Abstract
Background: Oral iron supplementation causes gastrointestinal side effects. Short-term alterations in dietary iron exacerbate inflammation and alter the gut microbiota, in murine models of colitis. Patients typically take supplements for months. We investigated the impact of long-term changes in dietary iron on colitis and the microbiome in mice. Methods: We fed mice chow containing differing levels of iron, reflecting deficient (100 ppm), normal (200 ppm), and supplemented (400 ppm) intake for up to 9 weeks, both in absence and presence of dextran sodium sulphate (DSS)-induced chronic colitis. We also induced acute colitis in mice taking these diets for 8 weeks. Impact was assessed (i) clinically and histologically, and (ii) by sequencing the V4 region of 16S rRNA. Results: In mice with long-term changes, the iron-deficient diet was associated with greater weight loss and histological inflammation in the acute colitis model. Chronic colitis was not influenced by altering dietary iron however there was a change in the microbiome in DSS-treated mice consuming 100 ppm and 400 ppm iron diets, and control mice consuming the 400 ppm iron diet. Proteobacteria levels increased significantly, and Bacteroidetes levels decreased, in the 400 ppm iron DSS group at day-63 compared to baseline. Conclusions: Long-term dietary iron alterations affect gut microbiota signatures but do not exacerbate chronic colitis, however acute colitis is exacerbated by such dietary changes. More work is needed to understand the impact of iron supplementation on IBD. The change in the microbiome, in patients with colitis, may arise from the increased luminal iron and not simply from colitis.
Keywords: iron, diet, intestinal inflammation, inflammatory bowel disease, fecal microbiota
1. Introduction
Inflammatory bowel disease (IBD) is a debilitating, relapsing-remitting long-term condition of the gastrointestinal tract that affects around 240,000 people in the UK [1,2,3]. Approximately one-third of patients develop iron deficiency anemia because of intestinal bleeding and/or malabsorption [4,5,6,7]. Iron deficiency is associated with a poor quality of life, fatigue, breathlessness, chest pain, and restless legs, as well as poor concentration and subfertility [8]. Iron absorption and metabolism is tightly regulated as iron is toxic [9]. The absorption of iron is influenced by serum ferritin. Ferritin ought to be low in iron deficiency, however it is an acute phase protein and its level is increased by inflammation such as active IBD, furthermore, hepcidin expression is also increased: these changes impair iron absorption exacerbating iron deficiency and complicating its diagnosis [10].
Iron deficiency may be treated effectively by intravenous or oral iron replacement therapy [11]. Different routes of administration have different side effect profiles [12]. In general, 10–15% of oral iron is absorbed by the small bowel [13] depending on requirement for iron. Unabsorbed iron leads to gastrointestinal side effects by generating free radicals in the colon [14] and stimulating the growth of some bacteria, and host infection by key enteric pathogens, including Escherichia coli pathovars [15,16,17].
Others have reported that, in mice, a low iron diet alters the abundance of the Prevotellaceae and Porphyromonadaceae families [18]. Thus, it appears that both low and high levels of luminal iron may influence the microbiome.
Unabsorbed oral iron supplements and gastrointestinal bleeding may result in an increase in luminal iron which may exacerbate IBD [19] and lead to increased proliferation and virulence of some bacteria [20,21,22]. Abnormal microbiota composition and decreased complexity of the gut microbiota are common features of patients with IBD, including both Crohn’s disease or ulcerative colitis [23]. Bacterial dysbiosis within the intestine is also associated with relapse of IBD [24,25] but it is not clear whether relapsing inflammation leads to dysbiosis by modulating luminal iron [26].
We hypothesized that iron supplementation (and or bleeding) in IBD patients could change the composition of the gut microbiota and potentially influence the natural history of IBD. To investigate this, we assessed the effects of long-term alterations of dietary iron on intestinal microbiota in murine models of colitis to eliminate any confounding factors based on background genetics that would be inevitable in a human population.
2. Results
2.1. Chronic Colitis in C57BL/6 Mice Is Not Influenced by Altering Dietary Iron
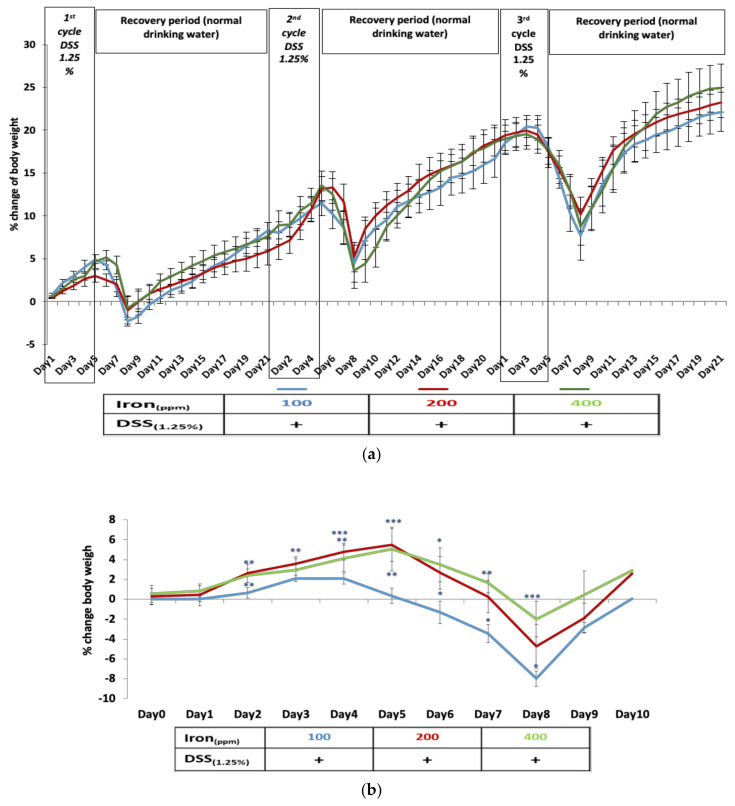
Chronic colitis was induced in C57BL/6 mice by three treatment cycles of 1.25% w/v DSS in their drinking water. At autopsy, all mice showed histological evidence of mild chronic colitis. Chronic colitis-induced weight loss in C57BL/6 mice was not influenced by altering dietary iron: all mice, irrespective of the iron content of their diet, lost significant body weight from day 6, with maximal weight loss occurring at day 8 of each of the DSS treatment cycles (Figure 1a). Although mice receiving an iron-deficient diet (100 ppm iron) appeared to lose more weight than other groups, this difference was not significant (Figure 1a). Non-DSS treated control mice, irrespective of dietary iron content, all showed steady increases in body weight. However, mice fed a 400 ppm iron supplemented diet showed significantly greater weight gain throughout the 63-day study; all sampling points p < 0.05; n = 4, Kruskal–Wallis test (Supplementary Information, Figure S2).
Figure 1.
Dextran sulfate sodium (DSS) colitis – induced body weight changes in mice consuming diets of differing dietary iron levels. (a) Percentage weight loss observed in mice with chronic colitis induced by three cycles of 1.25% w/v DSS during the 63-day period was not influenced by consumption of an iron-deficient diet (100 ppm iron [blue]), standard chow diet (200 ppm iron [red]) nor an iron supplemented diet (400 ppm iron [green]); Data presented as mean ± standard error of the mean (SEM). No statistical differences were seen between groups (n = 8 mice per group). (b) Percentage body weight loss in mice resulting from DSS-induced acute colitis on 63 days on deficient and supplemented iron diets compared with standard chow (n = 4 female mice per group). Statistical differences * p < 0.05, ** p < 0.01, *** p < 0.001; Kruskal–Wallis test followed by multiple comparison tests.
