Abstract
Porphyrinic compounds are widespread in nature and play key roles in biological processes such as oxygen transport in blood, enzymatic redox reactions or photosynthesis. In addition, both naturally derived as well as synthetic porphyrinic compounds are extensively explored for biomedical and technical applications such as photodynamic therapy (PDT) or photovoltaic systems, respectively. Their unique electronic structures and photophysical properties make this class of compounds so interesting for the multiple functions encountered. It is therefore not surprising that optical methods are typically the prevalent analytical tool applied in characterization and processes involving porphyrinic compounds. However, a wealth of complementary information can be obtained from NMR spectroscopic techniques. Based on the advantage of providing structural and dynamic information with atomic resolution simultaneously, NMR spectroscopy is a powerful method for studying molecular interactions between porphyrinic compounds and macromolecules. Such interactions are of special interest in medical applications of porphyrinic photosensitizers that are mostly combined with macromolecular carrier systems. The macromolecular surrounding typically stabilizes the encapsulated drug and may also modify its physical properties. Moreover, the interaction with macromolecular physiological components needs to be explored to understand and control mechanisms of action and therapeutic efficacy. This review focuses on such non-covalent interactions of porphyrinic drugs with synthetic polymers as well as with biomolecules such as phospholipids or proteins. A brief introduction into various NMR spectroscopic techniques is given including chemical shift perturbation methods, NOE enhancement spectroscopy, relaxation time measurements and diffusion-ordered spectroscopy. How these NMR tools are used to address porphyrin–macromolecule interactions with respect to their function in biomedical applications is the central point of the current review.
Keywords: porphyrin, NMR spectroscopy, interaction, phospholipids, proteins, nucleic acids, drug delivery, polymer, cyclodextrin, surfactant, micelles
1. Introduction
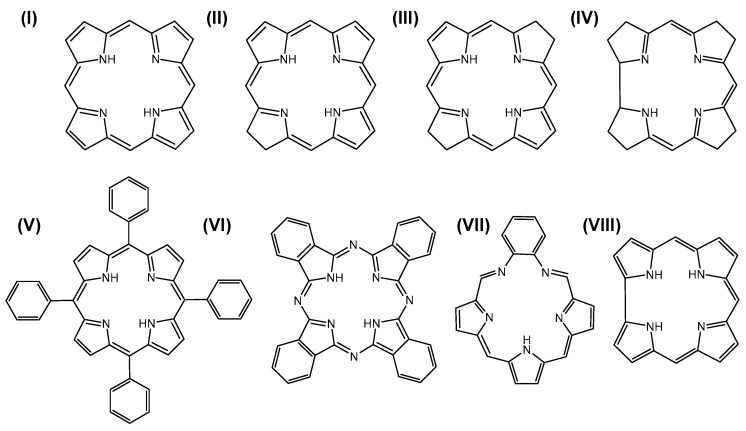
Porphyrinic compounds stand out among the organic molecules found in nature due to their unique properties associated with their common scaffold, a planar macrocycle consisting of four pyrrole rings linked by methine bridges [1]. The tetrapyrrole ring system forms a cavity that can accommodate many different metal ions, typically forming bidentate complexes that give rise to the widespread metalloporphyrins [2,3]. It is remarkable that a wide range of living systems including animals, plants and microorganisms make use of this common concept for fulfilling key functions in biological processes [4,5,6]. The major classes of naturally occurring porphyrinic compounds can be subdivided into porphyrins (I) in a narrower sense (often the whole group of porphyrinic compounds is referred to as porphyrins), chlorins (II), bacteriochlorins (III), and corrins (IV) (Figure 1) [3,4]. Heme forms the iron complex of protoporphyrin IX (PPIX) and its protein complex hemoglobin is the major constituent of red blood cells, imparting them their red color. The ability of hemoglobin and myoglobin to bind molecular oxygen plays an important role in oxygen transport and storage in living systems. Heme also functions as cofactor or prosthetic group in a vast number of hemoprotein-based enzymes such as the cytochromes that are involved in electron transfer and catalytic reactions [7,8]. Chlorins are partly reduced dihydro-porphyrins and their magnesium complexes form the core structure of chlorophylls, rendering the green color to plants [4]. The chlorophylls are part of the light-harvesting complexes of all photosynthetic organisms and thus fulfill one of the most important functions in life with their ability to use solar energy and transferring it to reaction centers so that molecular oxygen can be formed [9,10]. In bacteriochlorins, two opposite pyrrole rings are reduced, leading to tetrahydro-porphyrins (two adjacently reduced pyrrole rings yield isobacteriochlorins). They are part of bacteriochlorophylls in anoxygenic phototropic bacteria in the form of their Mg or Zn complexes [9,11]. Finally, the corrins have to be pointed out as modified tetrapyrroles whose Co(III) complexes forming vitamin B12 (cobalamin) are part of key metabolic enzymes [12].
Figure 1.
Structures of porphyrins (I), chlorins (II), bacteriochlorins (III), corrins (IV), tetraphenylporphyrins (V), phthalocyanines (VI), texaphyrins (VII), and corroles (VIII).
The many different aspects of porphyrins such as synthesis, coordination chemistry, biochemistry, and applications have been compiled in a detailed compendium of numerous volumes devoted to this class of compounds, “The Porphyrin Handbook” [13]. Owing to their unique electronic structures, redox-, photochemical and photophysical properties, porphyrinic compounds have gained much interest in a broad range of applications in technology and biomedicine. Technical applications mainly rely on the capability of porphyrins for energy conversion, making them attractive materials for photovoltaic systems, photocatalysts and energy storage systems [14,15,16].
The most prevalent applications of porphyrinic compounds can be found in the biomedical field [17,18,19]. This is mainly due to the fact that porphyrins combine many advantageous properties such as light absorption in the visible and near-infrared wavelength region, intense fluorescence, ability to form toxic singlet oxygen from excited electronic triplet states following light irradiation (phototoxicity), preferential accumulation in tumor tissue, low dark toxicity, stability under physiologic conditions, and their ability to form complexes with various metal ions [20,21]. In addition, the tetrapyrrolic scaffold offers many possibilities for structural modifications, for example by introducing side chains onto the macrocycle, different metal ions into the core and expanding or reducing the ring system in order to tune the properties according to the desired features [22,23]. In addition to the naturally derived porphyrinic compounds given in Figure 1 (I–IV), the classes can be expanded towards modified synthetic derivatives such as the tetraphenylporphyrins (V) [24], phthalocyanines (VI) [25], porphyrins with extended ring systems such as texaphyrins (VII) [26], and corroles (VIII) [27] (Figure 1). Porphyrin-based drugs are used in both therapeutic and diagnostic areas [19]. For many decades now, the phototoxicity of porphyrins has been used in photodynamic therapy (PDT) of various oncologic and non-oncologic diseases [28] and PDT is the most important medical application of porphyrins to date. In PDT, the phototoxic reaction that leads to a selective tissue destruction is based on the formation of singlet oxygen and reactive oxygen species (ROS) via light excitation of the porphyrinic photosensitizer (PS) in the target tissue [21,29]. The same mechanism is also applied in the treatment of inflammations inactivating microorganisms in antimicrobial PDT [30,31]. Photodynamic diagnosis (PDD) measures the fluorescence of the PS enriched in tumor tissue or lesions [32,33,34]. Further imaging techniques make use of specific paramagnetic or radiolabeled metalloporphyrins as contrast agents for magnetic resonance imaging (MRI) [35,36], and positron emission tomography (PET) [37,38] or near-infrared (NIR)-absorbing porphyrins for photoacoustic imaging (PAI) [39,40] of tissue. Concomitant with the development of multimodal drugs, more recently, porphyrins are investigated as potential theranostics combining therapeutic and diagnostic functions [19,41,42]. Finally, the application of porphyrinic compounds in drug delivery should be mentioned in connection with the design of smart, stimuli-responsive platforms for controlled drug release. This includes for example photochemical internalization (PCI) [43,44] or porphyrin-based metal–organic frameworks (MOFs) [45]. Nanoplatforms have become an inevitable part in the application of porphyrinic drugs for solubilization, enhancing their efficiency and in vivo stability, and for passive and active tumor targeting [46,47,48,49]. Different strategies are applied in drug delivery of porphyrinic compounds, ranging from chemical binding to carrier or targeting molecules to physical entrapment into nanoparticles (NPs). Suitable NPs approved or under investigation consist of inorganic compounds [50], biodegradable carriers such as liposomes [51,52,53] or block copolymer micelles (BCMs) [54,55], molecular networks such as polyvinylpyrrolidone (PVP) [56,57] or cyclodextrins (CDs) [58,59] and numerous other materials [47,49]. In this vast range of biomedical applications, porphyrins are constantly exposed to macromolecules, either by the building blocks of their carrier materials or by the in vivo biological components such as plasma proteins or phospholipids in membranes. The interactions with these surrounding macromolecules form an important aspect as they often determine the stability, in vivo fate, efficiency and mechanisms of action of the porphyrinic agent. Analytical methods are therefore essential to address the different facets of interactions and nuclear magnetic resonance (NMR) spectroscopy can make an important contribution to this. To limit the scope of the current review, the focus lies on porphyrin derivatives investigated as medical drugs.
In general, most spectroscopic methods are well suited for porphyrin characterization. However, owing to their specific aromatic structure, porphyrins are predestined for spectrophotometric methods. The highly conjugated macrocyclic aromatic core of porphyrin molecules gives rise to strong light absorption with the characteristic Soret and Q-bands in the electron absorption spectra. From excited electronic states, radiative relaxation processes typically yield intense porphyrin fluorescence in the wavelength region of 620–700 nm [60]. Although the high sensitivity of the corresponding optical spectrometric instruments is beneficial for the analysis of porphyrin solutions at concentrations in the nano- and micro-molar range, at which most porphyrinic compounds exist in monomeric form, the structural and dynamic information important for comprehensive understanding of the interactions within the host–guest ensemble on a molecular or atomic level is rather limited.
NMR spectroscopy was discovered in 1946, and was introduced as a routine analytical tool in chemistry approximately 15 years later [61]. The first NMR investigations on porphyrin molecules were reported in 1959 [62] and since then the use of NMR for investigating porphyrin molecules steadily increased and continues to increase nowadays. Technical (hardware, computers) and methodological developments (democratization of 2D NMR spectroscopy and of pulsed field gradients (PFGs), new NMR pulse sequences) and technological breakthroughs (cryogenically cooled magnets and probeheads) have advanced NMR spectroscopy to a powerful analytical tool with increasing sensitivity and ability to address structural and dynamic questions in recent years. Therefore, these days, NMR spectroscopy is undoubtedly one of the most important analytical tool to study the structure of porphyrinic compounds and their metal complexes at the atomic level [63].
Our aim with this review is to provide the readers with an overview of several NMR techniques applied for characterizing biomedical porphyrinic compounds, focusing on their interaction with biological and synthetic macromolecules in solution. Essentially, these investigations are undertaken with the aim of getting deeper insights into the underlying mechanisms of interactions of these porphyrinic compounds. In particular, we wish to point out how NMR spectroscopic methods have contributed to the current knowledge in this field and why NMR spectroscopy is unique and complementary to other spectroscopic techniques. In Section 1, the most important NMR parameters and methods are briefly described with a short theoretical background. In Section 2, applications of these methods are presented in the context of porphyrins interacting with biomolecules such as proteins, phospholipids, or nucleic acids and with polymeric carrier systems. This overview is not exhausting but will rather point out the wide range of applications and the versatility of NMR spectroscopic methods in the field.
A list of abbreviations in alphabetical order is given in Appendix A at the end of this review.
2. NMR Parameters and Methods for Studying Porphyrinic Compounds and Their Surroundings
2.1. NMR Basics
NMR spectroscopy is a non-invasive technique for qualitative and quantitative analysis of a vast number of compounds. Similar to other spectroscopic methods, NMR spectroscopy finds its roots in the interactions between matter—specifically atomic nuclei with an active nuclear spin—and radiation—specifically electromagnetic fields. When a nuclear spin is placed into a strong static magnetic field (in an NMR spectrometer), the response of the spin polarization is to move around the field, at a specific precession rate, the Larmor frequency ν0. This Larmor frequency ν0,i of a given nucleus i depends on the external magnetic field B0 and the gyromagnetic ratio γi, a nucleus-specific constant which correlates the magnetic moment of a nucleus to its angular momentum (Equation (1)). If we consider a sample with N nuclear spins, a stable anisotropic distribution of nuclear spin polarizations gradually takes place (the buildup and decay of longitudinal spin magnetization follows an exponential process, governed by T1, the longitudinal relaxation time) and leads to a small net magnetic longitudinal moment (Mz) along the field B0 (z-direction). Since this longitudinal nuclear spin magnetization is nearly undetectable, the magnetization perpendicular (Mx or My) to the external field is measured. A perpendicular magnetization is obtained by applying a radiofrequency (RF) pulse of appropriate frequency ν1, equal to the Larmor frequency ν0 of the nuclei that one intends to observe, and of appropriate duration [64].
| (1) |
One of the strengths of NMR spectroscopy is that the Larmor frequency ν0,i of a given nucleus i or its chemical shift δi does not actually depend on the external magnetic field B0, but on the effective field Beff, which is the external magnetic field B0 corrected by a contribution σi (shielding constant), a measure of the shielding of the nucleus i originating from the movement of the surrounding electrons (Equations (2) and (3)).
| (2) |
| (3) |
As such, for a given molecule, the different shielding constants σi and thus the different Larmor frequencies ν0,i are detectable by the NMR spectrometer [65,66] and give rise to NMR spectra in which all non-equivalent nuclei appear with distinguishable resonance frequencies.
In practice, the measurement and labeling of the absolute frequencies have rapidly appeared as unpractical, since they depend on the external magnetic field B0. Therefore, a new, B0-independent parameter, the chemical shift δ, was introduced. Indeed, the chemical shift δ is the relative deviation (offset) from the reference (onset) frequency with respect to the reference frequency, given in ppm (Equation (4)). In proton and carbon spectra, the chemical shift values are referenced to the resonance of tetramethylsilane (TMS, δ = 0 ppm) for organic solvents or alternatively of 2,2-dimethyl-2-silapentane-5-sulfonate (DSS, δ (Trimethylsilyl) = 0 ppm) for aqueous solutions.
| (4) |
In addition to the chemical shift, the scalar-scalar or indirect coupling J and the direct or through-space coupling (Nuclear Overhauser Effect, NOE, see Section 2.3) between nuclear spins provide additional valuable information on the chemical environment of a given nucleus. Specifically, the J-coupling splits the resonance (multiplicity) of the observed nucleus according to the number of NMR active nuclei present in the direct vicinity (commonly up to five chemical bonds, but possible up to seven bonds). Additionally, the value of the coupling constant J can provide useful information about the structure and the stereochemistry of the investigated molecule. The most useful coupling constants are undoubtedly the vicinal (3J) coupling constants. Their values are dependent on the dihedral angle Θ, and for protons in a typical H1-X-X-H2 fragment, range from ~0 Hz (Θ ~ 90°) to ~15 Hz (Θ ~ 0° or 180°), following the Karplus equation [67,68,69]. This relationship is valuable for determining the stereochemistry of molecules, particularly of sugars, and for determining the backbone torsion angles in protein NMR studies.
Thus, relying on the NMR phenomena important parameters can be obtained for each NMR active nucleus (spin quantum number ≠ 0) within a molecule such as the chemical shift δ (a normalized measurement of the Larmor frequency ν0, independent of the external magnetic field B0), the scalar coupling J or through space spin polarization transfer (NOE).
2.2. Induced Spectral Perturbation
2.2.1. The Porphyrin Ring Current Effect
The overall shielding constant σ for each group described in Equation (3) is formed as the sum of the following contributions (Equation (5)):
| σ = σdia + σpara + σR + σext | (5) |
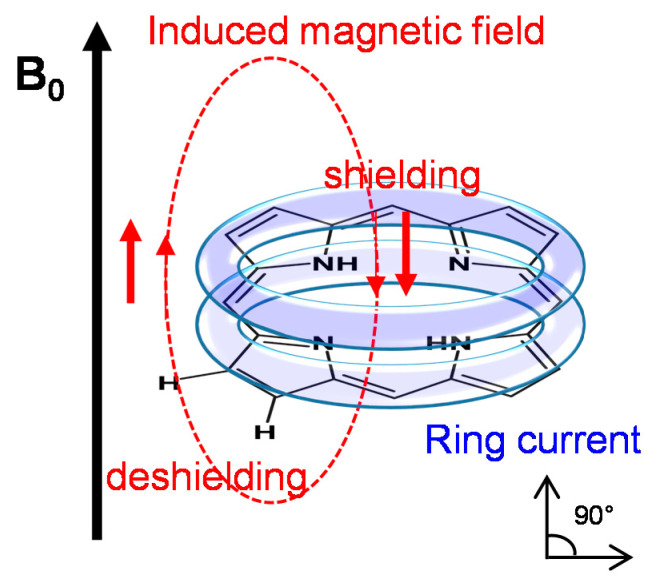
The diamagnetic contribution σdia derives from the electron distribution in spherically symmetrical orbitals surrounding the nucleus (s orbitals). The paramagnetic shielding term σpara arises from magnetic fields generated by non-spherically distributed electrons. It originates from excited electronic states that appear upon interaction of the electron orbitals with the applied magnetic field and generally becomes more important for other non-proton nuclei like, e.g., in 13C-NMR [65,66]. Of particular interest for NMR spectra of porphyrins and interacting molecules are the ring current term σR and the external (intermolecular) shielding term σext derived from interactions with the neighboring molecules. Local magnetic anisotropy and unusual chemical shifts for the nuclei in the porphyrin core are well described by the ring current model. Mainly, protons inside (in the center) or above the porphyrin ring are in the shielding region while the protons placed in the porphyrin plane periphery are in the deshielding region of the ring current effect (Figure 2) [70]. These interactions, which are diamagnetic in origin, are generated by the neighboring groups in the vicinity such as the delocalized π-systems or the carbonyl groups and depending on the orientation and distance regarding the observed core can produce shielding or deshielding contributions [70].
Figure 2.
Scheme of porphyrin ring current.
This concept of ring current effect was introduced by Pauling, elaborating the appearance of anisotropic magnetic susceptibility of the benzene core, ascribing the magnetic anisotropy to the ring current effect, i.e., to induced circulation of the delocalized π- electrons by the magnetic field [71]. Further, empirical description and application of the ring current model for the NMR calculations (chemical shift) were reported by Pople, explaining the unusual chemical shift of the cyclic aromatic molecules compared to the alkanes [72]. Later, the shortfalls of the Pople (dipolar) model were overcome with the ring current models reported by Fassenden and Waugh [73,74] and Johnson and Bovey [75], modelled in terms of electron flow in loops located above and below the aromatic plane. Recent models use either five-loop models (four for the pyrrole ring and one for the macrocycle) or eight-loop models (the four pyrrole rings and the four hexagons between the central metal atom, neighboring pyrrole atoms and the meso position). Current models are approximations of either the current loop model of Johnson and Bovey [75] or the dipole model developed by Abraham et al. [76,77,78] and they are in good agreement to the observed NMR shifts.
2.2.2. Induced Changes onto the NMR Spectrum of the Porphyrin
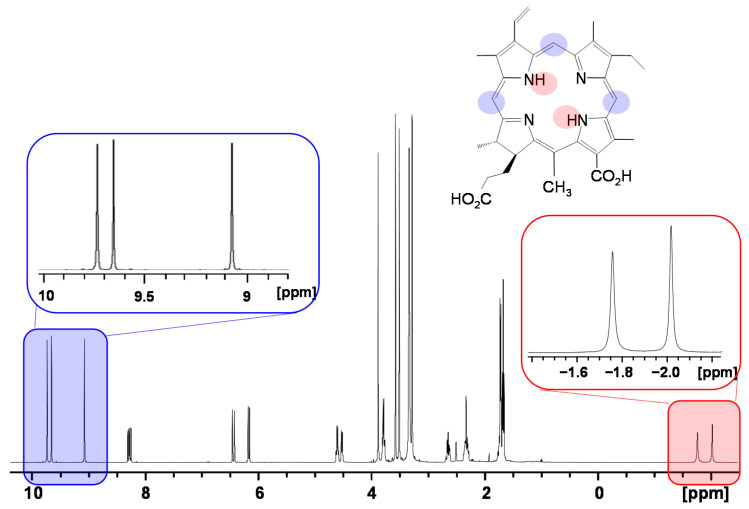
As outlined above, owing to their unique structure porphyrinic compounds give rise to unusual 1H NMR spectra in solution [79]. The ring current effect induces a large spread of the 1H NMR resonances shifting the inner NH-resonances of free-base porphyrins upfield to values of approximately −2 ppm and the pyrrole- or meso proton resonances located in the periphery of the macrocycle plane downfield to values of approximately 8 ppm and 9–10 ppm, respectively (Figure 3). Since most synthetic polymers as well as bio-macromolecules such as polysaccharides and lipids are devoid of aromatic protons, this spectral region is of specific diagnostic value when studying interactions due to non-overlapping NMR resonances.
Figure 3.
1H NMR spectrum of chlorin e4 in DMSO-d6.
In addition to the local magnetic anisotropy that occurs in the frame of a single molecule, the ring current effect also profiles the local magnetic field of the adjacent molecules due to existence of intense intermolecular π–π interactions. Porphyrins exist as monomeric species mostly in unpolar or polar aprotic solvents such as dimethylformamide (DMF) or dimethylsulfoxide (DMSO) and give rise to sharp 1H NMR resonances (Figure 3). However, in aqueous solution porphyrin self-aggregation is a well-known phenomenon. The main cause is mostly attributed to π–π interactions between the porphyrin macrocycles and the geometry of the resulting aggregates is determined by the charge distribution in the π system according to the well-known model by Hunter and Sanders [80]. However, substituent effects can also be the dominant forces for porphyrin aggregation. In the early eighties solution structures of porphyrin aggregates have been extensively studied by Abraham, Smith and coworkers applying theoretic models based on ring current-induced NMR aggregation shifts [76,81,82]. Three-dimensional aggregate structures, e.g., formation of J- or H-aggregates, interplanar distances, orientation, and aggregation maps could be deduced form 1H NMR aggregation shift data [83,84]. Thus, depending on the aggregation extent and the magnitude of intermolecular interactions, a single observed nucleus can give rise to resonances at different Larmor frequencies. Therefore, the interpretation of porphyrin NMR spectra and structural elucidation need to be approached with caution. In addition to the large induced shifts, there is a significant effect on the linewidths of the NMR resonances. Both changes in porphyrin ring current-induced shifts (RISs) and line broadening offer measures for interactions with macromolecules.
2.2.3. Induced Changes onto the NMR Spectrum of the Macromolecule
The electron density around the nucleus can be increased or decreased through interactions via chemical bonds or directly through space. The latter type of interaction (through space) allows the detection of nuclei from other molecules in proximity by perturbation of the NMR spectrum of the compound of interest. Monitoring the chemical shifts of the macromolecule (host) upon titration with the small guest molecule provides thus a simple method to probe for intermolecular interactions resolved to molecular segments or even atoms. This chemical shift perturbation (CSP) technique has been widely used for mapping protein-binding sites of ligands [85,86]. The larger the impact of the chemical shift perturbator, the more sensitive is the detection power of the CSP method. The magnitude of perturbation depends on the distance r between the interacting species (function of r−3) and on the physical properties of the small molecule being added. Enhancement of induced changes can be achieved for example by paramagnetic molecules [87] such as lanthanide shift reagents [88] or by molecules with a pronounced magnetic anisotropy as it is encountered in porphyrins [89]. The ring current effect provides a “built-in chemical shift reagent” [79] allowing for a very sensitive detection of interactions with nearby molecules. Based on RISs the spectral perturbation of surrounding macromolecules gives information about sub-molecular sites of interaction and on the relative orientation of the porphyrin macrocycle plane towards its binding partner.
