Abstract
Background
passive immunotherapy is a therapeutic alternative for patients with COVID-19. Equine polyclonal antibodies (EpAbs) could represent a source of scalable neutralizing antibodies against SARS-CoV-2.
Methods
we conducted a double-blind, randomized, placebo-controlled trial to assess efficacy and safety of EpAbs (INM005) in hospitalized adult patients with moderate and severe COVID-19 pneumonia in 19 hospitals of Argentina. Primary endpoint was improvement in at least two categories in WHO ordinal clinical scale at day 28 or hospital discharge (ClinicalTrials.gov number NCT04494984).
Findings
between August 1st and October 26th, 2020, a total of 245 patients were enrolled. Enrolled patients were assigned to receive two blinded doses of INM005 (n = 118) or placebo (n = 123). Median age was 54 years old, 65•1% were male and 61% had moderate disease at baseline. Median time from symptoms onset to study treatment was 6 days (interquartile range 5 to 8). No statistically significant difference was noted between study groups on primary endpoint (risk difference [95% IC]: 5•28% [-3•95; 14•50]; p = 0•15). Rate of improvement in at least two categories was statistically significantly higher for INM005 at days 14 and 21 of follow-up. Time to improvement in two ordinal categories or hospital discharge was 14•2 (± 0•7) days in the INM005 group and 16•3 (± 0•7) days in the placebo group, hazard ratio 1•31 (95% CI 1•0 to 1•74). Subgroup analyses showed a beneficial effect of INM005 over severe patients and in those with negative baseline antibodies. Overall mortality was 6•9% the INM005 group and 11•4% in the placebo group (risk difference [95% IC]: 0•57 [0•24 to 1•37]). Adverse events of special interest were mild or moderate; no anaphylaxis was reported.
Interpretation
Albeit not having reached the primary endpoint, we found clinical improvement of hospitalized patients with SARS-CoV-2 pneumonia, particularly those with severe disease.
Research in context.
Evidence before this study
Only two drugs, dexamethasone and remdesivir, have shown positive clinical impact in the treatment of severe COVID-19 disease in randomized clinical trials (RCT). Passive immunotherapy appears as an attractive strategy and is currently an active area of research. We searched PubMed Library for articles published until January 15, 2021, using various combinations of the terms “SARS-CoV” “Covid-19″, “SARS-CoV-2″, “clinical trial”, “immunotherapy”, and “convalescent plasma” with no language restrictions.
The enhancement of the humoral immune response using convalescent plasma (CP) and a monoclonal antibody against the SARS-CoV-2 receptor-binding domain (RBD) (bamlanivimab) failed to show any benefit in hospitalized patients with COVID-19 disease in published RCTs. However, high antibody titer CP was effective in elderly patients when administered within 72 h of symptoms onset, as it was the mentioned monoclonal antibody in out-patients at risk for complications. Aiming to improve the passive immunotherapy approach, purified F(ab’)2 fragments were obtained from horses immunized with the RBD domain of the viral spike protein. These equine polyclonal antibodies (EpAbs) displayed high in vitro activity in viral neutralization SARS-CoV-2 assays.
Added value of this study
This is the first human trial using EpAbs (INM005). Two intravenous doses (4 mg/kg) of INM005 or placebo were administered at baseline and at 48 h to 243 patients with moderate or severe COVID-19 disease (critically ill ones were excluded). Even though there was no difference between INM005 and placebo in the improvement of at least 2 categories on the ordinal clinical status scale or hospital discharge at day 28 (primary end-point), this analysis favored patients in the INM005 group at days 7 to 21. Also, a statistically significant less time to improvement (at least two ordinal categories or hospital discharge) was noted in the INM005 group. Among those with severe disease at baseline, a not statistically significant difference in mortality was observed among patients receiving INM005 versus those receiving placebo. A greater benefit from INM005 was observed in those with non-reactive antibodies versus those with positive antibodies at baseline. Importantly, INM005 displayed a good safety profile and no serious adverse reactions were associated with it; none of the patients developed anaphylaxis.
Implications of all the available evidence
Effective therapeutic approaches for patients with severe COVID-19 disease are urgently needed. The administration of INM005 was associated with clinical benefits, especially in the subgroup of patients with severe COVID-19 disease as well as in those with delayed immune response measured by the absence of IgG anti-SARS-CoV-2 at study entry. Future studies will help to define the final role of this treatment in the context of current COVID-19 pandemic.
Alt-text: Unlabelled box
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), is currently generating a global pandemic with more than 91 million infections and 1•9 million fatalities (as of January 2021) [1]. In Argentina, SARS-CoV-2 has caused more than 1•7 million infections and around 44,000 deaths. So far, dexamethasone [2,3] and remdesivir [4], [5], [6] have shown efficacy in adequately powered clinical trials. In addition, passive immunotherapy appears as a promising therapeutic approach, particularly for early stages of the disease in which patients have not yet established their specific immune response. To date, convalescent plasma (CP) has been the only antibody-based therapy widely available for COVID-19 patients, mainly through extended and compassionate use. This strategy has consistently shown an adequate safety profile, although no effect has been demonstrated in the treatment of patients with severe pneumonia, while it may have a role in the treatment of elder patients within 72 h of initiation of symptoms of COVID-19 [7], [8], [9]. CP poses the additional difficulties of the donor selection process and apheresis, the need of high titers of neutralizing antibodies (NAbs) and the potential limitation for scalability.
As an alternative approach for immune therapy, different anti-receptor binding domain (RBD) human monoclonal antibodies (mAbs) have been evaluated in the treatment of COVID-19 [10]. Although some degree of activity was observed in patients with mild disease, no consistent effect has been demonstrated so far in hospitalized patients with moderate and severe disease [11].
It has been previously shown that the RBD from the viral spike glycoprotein elicits high titers of NAbs against SARS-CoV-2 when used as immunogen in horses [12]. In this regard, equine polyclonal antibodies (EpAbs) can represent a practical and efficient source of NAbs. EpAbs are composed of F(ab)’2 fragments generated by pepsin digestion. These fragments retain the bivalent binding capacity of IgG immunoglobulins but lack the constant region (Fc), responsible for serum sickness reactions and Fc-triggered side effects. EpAbs recognize a vast array of epitopes (limiting the risk of viral escape mutations) and tend to develop greater avidity than mAbs for their cognate antigens. In addition, EpAbs are relatively easy to manufacture allowing a fast development and scaling up for a treatment. We have previously described the development and in vitro characterization of a therapeutic based on purified equine anti-RBD F(ab´)2 fragments, called INM005 [12]. INM005 shows a very high serum neutralization titer against SARS-CoV-2 and its format, devoid of Fc domains, may prove preferable for its capacity to avoid potentially negative Fc-related effects [13]. A more detailed description of the technique used in the preparation of INM005 is provided in the Supplementary Appendix. The aim of our study was to assess the safety and clinical efficacy of intravenous administration of two doses of INM005 versus placebo in hospitalized adult patients with moderate and severe COVID-19 pneumonia.
