Abstract
Background
Fosfomycin has been proven to be a vital choice to treat infection caused by multidrug resistance bacteria, especially carbapenem-resistant Klebsiella pneumoniae (CRKP). However, fosfomycin resistant cases has been reported gradually. In this study, we reported the fosfomycin-resistant rate in CRKP strains and further revealed the molecular mechanisms in resistance gene dissemination.
Results
A total of 294 non-duplicated CRKP strains were collected. And 55 fosfomyin-resistant strains were detected, 94.5% of which were clustered to sequence type (ST) 11 by PCR followed up sequencing. PFGE further revealed two major groups and four singletons. The positive rates of genes responsible to fosfomycin and carbapenem resistance were 81.8% (fosA3), 12.7% (fosA5) and 94.5% (blaKPC-2), respectively. Genomic analysis confirmed insertion sequence (IS) 26 was the predominant structure surrounding fosA3. The fosA3 genes in six isolates were located on plasmids which were able to transfer to E. coli J53 recipient cells by means of conjugation.
Conclusions
Although the resistant rate of CRKP to fosfomycin is relatively low in our area, considering its gene is located on transferrable plasmid and inserted in IS structure, continuous monitoring is still needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-021-02165-7.
Keywords: Carbapenem resistance, Klebsiella pneumoniae, Fosfomycin, fosA3
Introduction
Carbapenem resistant Klebsiella pneumoniae (CRKP) has become a great threat to public health. The dissemination of CRKP causes severe morbidity and mortality, due to few antibiotics available for the treatment [1].
Fosfomycin is a bactericidal antibiotic which inhibits the biosynthesis of cell wall by irreversibly binding with UDP-N-acetylglucosenol acetonyl transferase (MurA), an essential enzyme for peptidoglycan biosynthesis. Fosfomycin is commonly used in uncomplicated urinary tract infection caused by susceptible organisms [2, 3]. In recent years, fosfomycin has been proven to be effective against multidrug-resistance bacteria and recommended as alternative option for treatment of CRKP [4].
During the medical application of fosfomycin, resistant strains has been continually reported [5]. Three resistance mechanisms to fosfomycin have been reported, including the fosfomycin modified enzymes, amino acid substitutions of the antibiotic MurA target and mutations of fosfomycin transport system (GlpT and UhpT) and its regulatory genes [5–7]. The resistance mechanisms exhibit divergences among different regions [5]. The fosfomycin modified enzymes is the predominant mechanism of fosfomycin resistance in China. More than ten fos genes have been identified [8, 9]. Gene fosA3 is the most prevailing variant, mainly distributed in Asia, and can spread horizontally [10]. Therefore, the monitor of fosfomycin resistance is necessary to maintain fosfomycin effectiveness.
In the present study, we intended to investigate the in vitro antibacterial activity against CRKP from two teaching hospitals in China and further explore the resistance mechanism.
Result
Antibiotic susceptibility profiles
Among the 294 tested CRKP strains, 55 strains were resistant to fosfomycin (MIC ≥256 μg/mL). The fosfomycin resistant rates for two hospitals were 14.3 and 18.9%, respectively. All the fosfomycin resistance strains were highly resistant to tested antibiotics, including amikacin (AK), aztreonam (ATM), cefotaxime (CTX), cefotaxime (CRO) and ceftriaxone (FEP), with the minimum inhibitory concentration (MIC) at which 50% isolates were inhibited (MIC50) were greater than or equal to 256 μg/mL. All the strains were susceptible to polymyxin B (PB). The antibiotic susceptibility results were showed in Table 1.
Table 1.