2.3. Nuclear Overhauser Enhancement Spectroscopy
The NOE allows detecting through space interactions dij between nuclear spins in close proximity and describes the transfer of polarization between nuclear spin populations, mostly 1H, via the so-called cross-relaxation phenomenon. In spectra, the NOE manifests itself as the change in the integrated intensity (positive or negative) of one NMR resonance that occurs when another is saturated by irradiation with an RF field. This change in resonance intensity of a nucleus is a consequence of the nucleus being close in space to those directly affected by the RF perturbation [90]. The NOE experiment is therefore a unique method which depends on the spatial proximity between nuclei and measures dipolar couplings as opposed to other NMR techniques which mostly measure scalar couplings (J-couplings) among the spins connected through chemical bonds. It can be run as one-dimensional experiment with selective saturation of a single resonance or as two-dimensional experiment (NOE SpectroscopY, NOESY) covering the whole spectral range with the corresponding 2D NOESY pulse sequence [91,92,93]. In small molecules, NOEs may be observed between spins which are up to 4 Å apart [94,95], while the upper limit for large molecules is approximately 5 Å [96]. For large molecules, care must always be taken to avoid the so-called spin-diffusion regime, during which polarization may continue to propagate diffusively among spins until the extra polarization is lost to the lattice (thermal motions) via the spin-lattice relaxation (T1) process. While being useful for several applications, one disadvantage of spin diffusion is that the size of the observed NOEs is no longer dependent on the spatial proximity between the spins [90,92]. To avoid spin diffusion effects, truncated NOE or transient NOE experiments are performed at short saturation or mixing times enabling to measure NOE build up while minimizing the indirect NOEs [90]. Furthermore, the magnitude and sign of the NOE are determined by the correlation time τc, which in turn is a function of the molecular weight (MW). For small molecules the NOE is positive, whereas, for large molecules, it becomes negative. Consequently, at room temperature (298 K) and for intermediate NMR spectrometers, compounds with an intermediate MW of approximately 1000 Da possess a correlation time close to τc = 1/ω0 (with ω0 being the circular resonance frequency), for which the NOE is very weak or can even be zero. In these cases, the rotating frame NOE spectroscopy (ROESY) experiment must be used, as the ROE is always positive; it ranges from maximal 35% (small molecules) to 65% (large molecules) and amounts ~50% when the corresponding NOE is 0 at τc = 1/ω0 [97,98,99].
In summary, the NOE plays an important role in the assignment of NMR resonances and in the determination of the inter- or intra-molecular distances between spins. It is especially useful in the elucidation of structures, steric conformations of organic and biological molecules or host–guest interactions.
2.4. NMR Relaxation Times (T1 and T2)
NMR relaxation phenomena describe the processes by which excited nuclei return to their equilibrium (ground state) distribution. These exponential decay processes can be described and measured using the longitudinal or spin-lattice (T1) and the transverse or spin–spin (T2) relaxation times that refer to the recovery of magnetization parallel and decay to zero perpendicular to the direction of the external magnetic field B0, respectively. The relaxation times of nuclear spins depend on dynamic properties of the corresponding molecules and their interaction with the immediate environment [66]. In longitudinal relaxation (T1), energy can be transferred to the environment (“lattice”) of the corresponding nucleus by different relaxation mechanisms that can be of paramagnetic, dipolar, or chemical shift anisotropy (CSA) origin [64]. For example, the principle of MRI contrast agents including metalloporphyrins is based on the paramagnetic relaxation enhancement of nearby proton nuclei (shortening of T1 relaxation time) in biological tissue [36]. T2 relaxation concerns the loss of transverse magnetization or phase coherence of spins that can be caused through spin–spin interactions and fluctuating magnetic fields. These in turn depend on molecular size and tumbling (Brownian motion), which is characterized by the rotational correlation time τc. For macromolecules, molecular motion is slow and τc is large, leading to efficient spin–spin relaxation, i.e., short T2 relaxation times (while the T1 relaxation time goes through a minimum with increasing τc). On the other hand, small fast tumbling molecules with small τc have slow relaxation rates (both T1 and T2 relaxation times are similar and high) [64].
The relationship between T2 and τc is of particular value for describing the interactions between small and large molecules. Thus, the interaction of porphyrins with biomolecules or the polymeric encapsulation of a porphyrin molecule will lead to a shortening of the spin–spin T2 relaxation times and can be easily monitored. However, a serious drawback is associated with this interesting property of T2 relaxation times: Since the observed linewidths of the resonances are directly proportional to the inverse of T2 (linewidth at half-height ν1/2 = 1/πT2), porphyrins interacting with biomolecules exhibit very broad lines, which reduces sensitivity and can make it very difficult to observe resonances.
2.5. Diffusion-Ordered Spectroscopy
The fact that molecular diffusion and diffusion coefficients can be easily studied by NMR methods was realized in the early days of NMR spectroscopy. The easiest and most practical pulse sequence for measuring diffusion coefficients by NMR spectroscopy is the PGSE (Pulsed Gradient Spin Echo) sequence, introduced by Stejskal and Tanner in 1965 [100], actually long before the introduction of 2D NMR spectroscopy. Diffusion NMR measurements have increasingly been used, and the possible applications in solution were summarized in many semantical reviews [101,102,103]. Briefly, the diffusion coefficient, in accordance to the Stokes–Einstein equation (Equation (6)), allows the determination, mostly an approximation, of the hydrodynamic radius, i.e., solvation shell and the size of molecules in solution
| (6) |
where kB is the Boltzmann’s constant; T the absolute temperature; η the dynamic viscosity; and r the radius of the spherical particle.
In 1992, it was realized that two-dimensional diffusion spectra can be obtained by incrementing the areas of the gradient pulses in PFG-NMR experiments and transforming the NMR signal amplitudes with respect to the square of this area, resulting in an experiment known as diffusion-ordered NMR spectroscopy (DOSY) [102,104]. DOSY experiments can resolve multiple components based on their different diffusion coefficients giving rise to pseudo-2D spectra correlating the chemical shift (1D spectra) of the components given on the abscissa with the corresponding diffusion coefficients projected on the ordinate. Diffusion coefficients represented on the ordinate are calculated according to the known Stejskal-Tanner equation [100] (Equation (7))
| (7) |
where I0 and I are the initial and attenuated NMR signal intensities, D the translational diffusion coefficient, γ the gyromagnetic ratio, g the gradient strength, δ the gradient pulse length and Δ the diffusion time. The DOSY experiment has become a well-established and useful technique for investigating multicomponent mixtures by disentangling the components according to MW and size [105,106,107,108]. Most importantly for the subject of the current review, DOSY can detect intermolecular interactions and the formation of supramolecular systems [101,108,109,110]. This includes homo-association of same molecules as for example in polymeric micelles [103,111] or multi-porphyrin assemblies [112,113] as well as hetero-association of different molecular species. The latter is often applied to monitor the binding of small molecules to larger polymer or protein hosts through alterations in the corresponding diffusion properties. DOSY can thus provide valuable information on complex formation such as the encapsulation of small drugs into polymeric delivery systems [108,114,115]. Here, also, note that the very short T2 relaxation times of embedded porphyrins and the resulting broad resonances can make it very difficult to record such diffusion experiments.
2.6. Heteronuclear NMR Spectroscopy
In addition to the most common observation of 1H nuclei in NMR spectroscopy of solutions, there are numerous NMR-active nuclei across the periodic table that can be measured [116,117]. The prerequisite for nuclei to be observable by NMR is that their spin quantum number I is unequal to zero, which is the case for all nuclei with an odd number either of both or of the sum of protons and neutrons. Moreover, the suitability of heteronuclear NMR spectroscopy depends on the sensitivity of detection for a given nucleus that is determined by its natural abundance, gyromagnetic ratio and spin quantum number. While nuclei with an asymmetric charge distribution (quadrupolar nuclei, I > ½) often give rise to broad NMR resonances, nuclei with half integer spins I = ½ are best suited as for example 1H, 13C, 15N, and 31P [118]. Since these atoms (H, C, N, and P) belong at the same time to the main constituents in biological material, the corresponding nuclei are most useful in NMR applications of biomolecules such as proteins [119], lipids [120], and carbohydrates [121]. To compensate the low natural abundance of 13C (1.1%) or of 15N (0.36 %) [116], isotope labeling is often applied, i.e., the enrichment with the corresponding NMR-active nucleus. In addition, the development of NMR hardware (magnets and probeheads) has achieved significant increase in sensitivity for nuclei such as 13C or 15N [122].
In the study of small molecules interacting with macromolecules, it can be very useful to monitor NMR-active-sensitive nuclei such as 19F (I = ½) that are not endogenous in biological soft tissue [123], since, compared to 1H, the chemical shift range of 19F and hetero-nuclei is much larger. For porphyrinic compounds, fluorine substitution [124] or NMR observation of the central metal nuclei in metalloporphyrins [125] thus allows the selective detection of the porphyrin in mixtures with biological or other polymeric compounds without the overload of background signals deriving from the macromolecules.
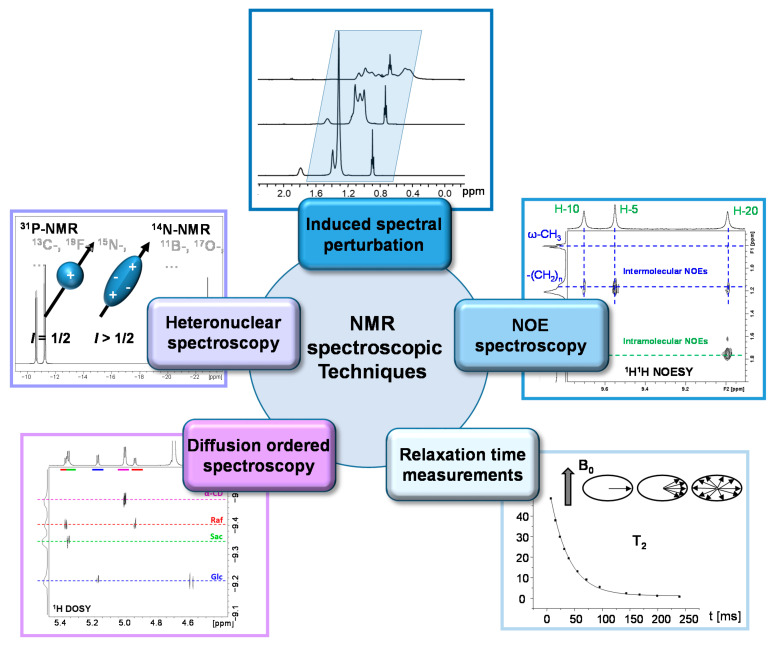
In Figure 4, an overview of the NMR methods explained in Section 2.2, Section 2.3, Section 2.4, Section 2.5 and Section 2.6 is shown. The selected experiments are not exhaustive but represent some of the most frequently applied NMR methods in the study of porphyrinic compounds interacting with macromolecules.
Figure 4.
Overview of selected NMR techniques to study porphyrin–macromolecule interactions.
3. Applications to Study Porphyrin–Macromolecule Interactions
3.1. Biomolecules
3.1.1. Phospholipids (Membrane Models and Liposomal Drug Delivery Vehicles)
Phospholipid membranes belong to the preferential localization sites of porphyrinic PSs in living systems [126,127]. Therefore, interactions with membrane models have been of great interest in the context of PDT. Small unilamellar vesicles (SUVs) composed of phospholipids (PLs) have been used as suitable simplified membrane models providing access to PL-bilayers for solution NMR studies. SUVs are easy to prepare and are small (< 50 nm), fast tumbling systems with some PL mobility within the bilayer that typically give rise to relatively well-resolved proton resonances [128,129]. However, tumbling and in-bilayer mobility are often not fast enough for sufficient averaging of dipolar coupling and direct NMR visibility of small molecules interacting with the PLs. Another contribution originates from strong resonance broadening due to exchange processes between free and PL-bound molecules at intermediate rates on the NMR time scale. The exchange broadening can be reduced by modifications of the conditions (pH, concentration ratios) in solution that alter the exchange kinetics resulting in enhanced NMR visibility of porphyrins associated with PLs in SUVs [130].
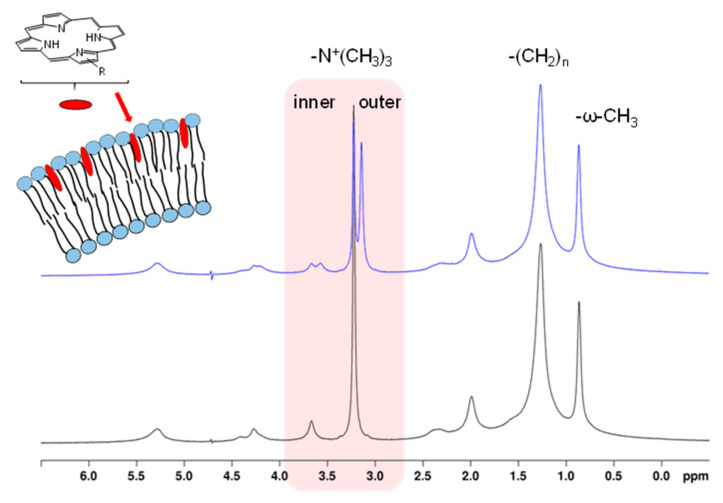
Moreover, indirect changes on the PL resonances such as chemical shift perturbation can provide valuable information on the interactions between porphyrinic molecules and PL-bilayers. Interactions of amphiphilic chlorin e6 (Ce6) derivatives with SUVs consisting of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) resulted in pronounced upfield shifts and splits of the DOPC choline 1H resonances due to the porphyrin ring current effect of Ce6 molecules nearby the outer PL bilayer head groups (Figure 5) [131]. This allowed not just the NMR detection of porphyrin membrane association but also of the preferential localization sites along the PL molecules as well as the discrimination between outer and inner PL layers (Figure 5) similar to the impact of lanthanide shift reagents [132]. The magnitude of the N-methyl choline resonance split correlated with chlorin concentration up to a level where saturation was reached. The possibility to distinguish between the outer and inner PL monolayers allowed monitoring slow transmembrane kinetics of the Ce6 derivatives by convergence of the split choline resonances over time, which was described as a flip-flop process [131]. The transmembrane kinetics were shown to be strongly pH-dependent for Ce6 derivatives with ionizable carboxylic side chains [133]. Protonation of the carboxylate groups significantly accelerated the transmembrane distribution, whereas Ce6 derivatives bearing carboxylate groups with low pKa values were retained in the outer monolayer supported by electrostatic interactions. Accordingly, individual pKa values of acidic substituents had a special importance for membrane translocation and could be determined by 13C NMR chemical shift titration of the chlorin carboxylate groups [133]. The indirect NMR chemical shift perturbation analysis of 15 different porphyrinic compounds interacting with DOPC vesicles was used to make a classification according to four differently induced NMR patterns of the DOPC -N+(CH3)3- -(CH2)n-, and -ω-CH3-resonances and their time evolutions. From this a relationship between porphyrin structure and type of PL bilayer interaction was proposed where symmetry of substitution, amphiphilicity and overall lipophilicity of the porphyrinic compound were the key factors governing membrane interactions [134]. Another NMR study based on ring current-induced shifts onto PL choline resonances revealed that the porphyrin aggregate structure formed in aqueous solutions has a significant contribution to the initial association with the bilayer surface that is supported by the free accessibility of charged or polar substituents [84].
Figure 5.
1H NMR spectrum (500 MHz) of DOPC SUVs in water before (bottom) and after (top) addition of a Ce6 derivative. The interaction of the chlorin with the DOPC bilayer induces a split of the DOPC choline resonances so that the outer and inner PL layers become distinguishable.
Unilamellar PL vesicles, also called liposomes, have not just been used as membrane models but also as liposomal delivery vehicles for lipophilic porphyrinic PSs [135,136,137]. Ikeda and coworkers have used 1H NMR spectroscopy to monitor the successful encapsulation of a series of tetraphenylporphyrins (TPPs) into the bilayer of DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) or egg- phosphocholine (PC) liposomes. They applied an exchange method for improved liposomal drug loading based on a transfer of the porphyrin from a 1:2 CD complex to the liposome. While TPPs forming inclusion complexes with CDs give rise to relatively sharp 1H NMR resonances in the aromatic spectral region (see Section 3.2.2), disappearance due to strong resonance broadening upon membrane intercalation (reduced mobility) was used as indicator for successful transfer, which was best for lipophilic TPPs [138,139]. Here, the indirect chemical shift perturbation of the PLs evaluating the relative upfield shifts of the -N+(CH3)3-, -(CH2)n-, and -ω-CH3-resonances was also used to determine the location of the TPPs in the PL bilayer [139].
3.1.2. Proteins
Numerous NMR spectroscopic studies have been applied and reported in the literature for elucidating the structure and function of porphyrin-containing proteins such as hemoglobin, cytochromes and chlorophylls. Early studies of heme proteins have made use of the large hyperfine shifts of paramagnetic metal complexes that are sensitive towards changes in their electronic environment [140]. However, as mentioned in the introduction, this review limits its scope to porphyrinic compounds used as biomedical agents.
For instance, Stojanovic et al. have investigated the interactions between the porphyrin ring and the protein part of porphyrin-containing proteins to better understand their stabilizing role. This study shows that stabilization centers are composed predominantly from nonpolar amino acid residues [141].
Klein-Seetharaman et al. showed using fluorescence and 1H and 19F NMR spectroscopy that Ce6 weakly binds to rhodopsin with μM affinity. Furthermore, numerous chemical shift changes in the 1H-15N NMR heteronuclear single quantum coherence (HSQC) spectra of 15N-Trp-labeled rhodopsin revealed that Ce6 binding perturbs the entire structure [142].
Two Ce6 derivatives and the barrier function of drug delivery systems towards binding to the serum proteins human serum albumin (HSA) and transferrin (Tf) were monitored by 1H NMR spectral appearance of the Ce6 resonances in the aromatic region. Chlorin association with HSA or Tf lead to severe resonance line broadening that was prevented by PVP encapsulation. Block copolymer micelles protected the chlorins from binding to Tf but released them in favor of binding to HSA [143].
3.1.3. Nucleic Acids (DNA, RNA)
Owing to their structures, porphyrins are prone to interact with DNA and RNA, and already in 1979 experimental evidence from binding isotherms, thermal melting profiles, and circular dichroism measurements showed that meso-tetrakis (4-N-methylpyridyl) porphine (TMPyP) binds to DNA by intercalation [144,145,146]. In 1983, Banville et al. showed that the intercalators TMPyP and Ni(II)TMPyP induced a broad downfield peak in the 31P NMR spectrum of DNA and a slight upfield shift of the main peak, while none of these characteristic changes were present in the NMR spectrum of DNA after treatment with the outside-binding porphyrin, Zn(II)TMPyP [147]. Several similar studies involving 1H and 31P NMR spectroscopy have been summarized by Fiel [148].
More recently, several studies have highlighted how double-stranded DNA or G-quadruplex DNA participates in the reaction with porphyrins, which have contributed to a better understanding of the chemistry of porphyrin models [149].
Another interesting application of NMR is the investigation of the modulation of the PS-quencher unit, which promotes the PDT development, by assessing their interactions with DNA [150,151]. For instance, Hirakawa et al. synthesized a series of water-soluble porphyrin derivatives that target DNA: meso-anthryl-tris(p-pyridyl)porphyrin (AnTPyP) [152], meso-pyrenyl-tris(N-methyl-p-pyridinio)porphyrin (PyTMPyP) [153], meso-(9-anthryl)-tris(N-methyl-p-pyridinio)porphyrin (AnTMPyP) [154], meso-(naphthyl)-tris(N-methyl-p-pyridinio)porphyrin (NapTMPyP), and TMPyP [155]. In addition to 1O2, these photosensitization processes could also generate other ROS. Both type I and type II photosensitization processes could occur, depending on the mode that the PS is bound to DNA, i.e., the distance between the PSs and base pairs.
In Table 1, the NMR applications to study porphyrin interactions with biomolecules discussed in Section 3.1.1, Section 3.1.2 and Section 3.1.3 are summarized.
Table 1.
Summary of NMR interaction studies between porphyrins and biomolecules.
| Porphyrin (Guest) |
Macromolecule (Host) |
NMR Technique | Result | Ref |
|---|---|---|---|---|
| Phospholipids | ||||
| Ce6 Ce6 derivatives |
DOPC-SUVs |
1H NMR chem. shift perturbation of host Time-dependent 1H NMR chem. shift perturbation of host |
Ce6 attached to PL-bilayer head group Transmembrane kinetics of Ce6 (flip-flop) pH dependence of kinetics |
[131,133] |
| Ce6, Ce6 derivatives PPIX, DPIX, HPIX and derivatives |
DOPC-SUVs | 1H NMR chem. shift perturbation of host | Porphyrin aggregate structure determines membrane interaction | [84] |
| Ce6 derivatives PPIX, DPIX, HPIX and derivatives TPP derivatives |
DOPC-SUVs |
1H NMR chem. shift perturbation of host Time-dependent 1H NMR chem. shift perturbation of host |
Different patterns of bilayer localization and transmembrane kinetics depending on porphyrin structure and substitution Patterns used for classification of membrane interactions |
[134] |
| TPP Zn-TPP |
DMPC liposomes | 1H NMR spectral appearance of guest | Transfer from CD complex to liposome | [138] |
| TPP | Egg-PC liposomes | 1H NMR chem. shift perturbation of host | Liposomal localization (hydrophobic core) | [139] |
| Proteins | ||||
| Ce6 | Bovine rhodopsin 19F-/15N-Trp-labeled rhodopsin |
1H-, 19F- and 15N-NMR chem. shift perturbation of host 1H-15N NMR HSQC 1H NMR spectral appearance of guest |
Weak binding of Ce6 to rhodopsin localized at cytoplasmic domain | [142] |
| Ce6 SerCe |
HSA Tf |
1H NMR spectral appearance of guest | Binding to both HSA and Tf PVP encapsulation prevents binding BCM encapsulation prevents only Tf binding |
[143] |
| Nucleic acids | ||||
| TMPyP, Ni(II)TMPyP, Zn(II)TMPyP | DNA | 31P NMR chem. shift perturbation of host | TMPyP, Ni(II)TMPyP intercalate, Zn(II)TMPyP binds to the outside of DNA | [147] |
| Cationic TMPyP derivatives | DNA | 31P-, 1H NMR chem. shift perturbation of host | Review: Three binding modes (intercalation, outside binding, outside binding with self-stacking) | [148] |
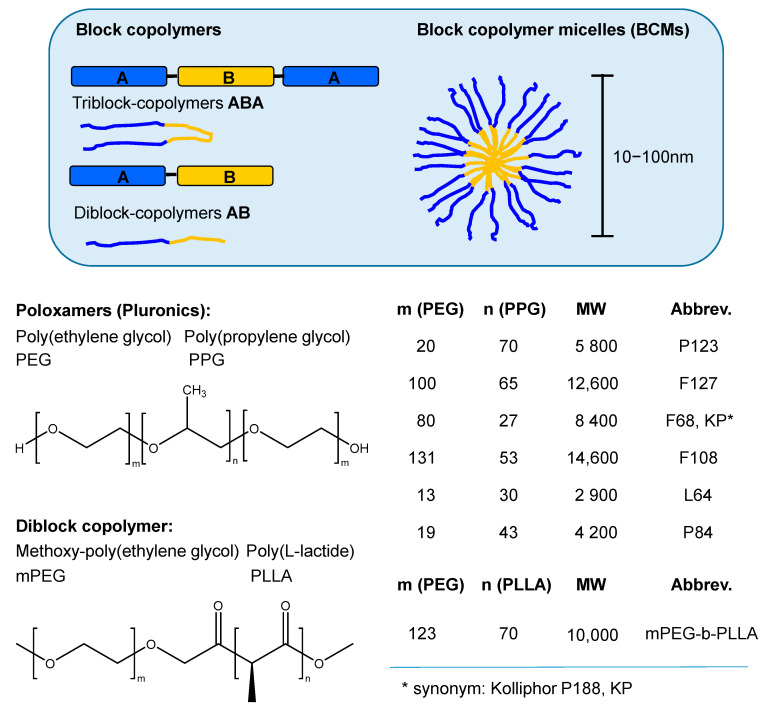
3.2. Carrier Polymers
Carrier polymers play an essential role as drug delivery vehicles in the formulation of porphyrinic drugs. NMR spectroscopy is an efficient tool to monitor the conjugation or physical entrapment of small molecular drugs into macromolecular carriers forming NPs. These polymeric delivery systems are mostly NPs with sizes up to 100 nm and their dynamic properties (molecular tumbling and internal motion) are sufficient that they usually give rise to 1H NMR spectra in aqueous solution with intense well-resolved resonances.
