Abstract
Simple Summary
Prostate carcinoma is the most common visceral cancer for men and the second most common cause of death. The early detection of micrometastasis may improve clinical outcome due to individual treatment approaches like early intensified therapy. Imaging using prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT) has a high potential of detecting even small metastases. Therefore, the present study aimed to analyze data of 335 men with primary diagnosed prostate cancer and available PSMA-PET/CT with regard to characteristic PET-parameters and the detection of metastases. We observed that an increased accumulation of the PET-tracer measured in the primary tumor significantly correlates with the presence of distant metastases. The current results may be helpful in decision making of individual treatment escalation for a variety of men with aggressive disease which should improve clinical outcome.
Abstract
Men diagnosed with aggressive prostate cancer are at high risk of local relapse or systemic progression after definitive treatment. Treatment intensification is highly needed for that patient cohort; however, no relevant stratification tool has been implemented into the clinical work routine so far. Therefore, the aim of the current study was to analyze the role of initial PSMA-PET/CT as a prediction tool for metastases. In total, 335 men with biopsy-proven prostate carcinoma and PSMA-PET/CT for primary staging were enrolled in the present, retrospective study. The number and site of metastases were analyzed and correlated with the maximum standardized uptake value (SUVmax) of the intraprostatic, malignant lesion. Receiver operating characteristic (ROC) curves were used to determine sensitivity and specificity and a model was created using multiple logistic regression. PSMA-PET/CT detected 171 metastases with PSMA-uptake in 82 patients. A statistically significant higher SUVmax was found for men with metastatic disease than for the cohort without distant metastases (median 16.1 vs. 11.2; p < 0.001). The area under the curve (AUC) in regard to predicting the presence of any metastases was 0.65. Choosing a cut-off value of 11.9 for SUVmax, a sensitivity and specificity (factor 1:1) of 76.0% and 58.4% was obtained. The current study confirms, that initial PSMA-PET/CT is able to detect a relatively high number of treatment-naïve men with metastatic prostate carcinoma. Intraprostatic SUVmax seems to be a promising parameter for the prediction of distant disease and could be used for treatment stratification—aspects which should be verified within prospective trials.
Keywords: prostate cancer, PSMA, PET, metastases, intraprostatic SUV
1. Introduction
With an estimated 1.93 million deaths from cancer in Europe in 2018 [1], the treatment of patients with tumor diseases is still demanding. For men, carcinoma of the prostate is the most common visceral cancer with estimated 449,800 new cases in 2018 [1]. Although many cases of prostate cancer have excellent prognosis or stay indolent for a lifetime, the malignant tumor remains a heterogeneous disease varying from subclinical to highly aggressive.
Therefore risk-adopted diagnosis and treatment of prostate cancer is challenging to avoid overtreatment for indolent disease and insufficient therapy for carcinomas with a high risk for local or systemic progression. By current estimates, high-risk disease is diagnosed in up to 20% of patients undergoing definitive treatment [2,3,4,5]. Active and highly effective therapy is of utmost necessity for this patient cohort. However, for high-risk prostate cancer local progression and/or distant metastases were observed in 11–40% after definitive radiotherapy or radical prostatectomy, respectively [6,7,8]. These patients are candidates for treatment intensification to prolong survival.
The RTOG 0521 trial included 612 men with high risk prostate cancer undergoing definitive radiotherapy with long-term androgen deprivation therapy (ADT). The trial resulted in improved overall survival (OS), disease-free survival (DFS) and reduction in the rate of distant metastasis for the cohort with intensified systemic therapy with adjuvant docetaxel after a median follow-up of 5.7 years [8]. In contrast, two Scandinavian trials did not observe any relevant, clinical benefit by adding chemotherapy. Both, the Scandinavian Prostate Cancer Group (SPCG)-12 and -13, were not able to show a significant benefit in DFS by adding adjuvant chemotherapy [9,10]. These differences clearly demonstrate, that there is an urgent need for an appropriate risk classification which is able to identify candidates for treatment intensification.
Nowadays, prostate-specific membrane antigen (PSMA)-positron emission tomography/computed tomography (PET/CT) is an important element of prostate carcinoma imaging. PSMA is an extracellular, membrane-bound antigen that is not secreted, but is overexpressed on almost all prostate tumors [11,12,13,14]. In only about 5–10% of all prostate carcinomas there is no PSMA expression [15,16]. By using radioactively labeled substances that specifically bind to PSMA, malignant prostate cells which excessively express PSMA can be detected with a sensitivity of 76.6–84% and a specificity of 82–100% [17,18,19,20]. The majority of the low molecular weight PSMA ligands are based on a glutamate-urea-lysine structure and two other units [17]. The radioactive unit is used for imaging and consists of a chelator molecule, which forms a covalent bond with a radioactive molecule (as in 68Ga) or from a group of fluorinated molecules (as in 18F) [18]. A chelator serves to stabilize the radioactive molecule via a covalent bond by fixing it [19]. The radioactive unit in turn is coupled via a connector to a binding group which can bind in the active center of the PSMA molecule. The binding motif best described in science consists of the combination glutamate-urea-lysine (Glu-urea-Lys) [18].
