Abstract
A novel strategy involving Olive Leaf Extract (OLE) and Cold Atmospheric Plasma (CAP) was developed as a green antimicrobial treatment. Specifically, we reported a preliminary investigation on the combined use of OLE + CAP against three pathogens, chosen to represent medical and food industries (i.e., E. coli, S. aureus and L. innocua). The results indicated that a concentration of 100 mg/mL (total polyphenols) in OLE can exert an antimicrobial activity, but still insufficient for a total bacterial inactivation. By using plain OLE, we significantly reduced the growth of Gram positive S. aureus and L. innocua, but not Gram-negative E. coli. Instead, we demonstrated a remarkable decontamination effect of OLE + CAP in E. coli, S. aureus and L. innocua samples after 6 h. This effect was optimally maintained up to 24 h in S. aureus strain. E. coli and L. innocua grew again in 24 h. In the latter strain, OLE alone was most effective to significantly reduce bacterial growth. By further adjusting the parameters of OLE + CAP technology, e.g., OLE amount and CAP exposure, it could be possible to prolong the initial powerful decontamination over a longer time. Since OLE derives from a bio-waste and CAP is a non-thermal technology based on ionized air, we propose OLE + CAP as a potential green platform for bacterial decontamination. As a combination, OLE and CAP can lead to better antimicrobial activity than individually and may replace or complement conventional thermal procedures in food and biomedical industries.
Keywords: polyphenols, olive tree, green technology, antibacterial, food contamination, S. aureus, E. coli, L. innocua
1. Introduction
There is a growing interest in sustainable industrial routes, with special emphasis in the food and biomedical sectors, for the manufacture of safe yet sustainable packaging for edible products as well as surgical devices [1,2]. Consequently, the attention towards less impactful processing technologies capable of replacing conventional decontamination methods is increasing. To this end, natural antimicrobial compounds, including vegetable bioactive molecules, offer an emerging strategy to control microbial contamination. Indeed, some molecules and plant-derivatives demonstrate good antimicrobial activity against pathogenic bacteria found in packaging or processing phases in the food industry [1], as well as in biomedical devices, such as surgical tools and supporting equipment, the latter being very important in healthcare, since inaccurate sterilization is responsible for at least 1.5%–7.2% of post-operative complications [2]. The antibacterial properties of plants have been widely investigated [3]. In fact, a great variety of plant species containing components which exhibit antimicrobial activity against a wide range of Gram-positive and Gram-negative bacteria has been reported, including Hibiscus, Rosmarinus officinalis, Thymus vulgaris, Malva sylvestris and Allium sativum, among others [4,5,6,7,8,9,10,11,12,13,14]. Plant antimicrobials are more attractive than the synthetic food preservatives, as they are generally recognized as safe and capable of benefiting human health, including essential oils [15]. Olive Leaf Extract (OLE), an agricultural by-product obtained during the harvesting or pruning process of olive fruits, can be considered as a plant derivative entitled with both antimicrobial and antioxidant activities [16]. The leaves of olive trees (i.e., Olea europaea, Oleaceae) together with small and large branches produced from the cultivation and harvesting of olives represent waste biomasses, usually burned by farmers with consequent production of greenhouse gases [17]. As such, their conversion into higher-value products can represent a sustainable and eco-friendly alternative to their current disposal. To obtain OLE from olive oil leaves, a water-based extraction method can be applied in place of organic solvent solutions [18]. In fact, widely used OLE extraction procedures based on ethanol and methanol aqueous solutions have shown shortcomings such as low extraction efficiency, prolonged extraction times and high energy consumption for heating [19]. OLE is used in traditional medicine as a dietary supplement and an over-the-counter drug for a variety of beneficial effects on human health, such as lowering blood pressure and supporting the cardiovascular and immune systems [20]. OLE and the other parts of the olive tree contain considerable amounts of polyphenols [18]. Specifically, OLE contains oleuropein, the main phenolic compound found in unprocessed olive fruits and leaves, up to 140 mg/g and 60–90 mg/g, respectively [21]. Oleuropein is a glycosylated seco-iridoid glucoside composed of elenolic acid and hydroxy-tyrosol; it has an oleosidic skeleton that is common within the seco-iridoid glucosides of Oleaceae, mainly in its aglycone form, which makes the sugar moiety insoluble in oil [22].According to some studies, OLE exerts antimicrobial effects due to its high phenolic content [16,23,24]. They report the growth inhibition in some bacteria species, reported in Table 1. However, these findings are contradictory and may depend on the concentration and/or the bacteria used. For example, Sudjana et al. showed that OLE had appreciable antimicrobial activity only against C. jejuni, H. pylori and Staphylococcus spp., but poor effect against B. subtilis, Candida spp., E. coli, K. pneumoniae, P. aeruginosa and S. marcescens [25]. Djanane et al. showed that 5% OLE was more efficient as compared to 1% OLE against the pathogens Salmonella and E. coli O157:H7 in Halal meat [26]. Albertos et al. reported that edible films with OLE at 5.6% (w/w) reduced L. monocytogenes growth on smoked salmon [27]. Furthermore, in a study by Pereira et al., OLE was screened for its antimicrobial activity against B. cereus, B. subtilis, S. aureus, E. coli, P. aeruginosa, K. pneumoniae bacteria and against the fungi C. albicans and C. neoformans, showing B. cereus and C. albicans to be the most sensitive to OLE [28]. Therefore, the use of OLE as antimicrobial treatment is still a matter of debate due to the contradictory results of the insufficient number of systematic studies conducted so far [29,30].
Table 1.
