associated side effects, including acupuncture [12], aerobic exercise [26], omega fatty acids [27] and the SNRI duloxetine [28]. A recent study found that obese patients with AIMSS obtained improved analgesic benefit from duloxetine when compared to non-obese patients [29]. In our study, patients taking SSRIs or gabapentin had statistically significant decreased rates of musculoskeletal symptoms on multivariate analysis. To the best of our knowledge, there have been no prior studies looking at the impact of SSRI or gabapentin use on AIMSS. We believe our findings warrant further study, including additional analysis of comorbidities leading to SSRI and gabapentin use and the interplay between those factors and subsequent AIMSS symptom reporting.
Our study has several limitations. It is a retrospective study with a relatively small sample size performed at a single institution tertiary referral center, which may limit generalizability. There may also be bias in the symptoms elicited and recorded in the medical record during therapy, as patients are more likely to endorse symptoms if they are asked directly about certain side effects. Additionally, studies have shown that the documentation of side effects when reviewed retrospectively tends to underestimate those side effects [30,31]. However, this bias would result in a frequency of AIMSS that is potentially higher than that reported herein, which would strengthen rather than lessen our finding that despite high AI side effect rates in gynecologic cancer patients, discontinuation rates are low. Furthermore, there is likely bias in the patients for whom AI therapy is recommended by their provider, including factors such as patient performance status and patient perspective on quality of life factors. Less than 5% of patients were prescribed exemestane, limiting potential conclusions about the side effect profile of this AI as compared to others. To begin to address these limitations, we believe that additional prospective study of the efficacy of potential interventions to reduce AIMSS in gynecologic cancer patients is warranted. Indeed, our study provides important baseline, retrospective side effect rates to aid in study design. Furthermore, we advocate for—and are now implementing in our clinic setting—the prospective collection of patient-reported outcomes (PROs) of AIMSS. Validated instruments for assessing these PROs include the Brief Pain Inventory (BPI) and the Breast Cancer Prevention Trial Symptom Scale-Musculoskeletal (BCPT-MS) subscale [32,33].
Despite the above study limitations, there are also several strengths to our study and analysis. The heterogeneity of the patient population with respect to tumor type, initial stage and grade, allows for broad assessment of AI-associated side effects across tumor types. We also reviewed multiple visits for each patient, allowing assessment of symptoms and potentially associated factors at the initiation of AI therapy, during AI therapy and at its discontinuation.
In conclusion, patients on AI therapy for treatment of gynecologic cancer have similar rates of side effects compared to breast cancer patients on AI treatment, but have significantly lower rates of discontinuation due to the side effects. Gabapentin and SSRIs were found to be statistically significant in reducing rates of side effects among gynecologic oncology patients on AI therapy. These findings highlight the importance of assessing patient-reported symptoms on AI therapy and the importance of future work to investigate and adopt strategies to mitigate these symptoms.
HIGHLIGHTS.
Over half of gynecologic cancer patients reported aromatase inhibitor side effects, and almost one-third had arthralgias
Only 5% of patients discontinued aromatase inhibitor therapy due to side effects
Patients with measurable cancer burden less frequently reported aromatase inhibitor musculoskeletal side effects
Gabapentin or SSRI use was associated with decreased musculoskeletal side effects during aromatase inhibitor therapy
Acknowledgements
The authors would like to thank the Michigan Medicine Gynecologic Oncology Workgroup for their thoughtful input on this project. The authors thank Sarah Block for her assistance with manuscript preparation.
Financial support
Funding for this work has been provided in part by the Department of Defense Ovarian Cancer Academy Early-Career Investigator Award to KM (DOD W81XWH-15-1-0194).
Footnotes
Declaration of Competing Interest
The authors report no conflicts of interest.
