Abstract
BACKGROUND:
Poor physical function impairs fitness and exercise and is associated with worse cardiovascular outcomes and all-cause mortality. Joint pain and stiffness limit physical function.
OBJECTIVE:
To determine if eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation improves physical function and exercise in coronary artery disease (CAD) patients.
METHODS:
291 subjects with stable CAD were randomized to either Lovaza (1.86 g of EPA and 1.5 g of DHA daily) or no Lovaza (control) for 1 year. Change in pain, stiffness and physical function was assessed by the Western Ontario and McMaster Universities Arthritis Index (WOMAC). Minutes of exercise per week were recorded, and musculoskeletal events were reported.
RESULTS:
Mean age (SD) was 63.3 (7.6) years. In the intention-to-treat analysis, compared to controls, those on Lovaza had better physical function (mean difference, −11.0%, 95% CI −18.5% to −3.5%, P = .004), better total WOMAC scores (mean difference, −9.8%, 95% CI −16.6% to −3.0%, P = .005), more exercise per week (135 minutes versus 197 minutes, respectively, P = .028) and less joint replacement (11 vs 1, respectively, P = .002). Pain and stiffness showed a trend toward significance (P = .06). The per-protocol analysis also showed less stiffness compared to controls (mean difference, −11.5%, 95% CI −22.9% to −0.1%, P = .048).
CONCLUSION:
High-dose EPA and DHA may benefit CAD patients by preserving physical function, increasing amount of exercise and reducing joint replacement. EPA and DHA may be a safe preventative strategy against musculoskeletal symptoms in CAD patients.
Keywords: Omega-3 fatty acids, Eicosapentaenoic acid, EPA, Docosahexaenoic acid, DHA, Physical function, Exercise, Coronary artery disease, Fish oil
Introduction
Coronary artery disease (CAD), the leading cause of death in industrialized countries, is characterized by chronic inflammation of the coronary arterial wall in which leukocytes, including neutrophils and monocytes, are recruited to the arterial wall and play a key role in the initiation and progression of coronary atherosclerosis.1 Aerobic exercise capacity and amount of exercise are inversely related with cardiovascular morbidity and mortality and all-cause mortality in men and women.2,3 For every 1 metabolic equivalent of task (MET) increase during graded exercise treadmill testing, total mortality is 16% lower in men without CAD, 9% lower in men with CAD and 17% lower in women without CAD.4,5 Potential barriers to exercise include the development of age-related joint disease leading to joint pain, stiffness and poor physical function. Non-steroidal anti-inflammatory drugs (NSAIDs) are efficacious in reducing joint symptoms mainly through their anti-inflammatory effect.6 However, NSAIDs are associated with increased adverse cardiovascular outcomes especially in patients with established cardiovascular disease.7,8 Thus, the search for analgesic/anti-inflammatory options which are safe in patients with CAD is ongoing.
Omega-3 fatty acids are a dietary component which can be obtained from fatty fish or as supplements. Furthermore, omega-3 fatty acids have been shown in some studies to have cardioprotective effects, particularly in those with established CAD.9,10 In patients with various forms of arthritis, omega-3 fatty acids showed significant improvement in pain, stiffness and physical function as well as a significant reduction in the use of NSAIDs11,12 However, no study has examined the effect of omega-3 fatty acids on physical function and musculoskeletal symptoms or amount of exercise in CAD patients. Our objective was to examine the effect of very long-chain omega-3 fatty acids - eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) - on maintaining physical function, preventing pain and stiffness and duration of exercise in patients with stable CAD.
Material and methods
The study design, participants and randomization have been described previously.13,14 Briefly, the trial is a randomized, parallel study of subjects with stable CAD aged 21 to 80 years at time of enrollment and had stable CAD. Inclusion criteria also included a body mass index (BMI – weight in kilograms divided by height in meters squared) ≥ 27 kg/m2 or a BMI of 25 to 26.9 with either an increased waist circumference or a history of at least 2 components of the metabolic syndrome which included triglyceride ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL if male or <50 mg/dL if female, glucose ≥ 100 mg/dL or treated hypertension or blood pressure ≥ 130/85 mm Hg.15 Additional inclusion criteria included stable use of 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor (statin) and estimated creatinine clearance as measured by the Cockcroft-Gault equation ≥ 60 ml/min/1.73 m2. Exclusion criteria for CCTA were BMI >35 kg/m2 (females) or >40 kg/m2 (males), contraindication to iodinated contrast agents and serum creatinine >1.5 mg/dL.
Participants were randomly assigned to receive either open-label omega-3 ethyl esters (Lovaza) 4 capsules daily or no Lovaza (termed control). All subjects were counseled not to take over-the-counter fish oil. All subjects were recommended statin and aspirin. Subjects in the Lovaza group received 3.36 g of Lovaza daily as 4 soft gels, each containing predominantly 465 mg EPA and 375 mg DHA for a total daily dose of 1.86 g EPA and 1.5 g DHA for 1 year at which time the current data were collected. Study subjects continued in their respective assignments for an additional 18 months. Study subjects returned unused Lovaza capsules at each visit to measure compliance. A detailed medication list was obtained during each visit to screen for use of over-the-counter omega-3 fatty acids. Subjects recorded the type and minutes of exercise daily. All subjects were encouraged to exercise at least 30 minutes at least 5 days per week.
Outcomes and data collection
The primary endpoint of the trial is the effect of Lovaza on progression of coronary arterial plaque at 30 months of follow-up and has been reported.14 A prespecified secondary outcome, urine albumin/creatinine ratio stratified by diabetes status, was recently published.13 Another prespecified secondary outcome is change in pain, stiffness and physical function over a 1-year period. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) (Likert version 3.1), which is among the most widely used assessments in arthritis research,16 was used to evaluate pain, stiffness and physical function at baseline and 1-year follow-up. The WOMAC has been validated in osteoarthritis16 but has also been used and proven useful in assessing pain, stiffness and physical function in patients without diagnosed arthritis.17,18 The WOMAC is a multidimensional, self-administered health status instrument composed of a set of 24 items. The unweighted mean of 5 items results in the pain score; the mean of 2 items, in the stiffness score; and the mean of 17 items, in the physical function score. A total WOMAC score is calculated by adding value of all 24 items.
