Abstract
The use of probiotics in reproductive-related dysbiosis is an area of continuous progress due to the growing interest from clinicians and patients suffering from recurrent reproductive microbiota disorders. An imbalance in the natural colonization sites related to reproductive health—vaginal, cervicovaginal, endometrial, and pregnancy-related altered microbiota—could play a decisive role in reproductive outcomes. Oral and vaginal administrations are in continuous discussion regarding the clinical effects pursued, but the oral route is used and studied more often despite the need for further transference to the colonization site. The aim of the present review was to retrieve the standardized protocols of vaginal probiotics commonly used for investigating their microbiota modulation capacities. Most of the studies selected focused on treating bacterial vaginosis (BV) as the most common dysbiosis; a few studies focused on vulvovaginal candidiasis (VVC) and on pretreatment during in vitro fertilization (IVF). Vaginal probiotic doses administered were similar to oral probiotics protocols, ranging from ≥107 CFU/day to 2.5 × 1010 CFU/day, but were highly variable regarding the treatment duration timing. Moderate vaginal microbiota modulation was achieved; the relative abundance of abnormal microbiota decreased and Lactobacillus species increased.
Keywords: vaginal probiotics, reproductive dysbiosis, bacterial vaginosis, VVC, IVF
1. Introduction
1.1. Microbiota Colonization Sites in Women’s Reproductive System
The taxa composition of the microbiota appears to exert a relevant role in reproductive and hormonal health, determining states of eubiosis versus dysbiosis [1]. The effects of microbiota imbalance seem to contribute to trigger reproductive [2,3], hormonal [4], and metabolic disorders [5,6]. Similarly, the reproductive-site microbiota can be affected by hormones or endocrine disruptor chemicals [7]. Conversely, if microbial dysbiosis occurs, subsequent decreased enzymes levels may diminish circulating estrogens and lead to recurrent reproductive pathologies [4,8]. Special attention has been paid to the following dysbiosis sites: vaginal, cervicovaginal, endometrial, and, indirectly, placental microbiotas. They are described in detail below.
The vaginal microbiota shows a specific colonization pattern for each woman. In the vaginal microbiota, the Lactobacillus genus is dominant and determinant during the establishment of a healthy microbiome community [9]. Recently, certain authors have postulated on the specific colonization of the endometrium. Specifically, a decrease in the Lactobacillus population appeared to be linked to implantation failures or early miscarriage in in vitro-fertilization patients [10,11]. However, there is controversy in these results and in determining the ratio of dominant microorganisms associated with health/dysbiosis. The theory of the existence of microbes in the placenta against the dogma of sterility has been experimentally approached by different authors. There is controversial research on the presence of specific microbiota in human uterine and placental sites and its effect on pregnancy and the fetus [12,13]. The hypothesis regarding the existence of microbiota in the placenta is generally considered disproven, as rigorously controlled studies found either pathogenic infections or no bacterial presence [12,13]. The formation and conservation of placental integrity and utility are known to be critical to fetal progress, and survival [14].
1.2. Microbial Dysbiosis Associated with Reproductive System Diseases
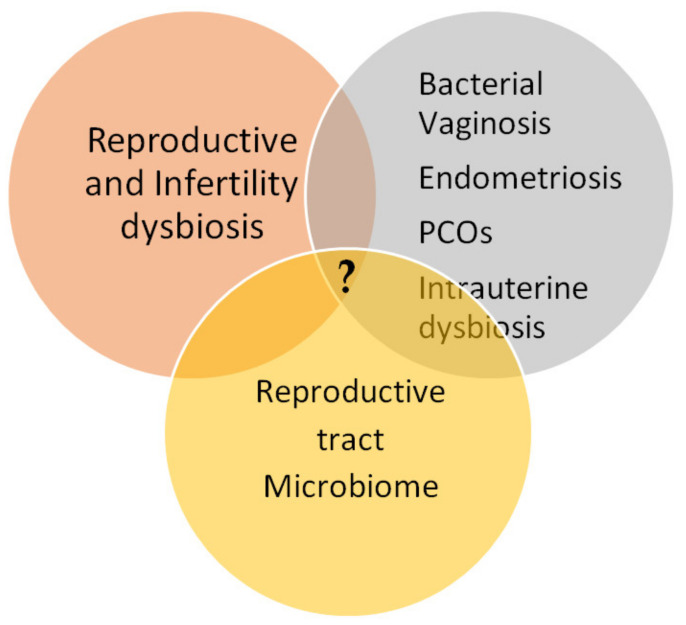
The reproductive tract microbiota’s composition and their variable patterns seem to be associated with alterations in reproductive disorders (Figure 1). Moreover, several recent studies have demonstrated that microbial dysbiosis could be linked to long-term recurrent reproductive modifications.
Figure 1.
Intersection gap knowledge of reproductive system disorders, unexplained infertility, microbiome dysbiosis, and recurrent reproductive pathogenesis.
Bacterial vaginosis (BV) is the most prevalent reproductive disorder and is linked with gynecological complications, like spontaneous preterm labor, abortion, and endometriosis. It can be cured by restoring the representative vaginal components of the microbiota with probiotic formula, usually species of the genus Lactobacillus [15,16]. Salah et al. [17] postulated that BV is strongly implicated in underestimated causes of unexplained infertility. They found that BV detection and treatment improves the pregnancy rate in women [17]. Furthermore, van Oostrum et al. [18] claim that BV is meaningfully linked with preclinical pregnancy loss. They claimed that infertility is generally related to BV and atypical microbiota at lower genital tract, estimating that one in every five infertile patients suffers from BV and at least one in every three has an altered vaginal taxa microbiota composition. Thus, they suggested that BV might be involved in the etiology and irregular pregnancies of these patients.
In addition, among women of reproductive age, there are other common dysbiosis such as endometriosis, that have been linked to an unfavorable effect on fertility; 30% to 71% of women suffering infertility showed endometriosis and 30% to 50% of women with endometriosis are infertile [19]. Moreover, polycystic ovary syndrome (PCOS), linked to multiple physiological risk factors (obesity, hypertension, dyslipidemia, and insulin resistance), has also been associated with reproductive disorders [2,20]. Furthermore, spontaneous abortions and preterm deliveries, including non-implantation of the embryo, could be highly related to episodes of microbial dysbiosis; these could be modulated by restoring the disrupted microbiota [21,22,23,24]. Therefore, one strategy to counteract this bacterial misbalance involves the administration of probiotics, which are in indicative cases less harmful, safer and more natural than using antibiotics [22]. However, in general, studies of probiotics in relation to pregnancy complications generally require statistically significant sample sizes or complete data with a superior number of clinical trials and the determination of microbiota at the species and strains level [24].
1.3. Probiotics for Reproductive Health Interventions
The administration of probiotics for reproductive clinical translational studies are continuously progressing due to the growing interest in the previous scientific evidence reported for demonstrating the beneficial effects related to the restoration of natural microbiome colonization in reproductive sites.
