Abstract
Background:
Blue light photodynamic therapy (PDT) is effective for actinic keratosis, but many patients experience stinging pain during illumination.
Objective:
To compare a conventional regimen (1 hour of 5-aminolevulinic acid [ALA] preincubation, followed by blue light) versus a new modified regimen in which blue light is started immediately after ALA application.
Methods:
A clinical trial with a bilaterally controlled, intrapatient study design was conducted with 23 patients. Topical 20% ALA was applied to the entire face and/or scalp. On 1 side of the body, blue light was started immediately and continued for either 30, 45, or 60 minutes (simultaneous PDT). On the contralateral side, the blue light began 1 hour after ALA application and lasted 1000 seconds (conventional PDT). Pain was evaluated on a scale from 0 to 10. Actinic keratosis lesion counts were determined by clinical examination and photography.
Results:
All patients experienced significantly less pain during simultaneous illumination than during the conventional regimen. At 3 months after treatment, lesion clearance was nearly identical on the 2 sides, as determined by statistical testing of noninferiority ± 15% margin.
Limitations:
Although bilaterally controlled, the study was relatively small. Additional studies are recommended.
Conclusion:
The modified PDT regimen is essentially painless, yet it provides treatment efficacy similar to a conventional regimen. (J Am Acad Dermatol https://doi.org/10.1016/j.jaad.2019.09.010.)
Keywords: clinical research, oncology, phototherapy, skin cancer, therapeutics
Photodynamic therapy (PDT) is a field treatment that uses a photosensitizing drug and visible light to treat skin cancers and precancers. PDT is approved by the US Food and Drug Administration for actinic keratoses (AKs)1 and in Europe for basal cell carcinoma and squamous cell carcinoma.2 PDT is performed by applying topical 5-aminolevulinic acid (ALA) or its methyl ester to lesional skin; these prodrugs are then preferentially taken up by neoplastic cells and converted into protoporphyrin IX (PpIX) within mitochondria.3 Exposure to visible light activates PpIX, damaging the mitochondria and triggering the cell.4 PDT has important clinical advantages, including the ability to target multiple AK lesions simultaneously and eliminate them without scarring. 1,2,4 However, one downside of PDT is that patients often experience stinging pain during illumination. 5,6 PDT-induced pain, experienced with either blue light or red light, is not alleviated by any topical anesthetic agent (other than ice and evaporative cooling), and it can be so severe that patients refuse to complete the treatment or decline to undergo any future PDT.5,6 Therefore PDT-related pain represents a significant barrier to achieving optimal therapeutic outcomes.
PDT-associated pain is positively correlated with the length of time that ALA resides on the skin (ALA incubation time). The original protocol for blue light PDT approved by the US Food and Drug Administration specified 14 to 18 hours of ALA incubation before initiation of blue light exposure.1 Over the past 2 decades, practicing physicians have gradually reduced the ALA incubation period and found that good treatment efficacy is still achievable using shorter incubations.7,8 For example, a recent PDT clinical trial showed similar AK lesion clearance rates when using ALA incubation times of 1, 2, or 3 hours.8 The kinetics of PpIX synthesis within AK lesions is linear, and PpIX accumulation is first detectable within minutes after ALA application,9 suggesting that it might be possible to shorten ALA incubation times even further. Intriguingly, daylight PDT, a technique in which methyl ester ALA cream is applied to lesions and is followed within 30 minutes by exposure to sunlight for approximately 2 hours, yields therapeutic results similar to PDT with artificial blue or red lights yet reportedly causes little to no pain during sunlight exposure.10–12 Based on these observations and other considerations (see Discussion), we designed a clinical trial to ask whether topical ALA application followed immediately by exposure to blue light might provide good clinical efficacy for AK lesions of the face and scalp while minimizing the pain experienced during illumination.
