Abstract
Parthenolide, a strong cytotoxic compound found in different parts of Tarchonanthus camphoratus which motivated the authors to develop an optimized microwave-assisted extraction (MEA) method using Box–Behnken design (BBD) for efficient extraction of parthenolide from the stem of T. camphoratus and its validation by high-performance thin-layer chromatography (HPTLC) and cytotoxic analysis. The optimized parameters for microwave extraction were determined as: 51.5 °C extraction temperature, 50.8 min extraction time, and 211 W microwave power. A quadratic polynomial model was found the most suitable model with R2 of 0.9989 and coefficient of variation (CV) of 0.2898%. The high values of adjusted R2 (0.9974), predicted R2 (0.9945), and signal-to-noise ratio (74.23) indicated a good correlation and adequate signal, respectively. HPTLC analyzed the parthenolide (Rf = 0.16) content in T. camphoratus methanol extract (TCME) at λmax = 575 nm and found it as 0.9273% ± 0.0487% w/w, which was a higher than expected yield (0.9157% w/w). The TCME exhibited good cytotoxicity against HepG2 and MCF-7 cell lines (IC50 = 30.87 and 35.41 µg/mL, respectively), which further supported our findings of high parthenolide content in TCME. This optimized MAE method can be further applied to efficiently extract parthenolide from marketed herbal supplements containing different Tarconanthus species.
Keywords: Box–Behnken design, parthenolide, T. camphoratus, HPTLC analysis, cytotoxicity
1. Introduction
Parthenolide (sesquiterpene lactone, Figure 1) is a potent cytotoxic agent [1] and is present in large amounts in Chrysanthemum parthenium (feverfew; 85% w/w). Parthenolide reportedly prevents platelet clumping and serotonin and inflammatory mediators release [2]. Also, parthenolide has been demonstrated to possess anticancer and cytotoxic activities against several human cancer cell lines, including HeLa cells (cervical carcinoma), h9c2 cells (cardiomyoblasts), HT-29 cells (colon cancer), PC-3 and DU−145 cells (prostate cancer), and MDA-MB-231 and MCF7 cells (breast cancer) [3,4,5,6]. Therefore, the authors of this study sought to explore alternatives to feverfew for parthenolide content. Tarchonanthus camphoratus, belonging to the Asteraceae family, is considered to be a prominent substitute of feverfew for parthenolide content, especially in its stem and leaves [7].
Figure 1.

Chemical structure of parthenolide.
Uneven distribution of phytoconstituents in different plant parts, degree of solubility of these phytoconstituents in various solvents, and extraction temperature require optimization to extract the desired phytoconstituents better. Sesquiterpene lactones such as parthenolide were first extracted in 1970 using the conventional extraction method of chloroform and petroleum-ether extraction solvents [8]. Subsequently, several other extraction methods were developed for parthenolide extraction, such as high-performance liquid chromatography (HPLC) [9] and the Soxhlet extraction method [10]. Recently, a progressive change has been observed in extraction technology to develop simple and effective sample preparation methods. The microwave-assisted extraction (MAE) technique, being inexpensive, simple, and efficient, is a promising technique for increasing the extraction of compounds from plants [11]. A substantial increase has been achieved in the yields of medicinal plant extraction by using microwave irradiation. In the event of microwave irradiation on natural material, electromagnetic waves are absorbed selectively by media with a high dielectric constant, resulting in more effective heating. In the course of absorption, the microwaves’ energy is converted into kinetic energy, thus allowing the selective heating of the microwave-absorbent plant material. The subsequent volume increase causes cells to explode, releasing their content into the liquid phase. When the liquid absorbs the microwaves, its molecules’ kinetic energy increases; consequently, the diffusion rate increases, resulting in faster mass transfer [12].
Response surface methodology (RSM) is an efficient method for optimizing the complex extraction processes comprising numerous variables [13]. The Box–Behnken design (BBD) of RSM operates at three levels (low, medium and high) and requires few experiments [14]. Several research groups have reported the various methods used to analyze parthenolide in Feverfew such as infrared spectrometry [15] and HPLC [16,17].
In this study, the authors applied the BBD method to optimize parthenolide extraction from T. camphoratus stems using MAE technique with three extraction variables (extraction temperature, extraction time and microwave power). The obtained BBD optimized extract was analyzed for parthenolide content by high-performance thin-layer chromatography (HPTLC) and assayed against two cancer cell lines (HepG2 and MCF-7).
2. Materials and Methods
2.1. Plant Material
The T. camphoratus stems (voucher specimen #15451) were collected from Wadi gamma (Saudi Arabia) and authenticated by field taxonomist Dr. Mohamed Yusuf. Post collection, the plant part was cut into small pieces, washed and dried in the plant drying room, and stored in a clean glass jar. A specimen was deposited in herbarium of Pharmacognosy Department, Pharmacy College, KSU, Saudi Arabia.