A detailed history, physical examination, height, weight, waist measurement and blood pressure measurement were obtained at baseline and 1-year follow-up. Blood samples were obtained after a 12-hour fast. Glucose, hemoglobin A1c (HbA1c), chemical profile, total white blood cell (WBC) count, absolute neutrophil, lymphocyte, monocyte and platelet counts and lipid panel were measured at Quest Diagnostics (Cambridge, MA). High-sensitivity C-reactive protein (hs-CRP) was measured by immunoturbimetric assays using an automated, standardized, high throughput method with coefficient of variation below 5% at Boston Heart Diagnostics (a College of American Pathologists-accredited, CLIA-certified clinical laboratory; Framingham, MA). All subjects underwent a symptom-limited, graded exercise treadmill test at baseline with metabolic equivalents of exercise determined. Subjects reported the number of minutes of exercise per day for a particular exercise and the number of days of exercise per week.
Statistical analyses
Change in pain, stiffness and physical function in those on Lovaza was compared to those not taking Lovaza at 1 year compared to baseline. The analysis was performed following intention-to-treat principles. To assess the biological effects of Lovaza, we also performed a prespecified per-protocol analysis excluding participants who were non-adherent to their treatment assignment. Categorical variables were compared using Chi-square or Fisher’s exact tests. Normality tests were conducted using the Shapiro-Wilk test. Continuous variables were reported as the mean (standard deviation [SD]) for normally distributed variables and as median (interquartile range [IQR]) for variables which were not normally distributed. Continuous variables were compared using paired (within group comparisons) and unpaired (control versus Lovaza) t-tests for normally distributed variables. Non-normally distributed variables were compared using the signed-rank test (within group comparisons) and Wilcoxon-Mann-Whitney test (control versus Lovaza). Percent change in baseline characteristics and WOMAC scores were reported as mean and 95% confidence interval (95% CI). Correlations were determined using Pearson correlation coefficient for normally distributed variables and Spearman’s rank correlation coefficient for non-normally distributed variables. All analyses were conducted using SPSS version 20.0 (IBM Corp. Armonk, NY). All tests were 2-sided, and a P < .05 was considered statistically significant.
Results
Patient population
A total of 291 subjects were randomized to Lovaza (n = 147) or no Lovaza (n = 144) (Figure 1). Twenty-three patients discontinued the intervention. The remaining 268 participants, 138 in the Lovaza group and 130 in control, were included in the intention-to-treat analysis. Eighteen participants from the control group who were taking ≥900 mg of over-the-counter EPA and DHA, and 5 of the Lovaza group who did not take daily Lovaza were excluded in the per-protocol analysis.
Figure 1.
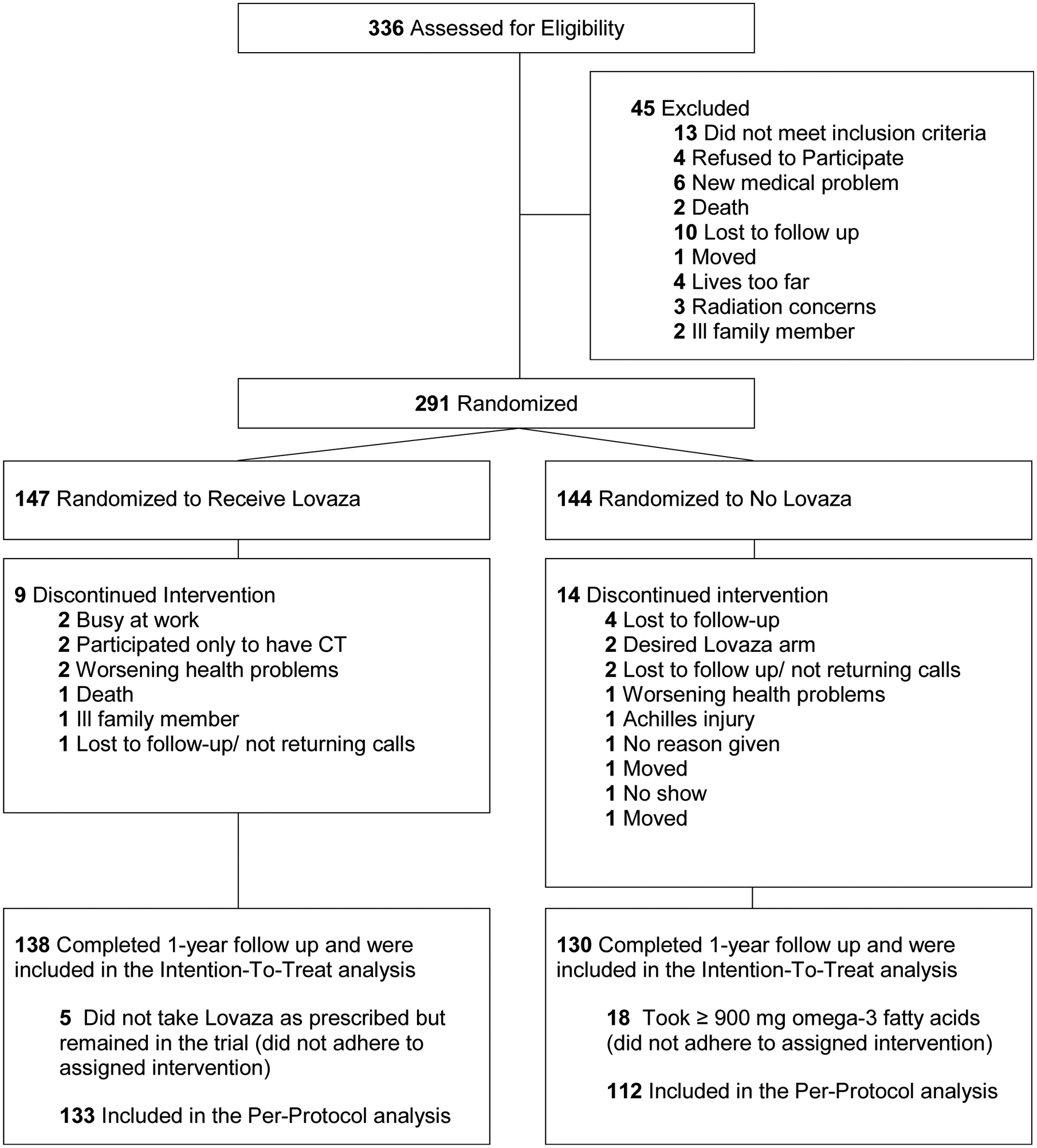
Consolidated Standards of Reporting Trials (CONSORT) Diagram.
For the total group, mean age (SD) was 63.3 (7.6) years. Forty-five (16.8%) were women, and 79 (29.5%) had diabetes. There were no significant differences in baseline characteristics by treatment group in the intention-to-treat or per-protocol analyses (Table 1). Approximately 40% of subjects had documented osteoarthritis or joint symptoms, but fewer than 10% of subjects used NSAIDs at baseline and only as-needed.
Table 1.
