Abstract
Background:
Macroglossia, a cardinal feature of the (epi)genetic disorder Beckwith-Wiedemann syndrome, is associated with obstructive sleep apnea, speech and/or feeding difficulties, and dental or jaw malalignment. These sequelae may be treated and/or prevented with tongue reduction surgery; the authors sought to determine whether certain Beckwith-Wiedemann syndrome patients may benefit from early surgical intervention before age 12 months.
Methods:
The authors conducted a retrospective review of patients with Beckwith-Wiedemann syndrome who underwent tongue reduction from 2014 to 2019. The authors assessed primary outcomes of change in obstructive sleep apnea by polysomnography, respiratory support required, and feeding route before and after tongue reduction, and reviewed postoperative complications and the need for repeated tongue reduction.
Results:
Of the 36 patients included, the median age at tongue reduction was 9.5 months (interquartile range, 3.8 to 22.8 months). For those with severe obstructive sleep apnea, there was a significant reduction in the obstructive apnea hypopnea index from 30.9 ± 21.8 per hour to 10.0 ± 18.3 per hour (p = 0.019) and improvement in nadir oxyhemoglobin saturation from 72 ± 10 percent to 83 ± 6 percent (p = 0.008). Although there was no significant change in overall supplemental feeding tube or respiratory support, there were specific patients who experienced clinically meaningful improvement. Of note, these positive outcomes applied equally to those who underwent surgery at a younger age (<12 months). To date, only one patient required a repeated tongue reduction.
Conclusion:
Based on improved polysomnographic findings and rarity of surgical complications or repeated surgery, the authors’ data support the safety and efficacy of this early intervention when clinical indications are present and an experienced multidisciplinary team is available for consultation.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, IV.
Beckwith-Wiedemann syndrome is an overgrowth disorder caused by an imbalance of genes controlling growth; one feature of the syndrome is macroglossia, noted in 80 to 85 percent of children.1 The specific mechanism of tongue overgrowth in Beckwith-Wiedemann syndrome is not currently known. The range in severity of macroglossia presents a challenge to standardization of treatment. In the case of mild macroglossia, multidisciplinary and conservative treatment is preferred.2–4 Accepted indications for surgical intervention include airway obstruction, feeding difficulty, language delay, dental deformities, and cosmetic concern.5
Previous literature has reported tongue reduction in Beckwith-Wiedemann syndrome patients, but with varying degrees of data in the clinical realms of respiratory, feeding, and dental outcomes. We have previously shown that children with Beckwith-Wiedemann syndrome and macroglossia are at high risk for obstructive sleep apnea, especially those younger than 6 months.6 Variable efficacy has been reported regarding treatment of obstructive sleep apnea by tongue reduction.7 Furthermore, there are no systematic and quantitative investigations examining a cohort of children with Beckwith-Wiedemann syndrome undergoing tongue reduction using preoperative and postoperative polysomnography. In the literature, feeding has been largely qualitatively assessed,2,8,9 and variable efficacy regarding speech outcomes postoperatively have been documented.3,5,10–12
The surgical methods for tongue reduction can be classified into six general categories,13 with earlier techniques focused on nonspecific debulking and more recent techniques emphasizing functional integrity.14 A meta-analysis of available reported data for tongue reduction in children with Beckwith-Wiedemann syndrome reveals central tongue reduction2,5,10–13,15–34 and anterior wedge resections10,19,20,21,26,27,35–43 are the most common techniques.
Based on current literature, the optimal timing for tongue reduction in children with Beckwith-Wiedemann syndrome has not been systematically addressed in a large detailed cohort. In general, studies recommend avoiding tongue reduction before age 6 months, stating that early intervention carries the risk for surgical complications and tongue regrowth.14,44 Some studies suggest that tongue reduction should be delayed until at least 1 year of age, ideally between 2 and 3 years.18 Others recommend early intervention to avoid progressive orofacial and dental deformities, most commonly prognathism, anterior open bite, and Angle class III malocclusion, and to restore normal facial aesthetics.17,18,33,38 Early tongue reductions have been mainly performed in cases of severe macroglossia, with the earliest reported case in the literature completed on a young girl at 12 days of age because of severe airway compromise.45 Current literature provides little evidence to support these conclusions and recommendations.
Our study investigates the benefits of early tongue reduction in Beckwith-Wiedemann syndrome patients who present with macroglossia, by systematically quantifying data from a large cohort of cases performed at a single institution. By examining preoperative and postoperative data, we have sought to determine the efficacy and safety of early tongue reduction surgery.
PATIENTS AND METHODS
Data Collection
This research was approved by the Institutional Review Board at Children’s Hospital of Philadelphia (no. 13–010658). Data were extracted from a registry database built to house clinical information for patients with Beckwith-Wiedemann syndrome. Signed consent to participate in that registry study was obtained for all participants and consents for publication of photographs were obtained.
From 2014 to 2019, 244 patients at our institution received a clinical genetics consultation for concern for classic features of Beckwith-Wiedemann syndrome. Of these, 41 (17 percent) underwent tongue reduction at Children’s Hospital of Philadelphia. Inclusion criteria for our study were as follows: patient underwent a tongue reduction performed at the physician-recommended time by a single plastic surgeon (J.A.T.), and was either followed clinically for at least 1 month postoperatively, or reached the study endpoint outcomes by hospital discharge. Endpoint outcomes were defined as requiring no supplemental tube feeding and no respiratory support. Thirty-six patients met inclusion criteria.
