Abstract
Bcl-2 family proteins are considered as one of the major regulators of apoptosis. Indeed, this family is known to control the mitochondrial outer membrane permeabilization (MOMP): a central step in the mitochondrial pathway of apoptosis. However, in recent years Bcl-2 family members began to emerge as a new class of intracellular calcium (Ca2+) regulators. At mitochondria-ER contacts (MERCs) these proteins are able to interact with major Ca2+ transporters, thus controlling mitochondrial Ca2+ homeostasis and downstream Ca2+ signalling pathways. Beyond the regulation of cell survival, this Bcl-2-dependent control over the mitochondrial Ca2+ dynamics has far-reaching consequences on the physiology of the cell. Here, we review how the Bcl-2 family of proteins mechanistically regulate mitochondrial Ca2+ homeostasis and how this regulation orchestrates cell death/survival decisions as well as the non-apoptotic process of cell migration.
Keywords: Bcl-2 proteins, mitochondrial calcium homeostasis, VDAC, IP3R, apoptosis, cell migration
1. Introduction
Apoptosis is a form of regulated cell death by which complex multicellular organisms orchestrate the regulated removal of unwanted or damaged cells. It is well established that apoptosis plays critical roles in development, tissue homeostasis and the response to cellular stress [1]. Aberrations in the apoptotic program contribute to the aetiology of a broad range of human pathologies including cancer and neurodegenerative diseases [2,3].
Mitochondria play a central role in apoptosis execution. Actually, these genuine intracellular powerhouses contain in their intermembrane space (IMS) several cytotoxic proteins including Omi, SMAC/Diablo, and cytochrome c [4,5,6,7,8]. Following cellular stress and apoptosis induction, the outer mitochondrial membrane (OMM) is permeabilized, leading to their release into the cytosol and subsequent activation of cysteine-aspartic proteases, called caspases [4].
This mitochondrial outer membrane permeabilization (MOMP) is under the tight control of the Bcl-2 family of proteins [9]. Initially discovered within the chromosomal translocations of follicular lymphomas, the Bcl-2 proteins (an acronym for B-cell lymphoma 2 gene) are considered as one of the main MOMP regulators [10,11,12,13,14]. These intracellular proteins possess one or up to four conserved sequences called Bcl-2 homology (BH) domains or motifs [14,15]. As MOMP regulators, they are divided into three groups: multidomain pro-apoptotic Bax-like, which have pore-forming activity and induce MOMP, multidomain anti-apoptotic Bcl-2-like, which bind to Bax-like thus repressing MOMP, and pro-apoptotic BH3-only proteins. Structurally, Bax-like and Bcl-2-like family members are related as they possess four BH motifs (BH1 to 4). The sequence spanning between BH1 to BH3 organizes into the canonical BH3-binding groove where a BH3 motif can bind. In this regard, BH3-only proteins are considered pro-apoptotic as interaction between their BH3 motifs and the BH3-binding groove results in activation of Bax-like or repression of Bcl-2-like proteins, shifting the balance towards MOMP [14,16].
Bcl2-related genes are found only in multicellular animals and thus they are referred to as markers of multicellularity, evolutionarily selected to regulate apoptotic cell removal in development and sustain tissue homeostasis in metazoans [17,18]. This was first demonstrated in C. elegans in which the Bcl-2 homolog CED-9 was mutated. Loss-of-function of the ced9 gene resulted in widespread death of embryonic cells [19,20]. Subsequent observations in knockout (KO) mice for bcl2 homologs solidified their critical role in apoptosis regulation [21,22,23]. However, more recent experiments have demonstrated that Bcl-2 family members are actually multifunctional proteins involved in non-MOMP related processes [24,25,26,27,28]. Indeed, many Bcl-2-related proteins have a C-terminal hydrophobic transmembrane (TM) motif allowing them to be anchored not only to mitochondria but also to the endoplasmic reticulum (ER) [29,30,31,32,33]. At the level of these internal membranes, Bcl-2 family members dynamically control the exchange of Ca2+ ions [34,35,36,37].
Ca2+ ions are important secondary messengers participating in many cellular functions [38]. Ca2+ is able to enhance mitochondrial bioenergetics by promoting the activities of pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase [39]. In contrast, mitochondrial Ca2+ is also required for the efficient execution of apoptosis, while Ca2+ overload induces the opening of the elusive mitochondrial permeability transition pore (mPTP), leading to necrotic cell death [40,41]. The role of Ca2+ in the balance between life and death underlies the need for tight regulation of mitochondrial Ca2+ pools. Bcl-2 family of proteins participates in this process through direct interactions with various intracellular Ca2+ transporters or channels, which have profound consequences for mitochondrial Ca2+ homeostasis and downstream Ca2+ signalling pathways. Here, we review the role of Bcl-2 proteins in mitochondrial Ca2+ homeostasis and how this regulation orchestrates not only survival/death decisions but also non-apoptotic processes like cell migration.
2. Mitochondria-ER Contacts (MERCs): A Signalling Platform for Mitochondrial Ca2+ Homeostasis
Mitochondria are dynamic intracellular organelles that can store and exchange with the surrounding environment substantial amounts of Ca2+ ions [42]. As mitochondria are encompassed by a double membrane, Ca2+ is required to cross both layers in order to reach the matrix. The inner mitochondrial membrane (IMM) mitochondrial Ca2+ uniporter (MCU) has a low affinity for Ca2+, with a Kd of 10 µM [43]. This therefore means that mitochondria are unable to uptake Ca2+ directly from the cytosol, but rather require the direct transfer of Ca2+ from other stores through membrane contact sites (MCS), in order to be able to maintain their Ca2+ pools [44]. While lysosomes [45] can directly transfer Ca2+ to mitochondria, the most understood pathway is the transfer of Ca2+ from the ER to mitochondria at mitochondria-ER contacts (MERCs) [46]. Mitochondria engage in MCS with the ER forming specialized structures known as MERCs or mitochondria-associated ER membranes (MAMs). Around 20% of the mitochondrial surface is involved in ER contacts, with average inter-organelle distances ranging around 10 to 50 nm [47,48]. MERCs are formed and stabilized by tethering proteins, such as Mitofusin-2 (MFN2) [49] or PDZ domain-containing 8 (PDZD8) [50] in mammals regulating the optimal distance between both organelles. This mitochondria-ER interface is essential for several other processes including the synthesis and exchange of lipids, autophagosome formation and mitochondrial dynamics, thus providing a signalling platform to coordinate cell fate [51,52,53].
