Abstract
Simple Summary
Due to the rapid increase of primary liver cancer incidence and the poor prognosis, it is imperative to identify new modifiable factors such as diet and nutrition for the prevention of liver cancer. Diet high in saturated fatty acids (SFA) has been hypothesized to be associated with increased risk of cancers. However, the associations between dietary fatty acids and liver cancer are not consistent. We aimed to examine the association between dietary total fat, its major components, serum cholesterol, and risk of liver cancer combining current evidence from prospective studies. Our meta-analyses provided new evidence on associations between dietary fats, serum cholesterol, and liver cancer risk. Higher intake of dietary SFA was associated with higher risk of liver cancer while higher serum levels of cholesterol and high-density lipoprotein (HDL) were associated with a lower risk of liver cancer with high between-studies variability. Based on our findings, reducing dietary SFA may help to prevent the development of liver cancer.
Abstract
To quantify the associations between dietary fats and their major components, as well as serum levels of cholesterol, and liver cancer risk, we performed a systematic review and meta-analysis of prospective studies. We searched PubMed, Embase, and Web of Science up to October 2020 for prospective studies that reported the risk estimates of dietary fats and serum cholesterol for liver cancer risk. We carried out highest versus lowest intake or level and dose-response analyses. Higher intake of dietary saturated fatty acids (SFA) was associated with a higher liver cancer risk in both category analysis (relative risk [RR]highest vs. lowest intake = 1.34, 95% confidence interval [CI]: 1.06, 1.69) and dose-response analysis (RR1% energy = 1.04, 95%CI: 1.01, 1.07). Higher serum total cholesterol was inversely associated with liver cancer but with large between-studies variability (RR1 mmol/L = 0.72, 95%CI: 0.69, 0.75, I2 = 75.3%). The inverse association was more pronounced for serum high-density lipoprotein (HDL) cholesterol (RR1 mmol/L = 0.42, 95%CI: 0.27, 0.64). Higher intake of dietary SFA was associated with higher risk of liver cancer while higher serum levels of cholesterol and HDL were associated with a lower risk of liver cancer with high between-studies variability.
Keywords: meta-analysis, liver cancer, dietary fat, cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, epidemiological study
1. Introduction
Primary liver cancer is the sixth most frequently diagnosed cancer and the fourth leading cause of cancer-related death worldwide in 2018 [1]. Current known risk factors for liver cancer include chronic infections (e.g., hepatitis B virus, hepatitis C virus), metabolic diseases (e.g., type 2 diabetes, obesity), behavioral factors (e.g., alcohol consumption, tobacco), and aflatoxin-contaminated foods [2]. In the past several decades, we have witnessed an increase in the incidence of liver cancers in Western countries [3]. However, these established risk factors combined can only explain less than 60% of all liver cancers in the U.S. [4]. Therefore, it is imperative to identify other modifiable factors, such as diet and nutrition.
The liver is the main organ for the synthesis and circulation of fatty acids and cholesterol in the human body. The relationship between dietary fat intake and liver cancer risk has been long of interest to researchers. Experimental and animal studies from the last several decades have demonstrated that high-fat diets can increase the risk of steatohepatitis and liver cancer in mice via the accumulation of fat and cholesterol [5]. However, limited prospective studies investigated the associations between dietary fat and liver cancer risk and reported inconsistent results [6,7,8]. In addition, any influence on the development of liver cancer may depend on the types of fatty acids. Due to the lower power of individual studies with a limited number of liver cancer cases, combining all the available evidence using meta-analysis methods will enhance the power to detect significant associations with liver cancer not only for dietary fats but also for different types of fatty acids.
A high blood level of total cholesterol, which could be affected by dietary saturated fatty acids (SFA), is a well-established risk factor for coronary heart disease and stroke [9]. However, the relationship between total cholesterol and the risk of cancer remains uncertain [10]. The different types of serum cholesterol, the high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, may play different roles in etiology of liver cancer according to their functions in cholesterol metabolism. Recently, several studies showed the associations between serum cholesterol and liver cancer risk in different populations and reported inverse associations but with different magnitudes [11,12].
Hence, we examined the association between dietary total fat and its major components including SFA, monounsaturated fatty acids (MUFA), N-3 and N-6 polyunsaturated fatty acids (PUFA), serum cholesterol including HDL and LDL cholesterol, and risk of liver cancer combining current evidence from prospective studies. We hypothesized that dietary SFA, but not MUFA and PUFA would be associated higher risk of liver cancer. We also hypothesized that higher level of cholesterol, if driven by HDL rather than LDL cholesterol, would be inversely associated with liver cancer risk.
2. Materials and Methods
The current review was conducted and reported in accordance with the standard criteria (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PRISMA) [13].
2.1. Search Strategy
We searched PubMed, Embase, and Web of Science up to October 2020. Details of the search terms are provided in Table S1. We did not apply any year, language, or publication status restrictions to the selection of articles for inclusion. We also searched the reference of the retrieved studies for any additional studies.
2.2. Study Selection and Inclusion Criteria
Two researchers (C.D. and Z.L.) independently performed the study selection. First, we scanned the title and abstract to obtain the relevant literature for further full-text review. Second, we downloaded the identified literature and read the full text carefully to ascertain the target literature through our inclusion criteria. The inclusion criteria included as follows: (1) the exposure of interest was dietary fat intake or its subtypes or serum cholesterol level; and (2) the outcome of interest was incidence of primary liver cancer or hepatocellular carcinoma or intrahepatic bile duct carcinoma; and (3) the study design was cohort design or nested case-control design, or case-cohort design; and (4) the study reported adjusted relative risk (RR) estimates and 95% confidence intervals (CI). An exclusion list of full-text review was provided in Table S2.
2.3. Data Extraction and Quality Assessment
Two researchers (C.D. and Z.L.) performed the data extraction independently and the discrepancy was solved with another researcher (L.Z.). We used a priori abstract table to obtain the information including last name of first author, publication year, country, name of the study, follow-up period, cohort size or numbers of participants, liver cancer cases, sex, baseline age, population exclusion criteria, type of outcome and its ascertainment, type of fat or serum cholesterol and its assessment, amount or frequency of exposures, RRs and 95%CI, variables adjusted for in the analysis.
