Abstract
Zeylanicobdella arugamensis (Hirudinea), a marine parasitic leech, not only resulted in the mortality of the host fish (Groupers) but also caused economic losses. The current study aimed to elucidate the antiparasitic efficacy of the aqueous extract of the Azadirachta indica leaves against Z. arugamensis and to profile the composition via LC-Q Exactive HF Orbitrap mass spectrometry. Different concentrations (25, 50 and 100 mg/mL) of A. indica extract were prepared and tested on the parasitic leeches. The total mortality of leeches was noticed with an exposure to the A. indica aqueous extract. The average times required for the aqueous extract at concentrations of 25, 50 and 100 mg/mL to kill the leeches were 42.65 ± 9.20, 11.69 ± 1.11 and 6.45 ± 0.45 min, respectively, in a dose-dependent manner. The Orbitrap mass spectrometry analysis indicated the presence of five flavonoids (myricetin 3-O-galactoside, trifolin, isorhamnetin, quercetin and kaempferol), four aromatics (4-methoxy benzaldehyde, scopoletin, indole-3-acrylic acid and 2,4-quinolinediol), three phenolics (p-coumaric acid, ferulic acid and phloretin) and two terpenoids (pulegone and caryophyllene oxide). Thus, our study indicates that A. indica aqueous extract is a good source of metabolites with the potential to act as a biocontrol agent against the marine parasitic leech in aquaculture.
Keywords: LC-Q Exactive HF Orbitrap MS, anti-parasitic, metabolites, aquaculture, grouper, control, Zeylanicobdella arugamensis
1. Introduction
In Malaysia, parasitic infestation is a serious problem for different types of fish species, and several parasites have been reported to be reared in open floating net-cages [1,2,3]. Aquacultured groupers showed a greater variety of parasites and higher intensities of infestation than wild groupers [2,4]. Some of the commonly found parasites in Malaysian cage aquacultures are monogeneans (Benedenia spp., Neobenedenia spp.), copepods (Caligus epidemicus, Caligus spp.) and piscicolids [1,3,4,5].
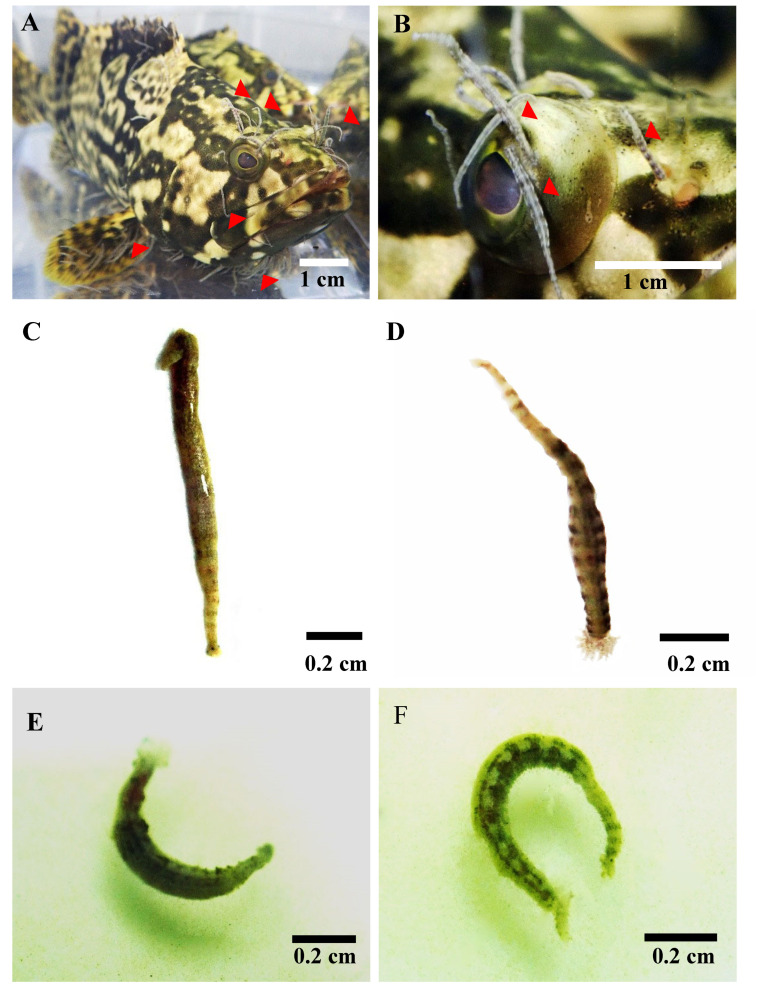
The infestation of the marine parasitic leech Zeylanicobdella arugamensis (Annelida: Hirudinea: Piscicolidae) (Figure 1A,B) spread rapidly in Southeast Asian countries [3,4,5,6]. In Malaysia, the marine leech was first reported in a grouper (Epinephelus coioides) reared in floating cages with a 0.4-percent prevalence [3]; later on, the leeches were frequently isolated from various major species of marine fish reared in cages such as hybrid groupers (Epinephelus fuscoguttatus × E.lanceolatus), groupers (E. fuscoguttatus, E. lanceolatus), snappers (Lutjanus johnii, L. argentimaculatus and L. stellatus) and sea bass (Lates calcarifer) [3,4,5]. Further, around 60 percent of moribund sea bass fingerlings nurtured in cages were found to be infested with Z. arugamensis [7]. The ectoparasitic leech also served as a vector for the transfer of pathogens and resulted in the mortality of the host fishes in a short period [8]. The control of leech infestation is vital for the management of the aquaculture industry. An expensive and harmful chemical, especially formalin, is used for the control of leech infestation, which is not conducive for an ecofriendly aquaculture policy [9]. The best alternative is the application of the natural product as a biocontrol agent due to the presence of various metabolites with less or zero toxicity [10].
