Abstract
Background
In retrospective series, mechanical and oral antibiotic bowel preparation (MOABP) has been reported to reduce surgical-site infections (SSIs) after colectomy compared with no bowel preparation (NBP).
Method
This was a subgroup analysis of a multicentre randomized trial that included patients scheduled for elective colectomy. The MOABP group underwent mechanical bowel preparation, and took 2 g neomycin and 2 g metronidazole orally during the day before surgery. The NBP group did not undergo bowel preparation. Patients were categorized according to the side of resection (right versus left colectomy), and these subgroups compared for postoperative outcomes.
Results
Among 217 patients undergoing right colectomy (106 in MOABP and 111 in NBP group), SSI was detected in seven (7 per cent) and 10 (9 per cent) patients (odds ratio (OR) 0.71, 95 per cent c.i. 0.26 to 1.95; P = 0.510), anastomotic dehiscence in two (2 per cent) and two (2 per cent) patients (OR 1.05, 0.15 to 7.58; P = 1.000), and the mean(s.d.) Comprehensive Complication Index (CCI) score was 9.4(12.9) and 10.5(18.0) (mean difference –1.09; 95 per cent c.i. –5.29 to 3.11; P = 0.608) in the MOABP and NBP groups respectively. Among 164 patients undergoing left colectomy (84 in MOABP and 80 in NBP group), SSI was detected in five (6 per cent) and eight (10 per cent) patients (OR 0.57, 0.18 to 1.82; P = 0.338), anastomotic dehiscence in four (5 per cent) and five (6 per cent) patients (OR 0.75, 0.19 to 2.90; P = 0.742), and the CCI score was 10.2(13.1) and 6.5(11.0) (mean difference 3.68, –0.06 to 7.42; P = 0.053) in the MOABP and NBP groups respectively.
Conclusions
MOABP did not decrease the rate of SSI or complications in patients undergoing either right or left colectomy compared with NBP.
Mechanical preparation in colorectal surgery
Introduction
Surgical-site infection (SSI), including anastomotic dehiscence, is still a major problem after colorectal surgery. Mechanical and oral antibiotic bowel preparation (MOABP) has emerged for debate as several recent large retrospective studies1–6 using data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and a prospective cohort study from the European Society of Coloproctology (ESCP) have suggested a beneficial effect on SSIs after colorectal surgery. Based on these non-randomized data, the American Society of Colon and Rectal Surgeons, American Society of Enhanced Recovery, and Society of American Gastrointestinal and Endoscopic Surgeons have updated their guidelines to recommend MOABP7–9. These studies and changes in the recommendation have sparked a lively debate about whether MOABP should be performed in every patient undergoing colorectal surgery10–12. Advocates of no bowel preparation (NBP) have pointed out that, although there are RCTs comparing MOABP with mechanical bowel preparation alone showing the benefit of MOABP in colorectal surgery13–16, until very recently there have been no data from an RCT comparing MOABP with NBP directly. The MOBILE (Mechanical and Oral Antibiotic Bowel Preparation Versus no Bowel preparatIon for eLEctive Colectomy) trial (NCT02652637)17 was the first RCT to directly compare MOABP with NBP in patients undergoing colonic surgery. The main finding of the trial was that MOABP did not reduce the rate or severity of SSIs, or overall postoperative complications. The trial was rightly criticized for including low-risk right colectomies, and not reporting right- and left-sided colectomies separately18–20. Indeed, right- and left-sided procedures may have different complication and SSI profiles, and may be affected differently by MOABP versus NBP21. The variation in complication profile between right- and left colectomies might stem from differences in anastomoses (ileocolonic versus colocolonic) and microbiome.
To address this shortcoming of the MOBILE trial17, in the present post hoc subgroup study, the outcomes of patients randomized to either MOABP or NBP were analysed separately according to the location of the colectomy (right or left side).