We used the Newcastle–Ottawa scale (NOS) to assess the risk of bias of the included studies based on selection bias, comparability, and outcome assessment [14]. We considered studies with 0–3, 4–6, and 7–9 points to represent low-, medium-, and high-quality studies, respectively (Table S3).
2.4. Patient Involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results.
2.5. Statistical Methods
2.5.1. Statistical Synthesis
We conducted two types of analyses: associations for the highest versus lowest categories and dose-response analyses using the random effects model by DerSimonian and Laird, which considers variation both within and between studies [15]. For dose-response analysis, we estimated the RR for one unit of exposure as follows: for serum cholesterol, HDL, LDL, per 1 mmol/L; for dietary cholesterol, per 100 mg/day; for total fat, per 5% of energy from total fat; for subtypes of fatty acids, per 1% of energy from a specific type of fat; for N-6/N-3 PUFA ratio, per 1 unit change; for other fat ratios, per 0.1-unit change. We both estimated the RR and 95%CI for each increment of per one-unit increase and explored the nonlinear associations if appropriate. For each study, the trend from the correlated log RRs across categories of fat intake or cholesterol level was calculated using the method proposed by Greenland et. al. [16]. We assigned the midpoint of fat intake or cholesterol level of each category to the corresponding risk estimates of each study. If the upper bound in the highest category was not available, we assumed that it had the same amplitude as the preceding one. For nonlinear associations, we applied a two-stage, random-effect dose-response meta-analysis by modeling fat intake or cholesterol level using restricted cubic splines with three knots at fixed percentiles (5%, 50%, and 95%) of the distribution. We first fitted a restricted cubic spline model into each set of RRs within a specific study and then combined the two regression coefficients and the variance/covariance matrices for each study using a multivariate random-effects model. p value for nonlinearity was calculated by testing whether the coefficient of the second spline was equal to zero [17].
2.5.2. Heterogeneity
We evaluated heterogeneity by estimating the variance between studies using Cochran’s Q test and the I-squared (I2) statistic. We used p < 0.10 for Q test or I2 > 50% as statistically significant. I2 is the amount of total variation explained by variation between studies [18]. We did not conduct meta-regression analyses because of the limited number of studies for each exposure.
2.5.3. Sensitivity Analyses and Publication Bias
Influential analyses by excluding one study at a time from each analysis were conducted to investigate the robustness of the findings. Indication of small study effects was evaluated based on Egger’s regression asymmetry test (p = 0.10) [19]. If there was evidence of publication bias, we used the trim-fill methods to reanalyze the data.
2.5.4. Software Used
All statistical analyses were performed using the R program (Version 3.5.0, R core team, Vienna, Austria). A two-sided p value less than 0.05 was considered as statistical significance if not specified.
3. Results
3.1. Literature Research and Data Abstraction
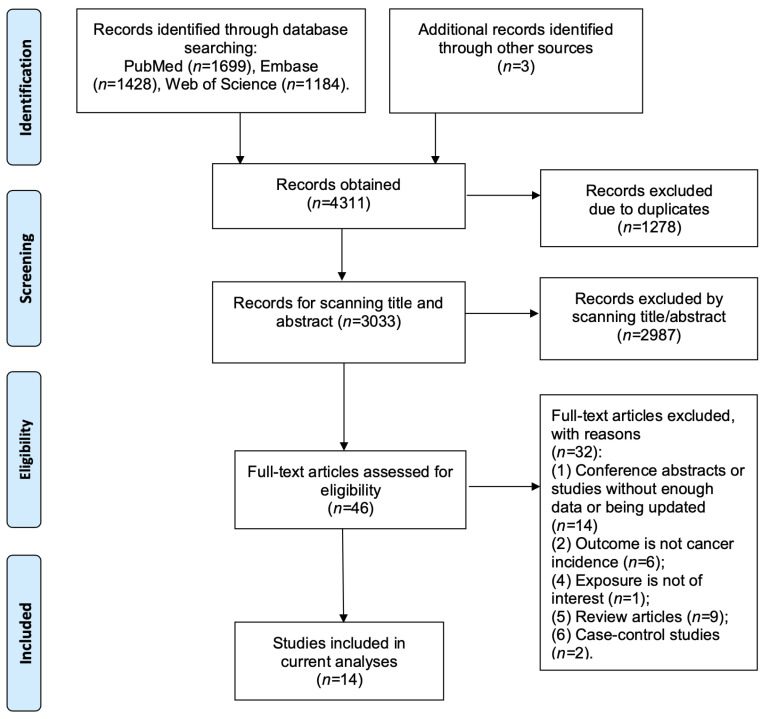
We identified 3033 records by searching PubMed, Embase, and web of science after removing the duplicates (Figure 1). We identified 46 articles that needed further full-text review, of which 32 articles were excluded (Table S2). Finally, we included 15,890 liver cancer cases from 14 prospective studies in the current meta-analysis [6,7,8,11,12,20,21,22,23,24,25,26,27,28] (Table 1).
Figure 1.
Follow chart of study selection.
Table 1.
Characteristics of prospective cohort studies included in meta-analysis on associations between dietary fats and serum cholesterol and liver cancer.