Figure 1.
(A,B) = Hybrid grouper (Epinephelus fuscoguttatus ×E.lanceolatus), highly infested with Z. arugamensis, indicated by the arrow (The parasites were isolated and used for the current research), (C) = normal parasite (exposed to seawater only, untreated with extract or chemicals, no mortality was noticed), (D) = formalin-exposed parasite (exposed to 0.25% of formalin solution in seawater, resulted in mortality of the parasites) (E,F) = A. indica aqueous extract-exposed parasites (exposed to low, medium and the higher concentration of plant extract prepared in seawater, resulted in mortality of the parasites with a wrinkled body).
Extracts and essential oils from a natural product can act as herbicide, antimicrobial, anticancer and antiparasitic agents [11,12,13,14]. Azadirachta indica (neem plant) belongs to the family Meliaceae, commonly found in the Indo-Malayan region and other parts of the world [15,16]. Various parts of the plant, such as fruits, seeds, leaves, bark and roots, are a good source of bioactive compounds with antimicrobial, antipyretic, anti-inflammatory and antiparasitic properties [15,16,17]. The antimicrobial activity of A. indica leaves’ solvent extracts against fish pathogenic bacteria (Aeromonas veronii, Aeromonas hydrophila, Acinetobacter junii, Acinetobacter tandoii, Acinetobacter spp. and Pseudomonas stutzer) isolated from Blackspot barb (Dawkinsia filamentosa) have been reported [18]. The aqueous extract of the plant leaf at a concentration of 150 mg/l has been reported to be effective against the pathogenic infection caused by Citrobacter freundii in tilapia [19]. The essential oils of A. indica have been reported to have an antimicrobial property against Enterococcus faecalis, Aerococcus viridans, Pseudomonas aeruginosa, Proteus mirabilis and Escherichia coli and an antiparasitic activity against caligid parasites on seabass [20,21]. The data have revealed that A. indica extracts are toxic to over 400 species of insect pests, some of which have developed resistance to chemically prepared pesticides [15,22]. The plant is also used for the removal of intestinal worms [15]. Currently, no data is available regarding the antiparasitic properties of A. indica aqueous extracts against the marine parasitic leech. Hence, this study aimed to elucidate the antiparasitic efficacy of the aqueous extract of A. indica against Z. arugamensis and to profile the composition using LC-Q Exactive HF Orbitrap mass spectrometry.
2. Results
2.1. Physiochemical Parameters
The water quality parameters of the controls and plant-treated groups are provided in Table 1. Slight changes in the pH of the plant solutions were noticed when compared to the control groups, which could be due to the presence of metabolites with an acidic nature in the extract while the rest of the parameters remained constant.
Table 1.
Water quality parameters of the solution for the treatment of parasitic leeches.
| No. | Water Parameters | Concentrations | ||||
|---|---|---|---|---|---|---|
| Groups | Normal Control | Positive Control (Formalin 0.25%) (v/v) | Azadirachta indica (mg/mL) | |||
| (25) | (50) | (100) | ||||
| 1 | Temperature (°C) | 24.6 | 25.3 | 24.7 | 24.9 | 25.1 |
| 2 | pH | 7.80 | 7.24 | 5.56 | 5.4 | 4.03 |
| 3 | Salinity (ppt) | 30.0 | 30.9 | 31 | 30.9 | 30.9 |
| 4 | Dissolved oxygen (mg/L) | 7.0 | 6.5 | 6.8 | 7.1 | 7.0 |
2.2. Antiparasitic Properties of the Aqueous Extract of A. indica
The mortality time and percentage of the leeches treated with aqueous extracts of the plant were calculated (Table 2, Figure 1C–F). A total mortality of the leeches was noticed in negative control (Figure 1D) and all the plant-treated groups (Figure 1E–F), and the time taken was from 6.45 to 42.65 min. No mortality of the leeches was noticed in the normal control group (Figure 1C) until 720 min (12 h).
Table 2.
Mortality time of parasitic leeches treated with different concentrations of aqueous extract of A. indica.
| No | Group | Mortality Time (min) Mean ± S. D | Mortality (%) |
|---|---|---|---|
| 1 | Normal control | 720.00 ± 00 | 0 |
| 2 | Positive control (Formalin 0.25%) (v/v) | 3.77 ± 0.25 # | 100 |
| 3 | A. indica (25 mg/mL) | 42.65 ± 9.20 #,$ | 100 |
| 4 | A. indica (50 mg/mL | 11.69 ± 1.11 #,$,%, | 100 |
| 5 | A. indica (100 mg/mL) | 6.45 ± 0.45 #,$,%,* | 100 |
Each value represents the mean ± S.D. of six parasitic leeches per group. # Significance at p < 0.05 compared with the control group. $ Significance at p < 0.05 compared with the formalin-treated group (0.25% v/v); % Significance at p < 0.05 compared with A. indica (25 mg/mL); * Significance at p < 0.05 compared with A. indica (50 mg/mL).