Currently, two groups of 68Ga-coupled PSMA ligands (such as 68Ga-PSMA-11, 68Ga-PSMA-I + T and 68Ga-PSMA-617) and 18F-coupled ligands (such as 18F-DCPyL, 18F-PSMA-1007) are used world wide. While PSMA-PET/CT imaging was mainly used for patients with biochemical recurrence for a long time, the number of PET/CTs using PSMA-ligands as a primary imaging probe rapidly increased. Results of a recently published, randomized prospective study clearly underlined its importance also for first-line staging of prostate cancer patients before curative-intent surgery or radiotherapy [20]. Therefore, the current study aimed to evaluate the role of PSMA-PET/CT for the prediction of metastases.
2. Materials and Methods
2.1. Study Design
This monocentric and retrospective study, which was approved by the local ethics committee (S-093/2019) and conducted in accordance with the Helsinki Declaration and its amendments. This present study enhances and analysis new aspects of a patient cohort partially previously reported [21]. In total, 335 men with histologically proven, treatment-naïve prostate cancer met the inclusion criteria (age of 18 or older, sufficient clinical data, no previous therapy like ADT, high-intensity focused ultrasound (HIFU) or transurethral resection of the prostate (TURP)) and were included in the current trial.
2.2. Prostate-Specific Membrane Antigen-Positron Emission Tomography/Computed Tomography (PSMA PET/CT) Imaging and Image Evaluation
All patients were hydrated with 500 mL water two hours before imaging. Imaging was performed by Siemens mCT flow, Siemens Biograph 6 and Siemens Biograph 20 mCT scanners. Low-dose CTs without contrast were performed for all patients (5 mm slices). For 68Ga-PSMA-11, two to three MBq per kilogram body weight were applied and the images were taken according to the first clinical description one hour after application [22]. The median uptake time was 60 min between application and acquisition. For 18F-PSMA-1007, an effective dose of approximately 4.4–5.5 mSv per 200–250 MBq examination was applied [23]. The median dose of 68Ga-PSMA-11 was 223 MBq and 254 MBq for 18F-PSMA-1007, respectively. Imaging acquisition was performed after 60 min for 68Ga-PSMA-11 and 90–120 min for 18F-PSMA-1007 after application [24].
Image evaluation was done using the “syngo TrueD” software (Siemens Healthineers, Erlangen, Germany). The subsequent determination of the maximum standardized uptake values (SUVmax) was performed via a semi-automatic region of interest (ROI). All scans were evaluated by two certified nuclear physicians as well as one certified radiooncologist. Any tracer accumulation larger than 3 mm diameter that did not correspond to a physiological uptake and was observed outside the prostate was considered primarily tumor. All these findings were collected in a common consensus. Due to several publications [18,21,24,25] a direct correlation of histopathology and PSMA-imaging findings has been assumed in this study.
2.3. Statistical Analysis
Statistical analysis was performed using Microsoft Excel and SPSS Statistics version 26 (IBM, Armonk, NY, USA). The descriptive analysis of the SUVmax values between patients with and without distant metastases was displayed using box plots. Univariable and multivariable binary logistic regression models were calculated. The dependent variable in all cases was: metastasis occurred yes or no. Univariable models were calculated for four variables, SUVmax (continuous), initial prostate-specific-antigen (PSA) (continuous), World Health Organization (WHO) grading (categorical, 5 classes) and d’Amico classification (categorical, 3 classes). The multivariable model includes all four variables [25]. Results are expressed as odds ratios with 95% confidence intervals for each variable, as well as the area under the curve (AUC) statistic for the whole model. These receiver operating characteristic (ROC) curves were used to determine sensitivity and specificity as a function of the association between the binary characteristic “any metastasis occurred” and the quantitative characteristic “SUVmax”. From the variables of the multiple logistic regression model, the variable “probability of occurrence p” could be determined by transforming the regression equation. The resulting variable “probability of occurrence p” could again be examined in terms of a ROC curve (Receiver Operating Characteristic) in relation to the binary variable of “metastasis that has occurred”. The area under the curve (AUC) values were again determined on the basis of the curve thus calculated.