Baseline characteristics in the control and Lovaza groups
| Intention-to-Treat | Per-Protocol | |||
|---|---|---|---|---|
| Controls (n = 130) | Lovaza (n = 138) | Controls (n = 112) | Lovaza (n = 133) | |
| Demographic characteristics | ||||
| Age (y), mean±SD | 63.9±7.4 | 62.8±7.8 | 63.2±7.4 | 62.6±7.8 |
| Male Sex (%) | 108 (83.1) | 115 (83.3) | 94 (83.9) | 112 (84.2) |
| Inclusion criteria (may have > 1) | ||||
| History of MI (%) | 55 (42.3) | 68 (49.3) | 52 (46.4) | 66 (49.6) |
| History of PCI (%) | 78 (60.0) | 87 (63.0) | 67 (59.8) | 83 (62.4) |
| History of CABG (%) | 38 (29.2) | 28 (20.3) | 29 (25.9) | 27 (20.3) |
| Cardiovascular risk factors | ||||
| Hypertension (%) | 115 (88.5) | 110 (79.7) | 100 (89.3) | 106 (79.7) |
| Diabetes (%) | 40 (30.8) | 39 (28.3) | 33 (29.5) | 38 (28.6) |
| Osteoarthritis (%) | 54 (41.5%) | 54 (39.1%) | 42 (37.5%) | 53 (39.9%) |
| Anthropometrics and blood pressure, mean±SD | ||||
| Weight (kg) | 90.7±15.3 | 91.9±13.5 | 90.9±15.4 | 92.4±13.3 |
| Body mass index (kg/m2)* | 30.6±3.7 | 30.8±3.6 | 30.5±3.7 | 30.8±3.6 |
| Waist circumference (cm) | 106.7±11.2 | 106.9±10.2 | 107.0±11.4 | 107.0±10.4 |
| Systolic BP (mmHg) | 124.4±14.8 | 124.9±14.6 | 124.1±14.9 | 124.9±14.7 |
| Diastolic BP (mmHg) | 72.5±8.9 | 73.1±9.9 | 72.4±9.1 | 73.1±10.0 |
| Metabolic Equivalents† | 9.0±3.3 | 9.3±2.9 | 9.1±3.2 | 9.3±2.9 |
| Inflammatory markers, mean±SD | ||||
| hs-CRP, median [IQR] | 0.8 [0.4, 2.5] | 0.9 [0.5, 2.7] | 0.8 [0.4, 2.8] | 0.9 [0.5, 2.8] |
| WBC (109 cells/L) | 6.6±1.9 | 6.8±2.4 | 6.7±2.0 | 6.7±2.4 |
| Monocytes (cells/μL) | 541±169 | 516±168 | 542±175 | 513±160 |
| Neutrophils (cells/μL) | 4215±1721 | 4169±1322 | 4262±1755 | 4151±1314 |
| Lymphocytes (cells/μL) | 1629±539 | 1840±1806 | 1654±563 | 1841±1839 |
| Platelets (cells/μL) | 195±49 | 190±54 | 198±48 | 189±54 |
| Lipids, mean±SD‡ | ||||
| Total cholesterol (mg/dL) | 151.2±37.1 | 151.8±34.0 | 152.4±37.9 | 152.0±34.2 |
| Triglyceride (mg/dL), median [IQR] | 116 [79, 162] | 121 [81, 179] | 115 [ 81, 164] | 121 [81, 177] |
| HDL-C (mg/dL) | 46.6±14.4 | 46.8±14.3 | 46.8±14.9 | 47.0±14.3 |
| LDL-C (mg/dL) | 78.2±28.3 | 77.7±26.9 | 79.0±29.2 | 78.1±27.1 |
| Biochemical profile, mean±SD | ||||
| Glucose (mg/dL) | 108.6±37.3 | 104.6±27.2 | 107.8±35.3 | 104.6±27.4 |
| HbA1c (%) | 6.3±1.1 | 6.1±0.8 | 6.3±1.2 | 6.1±0.8 |
| Medications | ||||
| Statin (%) | 123 (94.6) | 133 (96.4) | 106 (94.6) | 128 (96.2) |
| Aspirin (%) | 124 (95.4) | 133 (96.4) | 106 (94.6) | 128 (96.2) |
| ACE-I (%) | 74 (56.9) | 76 (55.1) | 63 (56.3) | 73 (54.9) |
| ARB (%) | 25 (19.2) | 23 (16.7) | 21 (18.8) | 22 (16.5) |
| Hydrochlorothiazide (%) | 26 (20.0) | 25 (18.1) | 23 (20.5) | 24 (18.0) |
| Furosemide (%) | 17 (13.1) | 9 (6.5) | 14 (12.5) | 8 (6.0) |
| Calcium channel blocker (%) | 33 (25.4) | 35 (25.4%) | 27 (24.1) | 35 (26.3) |
| Beta blockers (%) | 96 (73.8) | 99 (71.7) | 83 (74.1) | 96 (72.2) |
| NSAIDs (intermittent use) (%) | 12 (9.2) | 13 (9.4) | 10 (8.9) | 12 (9.0) |
ACE-I, angiotensin converting enzyme inhibitor; BP, blood pressure; CABG, coronary artery bypass grafting; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low density lipoprotein cholesterol; MI, myocardial infarction; NSAIDs, non-steroidal anti-inflammatory drugs; PCI, percutaneous coronary intervention; WBC, white blood cell count.
Calculated as weight in kilograms divided by height in meters squared.
Determined by symptom-limited, graded exercise treadmill test.
Multiply by 0.02586 to convert cholesterol values to mmol/L and by 0.01129 to convert triglyceride to mmol/L.
Clinical and laboratory results
Table 2 shows the change in clinical and laboratory results at 1-year follow-up in the intention-to-treat analysis. Compared to those not on Lovaza, those on Lovaza had a significant reduction in triglyceride level (mean difference in % change = −15.9%, 95% CI −24.0% to −7.9%, P < .001) and significant reductions in hs-CRP (median % change from baseline, 19.2% versus −11.1%, P = .023), WBC count (mean difference in % change = −5.2%, 95% CI −9.8% to −0.5%, P = .029) and neutrophil count (mean difference in % change = −9.5%, 95% CI −16.4% to −2.7%, P = .007). Similar results were observed in the per-protocol analysis (Table 1 in the Supplement).
Table 2.