Antimicrobial susceptibility results of 55 CRKP clinical isolates
| Antimicrobial agents | MIC (μg/mL) | number of isolates (%) | ||||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | S | I | R | |
| CTX | 256–> 256 | > 256 | > 256 | 0 (0.0) | 0 (0.0) | 55 (100) |
| CRO | 256–> 256 | > 256 | > 256 | 0 (0.0) | 0 (0.0) | 55 (100) |
| FEP | 128 – > 256 | > 256 | > 256 | 0 (0.0) | 0 (0.0) | 55 (100) |
| MEM | 8–> 256 | 256 | > 256 | 0 (0.0) | 0 (0.0) | 55 (100) |
| ATM | 2–> 256 | > 256 | > 256 | 1 (1.8) | 0 (0.0) | 54 (98.2) |
| FOS | 256–> 512 | > 512 | > 512 | 0 (0.0) | 0 (0.0) | 55 (100) |
| AK | 1–> 256 | > 256 | > 256 | 4 (7.3) | 0 (0.0) | 51 (92.7) |
| TGC | 1–8 | 2 | 8 | 22 (40.0) | 8 (14.5) | 25 (45.5) |
| PB | 0.25–1 | 0.5 | 0.5 | 55 (100) | 0 (0.0) | 0 (0.0) |
MIC50, minimum inhibitory concentration for 50% of the isolates; MIC90, minimum inhibitory concentration for 90% of the isolates
S susceptibility, I intermediate, R resistance
CTX cefotaxime, CRO ceftriaxone, FEP cefepime, MEM meropenem, ATM aztreonam, FOS fosfomycin, AK amikacin, TGC tigecycline, PB polymyxin B
The susceptibility profiles were analyzed according to the CLSI guidelines for CTX, CRO, FEP, MEM, ATM, FOS and AK, and EUCAST for PB and TGC
Screening for carbapenem and fosfomycin resistance genes
For carbapenem-resistance genes, the detection rate of blaKPC was 94.5% (52/55) in the CRKP isolates and all blaKPC belonged to blaKPC-2. None blaNDM and blaOXA-48 were identified in our study.
Among the 55 fosfomycin-resistant strains, 45 (81.8%) were positive with fosA3 gene, 7 (12.7%) were fosA5, while none harbored with fosA or fosC2 genes. For three strains which were negative for fosfomycin-resistance genes tested in our study, an amino acid substitution in Thr287Asn was discovered in fosfomycin target murA.
Bacterial genotype
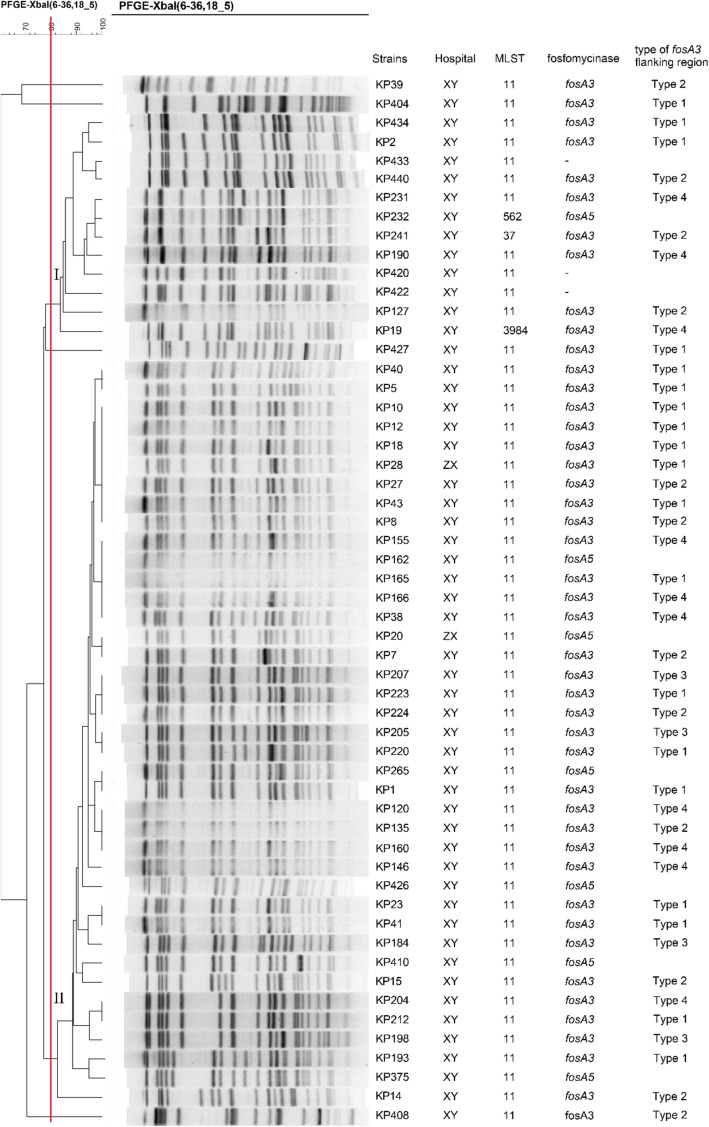
The dendrogram map conducted by pulsed-field gel electrophoresis (PFGE) revealed the genetic relationship between the fosfomycin-resistant strains. Two major groups (Group I and Group II) and four singletons were identified (Fig. 1). Group II was predominant that comprised 39 strains that were isolated in two hospitals.
Fig. 1.