2.2. Acute Colitis Is More Severe in Mice Fed Long-Term on an Iron Deficient Diet
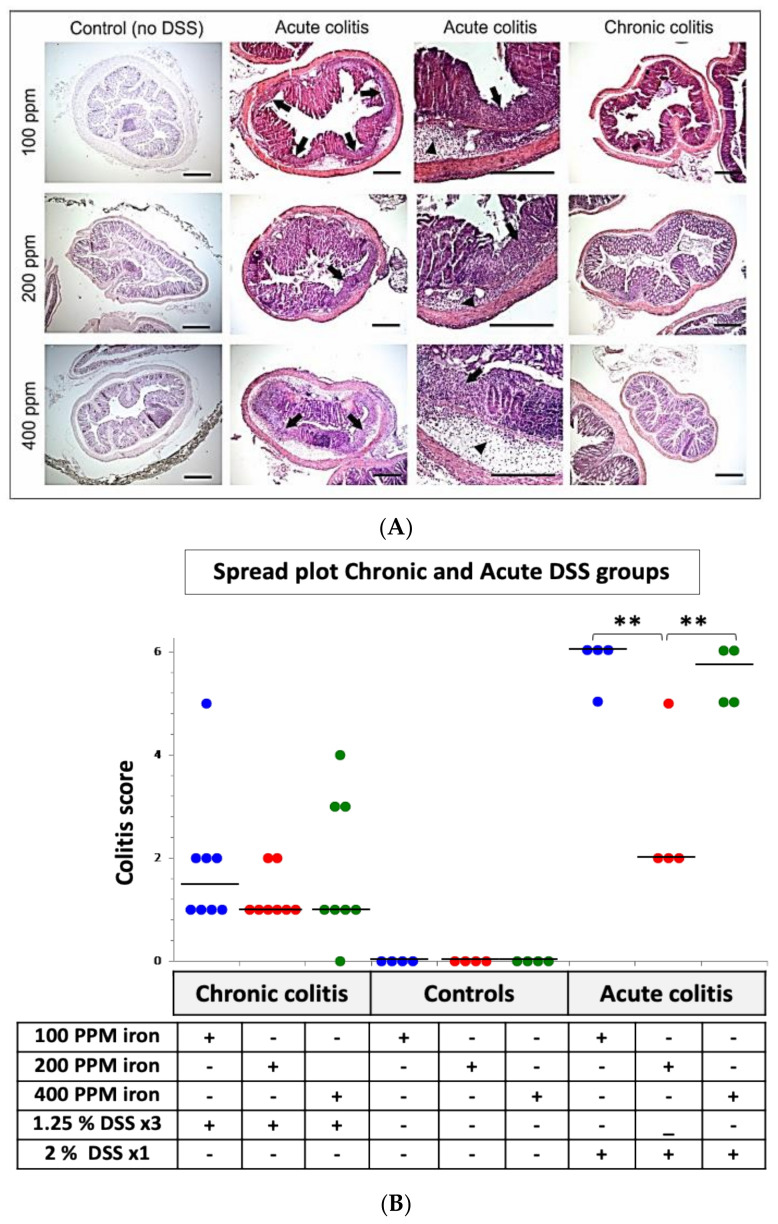
Following 53-days consuming one of three diets of differing iron content, mice ingesting 2% w/v DSS for 5-days in their drinking water developed moderately severe acute colitis. Mice ingesting 2% w/v DSS for 5-days in their drinking water also lost significant body weight with acute colitis. Weight loss began earlier (day 4) in those ingesting an iron-deficient (100 ppm) diet compared to mice consuming standard (200 ppm) and iron-supplemented (400 ppm) chow (p < 0.001; n = 4; Kruskal–Wallis test (Figure 1b). Indeed, mice fed the iron-deficient diet lost significantly more weight (peak weight loss at day 8, p < 0.001; n = 4, Kruskal–Wallis test) than the other treated groups from day 4 to day 8. The colons of control mice, that had not received DSS, appeared histologically normal (see Figure 2A). The colitis scores were significantly greater in mice that had been treated with 2% w/v DSS after consuming either the iron deficient (100 ppm) diet or the iron supplemented (400 ppm) diet, compared to mice on standard chow (p < 0.01; n = 4, Kruskal–Wallis test) and compared to mice that received three cycles of 1.25% w/v DSS (Figure 2B).
Figure 2.
Histological analysis of colon from DSS-treated mice in consuming diets of differing dietary iron levels. (A) Representative hematoxylin and eosin-stained segments of distal colon from control C57BL/6J mice ingesting 100, 200 or 400 ppm iron diets alone (n = 4 mice in total), C57BL/6 with acute (n = 4) and chronic DSS-induced colitis (n = 8) administered with 100, 200 or 400 ppm iron diets as indicated. Arrowheads highlight submucosal oedema; arrows highlight almost complete loss of colonic epithelium and leukocyte infiltration. Scale bar: 200 µm. (B): Inflammation (colitis) scores for all groups’ DSS-treated (24 (63-days) and 12 (10-days) mice per group) and untreated (controls) 12 (63-days) mice on different iron diets. Horizontal lines at the median. an iron deficient diet (100 ppm iron [blue]), standard chow diet (200 ppm iron [red]) nor an iron supplemented diet (400 ppm iron [green]) Differences tested by Kruskal–Wallis test followed by multiple comparison tests ** p < 0.01.
2.3. Intestinal Fibrosis Is Increased in Mice with Chronic Colitis Fed an Iron Deficient Diet
Masson’s trichrome staining was used to assess the level of fibrosis in the colons of mice with DSS-induced chronic colitis. As expected, the colon of all mice with chronic colitis showed increased levels of fibrosis, as evidenced by increased presence of collagen and proteoglycan within the mucosa and submucosa) compared to non-DSS treated animals (p < 0.01, n = 8 mice per group; Kruskal–Wallis test). Mice with chronic colitis fed the iron-deficient diet (100 ppm iron) had markedly more fibrosis evident (p < 0.05, n = 8; Kruskal–Wallis test) than those the mice treated with DSS consuming standard and iron supplemented diets (see Supplementary Information—Figure S3).
2.4. Fecal Calprotectin Changes in Colitic Mice Fed Differing Iron Diets
A trend of increasing level of calprotectin detected in feces was seen following each cycle of 1.25% w/v DSS treatment in mice consuming altered (deficient and supplemented) iron diets for 63-days compared to those mice consuming standard chow. However, significant differences were only seen in colitic mice consuming the high iron supplemented (400 ppm) chow at day-21 and day-63; both p < 0.01, n = 8 per group; Kruskal–Wallis test (Supplementary Information—Figure S4). Following 53-days consuming one of three diets of differing iron levels, induction of moderately severe acute colitis over the following 10-days led to significantly increased calprotectin in the feces. The change in fecal calprotectin appears to be greater in mice with acute colitis consuming the 100 ppm iron-deficient diet than seen in the other experimental groups (Supplementary Information—Figure S4). No changes were seen in non-DSS-treated mice on the three respective diets.