Characteristic and outcome data were analyzed for these 36 patients. The indication for referral and the molecular Beckwith-Wiedemann syndrome diagnoses were recorded. Postoperative complications were noted, including wound dehiscence and need for repeated surgery. Characteristic data included gestational age at birth, age at first evaluation by a plastic surgeon at Children’s Hospital of Philadelphia, time elapsed between first evaluation and surgery, age at tongue reduction (corrected to 37 weeks’ gestational age for premature infants), duration of postoperative follow-up, age at most recent follow-up, and average number of days from tongue reduction to discharge home if the patient was admitted specifically for the procedure. Tongue reduction at younger than 12 months was considered to be early, and reduction at older than 12 months was considered to be late. Preoperative and postoperative outcome data included the following: full in-laboratory diagnostic polysomnography (including the obstructive apnea hypopnea index and oxyhemoglobin saturation nadir), respiratory support needs, enteral feeding tube requirement, and plastic surgery notes detailing dental and jaw alignment. Beckwith-Wiedemann syndrome phenotypic features were analyzed to calculate clinical score based on the international consensus guidelines.46 Factors considered to potentially confound a patient’s respiratory or feeding status were recorded.
The need for respiratory support both preoperatively and postoperatively was assessed, including (1) endotracheal or tracheostomy tube to bypass upper airway obstruction, (2) supplemental oxygen, (3) noninvasive positive pressure, or (4) invasive mechanical ventilation. Dental outcomes included Angle class of occlusion, buccal inclination of teeth, anterior open bite, tongue protrusion, and other jaw deformity. Feeding outcomes included need for supplemental gastrostomy or nasogastric tube feeds.
Surgical Technique
The technique used for all procedures was a modified W-shaped pattern with keyhole. The surgeon marked the W-shaped pattern with keyhole with a marking pen (Fig. 1, above, third from left) followed by injection of local anesthesia containing epinephrine into the tongue. We use the “modified W excision with keyhole” to tailor the reduction of tongue bulk, both length and width, to fit within the borders of the lingual surface of the mandible while placing the neurovascular bundles, which course from ventrolaterally to dorsomedially, at low risk.
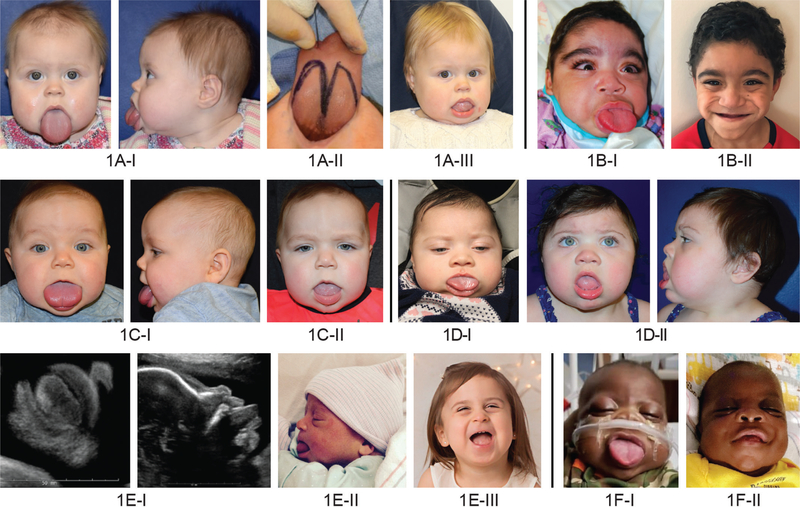
Fig. 1.
Photographs of Beckwith-Wiedemann syndrome patients presenting with macroglossia, who underwent tongue reduction surgery at Children’s Hospital of Philadelphia from 2014 to 2019. The patients shown above and second row, left and second row, second and third from left have a molecular diagnosis of paternal uniparental isodisomy. The patient shown in second row, right and second row, second and third from right has a deletion at imprinting control region 1. The patients shown below have a molecular diagnosis of loss of methylation at imprinting control region 2. Above, left, above, second from left, above, second from right; center, left and second from left, center, third from right; below, third from left, and below, second from right are before tongue reduction. Above, third from left is intraoperative. Below, left and second from left are intrauterine from a fetal ultrasound. Above, third from right, 16 months old; above, right, 5 years old; center, third from left, 10 months old; center, right and second from right, 11 months old; below, third from right, 35 months old; and below, right, 5 months old, postoperatively.
Bovie electrocautery on “cut” mode is used to cut all mucocutaneous incisions, including the keyhole. Then, Bovie electrocautery on “coagula-tion” mode is used to resect the muscularis. Next, hemostasis is checked and obtained with Bovie electrocautery. Layered closure of the tongue is performed with interrupted 4–0 Vicryl (Ethicon, Inc., Somerville, N.J.) sutures followed by a running 4–0 Vicryl suture to oversew each suture line. The average duration of tongue reduction surgery was determined for patients who had individual surgical times recorded (n = 33).
Clinical Protocols
Our standard postoperative extubation protocol included intubation for 5 to 7 days postoperatively in all patients younger than 1 year, to protect the airway during the period of intense postoperative swelling of the tongue. Patients older than 1 year were extubated earlier, generally 1 to 3 days postoperatively. For feeding, our standard immediate postoperative management includes gastrostomy feeds (nasogastric or gastrostomy tube if the patient previously had one), followed by a return to oral feeding if the patient was previously without supplemental enteral feeds, typically by 1 month postoperatively.
Statistical Analysis
Descriptive analysis for continuous variables was reported as median (interquartile range) or mean ± standard deviation. Linear regression determined whether Beckwith-Wiedemann syndrome clinical score correlated with the age at which a tongue reduction was performed. Paired t tests determined whether there was a significant difference between preoperative and postoperative obstructive sleep apnea parameters for those patients who had both preoperative and postoperative data available. If the patient had more than one postoperative evaluation, the single most recent postoperative data point was used for analysis. Fisher’s exact test determined whether there was a difference in respiratory and feeding needs preoperatively and postoperatively. STATA IC 15.1 (StataCorp, College Station, Texas) was used for all statistical analyses, and significance was set at p < 0.05.
