Abstract
Globally the proportion of tuberculosis cases caused by drug-resistant strains is increasing. Interruptions in the drug supply, improper drug prescription and nonadherence to treatment protocols promote drug resistance through mechanisms that are now well understood. The treatment of tuberculosis must take into account the possibility of drug resistance and include at least 2 drugs, preferably 3, to which the isolate is proven or anticipated to be susceptible.
In the absence of effective interventions, a tuberculosis (TB) epidemic is believed to have a life span within a community of several hundred years. Since the middle of the 20th century, antituberculous drugs have been accelerating the natural decline in the incidence of the disease within epidemics. Latterly, 2 forces have conspired to reverse this trend. One is a natural phenomenon &mdash% HIV. By destroying the 2 cells most important to the containment of tubercle bacilli (macrophages and CD4-receptor-bearing lymphocytes), HIV vigorously promotes progression of recent or remotely acquired TB infection to active disease. The other force &mdash% drug-resistant TB &mdash% is, like the discovery of the drugs themselves, a purely man-made event.1
A patient is said to have drug-resistant TB if the strain causing the disease is resistant to one or more of the first-line drugs (isoniazid, rifampin, pyrazinamide, ethambutol and streptomycin). First-line drugs are the most effective agents; all but one, low-dose ethambutol, are bactericidal. Epidemiologically, drug resistance in TB is classified into 3 types:2
· Primary: Previously untreated patients are found to have drug-resistant organisms, presumably because they have been infected from an outside source of resistant bacilli.
· Acquired: Patients initially have drug-susceptible tubercle bacilli that later become resistant because of inadequate, inappropriate or irregular treatment or, more important, because of nonadherence to treatment protocols.
· Initial: This type of drug resistance applies to patients who deny previous treatment but whose prior drug use history cannot be verified. In reality this category consists of true primary resistance and an unknown amount of undisclosed acquired resistance.
Unless they have travelled abroad to countries with high prevalence rates, Canadian-born TB patients are unlikely to have primary drug resistance. Drug resistance in foreign-born patients who deny previous drug use is best classified as initial rather than primary, because their drug use histories cannot usually be verified.
Finally, in regions of the world where TB is epidemic and drug susceptibility testing is not always available, the term ”acquired drug resistance” may be misleading, as it will apply to patients infected with strains that truly acquired drug resistance during treatment as well as patients who were initially infected with or reinfected with a drug-resistant strain.3 In such regions the term ”drug resistance in previously treated cases” may be more accurate.
Why is drug-resistant tuberculosis an issue?
Globally the proportion of TB cases that are caused by drug-resistant strains is increasing.1 In developed countries such as Canada, the shift to outpatient care in the late 1960s may have reduced patient adherence to treatment protocols and increased the likelihood of treatment failure, relapse and acquired drug resistance. In developing countries, where resources are scarce and access to health care is limited, acquired resistance is even more likely to occur. In those countries, resistant strains may circulate to people who later immigrate to Canada; when and if reactivation TB develops in these immigrants, it will be drug-resistant.
What‚s new that will help in the management of drug-resistant TB?
An understanding of drug-resistance theory and when to suspect drug resistance will aid in the management of drug-resistant TB.
Drug-resistance theory
In any large population of tubercle bacilli, there will be several naturally occurring drug-resistant mutants.4 Random mutation that confers resistance to 4 of the first-line antituberculous agents occurs at predictable frequencies in untreated populations of tubercle bacilli: isoniazid, 1 in 106; streptomycin, 1 in 106; ethambutol, 1 in 106; and rifampin, 1 in 108.
A tuberculous cavity harbouring 107-109 bacilli may contain a few (10-1000) bacilli resistant to isoniazid, a few (&le%10) resistant to rifampin, a few (10-1000) resistant to ethambutol and a few (10-1000) resistant to streptomycin, and so on. This does not imply that when a sample of this population of bacilli is cultured in the laboratory it will be found to be resistant to these drugs; for resistance to be reported in the laboratory, &ge% 1/100 of the bacilli must be resistant to the drug.2
The sites of resistance within the mutants are chromosomally located (not plasmid) and are not linked. Accordingly, the likelihood of a bacillus spontaneously developing resistance to 2 unrelated agents is the product of probabilities; for example, for resistance to isoniazid and rifampin, 1 in 106 &khcy% 1 in 108 equals 1 in 1014. Because the total number of bacilli in the body, even in cases of advanced cavitary disease, rarely approaches this number (1014), spontaneous evolution of a multidrug-resistant bacillus is exceedingly rare.