2.5. Fecal Iron Changes in Colitic Mice Fed Differing Iron Diets
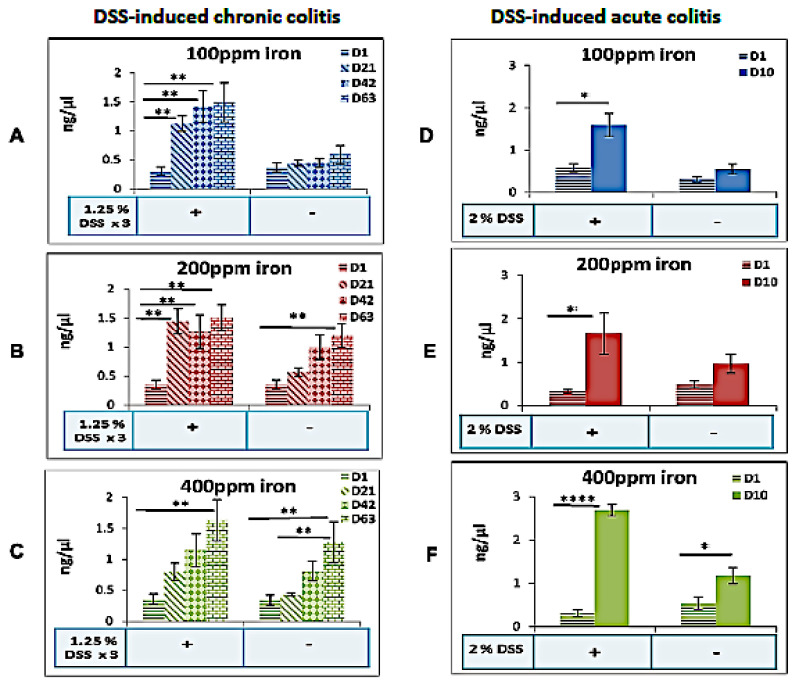
In the chronic colitis study, DSS-treated mice consuming a high (400 ppm) supplemented iron diet showed an increase in fecal iron levels at day-63 compared to day-1 (2.6-fold increase). Groups ingesting the iron deficient (100 ppm) and standard iron (200 ppm) diets, and treated with DSS to induce colitis, both showed significant increase in fecal iron levels at day-21, day-42, and day-63 compared to levels measured on day-1 (Figure 3), consistent perhaps with the presence of luminal iron from bleeding resulting from colitis. In control mice, fecal iron was only seen to be significantly increased at day-63 when ingesting standard (200 ppm) and supplemented (400 ppm) diets, and not in mice consuming the low (100 ppm) iron diet (Figure 3).
Figure 3.
Fecal iron changes in mice fed differing iron diets in the presence or absence of colitis. Fecal iron concentrations in the presence or absence of DSS-induced chronic DSS (day-1 vs. day-21, day-42, and day-63; n = 8 mice per group) or acute colitis DSS (day-1 vs. day-10; n = 4 mice per group), for mice consuming a (A,D) deficient (100 ppm iron), (B,E) standard (200 ppm iron) or (C,F) supplemented (400 ppm iron) chow diet respectively. Data are presented as a mean ± standard error of the mean (SEM). Differences were tested by Kruskal–Wallis test followed by multiple comparison Dunn’s test; * p < 0.05, ** p < 0.01, **** p < 0.0001.
Following induction of acute colitis, fecal iron concentration increased significantly in all dietary study groups; all p < 0.05 (Figure 3). This was more pronounced in mice ingesting higher levels of iron, i.e., the iron supplemented (400 ppm) diet.
2.6. Bacterial Diversity in Mice Fed Differing Levels of Dietary Iron and Following Induction of Chronic Colitis
Tables of rarefied OTU data were prepared, and three measures of alpha diversity were estimated: chao1, the observed number of species, and the phylogenetic distance. These estimates were plotted as rarefaction curves using Qiime (see Supplementary Information—Figure S5). Weighted and unweighted pairwise UniFrac matrices UPGMA trees were prepared for measure of beta-diversity (Supplementary Information—Figure S6).
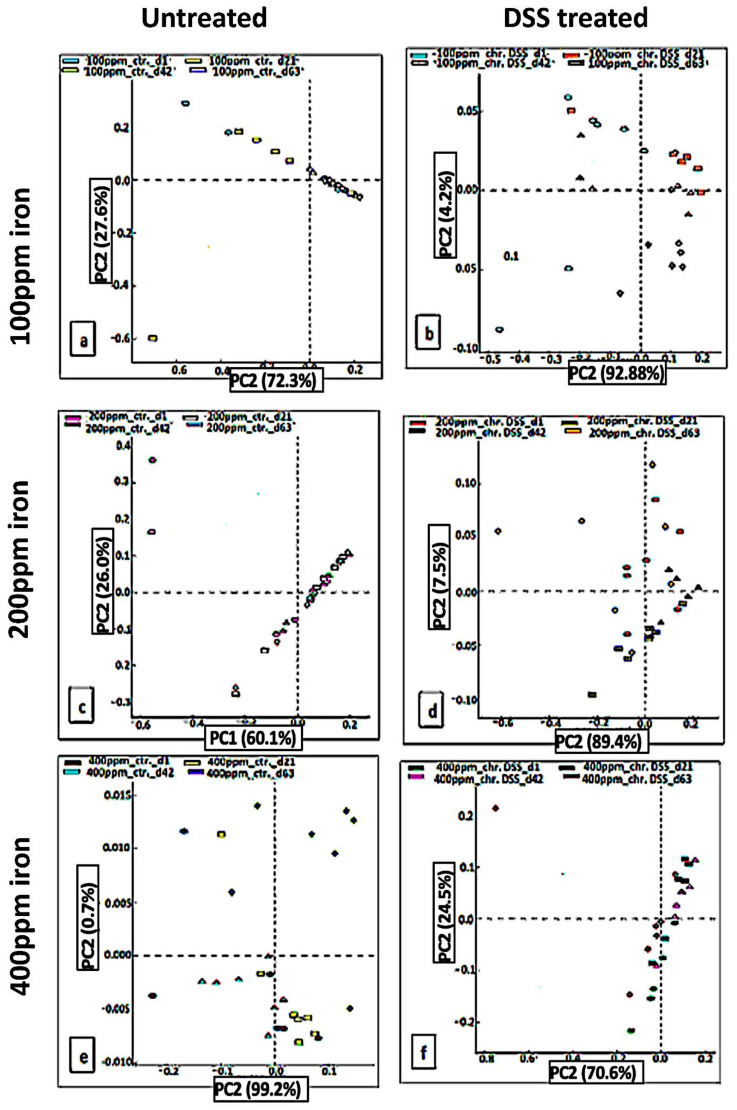
Principal component analysis (PCA) identified linear combinations of fecal microbial taxa associated with the duration of the test diets (Figure 4). Data showed overlap in identified taxa within fecal samples obtained from mice fed the low iron (100 ppm) diet, mice on the standard iron (200 ppm) diet, and in mice with chronic colitis induced by DSS ingesting the standard iron diet, from days 1, 21, and 42 to day-63 (Figure 4a,c,d). However, there was clustering with little separation of samples pre- and post-DSS treatment who were fed the low iron (100 ppm) diet and the high (400 ppm) iron diet, as well as mice fed the high iron (400 ppm) diet alone (Figure 4b,e,f). The high iron (400 ppm) diet altered the microbial community significantly in both DSS-treated and untreated mice.