2.3. LC-Q Exactive HF Orbitrap Mass Spectrometry Analysis of the Aqueous Extract of A. indica
In the current study, a total of 42 compounds were identified using Q Exactive HF Orbitrap mass spectrometry (Table 3). Among these 42 compounds, there are five flavonoids (myricetin 3-O-galactoside, trifolin, isorhamnetin, quercetin and kaempferol), four aromatics (4-methoxy benzaldehyde, scopoletin, indole-3-acrylic acid and 2,4-quinolinediol), three phenolics (p-coumaric acid, ferulic acid and phloretin) and two terpenoids (pulegone and caryophyllene oxide).
Table 3.
Compounds identified from the aqueous extract of A. indica using Q Exactive HF Orbitrap mass spectrometry and Compound Discoverer 3.0. Number shows the respective compound structures can be referred in supplementary Figure S1.
| No | Identified Compounds | Class | Retention Time | Formula |
|---|---|---|---|---|
| 1 | Glutamic acid | Amino acid | 0.623 | C5H9NO4 |
| 2 | Arginine | Amino acid | 0.687 | C6H14N4O2 |
| 3 | Histidine | Amino acid | 0.689 | C6H9N3O2 |
| 4 | γ-Aminobutyric acid | Amino acid | 0.734 | C4H9NO2 |
| 5 | Valine | Amino acid | 0.752 | C5H11NO2 |
| 6 | Tyrosine | Amino acid | 0.845 | C9H11NO3 |
| 7 | N3, N4-Dimethylarginine | Amino acid | 0.854 | C8H18N4O2 |
| 8 | Leucine | Amino acid | 0.896 | C6H13NO2 |
| 9 | Phenylalanine | Amino acid | 1.202 | C9H11NO2 |
| 10 | 1-Aminocyclohexanecarboxylic acid | Amino acid | 1.203 | C7H13NO2 |
| 11 | 4-Methoxybenzaldehyde | Aromatic | 3.504 | C8H8O2 |
| 12 | Scopoletin | Aromatic | 4.351 | C10H8O4 |
| 13 | 2-Methylcyclohexan-1,3-dione | Cyclic ketone | 1.502 | C7H10O2 |
| 14 | Jasmone | Cyclic ketone | 7.041 | C11H16O |
| 15 | 3-Hexenoic acid | Fatty acyl | 1.873 | C6H10O2 |
| 16 | 9S,13R-12-Oxophytodienoic acid | Fatty acyl | 7.369 | C18H28O3 |
| 17 | Decanamide | Fatty acyl | 8.086 | C10H21NO |
| 18 | Myricetin 3-O-galactoside | Flavonoid | 4.717 | C21H20O13 |
| 19 | Trifolin | Flavonoid | 5.412 | C21H20O11 |
| 20 | Isorhamnetin | Flavonoid | 5.54 | C16H12O7 |
| 21 | Quercetin | Flavonoid | 5.032 | C15H10O7 |
| 22 | Kaempferol | Flavonoid | 5.303 | C15H10O6 |
| 23 | Pyroglutamic acid | Heterocyclic | 0.827 | C5H7NO3 |
| 24 | β,β-Dimethyl-γ-methylene-γ-butyrolactone | Heterocyclic | 1.612 | C7H10O2 |
| 25 | Pipecolic acid | Heterocyclic | 1.049 | C6H11NO2 |
| 26 | 6-Methyl-2-pyridinemethanol | Heterocyclic | 1.084 | C7H9NO |
| 27 | Indole-3-acrylic acid | Heterocyclic aromatic | 1.935 | C11H9NO2 |
| 28 | 2,4-Quinolinediol | Heterocyclic aromatic | 3.241 | C9H7NO2 |
| 29 | Valylproline | Peptide | 1.464 | C10H18N2O3 |
| 30 | Prolylleucine | Peptide | 1.531 | C11H20N2O3 |
| 31 | p-Coumaric acid | Phenolic | 3.205 | C9H8O3 |
| 32 | Ferulic acid | Phenolic | 3.705 | C10H10O4 |
| 33 | Phloretin | Phenolic | 5.399 | C15H14O5 |
| 34 | Guanine | Purine | 0.787 | C5H5N5O |
| 35 | Adenine | Purine | 0.804 | C5H5N5 |
| 36 | 1-Methyladenine | Purine | 0.83 | C6H7N5 |
| 37 | 2′-Deoxyadenosine | Purine | 1.192 | C10H13N5O3 |
| 38 | 2′-O-Methyladenosine | Purine | 1.608 | C11H15N5O4 |
| 39 | Pulegone | Terpenoid | 3.61 | C10H16O |
| 40 | Caryophyllene oxide | Terpenoid | 6.354 | C15H24O |
| 41 | Nicotinic acid | Vitamin B3 | 0.836 | C6H5NO2 |
| 42 | Nicotinamide | Vitamin B3 | 0.894 | C6H6N2O |
3. Discussion
The marine leech Z. arugamensis is a notorious ectoparasite and distributed throughout the Indian Ocean [3,4,5,23,24]. For many years, fish farmers used toxic antiparasitic chemotherapeutics and insecticides to prevent or control parasitic infestations in aquaculture [25,26,27]. The accumulation of these chemical residues in water has caused impacts on the environment and may have lethal or sublethal effects on nontarget organisms [28]. For example, in Norway, when Neguvon and Nuvon pesticides were applied to control the copepod Lepeophtheirus salmonis (Caligidae) in salmon net-pen farming, there were harmful effects on several crustaceans near the farms [29]. However, plants are a good alternative and can be applied to control parasitic infestation as a natural remedy for sustainable aquaculture to avoid the negative effects of pesticides [10]. They show zero or less toxicity to the environment due to their biodegradability and are a great source of bioactive compounds [30,31].