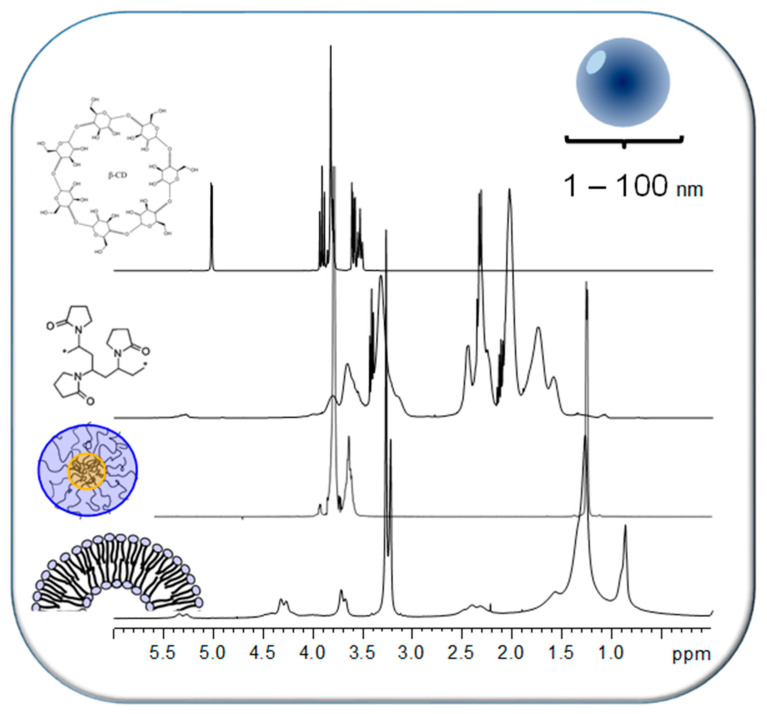
Figure 6 depicts representative examples including phospholipid SUVs, triblock copolymer micelles with polyethylene glycol (PEG) and polypropylene glycol (PPG) blocks, PVP and β-CD. A comprehensive review on the various NMR techniques useful to characterize nanosystems and their interactions with encapsulated drugs as well as with external biologically relevant ligands has been given by Lopez-Cebral et al. [156]. In the subsequent sections, the different polymeric carriers that have been investigated in combination with porphyrinic compounds applying NMR spectroscopic methods are discussed providing a brief overview of studies for each system.
Figure 6.
1H NMR spectra of selected polymeric nanoparticles used for drug delivery in aqueous solutions, from bottom to top: DOPC SUVs, triblock copolymer (PEG-PPG-PEG) micelles, polyvinylpyrrolidone (PVP) and β-cyclodextrin (CD).
3.2.1. Polyvinylpyrrolidone (PVP)
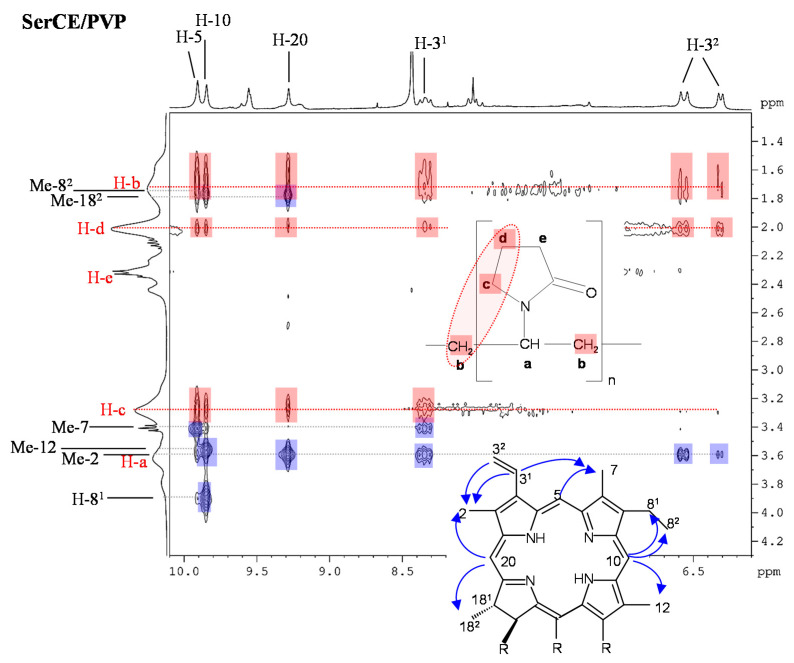
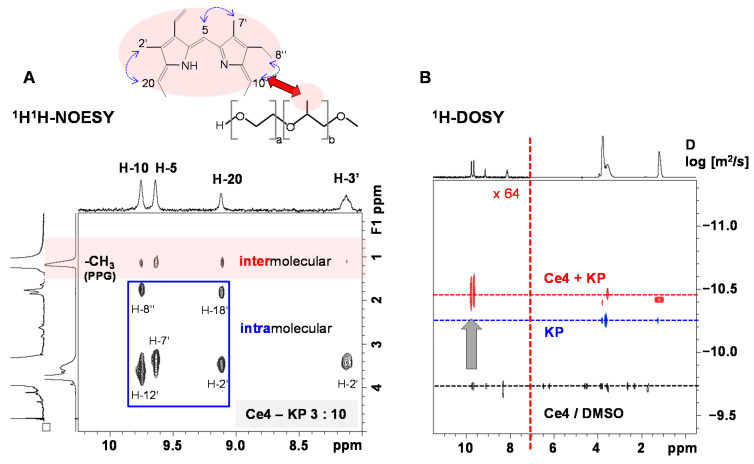
PVP is a widespread polymer used among numerous other applications [157] in the formulation of drugs because of its good water solubility, non-toxicity, inertness and high biocompatibility [158,159]. PVP exists with different MWs and degrees of cross-linking [157] and contains both hydrophilic and hydrophobic functional groups, giving rise to its pronounced versatility with respect to drugs that can be associated with PVP. It is being investigated for the formulation of various porphyrinic PSs. Among those, many studies have focused on the combination of Ce6 with PVP that showed significant enhancement of photodynamic efficiency compared to Ce6 alone and a Ce6–PVP conjugate was approved for PDT under the name Photolone [160]. To determine the origin of PDT enhancement of Ce6–PVP, the interactions between Ce6 and PVP have been analyzed in detail by spectroscopic methods including NMR spectroscopy [161,162,163,164]. In the presence of PVP, pronounced and selective downfield shifts of individual Ce6 1H NMR resonances were observed that indicated disaggregation of the chlorin upon interacting with PVP in aqueous solutions where Ce6 exists as aggregates in the absence of PVP. Chemical shift perturbation of the PVP resonances revealed that Ce6 mainly interacts with the hydrophobic vinyl-backbone of PVP [161,163]. The results could be confirmed by molecular modelling studies based on molecular dynamics of PVP, partial charge distribution in Ce6 and docking analysis of the Ce6–PVP system. In this study, the method for evaluating charge distribution was selected based on linear correlations with experimental 1H and 13C NMR chemical shifts [162]. PVP was found to have similar disaggregating capability for a series of amino acid derivatives of Ce6 (xCE) bearing either one serine, lysine, tyrosine or arginine residue at the carboxylic acid function of Ce6 as well as for Ce6 mono-6-amino-hexanoic acid amide. All Ce6 derivatives showed aggregation in aqueous solutions to different extents as revealed by their 1H NMR spectra, ranging from partly broadened and upfield shifted to strongly broadened or completely disappeared resonances. Interaction with PVP was each indicated by appearance and downfield shifts of the xCE resonances. NMR titration, i.e., monitoring the increasing downfield shifts of the chlorin meso protons as function of PVP concentration, was used to calculate binding constants. Moreover, 2D 1H DOSY could prove association of each Ce6 derivative with PVP based on the detection of a common diffusion coefficient for xCE and PVP that was significantly lower than that for the chlorin alone [163]. The observation that xCE and PVP adopt the same dynamic properties with respect to translational motion was also reflected in changes of the transverse relaxation times T2 and the correlated 1H NMR resonance linewidths (inverse proportional to T2). Another contribution was suggested to derive from motional restriction of the PVP-encapsulated chlorin [163,165]. Regions of preferential intermolecular interactions were derived from 2D 1H1H NOESY experiments of xCE–PVP mixtures that revealed intermolecular NOE cross peaks between chlorin and PVP resonances. From NOESY-based inter-proton distance calculations it could be concluded that the xCE–PVP complex formation is mainly driven by hydrophobic interactions with participation of the chlorin H-5 and H-10 meso-protons and the PVP H-b and H-c protons (Figure 7) [163].
Figure 7.
2D 1H1H NOESY (excerpt) of chlorin e6 serine amide (SerCe) associated with PVP at a SerCe/PVP molar ratio of 3:20 in phosphate buffered saline (PBS). (Reprinted with permission from: M. Hädener et al., J. Phys. Chem. B 2015, 119, 12117−12128. [163] Copyright © 2015, American Chemical Society).
Similar to the Ce6 amino acid derivatives, the more hydrophobic strongly aggregating chlorin e4 (Ce4) was well encapsulated into PVP, forming stable Ce4–PVP complexes, as was shown by 1H NMR chemical shift titration (appearance and increasing upfield shift of Ce4 resonances) and 2D 1H DOSY (Ce4 and PVP had a common diffusion coefficient). The PVP-encapsulated Ce6 derivatives Ce4 and serine-amide of Ce6 (SerCe) were also shown to be protected from binding to the external proteins Tf and HSA (see Section 3.1.2) as was indicated by unchanged chlorin 1H NMR resonances upon protein addition, whereas severe line broadening occurred in the absence of PVP [143].
In addition to Ce6 derivatives, other porphyrinic compounds as potential PSs have been probed in combination with PVP by NMR spectroscopy. The interaction of the amphiphilic hematoporphyrin derivative (dimegin, DMG) with PVP was studied by induced 1H NMR chemical shifts compared to the NMR spectra of the single components. Downfield shifts of the DMG meso-proton resonances indicated disaggregation of the porphyrin that correlated with increased photoactivity. Moreover, it was concluded from PVP chemical shift changes (upfield shifts of all PVP resonances) that not just hydrophobic interactions, that were most pronounced, participated in the binding but also hydrophilic [166,167]. In another study, a series of six different porphyrin derivatives derived either from hematoporphyrin IX (HPIX) or PPIX was monitored with respect to PVP encapsulation by 1H NMR spectroscopic analyses of the porphyrin resonances. The results were compared to those obtained from Ce6 derivatives and revealed that PVP has an overall higher capability for disaggregating Ce6 derivatives [165]. This was related to the different aggregate structures and the resulting stability of aggregates that had been deduced for Ce6 and porphyrin derivatives from NMR-derived aggregation maps. The asymmetric substitution pattern of polar and non-polar substituents as well as the nature of the substituents of the Ce6 derivatives lead to aggregate structures that were more easily disrupted than those of the porphyrinic derivatives [84]. Among the investigated porphyrin derivatives, HPIX, deuteroporphyrin IX 2,4-disulfonic acid (DPIXDS), and deuteroporphyrin IX 2,4-disulfonic acid dimethyl ester (DPIXDSME) exhibited relatively good PVP encapsulation based on 1H NMR chemical shift titration, 1H DOSY and T2 relaxation time measurements [165].
With the development of theranostic PDT agents, porphyrinic PSs bearing fluorine substituents are very attractive adding the potential for 19F-MRI diagnostics. For this purpose, the synthesis of a Zn-phthalocyanine (ZnPc) was reported with 24 fluorine substituents that gave rise to two proximate intense 19F NMR signals from the magnetically pseudo-equivalent fluorine atoms of the isomeric mixture. PVP formulation of this hydrophobic F-substituted ZnPc, however, lead to a collapse of the intense 19F resonance into a low-intensity very broad signal, which indicated that the ZnPc exists as aggregates in the PVP environment [168].
In Table 2, the NMR applications to study porphyrin interactions with PVP discussed in this section are summarized.
Table 2.
Summary of NMR interaction studies between porphyrins and PVP.
| Porphyrin (Guest) |
Macromolecule (Host) |
NMR Technique | Result | Ref |
|---|---|---|---|---|
| Ce6 | PVP (MW 25 kDa) |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Ce6 mainly interacts with the hydrophobic vinyl-backbone of PVP Disaggregation upon interaction with PVP |
[161] |
| Ce6 |
PVP (MW 40 kDa) |
1H NMR spectral appearance of guest | Disaggregation upon interaction with PVP | [164] |
| Ce6 SerCe LysCe TyrCe ArgCe Ce6-amino-hexanoic amide |
PVP (MW 10 kDa) |
1H NMR spectral appearance of guest 1H NMR chem. shift titration with host 1H DOSY of host–guest mixture 2D 1H1H NOESY of host–guest mixture |
Disaggregation upon interaction with PVP Determination of binding constant Host and guest have same diffusion properties Identification of host and guest protons in close proximity |
[163] |
| SerCe | PVP (MW 10 kDa) |
T2 relaxation time measurements of host and guest | Change and assimilation of dynamic properties of host and guest Motional restriction of guest |
[163,165] |
| Ce4 | PVP (MW 10 kDa) |
1H NMR spectral appearance of guest 1H NMR chem. shift titration with host 1H DOSY of host–guest mixture |
Disaggregation upon interaction with PVP Determination of binding constant Host and guest have same diffusion properties |
[143] |
| Ce4, SerCe | PVP (MW 10 kDa), HSA, Tf | 1H NMR spectral appearance of guest | PVP-encapsulated guest is protected from protein binding | [143] |
| DMG | PVP (MW 40 kDa) |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host |
Disaggregation upon interaction with PVP Hydrophobic and hydrophilic interactions between host and guest |
[166,167] |
| PPIX, DPIX, HPIX and derivatives | PVP (MW 10 kDa) |
1H NMR spectral appearance of guest | Different extent of disaggregation upon PVP interaction | [165] |
| HPIX, DPIXDS, DPIXDSME | PVP (MW 10 kDa) |
1H NMR chem. shift titration with host 1H DOSY of host–guest mixture T2 relaxation time measurements of host and guest |
Determination of binding curves Host and guest have same diffusion properties Restricted mobility of encapsulated guest |
[165] |
| Fluorinated ZnPc (ZnPcF24) | PVP | 19F NMR spectral appearance of guest | Guest exists as aggregate in PVP | [168] |
3.2.2. Cyclodextrins (CDs)
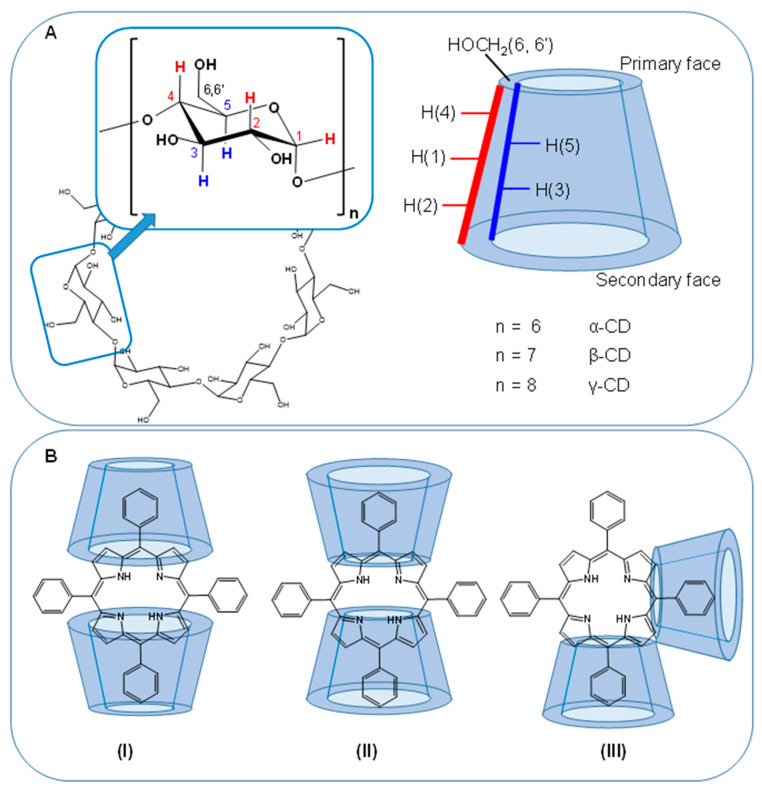
CDs are circular oligosaccharides typically consisting of 6 (α-CDs), 7 (β-CDs) or 8 (γ-CDs) α-1,4-D-glucopyranose units. The supramolecular cone-shaped structures form a hydrophobic cavity with the protons H-3 and H-5 on the interior and a hydrophilic surface with H-1, H-2, and H-4 pointing to the outside rendering the CDs water soluble (Figure 8A). The size of the hydrophobic cavity on the primary and secondary face of the cone depends on the number of sugar units and is well suited to form inclusion complexes with small molecules or parts of them (Figure 8B). These features have made CDs very attractive as solubilizers and carriers for drug delivery of a wide range of drugs. Owing to their relatively small size, CDs give rise to well-resolved intense 1H NMR spectra (Figure 6, top row). It is therefore not surprising that already three decades ago NMR spectroscopy was extensively applied for characterizing drug-CD inclusion complexes. Schneider et al. have given a comprehensive overview about the various NMR techniques applied to study the structure and binding modes in CD host–guest systems [169].
Figure 8.
(A) Structure of cyclodextrin and the α-1,4-d-glucopyranose unit with atom numbering; sketch of the cone-shaped structure of CDs in which the protons H(3) and H(5) point to the hydrophobic interior of the cavity (blue), the protons H(1), H(2) and (H4) point to the hydrophilic exterior (red), and the protons H(6,6′) are located at the rim of the primary face. (B) Examples of possible 2:1 inclusion complexes formed with tetraphenylporphyrins (TPPs): (I) inclusion via secondary face, (II) inclusion via primary face, opposite (anti) conformation, and (III) complex with adjacent (syn) conformation.
While the porphyrinic macrocycle is too large for inclusion, peripheral aromatic ring substituents fit well into the hydrophobic cavities of CDs (Figure 8B). Thus, TPPs are predestined to form complexes with CDs and there are numerous studies in the literature that have addressed the interactions between TPP derivatives and CDs by NMR spectroscopic methods. With its atomic resolution, selective changes on the CD nuclei either located in the inner hydrophobic cavity or at the outer hydrophilic surface, NMR spectroscopy is a sensitive method for detecting binding sites of the porphyrinic guest molecules. Depending on the symmetry of the TPP–CD complexes, the TPP pyrrole protons give rise to different numbers of resonance frequencies allowing, e.g., to distinguish oppositely (2 pyrrole signals) or adjacently (4 pyrrole signals) capped TPP (Figure 8B, II and III). Further, the detection of intermolecular NOEs is an important tool for understanding the spatial arrangement of the porphyrin–CD complexes. Since the MW of porphyrin–CD complexes typically falls into the intermediate regime with MW = 700–1500 Da where the NOE goes through zero, most NMR studies have used the ROESY experiment instead because in ROESY, the NOE is always positive and unequal to zero (see Section 2.3) [169].