METHODS
Study design
This prospective, randomized clinical trial was conducted at a single academic center. The study was bilaterally controlled, using a contralateral split-body design. Participants were recruited from outpatient clinics of the Dermatology Department, Cleveland Clinic, and gave informed consent before enrollment. The study was approved by the Cleveland Clinic’s institutional review board and registered with ClinicalTrials.gov (NCT02124733).
Study population
Eligible patients had at least 8 nonhypertrophic AKs, with at least 4 lesions on each side of the face or scalp. Candidates using other treatments for AK were excluded. Patients taking doxycycline or topical retinoids were asked to stop at least 2 weeks before PDT.
Randomization
In this bilateral study, the left and right halves of the body were compared. Side A, receiving the experimental (simultaneous) blue light regimen, was chosen via a computer-generated block randomization scheme to ensure an equal left/right distribution. The contralateral side was designated as side B (the control, or conventional PDT, side).
Interventions
On day 1, ALA 20% solution (Levulan Kerastick; Sun/DUSA Pharmaceuticals, Wilmington, MA) was applied to the entire face and scalp. Side B was shielded with a dark cloth, and side A was immediately exposed to blue light (Blu-U, Sun/DUSA Pharmaceuticals, 10 mW/cm2). The light dose for side A was given per a dose escalation scheme, wherein the first patient cohort received 30 minutes, the second cohort received 45 minutes, and the third cohort received 60 minutes of blue light. Beginning 60 minutes after ALA application, side A was shielded and side B was illuminated for 1000 seconds (16 minutes 40 seconds). After this light exposure, a soothing emollient (Aquaphor; Beiersdorf, Hamburg, Germany) was applied to the treated areas, and the patient was sent home wearing a wide-brim hat and instructions to avoid sunlight for 48 hours.
Outcome measures
The primary endpoint was therapeutic efficacy (AK lesion clearance), defined as reduction in the number of AK lesions between the baseline and month 3 visits. At each visit, nonhypertrophic AK lesions (Olsen grade 1 or 2)13 were identified by a study physician using clinical criteria (scale, erythema, roughness to palpation), and each lesion marked with a black pen. Marked lesions were digitally photographed for a permanent record. From the photographs, AK lesions were counted within site-specific regions (face and scalp; right and left of midline) by 2 investigators unaware of the treatment assignment. For balding patients, the location of the original hairline was drawn onto photographs to facilitate accurate counting of lesions on the face versus scalp.
Several secondary endpoints were measured. For pain assessment, patients were asked every 5 to 10 minutes during illumination to report their pain on a 0-to-10 visual analog scale (VAS). Erythema was recorded photographically at day 1 and on day 4 after PDT. An adverse effect profile was recorded by each patient in a daily home questionnaire to document the presence or absence of 11 different signs and symptoms in the 4 days immediately after PDT.
Statistical analysis
The primary endpoint was AK lesion reduction. For each patient, we first computed the lesion reduction rates on the 2 sides treated by the experimental and conventional regimens, respectively. We then computed the difference of the 2 reduction rates. The t test was used to compare the reduction rate difference against a noninferiority margin of 15%, that is, the intervention regimen was considered noninferior to the conventional regimen if its reduction rate was not 15% lower than the conventional regimen. Subgroup analyses were performed for each experimental cohort (30 minutes, 45 minutes, or 60 minutes), and the same analyses were applied to the scalp data. The secondary endpoint of the posttreatment pain scale was analyzed with a linear mixed-effect model because the VAS scale was assessed up to 5 times on the side receiving the experimental regimen and up to 3 times on the side receiving the conventional regimen. The model included a random effect to account for the repeated measurements and the paired design. Adverse events were summarized as descriptive statistics. Statistical significance was considered at a P value of less than .05. All analyses were performed with R 3.5.1 (R Core Team, Vienna, Austria; cran.r-project.org).
Sample size justifications.