Probiotics remain an important complementary intervention resource to modulate dysbiosis of the microbiota, which were associated with various metabolic disorders and diseases [25,26]. Therefore, specific doses of certain probiotic strains could modulate the microbiota toward a healthier state, that is, to recover the state of eubiosis [27,28]. Conversely, the inappropriate use of probiotics might pose some risks and safety concerns in immunologically compromised individuals [29].
1.4. Administration Routes of Probiotics in Reproductive Dysbiosis
Most clinical trials on the modulation of reproductive dysbiosis have been carried out using oral probiotics [30]. However, oral administration requires transfer of the probiotic bacteria to the site of colonization to promote a specific clinical effect, which implies that the probiotics have to subsist to the low pH of the upper gastrointestinal region. This transfer is generally demonstrated by the recovery of the specific microorganisms from fecal samples [25,31]. Specifically, in microbiota reproductive dysbiosis, probiotics should be transferred to the dysbiotic colonization sites, such as the vagina (vaginosis), the endometrium (endometritis), or the breast (mastitis). This transfer can be achieved via the physical ascending pathway, hematogenous route or lymph node transfer [32]. Presently, there are scientific results that prove benefits of probiotic microorganisms on reproductive health outcomes, such as the modulation of vaginosis [33], PCOS [20], and mastitis [34].
Vaginal administration of Lactobacillus can restore the vaginal microbiota by controlling the Nugent index within the range of normal values (0–3). Furthermore, Lactobacillus colonization is inversely correlated with the concentration of bacteria associated with bacterial vaginosis [35]. Moreover, to treat vulvovaginal infections, probiotics can be administered, preferably vaginally, to control the recolonization of Lactobacillus without any transfer needs or survival concerns towards the site of action [31].
The Lactobacillus-dominated endometrium may also benefit embryo implantation. However, there is controversy in these results and in determining the ratio of dominant microorganisms associated with health/dysbiosis status [36]. Furthermore, the same authors claimed that further taxonomical analysis of the endometrial microbiota may be necessary to identify and discern between the beneficial and/or pathogenic bacteria involved in embryo implantation. This would avoid multiple interventions against the anomalous microbiota that were not dominated by Lactobacillus.
Additionally, in a recent research, the link between endometrial microbiota composition and pregnancy outcomes in in vitro-fertilization (IVF) patients was examined. Remarkably, Moreno et al. [37] found an association between an endometrial microbial composition that was limited in Lactobacillus strains and ultimately adverse pregnancy outcomes. It was concluded that the negative effects of endometrial microbiota that are not dominated by Lactobacillus should be related with negative reproductive outcomes, such as implantation failure and pregnancy loss [38,39,40]. According to this, vaginal administration of probiotics could allow a direct, quicker, and targeted colonizing action to restore the altered vaginal microbiota compared to the long-term effects obtained by oral probiotics.
The main objective of the present work was to collect, scrutinize, and extract the most recent information from the high-quality and relevant scientific literature on probiotics administered vaginally and their possible qualitative and quantitative modulation capacities in reproductive-health-related dysbiosis.
2. Materials and Methods
2.1. Eligibility Criteria and Search Strategy
All interventional studies compiling data on specific probiotic microbial strains and dosages administered for human reproductive microbiota-related dysbiosis were included. Two reviewers, ALM and MA, screened titles, abstracts, and then full-text papers independently against inclusion criteria according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [41].
These four criteria were applied for the selection of the study: (1) being published within the last fifteen years, specifying (2) the probiotic strain used, (3) the dose, and (4) the time/period of administration. The specific data on population, intervention, comparison, and outcome criteria for inclusion in the comprehensive review are described in Table 1.
Table 1.
Population, intervention, comparison, and outcome (PICO) criteria for inclusion of studies.
| Parameters | Inclusion Criteria |
|---|---|
| Population | Human |
| Intervention | Probiotics strains and doses |
| Comparison | Vaginal probiotics versus placebo |
| Outcome | Fertility parameters |
| Setting | Clinical trials (CTs) |
Non-English-language manuscripts and documents or studies without specific data on fertility and reproductive dysbiosis biomarkers were excluded.
Each eligible article identified was reanalyzed by its title and abstract, and the eligible articles were selected for complete reading. The initial selection was done based on a designed term search through title and abstract screening, and the second selection was based on a full-text screening, where the two independent reviewers revised the publications with specific reference to the inclusion criteria. The study selection interrater agreement between the two reviewers was calculated as the proportion of positive agreement (PA) [42].
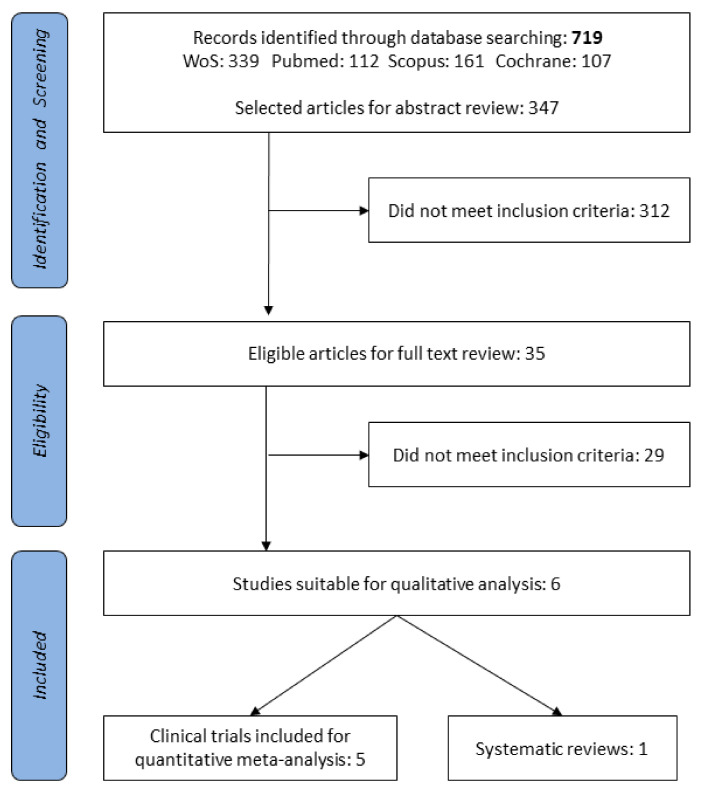
Literature search and review were carried out under the stepwise search procedure. The systematic review was developed in collaboration with University of Granada library support using search keywords/terms (described below) and medical subject headings (MeSH). MEDLINE/PubMed [43], Web of Science (Thomson Reuters Scientific, Philadelphia, PA, USA), Scopus (Elsevier, Amsterdam, The Netherlands), and Cochrane Library [44] were the databases used. A PRISMA flow diagram of the literature search condenses the selection of the studies comprising the two screening phases (Figure 2).