2. Methods
2.1. Study design
CT-INM005–01 was a phase 2/3, double-blind, placebo-controlled, multicenter clinical trial that analyzed the safety and efficacy of specific anti SARS-COV-2 EpAbs in hospitalized patients with moderate and severe COVID-19 disease in nineteen clinical sites of Argentina (full description of participating sites is available in Supplementary Appendix). Eligible participants were randomized to receive either two doses of specific INM005 or placebo with an interval of 48 h.
This was a parallel group study with adaptive design. A blinded interim analysis was planned when about 60% of the enrollment was reached. Based on the rate of events in the control group, the Data and Safety Monitoring Board (DSMB) could determine whether the sample size had to be increased or if the criteria for futility had been met.
The study protocol was approved by the Institutional Review Boards of all participant clinical sites as well as regional or jurisdictional Ethics Committees as applicable. The Argentinean National Administration of Medicines, Food and Medical Technology (ANMAT) also approved the study protocol. Written informed consent was obtained from all participants and the study was conducted in accordance with the principles stated in the Declaration of Helsinki and Good Clinical Practice guidelines. A CONSORT checklist has been completed and we declare fully adherence.
3. Patients
3.1. Inclusion and exclusion criteria
Hospitalized adult patients were eligible if they had a positive reverse-transcriptase-polymerase-chain reaction (RT-PCR) for SARS-CoV-2, were between 18 and 79 years old, within 10 days from the initiation of symptoms, were hospitalized with a diagnosis of moderate or severe COVID-19 disease and provided a voluntary undelegated written informed consent.
Exclusion criteria were: pregnant women or during lactation period, history of treatment with SARS-CoV-2 convalescent plasma, participation in other therapeutic clinical trial for COVID-19, history of anaphylaxis, severe allergic reaction to equine sera or to contact or exposure to horse proteins, hospitalization in ICU and/or requirement of mechanical ventilation, likelihood of death due to clinical reasons other than COVID-19 within the following 30 days, or expected transfer to other healthcare institution.
In accordance with the disease categorization proposed by the National Institutes of Health, [14] moderate illness was defined as patients who had any of the various signs and symptoms of COVID-19 plus evidence of lower respiratory disease during clinical assessment or imaging and who had oxygen saturation (SpO2) ≥94% on room air at sea level. Severe illness was defined for individuals who had SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, respiratory frequency >30 breaths per minute, or lung infiltrates >50%.
3.2. Randomization and masking
Patients were randomized in a 1:1 manner to receive either active treatment (INM005) or placebo. Permuted block randomization was performed with a block size of 6 in a mixed sequential fashion. Randomization was centrally performed through an allocation system based upon a R free software environment. For the initial participants, random allocation was supervised by an unblinded statistician specifically designated by the Sponsor that did not participate in any patient-related activities and who was in either phone and email contact with a designated unblinded pharmacist from each participating site. Unblinded site personnel accessed the database with a non-delegable individual user and password in order to receive assignment information. As such, randomization results were concealed to the rest of the site research members. This procedure was maintained until the first twelve subjects were randomized in the study. From patient thirteen onwards randomization was stratified per participating site and directly performed by the unblinded pharmacist designated at each participating site through a close envelope method maintained in random order. Closed envelopes were only accessible to unblinded pharmacists and unblinded statisticians and concealed to all other personnel. Site pharmacist was responsible for properly masking the intervention, handing the corresponding optically indistinguishable infusion bag to the blinded clinical team.
3.3. Study product and procedures
Specific equine hyperimmune sera for the treatment of COVID-19 was developed using the RBD domain of the SARS-CoV-2 spike protein as immunogen [12,15]. RBD-immunized horses elicit a large amount of anti-SARS-CoV-2 neutralizing antibodies, which are capable to neutralize in vitro the virus with very high potency (serum neutralization titer of around 1:20,000). Processed and purified F(ab’)2 fragments were used as passive immunotherapy for the current trial, supplying them in vials manufactured with GMP standards and labelled as INM005 [12]. Additional information regarding the intervention is provided in the Supplementary Appendix.
Starting within 24 h from patient enrollment, two INM005 doses of 4 mg/kg (or matching placebo) each were administered as intravenous infusion of 100 ml over a period of fifty minutes with an interval of 48 h between them. All patients received supportive care according to the standard of care of each participating hospital. Patients were followed up during a 28-days period after the first dose.
3.4. Outcomes
The primary objective was to demonstrate the efficacy and safety of INM005 in COVID-19 in terms of clinical improvement at 28 days after initiation of treatment versus placebo. Primary efficacy endpoint was the proportion of patients that showed improvement 28 days after the administration of the first dose of at least two categories based upon the WHO 8-points ordinal clinical scale or hospital discharge [16].
The categories were as follows: 0, no evidence of infection; 1, not hospitalized and no limitations of activities; 2, not hospitalized, with limitation of activities; 3, hospitalized, not requiring supplemental oxygen; 4, hospitalized, requiring supplemental oxygen; 5, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices; 6, hospitalized, receiving invasive mechanical ventilation; 7, hospitalized, receiving invasive mechanical ventilation and additional organ support-extracorporeal membrane oxygenation, pressers, or renal replacement therapy; and, 8, death.
Secondary and exploratory outcomes included pharmacokinetic behavior of INM005, risk and time to disease progression between study groups, viral load modification in time, improvement in ordinal clinical scale at day 28, time to achieve improvement in at least two categories on the ordinal clinical scale, time until discharge, time until discharge from ICU, proportion of patients presenting an improvement of at least two categories in the WHO 8-point ordinal clinical scale at 7 and 14 days from onset of treatment, proportion of patients with discharge at 28 days, proportion of patients requiring ICU admission, proportion of patients requiring invasive mechanical ventilation, overall mortality, change in viral load from baseline to 7 and 21 days after the start of treatment and INM005 concentration in serum at different times after treatment administration. Pre-specified subgroup analyses included severity of disease as well as presence of specific IgG and/or IgM antibodies at baseline.