The study was powered to evaluate the primary endpoint of AK lesion clearance. We estimated the treatment effect size (AK lesion clearance) and variability from a previous study.14 If the difference of lesion clearance between the 2 sides is 0% and the standard deviation for the difference is 24%, the sample size of 23 patients has a power of 87% for a noninferiority margin of 15% when the 1-sided alpha value is equal to 0.05. The power is 80% if the clearance rate on side A is even 2% higher than on side B.
RESULTS
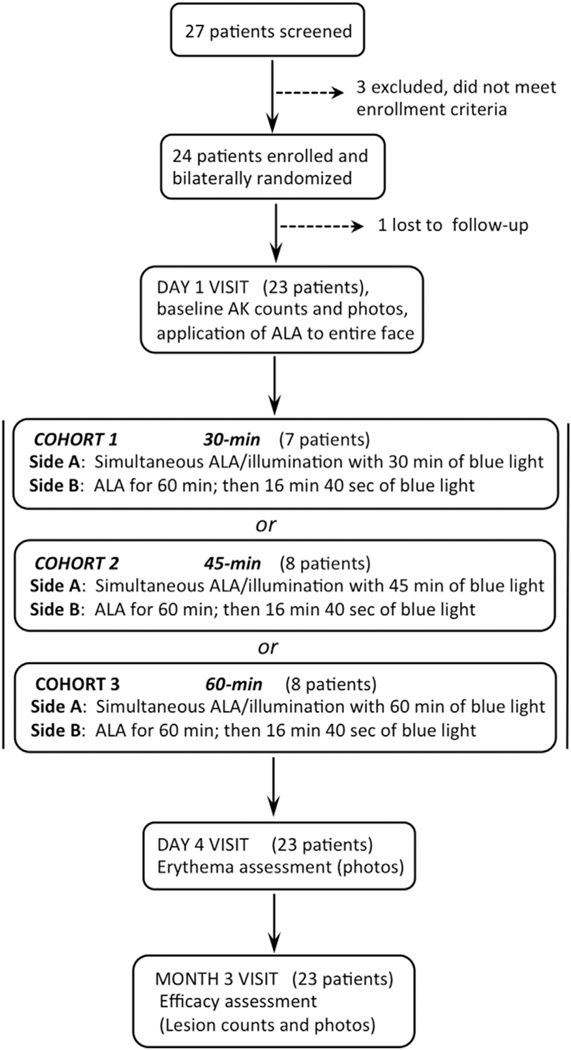
Twenty-seven patients were screened between May 2014 and October 2016. Of these, 3 did not meet enrollment criteria and 1 was lost to follow-up (Fig 1). Amongst the remaining 23 patients, 21 were elderly white men (mean age, 69.7 years; range, 58–88 years) and 2 were women (ages 50 and 73 years). Overall, this population had abundant AK lesions, with total counts of 23 to 70 on the face and 0 to 123 on the scalp. Only 7 patients had no AK lesions on the scalp.
Fig 1.
Study design and patient enrollment. AK, Actinic keratosis; ALA, 5-aminolevuline acid.
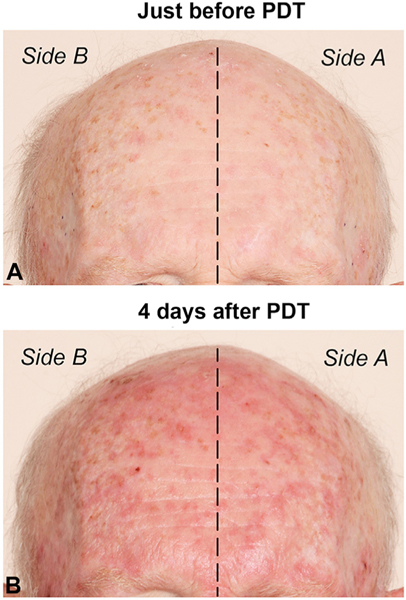
At the outset, the consequences of a long blue light exposure to the face were unknown, so a light-dose escalation scheme was used to ensure patient safety (Fig 1). For the first patient cohort, the duration of simultaneous blue light was limited to 30 minutes. The adverse event profile among this group was acceptable, so subsequent cohorts received 45 minutes (8 patients) or 60 minutes (8 patients) on side A. All patients received a conventional dose of 1000 seconds of blue light to side B. Erythema, an early indicator of PDT response, was typically very mild at the conclusion of treatment but increased markedly over the next 1 to 4 days. As illustrated in Fig 2, spotty erythema (corresponding to inflamed AK lesions) appeared to be approximately equal on side A and side B; this was true for the majority of patients.