Dendrogram of relationships among 55 fosfomycin-resistant CRKP via the unweighted pair group method. Red line represents Dice coefficient equal to 80%
In addition to multilocus sequence typing (MLST), 52 (94.5%) strains belonged to ST11. The rest of three strains belonged to ST 562, ST 37 and a new ST type, ST 3984, respectively.
Genetic environment surrounding fosA3 gene
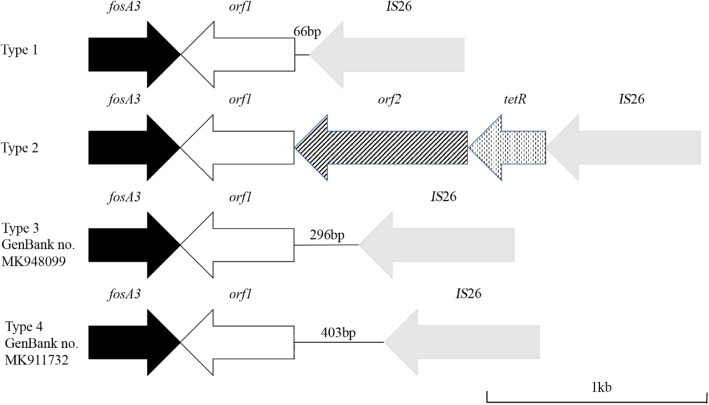
The genetic environment adjacent to fosA3 was determined by PCR mapping. All the fosA3 genes were located between two IS26 oriented in the opposite direction. The structure between fosA3 and upstream IS26 was the same in all 45 strains. The length of intergenic region between upstream IS26/fosA3 was 386 bp. However, four different downstream regions of fosA3 were discovered and designated as type 1 to 4, with variable lengths between fosA3 and the downstream IS26 (589, 819, 926 and 1811 bp). Type 1, accounting for 20 strains, consisted of fosA3-orf1-IS26 and shared 99.4% identity with the corresponding region of plasmid pKP 19–2029-KPC2 from K. pneumoniae strain KP19–2019 (GenBank no. CP047161). Twelve strains belonged to type 2, with a genetic background of fosA3-orf1-orf2-tetR-IS26, which was similar with that on plasmid p116753-KPC from K. pneumoniae strain 116,753 (GenBank no. MN891682). Two new types of fosA3 downstream sequence were found in our study, namely type 3 and 4, accounting for four and nine strains, respectively, and registered as MK948099 and MK911732 in the GenBank. The schematic map for four types was shown in Fig. 2.
Fig. 2.
Schematic maps of four type of genetic environments between gene fosA3 and downstream IS26
Conjugation experiments and plasmid analysis
Among the 45 fosA3 positive strains, 6 (13.3%) fosA3 genes were transferable to E. coli J53 recipient. For the antibiotic susceptibility profiles, four transconjugants showed highly resistant to antibiotics tested, compared to E. coli J53 recipient. However, two transconjugants (TC5 and TC18) only showed an increase in the MIC value of fosfomycin and amikacin (Table 2). PCR confirmed the blaKPC-2 was absent in TC5 and TC18 (Figure S1).
Table 2.
Antimicrobial susceptibility results of 6 fosA3 isolates with capability of transconjugation and their transconjugants
| Isolate | MIC (μg/mL) | plasmid type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTX | CRO | FEP | MEM | ATM | FOS | AK | TGC | PB | ||
| KP5 | > 256 | > 256 | > 256 | > 256 | > 256 | > 512 | > 256 | 4 | 0.5 | IncN |
| KP18 | > 256 | > 256 | > 256 | > 256 | > 256 | > 512 | > 256 | 4 | 0.5 | IncN, IncF |
| KP165 | > 256 | > 256 | > 256 | 256 | > 256 | > 512 | > 256 | 4 | 0.5 | ND |
| KP190 | > 256 | > 256 | > 256 | > 256 | > 256 | > 512 | > 256 | 1 | 0.5 | IncL/M |
| KP212 | > 256 | > 256 | > 256 | 256 | > 256 | > 512 | > 256 | 4 | 0.5 | ND |
| KP223 | > 256 | > 256 | > 256 | 256 | > 256 | > 512 | > 256 | 4 | 0.5 | ND |
| TC5 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | ≤0.25 | > 512 | 2 | ≤0.25 | ≤0.25 | IncN |