3.5. Safety endpoints
Safety outcome measures included any type of adverse events, as well as serious, emergent treatment-related, and adverse events of special interest, such as injection-site reactions or hypersensitivity reactions, that occurred during the 28 day follow up period of the trial, discontinuation or temporary suspension of infusions, and changes in assessed laboratory values over time.
Throughout the study, adverse events were monitored and recorded in electronic case report forms (eCRF), including event description, start and end date, seriousness, severity, action taken and relationship to the investigational product. All events were followed-up until its resolution or stabilization, and its outcome was documented in the eCRF. If a serious adverse event (SAE) occurred, the investigator had 24 h to report it to the Sponsor using a SAE form and regardless of their relationship with the study drug.
The DSMB periodically reviewed the safety results of the study and reported recommendations in real-time. A more detailed description of these interim analyses is provided in Supplementary Appendix.
3.6. Total and neutralizing antibody measurements and additional laboratory evaluations
All participants were tested for specific IgG and IgM antibodies against SARS-CoV-2 spike protein at baseline (prior to first dose) and at days 7 and 21. In addition specific anti INM005 antibodies were measured at baseline and at day 21. Viral load as well as other laboratory measurements including troponin T, D-Dimer, ferritin, LDH, and C-reactive protein were performed at baseline and at days 7 and 21.
Pharmacokinetic analysis measurements were performed in a subgroup of 19 patients, 9 received INM005 and 10 received placebo. Plasmatic concentration of INM005 was measured by means of a quantitative sandwich ELISA for equine immunoglobulins. This assay was developed and validated by Inmunova S.A.
3.7. Clinical follow-up
Patients were assessed daily during their hospitalization, from day 1 to 5, then on days 7, 14, 21 and 28. During scheduled visits clinical status was assessed on the WHO 8-point ordinal clinical scale and laboratory investigations were conducted. All adverse events, serious adverse events and suspected drug-related hypersensitivity reactions were recorded.
3.8. Statistical analysis
Assuming an event rate of 70% for standard treatment and an absolute effect size of 15 percentage points (target difference: 85% in treated arm vs 70% in placebo arm), a total sample size of 242 individuals (121 in each treatment group) was estimated to achieve 80% of statistical power and α error of 0•025 (one-tailed analysis).
An interim analysis for safety and efficacy was planned when about 60% of the total recruitment was achieved. The DSMB analyzed the event rate in the group under “standard of care” (placebo) and could recommend: 1) the modification of the sample size up to 314 patients, based on the observed event rate, or 2) the interruption of the study if: a. it was judged not feasible to continue with the study since an excessively large sample size would be required, or b. it was considered futile because an event rate of ≥ 95% was met in the placebo group.
Full analysis set population was defined as all patients included in the study and randomized to receive a protocol-defined intervention. Modified intent-to-treat (mITT) population was defined as all patients randomized to receive a protocol-defined intervention who received at least one dose of treatment and fulfilled all major inclusion/exclusion criteria. This was the primary population for efficacy analyses. Per protocol population (PP) was defined as all mITT patients that did not have a major protocol deviation. This population was selected for sensitivity analyses.
Safety population was defined as all patients that received at least one intervention dose. This population was selected for basal demographics and whole safety analyses. Adverse events were coded by PT (Preferred Term) using the Medical Dictionary for Regulatory Activities (MedDRA). The incidence of adverse events related to INM005 and of adverse events of special interest during the study period was compared between the groups using a χ2 test.
The proportion of “responders”, the primary endpoint of the study, was compared between groups by using a one-tailed Z-test with the continuity correction. A critical value of α of 0•025 was used for the assessment of the superiority of the active treatment. Survival curves were fitted by the Kaplan Meier procedure for the secondary endpoints. Hazard Ratios (HR) were calculated using the Cox Proportional Hazards Regression method. Mean time to the events was calculated by the "restricted mean survival time" method, using 28 days as the horizon. No allowance for multiplicity was made. The analysis of the variation in the clinical ordinal scale over time was performed by using Generalized Estimation Equations (GEE), and the differences between groups at days 7, 14, 21, and 28 were analyzed by ordinal logistic regression, adjusting for multiple comparisons. Additional information about the statistical analysis plan and the interim analysis are provided in the Supplemental Appendix.
3.9. Role of the funding source
The funder of the study (Inmunova S.A.) participated in study design, data collection, data analysis, data interpretation and writing of the report. Inmunova S.A. was also responsible for sites and principal investigator selection and contracts, project management and supervision of sites monitoring through a Contract Research Organization. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
4. Results
4.1. Patients
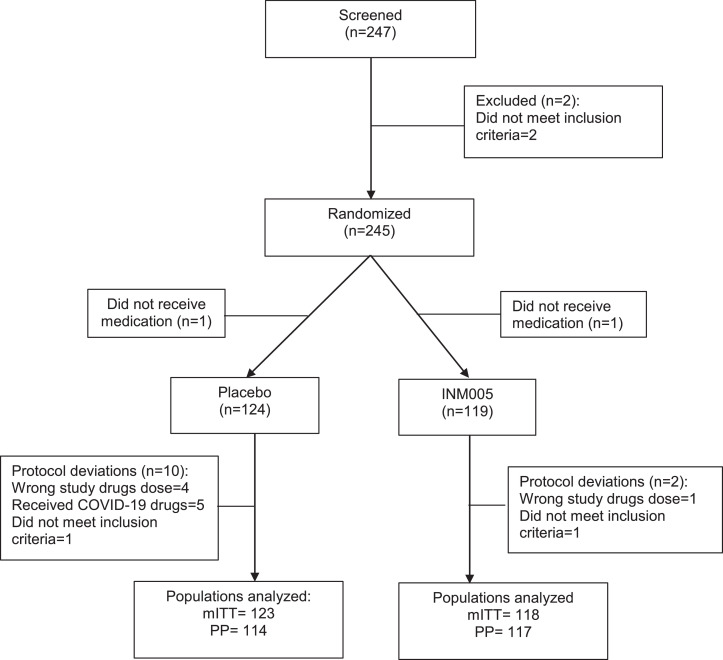
Between August 1st and October 26th, 2020, 247 patients were assessed for participation in the present study of which 245 were enrolled. One participant in each study group did not receive the protocol-defined intervention and consequently, 119 in the INM005 and 124 participants in the placebo group constituted the safety population. Of those, nine patients in the placebo group and one patient in the INM005 group had protocol deviations that precluded the completion of the study intervention, thus 118 in the INM005 and 123 participants in the placebo group constituted the mITT population whereas 114 patients in the placebo group and 117 patients in the INM005 group were included in the Per Protocol population (Fig. 1). No patient was lost to follow up, neither discontinued study treatment due to a treatment emergent adverse event.