Fig 2.
An example of AK lesions on the forehead, (A) at baseline (just before PDT) and (B) at 4 days after PDT. Note that the extent of post-PDT erythema appears to be very similar on sides A and B. PDT, Photodynamic therapy.
The primary endpoint, clinical efficacy, was defined as percent reduction in AK lesion counts at 3 months. Data in Table I and Supplemental Table I (available at https://doi.org/10.17632/rp7wn5kpzy.1) show that clinical efficacy was nearly identical on both sides of the face and scalp. This was confirmed statistically by a test of noninferiority for both face and scalp, indicating that simultaneous PDT and conventional PDT provide essentially identical treatment efficacy. In addition, there was no statistical difference in efficacy between the 3 simultaneous PDT cohorts (30, 45, and 60 minutes) for either face or scalp (Table I).
Table I.
Clinical response* of AK lesions at 3 months after simultaneous or conventional PDT
| Group | Simultaneous, % decrease† | Conventional, % decrease‡ | Difference, %§ | P value¶ |
|---|---|---|---|---|
| Lesions on face | ||||
| All patients | 57.7 | 59.1 | −1.4 (−13.8 to 10.9) | .016 |
| 30-minute cohort| | 55.4 | 57.1 | −1.7 (−25.1 to 21.7) | .133 |
| 45-minute cohort| | 59.8 | 58.6 | 1.1 (−20.8 to 23.0) | .074 |
| 60-minute cohort| | 57.5 | 61.2 | −3.8 (−25.6 to 18.1) | .157 |
| Lesions on scalp | ||||
| All patients | 43.8 | 41.9 | 1.9 (−6.9 to 10.8) | <.001 |
| 30-minute cohort| | 44.8 | 46.0 | −1.2 (−18 to 15.6) | .054 |
| 45-minute cohort| | 41.2 | 38.2 | 3 (−13.8 to 19.8) | .018 |
| 60-minute cohort| | 45.2 | 41.5 | 3.7 (−11.7 to 19.0) | .009 |
AK, Actinic keratosis; PDT, photodynamic therapy.
Clinical response is defined as the percent decrease in AK lesion counts at 3 months (visit 2) relative to baseline (visit 1).
Simultaneous indicates the side of the body receiving simultaneous ALA incubation and blue light exposure (side A).
Conventional indicates the side of the body receiving 1 hour of ALA incubation followed by blue light (side B).
Difference between the 2 preceding columns, with confidence intervals shown in parentheses.
One-sided P values from noninferiority tests, using a margin of 15%. The test is significant for the hypothesis that simultaneous PDT is noninferior to conventional PDT.
Note that lesion reductions after simultaneous PDT do not differ among the 3 incubation time cohorts. When testing the hypothesis that the 3 cohorts are different, the test fails for the face (P = .95) and for the scalp (P = .91), respectively.