RESULTS
For the 36 participants included, 13 (36 percent) presented to medical attention because of prenatal findings consistent with Beckwith-Wiedemann syndrome (i.e., macroglossia or omphalocele). The molecular diagnoses for the cohort showed 67 percent had the most common type of Beckwith-Wiedemann syndrome, loss of methylation at imprinting control region 2. [See Figure, Supplemental Digital Content 1, which shows characteristic data for entire cohort of tongue reduction patients with Beckwith-Wiedemann syndrome at a single institution from 2014 to 2019. (Above) Pie chart demonstrating the initial reason for genetics and plastic surgery consultations. BWS, Beckwith-Wiedemann syndrome. (Below) Pie chart demonstrating the relative prevalence of Beckwith-Wiedemann syndrome molecular diagnoses for patients in the cohort. IC2, imprinting control region 2; LOM, loss of methylation; pUPD, paternal uniparental isodisomy; IC1, imprinting control region 1; GOM, gain of methylation, http://links.lww.com/PRS/E22.] Characteristic data for the cohort are detailed in Table 1. The age at tongue reduction ranged from age 1 day, by corrected age (day of life 20), to age 51.3 months. The earliest tongue reduction was conducted at 7 days of life in a full-term infant (Fig. 1, below, third from left). Timing of tongue reduction was as follows: eight patients, younger than 3 months; three patients, age 3 to 6 months; 10 patients, age 6 to 12 months; and 15 patients, older than 12 months. [See Figure, Supplemental Digital Content 2, which shows the age at tongue reduction for patients who underwent surgery performed by a single surgeon, at a single institution, at the physician-recommended time. Histogram demonstrating the age at tongue reduction (months) corrected to 37 weeks’ gestation if premature (<37 weeks) (n = 36; median, 9.5 months; interquartile range, 3.8 to 22.8 months). TR, tongue reduction; GA, gestational age, http://links.lww.com/PRS/E23.] Length of surgery for tongue reduction (n = 33) was 36 ± 13 minutes. Length of stay after tongue reduction if the patient was admitted specifically for the procedure and was discharged to home (n = 27) was 7.8 ± 5.5 days.
Table 1.
Characteristic Data of Patients Who Underwent Tongue Reduction by a Single Surgeon, at a Single Institution, at the Physician-Recommended Time
| Characteristic | Value |
|---|---|
| No. of patients | 36 |
| Gestational age, wk | |
| Median | 35.2 |
| IQR | 31.9–38.4 |
| Length of follow-up, mo* | |
| Median | 7 |
| IQR | 2.5–26 |
| Age at first evaluation by any plastic surgeon, mo* | |
| Median | 5 |
| IQR | 1–9.5 |
| Time elapsed between first evaluation by single surgeon and tongue reduction surgery, mo* | |
| Median | 2 |
| IQR | 0–8 |
| Age at last follow-up with physician, mo | |
| Median | 27.5 |
| IQR | 13–43 |
| Age at tongue reduction, mo*† | |
| Median | 9.5 |
| IQR | 3.8–22.8 |
| BWS clinical score | |
| Median | 10 |
| IQR | 8.5–11 |
IQR, interquartile range; BWS, Beckwith-Wiedemann syndrome.
Skewness/Kurtosis test for normality demonstrated that these data points did not follow a normal distribution.
Age corrected to 37 weeks’ gestation if premature (<37 weeks).
Six patient cases representing the various Beckwith-Wiedemann syndrome molecular diagnoses are depicted with preoperative and postoperative photographs in Figure 1. The patient depicted in Figure 1, above, second from right and right underwent two additional tongue reductions following surgery at Children’s Hospital of Philadelphia, demonstrating the potential severity of macroglossia, and how a single tongue reduction may not always sufficiently manage symptoms.
The average Beckwith-Wiedemann syndrome clinical score46 for the cohort was 9.9, and there was no correlation between clinical score and age at tongue reduction by linear regression analysis for either chronologic age (R2 = 0.004; p = 0.73) or corrected-for-gestational age (R2 = 0.004; p = 0.72). The range of clinical scores was 6 to 15; a large proportion had scores of 10 or greater, demonstrating that, in general, patients requiring a tongue reduction have a more severe overall Beckwith-Wiedemann syndrome phenotype.