2.2. Apparatus and Reagents
The standard compound parthenolide (≥98%) was procured from Sigma-Aldrich (St. Louis, MI, USA). Methanol, ethyl acetate, and n-hexane of analytical grade were procured from WINLAB (Unit 13, Courtyard Workshops, Bath Street, Market Harborough LE16 9EJ, UK) and DMSO used in the cytotoxic analysis was procured from Sigma-Aldrich (St. Louis, MI, USA). Silica gel 60F254-precoated HPTLC plates (20 × 10 cm) were purchased from Merck (Darmstadt, Germany), and standard compounds along with extracts were applied (band wise) to plates using a CAMAG automatic TLC sampler-4 (ATS-4) and developed in a CAMAG automatic development chamber (ADC 2). Finally, the developed plates were documented using a CAMAG TLC Reprostar 3 and scanned using a CAMAG ATS 4 (CATS 4; CAMAG, Muttenz, Switzerland).
2.3. Microwave-Assisted Extraction of T. camphoratus Stems
The extraction of T. camphoratus (TC) stem powder (1 g) was performed in a closed container in a microwave (MARS 5, Matthews, NC, USA) using methanol as solvent. After the completion of the extraction procedure, the T. camphoratus methanol extract (TCME) was cooled and filtered. The residue was washed again with methanol thrice to get the final volume of TCME, filtered through a syringe filter (PTFE membrane, 0.45 µm, Phenomenex) and dried at reduced pressure using rotavapor (R-300, Buchi, Switzerland) to achieve the final percentage yield.
2.4. High-Performance Thin-Layer Chromatography (HPTLC) Analysis of TCME
2.4.1. TLC Instrumentation and Chromatographic Conditions
In the process of analysis of parthenolide content in TCME by HPTLC methods, the TCME samples were applied on a 20 × 10 cm glass-backed HPTLC plate (coated with silica gel 60 F254) with a Hamilton Gastight Syringe (25 µL) fitted in Automatic TLC Sampler-4 at a speed of 160 nL/s. Afterward, the plate development took place in previously saturated ADC 2 for 20 min at 22 °C, with a mobile phase of n-hexane and ethyl acetate at the ratio of 3:1 (v/v). Further, the developed plate’s derivatization was done using reagent p-anisaldehyde, then the plate was dried gently, and scanned with CATS 4 (slit dimension: 4.00 × 0.45 mm; speed: 20 mm/s).
2.4.2. Preparation of the Standard Stock Solution
A stock solution of parthenolide (1 mg/mL) was prepared by dissolving 1 mg of parthenolide in 1 mL of methanol and further diluted with methanol to make a final working concentration of 0.1 mg/mL. For calibration, 1–7 µL of working standard solution was applied to the HPTLC plate to provide a 100–700 ng/band concentration range.
2.4.3. HPTLC Method Development and Validation
Several combinations of different solvents were used to select the most appropriate mobile phase to obtain the best resolution on chromatograms of standard and test extracts. The same mobile phase [n-hexane:ethyl acetate (3:1, v/v)] was used for efficient separation of various constituents of TCME samples. The developed HPTLC method was validated according to the International Council for Harmonisation of Technical Requirements for precision, accuracy, LOD (limit of detection), LOQ (limit of quantification), and robustness [18]. The LOD and LOQ for parthenolide were calculated by using the following equations 1 and 2, respectively:
| LOD = (3.3 × SD)/α | (1) |
| LOQ = (10 × SD)/α | (2) |
where SD is the least standard deviation and α is the slope of the curve.
2.4.4. Quantitative Analysis of Parthenolide in TCME Samples
For quantitative analysis, the TCME samples along with standards were spotted on HPTLC plates, and the parthenolide content (% w/w) in TCME samples was determined by measuring the area.
2.5. BBD Experimental Design
2.5.1. Single-Factor Experimental Design
The range of different extraction variables (extraction temperature, time and microwave power) was selected based on observation of single-factor effect on the total extraction yield, which was used to optimize all extraction variables by Box–Behnken design (BBD) method to obtain the maximum parthenolide content from TCME. The impact of a single factor on total extraction yield was evaluated by varying the extraction temperature (30–90 °C), extraction time (20–80 min), and microwave power (50–600 W) while keeping extraction temperature (40 °C), extraction time (40 min), and microwave power (300 W) constant when another factor is variable.
2.5.2. Optimization of Extraction Variables Using BBD Method
A 3 factorial (33) Box–Behnken design (BBD; Design Expert Software, Trial version 12, Stat-Ease Inc., Minneapolis, MN, USA) of Response Surface Methodology (RSM) was used to optimize the three independent variables, namely, extraction temperature (P1), extraction time (P2), and microwave power (P3), with low (−1), medium (0), and high levels (+1) for each variable (Table 1). The BBD method had generated 17 experimental runs comprising five central points (Table 2). The response value was denoted by the total yield of parthenolide (R), and the results were fit into a second-order polynomial Equation (3):
| (3) |
where Y = Total parthenolide yield (R), q0, qi, qii, and qij = the regression coefficients of the intercept, linearity, square, and interaction, respectively. Pi, Pj = independent variables.
Table 1.