Methods
The MOBILE trial was a national, multicentre, single-blinded, parallel-group, randomized superiority trial comparing MOABP with NBP in patients undergoing elective colonic surgery17. Briefly, the trial was carried out in four Finnish hospitals: two university hospitals (Helsinki University Hospital and Oulu University Hospital) and two community hospitals (Central Finland Central Hospital and Seinäjoki Central Hospital). Patients who were scheduled for colonic resection in participating centres were assessed for eligibility. Exclusion criteria were: emergency surgery; bowel obstruction; colonoscopy planned to be undertaken during surgery; other indication for, or contraindication to, mechanical preparation; allergy to drugs used in the trial (polyethylene glycol, neomycin, metronidazole); and age under 18 years or over 95 years. Patients were allocated randomly to either MOABP or NBP. The patients could not be masked to mechanical bowel preparation, but the recruiters, treating physicians, operating surgeons, data collectors, and analysts were blinded to the allocation group. Patients allocated to MOABP were instructed to undertake bowel preparation with 2 litres of polyethylene glycol (Moviprep®; Norgine, Amsterdam, the Netherlands) and 1 litre of clear fluids the day before surgery, and after bowel preparation to take 2 g neomycin orally at 19.00 hours and 2 g metronidazole orally at 23.00 hours the evening before surgery. Patients in NBP group were instructed not to prepare the bowel. Prophylactic intravenous antibiotics (1500 mg cefuroxime and 500 mg metronidazole) were administered at the induction of anaesthesia and readministered if the operation lasted more than 3 h from the first antibiotic dose or if blood loss exceeded 1.5 litres.
In this subgroup study, all outcomes were analysed separately for right and left colectomies. Right colectomies were those with ileocolic anastomoses; all colocolic anastomoses were included in the left colectomy group. The primary and secondary outcome measures were the same as those in the original trial. The primary outcome was the rate of SSI within 30 days after surgery as well as subcategories of SSI (superficial incisional, deep incisional, or organ/space), as defined by Centers for Disease Control and Prevention22. Secondary outcomes included overall morbidity measured using the Comprehensive Complication Index (CCI) score23, anastomotic dehiscence rate, reoperations, readmission, mortality, and adverse effects of antibiotics, all within 30 days after surgery, as well as duration of hospital stay and the rate of adjuvant therapy (number of patients receiving adjuvant therapy divided by number needing adjuvant therapy). The trial was approved by the Finnish National Committee on Medical Research Ethics and Finnish Medicines Agency, and further approved by the local ethics committee of Helsinki University Hospital and by each participating centre’s institutional review board (Helsinki University Hospital, Oulu University Hospital, Central Finland Central Hospital, and Seinäjoki Central Hospital).
Statistical analysis
Categorical variables were compared using the χ2 test, or Fisher’s exact test if expected numbers in one cell were fewer than five. The effect size for categorical variables was estimated using odds ratios (ORs) with 95 per cent confidence intervals. Where zeros caused problems with computation of the OR, 0.5 was added to all cells. Continuous variables were reported as mean(s.d.) and compared using Student’s t test. Continuous variables with non-normal distribution are reported as medians with IQRs and compared using Mann–Whitney-U-test. The effect size for such variables was estimated as mean differences with 95 per cent confidence intervals. Ordinal variables with a non-normal distribution were compared using the Mann–Whitney U test. Statistical significance was set at a two-sided alpha level of 0.05. Patients with missing values were excluded from analyses of that particular variable, and missing values were not imputed. Outcomes were analysed using the modified intention-to-treat principle, with inclusion of all patients who were randomly allocated to and underwent elective colonic resection with an anastomosis. Patients who did not have surgery or who underwent emergency surgery while waiting for scheduled elective operation, those in whom an anastomosis was not created, and patients who underwent only explorative laparoscopy/laparotomy were excluded from the analyses. Statistical analyses were performed using SPSS® version 25 (IBM, Armonk, New York, USA).