3. Results
3.1. Cohort Characteristics
The cohort included 335 men with histologically confirmed prostate carcinoma after biopsy. All patients had no previous therapy. Median age of the entire cohort was 67 years (range 38–84 years) and a median PSA of 11 ng/mL (range 1.2–511 ng/mL) was found at initial diagnosis. Histological examinations resulted in 148 patients (44.2%) with WHO grading 4 or 5 carcinomas. Most patients (65.4%) were classified as high-risk according to the d’Amico risk classification [16]. Patient’s characteristics are summarized in Table 1.
Table 1.
Patient’s characteristics.
| Total Number of Patients | n = 335 |
|---|---|
| age at PSMA-PET/CT (years), median (range) | 67 (38–84) |
| WHO grading, n = 326 | |
| 1 | 36 (10.7%) |
| 2 | 85 (25.4%) |
| 3 | 57 (17.0%) |
| 4 | 58 (17.3%) |
| 5 | 90 (26.9%) |
| PSA at initial diagnosis (ng/mL), median (range), n = 331 | 11 (1.2–511) |
| <10 | 153 (45.7%) |
| 10–20 | 84 (25.1%) |
| >20 | 94 (28.1%) |
| risk classification according d’Amico, n = 335 | |
| low risk | 15 (4.5%) |
| intermediate risk | 101 (30.1%) |
| high risk | 219 (65.4%) |
| PSMA-PET/CT tracer | |
| 68Ga-PSMA-11 | 272 (81.2%) |
| 18F-PSMA-1007 | 63 (18.8%) |
| N staging according to PSMA-PET/CT, n = 335 | |
| cN0 | 254 (75.8%) |
| cN1 | 81 (24.2%) |
| M staging according to PSMA-PET/CT, n = 335 | |
| cM0 | 253 (75.5%) |
| cM1a | 18 (5.4%) |
| cM1b | 39 (11.6%) |
| cM1c | 25(7.5%) |
3.2. Imaging Evaluation
PSMA imaging detected 81 patients (24.2%) with regional nodal metastases. In total, 82 patients (24.7%) with 171 distant, PSMA-positive lesions were diagnosed. Most metastases were located in extrapelvic nodes (n = 60) and bone (n = 103), the rate of visceral metastases was low with eight lesions with tracer uptake (Table 2). Median SUVmax of malignant intraprostatic lesions was 12.5 (range 3–109) for the entire cohort. 48 out of 82 patients with distant metastases also had regional, nodal metastases with PSMA-uptake.
Table 2.
Overview of site of metastases detected by PSMA-PET/CT.
| Total Number of Distant Metastases with PSMA-Uptake | n = 173 |
|---|---|
| extrapelvic nodal metastases | |
| total lesions | 60 (35.1%) |
| abdominal | 42 (70.0%) |
| Thoracic | 15 (25.0%) |
| Cervical | 1 (1.7%) |
| others | 2 (3.3%) |
| bone metastases | |
| total lesions | 103 (60.2%) |
| Pelvic | 43 (41.7%) |
| abdominal/thoracic | 49 (47.6%) |
| Extremities | 8 (7.8%) |
| head/cervical | 3 (2.9%) |
| organ metastases | |
| total lesions | 10 (5.7%) |
| Intrahepatic | 4 (40.0%) |
| Pulmonal | 4 (40.0%) |
| others | 2 (20.0%) |
3.3. Association between PSMA-PET/CT and the Presence of Metastases
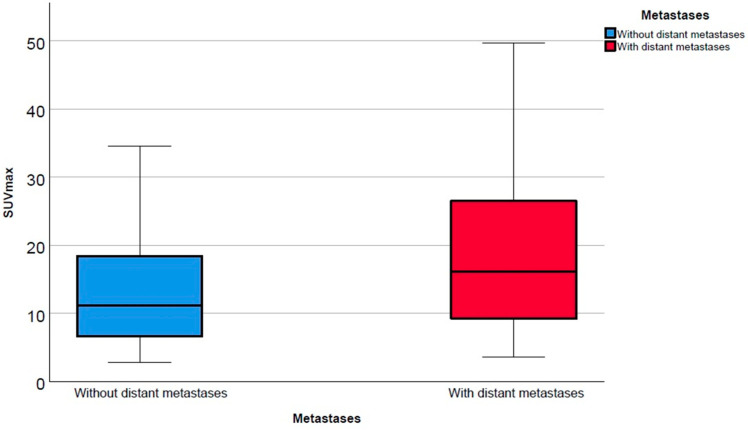
Correlating the presence of metastases and intraprostatic SUVmax in PSMA imaging, a higher SUVmax was observed for men with metastatic disease than for those without metastases (median 11.2 vs. 16.1; p < 0.001) (Figure 1).