In this study, we selected A. indica due to its antimicrobial, anti-inflammatory, antipyretic, insecticidal and acaricidal nature [15,16,17,22] and determined the antiparasitic potential of the aqueous extracts of A. indica leaves. The exposure of the aqueous extract resulted in the total mortality of leeches in a dose-dependent manner (Figure 1). In three different doses used, all leeches were killed in an average period of 6.45 ± 0.45 min (100 mg/mL), 11.69 ± 1.11 min (50 mg/mL) and 42.65 ± 9.20 min (25 mg/mL). Previous studies have shown the effect of plant extracts on different fish parasites. The methanol extract of Dillenia suffruticosa (Dilleniaceae, tropical shrub) was applied against Z. arugamensis at a concentration of 100 mg/mL, and it took 14.39 and 4.88 min to kill all the leeches [32]. However, our treatment using the aqueous extract of A. indica took less time than D. suffruticosa to kill all the leeches. Further, the exposure of the methanol extract and chromatographic fraction (fraction 3) of Nephrolepis biserrata (Nephrolepidaceae, perennial fern) was applied against Z. arugamensis at a concentration of 100 and 2.5 mg/mL and killed all the leeches in 4.88 and 1.92 min, respectively [33,34]. Similarly, the methanol extracts of Allium sativum (Amaryllidaceae, garlic) (600 μg/mL) were tested against the aquatic leech Limnatis nilotica (Hirudinidae) and took 144.55 min for total mortality [35]. The essential oil of A. indica was applied against a crustacean parasite Caligus (Caligidae) infestation on the Asian seabass Lates calcarifer (Latidae) and showed a 100% mortality of Caligus within 5760 min (96 h) at a rate of 10 ppm [21]. The extract of Artemisia annua (Asteraceae, aromatic herbaceous plant, Sweet Annie) was reported to be effective in 30 to 180 min against monogenean parasites of the cultured airbreathing catfish Heterobranchus longifilis at concentrations ranging from 50 to 200 mg/L [36]. Thus, in comparison to the above-mentioned plant, the aqueous extract of A. indica resulted in the total mortality of leeches in less than 7 min at a higher concentration. Furthermore, we explained the principle behind the antiparasitic nature of the plant via the identification of the different responsible metabolites using Q Exactive HF Orbitrap mass spectrometry.
Among the compounds identified by Orbitrap mass spectrometry, some of them have been reported to have antiparasitic properties, especially on mammalian parasites, including quercetin, kaempferol, phloretin, trifolin, caryophyllene oxide and nicotinamide. The metabolites have also been reported in the plant extracts of Trigonella foenum-graecum [37], Moringa oleifera [38], Golden delicious apple pomace [39], Zanthoxylum bungeanum [40], Pinus eldarica [41] and Oryza sativa [42]. Some reports revealed that quercetin was effective against several mammalian parasites such as Cryptosporidium parvum (Cryptosporidiidae) and Encephalitozoon intestinali (Unikaryonidae), responsible for diarrheal diseases [43], Leishmania amazonensis (Trypanosomatidae), responsible for leishmaniasis [44], and Trypanosoma sp. (Trypanosomatidae), responsible for vector-borne disease and trypanosomiasis [45,46]. Quercetin directly induced apoptosis of Trypanosoma brucei gambiense without affecting the viability of the host cell [46]. Kaempferol inhibited the growth of the parasite Entamoeba histolytica (Entamoebidae), responsible for amoebiasis, by altering cytoskeleton proteins [47]. It is also well known for its antiproliferative [48], antidiabetic [49], antioxidative [50], anti-inflammatory [51] and anticancer properties [52]. Thus, we believe that the metabolites found in the aqueous extracts of A. indica could be responsible for the antiparasitic effect. Hence, we suggest that a natural-based treatment could be a viable alternative to chemicals and is effective for eco-friendly and sustainable aquaculture.
4. Materials and Methods
4.1. Chemicals
Formalin (37% aqueous formaldehyde solution) and sodium bicarbonates were obtained from Sigma, Leica, Microsystem, and Germany. Methanol (HPLC grade) was purchased from Merck (Darmstadt, Germany). LCMS-grade acetonitrile, water and formic acid were obtained from Fisher Scientific (Thermo Fisher Scientific, Waltham, MA, USA). Regenerated cellulose syringe filters with a 0.22-µm pore size and 13-mm diameter were obtained from Thermo Scientific (Thermo Fisher Scientific, Waltham, MA, USA).