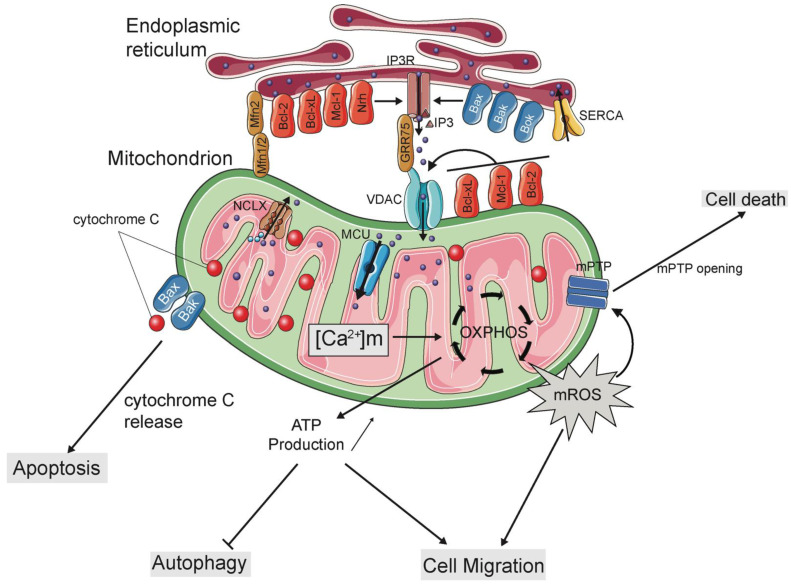
At the MERCs, a specialized subdomain exists to enable the efficient ER to mitochondria Ca2+ transfer. The ER-localized inositol 1,4,5-trisphosphate receptor (IP3R) and OMM-localized voltage-dependent anion channel (VDAC) are bridged by the glucose regulated protein-75 (GRP75), forming a tethering complex between both organelles [54,55]. Upon stimulation, the natural ligand IP3 binds to IP3R leading to opening of the channel and subsequent Ca2+ release into cytosol and mitochondria, through VDAC [54] (Figure 1). Ca2+ transfer at the MERCs generates a high local concentration of Ca2+, called Ca2+ microdomains, enabling the MCU complex to uptake Ca2+ into the mitochondrial matrix [43]. The formation of this functional Ca2+ signalling platform at MERCs organizes all of the appropriate machinery required to efficiently transfer Ca2+ from the ER to mitochondria. The high local concentration of Ca2+ generated at this interface enables the IMM- and MERCs-localized MCU [56] to allow the entry of Ca2+ into the matrix. The spatial organization and coordination of the ER-, OMM- and IMM-localized Ca2+ channels/receptors are therefore crucial in order for mitochondria to efficiently uptake Ca2+.
Figure 1.
Schematic representation of ER to mitochondria Ca2+ regulation by Bcl-2 proteins. Members of the Bcl-2 family including pro- and anti-apoptotic proteins are found at the mitochondria-endoplasmic reticulum contacts (MERCs). At this interface, they control mitochondrial Ca2+ trafficking via the interaction with ER- and mitochondria-localized Ca2+ channels and transporters, which has an important implication in mitochondrial Ca2+-dependent processes. Through mitochondrial Ca2+ pools regulation, Bcl-2 proteins control bioenergetics, ATP production and reactive oxygen species (ROS), thus influencing cell fate decisions including apoptosis, cell survival and cell migration. At the ER, the anti-apoptotic proteins Bcl-2, Bcl-xL, Mcl-1 and Nrh interact with IP3R to decrease ER-Ca2+ release into mitochondria to sustain mitochondrial bioenergetics and to protect from Ca2+-induced cell death. At the mitochondria, Bcl-2, Bcl-xL and Mcl-1 interact with VDACs to promote or inhibit mitochondrial Ca2+ uptake, depending on cell types and the cellular metabolic state. In contrast, the pro-apoptotic members Bax and Bax can also localize to the ER where they promote ER-Ca2+ release and cell death. Recently, ER-localized Bok has been shown to directly regulate MERCs number and to interact with IP3R promoting ER-Ca2+ release and mitochondrial Ca2+ uptake required for cell death.
Interestingly, biochemical subcellular fractionation studies have shown the presence of the anti-apoptotic proteins Bcl-2 and Bcl-xL at MERCs at steady state [57], with the recruitment of Bcl-2 to this specific interface, mediated by TOM20, being enhanced upon apoptotic stimulations [58]. In addition, microscopy analyses revealed that the apoptosis accelerator Bax is recruited to MERCs during tBid-induced apoptosis [59]. Upon mild stress induced by thapsigargin in Chinese hamster ovary (CHO) cells, Bcl-xL can also translocate specifically to MERCs promoting the increase of mitochondrial Ca2+ by regulating IP3R-induced ER-Ca2+ release and cellular bioenergetics [60].
While the subcellular localization of Bcl-2 proteins at this ER-mitochondria interface allows direct interaction with components of the Ca2+ homeostasis machinery, there is little evidence showing that Bcl-2 proteins can directly regulate ER and mitochondria membranes apposition. Recently, it has been shown that the pro-apoptotic member Bok was localized at MERCs where it controls the optimal distance between the two membranes for an efficient ER to mitochondria Ca2+ transfer to control cell death [61]. These results are in accordance with recent evidence indicating that overexpression of both Mcl-1 and Bok TMs leads to an increase of MERCs number and cell death [32]. An interaction between Bcl-2 and Bcl-xL with GRP75 was also identified, and it may be plausible that this interaction could regulate MERCs by controlling the IP3R-GRP75-VDAC tethering complex [62]. Together, these data suggest that Bcl-2 proteins are not only localized to MERCs but could directly regulate them to sustain efficient ER to mitochondria Ca2+ transfer (Figure 1).
In the next sections, we will describe how Bcl-2 proteins regulate Ca2+ transients at the ER and mitochondrial interface to promote the uptake of mitochondrial Ca2+ required for cell death and the complex process of cell migration.
3. Regulation of Mitochondrial Ca2+ Uptake Machinery by Bcl-2 Family Proteins
The OMM is highly permeable to ions and low molecular weight molecules, due to the presence of VDACs, whereas the IMM-localized MCU complex enables Ca2+ uptake into the matrix [63,64,65]. Three VDAC isoforms are found in vertebrates (VDAC1–3), representing the most abundant proteins of the OMM [66]. They can adopt two conformational stages: an open state, observed at low membrane potential (−10 mV to +10 mV), which is permeable for cations and small anionic metabolites, and a closed state at high mitochondrial membrane potential exhibiting only cation permeability [67]. All three VDAC isoforms are able to transfer Ca2+ ions through the OMM, however, functional implications differ. For instance, VDAC1 allows the passage of the low-amplitude apoptotic Ca2+ signals following IP3R stimulation [68], whereas VDAC2 is involved in transfer of Ca2+ from sarcoplasmic reticulum (SR) and in the rhythmicity of cardiomyocytes [69].
Bcl-2 family members are mainly OMM-resident proteins, so they exert a control over mitochondrial Ca2+ uptake mainly through the control of VDACs permeability (Figure 1), however this regulation is still a matter of debate. The first evidence for the implication of a Bcl-2 family member in VDAC permeability came from Craig Thompson’s lab in the late 90s. Vander Heiden and collaborators demonstrated that following growth factor deprivation, cells overexpressing Bcl-xL survive by sustaining ATP/ADP exchanges in the mitochondria, suggesting that Bcl-xL maintains VDAC in an open state [36,70]. Supporting this model, the dephosphorylation of the BH3-only protein Bad, which causes its translocation to the OMM, disrupts the interaction between Bcl-xL and VDAC leading to mitochondrial Ca2+ overload [71]. However, using liposomes embedded with purified VDAC proteins, Shimizu and colleagues demonstrated that Bcl-xL binds to and inhibits VDAC opening [72]. Interestingly, in this latter experimental system pro-apoptotic Bax and Bak have the opposite effect and lead to VDAC opening [72].
More recently, the team of Chi Li demonstrated that bclx KO mouse embryonic fibroblasts (MEFs) uptake less Ca2+ into the mitochondria compared to control cells [73]. Notably, mitochondrial Ca2+ uptake was restored when KO MEFs were complemented with exogenous Bcl-xL targeted to the mitochondria but not to the ER. It has then been proposed that Bcl-xL promotes mitochondrial Ca2+ entry via direct interaction with the VDAC1 and VDAC3 channels [74], and that its N-terminal BH4 motif was required for this interaction and therefore Ca2+ regulation [57]. In this respect, a peptide corresponding to the BH4 motif of Bcl-xL reduces agonist-induced mitochondrial Ca2+ uptake and protects cells from apoptosis [57,75].