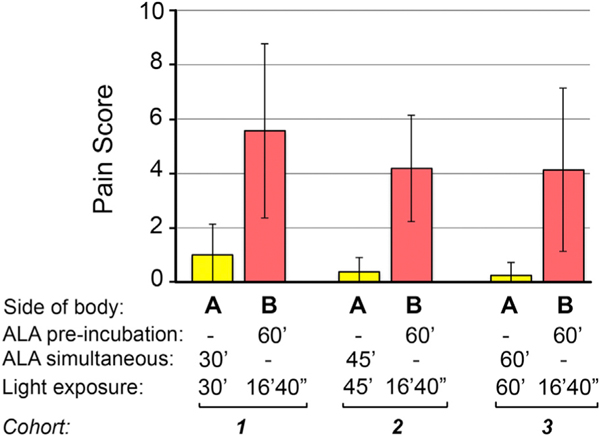
Pain during blue light illumination was a secondary endpoint. When VAS pain scores (reported every 5 to 10 minutes by the patients, as listed in Supplemental Table II, available at https://doi.org/10.17632/rp7wn5kpzy.1) were averaged, the mean scores were 0.52 (95% confidence interval = 0–1.09) on side A versus 3.57 (95% CI = 2.97–4.16) on side B. This difference was statistically significant, with a P value of less than .001. As additional documentation, maximum pain scores are reported in Fig 3, which further illustrates the vast reduction in pain experienced with simultaneous PDT versus the conventional regimen.
Fig 3.
Maximum pain scores reported by patients during PDT on the side receiving simultaneous PDT (side A) and on the side receiving conventional PDT (side B). Bars represent mean ± standard error of the mean from patients in each of the 3 cohorts. ALA, 5-aminolevulinic acid; PDT, photodynamic therapy.
We also examined the inflammatory adverse effects that patients experienced during the first few days after PDT. Supplemental Table III (available at https://doi.org/10.17632/rp7wn5kpzy.1) shows the aggregate results of the daily questionnaire completed by every patient during the first 4 days after treatment. Post-PDT inflammatory responses developed gradually, as previously reported for conventional PDT.14 Maximum values for burning, itching, redness, stinging, and swelling were observed by day 2 after PDT. At days 3 and 4, those characteristics began to decrease and were supplanted by increased crusting and peeling, reflecting the normal course of resolution after PDT. No noticeable differences among the major inflammatory parameters were observed when comparing the 2 treated sides. These results confirm that simultaneous PDT, although initially much less painful than the conventional regimen, eventually produces a similar inflammatory response and similar AK lesion clearance. The mechanism for this remains to be determined (see Discussion).
DISCUSSION
Here, we report results from a clinical trial designed to ask whether blue light PDT, after a simple timing modification, can clear AK lesions while ameliorating the stinging pain often felt by patients during conventional blue light therapy. In our study, the ALA preincubation (typically 1–3 hours, an effective regimen8) was eliminated, and instead, blue light exposure was started immediately after topical ALA application. The trial was bilaterally controlled so that each patient served as his/her own control. Results showed that pain was drastically reduced on the side receiving the simultaneous PDT regimen. Equally important, therapeutic response (lesion clearance) was statistically indistinguishable between the sides receiving simultaneous versus conventional PDT.