Results
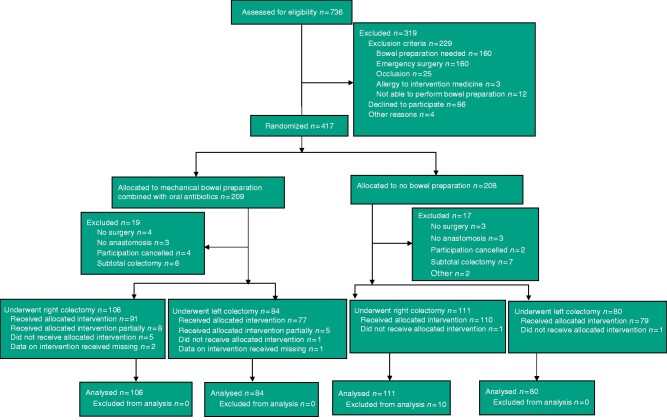
There were 381 patients in the intention-to-treat analysis: 217 patients (106 in MOABP group and 111 NBP group) who underwent right colectomy, and 164 (84 in MOABP group and 80 in NBP group) who underwent left colectomy (Fig. 1).
Fig. 1.
Trial profile
*One patient had emergency surgery and three cancelled participation. †One underwent operation in other hospital and one cancelled participation. ‡One caecal resection and one reversal of Hartmann’s procedure. §Four took bowel preparation with polyethylene glycol (PEG) only partially, one took antibiotics before bowel preparation, two took antibiotics only partially, and one did not take antibiotics. ¶Four took bowel preparation with PEG only partially and one took antibiotics only partially. #Bowel preparation with PEG.
Among patients undergoing right colectomy, baseline characteristics were similar between MOABP and NBP groups except that metastatic malignancy was more frequent in the NBP group (Table 1). Among patients undergoing left colectomy, patients in the MOABP group were slightly older and had more coronary and peripheral vascular disease, but baseline characteristics were otherwise similar in the two groups. The duration of operation, timing of preoperative intravenous antibiotics, and blood loss were similar in both groups among patients undergoing right or left colectomies (Table 2).
Table 1.
Baseline characteristics
| Right colectomy |
Left colectomy |
|||
|---|---|---|---|---|
|
Mechanical and oral antibiotic bowel preparation
(n = 106) |
No bowel preparation
(n = 111) |
Mechanical and oral antibiotic bowel preparation
(n = 84) |
No bowel preparation
(n = 80) |
|
| Age (years)* | 70.9 (63.0–77.4) | 74.0 (66.0–78.2) | 67.6 (59.2–75.0) | 64.2 (57.1–71.1)# |
| Sex ratio (F : M) | 50 : 56 | 61 : 50 | 39 : 45 | 39 : 41 |
| BMI (kg/m2)† | 26.7(4.4) | 26.6(4.7) | 27.4(4.2)* | 27.8(5.2) |
| Mean albumin g/l† | 36.1(3.8)† | 34.9(5.2)‡ | 37.0(6.6)§ | 37.0 (3.3)† |
| Smoking | 5 (4.8)† | 5 (4.7)¶ | 13 (16.3)§ | 10 (13.2)§ |
| ASA physical status grade | ||||
| I | 7 (7) | 7 (6) | 12 (14) | 15 (19) |
| II | 40 (40) | 41 (37) | 33 (39) | 40 (50) |
| III | 53 (50) | 53 (48) | 34 (40) | 24 (30) |
| IV | 7 (6) | 10 (9) | 5 (6) | 1(1) |
| Co-morbidities | ||||
| Myocardial infarction | 7 (7) | 5 (5) | 5 (6) | 2 (3) |
| Congestive heart failure | 5 (5) | 9 (8) | 9 (11) | 2 (3)# |
| Coronary disease (not infarction) | 13 (12) | 16 (14) | 14 (17) | 3 (4)# |
| Hypertension | 49 (46) | 50 (45) | 37 (44) | 31 (39) |
| Atrial fibrillation | 14 (13) | 20 (18) | 11 (13) | 7 (9) |
| Peripheral vascular disease | 5 (5) | 6 (5) | 9 (11) | 2 (3)# |
| Cerebrovascular disease | 9 (8) | 6 (5) | 5 (6) | 4 (5) |
| Hemiplegia | 1 (1) | 1 (1) | ||
| Dementia | 1 (1) | 3 (3) | 0 (0) | 0 (0) |
| COPD or asthma | 22 (21) | 18 (16) | 11 (13) | 8 (10) |
| Connective tissue disease | 2 (2) | 3 (3) | 5 (6) | 2 (3) |
| Liver disease | ||||
| Mild | 2 (2) | 3 (3) | 0 (0) | 0 (0) |