| TC18 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | ≤0.25 | > 512 | 2 | ≤0.25 | ≤0.25 | IncN |
| TC165 | > 256 | > 256 | 64 | 4 | 256 | > 512 | > 256 | ≤0.25 | ≤0.25 | ND |
| TC190 | 8 | > 256 | 8 | 2 | 128 | > 512 | > 256 | ≤0.25 | ≤0.25 | IncL/M |
| TC212 | > 256 | > 256 | 32 | 4 | 128 | > 512 | > 256 | ≤0.25 | ≤0.25 | ND |
| TC223 | > 256 | > 256 | 32 | 4 | 256 | 512 | > 256 | ≤0.25 | ≤0.25 | ND |
| EC J53 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | ≤0.25 | 2 | 1 | ≤0.25 | ≤0.25 | / |
KP K. pneumoniae, TC transconjugant, EC E. coli, ND not detected
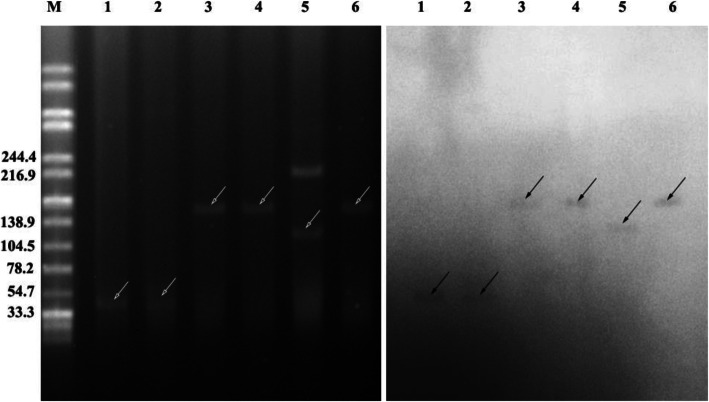
S1-PFGE, southern blotting and PCR-based replicon typing were used for plasmid analysis. S1-PFGE demonstrated that five in six transconjugants harbored single plasmid. The plasmids harbored in six transconjugants were assigned to the following incompatibility groups: IncN (n = 2), IncL/M (n = 1) and not determined (ND, n = 3) (Table 2). Southern blot analysis confirmed that the fosA3 genes were located in the plasmids of different sizes (~ 40, 100 and 140 kb) in the transconjugants (Fig. 3).
Fig. 3.
S1-PFGE and Southern blot hybridization of fosA3 transconjugants. Bands with black arrows pointing showed the positive signals in Southern blot with fosA3 probes. S1-PFGE was shown in left and Southern blot was shown in right. M = DNA ladder of Salmonella serotype Braenderup H9812 strain digested by XbaI. 1, E. coli J53 (KP5 plasmid); 2, E. coli J53 (KP 18 plasmid); 3, E. coli J53 (KP 165 plasmid); 4, E. coli J53 (KP 190 plasmid); 5, E. coli J53 (KP 212 plasmid); 6, E. coli J53 (KP 223 plasmid)
For the rest 49 strains, the plasmids were assigned to the following incompatibility groups: IncF (n = 42) and ND (n = 3). Multiple replicons were detected in 4 strains (the data were shown in supplemental table).
Discussion
Our study investigated the prevalence of fosfomycin resistance genes among 294 non-duplicate CRKP strains from two tertiary hospitals in two provinces. We have reported a resistance rate of 18.7%, indicating a relatively low resistant rate to fosfomycin, compared to a study conducted by Chen et al. (28.7%, 29/101 strains of CRKP) [11]. Probably because fosfomycin is not commonly used in the two settings. However, recently studies reported severe resistance rates to fosfomycin among CRKP in China between 2015 to 2020, ranging from 48.5 to 80% [12, 13]. The fast spread of fosfomycin resistance present further medical challenge for CRKP treatment, due to few antibiotics available. Another factor, which may impede the application of fosfomycin, is the difference between CLSI and EUCAST on MIC breakpoint. CLSI considers a MIC greater than or equal to 256 μg/mL to fosfomycin as resistance. However, EUCAST chooses 32 μg/mL as the breakpoint for fosfomycin to discriminate resistance. Furthermore, the CLSI breakpoint for fosfomycin only applies to E. coli urinary trait isolates. There is an urgent need for more clinical studies to determine the breakpoints for fosfomycin on K. pneumoniae systematic infection.