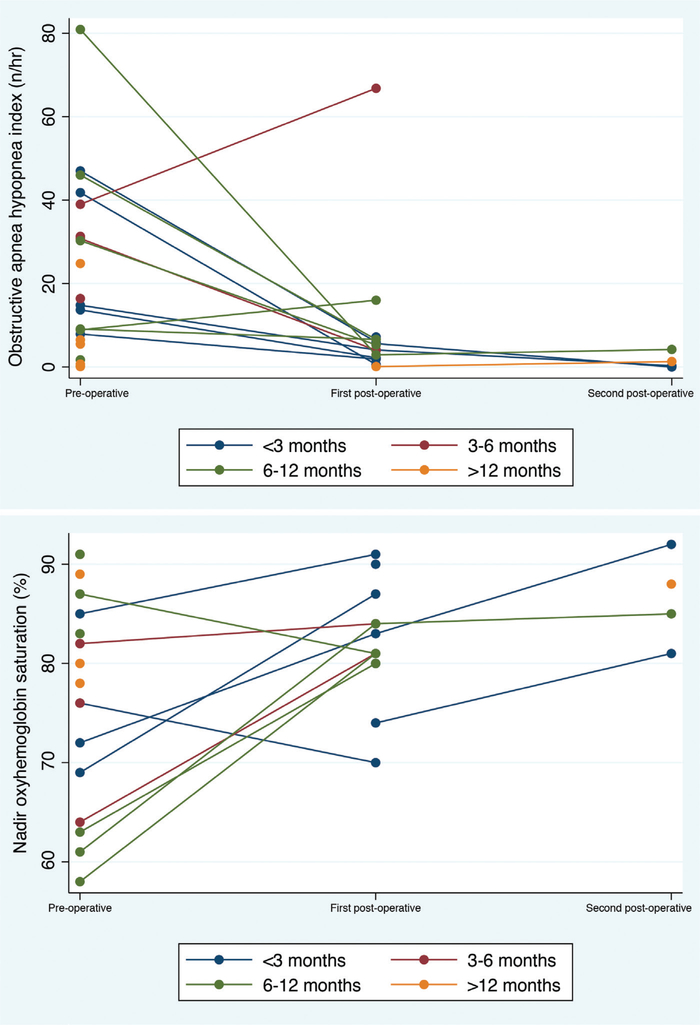
Severe obstructive sleep apnea was the main indication for early tongue reduction, and parameters improved for the majority of patients in the cohort, both in terms of obstructive apnea hypopnea index and nadir oxyhemoglobin saturation (Fig. 2). The incidence of sleep apnea in patients aged younger than 12 months was as follows: 14 patients with preoperative obstructive sleep apnea, two with a negative obstructive sleep apnea diagnosis, and five without evaluation. In those aged older than 12 months, three were diagnosed with obstructive sleep apnea, five were deemed to not have obstructive sleep apnea, and seven were without a diagnosis in either direction. For the 12 patients with available preoperative and postoperative data, there was a significant reduction in obstructive apnea hypopnea index from 30.9 ± 21.8 per hour to 10.0 ± 18.3 per hour (p = 0.019). For the 10 patients whose preoperative and postoperative nadir oxyhemoglobin saturation data were available, there was significant improvement between nadir saturation from preoperatively (72 ± 10 percent) to postoperatively (83 ± 6 percent) (p = 0.008). Interestingly, when further stratifying the early tongue reduction cohort by age at surgery, the group who underwent surgery at younger than 3 months (n = 5) experienced the greatest percentage change in their obstructive apnea hypopnea index, with a preoperative mean of 25 ± 18 per hour and a postoperative mean of 2.2 ± 2.3 per hour (p = 0.0225), reflecting an average percentage change of 89 ± 10. These data show that it is possible to perform the surgery successfully at this early age; however, a larger cohort of this age group is needed before official recommendations can be made.
Fig. 2.
Two-way connected plots representing polysomnographic reports for each individual patient at various time points related to their surgery; plots are stratified by age at tongue reduction and coded by color. (Above) Obstructive apnea hypopnea index on polysomnography. (Below) Nadir oxyhemoglobin saturation on polysomnography.
Dental and plastic surgery physical examination findings for the 36 patients preoperatively and postoperatively demonstrate an overall increase in Angle class I occlusion and decrease in the following: Angle class III occlusion, anterior open bite, and out-of-mouth tongue posture (Table 2). Of note, some patients required additional non– tongue reduction operations, including mandibular distraction (two patients) or tonsillectomy and/or adenoidectomy (six patients). One patient required the mandibular distraction before tongue reduction, the other patient after tongue reduction, and both mandibular distractions were performed after microlaryngoscopy/bronchoscopy revealed additional tongue-based upper airway obstruction. Two patients underwent tonsillectomy and/or adenoidectomy before tongue reduction; the other four patients underwent tonsillectomy and/or adenoidectomy after tongue reduction.
Table 2.
Description of Plastic Surgery Physical Examination Findings, Immediately before Tongue Reduction Compared to Postoperatively at the Patienťs Most Recent Follow-Up
| Dental Features* | Immediately Preoperatively | Most Recent Postoperative Follow-Up |
|---|---|---|
| No. of patients | 32 | 25 |
| Angle class I occlusion | 4 | 10 |
| Angle class III occlusion | 6 | 3 |
| Anterior open bite | 7 | 3† |
| Out-of-mouth tongue posture/tongue protrusion | 14 | 2 |
| Jaw deformity or retrognathia/micrognathia/prognathia or mandibular dentoalveolar protrusion | 7 | 5 |
| Tongue-tie | 2 | NA |
| Buccal inclination of teeth | 2 | 1 |
Specific dental documentation was available for only a subset of patients in the cohort.
One patient’s anterior open bite was presumed to be caused by thumb-sucking, not macroglossia.
The overall requirement for any type of respiratory support preoperatively compared to postoperatively was not statistically different (p = 0.31). However, for individual patients, the burden of respiratory support was reduced greatly postoperatively. For instance, two patients with severe obstructive sleep apnea requiring tracheostomy were decannulated following tongue reduction.
The average time to extubation immediately following surgery in the 29 non–tracheostomy-dependent participants was 5 ± 5 days. [See Figure, Supplemental Digital Content 3, which shows a histogram demonstrating when extubation occurred following tongue reduction surgery for those patients who were not preoperatively tracheostomy-dependent (n = 29; mean ± SD, 5 ± 5 days), http://links.lww.com/PRS/E24.]
In a similar manner to the respiratory support outcomes observed, the collective difference in the need for enteral feeding support showed qualitative improvement. Twenty-two patients did not require feeding support before surgery and returned to their baseline after surgery. Of the 14 patients requiring feeding support preoperatively, five patients were able to eliminate feeding support postoperatively; the others are currently weaning off of support. For both feeding and respiratory support, there are multiple cofounding variables for many of these patients—pulmonary hypoplasia secondary to giant omphalocele, chronic lung disease because of prematurity, and micrognathia or retrognathia of the jaw, which may account for their continuation of respiratory and/or feeding support postoperatively.
Postoperative complications were rare. There were no perioperative infections, and no patients required blood transfusion. One patient experienced wound dehiscence and required a return to surgery for reclosure. One other patient required additional tongue reduction operations (Fig. 1, above, second from right and above, right), and this patient represents the most severe case of macroglossia in this series. No patient developed tongue paralysis or loss of tongue sensibility.