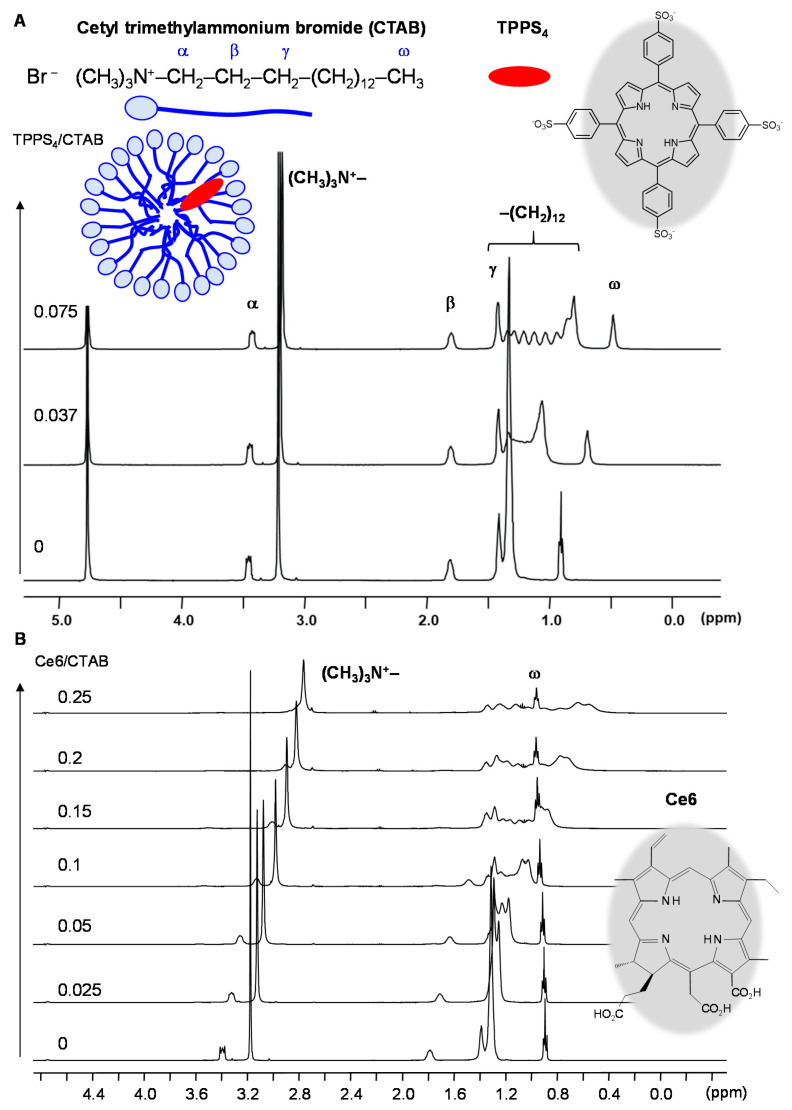
Initial studies on phenyl-substituted porphyrin-CD inclusion complexes were inspired by mimicking photosynthetic reaction centers [170] or protein containing porphyrins such as hemoglobin, myoglobin or cytochromes where the CD cavity mimics the protein hydrophobic pockets to obtain water-soluble artificial analogues for mechanistic investigations [171,172,173,174]. Numerous studies have addressed the CD inclusion of meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS4) and its metal complexes that exhibit aggregation in aqueous solutions [175,176]. Based on analyses of 1H NMR chemical shift changes of the CD- and the TPPS4 resonances as well as on intermolecular NOEs it could be concluded that no inclusion complex was formed with α-CD, whereas 2:1 inclusion complexes were formed with β- and γ-CD. In these complexes, the TPPS4 sulfonatophenyl substituents were shown to enter the CD cavity through the secondary face in the case of β-CD and through the primary face in the case of γ-CD resulting in different geometries according to the examples shown in Figure 8B (I) and (II), respectively. In γ–CD complexes, ring-current-induced shifts for H-5 and H-6,6′ were larger compared to β-CD, which in turn had larger shifts of the H-3 protons. Further proof that the TPPS4 macrocycle was closer located to the primary face in γ-CD was obtained from ROESY data: In addition to strong NOEs between TPPS4 phenyl and inner H-3 and H-5 protons, weak NOEs were also and only detected in γ-CD with H(6,6′) located at the rim of the primary face [176]. Similarly, formation of CD-inclusion complexes could be shown by NMR spectroscopic methods (induced shifts and/or NOE) for the metal complexes Mn(III)TPPS4 [177], Zn(II)TPPS4 [178,179], and Pd(II)TPPS4 [179] with respect to α, β and γ-CD binding affinity and geometry [179]. In these studies, selective changes were also reported for the 13C NMR shifts of the inner CD carbon nuclei (C-5 and C-3) of the CD-inclusion complexes [177,179]. Based on weak NOEs between porphyrin protons and protons from the CD exterior surface, non-specific binding of TPPS4 monomers or aggregates to the CD outer surface was postulated to coexist with the main inclusion complex.
Compared to the native α-, β- and γ-CDs the corresponding hydroxypropyl (HP) derivatives HP-α-, β- and γ-CDs each yielded similar binding modes but the binding force was stronger in HP-CDs, resulting in more stable complexes with reduced dynamics, i.e., slow dissociation on the NMR time scale [179]. Kano et al. have demonstrated the impact of per-methylation of β-CD using trimethyl-β-CD (TMe-β-CD) that—combined with TPPs bearing anionic substituents in the periphery—formed very stable 2:1 complexes. TPPS4 complexation induced much stronger changes in the 13C NMR shifts in particular for C-1 and C-4 of TMe-β-CD compared to β-CD. This was related to a higher flexibility of methylated CDs due to the lack of hydrogen-bonds enhancing the binding capability for guest molecules by the “induced fit-type complexation”. 13C-T1 relaxation time measurements provided further insights into the dynamics of the TPPS4 (TMe-β-CD)2 complex: Slight decrease in the CD- 13C-T1 values indicated reduced motion of the CD scaffold and the rotation of the outer TPPS4 phenyl substituents was more inhibited by the two CD-caps than the included ones [180]. Methylated β-CDs (Me-β-CD, HP-β-CD) were also shown by NMR ROESY to form inclusion complexes with TPPS4 under acidic conditions where strong J-aggregation dominates. However, for Me-β-CD, an inclusion via the primary face was postulated based on more pronounced changes of the corresponding proton resonances [181]. In the series of meso-tetrakis(phenyl)porphyrins with mixed phenyl- and sulfonatophenyl substituents TPPS3 and TPPS2o (o = opposite), inclusion complexes with β- and γ-CDs were only formed by insertion of the sulfonatophenyl- but not with the hydrophobic phenyl substituents as could be shown by ROESY experiments. This was explained by intermolecular hydrophobic interactions between phenyl substituents forming lateral porphyrin homo-aggregates that CDs cannot break up as opposed to porphyrin aggregates formed by π–π-stacking. This was evidenced by NMR shift titration and temperature-dependent experiments showing an incomplete disaggregation effect (dissolution of stacked aggregates) for TPPS3 and TPPS2o as opposed to TPPS4. Type and intensity of intermolecular NOEs were consulted to derive the geometries of the inclusion complexes: In the case of TPPS3, both adjacent and opposite sulfonatophenyl substituents were enclosed by CD (exemplified in Figure 8B), and for TPPS2o a slightly tilted CD arrangement was postulated due to coexisting TPP homo-associates via phenyl-ring overlapping. TPPS2a (a = adjacent) did not yield any CD inclusion (absence of NOEs) that was explained by the relatively strong contribution of the two adjacent phenyl substituents to aggregate formation [182]. The presence of adjacently (“syn”) and oppositely (“anti”) capped TPPS4-CD inclusion complexes and mixtures thereof could be shown with the application of CD dimers connected through a flexible linker. The different arrangements were deduced from the symmetry/asymmetry of the resulting complexes with adjacent giving rise to four and opposite to two TPPS4 -pyrrole proton resonances. In this study, the presence of a 2:2 complex (2 TPPS4 and 2 CD dimers with bi-pyridyl-linkers) was also proposed and the structure deduced from NMR-symmetry and dynamic considerations [183]. In the same way, the opposite CD arrangement of a TPPS4-TMe-β-CD dimer with a pyridyl-linker serving as hemoglobin model could be confirmed [173].
While most studies focused on CD interactions with TPPS4, similar NMR-derived results were obtained for the analogous meso-tetrakis(4-carboxyphenyl) porphyrin (TPPC4) bearing carboxylate instead of sulfonate substituents [174,179,180]. The importance of charge on the TPP-guest molecule was addressed by several researchers. For TMPyP with four positive charges in the periphery, no inclusion complexes with CDs could be detected [182,184]. However, based on NOEs between TMPyP and the external protons of native and permethylated CDs an external binding mode was suggested. In this study, it was also shown that positive charges on the pyrrole nitrogen atoms of anionic TPPS4H22+ still enabled the formation of inclusion complexes with CD indicating that only the charge in the periphery determines CD inclusion promoted by electrostatic interactions between anionic substituents and the positively polarized CD cavity [184]. As opposed to TMPyP, the also positively charged corresponding N-ethylpyridyl-porphyrin (TEPyP) was clearly shown by NMR-induced chemical shift changes and ROESY data to form inclusion complexes with β-CD and HP-β-CD from the primary face [185]. However, binding affinity to HP-β-CD was much stronger compared to β-CD. Likewise, inclusion of the cationic p-phenyl-O-(CH2)3-Py+-TPP (TPPOC3Py) into TMe-β-CD was proved by NMR [180]. Moreover, the usage of CD derivatives bearing anionic groups, e.g., sulfonate-CD [184] or sulfobutylether-CD (SBE-CD) [185,186], further promotes binding to cationic TPP derivatives by electrostatic interactions among which NMR data proved the existence of inclusion complexes in the case of SBE-CD.
Hydrophobic interactions were postulated to play a major role in the CD inclusion of TPP derivatives with neutral substituents. In these studies, interactions (NOEs and induced chemical shift changes) were more pronounced with methylated CDs with a more apolar cavity as compared to non-methylated CDs. This could be shown for 5-pyridine-10,15,20-tris-(p-chlorophenyl)porphyrin (PyTPP) that hardly interacted with β-CD but formed 1:1 inclusion complexes with TMe-β-CD [187]. A comparative study with di- and tri-methyl CD yielded more intense interactions of 5,10,15,20-tetrakis(4-pyridyl)porphyrin (TPyP) with the trimethylated CD forming a 1:1 complex from the secondary face based on NMR NOE and chemical shift asymmetry indications [188]. Formation of a 1:2 TPyP TMe-β-CD inclusion complex was obtained and confirmed by 1H NMR TPP shifts that clearly indicated the symmetry of a bicapped complex with opposite CDs [189]. Similar 1:2 TPP-TMe-β-CD structures were described for neutral TPP derivatives with p-hydroxy-phenyl- and p-methoxy-phenyl substituents [190] as well as for a TPP bearing an octa-arginine chain on one phenyl ring in which the two opposite non-substituted phenyl rings were included [191].
In the past ten years, most porphyrin-CD related research has converted to the covalent linkage of CD moieties to the porphyrin periphery and NMR spectroscopic methods have been applied for structure characterization and verification. Peripheral CD units can be used to accommodate other molecules combining multiple functions within one complex [192,193,194]. Covalent linkage of several CD units allows to create larger supramolecular structures that—depending on the substitution pattern, symmetry of substitution and nature of substituents—can form larger networks or nanorods suitable for drug delivery [195]. Further, efficiency enhancement could be reached by using CD inclusion complexes as TPP-multiplying unit preventing aggregation at the same time [186,196]. The different binding affinities of TPPS4 for β-CD and TMe-β-CD was the basis for the formation of different nano-architectures consisting of alternating TPP units with 4 covalently bound CD units in the periphery bridged byTPPS4 units included on opposite sides into free CD cavities. The single tetra-CD-substituted TPP units formed partial, oppositely arranged intramolecular self-inclusion complexes by rotation of the glucose unit directly bound to TPP leaving two free CD cavities as was evidenced by NMR spectroscopic methods. The TPP 1H NMR shifts exhibited the pattern of a symmetrical bicapped inclusion complex and NOEs were detected between TPP and CD protons indicating that the CD secondary face was close to the TPP ring. Only in the case of TMe-β-CD the self-inclusion complex was dissolved in favor of TPPS4 inclusion (disappearance of intra- and appearance of intermolecular NOEs), providing four binding sites and yielding networks rather than nanorods as in the case of β-CD [197]. Similar self-inclusion complexes of tetra-TMe-CD-TPP derivatives were constructed to obtain water-soluble nanospheres with improved photophysical properties since the nanostructures prevent porphyrin stacking [198].
In Table 3, the NMR applications to study porphyrin interactions with CDs discussed in this section are summarized.
Table 3.
Summary of NMR interaction studies between porphyrins and cyclodextrins.
| Porphyrin (Guest) |
Macromolecule (Host) |
NMR Technique | Result | Ref |
|---|---|---|---|---|
| TPPS4 | α-, β-, γ-CD |
1H NMR chem. shift perturbation of host 2D 1H1H ROESY of host–guest mixture |
2:1 (CD:TPPS4) inclusion complexes with β- and γ-CD, no complex with α-CD β-CD: through secondary face γ -CD: through primary face |
[176] |
| TPPS4, Mn(III)TPPS4 | α-, β-, γ-CD |
1H and 13C NMR chem. shift perturbation of host 2D 1H1H ROESY of host–guest mixture |
Strongest binding for β-CD | [177] |
| Zn(II)TPPS4 | β-CD | 1H NMR chem. shift perturbation of host | Formation of inclusion complex | [178] |
| Zn(II)TPPS4 Pd(II)TPPS4 TPPC4 |
β-, γ-CD HP-β-CD HP-γ-CD |
1H and 13C NMR chem. shift perturbation of host 2D 1H1H ROESY of host–guest mixture |
Formation of inclusion complexes: (HP)β-CD: through secondary face (HP)γ -CD: through primary face Weak binding to CD exterior |
[179] |
| TPPS4 TPPOC3PSa TPPC4 TPPOC3Py |
β-Cd TMe-β-CD |
1H and 13C NMR chem. shift perturbation of host 2D 1H1H ROESY 1H NMR spectral appearance of guest 13C-T1 relaxation time of host and guest |
β-CD, TMe-β-CD: anionic porphyrin guests binding more favorable than cationic Disaggregation; formation of trans-type 2:1 complexes with TMe-β-CD TPPS4: stronger binding to TMe-β-CD than to β-CD Motional restriction of CD and TPP-phenyl rings (less pronounced inside cavity) |
[180] |
| TPPS4 Acidic conditions |
β-CD Me-β-CD HP-β-CD |
1H and 13C NMR chem. shift perturbation of host 2D 1H1H ROESY 1H NMR spectral appearance of guest |
β-CD, HP-β-CD: inclusion via secondary face Me-β-CD: inclusion via primary face Disaggregation of J-aggregates upon complexation |
[181] |
| TPPS4 TPPS3 TPPS2o TPPS2a TMPyP |
β-, γ-CD |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host 2D 1H1H ROESY |
Inclusion complexes formed with all but TPPS2a and TMPyP TPPS4, TPPS3, TPPS2o: Partial disaggregation Sulfonatophenyl- but not phenyl-group included in the case of mixed substituents Inclusion via secondary face for β-CD and primary for γ-CD |
[182] |
| TPPS4 TPPC4 |
CD dimers with flexible spacers |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host |
Adjacently (“syn”) and oppositely (“anti”) capped TPPS4 | [183] |
| TMPyP TPPS4H22+ |
α-, β-, γ-Cd TMe-β-CD SO3-β-CD DiMeSO3-β-CD |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest 1H NMR chem. shift titration with host 2D 1H1H ROESY |
TMPyP: External binding to native CDs and TMe-β-CD TPPS4H22+: Inclusion with β-CD via secondary and with γ-CD via primary face Fast exchange between free/complex form |
[184] |
| TEPyP | β-CD HP-β-CD SBE-CD |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest 2D 1H1H ROESY |
Inclusion with β-CD and HP-β-CD from the primary face | [185] |
| TMPyP | SBE-CD | 1H NMR spectral appearance of guest | Complex formation with SBE-CD Fast exchange between free/complex form |
[186] |
| PyTPP | β-CD Tme-β-CD |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Inclusion with TMe-β-CD (from the primary face) but hardly with β-CD | [187] |
| TPyP | HP-β-CD Di-Me-β-Cd TMe-β-CD |
1H NMR spectral appearance of guest 2D 1H1H NOESY |
1:1 complex with TMe-β-CD from the secondary face | [188] |
| TPyP | TMe-β-CD | 1H NMR spectral appearance of guest at different pH | 1:2 (TPyP:CD) complex pH-dependent release (acidic condition) |
[189] |
| TPP, TPPC4 THPPb, TAPPc TMeOPPd |
TMe-β-CD | 1H NMR spectral appearance of host–guest mixture | All form 1:2 (TPP:CD) inclusion complexes with TMe-β-CD | [190] |
| octa-arginine-TPP (R8-TPP) |
TMe-β-CD |
1H NMR chem. shift titration with host 1H NMR spectral appearance of guest 2D 1H1H ROESY |
Trans-type 1:2 (TPP:CD) inclusion complex with the non-substituted phenyl groups via secondary face Slow free/complex exchange rate |
[191] |
| TPPS4 | ZnTPP- DAPM-β-CDe |
1H NMR spectral appearance of host–guest mixture 2D 1H1H NOESY 1H-DOSY |
ZnTPP- β-CD forms self-inclusion and inclusion complexes with TPPS4 yielding vesicles and networks | [195] |
| Mn(III)TPP PEGylated |
bridged bis(TMe-β-CD) | 2D 1H1H NOESY | Inclusion complexes, formation of supramolecular polymers | [196] |
| TPPS4 | ZnTPP-, DAPM-β-CDe DAPM-TMe-β-CDe |
1H NMR spectral appearance of host–guest mixture 2D 1H1H NOESY |
ZnTPP- β-CD and -TMe-β-CD form self-inclusion and inclusion complexes; the latter are dissolved in favor of TPPS4 inclusion Formation of networks and nanorods |
[197] |
a TPPOC3PS: p-phenyl-O-(CH2)3-p-phenyl-(SO3)−-tetra-phenylporphyrin; b THPP: meso-tetrakis(4-hydroxyphenyl) porphyrin; c TAPP: meso-tetrakis(4-aminophenyl) porphyrin; d TMeOPP: meso-tetrakis(4-methoxyphenyl) porphyrin; e DAPM: 6-deoxy-6-azidopermethyl.
3.2.3. Surfactant Micelles
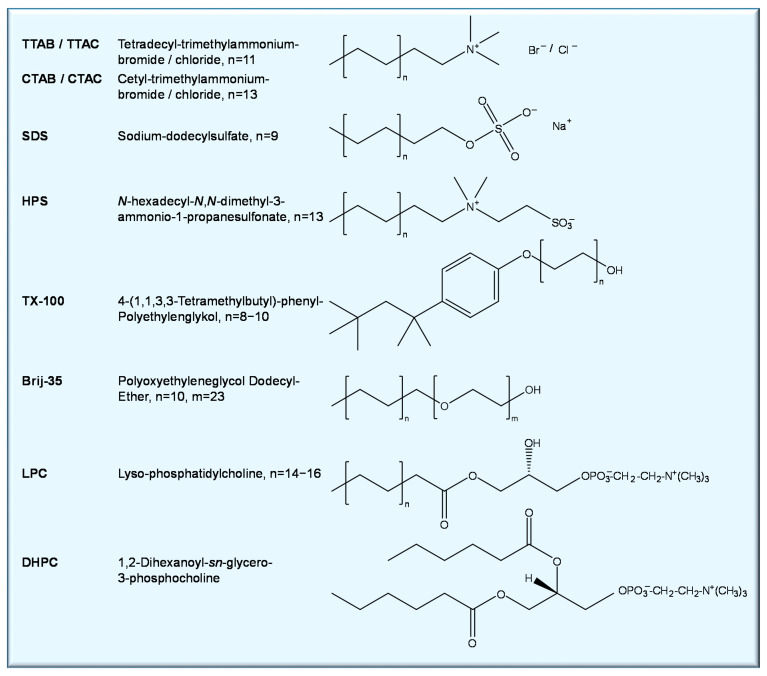
Micelles are small spheroidal molecular assemblies that spontaneously form from amphiphilic surfactants in aqueous solutions (or in non-polar solutions as reversed micelles) above their characteristic critical micelle concentrations (cmc). Rapid tumbling and high intrinsic mobility in micelles typically give rise to well-resolved, sharp proton resonances. Therefore, NMR spectroscopic techniques are well suited to characterize surfactant micelles and their solutes. For example, the transitions from surfactant monomers to micelles impose changes in the chemical environment and in the dynamic properties that can be monitored by various NMR parameters such as 1H- and 13C-chemical shifts, T1- and T2 relaxation times and diffusion coefficients measured by DOSY experiments [199,200,201].
The diverse application of surfactant micelles includes solubilization of hydrophobic molecules and mimicking the anisotropy of biological membranes serving as simplified membrane models [199]. The most frequently applied surfactants have hydrocarbon chains of 12–16 carbon atoms with hydrophilic heads that are either non-ionic, cationic, anionic, or zwitterionic (Figure 9).
Figure 9.
Structures of micelle forming surfactants.
In analogy to the TPP–CD complexes (see Section 3.2.2), extensive NMR spectroscopic studies (in the 90ies) have been conducted on heme porphyrins incorporated into CTAB, SDS, or TX-100 (Figure 9) surfactant micelles as models for hemoproteins or enzymes enabling heme studies in a protein-like environment without facing problems of porphyrin aggregation [202,203,204,205,206].
Utilizing CTAB, SDS and TX-100 micelles as simple membrane models Kadish et al. have shown that the tetra-anionic TPPS4 and its metal complexes are solubilized and disaggregated by the cationic CTAB and non-ionic TX-100 micelles but not by anionic SDS micelles demonstrating the importance of electrostatic in addition to hydrophobic interactions. This was evidenced from 1H NMR chemical shifts of the TPP and the micellar protons that remained unchanged in the presence of SDS, whereas, for TX-100 and CTAB, 1H NMR resonances of TPPS4 were downfield shifted and the β-pyrrole resonances coalesced into one single resonance instead of two, which indicated a faster rate of NH-tautomerism of the TPP central NH-groups in the micellar environment. Characteristic ring-current-induced upfield shifts of the TX-100 and CTAB resonances belonging to the hydrophobic core further proved porphyrin insertion [175]. Prominent changes are particularly observed for the CTAB methylene -(CH2)12 envelope that splits upon TPPS4 interaction into single resolved CH2 peaks [175,207,208] as is visualized in Figure 10A. This split is also observed in the presence of Ce6. However, as opposed to TPPS4, Ce6 exhibits more interactions with the polar head region and does not affect the CTAB terminal methyl-group indicating a micellar insertion of Ce6 that reaches into the hydrophobic region but is more oriented towards the micelle-water interface (Figure 10B, own unpublished data). An analogous NMR study of the cationic TMPyP only yielded insertion into anionic SDS but not into CTAB and TX-100 micelles [209]. Similar NMR results were obtained for a tetra-cationic Pt complex of TMPyP [210]. The lack of interactions with the non-ionic micelles was suggested to be due to the charge delocalization over the entire porphyrin ring in TMPyP reducing the hydrophobic character [209].
Figure 10.
1H NMR spectra of CTAB micelles (A) in the presence of TPPS4 and (B) in the presence of chlorin e6 (Ce6) at increasing molar ratios TPPS4/Ce6 : CTAB in aqueous buffer solutions (pH = 7.2, CTAB constant concentration 40 mM) (unpublished data).
The importance of charge and hydrophobic properties for interacting with ionic and non-ionic micelles was also demonstrated on a series of water insoluble TPPs with only one substituted phenyl-ring bearing either a carboxy-, hydroxy-, amino- or nitro group in para position. Detailed analysis of micellar 1H NMR chemical shifts and the (non-)uniformity, direction and magnitude of induced changes revealed that (i) a certain polarity was required for solubilization in all three micelle types (nitro-phenyl-TPP was not incorporated), (ii) electrostatic repulsive forces inhibited micelle insertion, (iii) peripheral hydroxy-groups promoted micelle insertion, since hydroxy-phenyl-TPP reached highest intra-micellar concentration and was more oriented towards the micelle water interface compared to the other TPP derivatives [211].
The interaction of TPPS4 as free base or metal complex with cationic and zwitterionic micelles was the subject of further comprehensive NMR spectroscopic studies. By varying the surfactant concentration below and above the cmc it could be shown that TPPS4 forms premicellar aggregates with CTAB, whereas micellized monomers exist above cmc [212]. Similar premicellar aggregates were also reported for cationic TMPyP and pyridiniumpropoxy-TPP in the presence of SDS below cmc [210,213].
Based on 1H NMR chemical shift, T1 relaxation time and NOE data, it was shown that TPPS4 localizes in the hydrophobic core of cationic CTAC and zwitterionic HPS micelles, whereas, in LPC micelles (for the surfactant structures see Figure 9), the polar head region is also involved in TPP interactions. The split methylene resonance (as exemplified in Figure 10A) allowed the determination of NOE buildup rates for TPP protons through dipolar interactions with CTAC protons in different chain positions in addition to the choline and terminal methyl-group in truncated and transient NOE experiments. Taking intramolecular differences in surfactant mobility (rotational correlation times) into account, the different NOE rates could provide an interaction profile along the surfactant molecules [207]. Metal complexes of TPPS4 (Fe(III), Zn(II)) exhibited similar localization sites in CTAC, HPS and in the non-ionic TX-100 and Brij-35 (Figure 9) micelles evidenced by largest upfield shifts for the terminal surfactant protons [208,214]. More recent studies have reported the insertion of diaxial Sn(IV)– and Co(III)–TPPS4 complexes into the hydrophobic region of CTAB micelles as concluded from selective 1D NOE experiments [215,216].