Fig. 1.
Study patients consort flowchart.
The median age of the population was 54 years old (interquartile range 44 to 63). 65•1% were male and 81•3% were self-reported caucasic. Median BMI was 30•1 kg/m2 (interquartile range 26•8 to 34•7 kg/m2). In this regard 87•1% of the whole population had BMI of 25 or more, and 51•5% had obesity. A total of 192 (79•7%) of the included patients had at least one coexisting condition at study entry and 114 (59•4%) had at least two comorbidities (Table 1). Other than obesity, the most frequent coexisting clinical conditions of patients at baseline were cardiovascular disease (44•8%) where 32•8% had arterial hypertension, diabetes (23•7%) and lung disease (10•4%).
Table 1.
Demographic and clinical characteristics of the patients at baseline (mITT).
|
All (N = 241) |
INM005 (N = 118) |
Placebo (N = 123) |
|
|---|---|---|---|
| Characteristics | |||
| Age, years* | 54 (44 to 63) | 54 (43 to 63) | 54 (45 to 65) |
| Male sex | 157 (65•1%) | 80 (67•8%) | 77 (62•6%) |
| Ethnicity | |||
| Caucasian | 196 (81•3%) | 93 (78•8%) | 103 (83•7%) |
| Hispanic / Latino | 27 (11•2%) | 18 (15•3%) | 9 (7•3%) |
| Native American | 15 (6•2%) | 6 (5•.1%) | 9 (7•3%) |
| Asian | 3 (1•2%) | 1 (0•8%) | 2 (1•6%) |
| BMI | |||
| Median (Interquartile range) | 30•1 (26•8 to 34•7) | 30.1 (26•.8 to 35•6) | 30.3 (26•8 to 34•3) |
| Patients with BMI 30–35 | 65 (27•0%) | 27 (22•9%) | 38 (30•9%) |
| Patients with BMI >35 | 59 (24•5%) | 33 (28•0%) | 26 (21•1%) |
| Number of coexisting conditions- No./total No: (%)⁎⁎ | |||
| None | 49 (20•3%) | 24 (20•3%) | 25 (20•3%) |
| One | 78 (32•3%) | 39 (33•0%) | 39 (31•7%) |
| Two or more | 114 (47•3%) | 55 (46•6%) | 59 (47•9%) |
| Baseline characteristics | |||
| Days from symptoms onset to study treatment* | 6 (5 to 8) | 6 (5 to 8) | 7 (5 to 8) |
| Score on ordinal scale* | 4 (3 to 4) | 4 (3 to 4) | 4 (3 to 4) |
| Hospitalized, not requiring oxygen (category 3) | 109 (45•2%) | 54 (45•8%) | 55 (44•7%) |
| Hospitalized, requiring supplemental oxygen (category 4) | 125 (51•9%) | 61 (51•7%) | 64 (52•0%) |
| Hospitalized, receiving noninvasive mechanical ventilation or high-flow oxygen devices (category 5) | 7 (2•9%) | 3 (2•5%) | 4 (3•3%) |
| COVID-19 classification | |||
| Moderate | 147 (61•0%) | 74 (62•7%) | 73 (59•3%) |
| Severe | 94 (39•0%) | 44 (37•3%) | 50 (40•7%) |
| Concomitant COVID-19 therapeutic interventions | |||
| Dexamethasone | |||
| Moderate (n = 148) | 66 (44•6%) | 31 (41•9%) | 35 (47•9%) |
| Severe (n = 93) | 72 (77•4%) | 34 (77•3%) | 38 (76•0%) |
All data are n (%), except for:.
Data are in median (interquartile range 25:75);.
number of coexisting conditions data are in No./total No: (%). No means number.
According to the NIH clinical classification 61 and 39% of the participants had moderate and severe disease respectively at study entry; and based upon the WHO ordinal clinical status scale, 45•2% were at stage 3, 51•9% at stage 4, and 2•9% at stage 5 at baseline. The median reported time from the onset of COVID-19 symptoms to the administration of the first dose of intervention was 6 days (interquartile range 25–75 from 5 to 8 days). Concomitant use of dexamethasone was highly prevalent in the whole population, particularly in patients with severe disease, and there were no differences between groups (Table 1).
An interim analysis on efficacy was performed when 156 patients reached 28 days of follow up. Neither futility nor early termination criteria were reached, nor sample re-estimation was required. The Board recommended the continuation of the study as planned, maintaining the original estimation of the sample size. The extended results of the blinded Interim analysis are in Supplementary Appendix.
4.2. Primary outcome
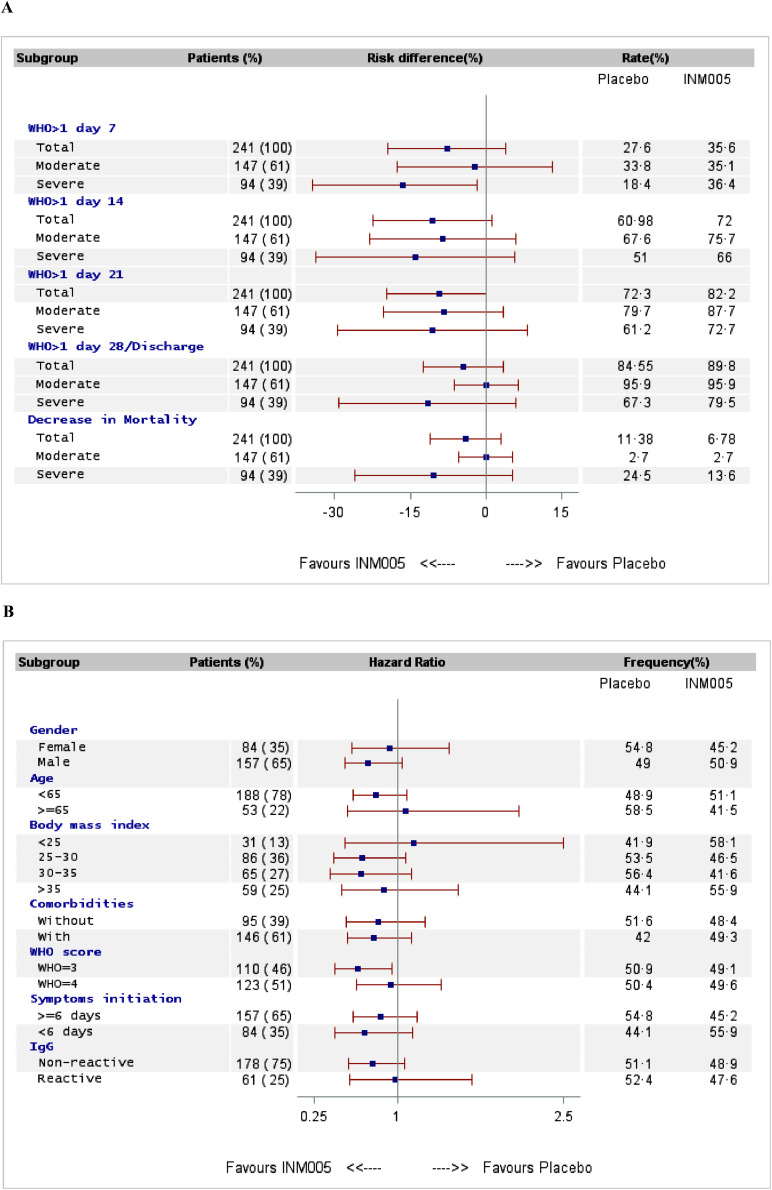
At day 28 no statistically significant difference was noted between study groups on improvement in at least two categories in ordinal clinical status scale or hospital discharge (risk difference [95% IC]: 5•28% [-3•95; 14•50]; p = 0•15); Table 2; Figs. 2A and S1).