As Iseman and Madsen5 articulated so well,
This is the salient principle of modern tuberculosis treatment. Because naturally occurring two-drug resistance is very uncommon, therapy with two (or more) drugs prevents the emergence of progressive resistance in the following manner: some organisms in the population will be resistant to drug A, and some others will be resistant to drug B, but none will be simultaneously resistant to both drugs. Thus drug B will kill those organisms resistant to drug A, whereas drug A will kill those resistant to drug B. In principle this means a two drug regimen should be adequate to treat the usual case of drug-susceptible tuberculosis. Owing to the relative weakness of streptomycin and para-aminosalicylic acid, triple rather than double therapy was the standard until the advent of rifampin. The success of the two-drug (isoniazid and rifampin) ”Arkansas” regimen substantially validated the aforementioned model for drug-susceptible tuberculosis.6
The emergence of drug resistance is due to the selection of pre-existing resistant mutants in the original bacterial population by ”drug pressure.” For example, if isoniazid alone is prescribed (or is the only drug adhered to in a multidrug regimen) for cavitary pulmonary TB, it will kill all of the organisms susceptible to it, including those random mutants resistant to drugs such as rifampin, ethambutol and streptomycin, but it will not kill isoniazid-resistant mutants. These will continue to multiply and will eventually dominate the population, and isoniazid will be lost to the armamentarium. The likelihood of this occurring is influenced by the duration of such monotherapy: 25% among people receiving isoniazid alone for 2 weeks, 60% among those receiving it for 6 months and 80% among those receiving it for 2 years.7 If rifampin alone is now added to the regimen, then by the same mechanism, a multidrug-resistant strain (i.e., resistant to both isoniazid and rifampin) will emerge; rifampin will kill all bacteria resistant to isoniazid, but it will not kill those few random mutants in the new population that are resistant to both isoniazid and rifampin.
This classic theory of drug resistance in TB posits a sequence of events in which the patient effectively receives monotherapy. It does not explain how drug resistance may emerge solely because of irregularity in drug-taking and without monotherapy. Other mechanisms have been proposed to explain resistance under these circumstances.8,9 >In essence, they require several cycles of killing (when drugs are taken) and regrowth (when drug-taking stops). In each of these cycles, there is selection favouring the resistant mutants relative to the susceptible bacterial population. Regrowth to the size of the original population may occur and result in an increasing proportion of resistant bacilli at the start of each cycle.
When to suspect drug resistance
A case of TB should be suspected of being drug-resistant under the following circumstances:
· The patient was born outside of Canada and is from a country with a high prevalence of TB, or the patient was born in Canada but recently resided in a country with a high prevalence of TB.10,11,12 As the proportion of people who fall into the first group increases in Canada, so too will the proportion of TB cases that are drug-resistant.
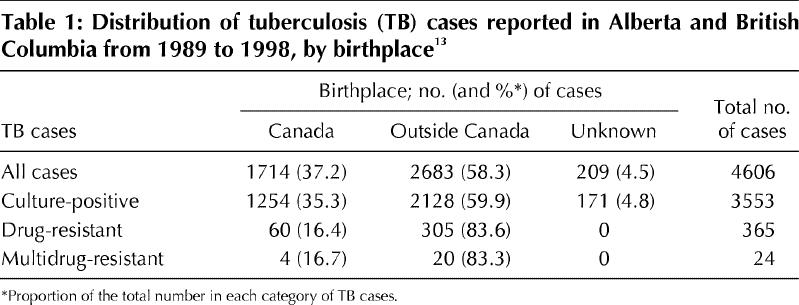
· The patient has previously received antituberculous drugs. Multidrug-resistant TB is still uncommon in Canada (Table 1), but it should be suspected in immigrants recently arrived from TB-endemic countries who have a history of antituberculous drug use in their country of origin.13
Table 1
· The patient is thought to have become infected through exposure to a person known to have drug-resistant TB.
· The disease is cavitary pulmonary TB.14,15 Presumably such cases are more apt to be drug-resistant because they harbour greater numbers of bacilli.