| Author, Year (Reference) | Country, Study Design | Baseline Years | Median Follow-Up (Years) | Age (Mean/Median, Years) | Sex | Population Exclusion | Cohort Size | Cases | Exposure | Exposure Assessment | Outcome | Outcome Assessment | Adjustment for Confounding Variables | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li, 2020 [12] | China, 4C Cohort | 2010–2014 | 3.8 | 56.9 | Both | Participants diagnosed as cancer within 6 months from baseline were excluded. | 137,884 | 156 | LDL cholesterol | Serum level | Liver cancer | Medical records | Age, sex, BMI, family history of cancer, smoking, drinking, education status, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, and systolic blood pressure. | 8 |
| Yang, 2020 [8] | U.S., NHS/HPFS | 1980 | 26.6 | Median = 64–68 | Both | Cancer diagnosed before baseline except for non-melanoma skin cancer were excluded. | 138,483 | 160 | Total fat, SFA, MUFA, PUFA, Trans fat, cholesterol, N3 PUFA, N6 PUFA, N6/N3 ratio, P:S ratio, M:S ratio, (M + P):S ratio | Validated FFQ | HCC | Biennial questionnaires, medical records and pathological reports confirmed by a study physician, state cancer registries, the National Death Index, death certificate | Age, sex, race, physical activity, BMI, smoking status, aspirin use, type 2 diabetes, alcohol intake, total coffee intake, and total energy intake. | 8 |
| Nderitu, 2017 [26] | Sweden, Swedish AMORIS | 1985 | 20.03 | 44 | Both | Participants with benign liver tumors, primary liver cancer or cirrhosis at baseline were excluded. | 509,436 | 766 | Cholesterol, HDL cholesterol, LDL cholesterol | Serum level | Liver cancer | Linkage with Swedish national registries | Age, sex, SES, triglycerides, cholesterol, raised glucose, diabetic status and history of liver disease (Cholesterol not adjusted for total cholesterol; HDL-C, LDL-C not adjusted for triglycerides). | 7 |
| Guan, 2017 [23] | China, Kailuan Cohort Study | 2006 | approximately 8 years | 51.05 | Male | Participants with history of cancer and cardiovascular disease were excluded. | 68,759 | 205 | Cholesterol, LDL cholesterol | Serum level | Liver cancer | Medical insurance, hospital records/death confirmed by death certificates | Age, cigarette smoking, alcohol consumption, physical activity, hypertension, diabetes mellitus, BMI. | 7 |
| Koh, 2016 [7] | Singapore, SCHS | 1993 | 14 | 56.4 | Both | Individuals who had history of invasive cancer at baseline (except non-melanoma skin cancer) were excluded. | 60,298 | 488 | Total fat, SFA, MUFA, N3 PUFA, N6 PUFA, N6/N3 ratio | Validated FFQ | HCC | Record linkage analysis of the cohort database with databases of the population-based Singapore Cancer Registry and Singapore Registry of Births and Deaths | Age, sex, dialect, year of interview, educational level, BMI, smoking status, alcohol use, coffee drinking status, baseline history of self-reported diabetes, total energy and dietary protein. Fat subtype intakes are mutually adjusted. | 9 |
| Duarte-Salles, 2015 [6] | Europe, EPIC | 1992 | 11.4 | 51.2 | Both | Generally healthy population across centers. | 477,206 | 191 | Total fat, SFA, MUFA, PUFA, P:S ratio, M:S ratio, (P + M):S ratio | Validated FFQ and 24-h dietary recall | HCC | Record linkage with cancer registries/a combination of methods. | Baseline alcohol intake and non-alcohol total energy intake, sex-specific physical activity level, BMI, smoking status, lifetime alcohol intake pattern, coffee intake, and intake of dietary fiber. Fat subtype intakes are mutually adjusted. | 9 |
| Sawada, 2012 [27] | Japan, JPHC | 1990 | 11.2 | 40–69 | Both | Subjects who had been diagnosed with cancer before the starting point were excluded. | 90,296 | 398 | N3 PUFA | Validated FFQ | HCC | Data linkage of major local hospitals with cancer registries/death certificate | Age, area, sex, smoking status, alcohol frequency, BMI, past history of diabetes mellitus, intake of coffee, soy foods, vegetables, vegetable oil, protein, and iron. | 9 |
| Borena, 2011 [21] | Europe, Me-Can cohort | 2006 | 12 | 44 | Both | Malignant cancer before the health examination were excluded. | 578,700 | 266 | Cholesterol | Serum level | Liver Cancer | National cancer registries | Age, smoking status, cohort, birth year and sex, BMI. | 8 |