Figure 2.
Vaginal probiotics Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Complementary oral probiotics PRISMA was previously performed [45].
The collective search approach was carried out using MeSH and free text search terms detailed as follows: (probiotic* and infertility and doses); (probiotic* and microbiota and fertility); (probiotic* and microbiota and infertility); (probiotic* and “vaginal microbiota” and infertility); (probiotic* and endometriosis); (probiotic* and endometriosis and fertility); (probiotic* and endometriosis and infertility); (probiotic* and “endometrial microbiota” and infertility); (probiotic* and endometrium and infertility); (probiotic* and endometrium and fertility); (probiotic* and microbiota and “*vaginal administration”); (probiotic* and ovules); (probiotic* and reproductive and “*vaginal administration”); (probiotic* and “Polycystic Ovary Syndrome”).
2.2. Data Extraction, Analysis, and Risk of Bias (Quality) Assessment
The resulting data were extracted from all the selected clinical studies: publication year, study design, characteristics of the population and sample size (n) in the intervention group, sex, and age; microorganism probiotic strains; doses and pattern of administration; modification of the main clinical outcomes, Nugent score, or alterations in several fertility-related parameters. The main data results extracted from CT were qualitatively compiled and organized into form of table detailed below in the results.
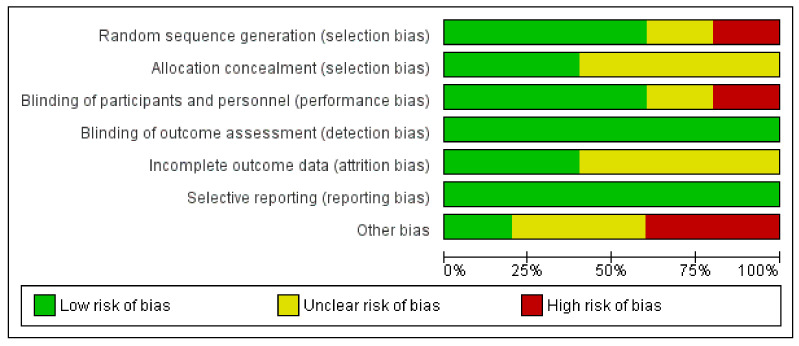
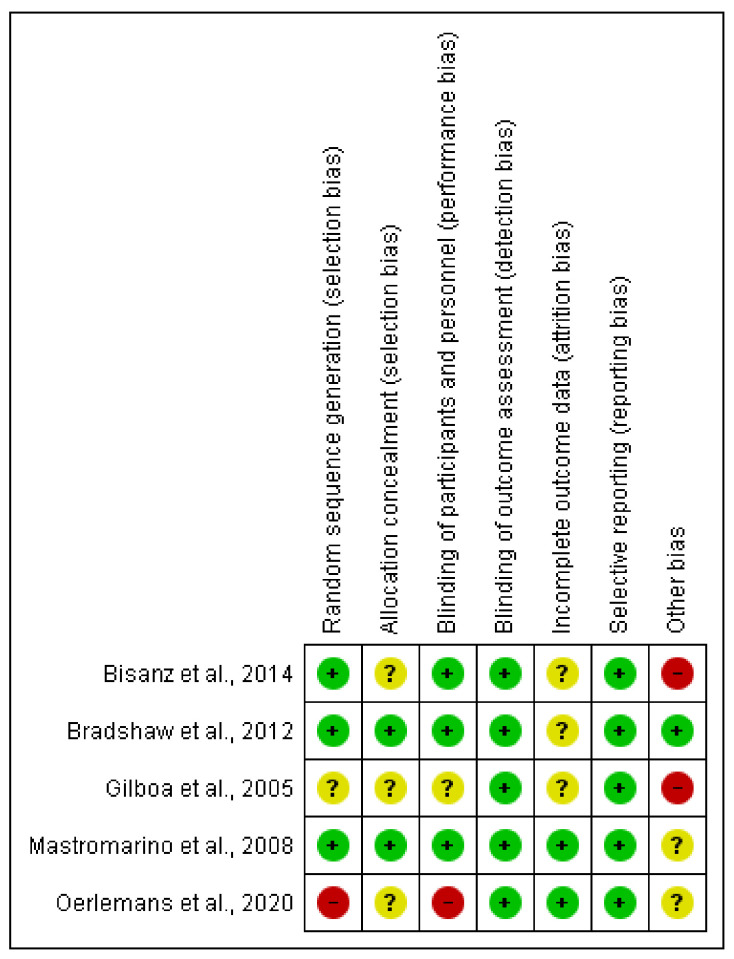
The risk of bias for each clinical trial selected was assessed independently by the authors using the Cochrane collaboration methodology [44]. The risk of bias was tabulated for each study (Figure 3 and Figure 4). Each item evaluated was classified as low risk, high risk, or unclear risk according to the quality recommendations described in Chapter 8 of the Cochrane Handbook of Systematic Reviews of Interventions [44]. Analysis and corresponding figures were generated in RevMan 5.3 Review Manager (RevMan Computer program) Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2019, available at (revman.cochrane.org accessed on 20 January 2021).
Figure 3.
Risk of bias graph of clinical trial (CT): review authors’ judgments about each item as percentages across all included studies.
Figure 4.
Risk of bias summary of CT: authors’ judgments about each risk of bias item for each included study low risk (+, green circle), high risk (−, red circle), or unclear risk (?, yellow circle).
2.3. Statistical Analysis
To compute the global quantitative effect for each relevant study analyzed regarding the modulation of vaginal microbiota capacities, the subsequent phases were carried out: (1) Extraction data regarding the baseline value in treatment group, baseline value in placebo group, endpoint in treatment group, and endpoint value in placebo group. When baseline values were not stated, only the endpoints were used. (2) Value change ± SD from baseline was calculated for the treatment and placebo groups, separately. (3) The mean variance between data from baseline in probiotics group versus placebo group was calculated and used as the overall effect size.
Alignment calculations and Hedges’ adjusted g were used to calculate the effect size. A random-effects model pooled the calculated effect sizes. Heterogeneity was explored using the I2 test and considering I2 > 75% high heterogeneity and I2 < 25% low heterogeneity. Heterogeneity between subgroups was calculated using a fixed-effects model. Sensitivity analysis was executed by omitting one study at a time to detect any significant changes in the results obtained. Begg’s rank correlation test and Egger’s regression asymmetry test were used to assess publication bias.
3. Results
Reproductive disorders are an increasing global health concern. Therefore, the plausible role of the microbiota in reproductive and hormonal health has promoted studies administering vaginal probiotics. Accordingly, an initial search with the keywords “probiotics and fertility” showed a triplication of available studies over the last fifteen years. A total of 719 documents were retrieved as a result of applying the selection criteria. A total of 35 clinical studies eligible for vaginal probiotics were selected for full-text review (Figure 2). The positive agreement (PA) value was 0.85 for titles and 0.90 for abstracts. When the full texts were analyzed for the specific strain, doses, and patterns, only six articles (clinical trials (n = 5) and a systematic review (n = 1)) fulfilled the inclusion criteria. The carefully chosen and included studies were extensively analyzed and the relevant qualitative outcomes are shown in Table 2.