To study the interactions with a series of Ce6, HPIX and PPIX derivatives, short-chain PL micelles obtained from DHPC (Figure 9) were used as mobile PL-membrane models with enhanced dynamics, making it possible to observe encapsulated porphyrin resonances by 1H NMR spectroscopy in the non-overlapping aromatic region. Based on the appearance of the 1H NMR chemical shifts of the porphyrinic compounds it could be concluded that DHPC-micelles have a high capability to include and break up even strongly aggregating Ce6 derivatives, whereas this was not the case for the porphyrin compounds. This indicated that not the extent but the structure of aggregates as well as the accessibility of peripheral polar substituents play key roles in porphyrin membrane insertion [84].
In Table 4, the NMR applications to study porphyrin interactions with surfactant micelles discussed in this section are summarized.
Table 4.
Summary of NMR interaction studies between porphyrins and surfactant micelles.
| Porphyrin (Guest) |
Macromolecule (Host) |
NMR Technique | Result | Ref |
|---|---|---|---|---|
| TPPS4 Zn(II)TPPS4 Cu(II)TPPS4 VO2+TPPS4 |
CTAB SDS TX-100 |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Encapsulation in hydrophobic core of CTAB and TX-100 micelles Disaggregation in CTAB, TX-100 SDS promotes aggregation, no insertion |
[175] |
| TPPS4 | CTAC HPS LPC |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest 1D NOE spectroscopy 1H T1 relaxation times |
CTAC and HPS micelles: TPPS4 localizes in the hydrophobic core Reduced molecular mobility of TPPS4 LPC micelles: TPPS4 intercalates involving the polar head region Fast exchange between free and bound states |
[207] |
| TPPS4 Fe(III)TPPS4 Zn(II)TPPS4 |
CTAC LPC |
1H NMR chem. shift perturbation of host; pH dependence 1H NMR spectral appearance of guest 1H T1 relaxation time |
TPPS4, Zn(II)-, Fe(III)TPPS4: Incorporate into CTAC and LPC micelles in the core region pH-dependent aggregation of Fe(III)TPPS4 in micelles |
[208] |
| TMPyP Zn(II)TMPyP Cu(II)TMPyP VO2+TMPyP |
CTAB SDS TX-100 |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
All solubilized by SDS micelles but not by CTAB and TX-100 Monomerization in SDS |
[209] |
| Pt(Cy2dim)Me]4(TpyP) a | SDS TX-100 |
1H NMR chem. shift perturbation of host | Location in hydrophobic region of SDS micelles; Low solubility in TX-100 | [210] |
|
p-(OH)-phenyl-TPP p-(COOH)-phenyl-TPP p-(NH2)-phenyl-TPP p-(NO2)-phenyl-TPP |
TTAB SDS TX-100 |
1H NMR chem. shift perturbation of host 1H NMR chem. shift titration with guest 1H NMR spectral appearance of guest |
Insertion inhibited by electrostatic repulsion p-(NO2)-TPP not inserted in any micelles p-(OH)-phenyl-TPP inserted in all micelles most efficiently |
[211] |
| TPPS4 | CTAB |
1H NMR spectral appearance of guest 1H NMR chem. shift titration with host |
Below cmc: Premicellar aggregates Above cmc: Micellar insertion and monomerization |
[212] |
| TPPOC3Py | SDS | 1H NMR chem. shift perturbation of host | Below cmc: Premicellar aggregates Above cmc: Intercalation among SDS chains, monomerization |
[213] |
| Fe(III)TPPS4 Zn(II)TPPS4, |
CTAC HPS Brij-35 TX-100 |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Fe(III)TPPS4, Zn(II)TPPS4, embedded in hydrophobic core of the micelles | [214] |
| Co(III)TPPS4(imidazole)2 | CTAB | 1D selective NOE spectroscopy | Closer location near the CTAB micellar core | [215] |
| Sn(IV)TPPS4(OH)2 Sn(IV)TPPS4(Met)2 Sn(IV)TPPS4(Tyr)2 |
CTAB | 1D selective NOE spectroscopy | Closer location near the CTAB micellar core | [216] |
| Ce6, Ce4 Ce6 derivatives DPIX, PPIX, HPIX and derivatives |
DHPC | 1H NMR spectral appearance of guest | Chlorin derivatives: Monomerized in DHPC micelles Porphyrin derivatives: Only weak interactions, no disaggregation |
[84] |
a Cy2dim: dicyclohexyldiimine.
3.2.4. Block Copolymer Micelles (BCMs)
BCMs have gained much interest as versatile delivery systems for a wide range of drugs including porphyrinic PSs [54,55] and several recent comprehensive review articles have been devoted to the chemistry, properties and applications of BCMs [54,217,218,219,220]. The BCM forming units are biocompatible, mostly synthetic polymers consisting of a hydrophilic block forming the micellar shell and a hydrophobic block forming the micellar core (Figure 11). The blocks can be arranged in two (A-B-type diblock copolymers) or three alternating segments (A-B-A-type triblock copolymers) and their chain lengths, ratio and overall MW determine the size and morphology of BCMs. Owing to their dynamic properties as relatively small fast tumbling systems with a high degree of internal motional freedom of the flexible polymer chains [221], BCMs are very well applicable for NMR spectroscopic characterizations giving rise to intense 1H NMR resonances in aqueous solution (Figure 6). The micelle formation process from block copolymer unimers induces changes not just in the diffusion properties but also in the 1H- and 13C-chemical shifts, resonance linewidths and relaxation rates of the polymeric nuclei rendering NMR a suitable method to monitor micellization [222,223,224,225,226]. Several studies either probing newly designed or commercial block copolymers for PS delivery have exploited NMR spectroscopy to verify polymer structures and to determine block sizes and MW of the polymers by quantitative NMR peak integration [227]. However, since the current review focuses on interaction studies, the corresponding work will not be included here. Since physical entrapment is frequently the mechanism of drug loading, polymer–drug interactions are of utmost importance for the loading capability, stability of the system and drug release. For this, NMR techniques including 1D and 2D NOE spectroscopy, NMR relaxation time and diffusion (DOSY) measurements are particular powerful tools in the description of the intermolecular interactions.
Figure 11.
Structures of block copolymers forming micelles discussed in the current review.
PEG is the most often encountered hydrophilic component of BCMs and in particular the triblock copolymers PEG-PPG-PEG, the so-called poloxamers or Pluronics (BASF trade name) [228], have been extensively explored as carriers for porphyrinic PSs [54,55,161,162,166,229,230,231]. BCM encapsulation is a strategy to enhance porphyrin delivery towards targeted tissue or profile the pharmacokinetics and pharmacodynamics of bioactive molecules. PEG grafting reduces mechanisms of biological recognition that lead to fast clearance via opsonization by the reticuloendothelial system (RES) [232,233]. In addition to its utilization in micellar form as carrier for low-MW drugs, PEGylation is also used as covalent modifier of biological macromolecules [232,234,235].
Steinbeck et al. have conducted extensive NMR studies on the interaction of TPPS4 with Pluronic P123 (PEG20PPG70PEG20, Figure 11) micelles at different temperatures. They analyzed 1H NMR chemical shift changes of TPPS4-P123 mixtures to determine the localization of the porphyrin along the polymer chains. Since the PPG methine and methylene resonances partially overlap with the PEG methylene peak they applied 1H13C HSQC for obtaining a better resolution. At low temperatures (<20 °C) TPPS4 exhibited strong interactions with the PPG-blocks, whereas, at higher temperatures (>35 °C), stronger interactions with the PEG part were observed. Sites of interactions were indicated by selective upfield shifts as well as—in part—inhomogeneous broadening either of the PEG- or PPG-polymer resonances and simultaneous downfield shifts of the TPPS4 resonances. The interactions were confirmed by corresponding intermolecular rotating frame NOEs (ROEs). The temperature dependence of interactions was explained by a shift towards a dehydrated, more hydrophobic nature of the polymer chains with increasing temperature. Detailed diffusion data including HSQC-resolved diffusion measurements at different temperatures and diffusion times lead to the conclusion that at <20 °C small TPPS4-P123 aggregates were formed, which at higher temperatures were in fast exchange with TPPS4 present in the larger micelles [236].
In analogy to TPPS4, the corresponding tetra-sulfonated Pc, ZnPcS4, as well as the perfluorinated ZnPcF16 were reported to preferentially localize in the PEG corona region of mPEG-b-PLLA (Figure 11) diblock copolymer micelles as was derived from induced 1H NMR chemical shift changes of the PEG moiety and NOE data (in the case of ZnPcS4). On the contrary, the non-substituted congener ZnPc lacked interactions with PEG and was localized in the hydrophobic micellar core. The mPEG-b-PLLA micelles were suggested to form a relatively rigid core region since the PLLA and the encapsulated ZnPc proton resonances were strongly broadened and only became NMR-visible after dissolution of the assembly by acetone addition [237].
The interaction of the hematoporphyrin derivative DMG [166] and Ce6 [161] with F127 (Figure 11) Pluronic micelles or solely with PEG as homo-polymer was each probed by 1H NMR spectroscopy. Both porphyrinic compounds exhibited disaggregation in the presence of the BCMs or PEG alone as was indicated by downfield shifts of the porphyrin/chlorin meso-proton, propionic acid and vinyl side chain protons, respectively. Both compounds were suggested to localize preferentially in the hydrophobic core regions of the BCMs due to induced upfield shifts and partial broadening specifically of the F127-PPG resonances. However, disaggregation capability and observed upfield shifts of the PEG resonance in the case of mixing with the sole homo-polymer also proved a significant affinity of both DMG and Ce6 for the more hydrophilic PEG blocks so that a micellar location close to the PEG-PPG interface was suggested [161,166].
In a comparative NMR study, BCMs formed by the poloxamer Kolliphor P188 (KP, Figure 11) also exhibited good disaggregation capability for Ce6 and four of its amino acid derivatives SerCe, lysine-, tyrosine- and arginine-amide of Ce6 (LysCe, TyrCe, and ArgCe) but a divergent one for porphyrinic compounds. Unlike HPIX, DPIXDS and its methylester DPIXDSME, DPIX, PPIX and iso-HPIX (iHPIX) were not disaggregated. The results were similar to those obtained for PVP and were attributed to the more stabilized aggregate structures of the porphyrinic compared to the Ce6 compounds. Chemical shift titration of the Ce6 derivatives resulted in progressive downfield shifts of selected (meso- and vinyl-) Ce6 1H-resonances with increasing KP concentration yielding uptake curves that levelled off at molar ratios of 3:10 (Ce6/KP). DOSY measurements of mixtures with KP revealed a dynamic exchange of Ce6 derivatives between the bulk phase and the micellar environment, since the Ce6 resonances had D values representing a weighted average between free and BCM-associated chlorin molecules. With increasing KP concentration this equilibrium was shifted towards micellized Ce6 compounds as the corresponding D values gradually decreased and levelled off. In this way, DOSY titration could be used to determine the BCM loading process and to derive uptake curves similar to the chemical shift titration experiments. The enhanced exchange dynamics were assumed to be responsible for the fact that only very weak intermolecular NOEs between the Ce6 derivative SerCe and the PPG methyl protons were observable. However, a strong and SerCe-concentration-dependent selective perturbation of the PPG methyl resonance (upfield shift and line broadening) clearly indicated the micellar core region as main interaction site [165].
Similar NMR studies with Ce4 proved that the micellar host–guest system (KP micelles as host and Ce4 as guest) could be stabilized by increased hydrophobicity and reduced water solubility of the guest molecule significantly repelling the dynamic exchange process [143]. In this system, more intense NOEs could be observed (Figure 12A). The three NOE cross peaks shown in Figure 12A, which arose from the interaction of the PPG methyl group of KP with the meso-H from Ce4, indicated localization in the micellar hydrophobic core with the hydrophobic part of the porphyrin molecule contributing to a stabilization effect of the Ce4–KP micellar assembly in aqueous medium. Moreover, the detection of negative NOEs (at a magnetic field strength of 11.74 T) demonstrated that the chlorin molecules behaved like large, slowly tumbling systems, proving their micellar association [90]. In addition, DOSY experiments not just confirmed the encapsulation of Ce4 in KP micelles but also indicated a lack of dynamic exchange (Figure 12B). The same diffusion coefficient (red line) was measured for the meso-protons from Ce4 (9.5 ppm) and for the -CH2- and CH3-groups (3.7 and 1.2 ppm, respectively) from KP showing that the Ce4–KP complex diffused as a single particle. Moreover, the corresponding d value of Ce4–KP (red line) was smaller than the d value of pure KP (blue line) as well as Ce4 (black line) alone, pointing out an increased hydrodynamic radius (according to Stokes–Einstein equation, Equation (6)) of Ce4-loaded micelles [143].
Figure 12.
(A) 1H 1H-NOESY spectrum of Ce4–KP in phosphate buffered saline (PBS, molar ratio 3:10); intramolecular NOE cross peaks are visible for the Ce4 meso proton resonances (marked by the blue square) and intermolecular NOEs are visible between the Ce4 meso resonances and the methyl resonance of KP (highlighted in red and indicated in the structures); (B) overlay of 1H-DOSY spectra of 3 mM Ce4 in DMSO (shown in black), 10 mM KP in PBS (shown in blue), and Ce4–KP in PBS at a molar ratio of 3:10 (shown in red). For Ce4–KP (3:10) the DOSY spectrum and the projection spectrum are scaled up by a factor of 64 in the region between 7 and 11 ppm. T = 310 K. (Reprinted from Gjuroski et al. Journal of Controlled Release, 316, (2019), 150-167 [143], © 2019 Published by Elsevier B.V., with permission from Elsevier).
Detailed NMR studies with five Ce6 derivatives of increasing hydrophobicity from SerCe, Ce4, Ce6 monoethylene diamine monoamide (CeMED), Ce6 dimethylester (CeDM) towards Ce6 trimethylester (CeTM) demonstrated the limitation of the poloxamer BCM encapsulation capability with respect to very hydrophobic strongly aggregating Ce6 compounds. Five different BCM forming Pluronic polymers with increasing HLB (hydrophilic-lipophilic balance) values and different block ratios and MWs were probed applying KP, F108, F127, L64 and P84 (Figure 11). Quantitative chlorin encapsulation was assessed by appearance and integration of the chlorin 1H resonances in the aromatic spectral region. Loading efficiency inversely correlated with chlorin hydrophobicity for each type of polymer. However, there was no correlation with the HLB value of the polymers, whereas the number of PPG blocks independent of the PEG part played a role as well as within certain limits the size of the PEG corona. Again, upfield shifts, broadening, asymmetric shapes, and the extent of chemical shift perturbation of the polymer PPG nuclei were very sensitive towards chlorin interaction and only in L64 and P84 BCMs also the PEG resonance was shifted indicating contributions from the micellar corona region. Dynamic properties of the chlorin BCM systems were addressed by DOSY, T1 and T2 relaxation time measurements. According to the DOSY spectra, a part of the systems exhibited dynamic exchange as indicated by fraction-weighted average d values for the chlorin nuclei in mixtures, whereas, for another part, the chlorin was more tightly bound to the micellar core with the chlorin molecules adopting the same diffusion properties as their micellar hosts [226].
Chlorin T1 and T2 relaxation times exhibited an overall reduction upon interaction with any of the BCM types due to an increase in their molecular correlation time τc [226]. Binding to the host molecule (the BCMs) increases the relaxation rates of the guest (the Ce6 derivatives) due to imposed tumbling motion from the host, i.e., longer correlation time resulting in shorter T2 [238]. However, not only the tumbling motion, also the spins in the vicinity and the distance between the spins in interactions influence the spin relaxation dynamics [239]. While BCM encapsulation was accompanied by chlorin disaggregation, the differential T2 values of the chlorin protons were equalized as they entered the homogeneous micellar environment. In chlorin aggregates protons of overlapping regions had strongly reduced relaxation times compared to those not experiencing the ring current of neighboring chlorin macrocycles. Similarly, for the pure poloxamer micelles, T2 values of the PPG spins were an order of magnitude shorter than those of the PEG units reflecting the spatial crowding in the micellar core region, whereas, in the outer corona, motional freedom is more and spin–spin interactions are less pronounced.
Chlorin encapsulation induced slight increases in the polymer T1 and T2 relaxation times that was explained by a disturbance of the molecular order. Further, it was postulated that the internal BCM flexibility as reflected in relaxation rates conduced to loading efficiency but not to stability (stronger binding) [226].
This was also supported by a comparison of PVP and KP micelles used as carriers: Although KP micelles had a smaller diffusion coefficient, i.e., larger hydrodynamic radius compared to PVP, implying longer molecular correlation times (τc) and shorter T2, chlorin association to the PVP matrix lead to stronger drop in chlorin T2 values as compared to KP. This was ascribed to the stiffness of the PVP matrix for which despite a smaller size than KP-BCMs dipole–dipole interactions were more pronounced. In accordance with this, chlorin-binding constants were an order of magnitude higher for PVP than for KP [165].
In Table 5, the NMR applications to study porphyrin interactions with block copolymer micelles discussed in this section are summarized.
Table 5.
Summary of NMR interaction studies between porphyrins and block copolymer micelles.
| Porphyrin (Guest) |
Macromolecule (Host) |
NMR Technique | Result | Ref |
|---|---|---|---|---|
| TPPS4 | P123 |
1H NMR chem. shift perturbation of host 1H13C HSQC 1H NMR spectral appearance of guest 1D ROE spectroscopy DOSY (HSQC-res.) T-dependent NMR |
<20 °C: TPPS4 strong interactions with PPG units Small TPPS4-P123 aggregates >35 °C: stronger interactions with PEG units TPPS4-P123 micelles in fast exchange with smaller TPPS4-P123 aggregates |
[236] |
| ZnPc ZnPcS4 ZnPcF16 |
mPEG-b-PLLA |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest 1D NOE spectroscopy |
ZnPc localized in micellar core ZnPcS4 and ZnPcF16 localize in micellar corona |
[237] |
| DMG | F127 pure PEG |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Disaggregation DMG localizes in hydrophobic core of F127 micelles DMG forms complex with PEG |
[166] |
| Ce6 | F127 pure PEG |
1H NMR chem. shift perturbation of host 1H NMR spectral appearance of guest |
Disaggregation Ce6 localizes in the PEG-PPG-interface region of F127 micelles |
[161] |
| Ce6 SerCe, LysCe, TyrCe, ArgCe DPIX DPIXDS, DPIXDSME PPIX HPIX, iHPIX |
KP |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host 1H NMR chem. shift titration with host 1H DOSY 1D NOE spectroscopy |
Chlorins: disaggregation Porphyrins: only HPIX, DPIXDS, DPIXDSME disaggregate Determination of binding curves Dynamic exchange between free and micellar state Preferential localization in micellar core |
[165] |
| Ce4 | KP |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host 1H NMR chem. shift titration with host 1H DOSY 2D NOESY |
Disaggregation Localization in hydrophobic core of KP micelles Lack of dynamic exchange |
[143] |
| SerCe Ce4 CeMED CeDM CeTM |
KP F108 F127 L64 P84 |
1H NMR spectral appearance of guest 1H NMR chem. shift perturbation of host 1H DOSY 1H T1 / T2 relaxation |
Loading efficiency inversely correlated with chlorin hydrophobicity Interaction mainly with hydrophobic core Differently pronounced dynamic exchange Disaggregation accompanied by changes in the dynamics |
[226] |
4. Conclusions
The interest in porphyrins as medical drugs is still growing and polymeric nanoplatforms have become an inevitable part of efficient delivery to the target tissue. With the development of theranostic agents, the systems have become complex and their characterization at the atomic level more demanding. While the optical properties of porphyrin-based drugs as phototoxic agents, i.e., their light absorbance and energy conversion, will remain the major focus in the analytical assessment, interactions with macromolecules are of equal high importance. Such interactions can significantly modify the photophysical properties and also determine the thermodynamic and kinetic stability of porphyrin formulations.
With this review, our aim was to provide an overview of studies that have applied NMR spectroscopy to address the interactions of biomedical-relevant porphyrinic compounds with macromolecules in solution. We have shown that NMR spectroscopy can offer a wealth of complementary information, thanks to its versatility, its accessibility to a vast range of dynamic properties, and its possibility to measure interactions with atomic resolution. For instance, the strong ring current inherent to porphyrins makes this class of compounds particularly suited for easy NMR detection of induced changes upon interaction with their large host molecules. In addition, NMR spectroscopy is non-destructive, highly reproducible and can be directly applied even to complex mixtures without the need of sample preparation steps except for the dissolution in an adequate solvent. Nevertheless, NMR spectroscopy also has several limitations: A low sensitivity compared to mass spectrometry or fluorescence spectroscopy and severe resonance line broadening can make certain large molecules or components with restricted, anisotropic mobility non-observable by NMR spectroscopy of solutions. Here, solid-state NMR techniques using magic angle spinning (MAS) and/or high resolution MAS (HR-MAS) NMR of semi-solids are better suited, but these two techniques have not been treated in this review in order to limit its scope and length. Yet, we hope that readers can realize through the examples given in this review, such as porphyrins interacting with CDs and surfactant micelles, that most nanoparticles can be studied by high resolution NMR in solution. It is a matter of fact, confirmed by a survey of the recent literature, that NMR spectroscopy is still rather underrepresented despite its enormous potential. This review may promote and encourage future studies involving porphyrins or any other type of small-molecule drugs interacting with macromolecules to make use of the advantages of NMR spectroscopy as powerful and versatile analytical tool.