| Moderate/severe | 1 (1) | 1 (1) | 1 (1) | 0 (0) |
| Diabetes mellitus | ||||
| Without complications | 19 (18) | 29 (26) | 14 (17) | 13 (16) |
| With complications | 5 (5) | 1 (1) | 0 (0) | 0 (0) |
| Kidney disease (moderate/severe) | 6 (6) | 4 (4) | 1 (1) | 4 (5) |
| Cancer | 88 (83) | 85 (77) | 51 (61) | 43 (54) |
| Metastatic malignancy | 3 (3) | 13 (12)# | 5 (6) | 7 (9) |
| No comorbidities | 6 (6) | 5 (5) | 15 (18) | 17 (21) |
| Charlson Co-morbidity Index score† | 2.8 (1.7) | 3.1 (2.1) | 2.3 (2.0) | 2.1 (2.0) |
| 0–2 (mild) | 60 (57) | 59 (53) | 51 (61) | 52 (65) |
| 3–4 (moderate) | 30 (24) | 30 (27) | 22 (26) | 20 (25) |
| ≥5 (severe) | 16 (15) | 22 (20) | 11 (13) | 8 (10) |
| Medication | ||||
| Aspirin | 15 (14) | 20 (18) | 14 (17) | 11 (14) |
| Clopidogrel | 3 (3) | 4 (4) | 6 (7) | 0 (0) |
| Warfarin | 11 (10) | 13 (12) | 4 (5) | 6 (8) |
| LMWH | 8 (8) | 2 (2) | 1(1) | 0 (0) |
| DOAC | 2 (2) | 3 (3) | 5 (6) | 1 (1) |
| ≥2 medications affecting thrombosis (anticoagulant or antithrombotic) | 2 (2) | 2 (2) | 0 (0) | 2 (3) |
| Corticosteroid or immunosuppressive medication | 3 (3) | 4 (4) | 5 (6) | 2 (3) |
| No high-risk medication | 62 (58) | 63 (57) | 49 (58) | 58 (73) |
| Previous abdominal/inguinal operation | 57 (54) | 54 (49) | 37 (44) | 45 (56) |
Values in parentheses are percentages unless indicated otherwise; values are *median (i.q.r.) and †mean(s.d.). COPD, chronic obstructive pulmonary disease; LMWH, low molecular weight heparin; DOAC, direct oral anticoagulant. Data missing for
one,
three,
six,
four, and
five patients.
P < 0.050 versus mechanical and oral antibiotic bowel preparation, same side (χ2 or Fisher’s exact test for categorical variables, Mann-Whitney U for age).
Table 2.
Operative characteristics
| Right colectomy |
Left colectomy |
|||
|---|---|---|---|---|
|
Mechanical and oral antibiotic bowel preparation
(n = 106) |
No bowel preparation
(n = 111) |
Mechanical and oral antibiotic bowel preparation
(n = 84) |
No bowel preparation
(n = 80) |
|
| Operation type | ||||
| Ileocaecal resection | 3 (3) | 2 (2) | – | – |
| Right hemicolectomy | 103 (97) | 109 (98) | – | – |
| Resection of transverse colon | – | – | 5 (6) | 1 (1) |
| Left hemicolectomy | – | – | 38 (45) | 38 (48) |
| Sigmoid resection | – | – | 37 (44) | 35 (44) |
| Anterior resection | – | – | 4 (5) | 6 (8) |
| Surgical approach | ||||
| Open | 14 (13) | 17 (15) | 7 (8) | 4 (5) |
| Laparoscopic | 83 (78) | 87 (78) | 67 (80) | 69 (86) |
| Laparoscopic converted to open | 9 (8) | 7 (6) | 10 (12) | 7 (9) |
| Timing of preoperative i.v. antibiotic before incision (min)* | 39.2(19.7)† | 39.7(28.0)‡ | 46.9(26.0) | 44.2 (26.5) |
| Duration of operation (min)* | 150.5(52.1)§ | 151.1(50.5) | 172.2(66.5) | 170.7(53.1) |
| Intraoperative blood loss (ml)* | 107.1(172.6) | 103.8(114.9)§ | 139.3(206.0) | 123.5(119.9)‡ |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). Data missing for
three,
two, and
one patients. i.v., Intravenous. There were no significant differences between treatment groups.