Bacterial growth inhibition studies with olive leaf extract (OLE) treatment on bacteria strains.
| Bacterial Species | Olive Variety and Origin | OLE Extraction Method | OLE Concentration | Reference |
|---|---|---|---|---|
| K. pneumoniae | Olea europaea (Turkey, west Anatolian) | Aqueous | 30 µL OLE; 15% (w/v) | [22,30] |
| S. aureus | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28,29] |
| B. cereus | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28] |
| B. subtilis | Olea europaea | Ethanol | 27.2 ± 0.99 mg/g | [28] |
| P. aeruginosa | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28] |
| C. jejuni | Olea europaea (Australia) | n.a. | n.a. | [27] |
| H. pylori | Olea europaea (Australia) | n.a. | n.a. | [27] |
| E. coli | Olea europaea (Several countries) | Water, ethanol. | Variable | [28,30,31] |
| S. enterica | Olea europaea subsp. europaea var. Sylvestris (Algeria) | Methanol/water | 198.7 ± 3.6 mg GAE/g | [27,28,30] |
| L. monocytogenes | Commercial extract (USA) | Water/ethanol | 62.5 mg/mL | [27,30] |
Another emerging non-thermal technology with potential applications in several different industries, including safe and sustainable food production, is Cold Atmospheric Plasma (CAP). Plasma is commonly referred to as the fourth state of matter, namely, an ionized state of gas which exhibits unique properties. The plasma state is pervasive, being found in diverse entities (e.g., stars, interstellar space, lighting technology). Cold plasma (CP) is commonly obtained by application of a strong electromagnetic field to a neutral gas that induces ionization. CP is composed of ions, electrons, free radicals, excited atoms/molecules and photons of various wavelengths. CAP treatment in the presence of air generates several reactive short and long-lived species including reactive oxygen species (ROS) and reactive nitrogen species (RNS), which have been shown to play a dominant role in antibacterial and biological activity [31,32,33]. CAP, due its non-thermal nature, is a potential alternative to conventional methods such as the use of chlorine and thermal processes (i.e., drying, chilling, freezing and pasteurization) to improve microbiological safety in food packaging [34].
In the biomedical field, this technique can be applied in surface disinfection, sterilization of surgical instruments and decontamination of devices [2]. Moreover, plasma devices, such as indirect argon plasma (e.g., MicroPlaSter alpha and beta), have been applied in a clinical trial in patients with chronic infected wounds [2]. Some studies reported that CAP exhibited excellent antibacterial efficacy against target food pathogens, their spores and biofilms [35]. In addition, new information has been elucidated explaining the effective application of CP in functional packaging, for elimination of toxins and degradation of pesticides [36]. Plasma has also been investigated as a pre-treatment step to activate or modify material surfaces, since it can improve the efficiency of post-grafting or the incorporation of antimicrobial components onto the surface [37]. Chang et al. used plasma pre-treatment to promote the grafting of chitosan on polyester fabrics to obtain antibacterial activity against B. subtilis and S. aureus [37]. In this study, fabrics were previously pre-treated by an argon/oxygen (Ar/O2) dielectric barrier discharge (DBD) plasma for surface activation, subsequently exposed to the atmosphere for further oxidization and, finally, immersed in a chitosan solution for chitosan grafting. Other natural compounds, such as nisin peptides, thymol and herbs, have also been grafted onto plasma pre-treated polymer surfaces to obtain an antibacterial material [38,39,40]. Different types of plasma pre-treatments, namely N2 and Ar/O2 plasma modifications, and plasma-induced grafting of acrylic acid have been used to incorporate nisin peptides onto the surface of low density poly-ethylene films [40]. Other interesting developments using whey protein formulations as coating strategies on polyethylene terephthalate films, pre-treated by corona discharge of CAP, resulted in excellent barrier properties, making the packaging efficacy comparable to the ethylene vinyl alcohol copolymers barrier layer, conventionally used in food packaging composites [41]. Similarly, in another study, the application of plasticized corn–zein coating on corona-discharge-treated polypropylene films for the flexible packaging industry showed more than three orders of reduction in oxygen permeability [42]. Hence, although the antimicrobial properties of plain OLE is still debated, OLE can be used in combination with CAP treatment (OLE + CAP) to empower antimicrobial activity against bacteria present in the food and biomedical industries. In this way, it is expected to replace or complement conventional sterilization and decontamination processes that use high energy with those of lower environmental impact [43,44]. Overall, both OLE and CAP are sustainable technologies for bacterial decontamination purposes; however, singularly, their action is not as strong and efficient as heat sterilization. The OLE + CAP combination represents a novel approach that involves the bioactive molecules of OLE and ROS/RNS generated in situ by CAP. In this study, the antibacterial activity of OLE, CAP and OLE + CAP against common bacterial species affecting both food and medical devices was preliminarily investigated to determine potential synergistic effects between the two approaches. Having a combined green technology for bacterial decontamination would allow more sustainable and better management of human health.
2. Results
2.1. Effect of OLE on Bacterial Strains
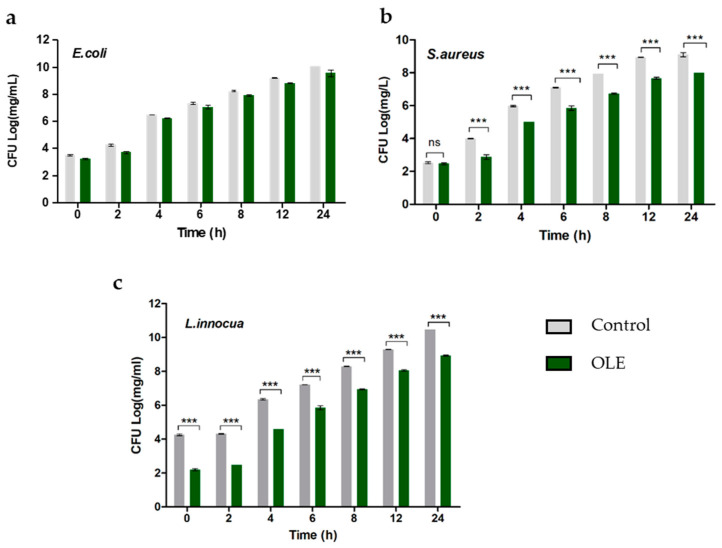
The OLE used was extracted from Olea europaea var. Olivastra seggianese in Tuscan cultivar [45]. The antibacterial activity was evaluated in vitro against gram-negative E. coli and gram-positive S. aureus and L. innocua strains. Each resuspended E. coli, S. aureus and L. innocua sample was separately treated with OLE at 100 mg/mL total polyphenols (TPs), as this concentration is within the range of efficiency [46]. The effect of OLE on the different bacteria is illustrated in Figure 1. OLE composition is given in Table 2.