Table 2.
Clinical outcomes in patients who received INM005 as compared with placebo.
| Outcomes | INM005 (N = 118) | Placebo (N = 123) | Risk difference or Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Primary outcome | ||||
| Improvement in at least two categories in WHO ordinal clinical scale at day 28 or discharge | 106 (89•8%) | 104 (84•5%) | Risk difference, 5•28% (−3•95 to 14•50) | 0•15 |
| Secondary outcomes | ||||
| Time to achieve improvement in at least two categories on the ordinal clinical scale (days) | 14•2 ± 7 | 16•3 ± 0•7 | 1•31 (1•00 to 1•74) | 0•05 |
| Improvement in at least two categories in WHO ordinal clinical scale at day 28* (%) | 87•3 ± 3•1 | 79•7 ± 3•6 | •• | 0•08 |
| Improvement in at least two categories in WHO ordinal clinical scale or discharge at day 7* (%) | 64•1 ± 4•4 | 58•3 ± 4•5 | •• | 0•26 |
| Improvement in at least two categories in WHO ordinal clinical scale or discharge at day 14 *(%) | 87•3 ± 3•1 | 79•7 ± 3•6 | •• | 0•05 |
| Time until discharge (days) | 8•7 ± 0•6 | 10•2 ± 0•7 | 1•26 (0•96 to 1•66) | 0•09 |
| Improvement in the ordinal scale for clinical status scale (AUC)⁎⁎ | 60•5 ± 41•7 | 73•7 ± 49•4 | -13•14 (-1•56 to -24•72) | 0•02 |
| Mean category at day 7⁎⁎⁎ | 3•1 ± 1•7 | 2•7 ± 1•7 | 0•63 (0•36 to 1•13) | 0•19 |
| Mean category at day 14⁎⁎⁎ | 2•4 ± 2•2 | 1•7 ± 1•8 | 0•52 (0•29 to 0•96) | 0•03 |
| Mean category at day 21⁎⁎⁎ | 2•1 ± 2•3 | 1•5 ± 1•9 | 0•54 (0•30 to 0•99) | 0•05 |
| Mean category at day 28⁎⁎⁎ | 1•9 ± 2•5 | 1•4 ± 2•1 | 0•80 (0•44 to 1•46) | 0•99 |
| Time until discharge from ICU (days) | 24•7 ± 0•8 | 23•6 ± 0•8 | 0•67 (0•35 to 1•28) | 0•22 |
| Patients requiring ICU admission at day 28* (%) | 12•7 ± 3•1 | 17•8 ± 3•5 | •• | 0•11 |
| Patients requiring invasive mechanical ventilation at day 28* (%) | 9•3 ± 2•6 | 13•9 ± 2•9 | •• | 0•20 |
| Overall mortality* (%) | 6•9 ± 2•3 | 11•4 ± 2•9 | •• | 0•19 |
| Risk to disease progression⁎⁎⁎ | 17 (14•4%) | 29 (23•5%) | 0•54 (0•28 to 1•05) | 0•07 |
The rates of events in the INM005 and placebo groups were obtained from the Kaplan-Meier survival curves. Therefore, risk ratios cannot be calculated. ** Mean ± standard deviations of the 0-to-28-day Area Under the Curve are provided. The between group difference and its 95% confidence interval is also provided.
Mean ± standard deviations at each time period. Proportional odds ratio, as calculated by an ordinal logistic regression model are provided. Confidence intervals and p-values were adjusted for multiple comparisons.
Fig. 2.
Forest plot of risk difference for changes in WHO scale at days 7 to 28 and mortality in patients following INM005 relative to placebo (A). Forest plot comparing INM005 vs placebo assessing changes in WHO scale in at least two categories and/or discharge at day 28 stratified by key predictors factors of response (B). The square denotes the effect size for the outcome for all subgroups, and the width of the square depicts the overall 95% CI. Data are in n (%).
4.3. Secondary and exploratory outcomes
At day 28 the area under the curve (AUC) of the ordinal clinical scale values between study groups measured showed a 18% mean difference in favor of patients that received INM005 (hazard ratio −13•14 (−1•56 to −24•72) (Table 2; Fig. S2). Improvement in two categories of the ordinal clinical scale or discharge from hospital started to diverge between study arms at day 7 and favored patients in INM005 group at days 14 and 21 (odds ratio [95% CI]: 0•63 [0•36 to 1•13]; 0•52 [0•29 to 0•96], and 0•54 [0•30 to 0•99], respectively; Table 2; Fig. S2).
At 28 days of follow-up, 105 patients in the INM005 group and 103 patients in the placebo group were discharged from hospital (hazard ratio [IC95%] = 1•265 [0•963–1•661]). A statistically significant difference was noted in the time to improvement in at least two ordinal categories or hospital discharge: 14•2 (± 0•7) days in the INM005 group and 16•3 (± 0•7) days in the placebo group (hazard ratio [95% CI]: 1•31 [1•0 to 1•74]).