Extraction variables selected for Box-Bohnken Design (BBD) optimization.
| Independent Variable | Factor Level | Dependent Variable | Goal | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Extraction temperature (°C) (P1) | 40 | 50 | 60 | Parthenolide yield (% w/w) (R) |
Maximized |
| Extraction time (min) (P2) | 35 | 45 | 55 | ||
| Microwave power (W) (P3) | 100 | 200 | 300 | ||
Table 2.
Experimental parameters of Box–Behnken design and result of R (parthenolide).
| Run | Factor (Coded) | Actual Variables | Parthenolide Yield (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (P1) (°C) |
(P2) (min) |
(P3) (mL/g) |
(P1) (°C) |
(P2) (min) |
(P3) (W) |
Experimental (% w/w) |
Predicted (% w/w) |
Residual | |
| 1 | 1 | 0 | 1 | 60 | 45 | 300 | 0.8758 ± 0.039 | 0.8767 | −0.0009 |
| 2 | 0 | −1 | −1 | 50 | 35 | 100 | 0.8149 ± 0.033 | 0.8143 | 0.0006 |
| 3 | 0 | 0 | 0 | 50 | 45 | 200 | 0.8967 ± 0.041 | 0.8946 | 0.0020 |
| 4 | 0 | 1 | −1 | 50 | 55 | 100 | 0.8666 ± 0.044 | 0.8678 | −0.0012 |
| 5 | −1 | 0 | −1 | 40 | 35 | 100 | 0.7714 ± 0.04 | 0.7705 | 0.0009 |
| 6 | 0 | 0 | 0 | 50 | 45 | 200 | 0.8939 ± 0.037 | 0.8946 | −0.0008 |
| 7 | −1 | 1 | 0 | 40 | 55 | 200 | 0.8093 ± 0.031 | 0.8090 | 0.0003 |
| 8 | −1 | −1 | 0 | 40 | 35 | 200 | 0.7530 ± 0.048 | 0.7545 | −0.0015 |
| 9 | 1 | 0 | −1 | 60 | 45 | 100 | 0.8503 ± 0.053 | 0.8506 | −0.0003 |
| 10 | 0 | 0 | 0 | 50 | 45 | 200 | 0.8983 ± 0.058 | 0.8946 | 0.0037 |
| 11 | 0 | 0 | 0 | 50 | 45 | 200 | 0.8933 ± 0.051 | 0.8946 | −0.0013 |
| 12 | 0 | −1 | 1 | 50 | 35 | 300 | 0.8462 ± 0.042 | 0.8450 | 0.0012 |
| 13 | 0 | 1 | 1 | 50 | 55 | 300 | 0.8631 ± 0.039 | 0.8637 | −0.0006 |
| 14 | 1 | −1 | 0 | 60 | 35 | 200 | 0.8654 ± 0.062 | 0.8657 | −0.0003 |
| 15 | −1 | 0 | 1 | 40 | 45 | 100 | 0.7714 ± 0.022 | 0.7711 | 0.0003 |
| 16 | 1 | 1 | 0 | 60 | 55 | 200 | 0.8849 ± 0.054 | 0.8834 | 0.0015 |
| 17 | 0 | 0 | 0 | 50 | 45 | 200 | 0.8911 ± 0.038 | 0.8946 | −0.0036 |
P1: extraction temperature, P2: extraction time, P3: microwave power.
To interpret the effects of all independent variables on parthenolide yield, three-dimensional response surface plots were constructed. The “biggest-is-best” principle was applied for each response to maximize the outcome of parthenolide extraction. Finally, to confirm the study, three parallel experiments were carried out using the optimal extraction conditions with the maximum interest for practically authenticating the quality characteristic’s progress.
2.5.3. BBD Model and Validity Testing
Box–Behnken design of response surface methodology was used for the analysis of the experimental results and optimization of three independent extraction variables, extraction temperature (P1), extraction time (P2), and microwave power (P3); p-values ≤ 0.05 were considered to be significant. The extraction of a sample using optimized independent extraction variables was carried out in triplicate (n = 3), and the experimental yield was compared with predicted values for model validation.
2.6. Cell Culture and Cytotoxicity Assay of BBD Run TCME Samples
In this study, two different human cancer cells MCF-7 (breast) and HepG2 (liver), were used. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with bovine calf serum (10%; Invitrogen, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C with 5% CO2 supply in a humid chamber. The MCF-7 and HepG2 cells were plated (1 × 105 cells/mL/well) in 24-well tissue culture plates 1 day before treatment. Stocks of TCME (n = 17) were prepared first in 100 μL DMSO (Sigma, St. Louis, MI, USA), and then in DMEM (100 mg/mL) following reconstitution of four working concentrations (100, 50, 25, and 10 μg/mL) in DMEM. The final concentration of DMSO never exceeded 0.1% in the treatment doses. After 24 h, the cells were treated with triplicated doses of each extract, including an untreated control (0.1% DMSO), and incubated for 48 h at 37 °C. Next, 100 µL of MTT (5 mg/mL; TACS MTT Cell Proliferation and Viability Assay Kit, BioTekhne, Minneapolis, MN, USA) was added to each well and incubated for 24 h. After incubation, 1 mL of 0.01 N HCL/isopropanol was added to the wells to solubilize the formazan on the shaker for 10 min. The absorbance of converted MTT was measured at λ = 490 nm with a microplate reader (Bio-Tek, Winooski, VT, USA). Vinblastine was used as a positive control. For each extract tested, the IC50 (concentration of tested compound needed to inhibit cell growth by 50%) was generated from the dose-response curves.