Figure 1.
Boxplot of intraprostatic maximum standardized uptake values (SUVmax) according to M-stage.
The AUC in regard to predicting the presence of metastases was 0.65. In subgroup analyses, the best AUC was obtained for patients with intermediate-risk prostate cancer (0.71; low-risk: 0.29; high-risk: 0.56) and after exclusion of International Society of Urological Pathology (ISUP) grade group 5 (0.66). Moreover, the AUC was significantly better for PET scans using 18F-PSMA ligands compared to those with 68Ga-PSMA ligands (0.71 vs. 0.63—Supplementary File 1 and Supplementary File 2).
Comparing different combinations of intraprostatic SUVmax, PSA, WHO grading and d’Amico risk classification in a multiple binary logistic regression model, all variables showed a high, statistical significance with regard to distant metastases (p < 0.001). Multiple logistic regression model calculations of all parameters lead to a slightly better AUC of 0.69 (Table 3). The multiple logistic regression showed a highly significant (p = 0.004) influence of SUVmax on the occurrence of distant metastasis.
Table 3.
Multiple logistic regression.
| Variables | - | Regression Coefficient B | Standard Error | p-Value | Odds Ratio | 95% Confidence Intervals for Odds Ratio | |
|---|---|---|---|---|---|---|---|
| - | - | - | - | - | - | Lower value | Upper value |
| SUVMax | - | 0.026 | 0.009 | 0.004 | 1.026 | 1.006 | 1.046 |
| initial PSA | - | 0.008 | 0.004 | 0.051 | 1.008 | 1.000 | 1.016 |
| WHO 1 | - | - | - | 0.264 | - | - | - |
| - | WHO (1) | −1.374 | 0.819 | 0.093 | 0.253 | 0.054 | 1.344 |
| - | WHO (2) | −0.798 | 0.456 | 0.316 | 0.639 | 0.200 | 1.191 |
| - | WHO (3) | −0.448 | 0.446 | 0.316 | 0.639 | 0.252 | 1.479 |
| - | WHO (4) | −0.510 | 0.383 | 0.183 | 0.601 | 0.288 | 1.292 |
| d’Amico 1 | - | - | - | 0.476 | - | - | - |
| - | d’Amico (1) | −0,332 | 0.064 | 0.800 | 0.717 | 0.054 | 9.094 |
| - | d’Amico (2) | −0.559 | 0.459 | 0.224 | 0.572 | 0.204 | 1.280 |
1 Reference categories have been “WHO5” and “d’Amico high-risk.
The maximum for the sum of sensitivity and specificity (weight factor 1:1) was observed using a cut-off value of 11.9 for SUVmax of malignant intraprostatic lesions, resulting in a sensitivity and specificity of 76.0% and 58.4%, respectively. Assuming a prevalence of 24.5% for metastases, the negative predictive value (NPV) and positive predictive value (PPV) could be stated as 85.3% and 33.1%.
4. Discussion
The present study confirmed, that a relatively large number of men with prostate cancer are at high risk for metastases. Distant, malignant lesions occurred in almost one in four patients in the current cohort, even though, considering the “negative” selection of men undergoing PSMA-PET/CT as primary staging (219 out of 335 with high-risk disease). However, while 5-year survival rates are excellent for localized prostate cancer, lifespan is limited for patients with distant tumor burden. Siegel et al. reported on a 5-year relative survival rate of 31% in men with metastatic disease [26]. Thus, treatment intensification strategies are of great interest for selected prostate cancer patients.