Figure 1.
Bar graphs showing the effect of OLE administration at 100 mg/mL total polyphenols (TPs) on the growth of different bacterial strains: (a) E. coli, (b) S. aureus, (c) L. innocua, up to 24 h. The values are reported as mean ± standard error of the mean (SEM) (n = 3), significance at p < 0.05 by One-way ANOVA and Tukey′s HSD post hoc test; *** p < 0.0001, n.s. not significant.
Table 2.
Concentration of the main polyphenols in OLE.
| OLE Composition | Concentration (mg/g OLE) |
|---|---|
| Oleuropein | 32.64 ± 3.06 |
| Luteolin-7-O-glucoside | 6.97 ± 0.24 |
| Rutin | 3.37 ± 0.33 |
| Apigenin-7-O-glucoside | 1.97 ± 0.17 |
| Hydroxy-tyrosol | 0.85 ± 0.08 |
| Caffeic acid | 0.18 ± 0.02 |
For E. coli, OLE did not exhibit any significant antimicrobial effect (Figure 1a). Conversely, in S. aureus and L. innocua statistically significant antimicrobial effects were observed over time (Figure 1b,c). In particular, for L. innocua OLE showed a reduction in the number of colonies even if inactivation was not reached (Figure 1c).
2.2. Effect of OLE + CAP on Bacterial Strains
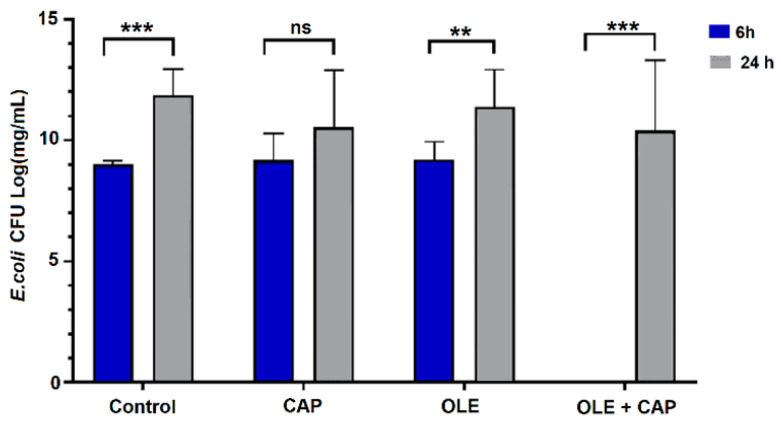
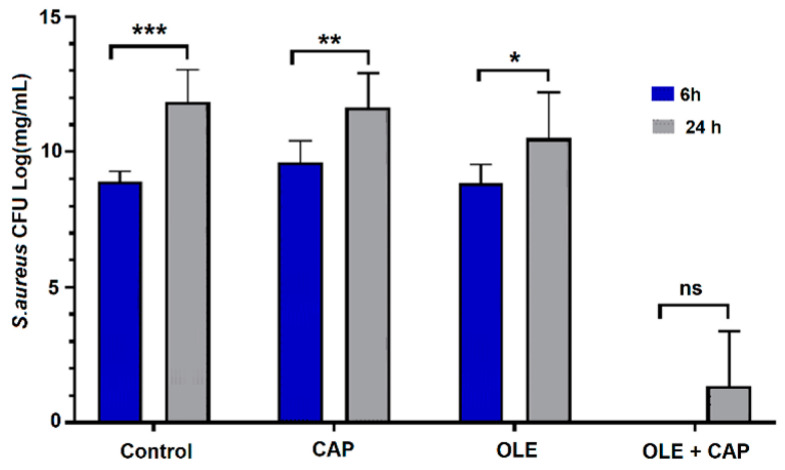
The potential synergistic effect of OLE and CAP was evaluated at two relevant time points, 6 h and 24 h after application of OLE, CAP or OLE + CAP, using previously published methods [47,48,49]. The comparison of results obtained after 6 h and 24 h using the single treatment and their combination is reported in Figure 2, Figure 3 and Figure 4. Using the combined OLE + CAP treatment, E. coli had significant inhibition at 6 h, while at 24 h the bacteria reproduced, but still with a significant reduction with respect to the control and individual treatments (Figure 2). The most remarkable effect was obtained against S. aureus (Figure 3).
Figure 2.
The effects of Cold Atmospheric Plasma (CAP) (1 min exposure time) and/or OLE on E. coli. The values are reported as mean ± standard error of the mean (SEM) (n = 3), significance at p < 0.05 by One-way ANOVA and Tukey′s HSD post hoc test; ** p < 0.001, *** p < 0.0001, n.s. not significant.
Figure 3.
The effects of CAP (1 min exposure time) and/or OLE on S. aureus. The values are reported as mean ± standard error of the mean (SEM) (n = 3), significance at p < 0.05 by One-way ANOVA and Tukey′s HSD post hoc test; * p < 0.01, ** p < 0.001, *** p < 0.0001, n.s. not significant.