2.7. Statistical Analysis
All experiments were carried out with three independent replicates and values are presented as mean ± standard error of the mean (SEM). Data were statistically analyzed using the Student’s t-test to compare the means applying a significance level of p < 0.05.
3. Results
3.1. Effect of Single-Factor Tests on the Total Extraction Yield of T. camphoratus Stems
3.1.1. Extraction Temperature Effect
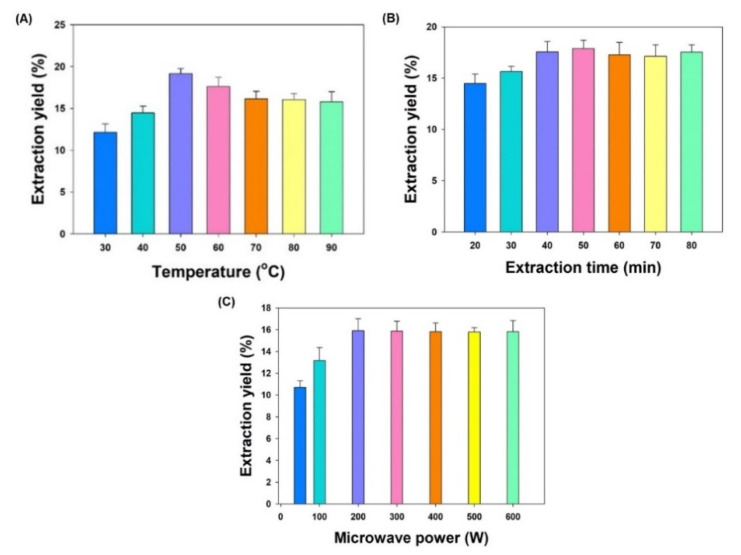
To investigate extraction temperature effect on total extraction yield, the microwave temperature was varied from 30 °C to 90 °C, while the extraction time (40 min) and microwave power (300 W) were kept constant. It is clear from Figure 2A that the total extraction yield obtained at 30 °C was the lowest and highest at 50 °C. On the other hand, setting higher temperatures did not produce a significant change in the yield. Temperature is the most widely varied parameter in MAE investigation. The increase in temperature causes decrease in viscosity and surface tension of the solvent, increasing its matrix penetration power and resulting in enhanced extraction. Based on the observation of this experiment a temperature range of 40–60 °C was selected to be applied for BBD method optimization.
Figure 2.
The effects of single factors on the total extraction yield of TCME. (A) Extraction temperature effect; (B) Extraction time effect; (C) Microwave power effect. Each value represents a mean ± SD (n = 5).
3.1.2. Extraction Time Effect
The extraction time impact on the total extraction yield was investigated by varying the extraction time from 20 to 80 min, keeping the extraction temperature (40 °C) and microwave power (300 W) constant. The result obtained in this experiment clearly indicated that the total extraction yield increases with time of extraction from 20 to 40 min but no significant change was observed in extraction yield beyond 40 min of extraction time (Figure 2B). Hence, a range of extraction time from 35 to 55 min was selected to optimize the extraction method using BBD.
3.1.3. Microwave Power Effect
The microwave power effect on the total extraction yield was investigated by adjusting the microwave power from 50 to 600 W, at constant extraction temperature (40 °C) and extraction time (40 min). It is evident from Figure 2C that the total extraction yield was lowest at microwave power 50 W and maximum at 200 W. In contrast, no significant increase in total extraction yield was observed after increasing the microwave power beyond 200 W. A low or moderate power for a longer time is typically preferred to elude a “bumping” incident generating a high power output. Based on the observation of this experiment, a microwave power ranged from 100 to 300 W was selected to be applied for extraction optimization by BBD method.