DISCUSSION
Because of limited systematic and quantitative data in the literature regarding optimal timing and outcomes for tongue reduction in Beckwith-Wiedemann syndrome patients, our study sought to address efficacy and outcomes using measures in a number of clinically relevant domains. Hesitancy regarding early tongue reduction surgery stems from prior published literature stating that early tongue reduction may have higher surgical complication rates and may later present with regrowth of tongue tissue and thus a need for additional operations.14
In our cohort, we found the need for additional surgery to occur in only one of the 21 patients who underwent a tongue reduction at younger than 12 months. This patient interestingly harbors the molecular diagnosis of paternal uniparental isodisomy seen in 19 percent of our cohort, which may have led to greater severity of phenotype, a phenomenon noted by previous studies.47 Somers and Samson also note postoperative complications14; however, our cohort experienced only one complication in 36 patients—wound dehiscence in the setting of an unplanned extubation event that required reoperation.
Indications for early tongue reduction include the need for noninvasive or invasive respiratory support, moderate to severe obstructive sleep apnea, feeding difficulties requiring supplemental enteral tube feeds, and jaw deformation. Our data show that tongue reduction surgery is a successful intervention for diminishing or eliminating the need for invasive respiratory support to treat obstructive sleep apnea.
In patients with Beckwith-Wiedemann syndrome who have severe upper airway obstruction or other pulmonary comorbidities, tracheostomy may bypass macroglossia or provide continuous positive-pressure ventilation. Of the seven patients treated with a tracheostomy before tongue reduction, most exhibited additional clinical factors beyond macroglossia that led to a ventilation requirement. These factors included chronic lung disease, giant omphalocele, and/or an additional chromosome rearrangement. Importantly, none of the 29 remaining patients required an increase in respiratory support or a tracheostomy postoperatively. Furthermore, our data show that if the macroglossia contribution is surgically corrected, this can help lead to successful decannulation of tracheostomies, demonstrated by two patients who were successfully decannulated. This change may positively impact a patient’s and family’s quality of life. Larger studies are needed, but it appears that in the absence of significant confounding medical factors, early tongue reduction may be a viable alternative to a tracheostomy. One patient with an initial obstructive apnea hypopnea index of 80.9 per hour underwent tracheostomy but was able to be decannulated after tongue reduction with an obstructive apnea hypopnea index of only 4.2 per hour; this is a notable observation of this individual’s clinical success.
Obstructive sleep apnea is a health concern that has significant consequences in children including, but not limited to, pulmonary hypertension, cognitive deficits, behavioral abnormalities, and cardiovascular changes48 if not adequately treated.49 Children with Beckwith-Wiedemann syndrome and macroglossia are a high-risk group for obstructive sleep apnea, especially those younger than 6 months.6 Although continuous positive airway pressure is highly effective in treating obstructive sleep apnea in children, it can be challenging for some families because the device must be worn consistently and often requires desensitization. Furthermore, the additional medical issues encountered by Beckwith-Wiedemann syndrome patients such as continuous nighttime feeds in instances of hyperinsulinism can create an even greater challenge to continuous positive airway pressure use, as nighttime nursing may be required. Our large cohort of infants and young children with Beckwith-Wiedemann syndrome demonstrates significant improvement in severe obstructive sleep apnea following tongue reduction surgery. For the one patient whose obstructive sleep apnea seemingly worsened postoperatively, the result may be related to technical limitations in the preoperative polysomnogram.
Enteral supplemental tube feeding can be helpful in optimizing growth and nutrition in patients with macroglossia and Beckwith-Wiedemann syndrome; however, tube feeding can present a burden to families, and long-term dependency may disrupt development of oral feeding skills.50 Although our data do not show a quantitative, significant change in the need for feeding tube supplementation postoperatively, qualitative review of records demonstrates improvement in oral feeding skills for many of those who had difficulties preoperatively. Further systematic prospective research is required to determine how tongue reduction surgery impacts oral feeding skills, swallowing function, and expressive speech articulation.
There are conflicting reports regarding tongue reduction impacting dental outcomes and dentoalveolar development. Recent case reports show improvement in tongue protrusion,15 whereas an older study supports nonsurgical conservative management by stating that with growth over time, the oral cavity may later accommodate the macroglossia seen in Beckwith-Wiedemann syndrome patients.44 Conversely, the tongue positioning and size can adversely impact the development of the jaw and dental anatomy, and our data qualitatively demonstrate the improvement that can begin to occur as a result of surgical intervention. There are, however, some patients that remain with jaw development issues postoperatively, and the data do not demonstrate a clear correlation between the timing of tongue reduction and whether or not improvement is achieved, which may explain the previous variability and controversy reported in the literature.2,8,12,13,17,18,22–24,29,32,33,38,42,51–55 In our cohort, of those with sufficient preoperative and postoperative dental data, five patients showed improved occlusion or other dental outcomes in the early tongue reduction cohort (aged <12 months), and three patients showed improved outcomes in the delayed tongue reduction cohort (aged >12 months). Longer follow-up postoperatively is required to determine definitive improvement in jaw alignment in this patient population.
This study has several important limitations. It is a retrospective study, and thus we cannot comment on causality but rather only on associations. There may have been inherent selection biases impacting the data. Because Beckwith-Wiedemann syndrome represents a broad spectrum of disease, there are a number of confounding clinical factors and comorbidities, and we have made a significant effort to report these confounders in a transparent manner. For instance, some of the respiratory support needs of the patients in our cohort were confounded by nonmacroglossia factors including pulmonary hypoplasia secondary to giant omphalocele, chronic lung disease caused by prematurity, and micrognathia or retrognathia of the jaw. Each Beckwith-Wiedemann syndrome patient must be individually assessed because of these clinical confounding factors, as they affect and therefore contribute to the surgical decision for an individual patient. Lastly, this is an immature series with limited follow-up. As we continue to follow these patients, our understanding of the risk-to-benefit ratio of early tongue reduction may change.