The hypothesis that ALA-PDT can work without any ALA preincubation seems counterintuitive. Nevertheless, we pursued the idea based on several available lines of evidence. First, investigators in Europe had reported that PDT with continuous exposure to relatively low-intensity sunlight (daylight PDT) causes very little pain yet substantially reduces AK lesions on the face and scalp.10–12 In the United States, a split-face study in 3 patients in 2016 showed that 15-minute ALA preincubation followed by 60 minutes of blue light was relatively painless and reduced AK lesion counts by 52%,15 and a case report in 2017 reported that immediate blue light PDT illumination was painless and reduced the patient’s AK lesions.16 One proposed explanation for reduced pain is that when illumination is started immediately, photons continuously degrade PpIX within lesions (photobleaching) and, therefore, PpIX never builds up to the high levels that drive its diffusion into neighboring nerves.17 In terms of efficacy, basic science experiments showed that PDT with continuous ALA infusion and low-intensity illumination over many days (so-called metronomic PDT) can shrink malignant brain tumors in rats.18 This appears similar to the situation in daylight PDT, where PpIX at very low concentrations is continuously activated with relatively low-intensity light (“Go low, go slow”). To ask whether unintentional daylight PDT may be a factor in patients having routine blue light PDT, we performed a pilot study in which 9 patients with facial AK received topical 5-ALA application but subsequently did not have blue light exposure (Supplemental Table IV, available at https://doi.org/10.17632/rp7wn5kpzy.1). When the patients returned at 3 months, AK lesion counts had declined in 8 of 9 patients (with a 9% average decrease for the entire cohort), suggesting that ambient light exposure can indeed exert metronomic PDT effects, although the effect varies widely among patients. Possible mechanisms by which simultaneous ALA-PDT leads to clearance of neoplastic cells, such as direct cellular damage or induction of a local immune response, are currently being investigated in animal models.19,20
Our findings have important clinical implications. The ability to perform PDT painlessly, while achieving good lesion clearance, should help optimize therapeutic responses in several ways. First, a single PDT treatment is seldom sufficient for complete AK clearance; thus, clinical trials to maximize efficacy typically use 2 treatments spaced 8 weeks apart,1,8 and European PDT protocols generally recommend 2 treatments.21 Our simultaneous regimen may help convince patients to have multiple PDT sessions because pain is no longer an issue. Another potential benefit is that combination agents can be added without fear of creating intolerable adverse effects. For example, a 1-week pretreatment with daily 5-fluorouracil cream before ALA-PDT nearly doubles lesion clearance rates, without any notable increase in adverse effects.14 Another approach is to apply topical calcipotriol before PDT; however, this option is off label in the United States.22 The simultaneous PDT regimen significantly reduces office wait times. Instead of several hours for a traditional PDT visit, a simultaneous blue light session may take only 45 to 60 minutes even when allowing for careful explanation of after-care instructions, as we have described elsewhere.23
Our study has some limitations. Although relatively small (23 patients), the trial was still able to reach statistical significance because of the bilateral design that allowed intrapatient paired comparisons. A bilateral study design might present problems for interpretation if a systemic immunologic effect, rather than local effects, were the main driver for PDT-induced lesion resolution. The former possibility seems less likely now because we have treated more than 100 additional patients using a simultaneous 30-minute PDT regimen and continue to see lesion clearance rates very similar to those reported here (data not shown).
CONCLUSIONS
ALA-PDT of extensive AK lesions of the face and scalp, using a new simultaneous incubation-illumination regimen, causes minimal pain yet provides clinical efficacy comparable to traditional PDT regimens. This new simultaneous approach should significantly improve patient care by reducing pain, discomfort, and patient anxiety. Additionally, the regimen should decrease the length of time that patients spend in the doctor’s office.
Supplementary Material
CAPSULE SUMMARY.
Conventional blue light photodynamic therapy (PDT) for actinic keratoses (AKs) of the face and scalp can be very painful for patients.
This study defines a simple modification of the PDT regimen that nearly eliminates pain during illumination yet provides the same clinical benefit in terms of AK lesion clearance.
Acknowledgments
The authors would like to thank Tayyaba Hasan, PhD, for her generous conceptual and financial support over the years and Stuart Marcus, MD, PhD, for his valuable perspectives on blue light PDT.
Funding sources: Supported by the Cleveland Clinic Research Protocol Committee fund (to Dr Maytin), Sun/DUSA Pharmaceuticals, investigator-initiated grant (to Dr Maytin), and grant number P01CA084203 (Drs Hasan and Maytin) from the National Cancer Institute of the National Institutes of Health, USA.
Abbreviations used:
- AK
actinic keratosis
- ALA
5-aminolevulinic acid
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
Footnotes
Conflicts of interest: None disclosed.