Table 2.
Effects of vaginal probiotic strains administered in clinical trials in reproductive and fertility-related disorders and their relevant clinical results.
| Reference | Population Sample (n) |
Probiotic Strains | Probiotic Doses | Probiotic Administration Time (Weeks) |
Disorders/Diseases | Clinical Effects and Health Parameter Modifications |
|---|---|---|---|---|---|---|
| Oerlemans et al. [48] | 20 women with vulvovaginal candidiasis (VVC) | Lactobacillus pentosus KCA1, Lactobacillus plantarum WCFS1, and Lactobacillus rhamnosus GG | 2.5 × 109–2.5 × 1010 CFU/day | 1.5 | Vulvovaginal candidiasis | Probiotic formulation restores the vaginal microbiota in 45% of women. The other 55% of women needed rescue medication (fluconazole), but, at the end of the study, these women presented a larger reduction in the amount of Lactobacillus sp. compared to the other group. |
| Mastromarino et al. [49] | 39 women | Florisia®: Lactobacillus brevis (CD2), Lactobacillus salivarius subsp. salicinius (FV2), and L. plantarum (FV9) | ≥109 CFU/day | 1 | Bacterial vaginosis | This probiotic product of exogenous strains of Lactobacillus spp. administered intravaginally restored the healthy vaginal microbiota and it can be administered to treat bacterial vaginosis (BV) disorders. |
| Bisanz et al. [50] | 14 postmenopausal women | L. rhamnosus GR-1 and Lactobacillus reuteri RC-14 | 2.5 × 109 CFU/day | 3 (day) | Bacterial vaginosis | Total Lactobacillus increased and the proportion of Atopobium decreased. In addition, there was a trend for Gardnerella and Prevotella reduction. No changes in Nugent score and host metabolome. |
| Bradshaw et al. [51] | 450 healthy women | Lactobacillus acidophilus KS400 | >107 CFU/day | 12 | Recurrent bacterial vaginosis | Lactobacillus acidophilus KS400 administered vaginally in combination of oral metronidazole during an extended course did not cure recurrent bacterial vaginosis. |
| Gilboa et al. [52] | 117 women | Probiotic Femina®: Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum | 6 × 109 CFU/treatment | 1 (day) | In vitro fertilization (IVF)–embryo transfer cycle | Probiotic Femina® did not affect the vaginal colonization of Lactobacillus during oocyte retrieval or embryo transfer and did not improve the pregnancy rate. |
The qualitative comparative data were extracted based on the following categories: sample number, population characteristics, probiotic strain(s), dosage and administration patterns, intervention period (weeks), disorder treated, and modulation data of clinical outcomes related with fertility disorders (Table 2).
The quality of the selected clinical studies was guaranteed based on the comprehensive method applied in the selection of the final documents and their outcomes in order to obtain comparative and useful conclusions. To evaluate the five clinical trials (CTs) in terms of their design, execution, and outcomes, the risk of bias was evaluated (Figure 3 and Figure 4), increasing the classification of the quality standards and giving extra significance to the selected CTs, as well as allowing the validation of the revised results. The disorders treated in the selected CTs were one of vulvovaginal candidiasis, two of BV, one of recurrent BV, and one of IVF (Table 2). The selected systematic review [46] only contained one article [47] that met the established inclusion criteria; however, the available data did not contain enough specifications to be incorporated into the quantitative analyses. The qualitative information extracted was aligned with normal microbiota restoration effects. The administration of L. acidophilus KS400 (Gynoflor®) to 360 women with BV did not show a significant improvement in BV symptoms, although the normal flora index (NFI) augmented meaningfully in the treated group. Further limitations beyond the restrictive design of the systematic review include the lack of studies fulfilling the strict inclusion criteria.
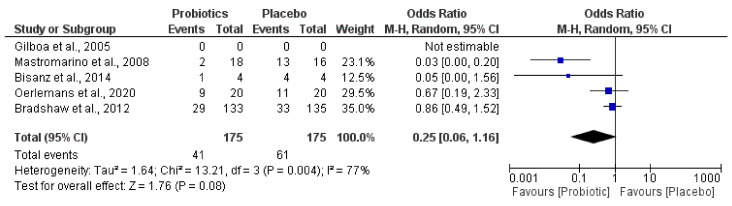
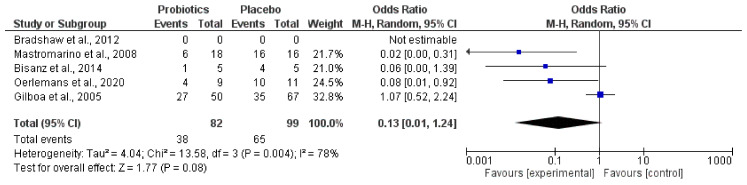
Furthermore, the most relevant changes and modulation capacities of vaginal probiotics administered on the abnormal microbiota (Figure 5) and the Lactobacillus genus amount (Figure 6) were revealed by quantitative examination through forest plot assessments, where the statistical impact on clinically significant parameters was verified.
Figure 5.
Effect of vaginal probiotics for modulation reduction in abnormal microbiota.
Figure 6.
Effect of vaginal probiotics for modulation increase in Lactobacillus spp.
The quantitative outcome promoted by the diverse probiotics administered in each population studied in relation to the capacity for a reduction in abnormal microbiota and the increase in the Lactobacillus genus amount is indicated by black diamonds.
Interestingly, the meta-analysis showed that probiotics groups could reduce the amount of abnormal microbiota (Gardnerella and Atopobium) (Figure 5) and increase in parallel the quantity of species belonging to the Lactobacillus genus (Figure 6).
4. Discussion
In the last several decades, reproductive disorders and infertility cases have increased. This seems to be the result of multiple factors and hormonal imbalances triggered by different etiologies including polycystic ovary syndrome (PCOS), endometriosis, obesity or metabolic syndrome, bacterial vaginosis, infections, and even some cancers [2]. Recently, most of these metabolic disorders have been concomitantly linked to reproductive microbiota dysbiosis [2]. Consequently, many studies were conducted to establishing the healthy female reproductive microbiota, and its role in fertility dysbiosis [8,9,10]. Healthy microbiota at reproductive sites contains lactobacilli as the most represented bacteria, but other anaerobic genera might be present such as the genera Prevotella, Gardnerella, Atopobium, Megasphaera, Sneathia, and Anaerococcus [53,54,55,56]. All these bacteria seem to also be involved in diverse phases of reproduction such as gamete formation, fertilization, gestation establishment, and maintenance, and also in the bacterial transfer mother-newborn [56,57]. There are multiple factors that act modifying the reproductive tract microbiome equilibrium, mainly triggering bacterial vaginosis as the most reported dysbiosis [58]. However, we highlight the misuse of antibiotics, together with cumulative exposure to several xenobiotics, and endocrine disruptors, which can also influence the healthy microbiome [7], especially when exposure occurred via direct contact with a high level of contaminants in hygiene products [59,60].