| Kitahara, 2011 [11] | Korea, KCPS | 1992–1995 | 12.7 | 44.9 (men), 49.3 (women) | Both | Participants who reported having cardiovascular disease, cancer, liver disease, or a respiratory disease at or before the initial visit, or who had extremely low levels of BMI, with missing or implausibly high or low total cholesterol levels were excluded. | 1,189,719 | 10,161 | Cholesterol | Serum level | Liver cancer | Medical records | Cigarette smoking, alcohol drinking, BMI, fasting serum glucose, hypertension, and physical activity. | 9 |
| Freedman, 2010 [22] | U.S., NIH-AARP | 1995 | NR | Median = 62.6 | Both | Participants who developed cancer or died before their questionnaires were scanned were excluded. | 495,006 | 338 | Total fat, SFA, MUFA, PUFA | Validated FFQ | HCC | State cancer registries | Age, sex, alcohol, BMI, cigarette smoking, diabetes, education, fruit intake, vegetable intake, marital status, race and/or ethnicity, total energy from nonalcohol sources, usual physical activity throughout the day, and vigorous physical activity. | 7 |
| Ahn, 2009 [20] | U.S., ATBC | 1985 | 14.9 | 50–69 | Male | Participants with history of cancer other than nonmelanoma skin cancer or carcinoma in situ, severe angina pectoris, chronic renal insufficiency, liver cirrhosis, chronic alcoholism, anticoagulant therapy, other medical problems that might have limited long-term participation were excluded. | 29,093 | 191 | Cholesterol, HDL cholesterol | Serum level | Liver cancer | Medical records | Age, intervention, level of education, systolic blood pressure, BMI, physical activity, duration of smoking, number of cigarettes smoked per day, saturates fat intake, polyunsaturated fat intake, total calorie, alcohol consumption, and serum HDL cholesterol. | 9 |
| Ioannou, 2009 [24] | U.S., NHANES | 1971 | 13.3 | 48.8 | Both | Participants who reported at baseline ever being told by a physician that they had jaundice, hepatitis, or a malignant tumor, who had hepatomegaly or splenomegaly at baseline examination, or whose level of serum albumin was less than 3 g/dL were excluded. | 9221 | 123 | Total fat, SFA, cholesterol | 24-h dietary recall questionnaire | Cirrhosis and liver cancer | Hospitalization records death certificates | Energy from other macronutrients, daily alcohol consumption, coffee or tea, gender, race, age, education, region, diabetes, BMI, and subscapular-to-triceps skinfold ratio. | 6 |
| Iso, 2009 [25] | Japan, JPHC | 1990 | 12.4 | 40–69 | Both | Participants with history of cardiovascular disease were excluded. | 33,368 | 125 | Cholesterol | Serum level | Liver cancer | Medical records and/or cancer registries | Age, BMI, pack years of smoking, ethanol intake, hypertension, diabetes, hyperlipidemia medication use, total vegetable intake, coffee intake and public health center. | 9 |
| Strasak, 2009 [28] | Australia, VHM and PP | 1985 | 11.6 | 41.6 | Both | Healthy participants, free of cancer at baseline. | 172,210 | 2322 | Cholesterol | Fasting blood sample | Malignant neoplasms of digestive organs | Vorarlberg Cancer Registry | Age, BMI, smoking status, occupational status, and year of entry into the cohort. | 8 |
Abbreviations: 4C, the China Cardiometabolic Disease and Cancer Cohort Study; AMORIS, The Swedish Apolipoprotein Mortality Risk Study; ATBC, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort; BMI, body mass index; EPIC, the European Prospective Investigation into Cancer and Nutrition cohort; FFQ, food frequency questionnaire; H-B, Hospital based; HCC, hepatocellular carcinoma; HDL, high-density lipoproteins; HPFS, the Health Professional Follow-up Study; JPHC, the Japan Public Health Center-based prospective study; KCPS, the Korean Cancer Prevention Study; LDL, low-density lipoproteins; M:S ratio, the ratio of MUFA and SFA; (M + P):S ratio, the ratio of unsaturated fat and saturated fat; MUFA, monounsaturated fatty acids; NHANES, the National Health and Nutrition Examination Survey; NHS, the Nurse Health Study; NOS, Newcastle-Ottawa Quality Assessment Scale; NR, not reported; SCHS, the Singapore Chinese Health Study; SES, social economic status; SFA, saturated fatty acids; PUFA, polyunsaturated fatty acids; P:S ratio, the ratio of PUFA and SFA; VHM and PP, the Vorarlberg Health Monitoring and Promotion Program.
3.2. Dietary Fats and Liver Cancer Risk
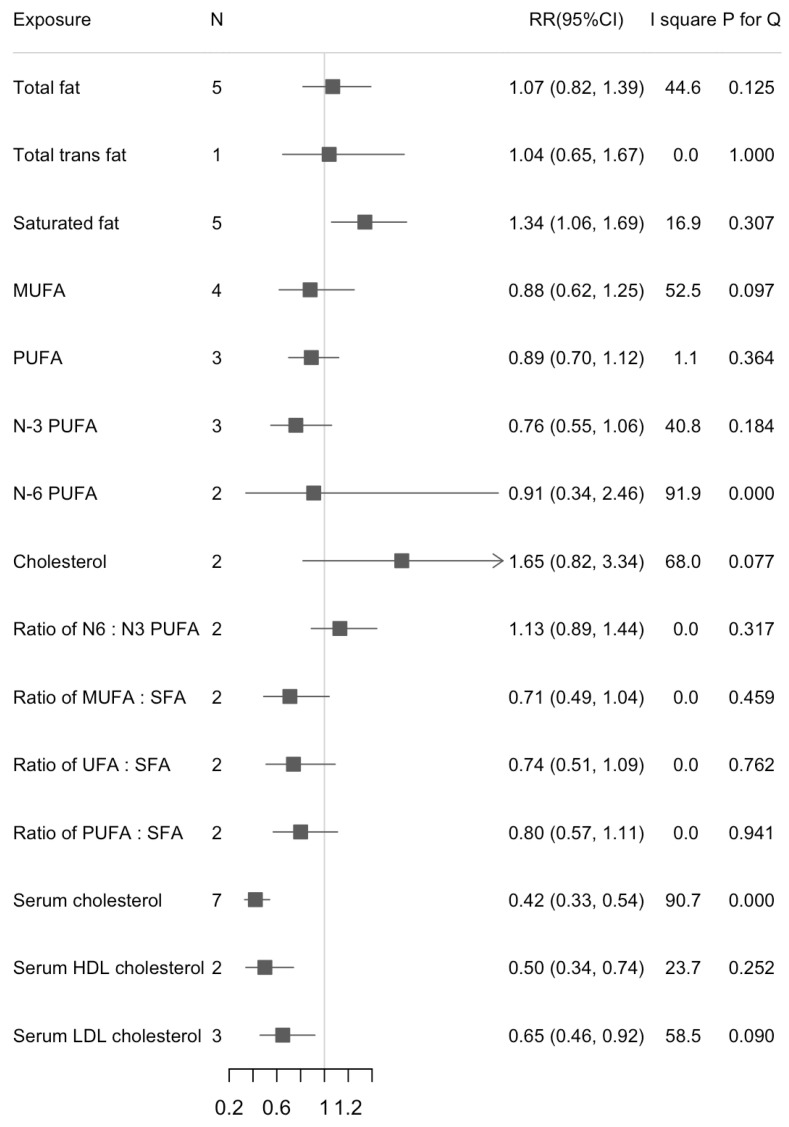
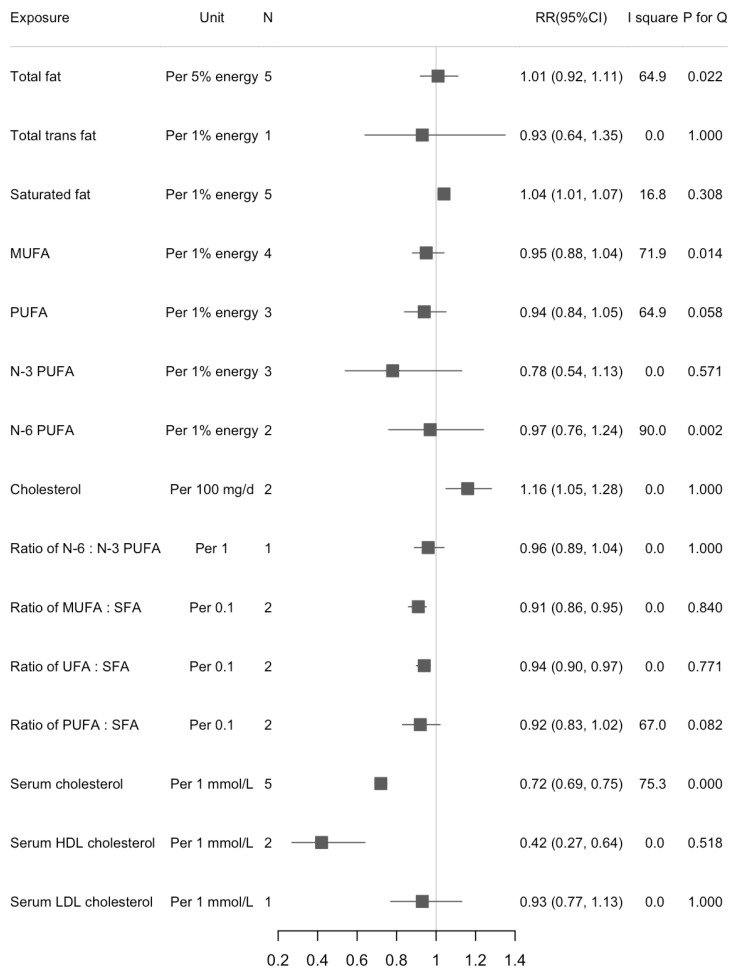