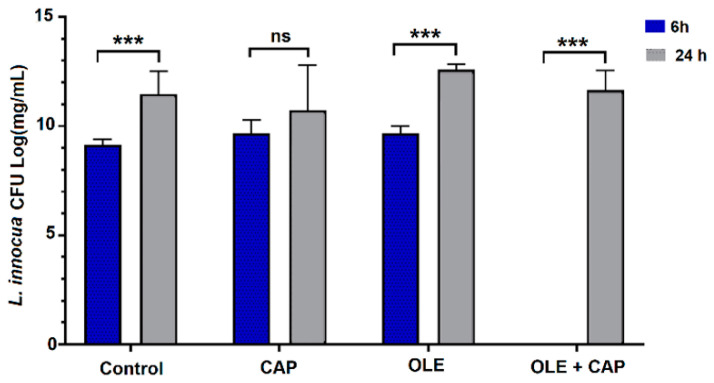
Figure 4.
The effects of CAP (1 min exposure time) and/or OLE on L. innocua. The values are reported as mean ± standard error of the mean (SEM) (n = 3), significance at p < 0.05 by One-way ANOVA and Tukey’s HSD post hoc test; *** p < 0.0001, n.s. not significant.
S. aureus samples subjected to OLE + CAP treatment significantly decreased after 6 h and 24 h compared to control and single treatments. The effectiveness of the combined treatment was confirmed by a very low replication of residual bacteria at 24 h.
The case of L. innocua was more complex. After a high eradication, at 6 h the bacteria started reproducing and grew up to control levels (Figure 4).
For this strain, the plain OLE was capable of controlling bacterial growth over time. In L. innocua, bacterial decontamination was observed after 6 h, but the effect was recovered after 24 h, reaching ~7∙Log [CFU]. Interestingly, and in accordance with the results reported in Figure 1, OLE alone demonstrated improved effectiveness in containing L. innocua growth.
Overall, the combined treatment (OLE + CAP) showed a synergistic antimicrobial effect against E. coli, S. aureus and L. innocua at 6 h. However, the remaining bacteria in small numbers were able to grow over longer time. The combined OLE + CAP treatment allowed a remarkable bacterial inactivation in terms of colony forming units (CFUs) during the exponential phase (6 h) for all the strains. In the stationary phase (24 h), the OLE + CAP treatment was sufficiently effective on E. coli, which grew by reaching ~7∙Log [CFU] and was still remarkably effective on S. aureus, which grew up to ~3∙Log [CFU], thus confirming the overall synergistic effect of OLE + CAP treatment. Differently, the individual treatments did not demonstrate relevant decontamination activity under our experimental conditions.
3. Discussion
The search for novel technologies for bacterial decontamination is a topic of intense research, which encompasses the use of chemical, thermal, radiation and combined treatments. A number of sectors can benefit from safe and green methods, such as bactericidal agents, in particular the food and health industries [1,2,3]. In time, microbes have started to become resistant to many decontamination technologies used on a large scale, thus leading to the need for newer and more powerful antibacterial agents. On the other hand, health and environmental factors are driving the industry towards less aggressive and better sustainable methods to treat their products [17,18,19,34]. However, low energy approaches are often less effective than their conventional counterparts. As several plants are known to possess antimicrobial properties, new research is also focusing on analyzing and using plant extracts with antibacterial purposes along with combined treatments, for application on an industrial scale [15,40]. The research on this topic is still fragmented, incomplete and controversial, due to the fact that biological derivatives, including OLE, greatly vary in their outcomes [29,30]. As such, better detailed and more systematic investigations are necessary to combine plant derivatives with other technologies to enhance antimicrobic efficacy.
We reported a preliminary investigation on the combined use of OLE + CAP against three pathogens, chosen to represent medical and food industries (i.e., E. coli, S. aureus and L. innocua). Since OLE derives from a bio-waste and CAP is a non-thermal technology based on ionized air, we propose OLE + CAP as a novel green approach potentially useful for bacterial decontamination.
It important to consider that OLE has a high variability in the concentration of bioactive compounds, as a consequence of various factors, such as the raw material and the extraction process, among others [50]. Moreover, the final composition of the OLE has great importance for its antimicrobial efficacy. Furthermore, broad-spectrum polyphenols have been found in OLE at small concentrations (Table 2), which can probably exert an antimicrobial effect together with the most abundant compound, oleuropein. In our study based on the surface spread method, the bacterial inactivation of plain OLE was evident (p < 0.0001) in S. aureus and L. innocua at all the time points up to 24 h, while E. coli did not show a considerable susceptibility to OLE. As a gram-negative bacterium, E. coli is in fact more resistant to conventional methods regarding its eradication [27]. The obtained results indicated that the presence of several phenolic compounds in 100 mg/mL TPs can exert an antimicrobial activity, but still insufficient to obtain a total or significant bacterial inactivation. It is reported that the polyphenols in OLE, or possibly the synergistic effects among them [16], may be responsible for OLE antimicrobial activity by inducing membrane permeability in bacteria, further inhibition of biochemical pathways and, finally, disintegration of the outer membrane leading to bacterial cell death [51].
The CAP effluent works at room temperature (RT) and atmospheric pressure, which eliminates the need for expensive noble gases, making it economically feasible on an industrial scale. CAP application leads to chemical species, such as ROS/RNS, which in combination with the polyphenolic compounds of OLE, mainly oleuropein, are supposed to inactivate the microorganisms. Recent investigations have shown that CAP efficacy is directly correlated to bacterial cell wall thickness in several species [52,53]. Gram-negative species, such as P. aeruginosa, were almost completely eradicated due to their thin cell membrane (2.4 nm cell wall), while Gram-positive species, such as B. subtilis, displayed the highest resistance to CAP, possessing thicker membranes (e.g., 55.4 nm cell wall). E. coli have a thinner outer membrane compared to the Gram-positive S. aureus and L. innocua. However, no clear trend is apparent from this and other studies, since complex interactions with the system, process, surface or medium may also impact on CAP efficacy in combination with cell type.