4.2. Plant collection
The leaves of the plant A. indica (Figure 2) were collected from Universiti Malaysia Sabah, Kota Kinabalu (5.7346° N, 115.9319° E), Sabah, East Malaysia. The identification of the plant was carried out at the Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu.
Figure 2.
Azadirachta indica plant leaves.
4.3. Extraction
The leaves of the plant were rinsed with distilled water and dried in an oven at 37 °C. The dried plant was ground separately in a heavy-duty grinder. About 100 g of the dry plant powder was boiled with distilled water for 10 min with a 1:10 ratio (sample to the amount of distilled water) using a stirring hot plate. The decoctions were removed and allowed to cool at room temperature for 1 h. Further, the extracts were filtered using a strainer to remove coarse residues, and then the filtrate was filtered again using Whatman No. 1 filter paper. The pure filtrate was kept at −80 °C for 24 h and then lyophilized using a freeze drier.
4.4. Source of Marine Leech Z. arugamensis
The marine leeches Z. arugamensis (1–1.5 cm) were procured from the aquaculture facilities. An infested hybrid grouper (Epinephelus fuscoguttatus × E. lanceolatus) (15–340 g) (diameter: 15–20 cm) with marine leeches (Figure 1A,B) was placed in a small tank containing seawater from the cage, and the leeches were removed individually by hand. The leeches were transferred into a container containing filtered seawater and incubated at 27 °C for 24 h.
4.5. Antiparasitic Bioassay
Adult parasites were selected, divided into five groups, and each group was provided with six parasites in a Petri dish. Group 1 served as a negative control (Figure 1C), treated with seawater only, and group 2 served as a positive control, treated with 0.25% formalin solution (Figure 1D). In contrast, groups 3, 4 and 5 were challenged with 25, 50 and 100 mg/mL of the aqueous extract of A. indica, respectively (Figure 1E,F). During the challenge, parasites’ inactivity and death were recorded using a stopwatch.
4.6. Liquid Chromatography
Liquid chromatography (LC) was performed using the Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled with a Thermo Syncronis C18 column (2.1 mm × 100 mm × 1.7 μm; Thermo Fisher Scientific, Waltham, MA, USA), which was maintained at 55 °C at a flow rate of 450 µL/min during analysis. The mobile phases were composed of solvent A (water added with 0.1% formic acid) and solvent B (acetonitrile added with 0.1% of formic acid). The gradient elution program was initiated at 0.5% of solvent B for 1 min, then from 0.5% to 99.5% of solvent B for 15 min and maintained for 4 min. Later, the columns were conditioned as initial for 2 min before the next injection. The injection volume was set at 2 µL.
4.7. Data Acquisition
MS and MS/MS data were acquired using the Thermo Scientific Q Exactive HF Orbitrap mass spectrometry system (Thermo Fisher Scientific, Waltham, MA, USA), which was equipped with a heated electrospray ionization (HESI) probe. The data acquisition was set between an m/z of 100–1000 for MS and 200–1000 for MS/MS. The resolutions of the MS and MS/MS data were acquired at 60 k and 15 k, respectively. Positive and negative HESI were both deployed at 3.5 kV and 3.0 kV, respectively. The ion source conditions were set as follows: capillary temperature of 320 °C, sheath gas flow rate of 50, aux gas flow rate of 18, sweep gas flow rate of 0 and aux gas heater temperature of 300 °C. Calibrations were performed using Pierce LTQ ESI Positive Calibrations solution and Pierce LTQ ESI Negative Calibrations solution (Thermo Fisher Scientific, Waltham, MA, USA) before sample analysis.
4.8. Data Analysis
The acquired data were processed and analyzed using Thermo Scientific Compound Discoverer 3.0 software (Thermo Fisher Scientific, Waltham, MA, USA) with the default settings in order to perform compound identification. Briefly, the default workflow includes background subtraction with blank data, retention time alignment, feature detection, elemental composition determination, libraries matching and fragment ion search (FISh) scoring. The identification of compounds was primarily based on the matching of MS/MS data against mzCloud and mzVault databases. Unmatched signals were attempted with the ChemSpider database using MS data and supported with a FISh scoring above 50.
4.9. Statistical Analysis
Data analysis was carried out using the IBM SPSS Statistics 25 Window package (IBM, Armonk, NY, USA). Significant differences between groups were determined using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. All results were presented as the mean ±standard error of the mean (S.E.). p values under 0.05 were regarded as significant.
5. Conclusions
In the present study, it was proven that the application of various concentrations of A. indica aqueous extract indicated a strong antiparasitic activity with 100% mortality against the marine parasitic leech Z. arugamensis in an average time ranging from 6.45 to 42.65 min. The Q Exactive HF Orbitrap mass spectrometry analysis indicated the presence of five flavonoids (myricetin 3-O-galactoside, trifolin, isorhamnetin, quercetin and kaempferol), four aromatics (4-methoxy benzaldehyde, scopoletin, indole-3-acrylic acid and 2,4-quinolinediol), three phenolics (p-coumaric acid, ferulic acid and phloretin) and two terpenoids (pulegone and caryophyllene oxide). Some of the compounds were reported to have strong antiparasitic properties. This demonstrates that A. indica aqueous extract has the potential to act as a biocontrol agent against Z. arugamensis infestation. The antiparasitic properties of the aqueous extract of A. indica could be due to the presence of the above-mentioned metabolites. However, further investigation of the purification and isolation of the pure metabolites responsible for the antiparasitic properties is vital.