Figure 4.
PCA to show unweighted UniFrac diseases after DSS treatment. In chronic DSS, PCA plots of the unweighted UniFrac distances of pre-and post-DSS-intervention stool samples from chronic (3 cycles) DSS – treated mice (b,d,f) and (a,c,e) untreated mice at phylum-level, phylogenetic classification of 16S rRNA gene sequences. Symbols represent data from individual mice, color-coded by the indicated metadata. Statistical differences were assessed by Kruskal–Wallis H-test followed by Storey’s FDR multiple test correction.
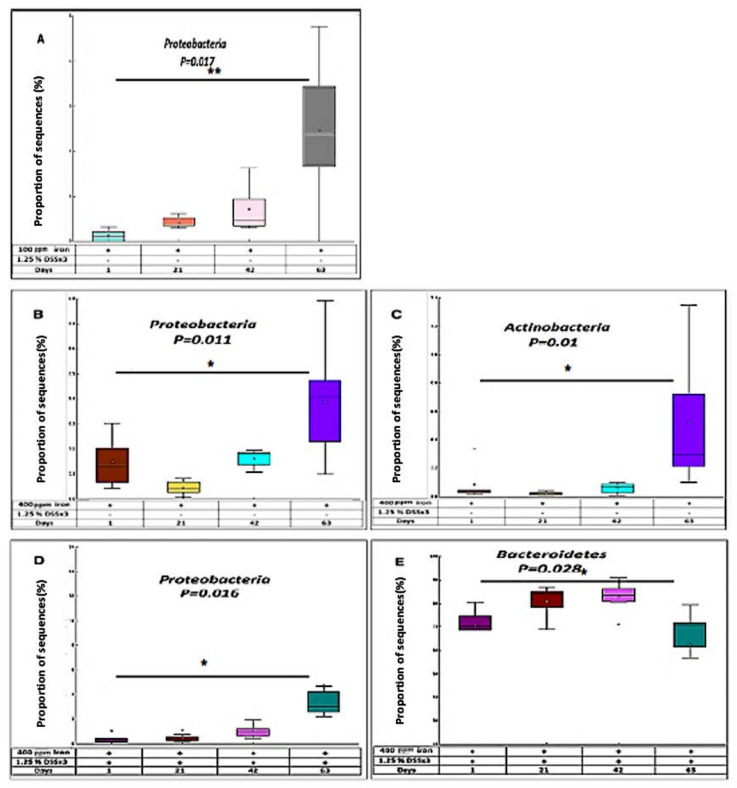
Post-hoc tests revealed a significant difference in the amount of Proteobacteria in chronic DSS-treated mice ingesting a low (100 ppm) iron diet when day-1 and day-63 were compared (p < 0.017, Kruskal–Wallis H-test) (Figure 5A). In control mice fed the high (400 ppm) iron diet, there was a significant increase in two key phyla, Proteobacteria and Actinobacteria, when comparing day-1 and day-63 samples (p < 0.05 for both) (Figure 5B,C). The analysis of fecal samples from mice treated with DSS fed the high (400 ppm) iron diet showed alterations in abundance of Bacteroidetes and Proteobacteria comparing day-1 and day-63; with a significant decrease observed in Bacteroidetes (p < 0.05) and increase in Proteobacteria (p < 0.05) (Figure 5D,E).
Figure 5.
Distribution of bacteria in response to chronic DSS. (A) In chronic DSS, box plot showing the distribution in the proportion of Proteobacteria assigned to samples at day-1, 21, 42, and 63 from 100 ppm iron DSS-treated mice. In chronic DSS, box plot showing the distribution in the proportion of two phyla (Proteobacteria (B) and Actinobacteria (C)) assigned to samples from 400 ppm iron untreated mice. In chronic DSS, box plot showing the distribution in the proportion of two phyla (Proteobacteria (D) and Bacteroidetes (E)) assigned to samples from 400 ppm iron DSS-treated mice.
Using STAMP software encourages the use of effect sizes and confidence intervals to identify significance [27]. Further bioinformatics analysis identified 4 phyla (Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria) and 15 taxa of interest. The results of the relative abundances of various phyla and identified genera were found only for iron-deficient diet, DSS-treated group, and for high iron diet groups (both DSS-treated and untreated) are summarized in Table 1a–c. Of the four phyla identified, Firmicutes were highly abundant within samples from all dietary and treatment groups, whilst Actinobacteria were seen to be less abundant. In the DSS-treated mice fed the iron deficient (100 ppm) diet, we found marked reduction in six genera, including Lactobacillus (corrected p = 0.002), Dorea, Clostridium, Bacteroides, Odoribacter (all p < 0.05) and Bilophila (p = 0.008), and an increase in Prevotella (p = 0.008). In the DSS-treated group fed the high (400 ppm) iron diet, a significant reduction was observed in the relative abundance of Lactobacillus (p = 0.01). The only control group where significant differences in the relative abundance of taxa were found was the supplemented (high) 400 ppm iron group, where with the seven genera showed a statistically significant difference. Increases were shown in Lactobacillus (corrected p = 0.02), Oscillospira (p = 0.03), Adlercreutzia and Candidatus Arthromitus (both p = 0.04), whereas reductions were observed for Bacteroides (p = 0.02), Bilophila (p = 0.03) and Ruminococcus (p = 0.04) Table 1a–c.
Table 1.
(a): Genus-level taxonomic composition of fecal samples from 100 ppm iron DSS-treated mice (Day-1 vs. 21, 42, and 63 samples), (b) Genus-level taxonomic composition of fecal samples from 400 ppm iron DSS-treated mice (Day-1 vs. 21, 42, and 63 samples), (c) Genus-level taxonomic composition of fecal samples from 400 ppm iron untreated mice (Day-1 vs. 21, 42, and 63 samples).
| (a) | |||
| 100 ppm iron DSS-treated group | |||
| Taxon | p-values | p-values (corrected) | Effect size |
| p_Bacteroidetes; g_Bacteroides | 0.003 | 0.047 | 0.496 |
| p_Bacteroidetes; g_Odoribacter | 0.002 | 0.04 | 0.620 |
| p_Bacteroidetes; g_Prevotella | 0.0002 | 0.008 | 0.669 |
| p_Firmicutes; g_Clostridium | 0.002 | 0.04 | 0.431 |
| p_Firmicutes; g_Dorea | 0.003 | 0.047 | 0.138 |
| p_Firmicutes; g_Lactobacillus | 0.00002 | 0.002 | 0.880 |
| p_Proteobacteria; g_Bilophila | 0.0002 | 0.008 | 0.766 |
| (b) | |||
| 400 ppm iron DSS-treated group | |||
| Taxon | p-values | p-values (corrected) | Effect size |
| p_Firmicutes; g_Lactobacillus | 0.0001 | 0.01 | 0.74 |
| (c) | |||
|
400 ppm iron untreated group
(Controls) | |||
| Taxon | p-values | p-values (corrected) | Effect size |
| p_Actinobacteria; g_Adlercreutzia | 0.002 | 0.04 | 0.49 |
| p_Bacteroidetes; g_Bacteroides | 0.0005 | 0.02 | 0.68 |
| p_Firmicutes; g_Candidatus Arthromitus | 0.003 | 0.04 | 0.54 |
| p_Firmicutes; g_Lactobacillus | 0.0002 | 0.02 | 0.77 |
| p_Firmicutes; g_Oscillospira | 0.001 | 0.03 | 0.61 |
| p_Firmicutes; g_Ruminococcus | 0.002 | 0.04 | 0.46 |
| p_Proteobacteria; g_Bilophila | 0.001 | 0.03 | 0.55 |
3. Discussion
Induction of colitis in mice using DSS is a popular surrogate model for the study of human ulcerative colitis [28]. The mechanism of action of DSS is not well defined and likely a combination of actions, including direct toxic activity on the colonic epithelium [29], induced mucosal infiltration of macrophages [30] and/or alteration of the intestinal microbiota [31,32]. More common has been the use of DSS to induce an acute colitis; however, Okayasu and colleagues have described a chronic colitis model in mice, which may more closely mirror chronic IBD in humans [28]. We recently reported effects for short-term dietary iron modification in the DSS acute colitis model, using iron deficient (100 ppm) and iron supplemented (400 ppm) diets compared to standard chow (200 ppm dietary iron), where both deficient and supplemented iron diets were associated with more severe colitis than seen with a standard chow diet [19]. Here, we report the effects of the same dietary modifications in a model of DSS-induced chronic colitis, and also the effects of long-term prior dietary modification on a model of acute colitis, so as to mimic relapse of IBD following iron supplementation or deficiency in patients.