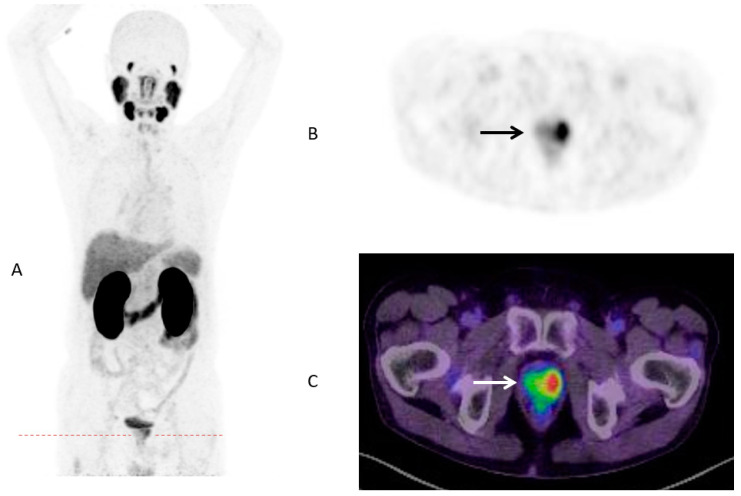
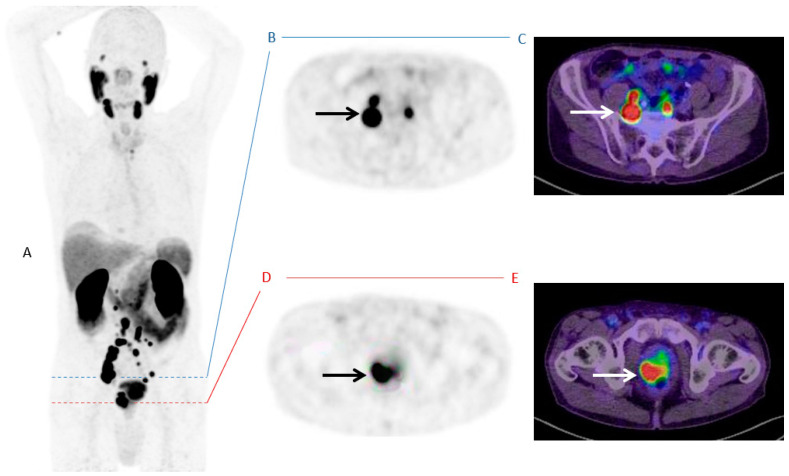
Until now, the identification of patient subgroups suitable for treatment escalation leading to improved survival is still challenging. Although numerous pretreatment risk stratification tools like the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram or the Cancer of the Prostate Risk Assessment (CAPRA) score are available [27], there is a lack of evident data with regard to individual therapeutic consequences. Increasing numbers of PSMA-PET/CTs for primary staging around the globe confirms a great potential to that molecular imaging methodology. As published a few years ago, SUVmax of intraprostatic, malignant lesions was highly correlated with several clinical parameters like Gleason Score (GS) or PSA [28]. The present study observed a statistically significant higher SUVmax for men with metastatic disease (Figure 2 and Figure 3).
Figure 2.
PSMA-PET/CT in maximum intensity projection (MIP) (A) of a 70-years old patient with prostate cancer (GS 7b/group grade 3, PSA 4.53 ng/mL) and low intraprostatic SUVmax (3.45) and no metastases (B) PSMA-PET Dx; (C) PSMA PET/CT Dx.
Figure 3.
PSMA-PET/CT in MIP (A) of a 83-years old patient with prostate cancer (GS 8/group grade 4; PSA 32 ng/mL) and high intraprostatic SUVmax (49.63) and several metastases; Level of prostate (B) PSMA-PET Dx; (C) PSMA PET/CT Dx; Level of nodal metastases (D) PSMA-PET Dx; (E) PSMA PET/CT Dx.
Choosing a cut-off value of 11.9, a relatively high sensitivity and specificity can be reached with regard to the presence of metastases leading to an estimated NPV and PPV of 85.3% and 33.1%, respectively, for the current cohort. Although subgroup analysis demonstrated a higher correlation for patients undergoing 18F-PSMA-1007 PET/CT compared with 68Ga-PSMA11-PET/CT, this cut-off value was determined for both tracers considering the relatively low number of patients who received 18F-PSMA-1007. A collection of two separate cut-off values did not seem sensible in this context. Nevertheless, the standard-PSA-blood test for prostate cancer screening provides a NPV and PPV of 15.5% and 25%, respectively [29], which is considerably lower compared to the current results. Thus, our findings suggest a high potential for PSMA-PET/CT predicting metastasis which might be integrated in clinical decision making for individual treatment escalation. In support of this hypothesis Komek et al. reported an association between SUV parameters from PSMA-imaging and survival outcome. In a cohort of 148 men with advanced prostate cancer undergoing 68Ga-PSMA-PET/CT, several SUV parameters like bone SUV >10.7 were significant in the determination of increased mortality risk [30]. Hence, SUV from PSMA-PET/CT seems to be a promising parameter for prostate cancer risk stratification in daily clinical practice.
In this context it is interesting to note, that for the present trial a stronger correlation was obtained when using 18F-PSMA-1007 compared with 68Ga-PSMA-11 (both ROC curves are depicted in the Supplementary File 2). Due to the retrospective nature of the current study including patients who have been investigated over a longer period, the 68Ga-PSMA-11-tracer was initially more widespread. Over time, the 18F-PSMA-tracer was later introduced and more frequently utilized at the Department of Nuclear Medicine due to improved biological properties explaining the analysis of both tracers. This leads to the fact that both subgroups were not well-balanced. However, similar differences were observed in literature: a head-to-head comparison of 68Ga-PSMA-11 and 18F-PSMA-1007 resulted in excellent identification of all dominant factors.