3.2. HPTLC Analysis of TCME
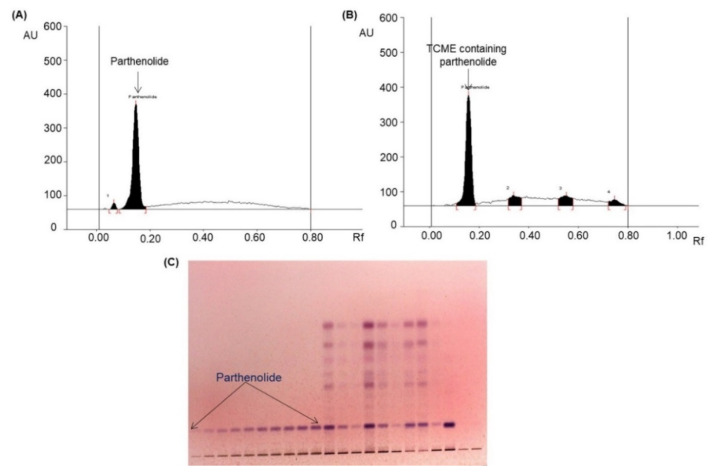
Various compositions of different solvents were used to develop a suitable mobile phase for parthenolide analysis. After examination of different compositions, n-hexane and ethyl acetate in the ratio of 3:1 (v/v) was chosen as best mobile phase, which afforded a strong parthenolide peak (Rf = 0.16; Figure 3A), and it efficiently separated various phytoconstituents present in the TCME (Figure 3B) in the absorbance mode at λmax of 575 nm.
Figure 3.
Quantification of parthenolide in BBD-run TCME sample by HPTLC [mobile phase—n-hexane:ethyl acetate (3:1, v/v); λmax = 575 nm]. (A) Chromatogram of standard parthenolide (Rf = 0.16); (B) chromatogram of the TCME sample (parthenolide, spot 1, Rf = 0.16); (C) pictogram of TLC plate derivatized with p-anisaldehyde in daylight.
The developed HPTLC method furnished high linearity (R2 = 0.9928) in 100–700 ng/band of linearity range, and low LOD (28.46 ng) and LOQ (86.24 ng) for parthenolide (Supplementary Table S1). The recovery of parthenolide was ranged from 97.64% to 98.97% (Supplementary Table S2). The precision for parthenolide at different concentration levels was indicated by % RSD (relative standard deviation) and listed in Supplementary Table S3. It was ranged from 1.53% to 1.81% (intra-day) and 1.46% to 1.71% (inter-day). As presented in Supplementary Table S4, low values of % RSD (1.79–1.84) indicated that the proposed HPTLC method was robust.
3.3. BBD Method Optimization of Extraction Conditions
The ranges for three extraction variables, namely, extraction temperature (P1), extraction time (P2) and microwave power (P3) at three level (+1, 0, −1) for extraction parameters optimization by BBD method, was selected based on the observation of a single-factor experiment.
3.3.1. Statistical Analysis and Model Fitting
In Table 2, the results of 17 experimental combinations of three extraction variables were recorded in terms of percentage parthenolide yield (R). These experimental combinations of different extraction variables were carried out to know their impact on parthenolide yield. The results were fitted into Equation (3) (second-order polynomial equation) to generate the following equation with coded factors for R:
| Rparthenolide = 0.8946 + 0.0464 P1 + 0.0181 P2 + 0.0067 P3 − 0.0092 P1P2 + 0.0064 P1P3 − 0.0087 P2P3 − 0.0485 P12 − 0.018 P22 − 0.029 P32. |
(4) |
For this BBD-based experimental design, a quadratic model with R2 value of 0.9989 was emerged as the best fit model. In Table 3, the regression analysis and response regression equation data for the suggested model have been listed.
Table 3.
Regression analysis and response regression equation results for the final proposed model.
| Dependent Variables | Source | R 2 | Adjusted R2 | Predicted R2 | SD |
|---|---|---|---|---|---|
| R | Linear | 0.5424 | 0.4368 | 0.3041 | 0.0362 |
| 2FI | 0.5640 | 0.3024 | −0.1510 | 0.0403 | |
| Quadratic | 0.9989 | 0.9974 | 0.9945 | 0.0025 | |
| Cubic | 0.8791 | 0.7665 | − | − |
The observed R2 (0.9989) and predicted R2 (0.9845) values for parthenolide were very close to 1, indicating that observed and predicted values were highly correlated. Also, the adjusted R2 and predicted R2 had a difference of less than 2, which was required for the fit model. The determined adequate precision (74.23) was greater than 4, which indicated that the model was fit. In Table 4, the ANOVA results (analysis of variance) for the model terms are listed.
Table 4.
ANOVA of the reduced quadratic model for extraction yields of parthenolide.
| Dependent Variable | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|---|
| R | Model | 0.0372 | 9 | 0.0041 | 681.78 | <0.0001 | Significant |
| Residual | 0.0001 | 7 | 6.064 × 10−6 | − | − | ||
| Lack of fit | 9.597 × 10−6 | 3 | 3.199 × 10−6 | 0.3895 | 0.7677 | Insignificant | |
| Pure error | 0.0001 | 4 | 8.212 × 10−6 | − | − |
The high F-value (681.78) and low p-value (<0.05) for the proposed model suggested that the developed model was significant and there is only a 0.01% chance that an F-value this large could occur due to noise. The F-value of 0.3895 and p-value of 0.7677 imply that Lack of Fit is not significant relative to the pure error (>0.05), which suggested a 76.77% chance that a Lack of Fit F-value this large could occur due to noise. Non-significant lack of fit is suitable for the model to be fit and sufficient for predicting the responses.