Our study highlights the probiotic modulation capacities in relation to bacterial vaginosis, which showed the restoration of a healthy microbiome. Lactobacillus spp. were the dominant colonizers in reproductive sites [38] and defended these sites against abnormal or pathogenic microorganisms [61]. Accordingly, more probiotic interventional studies have been conducted on reproductive failures with lactobacilli imbalance, such as adverse pregnancy outcomes [16], a significant decrease in endometrial implantation [36], and altered IVF outcomes [62,63]. The Lactobacillus genus has optimal probiotic properties, including high hydrophobicity and self-regulation, adhesion to epithelial cells and acid production [64], and restoration of healthy urogenital microbiota [65,66,67]. In this systematic review, combinations of Lactobacillus strains were also administered in most studies, as L. acidophilus KS400 was present in all formulae administered as single probiotic strain. Importantly, this strain produced bacteriocins with antimicrobial activity against relevant urogenital pathogens [68]. In addition, a combination of strains were administered orally and vaginally, similar to L. rhamnosus GR-1 and L. reuteri RC-14 [50,69,70], with the aim of reducing abnormal microbiota and recurrent dysbiosis.
Other probiotic genera administered orally, such as Bifidobacterium spp., can be used in fertility disorders. Zhang et al. [20] managed to modulate the levels of sex hormones in patients with PCOS through the intestine–brain axis with the probiotic strain Bifidobacterium lactis V9. In this review, only one article using Bifidobacterium in combination with Lactobacillus strains showed a specific impact on embryo transfer success; however the authors claimed that the supplementation of probiotics after oocyte retrieval did not improve vaginal colonization or pregnancy rate [52]. The use of the specific probiotic strains for such dysbiosis was corroborated through modifying bacterial vaginosis parameters [46,48,49,50]. There was a probiotic strain in combination with oral metronidazole that was not able to reduce BV recurrence [51]. The lack of success in clinical trials with probiotics as modulators of fertility disorders may be due to the efficacy of the probiotic being strain- and disease-dependent, as well as highly reliant on the dose, duration, administration method, and host state [71,72].
In agreement with the qualitative outcomes retrieved, we found a wide variation in the administration pattern of the probiotics used in the investigations selected. The doses administered in the studies collected for the systematic review ranged from ≥107 CFU/day [46] to 2.5 × 1010 CFU/day [48]. We found large variations in the administration time between the administration of the probiotic for 1 day [52] and its administration for 12 weeks [51]. Except for these two studies [51,52], the mean administration time found in the review was 1 week. When we compare these data with the oral administration of probiotics in reproductive disorders [45], it is observed that the range of CFU/day administered is similar to that of vaginal probiotics, from 1 × 106 CFU/day to 3 × 1010 CFU/day. However, in terms of treatment duration, the differential range is much more pronounced, varying from 3 to 24 weeks; this could be due to oral probiotics needing a longer duration to reach the natural reproductive site of colonization compared to the more localized site-direct administration for vaginal probiotics. As expected, there were more clinical trials in which probiotics were administered orally (10) than vaginally (5), which fulfilled the high-quality standards for fertility disorders. Until now, bacterial vaginosis has been the most common vaginal syndrome treated by local probiotics, but we consider that well-designed clinical studies would better support and explore the use of vaginal probiotics as therapeutic complementary solutions on reproductive site dysbiosis in relation to unexplained infertility cases.
The meta-analysis outcomes corroborated a slightly modulated vaginal probiotic capacity on the relative abundance of abnormal microbiota. This seemed to be associated with a tendency for microbiome restauration by the level of Lactobacillus species. Heterogeneity data were also similar for abnormal microbiota reduction (77%) and restoration of Lactobacillus (78%). The data analyzed were in agreement with postulates on the presence of abnormal vaginal microbiota as a factor of recurrent dysbiosis. Vaginal microbiotas of patients with BV contain more diverse and higher counts of Gardnerella, Prevotella, Atopobium, Mobiluncus, Peptostreptococcus, Sneathia, Leptotrichia, and Mycoplasma, whereas Lactobacillus are found in lower quantity and less frequently [58]. The combination of these microbial modifications can synthetize amino compounds and rise the vaginal pH, thus generating a site colonization more prone to several pathogenic infections and vulnerable to unhealthy disorders, including reproductive results [73]. Remarkably, reproductive site Lactobacillus species promotion, together with a proportionally decreasing abnormal microbiota, was also supported by in vitro studies that showed lactobacilli inhibiting the colonization of Gardnerella vaginalis to the vaginal epithelium tissues and producing bacteriocins, lactic acid, and/or H2O2, which inhibit the bacteria that cause BV [46].
The limitations of this review are based on the few comparative and qualitative clinical data available because of the low number of eligible studies and limited sample size population. Furthermore, there is no standardized probiotic administration, and there are different doses and several probiotic strains. International guidelines or protocols on probiotics for reproductive-related disease prevention and treatments are required. This will allow for a more significant and unified clinical effect comparison and provide robust meta-analysis outcomes.
In future studies, all probiotics used must be beneficial, safe and harmless to the target patients [74]. In this sense, next-generation probiotics (NGP) have been extensively characterized regarding their physiological interaction with the host [75,76]; therefore, new clinical studies with NGP might better modulate reproductive dysbiosis. Another innovative therapeutic method for reproductive site microbiota modulation could be vaginal microbiota transplantation (VMT) [77,78,79]. This method opens the door to a BV treatment that requires more research to advance it from conceptual analysis to clinical application.
5. Conclusions
The present study revealed that only a few clinical trials administering vaginal probiotics for fertility-related dysbiosis applied harmonized protocols for the most common reproductive disorder, bacterial vaginosis. Lactobacillus acidophilus remains the first election probiotic species to be vaginally administered. The impact of quantitative microbiota modulation capacities in reproductive-health-related dysbiosis was similar within the selected studies, as proved by the meta-analysis outcomes in which the administration of vaginal probiotics moderately modulated the relative abundance of abnormal microbiota, coinciding with an increase in Lactobacillus species. The variety of fertility disorders treated with vaginal probiotics found was significantly low compared to oral administration of probiotics. Hence, future vaginal intervention studies with next-generation probiotics could redirect the effort to obtain not only modulation of microbial biomarkers, but also better holistic reproductive health effects.
Acknowledgments
The authors acknowledge support from the Programs IniciaTC 2019, and INV 2019–2021 from the Plan Propio of the University of Granada. Part of results is from Ana López-Moreno doctoral thesis, Biomedicine Doctorate Program of the University of Granada.