We found that when acute colitis was induced after 7 weeks of dietary iron modification, mice consuming the iron-deficient (100 ppm) or iron supplemented (400 ppm) diet also developed more severe colitis than mice ingesting standard chow. Both clinical and histological data were concordant for the iron deficient (100 ppm) group. Our previous work had shown that reduced dietary iron intake was associated with increased weight loss and more severe colitis following the induction of acute colitis using DSS [19]. In contrast, mice in which chronic colitis was induced using DSS, whether consuming dietary iron levels of 100 ppm, 200 ppm, or 400 ppm, showed only modest weight loss and histological colitis. We must acknowledge that the differences in weight may be a reflection of iron deficiency per se, and not entirely due to colitis.
Fecal calprotectin changes were seen in our colitic mice fed differing iron diets, being greater in those animals consuming an iron-deficient diet in the acute colitis model, and with the iron supplemented diet in the chronic colitis model, most likely reflecting an enhanced protective response against inflammation-induced damage [33].
As expected, supplementation of dietary iron led to an increase in fecal iron levels and following induction of chronic colitis, fecal iron was increased in all mice irrespective of levels of dietary iron intake. In the acute DSS experiment, mice ingesting the iron supplemented (400 ppm) diet showed the most marked increases in fecal iron levels. There is an obvious paradox: reducing dietary iron was associated with an increase in loss of iron in feces. The mechanism appears to be exacerbating DSS-induced colitis. We speculate that the iron-deficient diet led to more severe colitis, which secondarily led to an increase in bleeding and hence the observed increased in fecal iron levels. Therefore, iron deficiency and DSS-induced colitis pathogenetic mechanisms are synergistic [27]. However, it is necessary to further explore the specific mechanism.
Changing dietary iron concentrations led to a significant alteration in the intestinal microbiota, both in mice with chronic colitis fed the iron-deficient (100 ppm) and the iron supplemented (400 ppm) diet, and in control (non-colitic) mice ingesting the high iron (400 ppm) diet. Previous research has established a reduction in the biodiversity of commensal bacteria in IBD [34,35,36,37]. In mouse experiments, changes in bacterial composition have been observed as a consequence of colonic inflammation and infection [17,38], with particular intestinal pathogens (species from within the phylum Proteobacteria) appearing to take advantage of this inflammatory environment. This observation is in agreement with the ‘food hypothesis’ and ‘differential killing’ hypothesis [39]. These two mechanisms are likely to contribute to the loss of colonization resistance in the inflamed bowel [39]. The post-hoc analysis of our data revealed that Proteobacteria were increased significantly, particularly in fecal samples obtained from mice with chronic colitis fed an iron deficient (100 ppm) or iron-supplemented (400 ppm) diet or fed the latter diet in the absence of inflammation. In other words, there was a significant association between fecal iron levels and abundance of Proteobacteria. Meanwhile, a relative abundance of Bacteroidetes was observed to be lower in colitic mice fed on the high iron (400 ppm) diet. Together these data suggest that Proteobacteria are dependent on luminal iron, but Bacteroidetes are suppressed by inflammation and/or luminal iron. However, acute DSS groups post-hoc analysis did not reveal any significance this could be, due to either low number of mice (4 per each group) or the detergent effect of 2% DSS on microbiota which caused desctuction to those microorganisms.
Investigation of the effects of dietary iron upon the murine gut microbiota has been previously described by the Haller group [40], where they identified eight bacterial families and nine genera were significantly affected by luminal iron deficiency: Bifidobacterium (p < 0.0018), Succinivibrio, Turicibacter, and Clostridium genera were significantly increased in mice fed an iron-depleted diet, whereas the genera Desulfovibrio, Dorea, and Bacteroides were greatly reduced [41]. Our data analysis showed that the relative abundance of seven genera were significantly altered with respect to dietary iron and colitis. Mice with chronic colitis-induced by DSS and fed either low (100 ppm) or high (400 ppm) iron diet, showed marked differences in seven genera belonging to three phyla Firmicutes, Bacteroidetes, and Proteobacteria within the fecal microbiota. Reduction in Lactobacillus was seen with both diets, whilst reductions in genera Dorea, Clostridium, Bacteroides, Odoribacter, and Bilophila were observed only with the iron-deficient diet, along with a significant increase in Prevotella. Impact on the fecal microbiota of dietary iron alone was only seen with mice fed the high iron (400 ppm) diet, with seven genera (from four phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria) showing marked change, including increase in Lactobacillus, Oscillospira, Adlercreutzia, and Candidatus Arthromitus, and reduction observed for Bacteroides, Bilophila, and Ruminococcus. Haller et al. concluded that all significant differences in bacterial abundance in wild-type mice likely appeared as a result of the interaction between treatment and host-mediated inflammation [40,42]. There are several key differences between that paper and our own: they investigated cecal contents, not feces; they induced ileitis, not colitis and they did not measure fecal iron concentration. Studies conducted by Sartor et al. highlight that changes in dietary iron intake likely modify the biological structure of the intestinal microbiota and level of inflammation [43], and agree well with our work presented here.
In the presence of colitis, dietary iron at 400 ppm resulted in a significant reduction in fecal abundance of Firmicutes and Bacteroidetes, and increase of Proteobacteria, changes which were not observed with lower dietary intake of iron at 100 ppm. Overall, altering dietary iron intake exacerbated DSS-induced colitis; increasing the iron content of the diet also led to changes in intestinal bacteria diversity and composition after colitis was induced with DSS. The successful and reproducible induction of DSS-induced colitis was achieved in our previous work [19] allowed us to obtain fecal samples to assess microbiota disturbances at various time-points. Therefore, we were encouraged to observe long-term changes in nutritional luminal iron and their effect on colitis as well as dysbiosis in mice with acute or chronic colitis.