3.3.2. Effect of Extraction Parameters (P1, P2, and P3) on Parthenolide Yield (R)
The contributions of each independent variable [extraction temperature (P1), extraction time (P2), and microwave power (P3)] as well their different interactions on R are listed in Table 5. The linear variables (P1, P2, P3), interaction variables (P1P2, P1P3, and P2P3), and quadratic variables (P12, P22, and P32) were found to be significant (p < 0.05) and to affect the yield of parthenolide R. The R2 value (0.9989) and % CV (0.2898) indicated good precision and reliability of the experimental values [19]. Figure 4 is the three-dimensional (3D) response surface plot that illustrates the key collaborative effects generated for each pair of factors. Each panel illustrates the impact of two factors on the extraction yields, while the third factor was fixed at the zero level, which was 50 °C for P1, 45 min for P2, and 200 W for P3.
Table 5.
The significance of each response variable effect shown by using the F ratio and p-value in the nonlinear second-order model.
| Dependent Variable | Independent Variables | SS a | DF b | MS c | F-Value | p-Value d |
|---|---|---|---|---|---|---|
| Linear effects | ||||||
| P 1 | 0.0172 | 1 | 0.0172 | 2842.99 | <0.0001 | |
| P 2 | 0.0026 | 1 | 0.0026 | 430.20 | <0.0001 | |
| P 3 | 0.0004 | 1 | 0.0004 | 58.63 | 0.0001 | |
| Quadratic effects | ||||||
| R | P 1 2 | 0.0099 | 1 | 0.0099 | 1632.69 | <0.0001 |
| P 2 2 | 0.0014 | 1 | 0.0014 | 224.96 | <0.0001 | |
| P 3 2 | 0.0035 | 1 | 0.0035 | 582.25 | <0.0001 | |
| Interaction effects | ||||||
| P 1 P 2 | 0.0003 | 1 | 0.0003 | 56.14 | 0.0001 | |
| P 1 P 3 | 0.0002 | 1 | 0.0002 | 26.79 | 0.0013 | |
| P 2 P 3 | 0.0003 | 1 | 0.0003 | 49.86 | 0.0002 |
a Sum of squares; b Degree of freedom; c Mean Square; d p-values < 0.05 were considered to be significant.
Figure 4.
Response surface model 3D plots showing the effects of P1, P2, and P3 on R. (A) Effect of P1 and P2 on R, (B) effect of P1 and P3 on R, and (C) effect of P2 and P3 on R.
The influences of P1 and P2 (Figure 4A), P1 and P3 (Figure 4B), and P2 and P3 (Figure 4C) on R were recorded. As illustrated in Figure 4A, the extraction yield was highest at P1 of 51.5 °C and P2 of 50.8 min at a fixed microwave power of 200 W. When P1 exceeded 51.5 °C, the yield decreased. Figure 4B illustrated the effect of P1 and P3 on R which demonstrated that the yield was increased significantly at P3 of 211 W at a fixed extraction time of 45 min. Figure 4C illustrated the impact of P2 and P3 interaction on R at a fixed extraction temperature of 50 °C, which demonstrated that no significant change was observed in R with P3 increase, but a substantial rise in R was observed with an increase in P2. From this observation, we concluded that the maximal R could be extracted from T. camphoratus stems using microwave extraction at an extraction temperature of 51.5 °C, an extraction time of 50.8 min, and a microwave power of 211 W.
3.3.3. BBD Method Validation
For the P1, P2, and P3 checkpoints, the yield evaluation was found to be within the detection limits. The experimental value and predicted value of the response were compared to validate P1, P2, and P3 results. The small percentage prediction error facilitated establishing the rationality of the generated polynomial equation and relating BBD model application. The linear correlation plot between actual and predicted values demonstrated a high R2 value (0.9989), indicating excellent goodness of fit (p < 0.0001) (Figure 5).
Figure 5.
Linear correlation plot between actual and predicted values for R.
3.3.4. Optimization and Verification of Microwave-Assisted Extraction Conditions
The selected factors exhibited different effects on parthenolide yield (Table 6). The predicted optimal conditions for parthenolide extraction were as follows: extraction temperature of 51.5 °C (P1), extraction time of 50.8 min (P2), and microwave power of 211 W (P3). The low residual percentage (1.26%) calculated between predicted (0.9157% w/w) and observed responses (0.9273% w/w) indicated that the model was reliable.
Table 6.
Observed and predicted levels for optimal extraction conditions.
| Factor | Optimal Level | ||
|---|---|---|---|
| P1 (°C) | 51.5 | ||
| P2 (min) | 50.8 | ||
| P3 (W) | 211 W | ||
| Response | Predicted (% w/w) | Experimental (% w/w, n = 3) | Residual (%) |
| Parthenolide (% w/w) | 0.9157 | 0.9273 ± 0.0487 | 1.26 |
Residual (%) = (observed value − expected value)/expected value × 100.