Prostatic lesions for both tracers, however, SUVmax was subject to significant variations depending on tracer type. For almost all patients, SUVmax obtained by 18F-PSMA-1007 was significantly higher than by 68Ga-PSMA-11 (factor 1.1–3.9) [31].
Even though to the best of our knowledge the present study is the first to evaluate the correlation of SUVmax of intraprostatic malignant lesion and the prediction of metastases, some limitations need to be highlighted. Although the number of patients included in the current study was relatively high, the trial had a retrospective design and was performed at only one institution. This makes it prone for patient selection biases only to be overcome by prospective trials. Moreover, the size of the individual metastases was not explicitly recorded and confounders like inflammation of the prostate may influence measurements of SUVmax affecting the interpretation of hybrid imaging results [32]. Although the current study did not include a correlation with histopathological data, the conclusions seem to be legitimate. Many analyses have already focused on the correlation of PSMA-PET/CT and histopathological data. In a cohort of 319 men who underwent 68Ga-PSMA-11 PET/CT after PSA relapse, PSMA-imaging demonstrated excellent histopathological correlation [33]. Moreover, a sensitivity of 94.7% for nodal disease using 18F-PSMA-1007 for primary staging was also observed [23]. Several other trials confirmed these observations after correlation of data obtained by PSMA-PET/CT and results from surgery [34,35]. Sensitivity and specificity ranged from 75–99% for both. Due to strong published results, a direct correlation of histopathology and PSMA-imaging findings was assumed in the current analysis.
In summary, the present study demonstrated promising results and provides an excellent basis for prospective trials evaluating the integration of PSMA-PET/CT as a stratification tool for prostate cancer patients with a high risk for systemic disease. Further research is required to assess the role of different tracers and evaluate the impact on survival outcome obtained by PSMA-guided treatment intensification.
5. Conclusions
The present study confirms, that there is a high rate of treatment-naïve prostate cancer patients with distant disease identified by PSMA-PET/CT. Including SUVmax of malignant, intraprostatic lesions, may be helpful in decision making of individual treatment escalation for a variety of men with aggressive disease which should improve clinical outcomes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/7/1508/s1, File 1: Univariate analysis of binary logistic regression SUVmax, WHO, PSA, d’Amico-Score; File 2: ROC curves of 68Ga and 18F.
Author Contributions
S.A.K. and F.L.G.: conceptualization; S.A.K., J.B., and F.L.G.: methodology; C.K. and E.W.: software; I.S. and J.R., T.H.-L. and K.K.: validation; J.B., S.A.K. and F.L.G.: formal analysis; S.A.K. and J.B.: writing—original draft preparation; K.K., K.H., D.J., M.H., J.D., U.H. and F.L.G.: writing—review and editing; L.H. and S.Z.: visualization; T.H.-L., J.D. and U.H.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee of Heidelberg University (“S-093/2019” from 25 February 2019).
Informed Consent Statement
Patient consent was waived due to the monocentric and retrospective nature of the study using available, de-identified data with permission from the local ethics committee.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
S.A.K. reports grants from Viewray Inc., outside the submitted work. U.H., C.K. and F.L.G. have a patent application for quinolone-based FAP-targeting agents for imaging and therapy outside of this work. U.H., C.K. and F.L.G. also have shares of a consultancy group for iTheranostics outside of this work. F.L.G. is a medical advisor for ABX Advanced Biochemical Compound, Sofie Biosciences and Telix Pharmaceuticals and patent application has been filed for PSMA-1007 by U.H. and F.L.G. J.D. reports grants from Viewray Inc., grants from CRI The Clinical Research Institute GmbH, grants from Accuray International Sari, grants from RaySearch Laboratories AB, grants from Vision RT Limited, grants from Merck Serono GmbH, grants from Astellas Pharma GmbH, grants from Astra Zeneca GmbH, grants from Siemens Healthcare GmbH, grants from Solution Akademie GmbH, grants from Egomed PLC Surrey Research Park, grants from Quintiles GmbH, grants from Pharmaceutical Research Associates GmbH, grants from Boehringer Ingelheim Pharma GmbH and CoKG, grants from PTW-Freiburg Dr. Pychlau GmbH, grants from Nanobiotix S.A, outside the submitted work. KK is SAB member of Telix Pharmaceuticals and coinventor on patents of PSMA-617 and PSMA-1007. All other authors have no relevant financial relationships to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Dyba T., Randi G., Bettio M., Gavin A., Visser O., Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Zagars G.K., Ayala A.G., von Eschenbach A.C., Pollack A. The prognostic importance of Gleason grade in prostatic adenocarcinoma: A long-term follow-up study of 648 patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:237–245. doi: 10.1016/0360-3016(94)00323-D. [DOI] [PubMed] [Google Scholar]