CONCLUSIONS
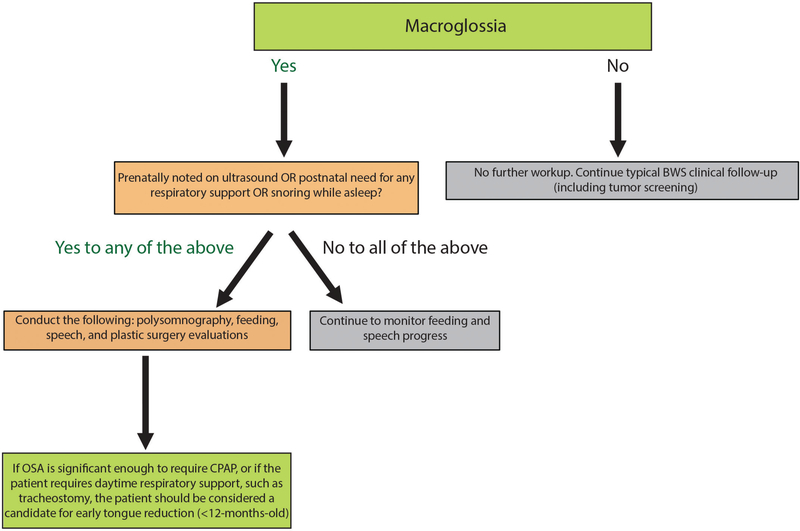
Our study systematically examined 36 patients who underwent a tongue reduction using a modified W-shaped pattern with keyhole, performed by a single surgeon, at a single institution, and demonstrated overall efficacy, especially in the domain of obstructive sleep apnea, with rare complications or need for repeated tongue reduction. Our data demonstrate that in the short-term, early tongue reduction is an effective and safe option for patients with Beckwith-Wiedemann syndrome. We propose that patients who present with severe macroglossia (determined either by prenatal presentation, significant obstructive sleep apnea in the neonatal period, or significant respiratory support requirements) should undergo formal polysomnography, feeding specialist evaluation, and plastic surgery evaluation, to determine the safety and optimal timing of tongue reduction for that patient (Fig. 3). Patients who present with prenatal macroglossia, postnatal obstructive sleep apnea requiring continuous positive airway pressure, or daytime respiratory support requirements such as tracheostomy, would likely benefit from tongue reduction surgery before age 12 months. Therefore, we recommend that any patient with Beckwith-Wiedemann syndrome and severe macroglossia undergo multidisciplinary evaluation for tongue reduction at the initial notion of respiratory distress based on objective data. The timing of the procedure should be based on symptomatology rather than the patient’s chronologic age. It is imperative, however, that an experienced multidisciplinary team of clinicians evaluate these patients before pursuing surgical intervention and that the patient has been deemed medically stable from all other perspectives. This algorithm will help ensure that proper evaluations are conducted to determine the severity and thus optimal timing of surgical intervention for each individual patient.
Fig. 3.
Proposed algorithm for clinical management of Beckwith-Wiedemann syndrome patients with macroglossia, and determination of the need for surgical intervention. BWS, Beckwith-Wiedemann syndrome; OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure.
Supplementary Material
ACKNOWLEDGMENTS
This research was approved by the Institutional Review Board at Children’s Hospital of Philadelphia (13–010658), and consent to participate in the Beckwith-Wiedemann syndrome registry at Children’s Hospital of Philadelphia was obtained from all participants and/or their guardians. Photography consents for publication were obtained where applicable. The authors would like to thank the patients and their families for their participation in this research and the clinicians who helped care for these patients.
Footnotes
Disclosure: Dr. Kalish is supported by Alex’s Lemonade Stand Foundation, St. Baldrick’s Foundation, and National Institutes of Health grant K08 CA193915. Dr. Cielo is supported by National Institutes of Health grant K23 HL135346 and a Francis Family Foundation fellowship award. Dr. Cohen was a one-time consultant for Sobi, Inc., on hereditary tyrosinemia type I (unrelated to the work in this article). All other authors have no financial disclosures to report.
PATIENT CONSENT
Parents or guardians provided written consent for use of patients’ images.
REFERENCES
- 1.Maas SM, Vansenne F, Kadouch DJ, et al. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A 2016;170:2248–2260. [DOI] [PubMed] [Google Scholar]
- 2.Naujokat H, Möller B, Terheyden H, et al. Tongue reduction in Beckwith-Wiedemann syndrome: Outcome and treatment algorithm. Int J Oral Maxillofac Surg. 2019;48:9–16. [DOI] [PubMed] [Google Scholar]
- 3.Kadouch DJ, Maas SM, Dubois L, van der Horst CM. Surgical treatment of macroglossia in patients with Beckwith-Wiedemann syndrome: A 20-year experience and review of the literature. Int J Oral Maxillofac Surg. 2012;41:300–308. [DOI] [PubMed] [Google Scholar]
- 4.Heggie AA, Vujcich NJ, Portnof JE, Morgan AT. Tongue reduction for macroglossia in Beckwith Wiedemann syndrome: Review and application of new technique. Int J Oral Maxillofac Surg. 2013;42:185–191. [DOI] [PubMed] [Google Scholar]
- 5.Hettinger PC, Denny AD. Double stellate tongue reduction: A new method of treatment for macroglossia in patients with Beckwith-Wiedemann syndrome. Ann Plast Surg. 2011;67:240–244. [DOI] [PubMed] [Google Scholar]
- 6.Cielo CM, Duffy KA, Taylor JA, Marcus CL, Kalish JM. Obstructive sleep apnea in children with Beckwith-Wiedemann syndrome. J Clin Sleep Med. 2019;15:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cielo CM, Duffy KA, Vyas A, Taylor JA, Kalish JM. Obstructive sleep apnoea and the role of tongue reduction surgery in children with Beckwith-Wiedemann syndrome. Paediatr Respir Rev. 2018;25:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto K, Morita K, Jinno S, Omura K. Sensory changes after tongue reduction for macroglossia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e1–e2. [DOI] [PubMed] [Google Scholar]