3.4. Cytotoxic Assay of BBD-Run TCME Samples
The in vitro anti-cell proliferative activities of 17 BBD-run TCME samples (Runs 1–17) tested against cultured HepG2 and MCF-7 cells exhibited promising cytotoxicity, with estimated IC50 values ranging from 33.92 to 71.83 μg/mL and from 39.57 to 75.44 μg/mL, respectively (Figure 6).
Figure 6.
The estimated IC50 values (µg/mL) of BBD-run TCME samples on HepG2 and MCF-7 cell lines. (ctrl: control).
Bioactive parthenolide derived from different plant sources are demonstrated to have in vitro anticancer potential on a range of human cancer cell lines. In line with this, the T. camphoratus extract obtained by using the BBD optimized microwave extraction conditions exhibited marked cytotoxic effect on HepG2 cells (IC50: 30.87 µg/mL) and MCF-7 cells (IC50: 35.41 µg/mL) which further supported our HPTLC finding of high parthenolide content (0.9273% w/w) in TCME.
4. Discussion
The effect of different extraction parameters (extraction temperature, extraction time and microwave power) on the parthenolide extraction from the stems of T. camphoratus using microwave extraction technique was evaluated and optimized by Box–Behnken design (BBD) of Response Surface Methodology (RSM). Initially, the range for BBD optimization of different extraction parameters was set based on observing single-factors’ effect on the total extraction yield of T. camphoratus. The result of single-factor effect revealed that the total yield significantly increased when extraction temperature, extraction time and microwave power reached up to 50 °C, 50 min and 200 W, respectively, and afterward there was no significant increase in the yield was observed upon increase in temperature, time and microwave power. The increased temperature of extraction increases the mass transfer rate and causes higher molecular diffusion, resulting in high extraction of plant material [20]. Similarly increase in microwave power increases the total yield which might be due to an increase in mass transfer driving force. Based on the observation of the single-factor effect on the total yield, the range of extraction temperature (40 °C–60 °C), extraction time (35–45 min), and microwave power (100–300 W) was selected for optimization by BBD method.
During the optimization of different extraction parameters by BBD method, it furnished 17 runs analyzed for the parthenolide content by validated HPTLC method. For BBD analysis, a quadratic model was found as the best fit model. The observed R2 and predicted R2 were found close to 1 and their difference was less than 2, indicating a good correlation between them and the model fit. The higher value of adequate precision (>4) suggested that the signal was fair, which could be used to navigate the design space, while low lack of fit value was found not significant, indicating the validity of BBD results. The high model F-value for parthenolide implied that the model was significant. In this study, the low value of percentage residual and considerable value of R2 supported the high predictive ability of BBD analysis.
The significance of each extraction variable (P1, P2, and P3) on parthenolide extraction and the BBD analysis was evaluated. When P1 and P2, P2 and P3 interacted, they negatively affected the parthenolide yield. Similarly, the square root of all extraction variables produces a negative impact on parthenolide yield. Only the interactions between P1 and P3 produced a positive impact on parthenolide extraction. From the 3D plot, it was clear that P1 (extraction temperature) had a more significant effect on the parthenolide extraction than the other extraction parameter. The parthenolide content was found maximum at an optimized temperature of 51.5 °C, demonstrating that a lower temperature helped enhance the compound yield. High extraction temperature would result in decreased parthenolide yield. Hence, to extract maximum parthenolide from stems of T. camphoratus, the optimum extraction condition was found as 51.5 °C, 50.8 min, and 211 W, respectively.
5. Conclusions
The experimental findings suggested that the BBD method is very promising in optimizing the various extraction conditions used in microwave extraction to get the maximum yield of parthenolide from T. camphoratus stems. The model prediction can be used to optimize the yield within the limits of the experimental variables. The experimental yield obtained after extracting the sample under optimized extraction conditions [temperature (51.5°C), time of extraction (50.8 min), and microwave power (211 W)] was found as 0.9273% ± 0.0487% w/w, which agreed closely with the predicted value of 0.9157% w/w. The quadratic polynomial model was most appropriate concerning parthenolide, and the high values of adjusted R2 and predicted R2 indicated good correlation and model fitting, while a high value of signal-to-noise ratio indicated an adequate signal that might be applied to navigate the design space. In the future, this optimized MAE method can be further used to extract parthenolide efficiently from the marketed herbal supplements containing different species of Tarconanthus.
Supplementary Materials
The following are available online. Table S1: Rf, Linear regression data for the calibration curve of parthenolide (n = 6), Table S2: Recovery as accuracy studies of the proposed HPTLC Method (n = 6), Table S3: Precision of the proposed HPTLC method (n = 6), Table S4: Robustness of the proposed HPTLC Method (n = 6).