Actually, within the Bcl-2 family, VDACs participate in functional interactions with Bcl-2 and Mcl-1 as well. Several studies have shown that Bcl-2 interacts with VDAC1 N-terminal α-helix, thereby leading to a reduction in mitochondrial Ca2+ uptake [76]. This probably also requires the BH4 motif of Bcl-2 because peptides corresponding to this region close VDAC and suppress pro-apoptotic stimuli [77]. In contrast, overexpression of Bcl-2 in neurons and myotubes has opposite effects, leading to an increase of mitochondrial Ca2+ [78,79]. Finally, Mcl-1 has also been shown to directly interact with VDACs to promote mitochondrial Ca2+ uptake and bioenergetics in a non-small cell carcinoma cell line [80].
The discrepancies regarding mitochondrial Ca2+ trafficking highlight the complex interactions between anti-apoptotic Bcl-2 proteins and VDACs. Due to their role in cell survival and death, it can be hypothesized that under physiological conditions, anti-apoptotic Bcl-2 members enhance mitochondrial Ca2+ uptake to regulate mitochondrial metabolism and bioenergetics, whereas upon apoptotic stimulations they protect mitochondria from deleterious massive Ca2+ overload by interacting with VDACs. This hypothesis is notably supported by findings in the heart of transgenic mice describing that Bcl-2 decreases mitochondrial Ca2+ efflux via the Na+/Ca2+ exchanger, NCLX, to maintain mitochondrial ATP production [81].
4. Remote Control of Mitochondrial Ca2+ Signalling by ER-Based Bcl-2 Proteins
The ER is the major storage organelle for cellular Ca2+. ER-dependent Ca2+ release controls basal cytosolic Ca2+ levels and mitochondrial Ca2+ uptake through the direct transfer of Ca2+ ions at MERCs [42,48,82]. At the level of the ER, this occurs via the release of Ca2+ through ER-Ca2+ channels. IP3Rs and ryanodine receptors (RyRs) are the two major families of ER Ca2+ channels [83]. In vertebrates, there are three IP3R isoforms (IP3R1-3), which are often co-expressed in most mammalian cell types. The three isoforms differ in their affinity for the IP3 ligand; IP3R2 exhibiting the highest sensitivity while IP3R3 has the weakest [84]. Interestingly, IP3R2 isoform is the most effective in delivering Ca2+ to the mitochondria [55]. IP3, the natural ligand for IP3R, is produced upon G-protein coupled receptor (GPCR) activation by ligands such as histamine or ATP at the plasma membrane. GPCR activation leads to hydrolysis of phosphatidyl inositol-4,5-bisphosphate (PIP2) by phospholipase C resulting in the production of IP3. IP3 then diffuses through the cell and binds to the IP3-binding domain of IP3R oligomers resulting in the opening of the Ca2+ channel, which subsequently allows Ca2+ flux into the cytosol and mitochondria [85,86].
Actually, many anti-apoptotic Bcl-2 proteins including Bcl-2, Bcl-xL, Mcl-1 and Nrh, possess dual mitochondrial and ER localizations and are able to interact with IP3R [34,35,87] (Figure 1). Although these interactions regulate IP3R-dependent Ca2+ release, the binding sites and the functional consequences of this regulation are different. For instance, Bcl-2 is able to bind to the central modulatory and transducing domain II (MTD II) of IP3R, which requires the N-terminal BH4 motif of Bcl-2 [34,88]. This interaction lowers Ca2+ release from the ER and inhibits the transfer of toxic Ca2+ insults to the mitochondria. Conversely, Bcl-xL interacts with IP3R through its BH3-binding groove [89]. Indeed, Yang and colleagues identified two new BH3-like helices in the IP3R C-terminus that are able to bind to Bcl-xL with high affinity. This interaction leads to IP3R opening and subsequent ER-Ca2+ release. Interestingly, the mode of action of Bcl-xL appears to be concentration-dependent because increasing Bcl-xL levels lead to a secondary IP3R inhibition, which occurs through the binding of Bcl-xL at the Bcl-2 interaction site in the MTDII domain [89]. Thus, the regulation of IP3R by Bcl-xL seems to be biphasic. At low levels, ER-based Bcl-xL favors the release of Ca2+ ions from IP3R and transfer to the mitochondria thus enhancing mitochondrial bioenergetics by activating the Ca2+-dependent dehydrogenases of the Krebs cycle. In contrast, at high protein concentration levels, Bcl-xL inhibits IP3R-dependent Ca2+ release and subsequent apoptosis initiation [89,90]. Of note, as described previously, the BH4 motif of Bcl-xL does not interact with IP3R but preferentially binds to VDAC1, controlling its permeability [57]. The difference between Bcl-2 and Bcl-xL BH4 motifs can be explained by subtle differences in their respective amino acid compositions. Indeed, in the BH4 motif of Bcl-2 a lysine residue at position 17 (Lys17) is critical for its interaction with IP3R. The corresponding residue in Bcl-xL is an aspartate at position 11 (Asp11). Mutating Lys17 into Asp in Bcl-2 leads to complete loss of IP3R binding capacity, whereas changing of Asp11 into Lys in BH4 of Bcl-xL converts Bcl-xL into an IP3R binder and inhibitor [57].
Mcl-1 is another IP3R interactor shown to control mitochondrial Ca2+ uptake. Actually, Mcl-1 and Bcl-xL seem to behave in a similar manner. Both proteins bind with comparable affinities to the C-terminus of all three IP3R isoforms suggesting that Mcl-1, like Bcl-xL, requires its BH3-binding groove to interact with IP3R channels [91]. In addition, the BH4 motif of Mcl-1 has a pronounced tropism for the OMM, where it inhibits mitochondrial Ca2+ signalling [92].
An outsider of this Bcl-2-IP3R interaction group is the Bcl-2 homolog Nrh (also referred to as Bcl-B or BCL2L10). In breast cancer (BC) cells, Nrh is exclusively found at the ER where it is able to interact with the N-terminal IP3 binding domain of the IP3R1 via its BH4 motif [87]. This interaction prevents IP3R1 opening, which in turn dampens the unfolded protein response (UPR). Actually, the UPR is an adaptive reaction that prevents the accumulation of misfolded proteins in the ER lumen to maintain cell viability. If stressful conditions persist, the UPR can prime cells for cell death through the activation of the BH3-only protein Bim [93,94]. The UPR is often suppressed in tumor cells in order to promote protein synthesis and cell survival. In this regard, Nrh expression in BC cells inhibits the UPR and induces drug resistance, whereas Nrh silencing makes BC cells more sensitive to drugs currently used in chemotherapy [87]. Interestingly, at MERCs, Nrh and IRBIT, another IP3 binding domain protein, exert an additive inhibitory effect over IP3R at resting states [95]. However, upon apoptotic stress, IRBIT is dephosphorylated, thus inhibiting Nrh and leading to Ca2+ accumulation in the mitochondria and subsequent apoptosis [95].
Finally, Bcl-2 proteins have also been proposed to interact with other ER-Ca2+ channels [96]. Both Bcl-2 and Bcl-xL can interact with the ryanodine receptor (Ryr) via their BH4 domains and decrease their activity [97,98]. Indeed, overexpression of Bcl-xL inhibits caffeine-induced Ryr-dependent Ca2+ release into the mitochondria [98]. Together, by direct interaction with ER-Ca2+ channels, Bcl-2 proteins tightly control ER to mitochondria Ca2+ transfer required for cell fate decisions.