· Treatment is failing. Failure of TB treatment has been defined by the World Health Organization as occurring in the following circumstances: a patient who, while receiving treatment, remains or becomes again smear-positive 5 months or more after commencing treatment. It also occurs in a patient who was initially smear-negative before starting treatment and who becomes smear-positive after the second month of treatment.16 The American Thoracic Society considers failure to have occurred if cultures are still positive 5-6 months into treatment.17 When cultures are available, as is the case in Canada, they are considered the best determinant of the bacteriologic response to treatment.18 Treatment failure can almost always be explained by 1 or more of 5 mechanisms: improper drug prescription, nonadherence to the prescribed therapy, drug resistance, drug malabsorption and exogenous reinfection with a drug-resistant strain during treatment of the original disease.18,19
Although concomitant HIV infection is a risk factor for drug resistance in the United States,18 it has so far not been demonstrated to be the case in Canada.9,13,20,21,22
What‚s the bottom line?
In TB control programs, priority must be given to the prevention, not the management, of drug-resistant disease. To prevent resistance it is necessary to (a) prescribe, in proper dosage, at least 2 drugs, preferably 3, to which the isolate is proven or anticipated to be susceptible, (b) provide assurances that the prescribed regimen is adhered to and that those who abscond from treatment are identified early (best done by supervising the ingestion of each dose), and (c) never introduce a single drug to a failing regimen.
The treatment of drug-resistant TB assumes the availability of state-of-the-art drug-susceptibility testing and an uninterrupted supply of a wide range of drugs. Canada is fortunate to have both. Of the 5 first-line drugs, testing for susceptibility to isoniazid, rifampin and ethambutol is performed routinely on all initial isolates and testing for susceptibility to streptomycin and pyrazinamide is performed on most initial isolates.
Isoniazid-resistant TB
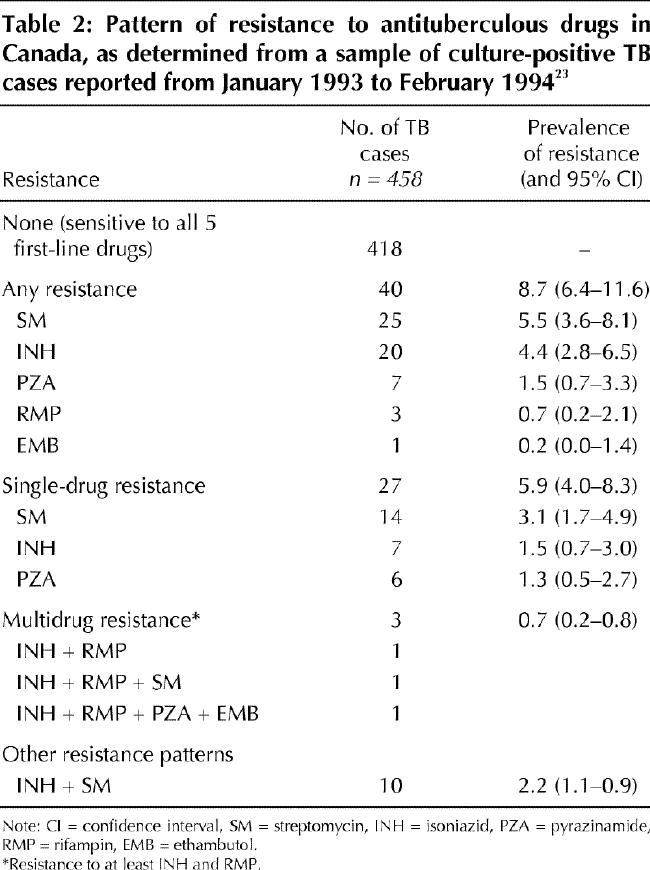
If an isolate is found to be resistant to a first-line drug, it will most likely be resistant to either isoniazid or streptomycin, or both, because these drugs have been in use the longest. Drug susceptibility data for a sample of culture-positive TB cases reported from Canada between January 1993 and February 1994 are representative of patterns of drug resistance in Canada (Table 2).23 Because streptomycin can be administered only parenterally and has largely been replaced by ethambutol, resistance to it is not usually an important consideration. Streptomycin resistance may become relevant when other treatment options are limited because of resistance to or toxicity from the oral agents. On the other hand, resistance to isoniazid is always important because the drug is arguably the single most effective antituberculous agent available. Fortunately, TB cases that are resistant to isoniazid, regardless of their resistance to streptomycin, may be cured by a number of treatment alternatives. These include the following:
Table 2
· Initial phase: daily treatment with rifampin, pyrazinamide and ethambutol for 2 months. Continuation phase: daily or intermittent therapy with rifampin, pyrazinamide and ethambutol for 7 months.24,25
· Initial phase: daily treatment with rifampin, pyrazinamide and ethambutol for 2 months (pyrazinamide is recommended here, but in most field trials26,27,28 this drug was not included in the regimen). Continuation phase: daily or intermittent therapy with rifampin and ethambutol for 10 months.26,27,28