Author Contributions
Conceptualization, investigation, writing—original draft preparation, P.A.; methodology, A.H.; software, writing—review and editing, A.A. and M.T.R.; validation, formal analysis, N.A.S. and S.R.M.; resources, supervision, project administration, funding acquisition, M.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-150.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated and used is presented in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds may be made available on request to the corresponding author.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berry M.I. Feverfew faces the future. Pharm. J. 1984;232:611–614. [Google Scholar]
- 2.Retnik L.C., Skeget M., Knez Z. Separation of parthenolide from feverfew: Performance of conventional and high-pressure extraction techniques. Sep. Purif. Technol. 2005;41:13–20. doi: 10.1016/j.seppur.2004.03.011. [DOI] [Google Scholar]
- 3.Al-Fatlawi A.A., Irshad M., Rahisuddin A. Effect of parthenolide on growth and apoptosis regulatory genes of human cancer cell lines. Pharm. Biol. 2015;53:104–109. doi: 10.3109/13880209.2014.911919. [DOI] [PubMed] [Google Scholar]
- 4.Tsai T.Y., Chan P., Gong C.L., Wong K.L., Su T.H., Shen P.C., Leung Y.M., Liu Z.M. Parthenolide-Induced Cytotoxicity in H9c2 Cardiomyoblasts Involves Oxidative Stress. Acta Cardiol. Sin. 2015;31:33–41. doi: 10.6515/ACS20140422B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosio C., Tomasoni G., Martínez R., Olea A.F., Carrasco H., Villena J. Cytotoxic and apoptotic effects of leptocarpin, a plant-derived sesquiterpene lactone, on human cancer cell lines. Chem. Biol. Interact. 2015;242:415–421. doi: 10.1016/j.cbi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Alwaseem H., Frisch B.J., Fasan R. Anticancer activity profiling of parthenolide analogs generated via P450-mediated chemoenzymatic synthesis. Bioorg. Med. Chem. 2018;26:1365–1373. doi: 10.1016/j.bmc.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osman W., Ibrahim M., Adam M., Ahmad S., Mothana R., Mohammed M., Abdoon I., Basudan O., Garelnab E., Mohamed H., et al. Isolation and Characterization of Four Terpenoidal Compounds with Potential Antimicrobial Activity from Tarconanthus camphorantus L. (Asteraceae) J. Pharm. Bioallied Sci. 2019;11:373–379. doi: 10.4103/jpbs.JPBS_249_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshioka H., Renold W., Fischer N.H., Higo A., Mabry T.J. Sesquiterpene lactones from Ambrosia connfertiflora (compositae) Phytochem. 1970;9:823. doi: 10.1016/S0031-9422(00)85188-2. [DOI] [Google Scholar]
- 9.Marchand B., Behl H.M., Rodriguez E. Application of high performance liquid chromatography for analysis and isolation of sesquterpene lactones. J. Chromatogr. 1983;265:97. doi: 10.1016/S0021-9673(01)96702-0. [DOI] [Google Scholar]
- 10.Rey J.P., Levesque J., Pousset J.L. Extraction and high-performance liquid chromatographic methods for the gamma lactones parthenolide (Chrysanthemum parthenium Bernh.), marrubiin (Marrubium vulgare L.) and artemisinin (Artemisia annua L.) J. Chromatogr. 1992;605:124. doi: 10.1016/0021-9673(92)85036-S. [DOI] [Google Scholar]
- 11.Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- 12.Alupului A., Călinescu L., Lavric V. Microwave Extraction of Active Principles from Medicinal Plants. U.P.B. Sci. Bull. Ser. B. 2012;74:129–142. [Google Scholar]
- 13.Sahin S., Samli R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochemistry. 2013;20:595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Box G., Behnken D. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2:455–475. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- 15.Bloszyk E., Geppert B., Drozdz B. Quantitative Determination of Sesquiterpene Lactones in Plant Material by Infrared Spectroscopy. Planta Med. 1978;34:79–86. doi: 10.1055/s-0028-1097418. [DOI] [Google Scholar]
- 16.Fontanel D., Bizot S., Beaufils P. Dosage by HPLC of parthenolide content in the great Chamomille Tanacetum parthenium (L) Schulz-Bip. PIant Med. Phytother. 1990;24:231–237. [Google Scholar]
- 17.Awang D.V.C., Dawson B.A., Kindack D.G., Crompton C.W., Heptinstall S. Parthenolide content of feverfew (Tanacetum parthenium) assessed by HPLC and 1H-NMR spectroscopy. J. Nat. Prod. 1991;54:1516–1521. doi: 10.1021/np50078a005. [DOI] [Google Scholar]
- 18.International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Text and Methodology Q2 (R1), Complementary Guideline on Methodology Incorporated in November 2005 by the ICH Steering Committee. IFPMA; Geneva, Switzerland: 2005. [Google Scholar]
- 19.Chopra S., Motwani S.K., Iqbal Z., Talengaonkar S., Ahmad F.J., Khar R.K. Optimization of polyherbal gels for vaginal drug delivery by Box-Behnken statistical design. Eur. J. Pharm. Biopharm. 2005;67:120–131. doi: 10.1016/j.ejpb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Chang C.W., Yen C.C., Wu M.T., Hsu M.C., Wu Y.T. Microwave-Assisted Extraction of Cannabinoids in Hemp Nut Using Response Surface Methodology: Optimization and Comparative Study. Molecules. 2017;22:1894. doi: 10.3390/molecules22111894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated and used is presented in this article.