The multi-factorial mechanisms by which IBD-associated dysbiosis develops are not fully understood, and it is unclear whether this dysbiosis should be considered a cause or consequence of IBD. Luminal iron likely to contributes to the dysbiosis associated with IBD [44,45,46]. In a recent preliminary study, our group has shown that when luminal iron increased a dysbiosis occurs especially in IBD patients who have IDA (iron deficiency anemia) as well as in active IBD [47]. Proteobacteria was obviously an iron-responsive microbiota as presence of iron in the gut promotes their growth and apparently contribute to the excess of this phylum during relapse. However, in the same study, Bacteroidetes seemed to be independent of luminal iron, unlike Firmicutes. Though, a further investigation required clarification of the exact effect of lack as well as excess of iron upon the colonic microbiome [47].
Obtaining iron is an important factor for bacterial pathogenicity, and recent discoveries have connected this to the expression of virulence factors in normal gut microbiota that have become pathobionts [48]. Though, dietary iron clearly plays a role in modulating the susceptibility to DSS-induced colitis as described here, and in our previous study [27]. As it has been shown in our data, a lower amount of iron in the diet has exacerbated the gut microbiota as well as excess iron diet. This was partly agreed with other previous studies that showed iron fortification in children’s diet has a potential production of further pathogenic gut microbiota profile occurred, and this was linked with increased gut inflammation [42].
The data presented are based on rodents with a single model of colitis and we did not study humans. However, the impact on the microbiome is determined by the microbial community and it is plausible that similar changes in the intestinal bacteria will be seen in humans to those we report in mice. We also acknowledge that a single form of iron supplementation was used: it is likely that other forms of ferrous iron supplementation will have similar effects, but we cannot extrapolate the observation to newer ferric formulations.
4. Materials and Methods
4.1. Animals
Female C57BL/6 mice, aged between 8 to 9 weeks old, were purchased from Charles River Laboratories (Margate, UK). Mice initially received standard chow diet and water ad libitum, during an acclimatization period of at least one week. Animals were then individually caged in a room with controlled temperature, humidity, and a pre-set dark: light cycle (12 h:12 h) in a specific pathogen-free animal facility. For experiments, mice were matched for age and body weight. Six groups of 8 mice were studied: 3 control groups and 3 groups where colitis was chemically-induced, with each group receiving one of 3 diets of differing iron levels. All were maintained for up to 63-days. The care of, and experimentation on, mice was carried out in accordance with UK Home Office regulations (project license no: 70/8457) and the project was reviewed by the University of Liverpool Animal Welfare and Ethical Review Body (AWERB).
4.2. Diets
When eating a normal (standard) chow diet, mice were fed CRM (P) Rat and Mouse Breeder and Grower 10 mm compression pellets (Special Diets Services; Witham, Essex, UK) which contain 200 parts per million (ppm) iron (ferrous sulphate), i.e., 0.02% w/w iron. Two modifications applied to the standard chow: First, reducing the content of iron by 50% result in CRM (P) iron-deficient diet containing only 100 ppm iron, second, doubling the content of iron in diet giving us a CRM (P) iron supplemented diet of 400 ppm iron.
4.3. Induction of Colitis
Three groups of 8 mice, consuming deficient, standard, and supplemented iron diets respectively, were given a 1.25% w/v dextran sulfate sodium (DSS) (M.W. 36,000–50,000 Da; Catalogue number: 160110; Lot number: 6683K; MP Biomedicals, LLC., UK) in their drinking water for 5-days to induce colitis. Mice were allowed to recover for 16 days and then treatment was repeated, with a total of three cycles of DSS used to induce a chronic colitis that models chronic IBD [49], and which is associated with the development of fibrosis [50].
Eight mice in three control groups also received the standard, iron deficient or supplemented diets respectively for 63-days. After 53-days, each group was divided into two, half (n = 4) carried on as controls and the other half were treated with 2% w/v DSS for 5-days in drinking water, followed by 5-days of plain drinking water, to induce an acute colitis. All mice were euthanized on day-63. A schematic of treatments and diets can be found in Supplementary Information, Figure S1.
4.4. Histopathological Scoring of Colonic Inflammation
The distal colon was removed, fixed in 4% v/v neutral-buffered formalin, dehydrated, wax-embedded, and then 4 μm sections were cut by microtome. Sections were stained with hematoxylin and eosin (H and E) and evidence of colitis was reported using the inflammatory scoring system described by Bauer et al. [51]. Fibrosis (collagen and proteoglycan deposition) was evaluated using a NovaUltraTM Masson’s Trichrome Stain Kit (Fisher Scientific UK Ltd., Loughborough, UK) [52]. The experimenter was blinded to the treatment groups while all slides were assessed.
4.5. Assessment of Degree of Gut Inflammation through Measurement of Faecal Calprotectin
Feces was collected from each mouse, on day-1, 21, 42, and 63 in the chronic colitis study, and on day-1 and day-10 of the acute colitis study, and from control mice. Fecal calprotectin concentration was prepared and measured as per manufacturer instructions using a S100A8/S100A9 ELISA kit (Immundiagnostik AG; Bensheim, Germany).
4.6. Measurement of Fecal Iron
Using the same fecal pellets that were collected for calprotectin assessment, the level of iron (Fe2+ and Fe3+) was measured using an iron immunoassay kit (MAK025; Sigma-Aldrich Company Ltd., Gillingham, UK).
4.7. Fecal Bacterial DNA Extraction and Sequencing
Fecal samples (2 g) were also collected and bacterial DNA isolated using the PSP® Spin Stool DNA Plus Kit (STRATEC Molecular GmbH, Berlin, Germany) following the manufacturer protocol. Isolated DNA was provided to the Centre for Genomic Research (University of Liverpool, Liverpool, UK) to generate the 16S Metagenomic Sequencing Library using primers described by Caporaso et al. [41] to amplify the V4 region of 16S rDNA:
F: 5’CACTCTTTCCCTACACGACGCTCTTCCGATCTNNNNNGTGCCAGCMGCC GCGGTAA3′
and R: 5’GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTGGACTACHVGGGTW TCTAAT3’.
Five µg of DNA was used for first-round PCR with conditions of 20 s at 95 °C, 15 s at 65 °C, and 30 s at 70 °C for 10 cycles, then a final 5-min extension at 72 °C. Samples were purified using Axygen SPRI Beads. The second-round PCR was performed to incorporate Illumina sequencing adapter sequences: 15 cycles of PCR were performed using the same conditions. Samples were re-purified then quantified using Qubit and assessed using the fragment analyser. Successfully-generated amplicon libraries were sequenced [19].
The final libraries were pooled in equimolar amounts using the Qubit and fragment analyser data and 350--550 bp size-selected on the Pippin Prep. The quantity and quality of each pool were assessed by Bioanalyzer and subsequently by qPCR using the Illumina Library Quantification Kit from Kapa on a Roche Light Cycler LC480II according to manufacturer’s instructions. The pool of libraries was sequenced on one lane of the MiSeq at 2 × 250 bp paired-end sequencing. To help balance the complexity of the amplicon library 15%, PhiX was spiked in [19].