Another significant role in the mechanical disruption of the bacterial cell membrane is the effect of charged particles that could accumulate on the surface and cause electrostatic stress [53]. The reactive species produced in plasma react with the protein amino-acids and cause further structural changes in proteins, finally destroying the quiescent cells [54]. It is hypothesized that such morphological changes overcome the tensile strength of the cell membrane [55]. In fact, S. aureus demonstrated a size reduction of colonies (results not shown). Cell membrane perforation induced by etching enhances the diffusion of secondary reactive species that might be formed in the plasma discharge inside the cell [31]. CP, due to its complex composition and multiple different reactive components, is expected to play a role, independently or synergistically, in the inactivation of microbial targets. Generally, the efficacy of CP depends on the device design and system operating parameters, such as gas composition, flow rate, moisture, temperature, voltage, and frequency [31,50,56]. In addition, the ozone O3, present in CAP effluent could break structural bonds in the peptidoglycan component of the cell wall, such as C–O, C–N bonds, leading to cell wall destruction and, consequently, cell death [56,57].
On the basis of the results obtained, it can be deduced that CAP, containing ROS/RNS [58], combined with the action of the OLE polyphenols, exerts an enhanced antimicrobial activity by efficient damage and disruption of the bacterial membrane [52,53]. In fact, in combination with OLE, a better inactivation was also obtained with S. aureus, a Gram-positive bacterium, probably due to ROS-enhanced intracellular damage [53]. Efficacy of the combined effect of CAP and nisin against L. innocua, grown planktonically or as surface colonies in a food model, has recently been reported as another application for bacteria eradication [59], which is suggestive of a potentiated effect of CAP in combination with selected biomolecules. In fact, in our findings, OLE alone demonstrated improved effectiveness in containing L. innocua growth with respect to OLE + CAP, in which, after a first significant decrease, the bacteria grew again to control level. Especially in this case, higher time exposure to CAP or more concentrated OLE could be needed to show a more prolonged effect of bacterial eradication.
All in all, we demonstrated the remarkable effect of OLE + CAP in sample decontamination by E. coli, S. aureus and L. innocua after 6 h. This effect was best maintained up to 24 h using the S. aureus strain. On the other hand, E. coli and L. innocua grew again after 24 h. In the latter case, OLE alone was most effective by significantly reducing bacterial growth. As the most innovative approaches also consider developing material surfaces with intrinsic antimicrobial properties, e.g., by virtue of nanostructures inhibiting bacterial growth and biofilm formation [60,61], the addition of OLE to those surfaces followed by CAP effluent application could provide a robust antimicrobial strategy. As a combination, OLE and CAP can lead to better antimicrobial activity than individually and may replace or complement conventional thermal procedures in the food and biomedical industries. However, a multifactorial study that takes into account the type of bacteria, time and mode of exposure to CAP, content and type of polyphenols of OLE and overall cost-effectiveness, safety and sustainability is needed to optimize the process for industrial use. For enhanced safety, innovative intelligent labels could be applied to the OLE + CAP packaging to properly monitor the sterilization process as well as the storage conditions [62].
The availability of effective and low cost green technologies to disinfect edible and medical products would greatly impact the management of food- and healthcare-associated infections, overall estimated to affect 30 million people annually in Europe.
4. Materials and Methods
4.1. OLE Extraction and Characterization
OLE was extracted from Olea europaea var. Olivastra seggianese cultivar. The collection of the leaves from which OLE was extracted was performed at CNR-IVALSA, Follonica (GR), Italy. TP content was determined according to the Folin-Ciocalteu method using gallic acid as the standard equivalent (µg GAE/mg), purchased from Merk (Darmstadt, Germany) [45]. A high-performance liquid chromatography analysis (HPLC) was carried out to identify and quantify the major phenolic compounds of the obtained OLE.
4.2. CAP Technology
A dielectric barrier discharge reactor was used for CAP inactivation provided by Fourth State Medicine Ltd. To generate CP, the instrument was set up at a flow rate of 5 L/min air for 1 min. The samples were treated at RT (approx. 20 °C). The plasma power supply was set at 8 kV voltage and 20 kHz AC frequency.
4.3. In Vitro Tests
Inoculum was prepared from stock cultures of E. coli (ATCC 47076), S. aureus (ATCC 25923) and L. innocua (ATCC 33090), previously stored at −80 °C in Tryptic Soy Broth (TSB, Oxoid Ltd., UK), supplemented with 15% v/v glycerol (Merk, Darmstadt, Germany). More specifically, a loopful of thawed stock culture was inoculated in 15 mL TSB for 24 h at 37 °C. Subsequently, 20 μL was transferred to fresh 20 mL TSB and cultured at 37 °C for either 6 h or 24 h to obtain bacterial cells in the exponential or stationary phase, respectively, following a procedure reported in previous studies [48,49,50]. Thereafter, 1 mL taken from the 6 h and 24 h cultures was centrifuged at 10,000× g for 10 min at 23 °C and re-suspended in 1 mL of phosphate saline buffer (PBS; Merk). Each resuspended bacteria sample was separately treated with OLE at 100 mg/mL TPs, as it is higher than other concentrations demonstrating antibacterial activity as previously tested [45]. In a separate 24-well plate, 900 mL of treatment solution (composed by OLE dissolved in TBS) with 100 mg/mL TPs was added to 100 µL of bacterial inoculum. The effects of the OLE on the strains were evaluated at different times for a total duration of 24 h by plate counting (CFU/mL). The potential synergistic effect of OLE and CAP was evaluated at two relevant time points, 6 h and 24 h after application of OLE, CAP or OLE + CAP. The samples to be treated with CAP were exposed soon after OLE addition, for 1 min using air (5.0 L/min) at atmospheric pressure and RT. All experiments were carried out in triplicate to ensure statistical significance.