5. Bridging the Gap between Mitochondria and ER during Cell Death and Survival
Mitochondrial Ca2+ plays a pivotal role in the balance between cell survival and cell death events [99]. While a minimal amount of Ca2+ is required to maintain mitochondrial bioenergetics and metabolism, larger and toxic mitochondrial Ca2+ levels have been proposed to facilitate apoptosis [100] and to trigger mPTP opening [41]. As already described, the anti-apoptotic Bcl-2 proteins are key executioners regarding the control of mitochondrial Ca2+ homeostasis as well as the cell death and survival balance. During apoptosis, the number of mitochondria-ER contact sites increases [59,101], fostering mitochondrial Ca2+ uptake [59,95], which has been associated with IMM remodelling and OPA1-dependent cristae reorganization, thus facilitating cytochrome c release and apoptosis [59]. Mitochondrial Ca2+ overload may also lead to IMM cardiolipin oxidation, increasing ROS production and mPTP opening [102]. Due to their roles in ER to mitochondria Ca2+ fluxes described above, anti-apoptotic Bcl-2 proteins have been widely shown to inhibit apoptosis by decreasing ER-induced Ca2+ release or decreasing VDAC1-dependent Ca2+ uptake [103] (Figure 1). In recent years, different peptides derived from their BH4 domain have been developed and their effects have been characterized in different cancer cell models [104]. Such peptides are able to disrupt the interactions between IP3R and several Bcl-2 proteins and impact on the apoptotic Ca2+ signals transfer to the mitochondria. For instance, a BH4-domain-targeting peptide of Bcl-2, called Bcl-2/IP3 receptor disrupter-2 (BIRD-2), has been shown to have cell death-inducing effects in different cancer cell lines [34,105,106,107,108]. Interestingly, such cell death has been shown to depend on ER-induced mitochondrial Ca2+ overload and caspase activation [109].
While the role of the pro-apoptotic proteins in basal mitochondrial Ca2+ homeostasis has been less described, there is evidence supporting their contribution to the Ca2+-dependent apoptotic process (Figure 1). Bok is the only multidomain pro-apoptotic member which has been shown to interact with the IP3R coupling domain of both IP3R1 and IP3R2 via its BH4 domain [110,111]. This interaction has been initially reported to protect both IP3Rs and unbound Bok from proteolysis and proteasomal-dependent degradation, respectively, and to control mitochondrial morphology [112]; however, no ER or mitochondrial Ca2+ defects were observed in these KO cell lines. Interestingly, a study has recently shown that KO of Bok resulted in a deregulation of intracellular Ca2+ signalling [61]. Indeed, these Bok KO MEFs harbored a reduction of Ca2+ transfer from ER to mitochondria and of apoptosis [61]. This study also showed that Bok-KO induces a decrease of MERCs number observed by microscopy, and a mislocalization and decrease of MERCs-resident proteins [61], suggesting that Bok can directly control MERCs to maintain mitochondrial Ca2+ pools and sustain cell viability. Rescue experiments with a Bok mutant unable to interact with IP3R was shown to rescue the MERCs defect but not the mitochondrial Ca2+ phenotype [61]. Interestingly, restoring MERCs by an artificial tether, was insufficient to recuse the Ca2+ defects induced by Bok loss [61]. These data suggest a specific and mutually exclusive role of Bok in controlling IP3R-mediated Ca2+ release and MERCs number.
Although no direct interaction with ER-localized Ca2+ channels/receptors have been reported, Bax and Bak can also localize to the ER where they control Ca2+-dependent apoptosis [113,114,115]. Indeed, overexpression of Bax and Bak leads to an increase of ER-Ca2+ release and mitochondrial Ca2+ levels accompanied by cytochrome c release and cell death [113], suggesting that Bax/Bak at the ER can control ER to mitochondria Ca2+ fluxes. In addition, Bax/Bak DKO MEFs have reduced ER-Ca2+ content, leading to decreased mitochondrial Ca2+ uptake and apoptosis upon ER-Ca2+ stimulation [114]. Importantly, re-expression of SERCA or ER-targeted Bax/Bak was able to restore ER-Ca2+ content and efficient apoptosis, indicating that Bax and Bak directly control ER Ca2+ concentration [114]. Mechanistically, it has been proposed that this increased ER Ca2+ leak was associated to an increase of Bcl-2-IP3R1 interaction and protein kinase A-dependent IP3R1 phosphorylation in Bax and Bak DKO cells [116]. Other studies have confirmed the contribution of Bax and Bak regarding ER-induced Ca2+ release and cell death regulation following different cellular stresses [115,117]. Alternatively, reports have shown that Bax and Bak are able to permeabilize the ER membrane leading to the release of the ER lumen contents to the cytosol [118,119]. Indeed, the oligomerization of Bax and Bak on the ER membrane could lead to the formation of pores, similar to mitochondrial [120,121] and peroxisomal [122] pore formations, which could potentially allow the passage of Ca2+ in the cytosol during apoptosis. Finally, BH3 only proteins [123,124], including Bik [125], can also control Bax/Bak-dependent ER-Ca2+ release to enhance mitochondrial Ca2+ uptake and cell death. In hyperplastic cells, not only Bik disrupts the Bcl-2-IP3R complex to promote ER-Ca2+ release, but it can also activate and translocate Bak to the ER to form a complex with DAPK1 leading to an increase of MERCs and mitochondrial Ca2+ uptake, subsequently leading to cell death [126].
The complex regulation of Ca2+ by Bcl-2 proteins reflects the critical and opposing functions of Ca2+ about life and death decisions. Therefore, several modes of regulation must exist to tightly control mitochondrial Ca2+ levels, depending on environmental conditions.
6. Role of Bcl-2 Family Proteins in Ca2+-Dependent Cell Migration
Intracellular Ca2+ dynamics regulates many cellular processes including cytoskeleton remodelling and cell migration [37]. Most of these regulations occur by modifying the cytosolic Ca2+ signals, which has been reviewed extensively elsewhere [127,128]. The significance of Bcl-2 family proteins in cell migration and invasion during embryonic development and cancer progression, however, has only recently emerged.
Actually, the first evidence came from experiments conducted in the zebrafish model. In this vertebrate, a highly divergent Bcl-2 homolog, called Bcl-wav (acronym for Bcl-2 homolog found in water-living anamniote vertebrates) was identified [28]. Bcl-wav is a mitochondrial resident pro-apoptotic Bcl-2 homolog, the knockdown of which affects convergence and extension (C&E) movements during zebrafish embryogenesis [28]. C&E movements are critical for the establishment of the anterior-posterior and dorsoventral embryonic axes. Bcl-wav orchestrates these morphogenic movements through the control of intracellular Ca2+ trafficking. Indeed, bclwav knockdown was correlated with a decrease in mitochondrial Ca2+ levels and concomitant increase of cytosolic Ca2+ levels [28]. At the level of the mitochondria, Bcl-wav interacts with VDAC1 via its BH4 motif and enhances mitochondrial Ca2+ uptake thus controlling the kinetics of actin polymerization and blastomeres migration. Interestingly, C&E movements seem to be strongly depended on mitochondrial Ca2+ uptake since knockdown of mcu resulted in a similar phenotype [28].