We found a statistically significant association between dietary SFA and liver cancer risk in the highest versus lowest intake (RR = 1.34, 95%CI: 1.06, 1.69; n = 5, I2 = 16.9%) (Figure 2). The increased risk of liver cancer with higher dietary SFA was also found in the dose-response analysis (RR1% energy increase = 1.04, 95%CI: 1.01, 1.07; n = 5, I2 = 16.8%) (Figure 3). There were statistically inverse associations between per 0.1-unit increase in ratio of MUFA:SFA, unsaturated fatty acids (UFA):SFA, and liver cancer risk with RRs (95%CIs) of 0.91 (0.86, 0.95), and 0.94 (0.90, 0.97), respectively. An inverse association between dietary cholesterol intake and liver cancer risk was found in dose-response analyses but not in highest versus lowest analyses (RR100 mg/d = 1.16, 95%CI: 1.05, 1.28; n = 2, I2 = 0%). We did not find any significant associations between intake of dietary total fat, MUFA, and PUFA and risk of liver cancer. Results for highest versus lowest categories and dose-response analyses were provided in Figure 2 and Figure 3, respectively. The forest plots for the above associations were shown in Figures S5–S10, S13–S18. We did not detect any nonlinear associations between dietary total fat, SFA and liver cancer risk (Figures S1 and S2).
Figure 2.
Summary of associations between dietary fats and serum cholesterol and liver cancer (Highest versus lowest categories).
Figure 3.
Summary of associations between dietary fats and serum cholesterol and liver cancer (dose-response analysis).
3.3. Serum Cholesterol and Liver Cancer Risk
We found an inverse association between serum total cholesterol and liver cancer risk when comparing the highest versus lowest categories (RR = 0.42, 95%CI: 0.33, 0.54; n = 7, I2 = 90.7%) (Figure 2). Results were similar in separate analyses for men (RRH/L = 0.39, 95%CI: 0.27, 0.57) and women (RRH/L = 0.31, 95%CI: 0.26, 0.38). For the subtypes of cholesterol, serum HDL cholesterol appeared more strongly associated with liver cancer risk than serum LDL cholesterol (RRHDL = 0.50, 95%CI: 0.34, 0.74; n = 2, I2 = 23.7%; RRLDL = 0.65, 95%CI: 0.46, 0.92; n = 3, I2 = 58.5%). When we calculated the RRs for per 1 mmol/L increase in serum cholesterol, the associations were stable except for serum LDL cholesterol. Serum cholesterol was inversely associated with risk of liver cancer but with large between-study heterogeneity (RR1 mmol/L = 0.72, 95%CI: 0.69, 0.75; n = 7, I2 = 75.3%) (Figure 3). The RR decreased 58% for per 1 mmol/L increase of HDL cholesterol (RR1 mmol/L = 0.42, 95%CI: 0.27, 0.64; n = 2, I2 = 0.0%). Results were provided in Figure 2 and Figure 3. The forest plots for above associations were shown in Figures S4, S11 and S12. A significant nonlinearity was found between serum cholesterol and liver cancer risk, which indicates a L-curve with an inflection at about 6 mmol/L (p for nonlinearity < 0.001, Figure S3).
3.4. Publication Bias
We performed tests for small study effect and sensitivity analyses when the number of studies in each association was larger than six. We found the association between serum cholesterol and liver cancer risk might be affected by publication bias. The p values for Egger’s test from highest versus lowest categories and per 1 mmol/L increase of serum cholesterol were 0.064 and 0.005. Therefore, we used the trim-fill methods to adjust the publication bias. However, the results did not change materially compared with the main results (Figure S19a,b).
We also performed sensitivity analyses to explore the robustness of our main findings. We used the leave-one-out methods and found the main findings were stable and robust in general when we excluded one study one time (Table S4). When we excluded studies with a NOS less than seven (only one study), the results did not change significantly (data not shown). We also conducted analyses by excluding Li et al. [12] study due to its short follow-up time and found association between LDL and liver cancer became statistically nonsignificant (RRH/L = 0.78, 95%CI: 0.59–1.03, I2 = 0.0%).
4. Discussion
4.1. Principal Findings
For the first time, our study provided a comprehensive analysis synthesizing evidence on dietary fat intake, serum cholesterol, and liver cancer risk. We found an increased risk for dietary SFA with liver cancer using both category and dose-response analyses. Higher ratios of MUFA:SFA and UFA:SFA were associated with a lower risk of developing liver cancer. Higher serum cholesterol and HDL were associated with a lower risk of liver cancer with high between-studies variability. These findings were generally robust and stable in sensitivity analyses.