Acknowledgments
We thank all our UMS hatchery staff, manager and students for their kind support in collecting leeches.
Supplementary Materials
The following are available online, Figure S1: Structures of the identified compounds (1–42) from the extract of Azadirachta indica, compound names are given in Table 3, Figure S2: Base peak chromatogram (BPC) of the aqueous extract of Azadirachta indica.
Author Contributions
B.A.V.M. was involved in conceptualization; B.A.V.M., M.D.S., D.J.; sample collection; investigation; methodology; data curation; and formal analysis; M.D.S., J.K.T. and Y.S.Y. were involved in LCMS data analysis; B.A.V.M. and M.D.S. wrote the original draft; All authors reviewed and edited the draft; B.A.V.M. was involved in funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Niche project (SDN0073-2019), Universiti Malaysia Sabah; Fundamental Research Grant Scheme, Ministry of Education, Malaysia (FRG0486-2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Venmathi Maran B.A., Seng L.T., Ohtsuka S., Nagasawa K. Records of Caligus (Crustacea: Copepoda: Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedis Bassett-Smith, 1898. Zool. Stud. 2009;48:797–807. [Google Scholar]
- 2.Leong T.S., Wong S.Y. A comparative study of the parasite fauna of wild and cultured grouper (Epinephelus malabaricus Bloch et Schneider) in Malaysia. Aquaculture. 1988;68:203–207. doi: 10.1016/0044-8486(88)90353-5. [DOI] [Google Scholar]
- 3.Kua B.C., Azmi M.A., Hamid N.K.A. Life cycle of the marine leech (Zeylanicobdella arugamensis) isolated from sea bass (Lates calcarifer) under laboratory conditions. Aquaculture. 2010;302:153–157. doi: 10.1016/j.aquaculture.2010.02.029. [DOI] [Google Scholar]
- 4.Cruz-Lacierda E.R., Toledo J.D., Tan-Fermin J.D., Burreson E.M. Marine leech (Zeylanicobdella arugamensis) infestation in cultured orange-spotted grouper, Epinephelus coioides. Aquaculture. 2000;185:191–196. doi: 10.1016/S0044-8486(99)00356-7. [DOI] [Google Scholar]
- 5.Murwantoko M., Negoro S.L.C., Isnansetyo A. Short communication: Identification of marine leech and assessment of its prevalence and intensity on cultured hybrid groupers (Epinephelus sp.) Biodiversitas J. Biol. Divers. 2018;19 doi: 10.13057/biodiv/d190529. [DOI] [Google Scholar]
- 6.Azmey S., Taruna M., Taha H., Arai T. Prevalence and infestation intensity of a piscicolid leech, Zeylanicobdella arugamensis on cultured hybrid grouper in Brunei Darussalam. Vet. Parasitol. Reg. Stud. Reports. 2020;20:100398. doi: 10.1016/j.vprsr.2020.100398. [DOI] [PubMed] [Google Scholar]
- 7.Kua B.C., Abdullah S.Z., Abtholuddin M.F., Mohd N.F., Mansor N.N. Marine leech isolated from cage-cultured sea bass (Lates calcarifer) fingerlings: A parasite or vector? [(accessed on 10 March 2021)];Malay. Fish. J. 2009 Available online: https://ci.nii.ac.jp/naid/10027229044/
- 8.Burreson E.M. Fish Diseases and Disorders. Volume 1: Protozoan and Metazoan Infections. Volume 1. CABI; Wallingford, UK: 2006. Phylum Annelida: Hirudinea as vectors and disease agents; pp. 566–591. [Google Scholar]
- 9.Leal J.F., Neves M.G.P.M.S., Santos E.B.H., Esteves V.I. Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Rev. Aquac. 2018;10:281–295. doi: 10.1111/raq.12160. [DOI] [Google Scholar]
- 10.Wink M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruľová D., Caputo L., Elshafie H.S., Baranová B., De Martino L., Sedlák V., Gogaľová Z., Poráčová J., Camele I., De Feo V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules. 2020;25:595. doi: 10.3390/molecules25030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshafie H.S., Armentano M.F., Carmosino M., Bufo S.A., De Feo V., Camele I. Cytotoxic activity of origanum vulgare L. on Hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules. 2017;22:1435. doi: 10.3390/molecules22091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camele I., Elshafie H.S., Caputo L., De Feo V. Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential oils against phytopathogenic bacteria. Front. Microbiol. 2019;10:2619. doi: 10.3389/fmicb.2019.02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estevam E.B.B., De Deus I.P.B., Da Silva V.P., Da Silva E.A.J., Alves C.C.F., Alves J.M., Cazal C.M., Magalhães L.G., Pagotti M.C., Esperandim V.R., et al. In vitro antiparasitic activity and chemical composition of the essential oil from Protium ovatum leaves (Burceraceae) An. Acad. Bras. Cienc. 2017;89:3005–3013. doi: 10.1590/0001-3765201720170310. [DOI] [PubMed] [Google Scholar]
- 15.Biswas K., Chattopadhyay I., Banerjee R.K., Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr. Sci. 2002;82:1336–1345. [Google Scholar]