4.4. Statistical Analysis
Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) by conducting one-way analysis of variance (ANOVA, San Francisco, CA, USA) and the Tukey′s honestly significant difference (HSD) post hoc test to determine any statistically significant difference among samples. Significance was set at p < 0.05.
5. Conclusions
In this study, the antimicrobial effects of CAP, OLE and their combination against bacterial pathogens, i.e., E. coli, S. aureus and L. Innocua, was investigated. The combined OLE + CAP treatment had substantial antimicrobial activity against the bacterial species under study at early time points (6 h), whereas individual CAP and OLE treatments showed comparatively poor effects under the conditions surveyed. At the conclusion of this preliminary study on the combined antimicrobial effect of CAP and OLE, a synergistic effect during the exponential phase was evident, suggesting that the combination of these sustainable approaches could offer an innovative strategy for providing microbiological safety in the biomedical and food industry. We can hypothesize the use of OLE as a coating or polymer blend in food or medical packaging, followed by CAP treatment, to ensure a green and safely decontaminated environment.
Acknowledgments
Jasmine Esposito-Salsano, Maria Digiacomo and Marco Macchia (University of Pisa) are greatly acknowledged for their technical support to OLE characterization.
Author Contributions
Conceptualization, E.V. and J.G.D.l.O.; methodology, E.V., T.W. and H.E.K.; software, J.G.D.l.O.; validation, S.D., M.S. and E.V.; formal analysis, S.D., M.S. and E.V.; investigation, J.G.D.l.O. and J.G.-M.; resources, T.H. and E.V.; data curation, J.G.D.l.O.; writing—original draft preparation, J.G.D.l.O.; writing—review and editing, J.G.D.l.O., E.V., M.S., S.D., T.W.; visualization, H.E.K. and E.V.; supervision, J.G.-M. and E.V.; project administration, R.D.S., S.D. and E.V.; funding acquisition, E.V. and R.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Doctorate Degree Program of the Tuscany Region, Italy. The group has received support from the Department of Chemical and Process Engineering of the University of Surrey, Fourth State Medicine Ltd., the National Biofilm Innovation Centre UK, an Impact Acceleration Grant (IAA-KN9149C) of the University of Surrey, an IAA-EPSRC Grant (RN0281J) and the Royal Society. E.V. is grateful to the Royal Academy of Engineering for an Industrial Fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
T.W. and T.H. are Research & Innovation Manager and CTO respectively at Fourth State Medicine Ltd.
Sample Availability
Samples of OLE are available from the authors (J.G.D.l.O.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guillard V., Gaucel S., Fornaciari C., Angellier-Coussy H., Buche P., Gontard N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018;5:1–13. doi: 10.3389/fnut.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isbary G., Shimizu T., Li Y., Stolz W., Tomas H.M., Morfill G., Zimmermmann J. Cold atmospheric plasma devices for medical issues. Exp. Rev. Med. Dev. 2013;10:367–377. doi: 10.1586/erd.13.4. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Othman S., Jõudu I., Bhat R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules. 2020;25:510. doi: 10.3390/molecules25030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hashimi A.G. Antioxidant and antibacterial activities of Hibiscus sabdariffa L. extracts. Afr. J. Food Sci. 2012;6:506–511. doi: 10.5897/AJFS12.099. [DOI] [Google Scholar]
- 5.Amaral G.P., Mizdal C.R., Stefanello S.T., Mendez A.S.L., Puntel R.L., de Campos M.M.A., Soares F.A.A., Fachinetto R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2018;9:383–392. doi: 10.1016/j.jtcme.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moemenbellah-Fard M.D., Abdollahi A., Ghanbariasad A., Osanloo M. Antibacterial and leishmanicidal activities of Syzygium aromaticum essential oil versus its major ingredient, eugenol. Flavour Fragr. J. 2020;35:534–540. doi: 10.1002/ffj.3595. [DOI] [Google Scholar]
- 7.Fani M., Kohanteb J. In vitro antimicrobial activity of thymus vulgaris essential oil against major oral pathogens. J. Evidence-Based Complement. Altern. Med. 2017;22:660–666. doi: 10.1177/2156587217700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S., Moni B.M., Ahmed S., Gomes D.J., Shohael A.M. Comparative phytochemical, antioxidant, and antibacterial study of different parts of Doigota plants (Bixa orellana L.) Bull. Natl. Res. Cent. 2020;44:1–10. doi: 10.1186/s42269-020-00349-1. [DOI] [Google Scholar]
- 9.Sharifi-Rad M., Nazaruk J., Polito L., Morais-Braga M.F.B., Rocha J.E., Coutinho H.D.M., Salehi B., Tabanelli G., Montanari C., del Mar Contreras M., et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018;215:76–88. doi: 10.1016/j.micres.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Heck C.I., De Mejia E.G. Yerba mate tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007;77:R138–R151. doi: 10.1111/j.1750-3841.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 11.Razavi S.M., Zarrini G., Molavi G., Ghasemi G. Bioactivity of Malva sylvestris L., a medicinal plant from Iran. Iran. J. Basic Med. Sci. 2011;14:574–579. [PMC free article] [PubMed] [Google Scholar]
- 12.Fayera S., Babu G.N., Dekebo A., Bogale Y. Phytochemical investigation and antimicrobial study of leaf extract of Plantago lanceolata. Nat. Prod. Chem. Res. 2018;6:1000311. doi: 10.4172/2329-6836.1000311. [DOI] [Google Scholar]