Author Contributions
Conceptualization, M.A.; methodology, A.L.-M. and M.A.; writing—original draft preparation, A.L.-M.; review and editing, A.L.-M. and M.A. Funding Acquisition, M.A. Both Authors have read and agreed to the published version of the manuscript.
Funding
This research received no direct external funding. A.L.-M. was granted with the IniciaTC programme—OTRI-UGR. Infrastructure Reference Microbiota Laboratory funding projects FEDER-IE_2019-198 and EIN-2019-103082.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.García-Velasco J.A., Menabrito M., Catalán I.B. What Fertility Specialists Should Know about the Vaginal Microbiome: A Review. Reprod. Biomed. Online. 2017;35:103–112. doi: 10.1016/j.rbmo.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez F., Fernández-Blázquez A., García B. Vaginosis. Vaginal Microbiota. Enf. Infec. Microbiol. Clin. 2019;37:592–601. doi: 10.1016/j.eimc.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Torres P.J., Siakowska M., Banaszewska B., Pawelczyk L., Duleba A.J., Kelley S.T., Thackray V.G. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018;103:1502–1511. doi: 10.1210/jc.2017-02153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontané L., Benaiges D., Goday A., Llauradó G., Pedro-Botet J. Influence of the Microbiota and Probiotics in Obesity. Clin. Investig. Arterioscler. 2018;30:271–279. doi: 10.1016/j.arteri.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Rondanelli M., Faliva M.A., Perna S., Giacosa A., Peroni G., Castellazzi A.M. Using Probiotics in Clinical Practice: Where Are We Now? A Review of Existing Meta-Analyses. Gut Microbes. 2017;8:521–543. doi: 10.1080/19490976.2017.1345414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilera M., Gálvez-Ontiveros Y., Rivas A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.578007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ata B., Yildiz S., Turkgeldi E., Brocal V.P., Dinleyici E.C., Moya A., Urman B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019;9:2204. doi: 10.1038/s41598-019-39700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pramanick R., Mayadeo N., Warke H., Begum S., Aich P., Aranha C. Vaginal Microbiota of Asymptomatic Bacterial Vaginosis and Vulvovaginal Candidiasis: Are They Different from Normal Microbiota? Microb. Pathog. 2019;134:103599. doi: 10.1016/j.micpath.2019.103599. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Grau I., Perez-Villaroya D., Bau D., Gonzalez-Monfort M., Vilella F., Moreno I., Simon C. Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens. 2019;8:205. doi: 10.3390/pathogens8040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno I., Garcia-Grau I., Bau D., Perez-Villaroya D., Gonzalez-Monfort M., Vilella F., Romero R., Simón C. The First Glimpse of the Endometrial Microbiota in Early Pregnancy. Am. J. Obstet. Gynecol. 2020;222:296–305. doi: 10.1016/j.ajog.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Muñoz M.E., Arrieta M.-C., Ramer-Tait A.E., Walter J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Goffau M.C., Lager S., Sovio U., Gaccioli F., Cook E., Peacock S.J., Parkhill J., Charnock-Jones D.S., Smith G.C.S. Human Placenta Has No Microbiome but Can Contain Potential Pathogens. Nature. 2019;572:329–334. doi: 10.1038/s41586-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood R., Zehnder J.L., Druzin M.L., Brown P.O. Gene Expression Patterns in Human Placenta. Proc. Natl. Acad. Sci. USA. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohbot J.M., Cardot J.M. Vaginal Impact of the Oral Administration of Total Freeze-Dried Culture of LCR 35 in Healthy Women. Infect. Dis. Obstet. Gynecol. 2012;2012:503648. doi: 10.1155/2012/503648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhandari P., Prabha V. Evaluation of Profertility Effect of Probiotic Lactobacillus plantarum 2621 in a Murine Model. Indian J. Med. Res. 2015;142:79–84. doi: 10.4103/0971-5916.162127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salah R.M., Allam A.M., Magdy A.M., Mohamed A.S. Bacterial Vaginosis and Infertility: Cause or Association? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;167:59–63. doi: 10.1016/j.ejogrb.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Van Oostrum N., De Sutter P., Meys J., Verstraelen H. Risks Associated with Bacterial Vaginosis in Infertility Patients: A Systematic Review and Meta-Analysis. Hum. Reprod. 2013;28:1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 19.Halis G., Arici A. Endometriosis and Inflammation in Infertility. Ann. N. Y. Acad. Sci. 2004;1034:300–315. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Sun Z., Jiang S., Bai X., Ma C., Peng Q., Chen K., Chang H., Fang T., Zhang H. Probiotic Bifidobacterium Lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems. 2019;4 doi: 10.1128/mSystems.00017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R., Espinoza J., Chaiworapongsa T., Kalache K. Infection and Prematurity and the Role of Preventive Strategies. Semin. Neonatol. 2002;7:259–274. doi: 10.1053/siny.2002.0121. [DOI] [PubMed] [Google Scholar]
- 22.Basavaprabhu H.N., Sonu K.S., Prabha R. Mechanistic Insights into the Action of Probiotics against Bacterial Vaginosis and Its Mediated Preterm Birth: An Overview. Microb. Pathog. 2020;141:104029. doi: 10.1016/j.micpath.2020.104029. [DOI] [PubMed] [Google Scholar]
- 23.Moreno I., Simon C. Relevance of Assessing the Uterine Microbiota in Infertility. Fertil. Steril. 2018;110:337–343. doi: 10.1016/j.fertnstert.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 24.Krauss-Silva L., Moreira M.E.L., Alves M.B., Braga A., Camacho K.G., Batista M.R.R., Almada-Horta A., Rebello M.R., Guerra F. A Randomised Controlled Trial of Probiotics for the Prevention of Spontaneous Preterm Delivery Associated with Bacterial Vaginosis: Preliminary Results. Trials. 2011;12:239. doi: 10.1186/1745-6215-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner G.E., Heinemann C., Baroja M.L., Bruce A.W., Beuerman D., Madrenas J., Reid G. Oral Administration of the Probiotic Combination Lactobacillus Rhamnosus GR-1 and L. Fermentum RC-14 for Human Intestinal Applications. Int. Dairy J. 2002;12:191–196. doi: 10.1016/S0958-6946(01)00138-8. [DOI] [Google Scholar]