- 16.Girish K., Shankara B.S. Neem–A green treasure. Electron. J. Biol. 2008;4:102–111. [Google Scholar]
- 17.Aslam F., Ur-Rehman K., Asghar M., Sarwar M. Antibacterial activity of various phytoconstituents of neem. Pakistan J. Agric. Sci. 2009;46:209–213. [Google Scholar]
- 18.Kavitha M., Raja M., Kamaraj C., Karthik Raja R., Balasubramaniam V., Balasubramani G., Perumal P. In vitro antimicrobial activity of Azadirachta indica (leaves) against fish pathogenic bacteria isolated from naturally infected Dawkinsia filamentosa (blackspot barb) Med. Aromat. Plants. 2017;6:2–7. doi: 10.4172/2167-0412.1000294. [DOI] [Google Scholar]
- 19.Thanigaivel S., Vijayakumar S., Gopinath S., Mukherjee A., Chandrasekaran N., Thomas J. In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus) Aquaculture. 2015;437:252–255. doi: 10.1016/j.aquaculture.2014.12.008. [DOI] [Google Scholar]
- 20.Ngum W.L., Hortense G., Barthélémy N., Estella T., Ntungwen F.C. Activity, in vivo acute toxicity studies of the seed oil of Azadirachta indica (neem oil) in Wistar rats. Toxicology. 2019;5:31–38. doi: 10.15406/mojt.2019.05.00149. [DOI] [Google Scholar]
- 21.Khoa T.N.D., Mazelan S., Muda S., Shaharom-Harrison F. Use of neem oil (Azadirachta indica) to control caligid copepod infestation on Asian seabass (Lates calcarifer) Aquac. Res. 2019;50:1885–1892. doi: 10.1111/are.14074. [DOI] [Google Scholar]
- 22.Williams L.A.D., Mansingh A. The insecticidal and acaricidal actions of compounds from Azadirachta indica (A. Juss.) and their use in tropical pest management. Integr. Pest Manag. Rev. 1996;1:133–145. doi: 10.1007/BF00130672. [DOI] [Google Scholar]
- 23.De Silva P.H.D.H., Fernando C.H. Three marine leeches (Piscicolidae, Hirudinea) from the Malay Peninsula. Spol. Zeyl. 1965;30:227–232. [Google Scholar]
- 24.Kua B.C., Choong F.C., Leaw Y.Y. Effect of salinity and temperature on marine leech, Zeylanicobdella arugamensis (De Silva) under laboratory conditions. J. Fish Dis. 2014;37:201–207. doi: 10.1111/jfd.12087. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed S., Nagaraj G., Chua F.H.C., Wang Y.G. The use of chemicals in aquaculture in Malaysia and Singapore. In: Arthur J.R., Lavilla-Pitogo C.R., Subasinghe R.P., editors. Use of Chemicals in Aquaculture in Asia: Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996, Tigbauan, Iloilo, Philippines. Aquaculture Department, Southeast Asian Fisheries Development Center; Tigbauan, Philippines: 2000. pp. 127–141. [Google Scholar]
- 26.Pitten F.A., Kramer A., Herrmann K., Bremer J., Koch S. Formaldehyde neurotoxicity in animal experiments. Pathol. Res. Pract. 2000;196:193–198. doi: 10.1016/S0344-0338(00)80100-4. [DOI] [PubMed] [Google Scholar]
- 27.Woo P.T.K., Kurt B., editors. Fish Parasites: Pathobiology and Protection. 1st ed. CABI; Wallingford, UK: 2012. p. 384. [DOI] [Google Scholar]
- 28.Beveridge M.C.M., Brummett R.E. Freshwater Fisheries Ecology. John Wiley & Sons, Ltd.; Chichester, UK: 2015. Aquaculture and the environment; pp. 794–803. [Google Scholar]
- 29.Egidius E., Møster B. Effect of Neguvon® and Nuvan® treatment on crabs (Cancer pagurus, C. maenas), lobster (Homarus gammarus) and blue mussel (Mytilus edulis) Aquaculture. 1987;60:165–168. doi: 10.1016/0044-8486(87)90309-7. [DOI] [Google Scholar]
- 30.Kayser O., Kiderlen A.F., Croft S.L. Natural products as antiparasitic drugs. Parasitol. Res. 2003;90:S55–S62. doi: 10.1007/s00436-002-0768-3. [DOI] [PubMed] [Google Scholar]
- 31.Wunderlich A.C., Zica É.d.O.P., Ayres V.F.d.S., Guimarães A.C., Takeara R. Natural Remedies in the Fight Against Parasites. InTech; London, UK: 2017. Plant-derived compounds as an alternative treatment against parasites in fish farming: A review. [Google Scholar]
- 32.Shah M.D., Venmathi Maran B.A., Iqbal M., Ching F.F., Mohamad Lal M.T., Binti Othman R., Shapawi R. Antiparasitic activity of the medicinal plant Dillenia suffruticosa against the marine leech Zeylanicobdella arugamensis (Hirudinea) and its phytochemical composition. Aquac. Res. 2020;51:215–221. doi: 10.1111/are.14367. [DOI] [Google Scholar]