Regarding the outcomes of worsening clinical condition, 15 (12•7%) patients from the INM005 group and 23 (17•8%) patients from the placebo group were admitted to ICU (hazard ratio [95% CI]: 0•67 [0•35 to 1•28]), and 11 (9•3%) and 17 (13•9%) patients, respectively, required mechanical ventilation (hazard ratio [95% CI]: 0•67 [0•31 to 1•43]; Table 2). No statistically significant differences were observed in median time of ICU admission (8•5 days, interquartile range 25–75, 4 to 17 days for the INM005 group and 9 days, interquartile range 25–75, 3 to 14 days for the placebo group), neither in time to discharge from ICU, 24•7 ± 0•8 days in INM005 group and 23•6 ± 0•8 days in placebo group (hazard ratio [95% CI]: 0•67 [0•35 to 1•28]).
Forty-six patients had signs of disease progression at the discretion of the clinical researchers, 17 in the INM005 group and 29 in the placebo group (odds ratio [95% CI]: 0•54 [0•28 to 1•05]), and no statistically significant difference was noted between groups in time to disease progression (Table 2). Similar curves of viral load decay were observed in both study groups (Fig. S3). An accurate treatment effect on viral load could not be determined given that the earliest measurement was done after an average of 13–14 days of symptoms onset. Several inflammatory parameters were analyzed as shown in Table S1.
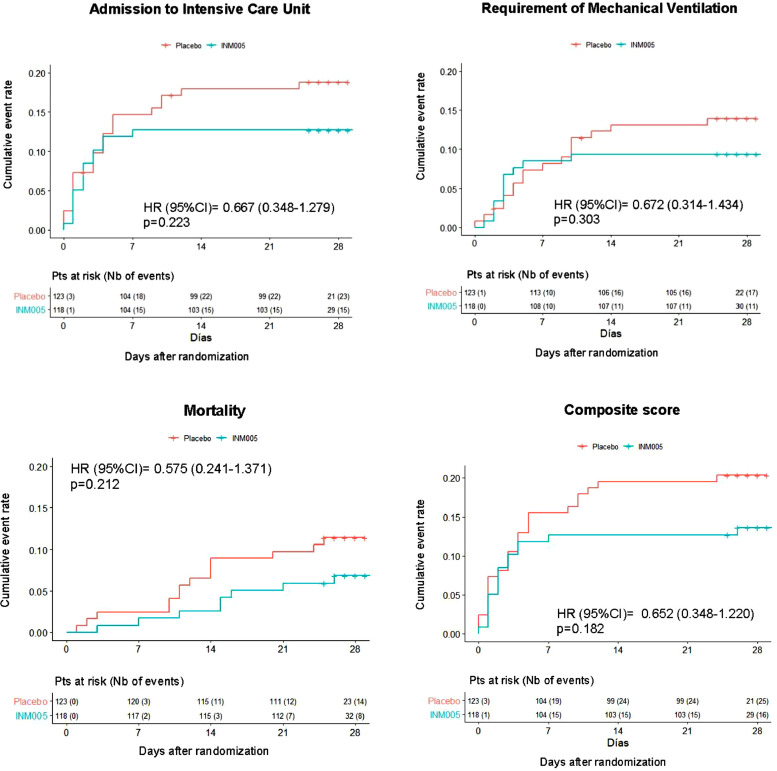
Mortality at 28 days was 9•1% (22 of 241 patients). Eight out of 118 (6•9%) patients from the INM005 group and 14 out of 123 (11•4%) patients from the placebo group died (hazard ratio [IC95%] 0•575 [0•241 to 1•371]; Table 2 and Fig. 3). A total of 16 patients in the INM005 group and 25 patients in the placebo group reached a composite endpoint of admission to ICU, requirement of mechanical ventilation or death at day 28 of follow up (hazard ratio [95% CI]: 0•65 [0•35 to 1•22]; Fig. 3).
Fig. 3.
Time to admission to Intensive Care Unit, requirement of mechanical ventilation, death or a composite outcome, defined as the occurrence of any of the three outcomes, in patients treated with INM005 or placebo. Hazard ratios (95% Confidence Intervals) as calculated by a Cox regression model are shown.
Nine patients in the INM005 group and 10 patients in the placebo group participated in the pharmacokinetic sub-analysis. None of the patients in the placebo group showed detectable levels of investigational drug. In patients receiving INM005, the product reached a Cmax1 of 84•6 mg/liter at 1 h and a Cmax2 of 102•4 mg/liter at 49 h, showing a T1/2 of 58•9 h (Table S2 and Fig. S4).
4.4. Subgroup analysis
Prespecified subgroup analysis according to baseline clinical status showed that no difference in primary outcome was noted in patients with moderate disease between groups (71 of 74 patients in INM005 group and 71 of 74 patients in placebo group) while an effect was noted in patients with severe disease (35 of 44 patients in the INM005 group and 33 of 50 patients in the placebo group) that did not reach statistical significance (odds ratio [95% CI]: 0•846 [0•45 to 1•58]; Fig. S5). A complete analysis of the prespecified subgroups can be found in Fig. 2A.
Mortality was 2•7% in both groups in patients with moderate disease (odds ratio [95% CI]: 0•97 [0•14 to 6•94]) whereas in patients with severe disease a trend was noted in favor of INM005 group (13•6% in the INM005 group vs 24•5% in the placebo group, odds ratio [95% CI]: 0•52 [0•19 to 1•39]) (Fig. 2A).
Pre-specified subgroup analysis by WHO clinical scale category at study entry did not show statistically significant differences in outcomes between patients with baseline category 3 or 4. Similarly, median reported time from initiation of symptoms, with cut-off point at 6 days, did not reveal any statistically significant difference between study groups. Additional subgroup analysis including BMI, age and comorbidities can be found in Fig. 2B.
At baseline, 178 patients (74•5%) did not have SARS-CoV-2 IgG antibodies. Treatment with INM005 did not interfere with the mounting of natural immune response against the virus (Table S3). No statistically significant difference was found in primary outcome between study groups analyzing patients with or without IgG specific antibodies at baseline (77 of 87 reactive patients receiving INM005 and 76 of 91 non-reactive patients receiving placebo, odds ratio [95% CI]: 0•94 [0•61 to 1•45]; 27 of 29 reactive patients receiving INM005 and 28 of 32 reactive patients receiving placebo, odds ratio [95% CI]: 0•95 [0•45 to 1•95]; Fig. 2B). AUC of the ordinal clinical scale values at day 21 of follow-up between study groups analyzing the subgroups by presence of IgG specific antibodies at baseline measured noted a 7% difference in favor of patients following INM005 between non-reactive patients (Fig. S6).
4.5. Safety outcomes
In the safety population, adverse events occurred in 52 of 119 patients (43•7%) in the INM005 group and in 55 of 124 (44•3%) in the placebo group. Serious adverse events occurred in 13•4 and 20•2% in the INM005 and the placebo groups, respectively. Emergent treatment adverse events of special interest occurred in 21 subjects in the INM005 group (17•6%) and in 12 in the placebo group (9•7%) (Table 3).