The associations between dietary fats and health outcomes have been a long-standing research topic of interest [29]. Although not entirely consistent, some evidence suggests that specific types of fat may play different roles in carcinogenesis, and replacing saturated fats with unsaturated fats may be beneficial to the prevention of some specific cancers including breast cancer [30], pancreatic cancer [31], prostate cancer [32], and lung cancer [33], but not colorectal cancer [34,35]. In general, our results provided new evidence for liver cancer by adding that higher intake of dietary SFA was associated with an increased risk of liver cancer. Additionally, we found inverse associations between ratio of MUFA:SFA, UFA:SFA, and liver cancer risk. However, due to the limited number of studies, these results should be interpreted with caution.
The positive association between dietary SFA and liver cancer risk is biologically plausible. In experimental studies, excess ingestion of SFA increases hepatic lipid storage, energy metabolism, and insulin resistance [36], which may directly contribute to development of nonalcoholic fatty liver disease (NAFLD), a risk factor for liver cancer [37]. Dietary nutrients such as fats and sugar are involved in the development of hepatic steatosis [38]. In addition, evidence has accumulated that higher dietary SFA was also associated with increased risk of type 2 diabetes and obesity [39,40], risk factors for liver cancer. When we evaluated the association between SFA and liver cancer, four [6,7,8,24] of five studies adjusted for total energy intake while the remaining one [22] additionally adjusted for other fatty acids types. A combination of studies replacing SFA with carbohydrate or protein or studies replacing SFA with all other types of macronutrients except for SFA yielded similar results (data not shown).
A large body of evidence indicates that higher intake of dietary SFAs increases blood levels of LDL cholesterol and the LDL to HDL ratio, both of which are associated with a higher risk of cardiovascular diseases [41]. The epidemiological evidence on associations between serum cholesterol and cancer risk has accumulated in recent decades [9,10,11,12]. Cholesterol is a unique lipid, essential for membrane biogenesis, cell proliferation, and cell differentiation. It is not only from the diet but is also mainly synthesized by the liver in humans and distributed throughout the body via LDL and HDL transporters. Previous studies indicate that serum cholesterol is positively associated with the risk of some specific cancers because it is the obligatory precursor of steroid hormones involved in tumor promotion and tumor death [10]. The positive associations of serum cholesterol were shown for risk of breast and prostate cancer but not for all cancer types [11].
In the current analysis, we found an inverse association between serum cholesterol and liver cancer risk although higher between-study variability was observed. The underlying mechanisms are largely unclear. First, unmeasured factors that could decrease the serum cholesterol level and lead to the development of liver cancer may be possible, such as unknown genotypes. For example, transmembrane 6 superfamily 2 (TM6SF2) rs5854292-T variant is a risk factor for NAFLD and liver cancer [42,43]. In animal studies, TM6DF2 rs5854292-T is associated with lower serum levels of total cholesterol and LDL [42]. Thus, some genetic susceptibility may influence the association between serum cholesterol and liver cancer, which requires further investigation. Other mutations such as rs738409-G (PNPLA3) and rs1800562-A (HFE) [44] could also influence the observed association between serum cholesterol and liver cancer. Second, undiagnosed liver diseases, such as NAFLD and cirrhosis, could influence cholesterol metabolism, possibly exaggerating the inverse association with liver cancer risk. Additionally, chronic hepatitis B virus infection is associated with both higher liver cancer risk and lower cholesterol concentration, which may provide qualitative confounding between blood cholesterol level and liver cancer [45]. In this context, the higher levels of cholesterol might be an indicator of liver function, rather than a causal factor for liver cancer risk. Among 14 studies we included in this meta-analysis, only 3 studies excluded participants who had cirrhosis or other liver diseases. Therefore, we cannot fully evaluate the impact of undiagnosed liver diseases on our results. Third, metabolic disorders are risk factors for liver cancer and are also associated with abnormal serum cholesterol level. It may have influenced the associations between serum cholesterol, especially HDL, and liver cancer risk [46]. Fourth, it is also possible that this association may be due to increased use of cholesterol-lowering drugs such as statin in patients with elevated cholesterol [47]. Stain use has been reported to be associated with a lower risk of liver cancer [48]. We could not fully evaluate the influence of statin use on our results because most studies on serum cholesterol-liver cancer have not adjusted for statin use. Nonetheless, statin use was not common before 1990 [49]. We thereby restricted our analyses for studies with a baseline survey before 1990 and found an inverse association between serum cholesterol level and liver cancer risk (ORH/L = 0.60, 95%CI: 0.46, 0.78, n = 3, I2 = 81.2%). Clearly, future studies with statin use data are warranted.
4.2. Implications
Our meta-analysis provides new evidence on associations between dietary fats, serum cholesterol, HDL, and liver cancer risk. Previous studies mainly elucidate the detrimental effect of dietary SFA intake and the benefits of replacing SFA with unsaturated fatty acids for the prevention of cardiovascular diseases. Based on our findings, reducing dietary SFA may also help to prevent the development of liver cancer. However, for other subtypes of fats such as N-3 and N-6 PUFA, more studies are needed because the current studies are limited. The inverse association between serum cholesterol and liver cancer is unexpected, which requires further investigation.
4.3. Strengths and Limitations
Our study has several strengths. First, we provided estimates for both category analysis and dose-response analysis. We also explored the potential nonlinearity between dietary fats, serum cholesterol, and liver cancer risk. Second, we only included prospective studies, which may increase the internal validity of our findings. Third, for most serum cholesterol studies (7 of 8 studies), the blood samples were collected at the cohort baseline and the follow-up time are over 10 years, which possibly minimized the potential concerns of reverse causation.