- 33.Shah M.D., Venmathi Maran B.A., Haron F.K., Ransangan J., Ching F.F., Shaleh S.R.M., Shapawi R., Yong Y.S., Ohtsuka S. Antiparasitic potential of Nephrolepis biserrata methanol extract against the parasitic leech Zeylanicobdella arugamensis (Hirudinea) and LC-QTOF analysis. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-79094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah M.D., Tani K., Yong Y.S., Ching F.F., Shaleh S.R.M., Vairappan C.S., Venmathi Maran B.A. Antiparasitic potential of chromatographic fractions of Nephrolepis biserrata and liquid chromatography-quadrupole time-of-flight-mass spectrometry analysis. Molecules. 2021;26:499. doi: 10.3390/molecules26020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eftekhari Z., Bahmani M., Mohsenzadeghan A., Gholami Ahangaran M., Abbasi J., Alighazi N. Evaluating the anti-leech (Limnatis nilotica) activity of methanolic extract of Allium sativum L. compared with levamisole and metronidazole. Comp. Clin. Path. 2012;21:1219–1222. doi: 10.1007/s00580-011-1268-6. [DOI] [Google Scholar]
- 36.Ekanem A.P., Andi Brisibe E. Effects of ethanol extract of Artemisia annua L. against monogenean parasites of Heterobranchus longifilis. Parasitol. Res. 2010;106:1135–1139. doi: 10.1007/s00436-010-1787-0. [DOI] [PubMed] [Google Scholar]
- 37.Sambandam B., Thiyagarajan D., Ayyaswamy A., Raman P. Extraction and isolation of flavonoid quercetin from the leaves of Trigonella foenum-graecum and their anti-oxidant activity. Int. J. Pharm. Pharm. Sci. 2016;8:120–124. [Google Scholar]
- 38.Makita C., Chimuka L., Steenkamp P., Cukrowska E., Madala E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. South African J. Bot. 2016;105:116–122. doi: 10.1016/j.sajb.2015.12.007. [DOI] [Google Scholar]
- 39.Zhang T., Wei X., Miao Z., Hassan H., Song Y., Fan M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016;10:47. doi: 10.1186/s13065-016-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong K., Li X.-J., Gou A.-N., Huang Y.-N., Bu Q., Gao H. Antioxidant and cytoprotective activities of flavonoid glycosides-rich extract from the leaves of Zanthoxylum bungeanum. J. Food Nutr. Res. 2014;2:349–356. doi: 10.12691/jfnr-2-7-4. [DOI] [Google Scholar]
- 41.Ghaffari T., Kafil H.S., Asnaashari S., Farajnia S., Delazar A., Baek S.C., Hamishehkar H., Kim K.H. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Pinus eldarica grown in Northwestern Iran. Molecules. 2019;24:3203. doi: 10.3390/molecules24173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi S., Kono Y., Kawarada A., Ota Y., Nakayama M. Nicotinamide as a plant growth regulator isolated from rice hulls. Agric. Biol. Chem. 1975;39:859–861. doi: 10.1271/bbb1961.39.859. [DOI] [Google Scholar]
- 43.Mead J.R., McNair N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006;259:153–157. doi: 10.1111/j.1574-6968.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 44.Fonseca-Silva F., Inacio J.D.F., Canto-Cavalheiro M.M., Almeida-Amaral E.E. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS ONE. 2011;6:e14666. doi: 10.1371/journal.pone.0014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodson H.C., Lyda T.A., Chambers J.W., Morris M.T., Christensen K.A., Morris J.C. Quercetin, a fluorescent bioflavanoid, inhibits Trypanosoma brucei hexokinase 1. Exp. Parasitol. 2011;127:423–428. doi: 10.1016/j.exppara.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamani-matsuda M., Malvy D., Thiolat D., Coves S., Courtois P., Vincendeau P., Mossalayi M.D., Andre S. Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrob. Agents Chemother. 2004;48:924–929. doi: 10.1128/AAC.48.3.924-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolaños V., Díaz-Martínez A., Soto J., Marchat L.A., Sanchez-Monroy V., Ramírez-Moreno E. Kaempferol inhibits Entamoeba histolytica growth by altering cytoskeletal functions. Mol. Biochem. Parasitol. 2015;204:16–25. doi: 10.1016/j.molbiopara.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Kim C.R., Kim H.S., Choi S.J., Kim J.K., Gim M.C., Kim Y.-J., Shin D.-H. Erucamide from radish leaves has an inhibitory effect against Acetylcholinesterase and prevents memory deficit induced by Trimethyltin. J. Med. Food. 2018;21:769–776. doi: 10.1089/jmf.2017.4117. [DOI] [PubMed] [Google Scholar]
- 49.Peng X., Zhang G., Liao Y., Gong D. Inhibitory kinetics and mechanism of Kaempferol on α-glucosidase. Food Chem. 2016;190:207–215. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 50.Liao W., Chen L., Ma X., Jiao R., Li X., Wang Y. Protective effects of Kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016;114:24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 51.Yeon M.J., Lee M.H., Kim D.H., Yang J.Y., Woo H.J., Kwon H.J., Moon C., Kim S.H., Kim J.B. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019;83:166–173. doi: 10.1080/09168451.2018.1528140. [DOI] [PubMed] [Google Scholar]
- 52.Kashyap D., Sharma A., Tuli H.S., Sak K., Punia S., Mukherjee T.K. Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods. 2017;30:203–219. doi: 10.1016/j.jff.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.