The importance of the MCU-dependent Ca2+ transport was further emphasized in the motility of cancer cells [129,130]. Indeed, mcu-silencing in highly invasive triple-negative breast cancer (TNBC) cell lines resulted in altered F-actin cytoskeleton dynamics, cell polarization loss and impairment of the focal adhesion proteins dynamics [129]. These processes are mediated by the reduction of Ca2+-dependent Calpain and Rho-GTPases activities [129]. In addition, Tosatto and collaborators showed that the knockdown of mcu also resulted in decreased cell motility and invasiveness as well as reduction of tumor growth [130]. However, they linked this phenotype to mitochondrial ROS (mtROS) production and downregulation of hypoxia-inducible factor-1α (HIF-1α) [130]. This suggests that mitochondrial Ca2+ uptake could probably control multiple downstream signalling pathways. High mtROS production is detrimental for cell survival, however, in cancer cells sub-lethal mtROS levels promote cell proliferation, migration and invasion [131,132]. In this respect, several studies have demonstrated that Bcl-2 family members control cancer cell motility via mtROS production, independently of their role in apoptosis [80,133,134]. For instance, Mcl-1 was proposed to promote migration in non-small cell lung carcinoma though its interaction with VDAC1 and 3 and its capacity to control mitochondrial Ca2+ homeostasis [80]. Indeed, mcl1-silencing or treatment with peptides that suppress VDAC-based Ca2+ uptake led to reduced mtROS generation. Bcl-xL and Bcl-2 were also shown to act as accelerators of cell motility, invasiveness and metastasis spreading. As it is the case for Mcl-1, mitochondrion-localized Bcl-xL, but not ER-based Bcl-xL, contributes to cell migration through the generation of reactive mtROS [133]. At the level of the mitochondria, Bcl-xL binds to VDAC1 via its BH4 motif thus promoting Ca2+ entry and mtROS production. Interestingly, one study linked this regulation with the effect of metalloprotease-processed CD95L (cl-CD95L) on TNBC accelerated metastatic dissemination and poor patient prognosis [134]. Actually, CD95-mediates Ca2+ release from the ER to mitochondria at MERCs. In this particular case, mitochondria-targeted Bcl-xL and ER-targeted Bcl-2 were proposed to increase Ca2+ transfer between the ER and the mitochondria, thus accelerating ATP production and mtROS generation [134]. Interestingly in this case, the use of BH3-mimetics was sufficient to decrease cell migration suggesting that these molecules may be useful not only to kill tumor cells but also to prevent metastatic dissemination [134].
7. Conclusions
The role of Bcl-2 family of proteins in the initiation of apoptosis has been well studied, which has led to our current understanding of how cells integrate stress signals at the level of the mitochondria, leading to initiation of the death program. The role of Ca2+ in mediating cell death decisions has also been emphasized, but recent evidence support additional functions for mitochondrial Ca2+ on top of mitochondrial bioenergetics and cell death. With their capacity to be localized at the mitochondria-ER interface and to interact with keys channels or receptors on both ER and mitochondrial membranes, Bcl-2 proteins have emerged as key regulators of intracellular and mitochondrial Ca2+ homeostasis, and subsequently to several other processes such as cell migration. Due to this connection, numerous studies are currently directly targeting Bcl-2-IP3R or Bcl-2-VDAC interactions to modulate Ca2+ signalling and to control cell fate in different types of cancer cell models. Together, future studies identifying precisely how mitochondrial Ca2+ is regulated by Bcl-2 proteins may identify new strategies for therapeutic intervention.
Acknowledgments
We would like to thank Mark Johnson and Nicola Morris for critically reading the manuscript.
Author Contributions
J.L.M., G.G., J.P. and N.P. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
J.L.M. is an MRC-funded Ph.D. student. G.G. lab is funded by Fondation ARC and INCa. J.P. and N.P. are supported by the Medical Research Council, UK (MC_ UU_00015/7) and by Ligue contre le Cancer, Comité du Rhône, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letai A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017;1:275–294. doi: 10.1146/annurev-cancerbio-050216-121933. [DOI] [Google Scholar]
- 3.Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1:120–130. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Kim C.N., Yang J., Jemmerson R., Wang X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 5.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 6.Verhagen A.M., Ekert P.G., Pakusch M., Silke J., Connolly L.M.E., Reid G., Moritz R.L., Simpson R.J., Vaux D.L. Identification of DIABLO, a Mammalian Protein that Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell. 2000;102:43–53. doi: 10.1016/S0092-8674(00)00009-X. [DOI] [PubMed] [Google Scholar]
- 7.Du C., Fang M., Li Y., Li L., Wang X. Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y., Imai Y., Nakayama H., Takahashi K., Takio K., Takahashi R. A Serine Protease, HtrA2, Is Released from the Mitochondria and Interacts with XIAP, Inducing Cell Death. Mol. Cell. 2001;8:613–621. doi: 10.1016/S1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 9.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujimoto Y., Cossman J., Jaffe E., Croce C.M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto Y., Finger L.R., Yunis J., Nowell P.C., Croce C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto Y., Croce C.M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Natl. Acad. Sci. USA. 1986;83:5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delbridge A.R.D., Grabow S., Strasser A., Vaux D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 14.Youle R.J., Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 15.Banjara S., Suraweera C.D., Hinds M.G., Kvansakul M. The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms. Biomolecules. 2020;10:128. doi: 10.3390/biom10010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kale J., Osterlund E.J., Andrews D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser A., Vaux D.L. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2017;25:13–20. doi: 10.1038/cdd.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green D.R., Fitzgerald P. Just So Stories about the Evolution of Apoptosis. Curr. Biol. 2016;26:R620–R627. doi: 10.1016/j.cub.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengartner M.O., Ellis R., Horvitz R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nat. Cell Biol. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner M.O., Horvitz H.C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 21.Veis D.J., Sorenson C.M., Shutter J.R., Korsmeyer S.J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-M. [DOI] [PubMed] [Google Scholar]
- 22.Knudson C.M., Tung K.S., Tourtellotte W.G., Brown G.A., Korsmeyer S.J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 23.Rinkenberger J.L., Horning S., Klocke B., Roth K., Korsmeyer S.J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genome Res. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Belguise K., Kersual N., Kirsch K.H., Mineva N.D., Galtier F., Chalbos D., Sonenshein G.E. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat. Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Alavian K.N., Lazrove E., Mehta N., Jones A., Zhang P., Licznerski P., Graham M., Uo T., Guo J., et al. A Bcl-Xl-Drp1 Complex Regulates Synaptic Vesicle Membrane Dynamics During Endocytosis. Nat Cell Biol. 2013;15:773–785. doi: 10.1038/ncb2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X., Zhang L.-S., Toombs J., Kuo Y.-C., Piazza J.T., Tuladhar R., Barrett Q., Fan C.-W., Zhang X., Walensky L.D., et al. Extra-mitochondrial prosurvival BCL-2 proteins regulate gene transcription by inhibiting the SUFU tumour suppressor. Nat. Cell Biol. 2017;19:1226–1236. doi: 10.1038/ncb3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popgeorgiev N., Bonneau B., Ferri K.F., Prudent J., Thibaut J., Gillet G. The Apoptotic Regulator Nrz Controls Cytoskeletal Dynamics via the Regulation of Ca2+ Trafficking in the Zebrafish Blastula. Dev. Cell. 2011;20:663–676. doi: 10.1016/j.devcel.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Prudent J., Popgeorgiev N., Bonneau B., Thibaut J., Gadet R., Lopez J., Gonzalo P., Rimokh R., Manon S., Houart C., et al. Bcl-wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nat. Commun. 2013;4:2330. doi: 10.1038/ncomms3330. [DOI] [PubMed] [Google Scholar]