- 9.Davalbhakta A, Lamberty BG. Technique for uniform reduction of macroglossia. Br J Plast Surg. 2000;53:294–297. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson JK, Morse SA, Bernard SP, Greensmith AL, Meara JG. Long-term outcomes of surgical tongue reduction in Beckwith-Wiedemann syndrome. Plast Reconstr Surg. 2007;119:992–1002. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz M, Mercan H, Karaman E, Kaytaz A. Tongue reduction in Beckwith-Wiedemann syndrome with CO(2) laser. J Craniofac Surg. 2009;20:1202–1203. [DOI] [PubMed] [Google Scholar]
- 12.Abeleira MT, Seoane-Romero JM, Outumuro M, Caamaño F, Suárez D, Carmona IT. A multidisciplinary approach to the treatment of oral manifestations associated with Beckwith-Wiedemann syndrome: A long-term case report. J Am Dent Assoc. 2011;142:1357–1364. [DOI] [PubMed] [Google Scholar]
- 13.Dos Santos VBD, de Assis GM, da Silva JSP, Germano AR. Partial glossectomy in a patient carrier of Beckwith-Wiedemann syndrome: Presentation of a case (in English). Rev Española Cir Oral Maxilofac. 2015;37:202–206. [Google Scholar]
- 14.Somers EH, Samson TD. Keyhole tongue reduction. Oper Tech Otol Head Neck Surg. 2015;26:127–130. [Google Scholar]
- 15.Harada T, Yamanishi T, Kurimoto T, Nishio J. Improved quality of life for children with Beckwith-Wiedemann syndrome following tongue reduction surgery. J Craniofac Surg. 2019;30:163–166. [DOI] [PubMed] [Google Scholar]
- 16.Roa Rojas P, Arango Fernández H, Rebolledo Cobos M, Harris Ricardo J. Surgical treatment of macroglossia in Beckwith-Wiedemann syndrome: Case report (in Spanish). Arch Argent Pediatr. 2018;116:e341–e345. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Rodriguez E, Gómez E, Martín M, Muñoz JM, Hernández-Godoy J, Burgueño M. Beckwith-Wiedemann syndrome: Open bite evolution after tongue reduction. Med Oral Patol Oral Cir Bucal. 2018;23:e225–e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda H, Tamura H, Tonoki M. Efficacy and optimal timing of tongue reduction surgery in three patients with Beckwith-Wiedemann syndrome. J Oral Maxillofac Surg Med Pathol. 2017;29:358–362. [Google Scholar]
- 19.Kamata S, Kamiyama M, Sawai T, et al. Assessment of obstructive apnea by using polysomnography and surgical treatment in patients with Beckwith-Wiedemann syndrome. J Pediatr Surg. 2005;40:E17–E19. [DOI] [PubMed] [Google Scholar]
- 20.Maturo SC, Mair EA. Submucosal minimally invasive lingual excision: An effective, novel surgery for pediatric tongue base reduction. Ann Otol Rhinol Laryngol. 2006;115:624–630. [DOI] [PubMed] [Google Scholar]
- 21.Kittur MA, Padgett J, Drake D. Management of macroglossia in Beckwith-Wiedemann syndrome. Br J Oral Maxillofac Surg. 2013;51:e6–e8. [DOI] [PubMed] [Google Scholar]
- 22.Choi JW, Kim HJ, Park HS, Kwon TG. Congenital macroglossia treated by 2-stage partial glossectomy. J Craniofac Surg. 2013;24:554–556. [DOI] [PubMed] [Google Scholar]
- 23.Kwon TG, Lee SH, Ryoo HM, Kim HJ, Park HS, Kim JB. Partial glossectomy for asymmetric tongue enlargement in Beckwith-Wiedemann syndrome. Asian J Oral Maxillofac Surg. 2005;17:190–194. [Google Scholar]
- 24.Van Lierde KM, Mortier G, Huysman E, Vermeersch H. Long-term impact of tongue reduction on speech intelligibility, articulation and oromyofunctional behaviour in a child with Beckwith-Wiedemann syndrome. Int J Pediatr Otorhinolaryngol. 2010;74:309–318. [DOI] [PubMed] [Google Scholar]
- 25.Van Lierde K, Galiwango G, Hodges A, Bettens K, Luyten A, Vermeersch H. Impact of tongue reduction on overall speech intelligibility, articulation and oromyofunctional behavior in 4 children with Beckwith-Wiedemann syndrome. Folia Phoniatr Logop. 2012;64:55–63. [DOI] [PubMed] [Google Scholar]
- 26.Van Borsel J, Van Snick K, Leroy J. Macroglossia and speech in Beckwith-Wiedemann syndrome: A sample survey study. Int J Lang Commun Disord. 1999;34:209–221. [DOI] [PubMed] [Google Scholar]
- 27.Shipster C, Morgan A, Dunaway D. Psychosocial, feeding, and drooling outcomes in children with Beckwith Wiedemann syndrome following tongue reduction surgery. Cleft Palate Craniofac J. 2012;49:e25–e34. [DOI] [PubMed] [Google Scholar]
- 28.Matsune K, Miyoshi K, Kosaki R, Ohashi H, Maeda T. Taste after reduction of the tongue in Beckwith-Wiedemann syndrome. Br J Oral Maxillofac Surg. 2006;44:49–51. [DOI] [PubMed] [Google Scholar]
- 29.Clauser L, Tieghi R, Polito J. Treatment of macroglossia in Beckwith-Wiedemann syndrome. J Craniofac Surg. 2006;17:369–372. [DOI] [PubMed] [Google Scholar]
- 30.Kacker A, Honrado C, Martin D, Ward R. Tongue reduction in Beckwith-Wiedemann syndrome. Int J Pediatr Otorhinolaryngol. 2000;53:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Dios PD, Posse JL, Sanromán JF, García EV. Treatment of macroglossia in a child with Beckwith-Wiedemann syndrome. J Oral Maxillofac Surg. 2000;58:1058–1061. [DOI] [PubMed] [Google Scholar]