- 13.Kolodziejczyk-Czepas J., Liudvytska O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2020 doi: 10.1007/s11101-020-09715-3. [DOI] [Google Scholar]
- 14.Fufa B.K. Anti-bacterial and anti-fungal properties of garlic extract (Allium sativum): A Review. Microbiol. Res. J. Int. 2019;28:1–5. doi: 10.9734/mrji/2019/v28i330133. [DOI] [Google Scholar]
- 15.Seow Y.X., Yeo C.R., Chung H.L., Yuk H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014;54:625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- 16.Lee O.-H., Lee B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Salomone R., Ioppolo G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily) J. Clean. Prod. 2012;28:88–100. doi: 10.1016/j.jclepro.2011.10.004. [DOI] [Google Scholar]
- 18.Lafka T.-I., Lazou A., Sinanoglou V., Lazos E. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods. 2013;2:18–31. doi: 10.3390/foods2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rombaut N., Tixier A.-S., Bily A., Chemat F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Bioref. 2014;8:530–544. doi: 10.1002/bbb.1486. [DOI] [Google Scholar]
- 20.Özcan M.M., Matthäus B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017;243:89–99. doi: 10.1007/s00217-016-2726-9. [DOI] [Google Scholar]
- 21.Nediani C., Ruzzolini J., Romani A., Calorini L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants. 2019;8:578. doi: 10.3390/antiox8120578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashmi M.A., Khan A., Hanif M., Farooq U., Perveen S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (Olive) Evidence-Based Complement. Altern. Med. 2015;2015:541591. doi: 10.1155/2015/541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Şahin S., Bilgin M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018;98:1271–1279. doi: 10.1002/jsfa.8619. [DOI] [PubMed] [Google Scholar]
- 24.Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- 25.Sudjana A.N., D’Orazio C., Ryan V., Rasool N., Ng J., Islam N., Riley T.V., Hammer K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents. 2009;33:461–463. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Djenane D., Gómez D., Yangüela J., Roncalés P., Ariño A. Olive leaves extract from Algerian oleaster (Olea europaea var. sylvestris) on microbiological safety and shelf-life stability of raw Halal minced beef during display. Foods. 2019;8:10. doi: 10.3390/foods8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertos I., Avena-Bustillos R.J., Martín-Diana A.B., Du W.X., Rico D., McHugh T.H. Antimicrobial Olive Leaf Gelatin films for enhancing the quality of cold-smoked Salmon? Food Packag. Shelf Life. 2017;13:49–55. doi: 10.1016/j.fpsl.2017.07.004. [DOI] [Google Scholar]
- 28.Pereira A.P., Ferreira I.C.F.R., Marcelino F., Valentão P., Andrade P.B., Eabra R., Estevinho L., Bento A., Pereira J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tranter H.S., Tassou S.C., Nychas G.J. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J. Appl. Bacteriol. 1993;74:253–259. doi: 10.1111/j.1365-2672.1993.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 30.Ghomari O., Sounni F., Massaoudi Y., Ghanam J., Drissi Kaitouni L.B., Merzouki M., Benlemlih M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019;23:e00347. doi: 10.1016/j.btre.2019.e00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourke P., Ziuzina D., Han L., Cullen P.J., Gilmore B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017;123:308–324. doi: 10.1111/jam.13429. [DOI] [PubMed] [Google Scholar]
- 32.Pan Y., Cheng J.-H., Sun D.-W. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr. Rev. Food Sci. Food Saf. 2019;18:1312–1326. doi: 10.1111/1541-4337.12474. [DOI] [PubMed] [Google Scholar]
- 33.Graves D. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012;45:263001. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 34.Djenane D., Aboudaou M., Djenane F., García-Gonzalo D., Pagán R. Improvement of the shelf-life status of modified atmosphere packaged camel meat using nisin and Olea europaea subsp. laperrinei leaf extract. Foods. 2020;9:1336. doi: 10.3390/foods9091336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J., Huang K., Wang J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control. 2015;50:482–490. doi: 10.1016/j.foodcont.2014.09.037. [DOI] [Google Scholar]
- 36.Cullen P.J., Lalor J., Scally L., Boehm D., Milosavljević V., Bourke P., Keener K. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018;15:1–11. doi: 10.1002/ppap.201700085. [DOI] [Google Scholar]
- 37.Chang Y., Tu P., Wu M., Hsueh T., Hsu S. A study on chitosan modification of polyester fabrics by atmospheric pressure plasma and its antibacterial effects. Fibers Polym. 2008;9:307–311. doi: 10.1007/s12221-008-0049-6. [DOI] [Google Scholar]
- 38.Nikiforov A., Deng X., Xiong Q., Cvelbar U., Degeyter N., Morent R., Leys C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016;49:204002. doi: 10.1088/0022-3727/49/20/204002. [DOI] [Google Scholar]
- 39.Karam L., Jama C., Mamede A.-S., Fahs A., Louarn G., Dhulster P., Chihib N.-E. Study of nisin adsorption on plasma-treated polymer surfaces for setting up materials with antibacterial properties. Reactive Funct. Polym. 2013;73:1473–1479. doi: 10.1016/j.reactfunctpolym.2013.07.017. [DOI] [Google Scholar]
- 40.Duday D., Vreuls C., Moreno M., Frache G., Boscher N.D., Zocchi G., Archambeau C., Van De Weerdt C., Martial J., Choquet P. Atmospheric pressure plasma modified surfaces for immobilization of antimicrobial nisin peptides. Surf. Coat. Technol. 2013;218:152–161. doi: 10.1016/j.surfcoat.2012.12.045. [DOI] [Google Scholar]