Baseline values and % change from baseline at 1-year follow-up in the control and Lovaza groups in the intention to treat analysis
| Control (n = 130) | Lovaza (n = 138) | Difference in % Change Lovaza minus Control | ||||
|---|---|---|---|---|---|---|
| Value at baseline Mean±SD | Value at 1-year Follow-up Mean±SD | Value at baseline Mean±SD | Value at 1-year Follow-up Mean±SD | Mean (95% CI) | P Value* | |
| Clinical Variable | ||||||
| Systolic BP (mmHg) | 124.4±14.8 | 122.9±15.5 | 124.9±14.6 | 124.2±15.4 | −1.4 (−4.5 to 1.7) | .39 |
| Diastolic BP (mmHg) | 72.5±8.9 | 69.5±9.0 | 73.1±9.9 | 71.1±10.0 | 0.0 (−3.5 to 3.5) | .99 |
| Waist circumference (cm) | 106.7±11.2 | 107.4±10.8 | 106.9±10.2 | 108.2±10.1 | −0.2 (−1.4 to 1.0) | .71 |
| Body mass index (kg/m2) | 30.6±3.7 | 30.2±3.9 | 30.8±3.6 | 31.3±7.0 | 0.0 (−1.8 to 1.9) | .98 |
| Inflammatory markers | ||||||
| hs-CRP (mg/L)† | 0.8 [0.4, 2.5]† | 0.9 [0.4, 2.9]† | 0.9 [0.5, 2.7]† | 1.2 [0.4, 2.7]† | −30.3‡ | .023 |
| WBC (109 cells/L) | 6.6±1.9 | 6.3±1.9 | 6.8±2.3 | 6.3±1.7 | −5.2 (−9.8 to −0.5) | .029 |
| Monocytes (cells/μL) | 541±169 | 506.1±178.2 | 516±168 | 490.1±142.9 | −3.1 (−8.8 to 2.7) | .29 |
| Neutrophils (cells/μL) | 4215±1721 | 4019.1±1610.9 | 4169±1322 | 3879.5±1409.3 | −9.5 (−16.4 to −2.7) | .007 |
| Lymphocytes (cells/μL) | 1629±539 | 1520.8±538.0 | 1840±1806 | 1697.7±822.2 | 3.0 (−1.8 to 7.7) | .22 |
| Platelets (cells/μL) | 195±49 | 180.1±45.2 | 190±54 | 177.9±46.0 | 8.0 (−11.8 to 27.8) | .43 |
| Lipid profile§ | ||||||
| Total cholesterol (mg/dL) | 151.2±37.1 | 151.1±37.5 | 151.8±34.0 | 150.6±42.4 | −2.9 (−7.3 to 1.5) | .19 |
| Triglyceride (mg/dL) | 116 [79, 162]† | 145.8±115.5 | 121 [81, 179]† | 122.4±77.7 | −15.9 (−24.0 to −7.9) | <.001 |
| HDL-C (mg/dL) | 46.6±14.4 | 45.2±14.2 | 46.8±14.3 | 46.9±14.7 | 1.9 (−2.3 to 6.1) | .38 |
| LDL-C (mg/dL) | 78.2±28.3 | 78.2±29.0 | 77.7±26.9 | 79.5±33.5 | −2.1 (−9.9 to 5.7) | .60 |
| Biochemical profile | ||||||
| Glucose (mg/dL) | 108.6±7.3 | 109.8±36.7 | 104.6±27.2 | 111.4±40.2 | 5.8 (−0.8 to 12.4) | .09 |
| HbA1c (%) | 6.3±1.1 | 6.3±1.3 | 6.1±0.8 | 6.2±1.1 | 1.1 (−1.4 to 3.6) | .38 |
BP, blood pressure; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low density lipoprotein cholesterol; WBC, white blood cell count.
P Value is for an unpaired t-test for the between group difference in % change for Lovaza minus Control except for hs-CRP which is calculated using a Mann-Whitney U due to % change in hs-CRP being abnormally distributed; therefore, the absolute difference in medians is shown and a 95% CI is not generated in this situation.
Values represented as median [Interquartile range].
Absolute difference in median % change between the two groups.
Multiply by 0.02586 to convert cholesterol values to mmol/L and by 0.01129 to convert triglyceride to mmol/L..
Pain, stiffness and physical function assessment with WOMAC
Figure 2 (data shown in Table 2 in the Supplement) shows the change in pain, stiffness, physical function and total WOMAC score at 1-year follow-up in the two treatment groups according to the intention-to-treat (Panel A) and per-protocol analysis (Panel B). A lower score or % change indicates less pain and stiffness and better physical function. At 1 year compared to baseline in the intention-to-treat analysis, those in the control group had significant worsening of pain (mean % change from baseline, 10.7%, 95% CI 4.0% to 17.4%, P = .002) and stiffness (mean % change from baseline, 15.6%, 95% CI 6.5% to 24.7%, P = .001) and worsening of physical function (mean % change from baseline, 11.5%, 95% CI 5.2% to 17.7%, P < .001) and worsening of total WOMAC score (mean % change from baseline, 10.2%, 95% CI 4.6% to 15.7%, P < .001) whereas those receiving Lovaza had no significant change from baseline. The difference in % change between the control and Lovaza groups was significant for physical function (mean difference in % change = −11.0%, 95% CI −18.5% to −3.5%, P = .004) and total WOMAC scores (mean difference in % change = −9.8%, 95% CI −16.6% to −3.0%, P = .005) with a trend toward significance for stiffness (P = .06) and pain (P = .06). Similar findings were observed in the per-protocol analysis and in addition, those on Lovaza had significantly less stiffness compared to controls (mean difference in % change = −11.5%, 95% CI −22.9% to −0.1%, P = .048).
Figure 2.