Several limitations also merit attention. First, the number of existing studies is relatively small, which led to lower statistical power to detect the associations and publication bias. For some associations such as the ratio of different subtypes of fatty acids, only one or two studies were retrieved. Second, our study is also subject to the limitations that affected the original studies. For example, dietary information in the included studies was mostly collected by FFQs, which may lead to measurement errors. Third, the information for HBV and HCV is not available in most studies. Therefore, we cannot evaluate the influence of HBV or HCV status on our results. However, the HBV/HCV seems unlikely to substantially confound the reported associations between diet and liver cancer [50]. Fourth, there is substantial between-study heterogeneity between serum cholesterol and liver cancer. We did not conduct meta-regression to explore the source of the heterogeneity due to the limited number of studies. Our findings also indicate that there is publication bias in associations between serum cholesterol and liver cancer. However, after applying a trim-filled method to adjust for the publication bias, our findings did not materially change. Lastly, in the current meta-analysis, 7 out of 8 studies for serum cholesterol used liver cancer as a main outcome and 5 out of 6 studies for dietary fat focused on HCC risk. Thus, our results apply largely to HCC, and we cannot fully evaluate the potential etiology heterogeneity by different subtypes of liver cancer.
5. Conclusions
Our meta-analysis supported that avoiding excess dietary SFA intake would be beneficial to the prevention of liver cancer. Higher ratio of MUFA:SFA and PUFA:SFA were associated with a lower risk of developing liver cancer. We also found higher serum levels of cholesterol and HDL cholesterol were associated with a lower risk of liver cancer with high between-study heterogeneity. The underlying mechanisms need to be further investigated.
Acknowledgments
We thank all the authors and the participants of the original studies included.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13071580/s1, Table S1: Search terms in current meta-analysis, Table S2: Studies reviewed in full text for eligibility (excluded reasons for meta-analysis), Table S3: Quality assessment of studies included in meta-analysis (Newcastle-Ottawa Quality Assessment Scale), Table S4: Sensitivity analyses of associations between dietary fat, serum cholesterol and liver cancer (number of studies ≥ 6), and Figure S1: Association between serum cholesterol and liver cancer, Figure S2: Association between total dietary fat and liver cancer, Figure S3: Association between dietary saturated fat and liver cancer, Figures S4–S10: Forest plots of associations between dietary fats and serum cholesterol and liver cancer (per 1-unit increase), Figures S11–S18: Forest plots of associations between dietary fats and serum cholesterol and liver cancer (Highest vs. Lowest categories), Figure S19: Trim-fill methods to adjust publication bias for associations indicating potential publication bias.
Author Contributions
The authors’ responsibilities were as follows: Study concept and design: L.Z., X.Z. Development and implementation of literature search: L.Z., C.D., Z.L. Data abstraction: C.D., Z.L. Data analysis: L.Z., C.D. Interpretation of results: L.Z., E.G., X.Z. Drafting of the manuscript: L.Z. Critical revision of the manuscript for important intellectual content: All authors. Study supervision: X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
X.Z. is supported by NIH K07 CA188126, R21 CA238651, American Cancer Society Research Scholar Grant (RSG NEC-130476), the Dana-Farber Harvard Cancer Center, and the Zhu Family Center at the Harvard T. H. Chan School of Public Health.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The full dataset and statistical code are available from the corresponding author.
Conflicts of Interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Makarova-Rusher O.V., Altekruse S.F., McNeel T.S., Ulahannan S., Duffy A.G., Graubard B.I., Greten T.F., McGlynn K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savard C., Tartaglione E.V., Kuver R., Haigh W.G., Farrell G.C., Subramanian S., Chait A., Yeh M.M., Quinn L.S., Ioannou G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte-Salles T., Fedirko V., Stepien M., Aleksandrova K., Bamia C., Lagiou P., Laursen A.S., Hansen L., Overvad K., Tjonneland A., et al. Dietary fat, fat subtypes and hepatocellular carcinoma in a large European cohort. Int. J. Cancer. 2015;137:2715–2728. doi: 10.1002/ijc.29643. [DOI] [PubMed] [Google Scholar]
- 7.Koh W.P., Dan Y.Y., Goh G.B., Jin A., Wang R., Yuan J.M. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36:893–901. doi: 10.1111/liv.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W., Sui J., Ma Y., Simon T.G., Petrick J.L., Lai M., McGlynn K.A., Campbell P.T., Giovannucci E.L., Chan A.T., et al. High dietary intake of vegetable or polyunsaturated fats is associated with reduced risk of hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters S.A., Singhateh Y., Mackay D., Huxley R.R., Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi: 10.1016/j.atherosclerosis.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Silvente-Poirot S., Poirot M. Cholesterol and cancer, in the balance. Science. 2014;343:1445–1446. doi: 10.1126/science.1252787. [DOI] [PubMed] [Google Scholar]
- 11.Kitahara C.M., Berrington de Gonzalez A., Freedman N.D., Huxley R., Mok Y., Jee S.H., Samet J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M., Lu J., Fu J., Wan Q., Wang T., Huo Y., Xu Y., Xu M., Zhao Z., Chen Y., et al. The association and joint effect of serum cholesterol, glycemic status with the risk of incident cancer among middle-aged and elderly population in china cardiometabolic disease and cancer cohort (4C)-study. Am. J. Cancer Res. 2020;10:975–986. [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 25 March 2021)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 17.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J., Lim U., Weinstein S.J., Schatzkin A., Hayes R.B., Virtamo J., Albanes D. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol. Biomarkers Prev. 2009;18:2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borena W., Strohmaier S., Lukanova A., Bjorge T., Lindkvist B., Hallmans G., Edlinger M., Stocks T., Nagel G., Manjer J., et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int. J. Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 22.Freedman N.D., Cross A.J., McGlynn K.A., Abnet C.C., Park Y., Hollenbeck A.R., Schatzkin A., Everhart J.E., Sinha R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J. Natl. Cancer Inst. 2010;102:1354–1365. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan X.M., Wu S.L., Yang X.L., Han X., Yang Y.H., Li X.T., Bin Waleed K., Yue D., Zhan S.Y., Liu Y., et al. Association of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol with atherosclerotic cardiovascular disease and cancer in a Chinese male population. Int. J. Cancer. 2018;142:1209–1217. doi: 10.1002/ijc.31149. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou G.N., Morrow O.B., Connole M.L., Lee S.P. Association between dietary nutrient composition and the incidence of cirrhosis or liver cancer in the United States population. Hepatology. 2009;50:175–184. doi: 10.1002/hep.22941. [DOI] [PubMed] [Google Scholar]