4.8. Bioinformatics
Initial processing and quality assessment of the sequence data was performed using an in-house pipeline. Base-calling and de-multiplexing of indexed reads were conducted by CASAVA version 1.8.2 (Illumina). The raw fastq files were trimmed to remove Illumina adapter sequences where any reads that matched the adapter sequence over at least three bp were trimmed off. The reads were further trimmed to remove low-quality bases (reads <10 bp were removed). Read pairs were aligned to produce a single sequence for each read pair that would entirely span the amplicon. Sequences with lengths outside the expected range were excluded [19]. The sequences passing the above filters for each sample were pooled into a single file. A metadata file was created to describe each sample. These two files were analyzed using Qiime, version 1.8.0 [53]. Similar sequences were clustered into groups, to define OTUs of 97% similarity. OTU-picking was performed using USEARCH7 [54]. The Greengenes database version 12.8 [55] was used for reference-based chimaera detection [19]. OTU tables were repeatedly sub-sampled (rarefied) and for each rarefied OTU table, three measures of alpha diversity were estimated: chao1, the observed number of species, and the phylogenetic distance. For inter-sample comparisons (beta-diversity), all datasets were rarefied and tables were used to calculate weighted and unweighted pairwise UniFrac matrices using Qiime. UniFrac matrices were then used to generate UPGMA (Unweighted Pair-Group Method with Arithmetic mean) trees and 2D principal coordinates plots [19].
4.9. Statistics
Normally distributed physiological and biochemical data were assessed by one-way analysis of variance (ANOVA) followed by selected pair-wise comparisons using Dunn’s test. Non-normally distributed data have been evaluated by Kruskal-Wallis test followed by pairwise multiple comparisons using StatsDirect v3.0.171; StatsDirect Ltd., Birkenhead, UK). For the bioinformatic analysis of microbiota data, Kruskal–Wallis H-test was used with the false discovery rate (FDR) Storey’s (multiple correction tests). The q-value is the adjusted p-value based on FDR calculation, where statistical significance was declared at p < 0.05.
5. Conclusions
Lowering iron content to 100 ppm in rodent diet significantly exacerbated acute colitis leading to an increase in fecal iron, whereas, increasing iron levels to 400 ppm resulted in significant alteration in gut microbiota. Consequently, this explains the important role that luminal iron plays in pathogenesis of inflammation in the gut. Additionally, the alteration in dietary iron over a longer period can significantly exacerbate susceptibility to DSS-induced intestinal inflammation, suggesting that the tenure of iron supplementation may also be crucial in aggravating colitis. Further studies will be necessary to investigate the relevance of our findings in humans.
Acknowledgments
The authors gratefully acknowledge the technical support of Dave Berry in the Gastroenterology Research Unit, staff of the Biomedical Services Unit (BSU) and staff at the Centre for Genomics Research, Institute of Integrative Biology, University of Liverpool.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22073646/s1, Figure S1: Schematic illustration of chronic DSS and control groups and the later subdivision at day 53 to start acute DSS colitis experiment, Figure S2: Percentage change in body weight in female C57BL/6 mice consuming diets differing in levels of iron over 63 day, Figure S3: Masson’s trichrome staining of the colonic tissues, Figure S4: Faecal calprotectin concentrations in the presence or absence of DSS-induced colitis, Figure S5: Rarefaction curves of the observed number of species metric for all samples (>500 reads), Figure S6: UPGMA (Unweighted Pair-Group Method with Arithmetic mean) trees.
Author Contributions
A.M. conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures and drafted the manuscript. M.D.B. analyzed data and contributed to writing of the manuscript. C.A.D. conceived and designed research, supported the animal studies, analyzed data and edited the manuscript. G.L.H. analyzed data, prepared figures and drafted the manuscript. B.J.C. conceived and designed research, supervised research, prepared figures, interpreted results of experiments and drafted the manuscript. D.M.P. conceived and designed research, supported the animal studies and edited the manuscript. C.S.P. conceived and designed research, supervised research, analyzed data, interpreted results of experiments and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
A.M. was supported by a grant from the Higher Education Ministry, Libyan Government; Tripoli-Libya.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Liverpool (protocol code 70/8457 and date of approval 2013).
Informed Consent Statement
Studies not involving humans.
Data Availability Statement
Data mainly presented and the rest stored at the University of Liverpool laboratories.
Conflicts of Interest
The authors have declared that no competing interest exists. The funder had no role in study design, data collection and analysis, decision to publish, nor in preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constante M., Fragoso G., Lupien-Meilleur J., Calvé A., Santos M.M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017;23:753–766. doi: 10.1097/MIB.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 3.Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., Mitton S., Orchard T., Rutter M., Younge L., et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 4.Manfred Wick W.P., Lehmann P. Clinical Aspects and Laboratory—Iron Metabolism. 6th ed. Springer; Vienna, Austria: 2011. [Google Scholar]
- 5.Kulnigg S., Teischinger L., Dejaco C., Waldhör T., Gasche C. Rapid Recurrence of IBD-Associated Anemia and Iron Deficiency After Intravenous Iron Sucrose and Erythropoietin Treatment. Am. J. Gastroenterol. 2009;104:1460–1467. doi: 10.1038/ajg.2009.114. [DOI] [PubMed] [Google Scholar]
- 6.Stein J., Dignass A.U. Management of iron deficiency anemia in inflammatory bowel disease—A practical approach. Ann. Gastroenterol. 2013;26:104–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Gasche C., Reinisch W., Lochs H., Parsaei B., Bakos S., Wyatt J., Fueger G.F., Gangl A. Anemia in Crohn’s disease. Importance of inadequate erythropoietin production and iron deficiency. Dig. Dis. Sci. 1994;39:1930–1934. doi: 10.1007/BF02088127. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez K., Kulnigg-Dabsch S., Gasche C. Management of Iron Deficiency Anemia. Gastroenterol. Hepatol. 2015;11:241–250. [PMC free article] [PubMed] [Google Scholar]
- 9.Waldvogel-Abramowski S., Waeber G., Gassner C., Buser A., Frey B.M., Favrat B., Tissot J.-D. Physiology of Iron Metabolism. Transfus. Med. Hemotherapy. 2014;41:213–221. doi: 10.1159/000362888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dignass A., Farrag K., Stein J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018;2018:9394060. doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulnigg S., Gasche C. Systematic review: Managing anaemia in Crohn’s disease. Aliment. Pharmacol. Ther. 2006;24:1507–1523. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 12.Rizvi S., Schoen R.E. Supplementation With Oral vs. Intravenous Iron for Anemia With IBD or Gastrointestinal Bleeding: Is Oral Iron Getting a Bad Rap? Am. J. Gastroenterol. 2011;106:1872–1879. doi: 10.1038/ajg.2011.232. [DOI] [PubMed] [Google Scholar]
- 13.Seyoum Y., Baye K., Humblot C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes. 2021;13:1–19. doi: 10.1080/19490976.2021.1874855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund E.K., Wharf S.G., Fairweather-Tait S.J., Johnson I.T. Oral ferrous sulfate supplements increase the free radical–generating capacity of feces from healthy volunteers. Am. J. Clin. Nutr. 1999;69:250–255. doi: 10.1093/ajcn/69.2.250. [DOI] [PubMed] [Google Scholar]