Polymerase chain reaction (PCR) screening revealed the plasmid gene fosA3 was the predominant resistance mechanism. Gene fosA3 was first discovered in a E. coli strain and transferred with resistance genes including CTX-M and rmtB, resulting in highly resistant to fosfomycin [14]. Previous studies have demonstrated that the plasmid carrying fosA3 were classified into incompatibility group IncF II, IncN, IncI1 IncB/O or not determined [15]. IncF plasmids are heterogeneous with variable size and frequently carry more than one replicon and resistance genes, contributing the fitness of the host [16]. In our study, IncF plasmid was also the predominant replicon type in all 55 strains. Interestingly, all the IncF plasmids were unable transferred to E. coli J53 by means of conjugation. Only six fosA3 genes were transferable to E. coli J53 recipient. However, two transconjugants showed no increase in MIC values of antibiotics tested in our study, except for fosfomycin and amikacin (Table 2). PCR and southern blot analysis confirmed the fosA3 genes of the two transconjugants were located in a plasmid around 40 kb (Fig. 3) and the absence of blaKPC-2 (Figure S1). The genes fosA3 and blaKPC-2 coexist on a plasmid and can spread together by means of plasmid transfer. Jiang et al. observed a plasmid co-harbored fosA3 and blaKPC-2 on different transposon systems [17]. Li et al. reported a IncP1 plasmid co-harbored fosA3 and blaKPC-2 in the same Tn1721-Tn3-like composite transposons [18]. The rmtB gene, which contributes to the resistance of aminoglycosides, is frequently located on plasmid with fosA3. So we also tested the rmtB gene among six transconjugants by PCR and confirmed that five transconjugants harbored rmtB gene (Figure S1). The coexistences of these resistance genes and the horizontal gene transfer may promote the spread of fosA3 and fosfomycin resistance by co-selection, due to the excessive use of carbapenems and aminoglycosides for treatment of bacterial infection.
As for the 3 strains which were negative for fosfomycin resistance genes, an amino acid change on murA may account for the resistance, which was also reported in other study [19]. However, no change in active binding site of fosfomycin (Cys115 residue) and three conserved positively charged residues (Lys22, Arg120 and Arg397) in murA was discovered, so further study is needed to reveal the influence of Thr287Asn in murA on fosfomycin susceptibility.
Insertion sequence IS26 surrounds fosA3 gene and plays an important role in the dissemination of fosA3. Different studies have reported a s IS26-fosA3-IS26-like structure, which was similar with the structures in our study, while the length between IS26 and fosA3 was variable [5]. The sequences of MK948099 and MK911732 showed some differences with that of plasmids known in GenBank. The sequence of MK948099 showed a difference of 47 base pairs compared with corresponding fragment of plasmid pHNGD46 (GenBank no KJ668701.1) in E. coli GDC46.
Based on the PFGE pattern, we disclosed that clone dissemination may play an important role in the spread of fosfomycin resistance, which is consistent with different studies on CRKP [20]. More importantly, group II contained strains from Cangzhou Central Hospital (KP28) and Xiangya hospital (KP10, KP12 and KP18), which shared similar PFGE bands and carried gene fosA3. It could be a clue that the spread of fosA3 among CRKP may attribute to clone expansion. So, it is urgent to monitor the fosfomycin resistance and use the antibiotics with caution to prevent further spread of fosfomycin resistance. According to MLST, ST11 was the predominant type in our study, which is in agreement with the fact the ST11 is primary sequence type in Asia for CRKP [21].
Conclusion
The fosfomycin resistance rate of CRKP strains is low in our study. The main mechanism of fosfomycin resistance is plasmid-mediated genes, which located on transferrable plasmid and inserted in IS structure, so further monitoring the fosfomycin resistance should be strengthened.
Materials and methods
Bacteria source
A total of 294 non-duplicate CRKP strains were collected from two tertiary hospitals (Cangzhou Central hospital from Hebei province and Xiangya hospital from Hunan province) in China between December 2016 and March 2019. The sample sources included blood (n = 48), sputum (n = 132), urine (n = 34), abscess (n = 22) and other samples (n = 58). CRKP was defined as strains with MIC values ≥4 μg/ml for IPM or MEM based on Clinical and Laboratory Standards Institute 2018 (CLSI) guidelines.
Bacteria identification and antimicrobial susceptibility test (AST)
The strains were identified by VITEK-2 Compact system (bioMérieux, Marcy L’Etoile, France) or Microflex™ MALDI-TOF MS system (Bruker Daltonik, Bremen, Germany).
Broth microdilution method with Mueller-Hinton broth (Oxoid, unipath, UK) was used for AST according to the CLSI 2018 guidelines [22]. Minimum inhibitory concentration (MIC) for fosfomycin was determined by agar dilution method with Mueller-Hinton agar supplemented with 25 μg/mL glucose-6-phosphate. The susceptibility profiles were analyzed according to the CLSI guidelines, and the European Committee on Antimicrobial Susceptibility Testing breakpoints (EUCAST, www.eucast.org) for polymyxin B and tigecycline.