- 3.Bott S.R., Freeman A.A., Stenning S., Cohen J., Parkinson M.C. Radical prostatectomy: Pathology findings in 1001 cases compared with other major series and over time. BJU Int. 2005;95:34–39. doi: 10.1111/j.1464-410X.2005.05245.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg M.R., Cowan J., Broering J.M., Carroll P.R. High-risk prostate cancer in the United States, 1990-2007. World J. Urol. 2008;26:211–218. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelefsky M.J., Eastham J.A., Cronin A.M., Fuks Z., Zhang Z., Yamada Y., Vickers A., Scardino P.T. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: A comparison of clinical cohorts adjusted for case mix. J. Clin. Oncol. 2010;28:1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal S.A., Hunt D., Sartor A.O., Pienta K.J., Gomella L., Grignon D., Rajan R., Kerlin K.J., Jones C.U., Dobelbower M., et al. A phase 3 trial of 2 years of androgen suppression and radiation therapy with or without adjuvant chemotherapy for high-risk prostate cancer: Final results of radiation therapy oncology group phase 3 randomized trial NRG oncology RTOG 9902. Int. J. Radiat. Oncol. Biol. Phys. 2015;93:294–302. doi: 10.1016/j.ijrobp.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciezki J.P., Weller M., Reddy C.A., Kittel J., Singh H., Tendulkar R., Stephans K.L., Ulchaker J., Angermeier K., Stephenson A., et al. A Comparison Between Low-Dose-Rate Brachytherapy With or Without Androgen Deprivation, External Beam Radiation Therapy With or Without Androgen Deprivation, and Radical Prostatectomy With or Without Adjuvant or Salvage Radiation Therapy for High-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:962–975. doi: 10.1016/j.ijrobp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Reichard C.A., Hoffman K.E., Tang C., Williams S.B., Allen P.K., Achim M.F., Kuban D.A., Chapin B.F. Radical prostatectomy or radiotherapy for high- and very high-risk prostate cancer: A multidisciplinary prostate cancer clinic experience of patients eligible for either treatment. BJU Int. 2019;124:811–819. doi: 10.1111/bju.14780. [DOI] [PubMed] [Google Scholar]
- 9.Ahlgren G.M., Flodgren P., Tammela T.L.J., Kellokumpu-Lehtinen P., Borre M., Angelsen A., Iversen J.R., Sverrisdottir A., Jonsson E., Sengelov L. Docetaxel Versus Surveillance After Radical Prostatectomy for High-risk Prostate Cancer: Results from the Prospective Randomised, Open-label Phase 3 Scandinavian Prostate Cancer Group 12 Trial. Eur. Urol. 2018;73:870–876. doi: 10.1016/j.eururo.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kellokumpu-Lehtinen P.-L., Hjälm-Eriksson M., Thellenberg-Karlsson C., Åström L., Franzen L., Fransson A.-S., Leskinen M.J., Zeke M., Huttunen T., Ginman C. Docetaxel Versus Surveillance After Radical Radiotherapy for Intermediate-or High-risk Prostate Cancer—Results from the Prospective, Randomised, Open-label Phase III SPCG-13 Trial. Eur. Urol. 2019;76:823–830. doi: 10.1016/j.eururo.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick D.G., Pacelli A., Blute M., Roche P., Murphy G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2256::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Mannweiler S., Amersdorfer P., Trajanoski S., Terrett J.A., King D., Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 13.Silver D.A., Pellicer I., Fair W.R., Heston W., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 14.Trover J.K., Beckett M.L., Wright Jr G.L. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 15.Budäus L., Leyh-Bannurah S.-R., Salomon G., Michl U., Heinzer H., Huland H., Graefen M., Steuber T., Rosenbaum C. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur. Urol. 2016;69:393–396. doi: 10.1016/j.eururo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Maurer T., Eiber M., Schwaiger M., Gschwend J.E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 2016;13:226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 17.Oh S.W., Cheon G.J. Prostate-specific membrane antigen PET imaging in prostate cancer: Opportunities and challenges. Korean J. Radiol. 2018;19:819. doi: 10.3348/kjr.2018.19.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiber M., Fendler W.P., Rowe S.P., Calais J., Hofman M.S., Maurer T., Schwarzenboeck S.M., Kratowchil C., Herrmann K., Giesel F.L. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 2017;58:67S–76S. doi: 10.2967/jnumed.116.186767. [DOI] [PubMed] [Google Scholar]