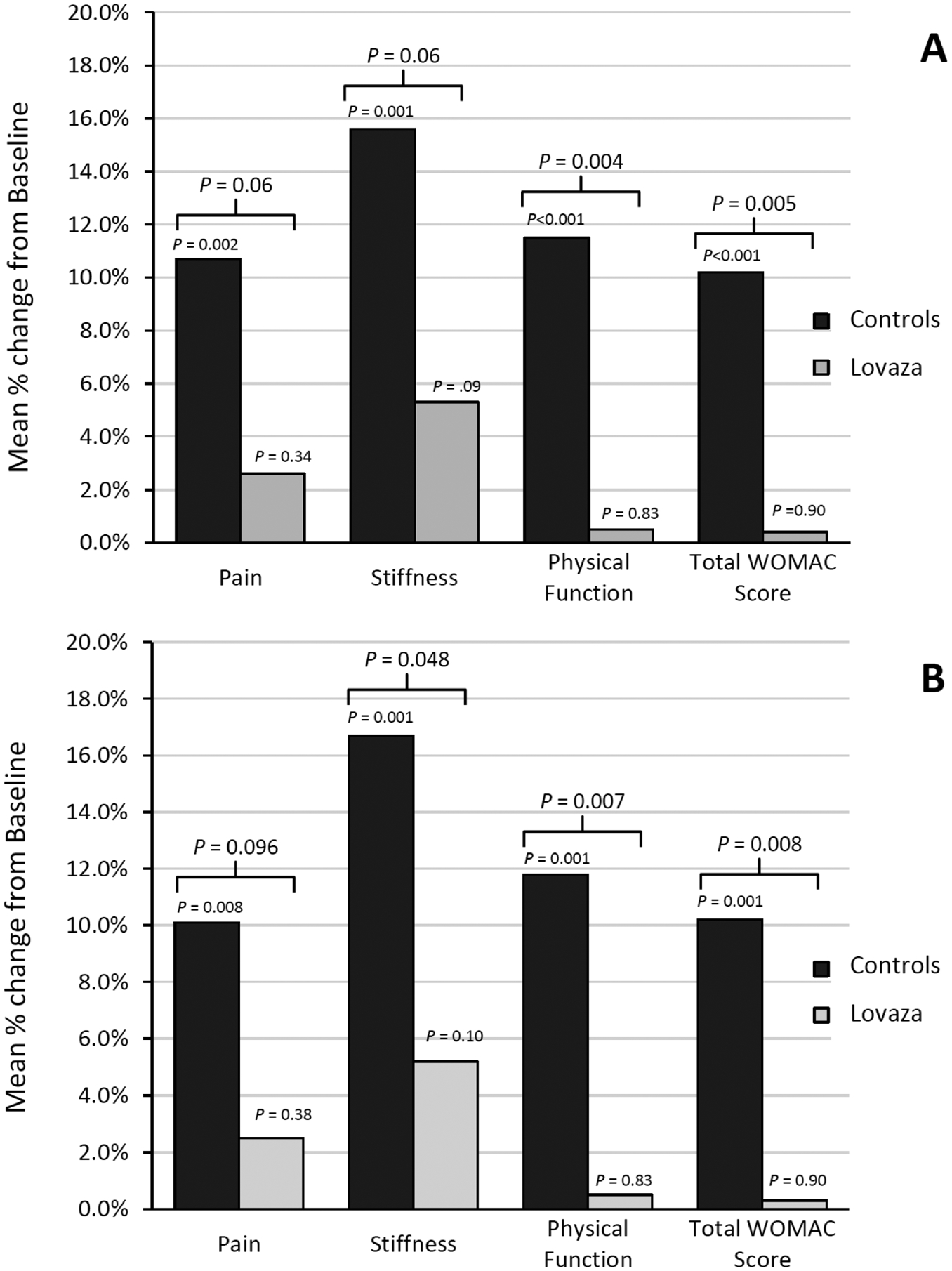
Mean percent change from baseline at 1-year follow-up for WOMAC scores aoccording to the intention-to-treat (panel A) and per-protocol analysis (Panel B) in the control and Lovaza groups. A lower % change indicates less pain and stiffness and better physical function. WOMAC indicates Western Ontario and McMaster Universities Arthritis Index.
Exercise assessment
At baseline, there was no difference in the number of minutes of exercise per week (Figure 3) and no difference in metabolic equivalents of exercise achieved with a graded exercise treadmill test in the control versus Lovaza groups (Table 1). At 1-year follow-up, those taking Lovaza had a 47 minute increase in exercise per week (197 minutes compared to 150 minutes at baseline, P = .044). Moreover, compared to controls, those taking Lovaza had a significantly higher number of minutes of exercise per week (median [IQR], 135 [0, 270] minutes versus 197 [60, 319] minutes, respectively, P = .028).
Figure 3.
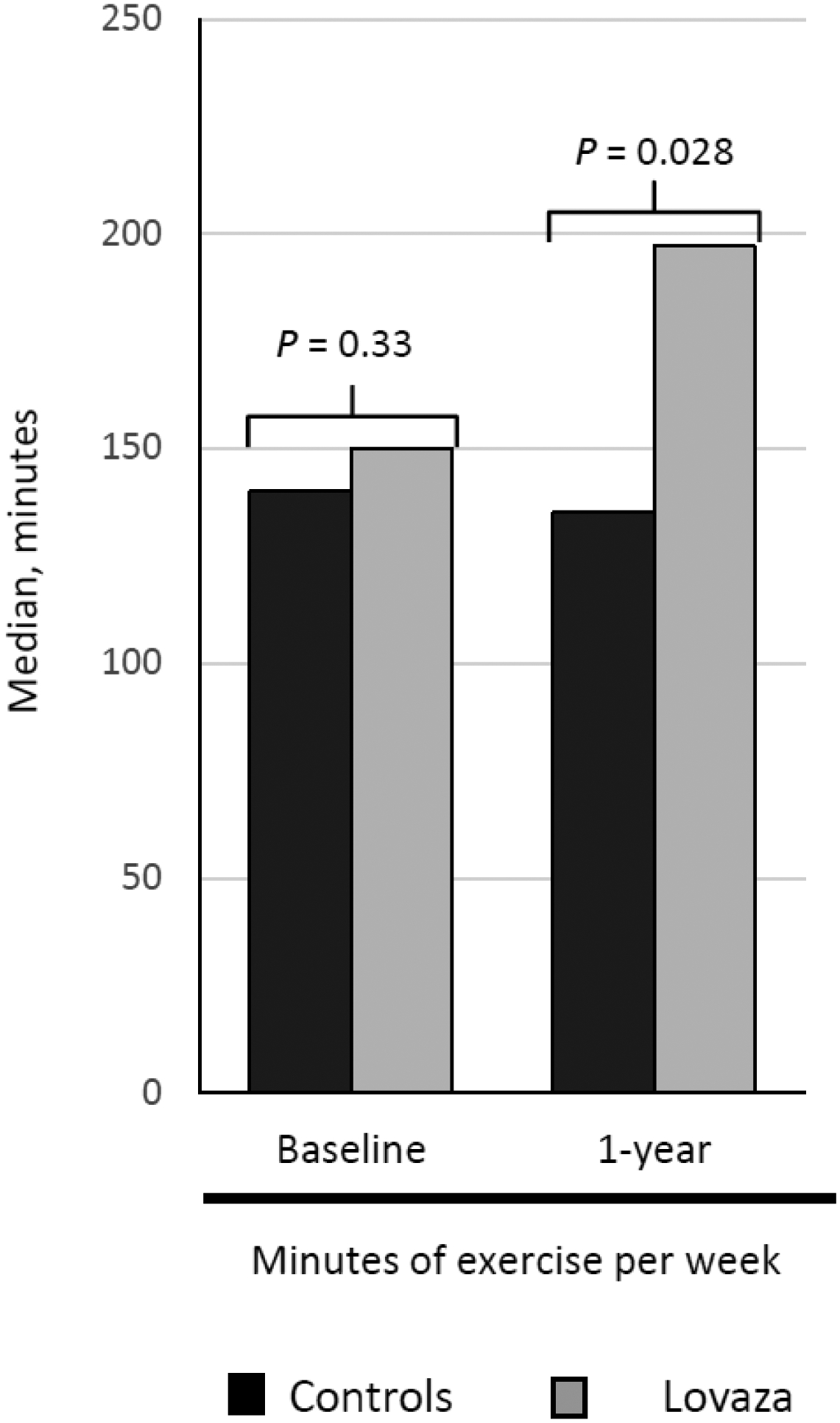
Median change in minutes of exercise per week at 1-year follow-up in the control and Lovaza groups.