Right colectomy
Among patients undergoing right colectomy, the rate of SSI was similar in the MOABP and NBP groups: seven (7 per cent) and 10 (9 per cent) patients respectively (OR 0.71, 95 per cent c.i. 0.26 to 1.95; P = 0.510) (Table 3). All secondary outcomes were similar between MOABP and NBP groups. There were nine reoperations (8 per cent) in the MOABP group and six (5 per cent) in the NBP group. The reoperations were for anastomotic dehiscence in two patients, suspected anastomotic dehiscence in one, fascial dehiscence in three, intestinal ischaemia in one, ileus in one, and haemorrhage in one patient in the MOABP group, and for postoperative haemorrhage in one, fascial rupture in one, anastomotic dehiscence in two, ileus in one, and intestinal ischaemia in one patient in the NBP group. There were three readmissions (3 per cent) in MOABP group and nine (8 per cent) in the NBP group. The reasons for readmission were fever in one patient, intraluminal haemorrhage in one, and ileus in one patient in the MOABP group, and anastomotic dehiscence in one patient, abscess in two, intraluminal bleeding in two, ileus in one, abdominal pain in two, and diarrhoea in one patient in the NBP group. Two patients died in the NBP group, one (ASA grade IV, Charlson Co-morbidity Index score 6) because of vomiting and pneumonia, and another patient (ASA grade III, Charlson Co-morbidity Index score 4) because of extensive postoperative intra-abdominal bleeding, two relaparotomies, myocardial infarction, and stroke.
Table 3.
Outcomes after right colectomies
|
Mechanical and oral antibiotic bowel preparation
(n = 106) |
No bowel preparation
(n = 111) |
P # | Effect size † | |
|
| ||||
| Surgical-site infection | 7 (7) | 10 (9) | 0.510 | 0.71 (0.26, 1.95) |
| Superficial§ | 0 (0) | 3 (3) | ||
| Deep§ | 3 (3) | 3 (3) | ||
| Organ/space infection§ | 4 (4) | 4 (4) | ||
| Comprehensive Complication Index score* | 9.4(12.9) | 10.5(18.0) | 0.608** | –1.09 (–5.29, 3.11)‡ |
| Anastomotic dehiscence | 2 (2) | 2 (2) | 1.000 | 1.05 (0.15, 7.58) |
| Reoperations | 9 (8) | 6 (5) | 0.370 | 1.62 (0.56, 4.73) |
| Readmissions | 3 (3) | 9 (8)¶ | 0.086 | 0.33 (0.09, 1.24) |
| Duration of hospital stay (days)* | 5.5(5.3) | 5.4(4.8) | 0.889** | 0.10 (–1.26, 1.45)‡ |
| 30-day mortality | 0 (0) | 2 (1.8) | 0.498 | |
| 90-day mortality | 0 (0) | 2 (1.8) | ||
| Any adverse effect of antibiotics | 3 (3) | 8 (7) | 0.142 | 0.38 (0.10, 1.45) |
| Diarrhoea | 2 (2) | 7(6) | ||
| Clostridium spp. infection | 0 (0) | 1 (1) | ||
| Candida spp. infection | 1 (1) | 0 (0) | ||
| Patients receiving adjuvant treatment as a proportion of those needing such treatment | 34 of 41 (83) | 44 of 55 (80) | 0.716 | 1.21 (0.43, 3.46) |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and †values in parentheses are 95 per cent confidence intervals. Effect sizes are shown as odds ratios, except ‡mean difference.
Only the most severe type of surgical-site infection reported here.
Data missing for one patient.
χ2 or Fisher’s exact test, except
Student’s t test.