Appendix A. The Following Abbreviations were used in the Text
AnTMPyP, meso-(9-anthryl)-tris(N-methyl-p-pyridinio)porphyrin; AnTPyP, meso-anthryl-tris(p-pyridyl)porphyrin; ArgCe, arginine-amide of Ce6; BCM, block copolymer micelle; Brij-35, polyoxyethylene glycol dodecyl-ether; CD, cyclodextrin; Ce4, chlorin e4; Ce6, chlorin e6; CeDM, chlorin e6 dimethylester; CeMED, chlorin e6 monoethylene diamine monoamide; CeTM, chlorin e6 trimethylester; cmc, critical micelle concentration; CSA, chemical shift anisotropy; CSP, chemical shift perturbation; CTAB, cetyl-trimethylammonium-bromide; CTAC, cetyl-trimethylammonium-chloride; Cy2dim, dicyclohexyldiimine; DAPM, 6-deoxy-6-azidopermethyl; DHPC, 1,2-Dihexanoyl-sn-glycero-3-phosphocholine; DMF, dimethylformamide; DMG, dimegin; DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; DMSO, dimethylsulfoxide; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOSY, diffusion-ordered spectroscopy; DPIXDS, deuteroporphyrin IX 2,4-disulfonic acid; DPIXDSME, deuteroporphyrin IX 2,4-disulfonic acid dimethyl ester; DSS, 2,2-dimethyl-2-silapentane-5-sulfonate; HLB, hydrophilic-lipophilic balance; HPIX, hematoporphyrin IX; HPS, N-hexadecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate; HP-β-CD, hydroxypropyl-β-cyclodextrin; HR-MAS, high resolution magic angle spinning; HSA, human serum albumin; HSQC, heteronuclear single quantum coherence; iHPIX, iso- hematoporphyrin IX; KP, Kolliphor P188; LPC, lyso-phosphatidylcholine; LysCe, lysine-amide of chlorin e6; MAS, magic angle spinning; Me-β-CD, methylated β-cyclodextrin; MOF, metal–organic frameworks; MRI, magnetic resonance imaging; MW, molecular weight; NapTMPyP, meso-(naphthyl)-tris(N-methyl-p-pyridinio)porphyrin; NIR, near infrared; NMR, nuclear magnetic resonance; NOE, nuclear Overhauser effect; NOESY, nuclear Overhauser effect spectroscopy; NP, nanoparticle; PAI, photoacoustic imaging; PBS, phosphate buffered saline; Pc, phthalocyanine; PCI, photochemical internalization; PC, phosphocholine; PDD, photodynamic diagnosis; PDT, photodynamic therapy; PEG, polyethylene glycol; PET, positron emission tomography; PFG, pulsed field gradient; PGSE, pulsed gradient spin echo; PL, phospholipid; PPG, polypropylene glycol; PPIX, protoporphyrin IX; PS, photosensitizer; PVP, polyvinylpyrrolidone; PyTMPyP, meso-pyrenyl-tris(N-methyl-p-pyridinio)porphyrin; PyTPP, 5-pyridine-10,15,20-tris-(p-chlorophenyl)porphyrin; RES, reticuloendothelial system; RF, radio frequency; RIS, ring current-induced shift; ROE, rotating frame nuclear Overhauser effect; ROESY, rotating frame nuclear Overhauser effect spectroscopy; ROS, reactive oxygen species; R8-TPP, octa-arginine-TPP; SBE-CD, sulfobutylether-cyclodextrin; SDS, sodium-dodecylsulfate; SerCe, serine-amide of chlorin e6; SUV, small unilamellar vesicle; TAPP, meso-tetrakis(4-aminophenyl) porphyrin; TEPyP, N-ethylpyridyl-porphyrin; Tf, transferrin; THPP, meso-tetrakis(4-hydroxyphenyl) porphyrin; TMe-β-CD, trimethyl-β-cyclodextrin; TMeOPP, meso-tetrakis(4-methoxyphenyl) porphyrin; TMPyP, meso-tetrakis (4-N-methylpyridyl) porphine; TMS, tetramethylsilane; TPP, tetraphenylporphyrin; TPPC4, meso-tetrakis (4-carboxyphenyl) porphyrin; TPPOC3PS, p-phenyl-O-(CH2)3-p-phenyl-(SO3)−-tetra-phenylporphyrin; TPPOC3Py, p-phenyl-O-(CH2)3-Py+-tetra-phenylporphyrin; TPPS4, meso-tetrakis (4-sulfonatophenyl) porphyrin; TPyP, 5,10,15,20-tetrakis(4-pyridyl)porphyrin; TX-100, Triton X-100, 4-(1,1,3,3-tetramethylbutyl)-phenyl-polyethylene glycol; TyrCe, tyrosine-amide of chlorin e6; xCE, amino acid derivatives of chlorin e6; ZnPc, zinc-phthalocyanine; ZnPcF16, perfluorinated zinc-phthalocyanine; ZnPcS4, tetra-sulfonated zinc-phthalocyanine.
Author Contributions
Writing—original draft preparation, M.V., I.G., J.F.; Writing—review and editing, M.V., I.G., J.F.; visualization, M.V.; supervision, M.V., J.F.; project administration, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lemberg R. Porphyrins in Nature. In: Ƶechmeister L., editor. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrés dans la Chimie des Substances Organiques Naturelles. Springer Vienna; Vienna, Austria: 1954. pp. 299–349. [Google Scholar]
- 2.Williams R.J.P. The Properties Of Metalloporphyrins. Chem. Rev. 1956;56:299–328. doi: 10.1021/cr50008a004. [DOI] [Google Scholar]
- 3.Boucher L.J. Coordination Chemistry of Porphyrins. In: Melson G.A., editor. Coordination Chemistry of Macrocyclic Compounds. Springer US; Boston, MA, USA: 1979. pp. 517–536. [Google Scholar]
- 4.Battersby A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000;17:507–526. doi: 10.1039/b002635m. [DOI] [PubMed] [Google Scholar]
- 5.Lesage S., Xu H.A.O., Durham L. The occurrence and roles of porphyrins in the environment: Possible implications for bioremediation. Hydrol. Sci. J. 1993;38:343–354. doi: 10.1080/02626669309492679. [DOI] [Google Scholar]
- 6.Bryant D.A., Hunter C.N., Warren M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020;295:6888–6925. doi: 10.1074/jbc.REV120.006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulos T.L. Heme enzyme structure and function. Chem. Rev. 2014;114:3919–3962. doi: 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latunde-Dada G.O. Iron: Biosynthesis and Significance of Heme. In: Caballero B., Finglas P.M., Toldrá F., editors. Encyclopedia of Food and Health. Academic Press; Oxford, UK: 2016. pp. 452–460. [Google Scholar]
- 9.Scheer H. An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. In: Grimm B., Porra R.J., Rüdiger W., Scheer H., editors. Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. Springer; Dordrecht, The Netherlands: 2006. pp. 1–26. [Google Scholar]
- 10.Tanaka A., Tanaka R. Chapter Six—The biochemistry, physiology, and evolution of the chlorophyll cycle. In: Grimm B., editor. Advances in Botanical Research. Volume 90. Academic Press; London, UK: 2019. pp. 183–212. [Google Scholar]
- 11.Hunter C.N., Thurnauer F.D.M.C., Beatty J.T. The Purple Phototrophic Bacteria. 1st ed. Springer; Dordrecht, The Netherlands: 2009. p. 1013. [Google Scholar]
- 12.Graham R.M., Deery E., Warren M.J. Tetrapyrroles: Birth, Life and Death. Springer; New York, NY, USA: 2009. Vitamin B12: Biosynthesis of the Corrin Ring; pp. 286–299. [Google Scholar]
- 13.Kadish K.M., Smith K.M., Guilard R. The Porphyrin Handbook. In: Kadish K.M., Smith K.M., Guilard R., editors. The Porphyrin Handbook. Academic Press; Amsterdam, The Netherlands: 2003. [Google Scholar]
- 14.Min Park J., Lee J.H., Jang W.-D. Applications of porphyrins in emerging energy conversion technologies. Coord. Chem. Rev. 2020;407:213157. doi: 10.1016/j.ccr.2019.213157. [DOI] [Google Scholar]
- 15.Costa E., Silva R., Oliveira da Silva L., de Andrade Bartolomeu A., Brocksom T.J., de Oliveira K.T. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow approaches. Beilstein J. Org. Chem. 2020;16:917–955. doi: 10.3762/bjoc.16.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Díaz M.V., de la Torre G., Torres T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010;46:7090–7108. doi: 10.1039/c0cc02213f. [DOI] [PubMed] [Google Scholar]
- 17.Huang H., Song W., Rieffel J., Lovell J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015;3 doi: 10.3389/fphy.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imran M., Ramzan M., Qureshi A.K., Khan M.A., Tariq M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors. 2018;8:95. doi: 10.3390/bios8040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsolekile N., Nelana S., Oluwafemi O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules. 2019;24:2669. doi: 10.3390/molecules24142669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: Part three-Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y., Zhou T., Bai R., Xie Y. Chemical approaches for the enhancement of porphyrin skeleton-based photodynamic therapy. J. Enzym. Inhib. Med. Chem. 2020;35:1080–1099. doi: 10.1080/14756366.2020.1755669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa L.D., Costa J.I.T., Tomé A.C. Porphyrin Macrocycle Modification: Pyrrole Ring-Contracted or -Expanded Porphyrinoids. Molecules. 2016;21:320. doi: 10.3390/molecules21030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey J.S., Hsu H.C., Schreiman I.C. Synthesis of tetraphenylporphyrins under very mild conditions. Tetrahedron Lett. 1986;27:4969–4970. doi: 10.1016/S0040-4039(00)85109-6. [DOI] [Google Scholar]
- 25.Lo P.-C., Rodríguez-Morgade M.S., Pandey R.K., Ng D.K.P., Torres T., Dumoulin F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020;49:1041–1056. doi: 10.1039/C9CS00129H. [DOI] [PubMed] [Google Scholar]
- 26.Mody T.D., Sessler J.L. Texaphyrins: A new approach to drug development. J. Porphyr. Phthalocyanines. 2001;05:134–142. doi: 10.1002/jpp.326. [DOI] [Google Scholar]
- 27.Teo R.D., Hwang J.Y., Termini J., Gross Z., Gray H.B. Fighting Cancer with Corroles. Chem. Rev. 2017;117:2711–2729. doi: 10.1021/acs.chemrev.6b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dougherty T.J. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 29.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 30.Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018;44:571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho C.M.B., Tomé J.P.C., Faustino M.A.F., Neves M.G.P.M.S., Tomé A.C., Cavaleiro J.A.S., Costa L., Alves E., Oliveira A., Cunha Â., et al. Antimicrobial photodynamic activity of porphyrin derivatives: Potential application on medical and water disinfection. J. Porphyr. Phthalocyanines. 2009;13:574–577. doi: 10.1142/S1088424609000528. [DOI] [Google Scholar]
- 32.Jocham D., Stepp H., Waidelich R. Photodynamic Diagnosis in Urology: State-of-the-Art. Eur. Urol. 2008;53:1138–1150. doi: 10.1016/j.eururo.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Zumbraegel A., Bichler K.-H., Krause F.S., Feil G., Nelde H.J. The Photodynamic Diagnosis (PDD) for Early Detection of Carcinoma and Dysplasia of the Bladder. In: Atala A., Slade D., editors. Bladder Disease, Part A: Research Concepts and Clinical Applications. Springer; Boston, MA, USA: 2003. pp. 61–66. [DOI] [PubMed] [Google Scholar]
- 34.Fritsch C., Lang K., Neuse W., Ruzicka T., Lehmann P. Photodynamic diagnosis and therapy in dermatology. Skin Pharmacol. Appl. Skin Physiol. 1998;11:358–373. doi: 10.1159/000029858. [DOI] [PubMed] [Google Scholar]
- 35.Cheng H.L., Haedicke I.E., Cheng W., Tchouala Nofiele J., Zhang X.A. Gadolinium-free T1 contrast agents for MRI: Tunable pharmacokinetics of a new class of manganese porphyrins. J. Magn. Reson. Imaging. 2014;40:1474–1480. doi: 10.1002/jmri.24483. [DOI] [PubMed] [Google Scholar]
- 36.Ni Y. Metalloporphyrins and Functional Analogues as MRI Contrast Agents. Curr. Med. Imaging Rev. 2008;4:96–112. doi: 10.2174/157340508784356789. [DOI] [Google Scholar]
- 37.Nasim V., Amir R.J. An Overview of Labeled Porphyrin Molecules in Medical Imaging. Recent Pat. Top. Imaging. 2015;5:3–12. doi: 10.2174/2451827105666150522235440. [DOI] [Google Scholar]
- 38.Yap S.Y., Price T.W., Savoie H., Boyle R.W., Stasiuk G.J. Selective radiolabelling with 68Ga under mild conditions: A route towards a porphyrin PET/PDT theranostic agent. Chem. Commun. 2018;54:7952–7954. doi: 10.1039/C8CC03897J. [DOI] [PubMed] [Google Scholar]
- 39.Merkes J.M., Zhu L., Bahukhandi S.B., Rueping M., Kiessling F., Banala S. Photoacoustic Imaging Probes Based on Tetrapyrroles and Related Compounds. Int. J. Mol. Sci. 2020;21:3082. doi: 10.3390/ijms21093082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abuteen A., Zanganeh S., Akhigbe J., Samankumara L.P., Aguirre A., Biswal N., Braune M., Vollertsen A., Röder B., Brückner C., et al. The evaluation of NIR-absorbing porphyrin derivatives as contrast agents in photoacoustic imaging. Phys. Chem. Chem. Phys. 2013;15:18502–18509. doi: 10.1039/c3cp52193a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Lovell J.F. Porphyrins as theranostic agents from prehistoric to modern times. Theranostics. 2012;2:905–915. doi: 10.7150/thno.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenni S., Sour A. Molecular Theranostic Agents for Photodynamic Therapy (PDT) and Magnetic Resonance Imaging (MRI) Inorganics. 2019;7:10. doi: 10.3390/inorganics7010010. [DOI] [Google Scholar]
- 43.Jerjes W., Theodossiou T.A., Hirschberg H., Høgset A., Weyergang A., Selbo P.K., Hamdoon Z., Hopper C., Berg K. Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J. Clin. Med. 2020;9:528. doi: 10.3390/jcm9020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norum O.-J., Selbo P.K., Weyergang A., Giercksky K.-E., Berg K. Photochemical internalization (PCI) in cancer therapy: From bench towards bedside medicine. J. Photochem. Photobiol. B: Biol. 2009;96:83–92. doi: 10.1016/j.jphotobiol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Chen J., Zhu Y., Kaskel S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2020 doi: 10.1002/anie.201909880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Jo Y.-U., Na K. Photodynamic therapy with smart nanomedicine. Arch. Pharmacal Res. 2020;43:22–31. doi: 10.1007/s12272-020-01214-5. [DOI] [PubMed] [Google Scholar]
- 47.Moret F., Reddi E. Strategies for optimizing the delivery to tumors of macrocyclic photosensitizers used in photodynamic therapy (PDT) J. Porphyr. Phthalocyanines. 2017;21:239–256. doi: 10.1142/S1088424617300014. [DOI] [Google Scholar]
- 48.Sztandera K., Gorzkiewicz M., Klajnert-Maculewicz B. Nanocarriers in photodynamic therapy-in vitro and in vivo studies. WIREs Nanomed. Nanobiotechnol. 2020;12:e1509. doi: 10.1002/wnan.1599. [DOI] [PubMed] [Google Scholar]
- 49.Calixto G.M., Bernegossi J., de Freitas L.M., Fontana C.R., Chorilli M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules. 2016;21:342. doi: 10.3390/molecules21030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montaseri H., Kruger C.A., Abrahamse H. Recent Advances in Porphyrin-Based Inorganic Nanoparticles for Cancer Treatment. Int. J. Mol. Sci. 2020;21:3358. doi: 10.3390/ijms21093358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Düzgüneş N., Piskorz J., Skupin-Mrugalska P., Goslinski T., Mielcarek J., Konopka K. Photodynamic therapy of cancer with liposomal photosensitizers. Ther. Deliv. 2018;9:823–832. doi: 10.4155/tde-2018-0050. [DOI] [PubMed] [Google Scholar]
- 52.Derycke A.S.L., de Witte P.A.M. Liposomes for photodynamic therapy. Adv. Drug Del. Rev. 2004;56:17–30. doi: 10.1016/j.addr.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Huang H.C., Mallidi S., Obaid G., Sears B., Tangutoori S., Hasan T. 23—Advancing photodynamic therapy with biochemically tuned liposomal nanotechnologies. In: Hamblin M.R., Avci P., editors. Applications of Nanoscience in Photomedicine. Chandos Publishing; Oxford, UK: 2015. pp. 487–510. [Google Scholar]
- 54.Demazeau M., Gibot L., Mingotaud A.-F., Vicendo P., Roux C., Lonetti B. Rational design of block copolymer self-assemblies in photodynamic therapy. Beilstein J. Nanotechnol. 2020;11:180–212. doi: 10.3762/bjnano.11.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento B.F.O., Pereira N.A.M., Valente A.J.M., Pinho E., Melo T.M.V.D., Pineiro M. A Review on (Hydro)Porphyrin-Loaded Polymer Micelles: Interesting and Valuable Platforms for Enhanced Cancer Nanotheranostics. Pharmaceutics. 2019;11:81. doi: 10.3390/pharmaceutics11020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pehlivan E.G., Ek Y., Topkaya D., Tazebay U.H., Dumoulin F. Effect of PVP formulation on the in vitro photodynamic efficiency of a photosensitizing phthalocyanine. J. Porphyr. Phthalocyanines. 2019;23:1587–1591. doi: 10.1142/S108842461950189X. [DOI] [Google Scholar]
- 57.Chin W.W.L., Heng P.W.S., Thong P.S.P., Bhuvaneswari R., Hirt W., Kuenzel S., Soo K.C., Olivo M. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur. J. Pharm. Biopharm. 2008;69:1083–1093. doi: 10.1016/j.ejpb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Mazzaglia A., Micali N., Scolaro L.M., Sciortino M.T., Sortino S., Villari V. Design of photosensitizer/cyclodextrin nanoassemblies: Spectroscopy, intracellular delivery and photodamage. J. Porphyr. Phthalocyanines. 2010;14:661–677. doi: 10.1142/S1088424610002562. [DOI] [Google Scholar]
- 59.Ben Mihoub A., Larue L., Moussaron A., Youssef Z., Colombeau L., Baros F., Frochot C., Vanderesse R., Acherar S. Use of Cyclodextrins in Anticancer Photodynamic Therapy Treatment. Molecules. 2018;23:1936. doi: 10.3390/molecules23081936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouterman M. 1—Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In: Dolphin D., editor. The Porphyrins. Academic Press; New York, NY, USA: 1978. pp. 1–165. [Google Scholar]
- 61.Ernst R.R., Bodenhausen G., Wokaun A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions. Clarendon Press; Oxford, UK: 1987. [Google Scholar]
- 62.Becker E.D., Bradley R.B. Effects of ‘‘Ring Currents’’ on the NMR Spectra of Porphyrins. J. Chem. Phys. 1959;31:1413–1414. doi: 10.1063/1.1730606. [DOI] [Google Scholar]
- 63.Scheer H., Katz J.J. Nuclear magnetic resonance spectroscopy of porphyrins and metalloporphyrins. In: Smith K.M., editor. Porphyrins and Metalloporphyrins. Elsevier; New York, NY, USA: 1975. pp. 399–524. [Google Scholar]
- 64.Keeler J. Understanding NMR Spectroscopy. 2nd ed. John Wiley and Sons; Chichester, UK: 2010. [Google Scholar]
- 65.Günther H. NMR Spectroscopy: Basic Principles, Concepts, and Applications in Chemistry. 3rd ed. Wiley-VCH; Weinheim, Germany: 2013. [Google Scholar]
- 66.Friebolin H. Basic One- and Two-Dimensional NMR Spectroscopy. 5th edition ed. Wiley-VCH; Weinheim, Germany: 2010. p. 418. [Google Scholar]
- 67.Karplus M. Contact Electron-Spin Coupling of Nuclear Magnetic Moments. J. Chem. Phys. 1959;30:11–15. doi: 10.1063/1.1729860. [DOI] [Google Scholar]
- 68.Karplus M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963;85:2870–2871. doi: 10.1021/ja00901a059. [DOI] [Google Scholar]
- 69.Minch M.J. Orientational dependence of vicinal proton-proton NMR coupling constants: The Karplus relationship. Concepts Magn. Reson. 1994;6:41–56. doi: 10.1002/cmr.1820060104. [DOI] [Google Scholar]
- 70.Pretsch E., Clerc T., Seibl J., Simon W. Tabellen zur Strukturaufklärung Organischer Verbindungen Mit Spektroskopischen Methoden. Springer; Berlin/Heidelberg, Germany: 1981. Protonenresonanzspektroskopie; pp. 108–181. [Google Scholar]
- 71.Pauling L. The Diamagnetic Anisotropy of Aromatic Molecules. J. Chem. Phys. 1936;4:673–677. doi: 10.1063/1.1749766. [DOI] [Google Scholar]
- 72.Pople J.A. Proton Magnetic Resonance of Hydrocarbons. J. Chem. Phys. 1956;24:1111. doi: 10.1063/1.1742701. [DOI] [Google Scholar]
- 73.Waugh J.S., Fessenden R.W. Nuclear Resonance Spectra of Hydrocarbons: The Free Electron Model. J. Am. Chem. Soc. 1957;79:846–849. doi: 10.1021/ja01561a017. [DOI] [Google Scholar]
- 74.Waugh J., Fessendn R. Additions and Corrections: Nuclear Resonance Spectra of Hydrocarbons: The Free Electron Model. J. Am. Chem. Soc. 1958;80:6697–6699. doi: 10.1021/ja01557a612. [DOI] [Google Scholar]
- 75.Johnson C.E., Bovey F.A. Calculation of Nuclear Magnetic Resonance Spectra of Aromatic Hydrocarbons. J. Chem. Phys. 1958;29:1012–1014. doi: 10.1063/1.1744645. [DOI] [Google Scholar]
- 76.Abraham R.J. A ring current model for the heme ring. J. Magn. Reson. 1981;43:491–494. doi: 10.1016/0022-2364(81)90063-9. [DOI] [Google Scholar]
- 77.Abraham R.J., Bedford G.R., Wright B. The NMR spectra of the porphyrins. 17—Metalloporphyrins as diamagnetic shift reagents, structural and specificity studies. Org. Magn. Reson. 1982;18:45–52. doi: 10.1002/mrc.1270180111. [DOI] [Google Scholar]
- 78.Abraham R.J., Bedford G.R., McNeillie D., Wright B. The NMR spectra of the porphyrins 16—zinc(II) meso-tetraphenylporphyrin (Zn TPP) as a diamagnetic shift reagent. A quantitative ring current model. Org. Magn. Reson. 1980;14:418–425. doi: 10.1002/mrc.1270140519. [DOI] [Google Scholar]