REFERENCES
- 1.Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140:41–46. [DOI] [PubMed] [Google Scholar]
- 2.Christensen E, Warloe T, Kroon S, et al. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J Eur Acad Dermatol Venereol. 2010;24:505–512. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275–292. [DOI] [PubMed] [Google Scholar]
- 4.Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. Bio-modulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012;326:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren CB, Karai LJ, Vidimos A, Maytin EV. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol. 2009;61:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang JM, Riaz IB, Kamal MU, Paragh G, Zeitouni NC. Photodynamic therapy and pain: a systematic review. Photodiagnosis Photodyn Ther. 2017;19:308–344. [DOI] [PubMed] [Google Scholar]
- 7.Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140:33–40. [DOI] [PubMed] [Google Scholar]
- 8.Pariser DM, Houlihan A, Ferdon MB, Berg JE, Group P-AI. Randomized vehicle-controlled study of short drug incubation aminolevulinic acid photodynamic therapy for actinic keratoses of the face or scalp. Dermatol Surg. 2016;42:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren CB, Lohser S, Wene LC, Pogue BW, Bailin PL, Maytin EV. Noninvasive fluorescence monitoring of protoporphyrin IX production and clinical outcomes in actinic keratoses following short-contact application of 5-aminolevulinate. J Biomed Opt. 2010;15:051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol. 2008;158:740–746. [DOI] [PubMed] [Google Scholar]
- 11.Wiegell SR, Fabricius S, Stender IM, et al. A randomized, multicentre study of directed daylight exposure times of 1(1/2) vs. 2(1/2) h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol. 2011;164:1083–1090. [DOI] [PubMed] [Google Scholar]
- 12.Wiegell SR, Wulf HC, Szeimies RM, et al. Daylight photodynamic therapy for actinic keratosis: an international consensus: International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:673–679. [DOI] [PubMed] [Google Scholar]
- 13.Olsen EA, Abernethy ML, Kulp-Shorten C, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol. 1991;24:738–743. [DOI] [PubMed] [Google Scholar]
- 14.Maytin EV, Anand S, Riha M, et al. 5-Fluorouracil enhances protoporphyrin IX accumulation and lesion clearance during photodynamic therapy of actinic keratoses: a mechanism-based clinical trial. Clin Cancer Res. 2018;24:3026–3035. [DOI] [PubMed] [Google Scholar]
- 15.Martin GM. In-office painless aminolevulinic acid photodynamic therapy: a proof of concept study and clinical experience in more than 100 patients. J Clin Aesthet Dermatol. 2016; 9:19–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Gandy J, Labadie B, Bierman D, Zachary C. Photodynamic therapy effectively treats actinic keratoses without pre-illumination incubation time. J Drugs Dermatol. 2017;16:275–278. [PMC free article] [PubMed] [Google Scholar]
- 17.Babes A, Sauer SK, Moparthi L, et al. Photosensitization in porphyrias and photodynamic therapy involves TRPA1 and TRPV1. J Neurosci. 2016;36:5264–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisland SK, Lilge L, Lin A, Rusnov R, Wilson BC. Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem Photobiol. 2004;80:22–30. [DOI] [PubMed] [Google Scholar]
- 19.de Souza ALR, LaRochelle E, Marra K, et al. Assessing daylight & low-dose rate photodynamic therapy efficacy, using biomarkers of photophysical, biochemical and biological damage metrics in situ. Photodiagnosis Photodyn Ther. 2017;20:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra K, LaRochelle EP, Chapman MS, et al. Comparison of blue and white lamp light with sunlight for daylight-mediated, 5-ALA photodynamic therapy, in vivo. Photochem Photobiol. 2018;94:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braathen LR, Szeimies RM, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology, 2005. J Am Acad Dermatol. 2007;56:125–143. [DOI] [PubMed] [Google Scholar]
- 22.Torezan L, Grinblat B, Haedersdal M, Valente N, Festa-Neto C, Szeimies RM. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol. 2018;179:829–835. [DOI] [PubMed] [Google Scholar]
- 23.Maytin EV, Warren CB. Photodynamic therapy. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. Available at: http://www.uptodate.com/contents/photodynamic-therapy. Accessed October 8, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.