Left colectomy
Among patients undergoing left colectomy, SSI was detected in five patients (6 per cent) in the MOABP group and eight (10 per cent) in the NBP group (OR 0.57, 95 per cent c.i. 0.18 to 1.82; P = 0.338) (Table 4). All secondary outcomes were similar between MOABP and NBP groups. Reoperation was required in six patients (7 per cent) in MOABP group and six (8 per cent) in the NBP group. The reoperations were for anastomotic dehiscence in four patients, a ureter lesion in one, and intra-abdominal bleeding in one patient in the MOABP group, and for anastomotic dehiscence in four and fascial dehiscence in two patients in the NBP group. There were nine readmissions (11 per cent) in the MOABP group and three (4 per cent) in the NBP group. The readmissions were for anastomotic dehiscence in one patient, fever in one, abdominal pain in one, ileus in two, pyelonephritis in two, urinary retention in one, and diarrhoea in one patient in the MOABP group, and for abdominal pain in one patient, anastomotic dehiscence in one, and ileus in one patient in the NBP group.
Table 4.
Outcomes after left colectomies
|
Mechanical and oral antibiotic bowel preparation
(n = 84) |
No bowel preparation
(n = 80) |
P ¶ | Effect size† | |
|---|---|---|---|---|
| Surgical-site infection | 5 (6) | 8 (10) | 0.338 | 0.57 (0.18, 1.82) |
| Superficial§ | 1 (1) | 1 (1) | ||
| Deep§ | 0 | 1 (1) | ||
| Organ /space infection§ | 4 (5) | 6 (8) | ||
| Comprehensive Complication Index score* | 10.2(13.1) | 6.5(11.0) | 0.053# | 3.68 (–0.06, 7.42)‡ |
| Anastomotic dehiscence | 4 (5) | 5 (6) | 0.742 | 0.75 (0.19, 2.90) |
| Reoperations | 6 (7) | 6 (8) | 0.930 | 0.95 (0.29, 3.07) |
| Readmissions | 9 (11) | 3 (4) | 0.087 | 3.08 (0.80, 11.82) |
| Duration of hospital stay (days)* | 4.8(2.6) | 4.8(3.7) | 0.859# | –0.09 (–1.06, 0.89)‡ |
| 30-day mortality | 0 (0) | 0 (0) | ||
| 90-day mortality | 0 (0) | 0 (0) | ||
| Any adverse effect of antibiotics | 8 (10) | 5 (6) | 0.438 | 1.58 (0.49, 5.05) |
| Diarrhoea | 7 (8) | 3 (4) | ||
| Clostridium spp. infection | 0 (0) | 1 (1) | ||
| Allergic reaction | 0 (0) | 1 (1) | ||
| Candida spp. infection | 1 (1) | 0 (0) | ||
| Patients receiving adjuvant treatment as a proportion of those needing such treatment | 23 of 28 (82) | 24 of 26 (92) | 0.423 | 0.38 (0.07, 2.18) |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
values in parentheses are 95 per cent confidence intervals. Effect sizes are shown as odds ratios, except ‡mean difference. §Only the most severe type of surgical-site infection reported here.
χ2 or Fisher’s exact test, except
Student’s t test.
Discussion
This was a subgroup analysis of the MOBILE trial that compared MOABP with NBP in patients undergoing colonic surgery. The original trial did not find any difference in outcomes between the MOABP and NBP groups in the whole cohort of patients undergoing either right or left colectomy. In this subgroup study, no difference was documented between MOABP and NBP in the rate of SSI or overall postoperative morbidity in patient subgroups undergoing either right or left colectomy. Although the overall postoperative morbidity rate was very similar in MOABP and NBP groups among patients undergoing right colectomy, more postoperative complications were observed in the MOABP group among patients undergoing left colectomy, but this difference did not reach statistical significance.
The effect of MOABP versus NBP was studied in previous retrospective series in both subcohorts of patients undergoing right or left colectomy. An ACS-NSQIP database study21 suggested an association with MOABP and decreased rate of SSI in patients undergoing either right or left colectomy, but this study had serious selection bias as patients in the MOABP group were younger, more often underwent minimally invasive surgery, and were less often taking preoperative steroids, malnourished or suffering from inflammatory bowel disease. Another retrospective series24 from the same database reported outcomes of patients undergoing left colectomy only, and also reported an association between MOABP and reduction in SSI. Furthermore, a meta-analysis25 indirectly estimated that MOABP diminishes SSI compared with NBP (OR 0.6, 95 per cent c.i. 0.45 to 0.79).