Musculoskeletal and joint replacement outcomes
At 1-year follow, knee or hip replacement occurred in 4 control subjects versus no Lovaza subjects (3.1% versus 0%, respectively, P = .056). Due to this strong trend, we also examined musculoskeletal outcomes at 30-month follow-up. A significantly lower incidence of serious musculoskeletal events in the Lovaza group was observed compared to control (5 versus 14 events, respectively, P = .034). Of these, 11 control subjects had total knee or hip replacements due to progressive pain and/or arthritis compared to only 1 in the Lovaza group (P = .002). This difference occurred in the setting of a similar prevalence of osteoarthritis in both groups at baseline (Table 1).
Correlations
At 1-year follow-up in the intention-to-treat analysis, the % change in total WOMAC score significantly directly correlated with % change in WBC, monocyte and lymphocyte counts (Table 3), % change in monocytes significantly directly correlated with % change in physical function, and % change in lymphocyte count significantly directly correlated with % change in WOMAC stiffness. In the per-protocol analysis, WBC significantly directly correlated with % change in physical function.
Table 3.
Correlation between % change in inflammatory markers and WOMAC components at 1-year follow-up for intention-to-treat and per-protocol analyses
| % Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | Monocytes | Neutrophils | Lymphocytes | hs-CRP | ||||||
| % Change in WOMAC scores | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value |
| Intention-to-Treat Analysis | ||||||||||
| Pain | 0.087 | .16 | 0.109 | .077 | 0.067 | .28 | 0.112 | .073 | 0.025 | .70 |
| Stiffness | 0.063 | .31 | 0.094 | .13 | 0.058 | .35 | 0.122 | .049 | −0.018 | .77 |
| Physical function | 0.114 | .065 | 0.138 | .025 | 0.087 | .16 | 0.113 | .069 | 0.060 | .34 |
| Total WOMAC score | 0.131 | .033 | 0.150 | .015 | 0.092 | .14 | 0.156 | .012 | 0.040 | .53 |
| Per-Protocol Analysis | ||||||||||
| Pain | 0.089 | .17 | 0.110 | .088 | 0.057 | .38 | 0.102 | .12 | 0.034 | .60 |
| Stiffness | 0.073 | .26 | 0.091 | .16 | 0.054 | .41 | 0.122 | .060 | −0.021 | .75 |
| Physical function | 0.136 | .035 | 0.145 | .024 | 0.097 | .13 | 0.108 | .097 | 0.067 | .31 |
| Total WOMAC score | 0.148 | .021 | 0.156 | .015 | 0.096 | .14 | 0.153 | .019 | 0.045 | .50 |
hs-CRP, high-sensitivity C-reactive protein; WBC, white blood cell count; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Discussion
In this randomized clinical trial of subjects with CAD, daily supplementation of high-dose EPA and DHA in the form of Lovaza over a 1-year period preserved physical function with a strong trend toward prevention of stiffness and pain. These beneficial changes may account for the higher amount of exercise observed in those taking Lovaza at 1-year follow-up. They may also account for the lower incidence of knee and hip replacement in those taking Lovaza, a finding which provides strong objective support for the subjective findings from the WOMAC instrument in this open label trial. This was an unexpected finding which may be a consequence of more limited symptoms and/or decreased progression of arthritis in those on Lovaza. The striking result of fewer joint replacements in the Lovaza group needs confirmation in a double-blind randomized trial.
Potential barriers to exercise include the development of age-related joint symptoms and loss of muscle mass leading to a greater likelihood of joint pain, stiffness and poorer physical function. Without treatment, these musculoskeletal symptoms can reduce physical function which may result in significant morbidity and impairment of quality of life in patients with CAD.19,20 Aerobic exercise capacity and amount of exercise are inversely related with cardiovascular morbidity and mortality and all-cause mortality in men and women.2–5,21,22 The current recommendation of the World Health Organization and 2008 physical activity guidelines from the U.S. Department of Health and Human Services is for 150 minutes of moderate intensity activity or 75 minutes of vigorous-intensity activity weekly.22,23 In the current study, Lovaza subjects exercised significantly more at 1-year follow-up compared to controls (197 versus 135 minutes, respectively). The increase of 47 minutes of exercise per week in the Lovaza group from a baseline of 150 minutes enabled subjects to exceed the recommended exercise duration of 150 minutes of moderate activity per week compared to controls who exercised less than the recommended duration. The mechanism for the increase in amount of exercise in the Lovaza group may be due to the preservation of physical function and attenuation of stiffness and pain with Lovaza observed in this study. To our knowledge, the current study is the first randomized trial reporting on a beneficial effect of EPA and DHA on pain, stiffness, physical function, amount of exercise and joint replacement in subjects with CAD.
The preservation of physical function with Lovaza in the current study may be especially important since age-associated declines in muscle mass and related physical function in CAD patients impair ability to exercise at maximal capacity. In a prior study, omega-3 fatty acids at a dose identical to that in the current study over a 6-month period in healthy 60–85 year-old subjects increased thigh muscle volume, handgrip strength, lower- and upper-body strength and average power during isokinetic leg exercises compared to corn oil.24 Although we did not measure physical strength in our study, the preservation of physical function could result from a similar mechanism. Therefore, omega-3 fatty acids should be considered as a therapeutic approach to prevent sarcopenia and maintain physical function in both older healthy adults and subjects with CAD.