- 32.Menard RM, Delaire J, Schendel SA. Treatment of the craniofacial complications of Beckwith-Wiedemann syndrome. Plast Reconstr Surg. 1995;96:27–33. [DOI] [PubMed] [Google Scholar]
- 33.Vasquez MP, Vacher C, Buis J, Schneid H, Martinez H, Le Bouc Y. Macroglossie congenitale et syndrome de Wiedemann-Beckwith: A propos de trente et une observations. Ann Pediatr (Paris) 1994;41:303–315. [Google Scholar]
- 34.Mixter RC, Ewanowski SJ, Carson LV. Central tongue reduction for macroglossia. Plast Reconstr Surg. 1993;91:1159–1162. [DOI] [PubMed] [Google Scholar]
- 35.Batra M, Valecha UK. Anesthetic management of tongue reduction in a case of Beckwith-Wiedemann syndrome. J Anaesthesiol Clin Pharmacol. 2014;30:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balaji SM. Reduction glossectomy for large tongues. Ann Maxillofac Surg. 2013;3:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas SM, Kadouch DJ, Masselink AC, Van Der Horst CM. Taste and speech following surgical tongue reduction in children with Beckwith-Wiedemann syndrome. J Craniomaxillofac Surg. 2016;44:659–663. [DOI] [PubMed] [Google Scholar]
- 38.Giancotti A, Romanini G, Di Girolamo R, Arcuri C. A less-invasive approach with orthodontic treatment in Beckwith-Wiedemann patients. Orthod Craniofac Res. 2002;5:59–63. [DOI] [PubMed] [Google Scholar]
- 39.Rimell FL, Shapiro AM, Shoemaker DL, Kenna MA. Head and neck manifestations of Beckwith-Wiedemann syndrome. Otolaryngol Head Neck Surg. 1995;113:262–265. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui A, Pensler JM. The efficacy of tongue resection in treatment of symptomatic macroglossia in the child. Ann Plast Surg. 1990;25:14–17. [DOI] [PubMed] [Google Scholar]
- 41.Patterson GT, Ramasastry SS, Davis JU. Macroglossia and ankyloglossia in Beckwith-Wiedemann syndrome. Oral Surg Oral Med Oral Pathol. 1988;65:29–31. [DOI] [PubMed] [Google Scholar]
- 42.Kveim M, Fisher JC, Jones KL, Gruer B. Early tongue resection for Beckwith-Wiedemann macroglossia. Ann Plast Surg. 1985;14:142–144. [DOI] [PubMed] [Google Scholar]
- 43.Shafer AD. Primary macroglossia. Clin Pediatr (Phila.) 1968;7:357–363. [DOI] [PubMed] [Google Scholar]
- 44.Friede H, Figueroa AA. The Beckwith-Wiedemann syndrome: A longitudinal study of the macroglossia and dentofacial complex. J Craniofac Genet Dev Biol Suppl. 1985;1:179–187. [PubMed] [Google Scholar]
- 45.Boku A, Tachibana K, Shinjo T, Hanamoto H, Takeuchi M, Kinouchi K. Perioperative management of tongue reduction surgery for macroglossia associated with Beckwith-Wiedemann syndrome: A retrospective evaluation of 14 patients (in Japanese). Masui 2013;62:416–420. [PubMed] [Google Scholar]
- 46.Brioude F, Kalish JM, Mussa A, et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat Rev Endocrinol. 2018;14:229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith AC, Shuman C, Chitayat D, et al. Severe presentation of Beckwith-Wiedemann syndrome associated with high levels of constitutional paternal uniparental disomy for chromosome 11p15. Am J Med Genet A 2007;143:3010–3015. [DOI] [PubMed] [Google Scholar]
- 48.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714–e755. [DOI] [PubMed] [Google Scholar]
- 49.McNicholas WT. Obstructive sleep apnoea and comorbidity: An overview of the association and impact of continuous positive airway pressure therapy. Expert Rev Respir Med. 2019;13:251–261. [DOI] [PubMed] [Google Scholar]
- 50.Krom H, de Winter JP, Kindermann A. Development, prevention, and treatment of feeding tube dependency. Eur J Pediatr. 2017;176:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hikita R, Kobayashi Y, Tsuji M, Kawamoto T, Moriyama K. Long-term orthodontic and surgical treatment and stability of a patient with Beckwith-Wiedemann syndrome. Am J Orthod Dentofacial Orthop. 2014;145:672–684. [DOI] [PubMed] [Google Scholar]
- 52.Kitamura R, Nishio J, Miyazaki T. A case report of Beckwith-Wiedemann syndrome. Ped Oral Maxillofac Surg. 1991;1:3–6. [Google Scholar]
- 53.Beltran J, Padwa BL, Ferraro N, August M. Effect of partial glossectomy on the dentofacial development of patients with Beckwith-Wiedemann syndrome. J Oral Maxillofac Surg. 2003;61(Suppl):63. [Google Scholar]
- 54.Miyawaki S, Oya S, Noguchi H, Takano-Yamamoto T. Long-term changes in dentoskeletal pattern in a case with Beckwith-Wiedemann syndrome following tongue reduction and orthodontic treatment. Angle Orthod. 2000;70:326–331. [DOI] [PubMed] [Google Scholar]
- 55.Okubu A, Arimura K, Kamikawa Y, Himeno N, Yamashita S. A case of Beckwith-Wiedemann syndrome undergoing tongue reduction. Jpn J Oral Maxillofac Surg. 1994;40:200–202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.