The ESCP collaborating group’s multicentre prospective audit6 of left colectomies reported anastomotic leak rates of 6.1 per cent for MOABP and 8.7 per cent for NBP, which are comparable to those in the present study (MOABP 5 per cent, NBP 6 per cent). The difference in the ESCP study was statistically significant only after adjustment in a multivariable model (OR 0.52, 0.30 to 0.92; P = 0.02). Although it was a prospective cohort study, it was not a randomized trial, and the estimates suffered similarly from selection bias as in previous retrospective series.
A recent randomized trial (SELECT)26 compared selective perioperative decontamination of the digestive tract using oral colistin, tobramycin, and amphotericin B versus no oral antibiotics in patients undergoing colorectal cancer surgery. All patients undergoing left colectomy, sigmoid or anterior resection underwent mechanical bowel preparation. The SELECT trial reported that oral antibiotics reduced infectious complications (especially SSI), but not anastomotic leakage. Because mechanical bowel preparation was done before all left-sided colectomies (and before none of the procedures on the right side), the results are not comparable to those of the MOBILE trial, which compared MOABP with NBP. Furthermore, the SELECT trial did not report patients undergoing right and left colectomy separately, and it is unclear whether the reduction in SSI rates applied to both right and left colectomies.
The original MOBILE trial was criticized for including low-risk right-sided anastomoses19, and this was one of the reasons for the present subgroup analysis. In contrast to the study hypothesis, MOABP was not more effective in preventing SSI or complications in patients undergoing left colectomy, but seemed to increase complications in left colectomy as the cumulative burden of postoperative complications was higher in MOABP group; however, this finding was not statistically significant.
This study has several limitations, including those of the original trial17. The original study was powered to detect an 8 per cent absolute difference in SSIs, and the post hoc subgroup analyses of patients undergoing right or left colectomy have even less statistical power. Absolute differences of 2 per cent in SSI rate in patients undergoing right colectomy, and 4 per cent among those undergoing left colectomy were documented. These differences were not significant, but the analysis may suffer from type II error. However, as in the original trial, the overall cumulative postoperative complications are more important for the patient. The differences in these (–1.1 CCI points for right colectomy and 3.7 CCI points for left colectomy) and their 95 per cent confidence intervals (up to 7.4 CCI points) do not suggest any benefit of MOABP over NBP in either right or left-sided resections, even if studied in larger cohorts of patients. A total of 10 CCI points is considered to be clinically significant as it reflects one Clavien–Dindo grade difference in complication burden27.
The study also has several strengths. It was a multicentre trial carried out in both university and non-university hospitals, increasing the external validity of the results. The patients were on average 70 years old and nearly half had an ASA physical status grade of III–IV, indicating that a real-life mix of patients was recruited into the trial. Postoperative morbidity was collected using the most sensitive method available, the CCI.
The main finding was that MOABP did not decrease the rate of SSI or overall postoperative morbidity in patients undergoing either right or left colectomy compared with NBP. Further larger RCTs are needed in these subgroups to confirm the results.
Funding
The study was funded by Vatsatautien Tutkimussäätiö Foundation, Mary and Georg Ehrnrooth’s Foundation, the Finnish Cancer Foundation, and Helsinki University Research Funds.
Conflict of intrests
The authors declare no conflict of interest
Contributor Information
L Koskenvuo, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
T Lehtonen, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
S Koskensalo, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
S Rasilainen, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
K Klintrup, Department of Surgery, Surgical Research Unit, Medical Research Centre, Oulu University Hospital, University of Oulu, Oulu, Finland.
A Ehrlich, Department of Surgery, Central Hospital of Central Finland, Jyväskylä, Finland.
T Pinta, Department of Surgery, Seinäjoki Central Hospital, Seinäjoki, Finland.