References
- [1].Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, et al. , Adjuvant exemestane with ovarian suppression in premenopausal breast cancer, N. Engl. J. Med. 371 (2014) 107–118, 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. , Tailoring adjuvant endocrine therapy for premenopausal breast Cancer, N. Engl. J. Med. 379 (2018) 122–137, 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. , Adjuvant endocrine therapy for women with hormone receptor—positive breast Cancer: American Society of Clinical Oncology clinical practice guideline focused update, J. Clin. Oncol. 32 (2014) 2255–2269, 10.1200/jco.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weelden WJ, Massuger LFAG, Pijnenborg JMA, Romano A, Anti-estrogen treatment in endometrial Cancer: a systematic review, Front. Oncol. 9 (2019) 359, 10.3389/fonc.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li YF, Hu W, Fu SQ, Li JD, Liu JH, Kavanagh JJ, Aromatase inhibitors in ovarian cancer: is there a role? Int. J. Gynecol. Cancer 18 (2008) 600–614, 10.1111/j.1525-1438.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- [6].Sieh W, Köbel M, Longacre TA, Bowtell DD, de Fazio A, Goodman MT, et al. , Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study, Lancet Oncol 14 (2013) 853–862, 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stasenko M, Plegue M, Sciallis AP, McLean K, Clinical response to antiestrogen therapy in platinum-resistant ovarian cancer patients and the role of tumor estrogen receptor expression status, Int. J. Gynecol. Cancer 25 (2015) 222–228, 10.1097/IGC.0000000000000334. [DOI] [PubMed] [Google Scholar]
- [8].Andersen CL, Sikora MJ, Boisen MM, Ma T, Christie A, Tseng G, et al. , Active estrogen receptor-alpha signaling in ovarian cancer models and clinical specimens, Clin. Cancer Res. 23 (2017) 3802–3812, 10.1158/1078-0432.CCR-16-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wiseman LR, Adkins JC, Anastrozole, Drugs Aging 13 (1998) 321–332, 10.2165/00002512-199813040-00008. [DOI] [PubMed] [Google Scholar]
- [10].Buzdar AU, Robertson JFR, Eiermann W, Nabholtz J-M, An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane, Cancer 95 (2002) 2006–2016, 10.1002/cncr.10908. [DOI] [PubMed] [Google Scholar]
- [11].Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N, Side effects of aromatase inhibitors versus tamoxifen: the patients' perspective, Am. J. Surg. 192 (2006) 496–498, 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- [12].Bae K, Yoo H-S, Lamoury G, Boyle F, Rosenthal DS, Oh B, Acupuncture for aromatase inhibitor—induced arthralgia: a systematic review, Integr. Cancer Ther. 14 (2015) 496–502, 10.1177/1534735415596573. [DOI] [PubMed] [Google Scholar]
- [13].Henry NL, Giles JT, Stearns V, Aromatase Inhibitor—Associated Musculoskeletal Symptoms: Etiology and Strategies for Management, Breast Cancer 22 (2008). [PubMed] [Google Scholar]
- [14].Gaillard S, Stearns V, Aromatase inhibitor-associated bone and musculoskeletal effects: new evidence defining etiology and strategies for management, Breast Cancer Res. 13 (2011) 205, 10.1186/bcr2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beckwée D, Leysen L, Meuwis K, Adriaenssens N, Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis, Support Care Cancer 25 (2017) 1673–1686, 10.1007/s00520-017-3613-z. [DOI] [PubMed] [Google Scholar]
- [16].Singer O, Cigler T, Moore AB, Levine AB, Hentel K, Belfi L, et al. , Defining the aromatase inhibitor musculoskeletal syndrome: a prospective study, Arthritis Care Res. 64 (2012) 1910–1918, 10.1002/acr.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, et al. , Treatment adherence and its impact on disease-free survival in the breast international group 1–98 trial of Tamoxifen and Letrozole, Alone and in Sequence, J. Clin. Oncol 34 (2016) 2452–2459, 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW, Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review, Breast Cancer Res. Treat. 134 (2012) 459–478, 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. , Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer, J. Clin. Oncol. 30 (2012) 936–942, 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hanauer DA, Mei Q, Law J, Khanna R, Zheng K, Supporting information retrieval from electronic health records: a report of University of Michigan’s nine-year experience in developing and using the electronic medical record search engine (EMERSE), J. Biomed. Inform. 55 (2015) 290–300, 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. , Results of the ATAC (Arimidex, Tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer, Lancet 365 (2005) 60–62, 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- [22].Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. , Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer, J. Clin. Oncol. 25 (2007) 3877–3883, 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- [23].Henry NL, Speth K, Lintermans A, Kidwell KM, Carlson R, Hayes DF, et al. , Associations Between Patient and Anthropometric Characteristics and Aromatase Inhibitor Discontinuation, Clin. Breast Cancer 17 (2017) 350–355, 10.1016/j.clbc.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. , Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors, Cancer 115 (2009) 3631–3639, 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hashem MG, Cleary K, Fishman D, Nichols L, Khalid M, Effect of concurrent prescription antiarthralgia pharmacotherapy on persistence to aromatase inhibitors in treatment-naive postmenopausal females, Ann. Pharmacother. 47 (2013) 29–34, 10.1345/aph.1R369. [DOI] [PubMed] [Google Scholar]
- [26].Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, et al. , Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors, J. Clin. Oncol. 33 (2015) 1104–1111, 10.1200/JCO.2014.57.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shen S, Unger JM, Crew KD, Till C, Greenlee H, Gralow J, et al. , Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927), Breast Cancer Res. Treat. 172 (2018) 603–610, 10.1007/s10549-018-4946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. , Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated Arthralgias in early-stage breast Cancer: SWOG S1202, J. Clin. Oncol. 36 (2018) 326–332, 10.1200/JCO.2017.74.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Henry NL, Lynn Henry N, Unger JM, Till C, Schott AF, Crew KD, et al. , Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202, Cancer (2019) 10.1002/cncr.32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, et al. , The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review, Support Care Cancer 24 (2016) 3669–3676, 10.1007/s00520-016-3297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tofthagen C, Threats to validity in retrospective studies, J Adv Pract Oncol 3 (2012) 181–183. [PMC free article] [PubMed] [Google Scholar]
- [32].Bennett MI, The brief pain inventory: revealing the effect of cancer pain, Lancet Oncol 10 (2009) 1020, 10.1016/S1470-2045(09)70114-7. [DOI] [PubMed] [Google Scholar]
- [33].Swenson KK, Nissen MJ, Henly SJ, Maybon L, Pupkes J, Zwicky K, et al. , Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors, Oncol. Nurs. Forum 40 (2013) 549–557, 10.1188/13.ONF.549-557. [DOI] [PubMed] [Google Scholar]