- 29.Krajewski S., Tanaka S., Takayama S., Schibler M.J., Fenton W., Reed J.C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: Residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 30.Yang T., Kozopas K.M., Craig R.W. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J. Cell Biol. 1995;128:1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann T., Schlipf S., Sanz J., Neubert K., Stein R., Borner C. Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J. Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucendo E., Sancho M., Lolicato F., Javanainen M., Kulig W., Leiva D., Duart G., Andreu-Fernández V., Mingarro I., Orzáez M. Mcl-1 and Bok transmembrane domains: Unexpected players in the modulation of apoptosis. Proc. Natl. Acad. Sci. USA. 2020;117:27980–27988. doi: 10.1073/pnas.2008885117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popgeorgiev N., Jabbour L., Gillet G. Subcellular Localization and Dynamics of the Bcl-2 Family of Proteins. Front. Cell Dev. Biol. 2018;6:13. doi: 10.3389/fcell.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong Y.-P., Aromolaran A.S., Bultynck G., Zhong F., Li X., McColl K., Matsuyama S., Herlitze S., Roderick H.L., Bootman M.D., et al. Targeting Bcl-2-IP3 Receptor Interaction to Reverse Bcl-2’s Inhibition of Apoptotic Calcium Signals. Mol. Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White C., Li C., Yang J., Petrenko N.B., Madesh M., Thompson C.B., Foskett J.K. The Endoplasmic Reticulum Gateway to Apoptosis by Bcl-X(L) Modulation of the Insp3r. Nat. Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Heiden M.G., Li X.X., Gottleib E., Hill R.B., Thompson C.B., Colombini M. Bcl-Xl Promotes the Open Configuration of the Voltage-Dependent Anion Channel and Metabolite Passage through the Outer Mitochondrial Membrane. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 37.Bonneau B., Prudent J., Popgeorgiev N., Gillet G. Non-apoptotic roles of Bcl-2 family: The calcium connection. Biochim. Biophys. Acta BBA Bioenerg. 2013;1833:1755–1765. doi: 10.1016/j.bbamcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 39.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta BBA Bioenerg. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Bernardi P., von Stockum S. The Permeability Transition Pore as a Ca(2+) Release Channel: New Answers to an Old Question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer T.M., Murphy E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020;126:280–293. doi: 10.1161/CIRCRESAHA.119.316306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffaello A., Mammucari C., Gherardi G., Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamer K.J., Mootha V.K. The molecular era of the mitochondrial calcium uniporter. Nat. Rev. Mol. Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 44.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 45.Peng W., Wong Y.C., Krainc D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA. 2020;117:19266–19275. doi: 10.1073/pnas.2003236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallese F., Barazzuol L., Maso L., Brini M., Calì T. ER-Mitochondria Calcium Transfer, Organelle Contacts and Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2019;1131:719–746. doi: 10.1007/978-3-030-12457-1_29. [DOI] [PubMed] [Google Scholar]
- 47.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 48.Csordás G., Várnai P., Golenár T., Roy S., Purkins G., Schneider T.G., Balla T., Hajnóczky G. Imaging Interorganelle Contacts and Local Calcium Dynamics at the ER-Mitochondrial Interface. Mol. Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Brito O.M., Scorrano L. Mitofusin 2 Tethers Endoplasmic Reticulum to Mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 50.Hirabayashi Y., Kwon S.-K., Paek H., Pernice W.M., Paul M.A., Lee J., Erfani P., Raczkowski A., Petrey D.S., Pon L.A., et al. ER-mitochondria tethering by PDZD8 regulates Ca2+dynamics in mammalian neurons. Science. 2017;358:623–630. doi: 10.1126/science.aan6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowland A.A., Voeltz G.K. Endoplasmic reticulum–mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–615. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prudent J., McBride H.M. The mitochondria–endoplasmic reticulum contact sites: A signalling platform for cell death. Curr. Opin. Cell Biol. 2017;47:52–63. doi: 10.1016/j.ceb.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/ebc20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartok A., Weaver D., Golenár T., Nichtova Z., Katona M., Bánsághi S., Alzayady K.J., Thomas V.K., Ando H., Mikoshiba K., et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-11646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De La Fuente S., Fernandez-Sanz C., Vail C., Agra E.J., Holmstrom K., Sun J., Mishra J., Williams D., Finkel T., Murphy E., et al. Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle. J. Biol. Chem. 2016;291:23343–23362. doi: 10.1074/jbc.M116.755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monaco G., Decrock E., Arbel N., van Vliet A.R., La Rovere R.M., De Smedt H., Parys J.B., Agostinis P., Leybaert L., Shoshan-Barmatz V., et al. The BH4 Domain of Anti-apoptotic Bcl-XL, but Not That of the Related Bcl-2, Limits the Voltage-dependent Anion Channel 1 (VDAC1)-mediated Transfer of Pro-apoptotic Ca2+ Signals to Mitochondria. J. Biol. Chem. 2015;290:9150–9161. doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lalier L., Mignard V., Joalland M.-P., Lanoé D., Cartron P.-F., Manon S., Vallette F.M. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021;12:1–11. doi: 10.1038/s41419-021-03471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prudent J., Zunino R., Sugiura A., Mattie S., Shore G.C., McBride H.M. MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Mol. Cell. 2015;59:941–955. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Williams A., Hayashi T., Wolozny D., Yin B., Su T.C., Betenbaugh M.J., Su T.P. The Non-Apoptotic Action of Bcl-Xl: Regulating Ca(2+) Signaling and Bioenergetics at the Er-Mitochondrion Interface. J. Bioenerg. Biomembr. 2016;48:211–225. doi: 10.1007/s10863-016-9664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carpio M.A., Means R.E., Brill A.L., Sainz A., Ehrlich B.E., Katz S.G. BOK controls apoptosis by Ca2+ transfer through ER-mitochondrial contact sites. Cell Rep. 2021;34:108827. doi: 10.1016/j.celrep.2021.108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena N., Katiyar S.P., Liu Y., Grover A., Gao R., Sundar D., Kaul S.C., Wadhwa R. Molecular Interactions of Bcl-2 and Bcl-Xl with Mortalin: Identification and Functional Characterization. Biosci. Rep. 2013;33:e00073. doi: 10.1042/BSR20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker T., Wagner R. Mitochondrial Outer Membrane Channels: Emerging Diversity in Transport Processes. BioEssays. 2018;40:e1800013. doi: 10.1002/bies.201800013. [DOI] [PubMed] [Google Scholar]