Table 3.
Overview of subjects who presented adverse events during the study.
| Total (N = 243) | INM005 (N = 119) | Placebo (N = 124) | |
|---|---|---|---|
| Subjects with any AE | 107 (44•0%) | 52 (43•7%) | 55 (44•3%) |
| Subjects with any SAE | 41 (16•9%) | 16 (13•4%) | 25 (20•1%) |
| Subjects with any related treatment-emergent SAE | 3 (1•2%) | 2 (0•8%) | 1 (0•8%) |
| Subjects with any treatment-emergent AESI | 11 (4•5%) | 9 (7•6%) | 2 (1•6%) |
| Subjects with a related TEAE | 33 (13•6%) | 21 (17•6%) | 12 (9•7%) |
| Subjects with any AE with fatal outcome* | 27 (11•1%) | 11 (9•2%) | 16 (12•9%) |
| Subjects with any related TEAE with fatal outcome | 0 (0•0%) | 0 (0•0%) | 0 (0•0%) |
| Subjects with any TEAE that required permanent treatment discontinuation | 0 (0•0%) | 0 (0•0%) | 0 (0•0%) |
Data are in n (%): amount and percentage of subjects with at least one TEAE. AE: Adverse event, TEAE: Treatment-emergent adverse event, SAE: Serious adverse event, AESI: Adverse event of special interest.
Data include deaths after day 28.
The incidence of adverse events was similar in the INM005 and the placebo group. No statistically significant differences were found in the overall incidence of adverse events or serious adverse events, and no anaphylaxis event was reported. No deaths were considered by the investigators to be related to the investigational product (Table 3). No differences were found between study groups in relation to changes in vital signs or laboratory parameters after the study treatment.
5. Discussion
This is the first clinical trial including patients with COVID-19 disease that evaluated polyclonal antibodies against the RBD-domain of the spike SARS-CoV-2 protein. This double-blind randomized, placebo-controlled trial showed a slight no statistically significant difference favoring INM005 in the primary endpoint at day 28, which did reach statistical significance at days 14 and 21 after treatment initiation. Among other secondary outcomes, we observed a no statistically significant decrease in the proportion of patients requiring ICU admission (29%), mechanical ventilation (33%), and overall mortality (39%) in patients treated with INM005.
Similar to other therapeutic approaches such as remdesivir, the potential benefits might be observed in those patients with severe instead of moderate disease [4]. In the group of patients with severe disease, we found an effect in favor of INM005 in the primary end-point at days 14 and 21. Accordingly, in this subgroup, we observed a decrease of 45% in mortality among those receiving INM005, although the effect was not statistically significant. The results in the severe population did not reach the statistical significance probably due to the low sample size in this subgroup. This study added initial evidence for the use of passive immunotherapy in patients with severe COVID-19 disease. These results were consistent among age groups, gender, comorbidities, and time to initiation of symptoms.
Convalescent plasma (CP) therapy in adult patients with severe COVID-19 disease did not show clinical improvement in a recent randomized clinical trial done in Argentina [7]. EpAbs anti-RBD F(ab´)2 fragments (INM005), as well as CP, contain anti-RBD neutralizing activity; however, some important differences between these two passive immunotherapies should be highlighted. First, EpAbs have a narrower binding specificity towards RBD and 50–100 fold higher potency than those usually observed with CP [12,15,17]. Second, since the Fc region of specific anti-spike IgG was associated with acute lung injury in SARS infection, [18] CP administration may be associated with this antibody-dependent enhancement (ADE) effect. Finally, INM005 retains the bivalent binding ability of IgG antibodies while, due to the lack of the Fc region, avoids the potential ADE consequence [19,20]. Therefore, in patients with severe disease, these differences might explain why INM005, and not CP therapy, might be associated with better outcomes in well controlled clinical trials. Supporting the linkage between the high in vitro neutralizing activity of INM005 and the clinical outcome, in a population at risk for severe COVID-19 disease, the titer of the anti-spike IgG of the CP was a major determinant for decreasing the progression of early SARS-CoV-2 infection [9].
Antibody response kinetics may have a major role in the outcome of COVID-19 disease. As delayed neutralizing antibodies responses were correlated with impaired viral control and lack of recovery [21], we hypothesize that seronegative patients would have higher clinical response using this treatment, as INM005 displayed more favorable effect in seronegative than in seropositive patients, suggesting that the delayed seroconversion and severe COVID-19 disease might be predictive factors of response.
The polyclonal nature of INM005 makes this passive immunotherapy less prone to lose efficacy against escape SARS-CoV-2 mutants. The neutralization capacity of INM005 can be monitored by sero-neutralization assays against new circulating mutants [22], [23], and the amino acid composition of the recombinant RBD used as immunogen can be adapted to these changes if needed.
INM005 was well tolerated and similar to placebo. Emergent treatment adverse events of special interest were mild or moderate, did not require interruption of study drug infusion nor discontinuation of second dose administration, and all resolved without sequelae. No statistically significant differences were found in the overall incidence of adverse events or serious adverse events. No anaphylaxis event was reported in any of the patients. The good safety profile of INM005 may be attributed to the manufacturing process that reduces the presence of complete equine immunoglobulins to practically undetectable levels. In addition, the elimination of the Fc region, which prevents complement activation, could reduce the intensity of immune complex formation responsible for the development of late reactions such as serum sickness.
This study had several limitations. Since the effect of INM005 was more noticeable in those with severe disease, the low number of patients included in this category and the variability observed at day 28 precluded us from reaching the primary endpoint with statistical significance. Another limitation of the study is that the overall age of the population was younger than that of other series of similar studies, making this a less generalizable finding. This clinical trial evaluated EpAbs in patients with moderate to severe COVID-19. INM005 could be tested in other clinical stages including early and critical disease. Another limitation includes the timing of the first viral load measurement after treatment initiation, as it was performed when most patients would have spontaneously reached low levels, precluding us from making an appropriate analysis of INM005 in this parameter.
In summary, as shown in this randomized clinical trial, INM005 appears as an attractive and safe agent for the treatment of patients with severe COVID-19 disease that deserves further evaluation. Future studies will help to define a more precise role of this treatment in the context of current COVID-19 pandemic.