Detection on resistance mechanisms of carbapenem and fosfomycin
Further confirmation test on the resistance genes was completed by means of PCR. The carbapenem-resistance genes, including blaKPC, blaNDM and blaOXA-48, and fosfomycin-resistance genes, such as fosA, fosA3, fosA5 and fosC2, were involved in our study according to previous reports [23–25]. We also analyzed the variants of the blaKPC genes by PCR and follow up sanger sequencing [26].
For strains which were negative for tested fosfomycin-resistance genes, murA gene were amplified according to previous work [19]. The products were sequenced and compared with the murA gene sequence of fosfomycin-sensitive K. pneumoniae K68 stains (GenBank no. KT334183) available at the National Center for Biotechnology Information website.
Bacteria homology analysis
PFGE was employed to analyze the genomic background among Fosfomycin-resistant CRKP strains according to the standard protocol [27]. Briefly, the genomic DNA was digested with XbaI restriction enzyme for 12 h and separated by running PFGE electrophoresis with 1% agarose gel at 12 °C and 5.5 V/cm, with alternating pulses at a 120° angle in 0.5–70 s pulse time gradient for 21 hs. BioNumerics software (Applied Maths) was used for dendrogram analysis using the dice similarity coefficient. Strains were classified as the same PFGE group if they possessed ≥80% genetic similarity [28]. Salmonella enterica H9812 was used as the size marker.
MLST was used to analyze ST type of CRKP. Seven house-keeping genes of K. pneumoniae (rpoB, gapA, mdh, pgi, phoE, infB and tonB) were amplified and the products were sequenced. The ST type was analyzed according to protocol of Pasteur website (http://bigsdb.web.pasteur.fr).
Conjugation experiment and plasmid typing
The conjugation experiments were used for the fosA3 strains to examine the transferring capability of plasmids. The sodium azide-resistant E. coli J53 was used as recipient strain and filter-mating method was performed according to reported procedures with 64 μg/mL fosfomycin and 200 μg/mL sodium azide [29]. PCR and antibiotic susceptibility tests for the transconjugants were conducted to confirm the transferred fosA3 and blaKPC genes.
Plasmid DNA of all strains was extracted by E.Z.N.A. Endo-free Plasmid DNA Mini Kit (OMEGA, USA). The plasmid incompatibility group was identified by PCR-based replicon typing according to previous work, including HI1, HI2, I1/Ir, X, L/M, N, FIA, FIB, W, Y, R, FIC, A/C, T, FIIA, F and K [30].
S1-PFGE and southern blotting
Southern blotting was employed to confirm the location of fosA3 gene. Total genomic DNA was digested with S1 nuclease and electrophoresed with a CHEF-Mapper XA PFGE system (Bio-Rad, USA) for 16 h at 14 °C and 6 V/cm, with alternating pulses in 2.16–63.8 s pulse time. The DNA fragments were transferred to nylon membranes (Millipore, USA) and hybridized with digoxigenin-labelled fosA3-specific probe. An NBT/BCIP color detection kit (Roche Applied Sciences, Germany) was employed to detect the fragments [31].
PCR mapping of the flanking region of fosA3 gene
The genetic environment around fosA3 gene was analyzed according to previous work [23]. The PCR products were sequenced and compared using the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences reported here have uploaded to NCBI website with accession numbers of MK948099 and MK911732.
Supplementary Information
Additional file 1: Figure S1. PCR analysis of genes blaKPC-2 (A) and rmtB (B) of fosA3 transconjugants. M = Marker. 1, E. coli J53 (KP5 plasmid); 2, E. coli J53 (KP 18 plasmid); 3, E. coli J53 (KP 165 plasmid); 4, E. coli J53 (KP 190 plasmid); 5, E. coli J53 (KP 212 plasmid); 6, E. coli J53 (KP 223 plasmid).
Additional file 2. Table S1. Isolate name, source, hospital, antimicrobial susceptibility results, homology analysis and PCR results of all isolates that were analysed in this study.
Acknowledgments
We thank all staff in the Microbiology Department of Xiangya Hospital and Cangzhou Central Hospital for their assistance with bacterial collection. We thank Ying Fu in Microbiology Lab of Clin Dep of Sir Run Run Shaw hospital for her aid in S1-PFGE and Southern blotting experiments.