- 26.Verna E.C., Lucak S. Use of Probiotics in Gastrointestinal Disorders: What to Recommend? Ther. Adv. Gastroenterol. 2010;3:307–319. doi: 10.1177/1756283X10373814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson K.S., Gibson G.R., Hutkins R., Reimer R.A., Reid G., Verbeke K., Scott K.P., Holscher H.D., Azad M.B., Delzenne N.M., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daliri E.B.-M., Lee B.H. New Perspectives on Probiotics in Health and Disease. Food Sci. Hum. Wellness. 2015;4:56–65. doi: 10.1016/j.fshw.2015.06.002. [DOI] [Google Scholar]
- 29.Doron S., Snydman D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015;60(Suppl. 2):S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Goffau M.C., Lager S., Salter S.J., Wagner J., Kronbichler A., Charnock-Jones D.S., Peacock S.J., Smith G.C.S., Parkhill J. Recognizing the Reagent Microbiome. Nat. Microbiol. 2018;3:851–853. doi: 10.1038/s41564-018-0202-y. [DOI] [PubMed] [Google Scholar]
- 31.Mombelli B., Gismondo M.R. The Use of Probiotics in Medical Practice. Int. J. Antimicrob. Agents. 2000;16:531–536. doi: 10.1016/S0924-8579(00)00322-8. [DOI] [PubMed] [Google Scholar]
- 32.McDonald B.D., Jabri B., Bendelac A. Diverse Developmental Pathways of Intestinal Intraepithelial Lymphocytes. Nat. Rev. Immunol. 2018;18:514–525. doi: 10.1038/s41577-018-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngugi B.M., Hemmerling A., Bukusi E.A., Kikuvi G., Gikunju J., Shiboski S., Fredricks D.N., Cohen C.R. Effects of Bacterial Vaginosis-Associated Bacteria and Sexual Intercourse on Vaginal Colonization with the Probiotic Lactobacillus Crispatus CTV-05. Sex. Transm. Dis. 2011;38:1020–1027. doi: 10.1097/OLQ.0b013e3182267ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández L., Cárdenas N., Arroyo R., Manzano S., Jiménez E., Martín V., Rodríguez J.M. Prevention of Infectious Mastitis by Oral Administration of Lactobacillus salivarius PS2 During Late Pregnancy. Clin. Infect. Dis. 2016;62:568–573. doi: 10.1093/cid/civ974. [DOI] [PubMed] [Google Scholar]
- 35.Tomusiak A., Strus M., Heczko P.B., Adamski P., Stefański G., Mikołajczyk-Cichońska A., Suda-Szczurek M. Efficacy and Safety of a Vaginal Medicinal Product Containing Three Strains of Probiotic Bacteria: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Drug Des. Dev. Ther. 2015;9:5345–5354. doi: 10.2147/DDDT.S89214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyono K., Hashimoto T., Kikuchi S., Nagai Y., Sakuraba Y. A Pilot Study and Case Reports on Endometrial Microbiota and Pregnancy Outcome: An Analysis Using 16S RRNA Gene Sequencing among IVF Patients, and Trial Therapeutic Intervention for Dysbiotic Endometrium. Reprod. Med. Biol. 2019;18:72–82. doi: 10.1002/rmb2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno I., Codoñer F.M., Vilella F., Valbuena D., Martinez-Blanch J.F., Jimenez-Almazán J., Alonso R., Alamá P., Remohí J., Pellicer A., et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 38.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S.K., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Bieda J., Chaemsaithong P., Miranda J., Chaiworapongsa T., Ravel J. The Vaginal Microbiota of Pregnant Women Who Subsequently Have Spontaneous Preterm Labor and Delivery and Those with a Normal Delivery at Term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero R., Chaiworapongsa T., Kuivaniemi H., Tromp G. Bacterial Vaginosis, the Inflammatory Response and the Risk of Preterm Birth: A Role for Genetic Epidemiology in the Prevention of Preterm Birth. Am. J. Obstet. Gynecol. 2004;190:1509–1519. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson P.F., Petrie A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology. 2010;73:1167–1179. doi: 10.1016/j.theriogenology.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Canese K. An Updated PubMed Is on Its Way. NLM Technical Bulletin. March–April. [(accessed on 20 January 2021)];2019 Available online: https://www.nlm.nih.gov/pubs/techbull/ma19/ma19_pubmed_update.html.
- 44.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons Ltd.; Chichester, UK: 2011. [(accessed on 20 January 2021)]. Version 5.1.0 (updated March 2011) The Cochrane Collaboration. Available online: https://handbook-5-1.cochrane.org/ [Google Scholar]
- 45.López-Moreno A., Aguilera M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients. 2020;12:757. doi: 10.3390/nu12030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falagas M.E., Betsi G.I., Athanasiou S. Probiotics for the Treatment of Women with Bacterial Vaginosis. Clin. Microbiol. Infect. 2007;13:657–664. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 47.Ozkinay E., Terek M.C., Yayci M., Kaiser R., Grob P., Tuncay G. The Effectiveness of Live Lactobacilli in Combination with Low Dose Oestriol (Gynoflor) to Restore the Vaginal Flora after Treatment of Vaginal Infections. Br. J. Obstet. Gynaecol. 2005;112:234–240. doi: 10.1111/j.1471-0528.2004.00329.x. [DOI] [PubMed] [Google Scholar]
- 48.Oerlemans E.F.M., Bellen G., Claes I., Henkens T., Allonsius C.N., Wittouck S., van den Broek M.F.L., Wuyts S., Kiekens F., Donders G.G.G., et al. Impact of a Lactobacilli-Containing Gel on Vulvovaginal Candidosis and the Vaginal Microbiome. Sci. Rep. 2020;10:7976. doi: 10.1038/s41598-020-64705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastromarino P., Macchia S., Meggiorini L., Trinchieri V., Mosca L., Perluigi M., Midulla C. Effectiveness of Lactobacillus-Containing Vaginal Tablets in the Treatment of Symptomatic Bacterial Vaginosis. Clin. Microbiol. Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 50.Bisanz J.E., Seney S., McMillan A., Vongsa R., Koenig D., Wong L., Dvoracek B., Gloor G.B., Sumarah M., Ford B., et al. A Systems Biology Approach Investigating the Effect of Probiotics on the Vaginal Microbiome and Host Responses in a Double Blind, Placebo-Controlled Clinical Trial of Post-Menopausal Women. PLoS ONE. 2014;9:e104511. doi: 10.1371/journal.pone.0104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradshaw C.S., Pirotta M., De Guingand D., Hocking J.S., Morton A.N., Garland S.M., Fehler G., Morrow A., Walker S., Vodstrcil L.A., et al. Efficacy of Oral Metronidazole with Vaginal Clindamycin or Vaginal Probiotic for Bacterial Vaginosis: Randomised Placebo-Controlled Double-Blind Trial. PLoS ONE. 2012;7:e34540. doi: 10.1371/journal.pone.0034540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilboa Y., Bar-Hava I., Fisch B., Ashkenazi J., Voliovitch I., Borkowski T., Orvieto R. Does Intravaginal Probiotic Supplementation Increase the Pregnancy Rate in IVF-Embryo Transfer Cycles? Reprod. Biomed. Online. 2005;11:71–75. doi: 10.1016/S1472-6483(10)61301-6. [DOI] [PubMed] [Google Scholar]