Ideally each of these regimens should be regarded as the minimum effective therapy and each should be fully supervised; certainly the continuation phase cannot be intermittent if it is not fully supervised. If the prevailing rate of resistance to isoniazid is 4% or greater among those without a history of prior antituberculous drug use, then initial treatment regimens should always include at least 4 first-line drugs.17
Disease resistant to other first-line drugs that is not multidrug-resistant TB
Although resistance to the first-line antituberculous drugs other than isoniazid is not common in Canada, the following generalizations regarding treatment apply. If isoniazid, rifampin and pyrazinamide are not included in the regimen, a 6-month short course of treatment is not recommended. Patients with isolates resistant to rifampin but susceptible to isoniazid must be treated for at least 12 months. Those with isolates resistant to pyrazinamide but susceptible to isoniazid and rifampin must be treated for at least 9 months. Although a fluoroquinolone, most commonly levofloxacin, is often used in place of ethambutol in those whose isolates are resistant to ethambutol or who cannot tolerate the drug, comparable efficacy of these 2 drugs has not been established, and therefore close monitoring of the clinical, radiologic and bacteriologic response to treatment is indicated.
Multidrug-resistant TB
Unfortunately, good data are not available on the relative effectiveness of various regimens and the necessary duration of treatment for patients with isolates resistant to both isoniazid and rifampin, with or without resistance to other drugs.29,30,31 Invariably, one must resort to the use of second-line drugs, which are more expensive, less effective and have many more side effects than the first-line drugs. Accordingly, they should be administered only by experienced staff, in specialized units, in close connection with a laboratory able to conduct cultures and reliable drug-susceptibility tests; otherwise, the emergence of incurable TB is possible.31
What does the future hold?
Molecular diagnostic techniques promise earlier detection of drug resistance through rapid identification of the genetic mutations scripting resistance to specific drugs. New drugs, currently under study, will help to simplify the treatment of multidrug-resistant TB. A global commitment of energy and resources will help to reduce the incidence of TB in developing countries. These advances will not, however, obviate the need for proper administration and adherence to drug therapy if further resistance is to be avoided.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Reprint requests to: Dr. Richard Long, Department of Medicine, Division of Pulmonary Medicine, University of Alberta Hospitals, Rm. 2E4.21, Walter Mackenzie Centre, 8440-112 St., Edmonton AB T6G 2B7; fax 780 407-6384; richard.long@ualberta.ca
References
- 1.Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F. Global surveillance for antituberculosis-drug resistance, 1994-1997. N Engl J Med 1998;338:1641-9. [DOI] [PubMed]
- 2.Gangadharam PRJ. Drug resistance in tuberculosis. In: Reichman LB, Hershfield ES, editors. Tuberculosis: a comprehensive international approach. New York: Marcel Dekker; 1993. p.293-328.
- 3.Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet 2000;356:22-5. [DOI] [PubMed]
- 4.Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med 1980;1:231-41. [PubMed]
- 5.Iseman MD, Madsen LA. Drug-resistant tuberculosis. Clin Chest Med 1989;10:341-53. [PubMed]
- 6.Dutt A, Stead W. Present chemotherapy for tuberculosis. J Infect Dis 1982; 146:698-705. [DOI] [PubMed]
- 7.Costello HD, Caras GJ, Snider DE Jr. Drug resistance among previously treated tuberculosis patients, a brief report. Am Rev Respir Dis 1980;121:313-6. [DOI] [PubMed]
- 8.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis 1998;2:10-5. [PubMed]
- 9.Iseman MD, editor. Drug-resistant tuberculosis. In: A clinician‚s guide to tuberculosis. New York: Lippincott Williams & Wilkins; 2000. p.323-54.