NSAIDs have been the major drugs used to alleviate pain and joint symptoms. Both nonselective NSAIDs and cyclooxygenase (COX)-2 selective inhibitors exert their analgesic effect by inhibiting 2-series prostaglandin production through inhibition of COX-2,25 which also inhibits the production of 2-series prostacyclins. Prostacyclins prevent the aggregation of platelets and cause vascular smooth muscle relaxation which leads to vasodilation; thus, prostacyclins are cardiovascular protective.26–28 Blocking prostacyclin production with NSAIDs leads to increased platelet aggregation, vasoconstriction and reduced nitric oxide production by the endothelium, thus leading to an augmented effect of vasoconstriction and increased risk of cardiovascular events in those with CAD (adjusted odds ratio = 1.23, 95% CI 1.12 to 1.35) and without pre-existing CAD (adjusted odds ratio = 1.14, 95% CI 1.03 to 1.25).7 Due to this increased risk of cardiovascular events, nonselective NSAIDs and COX-2 selective inhibitors are not recommended for CAD patients.29 Therefore, it’s of interest to find an alternative to NSAIDs for management of musculoskeletal symptoms.
Studies in patients with various forms of arthritis provide evidence that omega-3 fatty acids lower the use of NSAIDS and improve joint symptoms. In a meta-analysis of 17 randomized clinical trials of omega-3 fatty acids in patients with rheumatoid arthritis or joint pain secondary to inflammatory bowel disease, omega-3 fatty acid supplementation for 3 to 4 months significantly reduced patient-reported joint pain intensity (P = .03), minutes of morning stiffness (P = .003), number of painful and/or tender joints (P = .003) and NSAID consumption (P = .01).30 In a study of untreated patients with rheumatoid arthritis of <12 months duration, all were given methotrexate, sulphasalazine and hydroxychloroquine and then randomized to either high-dose EPA and DHA 5.5 g/day or 0.4 g/day (control).31 Subjects receiving high-dose EPA and DHA had a 76% lower failure rate of treatment (HR, 0.24, 95% CI 0.10 to 0.54, P = .0006), and a significantly greater rate of American College of Rheumatology remission compared with the control group (adjusted HR, 2.09, 95% CI 1.02 to 4.30, P = .04).
Omega-3 fatty acids have also shown benefit as assessed by the WOMAC in subjects with osteoarthritis. In a prospective, randomized, double-blind, placebo-controlled clinical trial, 81 patients with knee or hip osteoarthritis received either 3 capsules of Phytalgic, a food supplement containing n-3 and n-6 fatty acid from nettle with a total of 1350 mg of fish oil from cold water fish, or placebo.32 After 3-month follow-up, those on Phytalgic had significant reduction in WOMAC pain (86.5 versus 235.3, P < .001), WOMAC stiffness (41.4 versus 96.3, P < .001) and WOMAC physical function (301.6 versus 746.5, P < .001) scores and a significantly lower use of NSAIDs (defined daily doses per day = 0.5 versus 1.0, P = .02) and analgesic (500mg paracetamol equivalent tablets per week = 6.5 versus 16.5, P < .001) compared to placebo. In an 8-week study of 75 participants with knee osteoarthritis, subjects on either a low dose of EPA and DHA (400 mg and 200 mg, respectively) or high dose of EPA and DHA (800 mg and 400 mg, respectively) had significantly lower WOMAC pain (P < .001 and P < .001, respectively), stiffness (P < .001 and P < .001, respectively) and functional visual analog scores (P < .001 and P < .001, respectively) compared to placebo.33 Compared to those on the lower dose of EPA and DHA, those on the higher dose had significantly better WOMAC functional scores but did not have additional improvement in WOMAC pain or stiffness scores. Omega-3 fatty acids also decreased use of NSAIDs in subjects with discogenic pain.34 Based on these studies along with the results of our study and the fact that omega-3 fatty acids have been shown to reduce cardiovascular events and mortality in large randomized clinical trials and prospective analyses of subjects with CAD,9,10,35 high-dose omega-3 fatty acids may be an effective and safer alternative to NSAIDs in reducing musculoskeletal symptoms in subjects both with and without CAD.
In the current trial, inflammatory biomarkers including hs-CRP and WBC count were both significantly reduced in those receiving Lovaza. Epidemiological studies have shown that WBC count predicts CAD incidence and mortality in patients without CAD and predicts mortality in patients with CAD.36 In a meta-analysis of 5,337 subjects without CAD in 7 large studies, a high total WBC count at baseline was associated with a 1.4-fold increased risk of CAD (95% CI 1.3–1.5), comparable to that of the inflammatory marker, hs-CRP (1.45, 95% CI 1.25–1.68).37,38 The significant correlations between reduction in WBC count and its subsets and physical function, stiffness and total WOMAC scores in the current study suggest that the beneficial effect of EPA and DHA may be mediated via a reduction in inflammation. Anti-inflammatory actions of EPA and DHA include conversion to specialized pro-resolving lipid mediators with EPA and DHA being the respective precursors of the E-series and D-series resolvins.39 Resolvins resolve inflammation and reduce pain by reducing tissue infiltration of inflammatory cells, suppressing the production of inflammatory cytokines as interleukin (IL)-1-β, tumor necrosis factor-α, monocyte chemoattractant protein-1 and IL-6 from neutrophils39,40 and inhibiting the expression of nuclear factor-κB and COX-2 in the spinal cord and dorsal ganglia.40 Thus, the conversion of EPA and DHA to resolvins could be a significant contributor to the reduction of symptoms with omega-3 fatty acids reported in inflammatory diseases as rheumatoid arthritis30,31 and systemic lupus41,42 and reduction of reoccurrence rates in Crohn’s disease.43 In support of a relation between resolvins and pain, patients with arthritis who received omega-3 fatty acids had increased levels of resolvins in synovial fluid. Moreover, synovial fluid resolvin E2 correlated with a reduction in pain scores, a finding suggesting that resolvin E2 reduces pain in subjects with arthritis.44,45 Physiological levels of resolvin D1 have been reported in synovial fluid from subjects with rheumatoid arthritis and have been shown to attenuate chemotaxis of human neutrophils.44 In a mouse model of inflammatory arthritis, 17 R-resolvin D1 attenuated arthritis severity, cachexia, hind-paw edema and paw leukocyte infiltration and shortened the remission interval and stimulated chondrocyte matrix production and protection from cartilage degradation.44 These benefits were abolished in resolvin D1 receptor-deficient mice. Resolvins are also potent analgesics,46 and the precursor of resolvin D1 displays anti-hyperalgesic properties in adjuvant-induced arthritis in a rat model.47 Further studies should examine whether the conversion of omega-3 fatty acids to resolvins may be responsible for beneficial effects on pain and stiffness in clinical trials.
Strengths and limitations
Major strengths of the current study are the randomized, controlled design and the use of high-dose (3.36 g) EPA and DHA. A limitation of our trial is the open-label nature which gave subjects knowledge of their assignment and the reliance on self-reported exercise data; however, the WOMAC, which was used to assess symptoms, is a well-validated questionnaire.16–18 Moreover, joint replacement is a hard endpoint, and the remarkably lower rate of joint replacement in those receiving Lovaza would not be affected by knowledge of treatment assignment.