- 41.Schmid M., Dallmann K., Bugnicourt E., Cordoni D., Wild F., Lazzeri A., Noller K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012;2012:5–7. doi: 10.1155/2012/562381. [DOI] [Google Scholar]
- 42.Tihminlioglu F., Atik I.D., Özen B. Water vapor and oxygen-barrier performance of corn-zein coated polypropylene films. J. Food Eng. 2010;96:342–347. doi: 10.1016/j.jfoodeng.2009.08.018. [DOI] [Google Scholar]
- 43.Laroussi M., Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004;233:81–86. doi: 10.1016/j.ijms.2003.11.016. [DOI] [Google Scholar]
- 44.McKeen L. Introduction to food irradiation and medical sterilization. In: McKeen L., editor. Film Properties of Plastics and Elastomers. 3rd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2012. pp. 1–40. [Google Scholar]
- 45.De la Ossa J.G., Felice F., Azimi B., Salsano J.E., Digiacomo M., Macchia M., Danti S., Di Stefano R. Waste autochthonous tuscan olive leaves (Olea europaea var. olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019;20:5918. doi: 10.3390/ijms20235918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., McKeever L.C., Malik N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017;8:1–8. doi: 10.3389/fmicb.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costello K.M., Gutierrez-Merino J., Bussemaker M., Ramaioli M., Baka M., Van Impe J.F., Velliou E.G. Modelling the microbial dynamics and antimicrobial resistance development of Listeria in viscoelastic food model systems of various structural complexities. Int. J. Food Microbiol. 2018;286:15–30. doi: 10.1016/j.ijfoodmicro.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Costello K.M., Gutierrez-Merino J., Bussemaker M., Smet C., Van Impe J.F., Velliou E.G. A multi-scale analysis of the effect of complex viscoelastic models on Listeria dynamics and adaptation in co-culture systems. AIChE J. 2020;66:1–15. doi: 10.1002/aic.16761. [DOI] [Google Scholar]
- 49.Zhou J., Velliou E., Hong S.H. Investigating the effects of nisin and free fatty acid combined treatment on Listeria monocytogenes inactivation. LWT-Food Sci. Technol. 2020;133:110115. doi: 10.1016/j.lwt.2020.110115. [DOI] [Google Scholar]
- 50.Difonzo G., Russo A., Trani A., Paradiso V.M., Ranieri M., Pasqualone A., Summo C., Tamma G., Silletti R., Caponio F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods. 2017;31:63–70. doi: 10.1016/j.jff.2017.01.039. [DOI] [Google Scholar]
- 51.Mai-Prochnow A., Clauson M., Hong J., Murphy A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep38610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han L., Boehm D., Patil S., Cullen P.J., Bourke P. Assessing stress responses to atmospheric cold plasma exposure using Escherichia coli knock-out mutants. J. Appl. Microbiol. 2016;121:352–363. doi: 10.1111/jam.13172. [DOI] [PubMed] [Google Scholar]
- 53.Dobrynin D., Fridman G., Friedman G., Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009;11:115020. doi: 10.1088/1367-2630/11/11/115020. [DOI] [Google Scholar]
- 54.Surowsky B., Fröhling A., Gottschalk N., Schlüter O., Knorr D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014;174:63–71. doi: 10.1016/j.ijfoodmicro.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 55.Yang D.C., Blair K.M., Salama N.R. Staying in shape: The impact of cell shape on bacterial survival in diverse environments. Microbiol. Mol. Biol. Rev. 2016;80:187–203. doi: 10.1128/MMBR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Woedtke T., Metelmann H.-R., Weltmann K.-D. Clinical plasma medicine: State and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Phys. 2014;54:104–117. doi: 10.1002/ctpp.201310068. [DOI] [Google Scholar]
- 57.Stoffels E., Kieft I.E., Sladek R.E.J., Van Den Bedem L.J.M., Van Der Laan E.P., Steinbuch M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006;15:S169–S180. doi: 10.1088/0963-0252/15/4/S03. [DOI] [Google Scholar]
- 58.Yusupov M., Bogaerts A., Huygh S., Snoeckx R., Van Duin A.C.T., Neyts E.C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. C. 2013;117:5993–5998. doi: 10.1021/jp3128516. [DOI] [Google Scholar]
- 59.Costello K.M., Smet C., Gutierrez-Merino J., Bussemaker M., Van Impe J.F., Velliou E.G. The impact of food model system structure on the inactivation of Listeria innocua by cold atmospheric plasma and nisin combined treatments. Int. J. Food Microbiol. 2021;337:108948. doi: 10.1016/j.ijfoodmicro.2020.108948. [DOI] [PubMed] [Google Scholar]
- 60.Milazzo M., Gallone G., Marcello E., Mariniello M.D., Bruschini L., Roy I., Danti S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020;11:60. doi: 10.3390/jfb11030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danti S., Azimi B., Candito M., Fusco A., Sorayani Bafqi M.S., Ricci C., Milazzo M., Cristallini C., Latifi M., Donnarumma G., et al. Lithium niobate nanoparticles as biofunctional interface material for inner ear devices. Biointerphases. 2020;15:31004. doi: 10.1116/6.0000067. [DOI] [PubMed] [Google Scholar]
- 62.Romano L., Portone L., Coltelli M.B., Patti F., Saija R., Iatì M.A., Gallone G., Lazzeri A., Danti S., Marago O., et al. Intelligent non-colorimetric indicators for the perishable supply chain by non-wovens with photo-programmed thermal response. Nat. Commun. 2020;11:5991. doi: 10.1038/s41467-020-19676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.