Declaration of Competing Interest
MC, SS, VZ, LM, LS, FG received grants from Ministries of Science and Production of Argentina. MD, JF, GV, AB, FC, MFA, LB, RT, SL, DS, MI, VS, RS, PC, MMC, LA, HLL, AC, DC declare reimbursement for conduction of clinical trial as investigator of the study. PC, OS, YK report other funds from Inmunova S.A. EN, GL, WHB, SPLL report personal fees from Inmunova S.A. SM reports non-financial support from Inmunova S.A. AP, B de M, Gabriel L declare no competing interests. SPLL declare personal fees from Movement Disorders Society, Laboratorio Elea and Merck pharmaceuticals.
MC, SS, VZ, LM, LS are employed by Inmunova S.A.
Acknowledgments
Contributors
CL, WHB, MC, SS, VZ, LM, GL, SPLl, AP, OS, PC, LS y FG contributed to the formulation and general design of the study. CL, WHB, EN, MC, SS, VZ, LM, YK, LS and FG did the data curation. B de M, SPLL, YK conducted the formal analysis. LS y FG were responsible for the funding acquisition. CL, WHB, EN, MC, SS, MD, JF, GV, AB, FC, MFA, LB, RT, SL, DS, MI, VS, RS, PC, MMC, LA, HLL, AC, DC, SPLL, YK, AP, OS, PC, LS y FG were part of the conduction of the research and investigation process. MC, SS, VZ, LM provided management and coordination for the planning and execution of the research. Study material and analysis tools were provided by MC, SS, VZ, LM, LS y FG. Software implementation was done by MC. GL, WHB, MC, SS, LS y FG supervised the planning and research process. MC, VZ, LM, B de M, SPLl, SM accessed and verified the data. GL, WHB, EN, BdeM, SPLl and FG were part of the visualization, editing and data presentation. GL, WHB, EN, MC, SS, VZ, BdeM, SPLl, YK, AP, OS, PC, LS and FG critically reviewed the manuscript while GL, WHB, EN, FG also drafted the manuscript. All authors had full access to all of the data in the study and approved the final version. Article preparation was done by all study authors and the decision to submit the article for publication was made by all study authors. MC and FG verified these authors' contributions.
Funding
The study was funded by Inmunova S.A. and grants from the Ministries of Science and Production of Argentina: “Ministerio de Desarrollo Productivo - Programa soluciona. Reactivación de la economía del conocimiento” and “Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación del Ministerio de Ciencia, Tecnología e Innovación”.
Data sharing
Individual patient data collected during the trial is protected under the Personal Data Protection Act 25326 of Argentinian law. Participant data will not be available to others with identifiers under no circumstances. In case any patient data needs to be available, it will be provided in a de-identified manner. Study protocol, statistical analysis, informed consent form, and clinical study report containing patient de-identified data have been shared with the National Regulatory Authority (ANMAT). Study protocol, statistical analysis and informed consent form will become available by request to anyone who wishes to access the data immediately after publication with no end date. Any other data request will be assessed separately. Proposals should be directed to info@inmunova.com; to gain access, data requestors will need to sign a data access agreement.
Acknowledgments
We acknowledge Instituto Biológico Argentino S.A.I.C and mAbxcience, for their efforts in the scaling up of INM005. We thank Laboratorio de detección del virus SARS-CoV-2 de Plataforma de Servicios Biotecnológicos del Departamento de Ciencia y Tecnología de la Universidad Nacional de Quilmes and DominguezLab, for the different analyses in patient´s biological samples. We acknowledge Nubilaria for development of the electronic database. We thank both Servicio Virosis Respiratorias INEI-ANLIS Malbrán, Laboratorio Nacional de Referencia de Enfermedades Respiratorias Virales, Centro Nacional de Influenza de OMS, Buenos Aires and Instituto de Virología "Dr. J. M. Vanella", Facultad de Ciencias Médicas, Universidad Nacional de Córdoba for their contribution in the characterization of neutralizing titer of INM005. We also acknowledge the members of the DSMB: Esteban Nannini, MD; Angela Gentile, MD; Isabel Cassetti, MD; María Eugenia Socias, MD; and Tomás Orduna, MD. Part of the costs of this clinical trial were funded by grants and loans of the Ministerio de Desarrollo Productivo of Argentina through “Programa soluciona. Reactivación de la Economía del Conocimiento” and a grant from the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación of the Ministerio de Ciencia, Tecnología e Innovación de Argentina.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100843.
Appendix. Supplementary materials
References
- 1.https://www.worldometers.info/coronavirus/. COVID-19 coronavirus pandemic. (accessed January 15th, 2021).
- 2.Sterne J.A.C., Murthy S., Diaz J.V. Association between administration of systemic corticosteroids and mortality among critically III patients with COVID-19. JAMA. 2020;324:1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of COVID-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman J.D., Lye D.C.B., Hui D.S. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinner C.D., Gottlieb R.L., Criner G.J. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. JAMA. 2020;324:1048. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonovich V.A., Burgos Pratx L.D., Scibona P. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicenter randomized controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libster R., Pérez Marc G., Wappner D. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P., Nirula A., Heller B. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2020;384:229–237. doi: 10.1056/NEJMoa2029849. NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2020;384:905–914. doi: 10.1056/NEJMoa2033130. NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zylberman V., Sanguineti S., Pontoriero A.V. Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Medicina (B Aires) 2020;80(Suppl 3):1–6. [PubMed] [Google Scholar]
- 13.Boyer L., Degan J., Ruha A.M., Mallie J., Mangin E., Alagón A. Safety of intravenous equine F(ab’)2: insights following clinical trials involving 1534 recipients of scorpion antivenom. Toxicon. 2013;76:386–393. doi: 10.1016/j.toxicon.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Health. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Clin. Spectr. SARS-CoV-2 Infect. (accessed January 15th, 2021).
- 15.Pan X., Zhou P., Fan T. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral Res. 2020;182 doi: 10.1016/j.antiviral.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall J.C., Murthy S., Diaz J. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccoli L., Park Y.J., Tortorici M.A. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042. doi: 10.1016/j.cell.2020.09.037. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Wei Q., Lin Q. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F., Yan R., Liu M. medRxiv. 2020. Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: studies based on cellular and structural biology analysis. 2020.10.08.20209114. [Google Scholar]
- 20.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas C., Klein J., Sundaram M. Kinetics of antibody responses dictate COVID-19 outcome. medRxiv. 2020 2020.12.18.20248331. [Google Scholar]
- 22.Chan K.K., Tan T.J.C., Narayanan K.K., Procko E. Science Advances; 7: no. 8, eabf1738; 2021. An engineered decoy receptor for SARS-CoV-2 broadly binds protein S sequence variants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisblum Y., Schmidt F., Zhang F. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.