- 15.Weiss G., Wachter H., Fuchs D. Linkage of cell-mediated immunity to iron metabolism. Immunol. Today. 1995;16:495–500. doi: 10.1016/0167-5699(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg E.D. Iron availability and infection. Biochim. Biophys. Acta Gen. Subj. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Pi H., Jones S.A., Mercer L.E., Meador J.P., Caughron J.E., Jordan L., Newton S.M., Conway T., Klebba P.E. Role of Catecholate Siderophores in Gram-Negative Bacterial Colonization of the Mouse Gut. PLoS ONE. 2012;7:e50020. doi: 10.1371/journal.pone.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coe G.L., Pinkham N.V., Celis A.I., Johnson C., Dubois J.L., Walk S.T. Dynamic Gut Microbiome Changes in Response to Low-Iron Challenge. Appl. Environ. Microbiol. 2020;87 doi: 10.1128/AEM.02307-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahalhal A., Williams J.M., Johnson S., Ellaby N., Duckworth C.A., Burkitt M.D., Liu X., Hold G.L., Campbell B.J., Pritchard D.M., et al. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS ONE. 2018;13:e0202460. doi: 10.1371/journal.pone.0202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrier J., Aghdassi E., Platt I., Cullen J., Allard J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment. Pharmacol. Ther. 2001;15:1989–1999. doi: 10.1046/j.1365-2036.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- 21.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erichsen K., Ulvik R.J., Grimstad T., Berstad A., Berge R.K., Hausken T. Effects of ferrous sulphate and non-ionic iron-polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2005;22:831–838. doi: 10.1111/j.1365-2036.2005.02652.x. [DOI] [PubMed] [Google Scholar]
- 23.Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti M., Mino-Kenudson M., Mizoguchi E. Animal Models of Colitis-Associated Carcinogenesis. J. Biomed. Biotechnol. 2011;2011:342637. doi: 10.1155/2011/342637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comito D., Romano C. Dysbiosis in the Pathogenesis of Pediatric Inflammatory Bowel Diseases. Int. J. Inflamm. 2012;2012:687143. doi: 10.1155/2012/687143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi V.R.M., Etcheverría I., Carrera I., Cacabelos R., Chacón A.R. Prevention of Chronic Experimental Colitis Induced by Dextran Sulphate Sodium (DSS) in Mice Treated with FR91. J. Biomed. Biotechnol. 2012;2012:826178. doi: 10.1155/2012/826178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Califf K.G.A., Knight R., Caporaso J.G. The human microbiome: Getting personal. Microbe. 2014;9:410–415. [Google Scholar]
- 28.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-H. [DOI] [PubMed] [Google Scholar]
- 29.Laroui H., Ingersoll S.A., Liu H.C., Baker M.T., Ayyadurai S., Charania M.A., Laroui F., Yan Y., Sitaraman S.V., Merlin D. Dextran Sodium Sulfate (DSS) Induces Colitis in Mice by Forming Nano-Lipocomplexes with Medium-Chain-Length Fatty Acids in the Colon. PLoS ONE. 2012;7:e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I.-W., Myung S.-J., Do M.Y., Ryu Y.-M., Kim M.J., Do E.-J., Park S., Yoon S.M., Ye B.D., Byeon J.-S., et al. Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J. Gastroenterol. Hepatol. 2010;25:1785–1794. doi: 10.1111/j.1440-1746.2010.06332.x. [DOI] [PubMed] [Google Scholar]
- 31.Miyazawa F., Olijnyk O., Tilley C., Tamaoki T. Interactions between dextran sulfate and Escherichia coli ribosomes. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1967;145:96–104. doi: 10.1016/0005-2787(67)90658-2. [DOI] [PubMed] [Google Scholar]
- 32.Gao X., Cao Q., Cheng Y., Zhao D., Wang Z., Yang H., Wu Q., You L., Wang Y., Lin Y., et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA. 2018;115:E2960–E2969. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aranda C.J., Ocón B., Arredondo-Amador M., Suárez M.D., Zarzuelo A., Chazin W.J., Martínez-Augustin O., De Medina F.S. Calprotectin protects against experimental colonic inflammation in mice. Br. J. Pharmacol. 2018;175:3797–3812. doi: 10.1111/bph.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packey C.D., Sartor R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hold G.L., Smith M., Grange C., Watt E.R., El-Omar E.M., Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014;20:1192–1210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michail S., Durbin M., Turner D., Griffiths A.M., Mack D.R., Hyams J., Leleiko N., Kenche H., Stolfi A., Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 2012;18:1799–1808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupp C., Robertson M.L., Wickham M.E., Sekirov I., Champion O.L., Gaynor E.C., Finlay B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Stecher B., Hardt W.-D. The role of microbiota in infectious disease. Trends Microbiol. 2008;16:107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Werner T., Wagner S.J., Martínez I., Walter J., Chang J.-S., Clavel T., Kisling S., Schuemann K., Haller D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut. 2010;60:325–333. doi: 10.1136/gut.2010.216929. [DOI] [PubMed] [Google Scholar]
- 41.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., Fierer N., Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann M.B., Chassard C., Rohner F., N’Goran E.K., Nindjin C., Dostal A., Utzinger J., Ghattas H., Lacroix C., Hurrell R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010;92:1406–1415. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]
- 43.Ellermann M., Gharaibeh R.Z., Maharshak N., Peréz-Chanona E., Jobin C., Carroll I.M., Arthur J.C., Plevy S.E., Fodor A.A., Brouwer C.R., et al. Dietary iron variably modulates assembly of the intestinal microbiota in colitis-resistant and colitis-susceptible mice. Gut Microbes. 2020;11:32–50. doi: 10.1080/19490976.2019.1599794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee T., Clavel T., Smirnov K., Schmidt A., Lagkouvardos I., Walker A., Lucio M., Michalke B., Schmitt-Kopplin P., Fedorak R., et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2016;66:863–871. doi: 10.1136/gutjnl-2015-309940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constante M., Fragoso G., Calve A., Samba-Mondonga M., Santos M.M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 2017;8:1809. doi: 10.3389/fmicb.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ettreiki C., Gadonna-Widehem P., Mangin I., Coeffier M., Delayre-Orthez C., Anton P.M. Juvenile ferric iron prevents microbiota dysbiosis and colitis in adult rodents. World J. Gastroenterol. 2012;18:2619–2629. doi: 10.3748/wjg.v18.i21.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahalhal A., Subramanian S., Pritchard D.M., Probert C.S. PTU-043 Intestinal dysbiosis oc-curs in iron deficiency as well as active IBD. Gut. 2018;67:A193. [Google Scholar]
- 48.Buret A.G., Motta J.P., Allain T., Ferraz J., Wallace J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory dis-eases: A role for iron? J. Biomed. Sci. 2019;26:1–14. doi: 10.1186/s12929-018-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne F.R., Viney J.L. Mouse models of inflammatory bowel disease. Curr. Opin. Drug Discov. Dev. 2006;9:207–217. [PubMed] [Google Scholar]
- 50.Holvoet T., Devriese S., Castermans K., Boland S., Leysen D., Vandewynckel Y.-P., Devisscher L., Bossche L.V.D., Van Welden S., Dullaers M., et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology. 2017;153:1054–1067. doi: 10.1053/j.gastro.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Bauer C., Duewell P., Mayer C., Lehr H.A., Fitzgerald K.A., Dauer M., Tschopp J., Endres S., Latz E., Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 52.Ding S., Walton K.L., Blue R.E., McNaughton K., Magness S.T., Lund P.K. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen alpha1(I)-promoter-GFP reporter mice. PLoS ONE. 2012;7:e42568. doi: 10.1371/annotation/91f1d7f8-b09d-4067-943c-148e926b403b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 55.McDonald D., Price M.N., Goodrich J.K., Nawrocki E.P., DeSantis T.Z., Probst A.J., Andersen G.L., Knight R., Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2011;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data mainly presented and the rest stored at the University of Liverpool laboratories.