- 19.Aktories K., Forth W. Allgemeine und Spezielle Pharmakologie und Toxikologie: Für Studenten der Medizin, Veterinärmedizin, Pharmazie, Chemie, und Biologie sowie für Ärzte, Tierärzte und Apotheker; mit 303 Tabellen. Elsevier, Urban&FischerVerlag; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 20.Hofman M.S., Lawrentschuk N., Francis R.J., Tang C., Vela I., Thomas P., Rutherford N., Martin J.M., Frydenberg M., Shakher R. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 21.Koerber S.A., Stach G., Kratochwil C., Haefner M.F., Rathke H., Herfarth K., Kopka K., Holland-Letz T., Choyke P.L., Haberkorn U., et al. Lymph Node Involvement in Treatment-Naïve Prostate Cancer Patients: Correlation of PSMA PET/CT Imaging and Roach Formula in 280 Men in Radiotherapeutic Management. J. Nucl. Med. 2019;61:46–50. doi: 10.2967/jnumed.119.227637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afshar-Oromieh A., Malcher A., Eder M., Eisenhut M., Linhart H., Hadaschik B., Holland-Letz T., Giesel F., Kratochwil C., Haufe S. PET imaging with a [68 Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 23.Giesel F.L., Hadaschik B., Cardinale J., Radtke J., Vinsensia M., Lehnert W., Kesch C., Tolstov Y., Singer S., Grabe N., et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wondergem M., van der Zant F.M., Knol R.J., Lazarenko S.V., Pruim J., de Jong I.J. 18F-DCFPyL PET/CT in the detection of prostate cancer at 60 and 120 minutes: Detection rate, image quality, activity kinetics, and biodistribution. J. Nucl. Med. 2017;58:1797–1804. doi: 10.2967/jnumed.117.192658. [DOI] [PubMed] [Google Scholar]
- 25.Sperandei S. Understanding logistic regression analysis. Biochem. Med. 2014;24:12–18. doi: 10.11613/BM.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 27.Zelic R., Garmo H., Zugna D., Stattin P., Richiardi L., Akre O., Pettersson A. Predicting prostate cancer death with different pretreatment risk stratification tools: A head-to-head comparison in a nationwide cohort study. Eur. Urol. 2020;77:180–188. doi: 10.1016/j.eururo.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Koerber S.A., Utzinger M.T., Kratochwil C., Kesch C., Haefner M.F., Katayama S., Mier W., Iagaru A.H., Herfarth K., Haberkorn U. 68Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: Correlation of intraprostatic PSMA uptake with several clinical parameters. J. Nucl. Med. 2017;58:1943–1948. doi: 10.2967/jnumed.117.190314. [DOI] [PubMed] [Google Scholar]
- 29.Ried K., Tamanna T., Matthews S., Eng P., Sali A. New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Front. Oncol. 2020;10:582. doi: 10.3389/fonc.2020.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komek H., Can C., Yilmaz U., Altindag S. Prognostic value of 68 Ga PSMA I&T PET/CT SUV parameters on survival outcome in advanced prostat cancer. Ann. Nucl. Med. 2018;32:542–552. doi: 10.1007/s12149-018-1277-5. [DOI] [PubMed] [Google Scholar]
- 31.Kuten J., Fahoum I., Savin Z., Shamni O., Gitstein G., Hershkovitz D., Mabjeesh N.J., Yossepowitch O., Mishani E., Even-Sapir E. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J. Nucl. Med. 2020;61:527–532. doi: 10.2967/jnumed.119.234187. [DOI] [PubMed] [Google Scholar]
- 32.Shetty D., Patel D., Le K., Bui C., Mansberg R. Pitfalls in gallium-68 PSMA PET/CT interpretation—A pictorial review. Tomography. 2018;4:182. doi: 10.18383/j.tom.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afshar-Oromieh A., Avtzi E., Giesel F.L., Holland-Letz T., Linhart H.G., Eder M., Eisenhut M., Boxler S., Hadaschik B.A., Kratochwil C. The diagnostic value of PET/CT imaging with the 68 Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauscher I., Maurer T., Fendler W.P., Sommer W.H., Schwaiger M., Eiber M. 68 Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging. 2016;16:1–10. doi: 10.1186/s40644-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jilg C.A., Drendel V., Rischke H.C., Beck T.I., Reichel K., Krönig M., Wetterauer U., Schultze-Seemann W., Meyer P.T., Vach W. Detection rate of 18F-choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: Dependence on the size of tumor deposits in lymph nodes. J. Nucl. Med. 2019;60:971–977. doi: 10.2967/jnumed.118.220541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.