- 53.The Human Microbiome Project Consortium Structure, Function and Diversity of the Healthy Human Microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The Human Microbiome Project Consortium A Framework for Human Microbiome Research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González A., Vázquez-Baeza Y., Knight R. SnapShot: The Human Microbiome. Cell. 2014;158:690. doi: 10.1016/j.cell.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Shao X., Cheng H., Zhou J., Zhang J., Zhu Y., Yang C., Di Narzo A., Yu J., Shen Y., Li Y., et al. Prenatal Exposure to Ambient Air Multi-Pollutants Significantly Impairs Intrauterine Fetal Development Trajectory. Ecotoxicol. Environ. Saf. 2020;201:110726. doi: 10.1016/j.ecoenv.2020.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Argenio V. The Prenatal Microbiome: A New Player for Human Health. High-Throughput. 2018;7:38. doi: 10.3390/ht7040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Onderdonk A.B., Delaney M.L., Fichorova R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao C.-J., Kannan K. Phthalates, Bisphenols, Parabens, and Triclocarban in Feminine Hygiene Products from the United States and Their Implications for Human Exposure. Environ. Int. 2020;136:105465. doi: 10.1016/j.envint.2020.105465. [DOI] [PubMed] [Google Scholar]
- 60.Cherpes T.L., Hillier S.L., Meyn L.A., Busch J.L., Krohn M.A. A Delicate Balance: Risk Factors for Acquisition of Bacterial Vaginosis Include Sexual Activity, Absence of Hydrogen Peroxide-Producing Lactobacilli, Black Race, and Positive Herpes Simplex Virus Type 2 Serology. Sex. Transm. Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 61.Witkin S.S., Linhares I.M. Why Do Lactobacilli Dominate the Human Vaginal Microbiota? Br. J. Obstet. Gynaecol. 2017;124:606–611. doi: 10.1111/1471-0528.14390. [DOI] [PubMed] [Google Scholar]
- 62.Verstraelen H., Senok A.C. Vaginal Lactobacilli, Probiotics, and IVF. Reprod. Biomed. Online. 2005;11:674–675. doi: 10.1016/S1472-6483(10)61683-5. [DOI] [PubMed] [Google Scholar]
- 63.Bagga R., Arora P. Genital Micro-Organisms in Pregnancy. Front. Public Health. 2020;8:225. doi: 10.3389/fpubh.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melgaço A.C.C., Blohem Pessoa W.F., Freire H.P., Evangelista de Almeida M., Santos Barbosa M., Passos Rezende R., Timenetsky J., Miranda Marques L., Romano C.C. Potential of Maintaining a Healthy Vaginal Environment by Two Lactobacillus Strains Isolated from Cocoa Fermentation. BioMed Res. Int. 2018;2018:7571954. doi: 10.1155/2018/7571954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reid G., Beuerman D., Heinemann C., Bruce A.W. Probiotic Lactobacillus Dose Required to Restore and Maintain a Normal Vaginal Flora. FEMS Immunol. Med. Microbiol. 2001;32:37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 66.Reid G., Charbonneau D., Erb J., Kochanowski B., Beuerman D., Poehner R., Bruce A.W. Oral Use of Lactobacillus Rhamnosus GR-1 and L. Fermentum RC-14 Significantly Alters Vaginal Flora: Randomized, Placebo-Controlled Trial in 64 Healthy Women. FEMS Immunol. Med. Microbiol. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 67.Reid G., Burton J., Hammond J.-A., Bruce A.W. Nucleic Acid-Based Diagnosis of Bacterial Vaginosis and Improved Management Using Probiotic Lactobacilli. J. Med. Food. 2004;7:223–228. doi: 10.1089/1096620041224166. [DOI] [PubMed] [Google Scholar]
- 68.Gaspar C., Donders G.G., Palmeira-de-Oliveira R., Queiroz J.A., Tomaz C., Martinez-de-Oliveira J., Palmeira-de-Oliveira A. Bacteriocin Production of the Probiotic Lactobacillus Acidophilus KS400. AMB Express. 2018;8:153. doi: 10.1186/s13568-018-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMillan A., Rulisa S., Gloor G.B., Macklaim J.M., Sumarah M., Reid G. Pilot Assessment of Probiotics for Pregnant Women in Rwanda. PLoS ONE. 2018;13:e0195081. doi: 10.1371/journal.pone.0195081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anukam K.C., Duru M.U., Eze C.C., Egharevba J., Aiyebelehin A., Bruce A., Reid G. Oral Use of Probiotics as an Adjunctive Therapy to Fluconazole in the Treatment of Yeast Vaginitis: A Study of Nigerian Women in an Outdoor Clinic. Microbial Ecol. Health Dis. 2009;21:72–77. doi: 10.1080/08910600902907475. [DOI] [Google Scholar]
- 71.Sniffen J.C., McFarland L.V., Evans C.T., Goldstein E.J.C. Choosing an Appropriate Probiotic Product for Your Patient: An Evidence-Based Practical Guide. PLoS ONE. 2018;13:e0209205. doi: 10.1371/journal.pone.0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sivamaruthi B.S., Kesika P., Chaiyasut C. Effect of Probiotics Supplementations on Health Status of Athletes. Int. J. Environ. Res. Public Health. 2019;16:4469. doi: 10.3390/ijerph16224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyman R.W., Herndon C.N., Jiang H., Palm C., Fukushima M., Bernstein D., Vo K.C., Zelenko Z., Davis R.W., Giudice L.C. The Dynamics of the Vaginal Microbiome during Infertility Therapy with in Vitro Fertilization-Embryo Transfer. J. Assist Reprod. Genet. 2012;29:105–115. doi: 10.1007/s10815-011-9694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C.-J., Lin T.-L., Tsai Y.-L., Wu T.-R., Lai W.-F., Lu C.-C., Lai H.-C. Next Generation Probiotics in Disease Amelioration. J. Food Drug Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai K.-P., Chung Y.-T., Li R., Wan H.-T., Wong C.K.-C. Bisphenol A Alters Gut Microbiome: Comparative Metagenomics Analysis. Environ. Pollut. 2016;218:923–930. doi: 10.1016/j.envpol.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 76.O’Toole P.W., Marchesi J.R., Hill C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 77.Ma D., Chen Y., Chen T. Vaginal Microbiota Transplantation for the Treatment of Bacterial Vaginosis: A Conceptual Analysis. FEMS Microbiol. Lett. 2019;366:fnz025. doi: 10.1093/femsle/fnz025. [DOI] [PubMed] [Google Scholar]
- 78.DeLong K., Zulfiqar F., Hoffmann D.E., Tarzian A.J., Ensign L.M. Vaginal Microbiota Transplantation: The Next Frontier. J. Law Med. Ethics. 2019;47:555–567. doi: 10.1177/1073110519897731. [DOI] [PubMed] [Google Scholar]
- 79.Lev-Sagie A., Goldman-Wohl D., Cohen Y., Dori-Bachash M., Leshem A., Mor U., Strahilevitz J., Moses A.E., Shapiro H., Yagel S., et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019;25:1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]