- 64.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nat. Cell Biol. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L.A., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nat. Cell Biol. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raghavan A., Sheiko T., Graham B.H., Craigen W.J. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim. Biophys. Acta BBA Biomembr. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan W., Colombini M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta BBA Biomembr. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P., Rizzuto R. Vdac1 Selectively Transfers Apoptotic Ca2+ Signals to Mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimizu H., Schredelseker J., Huang J., Lu K., Naghdi S., Lu F., Franklin S., Fiji H.D., Wang K., Zhu H., et al. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. eLife. 2015;4:e04801. doi: 10.7554/eLife.04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vander Heiden M.G., Chandel N.S., Schumacker P.T., Thompson C.B. Bcl-Xl Prevents Cell Death Following Growth Factor Withdrawal by Facilitating Mitochondrial Atp/Adp Exchange. Mol. Cell. 1999;3:159–167. doi: 10.1016/S1097-2765(00)80307-X. [DOI] [PubMed] [Google Scholar]
- 71.Roy S.S., Madesh M., Davies E., Antonsson B., Danial N., Hajnóczky G. Bad Targets the Permeability Transition Pore Independent of Bax or Bak to Switch between Ca2+-Dependent Cell Survival and Death. Mol. Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu S., Narita M., Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nat. Cell Biol. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 73.Eno C.O., Eckenrode E.F., Olberding K.E., Zhao G., White C., Li C. Distinct Roles of Mitochondria- and Er-Localized Bcl-Xl in Apoptosis Resistance and Ca2+ Homeostasis. Mol. Biol. Cell. 2012;23:2605–2618. doi: 10.1091/mbc.e12-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang H., Hu X., Eno C.O., Zhao G., Li C., White C. An Interaction between Bcl-Xl and the Voltage-Dependent Anion Channel (Vdac) Promotes Mitochondrial Ca2+ Uptake. J. Biol. Chem. 2013;288:19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arbel N., Ben-Hail D., Shoshan-Barmatz V. Mediation of the Antiapoptotic Activity of Bcl-Xl Protein Upon Interaction with Vdac1 Protein. J. Biol. Chem. 2012;287:23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abu-Hamad S., Arbel N., Calo D., Arzoine L., Israelson A., Keinan N., Ben-Romano R., Friedman O., Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu S., Konishi A., Kodama T., Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl. Acad. Sci. USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiao J., Huang X., Feit-Leithman R.A., Neve R.L., Snider W., Dartt D.A., Chen D.F. Bcl-2 enhances Ca2+ signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basset O., Boittin F.-X., Cognard C., Constantin B., Ruegg U.T. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem. J. 2006;395:267–276. doi: 10.1042/BJ20051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang H., Shah K.H.A., Bradbury N., Li C., White C.M. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu L., Yu Y., Chua B.H., Ho Y.-S., Kuo T.H. Regulation of Sodium–Calcium Exchange and Mitochondrial Energetics by Bcl-2 in the Heart of Transgenic Mice. J. Mol. Cell. Cardiol. 2001;33:2135–2144. doi: 10.1006/jmcc.2001.1476. [DOI] [PubMed] [Google Scholar]
- 82.Csordás G., Weaver D., Hajnóczky G. Endoplasmic Reticulum–Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seo M.-D., Enomoto M., Ishiyama N., Stathopulos P.B., Ikura M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim. Biophys. Acta BBA Bioenerg. 2015;1853:1980–1991. doi: 10.1016/j.bbamcr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Newton C.L.A., Mignery G., Südhof T.C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem. 1994;269:28613–28619. doi: 10.1016/S0021-9258(19)61949-6. [DOI] [PubMed] [Google Scholar]
- 85.Taylor C.W., Tovey S.C. IP3 Receptors: Toward Understanding Their Activation. Cold Spring Harb. Perspect. Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ivanova H., Vervliet T., Missiaen L., Parys J.B., De Smedt H., Bultynck G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta BBA Bioenerg. 2014;1843:2164–2183. doi: 10.1016/j.bbamcr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Nougarède A., Popgeorgiev N., Kassem L., Omarjee S., Borel S., Mikaelian I., Lopez J., Gadet R., Marcillat O., Treilleux I., et al. Breast Cancer Targeting through Inhibition of the Endoplasmic Reticulum-Based Apoptosis Regulator Nrh/BCL2L10. Cancer Res. 2018;78:1404–1417. doi: 10.1158/0008-5472.CAN-17-0846. [DOI] [PubMed] [Google Scholar]
- 88.Rong Y.-P., Barr P., Yee V.C., Distelhorst C.W. Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochim. Biophys. Acta BBA Bioenerg. 2009;1793:971–978. doi: 10.1016/j.bbamcr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J., Vais H., Gu W., Foskett J.K. Biphasic Regulation of Insp3 Receptor Gating by Dual Ca2+ Release Channel Bh3-Like Domains Mediates Bcl-Xl Control of Cell Viability. Proc. Natl. Acad. Sci. USA. 2016;113:E1953–E1962. doi: 10.1073/pnas.1517935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li C., Wang X., Vais H., Thompson C.B., Foskett J.K., White C. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. USA. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eckenrode E.F., Yang J., Velmurugan G.V., Foskett J.K., White C. Apoptosis Protection by Mcl-1 and Bcl-2 Modulation of Inositol 1,4,5-Trisphosphate Receptor-dependent Ca2+ Signaling. J. Biol. Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minagawa N., Kruglov E.A., Dranoff J.A., Robert M.E., Gores G.J., Nathanson M.H. The Anti-apoptotic Protein Mcl-1 Inhibits Mitochondrial Ca2+ Signals. J. Biol. Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 93.Puthalakath H., O’Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N., et al. ER Stress Triggers Apoptosis by Activating BH3-Only Protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 94.Pihán P., Carreras-Sureda A., Hetz C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonneau B., Ando H., Kawaai K., Hirose M., Takahashi-Iwanaga H., Mikoshiba K. IRBIT controls apoptosis by interacting with the Bcl-2 homolog, Bcl2l10, and by promoting ER-mitochondria contact. eLife. 2016;5:e19896. doi: 10.7554/eLife.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vervliet T., Parys J.B., Bultynck G. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene. 2016;35:5079–5092. doi: 10.1038/onc.2016.31. [DOI] [PubMed] [Google Scholar]
- 97.Vervliet T., Decrock E., Molgó J., Sorrentino V., Missiaen L., Leybaert L., De Smedt H., Kasri N.N., Parys J.B., Bultynck G. Bcl-2 binds to and inhibits ryanodine receptors. J. Cell Sci. 2014;127:2782–2792. doi: 10.1242/jcs.150011. [DOI] [PubMed] [Google Scholar]
- 98.Vervliet T., Lemmens I., Vandermarliere E., Decrock E., Ivanova H., Monaco G., Sorrentino V., Kasri N.N., Missiaen L., Martens L., et al. Ryanodine receptors are targeted by anti-apoptotic Bcl-XL involving its BH4 domain and Lys87 from its BH3 domain. Sci. Rep. 2015;5:9641. doi: 10.1038/srep09641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhivotovsky B., Orrenius S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Danese A., Patergnani S., Bonora M., Wieckowski M.R., Previati M., Giorgi C., Pinton P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs) Biochim. Biophys. Acta BBA Bioenerg. 2017;1858:615–627. doi: 10.1016/j.bbabio.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 101.CsordásG G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hwang M.-S., Schwall C.T., Pazarentzos E., Datler C., Alder N.N., Grimm S. Mitochondrial Ca2+ influx targets cardiolipin to disintegrate respiratory chain complex II for cell death induction. Cell Death Differ. 2014;21:1733–1745. doi: 10.1038/cdd.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vervliet T., Clerix E., Seitaj B., Ivanova H., Monaco G., Bultynck G. Modulation of Ca2+ Signaling by Anti-apoptotic B-Cell Lymphoma 2 Proteins at the Endoplasmic Reticulum–Mitochondrial Interface. Front. Oncol. 2017;7:75. doi: 10.3389/fonc.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Ridder I., Kerkhofs M., Veettil S.P., Dehaen W., Bultynck G. Cancer cell death strategies by targeting Bcl-2’s BH4 domain. Biochim. Biophys. Acta BBA Bioenerg. 2021;1868:118983. doi: 10.1016/j.bbamcr.2021.118983. [DOI] [PubMed] [Google Scholar]