- 25.Iso H., Ikeda A., Inoue M., Sato S., Tsugane S., Group J.S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer. 2009;125:2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 26.Nderitu P., Bosco C., Garmo H., Holmberg L., Malmstrom H., Hammar N., Walldius G., Jungner I., Ross P., Van Hemelrijck M. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: A study in the Swedish AMORIS cohort. Int. J. Cancer. 2017;141:1148–1160. doi: 10.1002/ijc.30818. [DOI] [PubMed] [Google Scholar]
- 27.Sawada N., Inoue M., Iwasaki M., Sasazuki S., Shimazu T., Yamaji T., Takachi R., Tanaka Y., Mizokami M., Tsugane S., et al. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468–1475. doi: 10.1053/j.gastro.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Strasak A.M., Pfeiffer R.M., Brant L.J., Rapp K., Hilbe W., Oberaigner W., Lang S., Borena W., Concin H., Diem G., et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: A prospective 19-year follow-up study. Ann. Oncol. 2009;20:1113–1120. doi: 10.1093/annonc/mdn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig D.S., Willett W.C., Volek J.S., Neuhouser M.L. Dietary fat: From foe to friend? Science. 2018;362:764–770. doi: 10.1126/science.aau2096. [DOI] [PubMed] [Google Scholar]
- 30.Thiebaut A.C., Kipnis V., Chang S.C., Subar A.F., Thompson F.E., Rosenberg P.S., Hollenbeck A.R., Leitzmann M., Schatzkin A. Dietary fat and postmenopausal invasive breast cancer in the National Institutes of Health-AARP Diet and Health Study cohort. J. Natl. Cancer Inst. 2007;99:451–462. doi: 10.1093/jnci/djk094. [DOI] [PubMed] [Google Scholar]
- 31.Thiebaut A.C., Jiao L., Silverman D.T., Cross A.J., Thompson F.E., Subar A.F., Hollenbeck A.R., Schatzkin A., Stolzenberg-Solomon R.Z. Dietary fatty acids and pancreatic cancer in the NIH-AARP diet and health study. J. Natl. Cancer Inst. 2009;101:1001–1011. doi: 10.1093/jnci/djp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liss M.A., Al-Bayati O., Gelfond J., Goros M., Ullevig S., DiGiovanni J., Hamilton-Reeves J., O’Keefe D., Bacich D., Weaver B., et al. Higher baseline dietary fat and fatty acid intake is associated with increased risk of incident prostate cancer in the SABOR study. Prostate Cancer Prostatic Dis. 2019;22:244–251. doi: 10.1038/s41391-018-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J.J., Yu D., Takata Y., Smith-Warner S.A., Blot W., White E., Robien K., Park Y., Xiang Y.B., Sinha R., et al. Dietary fat intake and lung cancer risk: A pooled analysis. J. Clin. Oncol. 2017;35:3055–3064. doi: 10.1200/JCO.2017.73.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapkin R.S., Navarro S.L., Hullar M.A.J., Lampe J.W. Diet and Gut Microbes Act Coordinately to Enhance Programmed Cell Death and Reduce Colorectal Cancer Risk. Dig. Dis. Sci. 2020;65:840–851. doi: 10.1007/s10620-020-06106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen S., Li H., Yu D., Cai H., Gao J., Gao Y., Luu H., Tran H., Xiang Y.B., Zheng W., et al. Dietary Fatty Acids and Colorectal Cancer Risk in Men: A Report from the Shanghai Men’s Health Study and a Meta-Analysis. Int. J. Cancer. 2020 doi: 10.1002/ijc.33196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez E.A., Kahl S., Seelig A., Begovatz P., Irmler M., Kupriyanova Y., Nowotny B., Nowotny P., Herder C., Barosa C., et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017;127:695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Younossi Z.M., Otgonsuren M., Henry L., Venkatesan C., Mishra A., Erario M., Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips C.M., Kesse-Guyot E., McManus R., Hercberg S., Lairon D., Planells R., Roche H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012;142:824–831. doi: 10.3945/jn.111.153460. [DOI] [PubMed] [Google Scholar]
- 40.Van Dam R.M., Willett W.C., Rimm E.B., Stampfer M.J., Hu F.B. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 41.Mensink R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 42.Li T.T., Li T.H., Peng J., He B., Liu L.S., Wei D.H., Jiang Z.S., Zheng X.L., Tang Z.H. TM6SF2: A novel target for plasma lipid regulation. Atherosclerosis. 2018;268:170–176. doi: 10.1016/j.atherosclerosis.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Tang S., Zhang J., Mei T.T., Guo H.Q., Wei X.H., Zhang W.Y., Liu Y.L., Liang S., Fan Z.P., Ma L.X., et al. Association of TM6SF2 rs58542926 T/C gene polymorphism with hepatocellular carcinoma: A meta-analysis. BMC Cancer. 2019;19:1128. doi: 10.1186/s12885-019-6173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen V.L., Chen Y., Du X., Handelman S.K., Speliotes E.K. Genetic variants that associate with cirrhosis have pleiotropic effects on human traits. Liver Int. 2020;40:405–415. doi: 10.1111/liv.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z., Keech A., Collins R., Slavin B., Chen J., Campbell T.C., Peto R. Prolonged infection with hepatitis B virus and association between low blood cholesterol concentration and liver cancer. BMJ. 1993;306:890–894. doi: 10.1136/bmj.306.6882.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welzel T.M., Graubard B.I., Zeuzem S., El-Serag H.B., Davila J.A., McGlynn K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russo M.W., Hoofnagle J.H., Gu J., Fontana R.J., Barnhart H., Kleiner D.E., Chalasani N., Bonkovsky H.L. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology. 2014;60:679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islam M.M., Poly T.N., Walther B.A., Yang H.C., Jack Li Y.C. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers. 2020;12:671. doi: 10.3390/cancers12030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantor E.D., Rehm C.D., Haas J.S., Chan A.T., Giovannucci E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L., Zhang X. Comments on “one-carbon metabolism-related micronutrients intake and risk for hepatocellular carcinoma: A prospective cohort study”. Int. J. Cancer. 2021;148:252–253. doi: 10.1002/ijc.33184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset and statistical code are available from the corresponding author.