Abbreviations
- CRKP
Carbapenem resistant K. penumoniae
- AK
Amikacin
- ATM
Aztreonam
- CTX
Cefotaxime
- CRO
Ceftriaxone
- FEP
Cefepime
- TGC
Tigecycline
- PB
Polymyxin B
- MEM
Meropenem
- FOS
Fosfomycin
- CLSI
Clinical and Laboratory Standards Institute
- EUCAST
European Committee on Antimicrobial Susceptibility Testing breakpoints
- MIC
Minimum inhibitory concentration
- PCR
Polymerase chain reaction
- PFGE
Pulsed-field gel electrophoresis
- MLST
Multilocus sequence typing
- AST
Antimicrobial susceptibility test
Authors’ contributions
Study design: HCW and MXZ. Study conduct: HCW, CHM and JL. Data collection: CHM, TY and YMH. Data analysis: JL and MXZ. Data interpretation: YMH and QYD. Drafting manuscript: HCW and CHM. Revising manuscript content: MXZ. Approving the final version of the manuscript: HCW and MXZ. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Hunan Provincial Natural Science Foundation (No. 2019JJ50958 and 2020JJ5901). The authors declare that the funding body was not involved in study design, data collection, analysis, interpretation and writing of the study.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This research was conducted according to the recommendations of the Ethics Committee of Central South University (Changsha, Hunan Province, China) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol was approved by the Ethics Committee of Central South University (Changsha, Hunan Province, China) and written by participants or guardians prior to the study.
Consent for publication
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reyes J, Aguilar AC, Caicedo A. Carbapenem-resistant Klebsiella pneumoniae: microbiology key points for clinical practice. Int J Gen Med. 2019;12:437–446. doi: 10.2147/IJGM.S214305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz R. Fosfomycin: an old--new antibiotic. Clin Microbiol Infect. 2012;18(1):4–7. doi: 10.1111/j.1469-0691.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 3.Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in gram-negative pathogens. J Antimicrob Chemother. 2012;67(2):255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 4.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America. European Society for Microbiology and Infectious Diseases International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents. 2016;47(4):269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Couce A, Briales A, Rodriguez-Rojas A, Costas C, Pascual A, Blazquez J. Genomewide overexpression screen for fosfomycin resistance in Escherichia coli: MurA confers clinical resistance at low fitness cost. Antimicrob Agents Chemother. 2012;56(5):2767–2769. doi: 10.1128/AAC.06122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golla VK, Sans-Serramitjana E, Pothula KR, Benier L, Bafna JA, Winterhalter M, Kleinekathofer U. Fosfomycin permeation through the outer membrane porin OmpF. Biophys J. 2019;116(2):258–269. doi: 10.1016/j.bpj.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahata S, Ida T, Hiraishi T, Sakakibara S, Maebashi K, Terada S, Muratani T, Matsumoto T, Nakahama C, Tomono K. Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int J Antimicrob Agents. 2010;35(4):333–337. doi: 10.1016/j.ijantimicag.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Pakhomova S, Rife CL, Armstrong RN, Newcomer ME. Structure of fosfomycin resistance protein FosA from transposon Tn2921. Protein Sci. 2004;13(5):1260–1265. doi: 10.1110/ps.03585004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghamali M, Sedighi M, Zahedi Bialvaei A, Mohammadzadeh N, Abbasian S, Ghafouri Z, Kouhsari E. Fosfomycin: mechanisms and the increasing prevalence of resistance. J Med Microbiol. 2019;68(1):11–25. doi: 10.1099/jmm.0.000874. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wang D, Ding Y, Zhang L, Li X. Molecular epidemiology of plasmid-mediated fosfomycin resistance gene determinants in Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae isolates in China. Microb Drug Resist. 2019;25(2):251–257. doi: 10.1089/mdr.2018.0137. [DOI] [PubMed] [Google Scholar]
- 12.Liu P, Chen S, Wu ZY, Qi M, Li XY, Liu CX. Mechanisms of fosfomycin resistance in clinical isolates of carbapenem-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist. 2020;22:238–243. doi: 10.1016/j.jgar.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Cao M, Hu Y, Zhang R, Xiao Y, Chen G. Prevalence and mechanisms of fosfomycin resistance among KPC-producing Klebsiella pneumoniae clinical isolates in China. Int J Antimicrob Agents. 2021;57(1):106226. doi: 10.1016/j.ijantimicag.2020.106226. [DOI] [PubMed] [Google Scholar]