- 79.Kadish K.M., Smith K.M., Guilard R. NMR and EPR. 1st ed. Volume 5. Elsevier Science Publishing Co Inc.; San Diego, CA, USA: 1999. p. 360. [Google Scholar]
- 80.Hunter C.A., Sanders J.K.M. The nature of.pi.-.pi. interactions. J. Am. Chem. Soc. 1990;112:5525–5534. doi: 10.1021/ja00170a016. [DOI] [Google Scholar]
- 81.Abraham R.J., Smith K.M. NMR spectra of porphyrins. 21. Applications of the ring-current model to porphyrin and chlorophyll aggregation. J. Am. Chem. Soc. 1983;105:5734–5741. doi: 10.1021/ja00356a005. [DOI] [Google Scholar]
- 82.Abraham R.J., Rowan A.E., Smith N.W., Smith K.M. NMR spectra of the porphyrins. Part 42. The synthesis and aggregation behaviour of some chlorophyll analogues. J. Chem. Soc. Perkin Trans. 2. 1993:1047–1059. doi: 10.1039/p29930001047. [DOI] [Google Scholar]
- 83.Hynninen P.H., Lötjönen S. Effects of π−π interactions on the 1H-NMR spectra and solution structures of pheophytin a and a′ dimers. Biochim. Biophys. Acta. 1993;1183:374–380. doi: 10.1016/0005-2728(93)90242-8. [DOI] [Google Scholar]
- 84.Vermathen M., Marzorati M., Bigler P. Self-assembling properties of porphyrinic photosensitizers and their effect on membrane interactions probed by NMR spectroscopy. J. Phys. Chem. B. 2013;117:6990–7001. doi: 10.1021/jp403331n. [DOI] [PubMed] [Google Scholar]
- 85.Williamson M.P. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 2013;73:1–16. doi: 10.1016/j.pnmrs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Yu Z., Li P., Merz K.M. Using Ligand-Induced Protein Chemical Shift Perturbations To Determine Protein–Ligand Structures. Biochemistry. 2017;56:2349–2362. doi: 10.1021/acs.biochem.7b00170. [DOI] [PubMed] [Google Scholar]
- 87.Hunashal Y., Cantarutti C., Giorgetti S., Marchese L., Molinari H., Niccolai N., Fogolari F., Esposito G. Exploring exchange processes in proteins by paramagnetic perturbation of NMR spectra. Phys. Chem. Chem. Phys. 2020;22:6247–6259. doi: 10.1039/C9CP06950J. [DOI] [PubMed] [Google Scholar]
- 88.Mayo B.C. Lanthanide shift reagents in nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 1973;2:49–74. doi: 10.1039/cs9730200049. [DOI] [Google Scholar]
- 89.von Ammon R., Fischer R.D. Shift Reagents in NMR Spectroscopy. Angew. Chem. Int. Ed. 1972;11:675–692. doi: 10.1002/anie.197206751. [DOI] [Google Scholar]
- 90.Neuhaus D., Williamson M.P. The Nuclear Overhauser Effect in Structural and Conformational Analysis. Wiley; New York, NY, USA: 2000. p. 656. [Google Scholar]
- 91.Macura S., Huang Y., Suter D., Ernst R.R. Two-dimensional chemical exchange and cross-relaxation spectroscopy of coupled nuclear spins. J. Magn. Reson. 1981;43:259–281. doi: 10.1016/0022-2364(81)90037-8. [DOI] [Google Scholar]
- 92.Macura S., Ernst R.R. Elucidation of cross relaxation in liquids by two-dimensional N.M.R. spectroscopy. Mol. Phys. 1980;41:95–117. doi: 10.1080/00268978000102601. [DOI] [Google Scholar]
- 93.Kumar A., Ernst R.R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem. Biophys. Res. Commun. 1980;95:1–6. doi: 10.1016/0006-291X(80)90695-6. [DOI] [PubMed] [Google Scholar]
- 94.Butts C.P., Jones C.R., Towers E.C., Flynn J.L., Appleby L., Barron N.J. Interproton distance determinations by NOE—Surprising accuracy and precision in a rigid organic molecule. Org. Biomol. Chem. 2011;9:177–184. doi: 10.1039/C0OB00479K. [DOI] [PubMed] [Google Scholar]
- 95.Jones C.R., Butts C.P., Harvey J.N. Accuracy in determining interproton distances using Nuclear Overhauser Effect data from a flexible molecule. Beilstein J. Org. Chem. 2011;7:145–150. doi: 10.3762/bjoc.7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vögeli B., Segawa T.F., Leitz D., Sobol A., Choutko A., Trzesniak D., van Gunsteren W., Riek R. Exact Distances and Internal Dynamics of Perdeuterated Ubiquitin from NOE Buildups. J. Am. Chem. Soc. 2009;131:17215–17225. doi: 10.1021/ja905366h. [DOI] [PubMed] [Google Scholar]
- 97.Bothner-By A.A., Stephens R.L., Lee J., Warren C.D., Jeanloz R.W. Structure determination of a tetrasaccharide: Transient nuclear Overhauser effects in the rotating frame. J. Am. Chem. Soc. 1984;106:811–813. doi: 10.1021/ja00315a069. [DOI] [Google Scholar]
- 98.Bauer C.J., Frenkiel T.A., Lane A.N. A comparison of the ROESY and NOESY experiments for large molecules, with application to nucleic acids. J. Magn. Reson. 1990;87:144–152. doi: 10.1016/0022-2364(90)90092-N. [DOI] [Google Scholar]
- 99.Williamson M.P. Nuclear Magnetic Resonance Spectroscopy | Nuclear Overhauser Effect. In: Worsfold P., Poole C., Townshend A., Miró M., editors. Encyclopedia of Analytical Science. 3rd ed. Academic Press; Oxford, UK: 2019. pp. 264–271. [Google Scholar]
- 100.Stejskal E.O., Tanner J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965;42:288–292. doi: 10.1063/1.1695690. [DOI] [Google Scholar]
- 101.Cohen Y., Avram L., Frish L. Diffusion NMR Spectroscopy in Supramolecular and Combinatorial Chemistry: An Old Parameter—New Insights. Angew. Chem. Int. Ed. 2005;44:520–554. doi: 10.1002/anie.200300637. [DOI] [PubMed] [Google Scholar]
- 102.Johnson C.S. Diffusion ordered nuclear magnetic resonance spectroscopy: Principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999;34:203–256. doi: 10.1016/S0079-6565(99)00003-5. [DOI] [Google Scholar]
- 103.Groves P. Diffusion ordered spectroscopy (DOSY) as applied to polymers. Polym. Chem. 2017;8:6700–6708. doi: 10.1039/C7PY01577A. [DOI] [Google Scholar]
- 104.Morris K.F., Johnson C.S. Diffusion-ordered two-dimensional nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1992;114:3139–3141. doi: 10.1021/ja00034a071. [DOI] [Google Scholar]
- 105.Balayssac S., Delsuc M.-A., Gilard V., Prigent Y., Malet-Martino M. Two-dimensional DOSY experiment with Excitation Sculpting water suppression for the analysis of natural and biological media. J. Magn. Reson. 2009;196:78–83. doi: 10.1016/j.jmr.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 106.Simpson A.J. Determining the molecular weight, aggregation, structures and interactions of natural organic matter using diffusion ordered spectroscopy. Magn. Reson. Chem. 2002;40:S72–S82. doi: 10.1002/mrc.1106. [DOI] [Google Scholar]
- 107.Colbourne A.A., Morris G.A., Nilsson M. Local Covariance Order Diffusion-Ordered Spectroscopy: A Powerful Tool for Mixture Analysis. J. Am. Chem. Soc. 2011;133:7640–7643. doi: 10.1021/ja2004895. [DOI] [PubMed] [Google Scholar]
- 108.Pagès G., Gilard V., Martino R., Malet-Martino M. Pulsed-field gradient nuclear magnetic resonance measurements (PFG NMR) for diffusion ordered spectroscopy (DOSY) mapping. Analyst. 2017;142:3771–3796. doi: 10.1039/C7AN01031A. [DOI] [PubMed] [Google Scholar]
- 109.Cohen Y., Avram L., Evan-Salem T., Frish L. Diffusion NMR in Supramolecular Chemistry. In: Schalley C., editor. Analytical Methods in Supramolecular Chemistry. Wiley-VCH; Weinheim, Germany: 2006. pp. 163–219. [Google Scholar]
- 110.Kharlamov S.V., Latypov S.K. Modern diffusion-ordered NMR spectroscopy in chemistry of supramolecular systems: The scope and limitations. Russ. Chem. Rev. 2010;79:635–653. doi: 10.1070/RC2010v079n08ABEH004148. [DOI] [Google Scholar]
- 111.Bakkour Y., Darcos V., Li S., Coudane J. Diffusion ordered spectroscopy (DOSY) as a powerful tool for amphiphilic block copolymer characterization and for critical micelle concentration (CMC) determination. Polym. Chem. 2012;3:2006–2010. doi: 10.1039/c2py20054f. [DOI] [Google Scholar]
- 112.Khodov I.A., Alper G.A., Mamardashvili G.M., Mamardashvili N.Z. Hybrid multi-porphyrin supramolecular assemblies: Synthesis and structure elucidation by 2D DOSY NMR studies. J. Mol. Struct. 2015;1099:174–180. doi: 10.1016/j.molstruc.2015.06.062. [DOI] [Google Scholar]
- 113.Johnston M.R., Latter M.J. Characterization of porphyrin supramolecular complexes using NMR diffusion spectroscopy. J. Porphyr. Phthalocyanines. 2002;6:757–762. doi: 10.1142/S1088424602000877. [DOI] [Google Scholar]
- 114.Chatzigiannis C.M., Kiriakidi S., Tzakos A.G., Mavromoustakos T. 2D DOSYTwo-dimensional diffusion-ordered NMR spectroscopy (2D DOSY) NMR: A Valuable Tool to Confirm the Complexation in Drug Delivery Systems. In: Mavromoustakos T., Tzakos A.G., Durdagi S., editors. Supramolecules in Drug Discovery and Drug Delivery: Methods and Protocols. Springer; New York, NY, USA: 2021. pp. 235–246. [DOI] [PubMed] [Google Scholar]
- 115.Momot K.I., Kuchel P.W. Pulsed field gradient nuclear magnetic resonance as a tool for studying drug delivery systems. Concepts Magn. Reson. Part A. 2003;19:51–64. doi: 10.1002/cmr.a.10092. [DOI] [Google Scholar]
- 116.Bruker Corporation . Almanac—Bruker Corporation [Brochure]: Analytical Tables and Product Overview. Bruker Corporation; Billerica, MA, USA: 2012. Innovation with Integrity; Analytical Solutions; T8-T13. [Google Scholar]
- 117.Harris R.K. Nmr and the periodic table. Chem. Soc. Rev. 1976;5:1–22. doi: 10.1039/cs9760500001. [DOI] [Google Scholar]
- 118.Patching S.G. NMR active nuclei for biological and biomedical applications. J. Diagn. Imaging Ther. 2016;3:7–48. doi: 10.17229/jdit.2016-0618-021. [DOI] [Google Scholar]
- 119.Cavanagh J., Fairbrother W.J., Palmer A.G., Rance M., Skelton N.J. Chapter 7—Heteronuclear nmr experiments. In: Cavanagh J., Fairbrother W.J., Palmer A.G., Rance M., Skelton N.J., editors. Protein NMR Spectroscopy. 2nd ed. Academic Press; Burlington, NJ, USA: 2007. pp. 533–678. [Google Scholar]
- 120.Watts A. NMR of Lipids. In: Roberts G.C.K., editor. Encyclopedia of Biophysics. Springer; Berlin/Heidelberg, Germany: 2013. pp. 1727–1738. [Google Scholar]
- 121.Nieto L., Jiménez-Barbero J. Carbohydrate NMR Spectroscopy. In: Roberts G.C.K., editor. Encyclopedia of Biophysics. Springer; Berlin/Heidelberg, Germany: 2013. pp. 228–232. [Google Scholar]
- 122.Marion D. An introduction to biological NMR spectroscopy. Mol. Cell. Proteom. MCP. 2013;12:3006–3025. doi: 10.1074/mcp.O113.030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lindon J.C. Nuclear Magnetic Resonance Spectroscopy | Fluorine-19. In: Worsfold P., Poole C., Townshend A., Miró M., editors. Encyclopedia of Analytical Science. 3rd ed. Academic Press; Oxford, UK: 2016. pp. 141–151. [Google Scholar]
- 124.Goslinski T., Piskorz J. Fluorinated porphyrinoids and their biomedical applications. J. Photochem. Photobiol. C. 2011;12:304–321. doi: 10.1016/j.jphotochemrev.2011.09.005. [DOI] [Google Scholar]
- 125.Oz G., Pountney D.L., Armitage I.M. NMR spectroscopic studies of I = 1/2 metal ions in biological systems. Biochem Cell Biol. 1998;76:223–234. doi: 10.1139/o98-059. [DOI] [PubMed] [Google Scholar]
- 126.Moan J., Berg K., Kvam E., Western A., Malik Z., Rück A., Schneckenburger H. Intracellular Localization of Photosensitizers. In: Bock G., Harnett S., editors. Ciba Foundation Symposium 146—Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use. Wiley Online Library; Chichester, UK: 2007. pp. 95–111. [DOI] [PubMed] [Google Scholar]
- 127.Lavi A., Weitman H., Holmes R.T., Smith K.M., Ehrenberg B. The Depth of Porphyrin in a Membrane and the Membrane’s Physical Properties Affect the Photosensitizing Efficiency. Biophys. J. 2002;82:2101–2110. doi: 10.1016/S0006-3495(02)75557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Warschawski D.E., Arnold A.A., Beaugrand M., Gravel A., Chartrand É., Marcotte I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim. Biophys. Acta. 2011;1808:1957–1974. doi: 10.1016/j.bbamem.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 129.Da Costa G., Mouret L., Chevance S., Le Rumeur E., Bondon A. NMR of molecules interacting with lipids in small unilamellar vesicles. Eur. Biophys. J. 2007;36:933–942. doi: 10.1007/s00249-007-0186-7. [DOI] [PubMed] [Google Scholar]
- 130.Da Costa G., Chevance S., Le Rumeur E., Bondon A. Proton NMR detection of porphyrins and cytochrome C in small unilamellar vesicles: Role of the dissociation kinetic constant. Biophys. J. 2006;90:L55–L57. doi: 10.1529/biophysj.106.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vermathen M., Vermathen P., Simonis U., Bigler P. Time-dependent interactions of the two porphyrinic compounds chlorin e6 and mono-L-aspartyl-chlorin e6 with phospholipid vesicles probed by NMR spectroscopy. Langmuir. 2008;24:12521–12533. doi: 10.1021/la802040v. [DOI] [PubMed] [Google Scholar]
- 132.Barsukov L.I., Victorov A.V., Vasilenko I.A., Evstigneeva R.P., Bergelson L.D. Investigation of the inside-outside distribution, intermembrane exchange and transbilayer movement of phospholipids in sonicated vesicles by shift reagent NMR. Biochim. Biophys. Acta. 1980;598:153–168. doi: 10.1016/0005-2736(80)90273-4. [DOI] [PubMed] [Google Scholar]
- 133.Vermathen M., Marzorati M., Vermathen P., Bigler P. pH-dependent distribution of chlorin e6 derivatives across phospholipid bilayers probed by NMR spectroscopy. Langmuir. 2010;26:11085–11094. doi: 10.1021/la100679y. [DOI] [PubMed] [Google Scholar]
- 134.Marzorati M., Bigler P., Vermathen M. Interactions between selected photosensitizers and model membranes: An NMR classification. Biochim. Biophys. Acta. 2011;1808:1661–1672. doi: 10.1016/j.bbamem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 135.Allen T.M., Cullis P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 136.Temizel E., Sagir T., Ayan E., Isik S., Ozturk R. Delivery of lipophilic porphyrin by liposome vehicles: Preparation and photodynamic therapy activity against cancer cell lines. Photodiagnosis Photodyn. Ther. 2014;11:537–545. doi: 10.1016/j.pdpdt.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 137.Sadasivam M., Avci P., Gupta G.K., Lakshmanan S., Chandran R., Huang Y.Y., Kumar R., Hamblin M.R. Self-assembled liposomal nanoparticles in photodynamic therapy. Eur. J. Nanomed. 2013;5 doi: 10.1515/ejnm-2013-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ikeda A., Hino S., Mae T., Tsuchiya Y., Sugikawa K., Tsukamoto M., Yasuhara K., Shigeto H., Funabashi H., Kuroda A., et al. Porphyrin-uptake in liposomes and living cells using an exchange method with cyclodextrin. RSC Adv. 2015;5:105279–105287. doi: 10.1039/C5RA24985F. [DOI] [Google Scholar]
- 139.Nakaya T., Tsuchiya Y., Horiguchi B., Sugikawa K., Komaguchi K., Ikeda A. 1H NMR Determination of Incorporated Porphyrin Location in Lipid Membranes of Liposomes. Bull. Chem. Soc. Jpn. 2018;91:1337–1342. doi: 10.1246/bcsj.20180115. [DOI] [Google Scholar]
- 140.Bertini I., Turano P., Vila A.J. Nuclear magnetic resonance of paramagnetic metalloproteins. Chem. Rev. 1993;93:2833–2932. doi: 10.1021/cr00024a009. [DOI] [Google Scholar]
- 141.Stojanović S., Isenović E.R., Zarić B.L. Non-canonical interactions of porphyrins in porphyrin-containing proteins. Amino Acids. 2012;43:1535–1546. doi: 10.1007/s00726-012-1228-8. [DOI] [PubMed] [Google Scholar]
- 142.Mitchell J., Yanamala N., Tan Y.L., Gardner E.E., Tirupula K.C., Balem F., Sheves M., Nietlispach D., Klein-Seetharaman J. Structural and Functional Consequences of the Weak Binding of Chlorin e6 to Bovine Rhodopsin. Photochem. Photobiol. 2019;95:787–802. doi: 10.1111/php.13074. [DOI] [PubMed] [Google Scholar]
- 143.Gjuroski I., Girousi E., Meyer C., Hertig D., Stojkov D., Fux M., Schnidrig N., Bucher J., Pfister S., Sauser L., et al. Evaluation of polyvinylpyrrolidone and block copolymer micelle encapsulation of serine chlorin e6 and chlorin e4 on their reactivity towards albumin and transferrin and their cell uptake. J. Control. Release. 2019;316:150–167. doi: 10.1016/j.jconrel.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 144.Fiel R.J., Howard J.C., Mark E.H., Gupta N.D. Interaction of DNA with a porphyrin ligand: Evidence for intercalation. Nucleic Acids Res. 1979;6:3093–3118. doi: 10.1093/nar/6.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fiel R.J., Munson B.R. Binding of meso-tetra (4-N-methylpyridyl) porphine to DNA. Nucleic Acids Res. 1980;8:2835–2842. doi: 10.1093/nar/8.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carvlin M.J., Fiel R.J. Intercalative and nonintercalative binding of large cationic porphyrin ligands to calf thymus DNA. Nucleic Acids Res. 1983;11:6121–6139. doi: 10.1093/nar/11.17.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banville D.L., Marzilli L.G., Wilson W.D. 31P NMR and viscometric studies of the interaction of meso-tetra(4-N-methyl-pyridyl)porphine and its Ni(II) and Zn(II) derivatives with DNA. Biochem. Biophys. Res. Commun. 1983;113:148–154. doi: 10.1016/0006-291X(83)90444-8. [DOI] [PubMed] [Google Scholar]
- 148.Fiel R.J. Porphyrin—Nucleic Acid Interactions: A Review. J. Biomol. Struct. Dyn. 1989;6:1259–1274. doi: 10.1080/07391102.1989.10506549. [DOI] [PubMed] [Google Scholar]
- 149.Pratviel G. Porphyrins in complex with DNA: Modes of interaction and oxidation reactions. Coord. Chem. Rev. 2016;308:460–477. doi: 10.1016/j.ccr.2015.07.003. [DOI] [Google Scholar]
- 150.Wang Y., Dong Z., Hu H., Yang Q., Hou X., Wu P. DNA-modulated photosensitization: Current status and future aspects in biosensing and environmental monitoring. Anal. Bioanal. Chem. 2019;411:4415–4423. doi: 10.1007/s00216-019-01605-8. [DOI] [PubMed] [Google Scholar]
- 151.Zhao Y.-M., Lu Q.-Q., Yao S., Su H.-F., Liu H.-J., Wang Z.-J., Wu F.-S., Wang K. N-Methylpyridylporphyrin tailed with folate conjugate as a potential lysosomal-targeted photosensitizer: Synthesis, DNA interaction, singlet oxygen and subcellular localization. J. Porphyr. Phthalocyanines. 2019;23:679–684. doi: 10.1142/S1088424619500445. [DOI] [Google Scholar]
- 152.Hirakawa K., Hirano T., Nishimura Y., Arai T., Nosaka Y. Control of Singlet Oxygen Generation Photosensitized by meso-Anthrylporphyrin through Interaction with DNA. Photochem. Photobiol. 2011;87:833–839. doi: 10.1111/j.1751-1097.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 153.Hirakawa K., Harada M., Okazaki S., Nosaka Y. Controlled generation of singlet oxygen by a water-soluble meso-pyrenylporphyrin photosensitizer through interaction with DNA. Chem. Commun. 2012;48:4770–4772. doi: 10.1039/c2cc30880k. [DOI] [PubMed] [Google Scholar]
- 154.Hirakawa K., Nishimura Y., Arai T., Okazaki S. Singlet Oxygen Generating Activity of an Electron Donor Connecting Porphyrin Photosensitizer Can Be Controlled by DNA. J. Phys. Chem. B. 2013;117:13490–13496. doi: 10.1021/jp4072444. [DOI] [PubMed] [Google Scholar]
- 155.Hirakawa K., Taguchi M., Okazaki S. Relaxation Process of Photoexcited meso-Naphthylporphyrins while Interacting with DNA and Singlet Oxygen Generation. J. Phys. Chem. B. 2015;119:13071–13078. doi: 10.1021/acs.jpcb.5b08025. [DOI] [PubMed] [Google Scholar]
- 156.López-Cebral R., Martín-Pastor M., Seijo B., Sanchez A. Progress in the characterization of bio-functionalized nanoparticles using NMR methods and their applications as MRI contrast agents. Prog. Nucl. Magn. Reson. Spectrosc. 2014;79:1–13. doi: 10.1016/j.pnmrs.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 157.Haaf F., Sanner A., Straub F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985;17:143–152. doi: 10.1295/polymj.17.143. [DOI] [Google Scholar]
- 158.Luo Y., Hong Y., Shen L., Wu F., Lin X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech. 2021;22:34. doi: 10.1208/s12249-020-01909-4. [DOI] [PubMed] [Google Scholar]
- 159.Franco P., De Marco I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers. 2020;12:1114. doi: 10.3390/polym12051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Copley L., van der Watt P., Wirtz K.W., Parker M.I., Leaner V.D. Photolon™, a chlorin e6 derivative, triggers ROS production and light-dependent cell death via necrosis. Int. J. Biochem. Cell Biol. 2008;40:227–235. doi: 10.1016/j.biocel.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Zhiyentayev T.M., Boltaev U.T., Solov’eva A.B., Aksenova N.A., Glagolev N.N., Chernjak A.V., Melik-Nubarov N.S. Complexes of Chlorin e6 with Pluronics and Polyvinylpyrrolidone: Structure and Photodynamic Activity in Cell Culture. Photochem. Photobiol. 2014;90:171–182. doi: 10.1111/php.12181. [DOI] [PubMed] [Google Scholar]