Conclusions
In conclusion, the results of the current study show that high-dose omega-3 fatty acids preserve physical function and prevent stiffness and pain over a 1-year period in CAD patients. These beneficial changes may account for the higher amount of exercise and lower rate of joint replacement observed in those taking Lovaza. Given their cardiovascular benefit, lack of side effects and wide availability, omega-3 fatty acids may be a safe preventative strategy against musculoskeletal symptoms in CAD patients. These findings have a potential public health significance and should encourage research to further examine the effect of dietary supplemental omega-3 fatty acids on pain, mobility, exercise and joint replacement in future research.
Supplementary Material
Highlights:
EPA and DHA preserve physical function and total WOMAC scores in patients with CAD.
EPA and DHA also improve amount of exercise in CAD patients.
There is a strong trend toward reduction of pain and stiffness.
These beneficial effects may account for lower rates of joint replacement.
EPA and DHA may be a safe preventative strategy against musculoskeletal symptoms.
Acknowledgment
We thank the study subjects for participating in the trial.
Funding
This work was supported by the National Institutes of Health Specialized Center of Clinically Oriented Research (SCCOR) program grant to Dr. Welty: P50 HL083813 and supported by the Harvard Clinical and Translational Science Center Award, NIH UL1 TR001102. The study funders had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
None.
The data have been presented at the American Heart Association Scientific Sessions, November 9, 2015, in Orlando, FL.
References
- 1.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 3.Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Mathews CE. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–967. 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 5.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P; Americab College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 7.Lee TA, Bartle B, Weiss KB. Impact of NSAIDS on mortality and the effect of preexisting coronary artery disease in US veterans. Am J Med. 2007;120:98.e9–16. 10.1016/j.amjmed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bally M, Dendukur N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, Brophy J. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017;357:j1909. 10.1136/bmj.j1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F; GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. 10.1161/01.cir.0000014682.14181.F2 [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 11.Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012;43:356–362. 10.1016/j.arcmed.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Souza PR, Norling LV. Implications for eicosapentaenoic acid- and docosahexaenoic acid-derived resolvins as therapeutics for arthritis. Eur J Pharmacol. 2016;785:165–173. 10.1016/j.ejphar.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 13.Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes and coronary artery disease. J Am Heart Assoc. 2017;6:e004740, 10.1161/JAHA.116.004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients With Coronary Artery Disease: A Randomized Clinical Trial. J Am Heart Assoc. 2017; 6: e006981. 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 17.Lo T, Parkinson L, Cunich M, Byles J. Discordance between self-reported arthritis and musculoskeletal signs and symptoms in older women. BMC Musculoskelet Disord. 2016:17:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosales Ade L, Brito NL, Frucchi R, de Campos GC, Pailo AF, de Rezende MU. Obesity, osteoarthritis and clinical treatment. Acta Ortop Bras. 2014; 22:136–139. 10.1590/1413-78522014220300679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawker GA, Croxford R, Bierman AS, Harvey PJ, Ravi B, Stanaitis I, Lipscombe LL. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One. 2014;9:e91286. 10.1371/journal.pone.0091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokka T, Pincus T. Poor physical function, pain and limited exercise: risk factors for premature mortality in the range of smoking or hypertension, identified on a simple patient self-report questionnaire for usual care. BMJ Open. 2011;1:e000070. 10.1136/bmjopen-2011-000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. 10.1001/jama.1996.03540030039029. [DOI] [PubMed] [Google Scholar]
- 22.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. http://www.who.int/dietphysicalactivity/publications/9789241599979/en/. Accessed May 14, 2017. [PubMed] [Google Scholar]
- 24.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–122. 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulichino AM, Rowland S, Wu T, Clark P, Xu D, Mathieu MC, Riendeau D, Audoly LP. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J Pharmacol Exp Ther. 2006;319:1043–1050. 10.1124/jpet.106.110387. [DOI] [PubMed] [Google Scholar]
- 26.Moncada S, Vane JR. Prostacyclin and the vascular endothelium. Bull Eur Physiopathol Respir. 1981;17:687–701. [PubMed] [Google Scholar]
- 27.FitzGerald GA, Smith B, Pedersen AK, Brash AR. Increased prostacyclin biosynthesis in patients with severe atherosclerosis and platelet activation. N Engl J Med. 1984;310:1065–1068. 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- 28.FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2:879–890. 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 29.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Proudman SM, James MJ, Spargo LD, Metcalf RG, Sullivan TR, Rischmueller M, Flabouris K, Wechalekar MD, Lee AT, Cleland LG. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann Rheum Dis 2015;74:89–95. 10.1136/annrheumdis-2013-204145. [DOI] [PubMed] [Google Scholar]
- 32.Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11:R192. 10.1186/ar2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peanpadungrat P Efficacy and Safety of Fish Oil in Treatment of Knee Osteoarthritis. J Med Assoc Thai. 2015;98:Suppl 3:S110–4. [PubMed] [Google Scholar]
- 34.Maroon JC, Bost JW. Omega-3 fatty acids (fish oil) as an anti-inflammatory: an alternative to nonsteroidal anti-inflammatory drugs for discogenic pain. Surg Neurol. 2006;65:326–331. 10.1016/j.surneu.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of Eicosapentaenoic and Docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29. 10.1016/j.mayocp.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Madjid M, Fatemi O. Components of the Complete Blood Count as Risk Predictors for Coronary Heart Disease: In-depth review and update. Texas Heart Institute Journal. 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- 37.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N Engl J Med. 2004;350:1387–1397. 10.1056/NEJMoa032804 [DOI] [PubMed] [Google Scholar]
- 38.Danesh J, Collins R, Appleby P, Peto R. Association of Fibrinogen, C-reactive Protein, Albumin, or Leukocyte Count with Coronary Heart Disease: Meta-analyses of Prospective Studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477 [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton AJE, Snaith ML, Locniskar M, Cumberland AG, Morrow WJW, Isenberg DA. Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann Rheum Dis. 1991;50:463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosis. J.Rheumatol 2004;31:1551–1556. [PubMed] [Google Scholar]
- 43.Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s Disease. N Engl J Med 1996;334:1557–1560. 10.1056/NEJM199606133342401 [DOI] [PubMed] [Google Scholar]
- 44.Norling LV, Headland S, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CH, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1:e85922– . 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;107:24–29. 10.1016/j.plefa.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293. 10.1111/j.1476-5381.2011.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