- 14.Wachino J, Yamane K, Suzuki S, Kimura K, Arakawa Y. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother. 2010;54(7):3061–3064. doi: 10.1128/AAC.01834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Men S, Kong L, Ma S, Yang Y, Wang Y, Yuan Q, Cheng G, Zou W, Wang H. Prevalence of plasmid-mediated fosfomycin resistance gene fosA3 among CTX-M-producing Escherichia coli isolates from chickens in China. Foodborne Pathog Dis. 2017;14(4):210–218. doi: 10.1089/fpd.2016.2230. [DOI] [PubMed] [Google Scholar]
- 16.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 2010;65(12):2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Shen P, Wei Z, Liu L, He F, Shi K, Wang Y, Wang H, Yu Y. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents. 2015;45(1):66–70. doi: 10.1016/j.ijantimicag.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Zhang Y, Bi D, Shen P, Ai F, Liu H, Tian Y, Ma Y, Wang B, Rajakumar K, Ou HY, Jiang X. First report of a clinical, multidrug-resistant Enterobacteriaceae isolate coharboring fosfomycin resistance gene fosA3 and carbapenemase gene blaKPC-2 on the same transposon, Tn1721. Antimicrob Agents Chemother. 2015;59(1):338–343. doi: 10.1128/AAC.03061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu PL, Hsieh YJ, Lin JE, Huang JW, Yang TY, Lin L, Tseng SP. Characterization of fosfomycin resistance mechanisms and molecular epidemiology in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates. Int J Antimicrob Agents. 2016;48(5):564–568. doi: 10.1016/j.ijantimicag.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zou MX, Wang HC, Dou QY, Hu YM, Yan Q, Liu WE. An outbreak of infections caused by a Klebsiella pneumoniae ST11 clone coproducing Klebsiella pneumoniae carbapenemase-2 and rmtB in a Chinese teaching hospital. Chin Med J. 2016;129(17):2033–2039. doi: 10.4103/0366-6999.189049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute (CLSI) (2018). Performance standards for antimicrobial susceptibility testing. 28th informational supplement. CLSI document M100-S25 (ISBN 1-56238-839-8).
- 23.Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, Chen Z, Liu JH. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M beta-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother. 2012;56(4):2135–2138. doi: 10.1128/AAC.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho PL, Chan J, Lo WU, Lai EL, Cheung YY, Lau TCK, Chow KH. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J Med Microbiol. 2013;62(Pt 11):1707–1713. doi: 10.1099/jmm.0.062653-0. [DOI] [PubMed] [Google Scholar]
- 25.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Wolter DJ, Kurpiel PM, Woodford N, Palepou MF, Goering RV, Hanson ND. Phenotypic and enzymatic comparative analysis of the novel KPC variant KPC-5 and its evolutionary variants, KPC-2 and KPC-4. Antimicrob Agents Chemother. 2009;53(2):557–562. doi: 10.1128/AAC.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Zou M, Dou Q, Hu Y, Wang H, Yan Q, Liu WE. Characterization of clinical extensively drug-resistant Pseudomonas aeruginosa in the Hunan province of China. Ann Clin Microbiol Antimicrob. 2016;15(1):35. doi: 10.1186/s12941-016-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios E, Lopez MC, Rodriguez-Avial I, Culebras E, Picazo JJ. Detection of Escherichia coli ST131 clonal complex (ST705) and Klebsiella pneumoniae ST15 among faecal carriage of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae. J Med Microbiol. 2017;66(2):169–174. doi: 10.1099/jmm.0.000399. [DOI] [PubMed] [Google Scholar]
- 29.Zhong YM, Liu WE, Zheng ZF. Epidemiology and molecular characterization of mcr-1 in Escherichia coli recovered from patients with bloodstream infections in Changsha, Central China. Infect Drug Resist. 2019;12:2069–2076. doi: 10.2147/IDR.S209877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother. 2012;67(9):2114–2122. doi: 10.1093/jac/dks192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. PCR analysis of genes blaKPC-2 (A) and rmtB (B) of fosA3 transconjugants. M = Marker. 1, E. coli J53 (KP5 plasmid); 2, E. coli J53 (KP 18 plasmid); 3, E. coli J53 (KP 165 plasmid); 4, E. coli J53 (KP 190 plasmid); 5, E. coli J53 (KP 212 plasmid); 6, E. coli J53 (KP 223 plasmid).
Additional file 2. Table S1. Isolate name, source, hospital, antimicrobial susceptibility results, homology analysis and PCR results of all isolates that were analysed in this study.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.