- 162.Tsvetkov V.B., Solov’eva A.B., Melik-Nubarov N.S. Computer modeling of the complexes of Chlorin e6 with amphiphilic polymers. Phys. Chem. Chem. Phys. 2014;16:10903–10913. doi: 10.1039/C3CP55510K. [DOI] [PubMed] [Google Scholar]
- 163.Hädener M., Gjuroski I., Furrer J., Vermathen M. Interactions of Polyvinylpyrrolidone with Chlorin e6-Based Photosensitizers Studied by NMR and Electronic Absorption Spectroscopy. J. Phys. Chem. B. 2015;119:12117–12128. doi: 10.1021/acs.jpcb.5b05761. [DOI] [PubMed] [Google Scholar]
- 164.Solov’eva A.B., Khasanova O.V., Aksenova N.A., Chernyak A.V., Volkov V.I., Timofeeva V.A., Timashev P.S. The Influence of Effect of Polysaccharides and Polyvinylpyrrolidone on the Photocatalytic Activity of Chlorin e6 in Tryptophan Oxidation. Russ. J. Phys. Chem. A. 2019;93:2507–2514. doi: 10.1134/S0036024419110293. [DOI] [Google Scholar]
- 165.Gjuroski I., Furrer J., Vermathen M. How Does the Encapsulation of Porphyrinic Photosensitizers into Polymer Matrices Affect Their Self-Association and Dynamic Properties? Chemphyschem. 2018;19:1089–1102. doi: 10.1002/cphc.201701318. [DOI] [PubMed] [Google Scholar]
- 166.Aksenova N.A., Oles T., Sarna T., Glagolev N.N., Chernjak A.V., Volkov V.I., Kotova S.L., Melik-Nubarov N.S., Solovieva A.B. Development of novel formulations for photodynamic therapy on the basis of amphiphilic polymers and porphyrin photosensitizers. Porphyrin-polymer complexes in model photosensitized processes. Laser Phys. 2012;22:1642–1649. doi: 10.1134/S1054660X12100015. [DOI] [Google Scholar]
- 167.Solovieva A.B., Aksenova N.A. Porphyrin-Polymer Complexes in Model Photosensitized Processes and in Photodynamic Therapy. In: Berlin A.A., Rogovina S.Z., Zaikov G.E., editors. Additives in Polymers. Apple Academic Press; New York, NY, USA: 2015. pp. 77–123. [Google Scholar]
- 168.Küçük T., Alpugan S., Davarcı D., Pehlivan E.G., Bayır S., Tazebay U.H., Dumoulin F. Photoproperties, PVP formulation and 19F NMR of a Zn phthalocyanine with 24 magnetically pseudo-equivalent fluorine atoms. J. Porphyr. Phthalocyanines. 2019;23:611–618. doi: 10.1142/S1088424619500512. [DOI] [Google Scholar]
- 169.Schneider H.-J., Hacket F., Rüdiger V., Ikeda H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998;98:1755–1786. doi: 10.1021/cr970019t. [DOI] [PubMed] [Google Scholar]
- 170.Gonzalez M.C., Weedon A.C. Preparation and properties of a linked porphyrin–cyclodextrin. Can. J. Chem. 1985;63:602–608. doi: 10.1139/v85-099. [DOI] [Google Scholar]
- 171.Kuroda Y., Hiroshige T., Sera T., Shiroiwa Y., Tanaka H., Ogoshi H. Cyclodextrin-sandwiched porphyrin. J. Am. Chem. Soc. 1989;111:1912–1913. doi: 10.1021/ja00187a073. [DOI] [Google Scholar]
- 172.Dick D.L., Rao T.V.S., Sukumaran D., Lawrence D.S. Molecular encapsulation: Cyclodextrin-based analogs of heme-containing proteins. J. Am. Chem. Soc. 1992;114:2664–2669. doi: 10.1021/ja00033a046. [DOI] [Google Scholar]
- 173.Kano K., Kitagishi H., Dagallier C., Kodera M., Matsuo T., Hayashi T., Hisaeda Y., Hirota S. Iron Porphyrin−Cyclodextrin Supramolecular Complex as a Functional Model of Myoglobin in Aqueous Solution. Inorg. Chem. 2006;45:4448–4460. doi: 10.1021/ic060137b. [DOI] [PubMed] [Google Scholar]
- 174.Carofiglio T., Fornasier R., Lucchini V., Rosso C., Tonellato U. Very strong binding and mode of complexation of water-soluble porphyrins with a permethylated β-cyclodextrin. Tetrahedron Lett. 1996;37:8019–8022. doi: 10.1016/0040-4039(96)01814-X. [DOI] [Google Scholar]
- 175.Kadish K.M., Maiya G.B., Araullo C., Guilard R. Micellar effects on the aggregation of tetraanionic porphyrins. Spectroscopic characterization of free-base meso-tetrakis(4-sulfonatophenyl)porphyrin, (TPPS)H2, and (TPPS)M (M = zinc(II), copper(II), and vanadyl) in aqueous micellar media. Inorg. Chem. 1989;28:2725–2731. doi: 10.1021/ic00313a008. [DOI] [Google Scholar]
- 176.Ribó J.M., Farrera J.-A., Valero M.L., Virgili A. Self-assembly of cyclodextrins with meso-tetrakis(4-sulfonatophenyl)porphyrin in aqueous solution. Tetrahedron. 1995;51:3705–3712. doi: 10.1016/0040-4020(95)00085-M. [DOI] [Google Scholar]
- 177.Sur S.K., Bryant R.G. Spin-Lattice Relaxation Enhancement of Water Protons by Manganese Porphyrins Complexed with Cyclodextrins. J. Phys. Chem. 1995;99:4900–4905. doi: 10.1021/j100014a005. [DOI] [Google Scholar]
- 178.Mosseri S., Mialocq J.C., Perly B., Hambright P. Porhyrins-cyclodextrin 1 Photooxidation of zinc tetrakis(4-sulfonatophenyl)porphyrin in cyclodextrin cavities: The characterization of ZnTSPP dication Photolysis, radiolysis, and NMR studies. J. Phys. Chem. 1991;95:2196–2203. doi: 10.1021/j100159a021. [DOI] [Google Scholar]
- 179.Mosinger J., Kliment V., Jr., Sejbal J., Kubát P., Lang K. Host-guest complexes of anionic porphyrin sensitizers with cyclodextrins. J. Porphyr. Phthalocyanines. 2002;6:514–526. doi: 10.1142/S1088424602000646. [DOI] [Google Scholar]
- 180.Kano K., Nishiyabu R., Asada T., Kuroda Y. Static and Dynamic Behavior of 2:1 Inclusion Complexes of Cyclodextrins and Charged Porphyrins in Aqueous Organic Media. J. Am. Chem. Soc. 2002;124:9937–9944. doi: 10.1021/ja020253n. [DOI] [PubMed] [Google Scholar]
- 181.Ma H.L., Wu J.J., Liang W.J., Chao J.B. Study on the Association Phenomenon of Cyclodextrin to Porphyrin J-aggregates by NMR Spectroscopy. J. Incl. Phenom. Macrocycl. Chem. 2007;58:221–226. doi: 10.1007/s10847-006-9146-6. [DOI] [Google Scholar]
- 182.El-Hachemi Z., Farrera J.-A., Garcia-Ortega H., Ramirez-Gutierrez O., Ribo J.M. Heteroassociation of meso-sulfonatophenylporphyrins with β- and γ-cyclodextrin. J. Porphyr. Phthalocyanines. 2001;05:465–473. doi: 10.1002/jpp.346. [DOI] [Google Scholar]
- 183.Venema F., Rowan A.E., Nolte R.J.M. Binding of Porphyrins in Cyclodextrin Dimers. J. Am. Chem. Soc. 1996;118:257–258. doi: 10.1021/ja952401y. [DOI] [Google Scholar]
- 184.Mosinger J., Slavětínská L., Lang K., Coufal P., Kubát P. Cyclodextrin carriers of positively charged porphyrin sensitizers. Org. Biomol. Chem. 2009;7:3797–3804. doi: 10.1039/b908772a. [DOI] [PubMed] [Google Scholar]
- 185.Xiliang G., Shaomin S., Chuan D., Feng F., Wong M.S. Comparative study on the inclusion behavior between meso-tetrakis(4-N-ethylpyridiniurmyl)porphyrin and β-cyclodextrin derivatives. Spectrochim. Acta A. 2005;61:413–418. doi: 10.1016/j.saa.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 186.Khurana R., Kakatkar A.S., Chatterjee S., Barooah N., Kunwar A., Bhasikuttan A.C., Mohanty J. Supramolecular Nanorods of (N-Methylpyridyl) Porphyrin With Captisol: Effective Photosensitizer for Anti-bacterial and Anti-tumor Activities. Front. Chem. 2019;7 doi: 10.3389/fchem.2019.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo Y.-J., Chao J.-B., Pan J.-H. Study on the interaction of 5-pyridine-10,15,20-tris-(p-chlorophenyl)porphyrin with cyclodextrins and DNA by spectroscopy. Spectrochim. Acta A. 2007;68:231–236. doi: 10.1016/j.saa.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 188.Cosma P., Catucci L., Fini P., Dentuto P.L., Agostiano A., Angelini N., Scolaro L.M. Tetrakis(4-pyridyl)porphyrin supramolecular complexes with cyclodextrins in aqueous solution. Photochem. Photobiol. 2006;82:563–569. doi: 10.1562/2005-09-26-RA-694. [DOI] [PubMed] [Google Scholar]
- 189.Notsu S., Sugikawa K., Ikeda A. Reversible Supramolecular System of Porphyrin Exchange between Inclusion in Cyclodextrin and Intercalation in DNA by Change in pH. ChemistrySelect. 2018;3:5900–5904. doi: 10.1002/slct.201801070. [DOI] [Google Scholar]
- 190.Ikeda A., Satake S., Mae T., Ueda M., Sugikawa K., Shigeto H., Funabashi H., Kuroda A. Photodynamic Activities of Porphyrin Derivative–Cyclodextrin Complexes by Photoirradiation. ACS Med. Chem. Lett. 2017;8:555–559. doi: 10.1021/acsmedchemlett.7b00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kitagishi H., Hatada S., Itakura T., Maki Y., Maeda Y., Kano K. Cellular uptake of octaarginine-conjugated tetraarylporphyrin included by per-O-methylated β-cyclodextrin. Org. Biomol. Chem. 2013;11:3203–3211. doi: 10.1039/c3ob27248f. [DOI] [PubMed] [Google Scholar]
- 192.Králová J., Kejík Z., Bříza T., Poučková P., Král A., Martásek P., Král V. Porphyrin−Cyclodextrin Conjugates as a Nanosystem for Versatile Drug Delivery and Multimodal Cancer Therapy. J. Med. Chem. 2010;53:128–138. doi: 10.1021/jm9007278. [DOI] [PubMed] [Google Scholar]
- 193.Puglisi A., Purrello R., Rizzarelli E., Sortino S., Vecchio G. Spectroscopic and self-association behavior of a porphyrin-β-cyclodextrin conjugate. New J. Chem. 2007;31:1499–1506. doi: 10.1039/b703680a. [DOI] [Google Scholar]
- 194.Mineo P. A porphyrin/β-cyclodextrin conjugated nano-system having a pan–lid molecular structure for smart drug carrier applications. Org. Biomol. Chem. 2014;12:3663–3670. doi: 10.1039/c4ob00393d. [DOI] [PubMed] [Google Scholar]
- 195.Zhao J., Zhang H.Y., Sun H.L., Liu Y. Supramolecular nanoassemblies of an amphiphilic porphyrin-cyclodextrin conjugate and their morphological transition from vesicle to network. Chemistry. 2015;21:4457–4464. doi: 10.1002/chem.201405943. [DOI] [PubMed] [Google Scholar]
- 196.Sun M., Zhang H.-Y., Liu B.-W., Liu Y. Construction of a Supramolecular Polymer by Bridged Bis(permethyl-β-cyclodextrin)s with Porphyrins and Its Highly Efficient Magnetic Resonance Imaging. Macromolecules. 2013;46:4268–4275. doi: 10.1021/ma400806s. [DOI] [Google Scholar]
- 197.Liu Y., Ke C.-F., Zhang H.-Y., Cui J., Ding F. Complexation-Induced Transition of Nanorod to Network Aggregates: Alternate Porphyrin and Cyclodextrin Arrays. J. Am. Chem. Soc. 2008;130:600–605. doi: 10.1021/ja075981v. [DOI] [PubMed] [Google Scholar]
- 198.Zhang H., Zhang B., Zhu M., Grayson S.M., Schmehl R., Jayawickramarajah J. Water-soluble porphyrin nanospheres: Enhanced photo-physical properties achieved via cyclodextrin driven double self-inclusion. Chem. Commun. 2014;50:4853–4855. doi: 10.1039/C4CC01372G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cerichelli G., Mancinit G. NMR techniques applied to micellar systems. Curr. Opin. Colloid Interface Sci. 1997;2:641–648. doi: 10.1016/S1359-0294(97)80058-1. [DOI] [Google Scholar]
- 200.Söderman O., Stilbs P., Price W.S. NMR studies of surfactants. Concepts in Magnetic Resonance Part A. 2004;23A:121–135. doi: 10.1002/cmr.a.20022. [DOI] [Google Scholar]
- 201.Cui X., Mao S., Liu M., Yuan H., Du Y. Mechanism of Surfactant Micelle Formation. Langmuir. 2008;24:10771–10775. doi: 10.1021/la801705y. [DOI] [PubMed] [Google Scholar]
- 202.Medhi O.K., Mazumdar S., Mitra S. Proton NMR and optical spectra and magnetic properties of four-coordinated intermediate-spin, five-coordinated high-spin, and six-coordinated low-spin iron(II) hemes encapsulated in aqueous detergent micelles: Model for hemoproteins. Inorg. Chem. 1989;28:3243–3248. doi: 10.1021/ic00315a033. [DOI] [Google Scholar]
- 203.Mazumdar S. Proton and carbon-13 NMR studies on the structure of micelles encapsulating hemes in aqueous sodium dodecyl sulfate solutions. J. Phys. Chem. 1990;94:5947–5953. doi: 10.1021/j100378a062. [DOI] [Google Scholar]
- 204.Mazumdar S., Medhi O.K., Mitra S. Stability and characterization of iron(III) and iron(II) heme peptides encapsulated in aqueous detergent micelles: Proton NMR and UV-visible spectroscopic studies. Inorg. Chem. 1991;30:700–705. doi: 10.1021/ic00004a020. [DOI] [Google Scholar]
- 205.Mazumdar S., Mitra S. Structures and Biological Effects. Springer; Berlin/Heidelberg, Germany: 1993. Biomimetic chemistry of hemes inside aqueous micelles; pp. 115–145. [Google Scholar]
- 206.Maiti N.C., Mazumdar S., Periasamy N. Dynamics of Porphyrin Molecules in Micelles. Picosecond Time-Resolved Fluorescence Anisotropy Studies. J. Phys. Chem. 1995;99:10708–10715. doi: 10.1021/j100027a006. [DOI] [Google Scholar]
- 207.Gandini S.C.M., Yushmanov V.E., Borissevitch I.E., Tabak M. Interaction of the Tetra(4-sulfonatophenyl)porphyrin with Ionic Surfactants: Aggregation and Location in Micelles. Langmuir. 1999;15:6233–6243. doi: 10.1021/la990108w. [DOI] [Google Scholar]
- 208.Yushmanov V.E. Aggregation of Fe(III)TPPS4 on Biological Structures Is pH-Dependent, Suggesting Oxo-Bridging in the Aggregates. Inorg. Chem. 1999;38:1713–1718. doi: 10.1021/ic980377u. [DOI] [PubMed] [Google Scholar]
- 209.Kadish K.M., Maiya B.G., Araullo-McAdams C. Spectroscopic characterization of meso-tetrakis(1-methylpyridinium-4-yl)porphyrins, [(TMpyP)H2]4+ and [(TMpyP)M]4+, in aqueous micellar media, where M = VO2+, Cu(II), and Zn(II) J. Phys. Chem. 1991;95:427–431. doi: 10.1021/j100154a075. [DOI] [Google Scholar]
- 210.Monsú Scolaro L., Donato C., Castriciano M., Romeo A., Romeo R. Micellar aggregates of platinum(II) complexes containing porphyrins. Inorg. Chim. Acta. 2000;300–302:978–986. doi: 10.1016/S0020-1693(99)00617-9. [DOI] [Google Scholar]
- 211.Vermathen M., Louie E.A., Chodosh A.B., Ried S., Simonis U. Interactions of Water-Insoluble Tetraphenylporphyrins with Micelles Probed by UV−Visible and NMR Spectroscopy. Langmuir. 2000;16:210–221. doi: 10.1021/la990903+. [DOI] [Google Scholar]
- 212.Maiti N.C., Mazumdar S., Periasamy N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B. 1998;102:1528–1538. doi: 10.1021/jp9723372. [DOI] [Google Scholar]
- 213.Qiu W.-G., Li Z.-F., Bai G.-M., Meng S.-N., Dai H.-X., He H. Interaction of water-soluble cationic porphyrin with anionic surfactant. Spectrochim. Acta A. 2007;68:1164–1169. doi: 10.1016/j.saa.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 214.Gandini S.C.M., Yushmanov V.E., Tabak M. Interaction of Fe(III)- and Zn(II)-tetra(4-sulfonatophenyl) porphyrins with ionic and nonionic surfactants: Aggregation and binding. J. Inorg. Biochem. 2001;85:263–277. doi: 10.1016/S0162-0134(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 215.Mamardashvili G.M., Kaigorodova E.Y., Khodov I.y.A., Scheblykin I., Mamardashvili N.Z., Koifman O.I. Micelles encapsulated Co(III)-tetra(4-sulfophenyl)porphyrin in aqueous CTAB solutions: Micelle formation, imidazole binding and redox Co(III)/Co(II) processes. J. Mol. Liq. 2019;293:111471. doi: 10.1016/j.molliq.2019.111471. [DOI] [Google Scholar]
- 216.Mamardashvili G.M., Kaigorodova E.Y., Simonova O.R., Lazovskiy D.A., Mamardashvili N.Z. Interaction of the Sn(IV)-tetra(4-sulfonatophenyl)porphyrin axial complexes with cetyltrimethylammonium bromide: Aggregation and location in micelles, fluorescence properties and photochemical stability. J. Mol. Liq. 2020;318:113988. doi: 10.1016/j.molliq.2020.113988. [DOI] [Google Scholar]
- 217.Cabral H., Miyata K., Osada K., Kataoka K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 218.Biswas S., Kumari P., Lakhani P.M., Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016;83:184–202. doi: 10.1016/j.ejps.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 219.Sakai-Kato K., Nishiyama N., Kozaki M., Nakanishi T., Matsuda Y., Hirano M., Hanada H., Hisada S., Onodera H., Harashima H., et al. General considerations regarding the in vitro and in vivo properties of block copolymer micelle products and their evaluation. J. Control. Release. 2015;210:76–83. doi: 10.1016/j.jconrel.2015.05.259. [DOI] [PubMed] [Google Scholar]
- 220.Houdaihed L., Evans J.C., Allen C. Overcoming the Road Blocks: Advancement of Block Copolymer Micelles for Cancer Therapy in the Clinic. Mol. Pharm. 2017;14:2503–2517. doi: 10.1021/acs.molpharmaceut.7b00188. [DOI] [PubMed] [Google Scholar]
- 221.Fraenza C.C., Mattea C., Farrher G.D., Ordikhani-Seyedlar A., Stapf S., Anoardo E. Rouse dynamics in PEO-PPO-PEO block-copolymers in aqueous solution as observed through fast field-cycling NMR relaxometry. Polymer. 2018;150:244–253. doi: 10.1016/j.polymer.2018.07.027. [DOI] [Google Scholar]
- 222.Walderhaug H., Söderman O. NMR studies of block copolymer micelles. Curr. Opin. Colloid Interface Sci. 2009;14:171–177. doi: 10.1016/j.cocis.2008.10.001. [DOI] [Google Scholar]
- 223.Ma J.-H., Guo C., Tang Y.-L., Liu H.-Z. 1H NMR Spectroscopic Investigations on the Micellization and Gelation of PEO−PPO−PEO Block Copolymers in Aqueous Solutions. Langmuir. 2007;23:9596–9605. doi: 10.1021/la701221f. [DOI] [PubMed] [Google Scholar]
- 224.Ma J.-H., Guo C., Tang Y.-L., Wang J., Zheng L., Liang X.-F., Chen S., Liu H.-Z. Salt-Induced Micellization of a Triblock Copolymer in Aqueous Solution: A 1H Nuclear Magnetic Resonance Spectroscopy Study. Langmuir. 2007;23:3075–3083. doi: 10.1021/la063203v. [DOI] [PubMed] [Google Scholar]
- 225.Nilsson M., Håkansson B., Söderman O., Topgaard D. Influence of Polydispersity on the Micellization of Triblock Copolymers Investigated by Pulsed Field Gradient Nuclear Magnetic Resonance. Macromolecules. 2007;40:8250–8258. doi: 10.1021/ma071302p. [DOI] [Google Scholar]
- 226.Pfister S., Sauser L., Gjuroski I., Furrer J., Vermathen M. Monitoring the encapsulation of chlorin e6 derivatives into polymer carriers by NMR spectroscopy. J. Porphyr. Phthalocyanines. 2019;23:1576–1586. doi: 10.1142/S1088424619501815. [DOI] [Google Scholar]
- 227.Izunobi J.U., Higginbotham C.L. Polymer Molecular Weight Analysis by 1H NMR Spectroscopy. J. Chem. Educ. 2011;88:1098–1104. doi: 10.1021/ed100461v. [DOI] [Google Scholar]
- 228.Batrakova E.V., Kabanov A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cacaccio J., Durrani F., Cheruku R.R., Borah B., Ethirajan M., Tabaczynski W., Pera P., Missert J.R., Pandey R.K. Pluronic F-127: An Efficient Delivery Vehicle for 3-(1’-hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH or Photochlor) Photochem. Photobiol. 2020;96:625–635. doi: 10.1111/php.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mike Motloung B., Edward Sekhosana K., Managa M., Prinsloo E., Nyokong T. The photophysicochemical properties and photodynamic therapy activity of phenyldiazenyl phenoxy substituted phthalocyanines when incorporated into Pluronic® F127 micelles. Polyhedron. 2019;174:114157. doi: 10.1016/j.poly.2019.114157. [DOI] [Google Scholar]
- 231.Wenceslau A.C., Ferreira G.L.Q.C., Hioka N., Caetano W. Spectroscopic studies of pyridil and methoxyphenyl porphyrins in homogeneous and Pluronic®-based nanostructured systems. J. Porphyr. Phthalocyanines. 2015;19:1168–1176. doi: 10.1142/S1088424615500996. [DOI] [Google Scholar]
- 232.D’Souza A.A., Shegokar R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- 233.Zalipsky S. Chemistry of polyethylene glycol conjugates with biologically active molecules. Adv. Drug Del. Rev. 1995;16:157–182. doi: 10.1016/0169-409X(95)00023-Z. [DOI] [Google Scholar]
- 234.Harris J.M., Martin N.E., Modi M. Pegylation: A novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 2001;40:539–551. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 235.Swierczewska M., Lee K.C., Lee S. What is the future of PEGylated therapies? Expert. Opin. Emerg. Drugs. 2015;20:531–536. doi: 10.1517/14728214.2015.1113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steinbeck C.A., Hedin N., Chmelka B.F. Interactions of Charged Porphyrins with Nonionic Triblock Copolymer Hosts in Aqueous Solutions. Langmuir. 2004;20:10399–10412. doi: 10.1021/la048435d. [DOI] [PubMed] [Google Scholar]
- 237.Lamch Ł., Tylus W., Jewgiński M., Latajka R., Wilk K.A. Location of Varying Hydrophobicity Zinc(II) Phthalocyanine-Type Photosensitizers in Methoxy Poly(ethylene oxide) and Poly(l-lactide) Block Copolymer Micelles Using 1H NMR and XPS Techniques. J. Phys. Chem. B. 2016;120:12768–12780. doi: 10.1021/acs.jpcb.6b10267. [DOI] [PubMed] [Google Scholar]
- 238.Lepre C.A., Moore J.M., Peng J.W. Theory and applications of NMR-based screening in pharmaceutical research. Chem. Rev. 2004;104:3641–3676. doi: 10.1021/cr030409h. [DOI] [PubMed] [Google Scholar]
- 239.Bloembergen N., Purcell E.M., Pound R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948;73:679–712. doi: 10.1103/PhysRev.73.679. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article.