- 10.Long R, Manfreda J, Mendella L, Wolfe J, Parker S, Hershfield E. Antituberculous drug resistance in Manitoba from 1980 to 1989. CMAJ 1993;148:1489-95. [PMC free article] [PubMed]
- 11.Long R, Fanning A, Cowie R, Hoeppner V, FitzGerald M, and the Western Canada Tuberculosis Group. Antituberculous drug resistance in western Canada (1993-1994). Can Respir J 1997;4:71-6.
- 12.Manns BJ, Fanning EA, Cowie RL. Antituberculous drug resistance in immigrants to Alberta, Canada, with tuberculosis, 1982-1994. Int J Tuberc Lung Dis 1997;1:225-30. [PubMed]
- 13.Hersi A, Elwood K, Cowie R, Kunimoto D, Long R. Multidrug-resistant tuberculosis in Alberta and British Columbia, 1989 to 1998. Can Respir J 1999;6:155-60. [DOI] [PubMed]
- 14.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis 1965;92:687-703. [DOI] [PubMed]
- 15.Ben-Dov I, Mason GR. Drug-resistant tuberculosis in a southern California hospital: trends from 1969 to 1984. Am Rev Respir Dis 1987;135:1307-10. [DOI] [PubMed]
- 16.Maher D, Chaulet P, Spinaci S, Harries A, editors. Case definitions. In: Treatment of tuberculosis: guidelines for national programs. 2nd ed. Geneva: World Health Organization; 1997. p. 18-24.
- 17.Bass JB Jr, Farer LS, Hopewell PC, O‚Brien R, Jacobs RF, Ruben F. Treatment of tuberculosis and tuberculosis infection in adults and children. Am J Respir Crit Care 1994;149:1359-74. [DOI] [PubMed]
- 18.Long R, Chomyc S, Der E, Sitar D. ”Pseudo” treatment failure of pulmonary tuberculosis in association with a tuberculoma. Can Respir J 2000;7:79-83. [DOI] [PubMed]
- 19.Small PM, Shafer RW, Hopewell PC, Singh SP, Murphy MJ, Desmond E. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med 1993;328:1137-44. [DOI] [PubMed]
- 20.Moore M, Onorato IM, McCray E, Castro KG. Trends in drug-resistant tuberculosis in the United States, 1993-96. JAMA 1997;278:833-7. [PubMed]
- 21.Korzeniewska-Kosela M, FitzGerald M, Vedal S, Allen EA, Schechter MT. Spectrum of tuberculosis in patients with HIV infection in British Columbia: report of 40 cases. CMAJ 1992;146:1927-34. [PMC free article] [PubMed]
- 22.Rivest P, Tannenbaum T, Bédard L. Epidemiology of tuberculosis in Montreal. CMAJ 1998;158(5):605-9. Abstract available: www.cma.ca/cmaj/vol-158/issue-5/issue-5/0605.htm [PMC free article] [PubMed]
- 23.Farzad E, Holton D, Long R, et al. Drug resistance study of Mycobacterium tuberculosis in Canada. Can J Public Health. In press. [DOI] [PMC free article] [PubMed]
- 24.Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Am Rev Respir Dis 1979:119:579-85. [DOI] [PubMed]
- 25.Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis 1986; 133:423-30. [DOI] [PubMed]
- 26.Zierski M. Prospects of retreatment of chronic resistant pulmonary tuberculosis patients: a critical review. Lung 1977;154:91-102. [DOI] [PubMed]
- 27.A controlled clinical trial of daily and intermittent regimens of rifampicin plus ethambutol in the retreatment of patients with pulmonary tuberculosis in Hong Kong. A Hong Kong Tuberculosis Treatment Services/Brompton Hospital/ British Medical Research Council Investigation. Tubercle 1974;55:1-27. [DOI] [PubMed]
- 28.Hong Kong Tuberculosis Treatment Services/Brompton Hospital/British Medical Research Council. Results up to 30 months. Tubercle 1975;56:179-89.766340
- 29.Iseman MD. Treatment of multidrug-resistant tuberculosis. N Engl J Med 1993;329:784-91. [DOI] [PubMed]
- 30.Telzak A, Sepkowitz K, Alpert P, Mannheimer S, Medard F, El-Sadr W. Multidrug-resistant tuberculosis in patients without HIV infection. N Engl J Med 1995;333:907-11. [DOI] [PubMed]
- 31.Crofton J, Chaulet P, Maher D. Guidelines for the management of drug-resistant tuberculosis. Geneva: World Health Organization; 1997.