- 105.Zhong F., Harr M.W., Bultynck G., Monaco G., Parys J.B., De Smedt H., Rong Y.-P., Molitoris J.K., Lam M., Ryder C., et al. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2–IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akl H., Monaco G., La Rovere R., Welkenhuyzen K., Kiviluoto S., Vervliet T., Molgo J., Distelhorst C.W., Missiaen L., Mikoshiba K., et al. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greenberg E.F., McColl K.S., Zhong F., Wildey G., Dowlati A., Distelhorst C.W. Synergistic killing of human small cell lung cancer cells by the Bcl-2-inositol 1,4,5-trisphosphate receptor disruptor BIRD-2 and the BH3-mimetic ABT-263. Cell Death Dis. 2015;6:e2034. doi: 10.1038/cddis.2015.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lavik A.R., Zhong F., Chang M.-J., Greenberg E., Choudhary Y., Smith M.R., McColl K.S., Pink J., Reu F.J., Matsuyama S., et al. A synthetic peptide targeting the BH4 domain of Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma cells alone or in combination with agents targeting the BH3-binding pocket of Bcl-2. Oncotarget. 2015;6:27388–27402. doi: 10.18632/oncotarget.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kerkhofs M., La Rovere R., Welkenhuysen K., Janssens A., Vandenberghe P., Madesh M., Parys J.B., Bultynck G. BIRD-2, a BH4-domain-targeting peptide of Bcl-2, provokes Bax/Bak-independent cell death in B-cell cancers through mitochondrial Ca2+-dependent mPTP opening. Cell Calcium. 2021;94:102333. doi: 10.1016/j.ceca.2020.102333. [DOI] [PubMed] [Google Scholar]
- 110.Schulman J.J., Wright F.A., Kaufmann T., Wojcikiewicz R.J.H. The Bcl-2 Protein Family Member Bok Binds to the Coupling Domain of Inositol 1,4,5-Trisphosphate Receptors and Protects Them from Proteolytic Cleavage. J. Biol. Chem. 2013;288:25340–25349. doi: 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schulman J.J., Wright F.A., Han X., Zluhan E.J., Szczesniak L.M., Wojcikiewicz R.J.H. The Stability and Expression Level of Bok Are Governed by Binding to Inositol 1,4,5-Trisphosphate Receptors. J. Biol. Chem. 2016;291:11820–11828. doi: 10.1074/jbc.M115.711242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schulman J.J., Szczesniak L.M., Bunker E.N., Nelson H.A., Roe M.W., Wagner L.E., 2nd, Yule D.I., Wojcikiewicz R.J.H. Bok Regulates Mitochondrial Fusion and Morphology. Cell Death Differ. 2019;26:2682–2694. doi: 10.1038/s41418-019-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nutt L.K., Pataer A., Pahler J., Fang B., Roth J., McConkey D.J., Swisher S.G. Bax and Bak Promote Apoptosis by Modulating Endoplasmic Reticular and Mitochondrial Ca2+ Stores. J. Biol. Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 114.Scorrano L., Oakes S.A., Opferman J.T., Cheng E.H., Sorcinelli M.D., Pozzan T., Korsmeyer S.J. BAX and BAK Regulation of Endoplasmic Reticulum Ca2+: A Control Point for Apoptosis. Sci. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 115.Zong W.-X., Li C., Hatzivassiliou G., Lindsten T., Yu Q.-C., Yuan J., Thompson C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oakes S.A., Scorrano L., Opferman J.T., Bassik M.C., Nishino M., Pozzan T., Korsmeyer S.J. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.D’Orsi B., Kilbride S.M., Chen G., Alvarez S.P., Bonner H.P., Pfeiffer S., Plesnila N., Engel T., Henshall D.C., Düssmann H., et al. Bax Regulates Neuronal Ca2+ Homeostasis. J. Neurosci. 2015;35:1706–1722. doi: 10.1523/JNEUROSCI.2453-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X.E., Olberding K., White C., Li C. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 2010;18:38–47. doi: 10.1038/cdd.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanekura K., Ma X., Murphy J.T., Zhu L.J., Diwan A., Urano F. IRE1 prevents endoplasmic reticulum membrane permeabilization and cell death under pathological conditions. Sci. Signal. 2015;8:ra62. doi: 10.1126/scisignal.aaa0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salvador-Gallego R., Mund M., Cosentino K., Schneider J., Unsay J., Schraermeyer U., Engelhardt J., Ries J., García-Sáez A.J. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016;35:389–401. doi: 10.15252/embj.201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Große L.A., Wurm C., Brüser C., Neumann D., Jans D.C., Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hosoi K.-I., Miyata N., Mukai S., Furuki S., Okumoto K., Cheng E.H., Fujiki Y. The VDAC2–BAK axis regulates peroxisomal membrane permeability. J. Cell Biol. 2017;216:709–722. doi: 10.1083/jcb.201605002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Csordás G., Madesh M., Antonsson B., Hajnóczky G. tcBid promotes Ca2+ signal propagation to the mitochondria: Control of Ca2+ permeation through the outer mitochondrial membrane. EMBO J. 2002;21:2198–2206. doi: 10.1093/emboj/21.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klee M., Pallauf K., Alcalá S., Fleischer A., Pimentel-Muiños F.X. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mathai J.P., Germain M., Shore G.C. BH3-only BIK Regulates BAX,BAK-dependent Release of Ca2+ from Endoplasmic Reticulum Stores and Mitochondrial Apoptosis during Stress-induced Cell Death. J. Biol. Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- 126.Mebratu Y.A., Leyva-Baca I., Wathelet M.G., Lacey N., Chand H.S., Choi A.M.K., Tesfaigzi Y. Bik reduces hyperplastic cells by increasing Bak and activating DAPk1 to juxtapose ER and mitochondria. Nat. Commun. 2017;8:803. doi: 10.1038/s41467-017-00975-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prevarskaya N., Skryma R., Shuba Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 128.Clapham D.E. Calcium Signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 129.Prudent J., Popgeorgiev N., Gadet R., Deygas M., Rimokh R., Gillet G. Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016;6:36570. doi: 10.1038/srep36570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T.S., Berecz T., Duchen M.R., Rosato A., Bogeski I., Szabadkai G., et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF -1α. EMBO Mol. Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishikawa K., Koshikawa N., Takenaga K., Nakada K., Hayashi J.-I. Reversible regulation of metastasis by ROS-generating mtDNA mutations. Mitochondrion. 2008;8:339–344. doi: 10.1016/j.mito.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 132.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 133.Bessou M.J., Lopez R., Gadet M., Deygas N., Popgeorgiev D., Poncet A., Nougarede P., Billard I., Mikaelian P., Gonzalo R., et al. The Apoptosis Inhibitor Bcl-Xl Controls Breast Cancer Cell Migration through Mitochondria-Dependent Reactive Oxygen Species Production. Oncogene. 2020;39:3056–3074. doi: 10.1038/s41388-020-1212-9. [DOI] [PubMed] [Google Scholar]
- 134.Fouqué A., Lepvrier E., Debure L., Gouriou Y., Malleter M., Delcroix V., Ovize M., Ducret T., Li C., Hammadi M., et al. The apoptotic members CD95, BclxL, and Bcl-2 cooperate to promote cell migration by inducing Ca(2+) flux from the endoplasmic reticulum to mitochondria. Cell Death Differ. 2016;23:1702–1716. doi: 10.1038/cdd.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]