T Scheinin, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
V Sallinen, Department of Gastroenterological Surgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
References
- 1. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337 [DOI] [PubMed] [Google Scholar]
- 2. Klinger AL, Green H, Monlezun DJ, Beck D, Kann B, Vargas HD et al. The role of bowel preparation in colorectal surgery: results of the 2012-2015 ACS-NSQIP data. Ann Surg 2019;269:671–677 [DOI] [PubMed] [Google Scholar]
- 3. Koller SE, Bauer KW, Egleston BL, Smith R, Philp MM, Ross HM et al. Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann Surg 2018;267:734–742 [DOI] [PubMed] [Google Scholar]
- 4. Kiran RP, Murray ACA, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416–425 [DOI] [PubMed] [Google Scholar]
- 5. Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg 2015;261:1034–1040 [DOI] [PubMed] [Google Scholar]
- 6. 2017 European Society of Coloproctology(ESCP) collaborating group. Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis 2018;20(Suppl 6):15–32 [DOI] [PubMed] [Google Scholar]
- 7. Fry DE. Review of the American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. JAMA Surg 2020;155:80–81. [DOI] [PubMed] [Google Scholar]
- 8. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761–784 [DOI] [PubMed] [Google Scholar]
- 9. Holubar SD, Hedrick T, Gupta R, Kellum J, Hamilton M, Gan TJ et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beyer-Berjot L, Slim K. Colorectal surgery and preoperative bowel preparation: aren't we drawing hasty conclusions? Colorectal Dis 2018;20:955–958 [DOI] [PubMed] [Google Scholar]
- 11. Battersby CLF, Hajibandeh S, Hajibandeh S. Oral antibiotics as adjunct to systemic antibiotics and mechanical bowel preparation for prevention of surgical site infections in colorectal surgery. Do we really need more trials? Dis Colon Rectum 2018;61:e341–e442 [DOI] [PubMed] [Google Scholar]
- 12. Dellinger EP. Should a scheduled colorectal operation have a mechanical bowel prep, preoperative oral antibiotics, both, or neither? Ann Surg 2015;261:1041–1043 [DOI] [PubMed] [Google Scholar]
- 13. Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K, Nishitai R et al. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label, randomized trial. Ann Surg 2016;263:1085–1091 [DOI] [PubMed] [Google Scholar]
- 14. Anjum N, Ren J, Wang G, Li G, Wu X, Dong H et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum 2017;60:1291–1298 [DOI] [PubMed] [Google Scholar]
- 15. Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum 2016;59:70–78 [DOI] [PubMed] [Google Scholar]
- 16. Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg 2019;270:43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840–848 [DOI] [PubMed] [Google Scholar]
- 18. Alverdy JC, Shogan BD. Preparing the bowel for surgery: rethinking the strategy. Nat Rev Gastroenterol Hepatol 2019;16:708–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wexner SD, Yellinek S. Is preoperative bowel preparation needed before elective colectomy? Lancet 2019;394:808–810 [DOI] [PubMed] [Google Scholar]
- 20. Alverdy JC, Hyman N. Bowel preparation under siege. Br J Surg 2019;107:167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Midura EF, Jung AD, Hanseman DJ, Dhar V, Shah SA, Rafferty JF et al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 2018;163:528–534 [DOI] [PubMed] [Google Scholar]
- 22. National Healthcare Safety Network, Centers for Disease Controland Prevention. Surgical site infection (SSI) event. 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed Jan 25, 2021).
- 23. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7 [DOI] [PubMed] [Google Scholar]
- 24. Toh JWT, Phan K, Ctercteko G, Pathma-Nathan N, El-Khoury T, Richardson A et al. The role of mechanical bowel preparation and oral antibiotics for left-sided laparoscopic and open elective restorative colorectal surgery with and without faecal diversion. Int J Colorectal Dis 2018;33:1781–1791 [DOI] [PubMed] [Google Scholar]
- 25. Toh JWT, Phan K, Hitos K, Pathma-Nathan N, El-Khoury T, Richardson AJ et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Netw Open 2018;1:e183226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn-Schepens MLM, Budding AE et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019;106:355–363 [DOI] [PubMed] [Google Scholar]
- 27. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BPL, Breitenstein S et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–762 [DOI] [PubMed] [Google Scholar]